Abstract
Chronic peri-adolescent stress in humans increases risk to develop a substance use disorder during adulthood. Rats reared in social isolation during peri-adolescence (aSI; 1 rat/cage) period show greater ethanol and cocaine intake compared to group housed (aGH; 4 rats/cage) rats. In addition, aSI rats have a heightened dopamine response in the nucleus accumbens (NAc) to rewarding and aversive stimuli. Furthermore, single pulse electrical stimulation in slices containing NAc core elicits greater dopamine release in aSI rats. Here, we further investigated dopamine release kinetics and machinery following aSI. Dopamine release, across a wide range of stimulation intensities and frequencies, was significantly greater in aSI rats. Interestingly, subthreshold intensity stimulations also resulted in measurable dopamine release in accumbal slices from aSI but not aGH rats. Extracellular [Ca2+] manipulations revealed augmented calcium sensitivity of dopamine release in aSI rats. The readily releasable pools of dopamine, examined by bath application of Ro-04-1284/000, a vesicular monoamine transporter 2 (VMAT2) inhibitor, were depleted faster in aGH rats. Western blot analysis of release machinery proteins (VMAT2, Synaptogyrin-3, Syntaxin-1, and Munc13-3) showed no difference between the two groups. Tyrosine hydroxylase (TH) protein expression levels, however, were elevated in aSI rats. The greater dopamine release could potentially be explained by higher levels of TH, the rate-limiting step for dopamine synthesis. This augmented responsivity of the dopamine system and heightened dopamine availability post-aSI may lead to an increased risk of addiction vulnerability.
Keywords: peri-adolescent stress, social isolation, nucleus accumbens core, dopamine kinetics, dopamine availability, D2/D3 autoreceptors, tyrosine hydroxylase
Graphical Abstract
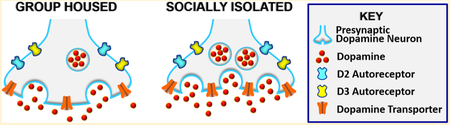
INTRODUCTION
Chronic stress exposure, especially during peri-adolescent phase, increases vulnerability to a myriad of neuropsychiatric and affective mental health disorders, such as generalized anxiety disorder, schizophrenia, and alcohol and substance use disorders.1-3 Self-report studies have shown that the number of adverse childhood experiences is positively correlated with increased prevalence of alcohol and cocaine dependence.4,5 Peri-adolescent social isolation (aSI) rearing in rats has been validated as a model of early life stress that engenders many behaviors linked with increased vulnerability to alcohol and substance use disorders.6 Behaviorally, rats exposed to aSI exhibit increased alcohol consumption7,8 and cocaine self-administration,9,10 elevated anxiety- and depressive-like behaviors,7,11,12 decreased social interaction,13 and reduced ability to adapt to environmental changes.14,15 Neurobiologically, aSI leads to morphological and synaptic alterations in many brain regions.6 In the current study, we chose to examine neurobiological alterations in dopamine release dynamics in the nucleus accumbens (NAc), as this region plays an integral role in the modulation of stress and anxiety-like behaviors.16 In addition, dopamine in the NAc plays a critical role in motivated and reward-seeking behaviors.17
Dopamine afferents into the NAc arise predominantly from the ventral tegmental area (VTA). Dopamine in the NAc is crucial for maintaining the overall excitatory and inhibitory balance of projections to other areas.18 The dopamine system goes through transformations within the mesocorticolimbic areas during adolescence; for example, there are alterations in dopamine receptor density during postnatal development—a majority of the studies have shown that dopamine receptor (D1-like and D2-like) expression in the NAc peaks during midadolescence and then declines in adulthood.19–23 In addition to receptor changes, developmental elevations in levels of tyrosine hydroxylase (TH) and basal dopamine have been reported during adolescence.24,25 Therefore, it is possible that exposure to stressful events during this time would interfere with the programmed maturation of the dopamine system resulting in its altered development.26,27 Indeed, it has been shown that repeated restraint stress exposure during the preadolescent period results in loss of D3 expression and function in the hippocampus.28 Together these studies indicate that the dopamine system continues to develop throughout adolescence and that exposure to stressful and aversive stimuli can profoundly alter the developmental trajectory of this neurotransmitter system.
Over the past few years, our group and others have shown profound and enduring changes in dopamine transmission in the NAc following aSI. For example, rats exposed to aSI have lower tonic levels of extracellular dopamine in the NAc compared to their group-housed (aGH) counterparts.29 However, dopamine tissue content levels and turnover have been reported to be greater in rats exposed to aSI.11,30,31 We have also repeatedly shown that stimulated dopamine release is greater and that reuptake of dopamine is faster in Asi rats.29,32,33 The augmented reuptake of dopamine and lower tonic levels mentioned above are likely due to an increase in dopamine transporter (DAT) protein expression levels.33 Further, we have shown an enhancement in cocaine induced evoked dopamine release in the NAc of aSI rats.33 In addition, in vivo studies have shown an augmentation of ethanol, cocaine, and foot-shock induced elevations in dopamine levels in aSI rats.11,34–37 Together, these studies suggest that, although tonic levels of dopamine are low following chronic stress exposure during adolescence, dopamine responses to salient stimuli are greater, possibly due to changes in dopamine neuron excitability or release-related mechanisms.
On the basis of the developmental data showing changes in the dopamine system, and our previous studies showing robust dysfunction in the dopamine transmission following chronic peri-adolescent stress, in the current study we sought to examine the mechanisms underlying enhanced electrically stimulated dopamine responses following aSI stress exposure. Because developmental studies show upregulation following pruning of dopamine receptors,19–23 experiments in the current study were designed specifically to test the function of autoreceptors involved in regulating dopamine release. Furthermore, we examined the probability of dopamine release and calcium sensitivity. Lastly, we examined dopamine availability, tyrosine hydroxylase (TH) levels, and expression levels of several proteins (VMAT2, synaptogyrin-3, syntaxin-1, munc13-3) involved in exocytotic dopamine release. These proteins were selected because of their involvement in vesicular release and packaging.
RESULTS AND DISCUSSION
Impact of aSI on Electrically Stimulated Dopamine Release in the NAc Core.
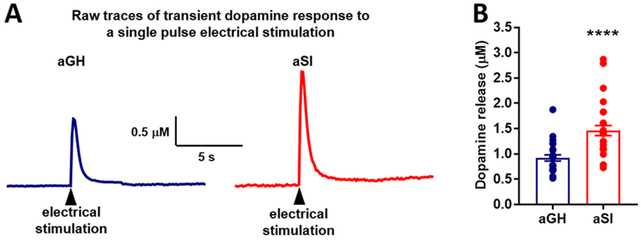
Following the aSI housing manipulation, electrically stimulated dopamine release was measured in slices containing the NAc core using fast scan cyclic voltammetry (FSCV). Dopamine was evoked using single pulse electrical stimulation. As shown in our previous studies, dopamine release was observed to be greater in recordings from aSI compared to aGH rats (Figure 1). Representative raw traces (Figure 1A) recorded from NAc core of a single aGH and aSI rat demonstrate enhanced dopamine release following aSI. An unpaired Student’s t-test between dopamine release in aGH and aSI rats revealed that electrically evoked dopamine responses were significantly augmented in aSI rats (Figure 1B; t51 = 4.49; p < 0.0001). Multiple studies in the past have shown that chronic stress exposure, especially during adolescence, dysregulates dopamine transmission,29,38,39 often resulting in a hypodopamine state. This hypodopamine state is characterized by lower tonic levels of dopamine and greater phasic (stimulated) dopamine levels, as shown by the current data and previous studies.29,40 In addition, a recent study showed that exposure to chronic and intermittent social defeat stress also results in augmented stimulated dopamine release.41 It is important to note, however, that this increase in stimulated dopamine release is only observed after aSI and not after adult social isolation.32 The following sections aim to understand the underlying mechanism that may drive this augmentation in dopamine response to electrical stimulation.
Figure 1.
Single electrical pulse evoked dopamine release in NAc core. (A) Raw representative traces illustrating transient dopamine response (peak height) to a single electrical pulse stimulation in NAc core of one aGH (blue) and one aSI (red) rat. (B) Population data showing a comparison of single electrical pulse-induced dopamine release in NAc core of aGH (blue) and aSI (red) rats. Evoked dopamine release was significantly greater in aSI compared to aGH rats. Group housed, aGH, n = 25; Socially isolated, aSI, n = 28; ****p < 0.0001.
Dopamine D2 and D3 Autoreceptor Function in NAc Core.
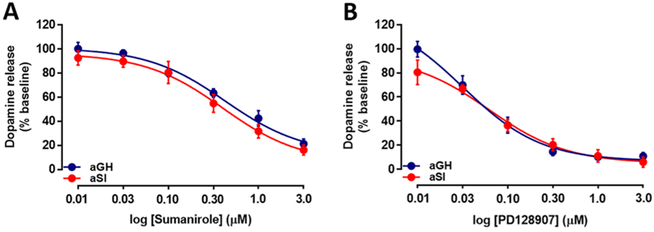
Dopamine D2-like autoreceptors (including D2 and D3 subtypes) reduce dopamine synthesis and release through inhibition of adenylyl cyclase, calcium channels, and other release-related mechanisms.42–46 At cell bodies, D2-type receptors also hyperpolarize dopamine neurons by activating G-protein-coupled inwardly rectifying potassium channels.46,47 Therefore, the enhanced release observed in aSI rats may indicate a disruption in D2 receptor signaling. The function of these D2-like (namely, D2 and D3) autoreceptors was examined by bath applying cumulative concentrations of selective D2 and D3 agonists to NAc core-containing slices from aGH and aSI rats (Figure 2; aGH: n = 4; aSI: n = 4 in both D2 and D3 experiments). A single-pulse electrical stimulation protocol was used here, because it is more sensitive to autoreceptor activation by application of exogenous ligand than longer stimulations, in which autoreceptors are activated within the stimulation.48 In addition, we documented autoreceptor changes with single-pulse stimulations in another study.49 A repeated measures two-way analysis of variance (ANOVA) showed that there was no significant difference in D2 autoreceptor function in aGH and aSI rats following bath application of cumulative concentrations of sumanirole (Figure 2A; 0.01–3.0 μM), a selective full agonist of the D2 autoreceptor. However, dopamine release was decreased in a concentration-dependent manner in both groups with application of sumanirole (F(5,30) = 127.3, p < 0.0001). Similarly, a repeated measures two-way ANOVA comparison of the data generated by cumulative concentration application of PD128907 (Figure 2B; 0.01–3.0 μM), a selective full agonist of the D3 autoreceptor, also revealed no significant difference of D3 autoreceptor function between aGH and aSI rats, but a PD128907 concentration-dependent reduction in dopamine release was observed in both groups (F(5,30) = 89.64, p < 0.0001). These data extend a previous study from our laboratory showing no difference in nonselective D2/D3 autoreceptor-mediated reduction in dopamine release between the two groups with application of quinpirole.32
Figure 2.
Dopamine D2 and D3 autoreceptor function in NAc core. (A) Activation of D2 autoreceptors with bath application of cumulative concentrations of sumanirole resulted in increased inhibition of dopamine release, but the function was not changed following housing manipulation. The magnitude of reduction was the same in aGH and aSI rats. (B) Activation of D3 autoreceptors with bath application of cumulative concentrations of PD128907 resulted in a similar reduction in stimulated dopamine release in both groups. Group housed, aGH, blue, n = 4; Socially isolated, aSI, red, n = 4.
Findings in the literature regarding stress effects are varied across distinct species and strains of rodents. For example, one study reported an increase in D2 receptor density, as measured by quantitative autoradiography, following repeated restraint stress exposure in the NAc of DBA/2 but not in C57BL/6 mice.50 Another study showed a reduction in D2 expression level in the NAc in mice reactive to chronic mild stress after five weeks of stress exposure.51 In the same study, stress-resilient mice showed a reduction in D2 expression following two weeks of chronic mild stress exposure, but this change was no longer observed after five weeks of chronic mild stress. These data suggest that the D2 receptor system may adapt to chronic mild stress exposure. Our data are also supported by another study that examined the effects of preadolescent exposure to repeated restraint stress on D3 expression in adulthood.28 This study showed that, while D3 expression levels and function were reduced in the hippocampus, no change was observed in the NAc. Thus, it is likely that this change in D3 expression and function is region-specific in our aSI model resulting in no change in the NAc. Alternatively, it is possible that the extended period of social isolation exposure in the current study results in D2/D3 autoreceptor adaptation such that the function recovers over time, occluding detection of any changes that may have been present after one or two weeks of social isolation. It is important to note, however, that studies generally do not differentiate between pre- and postsynaptic D2/D3 receptors, while the present study tested presynaptic D2 and D3 autoreceptors specifically.
Adolescent Social Isolation Stress Enhances Dopamine Release Potential.
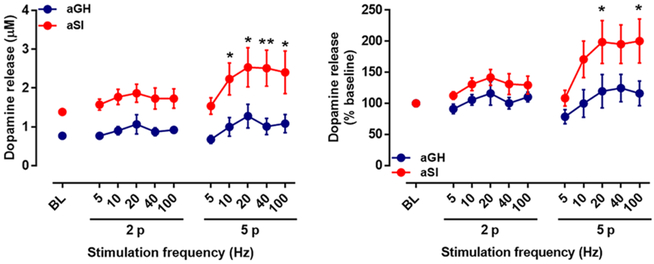
Exposure to stress has been shown to result in hyperexcitability of dopamine neurons in the VTA. For example, phasic dopamine neuron activity (high-frequency burst firing) in the VTA was increased in mice exposed to 10 d of chronic social defeat stress52 and in rats exposed to aSI.53 To examine the effects of aSI exposure on dopamine release in response to phasic stimulation, the NAc core in coronal slices was stimulated with multiple pulse stimulations at varying frequencies (two and five pulses at 5, 10, 20, 40, and 100 Hz), and dopamine release was measured (Figure 3; aGH: n = 5; aSI: n = 5). The stimulated dopamine levels at baseline (single pulse stimulation) were greater in aSI compared to aGH rats, as shown earlier. A comparison of multiple pulse stimulation (with varying frequencies)-induced dopamine release using a repeated measures two-way ANOVA between aSI and aGH rats revealed that dopamine release was significantly greater in aSI compared to aGH rats (Figure 3A; frequency variable: F(10,86) = 2.16, p < 0.05; housing variable: F(1,86) = 73.6, p < 0.0001). Bonferroni’s posthoc analysis revealed significant differences between the aGH and aSI rats at the five-pulse −10, 20, 40, and 100 HZ conditions. Percent baseline assessment using repeated measures two-way ANOVA also revealed that the stimulated dopamine release was significantly augmented in aSI compared to aGH rats (Figure 3B; frequency variable: F(10,88) = 3.21, p < 0.01; housing variable: F(1,88) = 25.8, p < 0.0001). Posthoc analysis showed elevated dopamine release in recordings from aSI rats in the five-pulse −20 and 100 HZ conditions. Ex vivo studies, including the current study, have shown that stimulated dopamine release is greater in rats exposed to chronic stress.29,32,33 In line with the current ex vivo results, in vivo studies have shown that salient stimuli, such as foot shock or systemic administration of ethanol, amphetamine, or cocaine, elicit a greater dopamine response in the NAc in animals with chronic or prolonged stress exposure.37,54–56 On the basis of these results, it is likely that chronic stress exposure augments dopamine release in response to salient stimuli. Therefore, in the next section, we aimed at examining dopamine response to low and high stimulation intensities.
Figure 3.
Dopamine release measured in NAc core mediated by multiple pulse stimulations at increasing frequencies. Dopamine release was evoked with two and five pulses at increasing frequencies. (A) Raw values of evoked dopamine release were significantly greater in aSI rats; an overall effect of frequency and housing was revealed with repeated measures two-way ANOVA. Bonferroni posthoc analysis revealed facilitated dopamine release with five pulses at high frequencies. (B) Percent baseline comparisons revealed increased stimulated dopamine release in aSI rats with an overall effect of frequencies and housing. Bonferroni posthoc analysis showed facilitated dopamine release in aSI rats with five pulse stimulations at high frequencies. Group housed, aGH, blue, n = 5; Socially isolated, aSI, red, n = 5; *p < 0.05; **p < 0.01.
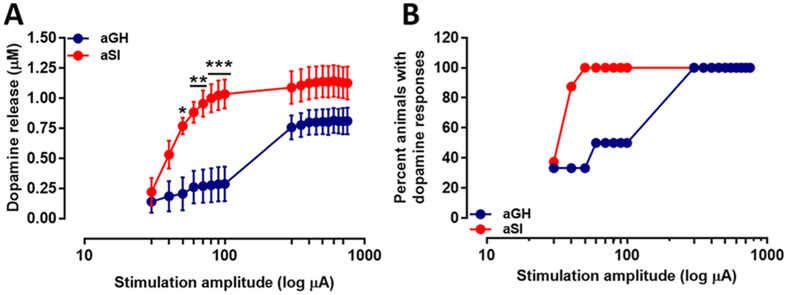
To examine dopamine release potential in response to low and high stimulus magnitudes, after baseline dopamine levels were stable, dopamine terminals in NAc core containing slices were electrically stimulated at varying intensities (30–750 μA), and dopamine release was measured. A repeated measures two-way ANOVA revealed a significant interaction between stimulation intensity and housing condition independent variables (Figure 4A; aGH: n = 6; aSI: n = 8; F(17,204) = 3.312, p < 0.0001). Specifically, while dopamine release was initially similar at low intensities, dopamine release increased at a greater rate as intensity increased in aSI rats. In addition, a significant effect of the independent variables, stimulus intensity (F(17,204) = 21.18, p < 0.0001), and housing condition (F(1,12) = 9.7, p < 0.01) was also observed. Posthoc analysis revealed significantly augmented dopamine release at 50, 60, 70, 80, 90, and 100 μA stimulus intensity in aSI rats. Therefore, chronic aSI stress exposure primes dopamine terminals so that more intense current injections produce greater dopamine release. In addition, we were also able to elicit a dopamine response at lower intensities (30–100 μA) in a greater percentage of accumbal slices from aSI rats (i.e., a greater percentage of aSI compared to aGH rats were responsive to low-intensity stimulation, as only one slice per rat was used in this experiment—Figure 4B). In these experiments, lower intensities (deemed subthreshold) often resulted in release failures. Failures to evoke release at lower intensities were observed more frequently in aGH than aSI rats (e.g., 4/6 failures in aGH vs 1/8 failures in aSI when stimulated at 40 μA). This effect is likely not due to dopamine release being below the detection limit, since nonfailures at low intensities produce release at 0.24 μM, which is an order of magnitude greater than that detected in slices using fast scan cyclic voltammetry (FSCV) in a recent study.57 This augmentation in dopamine release across increasing stimulation intensities indicates that it is possible that vesicular release probability is greater following aSI.
Figure 4.
Dopamine release elicited in NAc core by low and high stimulus intensities. (A) Dopamine release measured following single pulse electrical stimulation of low and high intensities. Dopamine release in aSI rats was measured to be significantly greater compared to dopamine release in aGH rats. Bonferroni posthoc analysis revealed a significant potentiation of dopamine release at the 50, 60, 70, 80, 90, and 100 μA stimulation intensities. (B) Percentage of slices (animals) in aGH and aSI groups in which dopamine was evoked at low and high stimulus intensities. Group housed, aGH, blue, n = 6; Socially isolated, aSI, red, n = 8; *p < 0.05; **p < 0.01; ***p < 0.001.
Facilitation of vesicular release probability could be driven by an increase in calcium sensitivity in aSI rats. In the following experiment, [Ca2+] in the aCSF was systematically varied (0.6–4.8 mM), and electrically stimulated (single pulse) dopamine release was measured (Figure 5; aGH: n = 5; aSI: n = 6) to examine calcium sensitivity. With a repeated measures two-way ANOVA, a significant interaction between [Ca2+] and housing condition was observed (Figure 5A; F(7,63) = 18.79, p < 0.0001); stimulated dopamine release was greater in aSI rats at supraphysiological [Ca2+]. In addition, a significant effect of [Ca2+] (F(7,63) = 100.9, p < 0.0001) and housing condition (F(1,9) = 30.04, p < 0.001) was observed. Posthoc analysis revealed that electrically stimulated dopamine release was greater at 1.8, 2.4, 3.0, 3.6, 4.2, and 4.8 mM [Ca2+]. Although no difference was observed at the physiological [Ca2+] (1.2 mM), when a repeated measures two-way ANOVA was used for comparison, an a priori Student’s t-test revealed that electrically stimulated dopamine release was significantly greater in the NAc core of aSI rats at the 1.2 mM [Ca2+] (Figure 5B; t9 = 3.52; p < 0.01). Because dopamine release at baseline was different in aGH and aSI rats, we normalized the data to directly compare dopamine response to [Ca2+] modulation in the two groups (Figure 5C). A percent baseline comparison using repeated measures two-way ANOVA revealed a significant interaction between [Ca2+] and housing condition independent variables (F(7,63) = 8.62, p < 0.0001). In addition, a main effect of [Ca2+] (F(7,63) = 74.42, p < 0.0001) and housing condition (F(1,9) = 8.06, p < 0.05) was observed. Posthoc analysis revealed that electrically stimulated dopamine release was greater at 2.4, 3.6, 4.2, and 4.8 mM [Ca2+]. This could potentially be due, at least in part, to altered calcium channels, a change in calcium sensitivity of dopamine release machinery, or an increase in the size of the dopamine readily releasable pool.
Figure 5.
Electrically (single pulse) stimulated dopamine release in the presence of increasing [Ca+2]. (A) After baseline levels of evoked dopamine release were stable, [Ca2+] in the aCSF was altered from 0.6 to 4.8 mM in incremental steps of 0.6 mM. Increasing concentrations of [Ca2+] lead to enhanced dopamine release in aSI compared to aGH rats. Bonferroni’s posthoc analysis revealed significant differences at 1.8, 2.4, 3.0, 3.6, 4.2, and 4.8 mM of [Ca2+]. (B) The physiological [Ca2+] is 1.2 mM. Because of small effect size the significance is lost in the process of running a repeated measures two-way ANOVA. Thus, we ran a Student’s t-test to test for group differences at 1.2 mM [Ca2+] concentration, to test our a priori hypothesis that stimulated dopamine release was greater in aSI rats at physiological levels of [Ca2+]. Indeed, the Student’s t-test showed this to be true. (C) A percent baseline comparison of evoked dopamine release between the two groups revealed significantly greater dopamine release with increasing [Ca2+] in slices from aSI rats. Group housed, aGH, blue, n = 5; Socially isolated, aSI, red, n = 6; **p < 0.01; ***p < 0.001; ****p < 0.0001.
Increase in Readily Releasable Dopamine Pool at the Terminal in NAc Core Following aSI.
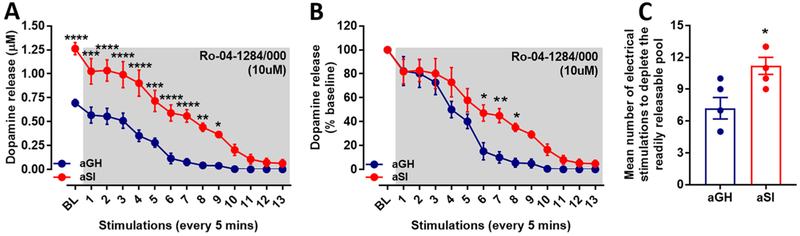
An increase in the readily releasable pool of dopamine could also contribute to an increased dopamine response to salient stimuli. Previous studies have shown that aSI results in increased dopamine content in NAc tissue samples.31 Such an increase in dopamine content could also be explained by a larger readily releasable pool, which further explains the augmented dopamine release observed in aSI rats. To examine the readily releasable pool and vesicular dopamine release, reserpine-like compound (Ro-04-1284/000) was bath-applied to slices containing the NAc core from aGH and aSI rats. Ro-04-1284/000 is a vesicular monoamine transporter 2 (VMAT2) blocker, and therefore it inhibits vesicular repackaging of dopamine following an occurrence of synaptic vesicular release and uptake. Ro-04-1284/000 was continuously perfused over slices containing the NAc core; single pulse electrical stimulation was applied once every 5 min to the NAc core, and dopamine release was measured. Overall, dopamine levels were depleted below the detection limit faster in slices from aGH compared to aSI rats (Figure 6; aGH: n = 5; aSI: n = 5). A repeated measures twoway ANOVA revealed an interaction between the number of stimulations to deplete the readily releasable pool and the housing condition (Figure 6A; raw values for dopamine levels; F(13,112) = 3.16, p < 0.001). In addition, a main effect of stimulation number to deplete the pool (F(13,112) = 41.94, p < 0.0001) and housing condition (F(1,112) = 183.4, p < 0.0001) were observed. Posthoc analysis revealed a significant difference between aGH and aSI groups in dopamine responses at baseline, first through ninth stimulations. A repeated measures two-way ANOVA of percent of baseline calculations (Figure 6B) between the two groups revealed a main effect of stimulation number to deplete the pool (F(13,112) = 49.44, p < 0.0001) and housing condition (F(1,112) = 31.63, p < 0.0001). Posthoc analysis revealed a significant difference between aGH and aSI groups in dopamine responses at the sixth (p < 0.05), seventh (p < 0.01), and eighth (p < 0.05) stimulations. A comparison between mean number of stimulations required to deplete the readily releasable pool of dopamine showed that a greater number of electrical stimulations was required to deplete the releasable pool storage in NAc core-containing slices from aSI compared to aGH rats (Figure 6C; t8 = 3.086; p < 0.05) concluding that aSI stress may increase vesicular dopamine releasable pools.
Figure 6.
VMAT2 blocker depletes readily releasable pools of dopamine faster in aGH compared to aSI rats. (A) Raw values of electrically stimulated dopamine release in the presence of Ro-04-128/000. Readily releasable pools deplete after eight or nine stimulations in NAc slices from aGH rats and ca. 11 to 12 stimulations in NAc slices from aSI rats. Posthoc analysis revealed significant differences between dopamine release in the two groups at baseline and first through the ninth stimulations. (B) Percent baseline representation of the data shown in (A). A lower number of stimulations was required to deplete readily releasable dopamine pools in aGH compared to aSI rats. Posthoc analysis revealed a significant difference in percent dopamine release at the sixth, seventh, and eighth stimulations. (C) Mean number of stimulations required to deplete the readily releasable dopamine pools were significantly greater in aSI compared to aGH rats. Group housed, aGH, blue, n = 5; Socially isolated, aSI, red, n = 5; *p < 0.05, **p < 0.01; ***p < 0.001; ****p < 0.0001.
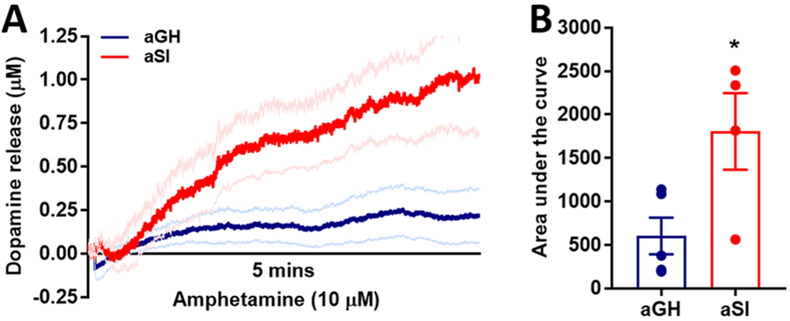
In this experimental protocol, because VMAT2s were blocked, repackaging of dopamine into synaptic vesicles did not occur. Therefore, any synaptic dopamine taken up by the DATs remained in the cytosol. To release this cytosolic dopamine, amphetamine was bath-applied to the slice following terminal depletion in the presence of Ro-04-1284/000, and dopamine release was measured in the NAc core (Figure 7; aGH: n = 5; aSI: n = 4). Amphetamine enters dopamine terminals through the DAT, resulting in DAT reversal so that dopamine is reverse-transported out of terminals.58,59 Amphetamine application resulted in a larger surge of dopamine in aSI compared to aGH rats (Figure 7A). Quantitative area under the curve comparison between aGH and aSI rats confirmed that dopamine released via reverse transport following amphetamine application over the slice was significantly greater in aSI rats (Figure 7B; t7 = 2.65; p < 0.05). These data indicate that the terminal content of dopamine was greater in aSI compared to aGH rats, possibly resulting in an elevation in release potential.
Figure 7.
Readily releasable dopamine pool size is larger in accumbal dopamine terminals in aSI compared to aGH rats. (A) Application of amphetamine, which reverses the function of the DAT causing dopamine to be transported exocytotically though the DAT. This experiment occurred in the same slices following the experiment in Figure 6. A greater amount of dopamine was released in aSI compared to aGH rats. (B) Area under the curve analysis of the graph in (A) shows that significantly more exocytotic dopamine released in aSI rats. Dopamine transporter, DAT; Group housed, aGH, blue, n = 5; Socially isolated, aSI, red, n = 4; *p < 0.05.
aSI Does Not Alter Expression of Release-Related Proteins in Dopamine Terminals.
Considering the increased release of dopamine across many forms of stimulation, we hypothesized that the levels of proteins involved in vesicular release and packaging might be increased following aSI. To examine these changes, we used Western blot hybridization and measured protein levels of VMAT2, synaptogyrin-3, syntaxin-1, and munc13-3. Since Western blot required additional tissue, NAc core and shell regions were considered together. Our comparison of dopamine release in shell and core with FSCV using this model showed that the two regions have similar adaptations. That is, electrically stimulated dopamine release (single-pulse) results in greater dopamine release in the core and shell of aSI rats compared to aGH rats.29 Therefore, although it is possible that these two regions have somewhat different expression of measured proteins, we would predict that proteins across regions are similarly modulated by aSI rearing.
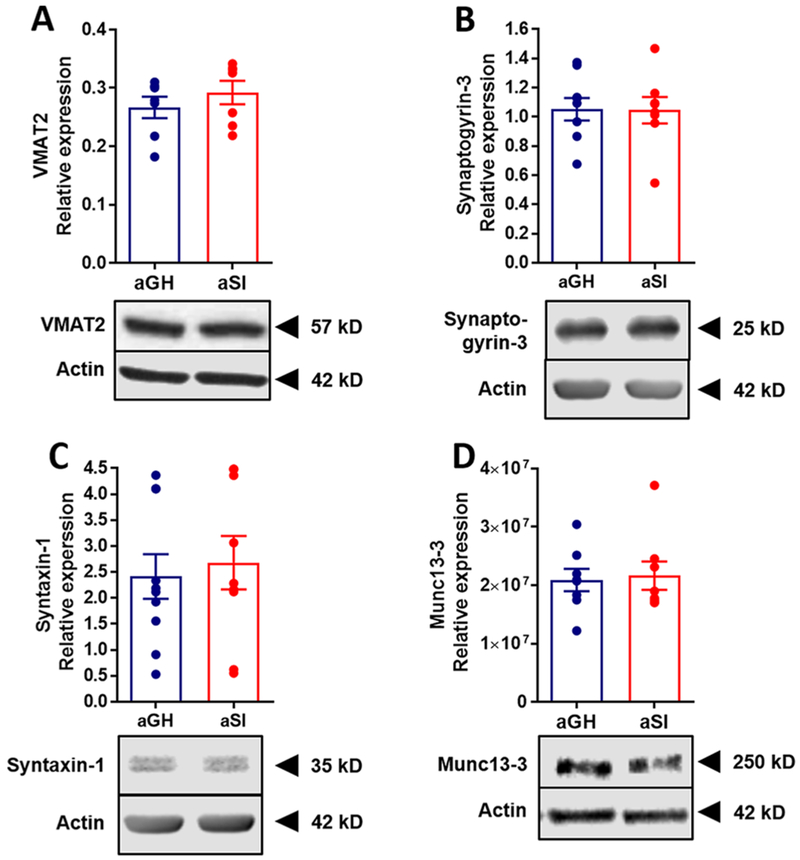
Dopamine packaging is primarily regulated by VMAT2, along with the DAT, by transporting cytosolic dopamine into synaptic vesicles within presynaptic terminals.60 VMAT2 activity largely dictates quantal size, ultimately affecting the size of subsequent dopamine release.61,62 Thus, perturbation of VMAT2 activity profoundly alters dopamine transmission. Because dopamine release was observed to be significantly greater in rats exposed to chronic stress during adolescence, and because inhibition of VMAT2 resulted in a differential temporal effect of electrical stimulation on dopamine depletion, we sought to examine VMAT2 expression levels in NAc tissue using Western blot hybridization (aGH: n = 7; aSI: n = 7). No significant differences between aGH and aSI VMAT2 expression levels were observed (Figure 8A; t12 = 0.936; p > 0.05) when compared using a Student’s t-test.
Figure 8.
Assessment of several terminal protein expression levels. Relative protein expression levels of (A) VMAT2 (n = 7 in both groups), (B) Synaptigyrin-3 (aGH: n = 6; aSI: n = 5), (C) Syntaxin-1 (aGH: n = 7; aSI: n = 6), and (D) Munc13-3 (aGH: n = 8; aSI: n = 8) were measured. None of these proteins had different expression levels in aGH and aSI rats. (insets) The representative Western blot images, with the respective protein and actin for comparison. Group housed, aGH, blue; Socially isolated, aSI, red.
Synaptogyrin-3 is another protein localized on synaptic vesicles63 that indirectly alters VMAT2 and DAT function by potentially increasing the interaction between these two proteins.64 Thus, we examined expression levels of synaptogyrin-3 in NAc tissue (aGH: n = 9; aSI: n = 8). Similar to VMAT2, however, we did not observe a significant difference between the two groups (Figure 8B; t15 = 0.051; p > 0.05). Next, we examined the expression of syntaxin-1 (aGH: n = 10; aSI: n = 9), another protein involved in exocytosis, and found no differences between expression levels in the NAc of aGH and aSI rats (Figure 8C; t17 = 0.403; p > 0.05). Finally, we examined the expression levels of Munc13–3 (aGH: n = 8; aSI: n = 8), as this protein is involved in priming and docking of synaptic vesicles.65 Considering the increased dopamine release probability observed in the current study, we expected to see an increase in Munc13-3 protein levels selectively in aSI rats. However, no difference was observed in NAc of aGH and aSI rats (Figure 8D; t14 = 0.238; p > 0.05).
The proteins examined in the current study are seldom examined in relation to stress exposure, especially during adolescence. A study has shown down-regulation of VMAT2 in the NAc following chronic forced swim stress for three weeks;66 however, this stress exposure occurred in adult rats, and this change may be age-dependent. We have shown that dopamine dynamics, and anxiety-like and ethanol intake behaviors, do not alter after social isolation in adulthood.8,32 Alternatively, it is possible that there are no changes in total expression levels, but an analysis of synaptosomes may have yielded some differences. Additionally, it is possible that some proteins may be altered selectively in dopamine terminals, whereas total tissue content expression levels consist of afferent dopamine and glutamate terminals along with interneurons. Another possibility is that protein function is altered with no change in expression.
Impact of aSI on Dopamine Synthesis.
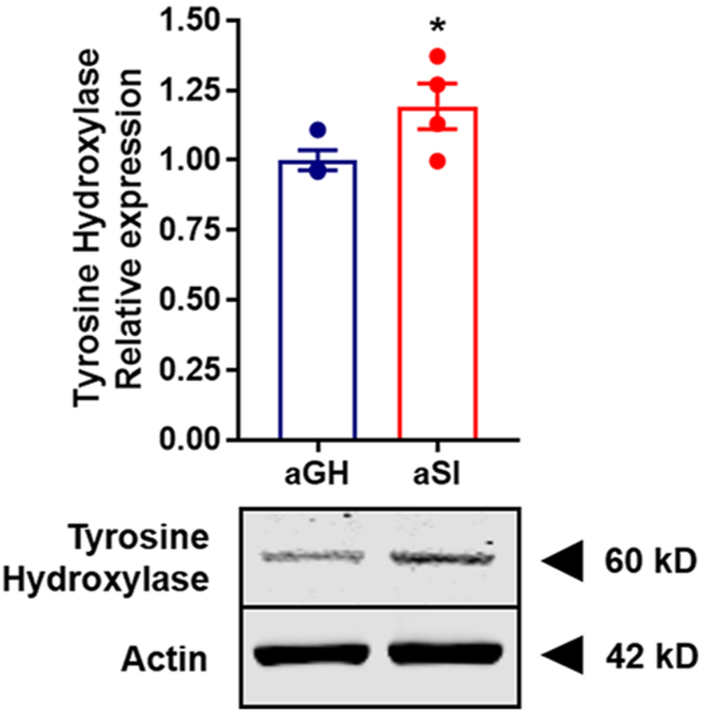
The enzyme tyrosine hydroxylase (TH) catalyzes hydroxylation of l-tyrosine to l-3,4-dihydroxyphenylalanine (L-DOPA), which is then converted to dopamine in a reaction catalyzed by DOPA decarboxylase. The conversion of l-tyrosine to L-DOPA is the rate-limiting step in dopamine synthesis. Therefore, we sought to examine TH protein expression levels in NAc tissue from aGH and aSI rats using a synaptoneurosomal preparation in conjunction with Western blot analysis (Figure 9; aGH: n = 4; aSI: n = 4). Synaptoneurosomes allow us to measure protein levels distinctly at pre- and postsynaptic sites.67,68 On the basis of the data in the current study showing increased dopamine availability, our a priori hypothesis was that TH protein expression levels would be greater in aSI compared to aGH rats. Indeed, a one-tailed t-test comparison revealed a significant difference between the two groups (Figure 9; t6 = 2.148; p < 0.05). These data indicate that the increased stimulated dopamine release and the augmented probability is likely driven, at least in part, by increased TH protein expression and corresponding production in dopamine. In addition, these data may also explain the increased dopamine content observed following aSI31 and the vesicular release experiment in the current study. Although there have been no studies that have measured TH protein expression levels in the NAc following chronic stress exposure, one study has reported elevated TH protein expression levels in the prefrontal cortex of rats exposed to chronic unpredictable stress.69 Because TH is the rate-limiting step in dopamine synthesis, an increase in TH likely results in increased dopamine content, which would further lead to a greater availability of dopamine for release. Therefore, it is possible for a greater magnitude of dopamine to be released in response to a given salient stimuli.
Figure 9.
Assessment of total TH protein expression using synaptosomal preparation. TH expression levels were significantly greater in aSI compared to aGH rats suggesting greater dopamine synthesis. (inset) Illustration of a Western blot image showing higher TH expression in aSI rats, with similar actin levels in aGH and aSI rats as the control. Tyrosine hydroxylase, TH; Group housed, aGH, blue, n = 4; Socially isolated, aSI, red, n = 4.
RESULTS SUMMARY
Taken together, data in the current study show that the potentiated evoked dopamine responses observed following aSI are largely driven by an increase in the size of the readily releasable pools, greater responsivity of release to [Ca2+], possibly due to enhanced calcium sensitivity of dopamine release, and augmented dopamine synthesis (shown by increased TH protein expression levels). Surprisingly, facilitated dopamine release appears to be driven from increases in TH as well as terminal excitability, whereas other release-related machinery and autoreceptor activity appear unaffected. It is unlikely that these changes in dopamine transmission are due to an increase in TH-containing neurons in the VTA, since neither the number of TH-positive neurons nor the volume of the VTA was altered following aSI.70 These findings suggest that the alterations observed are specifically occurring at the dopamine terminals in the NAc core. It is important to note that these changes were observed only in rats exposed to peri-adolescent social isolation. Adult social isolation does not produce such changes;32 however, social defeat stress exposure in adulthood does.41 This implies that varying degrees of stress and/or different stressors may engage this system differentially. Thus, while aSI can elicit changes during development of the catecholamine system, social isolation is not sufficient to alter the system once it has fully matured, as in adulthood. For such changes to occur in the VTA–NAc dopamine projection neurons during adulthood, a more severe stressor is necessary.
Implications for aSI-Induced Maladaptive Behaviors.
Postweaning rearing in social isolation leads to numerous maladaptive behaviors, including excessive consumption of ethanol7,8 and cocaine.9,10 In addition, rats exposed to social isolation during adolescence exhibit anxiety- and depressive-like behaviors in conjunction with cognitive inflexibility.7,11–15 These maladaptive behavioral perturbations may facilitate the development of addictive behaviors, elevating the risk of developing alcohol and substance use disorders. Results in the current study identify one major mechanism that likely contributes to the behavioral alterations mentioned above. Overall, we and others have shown that the mesolimbic dopamine system, specifically dopamine afferents from the VTA into the NAc, are hyper-responsive to external salient stimuli.11,34–37 This hyper-responsivity appears to be due to enhanced TH expression as well as augmented excitability of dopamine terminals, resulting in increased readily releasable vesicular pools and potentiated responsivity to high-frequency burst-firing. In ex vivo slice preparations, the hyper-responsivity is demonstrated by potentiated dopamine release in response to electrical stimulation and increasing concentrations of extracellular [Ca2+], suggesting that dopamine terminals may exhibit increased excitability, possibly due to altered expression of voltage-gated calcium channels. The ability of the dopamine system to have a facilitated response is driven, at least in part, due to augmented dopamine synthesis (shown by increased TH protein levels) resulting in greater dopamine availability following prolonged chronic stress exposure. Although expression of other release-related proteins (such as VMAT2, synaptogyrin-3, etc.) were not different between housing conditions, it is possible that the function of these proteins is altered due to post-translational modifications.
Burst-firing in dopamine neurons occurs in response to natural/drug rewards, cues associated with rewards, foot-shock, or any aversive stimuli, as well as reintroduction to previously exposed stress-inducing contextual cues, but responsivity is greater in stress-exposed rats.11,29,34–37,54 Notably, the enhanced dopamine responsivity observed in aSI rats corresponds with the heightened behavioral response to these external stimuli. Such a heightened response to external stimuli, especially substances of abuse, could potentially result in a shift in the valence of the perceived reward and push it more on the side of greater reward perception. Indeed, in the current model it has been shown that, while a 1 and 2 g/kg dose of ethanol administered intraperitoneally to aGH and/or stress naïve rats result in similar magnitude dopamine response in the NAc,71 the 2 g/kg dose of ethanol elicits a twofold increase in dopamine levels selectively in aSI rats.37 Note that aSI rats consume significantly more ethanol than aGH rats.8,29 Because a higher dose of ethanol (2 g/kg) results in augmented dopamine responses compared to a lower dose (1 g/kg) in aSI rats, and lower and higher doses of ethanol (1 and 2 g/kg) result in similar dopamine responses in aGH rats, it is possible that the greater ethanol consumption in aSI rats observed in previous studies occurs because ethanol is more reinforcing to aSI rats, whereas the reinforcing efficacy of ethanol to aGH rats is limited. It is important to note that the changes observed in the current study as well as previous studies occur only following peri-adolescent social isolation and not after isolation during adulthood.8,29,37
Other Regions and Other Stressors.
In the current study, we focused on the NAc core, as this is one of the striatal regions involved in and integral for stress, anxiety, depression, and motivation, and reinforcement-related processing.16,17 However, we would like to note that, in our previous studies, we observed increased dopamine release and uptake across the entire striatum—including NAc shell,29 NAc core,29,32,33 and dorsomedial striatum33 of aSI rats. In addition, using microdialysis, we observed ethanol-induced augmentation in dopamine responses in the basolateral amygdala of aSI rats.72
The peri-adolescent period during which we expose the rats to social isolation is rather long, and there may be a narrow window that could produce some of the phenotypes that we study.73,74 We chose a longer isolation period in the current study to be consistent with our earlier studies, which revealed profound and enduring behavioral and neurobiological adaptations using this model. In addition, in a previous study we showed that social isolation during adulthood does not affect electrically stimulated dopamine release and uptake measures.32 We hypothesize that other major stressors during peri-adolescence (such as restraint stress or predator odor exposure) would result in similar behavioral phenotypes and corresponding neurochemical changes and neural adaptations, as shown by previous studies in the medial prefrontal cortex.75,76 Thus, findings in the current study may provide additional valuable guidance for researchers interested in how chronic stress impacts mesolimbic dopamine signaling. Although we did not collect as much behavior, we believe that correlating behavioral disruptions with dopamine release, uptake, TH, or other neurobiological changes is an important task, and we will plan to do so in the future.
CONCLUSIONS
In conclusion, the results of the current study show that prolonged chronic social isolation stress exposure during peri-adolescence results in profound dysfunction of mesolimbic dopamine release dynamics. Neurobiological responsivity of this dopaminergic circuit to external stimuli is significantly heightened following aSI. Dopamine terminals in the NAc core are primed and possibly more sensitive to calcium influx thus leading to dopamine release at lower stimulation intensities. Furthermore, high-intensity and high-frequency stimulations result in greater dopamine release, indicating more dopamine availability, which was confirmed by elevated levels of TH. Together, these data suggest that adolescent stress primes the mesolimbic dopamine system, possibly leading to an increase in reward valency. These maladaptive alterations in dopamine signaling may further increase vulnerability to alcohol and drug addiction.
METHODS
Animals and Housing Conditions.
Male Long-Evans rats were purchased from Harlan at PD 21. Following a week of acclimation in standard housing conditions (four animals per cage, food and water ad libitum; 12 h light/dark cycle), at PD 28 rats were randomly assigned to one of two experimental groups: group housed (aGH; 4/cage; guinea pig cages) or socially isolated (aSI; 1/cage; rat cages). Housing procedures were identical to those used in previous studies.29,37,72 Rats were maintained in their respective housing conditions for at least eight weeks before ex vivo voltammetry and biochemical experiments were conducted. A total of 25 aGH rats and 28 aSI rats were used across voltammetry experiments. A total of 19 in each group were used across the biochemistry studies.
Ex Vivo Fast Scan Cyclic Voltammetry.
Ex vivo FSCV was used to characterize dopamine dynamics in the NAc core of aSI and aGH rats following the housing paradigm (between PD 84 and 110). Briefly, rats were sacrificed, and their brains were rapidly removed and cooled in ice-cold, preoxygenated (95% O2/5% CO2), artificial cerebrospinal fluid (aCSF) consisting of 126 mM NaCl, 2.5 mM KCl, 1.2 mM NaH2PO4, 2.4 mM CaCl2, 1.2 mM MgCl2, 25 mM NaHCO3, 11 mM glucose, and 0.4 mM l-ascorbic acid, and the pH was adjusted to 7.4. A vibrating tissue slicer (Leica VT1200S, Leica Biosystems) was used to prepare 400 μm thick coronal brain sections containing NAc. These slices were immersed in oxygenated aCSF (32 °C). During recording, slices were transferred to a submersion recording chamber perfused with oxygenated aCSF at the rate of 1 mL/min at 32 °C.
Endogenous dopamine release was evoked by single or multiple electrical pulse stimulation (monophasic+, 4 ms), which was applied to the tissue every 5 min. The exact stimulation parameters varied depending on the experimental protocol. All experimental protocols are described below (FSCV protocol 1–5). Extracellular dopamine concentration was recorded by applying a triangular waveform (−0.4 to +1.2 and back to −0.4 V, Ag vs AgCl) at the rate of 400 V/s. Extracellular concentrations of dopamine were assessed by comparing the current at the peak oxidation potential for dopamine with electrode calibrations of known concentrations of dopamine (3 μM). All FSCV data were analyzed using Demon Voltammetry and Analysis software.77 Dopamine-stimulated release parameters were determined from stabilized signals using a Michaelis–Menten kinetics-based algorithm.78
FSCV Protocol 1: D2 and D3 Autoreceptor Function.
Dopamine D2 and D3 autoreceptors are heavily involved in regulating exocytotic release of endogenous dopamine at the dopamine terminal. To test the D2 and D3 autoreceptor function, cumulative concentrations (0.01, 0.03, 0.10, 0.30, 1.0, 3.0 μM) of D2 autoreceptor full agonist, Sumanirole, and D3 autoreceptor full agonist, PD128907, were bath-applied to separate slices. Single pulse electrical stimulation, with an interstimulus interval of 5 min, was used to evoke dopamine release.
FSCV Protocol 2: Multiple Pulse Stimulation.
Once the extracellular dopamine response was stable for three consecutive stimulations, multiple pulse stimulations at various frequencies were applied to the slice, and endogenous dopamine release was measured. Specifically, two and five pulse stimulations were applied at 5, 10 20, 40, and 100 Hz. The interstimulus interval was maintained at 5 min.
FSCV Protocol 3: Stimulus Intensity Variation.
In a separate experiment, following stabilized baseline responses as described earlier, single pulse electrical stimulations of varying intensities were applied to the slice, and endogenous dopamine release was measured. The following stimulation intensities were used: 30, 40, 50, 60, 70, 80, 90, 100, 300, 350, 400, 450, 500, 550, 600, 650, 700, 750 μA. As in the previous experiment, the interstimulus interval was maintained at 5 min.
FSCV Protocol 4: [Ca2+] Manipulation.
In a third experiment, after baseline dopamine responses were stable, the aCSF was switched for an identical solution containing 0.6 mM CaCl2, rather than 2.4 mM. Following stabilization of electrically stimulated dopamine responses at 0.6 mM calcium, a cumulative concentration response curve of Ca2+ (1.2, 1.8, 2.4, 3.0, 3.6, 4.2, 4.8 mM) was run by bath applying CaCl2 to the slices. Dopamine responses were stabilized before adding the subsequent concentration. Interstimulus interval was maintained at 5 min.
FSCV Protocol 5: Dopamine Availability.
Vesicular monoamine transporters 2 (VMAT2) are involved in repackaging cytosolic dopamine into synaptic vesicles, which are then primed for dopamine release. To test whether aSI altered the vesicular dopamine release by affecting readily releasable pool VMAT2 blocker, a reserpine-like compound Ro-04-1284/000 (10 μM), was bath-applied to the slice. Electrical stimulations with an interstimulus interval of 5 min were applied to evoke dopamine release, and number of stimulations required to drain the readily releasable pool of dopamine in the terminal were measured. After the pools were completely drained (assumption made after zero evoked dopamine was detected), amphetamine (10 μM) was bath-applied to the slice, and dopamine was measured continuously for 5 min.
Tissue Harvesting for Western Blot Hybridization.
NAc tissue samples from a separate group of rats were extracted to analyze for VMAT2, syntaxin-1, and synaptogyrin-3 using Western blot hybridization. Following the housing paradigm, aGH and aSI rats were anesthetized with isoflorane, until unresponsive to toe pinch and rapidly decapitated by guillotine. Brains were removed and placed into ice-cold oxygenated artificial cerebrospinal fluid (aCSF; as described in “Ex Vivo Fast Scan Cyclic Voltammetry”) for ~1 min before slicing. The NAc (shell and core) was free-hand dissected from 400 μm thick slices of brain tissue (between Bregma +2.20 and 0.70 along the anterior-posterior axis) prepared using a vibrating tissue slicer (Leica VT1200S, Leica Biosystems). Tissue samples were placed in individual Eppendorf tubes, flash-frozen in isopentane (2-methylbutane, Fisher Scientific), and stored at −80 °C until further use. Total harvesting time between decapitation to flash freezing was 4 min.
Tissue Preparation and Western Blot Hybridization for VMAT2, Syanptogyrin-3, and Syntaxin-1.
All tissues were homogenized in RIPA buffer (150 mM NaCl, 1.0% Triton-X-100, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS), 50 mM Trizma base, pH 8.0) and centrifuged at 12 000g for 30 min. The pellet was discarded, and the supernatant was used for Western blot analysis for various proteins. Protein concentrations were determined with a commercially available BCA protein assay kit (23225; ThermoScientific) and Molecular Devices Spectra Max 384 Plus spectrophotometer utilizing the SoftMax Pro software.
Twenty micrograms of protein was loaded onto 4–12% NuPAGE Bis-Tris10-well precast gels (NP0321BOX; ThermoFisher Scientific) along with a MagicMark XP molecular weight ladder (LC5602; ThermoFisher Scientific) for VMAT2, synaptogyrin-3, and syntaxin-1. The membranes were blocked with a 1X solution of Tris buffered saline (TBS-T 20X concentrate; J640; Amresco) and 0.05% Tween-20 (BP337; Fisherbrand) containing 5% Carnation powdered nonfat dry milk (NFM) for 1 h at room temperature. Subsequently, blots were incubated with agitation for 2 h at room temperature in TBS-T/5% bovine serum albumin (05470; Sigma-Aldrich) solution containing the following primary antibody concentrations: VAMT2 (1:2000; AB1598P; Millipore Sigma); Synaptogyrin-3 (1:1000; ab106460; abcam); Syntaxin-1 (1:1000; ANR-002; Alomone laboratories). Following extensive washing with TBS-T, the blots were exposed to a goat antirabbit secondary antibody (1:5000; 4914; Sigma) in 5% NFM dissolved in TBS-T for 2 h at room temperature with agitation. Detection of bound secondary antibody was performed using enhanced chemiluminescence using Pierce ECL Western blotting substrate (32106; ThermoFisher Scientific). Band intensity was quantified with ImageJ densitometry software (National Institutes of Health). Samples from aGH and aSI animals were run on the same gel to facilitate direct comparisons. Note that the Western blots for VMAT2, Synaptogyrin-3 and Syntaxin-1 were run separately. Because of the similarities in the methods, information for all three is combined here.
Sample Preparation and Western Blot Hybridization for Munc13-3.
Homogenization buffer composed of Tissue Protein Extraction Reagent (T-PER; No. 78510; Thermo Fisher), protease inhibitors for mammalian tissue (P8340; Sigma), and phosphatase inhibitors (P5726 and PP0040; Sigma) was added to isolated rat Nac samples at 7uL/mg and disrupted by brief sonication. Following 2 h of incubation on a rotisserie mixer at 4 °C, samples were centrifuged at 15 000g to isolate the supernatant. Protein yield was quantified using Pierce BCA protein assay kit (PI23227; Thermo Fisher), SpectraMax 384 Plus Spectrophotometer, and SoftMax Pro Software (Molecular Devices).
Twenty micrograms of total protein was loaded onto 4–20% Criterion TGX 18-well precast gels (567-8094; Bio-Rad) along with Precision Plus Protein Dual Xtra Standards (161-0377; Bio-Rad), separated per manufacturer’s recommendations in a Bio-Rad Criterion Vertical Electrophoresis Cell, and transferred to nitrocellulose membrane using a semidry technique with a Bio-Rad Trans Blot Turbo Transfer System and preassembled membrane stacks (1704270; Bio-Rad). Total lane protein transfer was detected using Pierce Reversible Protein Stain Kit (PI24580; Thermo Fisher) prior to membrane blocking. The membranes were incubated in blocking buffer solution of Tris buffered saline (J640-4L; VWR International), 0.05% Tween-20 (P9416; Sigma) and 5% nonfat milk (Nestle S.A.) for 1 h at room temperature with agitation. Subsequently, the membranes were incubated with agitation at 4 °C overnight in a 1% solution of blocking buffer containing Munc13-3 primary antibody (126303; Synaptic Systems). Following extensive rinsing in Tris buffered saline/0.05% Tween-20, the membranes were exposed to peroxidase-labeled goat antirabbit secondary antibody (A4914; Sigma) for 1 h at room temperature with agitation. Detection of bound secondary antibody was performed using SuperSignal West Dura Extended Duration Substrate Enhanced Chemiluminescence (34076; Thermo Fisher). Immunoreactive band intensity was quantified from digital images captured on a charge-coupled device camera and normalized to total lane protein using a Bio-Rad Chemi-Doc XRS Imaging System and Image Lab Analysis software (Bio-Rad).
Synaptoneurosome Preparation for Western Blot Hybridization.
NAc synaptoneurosomes were prepared from age-matched aGH and aSI rats. NAc tissue was amalgamated in homogenization buffer (20 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPEs) pH 7.4, 5 mM ethylenediaminetetraacetic acid (EDTA) pH 8.0, and Halt, a protease and phosphatase inhibitor cocktail (Thermo Scientific)). The homogenate was filtered through a 100 μm nylon filter followed by a 5 μm filter and centrifuged at 14 000g for 20 min at 4 °C. The pellet was resuspended in RIPA buffer (150 mM NaCl, 10 mM Tris pH 7.4, 0.1% SDS, 1% Triton X-100, 1% deoxycholate, 5 mM EDTA and Halt) and centrifuged for an additional 20 min at 4 °C. The supernatant was utilized for Western blot analysis.
Proteins were separated by SDS-polyacrylamide gel electrophoresis (PAGE). Afterward, the gel was transferred to a 0.2 μm nitrocellulose membrane, blocked in 5% nonfat milk in a solution of tris-buffered saline with 0.1% Tween20 (TBST-0.1%) for 1 h, and incubated in blocking buffer with primary antibodies: mouse anti-TH (1:1000, abcam, ab129991) and mouse antiactin (1:10 000, Sigma, A1978) overnight at 4 °C. Each membrane was washed in TBST-0.1% three times for 15 min and incubated at room temperature for 45 min in a fluorescence-conjugated secondary antibody: goat polyclonal antimouse TH (1:4000, AF680 and AF800 LICOR). The membranes were washed as described earlier, with a final rinse in TBS for 10 min before being imaged on a LICOR Odyssey imaging system. Image Studio Lite and ImageJ (National Institutes of Health) were used for densitometry analyses of proteins and preparation of representative images, respectively.
Chemicals and Drugs.
Components of the artificial cerebrospinal fluid and neurotransmitter standards were of the highest quality obtainable from Sigma-Aldrich. Sumanirole, D2 receptor agonist, and PD128907, D3 autoreceptor agonist, were obtained from Sigma-Aldrich. Ro-04-1284/000, a reserpine-like VMAT2 blocker, was obtained from Hoffmann-La Roche Inc. Amphetamine was obtained from National Institute on Drug Abuse (NIDA) drug inventory.
Statistics.
All statistical analyses were conducted using GraphPad Prism 7 (GraphPad Software). Baseline dopamine release measures were compared using an unpaired Student’s t-test. D2 and D3 autoreceptor sensitivity, multiple pulse stimulations, varying stimulus intensity stimulations, [Ca2+] manipulation, and readily releasable pool examination experiments were all assessed using a two-way repeated measures ANOVA followed by Bonferroni’s posthoc test. All Western blot hybridization data sets were analyzed using a Student’s t-test. The significance level was set at p < 0.05.
ACKNOWLEDGMENTS
The authors would like to thank J. Locke, S. Albertson, and E. Carter for their technical support. Funding for the work included K01 AA023874 (A.N.K.); P50 AA026117 (A.N.K., S.R.J., J.L.W., B.A.M., K.F.R.); UL1 TR001420 (A.N.K., M.J.F.); R00 DA031791 (M.J.F.); R01 AA023999 (S.R.J., A.M.); U01 AA014091 (S.R.J.); R01 AA014445 (B.A.M.); R01 AA026551 (J.L.W.); R01 NS105005, IOS 1026527, W81XWH-14-1-0061 (K.R.G.).
Footnotes
Notes
The authors declare no competing financial interest.
REFERENCES
- (1).Scheller-Gilkey G, Moynes K, Cooper I, Kant C, and Miller AH (2004) Early life stress and PTSD symptoms in patients with comorbid schizophrenia and substance abuse. Schizophr Res. 69 (2–3), 167–74. [DOI] [PubMed] [Google Scholar]
- (2).Nugent NR, Tyrka AR, Carpenter LL, and Price LH (2011) Gene-environment interactions: early life stress and risk for depressive and anxiety disorders. Psychopharmacology 214 (1), 175–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Watt MJ, Weber MA, Davies SR, and Forster GL (2017) Impact of juvenile chronic stress on adult cortico-accumbal function: Implications for cognition and addiction. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 79, 136–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Dube SR, Anda RF, Felitti VJ, Edwards VJ, and Croft J (2002) Adverse childhood experiences and personal alcohol abuse as an adult. Addict Behav 27 (5), 713–25. [DOI] [PubMed] [Google Scholar]
- (5).Douglas KR, Chan G, Gelernter J, Arias AJ, Anton RF, Weiss RD, Brady K, Poling J, Farrer L, and Kranzler HR (2010) Adverse childhood events as risk factors for substance dependence: partial mediation by mood and anxiety disorders. Addict Behav 35 (1), 7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Butler TR, Karkhanis AN, Jones SR, and Weiner JL (2016) Adolescent Social Isolation as a Model of Heightened Vulnerability to Comorbid Alcoholism and Anxiety Disorders. Alcohol.: Clin. Exp. Res. 40 (6), 1202–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).McCool BA, and Chappell AM (2009) Early social isolation in male Long-Evans rats alters both appetitive and consummatory behaviors expressed during operant ethanol self-administration. Alcohol.: Clin. Exp. Res. 33, 273–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Skelly MJ, Chappell AE, Carter E, and Weiner JL (2015) Adolescent social isolation increases anxiety-like behavior and ethanol intake and impairs fear extinction in adulthood: Possible role of disrupted noradrenergic signaling. Neuropharmacology 97, 149–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Ding Y, Kang L, Li B, and Ma L (2005) Enhanced cocaine self-administration in adult rats with adolescent isolation experience. Pharmacol., Biochem. Behav. 82, 673–677. [DOI] [PubMed] [Google Scholar]
- (10).Baarendse PJ, Limpens JH, and Vanderschuren LJ (2014) Disrupted social development enhances the motivation for cocaine in rats. Psychopharmacology 231, 1695–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Hall FS, Wilkinson LS, Humby T, Inglis W, Kendall D, Marsden CA, and Robbins TW (1998) Isolation rearing in rats: pre- and postsynaptic changes in striatal dopaminergic systems. Pharmacol., Biochem. Behav. 59, 859–872. [DOI] [PubMed] [Google Scholar]
- (12).Kokare DM, Dandekar MP, Singru PS, Gupta GL, and Subhedar NK (2010) Involvement of alpha-MSH in the social isolation induced anxiety- and depression-like behaviors in rat. Neuropharmacology 58, 1009–1018. [DOI] [PubMed] [Google Scholar]
- (13).Green MR, Barnes B, and McCormick CM (2013) Social instability stress in adolescence increases anxiety and reduces social interactions in adulthood in male Long-Evans rats. Dev Psychobiol 55 (8), 849–59. [DOI] [PubMed] [Google Scholar]
- (14).Lapiz MD, Mateo Y, Parker T, and Marsden C (2000) Effects of noradrenaline depletion in the brain on response on novelty in isolation-reared rats. Psychopharmacology 152 (3), 312–20. [DOI] [PubMed] [Google Scholar]
- (15).Han X, Li N, Xue X, Shao F, and Wang W (2012) Early social isolation disrupts latent inhibition and increases dopamine D2 receptor expression in the medial prefrontal cortex and nucleus accumbens of adult rats. Brain Res. 1447, 38–43. [DOI] [PubMed] [Google Scholar]
- (16).Radke AK, and Gewirtz JC (2012) Increased dopamine receptor activity in the nucleus accumbens shell ameliorates anxiety during drug withdrawal. Neuropsychopharmacology 37 (11), 2405–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Sulzer D (2011) How addictive drugs disrupt presynaptic dopamine neurotransmission. Neuron 69 (4), 628–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Grace AA, Floresco SB, Goto Y, and Lodge DJ (2007) Regulation of firing of dopaminergic neurons and control of goal-directed behavior. Trends Neurosci. 30 (5), 220–227. [DOI] [PubMed] [Google Scholar]
- (19).Andersen SL, Thompson AT, Rutstein M, Hostetter JC, and Teicher MH (2000) Dopamine receptor pruning in prefrontal cortex during the periadolescent period in rats. Synapse 37 (2), 167–9. [DOI] [PubMed] [Google Scholar]
- (20).Andersen SL, Thompson AP, Krenzel E, and Teicher MH (2002) Pubertal changes in gonadal hormones do not underlie adolescent dopamine receptor overproduction. Psychoneuroendocrinology 27 (6), 683–691. [DOI] [PubMed] [Google Scholar]
- (21).Tarazi FI, and Baldessarini RJ (2000) Comparative postnatal development of dopamine D(1), D(2) and D(4) receptors in rat forebrain. Int. J. Dev. Neurosci. 18 (1), 29–37. [DOI] [PubMed] [Google Scholar]
- (22).Wahlstrom D, Collins P, White T, and Luciana M (2010) Developmental changes in dopamine neurotransmission in adolescence: behavioral implications and issues in assessment. Brain Cogn 72 (1), 146–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Doremus-Fitzwater TL, and Spear LP (2016) Reward-centricity and attenuated aversions: An adolescent phenotype emerging from studies in laboratory animals. Neurosci. Biobehav. Rev. 70, 121–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Mathews IZ, Waters P, and McCormick CM (2009) Changes in hyporesponsiveness to acute amphetamine and age differences in tyrosine hydroxylase immunoreactivity in the brain over adolescence in male and female rats. Dev. Psychobiol. 51 (5), 417–28. [DOI] [PubMed] [Google Scholar]
- (25).Philpot RM, Wecker L, and Kirstein CL (2009) Repeated ethanol exposure during adolescence alters the developmental trajectory of dopaminergic output from the nucleus accumbens septi. Int. J. Dev. Neurosci. 27 (8), 805–15. [DOI] [PubMed] [Google Scholar]
- (26).Andersen SL, and Teicher MH (2009) Desperately driven and no brakes: developmental stress exposure and subsequent risk for substance abuse. Neurosci. Biobehav. Rev. 33 (4), 516–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Burke AR, and Miczek KA (2014) Stress in adolescence and drugs of abuse in rodent models: role of dopamine, CRF, and HPA axis. Psychopharmacology 231 (8), 1557–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Seo JH, and Kuzhikandathil EV (2015) Dopamine D3 Receptor Mediates Preadolescent Stress-Induced Adult Psychiatric Disorders. PLoS One 10 (11), e0143908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Karkhanis AN, Rose JH, Weiner JL, and Jones SR (2016) Early-Life Social Isolation Stress Increases Kappa Opioid Receptor Responsiveness and Downregulates the Dopamine System. Neuropsychopharmacology 41 (9), 2263–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Heidbreder CA, Weiss IC, Domeney AM, Pryce C, Homberg J, Hedou G, Feldon J, Moran MC, and Nelson P (2000) Behavioral, neurochemical and endocrinological characterization of the early social isolation syndrome. Neuroscience 100 (4), 749–68. [DOI] [PubMed] [Google Scholar]
- (31).Miura H, Qiao H, and Ohta T (2002) Attenuating effects of the isolated rearing condition on increased brain serotonin and dopamine turnover elicited by novelty stress. Brain Res. 926 (1–2), 10–7. [DOI] [PubMed] [Google Scholar]
- (32).Yorgason JT, Espana RA, Konstantopoulos JK, Weiner J, and Jones SR (2013) Enduring increases in anxiety-like behavior and rapid nucleus accumbens dopamine signaling in socially isolated rats. Eur. J. Neurosci 37, 1022–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Yorgason JT, Calipari ES, Ferris MJ, Karkhanis AN, Fordahl SC, Weiner JL, and Jones SR (2016) Social isolation rearing increases dopamine uptake and psychostimulant potency in the striatum. Neuropharmacology 101, 471–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Jones GH, Hernandez TD, Kendall DA, Marsden CA, and Robbins TW (1992) Dopaminergic and serotonergic function following isolation rearing in rats: study of behavioural responses and postmortem and in vivo neurochemistry. Pharmacol., Biochem. Behav. 43 (1), 17–35. [DOI] [PubMed] [Google Scholar]
- (35).Fulford AJ, and Marsden CA (1998) Effect of isolation-rearing on conditioned dopamine release in vivo in the nucleus accumbens of the rat. J. Neurochem. 70 (1), 384–90. [DOI] [PubMed] [Google Scholar]
- (36).Howes SR, Dalley JW, Morrison CH, Robbins TW, and Everitt BJ (2000) Leftward shift in the acquisition of cocaine self-administration in isolation-reared rats: relationship to extracellular levels of dopamine, serotonin and glutamate in the nucleus accumbens and amygdala-striatal FOS expression. Psychopharmacology 151 (1), 55–63. [DOI] [PubMed] [Google Scholar]
- (37).Karkhanis AN, Locke JL, McCool BA, Weiner JL, and Jones SR (2014) Social isolation rearing increases nucleus accumbens dopamine and norepinephrine responses to acute ethanol in adulthood. Alcohol.: Clin. Exp. Res. 38, 2770–2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Broom SL, and Yamamoto BK (2005) Effects of subchronic methamphetamine exposure on basal dopamine and stress-induced dopamine release in the nucleus accumbens shell of rats. Psychopharmacology 181 (3), 467–76. [DOI] [PubMed] [Google Scholar]
- (39).Lucas LR, Wang CJ, McCall TJ, and McEwen BS (2007) Effects of immobilization stress on neurochemical markers in the motivational system of the male rat. Brain Res. 1155, 108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Mathews TA, Brookshire BR, Budygin EA, Hamre K, Goldowitz D, and Jones SR (2009) Ethanol-induced hyperactivity is associated with hypodopaminergia in the 22-TNJ ENU-mutated mouse. Alcohol 43, 421–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Deal AL, Konstantopoulos JK, Weiner JL, and Budygin EA (2018) Exploring the consequences of social defeat stress and intermittent ethanol drinking on dopamine dynamics in the rat nucleus accumbens. Sci. Rep. 8 (1), 332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Wolf ME, and Roth R H. (1990) Autoreceptor regulation of dopamine synthesis. Ann. N. Y. Acad. Sci. 604, 323–43. [DOI] [PubMed] [Google Scholar]
- (43).Tang L, Todd RD, and O’Malley KL (1994) Dopamine D2 and D3 receptors inhibit dopamine release. J. Pharmacol Exp Ther 270 (2), 475–9. [PubMed] [Google Scholar]
- (44).Missale C, Nash SR, Robinson SW, Jaber M, and Caron MG (1998) Dopamine receptors: from structure to function. Physiol. Rev. 78, 189–225. [DOI] [PubMed] [Google Scholar]
- (45).Phillips PE, Hancock PJ, and Stamford JA (2002) Time window of autoreceptor-mediated inhibition of limbic and striatal dopamine release. Synapse 44, 15–22. [DOI] [PubMed] [Google Scholar]
- (46).Ford CP (2014) The role of D2-autoreceptors in regulating dopamine neuron activity and transmission. Neuroscience 282, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Beaulieu JM, and Gainetdinov RR (2011) The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 63, 182–217. [DOI] [PubMed] [Google Scholar]
- (48).Kennedy RT, Jones SR, and Wightman RM (1992) Dynamic observation of dopamine autoreceptor effects in rat striatal slices. J. Neurochem. 59 (2), 449–55. [DOI] [PubMed] [Google Scholar]
- (49).Karkhanis AN, Rose JH, Huggins KN, Konstantopoulos JK, and Jones SR (2015) Chronic intermittent ethanol exposure reduces presynaptic dopamine neurotransmission in the mouse nucleus accumbens. Drug Alcohol Depend. 150, 24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Cabib S, Giardino L, Calzá L, Zanni M, Mele A, and Puglisi-Allegra S (1998) Stress promotes major changes in dopamine receptor densities within the mesoaccumbens and nigrostriatal systems. Neuroscience 84 (1), 193–200. [DOI] [PubMed] [Google Scholar]
- (51).Zurawek D, Faron-Górecka A, Kuśmider M, Kolasa M, Gruca P, Papp M, and Dziedzicka-Wasylewska M (2013) Mesolimbic dopamine D2 receptor plasticity contributes to stress resilience in rats subjected to chronic mild stress. Psychopharmacology 227 (4), 583–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Razzoli M, Andreoli M, Michielin F, Quarta D, and Sokal DM (2011) Increased phasic activity of VTA dopamine neurons in mice 3 weeks after repeated social defeat. Behav. Brain Res. 218 (1), 253–7. [DOI] [PubMed] [Google Scholar]
- (53).Fabricius K, Helboe L, Fink-Jensen A, Wörtwein G, Steiniger-Brach B, and Sotty F (2010) Increased dopaminergic activity in socially isolated rats: an electrophysiological study. Neurosci. Lett. 482 (2), 117–22. [DOI] [PubMed] [Google Scholar]
- (54).Lapiz MD, Fulford A, Muchimapura S, Mason R, Parker T, and Marsden CA (2003) Influence of postweaning social isolation in the rat on brain development, conditioned behavior, and neurotransmission. Neurosci. Behav. Physiol. 33, 13–29. [DOI] [PubMed] [Google Scholar]
- (55).Garcia-Keller C, Martinez SA, Esparza MA, Bollati F, Kalivas PW, and Cancela LM (2013) Cross-sensitization between cocaine and acute restraint stress is associated with sensitized dopamine but not glutamate release in the nucleus accumbens. Eur. J. Neurosci 37 (6), 982–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Han X, Albrechet-Souza L, Doyle MR, Shimamoto A, DeBold JF, and Miczek KA (2015) Social stress and escalated drug self-administration in mice II. Cocaine and dopamine in the nucleus accumbens. Psychopharmacology 232 (6), 1003–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Yorgason JT, Zeppenfeld DM, and Williams JT (2017) Cholinergic Interneurons Underlie Spontaneous Dopamine Release in Nucleus Accumbens. J. Neurosci. 37 (8), 2086–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Sulzer D, Maidment NT, and Rayport S (1993) Amphetamine and other weak bases act to promote reverse transport of dopamine in ventral midbrain neurons. J. Neurochem. 60 (2), 527–35. [DOI] [PubMed] [Google Scholar]
- (59).Hedges DM, Obray JD, Yorgason JT, Jang EY, Weerasekara VK, Uys JD, Bellinger FP, and Steffensen SC (2018) Methamphetamine Induces Dopamine Release in the Nucleus Accumbens Through a Sigma Receptor-Mediated Pathway. Neuropsychopharmacology 43 (6), 1405–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).German CL, Baladi MG, McFadden LM, Hanson GR, and Fleckenstein AE (2015) Regulation of the Dopamine and Vesicular Monoamine Transporters: Pharmacological Targets and Implications for Disease. Pharmacol. Rev. 67 (4), 1005–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Pothos EN, Larsen KE, Krantz DE, Liu Y, Haycock JW, Setlik W, Gershon MD, Edwards RH, and Sulzer D (2000) Synaptic vesicle transporter expression regulates vesicle phenotype and quantal size. J. Neurosci. 20 (19), 7297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Omiatek DM, Bressler AJ, Cans AS, Andrews AM, Heien ML, and Ewing AG (2013) The real catecholamine content of secretory vesicles in the CNS revealed by electrochemical cytometry. Sci. Rep. 3, 1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Belizaire R, Komanduri C, Wooten K, Chen M, Thaller C, and Janz R (2004) Characterization of synaptogyrin 3 as a new synaptic vesicle protein. J. Comp. Neurol. 470 (3), 266–81. [DOI] [PubMed] [Google Scholar]
- (64).Egaña LA, Cuevas RA, Baust TB, Parra LA, Leak RK, Hochendoner S, Peña K, Quiroz M, Hong WC, Dorostkar MM, Janz R, Sitte HH, and Torres GE (2009) Physical and functional interaction between the dopamine transporter and the synaptic vesicle protein synaptogyrin-3. J. Neurosci. 29 (14), 4592–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Südhof TC (2013) Neurotransmitter release: the last millisecond in the life of a synaptic vesicle. Neuron 80 (3), 675–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Zucker M, Weizman A, and Rehavi M (2005) Repeated swim stress leads to down-regulation of vesicular monoamine transporter 2 in rat brain nucleus accumbens and striatum. Eur. Neuropsychopharmacol. 15 (2), 199–201. [DOI] [PubMed] [Google Scholar]
- (67).Sosanya NM, Huang PP, Cacheaux LP, Chen CJ, Nguyen K, Perrone-Bizzozero NI, and Raab-Graham KF (2013) Degradation of high affinity HuD targets releases Kv1.1 mRNA from miR-129 repression by mTORC1. J. Cell Biol. 202 (1), 53–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Niere F, Namjoshi S, Song E, Dilly GA, Schoenhard G, Zemelman BV, Mechref Y, and Raab-Graham KF (2016) Analysis of Proteins That Rapidly Change Upon Mechanistic/ Mammalian Target of Rapamycin Complex 1 (mTORC1) Repression Identifies Parkinson Protein 7 (PARK7) as a Novel Protein Aberrantly Expressed in Tuberous Sclerosis Complex (TSC). Mol. Cell. Proteomics 15 (2), 426–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Qiu HM, Yang JX, Jiang XH, Hu XY, Liu D, and Zhou QX (2015) Enhancing tyrosine hydroxylase and tryptophan hydroxylase expression and improving oxidative stress involved in the antidepressant effect of sodium valproate on rats undergoing chronic unpredicted stress. NeuroReport 26 (18), 1145–1150. [DOI] [PubMed] [Google Scholar]
- (70).Wang YC, Ho UC, Ko MC, Liao CC, and Lee LJ (2012) Differential neuronal changes in medial prefrontal cortex, basolateral amygdala and nucleus accumbens after postweaning social isolation. Brain Struct. Funct. 217, 337–351. [DOI] [PubMed] [Google Scholar]
- (71).Yim HJ, Robinson DL, White ML, Jaworski JN, Randall PK, Lancaster FE, and Gonzales RA (2000) Dissociation between the time course of ethanol and extracellular dopamine concentrations in the nucleus accumbens after a single intraperitoneal injection. Alcohol.: Clin. Exp. Res. 24, 781–788. [PubMed] [Google Scholar]
- (72).Karkhanis AN, Alexander NJ, McCool BA, Weiner JL, and Jones SR (2015) Chronic social isolation during adolescence augments catecholamine response to acute ethanol in the basolateral amygdala. Synapse 69 (8), 385–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (73).Whitaker LR, Degoulet M, and Morikawa H (2013) Social deprivation enhances VTA synaptic plasticity and drug-induced contextual learning. Neuron 77 (2), 335–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (74).Lampert C, Arcego DM, de Sá Couto-Pereira N, Dos Santos Vieira A, Toniazzo AP, Krolow R, Garcia E, Vendite DA, Calcagnotto ME, and Dalmaz C (2017) Short post-weaning social isolation induces long-term changes in the dopaminergic system and increases susceptibility to psychostimulants in female rats. Int. J. Dev. Neurosci. 61, 21–30. [DOI] [PubMed] [Google Scholar]
- (75).Cuadra G, Zurita A, Lacerra C, and Molina V (1999) Chronic stress sensitizes frontal cortex dopamine release in response to a subsequent novel stressor: reversal by naloxone. Brain Res. Bull. 48 (3), 303–8. [DOI] [PubMed] [Google Scholar]
- (76).Matuszewich L, McFadden LM, Friedman RD, and Frye CA (2014) Neurochemical and behavioral effects of chronic unpredictable stress. Behav. Pharmacol. 25 (5–6), 557–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (77).Yorgason JT, Espana RA, and Jones SR (2011) Demon voltammetry and analysis software: analysis of cocaine-induced alterations in dopamine signaling using multiple kinetic measures. J. Neurosci. Methods 202, 158–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (78).Ferris MJ, Calipari ES, Yorgason JT, and Jones SR (2013) Examining the complex regulation and drug-induced plasticity of dopamine release and uptake using voltammetry in brain slices. ACS Chem. Neurosci. 4, 693–703. [DOI] [PMC free article] [PubMed] [Google Scholar]