Abstract
Background:
Antibiotics are among the most commonly prescribed medications for children; however, at least one-third of pediatric antibiotic prescriptions are unnecessary. National data on short-term antibiotic-related harms could inform efforts to reduce overprescribing and supplement interventions that focus on long-term benefits of reducing antibiotic resistance.
Methods:
Frequencies and rates of emergency department (ED) visits for antibiotic adverse drug events (ADEs) in children were estimated using adverse event data from the National Electronic Injury Surveillance System–Cooperative Adverse Drug Event Surveillance project and retail pharmacy dispensing data from QuintilesIMS (2011-2015).
Results:
Based on 6,542 surveillance cases, an estimated 69,464 ED visits (95% confidence interval: 53,488-85,441) were made annually for antibiotic ADEs among children aged ≤19 years from 2011-2015, accounting for 46.2% of ED visits for ADEs from systemic medications. Two-fifths (40.7%) of ED visits for antibiotic ADEs involved children aged ≤2 years and 86.1% involved allergic reactions. Amoxicillin was the most commonly implicated antibiotic among children aged ≤9 years. Accounting for dispensed prescriptions, rates of ED visits for antibiotic ADEs declined with increasing age for all antibiotics except sulfamethoxazole/trimethoprim. Amoxicillin had the highest rate of ED visits for antibiotic ADEs among children aged ≤2 years, whereas sulfamethoxazole/trimethoprim had the highest rate among children aged 10-19 years (29.9 and 24.2 ED visits per 10,000 dispensed prescriptions, respectively).
Conclusions:
Antibiotic ADEs lead to many ED visits, particularly among young children. Communicating risks of antibiotic ADEs could help reduce unnecessary prescribing. Prevention efforts could target pediatric patients with greatest risks of harm.
Keywords: antibiotics, antibiotic resistance, adverse drug event, allergic reaction, medication safety
Article Summary:
Nearly 70,000 estimated pediatric ED visits were made annually for antibiotic ADEs. Young children had the highest numbers and rates of ED visits for antibiotic ADEs. Interventions could be targeted to the pediatric patients with the greatest risk of ADEs.
Antibiotics are among the most commonly prescribed medications for children in the United States [1]. In 2011, 889 antibiotic prescriptions were dispensed from retail pharmacies for every 1,000 children aged ≤19 years, accounting for nearly 74 million prescriptions [2]. Antibiotic use drives development of antibiotic resistance, which is considered a major public health threat worldwide [3]. Antibiotic use also carries the risk of harm to individual patients. Antibiotic-related harms (ranging from mild gastrointestinal disturbances to life-threatening anaphylactic reactions) are a common cause of outpatient clinic visits and are the leading cause of emergency department (ED) visits for adverse drug events (ADEs) among children in the United States [4–6].
Recent efforts to reduce antibiotic resistance have largely focused on reducing inappropriate prescribing [7–9], and national clinical guidelines have been updated accordingly [10–12]. Although clinicians generally concur and are familiar with guideline recommendations [13], national data from 2010-2011 indicate that at least 29% of outpatient pediatric antibiotic prescriptions were unnecessary, and more were likely inappropriate in antibiotic selection, dosing, or duration of therapy [14]. Continued inappropriate prescribing has been attributed to factors such as perceived parent/caregiver expectation for antibiotics, and concern for parent/patient satisfaction [13, 15, 16]. Long-term societal risks of antibiotic resistance are also not prioritized in clinician decisions about prescribing or in parent/patient considerations about treatment [13, 17–19]. Data on the short-term individual risks of antibiotic ADEs could help clinicians, as well as parents/caregivers, weigh the risks and benefits of antibiotic treatment [20, 21].
We used nationally-representative public health surveillance data to (1) identify the antibiotics with the highest frequencies and rates of ED visits for ADEs and (2) to identify the pediatric patients with the highest risks in order to help inform and target prevention efforts.
METHODS
Data Sources
National estimates of ED visits for antibiotic ADEs were based on data from the National Electronic Injury Surveillance System-Cooperative Adverse Drug Event Surveillance (NEISS-CADES) project, a joint collaboration of the Centers for Disease Control and Prevention (CDC), the U.S. Consumer Product Safety Commission, and the U.S. Food and Drug Administration. NEISS-CADES is based on a nationally-representative, stratified probability sample of hospitals in the United States and its territories with at least 6 beds and a 24-hour ED, with 4 strata based on hospital size and 1 pediatric hospital stratum. From 2011-2015, the number of hospitals participating in NEISS-CADES ranged from 55-62, depending on the year.
As previously described, trained data abstractors at participating hospitals review all ED medical records to identify clinician-diagnosed ADEs and up to two medications implicated in each ADE [22, 23]. Abstractors then record patient demographics, clinical diagnoses, and narrative descriptions of the event, including precipitating circumstances (e.g., medication errors), clinical manifestations, treatments administered, and ED disposition. Narrative descriptions are then coded using the Medical Dictionary for Regulatory Activities (MedDRA), version 9.1.
National estimates of oral antibiotic prescriptions dispensed from outpatient retail pharmacies were based on data from the QuintilesIMS National Prescription Audit (NPA). The NPA includes new and refilled prescriptions from nearly 48,000 non-federal retail pharmacies across the United States, representing approximately 80% of retail prescription activity from pharmacies and food/mass merchandise stores. QuintilesIMS projects national estimates using proprietary methods.
Definitions
Cases included ED visits from 2011-2015 that the treating clinician attributed to use of systemic antibiotics by children aged ≤19 years. Systemic antibiotics, hereafter “antibiotics”, included oral or injectable formulations and excluded topical, ophthalmic, and otic formulations. ED visits for ADEs involving all other systemic medications were used for comparison. ADEs were classified as allergic reactions (immunologically-mediated effects, including severe hypersensitivity reactions such as Stevens-Johnson syndrome), adverse effects (undesirable pharmacologic or idiosyncratic effects at recommended doses), effects of excess dose, or other effects (e.g., injection site reactions, choking). Adverse event manifestations were categorized in a mutually exclusive and hierarchical manner based on severity (e.g., a case involving angioedema and mild nausea would be classified as a moderate-to-severe allergic reaction based on the angioedema). Hospitalizations included inpatient admission, observation admission or transfer to another facility. Cases in which a child aged ≤10 years accessed medication without caregiver oversight were excluded from analysis. Cases of drug therapeutic failures, non-adherence, substance use disorders, intentional self-harm, occupational exposures, and ADEs from treatments received in the ED were not included. Deaths occurring in or en route to the ED were also not included.
Statistical Analysis
Cases collected from the NEISS-CADES hospital EDs are weighted in order to calculate national estimates. Weights are assigned to each case based on the inverse probability of selection, adjusted for nonresponse and post-stratified to adjust for changes in the number of annual hospital ED visits [24]. National estimates of ED visits and corresponding 95% confidence intervals (CIs) were calculated using the SURVEYMEANS procedure in SAS, version 9.3 (SAS Institute) to account for sample weights and sample design. National estimates were annualized by dividing total estimates for the 5-year period by five. Estimates based on <20 cases or total estimates <1,200 are considered statistically unreliable and are not shown. Estimates with coefficients of variation greater than 30% may be statistically unreliable and are noted. Population-based rates were calculated by dividing the ED visit estimate for each age group (from NEISS-CADES) by the corresponding bridged-race population estimates from the U.S. Census Bureau [25]. Population estimates were considered free of sampling error.
Estimated numbers of dispensed retail prescriptions for oral antibiotics (QuintilesIMS NPA) were used to calculate rates of ED visits for ADEs from oral antibiotics relative to outpatient antibiotic use and number needed to harm (NNH). Rates were also estimated for specific drug products and patient age groups. Accompanying 95% CIs for rate estimates were calculated incorporating the variance of the numerator estimates of ED visits; because of the large sample size (approximately 3.5 billion dispensed prescriptions annually), the variance of QuintilesIMS estimates was considered to be negligible.
RESULTS
Based on 6,542 surveillance cases, an estimated 69,464 ED visits (95% CI: 53,488-85,441) were made annually in the United States for ADEs from antibiotics among children aged ≤19 years from 2011-2015 (Table 1). ED visits for antibiotic ADEs accounted for 46.2% (95% CI: 43.2%-49.1%) of all ED visits for ADEs from systemic medications in this age group. Among children aged ≤2 years, antibiotics were implicated in nearly two-thirds (63.9%; 95% CI: 60.0%-67.8%) of ED visits for ADEs from systemic medications, whereas among children aged 10-19 years, antibiotics were implicated in one-third (32.4%; 95% CI: 29.7%-35.2%) of ED visits for ADEs. Just over one-half (53.7%) of ED visits for antibiotic ADEs involved females, and 3.0% of ED visits for antibiotic ADEs resulted in hospitalization.
Table 1.
Emergency Department (ED) Visits for Adverse Drug Events (ADEs) from Systemic Medications, Children ≤19 Years, United States, 2011-2015a
| Patient and Case Characteristics | ED Visits for ADEs Involving Antibiotics | ED Visits for ADEs Involving Other Medicationsb | ||||
|---|---|---|---|---|---|---|
| Cases | Annual National Estimate | Cases | Annual National Estimate | |||
| No. | No. | % (95% CI) | No. | No. | % (95% CI) | |
| Age (Years) | ||||||
| <1-2 | 2,870 | 28,240 | 40.7 (38.5 - 42.8) | 1,637 | 15,959 | 19.7 (16.1 - 23.3) |
| 3-4 | 743 | 7,118 | 10.2 (8.9 - 11.6) | 889 | 7,101 | 8.8 (7.8 - 9.7) |
| 5-9 | 1,187 | 12,356 | 17.8 (16.0 - 19.5) | 1,535 | 12,662 | 15.6 (14.0 - 17.2) |
| 10-19 | 1,742 | 21,751 | 31.3 (29.2 - 33.4) | 4,476 | 45,329 | 55.9 (51.6 - 60.3) |
| Sex | ||||||
| Female | 3,411 | 37,292 | 53.7 (52.0 - 55.4) | 4,303 | 42,139 | 52.0 (50.2 - 53.8) |
| Male | 3,131 | 32,172 | 46.3 (44.6 - 48.0) | 4,234 | 38,911 | 48.0 (46.2 - 49.8) |
| Type of ADE | ||||||
| Allergic reaction | 5,763 | 59,776 | 86.1 (82.3 - 89.8) | 1,769 | 20,797 | 25.7 (22.8 - 28.5) |
| Adverse effect | 693 | 8,798 | 12.7 (9.0 - 16.4) | 3,176 | 23,269 | 28.7 (24.1 - 33.3) |
| Effects of excess dose | 37 | 373 | 0.5 (0.3 - 0.8) | 1,793 | 17,510 | 21.6 (19.2 - 24.0) |
| Other effectc | 49 | 517 | 0.7 (0.4 - 1.1) | 1,799 | 19,473 | 24.0 (20.0 - 28.0) |
| Documented Medication Errord | ||||||
| Yes | 63 | 651 | 0.9 (0.5 - 1.3) | 1,434 | 13,896 | 17.1 (14.4 - 19.9) |
| No | 6,479 | 68,813 | 99.1 (98.7 - 99.5) | 7,103 | 67,154 | 82.9 (80.1 - 85.6) |
| No. of Implicated Medications | ||||||
| 1 | 6,156 | 65,526 | 94.3 (93.3 - 95.3) | 7,393 | 72,609 | 89.6 (87.9 - 91.3) |
| 2 or more | 386 | 3,938 | 5.7 (4.7 - 6.7) | 1,144 | 8,440 | 10.4 (8.7 - 12.1) |
| Dispositione | ||||||
| Admitted, transferred, or held for observation | 265 | 2,056 | 3.0 (2.1 - 3.8) | 1,728 | 8,466 | 10.4 (6.1 - 14.8) |
| Treated/released or left against medical advice | 6,277 | 67,408 | 97.0 (96.2 - 97.9) | 6,808 | 72,580 | 89.5 (85.2 - 93.9) |
| Total | 6,542 | 69,464 | 100.0 | 8,537 | 81,050 | 100.0 |
Estimates of ED visits for ADEs based on the National Electronic Injury Surveillance System-Cooperative Adverse Drug Event Surveillance project (2011-2015). Data exclude cases of unsupervised ingestion in which children aged ≤10 years accessed medications without caregiver oversight. N/A=not applicable.
Includes prescription and over-the-counter medications, dietary supplements, homeopathic products, and vaccines that were administered by oral, sublingual, injectable, rectal, or transdermal routes.
Examples of other effects include injection site reactions, choking, and vaccination reactions.
Refers to errors in drug prescribing, dispensing or administration, including administration of another individual’s prescription medication, or accidental needle stick in individuals aged >10 years.
Missing for 1 case attributed to other medications.
Compared with ED visits for ADEs from other systemic medications, ED visits attributed to antibiotic ADEs more commonly involved children aged ≤2 years (40.7% [95% CI: 38.5%-42.8%] vs. 19.7% [95% CI: 16.1%-23.3%]) and more commonly involved allergic reactions (86.1% [95% CI: 82.3%-89.8%] vs. 25.7% [95% CI: 22.8%-28.5%]). Accounting for population, ED visits for antibiotic ADEs disproportionately involved young children. The population rate of ED visits for antibiotic ADEs was 4 times higher for children aged ≤2 years compared with children aged 10-19 years (23.8 ED visits per 10,000 population [95% CI: 17.8-29.8] vs. 5.2 ED visits per 10,000 population [95% CI: 4.0-6.4]).
In an estimated 95.9% (95% CI: 95.0%-96.7%) of pediatric ED visits for antibiotic ADEs, a single class of oral antibiotics was implicated in the ADE; few visits (2.2%) involved two antibiotics from different classes (Table 2). Only 1.9% of ED visits for antibiotic ADEs were attributed to only injectable antibiotics. Oral penicillins alone were implicated in an estimated 38,680 (95% CI: 30,311-47,048) ED visits annually, accounting for 55.7% of ED visits for antibiotic ADEs. The next most frequently implicated classes were oral cephalosporins (11.9%) and sulfonamides (11.1%) alone. Overall, oral sulfonamides and clindamycin had the highest rates of ED visits for antibiotic ADEs after accounting for estimates of prescriptions from retail pharmacies (18.0 ED visits and 16.6 ED visits per 10,000 dispensed prescriptions, respectively).
Table 2.
Emergency Department (ED) Visits for Adverse Drug Events (ADEs) from Antibiotics, by Drug Class, Children ≤19 Years, United States, 2011-2015a
| Antibiotic Drug Class | Cases | Annual National Estimate | ||
|---|---|---|---|---|
| ED Visits for Antibiotic ADEs | Rate per 10,000 Dispensed Prescriptionsb | |||
| No. | No. | % (95% CI) | Rate (95% CI) | |
| Oral Antibioticsc | 6,406 | 68,118 | 98.1 (97.4 - 98.7) | N/A |
| Penicillinsd | 3,904 | 38,680 | 55.7 (53.3 - 58.1) | 12.1 (9.5 - 14.8) |
| Cephalosporins | 733 | 8,260 | 11.9 (10.2 - 13.6) | 6.8 (4.6 - 9.0) |
| Sulfonamides | 623 | 7,707 | 11.1 (9.0 - 13.2) | 18.0 (13.3 - 22.7) |
| Macrolides | 378 | 5,249 | 7.6 (6.4 - 8.7) | 3.9 (2.8 - 5.0) |
| Lincomycins (Clindamycin) | 186 | 1,820 | 2.6 (2.2 - 3.1) | 16.6 (12.2 - 21.1) |
| Tetracyclines | 136 | 1,524 | 2.2 (1.6 - 2.8) | 3.9 (2.3 - 5.4) |
| Quinolones | 67 | 971 | 1.4 (0.9 - 1.9) | 13.2 (8.0 - 18.4) |
| Other or Unspecified Antibiotics | 220 | 2,390 | 3.4 (2.7 - 4.2) | N/A |
| Two Antibiotics from Different Classes | 159 | 1,518 | 2.2 (1.7 - 2.7) | N/A |
| Injectable Antibioticse | 136 | 1,346 | 1.9 (1.3 - 2.6) | N/A |
| Total | 6,542 | 69,464 | 100.0 | N/A |
Estimates of ED visits for ADEs based on the National Electronic Injury Surveillance System-Cooperative Adverse Drug Event Surveillance project (2011-2015); estimates of dispensed oral prescriptions from retail pharmacies based on the National Prescription Audit from QuintilesIMS (2011-2015). Data for specific drug classes represent only ED visits in which antibiotics from that single drug class was implicated. Data exclude cases of unsupervised ingestion in which children aged ≤10 years accessed medications without caregiver oversight. N/A=not applicable.
Rate estimates only calculated for ED visits attributed to a single, specified class of oral antibiotics. Rates were not calculated for ED visits attributed to injectable antibiotics, since the QuintilesIMS data on numbers of dispensed outpatient retail prescriptions does not reliably estimate use of injectable antibiotics, which are often administered in a clinical setting.
Includes 7 cases in which an oral antibiotic and injectable antibiotic from the same drug class were both implicated.
Includes penicillins in combination with beta-lactamase inhibitors (e.g., amoxicillin in combination with clavulanate).
Includes injectable cephalosporins (n=55 cases), penicillins (n=52 cases), other antibiotics (n=25 cases), and two injectable antibiotics from different classes (n=4 cases).
Mild allergic reactions (e.g., rash, pruritus) were the most common manifestation of antibiotic ADEs, ranging from half of visits involving oral quinolones alone (51.4%; 95% CI: 36.1%-66.7%) to four-fifths of visits involving penicillins alone (81.0%; 95% CI: 77.0%-84.9%) and sulfonamides alone (80.9%; 95% CI: 76.7%-85.1%) (Supplementary Table). Moderate-to-severe allergic reactions (e.g., anaphylaxis, angioedema) were documented in 30.0% (95% CI: 16.9%-43.1%) of ED visits for ADEs from oral quinolones alone and 19.8% (95% CI: 11.2%-28.3%) of visits from tetracyclines alone. The NNH for ED visits involving mild allergic reactions was lowest for oral sulfonamides and clindamycin (1 in 688 and 1 in 856 dispensed prescriptions, respectively). The NNH for ED visits involving moderate-to-severe allergic reactions was lowest for oral quinolones (1 in 2,525 dispensed prescriptions).
Without accounting for prescribing frequency, the oral antibiotics most commonly implicated in ED visits for ADEs were similar across age groups, but the relative frequency varied by age (Table 3). Amoxicillin was the most commonly implicated drug product in ED visits for antibiotic ADEs among children aged ≤9 years; however, the proportion of visits attributed to amoxicillin ADEs declined with increasing age (≤2 years [67.6%], 3-4 years [54.5%], and 5-9 years [44.7%]). A greater number of oral antibiotic products was implicated in ED visits for antibiotic ADEs among older children compared with younger children. For children ≤2 years, the three most commonly implicated antibiotics combined were implicated in 83.2% (95% CI: 80.9%-85.5%) of ED visits for antibiotic ADEs, (amoxicillin [67.6%], amoxicillin/clavulanate [8.5%], and cefdinir [7.6%]), while for children aged 10-19 years, the three most commonly implicated antibiotics combined were implicated in 55.0% (95% CI: 50.5%-59.6%) of ED visits for ADEs (sulfamethoxazole/trimethoprim [24.3%], amoxicillin [20.8%], and azithromycin [10.5%]).
Table 3.
Emergency Department (ED) Visits for Adverse Drug Events (ADEs) from the Most Commonly Implicated Oral Antibiotics, By Patient Age, Children ≤19 Years, United States, 2011-2015a
| Most Commonly Implicated Antibiotic Drug Products for Each Age Group | Cases | Annual National Estimate | ||
|---|---|---|---|---|
| ED Visits for Antibiotic ADEs | NNHb | |||
| No. | No. | % (95% CI) | ||
| Patients Aged <1-2 Years (Annual Estimate=27,871) | ||||
| Amoxicillin | 1,977 | 18,835 | 67.6 (63.6 - 71.5) | 334 |
| Amoxicillin/Clavulanate | 275 | 2,357 | 8.5 (6.8 - 10.1) | 723 |
| Cefdinir | 228 | 2,117 | 7.6 (5.3 - 9.9) | 851 |
| Azithromycin | 102 | 1,418 | 5.1 (3.9 - 6.3) | 1,458 |
| Cephalexin | 74 | 1,000 | 3.6 (2.2 - 5.0) | 566 |
| Sulfamethoxazole/Trimethoprim | 87 | 821 c | 2.9 (1.4 - 4.5) | 836c |
| Patients Aged 3-4 Years (Annual Estimate=7,067) | ||||
| Amoxicillin | 430 | 3,852 | 54.5 (49.5 - 59.5) | 958 |
| Amoxicillin/Clavulanate | 71 | 707 | 10.0 (6.2 - 13.8) | 1,474 |
| Cefdinir | 63 | 562 | 7.9 (4.5 - 11.3) | 1,873 |
| Azithromycin | 44 | 544 | 7.7 (5.1 - 10.3) | 3,126 |
| Sulfamethoxazole/Trimethoprim | 46 | 486 | 6.9 (3.9 - 9.8) | 1,039 |
| Cephalexin | 29 | 264 | 3.7 (2.1 - 5.4) | 1,870 |
| Patients Aged 5-9 Years (Annual Estimate=11,931) | ||||
| Amoxicillin | 566 | 5,330 | 44.7 (39.9 - 49.5) | 1,331 |
| Sulfamethoxazole/Trimethoprim | 155 | 1,768 | 14.8 (10.6 - 19.1) | 539 |
| Azithromycin | 94 | 1,024 | 8.6 (6.1 - 11.0) | 3,364 |
| Amoxicillin/Clavulanate | 82 | 876 | 7.3 (5.1 - 9.6) | 2,075 |
| Cefdinir | 83 | 793 | 6.6 (4.3 - 9.0) | 2,033 |
| Cephalexin | 59 | 624 | 5.2 (3.5 - 7.0) | 1,929 |
| Penicillin | 26 | 371 | 3.1 (1.6 - 4.6) | 707 |
| Clindamycin | 35 | 335 | 2.8 (1.4 - 4.2) | 646 |
| Patients Aged 10-19 Years (Annual Estimate=21,249) | ||||
| Sulfamethoxazole/Trimethoprim | 384 | 5,167 | 24.3 (20.3 - 28.4) | 413 |
| Amoxicillin | 366 | 4,414 | 20.8 (18.3 - 23.3) | 1,469 |
| Azithromycin | 153 | 2,225 | 10.5 (8.0 - 13.0) | 2,529 |
| Cephalexin | 127 | 1,711 | 8.1 (5.9 - 10.2) | 1,326 |
| Amoxicillin/Clavulanate | 112 | 1,419 | 6.7 (5.3 - 8.1) | 1,720 |
| Clindamycin | 126 | 1,304 | 6.1 (4.9 - 7.4) | 509 |
| Doxycycline | 78 | 845 | 4.0 (2.5 - 5.4) | 2,344 |
| Ciprofloxacin | 52 | 773 | 3.6 (2.2 - 5.1) | 700 |
| Minocycline | 58 | 686 | 3.2 (2.1 - 4.3) | 2,628 |
| Penicillin | 48 | 655 | 3.1 (2.1 - 4.1) | 1,114 |
| Cefdinir | 55 | 601 | 2.8 (1.4 - 4.2) | 2,017 |
| Metronidazole | 40 | 458 | 2.2 (1.2 - 3.1) | 1,082 |
Estimates of ED visits for ADEs based on the National Electronic Injury Surveillance System-Cooperative Adverse Drug Event Surveillance project (2011-2015); estimates of dispensed oral prescriptions from retail pharmacies based on the National Prescription Audit from QuintilesIMS (2011-2015). Drug products are not mutually exclusive; for some ED visits, more than one drug product was implicated in the ADE. Drug products are shown if they were implicated in ≥2% of estimated ED visits for antibiotic ADEs within each age group. Data exclude cases of unsupervised ingestion in which children aged ≤10 years accessed medications without caregiver oversight
Number needed to harm (NNH) calculated as the reciprocal of the estimated number of ED visits divided by the number of dispensed prescriptions.
Coefficient of variation >30%.
Accounting for prescribing frequency, estimated rates of ED visits for antibiotic ADEs per 10,000 dispensed retail prescriptions declined with age for all oral antibiotics except sulfamethoxazole/trimethoprim (Figure). Amoxicillin had the highest rate of ED visits for antibiotic ADEs among children aged ≤2 years (29.9 ED visits per 10,000 dispensed prescriptions); the rate of ED visits for amoxicillin ADEs declined to 10.4 ED visits per 10,000 dispensed prescriptions among children aged 3-4 years and further to 6.8 ED visits per 10,000 dispensed prescriptions among children aged 10-19 years. Sulfamethoxazole/trimethoprim (24.2 ED visits per 10,000 dispensed prescriptions) and clindamycin (19.6 ED visits per 10,000 dispensed prescriptions [95% CI: 14.1-25.2]) had the highest rates of ED visits for antibiotic ADEs among the oldest children (aged 10-19 years).
Figure. Rates of Emergency Department (ED) Visits for Adverse Drug Events (ADEs) from the Most Commonly Implicated Oral Antibiotics, by Patient Age, Children ≤19 Years, United States, 2011-2015.
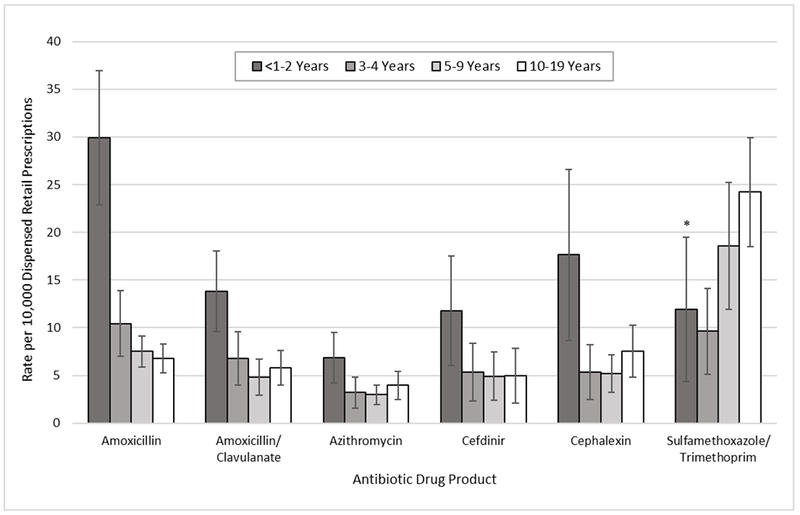
Estimates of ED visits for ADEs based on the National Electronic Injury Surveillance System-Cooperative Adverse Drug Event Surveillance project (2011-2015); estimates of dispensed oral prescriptions from retail pharmacies based on the National Prescription Audit from QuintilesIMS (2011-2015). Drug products are not mutually exclusive; for some ED visits, more than one antibiotic was implicated in the ADE. Data exclude cases of unsupervised ingestion in which children aged ≤10 years accessed medications without caregiver oversight. *Coefficient of variation >30%.
DISCUSSION
Antibiotic ADEs lead to nearly 70,000 estimated ED visits among children each year in the United States and should be a key area of focus for outpatient pediatric medication safety efforts. Antibiotics are implicated in nearly half of all ED visits for ADEs attributed to systemic medications among children of all ages, and are implicated in nearly two-thirds of ED visits for all ADEs among the youngest children (aged ≤2 years). Including age and drug-specific adverse event data in efforts to improve prescribing could help clinicians and parents/caregivers weigh risks of antibiotic treatment and reduce unnecessary prescribing.
High numbers of ED visits for antibiotic ADEs among younger children can be partially explained by greater prescription of antibiotics to younger children. Two-fifths (41%) of estimated ED visits for pediatric antibiotic ADEs involved children aged ≤2 years, and the population rate of ED visits for antibiotic ADEs is 4 times higher among children aged ≤2 years compared with children aged 10-19 years (23.8 vs. 5.2 ED visits per 10,000 children). The antibiotic prescribing rate has been reported to be nearly 2 times higher among children aged ≤2 years compared with children aged 10-19 years (1,287 vs. 691 antibiotic prescriptions per 1,000 children, respectively) [2]; however, 2-fold higher prescribing rates alone cannot account for a >4-fold higher population rate of ED visits for antibiotic ADEs among the youngest children.
Accounting for prescribing frequency, the risk of an ED visit for an antibiotic ADE was found to be higher for young children compared with older children for all antibiotics except sulfamethoxazole/trimethoprim. For example, the rate of ED visits for amoxicillin ADEs was 4 times higher for the youngest children (aged ≤2 years) compared with the oldest children (aged 10-19 years) (29.9 vs. 6.8 ED visits per 10,000 dispensed prescriptions, respectively). Several factors likely contribute to the increased risk of ED visits for antibiotic ADEs in young children. Most (86%) ED visits for antibiotic ADEs involved allergic reactions, and young children may be more susceptible to antibiotic allergy than older children [26]. Also, allergic reactions or other adverse effects following antibiotic exposure at a young age are typically documented so that future reactions are then avoided [27]. Finally, parents/caregivers might have a lower threshold for seeking emergency care for young children because they may perceive young children to be more vulnerable [26].
Pediatric antibiotic prescribing has declined in recent years following efforts to reduce unnecessary antibiotic use, but there is room for continued improvement [28–30]. An estimated 29% of outpatient antibiotic prescriptions for children in 2010-2011 were deemed unnecessary (i.e., prescribed for conditions, such as viral infections, for which national guidelines recommend against antibiotic use) [14]. While many antibiotic prescriptions are appropriate, and not all antibiotic ADEs can be avoided, minimizing unnecessary antibiotic prescribing can reduce the burden of acute antibiotic-related harms as well as help preserve antibiotic efficacy [20].
Detailed nationally-representative data on antibiotic ADE risks and patient populations at highest risk (young children) may help clinicians continue to reduce unnecessary prescribing. Efforts to reduce inappropriate prescribing have historically focused on the long-term benefits of limiting antibiotic resistance rather than the short-term risks of adverse events. However, focusing solely on limiting antibiotic resistance might not be sufficient to change prescribing behavior because inappropriate prescribing and subsequent antimicrobial resistance is often perceived as an external problem caused by other prescribers [17]. Instead, efforts to reduce inappropriate prescribing could place more focus on immediate risks to individual patients. In one recent study, 78% of parents did not recall any discussion of possible antibiotic harms during their child’s last doctor visit for an acute respiratory infection [19]. For example, the finding that each year, nearly 1 in 400 children aged ≤2 years are brought to an ED for an antibiotic ADE may help remind clinicians that antibiotic ADEs are near-term events that can be clinically significant and consequential for pediatric patients.
Incorporating up-to-date national data on harms into initiatives to improve antimicrobial prescribing, such as CDC’s Be Antibiotics Aware: Smart Use, Best Care educational program and the American Board of Internal Medicine (ABIM) Foundation’s Choosing Wisely series of recommendations by healthcare professional societies, might enhance the effectiveness of these initiatives [7, 8]. Additionally, providing support for clinical decisions is one recommendation in CDC’s Core Elements of Outpatient Antimicrobial Stewardship [9]; incorporating patient-specific information about the risks of antibiotic ADEs (e.g., NNH by age, drug, and sex) in clinical decision support systems, may help reinforce the potential for acute antibiotic harms when prescribing decisions are being made.
Similarly, communicating information on antibiotic ADE risks to parents/caregivers may help lessen the demand or expectation for antibiotics. Parents/caregivers often overestimate the benefits of antibiotics, which can lead to demand or expectation for antibiotic treatment [19, 31]. Clinicians may perceive parent/caregiver expectations and, coupled with a concern for parent/patient satisfaction, may overprescribe antibiotics [13, 15, 16]. However, in a recent study, few mothers of young children were familiar with severe antibiotic-associated ADEs and nearly all would have liked to receive this information when antibiotics were prescribed [21]. Engaging parents/caregivers in informed decision-making by discussing risks and benefits of antibiotic treatment and suggesting specific symptom relief strategies might help to reduce parent/caregiver demands and overprescribing by clinicians [9, 32, 33]. National estimates of the NNH (1 ED visit by a child aged ≤2 years for an amoxicillin ADE for every 330 prescriptions) could be used in these discussions as well as incorporated into educational materials, such as those available through CDC’s Be Antibiotics Aware: Smart Use, Best Care program [8].
These public health surveillance data have limitations. First, the burden of ADEs from outpatient antibiotic use is likely underestimated, since only ADEs resulting in ED visits were included. Antibiotic ADEs treated in other settings (e.g., urgent care, physician’s office), ADEs for which no treatment was sought, or ADEs that resulted in death were not included. An analysis of national surveillance data from 1995-2005 found that there were approximately twice as many estimated outpatient clinic visits for antibiotic ADEs as ED visits among children [4]. A more recent cohort study found that 3%-4% of children prescribed antibiotics had a clinician-documented adverse event and 25%-36% of children had a parent-reported adverse event [34]. Second, ADEs that are less likely to be diagnosed in the ED setting (e.g., Clostridium difficile infection) are not reliably included. Nonetheless, the ED setting is well-suited to identify well-recognized ADEs with acute onset, including those that result in serious harm. Third, it was not possible to assess which antibiotic prescriptions were appropriate since information on indication and prescribed dose or duration was not available. However, it has been estimated that 29% of pediatric antibiotic prescriptions are unnecessary, which suggests that a substantial number of these ED visits could have been avoided [14]. Fourth, diagnoses were made in an emergency setting in which patient management and symptom relief were the priority. Some rashes attributed to antibiotic allergic reactions may have been caused by viral exanthems, which are common in young children, but can be misdiagnosed as allergic reactions [35]. Additionally, some adverse event manifestations categorized as allergic may not have proven to be immune-mediated upon further investigation [36]. Documentation of true drug allergies is important to prevent repeat occurrences; however, overestimation of antibiotic allergies can lead to suboptimal prescribing (e.g., overuse of broad-spectrum agents) [27, 35, 37]. Lastly, only outpatient retail pharmacy data from QuintilesIMS were included in the analysis. Although less common, antibiotics can be obtained from other settings (e.g., mail order, federal, or specialty pharmacies), which were not included.
CONCLUSION
Antibiotic ADEs lead to nearly 70,000 estimated pediatric ED visits each year in the United States. Minimizing antibiotic overprescribing is important for reducing acute and clinically significant harms to individual patients as well as for reducing the societal risk of antibiotic resistance. Quantifying the risks of antibiotic ADEs can provide additional information to help clinicians and parents/caregivers weigh the risks and benefits of antibiotic treatment. Prevention efforts could target the pediatric patients with highest frequencies and rates of ED visits for antibiotic ADEs.
Supplementary Material
Acknowledgements:
We thank Dr. Nina Weidle from Eagle Medical Services, LLC (contractor to CDC), Ms. Katie Rose, Ms. Sandra Goring, Ms. Arati Baral, and Mr. Alex Tocitu, from Northrop Grumman (contractor to CDC), for assistance with data coding and programming. We also thank Mr. Tom Schroeder, Ms. Elenore Sonski, Mr. Herman Burney, and data abstractors from the U.S. Consumer Product Safety Commission, for their assistance with data acquisition. Lastly, we thank Dr. Ruth Moro from Northrop Grumman (contractor to CDC) for thoughtful review of the manuscript. No individuals named herein received compensation for their contributions.
Funding: This work was supported by the Federal government of the United States.
Abbreviations and Acronyms:
- ADE:
adverse drug event
- CDC:
Centers for Disease Control and Prevention
- CI:
confidence interval
- ED:
emergency department
- NEISS-CADES:
National Electronic Injury Surveillance System-Cooperative Adverse Drug Event Surveillance
- NNH:
number needed to harm
- NPA:
National Prescription Audit
Footnotes
Potential Conflicts of Interest: No conflicts of interest relevant to this article to disclose.
Publisher's Disclaimer: Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- 1.Chai G, Governale L, McMahon AW, Trinidad JP, Staffa J, Murphy D. Trends of outpatient prescription drug utilization in US children, 2002-2010. Pediatrics. 2012;130:23–31. [DOI] [PubMed] [Google Scholar]
- 2.Hicks LA, Bartoces MG, Roberts RM, Suda KJ, Hunkler RJ, Taylor TH Jr., et al. US outpatient antibiotic prescribing variation according to geography, patient population, and provider specialty in 2011. Clin Infect Dis. 2015;60:1308–16. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. https://www.cdc.gov/drugresistance/threat-report-2013/. Accessed November 8, 2017.
- 4.Bourgeois FT, Mandl KD, Valim C, Shannon MW. Pediatric adverse drug events in the outpatient setting: an 11-year national analysis. Pediatrics. 2009;124:e744–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shehab N, Lovegrove MC, Geller AI, Rose KO, Weidle NJ, Budnitz DS. US Emergency Department Visits for Outpatient Adverse Drug Events, 2013-2014. JAMA. 2016;316:2115–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shehab N, Patel PR, Srinivasan A, Budnitz DS. Emergency department visits for antibiotic-associated adverse events. Clin Infect Dis. 2008;47:735–43. [DOI] [PubMed] [Google Scholar]
- 7.American Board of Internal Medicine Foundation. Antibiotics: When you need them – and when you don’t. http://www.choosingwisely.org/patient-resources/antibiotics/. Accessed November 8, 2017.
- 8.Centers for Disease Control and Prevention. Antibiotic Prescribing and Use in Doctor’s Offices. https://www.cdc.gov/getsmart/community/index.html. Accessed November 8, 2017.
- 9.Sanchez GV, Fleming-Dutra KE, Roberts RM, Hicks LA. Core Elements of Outpatient Antibiotic Stewardship. MMWR Recomm Rep. 2016;65:1–12. [DOI] [PubMed] [Google Scholar]
- 10.Fashner J, Ericson K, Werner S. Treatment of the common cold in children and adults. Am Fam Physician. 2012;86:153–9. [PubMed] [Google Scholar]
- 11.Hersh AL, Jackson MA, Hicks LA, American Academy of Pediatrics Committee on Infectious D. Principles of judicious antibiotic prescribing for upper respiratory tract infections in pediatrics. Pediatrics. 2013;132:1146–54. [DOI] [PubMed] [Google Scholar]
- 12.Wald ER, Applegate KE, Bordley C, Darrow DH, Glode MP, Marcy SM, et al. Clinical practice guideline for the diagnosis and management of acute bacterial sinusitis in children aged 1 to 18 years. Pediatrics. 2013;132:e262–80. [DOI] [PubMed] [Google Scholar]
- 13.Sanchez GV, Roberts RM, Albert AP, Johnson DD, Hicks LA. Effects of knowledge, attitudes, and practices of primary care providers on antibiotic selection, United States. Emerg Infect Dis. 2014;20:2041–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fleming-Dutra KE, Hersh AL, Shapiro DJ, Bartoces M, Enns EA, File TM Jr, et al. Prevalence of Inappropriate Antibiotic Prescriptions Among US Ambulatory Care Visits, 2010-2011. JAMA. 2016;315:1864–73. [DOI] [PubMed] [Google Scholar]
- 15.Dempsey PP, Businger AC, Whaley LE, Gagne JJ, Linder JA. Primary care clinicians’ perceptions about antibiotic prescribing for acute bronchitis: a qualitative study. BMC Fam Pract. 2014;15:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mangione-Smith R, McGlynn EA, Elliott MN, Krogstad P, Brook RH. The relationship between perceived parental expectations and pediatrician antimicrobial prescribing behavior. Pediatrics. 1999;103:711–8. [DOI] [PubMed] [Google Scholar]
- 17.McCullough AR, Rathbone J, Parekh S, Hoffmann TC, Del Mar CB. Not in my backyard: a systematic review of clinicians’ knowledge and beliefs about antibiotic resistance. J Antimicrob Chemother. 2015;70:2465–73. [DOI] [PubMed] [Google Scholar]
- 18.Metlay JP, Shea JA, Crossette LB, Asch DA. Tensions in antibiotic prescribing: pitting social concerns against the interests of individual patients. J Gen Intern Med. 2002;17:87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coxeter PD, Mar CD, Hoffmann TC. Parents’ Expectations and Experiences of Antibiotics for Acute Respiratory Infections in Primary Care. Ann Fam Med. 2017;15:149–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Linder JA. Editorial commentary: antibiotics for treatment of acute respiratory tract infections: decreasing benefit, increasing risk, and the irrelevance of antimicrobial resistance. Clin Infect Dis. 2008;47:744–6. [DOI] [PubMed] [Google Scholar]
- 21.Roberts RM, Albert AP, Johnson DD, Hicks LA. Can Improving Knowledge of Antibiotic-Associated Adverse Drug Events Reduce Parent and Patient Demand for Antibiotics? Health Serv Res Manag Epidemiol. 2015;2:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Budnitz DS, Pollock DA, Weidenbach KN, Mendelsohn AB, Schroeder TJ, Annest JL. National surveillance of emergency department visits for outpatient adverse drug events. JAMA. 2006;296:1858–66. [DOI] [PubMed] [Google Scholar]
- 23.Jhung MA, Budnitz DS, Mendelsohn AB, Weidenbach KN, Nelson TD, Pollock DA. Evaluation and overview of the National Electronic Injury Surveillance System-Cooperative Adverse Drug Event Surveillance Project (NEISS-CADES). Med Care. 2007;45:S96–102. [DOI] [PubMed] [Google Scholar]
- 24.Schroeder T, Ault K. The NEISS sample (design and implementation) 1997 to present. http://www.cpsc.gov//PageFiles/106617/2001d011-6b6.pdf. Updated June 1, 2001. Accessed November 8, 2017.
- 25.Centers for Disease Control and Prevention. Bridged-race population estimates: 1990-2015. https://wonder.cdc.gov/bridged-race-population.html. Accessed November 8, 2017.
- 26.Marrs T, Fox AT, Lack G, du Toit G. The diagnosis and management of antibiotic allergy in children: Systematic review to inform a contemporary approach. Arch Dis Child. 2015;100:583–8. [DOI] [PubMed] [Google Scholar]
- 27.Langley J, Halperin S. Allergy to antibiotics in children: Perception versus reality. Can J Infect Dis. 2002;13:160–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention. Office-related antibiotic prescribing for persons aged ≤14 years--United States, 1993-1994 to 2007-2008. MMWR Morb Mortal Wkly Rep. 2011;60:1153–6. [PubMed] [Google Scholar]
- 29.Vaz LE, Kleinman KP, Raebel MA, Nordin JD, Lakoma MD, Dutta-Linn MM, et al. Recent trends in outpatient antibiotic use in children. Pediatrics. 2014;133:375–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention. Antibiotic Prescribing and Use in Doctor’s Offices: Measuring Outpatient Antibiotic Prescribing https://www.cdc.gov/antibiotic-use/community/programs-measurement/measuring-antibiotic-prescribing.html. Accessed November 8, 2017.
- 31.Vaz LE, Kleinman KP, Lakoma MD, Dutta-Linn MM, Nahill C, Hellinger J, et al. Prevalence of Parental Misconceptions About Antibiotic Use. Pediatrics. 2015;136:221–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mangione-Smith R, Zhou C, Robinson JD, Taylor JA, Elliott MN, Heritage J. Communication practices and antibiotic use for acute respiratory tract infections in children. Ann Fam Med. 2015;13:221–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Butler CC, Simpson SA, Dunstan F, Rollnick S, Cohen D, Gillespie D, et al. Effectiveness of multifaceted educational programme to reduce antibiotic dispensing in primary care: practice based randomised controlled trial. BMJ. 2012;344:d8173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gerber JS, Ross RK, Bryan M, Localio AR, Szymczak JE, Wasserman R, et al. Association of Broad- vs Narrow-Spectrum Antibiotics With Treatment Failure, Adverse Events, and Quality of Life in Children With Acute Respiratory Tract Infections. JAMA. 2017;318:2325–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Joint Task Force on Practice Parameters, American Academy of Allergy‚ Asthma and Immunology, American College of Allergy‚ Asthma and Immunology, Joint Council of Allergy‚ Asthma and Immunology. Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol. 2010;105:259–73. [DOI] [PubMed] [Google Scholar]
- 36.Vyles D, Adams J, Chiu A, Simpson P, Nimmer M, Brousseau DC. Allergy Testing in Children With Low-Risk Penicillin Allergy Symptoms. Pediatrics. 2017;140. [DOI] [PubMed] [Google Scholar]
- 37.Esposito S, Castellazzi L, Tagliabue C, Principi N. Allergy to antibiotics in children: an overestimated problem. Int J Antimicrob Agents. 2016;48:361–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


