Abstract
Extensive social networks are associated with better physical, mental, and cognitive health in aging, but the underlying brain substrates remain largely unexplored. Voxel-based morphometry and multivariate statistics were used to identify gray matter volume covariance networks associated with social networks in 86 older adults without dementia (M Age = 75.20 years, 53% women). Gray matter networks associated with the number of high-contact social roles and the total number of network members were identified after adjusting for age, sex, education, global health, and total intracranial volume – and shared nodes included medial, lateral and orbital prefrontal, hippocampal, precuneus, insular, and cingulate regions. Greater expression of these gray matter networks was associated with better memory scores on the Free and Cued Selective Reminding Test. A more distributed network was associated with high-contact social roles than total number of networks members – also extending into amygdala and entorhinal cortex. Thus, high-contact social roles and total number of network members in older adults are associated with gray matter networks composed of regions previously linked to memory and affected by both healthy aging and Alzheimer disease – and high-contact social roles are more strongly associated with brain structures than the total number of network members.
Keywords: Social Networks, Aging, Neuroimaging, Multivariate Statistics
Introduction
Extensive social networks and high levels of social support are associated with better physical, mental, and cognitive health outcomes in older adults (Barnes, De Leon, Wilson, Bienias, & Evans, 2004; Berkman, 1984; Ertel, Glymour, & Berkman, 2008; Fratiglioni, Paillard-Borg, & Winblad, 2004; Holt-Lunstad, Smith, & Layton, 2010; House, Landis, & Umberson, 1988; Kawachi & Berkman, 2001; McNeill, Kreuter, & Subramanian, 2006; Pillemer & Holtzer, 2016; Seeman, Lusignolo, Albert, & Berkman, 2001; Yeh & Liu, 2003). Yet, the brain substrates associated with social networks and social support remain largely unexplored, particularly in older adults. A social network is a social structure of interactions and relationships – including family members, friends, and coworkers – and social support is the perceived or actual level of support provided to an individual by their social network (Antonucci, 1990). Extensive social networks are associated with a reduced risk for morbidity and mortality (Berkman, 1984; Holt-Lunstad et al., 2010; House et al., 1988), and increased participation in physical activities (for a review see (McNeill et al., 2006)). Extensive social networks are also associated with reduced levels of depressive symptoms and anxiety (for a review see (Kawachi & Berkman, 2001)). In addition, extensive social networks and high levels of social support in older adults are associated with better cognitive functions, and a reduced risk for cognitive decline, Alzheimer’s disease (AD) and related dementias (Barnes et al., 2004; Bassuk, Glass, & Berkman, 1999; Ertel et al., 2008; Fratiglioni et al., 2004; Pillemer & Holtzer, 2016; Seeman et al., 2001; Yeh & Liu, 2003). A better understanding of the brain substrates associated with social networks and social support in older adults – potentially modifiable factors of physical, mental, and cognitive health – has the potential to inform the development of interventions for improving a number of different health outcomes in aging.
The brain substrates associated with social networks and social supports in older adults are not well-understood. Prior studies of predominantly young and middle-aged adults, however, have observed associations between social networks and social support and the structure of medial prefrontal cortex (Lewis, Rezaie, Brown, Roberts, & Dunbar, 2011; Powell, Lewis, Roberts, García-Fiñana, & Dunbar, 2012), the structure and function of the amygdala and the entorhinal cortex (Bickart, Wright, Dautoff, Dickerson, & Barrett, 2011; Von Der Heide, Vyas, & Olson, 2014), functional connectivity between amygdala and superior temporal sulcus, medial temporal lobe, anterior cingulate, orbitofrontal, and medial prefrontal cortex regions (Kevin C Bickart, Mark C Hollenbeck, Lisa Feldman Barrett, & Bradford C Dickerson, 2012), as well as white matter integrity of the genu of the corpus callosum (Molesworth, Sheu, Cohen, Gianaros, & Verstynen, 2015). These regions have been previously associated with a number of physical, mental, and cognitive processes in older adults, including physical exercise, depression, emotion, memory, social processing, cognitive decline and dementia (Bherer, Erickson, & Liu-Ambrose, 2013; Du et al., 2003; Gutchess, Kensinger, & Schacter, 2007; Pennanen et al., 2004; Rodrigue & Raz, 2004; St. Jacques, Dolcos, & Cabeza, 2010; Whalen, Shin, Somerville, McLean, & Kim, 2002).
We recently examined functional connectivity associated with social networks in 28 older adults without dementia (65–87 years (Pillemer, Holtzer, & Blumen, 2017)), using resting-state functional magnetic resonance imaging (fMRI) and the social network index (Cohen, Doyle, Skoner, Rabin, & Gwaltney, 1997). We found that functional connectivity in several well-established resting-state networks, previously linked to a number of visual, motor, speech and other language functions, were associated with the number of high-contact social roles (including spouse, parent, child, child-in-law, close relative, close friend, religious group member, student, employee, neighbor, volunteer and group member) and the total number of network members – including the sensory-motor, visual, vestibular/insular and left fronto-parietal resting-state networks. We also found that the number of high-contact social roles were more strongly associated with functional connectivity in the lateral prefrontal components of the left fronto-parietal resting-state network, while the total number of network members were more strongly associated with functional connectivity in the medial prefrontal components of the left fronto-parietal resting-state network. We concluded that a) linking social networks in older adults to functional connectivity in several resting-state networks is consistent with that extensive social networks and social support have been linked to a number of positive physical, mental, and cognitive health outcomes, and that b) the functional brain networks associated with the number of high-contact social roles and the total number of network members largely overlap, but also differ, particularly in the prefrontal cortex.
Although resting-state fMRI is a valuable tool for linking social networks in older adults to connectivity in well-established functional networks, normal and pathological aging is associated with structural deterioration in different brain regions that, in turn, are distributed across different functional networks (Kalpouzos et al., 2009; Raz et al., 1997; Thompson et al., 2003). We also know that the relationship between brain function and structure is not clear-cut or one-to-one (Kopell, Gritton, Whittington, & Kramer, 2014; Park & Friston, 2013). In normal aging, for example, the greatest gray matter atrophy is observed in prefrontal cortex regions – including dorsolateral and orbitofrontal regions – yet, considerable atrophy is also observed in sensorimotor, occipital, and insular regions (Kalpouzos et al., 2009; Raz et al., 1997). Entorhinal, hippocampal and amygdala regions, however, are relatively spared in normal aging (Thompson et al., 2003). By contrast, in older adults with AD, the greatest gray matter atrophy is observed in entorhinal, hippocampal, and other medial temporal regions, with relative sparing of frontal and sensorimotor regions until the later stages of the disease (Thompson et al., 2003). Thus, determining whether the structural brain substrates of social networks are particularly affected or relatively spared in normal aging and AD could inform us about who will be more or less likely to benefit from interventions aimed at strengthening social networks and social support. Further linking these brain substrates to key cognitive functions affected by normal aging and AD, including processing speed, memory, and executive functions, would provide additional information regarding this issue.
The aim of the current study was to examine the structural brain networks of social networks in older adults without dementia – and how they relate to processing speed, memory, and executive function. Voxel-based morphometry methods and multivariate covariance-based statistics were used to identify gray matter covariance patterns or ‘networks’ associated with both high-contact social roles and the total number of network members (Cohen et al., 1997). Unlike traditional univariate approaches to neuroimaging analysis that evaluate gray matter volume (or functional activation) on a voxel-by-voxel basis, multivariate covariance-based approaches to neuroimaging analysis evaluate the correlation or covariance between gray matter volume (or functional activation) between different brain regions, and can therefore be more straightforwardly interpreted as neural networks (Habeck & Stern, 2010). Multivariate covariance-based approaches to neuroimaging analysis also typically involves some form of data reduction (analogous to a principal components analysis) and do not require a priori modeling or hypotheses – thereby avoiding the multiple comparison problem of traditional univariate analyses and increasing statistical power (Ashby, 2011; Habeck et al., 2008; Habeck, Krakauer, et al., 2005; Habeck & Stern, 2010). In addition, multivariate covariance-based networks have been shown to be highly reproducible across different data sets of older adults (Bergfield et al., 2010; Habeck et al., 2008). Thus, multivariate covariance-based statistics offers a potentially sensitive and reproducible approach for identifying gray matter covariance networks associated with the number of high-contact social roles and the total number of network members in older adults without dementia. A network-based approach is also more appropriate than traditional univariate approaches because social networks and social support have been associated with a number of physical, mental and cognitive health outcomes in aging, and we hypothesized that both the number of high-contact social roles and the total number of network members would be associated with fairly distributed networks of gray matter volume. Our multivariate approach also permitted us to adjust for potential confounders, including age, sex, education, global health, and total intracranial volume. Finally, the expressions of these gray matter networks associated with the number of high-contact social roles and the total number of network members could also be correlated with neuropsychological assessments of processing speed, memory, and executive function.
Methods
Participants.
We examined gray matter covariance patterns related to the social network index (Cohen et al., 1997), and their associations with specific cognitive functions, in 86 community-dwelling of older adults without dementia who had undergone magnetic resonance imaging (MRI) from a larger study called the Central Control of Mobility in Aging (CCMA) study at Albert Einstein College of Medicine, Bronx, NY (Holtzer, Wang, & Verghese, 2014)]. Inclusion criteria for the larger CCMA study were 1) 65 years and older, 2) plan to be in the area for next three or more years, 3) able to speak English, and 4) ambulatory. Additional inclusion criteria for the current sub-study was willingness to undergo MRI. Persons with dementia were excluded from the CCMA study based on the telephone-based memory impairment screen ( score of <5 (Lipton et al., 2003) Ascertain Dementia 8-item Informant Questionnaire AD-8 score >1(Galvin et al., 2005) or clinical case conferences using the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (Association, 2000). Other exclusion criteria included, serious chronic or acute illness (e.g. cancer), any medical condition or chronic medication use (e.g., neuroleptics) that would compromise safety or affect cognitive functioning or terminal illness with life expectancy less than 12 months, progressive degenerative neurologic disease (e.g. Parkinson’s disease), hospitalized in the past 6 months for severe illness or surgery, severe auditory or visual loss, active psychoses or psychiatric symptoms and living in nursing home. Additional exclusion criteria for the current sub-study was MRI contraindication. Key MRI contraindications were vascular stents (7%), pacemakers (4%) and joint replacements (4%). The participant characteristics of the current sample are summarized in Table 1.
Table 1.
Demographic, social and cognitive characteristics of 86 older adults without dementia.
| M (SD) | Range | |
|---|---|---|
| Age (years) | 75.20 (5.60) | 65–91 |
| Gender (% female) | 53% (N/A) | N/A |
| Education (years) | 15.66 (3.56) | 7–28 |
| Global Health Status Score* | 1.24 (.99) | 0–4 |
| SNI-1 Score** | 5.26 (1.44) | 1–9 |
| SNI-2 Score*** | 22.98 (21.79) | 2–143 |
| Trails A | 44.35(16.77) | 23.72–108.5 |
| Trails B minus A | 77.05(55.59) | 16.13–261.9 |
| Total Free Recall | 30.88 (7.13) | 7–46 |
Global health status score (range 0–10) obtained from dichotomous rating (presence or absence) of diabetes, chronic heart failure, arthritis, hypertension, depression, stroke, Parkinson’s disease, chronic obstructive pulmonary disease, angina, and myocardial infarction.
SNI-1 score: total number of high-contact roles of a respondent (Cohen et al., 1997)
SNI-2 score: total number of individuals in a respondent’s social network (Cohen et al., 1997)
Social Network index.
The Social Network Index (SNI; (Cohen et al., 1997)) was used to assess the total number of high-contact social roles that a respondent interact with at least biweekly (SNI-1), and the total number of network members that a respondent interact with at least biweekly (SNI-2). The maximum number of high-contact social roles a respondent can have is 12 and these include: spouse, parent, child, child-in-law, close relative, close friend, religious group member, student, employee, neighbor, volunteer and group member (1 point is assigned for each type of relationship). The SNI-2 score reflects the total number of people whom the respondent has contact with at least bi-weekly, and is obtained by summing up the number of people across the 12 domains.
Measures and Covariates.
The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS (Randolph, Tierney, Mohr, & Chase, 1998) was used to assess overall cognitive function (Total Index). Processing speed and executive function was assessed with the Trail Making Test: Time to complete Part A (TMT:A (Reitan, 1978)), and Time to complete Part B minus time to complete Part A (TMT:B-A), respectively. Memory was assessed with the Free and Cued Selective Reminding Test: Total Free Recall (FCSRT (Buschke, 1973)), a reliable predictor of dementia (Grober, Lipton, Hall, & Crystal, 2000; Grober, Sanders, Hall, & Lipton, 2010). Finally, a global health status score (range 0 to 10) was obtained from dichotomous ratings (presence or absence) of physician diagnosed diabetes, chronic heart failure, arthritis, hypertension, depression, stroke, Parkinson’s disease, chronic obstructive pulmonary disease, and angina or myocardial infarction (Verghese, Holtzer, Lipton, & Wang, 2009).
MRI Data Acquisition.
MRI scanning was performed using a Philips 3T Achieva Quasar TX multinuclear MRI/MRS (Magnetic Resonance Spectroscopy) system equipped with a Dual Quasar High Performance Gradient System, 32-channel broadband digital RF system, Quadrature T/R Head Coil, RapidView reconstructor, Intera Achieva ScanTools Pro R2.5 Package, NetForum and ExamCards, and SENSE parallel imaging capability. Standard three-dimensional T1-weighted images were acquired using axial 3D-MP-RAGE (magnetic prepared rapid acquisition gradient echo) parameters over a 240 mm field of view (FOV) and 1.0 mm isotropic resolution, TE = 4.6 ms, TR = 9.9 ms, α = 8o, with SENSE factor 2.5. A neuroradiologist reviewed each MRI to verify that there were no clinically significant findings for any of the participants.
MRI Pre-Processing.
T1-weighted images were first manually re-oriented to the anterior commissure – posterior commissure line, and then pre-processed in using SPM8 (Wellcome Department of Cognitive Neurology) that was implemented with MATLAB R2016b (Mathworks, Natick, MA). Each structural MRI image was analyzed using Voxel-Based Morphometry (VBM) and segmented into Gray Matter (GM), White Matter (WM), and Cerebrospinal Fluid (CSF), using the unified segmentation procedure, Diffeomorphic Anatomical Registration Through Exponentiated Line Algebra (DARTEL; (Ashburner, 2007; Ashburner & Friston, 2005)). DARTEL ensures proper inter-subject alignment by modeling the shape of the brain using three parameters for each voxel. DARTEL simultaneously aligns gray matter and white matter to produce a study-specific and increasingly crisp template to which the data are iteratively aligned. For each participant, DARTEL produces a GM map, a WM map and a CSF map in the same space as the original T1-weighted image, where each voxel is assigned a probability. These probability maps were first manually examined to ensure proper segmentation, and then spatially normalized (Friston et al., 1995) into Montreal Neurologic Institute (MNI) space. Finally, these probability maps were spatially smoothed with an isotropic Gaussian kernel, full-width-at half-maximum = 8 mm. Only GM probability maps were used in the upcoming multivariate analyses.
Group-Level Covariance Analyses.
Multivariate analyses were performed to identify gray matter covariance patterns or networks associated with SNI-1 (total number of high-contact social roles) and SNI-2 (total number of network members), in two separate statistical models. Both models were adjusted by age, sex, education, total intracranial volume, and global health status (see Table 1). Multivariate analyses were implemented using the principal components analysis (PCA) suite, http://www.nitrc.org/projects/gcva_pca (Habeck, Rakitin, et al., 2005; Habeck & Stern, 2007; Moeller & Strother, 1991). Gray matter probability maps were first masked with a gray matter mask supplied by SPM to only include voxels with > 20% probability of being gray matter. A principal components analysis (PCA) was then performed after participant means were subtracted from each voxel, in order to generate a set of principal components and their associated participant-specific (or pattern) expression scores. Participant-specific expression scores reflect the degree to which a participant displays a particular component or pattern. The gray matter volume covariance patterns associated with the social network index were then computed by regressing the participant-specific factor scores from the best linear combination of principal components (PCs) – selected using the Akaike information criteria (Burnham & Anderson, 2002) – against SNI-1 or SNI-2. The stability of the voxels in each GM volume covariance pattern associated with a social network index were then tested using 1,000 bootstrap resamples (Efron & Tibshirani, 1994). Voxels with bootstrap samples of [Z] > + 1.96 or < −1.96, p < .05 (.025 in each tail) were considered significant. These group-level covariance analyses allowed us to identify key nodes in the gray matter volume covariance networks (Habeck, Krakauer, et al., 2005; Habeck & Stern, 2007; Steffener, Brickman, Habeck, Salthouse, & Stern, 2013) associated with SNI-1 and SNI-2.
Note that covariance patterns obtained from any multivariate analysis assigns positive and negative weightings (or loadings) to each voxel (or variable) included in the analysis (Habeck et al., 2008), and that both positively and negatively weighted regions contribute to the derived covariance patterns (Habeck et al., 2008; Spetsieris & Eidelberg, 2011; Steffener et al., 2013). Within the context of the current study, keep in mind that positively-weighted regions show relatively more volume with increasing scores on a social network index (total number of high-contact social roles or total number of network members), while negatively-weighted regions show relatively less volume with increasing scores on a social network index.
Results
The mean age of participants was 75.20 years (± 5.60). On average they had 15.66 years (± 3.56) of education, and 53% of participants were female. The mean total index score on the RBANS was 93.19 (± 13.20), indicative of average global cognition. The mean global health status score was 1.24 (±0.99), indicative of good health. On average, participants reported 5.26 (± 1.44) total number of high-contact social roles in their social network (SNI-1), and a total of 22.98 (± 21.79) individuals in their entire social network (SNI-2). There was no difference in SNI-1 and SNI-2 score as a function of gender: on average males reported 5.28 (± .26) total number of high-contact social roles and 22.13 (± .3.56) individuals in their entire social network, while females reported 5.26 (±.18) total number of high-contact social roles and 23.72 (±3.15) in their entire social network, t (84) = .04; p =.96 and t (84) = .33, p = .73, respectively.
Multivariate covariance-based analyses revealed a gray matter covariance network associated with both SNI-1 and SNI-2. These gray matter covariance networks were composed of both shared and distinct brain regions (see Figure 1a, Figure 1b and Table 2). We also found that greater expression of these gray matter covariance patterns linked to SNI-1 and SNI-2 were associated with better memory, as assessed with Total Free Recall on the FCSRT. We describe these results in more detail below.
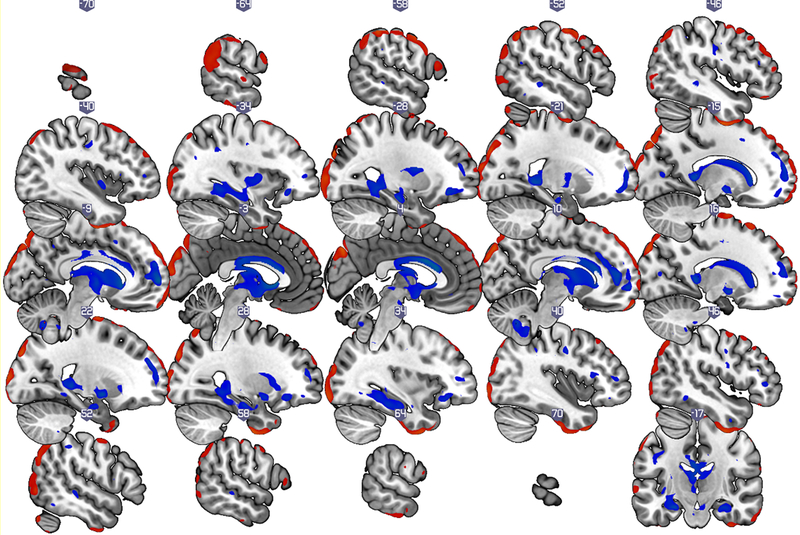
Figure 1a:
Gray matter covariance patterns associated with the SNI-1 score (total number of high-contact roles of a respondent) in older adults without dementia. Shown are thresholded Z-loadings at |Z|>1.96 p < .05 (.025 in each tail). Positively weighted regions are displayed in blue, implying relatively larger volumes with SNI1-score. Negatively weighted regions are displayed in red, implying relatively smaller volumes with SNI1–1 score. All results are adjusted for age, sex, education, total intracranial volume, and global health status.
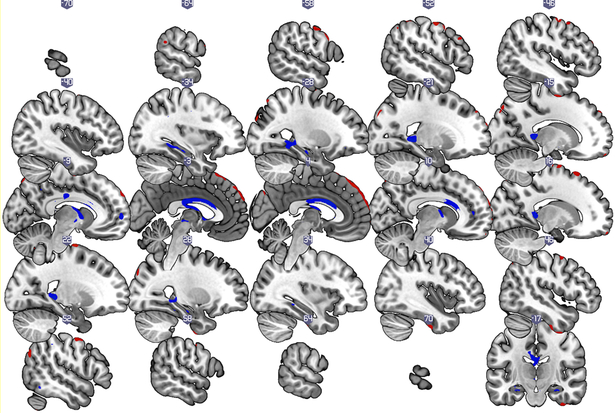
Figure 1b:
Gray matter covariance patterns associated with the SNI-2 score (total number of individuals in a respondent’s social network) in older adults without dementia. Shown are thresholded Z-loadings at |Z|>1.96 p < .05 (.025 in each tail). Positively weighted regions are displayed in blue, implying relatively larger volumes with SNI2-score. Negatively weighted regions are displayed in red, implying relatively smaller volumes with SNI-2 score. All results are adjusted for age, sex, education, total intracranial volume, and global health status.
Table 2.
Peak voxels and Corresponding Brain Regions of Clusters with Positive and Negative Pattern Weights. Positively-weighted regions show relatively more volume with increasing scores on a social network index (high-contact social relationships or overall social network size), while negatively-weighted regions show relatively less volume with increasing scores on a social network index. For large clusters (>5,000 voxels), the peak of each cluster is listed first, and then other regions within the cluster are named in parentheses. $$PARABREAKHERE$$ Threshold z = ± 1.96, p <.05, k >10 voxels.
| High-Contact Social Relationships (SNI-I) | |||||
| Brain Region(s) | X | Y | Z | z-value |
k
(cluster size) |
| Positive | |||||
| Superior Frontal Gyrus (medial; large cluster extending into cingulate cortex, caudate, putamen, globus pallidus, thalamus, hippocampal, entorhinal cortex and amygdala regions) |
−8 | 59 | 6 | 2.7900 | 73954 |
| Parahippocampal gyrus (large cluster extending into hippocampus, entorhinal cortex, and amygdala) |
15 | −36 | 4 | 2.5933 | 9368 |
| Cerebellum (VIII) | 9 | −61 | −41 | 2.4623 | 1947 |
| Precentral Gyrus | −45 | −18 | 28 | 2.2012 | 1022 |
| Middle Temporal Gyrus | −48 | −38 | −8 | 2.3611 | 670 |
| Cerebellum (VIII) | −9 | −64 | −43 | 2.4306 | 499 |
| Supramarginal Gyrus | 54 | −36 | 46 | 2.3558 | 479 |
| Precentral Gyrus | 41 | −6 | 38 | 2.3945 | 456 |
| Inferior Frontal Gyrus | 43 | 28 | 13 | 2.2894 | 445 |
| Brain Stem (Ventral Tegmental Area) | 6 | −18 | −19 | 2.1764 | 412 |
| Middle Temporal Gyrus | 55 | −30 | −4 | 2.3831 | 381 |
| Medial Frontal Gyrus (orbital) | 1 | 35 | −12 | 2.3583 | 336 |
| Inferior Temporal Gyrus | 47 | −48 | −13 | 2.1871 | 333 |
| Middle Cingulate Gyrus | 12 | 0 | 46 | 2.1936 | 289 |
| Calcarine Sulcus | −14 | −74 | 10 | 2.2368 | 270 |
| Precuneus | −33 | −45 | 33 | 2.2335 | 263 |
| Inferior Frontal Gyrus (lateral) | −44 | 33 | 20 | 2.2685 | 240 |
| Supplementary Motor Area | −13 | 14 | 47 | 2.3237 | 209 |
| Middle Frontal Gyrus | 48 | −1 | 21 | 2.3186 | 208 |
| Inferior Frontal Gyrus (lateral) | 46 | 38 | 3 | 2.2620 | 161 |
| Middle Frontal Gyrus (orbital) | 29 | 48 | −10 | 2.1721 | 140 |
| Superior Frontal Gyrus | 16 | 53 | −13 | 2.1423 | 128 |
| Cerbellum (IX) | −9 | −48 | −47 | 2.1600 | 111 |
| Precentral Gyrus | −13 | −24 | 54 | 2.2378 | 109 |
| Calcarine Sulcus | 15 | −76 | 12 | 2.2075 | 94 |
| Inferior Frontal Gyrus | 45 | 1 | −37 | 2.2726 | 76 |
| Middle Temporal Gyrus | −50 | −55 | 19 | 2.1592 | 74 |
| Precentral Gyrus | 20 | −33 | 52 | 2.2133 | 64 |
| Inferior Temporal Gyrus | −53 | −53 | −17 | 2.0404 | 64 |
| Inferior Parietal lobule | −48 | −47 | 47 | 2.0931 | 63 |
| Precuneus | −10 | −53 | 41 | 2.1673 | 53 |
| Middle Occipital Gyrus | −32 | −68 | 27 | 2.1778 | 51 |
| Insula | −37 | 20 | 8 | 2.1219 | 45 |
| Precentral Gyrus | 43 | −17 | 31 | 2.0984 | 44 |
| Superior Frontal Gyrus | 17 | 34 | 48 | 2.0631 | 44 |
| Brain Stem (pons) | 8 | −19 | −32 | 2.0244 | 43 |
| Posterior Cingulate Gyrus | 12 | −17 | 37 | 2.0524 | 35 |
| Superior Temporal Gyrus | −44 | −20 | −1 | 2.1107 | 34 |
| Inferior Frontal Gyrus (lateral) | −48 | 34 | 6 | 2.1159 | 27 |
| Calcarine Sulcus | −8 | −60 | 5 | 2.0647 | 26 |
| Calcarine Sulcus | 18 | −89 | 5 | 2.0209 | 26 |
| Pallidum | 18 | 7 | 1 | 1.9957 | 26 |
| Insula | 36 | −14 | −3 | 2.0065 | 22 |
| Precentral Gyrus | −18 | −33 | 53 | 2.1050 | 19 |
| Superior Parietal Gyrus | 34 | −45 | 60 | 2.1036 | 19 |
| Superior Frontal Gyrus (oribital) | −19 | 19 | −12 | 2.0547 | 14 |
| Superior Occiptal | −17 | −90 | 4 | 2.0197 | 14 |
| Putamen | −26 | 17 | −5 | 2.0054 | 13 |
| Supramarginal Gyrus | −58 | −37 | 33 | 2.0670 | 11 |
| Inferior Frontal Gyrus (lateral) | 52 | 13 | 11 | 2.0409 | 10 |
| Middle Cingulate Gyrus | −14 | −8 | 52 | 2.0217 | 10 |
| Negative | |||||
| Superior Occipital Gyrus (large cluster extending into cuneus and precuneus regions) | −24 | −90 | 32 | −2.7946 | 92005 |
| Inferior Temporal Gyrus | −26 | −9 | −51 | −2.6839 | 4395 |
| Inferior Temporal Gyrus | 26 | −6 | −49 | −2.6520 | 7715 |
| Cerebellum (Crus I) | 51 | −74 | −31 | −2.4052 | 1029 |
| Cerebellum (Crus I) | −54 | −67 | −34 | −2.3282 | 1007 |
| Inferior Parietal Lobule | −67 | −34 | −24 | −2.3267 | 1222 |
| Inferior Frontal Gyrus (lateral) | −56 | 23 | 17 | −2.3187 | 372 |
| Inferior Frontal Gyrus (lateral) | −47 | 47 | 10 | −2.3185 | 243 |
| Postcentral Gyrus | 67 | −13 | 24 | −2.3071 | 290 |
| Inferior frontal Gyrus (orbital, medial) | −6 | 22 | −29 | −2.2751 | 567 |
| Middle Frontal Gyrus | 44 | 2 | 59 | −2.2714 | 1650 |
| Inferior Temporal Gyrus | 68 | −27 | −26 | −2.2274 | 1703 |
| Postcentral Gyrus | 58 | −19 | 52 | −2.1976 | 177 |
| Inferior Occipital Gyrus | −52 | −71 | −2 | −2.1876 | 1177 |
| Superior Temporal Pole | −28 | 25 | −26 | −2.1767 | 75 |
| Inferior Frontal Gyrus (orbital) | 44 | 32 | −21 | −2.1621 | 131 |
| Middle Frontal Gyrus | 46 | 26 | 43 | −2.1362 | 729 |
| Inferior Frontal Gyrus (orbital) | 15 | 41 | −30 | −2.1339 | 78 |
| Inferior Frontal Gyrus (lateral) | −55 | 37 | 5 | −2.1308 | 156 |
| Inferior Frontal Gyrus (lateral) | 58 | 28 | 6 | −2.1274 | 128 |
| Inferior Parietal Lobule | 56 | −41 | 56 | −2.1171 | 237 |
| Inferior Frontal Gyrus (orbital) | −12 | 45 | −31 | −2.1047 | 38 |
| Inferior Frontal Gyrus (orbital) | 26 | 22 | −25 | −2.0948 | 25 |
| Postcentral Gyrus | −46 | −21 | 66 | −2.0938 | 22 |
| Precuneus | −2 | −56 | 35 | −2.0634 | 16 |
| Inferior Frontal Gyrus (orbital, medial) | 8 | 16 | −27 | −2.0578 | 50 |
| Supramarginal Gyrus | 66 | −26 | 39 | −2.0526 | 49 |
| Precentral Gyrus | 65 | 8 | 17 | −2.0458 | 75 |
| Precentral Gyrus | −68 | −17 | 29 | −2.0438 | 35 |
| Precentral Gyrus | −1 | −25 | 80 | −2.0433 | 41 |
| Inferior Temporal Gyrus | 66 | −47 | −13 | −2.0361 | 108 |
| Postcentral Gyrus | −49 | −22 | 64 | −2.0304 | 27 |
| Inferior Frontal Gyrus (orbital) | 31 | 66 | −14 | −2.0171 | 36 |
| Middle Temporal Gyrus | −61 | −65 | 2 | −2.0068 | 40 |
| Superior Temporal Gyrus | 71 | −18 | 5 | −1.9868 | 12 |
| Middle Frontal Gyrus (orbital) | −29 | 61 | −15 | −1.9820 | 20 |
| Inferior Temporal Gyrus | −67 | −48 | −12 | −1.9788 | 12 |
| Overall Social Network Size (SNI-II) | |||||
| Brain Region(s) | X | Y | Z | z-value | k |
| Positive | |||||
| Parahippocampal gyrus (large cluster extending into hippocampal regions) | −19 | −42 | 2 | 2.2970 | 19825 |
| Hippocampus | 20 | −35 | 1 | 2.2889 | 2336 |
| Superior Frontal Gyrus (medial) | −10 | 60 | 2 | 2.1813 | 336 |
| Medial Frontal Gyrus (orbital) | 8 | 59 | −3 | 2.0941 | 62 |
| Hippocampus | 28 | −17 | −19 | 2.0911 | 177 |
| Inferior Frontal Gyrus (orbital) | −29 | 35 | −11 | 2.0810 | 143 |
| Anterior Cingulate Gyrus | −7 | 32 | 23 | 2.0732 | 66 |
| Inferior Temporal Gyrus | 51 | −55 | −15 | 2.0703 | 139 |
| Precuneus | −9 | −53 | 42 | 2.0649 | 30 |
| Anterior Cingulate Gyrus | 10 | 47 | 10 | 2.0624 | 154 |
| Inferior Frontal Gyrus (lateral) | 48 | 0 | 22 | 2.0605 | 31 |
| Medial Frontal Gyrus (orbital) | 1 | 33 | −12 | 2.0547 | 61 |
| Supramarginal Gyrus | 52 | −38 | 46 | 2.0515 | 46 |
| Insula | −38 | −1 | −3 | 2.0510 | 65 |
| Precentral Gyrus | −36 | −10 | 38 | 2.0390 | 163 |
| Superior Frontal Gyrus | 20 | 53 | 24 | 2.0342 | 62 |
| Brain Stem (Ventral Tegmental Area) | 5 | −18 | −18 | 2.0279 | 34 |
| Cerebellum (VIII) | −7 | −65 | −43 | 2.0219 | 41 |
| Lingual Gyrus | 21 | −42 | −9 | 2.0102 | 16 |
| Middle Temporal Gyrus | 54 | −29 | −4 | 1.9987 | 10 |
| Superior Frontal Gyrus (orbital) | −16 | 52 | −12 | 1.9960 | 19 |
| Thalamus | 19 | −3 | −9 | 1.9921 | 27 |
| Superior Frontal Gyrus (orbital) | −19 | 58 | −2 | 1.9879 | 13 |
| Cerebellum (IX) | 8 | −61 | −45 | 1.9705 | 10 |
| Negative | |||||
| Superior Frontal Gyrus (supplementary motor area) | 22 | −6 | 76 | −2.2634 | 5200 |
| Precentral Gyrus | −48 | −4 | 57 | −2.2460 | 1456 |
| Superior Occipital Gyrus | −25 | −89 | 33 | −2.2375 | 662 |
| Inferior Temporal Gyrus | −44 | −5 | −49 | −2.1814 | 364 |
| Inferior Temporal Gyrus | 26 | −7 | −50 | −2.1635 | 440 |
| Middle Frontal Gyrus | 52 | −2 | 54 | −2.1611 | 728 |
| Angular Gyrus | 54 | −68 | 30 | −2.1595 | 275 |
| Superior Frontal Gyrus | 17 | 20 | 65 | −2.1374 | 378 |
| Inferior Temporal Gyrus | 43 | −13 | −41 | −2.1351 | 1138 |
| Superior Parietal Gyrus | −29 | −73 | 56 | −2.1341 | 151 |
| Inferior Frontal Gyrus (lateral) | −48 | 47 | 10 | −2.1108 | 104 |
| Precuneus | −12 | −59 | 74 | −2.1048 | 492 |
| Superior Frontal Gyrus | 19 | 59 | 37 | −2.0949 | 131 |
| Superior Parietal Gyrus | 14 | −76 | 57 | −2.0945 | 470 |
| Paracentral Lobule | −19 | −22 | 79 | −2.0835 | 528 |
| Inferior Frontal Gyrus (orbital,medial) | −14 | 62 | −21 | −2.0816 | 51 |
| Supplementary Motor Area | −5 | 7 | 71 | −2.0812 | 54 |
| Cerebellum (Crus II) | 53 | −60 | −47 | −2.0733 | 44 |
| Superior Frontal Gyrus | −15 | 7 | 72 | −2.0718 | 92 |
| Inferior Frontal Gyrus (orbital, medial) | 14 | 65 | −20 | −2.0689 | 91 |
| Superior Frontal Gyrus | −21 | 66 | 24 | −2.0640 | 86 |
| Superior Occipital Gyrus | 28 | −87 | 35 | −2.0554 | 113 |
| Angular Gyrus | 50 | −60 | 51 | −2.0530 | 185 |
| Middle Frontal Gyrus | −30 | 11 | 66 | −2.0459 | 111 |
| Superior Frontal Gyrus | −17 | 40 | 56 | −2.0407 | 17 |
| Middle Frontal Gyrus | −43 | 46 | 28 | −2.0407 | 107 |
| Middle Frontal Gyrus (lateral) | −51 | 27 | 33 | −2.0343 | 62 |
| Angular Gyrus | −51 | −61 | 48 | −2.0330 | 26 |
| Middle Frontal Gyrus | −45 | 10 | 55 | −2.0281 | 165 |
| Middle Frontal Gyrus (lateral) | 30 | 28 | 57 | −2.0242 | 37 |
| Superior Parietal Gyrus | 10 | −59 | 73 | −2.0202 | 175 |
| Cuneus | 2 | −78 | 36 | −2.0164 | 79 |
| Supramarginal Gyrus | −64 | −47 | 27 | −2.0141 | 73 |
| Postcentral Gyrus | −54 | −45 | 56 | −2.0122 | 59 |
| Superior Parietal Gyrus | −41 | −67 | 55 | −2.0110 | 19 |
| Superior Parietal Gyrus | −27 | −43 | 75 | −2.0083 | 22 |
| Inferior Parietal Gyrus | −57 | −29 | 53 | −2.0076 | 26 |
| Inferior Parietal Gyrus | −39 | −76 | 44 | −2.0015 | 12 |
| Superior Parietal Gyrus | −24 | −63 | 66 | −1.9998 | 15 |
| Inferior Temporal Gyrus | 33 | 4 | −52 | −1.9956 | 12 |
| Inferior Frontal Gyrus (orbital) | −23 | 69 | −8 | −1.9897 | 12 |
| Orbitofrontal Gyrus (medial) | 6 | 72 | −2 | −1.9853 | 63 |
| Middle Frontal Gyrus | 27 | 63 | 28 | −1.9848 | 15 |
Gray matter covariance network associated with total number of high-contact social roles (SNI-1).
The gray matter volume covariance pattern associated with SNI-1 scores was constructed from two principal components, and accounted for 17% of the variance in gray matter volume (R2 =.17). Positively weighted regions included superior, middle and inferior frontal (medial, lateral, and orbitofrontal) regions, inferior and middle temporal regions (including parahippocampal, hippocampal, entorhinal and amygdala) as well as precuneus, precentral (primary motor), insular, cingulate (anterior, posterior and mid-cingulate) thalamic, pallidum, brain stem (pons and ventral tegmental area) and cerebellar (VIII and IX) regions. Negatively weighted regions included superior and inferior occipital, superior and inferior temporal, middle and inferior frontal (particularly lateral inferior and orbitofrontal), as well as postcentral (primary somatosensory) and cerebellar (particularly Crus I and VIIb) regions. We also found that greater expression of this gray matter covariance pattern linked to SNI-1 was associated with better memory as assessed with Total Free Recall scores on the FCSRT, Pearson’s r =.28, p =.01, but not with processing speed (Trails A; Pearson’s r = .19, p =.09) or executive functions (Trails B-A, Pearson’s r =.16 p =.16).
Gray matter covariance network associated with total number of network members (SNI-2).
The gray matter volume covariance pattern associated with SNI-2 scores was constructed from two principal components, and had an R2 of .29 (i.e. accounted for 29 % of the variance in gray matter volume) Positively weighted regions included superior, middle and inferior frontal cortex (medial, lateral, and orbitofrontal), inferior temporal, and medial temporal (parahippocampal and hippocampal) regions, as well as precuneus, precentral, insular, cingulate (anterior and middle), thalamic, brain stem (ventral tegmental area) and cerebellar (VIII & IX) regions. Negatively weighted regions included supplementary motor, primary motor (precentral gyrus) and primary somatosensory (postcentral gyrus) regions, superior occipital, superior middle and inferior frontal gyrus (particularly medial and orbitofrontal), as well as inferior temporal, precuneus, and cerebellar (Crus II) regions. We also found that greater expression of this gray matter covariance pattern linked to SNI-2 was associated with better memory, Pearson’s r =.28, p =.01, but not with processing speed (Pearson’s r=.17, p =.12) or executive functions (Pearson’s r=.14, p =.22)
Discussion
The current study is the first to examine gray matter volume covariance patterns associated with social networks in a reasonably sized sample of older adults without dementia. We identified gray matter volume covariance patterns associated with total number of high-contact social roles and total number of network members that were composed of both shared and distinct regions (see Figure 1a and 1b). We also found that greater expression of these gray matter covariance patterns linked to total number of high-contact social roles and total number of network members were associated with better memory, but not with processing speed or executive function. We discuss the implications of these results in more detail next.
A distributed pattern of gray matter volume is associated with total number of high-contact social roles.
The gray matter volume of a widely distributed pattern of brain regions was associated with total number of high-contact social roles in the current study of older adults without dementia. Key nodes in this pattern were medial, lateral, and orbital prefrontal cortex, medial temporal (hippocampal, parahippocampal, entorhinal and amygdala), precuneus, insular cingulate (anterior, middle and posterior), and brain stem (pons and ventral tegmental area) regions (see Figure 1a and Table 2). Medial, lateral and orbital prefrontal, entorhinal, and amygdala regions were previously linked to social networks and social support in predominately young and middle-aged adults (Kevin C. Bickart, Mark C. Hollenbeck, Lisa Feldman Barrett, & Bradford C. Dickerson, 2012; Bickart et al., 2011; Lewis et al., 2011; Powell et al., 2012; Von Der Heide et al., 2014). Hippocampal and entorhinal regions are particularly affected in early Alzheimer’s disease (Thompson et al., 2003), while prefrontal cortex and insular regions are particularly affected in normal aging (Kalpouzos et al., 2009; Raz et al., 1997). Key functions associated with precuneus are visuospatial imagery, memory, and self-related mental representations (Cavanna & Trimble, 2006). The cingulate cortex plays an important role in a number of cognitive and emotional processes, including executive function, memory, and negative affect (Botvinick, Cohen, & Carter, 2004; Bush, Luu, & Posner, 2000; Shackman et al., 2011; Spaniol et al., 2009; Wagner, Shannon, Kahn, & Buckner, 2005). Finally, key functions associated with the insula are drives and emotions; yet recent studies suggest that insular regions are also important for maintaining executive function and attentional processes (Menon & Uddin, 2010), as well memory awareness in normal aging and AD (Cosentino et al., 2015). Taken together, these results suggest that the structural brain substrates associated with total number of high-contact social roles in older adults without dementia are composed of regions that undergo both age-related and AD-related changes, have been linked to social networks and social support in young and middle-aged adults, and are linked to a number of cognitive and emotional processes – including memory processes. Note also that many of these regions – including amygdala, hippocampal, orbitofrontal, medial prefrontal, and anterior cingulate regions - are part of the mesocorticolimbic dopamine system that originates in the ventral tegmental area, which was also a part of the network associated with total number of high-contact social roles (see Table 2). The mesocorticolimbic dopamine system is critical for reward-related learning and memory, and is also impaired in AD (Arias-Carrión, Stamelou, Murillo-Rodríguez, Menéndez-González, & Pöppel, 2010; Burns, Galvin, Roe, Morris, & McKeel, 2005; Gibb, Mountjoy, Mann, & Lees, 1989; Nobili et al., 2017).
A more restricted pattern of gray matter volume is associated with the total number of network members.
The gray matter volume of a similar, yet more restricted, pattern of brain regions was associated with total number of network members than total number of high-contact social roles in the current study of older adults without dementia. Shared nodes included medial, lateral and orbital prefrontal cortex, hippocampal, precuneus, insular and cingulate regions (see Figure 1b and Table 2). Unlike the total number of high-contact social roles, however, the total number of network members was not associated with gray matter volume in amygdala and entorhinal cortex regions. This finding tells us that the total number of high-contact social roles are more strongly associated with gray matter volume than the total number of network members. Nevertheless, total number of network members, like total number of high-contact social roles, were associated with gray matter volume in regions that undergo both age-related, AD-related changes to the structure of the brain, and have been previously linked to memory, social networks, and social support. (Kevin C. Bickart et al., 2012; Bickart et al., 2011; Burgess, Maguire, & O’Keefe, 2002; Kalpouzos et al., 2009; Lewis et al., 2011; Powell et al., 2012; Raz et al., 1997; Thompson et al., 2003; Von Der Heide et al., 2014).
Gray matter networks associated with the total number of high-contact social roles and the total number of network members are correlated with memory performance, but not with processing speed and executive function.
Greater expression of the gray matter covariance patterns associated with total number of high-contact social roles and total number of network members were associated with better memory performance in this study of older adults without dementia. This finding is consistent with that key nodes in these gray matter networks – particularly hippocampal regions, but also precuneus and anterior cingulate cortex – play important roles in memory (Burgess et al., 2002; Cavanna & Trimble, 2006; Spaniol et al., 2009). The expression of the gray matter covariance networks associated with the total number of high-contact social roles and the total number of network members, however, were not associated with a measure of processing speed (Trails A) and a measure of executive function (Trails B-A) in this sample of older adults without dementia. These findings are inconsistent with that key nodes in these gray matter networks, particularly medial, lateral and orbital prefrontal regions, have been linked to both executive functions and processing speed in general and performance on the trail making test in particular (Alvarez & Emory, 2006; Bowie & Harvey, 2006; Demakis, 2004; Eckert, Keren, Roberts, Calhoun, & Harris, 2010; Elderkin-Thompson, Ballmaier, Hellemann, Pham, & Kumar, 2008; Koechlin, Ody, & Kouneiher, 2003; Lee, Habeck, Razlighi, Salthouse, & Stern, 2016; Miller & Cohen, 2001). Future investigations are needed to determine the reliability and the generalizability of these correlations, and lack thereof - in different older adult populations, using the same, different, and potentially more challenging tests of processing speed, memory, and executive functions. Future investigations are also needed to determine if and how increasing the total number of high-contact social roles and/or total number of network members will be helpful for improving memory in normal and pathological aging.
Strengths, Limitations and Recommendations for Future Research.
There are important strengths, weaknesses, and implications of the current study findings. Identifying gray matter covariance networks associated with high-contact social roles and total number of network members in a physically-healthy and cognitively-normal sample of community-dwelling older adults with an appropriately adjusted, yet sensitive, multivariate analytic approach are the key strengths of the current study. This approach allowed us to identify gray matter covariance networks associated with both total number of high-contact social roles and total number of network members in older adults, which were composed of regions a) particularly affected in healthy aging and AD, b) previously linked to social networks and social support in young and middle-aged adults, and c) previously linked to a number of cognitive and emotional processes – including memory. This approach also allowed us to examine the relative distribution of the gray matter covariance patterns associated with total number of high-contact social roles and the gray matter covariance pattern associated with total number of network members. A more distributed pattern of gray matter volume was associated with total number of high-contact social roles than total number of network members, which tells us that high-contact social roles is more strongly associated with brain structure than total number of network members. Finally, this approach allowed us to examine the relationship between the expression of the gray matter covariance patterns associated with total number of high-contact social roles and total number of network members and different cognitive functions. The gray matter networks associated with total number of high-contact social roles and total number of network members in the current study of older adults were linked to a measure of memory, but not processing speed and executive functions.
Several limitations of the current study findings should also be considered. First, the results of the current study findings from older adults without dementia may not be generalizable to older adults with AD and related dementias. Thus, future studies of larger and more cognitively diverse populations of older adults are needed to determine the generalizability and/or define the boundaries of the current study findings. Second, the social network index employed in the current study did not consider if older adults had positive or negative relationships with the people in their social network. Although older adults are generally more satisfied with their social relationships than younger adults (Luong, Charles, & Fingerman, 2011), a recent study suggest that while positive social support from children is associated with a reduced risk of dementia, negative social support from children and other immediate family members is associated with an increased risk for the dementia (Khondoker, Rafnsson, Morris, Orrell, & Steptoe, 2017). Thus, additional studies are needed to explore potential similarities and differences between the brain substrates associated with positive and negative social relationships and social support in healthy aging and AD. Third, in the absence of longitudinal neuroimaging data the directionality of the relationship between gray matter volume, total number of high-contact social roles, and total number of network members remain unclear. It is also unclear what changes these brain substrates and social network indices undergo during healthy aging and AD, and how they may influence cognitive decline. Longitudinal studies that simultaneously examines trajectories of structural brain changes, cognitive function, total number of high-contact social roles, and total number of network members are needed to clarify these relationships.
Conclusions
This study suggest that total number of high-contact social roles and total number of network members in older adults are associated with gray matter networks, which include brain regions that are affected by both healthy and AD-related aging. This study also suggests that greater expression of these networks are associated with memory performance. Finally, this study suggests that the total number of high-contact social roles is more strongly associated with brain structure than the total number of network members.
References
- Alvarez JA, & Emory E (2006). Executive function and the frontal lobes: a meta-analytic review. Neuropsychology review, 16(1), 17–42. [DOI] [PubMed] [Google Scholar]
- Antonucci T (1990). Social support and social relationships Handbook of aging and the social sciences. Edited by: Binstock RH, George LK. 1990: San Diego, CA: Academic Press. [Google Scholar]
- Arias-Carrión O, Stamelou M, Murillo-Rodríguez E, Menéndez-González M, & Pöppel E (2010). Dopaminergic reward system: a short integrative review. International archives of medicine, 3(1), 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J (2007). A fast diffeomorphic image registration algorithm. Neuroimage, 38(1), 95–113. doi: 10.1016/j.neuroimage.2007.07.007 [DOI] [PubMed] [Google Scholar]
- Ashburner J, & Friston KJ (2005). Unified segmentation. Neuroimage, 26(3), 839–851. doi: 10.1016/j.neuroimage.2005.02.018 [DOI] [PubMed] [Google Scholar]
- Ashby FG (2011). Statistical analysis of fMRI data: MIT press. [Google Scholar]
- Association AP (2000). Diagnostic and statistical manual of mental disorders (revised 4th ed.). Washington, DC: American Psychiatric Association. [Google Scholar]
- Barnes LL, De Leon CM, Wilson RS, Bienias JL, & Evans DA (2004). Social resources and cognitive decline in a population of older African Americans and whites. Neurology, 63(12), 2322–2326. [DOI] [PubMed] [Google Scholar]
- Bassuk SS, Glass TA, & Berkman LF (1999). Social disengagement and incident cognitive decline in community-dwelling elderly persons. Annals of internal medicine, 131(3), 165–173. [DOI] [PubMed] [Google Scholar]
- Bergfield KL, Hanson KD, Chen K, Teipel SJ, Hampel H, Rapoport SI, … Alexander GE (2010). Age-related networks of regional covariance in MRI gray matter: reproducible multivariate patterns in healthy aging. Neuroimage, 49(2), 1750–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkman LF (1984). Assessing the physical health effects of social networks and social support. Annual review of public health, 5(1), 413–432. [DOI] [PubMed] [Google Scholar]
- Bherer L, Erickson KI, & Liu-Ambrose T (2013). A review of the effects of physical activity and exercise on cognitive and brain functions in older adults. Journal of aging research, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickart KC, Hollenbeck MC, Barrett LF, & Dickerson BC (2012). Intrinsic amygdala–cortical functional connectivity predicts social network size in humans. Journal of Neuroscience, 32(42), 14729–14741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickart KC, Hollenbeck MC, Barrett LF, & Dickerson BC (2012). Intrinsic Amygdala–Cortical Functional Connectivity Predicts Social Network Size in Humans. The Journal of Neuroscience, 32(42), 14729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickart KC, Wright CI, Dautoff RJ, Dickerson BC, & Barrett LF (2011). Amygdala Volume and Social Network Size in Humans. Nature neuroscience, 14(2), 163–164. doi: 10.1038/nn.2724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, & Carter CS (2004). Conflict monitoring and anterior cingulate cortex: an update. Trends in cognitive sciences, 8(12), 539–546. doi: 10.1016/j.tics.2004.10.003 [DOI] [PubMed] [Google Scholar]
- Bowie CR, & Harvey PD (2006). Administration and interpretation of the Trail Making Test. Nature Protocols, 1, 2277. doi: 10.1038/nprot.2006.390 [DOI] [PubMed] [Google Scholar]
- Burgess N, Maguire EA, & O’Keefe J (2002). The human hippocampus and spatial and episodic memory. Neuron, 35(4), 625–641. [DOI] [PubMed] [Google Scholar]
- Burnham KP, & Anderson DR (2002). Model selection and multimodel inference: a practical information-theoretic approach: Springer Science & Business Media. [Google Scholar]
- Burns J, Galvin J, Roe C, Morris J, & McKeel D (2005). The pathology of the substantia nigra in Alzheimer disease with extrapyramidal signs. Neurology, 64(8), 1397–1403. [DOI] [PubMed] [Google Scholar]
- Buschke H (1973). Selective reminding for analysis of memory and learning. Journal of Verbal Learning and Verbal Behavior, 12(5), 543–550. doi: 10.1016/S0022-5371(73)80034-9 [DOI] [Google Scholar]
- Bush G, Luu P, & Posner MI (2000). Cognitive and emotional influences in anterior cingulate cortex. Trends in cognitive sciences, 4(6), 215–222. [DOI] [PubMed] [Google Scholar]
- Cavanna AE, & Trimble MR (2006). The precuneus: a review of its functional anatomy and behavioural correlates. Brain, 129(3), 564–583. doi: 10.1093/brain/awl004 [DOI] [PubMed] [Google Scholar]
- Cohen S, Doyle WJ, Skoner DP, Rabin BS, & Gwaltney JM (1997). Social ties and susceptibility to the common cold. Jama, 277(24), 1940–1944. [PubMed] [Google Scholar]
- Cosentino S, Brickman AM, Griffith E, Habeck C, Cines S, Farrell M, … Stern Y (2015). The right insula contributes to memory awareness in cognitively diverse older adults. Neuropsychologia, 75, 163–169. doi: 10.1016/j.neuropsychologia.2015.05.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demakis GJ (2004). Frontal lobe damage and tests of executive processing: a meta-analysis of the category test, stroop test, and trail-making test. J Clin Exp Neuropsychol, 26(3), 441–450. [DOI] [PubMed] [Google Scholar]
- Du A, Schuff N, Zhu X, Jagust W, Miller B, Reed B, … Chui H (2003). Atrophy rates of entorhinal cortex in AD and normal aging. Neurology, 60(3), 481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert M, Keren N, Roberts D, Calhoun V, & Harris K (2010). Age-related changes in processing speed: unique contributions of cerebellar and prefrontal cortex. Front Hum Neurosci, 4(10). doi: 10.3389/neuro.09.010.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efron B, & Tibshirani RJ (1994). An introduction to the bootstrap: CRC press. [Google Scholar]
- Elderkin-Thompson V, Ballmaier M, Hellemann G, Pham D, & Kumar A (2008). Executive function and MRI prefrontal volumes among healthy older adults. Neuropsychology, 22(5), 626–637. doi: 10.1037/0894-4105.22.5.626 [DOI] [PubMed] [Google Scholar]
- Ertel KA, Glymour MM, & Berkman LF (2008). Effects of social integration on preserving memory function in a nationally representative US elderly population. American journal of public health, 98(7), 1215–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fratiglioni L, Paillard-Borg S, & Winblad B (2004). An active and socially integrated lifestyle in late life might protect against dementia. The Lancet Neurology, 3(6), 343–353. doi: 10.1016/S1474-4422(04)00767-7 [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Poline JB, Grasby PJ, Williams SC, Frackowiak RS, & Turner R (1995). Analysis of fMRI time-series revisited. Neuroimage, 2(1), 45–53. doi: 10.1006/nimg.1995.1007 [DOI] [PubMed] [Google Scholar]
- Galvin JE, Roe CM, Powlishta KK, Coats MA, Muich SJ, Grant E, … Morris JC (2005). The AD8: a brief informant interview to detect dementia. Neurology, 65(4), 559–564. doi: 10.1212/01.wnl.0000172958.95282.2a [DOI] [PubMed] [Google Scholar]
- Gibb W, Mountjoy C, Mann D, & Lees A (1989). The substantia nigra and ventral tegmental area in Alzheimer’s disease and Down’s syndrome. Journal of Neurology, Neurosurgery & Psychiatry, 52(2), 193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grober E, Lipton RB, Hall C, & Crystal H (2000). Memory impairment on free and cued selective reminding predicts dementia. Neurology, 54(4), 827–832. [DOI] [PubMed] [Google Scholar]
- Grober E, Sanders AE, Hall C, & Lipton RB (2010). Free and cued selective reminding identifies very mild dementia in primary care. Alzheimer disease and associated disorders, 24(3), 284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutchess AH, Kensinger EA, & Schacter DL (2007). Aging, self-referencing, and medial prefrontal cortex. Social Neuroscience, 2(2), 117–133. doi: 10.1080/17470910701399029 [DOI] [PubMed] [Google Scholar]
- Habeck C, Foster NL, Perneczky R, Kurz A, Alexopoulos P, Koeppe RA, … Stern Y (2008). Multivariate and univariate neuroimaging biomarkers of Alzheimer’s disease. NeuroImage, 40(4), 1503–1515. doi: 10.1016/j.neuroimage.2008.01.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habeck C, Krakauer JW, Ghez C, Sackeim HA, Eidelberg D, Stern Y, & Moeller JR (2005). A New Approach to Spatial Covariance Modeling of Functional Brain Imaging Data: Ordinal Trend Analysis. Neural Computation, 17(7), 1602–1645. doi: 10.1162/0899766053723023 [DOI] [PubMed] [Google Scholar]
- Habeck C, Rakitin BC, Moeller J, Scarmeas N, Zarahn E, Brown T, & Stern Y (2005). An event-related fMRI study of the neural networks underlying the encoding, maintenance, and retrieval phase in a delayed-match-to-sample task. Brain Res Cogn Brain Res, 23(2–3), 207–220. doi: 10.1016/j.cogbrainres.2004.10.010 [DOI] [PubMed] [Google Scholar]
- Habeck C, & Stern Y (2007). Neural network approaches and their reproducibility in the study of verbal working memory and Alzheimer’s disease. Clin Neurosci Res, 6(6), 381–390. doi: 10.1016/j.cnr.2007.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habeck C, & Stern Y (2010). Multivariate Data Analysis for Neuroimaging Data: Overview and Application to Alzheimer’s Disease. Cell Biochemistry and Biophysics, 58(2), 53–67. doi: 10.1007/s12013-010-9093-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt-Lunstad J, Smith TB, & Layton JB (2010). Social relationships and mortality risk: a meta-analytic review. PLoS medicine, 7(7), e1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzer R, Wang C, & Verghese J (2014). Performance variance on walking while talking tasks: theory, findings, and clinical implications. AGE, 36(1), 373–381. doi: 10.1007/s11357-013-9570-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- House JS, Landis KR, & Umberson D (1988). Social relationships and health. Science, 241(4865), 540. [DOI] [PubMed] [Google Scholar]
- Kalpouzos G, Chételat G, Baron J-C, Landeau B, Mevel K, Godeau C, … Desgranges B (2009). Voxel-based mapping of brain gray matter volume and glucose metabolism profiles in normal aging. Neurobiol Aging, 30(1), 112–124. doi: 10.1016/j.neurobiolaging.2007.05.019 [DOI] [PubMed] [Google Scholar]
- Kawachi I, & Berkman LF (2001). Social ties and mental health. Journal of Urban health, 78(3), 458–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khondoker M, Rafnsson SB, Morris S, Orrell M, & Steptoe A (2017). Positive and negative experiences of social support and risk of dementia in later life: An investigation using the English Longitudinal Study of Ageing. Journal of Alzheimer’s Disease, 58(1), 99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koechlin E, Ody C, & Kouneiher F (2003). The Architecture of Cognitive Control in the Human Prefrontal Cortex. Science, 302(5648), 1181–1185. doi: 10.1126/science.1088545 [DOI] [PubMed] [Google Scholar]
- Kopell NJ, Gritton HJ, Whittington MA, & Kramer MA (2014). Beyond the connectome: the dynome. Neuron, 83(6), 1319–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Habeck C, Razlighi Q, Salthouse T, & Stern Y (2016). Selective association between cortical thickness and reference abilities in normal aging. Neuroimage, 142, 293–300. doi: 10.1016/j.neuroimage.2016.06.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis PA, Rezaie R, Brown R, Roberts N, & Dunbar RIM (2011). Ventromedial prefrontal volume predicts understanding of others and social network size. NeuroImage, 57(4), 1624–1629. doi: 10.1016/j.neuroimage.2011.05.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton RB, Katz MJ, Kuslansky G, Sliwinski MJ, Stewart WF, Verghese J, … Buschke H (2003). Screening for dementia by telephone using the memory impairment screen. Journal of the American Geriatrics Society, 51(10), 1382–1390. doi: DOI 10.1046/j.1532-5415.2003.51455.x [DOI] [PubMed] [Google Scholar]
- Luong G, Charles ST, & Fingerman KL (2011). Better with age: Social relationships across adulthood. Journal of social and personal relationships, 28(1), 9–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeill LH, Kreuter MW, & Subramanian S (2006). Social environment and physical activity: a review of concepts and evidence. Social science & medicine, 63(4), 1011–1022. [DOI] [PubMed] [Google Scholar]
- Menon V, & Uddin LQ (2010). Saliency, switching, attention and control: a network model of insula function. Brain structure & function, 214(5–6), 655–667. doi: 10.1007/s00429-010-0262-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, & Cohen JD (2001). An integrative theory of prefrontal cortex function. Annual review of neuroscience, 24(1), 167–202. [DOI] [PubMed] [Google Scholar]
- Moeller J, & Strother S (1991). A regional covariance approach to the analysis of functional patterns in positron emission tomographic data. Journal of Cerebral Blood Flow & Metabolism, 11(1_suppl), A121–A135. [DOI] [PubMed] [Google Scholar]
- Molesworth T, Sheu LK, Cohen S, Gianaros PJ, & Verstynen TD (2015). Social network diversity and white matter microstructural integrity in humans. Social cognitive and affective neuroscience, 10(9), 1169–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobili A, Latagliata EC, Viscomi MT, Cavallucci V, Cutuli D, Giacovazzo G, … Federici M (2017). Dopamine neuronal loss contributes to memory and reward dysfunction in a model of Alzheimer’s disease. Nature communications, 8, 14727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H-J, & Friston K (2013). Structural and functional brain networks: from connections to cognition. Science, 342(6158), 1238411. [DOI] [PubMed] [Google Scholar]
- Pennanen C, Kivipelto M, Tuomainen S, Hartikainen P, Hänninen T, Laakso MP, … Soininen H (2004). Hippocampus and entorhinal cortex in mild cognitive impairment and early AD. Neurobiology of Aging, 25(3), 303–310. doi: 10.1016/S0197-4580(03)00084-8 [DOI] [PubMed] [Google Scholar]
- Pillemer S, & Holtzer R (2016). The differential relationships of dimensions of perceived social support with cognitive function among older adults. Aging & mental health, 20(7), 727–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillemer S, Holtzer R, & Blumen HM (2017). Functional connectivity associated with social networks in older adults: A resting-state fMRI study. Social Neuroscience, 12(3), 242–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell J, Lewis PA, Roberts N, García-Fiñana M, & Dunbar RI (2012). Orbital prefrontal cortex volume predicts social network size: an imaging study of individual differences in humans. Proceedings of the Royal Society of London B: Biological Sciences, 279(1736), 2157–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph C, Tierney MC, Mohr E, & Chase TN (1998). The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. Journal of clinical and experimental neuropsychology, 20(3), 310–319. [DOI] [PubMed] [Google Scholar]
- Raz N, Gunning FM, Head D, Dupuis JH, McQuain J, Briggs SD, … Acker JD (1997). Selective aging of the human cerebral cortex observed in vivo: differential vulnerability of the prefrontal gray matter. Cerebral Cortex, 7(3), 268–282. doi: 10.1093/cercor/7.3.268 [DOI] [PubMed] [Google Scholar]
- Reitan R (1978). Manual for Administration of Neuropsychological Test Batteries for Adults and Children: Tucson, AZ: Reitan Neuropsychology Laboratories. [Google Scholar]
- Rodrigue KM, & Raz N (2004). Shrinkage of the entorhinal cortex over five years predicts memory performance in healthy adults. Journal of Neuroscience, 24(4), 956–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman TE, Lusignolo TM, Albert M, & Berkman L (2001). Social relationships, social support, and patterns of cognitive aging in healthy, high-functioning older adults: MacArthur Studies of Successful Aging. Health Psychology, 20(4), 243–255. doi: 10.1037/0278-6133.20.4.243 [DOI] [PubMed] [Google Scholar]
- Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, & Davidson RJ (2011). The integration of negative affect, pain and cognitive control in the cingulate cortex. Nature Reviews Neuroscience, 12(3), 154–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaniol J, Davidson PS, Kim AS, Han H, Moscovitch M, & Grady CL (2009). Event-related fMRI studies of episodic encoding and retrieval: meta-analyses using activation likelihood estimation. Neuropsychologia, 47(8), 1765–1779. [DOI] [PubMed] [Google Scholar]
- Spetsieris PG, & Eidelberg D (2011). Scaled subprofile modeling of resting state imaging data in Parkinson’s disease: Methodological issues. NeuroImage, 54(4), 2899–2914. doi: 10.1016/j.neuroimage.2010.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. Jacques P, Dolcos F, & Cabeza R (2010). Effects of aging on functional connectivity of the amygdala during negative evaluation: A network analysis of fMRI data. Neurobiol Aging, 31(2), 315–327. doi: 10.1016/j.neurobiolaging.2008.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffener J, Brickman AM, Habeck C, Salthouse TA, & Stern Y (2013). Cerebral blood flow and gray matter volume covariance patterns of cognition in aging. Human brain mapping, 34(12), 3267–3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, De Zubicaray G, Janke AL, Rose SE, Semple J, … Doddrell DM (2003). Dynamics of gray matter loss in Alzheimer’s disease. Journal of Neuroscience, 23(3), 994–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verghese J, Holtzer R, Lipton RB, & Wang C (2009). Quantitative Gait Markers and Incident Fall Risk in Older Adults. Journals of Gerontology Series A: Biological Sciences & Medical Sciences, 64A(8), 896–901. doi: 10.1093/gerona/glp033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Der Heide R, Vyas G, & Olson IR (2014). The social network-network: size is predicted by brain structure and function in the amygdala and paralimbic regions. Social Cognitive and Affective Neuroscience, 9(12), 1962–1972. doi: 10.1093/scan/nsu009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, & Buckner RL (2005). Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci, 9(9), 445–453. doi: 10.1016/j.tics.2005.07.001 [DOI] [PubMed] [Google Scholar]
- Whalen P, Shin LM, Somerville LH, McLean AA, & Kim H (2002). Functional neuroimaging studies of the amygdala in depression. Paper presented at the Seminars in clinical neuropsychiatry. [DOI] [PubMed] [Google Scholar]
- Yeh S-CJ, & Liu Y-Y (2003). Influence of social support on cognitive function in the elderly. BMC Health Services Research, 3, 9–9. doi: 10.1186/1472-6963-3-9 [DOI] [PMC free article] [PubMed] [Google Scholar]