Abstract
Enabled by the discovery of new cinchinium salts and co-additives, a direct and efficient asymmetric access to trifluoromethylated γ-amino esters/lactones has been realized through the enantioselective and diastereoselective umpolung reaction of trifluoromethyl imines with acrylates or α,β-unsaturated lactones as carbon electrophiles. At 0.5 to 5.0 mol % catalyst loadings, the newly-developed catalytic system activates a variety of imine substrates as unconventional nucleophiles to mediate highly chemo-, regio-, diastereo- and enantioselective C-C bond forming reactions. The developed synthetic protocol represents an excellent strategy to target a seris of versatile and enantiromerically enriched γ-amino esters/lactones in good to excellent yields from the readily available starting materials. Additionally, we found that the epi-vinyl catalysts based on cinchonidine and quinine promote a similarly high enantioselective reaction generating the opposite configuration of chiral products in highly efficient manner, which allows the convenient access to either R or S-enantiomer of the chiral amine products in high yields and excellent enantioselectivities.
Graphical Abstarct

INTRODUCTION
Chiral γ-amino esters as a privileged structural scaffold are presented in a variety of natural products and biologically active molecules. Due to their important applications in the pharmaceutical industry and peptide chemistry, significant efforts have been devoted to the asymmetric synthesis of γ-amino acid derivatives.1–3 Meanwhile, the uniquely beneficial impact resulting from the presence of fluorine on organic molecules has led to a rapidly increasing demand for developing novel methodology to efficiently synthesize organofluorine compounds.4 Recently, γ-trifluoromethylated γ-amino acid motifs began to appear in many pharmaceutically appealing structures.5 A direct and general enantioselective method towards these chiral building blocks however is still less explored. Herein, we wish to disclose the discovery and development of new cinchona phase-transfer catalysts to promote the direct asymmetric generation of γ-trifluoromethylated γ-amino esters through the umpolung addition of various trifluoromethyl imines with readily available carbon electrophiles such as acrylates or α,β-unsaturated lactones.
RESULTS AND DISCUSSION
We6 and others7–9 demonstrated that the imine umpolung reactions through the catalytic activation of imines as nucleophiles could performed as a powerful alternative strategy for the efficient synthesis of chiral amino compounds. Inspired by these pioneering studies, our group recently discovered that the quinidium salts could efficiently activate trifluoromethyl imines 1 as nucleophiles to promote highly regio-, chemo-, diastereo-, and enantioselective umpolung reactions of imines 1 with α,β-unsaturated N-acyl pyrroles 13, and accordingly realized the enantioselective construction of γ,γ-disubstituted and β,γ-disubstituted γ-amino acid derivatives 15 in an efficient fashion (Scheme 1a).10 Following these interesting results, it would be highly desirable if we can extend this imine umpolung reactions to the readily available but significantly less reactive carbon electrophiles such as α,β-unsaturated esters 4 or α,β-unsaturated lactones 8, which would allow the straightforward access to chiral γ-amino esters/lactones in an efficient manner (Scheme 1b).
Scheme 1.
Imine Umpolung Reactions for Asymmetric Synthesis of γ-Trifluoromethylated γ-Amino Acids.
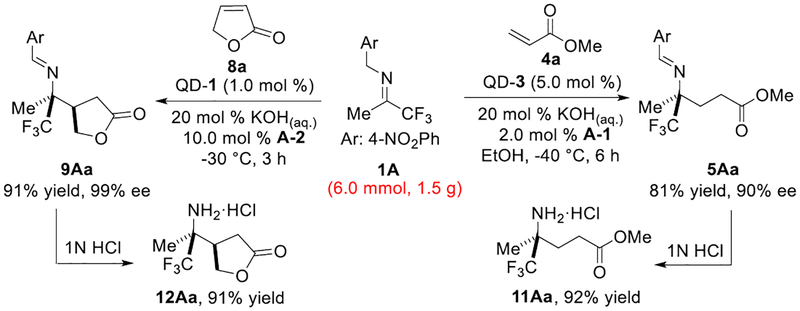
Compared with the reactive and unstable N-acyl pyrrole electrophiles, acrylates 4 were readily available but significantly less reactive. We started the investigation with the model reaction of trifluoromethyl imine 1A and methyl acrylate (4a) by utilizing our previously developed cinchona phase-transfer catalyst C-1. However, the substantially decreased electrophilicity of 4a posed considerable challenges.11 Specifically, we observed that the activated 2-azaallyanion intermediate 2A preferably underwent isomerization pathway to side product 6A and only a tiny amount of desired adduct 5Aa was obtained with poor enantioselectivity (5Aa/6A = 10/90, 15% ee of 5Aa, entry 1, Table 1). Moreover, a significant amount of [3+2] side product 7Aa was inevitably formed through the conjugate addition-Mannich reaction pathway (5Aa/7Aa = 79/21, entry 1, Table 1). Interestingly, we found that the quinidium catalyst QD-2 bearing the N-(9-methylanthracene) substituent demonstrated significantly superior control for the chemo-, and stereoselectivity, mediating efficient generation of the desired umpolung addition product 5Aa with good enantioselectivity, although a considerable amount of side products 6A and 7Aa were still observed (5Aa/6A = 58/42, 5Aa/7Aa = 80/20, 80% ee of 5Aa, entry 2). These initial results indicated that further catalyst development was required to achieve the desired umpolung adduct 5Aa with excellent enantioselectivity and high yield.
Table 1.
Development of Phase-Transfer Catalystsa

| entry | catyst | T (°C) | conv. (%)b | 5Aa/6A; 5Aa/7Aab | ee of 5Aa (%)c |
|---|---|---|---|---|---|
| 1d | C-1 | −20 | 100 | 10/90; 79/21 | 15 (S) |
| 2d | QD-2 | −20 | 69 | 58/42; 80/20 | 80 (S) |
| 3d | QD-3 | −20 | 95 | 66/34; 89/11 | 90 (S) |
| 4 | QD-3 | −30 | 92 | 71/29; 90/10 | 91 (S) |
| 5e | QD-3 | −30 | 100 | 83/17; 90/10 | 92 (S) |
| 6e,f | QD-3 | −30 | 100 | 93/7; 90/10 | 88 (S) |
| 7e,f | QD-3 | −40 | 100 | 95/5; 90/10 | 91 (S) |
| 8e,f | Q-3 | −40 | 32 | 75/25; 61/39 | 33 (R) |
| 9e,f | epiQ-3 | −40 | 100 | 97/3; 91/9 | 91 (R) |
Unless noted, reactions were performed with 1A (0.025 mmol), 4a (0.05 mmol), aqueous KOH (0.22 μL, 50 wt %, 10 mol %) in PhMe (0.25 mL) with catalyst (5.0 mol %) at the indicated temperature for 8 h.
Determined by 19F NMR analysis.
Determined by HPLC analysis.
Reaction time was 4 h.
2.0 mol % additive A-1 was added.
EtOH (2.5 μL) was added.
The 6’-methoxy group in catalyst QD-2 was an important structural modification position. The steric and electronic turning of 6’-OMe moiety represented an attractive route for the further catalyst optimizations.12 Following these considerations, we next prepared catalyst QD-3, in which the methoxy motif was replaced by a sterically more bulky and electronically more donating 6’-OiPr group. To our delight, QD-3 was found to be a more effective catalyst that promoted almost complete reaction in 4 h with excellent enantioselectivity and significantly improved chemoselectivity (5Aa/6A = 66/34, 90 % ee of 5Aa, entry 3). When the temperature was lowered to −30 °C, this reaction still progressed to high conversion with excellent steric control and slightly enhanced chemoselectivity (5Aa/6A = 71/29, 91 % ee of 5Aa, entry 4). Following our previous observations that the presence of phenol-type additives efficiently suppress the formation of side products 6A and 7Aa,6c,10 we found that the addition of 2 mol % phenol additive A-1 significantly accelerated the reaction rate, thereby promoting fully completed reaction with obviously improved chemoselectivity (5Aa/6A = 83/17, 5Aa/7Aa = 90/10, entry 5). Interestingly, we observed that the model reaction proceeded with the predominant formation of desired product 5Aa when a small amount of ethanol (2.5 μL) was also utilized (5Aa/6A = 93/7, 5Aa/7Aa = 90/10, entry 6). Further decreasing the reaction temperature to −40 °C, the desired adduct 5Aa was obtained in excellent chemo- and enantioselectivity (5Aa/6A = 95/5, 5Aa/7Aa = 90/10, 91% ee, entry 7).
We next examined catalyst Q-3, the pseudoenantiomer of QD-3. Unfortunately, we found that Q-3 failed to mediate the generation of opposite enantiomer of 5Aa with satisfactory results (-33% ee of 5Aa, 32% conv., entry 8). Building on our recent discovery that the epi-vinyl quinine- or epi-vinyl cinchonidine-derived derivatives could provide more efficient catalysis than that by the corresponding quinine- or cinchonidine-derived catalysts,13 we next prepared the epi-vinyl cinchona catalyst epiQ-3 and pleasantly found that it was a considerably improved catalyst, delivering the opposite enantiomer of product 5Aa with excellent enantioselectivity and high chemoselectivity in full conversion (91% ee, 5Aa/6A = 97/3, 5Aa/7Aa = 91/9, entry 9).
With the optimal catalysts in hand, we next explored the reaction scope for both trifluoromethyl imines 1 and acrylates 4. As shown in Table 2, the reactions of 1A with a series of acrylate derivatives 4a-e bearing simple and functionalized substituents consistently proceeded with good yields and high enantioselectivities (entries 1–5). We next examined various trifluoromethyl imines and found that a variety of simple and functionalized aliphatic imines of varying length 1B-E reacted efficiently with methyl acrylate (4a) in highly enantioselective fashion to form the desired products 5B-E in useful yield and ee (entries 6–9). Interestingly, the aldimine 1F was also well tolerated by the catalysts (entry 10). The reaction of branched imines such as 1G with methyl acrylate (4a) failed to deliver the desired addition products 5Ga due to the reduced reactivity (entry 11). Nevertheless, these sterically more hindered α or β-branched imines 1G-J were found to react with propargyl acrylate (4b) to furnish the products 5Gb-Jb in good yield with high enantioselectivity (entries 12–15).
Table 2.
Substrate Scope of Trifluoromethyl Imines with Various Acrylatesa

| entry | R1; 1 | 4 | t (h) | yield of 5 (%)b | ee of 5 (%)c |
|---|---|---|---|---|---|
| 1d | Me; 1A | 4a | 8 | 80 (85); 5Aa | 92(92) |
| 2e | Me; 1A | 4b | 3 | 89 (92); 5Ab | 91 (93) |
| 3d | Me; 1A | 4c | 20 | 70 (75); 5Ac | 85 (90) |
| 4d | Me; 1A | 4d | 20 | 50 (63); 5Ad | 81 (86) |
| 5e | Me; 1A | 4e | 8 | 91 (93); 5Ae | 94 (94) |
| 6d | Et; 1B | 4a | 5 | 71 (73); 5Ba | 87 (90) |
| 7 | n-Bu; 1C | 4a | 8 | 63 (68); 5Ca | 91 (91) |
| 8 | CH2=CH(CH2)3; 1D | 4a | 8 | 64 (68); 5Da | 90 (92) |
| 9 | Ph(CH2)2; 1E | 4a | 8 | 53 (55); 5Ea | 90 (88) |
| 10f | H; 1F | 4a | 4 | 62 (70); 5Fa | 90 (91) |
| 11 | Cy; 1G | 4a | 8 | -- (--); 5Ga | -- (--) |
| 12 | Cy; 1G | 4b | 8 | 65 (63); 5Gb | 91 (93) |
| 13 | CyCH2; 1H | 4b | 8 | 71 (72); 5Hb | 90 (93) |
| 14 | Bn; 1I | 4b | 8 | 63 (72); 5Ib | 90 (92) |
| 15 | 4-BrBn; 1J | 4b | 8 | 68 (66); 5Jb | 88 (91) |
Unless noted, reactions were performed with 1 (0.2 mmol), acrylates 4 (0.4 mmol), A-1 (2.0 mol %), ethanol (20 μL) and aqueous KOH solution (2.2 μL, 50 wt %, 10 mol %) in PhMe (2.0 mL) with catalyst QD-3 (5.0 mol %) at the indicated temperature; results in parentheses were obtained from reactions catalyzed by epiQ-3 (5.0 mol %) in same conditions.
Isolated yield of 5.
Determined by HPLC analysis.
Reaction was performed at −40 °C.
1.0 mol % catalyst was used.
Reaction was performed at −50 °C
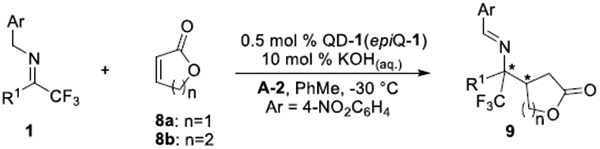
Chiral γ- and δ-lactones not only are commonly presented structural motifs in natural products but also serve as versatile synthetic intermediates to chiral tetrahydrofuran, tetrahydropyran, and hydroxycarboxylic acid derivatives.14 We therefore began to explore the imine umpolung reactions with α,β-unsaturated lactones. Our investigation commenced with the C-1 mediated reaction of trifluoromethyl imine 1L with γ-crotonolactone (8a) and found that the isomerized imine 6L was generated predominantly (9La/6L = 9/91, 9La/10La = 25/75, entry 1, Table 3). Interestingly, with the addition of phenol additive A-1, the reaction formed an increased amount of the desired product 9La with excellent enantioselectivity (9La/6L = 30/70, 9La/10La = 87/13, 98% ee, entry 2). Further screening of different phenol additives revealed that the addition of A-2 improved the reaction chemoselectivity considerably (9La/6L = 58/42, 9La/10La = 89/11, entry 3). Catalysts screening also revealed that the quinidium analogue QD-1 was a more powerful catalyst which promoted complete reaction with better chemoselectivity and excellent enantioselectivity (9La/6L = 79/21, >99% ee of 9La, entry 4). Notably, the reaction proceeded with obviously enhanced chemoselectivity when the amount of A-2 was changed to 50 mol % (9La/6L = 90/10, entry 5). The reaction still progressed to full conversion without any deterioration of chemo- and enantioselectivity (9La/6L = 90/10, >99% ee of 9La, entry 6) at a reduced 0.5 mol % catalyst loading. Once again, epiQ-1 proved to be better than Q-1 as a catalyst (entries 7 vs 8), affording the opposite enantiomer of 9La with good chemoselectivity and excellent optical purity (9La/6L = 90/10, 9La/10La = 84/16, >99% ee of 9La, entry 8).
Table 3.
Screening of Chiral Phase-Transfer Catalysts.

| entry | catalyst | additive | conv. (%)b | 9La/6L; 9La/10Lab | ee of 9La (%)c |
|---|---|---|---|---|---|
| 1 | C-1 | -- | 83 | 9/91; 25/75 | -- |
| 2 | C-1 | A-1 (100) | 100 | 30/70; 87/13 | 98 (S, R) |
| 3 | C-1 | A-2 (100) | 87 | 58/42; 89/11 | >99 (S, R) |
| 4 | QD-1 | A-2 (100) | 100 | 79/21; 81/19 | >99 (S, R) |
| 5 | QD-1 | A-2 (50) | 100 | 90/10; 82/18 | >99 (S, R) |
| 6d | QD-1 | A-2 (50) | 100 | 90/10; 82/18 | >99 (S, R) |
| 7d | Q-1 | A-2 (50) | 100 | 82/18; 78/22 | 99 (R, S) |
| 8d | epiQ-1 | A-2 (50) | 100 | 90/10; 84/16 | >99 (R, S) |
Unless noted, reactions were performed with 1L (0.025 mmol), 8a (0.05 mmol), additive (y mol %), aqueous KOH solution (0.22 μL, 50 wt %, 10 mol %) in PhMe (0.25 mL) with catalyst (x mol %) at −30 °C.
Determined by 19F NMR analysis.
Determined by HPLC analysis.
0.5 mol % catalyst was used.
We investigated the substrate scope with the optimized conditions. As summarized in Table 4, excellent diastereoselectivity and enantioselectivity for both enantiomers were readily accomplished for the aliphatic imines of varying length (1A, 1C, entries 1–2). For the sterically hindered cyclohexyl trifluoromethyl imine 1G, 2.0 mol % catalyst loading was sufficient for achieving highly enantioselective formation of 9Ga in good yields (entry 3). Such highly efficient catalytic control could also be extended to the reaction with the α,β-unsaturated alkenyl imine 1K (entry 4). The aryl substituted trifluoromethyl imines turned out to be more challenging substrates, as the addition of an increased amount of A-2 from 10 mol % to 50 mol % was necessary for suppressing the formations of side products 6 and 10. Under optimized conditions, the reactions of aryl substituted trifluoromethyl imines bearing either electron-donating or withdrawing groups proceeded efficiently in synthetically useful yields and high optical purities (entries 5–7). Notably, reaction of imine 1A with the six-membered δ-lactone 8b also delivered the chiral δ-lactone product 9Ab in high yield and excellent enantioselectivity (entry 8).
Table 4.
Substrate Scope of Trifluoromethyl Imines with α,β-Unsaturated Lactonesa
| entry | R1; 1 | 8 | t (h) | Yield of 9 (%)b | ee of 9 (%)c |
|---|---|---|---|---|---|
| 1d | Me; 1A | 8a | 1 | 93 (94); 9Aa | 99 (98) |
| 2d | n-Bu; 1C | 8a | 3 | 89 (90); 9Ca | 99 (98) |
| 3e | Cy; 1G | 8a | 5 | 84 (82); 9Ga | 99 (98) |
| 4 | PhCH=CH; 1K | 8a | 2 | 75 (71); 9Ka | 99 (98) |
| 5 | Ph; 1L | 8a | 2 | 70 (69); 9La | >99 (>99) |
| 6 | 3-OMeC6H4; 1M | 8a | 2 | 55 (51); 9Ma | >99 (>99) |
| 7 | 4-ClC6H4; 1N | 8a | 2 | 50 (48); 9Na | >99 (>99) |
| 8e | Me; 1A | 8b | 3 | 86 (91); 9Ab | 94 (91) |
Unless noted, reactions were performed with 1 (0.2 mmol), 8 (0.4 mmol), aqueous KOH solution (2.2 μL, 50wt %, 10 mol %), A-2 (10 mol % for entries 1–3 and 8, 50 mol % for entries 47) in PhMe (2.0 mL) with catalyst QD-1 (0.5 mmol %); results in parentheses were obtained from reactions catalyzed by epiQ-1 (0.5 mol %) in same conditions..
Isolated yield of 9, dr of 9 >95/5.
Determined by HPLC analysis.
1.0 mol % catalysts were employed.
2.0 mol % catalysts were employed.
As illustrated in Scheme 2, the reactions proceeded at gram-scale without any deterioration in efficiency and enantioselectivity. To further demonstrate the synthetic versatility of these new transformations, we converted products 5Aa and 9Aa into chiral γ-amino esters 11Aa and 12Aa, respectively in excellent yields. The absolute configuration of product 9Aa was established by X-ray crystallography.15
Scheme 2.
Gram-scale Reactions and Synthetic Applications.
CONCLUSION
We have identified new catalytic systems combing cinchona phase-transfer catalysts and phenol additives to realize highly diastereo- and enantioselective C-C bond-forming umpolung reaction of trifluoromethyl imines with less reactive but readily available carbon electrophiles such as acrylates or α,β-unsaturated lactones. Notably, these newly developed catalysts and co-catalysts resolve the issue of inadequate chemo-, regio-, and enantioselectivity mediated by existing catalysts, affording new access to either enantiomers of the highly versatile chiral γ-trifluromethylated γ-amino esters directly in good yields and excellent enantioselectivities from the readily available prochiral starting materials.
EXPERIMENTAL SECTIN
General Information.
1H and 13C {1H} NMR spectra were recorded on a Varian instrument (400 MHz and 100 MHz, respectively) and internally referenced to tetramethylsilane signal or residual protio solvent signals. Data for 1H NMR are recorded as follows: chemical shift (δ, ppm), multiplicity (s, singlet; d, doublet; t, triplet; q, quartet; m, multiplet), integration, coupling constant (J, Hz). Data for 13C {1H} NMR are reported in terms of chemical shift (δ, ppm). Infrared spectra were recorded on a Perkin Elmer FT-IR Spectrometer and are reported in frequency of absorption. High resolution mass spectra were recorded on either a Micromass 70-VSE-B instrument (EI, CI) or a Micromass Q-TOF instrument (ESI). Specific optical rotations were measured on a Jasco Digital Polar-imeter. All simple chemicals were purchased and used as received. High performance liquid chromatography (HPLC) analyses were performed on a Hewlett-Packard 1100 Series instrument equipped with a quaternary pump, using Daicel Chiralpak AD-H (250 × 4.6 mm), Daicel Chiralcel OJ-H Columns (250 × 4.6 mm). UV absorption was monitored at 210, 220 or 254 nm.
Preparation of Trifluoromethyl Imine Substrates (1A-1N).
Trifluoromethyl imines 1A-1N were obtained according to our previously reported procedures.6a,10
Preparation of 6’-Isopropoxy Cinchona Alkaloids Intermediates (S1–S3).
6’-isopropoxy quinidine (S1) and 6’-iso-propoxy quinine (S2) were synthesized according to the exactly same literature procedures.12b–d Epi-vinyl 6’-isopropoxy quinine (S3). The epi-vinyl 6’-isopropoxy quinine S3 was prepared according to the same method starting from epi-vinyl 6’-OH quinine13 as white foam (2.5 mmol scale reaction, 0.79 g, 90% yield); [α]D20 = −142.5 (c = 1.0, CHCl3); 1H NMR (400 MHz, CDCl3) δ 8.54 (d, J = 4.5 Hz, 1H), 7.83 (d, J = 9.0 Hz, 1H), 7.53 (d, J = 4.5 Hz, 1H), 7.21–7.09 (m, 2H), 5.88 (brs, 1H), 5.86–5.74 (m, 1H), 5.05 (d, J = 10.4 Hz, 1H), 4.99 (d, J = 17.3 Hz, 1H), 4.71–4.58 (m, 1H), 3.83 (brs, 1H), 3.24–3.06 (m, 2H), 2.79 (t, J = 12.6 Hz, 1H), 2.65 (dd, J1 = 13.0 Hz, J2 = 7.6 Hz, 1H), 2.28 (q, J = 8.6 Hz, 1H), 2.02 (t, J = 10.8 Hz, 1H), 1.82 (s, 1H), 1.78–1.67 (m, 1H), 1.66−1.51 (m, 1H), 1.28 (d, J = 5.8 Hz, 6H), 1.27−1.23 (m, 1H); 13C {1H} NMR (100 MHz, CDCl3) δ 155.9, 147.1, 146.8, 143.6, 139.2, 131.3, 126.2, 122.8, 118.6, 118.5, 115.5, 102.8, 69.9, 59.6, 55.7, 44.1, 38.4, 27.9, 25.6, 22.1, 21.4, 20.4; IR (CHCl3) υ 1618, 1240, 1215, 1111, 966, 744, 664, 639 cm−1; HRMS (ESI/[M+H]+) Calcd. for C22H29N2O2 m/z 353.2229, found m/z 353.2227.
General Procedure for the Preparation of Tertiary Amine Precursors (S5–S9).
(1S,2R,4S,5R)-2-((S)-((6-chloro-2,5-diphenylpyrimidin-4-yl)oxy)(6-methoxyquinolin-4-yl)methyl)-5-vinylquinuclidine (S5) and (1S,2S,4S,5R)-2-((R)-((6-chloro-2,5-diphenylpyrimidin-4-yl)oxy)(6-methoxyquin-olin-4-yl)methyl)-5-vinylquinuclidine (S6) were prepared according to our previously reported procedures.10,13 Tertial amine precusors S7–S9 were prepared according to the following general procedure. Under N2 atmosphere, to a solution of corresponding cinchona intermediates (S1–S3) (5.0 mmol, 1.0 equiv.) and 4, 6-dichloro-2, 5-diphenylpyrimidine (1.2 equiv.) in PhMe (0.1 M) was added grounded KOH powder (15.0 equiv.). The suspension was then refluxed for 30 mins. The resulting mixture was cooled to room temperature and diluted with water. The organic phase was separated and the aqueous layer was extracted with ethyl acetate. The combined organic extracts were successively washed with brine, dried over anhydrous Na2SO4 and concentrated under vacuum. The residue was purified through flash chromatography on silica gel (CH2Cl2/MeOH = 100/1 to 10/1) to afford desired cinchona tertiary amine precursors S7–S9 as white solid.
(1S,2R,4S,5R)-2-((S)-((6-chloro-2,5-diphenylpyrimidin-4-yl)oxy)(6-isopropoxyquinolin-4-yl)methyl)-5-vinylquinuclidine (S7). Cinchona tertiary amine precursor S7 was obtained according to the general procedure as white solid (5.0 mmol scale reaction, 2.53 g, 82% yield). [α]D20 = −101.7 (c = 0.5, CHCl3,); 1H NMR (400 MHz, CDCl3.) δ 8.70 (d, J = 4.5 Hz, 1H), 8.07–7.96 (m, 3H), 7.59−7.47 (m, 4H), 7.46−7.33 (m, 4H), 7.30 (d, J = 4.5 Hz, 1H), 7.24 (t, J = 7.6 Hz, 2H), 5.45−5.23 (m, 1H), 4.94 (d, J = 17.0 Hz, 1H), 4.88 (dd, J1 = 10.0 Hz, J2 = 1.4 Hz, 1H), 4.72−4.59 (m, 1H), 3.26−3.14 (m, 1H), 2.88 (s, 1H), 2.85 (s, 1H), 2.83−2.76 (m, 1H), 2.73−2.63 (m, 1H), 2.15 (q, J = 8.6 Hz, 1H), 1.83 (t, J = 11.0 Hz, 1H), 1.68 (s, 1H), 1.50–1.44 (m, 1H), 1.41 (d, J = 6.0 Hz, 3H), 1.38 (d, J = 6.0 Hz, 3H), 1.14 (dd, J1 = 7.5 Hz, J2 = 4.5 Hz, 1H); 13C {1H} NMR (100 MHz, CDCl3.) δ 166.4, 162.4, 160.1, 156.2, 147.3, 144.5, 135.6, 132.1, 132.0, 131.2, 129.9, 128.6, 128.5, 128.3, 128.2, 103.3, 70.1, 59.5, 50.0, 49.8, 40.2, 28.3, 26.1, 22.7, 22.0, 21.8, 18.0, 12.8; IR (CHCl3) υ 2940, 1570, 1517, 1407, 1215, 999, 744, 701 cm−1; HRMS (ESI/[M+H]+) Calcd. for C38H38N4O2Cl m/z 617.2683, found m/z 617.2677.
(1S,2S,4S,5R)-2-((R)-((6-chloro-2,5-diphenylpyrimidin-4-yl)oxy)(6-isopropoxyquinolin-4-yl)methyl)-5-vinylquinuclidine (S8). Cinchona tertiary amine precursor S8 was obtained according to the general procedure as white solid (3.0 mmol scale reaction, 1.44 g, 78% yield); [α]D20 = +212.0 (c = 1.0, CHQ3); 1H NMR (400 MHz, CDCl3) δ 8.68 (d, J = 4.6 Hz, 1H), 8.05 (d, J = 9.2 Hz, 1H), 7.97 (s, 1H), 7.95 (s, 1H), 7.60−7.31 (m, 8H), 7.28−7.18 (m, 3H), 6.96 (brs, 1H), 5.72−5.59 (m, 1H), 4.92 (d, J = 15.5 Hz, 1H), 4.88 (d, J = 7.5 Hz, 1H), 4.69 (brs, 1H), 3.27–3.14 (m, 1H), 3.14−2.99 (m, 2H), 2.72−2.54 (m, 2H), 2.22 (s, 1H), 1.69 (s, 1H), 1.63−1.50 (m, 2H), 1.42 (d, J = 6.2 Hz, 3H), 1.39 (d, J = 6.2 Hz, 3H), 1.34−1.24 (m, 1H), 1.09−0.97 (m, 1H); 13C {1H} NMR (100 MHz, CDCl3) δ 166.2, 162.5, 159.8, 156.3, 147.2, 144.4, 135.5, 132.1, 131.9, 131.2, 129.9, 128.6, 128.5, 128.2, 128.2, 126.4, 118.7, 103.2, 70.1, 59.3, 57.2, 43.2, 39.6, 27.5, 27.0, 22.4, 21.9, 21.7; IR (CHCl3) υ 1569, 1516, 1407, 1215, 1001, 969, 745, 699, 665 cm−1; HRMS (ESI/[M+H]+) Calcd. for C38H38N4O2Cl m/z 617.2683, found m/z 617.2680.
(1S,2S,4S)-2-((R)-((6-chloro-2,5-diphenylpyrimidin-4-yl)oxy)(6-isopropoxyquinolin-4-yl)methyl)-5-vinylquinuclidine (S9). The epi-vinyl cinchona tertiary amine precursor S9 was obtained according to the general procedure as white solid (2.0 mmol scale reaction, 1.05 g, 85% yield); [α]D20 = +180.3 (c = 0.5, CHCl3); 1H NMR (400 MHz, CDCl3) δ 8.68 (d, J = 4.6 Hz, 1H), 8.04 (d, J = 9.2 Hz, 1H), 7.97 (s, 1H), 7.95 (s, 1H), 7.63−7.31 (m, 8H), 7.28−7.18 (m, 3H), 6.91 (s, 1H), 5.87−5.72 (m, 1H), 4.97 (d, J = 9.2 Hz, 1H), 4.94 (d, J = 16.8 Hz, 1H), 4.69 (s, 1H), 3.28–3.15 (m, 1H), 3.12−2.92 (m, 2H), 2.62 (t, J = 11.0 Hz, 1H), 2.47 (dd, J1 = 12.8 Hz, J2 = 7.6 Hz, 1H), 2.24−2.11 (m, 1H), 1.77−1.59 (m, 2H), 1.58−1.47 (m, 2H), 1.42 (d, J = 6.2 Hz, 3H), 1.40 (d, J = 6.2 Hz, 3H), 0.98−0.86 (s, 1H); 13C {1H} NMR (100 MHz, CDCl3) δ 166.3, 162.5, 159.7, 156.3, 147.3, 144.4, 135.6, 132.1, 132.0, 131.2, 129.9, 128.6, 128.5, 128.3, 126.6, 122.9, 118.7, 103.4, 70.2, 59.2, 56.7, 44.0, 39.2, 28.0, 21.9, 21.8, 21.1; IR (CHCl3) υ 2932, 1569, 1515, 1405, 1218, 1110, 1000, 747, 699 cm−1; HRMS (ESI/[M+H]+) Calcd. for C38H38N4O2Cl m/z 617.2683, found m/z 617.2678.
Procedure for the Synthesis of Arylmethyl Bromides (S10-S11).
9-(bromomethyl)anthracene (S10). S10 was prepared according to the same literature procedures.16 5′-(bromomethyl)-2′ (tert-butoxy)-1,1′.3′,1″-terphenyl (S11). S11 was prepared according to our previously reported method.6a
General Procedure for Preparation of Cinchona Phase-Transfer Catalysts.
Cincona catalysts C-1 and QD-2 were synthesized according to our previously reported procedure.6a,10 Catalysts QD-1, epiQ-1, QD-3, Q-3, and epiQ-3 were synthesized by the following general procedures. To a solution of corresponding cinchona tertiary amine precursors (S5–S9) (1.0 equiv.) in CH3CN (0.1 M) was added the corresponding arylmethyl bromide (S10-S11) (1.0 equiv.) at room temperature. The reaction mixture was stirred overnight and then concentrated under vacuum. The residue was applied to flash chromatography (CH2Cl2/MeOH = 50/1 to 10/1) on silica gel to afford desired cinchona phase-transfer catalysts.
(1S,2R,4S,5R)-1-(anthracen-9-ylmethyl)-2-((S)-((6-chloro-2,5-diphenylpyrimidin-4-yl)oxy)(6-isopropoxyquinolin-4-yl)methyl)-5-vinylquinuclidin-i-ium bromide (QD-3). Catalyst QD-3 was obtained according to the general procedure as light yellow solid (0.96 g, 72% yield). [α]D20 = +244.8 (c = 1.0, CHCl3); 1H NMR (400 MHz, CDCl3) δ 9.64 (d, J = 9.1 Hz, 1H), 8.90 (d, J = 4.5 Hz, 1H), 8.62 (s, 1H), 8.25 (s, 1H), 8.15−7.84 (m, 7H), 7.79−7.60 (m, 5H), 7.58−7.40 (m, 4H), 7.39−7.18 (m, 4H), 6.47 (d, J = 13.2 Hz, 1H), 6.09 (t, J = 9.8 Hz, 1H), 5.70 (t, J = 10.6 Hz, 1H), 5.54 (d, J = 13.2 Hz, 1H), 5.48−5.37 (m, 1H), 4.86 (d, J = 10.2 Hz, 1H), 4.76 (d, J = 16.4 Hz, 1H), 4.71−4.59 (m, 1H), 3.39 (t, J = 10.4 Hz, 1H), 2.95 (t, J = 11.2 Hz, 1H), 2.55−2.36 (m, 2H), 2.16 (q, J = 10.6 Hz, 1H), 1.95 (q, J = 8.6 Hz, 1H), 1.79 (s, 1H), 1.73–1.62 (m, 1H), 1.55 (d, J = 6.0 Hz, 3H), 1.55−1.50 (m, 1H), 1.47 (d, J = 6.0 Hz, 3H); 13C {1H} NMR (100 MHz, CDCl3) δ 164.9, 163.4, 161.9, 157.0, 147.1, 144.7, 137.6, 134.8, 134.6, 132.8, 132.7, 132.1, 132.0, 131.6, 131.5, 130.9, 130.6, 129.7, 129.6, 129.5, 129.1, 128.6, 128.4, 127.7, 126.8, 126.2, 124.9, 119.0, 116.8, 106.5, 74.2, 71.1, 66.4, 56.9, 56.1, 54.5, 39.0, 26.7, 24.0, 23.0, 22.9, 21.8; IR (CHCl3) υ 2932, 1571, 1514, 1363, 1240, 1215, 1107, 998, 970, 844, 742, 701, 659 cm−1; HRMS (ESI/[M-Br]+) Calcd. for C53H48N4O2Cl m/z 807.3466, found m/z 807.3461.
(1S,2S,4S,5R)-1-(anthracen-9-ylmethyl)-2-((R)-((6-chloro-2,5-diphenylpyrimidin-4-yl)oxy)(6-isopropoxyquinolin-4-yl)methyl)-5-vinylquinuclidin-1-ium bromide (Q-3). Catalyst Q-3 was obtained according to the general procedure as light yellow solid (1.5 mmol scale reaction, 1.0 g, 75% yield). [α]D20 = −105.8 (c = 1.0, CHCl3,);1H NMR (400 MHz, CDCl3.) δ 9.46 (d, J = 9.2 Hz, 1H), 8.87 (d, J = 4.6 Hz, 1H), 8.54 (s, 1H), 8.17 (s, 1H), 8.09−7.92 (m, 5H), 7.90 (d, J = 4.6 Hz, 1H), 7.78 (t, J = 7.6 Hz, 1H), 7.68 (d, J = 9.2 Hz, 1H), 7.62−7.40 (m, 7H), 7.37 (t, J = 7.6 Hz, 1H), 7.27 (d, J = 8.0 Hz, 1H), 7.25 (d, J = 7.5 Hz, 1H), 7.18 (t, J = 7.6 Hz, 1H), 6.85 (d, J = 13.6 Hz, 1H), 6.08 (t, J = 8.7 Hz, 1H), 5.96−5.84 (m, 1H), 5.79 (d, J = 13.6 Hz, 1H), 5.27 (septet, J = 6.0 Hz, 1H), 5.21−5.13 (m, 1H), 5.13 (d, J = 17.1 Hz, 1H), 5.0 (d, J = 10.4 Hz, 1H), 3.57 (t, J = 11.5 Hz, 1H), 3.21 (t, J = 11.8 Hz, 1H), 2.38−2.17 (m, 3H), 2.02 (t, J = 12.7 Hz, 1H), 1.73 (s, 1H), 1.45 (d, J = 6.0 Hz, 3H), 1.39 (d, J = 6.0 Hz, 3H), 1.17−1.03 (m, 1H), 0.92−0.77 (m, 1H); 13C (1H) NMR (100 MHz, CDCl3) δ 165.2, 163.4, 161.3, 157.1, 147.1, 144.6, 137.9, 136.1, 134.7, 133.7, 132.6, 132.6, 132.2, 132.1, 131.4, 131.3, 130.9, 129.6, 129.4, 129.2, 128.6, 128.4, 126.6, 118.9, 117.2, 105.9, 73.4, 70.9, 66.5, 61.3, 55.1, 52.1, 38.4, 26.3, 24.5, 22.8, 22.7, 21.6; IR (CHCl3) υ 1517, 1409, 1214, 743, 704, 665 cm−1; HRMS (ESI/[M-Br]+) Calcd. for C53H4sN4O2Cl m/z 807.3466, found m/z 807.3458.
(1S,2S,4S)-1-(anthracen-9-ylmethyl)-2-((R)-((6-chloro-2,5-diphenylpyrimidin-4-yl)oxy)(6-isopropoxyquinolin-4-yl)methyl)-5-vinylquinuclidin-1-ium bromide (epiQ-3). Catalyst epiQ-3 was obtained according to the general procedure as light yellow solid (1.0 mmol scale reaction, 0.64 g, 72% yield). [α]D20 = −178.5 (c = 1.0, CHCl3.);1H NMR (400 MHz, CDCL.) δ 9.52 (d, J = 9.2 Hz, 1H), 8.86 (d, J = 4.6 Hz, 1H), 8.64 (s, 1H), 8.18 (s, 1H), 8.11 (d, J = 8.5 Hz, 1H), 8.07 (d, J = 9.3 Hz, 1H), 8.03 (d, J = 8.4 Hz, 1H), 8.00−7.92 (m, 3H), 7.72–7.78 (m, 2H), 7.74−7.45 (m, 8H), 7.40−7.29 (m, 2H), 7.22 (t, J = 7.7 Hz, 2H), 6.59 (d, J = 13.3 Hz, 1H), 6.24 (t, J = 9.1 Hz, 1H), 5.72 (t, J = 10.5 Hz, 1H), 5.65 (d, J = 13.3 Hz, 1H), 5.51−5.39 (m, 1H), 5.26−5.12 (m, 1H), 4.78 (d, J = 10.4 Hz, 1H), 4.58 (d, J = 17.2 Hz, 1H), 3.62−3.42 (m, 1H), 3.00−2.83 (m, 1H), 2.47−2.27 (m, 3H), 2.04−1.88 (m, 1H), 1.80 (s, 1H), 1.54 (d, J = 6.2 Hz, 3H), 1.43 (d, J = 6.2 Hz, 3H), 1.30−1.16 (m, 1H), 0.87−0.67 (m, 1H); 13C (1H) NMR (100 MHz, CDCl3) δ 165.0, 163.4, 161.1, 157.1, 146.9, 144.6, 137.6, 135.7, 134.5, 133.6, 132.8, 132.6, 132.1, 131.4, 131.3, 130.9, 129.6, 129.4, 129.2, 129.2, 129.1, 128.6, 128.4, 128.2, 127.6, 127.6, 126.6, 126.3, 126.2, 124.9, 122.7, 122.0, 120.4, 118.8, 117.4, 116.8, 105.4, 72.9, 70.9, 65.8, 61.2, 54.7, 52.2, 36.5, 27.8, 25.4, 22.9, 21.5, 19.7; IR (CHCl3) υ 1573, 1516, 1408, 1215, 1000, 743, 701, 663 cm−1; HRMS (ESI/[M-Br]+) Calcd. for C53H48N4O2Cl m/z 807.3466, found m/z 807.3455.
(1S,2R,4S,5R)-1-((2′-(tert-butoxy)-[1,1′:3′,1″-terphenyl]-5′-yl)methyl)-2-((S)-((6-chloro-2,5-diphenylpyrimidin-4-yl)oxy)(6-methoxyquinolin-4-yl)methyl)-5-vinylquinuclidin-1-ium bromide (QD-1). Catalyst QD-1 was obtained according to the general procedure as white solid (1.0 mmol scale reaction, 0.81 g, 82% yield). [α]D20 = −28.5 (c = 0.5, CHCl3);1H NMR (400 MHz, CDCl3) δ 8.75 (d, J = 4.6 Hz, 1H), 8.07 (d, J = 2.2 Hz, 1H), 8.07 (d, J = 9.3 Hz, 1H), 7.84 (d, J = 7.4 Hz, 2H), 7.80−7.55 (m, 7H), 7.58−7.30 (m, 13H), 7.20 (t, J = 8.0 Hz, 2H), 6.62 (d, J = 11.8 Hz, 1H), 5.80 (t, J = 10.8 Hz, 1H), 5.20 (t, J = 8.4 Hz, 1H), 5.15−5.01 (m, 2H), 4.97−4.86 (m, 1H), 4.45 (s, 3H), 4.17 (d, J = 11.8 Hz, 1H), 3.27 (t, J = 11.6 Hz, 1H), 3.00−2.84 (m, 2H), 2.41−2.21 (m, 2H), 2.00−1.85 (m, 2H), 1.78−1.67 (m, 1H), 1.44−1.31 (m, 1H), 0.57 (s, 9H).; 13C {1H} NMR (100 MHz, CDCl3) δ 164.7, 163.2, 161.4, 159.6, 153.4, 146.3, 145.0, 140.3, 139.5, 138.2, 135.1, 135.0, 134.5, 132.0, 131.9, 131.8, 130.1, 129.6, 129.3, 128.5, 128.4, 128.3, 127.6, 126.6, 123.5, 122.1, 119.7, 118.6, 102.2, 84.0, 72.4, 64.4, 60.7, 57.8, 55.9, 54.7, 38.2, 29.0, 27.4, 23.6, 23.3; IR (CHCl3) υ 1574, 1515, 1410, 1214, 1152, 1004, 743, 699, 665 cm−1; HRMS (ESI/[M-Br]+) Calcd. for C59H56N4O3Cl m/z 903.4041, found m/z 903.4031.
(1S,2S,4S,5R)-1-((2′-(tert-butoxy)-[1,1′:3′,1″-terphenyl]-5′-yl)methyl)-2-((R)-((6-chloro-2,5-diphenylpyrimidin-4-yl)oxy)(6-methoxyquinolin-4-yl)methyl)-5-vinylquinuclidin-1-ium bromide (Q-1). Catalyst Q-1 was obtained according to the general procedure as white solid (0.5 mmol scale reaction, 0.41 g, 82% yield). [α]D20 = +32.0 (c = 1.0, CHCl3);1H NMR (400 MHz, CDCl3) δ 8.73 (d, J = 4.6 Hz, 1H), 8.05 (d, J = 9.3 Hz, 1H), 7.92 (d, J = 2.1 Hz, 1H), 7.75−7.28 (m, 19H), 7.17 (t, J = 7.8 Hz, 2H), 5.88−5.74 (m, 1H), 5.37 (d, J = 13.0 Hz, 1H), 5.26 (d, J = 17.2 Hz, 1H), 5.09 (t, J = 9.4 Hz, 1H), 5.01 (d, J = 10.5 Hz, 1H), 4.37 (s, 3H), 4.27 (d, J = 11.7 Hz, 1H), 3.30 (dd, J1 = 12.6 Hz, J2 = 11.0 Hz, 1H), 3.10−2.90 (m, 1H), 2.55 (s, 1H), 2.25−2.13 (m, 1H), 1.99 (s, 1H), 1.81−1.69 (m, 1H), 1.60−1.47 (m, 1H), 1.43−1.30 (m, 1H), 0.56 (s, 9H); 13C {1H} NMR (100 MHz, CDCl3) δ 164.7, 163.1, 160.7, 159.6, 153.3, 146.2, 144.9, 140.2, 139.5, 138.1, 136.1, 135.1, 134.5, 132.0, 131.9, 131.6, 130.1, 129.6, 129.3, 129.2, 128.4, 128.4, 128.3, 127.5, 126.4, 123.4, 122.1, 119.7, 118.7, 118.5, 101.8, 84.0, 71.9, 64.7, 61.6, 60.0, 57.6, 50.8, 37.7, 29.0, 27.1, 25.2, 24.0; IR (CHCl3) υ 2932, 1573, 1514, 1408, 1365, 1216, 1152, 1027, 991, 743, 699, 661 cm−1; HRMS (ESI/[M-Br]+) Calcd. for C59H56N4O3Cl m/z 903.4041, found m/z 903.4036.
(1S,2S,4S)-1-((2′-(tert-butoxy)-[1,1′:3′,1″-terphenyl]-5′-yl)methyl)-2-((R)-((6-chloro-2,5-diphenylpyrimidin-4-yl)oxy)(6-methoxyquinolin-4-yl)methyl)-5-vinylquinuclidin-1-ium bromide (epiQ-1). Catalyst epiQ-1 was obtained according to the general procedure as white solid (1.0 mmol scale reaction, 0.79 g, 80% yield). [α]D20 = +2.8 (c = 0.5, CHCl3); 1H NMR (400 MHz, CDCl3) δ 8.74 (d, J = 4.6 Hz, 1H), 8.05 (d, J = 9.4 Hz, 1H), 8.03 (d, J = 2.4 Hz, 1H), 7.67−7.29 (m, 19H), 7.15 (t, J = 7.8 Hz, 2H), 6.65 (d, J = 11.8 Hz, 1H), 5.76 (t, J = 11.0 Hz, 1H), 5.53−5.42 (m, 1H), 5.30 (t, J = 9.4 Hz, 1H), 5.02 (d, J = 10.4 Hz, 1H), 4.92 (d, J = 17.0 Hz, 1H), 4.42 (s, 3H), 4.09 (d, J = 11.8 Hz, 1H), 2.93 (t, J = 7.8 Hz, 1H), 2.76 (q, J = 8.0 Hz, 1H), 2.59 (dd, J1 = 12.0 Hz, J2 = 8.8 Hz, 1H), 2.30 (t, J = 11.8 Hz, 1H), 1.94 (s, 1H), 1.72−1.57 (m, 2H), 1.24−1.17 (m, 1H), 0.56 (s, 9H); 13C {1H} NMR (100 MHz, CDCl3) δ 164.6, 163.2, 160.5, 159.6, 153.4, 146.2, 145.0, 140.3, 139.5, 138.1, 134.9, 134.9, 132.0, 130.1, 129.7, 129.1, 128.4, 128.4, 128.3, 127.6, 123.5, 122.0, 119.7, 118.4, 118.3, 102.1, 83.9, 72.2, 64.3, 61.2, 60.2, 57.7, 50.7, 36.3, 29.0, 28.6, 26.9, 20.3; IR (CHCl3) υ 1515, 1409, 1214, 1153, 992, 743, 700, 665 cm−1; HRMS (ESI/[M-Br]+) Calcd. for C59H56N4O3Cl m/z 903.4041, found m/z 903.4063.
Synthesis of Racemic Products (5 & 9).
Racemic samples of compounds 5 and 9 were obtained either from the reactions catalyzed by achiral phase-transfer catalyst tetra-n-butylammonium bromide (TBAB) following the same procedures as the ones catalyzed by chiral cinchona alkaloid-based phase-transfer catalysts or they were simply obtained from the mixture of two opposite enantiomers.
Monitoring of Reaction Crude Mixture.
The reaction was monitored by taking an aliquot of the reaction mixture (100 μL) which was filtered through a pipet pack with silica gel (0.5 cm), washed with ether, concentrated and subject to 1H and 19F NMR analysis. The conversion, chemo-and regio-selectivity were determined by 19F NMR analysis of the crude reaction mixture.
Preparation of deactivated silica.
The dry silica gel was packed in a column and washed with EtOH/MeOH/Et3N = 5/1/1 solution [2 column volume (CV)], followed by Et2O (5 CV) wash. The silica gel was then blown dry with compressed air and ready for use.
General Procedure for the Asymmetric Synthesis of Compound 5.
At indicated temperature, to a solution of trifluoromethyl imines 1 (0.2 mmol), acrylates 4 (0.4 mmol, 2.0 equiv.), phenol-additive A-1 (0.004 mmol, 2.0 mol%), ethanol (20.0 μL) and catalyst QD-3 or epiQ-3 (5.0 mol%) in PhMe (totally 2.0 mL) was added aqueous KOH solution (2.2 μL, 50 wt%, 0.02 mmol). The mixture was then vigorously stirred and turned purple immediately. After the reaction was completed or reached the maximum conversion (checked by 19F NMR), the reaction mixture was then passed through a small pad of deactivated silica (5 cm thick) and washed with Et2O (1.0 mL × 3). The filtrates were combined, concentrated, purified through flash chromatography with deactivated silica gel (Hexanes/Et2O) to afford the desired products.
Methyl (S)-5,5,5-trifluoro-4-methyl-4-((4-nitrobenzylidene)amino) pentanoate (5Aa). The product 5Aa was obtained as oil in 80% yield after flash chromatography with deactivated silica gel (Hexanes/Et2O = 100/1 to 10/1) from a reaction catalyzed by QD-3 (5.0 mol%), A-1 (2.0 mol%) and ethanol (20 μL) in PhMe (2.0 mL) at −40 °C for 8 h. 92% ee was determined by HPLC analysis [Daicel Chiralpak AD-H, Hexanes/IPA = 97/3, 1.0 mL/min, λ 254 nm, t (minor) = 12.78 min, t (major) = 17.88 min]. [α]D20 = −85.7 (c = 1.0, CHCl3); 1H NMR (400 MHz, CDCl3) δ 8.42 (s, 1H), 8.29 (d, J = 8.7 Hz, 2H), 7.97 (d, J = 8.7 Hz, 2H), 3.63 (s, 3H), 2.48–2.30 (m, 3H), 2.23–2.11 (m, 1H), 1.48 (s, 3H); 13C {1H} NMR (100 MHz, CDCl3) δ 173.1, 159.0, 149.6, 140.9, 129.3 (2C), 126.4 (q, JC-F = 283 Hz), 123.9 (2C), 65.8 (q, JC-F = 26 Hz), 51.8, 30.9, 28.3, 17.4; 19F NMR (376 MHz, CDCl3) δ −78.0; IR (CHCl3) υ 1734, 1650, 1603, 1523, 1345, 1273, 1145, 1098, 988, 838, 746, 688 cm−1; HRMS (ESI/[M+H]+) Calcd. for C14H16N2O4F3 m/z 333.1062, found m/z 333.1065. The product of opposite absolute configuration was obtained in 85% yield from a reaction catalyzed by epiQ-3 (5.0 mol%), A-1 (2.0 mol%) and ethanol (20 μL) in PhMe (2.0 mL) at −40 °C for 8 h. 92% ee was determined by HPLC analysis [t (major) = 12.86 min, t (minor) = 18.02 min]. [α]D20 = +62.0 (c = 0.5, CHCl3).
Prop-2-yn-1-yl (S)-5,5,5-trifluoro-4-methyl-4-((4-nitrobenzylidene)amino)pentanoate (5Ab). The product 5Ab was obtained as oil in 89% yield after flash chromatography with deactivated silica gel (Hexanes/Et2O = 100/1 to 4/1) from a reaction catalyzed by QD-3 (1.0 mol%), A-1 (2.0 mol%) and ethanol (20 μL) in PhMe (2.0 mL) at −30 °C for 3 h. 91% ee was determined by HPLC analysis [Daicel Chiralpak AD-H, Hexanes/IPA = 95/5, 1.0 mL/min, λ 254 nm, t (minor) = 14.88 min, t (major) = 19.86 min]. [α]D20 = −48.9 (c = 1.0, CHCl3). 13H NMR (400 MHz, CDCl3) δ 8.42 (s, 1H), 8.29 (d, J = 8.7 Hz, 2H), 7.97 (d, J = 8.7 Hz, 2H), 4.62 (d, J = 2.4 Hz, 2H), 2.55–2.32 (m, 4H), 2.22–2.11 (m, 1H), 1.49 (s, 3H); 13C {1H} NMR (100 MHz, CDCl3) δ 171.9, 159.1, 149.6, 140.9, 129.4 (2C), 126.4 (q, JC-F = 283 Hz), 123.9 (2C), 75.1, 65.7 (q, JC-F = 25 Hz), 52.2, 30.8, 28.3, 17.4; 19F NMR (376 MHz, CDCl3) δ −78.0; IR (CHCl3) υ 1740, 1523, 1346, 1147, 1098, 990, 838, 747, 687, 637 cm−1; HRMS (ESI/[M+H]+) Calcd. for C16H16N2O4F3 m/z 357.1062, found m/z 357.1064. The product of opposite absolute configuration was obtained in 92% yield from a reaction catalyzed by epiQ-3 (1.0 mol%), A-1 (2.0 mol%) and ethanol (20 μL) in PhMe (2.0 mL) at −30 °C for 3 h. 93% ee was determined by HPLC analysis [t (major) = 14.93 min, t (minor) = 20.01 min]. [α]D20 = +105.0 (c = 1.0, CHCl3).
Allyl (S)-5,5,5-trifluoro-4-methyl-4-((4-nitrobenzylidene)amino)pentanoate (5AC). The product 5Ac was obtained as oil in 70% yield after flash chromatography with deactivated silica gel (Hexanes/Et2O = 100/1 to 10/1) from a reaction catalyzed by QD-3 (5.0 mol%), A-1 (2.0 mol%) and ethanol (20 μL) in PhMe (2.0 mL) at −40 °C for 20 h. 85% ee was determined by HPLC analysis [Daicel Chiralpak AD-H, Hexanes/IPA = 97/3, 1.0 mL/min, λ 254 nm, t (minor) = 11.90 min, t (major) = 17.69 min]. [α]D20 = −72.0 (c = 1.0, CHCl3). 13H NMR (400 MHz, CDCl3) δ 8.42 (s, 1H), 8.29 (d, J = 8.8 Hz, 2H), 7.97 (d, J = 8.8 Hz, 2H), 5.93–5.80 (m, 1H), 5.33–5.19 (m, 2H), 4.52 (dt, J1 = 5.8 Hz, J2 = 1.2 Hz, 2H), 2.52–2.33 (m, 3H), 2.23–2.11 (m, 1H), 1.48 (s, 3H); 13C {1H} NMR (100 MHz, CDCl3) δ 172.4, 159.0, 149.6, 140.9, 131.9, 129.3 (2C), 126.4 (q, JC-F = 283 Hz), 123.9 (2C), 118.6, 65.8 (q, JC-F = 26 Hz), 65.4, 30.9, 28.5, 17.4; 19F NMR (376 MHz, CDCl3) δ −78.0; IR (CHCl3) υ 1732, 1650, 1603, 1523, 1345, 1273, 1145, 1098, 985, 837, 748, 689 cm−1; HRMS (ESI/[M+h]+) Calcd. for C16H18F3N2O4 m/z 359.1219, found m/z 359.1214. The product of opposite absolute configuration was obtained in 75% yield from a reaction catalyzed by epiQ-3 (5.0 mol%), A-1 (2.0 mol%) and ethanol (20 μL) in PhMe (2.0 mL) at −40 °C for 20 h. 90% ee was determined by HPLC analysis [t (major) = 11.90 min, t (minor) = 17.70 min]. [α]D20 = +33.8 (c = 0.5, CHCl3).
Ethyl (S)-5,5,5-trifluoro-4-methyl-4-((4-nitrobenzylidene)amino)pentanoate (5Ad). The product 5Ad was obtained as oil in 50% yield after flash chromatography with deactivated silica gel (Hexanes/Et2O = 100/1 to 10/1) from a reaction catalyzed by QD-3 (5.0 mol%), A-1 (2.0 mol%) and ethanol (20 μL) in PhMe (2.0 mL) at −40 °C for 20 h. 81% ee was determined by HPLC analysis [Daicel Chiralpak AD-H, Hexanes/IPA = 97/3, 1.0 mL/min, λ 254 nm, t (minor) = 11.07 min, t (major) = 16.73 min]. [α]D20 = −68.1 (c = 1.0, CHCl3); 13H NMR (400 MHz, CDCl3) δ 8.42 (s, 1H), 8.29 (d, J = 8.7 Hz, 2H), 7.97 (d, J = 8.7 Hz, 2H), 4.08 (q, J = 7.1 Hz, 1H), 2.46–2.27 (m, 3H), 2.23–2.10 (m, 1H), 1.48 (s, 3H), 1.22 (t, J = 7.1 Hz, 1H); 13C {1H} NMR (100 MHz, CDCl3) δ 172.7, 158.9, 149.5, 140.9, 129.3 (2C), 126.4 (q, JC-F = 283 Hz), 123.9 (2C), 65.8 (q, JC-F = 25 Hz), 60.7, 30.9, 28.6, 17.4, 14.2; 19F NMR (376 MHz, CDCl3) δ −78.1; IR (CHCl3) υ 1729, 1650, 1524, 1345, 1273, U46, 1097, 838, 747, 688 cm−1; HRMS (ESI/[M+H]+) Calcd. for C15H18N2O4F3 m/z 347.1219, found m/z 347.1211. The product of opposite absolute configuration was obtained in 63% yield from a reaction catalyzed by epiQ-3 (5.0 mol%), A-1 (2.0 mol%) and ethanol (20 μL) in PhMe (2.0 mL) at −40 °C for 20 h. 86% ee was determined by HPLC analysis [t (major) = 11.09 min, t (minor) = 16.72 min]. [α]D20 = +59.2 (c = 1.0, CHCl3).
Vinyl (S,E)-5,5,5-trifluoro-4-methyl-4-((4-nitrobenzylidene)amino)pentanoate (5Ae). The product 5Ae was obtained as oil in 91% yield after flash chromatography with deactivated silica gel (Hexanes/Et2O = 100/1 to 15/1) from a reaction catalyzed by QD-3 (1.0 mol%) and A-1 (2.0 mol%) and ethanol (20 μL) in PhMe (2.0 mL) at −30 °C for 8 h. 94% ee was determined by HPLC analysis [Daicel Chiralpak AD-H, Hexanes/IPA = 99/1, 1.0 mL/min, λ 254 nm, t (minor) = 17.73 min, t (major) = 24.73 min]. [α]D20 = −76.3 (c = 1.0, CHCl3); 13H NMR (400 MHz, CDCl3) δ 8.43 (s, 1H), 8.29 (d, J = 8.8 Hz, 2H), 7.96 (d, J = 8.8 Hz, 2H), 7.20 (dd, J1 = 13.9 Hz, J2 = 6.3 Hz, 1H), 4.86 (dd, J1 = 13.9 Hz, J2 = 1.7 Hz, 1H), 4.55 (dd, J1 = 6.3 Hz, J2 = 1.7 Hz, 1H), 2.61–2.37 (m, 3H), 2.24–2.13 (m, 1H), 1.50 (s, 3H); 13C {1H} NMR (100 MHz, CDCl3) δ 169.9, 159.2, 149.6, 141.0, 140.8, 129.4 (2C), 126.4 (q, JC-F = 283 Hz), 123.9 (2C), 97.9, 65.7 (q, JC-F = 25 Hz), 30.8, 28.4, 17.5; 19F NMR (376 MHz, CDCl3) δ −77.8; IR (CHCl3) υ 1752, 1648, 1523, 1344, 1146, 1096, 947, 837, 747, 688 cm−1; HRMS (ESI/[M+H]+) Calcd. for C15H16N2O4F3 m/z 345.1062, found m/z 345.1057. The product of opposite absolute configuration was obtained in 93% yield from a reaction catalyzed by epiQ-3 (1.0 mol%) and A-1 (2.0 mol%) and ethanol (20 μL) in PhMe (2.0 mL) at −30 °C for 8 h. 94% ee was determined by HPLC analysis [t (major) = 17.85 min, t (minor) = 24.80 min]. [α]D20 = +65.7 (c = 1.0, CHCl3).
Methyl (S)-4-((4-nitrobenzylidene)amino)-4-(trifluoromethyl)hexanoate (5Ba). The product 5Ba was obtained as oil in 71% yield after flash chromatography with deactivated silica gel (Hexanes/Et2O = 100/1 to 10/1) from a reaction catalyzed by QD-3 (5.0 mol%), A-1 (2.0 mol%) and ethanol (20 μL) in PhMe (2.0 mL) at −40 °C for 5 h. 87% ee was determined by HPLC analysis [Daicel Chiralpak AD-H, Hexanes/IPA = 98/2, 1.0 mL/min, λ 254 nm, t (minor) = 14.17 min, t (major) = 16.99 min]. [α]D20 = −10.5 (c = 0.3, CHCl3); 13H NMR (400 MHz, CDCl3) δ 8.44 (s, 1H), 8.29 (d, J = 8.7 Hz, 2H), 7.96 (d, J = 8.7 Hz, 2H), 3.66 (s, 3H), 2.50–2.42 (m, 2H), 2.30–2.22 (m, 2H), 1.94 (q, J = 7.6 Hz, 2H), 0.97 (t, J = 7.6 Hz, 3H); 13C {1H} NMR (100 MHz, CDCl3) δ 173.4, 158.8, 149.5, 141.1, 129.2 (2C), 126.8 (q, JC-F = 286 Hz), 123.9 (2C), 67.8 (q, JC-F = 24 Hz), 51.8, 28.4, 27.9, 26.4, 7.7; 19F NMR (376 MHz, CDCL,) δ −73.3; IR (CHCL.) υ 1734, 1649, 1603, 1523, 1344, 1256, 1164, 1113, 1092, 839, 748, 722, 689 cm−1; HRMS (ESI/[M+H]+) Calcd. for C15H18N2O4F3 m/z 347.1219, found m/z 347.1216. The product of opposite absolute configuration was obtained in 73% yield from a reaction catalyzed by epiQ-3 (5.0 mol%), A-1 (2.0 mol%) and ethanol (20 μL) in PhMe (2.0 mL) at −40 °C for 5 h. 90% ee was determined by HPLC analysis [t (major) = 13.79 min, t (minor) = 16.47 min]. [α]D20 = +77.0 (c = 0.5, CHCl3).
Methyl (S)-4-((4-nitrobenzylidene)amino)-4-(trifluoromethyl)octanoate (5Ca). The product 5Ca was obtained as oil in 63% yield after flash chromatography with deactivated silica gel (Hexanes/Et2O = 100/1 to 10/1) from a reaction catalyzed by QD-3 (5.0 mol%), A-1 (2.0 mol%) and ethanol (20 μL) in PhMe (2.0 mL) at −30 °C for 8 h. [α]D20 = +1.0 (c = 0.5, CHCl3). 91% ee was determined by HPLC analysis [Daicel Chiralpak AD-H, Hexanes/IPA = 98/2, 1.0 mL/min, λ 254 nm, t (minor) = 10.42 min, t (major) = 14.36 min]; 1H NMR (400 MHz, CDCl3) δ 8.44 (s, 1H), 8.29 (d, J = 8.7 Hz, 2H), 7.97 (d, J = 8.7 Hz, 2H), 3.66 (s, 3H), 2.50–2.42 (m, 2H), 2.31–2.23 (m, 2H), 1.86 (t, J = 7.9 Hz, 2H), 1.42–1.26 (m, 4H), 0.92 (t, J = 6.9 Hz, 3H); 13C {1H} NMR (100 MHz, CDCl3) δ 173.4, 158.6, 149.5, 141.1, 129.2 (2C), 126.7 (q, JC-F = 285 Hz), 123.9 (2C), 67.6 (q, JC-F = 23 Hz), 51.8, 28.4, 28.3, 25.1, 23.2, 13.9; 19F NMR (376 MHz, CDCl3) δ −73.3; IR (CHCl3) υ 1735, 1649, 1603, 1523, 1344, 1161, 1107, 854, 839, 748, 689 cm−1; HRMS (ESI/[M+H]+) Calcd. for C17H22N2O4F3 m/z 375.1532, found m/z 375.1530. The product of opposite absolute configuration was obtained in 68% yield from a reaction catalyzed by epiQ-3 (5.0 mol%), A-1 (2.0 mol%) and ethanol (20 μL) in PhMe (2.0 mL) at −30 °C for 8 h. 91% ee was determined by HPLC analysis [t (major) = 9.93 min, t (minor) = 13.65 min]. [α]D20 = +3.1 (c = 0.5, CHCl3).
Methyl (S)-4-((4-nitrobenzylidene)amino)-4-(trifluoromethyl)non-8-enoate (5Da). The product 5Da was obtained as oil in 64% yield after flash chromatography with deactivated silica gel (Hexanes/Et2O = 100/1 to 10/1) from a reaction catalyzed by QD-3 (5.0 mol%), A-1 (2.0 mol%) and ethanol (20 μL) in PhMe (2.0 mL) at −30 °C for 8 h. 90% ee was determined by HPLC analysis [Daicel Chiralpak AD-H, Hexanes/IPA = 98/2, 1.0 mL/min, λ 254 nm, t (minor) = 11.03 min, t (major) = 17.79 min]; [α]D20 = −3.3 (c = 0.5, CHCl3); 13H NMR (400 MHz, CDCl3) δ 8.43 (s, 1H), 8.29 (d, J = 8.7 Hz, 2H), 7.96 (d, J = 8.7 Hz, 2H), 5.83–5.67 (m, 1H), 5.08–4.95 (m, 2H), 3.66 (s, 3H), 2.49–2.41 (m, 2H), 2.32–2.22 (m, 2H), 2.08 (q, J = 7.0 Hz, 2H), 1.87 (t, J = 8.5 Hz, 2H), 1.54–1.38 (m, 2H); 13C {1H} NMR (100 MHz, CDCl3) δ 173.3, 158.8, 149.5, 141.0, 137.6, 129.2 (2C), 126.6 (q, JC-F = 285 Hz), 123.9 (2C), 115.6, 67.6 (q, JC-F = 23 Hz), 51.9, 33.8, 33.0, 28.3, 22.2; 19F NMR (376 MHz, CDCl3) δ −73.2; IR (CHCl3) υ 1735, 1648, 1603, 1523, 1344, 1158, 915, 839, 748, 689 cm−1; HRMS (ESI/[M+H]+) Calcd. for C18H22N2O4F3 m/z 387.1532, found m/z 387.1527. The product of opposite absolute configuration was obtained in 68% yield from a reaction catalyzed by epiQ-3 (5.0 mol%), A-1 (2.0 mol%) and ethanol (20 μL) in PhMe (2.0 mL) at −30 °C for 8 h. 92% ee was determined by HPLC analysis [t (major) = 10.87 min, t (minor) = 15.56 min]. [α]D20 = +1.8 (c = 0.5, CHCl3).
Methyl (S)-4-((4-nitrobenzylidene)amino)-6-phenyl-4-(trifluoromethyl)hexanoate (5Ea). The product 5Ea was obtained as oil in 53% yield after flash chromatography with deactivated silica gel (Hexanes/Et2O = 100/1 to 5/1) from a reaction catalyzed by QD-3 (5.0 mol%), A-1 (2.0 mol%) and ethanol (20 μL) in PhMe (2.0 mL) at −30 °C for 8 h. 90% ee was determined by HPLC analysis [Daicel Chiralpak AD-H, Hexanes/IPA = 97/3, 1.0 mL/min, λ 254 nm, t (minor) = 13.96 min, t (major) = 19.38 min]; [α]D20 = +1.0 (c = 1.0, CHCl3); 13H NMR (400 MHz, CDCl3) δ 8.49 (s, 1H), 8.29 (d, J = 8.7 Hz, 2H), 7.96 (d, J = 8.7 Hz, 2H), 7.33–7.13 (m, 5H), 3.66 (s, 3H), 2.78–2.16 (m, 2H), 2.54–2.46 (m, 2H), 2.40–2.32 (m, 2H), 2.17 (t, J = 8.7 Hz, 2H); 13C {1H} NMR (100 MHz, CDCl3) δ 173.2, 159.1, 149.6, 141.0, 129.3 (2C), 128.7 (2C), 128.2 (2C), 128.1 (2C), 126.6 (q, JC-F = 285 Hz), 126.4, 123.9 (2C), 67.6 (q, JC-F = 24 Hz), 51.9, 36.0, 29.6, 28.6, 28.4; 19F NMR (376 MHz, CDCl3) δ −72.9; IR (CHCl3) υ 1735, 1649, 1603, 1523, 1345, 1254, 1154, 841, 748, 701 cm−1; HRMS (ESI/[M+H]+) Calcd. for C21H22N2O4F3 m/z 423.1532, found m/z 423.1534. The product of opposite absolute configuration was obtained in 55% yield from a reaction catalyzed by epiQ-3 (5.0 mol%), A-1 (2.0 mol%) and ethanol (20 μL) in PhMe (2.0 mL) at −30 °C for 8 h. 88% ee was determined by HPLC analysis [t (major) = 13.88 min, t (minor) = 19.25 min]. [α]D20 = −3.8 (c = 1.0, CHCl3).
Methyl (S)-5,5,5-trfluoro-4-((4-nitrobenzylidene)amino)pentanoate (5Fa). The product 5Fa was obtained as oil in 62% yield after flash chromatography with deactivated silica gel (Hexanes/Et2O = 100/1 to 6/1) from a reaction catalyzed by QD-3 (5.0 mol%), A-1 (2.0 mol%) and ethanol (20 μL) in PhMe (2.0 mL) at −50 °C for 4 h. 90% ee was determined by HPLC analysis [Daicel Chiralpak AD-H, Hexanes/IPA = 94/6, 1.0 mL/min, λ 254 nm, t (minor) = 11.72 min, t (major) = 18.47 min]. [α]D20 = −77.0 (c = 0.5, CHCl3); 13H NMR (400 MHz, CDCl3) δ 8.41 (s, 1H), 8.30 (d, J = 8.8 Hz, 2H), 7.97 (d, J = 8.8 Hz, 2H), 3.93–3.80 (m, 1H), 3.66 (s, 3H), 2.48–2.13 (m, 4H); 13C {1H} NMR (100 MHz, CDCl3) δ 172.7, 164.0, 149.7, 140.2, 129.5 (2C), 124.9 (q, JC-F = 280 Hz), 124.0 (2C), 70.4 (q, JC-F = 28 Hz), 51.8, 29.4, 24.5; 19F NMR (376 MHz, CDCl3) δ −74.5 (d, JC-F = 8.0 Hz); IR (CHCl3) υ 1733, 1649, 1603, 1522, 1345, 1264, 1161, 1127, 1080, 1014, 853, 748, 688 cm−1; HRMS (ESI/[M+H]+) Calcd. for C13H14N2O4F3 m/z 319.0906, found m/z 319.0904. The product of opposite absolute configuration was obtained in 70% yield from a reaction catalyzed by epiQ-3 (5.0 mol%), A-1 (2.0 mol%) and ethanol (20 μL) in PhMe (2.0 mL) at −50 °C for 4 h. 91% ee was determined by HPLC analysis [t (major) = 11.76 min, t (minor) = 18.51 min]. [α]D20 = +80.2 (c = 1.0, CHCl3).
Prop-2-yn-i-yl (R)-4-cyclohexyl-5,5,5-trifluoro-4-((4-nitrobenzylidene)amino)pentanoate (5Gb). The product 5Gb was obtained as oil in 65% yield after flash chromatography with deactivated silica gel (Hexanes/Et2O = 100/1 to 15/1) from a reaction catalyzed by QD-3 (5.0 mol%), A-1 (2.0 mol%) and ethanol (20 μL) in PhMe (2.0 mL) at −30 °C for 8 h. 91% ee was determined by HPLC analysis [Daicel Chiralpak AD-H, Hexanes/IPA = 98/2, 1.0 mL/min, λ 254 nm, t (minor) = 13.89 min, t (major) = 17.55 min]. [α]D20 = +18.7 (c = 0.5, CHCl); 13H NMR (400 MHz, CDCl3) δ 8.41 (s, 1H), 8.29 (d, J = 8.7 Hz, 2H), 7.96 (d, J = 8.7 Hz, 2H), 4.70–4.66 (m, 2H), 2.56–2.19 (m, 5H), 1.94–1.75 (m, 4H), 1.70 (t, J = 11.3 Hz, 2H), 1.34–1.09 (m, 5H); 13C {1H} NMR (100 MHz, CDCl3) δ 172.3, 158.2, 149.5, 141.2, 129.2 (2C), 126.8 (q, JC-F = 289 Hz), 123.9 (2C), 75.1, 70.5 (q, JC-F = 22 Hz), 52.2, 44.2, 28.4, 27.5, 27.1, 26.9, 26.9, 26.2, 25.7; 19F NMR (376 MHz, CDCl3) δ −67.0; IR (CHCl3) υ 1740, 1524, 1345, 1216, 1155, 1108, 839, 746, 667, 633 cm−1; HRMS (ESI/[M+H]+) Calcd. for C21H24N2O4F3 m/z 425.1688, found m/z 425.1684. The product of opposite absolute configuration was obtained in 63% yield from a reaction catalyzed by epiQ-3 (5.0 mol%), A-1 (2.0 mol%) and ethanol (20 μL) in PhMe (2.0 mL) at −30 °C for 8 h. 93% ee was determined by HPLC analysis [t (major) = 13.58 min, t (minor) = 17.18 min]. [α]D20 = −44.8 (c = 0.5, CHCl3).
Prop-2-yn-1-yl (R)-4-(cyclohexylmethyl)-5, 5, 5-trifluoro-4-((4-nitrobenzylidene)amino)pentanoate (5Hb). The product 5Hb was obtained as oil in 71% yield after flash chromatography with deactivated silica gel (Hexanes/Et2O = 100/1 to 15/1) from a reaction catalyzed by QD-3 (5.0 mol%), A-1 (2.0 mol%) and ethanol (20 μL) in PhMe (2.0 mL) at −30 °C for 8 h. 90% ee was determined by HPLC analysis [Daicel Chiralpak AD-H, Hexanes/IPA = 98/2, 1.0 mL/min, λ 254 nm, t (minor) = 13.53 min, t (major) = 16.02 min]. [α]D20 = +2.0 (c = 1.0, CHCl). 13H NMR (400 MHz, CDCl3) δ 8.47 (s, 1H), 8.30 (d, J = 8.7 Hz, 2H), 7.95 (d, J = 8.7 Hz, 2H), 4.66 (d, J = 2.4 Hz, 2H), 2.58–2.51 (m, 2H), 2.47 (t, J = 2.4 Hz, 1H), 2.38–2.20 (m, 2H), 1.87–1.45 (m, 8H), 1.26–0.85 (m, 5H); 13C {1H} NMR (100 MHz, CDCl3) δ 172.2, 158.6, 149.5, 141.1, 129.2 (2C), 126.7 (q, JC-F = 286 Hz), 124.0 (2C), 75.1, 68.0 (q, JC-F = 23 Hz), 52.2, 41.9, 35.3, 35.2, 33.0, 29.3, 28.7, 26.3, 26.3, 26.0; 19F NMR (376 MHz, CDCl3) δ −72.8; IR (CHCl3) υ 2925, 1741, 1523, 1344, 1160, 853, 748, 688, 667 cm-; HRMS (ESI/[M+H]+) Calcd. for C22H26N2O4F3 m/z 439.1845, found m/z 439.1827. The product of opposite absolute configuration was obtained in 72% yield from a reaction catalyzed by epiQ-3 (5.0 mol%), A-1 (2.0 mol%) and ethanol (20 μL) in PhMe (2.0 mL) at −30 °C for 8 h. 93% ee was determined by HPLC data [t (major) = 13.45 min, t (minor) = 16.00 min]. [α]D20 = −9.9 (c = 1.0, CHCl3).
Prop-2-yn-1-yl (R)-4-benzyl-5,5,5-trifluoro-4-((4-nitrobenzylidene)amino)pentanoate (51b). The product 51b was obtained as oil in 63% yield after flash chromatography with deactivated silica gel (Hexanes/Et2O = 100/1 to 12/1) from a reaction catalyzed by QD-3 (5.0 mol%), A-1 (2.0 mol%) and ethanol (20 μL) in PhMe (2.0 mL) at −30 °C for 8 h. 90% ee was determined by HPLC analysis [Daicel Chiralpak AD-H, Hexanes/IPA = 93/7, 1.0 mL/min, λ 254 nm, t (major) = 10.14 min, t (minor) = 10.93 min]; [α]D20 = +62.8 (c = 0.5, CHCl); 13H NMR (400 MHz, CDCl3) δ 8.28 (d, J = 8.7 Hz, 2H), 7.86 (d, J = 8.7 Hz, 2H), 7.76 (s, 1H), 7.29–7.19 (m, 3H), 7.06–7.00 (m, 2H), 4.65 (d, J = 2.4 Hz, 2H), 3.18 (d, J = 13.6 Hz, 1H), 3.10 (d, J = 13.6 Hz, 1H), 2.65–2.52 (m, 1H), 2.47 (t, J = 2.4 Hz, 1H), 2.44–2.18 (m, 3H); 13C {1H} NMR (100 MHz, CDCl3) δ 172.0, 159.6, 149.5, 140.8, 133.5, 131.6 (2C), 129.1 (2C), 128.1 (2C), 127.5, 126.7 (q, JC-F = 285 Hz), 124.0 (2C), 75.1, 68.5 (q, JC-F = 23 Hz), 52.2, 39.8, 28.2, 26.3; 19F NMR (376 MHz, CDCl3) δ −73.8; IR (CHCl3) υ 1741, 1524, 1345, 1215, 1161, 1087, 745, 703, 667 cm−1; HRMS (ESI/[M+H]+) Calcd. for C22H20N2O4F3 m/z 433.1375, found m/z 433.1369. The product of opposite absolute configuration was obtained in 72% yield from a reaction catalyzed by epiQ-3 (5.0 mol%), A-1 (2.0 mol%) and ethanol (20 μL) in PhMe (2.0 mL) at −30 °C for 8 h. 92% ee was determined by HPLC analysis [t (minor) = 10.13 min, t (major) = 10.89 min]. [α]D20 = −66.0 (c = 0.5, CHCl3).
Prop-2-yn-1-yl (R)-4-(4-bromobenzyl)-5,5,5-trifluoro-4-((4-nitrobenzylidene)amino)pentanoate (5Jb). The product 5Jb was obtained as oil in 68% yield after flash/Et2O = 100/1 to 10/1) from a reaction catalyzed by QD-3 (5.0 mol%), A-1 (2.0 mol%) and ethanol (20 μL) in PhMe (2.0 mL) at −30 °C for 8 h. 88% ee was determined by HPLC analysis [Daicel Chiralpak AD-H, Hexanes/IPA = 93/7, 1.0 mL/min, λ 254 nm, t (minor) = 12.55 min, t (major) = 13.56 min]. [α]D20 = +80.2 (c = 1.0, CHCl3); 13H NMR (400 MHz, CDCl3) δ 8.30 (d, J = 8.7 Hz, 2H), 7.92 (s, 1H), 7.89 (d, J = 8.7 Hz, 2H), 7.36 (d, J = 8.4 Hz, 2H), 6.93 (d, J = 8.4 Hz, 2H), 4.65 (d, J = 2.4 Hz, 2H), 3.15 (d, J = 13.8 Hz, 1H), 3.08 (d, J = 13.8 Hz, 1H), 2.63–2.52 (m, 1H), 2.47 (t, J = 2.4 Hz, 1H), 2.44–2.35 (m, 1H), 2.29–2.16 (m, 2H); 13C {1H} NMR (100 MHz, CDCl3) δ 171.8, 159.6, 149.7, 140.6, 133.0 (2C), 132.7, 131.3 (2C), 129.2 (2C), 126.5 (q, JC-F = 285 Hz), 124.0 (2C), 121.7, 75.2, 68.4 (q, JC-F = 24 Hz), 52.3, 39.5, 28.2, 26.6; 19F NMR (376 MHz, CDCl3) δ −73.2; IR (CHCl3) υ 1525, 1346, 1163, 746, 667, 634 cm−1; HRMS (ESI/[M+H]+) Calcd. for C22H19N2O4F3Br m/z 511.0480, found m/z 511.0477. The product of opposite absolute configuration was obtained in 66% yield from a reaction catalyzed by epiQ-3 (5.0 mol%), A-1 (2.0 mol%) and ethanol (20 μL) in PhMe (2.0 mL) at −30 °C for 8 h. 91% ee was determined HPLC analysis [t (major) = 12.53 min, t (minor) = 13.63 min]. [α]D20 = −114.9 (c = 1.0, CHCl3).
General Procedure for the Asymmetric Synthesis of Compound 9.
At indicated temperature, to a solution of trifluoromethyl imines 1 (0.2 mmol), α,β-unsaturated lactones 8 (0.4 mmol, 2.0 equiv.), phenol-additive A-2 (10–50 mol%) and catalyst QD-1 or epiQ-1 (0.5 mol%) in PhMe (totally 2.0 mL) was added aqueous KOH solution (2.2 μL, 50 wt%, 0.02 mmol). The mixture was then vigorously stirred and turned purple immediately. After the reaction was completed or reached the maximum conversion (checked by 19F NMR), the reaction mixture was then passed through a small pad of deactivated silica (5 cm thick) and washed with Et2O (1.0 mL × 3). The filtrates were combined, concentrated, purified through flash chromatography with deactivated silica gel (Hexanes/Et2O) to afford the desired products 9.
(R)-4-((S)-1,1,1-trifluoro-2-((4-nitrobenzylidene)amino)propan-2-yl)dihydrofuran-2(3H)-one (9Aa). The product 9Aa was obtained as oil in 93% yield after flash chromatography with deactivated silica gel (Hexanes/Et2O = 5/1 to 1/2) from a reaction catalyzed by QD-1 (1.0 mol%) and A-2 (10 mol%) in PhMe (2.0 mL) at −30 °C for 1 h. 99% ee was determined by HPLC analysis [Daicel Chiralpak AD-H, Hexanes/IPA = 90/10, 1.2 mL/min, λ 254 nm, 40 °C, t (minor) = 13.95 min, t (major) = 16.41 min]. [α]D20 = −84.1 (c = 1.0, CHCl3); 1H NMR (400 MHz, CDCl3) δ 8.56 (s, 1H), 8.32 (d, J = 8.8 Hz, 2H), 7.99 (d, J = 8.8 Hz, 2H), 4.58 (t, J = 9.3 Hz, 1H), 4.49 (t, J = 8.6 Hz, 1H), 3.34–3.21 (m, 1H), 2.57 (dd, J1 = 17.2 Hz, J2 = 8.8 Hz, 1H), 2.41 (dd, J1 = 17.2 Hz, J2 = 10.9 Hz, 1H), 1.53 (s, 3H); 13C {1H} NMR (100 MHz, CDCl3) δ 175.1, 161.8, 149.9, 140.2, 129.6 (2C), 125.8 (q, JC-F = 285 Hz), 124.1 (2C), 68.5 (q, JC-F = 3.6 Hz), 65.7 (q, JC-F = 25 Hz), 41.5, 29.5, 17.4; 19F NMR (376 MHz, CDCL,) δ −75.6; IR (CHCl3) υ 1778, 1650, 1523, 1346, 1157, 1102, 1046, 1019, 837, 745, 668, 630 cm−1 HRMS (ESI/[M+H]+) Calcd. for C14N14N2O4F3·m/z 331.0906, found m/z 331.0909. The product of opposite absolute configuration was obtained in 94% yield from a reaction catalyzed by epiQ-1 (1.0 mol%) and A-2 (10 mol%) in PhMe (2.0 mL) at −30 °C for 1 h. 98% ee was determined by HPLC analysis [t (major) = 14.10 min, t (minor) = 17.01 min]. [α]D20 = +99.4 (c = 1.0, CHCl3).
(R)-4-((S)-i, i, i-trifluoro-2-((4-nitrobenzylidene)amino)hexan-2-yl)dihydrofuran-2(3H)-one (9Ca). The product 9Ca was obtained as oil in 89% yield after flash chromatography with deactivated silica gel (Hexanes/Et2O = 10/1 to 3/1) from a reaction catalyzed by QD-1 (1.0 mol%) and A-2 (10 mol%) in PhMe (2.0 mL) at −30 °C for 3 h. 99% ee was determined by HPLC analysis [Daicel Chiralpak AD-H, Hexanes/IPA = 90/10, 0.6 mL/min, λ 254 nm, 8 °C, t (major) = 24.05 min, t (minor) = 25.68 min]. [α]D20 = −52.1 (c = 1.0, CHCl3); 1H NMR (400 MHz, CDCl3) δ 8.58 (d, J = 1.2 Hz, 1H), 8.31 (d, J = 8.8 Hz, 2H), 7.96 (d, J = 8.8 Hz, 2H), 4.48–4.34 (m, 2H), 3.403.27 (m, 2H), 2.77 (dd, J1 = 17.1 Hz, J2 = 11.0 Hz, 1H), 2.56 (dd, J1 = 17.1 Hz, J2 = 8.7 Hz, 1H), 2.01–1.88 (m, 1H), 1.74–1.62 (m, 1H), 1.55–1.27 (m, 4H), 0.95 (t, J = 7.1 Hz, 3H); 13C (Ή) NMR (100 MHz, CDCl3) δ 175.5, 161.0, 149.9, 140.6, 129.3 (2C), 126.2 (q, JC-F = 288 Hz), 124.0 (2C), 68.7 (q, JC-F = 3.2 Hz), 67.2 (q, JC-F = 24 Hz), 39.5, 34.4, 29.3, 25.8, 23.2, 13.7; 19F NMR (376 MHz, CDCl3) δ −69.1; IR (CHCl3) υ 1779, 1648, 1524, 1346, 1216, 1163, 1106, 1022, 838, 746, 667 cm−1; HRMS (ESI/[M+H]+) Calcd. for C17H20N2O4F3 m/z 373.1375, found m/z 373.1371. The product of opposite absolute configuration was obtained in 90% yield from a reaction catalyzed by epiQ-1 (1.0 mol%) and A-2 (10 mol%) in PhMe (2.0 mL) at −30 °C for 3 h. 98% ee was determined by HPLC analysis [t (minor) = 21.65 min, t (major) = 23.42 min]. [α]D20 = +45.0 (c = 1.0, CHCl3).
(R)-4-((S)-1-cyclohexyl-2,2,2-trifluoro-1-((4-nitrobenzylidene)amino)ethyl)dihydrofuran-2(jH)-one (9Ga). The product 9Ga was obtained as oil in 84% yield after flash chromatography with deactivated silica gel (Hexanes/Et2O = 10/1 to 3/1) from a reaction catalyzed by QD-1 (2.0 mol%) and A-2 (10 mol%) in PhMe (2.0 mL) at −30 °C for 5 h. 99% ee was determined by HPLC analysis [Daicel Chiralpak AD-H, Hexanes/IPA = 90/10, 1.0 mL/min, λ 254 nm, 25 °C, t (minor) = 9.13 min, t (major) = 10.38 min]. [α]D20 = −42.8 (c = 0.5, CHCl3); 1H NMR (400 MHz, CDCl3) δ 8.47 (q, J = 2.1 Hz, 1H), 8.32 (d, J = 8.8 Hz, 2H), 7.96 (d, J = 8.8 Hz, 2H), 4.40–4.31 (m, 1H), 3.99 (t, J = 9.6 Hz, 1H), 3.49–3.37 (m, 1H), 3.14 (dd, J1 = 16.9 Hz, J2 = 11.6 Hz, 1H), 2.63 (dd, J1 = 16.9 Hz, J2 = 8.4 Hz, 1H), 1.95–1.83 (m, 2H), 1.83–1.67 (m, 3H), 1.57 (s, 1H), 1.41–1.10 (m, 5H); 13C (1H) NMR (100 MHz, CDCl3) δ 175.6, 160.0, 149.8, 140.6, 129.2 (2C), 126.6 (q, JC-F = 292 Hz), 124.1 (2C), 70.5 (q, JC-F = 21 Hz), 69.1 (q, JC-F = 4.3 Hz), 44.9, 39.4, 29.8, 28.0, 27.7, 27.5, 27.2, 26.3; 19F NMR (376 MHz, CDCl3) δ −63.4; IR (CHCl3) υ 1779, 1524, 1345, 1218, 1171, 1155, 1140, 1100, 1028, 836, 745, 685, 665 cm−1; HRMS (ESI/[M+H]+) Calcd. for C19H22N2O4F3 m/z 399.1532, found m/z 399.1544. The product of opposite absolute configuration was obtained in 82% yield from a reaction catalyzed by epiQ-1 (2.0 mol%) and A-2 (10 mol%) in PhMe (2.0 mL) at −30 °C for 5 h. 98% ee was determined by HPLC analysis [t (major) = 9.12 min, t (minor) = 10.35 min]. [α]D20 = +11.7 (c = 0.4, CHCl3).
(R)-4-((S,E)-1,1,1-trifluoro-2-((4-nitrobenzylidene)amino)-4-phenylbut-3-en-2-yl)dihydrofuran-2(3H)-one (9Ka). The product 9Ka was obtained as oil in 75% yield after flash chromatography with deactivated silica gel (Hexanes/Et2O = 10/1 to 3/1) from a reaction catalyzed by QD-1 (0.5 mol%) and A-2 (50 mol%) in PhMe (2.0 mL) at −30 °C for 2 h. >99% ee was determined by HPLC analysis [Daicel Chiralpak AD-H, Hexanes/IPA = 90/10, 1.0 mL/min, λ 254 nm, 25 °C, t (minor) = 19.03 min, t (major) = 25.91 min]. [α]D20 = −63.1 (c = 1.0, CHCl3);
1H NMR (400 MHz, CDCl3) δ 8.67 (s, 1H), 8.33 (d, J = 8.8 Hz, 2H), 8.02 (d, J = 8.8 Hz, 2H), 7.45–7.30 (m, 5H), 6.65 (d, J = 16.4 Hz, 1H), 6.17 (d, J = 16.4 Hz, 1H), 4.56–4.41 (m, 2H), 3.49 (pentet, J = 8.6 Hz, 1H), 2.76 (dd, J1 = 17.7 Hz, J2 = 9.1 Hz, 1H), 2.67 (dd, J1 = 17.7 Hz, J2 = 9.1 Hz, 1H); 13C {1H} NMR (100 MHz, CDCl3) δ 175.4, 163.7, 150.0, 140.2, 136.7, 134.9, 129.6 (2C), 129.2, 128.9 (2C), 126.8 (2C), 125.3 (q, JC-F = 288 Hz), 124.1 (2C), 122.1, 70.5 (q, JC-F = 24 Hz), 68.5 (q, JC-F = 3.3 Hz), 40.7, 30.1; 19F NMR (376 MHz, CDCl3) δ −70.9; IR (CHCl3) υ 1778, 1645, 1603, 1524, 1345, 1215, 1167, 1131, 1025, 974, 746, 689, 666 cm−1; HRMS (ESI/[M+H]+) Calcd. for C21H18N2O4F3 m/z 419.1219, found m/z 419.1229. The product of opposite absolute configuration was obtained in 71% yield from a reaction catalyzed by epiQ-1 (0.5 mol%) and A-2 (50 mol%) in PhMe (2.0 mL) at −30 °C for 2 h. 99% ee was determined by HPLC analysis [t (major) = 19.02 min, t (minor) = 26.29 min]. [α]D20 = +65.7 (c = 1.0, CHCl3).
(R)-4-((R)-2,2,2-trfluoro-1-((4-nitrobenzylidene)amino)-1-phenylethyl)dihydrofuran-2(3H)-one (9La). The product 9La was obtained as oil in 70% yield after flash chromatography with deactivated silica gel (Hexanes/Et2O = 10/1 to 3/1) from a reaction catalyzed by QD-1 (0.5 mol%) and A-2 (50 mol%) in PhMe (2.0 mL) at −30 °C for 2 h. >99% ee was determined by HPLC analysis [Daicel Chiralpak AD-H, Hexanes/IPA = 92/8, 1.0 mL/min, λ 254 nm, 25 °C, t (minor) = 27.53 min, t (major) = 30.28 min]. [α]D20 = −108.2 (c = 1.0, CHCl); 13 H NMR (400 MHz, CDCl3) δ 8.69 (q, J = 2.1 Hz, 1H), 8.32 (dt, J1 = 8.8 Hz, J2 = 2.1 Hz, 2H), 8.05 (dt, J1 = 8.8 Hz, J2 = 2.1 Hz, 2H), 7.45–7.38 (m, 3H), 7.32–7.27 (m, 2H), 4.53 (t, J = 9.0 Hz, 1H), 4.31 (dd, J1 = 9.5 Hz, J2 = 7.5 Hz, 1H), 3.80 (pentet, J = 8.3 Hz, 1H), 2.71 (dd, J1 = 17.8 Hz, J2 = 8.6 Hz, 1H), 2.63 (dd, J1 = 17.8 Hz, J2 = 9.0 Hz, 1H); 13C {1H} NMR (100 MHz, CDCl3) δ 175.5, 163.0, 150.1, 140.4, 135.0, 129.6 (2C), 129.2, 129.0 (2C), 127.3 (2C), 125.5 (q, JC-F = 289 Hz), 124.1 (2C), 72.1 (q, JC-F = 23 Hz), 68.6 (q, JC-F = 3.8 Hz), 41.7, 30.7; 19F NMR (376 MHz, CDCl3) δ −67.4; IR (CHCl3) υ 1777, 1525, 1346, 1216, 1151, 1037, 837, 746, 701, 666 cm−1; HRMS (ESI/[M+H]+) Calcd. for C19H16N2O4F3 m/z 393.1062, found m/z 393.1064. The product of opposite absolute configuration was obtained in 69% yield from a reaction catalyzed by epiQ-1 (0.5 mol%) and A-2 (50 mol%) in PhMe (2.0 mL) at −30 °C for 2 h. >99% ee was determined by HPLC analysis [t (major) = 27.18 min, t (minor) = 29.98 min]. [α]D20 = +52.3 (c = 1.0, CHCl3).
(R)-4-((R)-2,2,2-trfluoro-1-(3-methoxyphenyl)-1-((4-nitrobenzylidene)amino)ethyl)dihydrofuran-2(3H)-one (9Ma). The product 9Ma was obtained as oil in 55% yield after flash chromatography with deactivated silica gel (Hexanes/Et2O = 10/1 to 3/1) from a reaction catalyzed by QD-1 (0.5 mol%) and A-2 (50 mol%) in PhMe (2.0 mL) at −30 °C for 3 h. >99% ee was determined by HPLC analysis [Daicel Chiralpak AD-H, Hexanes/IPA = 80/20, 0.5 mL/min, λ 254 nm, 3 °C, t (minor) = 30.56 min, t (major) = 33.20 min]. [α]D20 = −86.5 (c = 1.0, CHCl3); 1H NMR (400 MHz, CDCL.) δ 8.68 (q, J = 2.0 Hz, 1H), 8.36 (d, J = 8.7 Hz, 2H), 8.04 (d, J = 8.7 Hz, 2H), 7.34 (t, J = 8.2 Hz, 1H), 6.93 (dd, J1 = 8.2 Hz, J2 = 2.3 Hz, 1H), 6.88 (d, J = 8.2 Hz, 1H), 6.80 (s, 1H), 4.52 (t, J = 8.7 Hz, 1H), 4.30 (dd, J1 = 9.2 Hz, J2 = 7.6 Hz, 1H), 3.79 (s, 3H), 3.75 (pentet, J = 8.4 Hz, 1H), 2.73 (dd, J1 = 17.8 Hz, J2 = 8.6 Hz, 1H), 2.43 (dd, J1 = 17.8 Hz, J2 = 9.0 Hz, 1H); 13C {1H} NMR (100 MHz, CDCl3) δ 175.5, 163.1, 159.9, 150.1, 140.4, 136.6, 130.1, 129.6 (2C), 125.5 (q, J = 291 Hz), 124.2 (2C), 119.4, 114.6, 113.3, 72.0 (q, JC-F = 24 Hz), 68.6 (q, JC-F = 3.6 Hz), 55.4, 41.8, 30.7; 19F NMR (376 MHz, CDCl3) δ −67.1; IR (CHCl3) υ 1776, 1603, 1523, 1345, 1256, 1166, 1144, 1034, 854, 837, 746, 729, 710, 688, 667 cm−1; HRMS (ESI/[M+H]+) Calcd. for C20H18N2O5F3 m/z 423.1168, found m/z 423.1151. The product of opposite absolute configuration was obtained in 51% yield from a reaction catalyzed by epiQ-1 (0.5 mol%) and A-2 (50 mol%) in PhMe (2.0 mL) at −30 °C for 3 h. >99% ee was determined by HPLC analysis [t (major) = 30.37 min, t (minor) = 33.66 min]. [α]D20 = +85.4 (c = 1.0, CHCl3).
(R)-4-((R)-1-(4-chlorophenyl)-2,2,2-trifluoro-1-((4-nitrobenzylidene)amino)ethyl)dihydrofuran-2(3H)-one (9Na). The product 9Na was obtained as oil in 50% yield after flash chromatography with deactivated silica gel (Hexanes/Et2O = 10/1 to 3/1) from a reaction catalyzed by QD-1 (0.5 mol%) and A-2 (50 mol%) in PhMe (2.0 mL) at −30 °C for 2 h. >99% ee was determined by HPLC analysis [Daicel Chiralpak AD-H, Hexanes/IPA = 90/10, 1.0 mL/min, λ 254 nm, 25 °C, t (minor) = 24.22 min, t (major) = 29.91 min]. [α]D20 = −35.9 (c = 0.5, CHCl3); 1H NMR (400 MHz, CDCl3) δ 8.68 (q, J = 2.0 Hz, 1H), 8.37 (d, J = 8.8 Hz, 2H), 8.04 (d, J = 8.8 Hz, 2H), 7.40 (d, J = 8.8 Hz, 2H), 7.23 (d, J = 8.8 Hz, 2H), 4.52 (dd, J1 = 9.6 Hz, J2 = 8.4 Hz, 1H), 4.28 (dd, J1 = 9.6 Hz, J2 = 7.3 Hz, 1H), 3.76 (pentet, J = 8.0 Hz, 1H), 2.69 (dd, J1 = 17.7 Hz, J2 = 8.4 Hz, 1H), 2.43 (dd, J1 = 17.7 Hz, J2 = 9.0 Hz, 1H); 13C {1H} NMR (100 MHz, CDCl3) δ 175.1, 163.2, 150.2, 150.1, 140.1, 135.6, 133.6, 129.7 (2C), 129.3 (2C), 128.8 (2C), 125.3 (q, J = 289 Hz), 124.3 (2C), 71.83 (q, JC-F = 23 Hz), 68.4 (q, JC-F = 3.6 Hz), 41.6, 30.6; 19F NMR (376 MHz, CDCl3) δ −67.4; IR (CHCl3) υ 1774, 1743, 1525, 1346, 1154, 1093, 1036, 839, 809, 746, 689, 666 cm−1; HRMS (ESI/[M+H]+) Calcd. for C19H15N2O4F3CI m/z 427.0672, found m/z 427.0655. The product of opposite absolute configuration was obtained in 48% yield from a reaction catalyzed by epiQ-1 (0.5 mol%) and A-2 (50 mol%) in PhMe (2.0 mL) at −30 °C for 2 h. >99% ee was determined by HPLC analysis [t (major) = 23.87 min, t (minor) = 31.01 min]. [α]D20 = +40.6 (c = 0.5, CHCl3).
(R)-4-((S)-1,1,1-trifluoro-2-((4-nitrobenzylidene)amino)propan-2-yl)tetrahydro-2H-pyran-2-one (9Ab). The product 9Ab was obtained as oil in 86% yield after flash chromatography with deactivated silica gel (Hexanes/Et2O = 5/1 to 1/2) from a reaction catalyzed by QD-1 (2.0 mol%) and A-2 (10 mol%) in PhMe (2.0 mL) at −30 °C for 3 h. 94% ee was determined by HPLC analysis [Daicel Chiralpak AD-H, Hexanes/IPA = 90/10, 1.2 mL/min, λ 254 nm, 40 °C, t (minor) = 15.42 min, t (major) = 20.69 min]. [α]D20 = −83.2 (c = 1.0, CHCl); 13 H NMR (400 MHz, CDCl3) δ 8.49 (s, 1H), 8.31 (d, J = 8.8 Hz, 2H), 7.97 (d, J = 8.8 Hz, 2H), 4.47–4.40 (m, 1H), 4.32–4.23 (m, 1H), 2.82–2.64 (m, 2H), 2.46 (dd, J1 = 16.6 Hz, J2 = 9.6 Hz, 1H), 2.08–1.94 (m, 2H), 1.49 (s, 1H); 13C {1H} NMR (100 MHz, CDCl3) δ 170.4, 160.6, 149.7, 140.5, 129.5 (2C), 126.1 (q, JC-F = 285 Hz), 124.0 (2C), 67.9, 67.8 (q, JC-F = 24 Hz), 37.5, 31.4, 24.3, 15.4; 19F NMR (376 MHz, CDCl3) δ −73.4; IR (CHCl3) υ 1732, 1649, 1603, 1522, 1345, 1299, 1267, 1173, 1124, 1076, 966, 837, 746, 684, 666 cm−1; HRMS (ESI/[M+H]+) Calcd. for C15H16N2O4F3 m/z 345.1062, found m/z 345.1058. The product of opposite absolute configuration was obtained in 91% yield from a reaction catalyzed by epiQ-1 (2.0 mol%) and A-2 (10 mol%) in PhMe (2.0 mL) at −30 °C for 3 h. 91% ee was determined by HPLC analysis [t (major) = 15.30 min, t (minor) = 20.62 min]. [α]D20 = +79.5 (c = 1.0, CHCl3).
Procedure for Gram-Scale Reaction to Compound 5Aa.
Methyl (S)-5,5,5-trifluoro-4-methyl-4-((4-nitrobenzylidene)amino) pentanoate (5Aa). At −40 °C, to a solution of trifluoromethyl imine 1A (1.5 g, 6.0 mmol), 4a (1.1 mL, 12.0 mmol, 2.0 equiv.), A-1 (19.0 mg, 0.12 mmol, 2.0 mol%), ethanol (0.6 mL) and catalyst QD-4 (266.0 mg, 0.3 mmol, 5.0 mol%) in PhMe (60.0 mL) was added aqueous KOH solution (100.0 μL, 50 wt%, 1.2 mmol, 0.2 equiv.). The mixture was then vigorously stirred and turned purple in a few minutes (The reaction mixture was sticky, make sure the stirring is good). After 6 h, the reaction was fully completed (checked with crude NMR). The reaction mixture was then passed through a pad of Celite (5 cm thick) and washed with CH2Cl2 (60.0 mL). The filtrates were combined, concentrated under vacuum and purified through flash chromatography (deactivated silica gel, Hexanes/Et2O = 50/1 to Hexanes/Et2O = 10/1) to afford the desired product 5Aa as light-yellow oil in 81% yield. 90% ee was determined by HPLC analysis [Daicel Chiralpak AD-H, Hexanes/IPA = 97/3, 1.0 mL/min, λ 254 nm, t (minor) = 13.01 min, t (major) = 18.13 min].
Procedure for Gram-Scale Reaction to Compound 9Aa.
(R)-4-((S)-1,1,1-trifluoro-2-((4-nitrobenzylidene)amino)propan-2-yl)dihydrofuran-2(3H)-one (9Aa). At −30 °C, to a solution of trifluoromethyl imine 1A (1.5 g, 6.0 mmol), 8a (0.85 mL, 12.0 mmol, 2.0 equiv.), A-2 (82.0 mg, 0.6 mmol, 10.0 mol%), and catalyst QD-4 (60.0 mg, 0.06 mmol, 1.0 mol%) in PhMe (60.0 mL) was added aqueous KOH solution (100.0 μL, 50 wt%, 1.2 mmol, 0.2 equiv.). The mixture was then vigorously stirred and turned purple in a few minutes. After 3 h, the reaction was fully completed (checked with crude NMR). The reaction mixture was then passed through a pad of Celite (5 cm thick) and washed with CH2Q2 (60.0 mL). The filtrates were combined, concentrated under vacuum and purified through flash chromatography (deactivated silica gel, Hexanes/Et2O = 10/1 to Hexanes/Et2O = 1/1) to afford the desired product 9Aa as clear oil in 91% yield (which could be solidified in ether solution at low temperature). 99% ee was determined by HPLC analysis [Daicel Chiralpak AD-H, Hexanes/IPA = 90/10, 1.2 mL/min, λ 254 nm, 40 °C, t (minor) = 14.34 min, t (major) = 16.87 min].
Synthetic Transformation to Compound (11Aa).
Methyl (S)-4-amino-5,5,5-trifluoro-4-methylpentanoate hydrogenchloride (11Aa). To a solution of 4Aa (1.0 mmol) in Et2O (10.0 mL) at room temperature was added 1N HCl aqueous solution (10.0 mL). The reaction mixture was then stirred at the same temperature for 3 h for the complete hydrolysis of imine 4Aa. After that, the aqueous layer was separated, and concentrated under vacuum to afford the desired product 11Aa. 1H NMR (400 MHz, CD3OD) δ 3.68 (s, 3H), 3.28–3.26 (m, 1H), 2.60–2.54 (m, 2H), 2.24–2.18 (m, 2H), 1.53 (s, 3H); 13C {1H} NMR (100 MHz, CD3OD) δ 173.6, 126.5 (q, JC-F = 284 Hz), 59.6 (q, JC-F = 29 Hz), 52.6, 29.2, 28.2, 17.7; 19F NMR (376 MHz, CD3OD) δ −80.0.
Synsthetic Transformation to Compound (12Aa).
(R)-4-((S)-2-amino-1,1,1-trifluoropropan-2-yl)dihydrofuran-2(3H)-one (12Aa). To a solution of 9Aa (1.0 mmol) in Et2O (10.0 mL) at room temperature was added 1N HCl aqueous solution (10.0 mL). The reaction mixture was then stirred at the same temperature for 3 h for the complete hydrolysis of imine 9Aa. After that, the aqueous layer was separated, and concentrated under vacuum to afford the desired product 12Aa. [α]D20 = −0.9 (c = 1.0, CHCL3). 1H NMR (400 MHz, CD3OD) δ 4.49 (t, J = 9.2 Hz, 1H), 4.34 (t, J = 9.2 Hz, 1H), 3.36 (quintet, J = 9.2 Hz, 1H), 2.83–2.66 (m, 2H), 1.61 (s, 3H); 13C (1H) NMR (100 MHz, CD3OD) δ 174.9, 124.8 (q, JC-F = 283 Hz), 66.6, 58.8 (q, JC-F = 29 Hz), 38.11, 28.91, 14.46; 19F NMR (376 MHz, CD3OD) δ −78.9; IR (MeOH) υ 2811, 2577, 1781, 1539, 1179, 1021 cm−1; HRMS (ESI/[MCl]+) Calcd. for C7HnNO2F3 m/z 198.0742, found m/z 198.0737.
Supplementary Material
ACKNOWLEDGMENT
We are grateful for the financial support from the National Institute of General Medical Science (GM-61591) and the Keck Foundation. We are grateful to Dr. Mark Bezpalko, and Prof. Bruce Foxman for X-ray crystallographic characterizations of structures. We also thank Dr. Shao-Liang Zheng from Harvard University for his help with the X-ray data collection
Footnotes
Supporting Information
This material is available free of charge on the ACS Publications website. X-ray crystallography data and CIF file. NMR spectra and HPLC (PDF) data of all new compounds
The authors declare no competing financial interest.
REFERENCES
- 1.For selected examples of biologically interesting natural products and analogues, see:Nicolaou KC; Yin J; Mandal D; Erande RD; Klahn P; Jin M; Aujay M; Sandoval J; Gavrilyuk J; Vourloumis D Total Synthesis and Biological Evaluation of Natural and Designed Tubulysins. J. Am. Chem. Soc 2016, 138, 1698–1708.Luesch H; Moore RE; Paul VJ; Mooberry SL; Corbett TH Isolation of Dolastatin 10 from the Marine Cyanobacterium Symploca Species VP642 and Total Stereochemistry and Biological Evaluation of Its Analogue Symplostatin 1. J. Nat. Prod 2001, 64, 907–910.Maderna A; Doroski M; Subramanyam C; Porte A; Leverett CA; Vetelino BC; Chen Z; Risley H; Parris K; Pandit J; Varghese AH; Shanker S; Song C; Sukuru SCK; Farley KA; Wagenaar MM; Shapiro MJ; Musto S; Lam M-H; Loganzo F; O’Donnell CJ Discovery of Cytotoxic Dolastatin 10 Analogues with N-Terminal Modifications. J. Med. Chem 2014, 57, 10527–10543.Colombo R; Wang Z; Han J; Balachandran R; Daghestani HN; Camarco DP; Vogt A; Day BW; Mendel D; Wipf P Total Synthesis and Biological Evaluation of Tubulysin Analogues. J. Org. Chem 2016, 81, 10302–10320.Khalil MW; Sasse F; Lunsdorf H; Elnakady YA; Reichenbach H Mechanism of Action of Tubulysin, an Antimitotic Peptide from Myxobacteria Chembiochem 2006, 7, 678–683.Palomo C; Oiarbide M; Garcia JM; Gonzalez A; Pazos R; Odriozola JM; Banuelos P; Tello M; Linden A A Practical Total Synthesis of Hapalosin, a 12-Membered Cyclic Depsipeptide with Multidrug Resistance-Reversing Activity, by Employing Improved Segment Coupling and Macrolactonization. J. Org. Chem 2004, 69, 4126–4134.
- 2.For selected examples of medical agents, see:Gulder TAM; Moore BS Salinosporamide Natural Products: Potent 20 S Proteasome Inhibitors as Promising Cancer Chemotherapeutics. Angew. Chem. Int. Ed 2010, 49, 9346–9367.Froestl W Chemistry and Pharmacology of GABAB Receptor Ligands. Adv. Pharmacol 2010, 58, 19–62.Silverman RB From Basic Science to Blockbuster Drug: The Discovery of Lyrica. Angew. Chem. Int. Ed 2008, 47, 3500–3504.
- 3.For selective examples of catalytic asymmetric synthesis of chiral γ-amino ester derivatives, see:Ordóñez M; Cativiela C; Romero-Estudillo I An Update on the Stereoselective Synthesis of γ-Amino Acids. Tetrahedron: Asymmetry 2016, 27, 999–1055.Shen L-T; Sun L-H; Ye S Highly Enantioselective γ-Amination of α,β-Unsaturated Acyl Chlorides with Azodicarboxylates: Efficient Synthesis of Chiral γ-Amino Acid Derivatives. J. Am. Chem. Soc 2011, 133, 15894–15897.Lundgren RJ; Wilsily A; Marion N; Ma C; Chung YK; Fu GC Catalytic Asymmetric C-N Bond Formation: Phosphine-Catalyzed Intra- and Intermolecular γ-Addition of Nitrogen Nucleophiles to Allenoates and Alkynoates. Angew. Chem. Int. Ed 2013, 52, 2525–2528.Fang Y-Q; Tadross PM; Jacobsen EN Highly Enantioselective, Intermolecular Hydroamination of Allenyl Esters Catalyzed by Bifunctional Phosphinothioureas. J. Am. Chem. Soc 2014, 136, 17966–17968.Gómez JE; Guo W; Gaspa S; Kleij AW Copper-Catalyzed Synthesis of γ-Amino Acids Featuring Quaternary Stereocenters. Angew. Chem. Int. Ed 2017, 56, 15035–15038.
- 4.Gillis EP; Eastman KJ; Hill MD; Donnelly DJ; Meanwell NA Applications of Fluorine in Medicinal Chemistry. J. Med. Chem 2015, 58, 8315–8359.Furuya T; Kamlet AS; Ritter T Catalysis for Fluorination and Trifluoromethylation. Nature 2011, 473, 470–477.Yang X; Wu T; Phipps RJ; Toste FD Advances in Catalytic Enantioselective Fluorination, Mono, Di-, and Trifluoromethylation, and Trifluoromethylthiolation Reactions. Chem. Rev 2015, 115, 826–870.Yerien DE; Bonesi S; Postigo A Fluorination methods in drug discovery. Org. Biomol. Chem 2016, 14, 8398–8427.Wang J; Sánchez-Roselló M; Aceña JL; del Pozo C; Sorochinsky AE; Fustero S; Soloshonok VA; Liu H Fluorine in Pharmaceutical Industry: Fluorine-Containing Drugs Introduced to the Market in the Last Decade (2001–2011). Chem. Rev 2014, 114, 2432–2506.Zhou Y; Wang J; Gu Z; Wang S; Zhu W; Acena JL; Soloshonok VA; Izawa K; Liu H Next Generation of Fluorine-Containing Pharmaceuticals, Compounds Currently in Phase II-III Clinical Trials of Major Pharmaceutical Companies: New Structural Trends and Therapeutic Areas. Chem. Rev 2016, 116, 422–518.
- 5.Pesenti C; Arnone A; Bellosta S; Bravo P; Canavesi M; Corradi E; Frigerio M; Meille SV; Monetti M; Panzeri W; Viani F; Venturini R; Zanda M Total Synthesis of a Pepstatin Analog Incorporating Two Trifluoromethyl Hydroxymethylene Isosteres (Tfm-GABOB) and Evaluation of Tfm-GABOB Containing Peptides as Inhibitors of HIV-1 Protease and MMP-9. Tetrahedron 2001, 57, 6511–6522.Dave R; Badet B; Meffre P γ-Fluorinated Analogues of Glutamic Acid and Glutamine. Amino Acids 2003, 24, 245–261.Chaume G; Van Severen M-C; Ricard L; Brigaud T Concise Access to Enantiopure (S)- and (R)-α-Trifluoromethyl Pyroglutamic Acids From Ethyl Trifluoropyruvate-based Chiral CF3-Oxazolidines (Fox). J. Fluorine Chem 2008, 129, 1104–1109.Berger AA; Voller JS; Budisa N; Koksch B Deciphering the Fluorine Code-The Many Hats Fluorine Wears in a Protein Environment. Acc. Chem. Res 2017, 50, 2093–2103.
- 6.Wu Y; Hu L; Li Z; Deng L Catalytic Asymmetric Umpolung Reactions of Imines. Nature 2015, 523, 445–450;Hu L; Wu Y; Li Z; Deng L Catalytic Asymmetric Synthesis of Chiral γ-Amino Ketones via Umpolung Reactions of Imines. J. Am. Chem. Soc 2016, 138, 15817–15820;Li Z; Hu B; Wu Y; Fei C; Deng L Control of Chemoselectivity in Asymmetric Tandem Reactions: Direct Synthesis of Chiral Amines Bearing Nonadjacent Stereocenters. Proc. Natl. Acad. Sci. U. S. A 2018, 115, 1730–1735.
- 7.Zhu Y; Buchwald SL Ligand-Controlled Asymmetric Arylation of Aliphatic α-Amino Anion Equivalents. J. Am. Chem. Soc 2014, 136, 4500–4503.Liu J; Cao C-G; Sun H-B; Zhang X; Niu D Catalytic Asymmetric Umpolung Allylation of Imines. J. Am. Chem. Soc 2016, 138, 13103–13106.Zhan M; Pu X; He B; Niu D; Zhang X Intramolecular Umpolung Allylation of Imines. Org. Lett 2018, 20, 5857–5860.
- 8.Tang S; Zhang X; Sun J; Niu D; Chruma JJ 2-Azaallyl Anions, 2-Azaallyl Cations, 2-Azaallyl Radicals, and Azomethine Ylides. Chem. Rev 2018, 118, 10393–10457.Niwa T; Yorimitsu H; Oshima K Palladium-Catalyzed Benzylic Arylation of NBenzylxanthone Imine. Org. Lett 2008, 10, 4689–4691.Liu X; Gao A; Ding L; Xu J; Zhao B Aminative Umpolung Synthesis of Aryl Vicinal Diamines from Aromatic Aldehydes. Org. Lett 2014, 16, 2118–2121.Qian X; Ji P; He C; Zirimwabagabo J-O; Archibald MM; Yeagley AA; Chruma JJ Palladium-Catalyzed Decarboxylative Generation and Asymmetric Allylation of α-Imino Anions. Org. Lett 2014, 16, 5228–5231.Li M; Berritt S; Walsh PJ Palladium-Catalyzed Regioselective Arylation of 1,1,3-Triaryl-2-azaallyl Anions with Aryl Chlorides. Org. Lett 2014, 16, 4312–4315.Li M; Yucel B; Jimenez J; Rotella M; Fu Y; Walsh PJ Umpolung Synthesis of Diarylmethylamines via Palladium-Catalyzed Arylation of N-Benzyl Aldimines. Adv. Synth. Catal 2016, 358, 1910–1915.Li M; Gutierrez O; Berritt S; Pascual-Escudero A; Yeşiçimen A; Yang X; Adrio J; Huang G; Nakamaru-Ogiso E; Kozlowski MC; Walsh PJ Transition-Metal-Free Chemo- and Regioselective Vinylation of Azaallyls. Nat. Chem 2017, 9, 997–1004.
- 9.Chen P; Yue Z; Zhang J; Lv X; Wang L; Zhang J Phosphine-Catalyzed Asymmetric Umpolung Addition of Trifluoromethyl Ketimines to Morita-Baylis-Hillman Carbonates. Angew. Chem. Int. Ed 2016, 55, 13316–13320.Chen P; Zhang J Phosphine-Catalyzed Asymmetric Synthesis of α-Quaternary Amine via Umpolung γ-Addition of Ketimines to Allenoates. Org. Lett 2017, 19, 6550–6553.
- 10.Hu B; Deng L Catalytic Asymmetric Synthesis of Trifluoromethylated γ-Amino Acids through the Umpolung Addition of Trifluoromethyl Imines to Carboxylic Acid Derivatives. Angew. Chem. Int. Ed 2018, 57, 2233–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mulliner D; Wondrousch D; Schüürmann G Predicting Michael-Acceptor Reactivity and Toxicity Through Quantum Chemical Transition-State Calculations. Org. Biomol. Chem 2011, 9, 8400–8412.Yoshida Y; Moriya Y; Mino T; Sakamoto M Regio- and Enantioselective Synthesis of α-Amino-δ-Ketoesters Through Catalytic Umpolung Reaction of α-Iminoesters with Enones. Adv. Synth. Catal 2018, 360, 4142–4146.
- 12.Marcelli T; van der Haas RNS; van Maarseveen JH; Hiemstra H Asymmetric Organocatalytic Henry Reaction. Angew. Chem. Int. Ed 2006, 45, 929–931.Berkessel A; Guixá M; Schmidt F; Neudörfl JM; Lex J Highly Enantioselective Epoxidation of 2-Methylnaphthoquinone (Vitamin K3) Mediated by New Cinchona Alkaloid Phase-Transfer Catalysts. Chem. Eur. J 2007, 13, 4483–4498.Du Z; Zhou C; Gao Y; Ren Q; Zhang K; Cheng H; Wang W; Wang J Expeditious Diastereoselective Construction of A Thiochroman Skeleton via a Cinchona Alkaloid-Derived Catalyst. Org. Biomol. Chem 2012, 10, 36–39.Du T; Du F; Ning Y; Peng Y Organocatalytic Enantioselective 1,3-Dipolar Cycloadditions between Seyferth-Gilbert Reagent and Isatylidene Malononitriles: Synthesis of Chiral Spirophosphonylpyrazoline-oxindoles. Org. Lett 2015, 17, 1308–1311.
- 13.Hu B; Bezpalko MW; Fei C; Dickie DA; Foxman BM; Deng L Origin of and a Solution for Uneven Efficiency by Cinchona Alkaloid-Derived, Pseudoenantiomeric Catalysts for Asymmetric Reactions. J. Am. Chem. Soc 2018, 140, 13913–13920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang EJ; Lee E Total Synthesis of Oxacyclic Macrodiolide Natural Products. Chem. Rev 2005, 105, 4348–4378.Lorente A; Lamariano-Merketegi J; Álbericio F; Alvarez M Tetrahydrofuran-Containing Macrolides: A Fascinating Gift from the Deep Sea. Chem. Rev 2013, 113, 4567–4610.
- 15.CCDC 1839921 (product 9Aa obtained from the reaction catalyzed by QD-1) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.
- 16.Lan P; Berta D; Porco JA; South MS; Parlow JJ Polymer-Assisted Solution-Phase (PASP) Suzuki Couplings Employing an Anthracene-Tagged Palladium Catalyst. J. Org. Chem 2003, 68, 9678–9686. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.