Abstract
An iron complex bearing a pyridine(dicarbene) pincer was designed to probe the requirements of Lewis acid enabled N2H4 capture and subsequent N-N bond cleavage. Appended boron Lewis acids were installed by two methods to circumvent the incompatibilities associated with Lewis acid/base quenching of free carbenes and boranes. N2H4 capture by borane Lewis acids is dependent on both Lewis acidity and the steric profile about boron. A substitutionally inert primary coordination sphere at iron prevents an Fe-N2H4 interaction as well as N-N bond homolysis upon reduction.
Graphical Abstract

INTRODUCTION
The reductive capabilities of nitrogenase enzymes are unparalleled, and exemplified by their ability to reduce dinitrogen to ammonia.1–3 The 6H+/6e− reduction of N2 to NH3 represents one of the largest scale reactions on earth,4 yet key mechanistic steps regarding the biological reduction pathways remain largely unknown.5,6 Well-defined small molecule complexes can aid in the elucidation of complex substrate reduction sequences because they can be engineered to test hypotheses regarding key redox transformations between nitrogenous substrates. Although at least two mechanistic pathways have been proposed for the N-N bond rupture event in N2 reduction,7,8 synthetic platforms capable of accommodating the multiple products derived from N2 reduction (NxHy, x = 1,2; y = 0–4) are rare, and necessarily require geometric and/or electronic flexibility.9,10 Another design strategy reminiscent of the enzyme is to modify the metal’s secondary sphere with acidic/basic sites in order to capture and stabilize the array of nitrogenous substrates.
Second sphere interactions within enzyme active sites serve a variety of roles to facilitate substrate reduction by: (a) orienting small molecule substrates,11–14 (b) stabilizing high energy intermediates,15 or (c) providing channels for catalytic turnover.16 Synthetic systems have been designed to replicate this overall design aspect, most commonly, by incorporating Brønsted (hydrogen bond) donors within a ligand framework.17–20 Although hydrogen-bonding interactions serve a prominent role to stabilize high-valent intermediates,21–26 the highly reducing conditions associated with N2 fixation are incompatible with Brønsted acidic groups. In contrast, Lewis acidic groups are a viable secondary sphere alternative to address the reductive incompatibilities of Brønsted acidic residues and achieve substrate capture/activation27 and/or stabilization of high energy intermediates.28,29
Recently, we reported the synthesis of a pyridine(dipyrazole) iron complex with flexible appended boron Lewis acids capable of capturing a single equivalent of hydrazine outside of iron’s primary coordination sphere.30 Reduction resulted in hydrazine N-N homolytic cleavage, with stabilization of the parent amido ligands augmented by appended acids. Crossover experiments confirmed N-N bond cleavage occurred at a single metal center, and notably, the appended 9-BBN Lewis acids were required for this transformation. To extend the scope of Lewis acid-assisted activation of small molecules beyond this ligand system, structure/function relationships are required. Importantly, the interplay between the electronic/steric requirements of the metal as well as the Lewis acid(s) needs to be carefully optimized to facilitate a closed-loop synthetic cycle that proceeds through: (a) substrate capture (independent or cooperatively with metal), (b) stabilization of intermediates, and (c) release/exchange of products. Boron Lewis acids are ideally suited to probe both the acidity and steric requirements of these reactions/equilibria. Whereas examples of redox chemistry with the pyridine(dipyrazole) framework are rare,31 pyridine(dicarbene) ligands are capable of stabilizing metal centers in multiple oxidation states.32,33 Herein, we describe the development of a highly modular pyridine(dicarbene) ligand platform with appended boron Lewis acids, the requirements of N2H4 capture, and consequence of a substitutionally inert primary coordination sphere on N-N bond cleavage.
RESULTS AND DISCUSSION
Appended BR2 Installment on CNC Framework
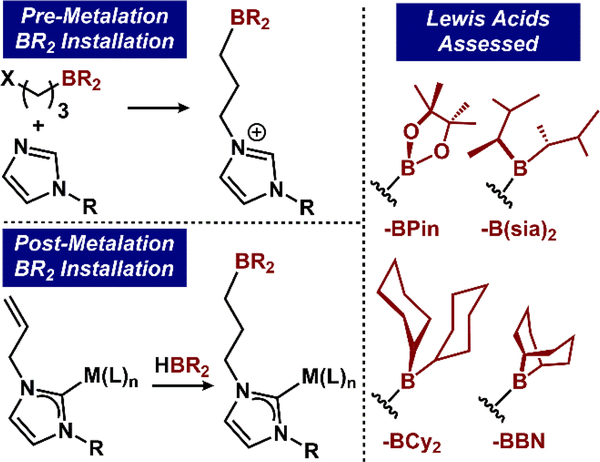
To probe the requirement of N2H4 capture by boron Lewis acids independent of the metal center, we sought to design a system that was coordinatively saturated at iron with substitutionally inert ligands. The 2,6-bis(carbene)pyridine system34–39 presents a robust ligand template with a similar bite-angle and steric profile to the previously studied pyridine(dipyrazole) species. Installment of 9-borabicyclo[3.3.1]nonyl (9-BBN) and 4,4,5,5-tetramethyl-1,2,2-dioxoboryl (BPin) Lewis acids on the 2,6-bis(imidazol-1-yl)pyridine (CNC) framework was achieved by two methods: 1) hydroboration of the previously reported 2,6-bis(N-allyl-imidazolium)pyridine,40 [allylH2CNC][BPh4]2, with 9-BBN in DCM to afford the anti-Markovnikov product, [BBNH2CNC][BPh4]2, and 2) nucleophilic addition of 2,6-bis(imidazol-1-yl)pyridine to Br(CH2)3BPin41 to afford [BPinH2CNC][BPh4]2 after anion metathesis.
Metalation of both [BPinH2CNC][BPh4]2 and [BBNH2CNC][BPh4]2 with Fe(N(SiMe3)2)2 was attempted (Figure 2). Treating an acetonitrile solution of Fe(N(SiMe3)2)2 with [BPinH2CNC][BPh4]2 affords low-spin [(BPinCNC)Fe(MeCN)3][BPh4]2 (1-BPin) as an orange powder. Under analogous reaction conditions, metalation of [BBNH2CNC][BPh4]2 did not afford a tractable iron containing species; instead, an inert Lewis acid/base pair formed between the free carbene and acidic 9-BBN (Figure 2).42 The identity of the quenched adduct was confirmed by independent deprotonation of [BBNH2CNC][BPh4]2 with nBuLi, which afforded a tetrahedral 11B NMR resonance at −15.44 ppm (C6D6), similar to the MeNHCMes·BEt3 adduct (−13.1 ppm, C6D6),43 as well as the absence of any imidazolium-CH resonances in the 1H NMR spectrum.
Figure 2.
Metalation of CNC ligand to afford compounds 1 and subsequent reduction to form 2-allyl and 2-BPin. Right: Molecular structures of 1-allyl and 2-allyl displayed with 50% probability ellipsoids. Hydrogen atoms not attached to allylic moiety are omitted for clarity.
To circumvent the acid/base quenching, we targeted a strategy of metalating the carbene ligand prior to installing the appended borane. Metalation of [allylH2CNC][BPh4]2 with Fe(N(SiMe3)2)2 in acetonitrile affords orange [(allylCNC)Fe(MeCN)3][BPh4]2 (1-allyl), analogous to 1-BPin. For each 1-allyl and 1-BPin, NMR spectroscopy (CD3CN) confirmed metalation by disappearance of the diagnostic imidazolium CH 1H-resonance (9.80 and 9.15 ppm, respectively) concomitant with a new carbene resonance in the 13C spectrum (201.90 and 200.29 ppm, respectively). Structural confirmation of 1-allyl was achieved by X-ray diffraction of single crystals, which revealed the expected mer-octahedral dicationic iron pincer species with pendent allylic moieties (Figure 2). Complex 1-allyl is structurally analogous to previously reported [(DIPPCNC)Fe(MeCN)3][BPh4]2 (DIPP = 2,6-diisopropylphenyl)39 but contains slightly longer Fe-C distances (1.975(2) and 1.970(2) Å).44
Carbonyls are strong field ligands that often impart substitutional inertness and also provide a spectroscopic handle to assess electronic changes to the primary coordination sphere.45 Carbonyl complexes were prepared by reduction of THF slurries of 1-allyl and 1-BPin with excess 0.4% Na(Hg) under a carbon monoxide atmosphere, affording (allylCNC)Fe(CO)2 (2-allyl) and (BPinCNC)Fe(CO)2 (2-BPin) as red powders (Figure 2). Upon reduction, the 13C NMR resonances assigned to the carbene for 2-allyl and 2-BPin shift downfield to 211.58 and 211.06 ppm, respectively, and infrared spectroscopy (KBr) confirmed the presence of carbonyl ligands (2-allyl: 1896, 1832 cm−1; 2-BPin: 1900, 1841 cm−1). The carbonyl ligands of complexes 2 are more activated than those in other dicarbonyl Fe(0) pincer compounds including (iPrPDI)Fe(CO)2 (1974, 1914 cm−1) (iPrPDI = 2,6-((DIPP)N=CMe)2-C5H3N; DIPP = 2,6-diisopropylphenyl),46 (PhPDPOSiMe3)Fe(CO)2 (1935, 1868 cm−1),31 and, notably, (DIPPCNC)Fe(CO)2 (1928, 1865 cm−1).38 Single crystal X-ray diffraction studies on 2-allyl confirmed a five-coordinate (τ5 = 0.52) iron dicarbonyl complex with the allylic substituents intact (Figure 2). Following reduction, the Fe-Ccarbene distances contract to 1.9194(15) Å and the Fe-Ccarbonyl distances (1.7457(15) Å) are identical to (DIPPCNC)Fe(CO)2 (1.746(3) and 1.767(4) Å).38 2-allyl is thermally stable in C6D6 in the presence of small molecule substrates such as N2H4 or H2 at 85 °C. Attempts to liberate the carbonyl ligands were unsuccessful: 2-allyl was unreactive toward both stoichiometric NaOH47 and N-methylmorpholine N-oxide48 at elevated temperature or under UV radiation and does not undergo ligand substitution reactions with trimethylphosphine or phenylisocyanide after 24 hr in C6D6. These experiments clearly demonstrate a highly inert primary coordination environment about iron in 2-allyl.
After establishing an inert primary coordination sphere, we targeted installment of the appended trialkylboranes. Anti-Markovnikov selective Lewis acid addition was achieved by treating THF solutions of 2-allyl with dicyclohexylborane, disiamylborane, and 9-BBN at room temperature for 16 hr to afford (BCy2CNC)Fe(CO)2 (2-BCy2), (sia2BCNC)Fe(CO)2 (2-sia2B), and (BBNCNC)Fe(CO)2 (2-BBN), respectively, as red powders (Figure 3). In each case, 1H NMR spectroscopy (C6D6) was employed to confirm consumption of the allylic resonances of 2-allyl and the 11B NMR spectra confirmed formation of the trialkylborane (2-BCy2 = 84.18; 2-sia2B = 81.10; 2-BBN = 87.73 ppm). Infrared (KBr) and NMR spectroscopy established that hydroboration has a minimal effect on the electronic environment at the iron: 13C-carbene resonances and υ(CO) vibrations (2-BCy2 = 210.77 ppm, 1902/1839 cm−1; 2-sia2B = 211.23 ppm, 1901/1839 cm−1; 2-BBN = 211.58 ppm, 1895/1835 cm−1) are comparable to 2-allyl.
Figure 3.
Top: hydroboration of 2-allyl to afford 2-BR2. Bottom: molecular structures of 2-BPin, 2-BBN, 2-sia2B, and 2-BCy2. 2-BPin and 2-sia2B are displayed with 30% probability ellipsoids while 2-BBN and 2-BCy2 are displayed with 50%. Hydrogen atoms have been removed for clarity and select carbon atoms are displayed in wireframe.
Structural analysis of single X-ray quality crystals of 2-BR2 revealed similar bonding metrics across the series (Figure 3, Table S14).49 For each, the appended boranes are non-interacting as noted by their planarity (ΣBα = 360°). The CNC ligands possess a bite angle (Ccarbene-Fe-Ccarbene = 157° for each) and geometry at iron (τ5 = 0.47–0.52) similar to (BBNPDPtBu)FeCl2 (Npyrazole-Fe-Npyrazole = 148.83°; τ5 = 0.37) confirming that compounds 2-BR2 are appropriate isostructural models to probe requirements of N2H4 capture and reduction.
Hydrazine Capture in Secondary Coordination Sphere
Given that the four appended Lewis acids within iron’s secondary coordination sphere do not perturb the electronic and steric environment at iron (vide supra), we assessed their relative Lewis acidity by determining their acceptor numbers (AN) using the Gutmann-Beckett method (Table S16).50 Model acids derived from styrene (PhCH2CH2BR2 (BR2 = BPin, sia2B, BCy2, BBN)), were investigated as proxies to simplify experimental determination by eliminating any competitive inter/intramolecular acid/base equilibria. The acid derived from 9-BBN affords the largest AN (24.9) and is similar to that reported for BEt3,51 while heteroatom stabilized PhCH2CH2BPin is the poorest acceptor (AN = 10.0). Surprisingly, the other two trialkylboranes, PhCH2CH2B(sia)2 and PhCH2CH2BCy2, also are best described as poor acceptors (AN = 10.2 and 13.0, respectively) by this method. The challenges of quantifying Lewis acidity is well documented51,52 and we propose the low acceptor numbers for PhCH2CH2B(sia)2 and PhCH2CH2BCy2 are derived from their larger steric encumbrance, compared to PhCH2CH2BBN. This steric congestion weakens the Lewis acid/base interaction for triethylphosphine oxide, a base with a larger cone angle than N2H4. Similar to a cone angle analysis, the steric contribution of the substituents on each acid was estimated by solid angle analysis, providing a relative ranking of steric accessibility at boron: BPin < BBN < BCy2 < B(sia)2 (Table S17).53 These data reinforce that Lewis acidity measurements are substrate-dependent and incorporate both steric accessibility and electrophilicity.
We interrogated the ability of the set of 2-BR2 compounds, containing boranes of varied acidity to sequester a single equivalent of N2H4. 2-BPin was unreactive toward N2H4, as determined by 1H NMR and IR spectroscopies, consistent with its low AN. Conversely, 2-sia2B and 2-BCy2 both react with a single equivalent of N2H4, despite their low AN, to afford 1:1 N2H4:Fe adducts (3-sia2B and 3-Bcy2; Figure 4). The captured N2H4 was confirmed in each case by NMR spectroscopy by a broad N2H4 resonance in the 1H-spectrum at 3.19 (3-sia2B, C6D6) and 4.42 ppm (3-Bcy2, THF-d8) as well as an upfield 11B resonance, consistent with a tetrahedral boron (3-sia2B = −6.04; 3-BCy2 = −8.41 ppm). IR spectroscopy (KBr) confirmed minimal perturbations to the iron center upon substrate capture by their similar υ(CO) vibrations (3-sia2B: 1901, 1836 cm−1; 3-BCy2: 1900, 1832 cm−1). Single, X-ray quality crystals of 3-BCy2 were investigated to probe the nature of the R3B-N2H4 interaction. Data refinement revealed a polymeric species in which borane Lewis acids from opposing molecules cooperatively sequester the N2H4 molecule (Figure 4). This bonding arrangement directly contrasts our previous findings for (BBNPDPtBu)MX2 (MX2 = FeCl2, FeBr2, ZnCl2) in which N2H4 capture occurred at a single ligand platform and was augmented by weak MX-H4N2 hydrogen bonding interactions. The similarity in bite-angle and geometry about iron between 2-BCy2 and (BBNPDPtBu)MX2 suggests the divergent mode of N2H4 capture (oligomeric vs. monomeric) is derived from the steric congestion imparted by the cyclohexyl substituents of 2-BCy2 (and sec-isoamyl substituents of 2-sia2B), which prevents monomeric N2H4 capture.54
Figure 4.
Capture of N2H4 with sterically encumbered borane Lewis acids, 2-sia2B and 2-BCy2. Bottom: molecular structure of 3-BCy2 displayed with 50% probability ellipsoids. Hydrogen atoms not attached to nitrogen are omitted and cyclohexyl groups are displayed in wireframe for clarity.
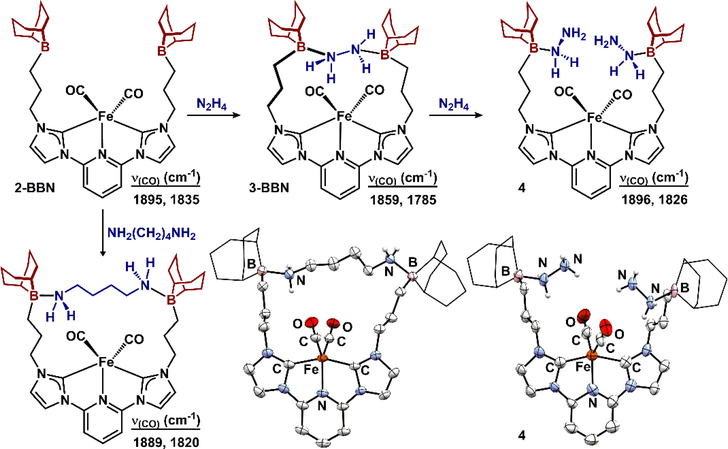
In contrast to the polymeric structure for 2-BCy2, addition of a single equivalent of N2H4 to a THF solution of 2-BBN results in precipitation of an orange solid assigned as (BBNCNC)Fe(CO)2(N2H4) (3-BBN) that displays a large shift in the υ(CO) (KBr, 1859, 1785 cm−1) that are broadened and bathochromically shifted, compared to 2-BBN (Δ = 36, 50 cm−1; Figure 5). The increased activation of the carbonyl ligands in 3-BBN is consistent with hydrogen bonding interactions55–57 between the captured hydrazine and carbonyl ligands in analogy to (BBNPDPtBu)FeCl2(N2H4). The insolubility of 3-BBN in common organic solvents precluded solution NMR characterization; however, 3-BBN gradually dissolved in DMSO concomitant with release of hydrazine (Figure S47). We propose the poor solubility is due to fewer degrees of freedom, compared to 2-BBN, as a result of increased rigidity upon capturing the N2H4. The assignment of 3-BBN is supported by the following data: 1) MALDITOF spectrometry is consistent with the (BBNCNC)Fe(CO)2 fragment (m/z = 647.370), 2) elemental analysis establishes the molecular formulation as (BBNCNC)Fe(CO)2(N2H4), 3) IR spectroscopy indicates the presence of N-H absorptions and CO bands (1859 and 1785 cm−1; shifted to lower energy to due hydrogen-bonding with the captured N2H4),56,57 4) release of N2H4 upon dissolution in DMSO to reform 2-BBN,58 5) synthesis of a more soluble variant, (BBNCNC)Fe(CO)2(1,4-diaminobutane) (vida infra), and 6) addition of a second equivalent of N2H4 affords the 2:1 N2H4:Fe complex (vida infra).
Figure 5.
Top: capture of one and two equivalents of N2H4. Bottom: synthesis of (BBNCNC)Fe(CO)2(1,4-diaminobutane) and molecular structures of 4 and (BBNCNC)Fe(CO)2(1,4-diaminobutane) displayed with 50% probability ellipsoids. Hydrogen atoms not attached to nitrogen are omitted for clarity and BBN substituents are displayed in wireframe.
Due to the insolubility of 3-BBN, we sought to develop a more soluble analogue to establish the entropic favorability of 1:1 Fe:substrate capture. We selected 1,4-diaminobutane as an extended diamine to impart higher solubility. Treating 2-BBN with a single equivalent of 1,4-diaminobutane afforded the 1:1 Fe:1,4-diaminobutane adduct, (BBNCNC)Fe(CO)2(1,4-diaminobutane) (Figure 5). Crystallographic characterization confirmed capture of the diamine between the two appended trialkylboranes forming a 19-atom macrocyclic structure (B-N = 1.657(3) and 1.654(3) Å). The flexibility of the attached trialkylboranes is highlighted by their ability to accommodate substrates of varied size: the B1-B2 distance in (BBNCNC)Fe(CO)2(1,4-diaminobutane) is 8.512 Å whereas the distance in (BBNPDPtBu)FeCl2(N2H4) is 4.191 Å. These studies demonstrate that, when the steric environment surrounding the boron is able to accommodate a substrate, intramolecular capture is favored over oligomeric capture (i.e. 3-BCy2).
The chemical composition of 3-BBN was further confirmed by addition of a second equivalent of hydrazine. Addition of N2H4 to an orange THF slurry of 3-BBN gradually affords a homogenous red solution from which a red powder was isolated and assigned as (BBNCNC)Fe(CO)2(N2H4)2 (4) (Figure 5). The IR spectrum (KBr) revealed υ(CO) absorptions (1896, 1826 cm−1) that are similar to complexes 2-BR2 and suggest diminished hydrogen bonding interactions between the hydrazine and carbonyl ligands. NMR spectroscopy (THF-d8) confirmed a tetrahedral boron (11B = −4.04 ppm) in 4 as well as two distinct NH2 resonances (1H: 3.38 (B-NH2), 5.70 (BNH2NH2)). The molecular structure of 4 by an XRD experiment confirmed its identity (Figure 5). The metrical parameters of the primary coordination sphere remain unchanged with respect to 2-BR2. The tetrahedral trialkylborane units (ΣBα = 337.6(8)°) each engage a separate molecule of N2H4 (B-N = 1.665(11) Å). The B-N distances in 4 are significantly shorter than those in 3-BCy2, consistent with smaller steric profile. In the solid state, each carbonyl ligand engages a separate N2H4 in moderate intramolecular hydrogen bonding interactions (Nproximal-O = 3.089, Ndistal-O = 3.016 Å) 59–61 that were not manifest in the IR spectra of the bulk samples.
The disparate modes of N2H4 capture between the four complexes, 2-BR2, suggests multiple factors, including Lewis acidity and steric profile, contribute to the ability to properly orient a small molecule substrate within the secondary coordination sphere. While 2-BPin is sterically accessible for acid/base interactions, it lacks the acidity to capture N2H4. Each trialkylborane variant, however, is sufficiently acidic. The large steric profile of 2-BCy2 and 2-sia2B, evident by their low AN’s, capably engage both N2H4 lone pairs, although this interaction requires an oligomeric conformation. Of the appended acids studied, only 2-BBN offers both adequate acidity and steric accessibility to allow monomeric N2H4 capture. Notably, 2-BBN is less reducing (Fe(0)/Fe(I) = −1.19 V vs. Fc/Fc+) than complexes of the BBNPDPtBu ligand class and may not be reducing enough on their own to reduce N2H4.
Reduction of 1:1 Hydrazine Adduct
The isolation of 3-BBN provided a platform to probe whether N2H4 coordination to the iron center was a requirement for N-N bond scission. The hypothetical product of hydrazine N-N bond scission, an amido-borane (R3B-NH2−), are typically synthesized by ammonia-borane deprotonation.62–64 We set out to assess whether isolation of these types of species was viable with this ligand platform. Addition of a THF solution of NH3 to 2-BBN affords the red ammonia-borane complex, (BBNCNC)Fe(CO)2(NH3)2 (5). Spectroscopic analysis revealed 5 is analogous to 4 with diagnostic NMR resonances (C6D6; 11B: −5.04; 1H: 0.58 ppm (B-NH3)) and infrared (KBr) υ(CO) absorptions (1889, 1821 cm−1). Addition of two equivalents of KN(SiMe3)2 to a freshly thawed THF solution of 5 affords the red amido-borane complex, [K(THF)]2[(BBNCNC)Fe(CO)2(NH2)2] (6). Investigation of 6 by 1H NMR spectroscopy (C6D6) revealed a C2v symmetric species—consistent with the amido-borane motif not interacting with iron—with the −NH2 resonance at −0.85 ppm. The 11B NMR resonance in 4 is shifted upfield, relative to 5, to −11.24 ppm. The infrared spectrum (KBr) reveals carbonyl ligands that are more activated (υ(CO) = 1871, 1801 cm−1) in comparison to 5. The additional carbonyl activation in 6 is cation dependent: υ(CO) absorptions shift to 1860 and 1794 cm−1 for the sodium analogue and upon half encapsulation of the potassium cations with 18-crown-6 to 1900 and 1839 cm−1.
Single X-ray quality crystals of both 5 and 6 (18-crown-6 encapsulated variant) were analyzed for comparison (Figure 6B). The metrical parameters for the primary sphere environment for both complexes are analogous (τ5 = 0.47 and 0.46 for 5 and 6, respectively). Each contain pyramidalized trialkylborane pendants (5: ΣB1α = 335.4(6), ΣB2α = 336.1(6); 6: ΣBα = 332.7(8)°) that interact with an ammonia (5: B-NH3 = 1.671(9) and 1.654(10 Å) or an amido (6: B-NH2 = 1.615(11) Å). The captured ammonia molecules in 5 engage in intermolecular hydrogen bonding interactions with THF solvate molecules while the amido substituent in 6 coordinates to the crown ether encapsulated potassium (N-K = 2.792(10) Å).
Figure 6.
A) Reduction of 3-BBN does not afford NHX equivalents. Synthesis of model amido-borane complex, 6. B) Molecular structures of 5 and 6 displayed with 50% and 30% probability ellipsoids, respectively.
We attempted to prepare the amido-borane complex, 6, through chemical reduction of captured hydrazine in 3-BBN. Addition of two equivalents of KC8 to a freshly thawed THF slurry of 3-BBN gradually afforded a homogenous red solution. 1H NMR spectroscopy revealed a mixture that does not contain either 5 or 6 (Figure 6A).65 To probe whether NH2− (or NH3) equivalents were formed, the reaction was quenched with HCl and the amount of NH4Cl produced was quantified by 1H NMR spectroscopy.66 Each molecule of 3-BBN can produce a maximum of two equivalents of NH2− if homolytic N-N cleavage occurs30,67 or between 4/3 and 2/3 equivalents for either limiting disproportionation pathways.68,69 Following reduction, quenching with HCl produced minimal NH4+ (0.08 equivalents, average of three runs, see SI). The digestion method was verified by quenching samples of 6. Treating samples of 6 with HCl followed by 1H NMR analysis confirmed each equivalent of 6 produces 1.83 equivalents of NH4+ (91.5% yield; average of 4 samples, see SI). These studies show that Lewis acid captured hydrazine cannot be reduced to NH2− equivalents if coordination to the metal center is inhibited.
CONCLUSION
Lewis acidic boranes were appended to a pyridine(dicarbene) ligand framework through late-stage hydroboration to circumvent the Lewis acid/base pair incompatibilities between the carbene and boron Lewis acid. The nature of the Lewis acid has consequences on the ability of the molecule to capture hydrazine: weakly acidic –BPin is incapable of sequestering N2H4, while sterically encumbered –BCy2 and –B(sia)2 Lewis acids favor oligomeric N2H4 capture. Only the moderately acidic and sterically accessible –BBN fragments enable hydrazine capture within a single ligand platform.
By incorporating substitutionally inert carbonyl ligand on the pincer dicarbene iron complex, insight into the reduction of the captured hydrazine was probed. Hydrazine reduction to NH3 equivalents was completely suppressed by eliminating the possibility for N2H4 coordination to the metal (Figure 7). These findings demonstrate that hydrazine coordination to the metal center is a requirement for productive bond scission. The lack of N-N homolysis in this system augments our prior study of the reductive N-N cleavage in (BBNPDPtBu)FeBr2(N2H4). In that case, upon reduction, the captured hydrazine molecule must migrate to the metal center to effect N-N cleavage. This reduction sequence requires dissociation of hydrazine from the appended borane concomitant with coordination to iron, and is only possible when employing moderately Lewis acidic fragments (9-BBN). Control of Lewis acidity is a critical design aspect necessary to enable both substrate capture and subsequent substrate/product release.
Figure 7.
Requirements of the primary coordination sphere to accommodate N-N homolysis of captured hydrazine.
Supplementary Material
Figure 1.
Methods used to install boron Lewis acids in this work.
SYNOPSIS.
Coordinatively saturated Fe(0) complexes bearing flexibly appended boron moieties synthesized by two methods. N2H4 capture by the Lewis acids is depended on both the acidity and steric profile about boron. A substitutionally inert primary coordination sphere hydrazine N-N bond cleavage upon reductions by preventing a Fe-N2H4 interaction.
ACKNOWLEDGMENT
This work was supported by the NIGMS of the NIH under award numbers 1R01GM111486–01A1 (N.K.S.) and F32GM126635 (J.J.K.). N.K.S. is a Camille Dreyfus Teacher–Scholar. X-ray diffractometers were funded by the NSF under award number CHE 1625543 (M.Z.).
Footnotes
The authors declare no competing financial interest.
ASSOCIATED CONTENT
Experimental procedures and spectroscopic characterization of all species are presented in the Supporting Information. The Supporting Information is available free of charge on the ACS Publications website.
REFERENCES
- (1).Dance I Nitrogenase: a general hydrogenator of small molecules Chem. Commun 2013, 49, 10893. [DOI] [PubMed] [Google Scholar]
- (2).Seefeldt LC; Yang Z-Y; Duval S; Dean DR Nitrogenase reduction of carboncontaining compounds Biochim. Biophys. Acta, Bioenerg 2013, 1827, 1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Yang Z-Y; Dean DR; Seefeldt LC Molybdenum Nitrogenase Catalyzes the Reduction and Coupling of CO to Form Hydrocarbons J. Biol. Chem 2011, 286, 19417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Smil V Enriching the Earth: Fritz Haber, Carl Bosch, and the Transformation of World Food Production; 1st ed.; MIT Press: Cambridge, MA, 2001. [Google Scholar]
- (5).Hoffman BM; Dean DR; Seefeldt LC Climbing Nitrogenase: Toward a Mechanism of Enzymatic Nitrogen Fixation Acc. Chem. Res 2009, 42, 609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Howard JB; Rees DC How many metals does it take to fix N2? A mechanistic overview of biological nitrogen fixation Proc. Nat. Acad. Sci. U.S.A 2006, 103, 17088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Rittle J; Peters JC An Fe-N2 Complex That Generates Hydrazine and Ammonia via Fe═NNH2: Demonstrating a Hybrid Distal-to-Alternating Pathway for N2 Reduction J. Am. Chem. Soc 2016, 138, 4243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Foster SL; Bakovic SIP; Duda RD; Maheshwari S; Milton RD; Minteer SD; Janik MJ; Renner JN; Greenlee LF Catalysts for nitrogen reduction to ammonia Nature Catalysis 2018, 1, 490. [Google Scholar]
- (9).Anderson JS; Moret M-E; Peters JC Conversion of Fe–NH2 to Fe–N2 with release of NH3 J. Am. Chem. Soc 2013, 135, 534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Saouma CT; Müller P; Peters JC Characterization of Structurally Unusual Diiron NxHy Complexes J. Am. Chem. Soc 2009, 131, 10358. [DOI] [PubMed] [Google Scholar]
- (11).Berglund GI; Carlsson GH; Smith AT; Szöke H; Henriksen A; Hajdu J The catalytic pathway of horseradish peroxidase at high resolution Nature 2002, 417, 463. [DOI] [PubMed] [Google Scholar]
- (12).Dunietz BD; Beachy MD; Cao Y; Whittington DA; Lippard SJ; Friesner RA Large Scale ab Initio Quantum Chemical Calculation of the Intermediates in the Soluble Methane Monooxygenase Catalytic Cycle J. Am. Chem. Soc 2000, 122, 2828. [Google Scholar]
- (13).Perutz MF; Fermi G; Luisi B; Shaanan B; Liddington RC Stereochemistry of cooperative mechanisms in hemoglobin Acc. Chem. Res 1987, 20, 309. [DOI] [PubMed] [Google Scholar]
- (14).Springer BA; Sligar SG; Olson JS; Phillips GN Jr. Mechanisms of Ligand Recognition in Myoglobin Chem. Rev 1994, 94, 699. [Google Scholar]
- (15).Zhang X; Houk KN Why Enzymes Are Proficient Catalysts: Beyond the Pauling Paradigm Acc. Chem. Res 2005, 38, 379. [DOI] [PubMed] [Google Scholar]
- (16).Hoffman BM; Lukoyanov D; Yang Z-Y; Dean DR; Seefeldt LC Mechanism of Nitrogen Fixation by Nitrogenase: The Next Stage Chem. Rev 2014, 114, 4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Sickerman NS; Peterson SM; Ziller JW; Borovik AS Synthesis, structure and reactivity of FeII/III-NH3 complexes bearing a tripodal sulfonamido ligand Chem. Commun 2014, 50, 2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Egbert JD; O’Hagan M; Wiedner ES; Bullock RM; Piro NA; Kassel WS; Mock MT Putting chromium on the map for N2 reduction: production of hydrazine and ammonia. A study of cis-M(N2)2 (M = Cr, Mo, W) bis(diphosphine) complexes Chem. Commun 2016, 52, 9343. [DOI] [PubMed] [Google Scholar]
- (19).Hartle MD; Delgado M; Gilbertson JD; Pluth MD Stabilization of a Zn(II) hydrosulfido complex utilizing a hydrogen-bond accepting ligand Chem. Commun 2016, 52, 7680. [DOI] [PubMed] [Google Scholar]
- (20).Creutz SE; Peters JC Exploring secondary-sphere interactions in Fe-NxHy complexes relevant to N2 fixation Chem. Sci 2017, 8, 2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Lee CH; Dogutan DK; Nocera DG Hydrogen Generation by Hangman Metalloporphyrins J. Am. Chem. Soc 2011, 133, 8775. [DOI] [PubMed] [Google Scholar]
- (22).MacBeth CE; Golombek AP; Young VG; Yang C; Kuczera K; Hendrich MP; Borovik AS O2 Activation by Nonheme Iron Complexes: A Monomeric Fe(III)-Oxo Complex Derived From O2 Science 2000, 289, 938. [DOI] [PubMed] [Google Scholar]
- (23).Matson EM; Park YJ; Fout AR Facile Nitrite Reduction in a Non-heme Iron System: Formation of an Iron(III)-Oxo J. Am. Chem. Soc 2014, 136, 17398. [DOI] [PubMed] [Google Scholar]
- (24).Wallen CM; Bacsa J; Scarborough CC Hydrogen Peroxide Complex of Zinc J. Am. Chem. Soc 2015, 137, 14606. [DOI] [PubMed] [Google Scholar]
- (25).Collman JP; Gagne RR; Reed C; Halbert TR; Lang G; Robinson WT Picket fence porphyrins. Synthetic models for oxygen binding hemoproteins J. Am. Chem. Soc 1975, 97, 1427. [DOI] [PubMed] [Google Scholar]
- (26).Syuhei Y; Akira W; Shigenori N; Teizo K; Koichiro J; Hideki M Thermal Stability of Mononuclear Hydroperoxocopper(II) Species. Effects of Hydrogen Bonding and Hydrophobic Field Chem. Lett 2004, 33, 1556. [Google Scholar]
- (27).Chen C-H; Gabbai FP Large-bite diboranes for the μ(1,2) complexation of hydrazine and cyanide Chem. Sci 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Henthorn JT; Agapie T Dioxygen Reactivity with a Ferrocene–Lewis Acid Pairing: Reduction to a Boron Peroxide in the Presence of Tris(pentafluorophenyl)borane Angew. Chem. Int. Ed 2014, 53, 12893. [DOI] [PubMed] [Google Scholar]
- (29).Miller AJM; Labinger JA; Bercaw JE Homogeneous CO Hydrogenation: Ligand Effects on the Lewis Acid-Assisted Reductive Coupling of Carbon Monoxide Organometallics 2010, 29, 4499. [Google Scholar]
- (30).Kiernicki JJ; Zeller M; Szymczak NK Hydrazine Capture and N–N Bond Cleavage at Iron Enabled by Flexible Appended Lewis Acids J. Am. Chem. Soc 2017, 139, 18194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Polezhaev AV; Chen C-H; Kinne AS; Cabelof AC; Lord RL; Caulton KG Ligand Design toward Multifunctional Substrate Reductive Transformations Inorg. Chem 2017, 56, 9505. [DOI] [PubMed] [Google Scholar]
- (32).Danopoulos AA; Wright JA; Motherwell WB; Ellwood S N-Heterocyclic “Pincer” Dicarbene Complexes of Cobalt(I), Cobalt(II), and Cobalt(III) Organometallics 2004, 23, 4807. [Google Scholar]
- (33).Yu RP; Darmon JM; Hoyt JM; Margulieux GW; Turner ZR; Chirik PJ High-Activity Iron Catalysts for the Hydrogenation of Hindered, Unfunctionalized Alkenes ACS Catalysis 2012, 2, 1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Pugh D; Wright JA; Freeman S; Danopoulos AA ‘Pincer’ dicarbene complexes of some early transition metals and uranium Dalton Trans. 2006, 775. [DOI] [PubMed] [Google Scholar]
- (35).Danopoulos AA; Tulloch AAD; Winston S; Eastham G; Hursthouse MB Chelating and ‘pincer’ dicarbene complexes of palladium; synthesis and structural studies Dalton Trans. 2003, 1009. [Google Scholar]
- (36).Darmon JM; Yu RP; Semproni SP; Turner ZR; Stieber SCE; DeBeer S; Chirik PJ Electronic Structure Determination of Pyridine N-Heterocyclic Carbene Iron Dinitrogen Complexes and Neutral Ligand Derivatives Organometallics 2014, 33, 5423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Pugh D; Wells NJ; Evans DJ; Danopoulos AA Reactions of ‘pincer’ pyridine dicarbene complexes of Fe(0) with silanes Dalton Trans. 2009, 7189. [DOI] [PubMed] [Google Scholar]
- (38).Danopoulos AA; Wright JA; Motherwell WB Molecular N2 complexes of iron stabilised by N-heterocyclic ‘pincer’ dicarbene ligands Chem. Commun 2005, 784. [DOI] [PubMed] [Google Scholar]
- (39).) Danopoulos AA; Tsoureas N; Wright JA; Light ME N-Heterocyclic Pincer Dicarbene Complexes of Iron(II): C-2 and C-5 Metalated Carbenes on the Same Metal Center Organometallics 2004, 23, 166. [Google Scholar]
- (40).Lake BRM; Willans CE Structural Diversity of Copper(I)–N-Heterocyclic Carbene Complexes; Ligand Tuning Facilitates Isolation of the First Structurally Characterised Copper(I)–NHC Containing a Copper(I)–Alkene Interaction Chem. Eur. J 2013, 19, 16780. [DOI] [PubMed] [Google Scholar]
- (41).Toure M; Chuzel O; Parrain J-L Synthesis and structure of Ag(i), Pd(ii), Rh(i), Ru(ii) and Au(i) NHC-complexes with a pendant Lewis acidic boronic ester moiety Dalton Trans. 2015, 44, 7139. [DOI] [PubMed] [Google Scholar]
- (42).Gott AL; Piers WE; McDonald R; Parvez M Synthesis of trifluoroborate functionalised imidazolium salts as precursors to weakly coordinating bidentate NHC ligands Inorg. Chim. Acta 2011, 369, 180. [Google Scholar]
- (43).Ono S; Watanabe T; Nakamura Y; Sato H; Hashimoto T; Yamaguchi Y Synthesis of N-heterocyclic carbene boranes via silver N-heterocyclic carbene complexes Polyhedron 2017, 137, 296. [Google Scholar]
- (44).Single crystal of 1-BPin were elusive, however, crystallization in the presence of two equivalents of 4-dimethylaminopyridine afforded single crystals of [(BPinCNC)Fe(MeCN)(DMAP)2][BPh4]2. Metrical parameters of this structure are presented in the supporting information.
- (45).Tondreau AM; Milsmann C; Lobkovsky E; Chirik PJ Oxidation and Reduction of Bis(imino)pyridine Iron Dicarbonyl Complexes Inorg. Chem 2011, 50, 9888. [DOI] [PubMed] [Google Scholar]
- (46).Bart SC; Lobkovsky E; Chirik PJ Preparation and Molecular and Electronic Structures of Iron(0) Dinitrogen and Silane Complexes and Their Application to Catalytic Hydrogenation and Hydrosilation J. Am. Chem. Soc 2004, 126, 13794. [DOI] [PubMed] [Google Scholar]
- (47).Farmery K; Kilner M Substitution reactions of dihydridotetracarbonyliron J. Chem. Soc. A: Inorg., Phys., Theo 1970, 634. [Google Scholar]
- (48).Luh T-Y Trimethylamine N-oxide—a versatile reagent for organometallic chemistry Coord. Chem. Rev 1984, 60, 255. [Google Scholar]
- (49).The molecular structure of 2-sia2B is of low quality. Full refinement details for each structure are provided in the supporting information.
- (50).Beckett MA; Strickland GC; Holland JR; Sukumar Varma K A convenient n.m.r. method for the measurement of Lewis acidity at boron centres: correlation of reaction rates of Lewis acid initiated epoxide polymerizations with Lewis acidity Polymer 1996, 37, 4629. [Google Scholar]
- (51).Sivaev IB; Bregadze VI Lewis acidity of boron compounds Coord. Chem. Rev 2014, 270–271, 75. [Google Scholar]
- (52).Plumley JA; Evanseck JD Periodic Trends and Index of Boron Lewis Acidity J. Phys. Chem. A 2009, 113, 5985. [DOI] [PubMed] [Google Scholar]
- (53).Guzei IA; Wendt M An improved method for the computation of ligand steric effects based on solid angles Dalton Trans. 2006, 3991. [DOI] [PubMed] [Google Scholar]
- (54).In the solid state, 3-BCy2 displays weak hydrogen bonding between the carbonyl ligand and N2H4 (O-N = 3.107 Å), but is not manifested by the υ(CO) (KBr, bulk sample).
- (55).Lokshin BV; Kazaryan SG; Ginzurg AG Hydrogen bonding in transition metal carbonyl complexes Bulletin of the Academy of Sciences of the USSR, Division of chemical science 1986, 35, 2390. [Google Scholar]
- (56).Hamley PA; Kazarian SG; Poliakoff M Hydrogen-Bonding and Photochemistry of Organometallics in Liquid Xenon Solution in the Presence of Proton Donors: A Low Temperature Infrared Study of the Interaction of (CF3)3COH with (C5Me5)M(CO)2L (M = Mn and Re; L = CO, N2, and H2) and with (C5Me5)V(CO)4 Organometallics 1994, 13, 1767. [Google Scholar]
- (57).Belkova NV; Besora M; Epstein LM; Lledós A; Maseras F; Shubina ES Influence of Media and Homoconjugate Pairing on Transition Metal Hydride Protonation. An IR and DFT Study on Proton Transfer to CpRuH(CO)(PCy3) J. Am. Chem. Soc 2003, 125, 7715. [DOI] [PubMed] [Google Scholar]
- (58).The B-N bond formation is reversible, as illustrated by the second addition of N2H4 to cleave one B-N bond to form 4. When 3-BBN is dissolved in dmso-d6, the sulfoxide (~14.1 M) is capable of outcompeting hydrazine.
- (59).Rettig SJ; Storr A; Trotter J Crystal and molecular structure of the di-μ-hydroxo-dimolybdenum compound, [Mo(CO)2(OH)(C4H7)(3,5-diMepzH)]2•C6H6 Can. J. Chem 1988, 66, 97. [Google Scholar]
- (60).Steiner T The Hydrogen Bond in the Solid State Angew. Chem. Int. Ed 2002, 41, 48. [DOI] [PubMed] [Google Scholar]
- (61).Braga D; Grepioni F Hydrogen-Bonding Interactions with the CO Ligand in the Solid State Acc. Chem. Res 1997, 30, 81. [Google Scholar]
- (62).Jacobs EA; Fuller A-M; Lancaster SJ; Wright JA The hafnium-mediated NH activation of an amido-borane Chem. Commun 2011, 47, 5870. [DOI] [PubMed] [Google Scholar]
- (63).Mountford AJ; Clegg W; Coles SJ; Harrington RW; Horton PN; Humphrey SM; Hursthouse MB; Wright JA; Lancaster SJ The Synthesis, Structure and Reactivity of B(C6F5)3-Stabilised Amide (M-NH2) Complexes of the Group 4 Metals Chem. Eur. J 2007, 13, 4535. [DOI] [PubMed] [Google Scholar]
- (64).Bernd W; Christine S; Rhett K; Germund G; Awal N Cyclic Hydroboration – The Structure of Perhydro-9b-boraphenalenes Z. Anorg. Allg. Chem 2016, 642, 922. [Google Scholar]
- (65).The two carbonyl ligands were confirmed to remain intact by IR spectroscopy (KBr): 1900 and 1838 cm−1.
- (66).Wickramasinghe LA; Ogawa T; Schrock RR; Müller P Reduction of Dinitrogen to Ammonia Catalyzed by Molybdenum Diamido Complexes J. Am. Chem. Soc 2017, 139, 9132. [DOI] [PubMed] [Google Scholar]
- (67).Yoo C; Lee Y A T-Shaped Nickel(I) Metalloradical Species Angew. Chem. Int. Ed 2017, 56, 9502. [DOI] [PubMed] [Google Scholar]
- (68).Schmidt EW Hydrazine and Its Derivatives: Preparation, Properties, Applications; 2nd ed.; Wiley: New York, 2001. [Google Scholar]
- (69).Umehara K; Kuwata S; Ikariya T N–N Bond Cleavage of Hydrazines with a Multiproton-Responsive Pincer-Type Iron Complex J. Am. Chem. Soc 2013, 135, 6754. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.