Abstract
We review the range of psychosocial stress factors/processes (e.g., chronic stress, distress states, coping, social adversity) as they relate to immune variables in cancer, and the studies of psychosocial interventions on these stress processes and immune measures in cancer populations. The review includes molecular, cellular and clinical research examining specifically the effects of stress processes and stress management interventions on immune variables (e.g., cellular immune function, inflammation), which may or may not be changing directly in response to the cancer or its treatment. Basic psychoneuroimmunologic research on stress processes (using animal or cellular/tumor models) provides leads for investigating biobehavioral processes that may underlie associations reported to date. The development of theoretically-driven and empirically-supported stress management interventions may provide important adjuncts to clinical cancer care going forward.
Keywords: Stress, Stress Management, Biobehavioral, Immune, Inflammatory, Adaptation
Condensed Abstract
We review the range of psychosocial stress factors/processes related to immune variables in cancer, and the effects of stress management interventions on these stress and immune measures in cancer populations. Molecular, cellular and clinical research on stress-immune associations provides leads for understanding the effects of stress management interventions on clinical health outcomes, which may provide important adjuncts to clinical cancer care going forward.
I. EFFECTS OF STRESS ON IMMUNE PROCESSES THAT AFFECT TUMOR PROGRESSION AND METASTASIS
Stress and Cancer
Cancer diagnosis, treatment, and survivorship can all be extremely stressful (1,2,3). Stress has been shown to affect tumor emergence, progression, and metastasis (for review see (4–5). The immune system is a crucial mediator through which stress affects the course of cancer (2,6), although many immune-independent mechanisms are also critical mediators of cancer-related effects of stress (7). This review will focus on the effects of stress (Section I) and stress management (Section II) on immune function in relation to cancer.
“Stress” is an intrinsic and familiar aspect of life, being a stimulant for some individuals, but a burden for many others. Most definitions of stress invoke an internal or external challenge, disturbance, or stimulus; or a perception of a challenge; or a physiological response to the challenge (8). An integrated definition states that “stress is a constellation of events, consisting of a stimulus (stressor), that precipitates a reaction in the brain (stress perception), that activates physiologic fight or flight systems in the body (stress response)” (9,10,19).
The way a stressor can affect brain or body is through the biological stress response which consists of the release of factors in the systemic circulation and locally within central and peripheral tissues. In the periphery, the stress response consists largely of the “big three” stress hormones: norepinephrine, epinephrine, and cortisol. Almost all cells in the body express receptors for stress hormones, which illustrates the importance of these hormones. The peripheral stress response also includes other neuroendocrine factors such as adrenocorticotropin (ACTH), vasopressin (11), and oxytocin (11), and “immunohormone-like” cytokines (12) such as inteleukin-1beta (IL-1β) (13) and IL-6 (14).
It is important to recognize that the stress response has adaptive, protective effects in the short run (10,18) although chronic stress can be harmful (9, 10, 15, 19). The duration of the biological effects of stress is an important characteristic (10): Short-term stress (e.g. job interview, speaking before an audience, exercise / sports activities) has been defined as “stress that lasts for a period of minutes to hours, and chronic stress (e.g. caregiving, relationship problems, prolonged financial hardship) as stress that persists for weeks, months, or years” (10). Dysregulation of the circadian cortisol rhythm often coincides with the beginning of the harmful effects of long-term stress (10, 16), while down-regulation of the glucocorticoid receptor (GR) in circulating inflammatory cells is another (17).
Inter-individual differences in stress perception, processing, appraisal, and coping (19) can have significant effects on the profile of the biological stress response. Studies in mice and rats have shown strain differences in stress hormone receptor levels, and in the reactivity and peak levels of the biological stress response (20,21), stress adaptation (22), and activation of mineralocorticoid and glucocorticoid receptors and corticosteroid-binding globulin concentrations (20,23). These studies suggest that genetic and environmental factors mediate differences in stress responses and their effects on the body (14,23). Humans can experience psychological stress in the absence of external stressors resulting in chronic stress and its harmful effects. It is now well-established that the magnitude and duration of stress-induced elevations in stress hormones can also affect immune cell trafficking and function, and can in turn affect health outcomes (9,10,19,24,25).
Effects of Chronic Stress on Immune Function – Relevance for Cancer
Immune responses are often categorized in terms of their cellular and molecular components (e.g. innate, adaptive, Th1, Th2, Th17, etc.). In addition to these mechanistic categories, it is also useful and physiologically relevant to investigate immune responses in terms of their functional end effects. Therefore, Dhabhar et al. proposed that while studying the immune system and its health-relevant effects, it is useful to investigate three functional categories of responses: Immuno-protective, immuno-pathological/inflammatory, and immuno-regulatory/suppressive (9,18,19). While these categories have previously been investigated in isolation, we suggest that an integrative and simultaneous examination is likely to be more useful and meaningful. Here we discuss mechanisms through which chronic stress is thought to influence immune responses that are critical for cancer patients, through: a) Suppression of protective immunity; b) Induction/exacerbation of chronic inflammation; and c) Enhancement of immuno-suppressive mechanisms.
Chronic stress-induced suppression of protective immune responses.
Protective immune responses include those that promote efficient healing of open as well as sterile wounds, eliminate infections and cancer, mediate cancer immunosurveillance, enable tumor immunotherapy, and drive vaccine effectiveness (9,18,19). Effective immuno-protection is mediated by robust immuno-surveillance, a rapid response to activation, clearance of the antigen/pathogen, and rapid and complete resolution of inflammation (9,18,19). Immuno-protective responses are crucial during the inflammatory phase of wound healing, which is critical for subsequent proliferative and remodeling phases to be completed effectively. Wound healing is important for open wounds, sterile wounds, and collateral tissue damage caused by activity of immune cells and cytokines (9,18,19). Innate, adaptive, Type-1, and Type-2 immune responses all confer immuno-protection.
Protective immunity is critical for eliminating immunogenic cancers such as squamous cell carcinoma (SCC) and basal cell carcinoma; virally-associated cancers such as human papillomavirus (HPV)-associated cervical, anal, and oral cancers; and for eliminating tumors that are made immunogenic through immunotherapy. Importantly, whether a tumor is immunogenic or not, immuno-protective responses are crucial for the health and survival of a cancer patient because they are essential for protecting the individual from wounds, opportunistic infections, and cancer-treatment induced tissue damage. Furthermore, immuno-protective responses are also essential for the success of any cancer treatment, including tumor immunotherapy, which aims to make a tumor immunogenic and to make it susceptible to elimination by immune cells. Thus, suppression of protective immunity is one critical pathway through which chronic stress can exacerbate tumor emergence, progression and metastasis.
Numerous studies have elucidated mechanisms and pathways through which chronic stress suppresses protective immunity (9,19). Catecholamine (7) and glucocorticoid (26) hormones have been identified as the major physiological mediators of chronic stress-induced suppression of protective immunity, although further research into other factors is necessary. One mechanism through which chronic stress suppresses immuno-protection is by decreasing/altering immune cell redistribution between different body compartments (10). In human and animal studies, chronic stress has also been shown to suppress different dimensions of protective immunity including: cell-mediated immunity (CMI), antibody responses, immune cell proliferation, graft rejection, T cell and NK driven anti-viral responses, and macrophage driven anti-mycobacterial activity (22,25,26,27). NK cell activity, CMI, and other types of immune responses are important for tumor surveillance, and for inhibiting tumor progression, invasion, and metastasis (28,6,29,30).
Chronic stress-induced suppression of multiple aspects of protective immunity mediates increased susceptibility to cancer. For example, in a naturalistic model of SCC in mice, the emergence and progression of precancerous and cancerous lesions, was accelerated by chronic stress. Chronic stress suppressed gene expression of cutaneous-T-cell-attracting-chemokine (CTACK/CCL27) that is critical for recruiting T cells to the site of immune activation. This was accompanied by suppression of infiltration by protective CD4+ and CD8+ T cells, and inhibition of T-helper type 1 (Th1) type cytokines (IL-12 and interferon-gamma [IFN-γ]) resulting in an overall suppression of immuno-protection. Interestingly, in addition to suppressing protective immune responses, chronic stress, enhanced immuno-suppression by increasing regulatory/suppressor T cell (CD4+CD25+) numbers in the tumor microenvironment and within the systemic circulation (6). Similar to the effects of chronic stress, a high-anxious behavioral phenotype measured and identified at baseline (before naturalistic tumor induction), was associated with a higher tumor burden throughout the time course of tumor development. High-anxious mice showed higher corticosterone concentrations indicating greater chronic stress. They also showed increased CCL22 expression and correspondingly regulatory T-cell (Treg) infiltration indicating greater immuno-suppression. This was accompanied by suppressed CTACK/CCL27, IL-12, and IFN-γ gene-expression, and CD4+ and CD8+ infiltration within and around tumors indicating suppressed protective immunity, and increased VEGF concentrations, indicating increased potential for tumor angiogenesis and metastasis (31). Taken together these results show that the tumor-exacerbating effects of high trait anxiety could be worsened by life stressors and cancer diagnosis and treatment related stressors which could in turn contribute to increased tumor progression and/or metastasis. These results also highlight the importance of investigating the targeted use of chemotherapy-compatible anxiolytic treatments immediately following cancer diagnosis, and during cancer treatment/survivorship.
Studies in humans with different types of advanced-stage cancers have shown chronic stress can suppress cancer relevant immune responses through catecholamine and glucocorticoid hormone-mediated mechanisms (4). Patients with metastatic breast cancer who showed higher average diurnal cortisol concentrations and more depressive symptoms, indicating a higher level of chronic stress, showed significantly suppressed CMI (32). Depression also relates to suppressed NK cell cytotoxicity (NKCC) and T-cell cytokine production within the tumor microenvironment in patients with ovarian cancer, while social support, thought to be a chronic stress buffer, is associated with higher NKCC in the tumor microenvironment and circulation in these patients (33,34). In ovarian cancer patients social support and vigor were associated with higher percentages of NKT cells within the tumor and in peripheral blood respectively (35), while social isolation was associated with higher tumor norepinephrine (36).
In breast cancer patients with early-stage disease, lower anxiety in the weeks after surgery was associated with greater production of IL-2 following anti-CD3 (T-cell receptor) stimulation, while greater positive mood was associated with increased IL-12 and IFN-γ production(37). Another study of early-stage breast cancer patients showed that increased stress was associated with lower NKCC, lower NK cell response to stimulation by IFN-γ, and lower T cell proliferation response to stimulation by lectins or anti-CD3 (38). A recent longitudinal analysis of this latter cohort revealed that decreases in stress and depression paralleled increases in NKCC over 18 months (39). In sum, across observational studies in humans with different cancers at early and more advanced stages, chronic stress/distress states appear associated with dampened immuno-protective responses, while factors such as social support, that are thought to buffer against the effects of chronic stress, are associated with more robust immuno-protective responses.
Chronic stress-induced exacerbation of chronic inflammation.
Chronic stress may induce a sustained increase in circulating proinflammatory factors such as IL-6, IL-1β and C-Reactive Protein (CRP), and to shift the cytokine balance from Th1 cytokines that promote tumor-protective CMI, to Th2 cytokines that promote antibody-mediated immunity (40–42). While short-term elevations in IL-6 and IL-1β are crucial for launching and sustaining immuno-protective responses, chronic elevation of these factors leads to chronic inflammation that is known to contribute to proinflammatory and autoimmune disorders and poorer cancer outcomes.
Chronic inflammation is thought to be critical factor for tumor initiation, progression, and metastasis (43–45) by multiple pathways including increased oxidative damage and oxidative stress, DNA mutations, release of factors such as matrix metalloproteases (MMPs) that enable tumor invasion, and factors that induce angiogenesis and metastasis. Evidence for chronic inflammation-induced tumor formation comes from studies showing increased incidence of cancers at sites of chronic inflammation. For example, inflammatory bowel disease, Crohn’s disease, and ulcerative colitis, are associated with colorectal cancers; cystisis and bladder inflammation with bladder cancer; gingivitis and lichen planus with oral SCC; chronic pancreatitis with pancreatic cancer; and skin inflammation with melanoma (43). In addition to exacerbating tumor progression and metastasis, chronic inflammation also contributes to cancer-related fatigue, depression and sleep disturbance, all of which can negatively affect the patient’s quality of life and disease progression (for reviews see (46–48).
Over the past several years, studies have examined how multiple indicators of adversity (cancer-specific distress/anxiety, negative affect, depressive symptoms, social adversity) relate to inflammation (serum cytokines, leukocyte gene expression, and upstream processes such as leukocyte nucleus NFκB DNA binding) in breast cancer patients (49–52). For instance, greater depressive symptoms relate to greater serum TNF-α, IL-1β and IL-6 in breast cancer patients in the weeks after surgery (51). In that cohort, greater post-surgical depressive symptoms also predicted shorter 11-yr overall survival (53), which is in line with prior reports in breast cancer patients(54–55). Greater negative affect and less positive affect in post-surgical breast cancer patients were also related to greater leukocyte gene expression for pro-inflammatory cytokines (e.g., TNFA, IL1B, and IL6), inflammatory chemokines and related receptors (CCL3, CCL3L1, CCL4L2, CCL7, CCL20, CXCL10, CXCR6, CXCR7), prostaglandin–synthesis enzyme COX2 (PTGS2), and tissue remodeling and epithelial-mesenchymal transition (EMT)-related genes (e.g., LMNA, MMP-9) (56). Gene Ontology analyses confirmed that genes upregulated with greater negative affect were disproportionately associated with pro-inflammatory and wound healing activities. Thus, chronic stress and adversity is related to immune cell behavior in ways that can influence inflammatory signaling and possibly metastatic potential in women under treatment for breast cancer.
Similar associations have been identified in other cancers. For instance, greater depressive symptoms were associated with greater leukocyte pro-inflammatory (cytokine, chemokine, COX2/PTGS2), and pro-metastatic gene expression in patients with metastatic renal cell carcinoma (RCC), consistent with increased activity of NFκB and STAT1 transcription factors, and factors involved in myeloid cell activation and differentiation (57). These results are remarkably similar to those noted above in breast cancer patients (48). In this study RCC patients with greater depressive symptoms also had greater numbers of CD68+ tumor associated macrophages and greater pro-inflammatory (COX2/PGE) and pro-metastatic (MMP2, MMP9) gene expression in tumor tissue (57). Greater depressive symptoms and HPA axis dysregulation (flatter di-urnal salivary cortisol slope) also predicted shorter survival time in this cohort (57).
In ovarian cancer, adversity indicators (depression, low social support, social isolation) have been related to greater inflammatory gene expression in ovarian tissue (58), greater levels of tumor promoters VEGF and IL-6 and increased macrophage expression for these tumor promoters (59), and shorter survival (60). In a recent study providing in-vivo analysis of dynamic changes in tumor exosomes (intact cell-derived vesicles from tumor cells involved in intracellular signaling, 61) prior to surgery, advanced ovarian cancer patients with low social support (vs high social support) showed upregulation of 67 genes with mesenchymal characteristics and down-regulation of 63 endothelial-characteristic genes in circulating tumor cells, which could support enhanced EMT (61). Secondary analyses implicated upregulation of genes associated with heightened SNS activity and NFκB family transcription factors (61). Thus greater adversity in the peri-surgical period for ovarian cancer, as in the case of breast cancer (56) and RCC (57), is associated with greater inflammatory signaling and processes favoring metastasis, and poorer clinical outcomes.
Chronic stress-induced enhancement of immuno-regulatory/suppressive mechanisms.
Recent studies indicate that chronic stress increases numbers of regulatory T-cells and B-cells (Tregs and Bregs), myeloid-derived suppressor cells (MDSCs), and tumor associated macrophages (TAMs) in the context of cancer (30,31,62). Tregs are known to be increased in the context of different types of cancer (30,63), are recruited to the tumor microenvironment and suppress protective anti-tumor immune responses, and are associated with poor prognosis and increased mortality (64). Similarly, Bregs have been shown to suppress anti-tumor immune responses and to be associated with poor prognosis (65,66). MDSCs represent another cell type that suppresses anti-tumor immunity, and are also thought to create conditions that promote tumor invasion and metastasis (e.g., EMT) (67,68), and indicate poor prognosis (69). However, in the case of MDSCs, it has also been shown that lower stress levels are related to higher numbers of MDSCs (70), thus suggesting a need for further research into the effects of chronic stress on MDSCs and on immune-regulatory/suppressive cell types in general.
Potentially Harnessing the Immuno-Enhancing Effects of Short-term Stress in the Context of Cancer
In contrast to the effects of chronic stress, short-term stress has been shown to enhance protective immune responses especially in compartments (e.g. skin) to which some leukocytes traffic during stress (9,19,26). Thus, short-term stress, when coupled with immune activation, enhances wound infiltration by leukocytes (71), innate/primary (72,73), and adaptive/secondary (26,74,75) immune responses. In contrast to these findings, several studies by Ben-Eliyahu et al. have shown that surgery stress, which can be considered to be a short-term stressor, results in suppressed immunity, and increased tumor metastasis, and that these effects can be reversed by prophylactic treatment with beta-adrenergic antagonists and COX-2 inhibitors.(76, 77). These findings suggest that there are conditions under which short-term stress does not enhance anti-tumor immunity.
In addition to the above-mentioned preclinical studies, clinical studies have also shown that patients who mount an a priori defined adaptive stress response during surgery, show significantly enhanced recovery compared to patients who fail to mount an adaptive stress response.(78) Furthermore, an elegant series of studies has shown that adjuvant effects of short-term psychological stress, or exercise stress, administered before vaccination, can enhance vaccine-induced immunity in human subjects (79, 80, 81). These findings have led to the suggestion that the short term stress response is Mother Nature’s endogenous adjuvant that kicks into gear during times of stress that are often accompanied by wounding and pathogen entry, and that require immune responses to be mounted in order to heal wounds and fight pathogens (9,18,19). Interestingly, in the context of skin cancer, it has recently been shown in a mouse model that short-term stress (2.5 hour restraint), administered three times a week, for three weeks, during a naturalistic UV-induced tumor induction protocol, results in lower SCC tumor incidence and fewer tumors (82). These effects are mediated by increased expression of CTACK/CCL27, RANTES, IL-12, and IFN-γ gene expression and, increased numbers of skin-infiltrating T cells (82).
Although the findings showing short-term stress induced enhancement of anti-tumor immunity need to be interpreted cautiously and replicated, they suggest that short-term stress could enhance anti-tumor immunity just as it enhances other aspects of innate and adaptive immunity. These findings also raise the intriguing possibility that the beneficial effects of exercise/physical activity in the context of cancer (83, 84), may at least partially be mediated by activation of short-term stress physiology and its adjuvant-like effects on anti-tumor immunity. We recognize that much work remains to be done, that stress-induced enhancement of immune function may not be applicable to all types of cancer, and that hypothesizing that short-term stress physiology could be harnessed to enhance anti-tumor immunity and cancer treatment may at first glance seem counter-intuitive and controversial. At this point we suggest more preclinical and clinical studies should be conducted to safely test this hypothesis, elucidate potential mechanisms, and if the results pan out, to design interventions that harness short-term stress physiology to enhance the effectiveness of cancer treatment.
II. STRESS MANAGEMENT AND IMMUNE PROCESSES IN CANCER PATIENTS
Because modifying stress processes might facilitate psychological adaptation to cancer and better health outcomes, randomized controlled trials (RCTs) have tested interventions that can be considered stress management (SM) at various points along the cancer continuum (1, 85). Despite an impressive number of psychological interventions tested to date few have experimentally demonstrated that SM can modulate psychological adaptation in cancer patients in parallel with: (a) changes in stress physiology (decreased or normalized SNS and HPA activity); (b) immune changes (decreased inflammation and improved CMI); and (c) long-term effects on QoL and disease course (recurrence, mortality). Such studies are rare because they require recruiting patients into an RCT at a specific juncture in the cancer continuum (e.g., during primary treatment or at the point of disease recurrence); inducing improvements in psychological distress and physiological stress responding (SNS and HPA markers) via SM; monitoring changes in immune parameters; and following cohorts for several years for clinical outcomes. We first present evidence that various SM approaches are associated with changes in stress/adversity, neuroendocrine and immune system variables over relatively short periods in cancer patients and survivors, and then highlight research showing long-term clinical benefits that appear tied to immunologic changes.
Stress Management Effects on Immune Parameters in Cancer Patients
Many SM interventions tested in cancer patients work by changing bodily tension and physiological activation through “physical” techniques such as muscle relaxation training, deep breathing, Yoga and Tai-Chi, massage, acupuncture and biofield therapies. Others work by increasing awareness and developing a non-judgmental attitude toward stressful thoughts via mindfulness techniques (86). Others work by teaching skills for changing cognitive appraisals of stressful stimuli and coping with stressors through cognitive behavioral therapy (CBT) techniques such as cognitive restructuring, and coping effectiveness training; and building interpersonal/communications skills to better access and maintain coping resources such as social support (87,88). Additional psychosocial/behavioral interventions have shown efficacy in cancer populations but will not be reviewed here because we explicitly target stress reduction approaches. These include: Supportive-Expressive Therapy (89) targeting existential issues; palliative care interventions targeting symptom management (90); and physical exercise interventions targeting physical activity, strength and fitness (91).
Physical-based SM approaches.
Meta-analyses show reliable effects of yoga on reducing distress, anxiety and depression in cancer patients (92). Breast cancer survivors assigned to 12 weeks of yoga showed decreased pro-inflammatory serum cytokines in two trials (93,94), and Tai-Chi showed decreased leukocyte inflammatory gene expression in breast cancer survivors in one trial (95). More passive physical SM approaches that involve external manipulation of skin, muscles, nerves and energy fields by trained interventionists show increased or stabilized NK cell counts or NKCC in cancer patients. These include massage (breast cancer patients undergoing radiation, 96), acupuncture with warmed needles (moxibustion) (colorectal cancer patients undergoing chemotherapy, 97) and Biofield therapy/ healing touch (cervical cancer patients receiving chemoradiation,98). All of these trials are based on small samples and short follow-up periods, and lack information on long-term clinical outcomes.
Mindfulness Based Stress Reduction (MBSR).
MBSR typically involves 4–8 weeks of training in meditation techniques (awareness-raising and mindful movement), mindfulness and stress physiology, and group support. MBSR reduces pain, anxiety and negative mood among patients with breast cancer (99). Two RCTs in breast cancer survivors found those assigned to 6 weeks of MBSR had greater post-intervention lymphocyte proliferative responses to mitogen stimulation (LPR) and increase in the ratio of Th1:Th2 cytokines produced vs controls (100,101). Among younger breast cancer survivors (≤ 50 yrs old) a 6-week (2hrs/week) group-based mindfulness intervention (vs wait-list control), showed decreased depression and perceived stress, and changes in expression of a 19-gene composite in leukocytes reflecting reduced NFκB activity and increased anti-inflammatory Glucocorticoid Receptor (GR) and IFN type-I gene expression (102). While these findings are encouraging, much of the work is limited by small sample sizes, short follow-ups and a lack of information on long-term clinical effects.
Cognitive-behavioral approaches.
A CBT-based SM group intervention that included relaxation and stress reduction exercises, coping skills training and health behavior change strategies provided over 12 months was tested in an RCT in women with Stage II – III breast cancer recruited in the period after surgery (87). Women in the intervention (vs standard care) showed increases in cellular immunity (LPR) and reported decreased distress, more healthy eating habits (avoiding high-fat foods), and reduced smoking rates, over the initial 4 months (87). At 12-month follow-up intervention participants evidenced better health status based on staff ratings, and reductions in distress at 4-months predicted better health at 12 months (103).
Another CBT-based SM approach, CBSM, is a 10-week group-based program that blends cognitive, behavioral and interpersonal skills training through in-session didactic and role playing activities and homework and daily practice (88). CBSM was shown in 2 RCTs of post-surgical Stage 0 – III breast cancer patients to improve cancer-specific distress, mood, social adversity, and QoL (104,105,106); decrease evening serum cortisol (107); and increase LPR and IL-2 and IFN-γ production (108,109). Showing reductions in evening cortisol is important because flatter di-urnal cortisol slopes (due partly to higher evening levels) have been associated with decreased survival in metastatic breast cancer (110]), non-small cell lung cancer (111]), and metastatic renal cell carcinoma (57). Intervention effects on Th1 cytokine (IL-2 and IFN-γ) production may be important for supporting cellular immune processes involved in tumor eradication. Those assigned to CBSM (vs control) also showed altered expression of 91 leukocyte genes at 6 – 12 month follow-up including down-regulation of 62 genes for pro-inflammatory cytokines (IL1B, IL6, TNF), inflammatory chemokines and their receptors, COX2/PGS2, and mediators of tissue remodeling and EMT; and upregulation of 29 genes related to CMI (anti-viral) responses including Type I IFN response, Type II IFN signaling, and IFN signal transduction (56). Bioinformatic analysis of CBSM effects suggested decreased activity of NFκB/Rel and the Globin Transcription Factor (GATA) family, and increased activity of IFN response factors, which have been linked to stress and SNS signaling in prior work (112). Those in CBSM also showed increased expression of GR-related genes relative to controls and an over-representation of GR response elements in the promoters of CBSM-up-regulated genes (56), suggesting that CBSM may reverse stress-induced GR desensitization (113) and subsequently down-regulate inflammatory signaling (114).
Do Stress Management Interventions Affect Long-Term Clinical Outcomes in Cancer Patients and Are These Effects Associated with Immune System Changes?
A meta-analysis of 15 randomized trials meeting Cochrane criteria for methodological quality involving nearly 3000 cancer patients indicated that while psychosocial interventions did not provide an overall survival benefit, those interventions delivered early in disease (in 6 trials with 1448 patients with non-metastatic disease) were associated with a 41% reduced risk of cancer mortality (115). We focus on two sets of studies in non-metastatic breast cancer patients showing post-surgical CBT-based SM interventions to modulate psychological adaptation, cellular immune and/or inflammatory processes and disease course.
Andersen et al tested the effects of a post-surgical group-based SM intervention described previously (87), and reported a significant reduction in overall and breast cancer specific mortality rates as well as a 45% reduced risk of cancer recurrence at a median of 11 years follow-up (116). In a subgroup of depressed women monitored over this follow-up period, those receiving the intervention showed decreases in immunologic markers consistent with active infection or chronic inflammatory conditions (total white blood cells [WBC] and neutrophils) compared to controls (117). Women whose cancer recurred revealed greater serum cortisol and inflammation (greater total WBC and neutrophils) 17 months prior to their recurrence (118). Women who experienced a distal recurrence at this point had weaker cellular immune responses (LPR, NKCC) and greater elevations in WBC compared to those experiencing a local recurrence (118). Thus one possible explanation for intervention effects on recurrence may be normalization of stress- and treatment-associated neuroendocrine and immunologic regulation during a critical period following treatment and preceding recurrence. This team also followed women after the point of disease recurrence and observed a reduced risk of death over an 80-month follow-up among those who had been assigned to the intervention arm (vs control) during primary treatment years ago (119). During the 12-months following recurrence, the intervention group also showed improved psychological adaptation (decreased negative mood and increased social support) and greater LPR and NKCC. This set of studies suggests a CBT-based SM intervention that improves psychological adaptation (decreased distress) may increase cellular immune function (LPR) early in treatment, prevent inflammatory changes during survivorship, and decrease the odds of mortality and recurrence. Having received this intervention during primary treatment may also promote persisting benefits in psychological adaptation, immune functioning and clinical outcomes even after disease recurs.
In another previously described RCT (104 – 109), women with Stage 0 – III breast cancer assigned to a 10-wk CBSM intervention vs a psychoeducational control after surgery showed lower odds of mortality and recurrence at 8–15yr (11-yr median) follow-up over and above the effect of age, time since surgery, stage, tumor receptor type, tumor size, and adjuvant therapy (120). In analyses of patients matched for the same stage (Stage II – III) of the patients enrolled in the Andersen et al trial (116), CBSM had even larger reduction in odds of breast cancer mortality and recurrence (120). Among long-term survivors in this trial, those who had been assigned to CBSM showed less depressive symptoms and better general, emotional and physical well-being vs controls at 11yr median follow-up, suggesting that maintaining psychological adaptation over survivorship may support better clinical outcomes (121).
Since research has implicated inflammation and leukocyte recruitment in breast cancer progression (122,123), these investigators next explored whether SM-related changes in leukocyte transcriptional activities during primary treatment could explain the effects of CBSM on increased time to recurrence in this cohort. To examine whether immunologic changes observed after CBSM over the initial 12 months of primary treatment predicted 11-yr disease free survival (DFS), investigators used a 53-gene composite which included 19 pro-inflammatory transcripts and 34 transcripts (inversely scored) related to Type I IFN responses and antibody synthesis, based on prior research on stress and adversity (4,5,56,62). Patients assigned to CBSM showed decreased gene expression composite vs controls, and greater magnitude of the gene expression composite score decrease over 12 months of primary treatment predicted greater 11yr DFS (124). It is plausible that other SM interventions shown to have positive effects on breast cancer disease recurrence (e.g.,116) may operate via similar mechanisms. This may also have implications for SM in other cancers, since greater expression of this same combination of genes also predicts increased relapse risk and decreased leukemia-free survival in recipients of hematopoietic stem cell transplant (HSCT) for acute myelogenous leukemia (125).
Despite being conducted by independent laboratories the two CBT-based SM trials reviewed here are comparable in terms of sample size, timing (post-surgical period prior to starting adjuvant therapy), intervention features (format: group; content: CBT-based SM; frequency: weekly for initial training), and follow-up intervals (psychological and immune measures over 12 months; disease outcomes over a median of 11yrs). In each trial, distress and/or cortisol decreases related to either increased frequency of relaxation practice (126) or increased confidence in using SM skills (127). This provides insight into possible “active ingredients” of these interventions. Although the disease stage range of the samples did differ, when stage was matched between the two trials (i.e., using Stage II – III cases only) very similar results emerged for intervention effects on survival and recurrence. Differences in the trials include a longer period of continuous intervention (12 months [116] vs 10 weeks [120]), and an additional focus on heath behavior change in one (116). This suggests that it may be possible to achieve long-term health benefits in the briefer program with a sole focus on SM skills training. Taken together, CBT-based SM might improve long-term health outcomes in breast cancer patients by modulating immune cell activities (e.g., inflammation and anti-viral immune signaling), which have the potential to communicate with cancer cells and stroma to promote progression and metastasis (4,5,6,122).
Emerging Trends in Stress Management Research
Effects of brief SM on stress and immune activity.
Since combined approaches that include relaxation training (RT), CBT, and Health Education (HE) (87); or RT and CBT (88); have been shown to improve psychological adaptation, physiological stress responses and health outcomes in cancer patients undergoing primary treatment, it is important to understand whether specific elements of these combined approaches are effective. This is key since interventions requiring 10 weeks to 12 months may not be feasible in the clinical oncology setting. One “dismantling” trial comparing the effects of 5-week RT vs 5-week CBT vs 5-week HE found that breast cancer patients assigned to either RT or CBT showed improved psychological adaptation (mood, cancer-specific distress) vs those in HE (128). Recent evidence shows that women assigned to either RT or CBT showed reduced inflammatory signaling (circulating s100A8/A9 levels and leukocyte nuclear NFκB DNA binding) vs those in HE (129, 52). This cohort is now being followed for long-term clinical outcomes. Thus post-surgical interventions as brief as 5 weeks, focused on either RT or CBT, can improve adaptation and reduce inflammatory signaling in ways possibly relevant for breast cancer disease progression.
Remotely-delivered SM intervention.
Attending face-to-face group meetings may present challenges to specific cancer populations as they navigate healthcare appointments while maintaining employment and child care responsibilities; in those hesitant to attend structured groups in institutional settings; or in patients who must be isolated for infection control following procedures such as chemotherapy and HSCT. Technological innovations make it now possible to offer interventions at home over a broadband platform (130). On-line programs that allow breast cancer patients to create personal blog sites have been shown in RCTs to improve mood (131). While these venues provide support resources less is known about the value of remotely-delivered empirically validated SM interventions known to affect stress and immune parameters (e.g., CBSM), though RCTs are underway examining their impact in breast cancer patients (132).
Pharmacological approaches to modulating stress pathways.
One pharmacologic approach targets stress physiology pathways more explicitly by using agents that antagonize SNS signaling (e.g., non-selective beta adrenergic blockade) and inflammation (e.g., COX2 inhibitors) since chronic use of beta blockers and COX inhibitors are associated with reduced risk of cancer metastasis in humans (133), and blocking these pathways in mice decreases metastasis (134, 135). The perioperative period involves release of catecholamines and prostaglandins due to psychological and surgical stress and tissue damage (135). A double-blind placebo-controlled trial in breast cancer patients showed that peri-surgical administration (beginning 5 days pre-surgery and extending 6 days post) of a combined propranolol and etodolac regimen was associated with decreased EMT signaling, and downregulation of leukocyte pro-inflammatory/pro-metastatic GATA and EGR family transcriptional factors. This regimen also mitigated increases in serum IL-6 and CRP, and declines in stimulated production of Th1 cytokines IL-12 and IFN-γ (136). The pattern of down-regulated pro-inflammatory and pro-metastatic, and up-regulated anti-viral signaling is very similar to what has been observed in breast cancer patients receiving CBT-based SM approaches such as CBSM after surgery (56,109,124). It may be plausible to test whether an initial pre-surgical propranolol-etodolac cocktail such as this, combined with brief CBT-based SM, in the post-surgical period provides an optimal SM adjunctive regimen to facilitate the success of cancer treatments.
Addressing understudied populations.
Ethnic minority groups suffer disproportionate morbidity and mortality and compromised QoL from cancers. While interventions such as CBSM have been efficacious in reducing stress and adversity in different patient groups including Black breast cancer survivors (137) and Hispanic men with prostate cancer (138), effects on immune activity and clinical outcomes are unknown. Another needed application of this work is in the context of opportunistic infections. For instance, treated cancer patients have 4 times the risk of influenza-related mortality (139). Because chronic stress can dampen the immune response to the influenza vaccine in older populations (140), one ongoing RCT examines the effects of a remotely-delivered CBSM intervention on stress and immunologic responses to the influenza vaccine in distressed older women undergoing primary treatment for breast cancer (132). With evidence that stress processes contribute to immunologic and tumor cell biology changes and poorer clinical outcomes in skin (31), lung (111), ovarian (60,61), renal (57), hepatocellular (141), and hematologic cancers (125,142), it is imperative that trials evaluate the effects of SM interventions on stress, immunologic and health outcomes in these and other cancers.
Practical and Clinical Considerations
With growing evidence that psychosocial interventions may influence psychological adaptation, stress-related biobehavioral processes and clinical health outcomes in cancer patients, contemporary questions move to when, where, and for whom these interventions might be used in clinical oncology settings. Regarding “when” in the post-diagnosis cancer continuum to intervene, most of the evidence on the effects of these interventions has been demonstrated in either post-surgical patients or cancer “survivors” who have completed primary treatment months prior. The potential impact of these interventions on psychological adaptation and quality of life is demonstrable in patients in both the early and later stages of cancer, and this should be a primary goal. While there is provocative evidence that these interventions can create changes in stress-related biobehavioral processes for periods up to 12 months in patients with early-stage non-metastatic disease, it remains to be determined whether they are able to modulate these biobehavioral processes in patients with advanced cancers.
In terms of clinical outcomes, there are two stress management trials that have shown effects on long-term recurrence and survival [116,120] in early-stage patients receiving intervention in the post-surgical period. Given the established effects of surgery on stress-related biobehavioral processes it is arguable that the peri-surgical period may be an important point to explore in further intervention trials with cancer patients. This could include recruiting patients just after biopsy-confirmed diagnosis, randomizing them to study conditions either prior to surgery (a period of heightened anxiety and stress), or in the weeks post-surgery, and examining relative changes in biobehavioral processes pre-post surgery, and again pre-post adjuvant therapy. This might illuminate the optimal timing of “early” interventions. We know from prior work that psychosocial interventions initiated prior to surgery are associated with improved 10-year survival in patients treated for gastrointestinal cancer (143), yet few trials have tested for immunologic effects of pre-surgical stress management in cancer patients. Importantly, one study showed a 2-session stress management intervention (teaching breathing, relaxing imagery and coping skills) offered to men prior to prostate cancer surgery related to decreases in mood disturbance and increases in NKCC one week pre- to 48 hrs post-surgery (144). In the future one could compare the relative benefits of these early administrations vs. delaying intervention to the post-adjuvant, early survivorship period, or beyond. In each case these cohorts of patients could be followed for longer term outcomes to determine the impact of early vs delayed intervention. One trial did observe that patients receiving a post-surgical stress management intervention showed greater survival after they recurred (119). Future work should test the effects of these interventions delivered in the period just after notification of recurrence—a very stressful point in time, which may be more stressful than the initial diagnosis of primary disease.
Aside from the “when” questions, the “where” questions concern the format for delivery of the interventions. As we have noted, extended interventions requiring weekly group attendance over several months may not be practical within the context of primary treatment, and ongoing trials are testing briefer forms and remote delivery platforms (using tablets and broadband connection) to determine if they show comparable effects to their longer and in-person versions. More work should also be conducted testing the effects of ‘embedding’ stress management interventions into adjuvant therapy settings such as the CTU, which could be delivered in-person, via DVD recordings, or through a remote connection. Given the long periods that patients must spend in receiving chemotherapy, there is an opportunity to test the efficacy of these interventions in large numbers of patients receiving specific regimens, with the potential of providing immediate clinical benefits such as relaxation, sense of control and self-efficacy during treatment, and lasting benefits between and beyond infusion visits. Another extension of this work would include testing the effects of stress management interventions embedded in the neoadjuvant chemotherapy or HSCT settings, among others.
In terms of the “who” question, we need more information on which subgroups of patients are likely to benefit the most from stress management interventions. While there is evidence that patients with greater cancer-specific distress (145), pessimism (104), and other psychosocial adversity indicators (146) show the greatest effects of these interventions on psychological adaptation, there is no evidence to date that these psychosocial characteristics can predict intervention effects on biobehavioral and long-term clinical health outcomes. Beyond psychosocial characteristics it is also important to explore biomedical (e.g., tumor phenotype and immune system status) and sociodemographic factors that can be used to identify patients most likely to show psychological, biobehavioral and health benefits. It would also be fruitful to test which host factors predict differential effects of one stress management approach over another (e.g., relaxation vs CBT vs beta-adrenergic blockade). This line of work could be facilitated by secondary analyses of prior trials, provided there is sufficient power, to identify leads, which would then require confirmation in future trials with targeted sampling of subgroups and a-priori hypotheses testing for relative effectiveness of different approaches between these subgroups.
As this field evolves it will be important to examine other issues such as the cost-effectiveness of stress management per se, as well as the relative cost-effectiveness of interventions varying in length and delivery format. As research informs us of the host factors that predict optimal intervention effects we can make use of in-depth yet efficient psychosocial screening with patient-reported outcomes (PROs) for depressive symptoms, cancer-specific distress, personality and coping style, and social/interpersonal factors that are most relevant. In the future the notion of “targeted stress management” for specific cancer patients may become part of mainstream precision oncology care.
Conclusions
Chronic stress and adversity are associated with neuroendocrine alterations (SNS, HPA), which can up-regulate inflammation and down-regulate CMI. Immune cells that have undergone such changes may not control cancer cells effectively, and may act as stromal cells communicating with the tumor microenvironment and circulating cancer cells to promote tumor growth mechanisms, invasiveness, extravasation into the circulation, and metastasis. Many RCTs demonstrate physical-, mindfulness- and CBT-based stress management interventions and pharmacologic blockade of stress-related pathways can affect immune parameters in cancer patients and survivors. Importantly, research has now linked stress management-associated changes in immune parameters early in treatment with long-term health outcomes in the context of early-stage breast cancer. Thus, in addition to reducing chronic stress and adversity, stress management intervention can also alter immune cell activity in a manner that may mitigate the biological impact of stress early in treatment, and potentially influence disease progression and clinical outcomes in cancer patients.
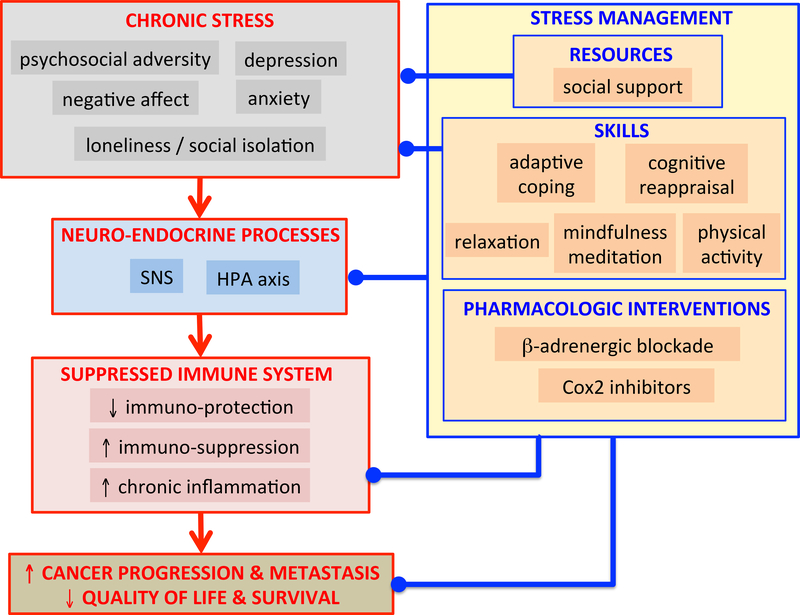
Figure 1. Impact of Psychosocial Stress and Stress Management on Cancer-Relevant Immune Responses.
This model summarizes the potential impact of chronic stress (chronic psychosocial adversity, depression, negative affect, anxiety, and loneliness/social isolation) on neuro-endocrine processes (sympathetic nervous system (SNS) and hypothalamic-pituitary-adrenal (HPA) axis activation, which are associated with altered immune system activity (decreased immuno-protection, increased immuno-suppression and increased chronic inflammation), which may in turn hasten cancer progression and metastasis, and decrease quality of life and survival. The model also summarizes stress management resources (social support), and skills (adaptive coping and cognitive reappraisal, relaxation, mindfulness meditation, physical exercise), and pharmacologic interventions (beta-adrenergic blockade and COX2 inhibitors), which may act to decrease chronic psychosocial adversity and/or modulate neuro-endocrine and/or immune system processes that could contribute to positive health outcomes (decreased cancer progression and metastasis, and increased quality of life and survival). (Figure by FSD.)
Acknowledgements
Studies mentioned here that were conducted in Firdaus Dhabhar’s laboratory were supported by: The NIH (AI48995, AR46299, CA107498), The Office of Naval Research (N00014–15-1–2116), The Dana Foundation, and The John D. and Catherine T. MacArthur Foundation. Studies conducted in Michael Antoni’s laboratory were supported by: NIH (CA064710, CA131451, HHSN261200800001E), Florida Department of Health (6BC06), and the Florida Breast Cancer Foundation.
Footnotes
Conflict of Interest
Dr. Antoni reports that he receives royalties from a Book on stress management in breast cancer patients. Dr. Dhabhar reports no conflicts.
REFERENCES
- 1.Antoni MH Psychosocial intervention effects on adaptation, disease course and biobehavioral processes in cancer. Brain, Behavior and Immunity. 2013; 30: S88–S89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gil F, Costa G, Hilker I, Benito L. First anxiety, afterwards depression: psychological distress in cancer patients at diagnosis and after medical treatment. Stress Health. 2012;28: 362–367 [DOI] [PubMed] [Google Scholar]
- 3.Linden W, Vodermaier A, Mackenzie R, Greig D. Anxiety and depression after cancer diagnosis: prevalence rates by cancer type, gender, and age. J Affect Disord. 2012;141: 343–351 [DOI] [PubMed] [Google Scholar]
- 4.Antoni MH, Lutgendorf S, Cole S, Dhabhar F, Sephton S, McDonald P, Stefanek M, & Sood A The influence of biobehavioral factors on tumor biology: pathways and mechanisms. Nature Reviews Cancer. 2006; 6: 240–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lutgendorf SK, Sood A & Antoni MH Host Factors and Cancer Progression: Biobehavioral Signaling Pathways and Interventions. Journal of Clinical Oncology. 2010; 28: 4094–4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saul AN, Oberyszyn TM, Daugherty C, et al. Chronic stress and susceptibility to skin cancer. J Nat Cancer Institute. 2005;97: 1760–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Armaiz-Pena GN, Cole SW, Lutgendorf SK, Sood AK. Neuroendocrine influences on cancer progression. Brain Behav Immun. 2013;30 Suppl: S19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldstein DS, McEwen B. Allostasis, homeostats, and the nature of stress. Stress. 2002;5: 55–58. [DOI] [PubMed] [Google Scholar]
- 9.Dhabhar FS. Effects of stress on immune function: the good, the bad, and the beautiful. Immunol Res. 2014;58: 193–210. [DOI] [PubMed] [Google Scholar]
- 10.Dhabhar FS, McEwen BS. Acute stress enhances while chronic stress suppresses immune function in vivo: A potential role for leukocyte trafficking. Brain Behavior & Immunity. 1997;11: 286–306. [DOI] [PubMed] [Google Scholar]
- 11.Jezova D, Jurankova E, Mosnarova A, Kriska M, Skultetyova I. Neuroendocrine response during stress with relation to gender differences. Acta Neurobiol Exp (Wars). 1996;56: 779–785. [DOI] [PubMed] [Google Scholar]
- 12.Steptoe A, Hamer M, Chida Y. The effects of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis. Brain Behav Immun. 2007;21: 901–912. [DOI] [PubMed] [Google Scholar]
- 13.Aschbacher K, Epel E, Wolkowitz OM, Prather AA, Puterman E, Dhabhar FS. Maintenance of a positive outlook during acute stress protects against pro-inflammatory reactivity and future depressive symptoms. Brain Behav Immun. 2012;26: 346–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Puterman E, Epel ES, O’Donovan A, Prather AA, Aschbacher K, Dhabhar FS. Anger Is Associated with Increased IL-6 Stress Reactivity in Women, But Only Among Those Low in Social Support. Int J Behav Med. 2014;21:936–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Antoni MH & Lutgendorf S Psychosocial Factors and Disease Progression in Cancer. Current Directions in Psychological Science. 2007; 16: 42–46. [Google Scholar]
- 16.Sephton S, Spiegel D. Circadian disruption in cancer: a neuroendocrine-immune pathway from stress to disease? Brain Behav Immun. 2003;17: 321–328. [DOI] [PubMed] [Google Scholar]
- 17.Miller GE, Chen E, Sze J, Marin T, Arevalo JM, Doll R. A functional genomic fingerprint of chronic stress in humans: blunted glucocorticoid and increased NF-kappaB signaling. Biol Psychiatry. 2008;64: 266–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dhabhar FS. Enhancing versus suppressive effects of stress on immune function: implications for immunoprotection and immunopathology. Neuroimmunomodulation. 2009; 16: 300–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dhabhar FS. The Short-Term Stress Response - Mother Nature’s Mechanism for Enhancing Protection and Performance Under Conditions of Threat, Challenge, and Opportunity. Frontiers in Neuroendocrinology. 2018; In Press, DOI: 10.1016/j.yfrne.2018.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dhabhar FS, McEwen BS, Spencer RL. Stress response, adrenal steroid receptor levels, and corticosteroid-binding globulin levels -- a comparison between Sprague Dawley, Fischer 344, and Lewis rats. Brain Research. 1993;616: 89–98. [DOI] [PubMed] [Google Scholar]
- 21.Sternberg EM, Hill JM, Chrousos GP, et al. Inflammatory mediator-induced hypothalamic-pituitary-adrenal axis activation is defective in streptococcal cell wall arthritis-susceptible Lewis rats. Proc. Natl. Acad. Sci. USA 1989;86: 2374–2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dhabhar FS, McEwen BS, Spencer RL. Adaptation to prolonged or repeated stress -- Comparison between rat strains showing intrinsic differences in reactivity to acute stress. Neuroendocrinology. 1997;65: 360–368. [DOI] [PubMed] [Google Scholar]
- 23.Dhabhar FS, Miller AH, McEwen BS, Spencer RL. Differential activation of adrenal steroid receptors in neural and immune tissues of Sprague Dawley, Fischer 344, and Lewis rats. J. Neuroimmunol 1995;56: 77–90 [DOI] [PubMed] [Google Scholar]
- 24.Lutgendorf SK, Sood AK. Biobehavioral factors and cancer progression: physiological pathways and mechanisms. Psychosom Med. 2011;73: 724–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dhabhar FS, Malarkey WB, Neri E, McEwen BS. Stress-induced redistribution of immune cells - From barracks to boulevards to battlefields: A tale of three hormones. Psychoneuroendocrinology. 2012;37: 1345–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dhabhar FS, McEwen BS. Enhancing versus suppressive effects of stress hormones on skin immune function. PNAS, USA. 1999;96: 1059–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fleshner M, Laudenslager ML, Simons L, Maier SF. Reduced serum antibodies associated with social defeat in rats. Physiol. & Behav 1989;45: 1183–1187. [DOI] [PubMed] [Google Scholar]
- 28.Whiteside TL, Vujanovic NL, Herberman RB. Natural killer cells and tumor therapy. Current topics in microbiology and immunology. 1998;230: 221–244. [DOI] [PubMed] [Google Scholar]
- 29.Whiteside T Immune modulation of T-cell and NK cell activities by TEXs (tumour-derived exosomes). Biochem Soc Trans.2013; 41: 245–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ben-Eliyahu S, Yirmiya R, Liebeskind JC, Taylor AN, Gale RP. Stress increases metastatic spread of a mammary tumor in rats: evidence for mediation by the immune system. Brain, Behav., Immun 1991;5: 193–205 [DOI] [PubMed] [Google Scholar]
- 31.Dhabhar FS, Saul AN, Holmes TH, et al. High anxiety is associated with higher chronic stress burden, lower protective immunity, and increased cancer progression. PLoS One. 2012;7: DOI: 10.1371/journal.pone.0033069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sephton SE, Dhabhar FS, Keuroghlian AS, et al. Depression, cortisol, and suppressed cell-mediated immunity in metastatic breast cancer. Brain Behav Immun. 2009;23: 1148–1155. [DOI] [PubMed] [Google Scholar]
- 33.Lutgendorf SK, Lamkin DM, DeGeest K, et al. Depressed and anxious mood and T-cell cytokine expressing populations in ovarian cancer patients. Brain, Behavior, and Immunity. 2008;22: 890–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lutgendorf SK, Sood AK, Anderson B, et al. Social support, psychological distress, and natural killer cell activity in ovarian cancer. J Clin Oncol. 2005;23: 7105–7113. [DOI] [PubMed] [Google Scholar]
- 35.Lamkin DM, Lutgendorf SK, McGinn S, et al. Positive psychosocial factors and NKT cells in ovarian cancer patients. Brain Behav Immun. 2008;22: 65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lutgendorf SK, DeGeest K, Dahmoush L, et al. Social isolation is associated with elevated tumor norepinephrine in ovarian carcinoma patients. Brain, Behavior, and Immunity. 2011;25: 250–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blomberg BB, Alvarez JP, Diaz A, et al. Psychosocial adaptation and cellular immunity in breast cancer patients in the weeks after surgery: An exploratory study. J Psychosom Res. 2009;67: 369–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Andersen BL, Farrar WB, Golden-Kreutz D, et al. Stress and immune responses after surgical treatment for regional breast cancer. J. Natl. Cancer Inst 1998;90: 30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Andersen BL, Goyal NG, Westbrook TD, Bishop B, Carson WE, 3rd. Trajectories of Stress, Depressive Symptoms, and Immunity in Cancer Survivors: Diagnosis to 5 Years. Clin Cancer Res. 2017;23: 52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gouin JP, Glaser R, Malarkey WB, Beversdorf D, Kiecolt-Glaser J. Chronic stress, daily stressors, and circulating inflammatory markers. Health Psychol. 2012;31: 264–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elliot AJ, Mooney CJ, Infurna FJ, Chapman BP. Associations of Lifetime Trauma and Chronic Stress With C-reactive Protein in Adults Ages 50 Years and Older: Examining the Moderating Role of Perceived Control. Psychosom Med. 2017;79: 622–630. [DOI] [PubMed] [Google Scholar]
- 42.Elenkov IJ, Chrousos GP. Stress hormones, proinflammatory and antiinflammatory cytokines, and autoimmunity. Ann N Y Acad Sci. 2002;966: 290–303. [DOI] [PubMed] [Google Scholar]
- 43.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420: 860–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lu H, Ouyang W, Huang C. Inflammation, a key event in cancer development. Mol Cancer Res. 2006;4: 221–233. [DOI] [PubMed] [Google Scholar]
- 45.II’yasova D, Colbert LH, Harris TB, et al. Circulating levels of inflammatory markers and cancer risk in the health aging and body composition cohort. Cancer Epidemiol Biomarkers Prev. 2005;14: 2413–2418. [DOI] [PubMed] [Google Scholar]
- 46.Bower JE, Lamkin DM. Inflammation and cancer-related fatigue: mechanisms, contributing factors, and treatment implications. Brain Behav Immun. 2013;30 Suppl: S48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Irwin MR. Depression and insomnia in cancer: prevalence, risk factors, and effects on cancer outcomes. Curr Psychiatry Rep. 2013;15: 404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Irwin MR, Olmstead RE, Ganz PA, Haque R. Sleep disturbance, inflammation and depression risk in cancer survivors. Brain Behav Immun. 2013;30 Suppl: S58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hughes S, Jaremka LM, Alfano CM, Glaser R, Povoski SP, Lipari AM, Agnese DM, Farrar WB, Yee LD, Carson WE 3rd, Malarkey WB, Kiecolt-Glaser JK. Social support predicts inflammation, pain, and depressive symptoms: Longitudinal relationships among breast cancer survivors. Psychoneuroendocrinology. 2014;42:38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Soygur H, Palaoglu O, Akarsu E, Cankurtaran E, Ozalp E, Turhan L, Ayhan I. Interleukin-6 levels and HPA axis activation in breast cancer patients with major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry 2007;31:1242–1247. [DOI] [PubMed] [Google Scholar]
- 51.Bouchard L, Antoni MH, Blomberg BB, Stagl J, Gudenkauf L, Jutagir D, Diaz A, Lechner S, Gluck S, Derhagopian R, & Carver CS Postsurgical depressive symptoms and proinflammatory cytokine elevations in women undergoing primary treatment for breast cancer. Psychosomatic Medicine. 2016;78: 36–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Antoni MH, Diaz A, Amiel C, Bouchard L, Jacobs J, Gudenkauf L, Jutagir D, Lechner S, Carver CS & Blomberg B (2017, March). Brief Stress Management Decreases Leukocyte Nuclear Inflammatory Protein Expression in Distressed Breast Cancer Patients Undergoing Primary Treatment. Presented at the American Psychosomatic Society Meeting, Sevilla, Spain [Google Scholar]
- 53.Antoni MH, Jacobs JM, Bouchard LC, Lechner SC, Jutagir DR, Gudenkauf L, Blomberg BB, Gluck S & Carver CS Post-surgical depressive symptoms and long-term survival in non-metastatic breast cancer patients at 11-year follow-up. General Hospital Psychiatry. 2017; 44: 16–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Giese-Davis J, Collie K, Rancourt KM, et al. Decrease in depression symptoms is associated with longer survival in patients with metastatic breast cancer: a secondary analysis. J. Clinical Oncology. 2011; 29: 413–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pinquart M & Duberstein PR Depression and cancer mortality: a meta-analysis. Psychological Medicine. 2010; 40: 1797–1810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Antoni MH, Lutgendorf S, Blomberg B, Carver CS, Lechner S, Diaz A, Stagl J, Arevalo J & Cole S Cognitive-behavioral stress management reverses anxiety-related leukocyte transcriptional dynamics. Biological Psychiatry. 2012; 71: 366–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cohen L, Cole SW, Sood AK, Prinsloo S, Kirschbaum C, Arevalo JM,…Pisters L Depressive symptoms and cortisol rhythmicity predict survival in patients with renal cell carcinoma: Role of inflammatory signaling. PLoS ONE. 2012; 7: e42324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lutgendorf SK, Lamkin DM, Jennings NB, Arevalo JMG, Penedo F, DeGeest K,…Sood AK Biobehavioral influences on matrix metalloproteinase expression in ovarian carci- noma. Clinical Cancer Research. 2008; 14: 6839–6846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lutgendorf SK, Johnsen EL, Cooper B, Anderson B, Sorosky JI, Buller RE, & Sood AK Vascular endothelial growth factor and social support in patients with ovarian carcinoma. Cancer. 2002; 95: 808–815. [DOI] [PubMed] [Google Scholar]
- 60.Lutgendorf SK, De Geest K, Bender D, Ahmed A, Goodheart MJ, Dahmoush L,…Sood AK Social influences on clinical outcomes of patients with ovarian cancer. Journal of Clinical Oncology. 2012; 30: 2885–2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lutgendorf S, Thaker P, Arevalo J, Goodheart M, Slavich G, Sood A & Cole W Biobehavioral modulation of the exosome transcriptome in ovarian carcinoma. Cancer. 2017, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sloan EK, Priceman SJ, Cox BF, et al. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res. 2011;70: 7042–7052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10: 942–949. [DOI] [PubMed] [Google Scholar]
- 64.Boucek J, Mrkvan T, Chovanec M, et al. Regulatory T cells and their prognostic value for patients with squamous cell carcinoma of the head and neck. J Cell Mol Med. 2010;14: 426–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li W, Song D, Li H, Liang L, Zhao N, Liu T. Reduction in Peripheral CD19+CD24hCD27+ B Cell Frequency Predicts Favourable Clinical Course in XELOX-Treated Patients with Advanced Gastric Cancer. Cell Physiol Biochem. 2017;41: 2045–2052. [DOI] [PubMed] [Google Scholar]
- 66.Schwartz M, Zhang Y, Rosenblatt JD. B cell regulation of the anti-tumor response and role in carcinogenesis. J Immunother Cancer. 2016;4: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Anani W, Shurin MR. Targeting Myeloid-Derived Suppressor Cells in Cancer. Adv Exp Med Biol. 2017;1036: 105–128. [DOI] [PubMed] [Google Scholar]
- 68.Ostrand-Rosenberg S, Fenselau C. Myeloid-Derived Suppressor Cells: Immune-Suppressive Cells That Impair Antitumor Immunity and Are Sculpted by Their Environment. J Immunol. 2018;200: 422–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Drews-Elger K, Iorns E, Dias A, et al. Infiltrating S100A8+ myeloid cells promote metastatic spread of human breast cancer and predict poor clinical outcome. Breast Cancer Res Treat. 2014;148: 41–59. [DOI] [PubMed] [Google Scholar]
- 70.Mundy-Bosse BL, Thornton LM, Yang HC, Andersen BL, Carson WE. Psychological stress is associated with altered levels of myeloid-derived suppressor cells in breast cancer patients. Cell Immunol. 2011;270: 80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Viswanathan K, Dhabhar FS. Stress-induced enhancement of leukocyte trafficking into sites of surgery or immune activation. PNAS, USA. 2005;102: 5808–5813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dhabhar FS, Viswanathan K. Short-Term Stress Experienced At The Time Of Immunization Induces a Long-lasting Increase In Immunological Memory. Am J Physiol Regul Integr Comp Physiol. 2005;289: R738–744. [DOI] [PubMed] [Google Scholar]
- 73.Viswanathan K, Daugherty C, Dhabhar FS. Stress as an endogenous adjuvant: augmentation of the immunization phase of cell-mediated immunity. International Immunology. 2005;17: 1059–1069. [DOI] [PubMed] [Google Scholar]
- 74.Dhabhar FS, McEwen BS. Stress-induced enhancement of antigen-specific cell-mediated immunity. J. Immunology 1996;156: 2608–2615 [PubMed] [Google Scholar]
- 75.Dhabhar FS, Satoskar AR, Bluethmann H, David JR, McEwen BS. Stress-Induced Enhancement of Skin Immune Function: A Role For IFNg. PNAS, USA. 2000;97: 2846–2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shaashua L, Rosenne E, Neeman E, Sorski L, Sominsky L, Matzner P, et al. Plasma IL-12 levels are suppressed in vivo by stress and surgery through endogenous release of glucocorticoids and prostaglandins but not catecholamines or opioids. Psychoneuroendocrinology. 2014;42:11–23. doi: 10.1016/j.psyneuen.2013.12.001. PubMed PMID: ; PubMed Central PMCID: PMC3959722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Melamed R, Rosenne E, Shakhar K, Schwartz Y, Abudarham N, Ben-Eliyahu S. Marginating pulmonary-NK activity and resistance to experimental tumor metastasis: suppression by surgery and the prophylactic use of a beta-adrenergic antagonist and a prostaglandin synthesis inhibitor. Brain Behav Immun. 2005;19(2):114–26. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 78.Rosenberger PH, Ickovics JR, Epel E, Nadler E, Jokl P, Fulkerson JP, Tillie JM, and Dhabhar FS. Surgery stress induced immune cell redistribution profiles predict short- and long-term postsurgical recovery: A prospective study. Journal of Bone and Joint Surgery. 2009; 91: 2783–2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Edwards KM, Burns VE, Reynolds T, Carroll D, Drayson M, and Ring C. Acute stress exposure prior to influenza vaccination enhances antibody response in women. Brain Behav Immun. 2006; 20: 159–68. [DOI] [PubMed] [Google Scholar]
- 80.Edwards KM, Burns VE, Adkins AE, Carroll D, Drayson M, and Ring C Meningococcal A vaccination response is enhanced by acute stress in men. Psychosom Med. 2008; 70: 147–51. [DOI] [PubMed] [Google Scholar]
- 81.Edwards KM, Burns VE, Carroll D, Drayson M, and Ring C. The acute stress-induced immunoenhancement hypothesis. Exerc Sport Sci Rev. 2007; 35: 150–5 [DOI] [PubMed] [Google Scholar]
- 82.Dhabhar FS, Saul AN, Daugherty C, Holmes TH, Bouley DM, Oberyszyn TM. Short-Term stress enhances cellular immunity and increases early resistance to squamous cell carcinoma. Brain, Behavior, and Immunity. 2010;24: 127–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Betof AS, Dewhirst MW, Jones LW. Effects and potential mechanisms of exercise training on cancer progression: a translational perspective. Brain Behav Immun. 2013;30 Suppl: S75–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Speck RM, Courneya KS, Masse LC, Duval S, Schmitz KH. An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv. 2010;4: 87–100. [DOI] [PubMed] [Google Scholar]
- 85.Lutgendorf S & Andersen B Biobehavioral approaches to cancer progression and survival. American Psychologist. 2015; 70 (2): 186–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Carlson LE, Speca M, Patel KD, & Goodey E Mindfulness‐based stress reduction in relation to quality of life, mood, symptoms of stress, and immune parameters in breast and prostate cancer outpatients. Psychosomatic Medicine. 2003; 65(4); 571–581 [DOI] [PubMed] [Google Scholar]
- 87.Andersen B, Farrar WB, Golden-Kreutz DM, Glaser R, Emery CF, & Crespin TR. Psychological, behavioral, and immune changes after a psychological intervention: A clinical trial. J Clin Oncol. 2004; 22: 3570–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Antoni MH. Stress Management Intervention for Women With Breast Cancer. Washington D.C.: American Psychological Association Press; 2003. [Google Scholar]
- 89.Spiegel D, Bloom JR, Kraemer HC, & Gottheil E Effect of psychosocial treatment on survival of patients with metastatic breast cancer. Lancet. 1989; 2: 888–891. [DOI] [PubMed] [Google Scholar]
- 90.Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363: 733–42. [DOI] [PubMed] [Google Scholar]
- 91.McNeely ML, Campbell KL, Rowe BH, Klassen TP, Mackey JR, Courneya KS. Effects of exercise on breast cancer patients and survivors: a systematic review and meta-analysis. CMAJ. 2006;175: 34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Buffart L, van Uffelen J, Riphagen I, Brug J, van Mechelen W, Brown W, & Chinapaw M Physical and psychosocial benefits of yoga in cancer patients and survivors, a systematic review and meta-analysis of randomized controlled trials. BMC Cancer. 2012; 12: 559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kiecolt-Glaser J, Bennett J, Andridge R, Peng J, Shapiro C, Malarkey W, Emery C, Layman R, Mrozek E & Glaser R Yoga’s impact on inflammation, mood and fatigue in breast cancer survivors: a randomized controlled trial. Journal of Clinical Oncology. 2014; 32 (10): 1040–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bower J, Greendale G, Croswell A, Garet D, Sternlieb B, Ganz P, Irwin M, Olmstead R, Arevalo J & Cole SW Yoga reduces inflammatory signaling in fatigued breast cancer survivors: A randomized controlled trial. Psychoneuroendocrinology. 2014; 43: 20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Irwin M, Olmstead R, Breen E, Witarama T, Carillo C, Sadeghi N, Arevelo J, Ma J, Nicassio P, Ganz P, Bower J & Cole SW Tai chi, cellular inflammation, and transcritome dynamics in breast cancer survivors with insomnia: A randomized controlled trial. JNCI Monograph. 2014; 50: 295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hernandez-Rief M, Field T, Ironson G, Beutler J, Vera Y, Hurley J, Fletcher MA, Schanberg S, Kuhn C & Fraser M Natural killer cells and lymphocytes increase in women with breast cancer following massage therapy. Int J. Neuroscience 2005; 115: 494–510. [DOI] [PubMed] [Google Scholar]
- 97.Pais I,Correia N, Pimentel I, Teles M, Neves E, Vasconcelos J, Guimaraes J, Azevedo N, Moreira Pinto A, Machado J, Efferh T & Greten H Effects of acupuncture on leucopenia, neutropenia, NK, and B cells in cancer patients: a randomized pilot study. Evidence-based Compl Alternative Med. 2014; 2014: 217397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lutgendorf SK., Mullen-Houser E, Russell D, DeGeest K, Jacobsen G, Hart L, Bender D, Buekers T, Goodheart M, Antoni MH, Sood A, & Lubaroff D Preservation of Immune Function in Cervical Cancer Patients during chemoradiation using a Novel Integrative Approach. Brain, Behav Immunity. 2010; 24: 1231–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wurtzen H, Dalton S, Elsass P, Sumbundu A, Steding-Jensen M, Larsen R, Andersen K, Flyger H, Pedersen A & Johansen C Mindfulness significantly reduces self-reported levels of anxiety and depression: results of a randomized controlled trial among 336 Danish women treated for stage I-III breast cancer. European Journal of Cancer. 2013; 49: 1365–1373. [DOI] [PubMed] [Google Scholar]
- 100.Witek-Janusek L, Albuquerque K, Chroniak KR, Chroniak C, Durazo-Arvizu R, & Mathews H Effect of mindfulness-based stress reduction on immune function, quality of life and coping in women newly diagnosed with early stage breast cancer. Brain Behav Immun. 2008; 22: 969–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lengacher CA, Kip KE, Post-White J, Fitzgerald S, Newton C, Barta M,…Johnson-Mallard V Lymphocyte recovery after breast cancer treatment and mindfulness-based stress reduction (MBSR) therapy. Biological Research for Nursing. 2013; 15(1): 37–47. [DOI] [PubMed] [Google Scholar]
- 102.Bower JE, Crosswell AD, Stanton AL, Crespi CM, Winston D, Arevalo J,…Ganz PA Mindfulness meditation for younger breast cancer survivors: a randomized controlled trial. Cancer. 2015; 121(8): 1231–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Andersen BL, Farrar WB, Golden-Kreutz D, et al. Distress reduction from a psychological intervention contributes to improved health for cancer patients. Brain Behav Immun. 2007; 21: 953–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Antoni MH, Lehman JM, Kilbourn KM, Boyers AE, Culver JL, Alferi SM, Carver CS Cognitive-behavioral stress management intervention decreases prevalence of depression and enhances benefit finding among women under treatment for early-stage breast cancer. Health Psychology. 2001; 20: 20–32. [DOI] [PubMed] [Google Scholar]
- 105.Antoni MH, Lechner SC, Kazi A, Wimberly S, Gluck S & Carver CS How stress management improves quality of life after breast cancer treatment. J Consult Clin Pyschol, 2006; 74, 1143–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Antoni MH, Wimberly SR, Lechner SC, Kazi A, Sifre T, Urcuyo KR, Phillips K, Smith RG, Petronis VM, Guellati S, Wells KA, Blomberg B, Carver CS Reduction of cancer-specific thought intrusions and anxiety symptoms with a stress management intervention among women undergoing treatment for breast cancer. Am J Psychiatry. 2006; 163(10): 1791–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Phillips KM, Antoni MH, Lechner SC, Blomberg BB, Llabre M, Avisar E, Gluck S, Dehragopian R & Carver C Stress management intervention reduces serum cortisol and increases relaxation during treatment for non-metastatic breast cancer. Psychosomatic Medicine. 2008; 70: 1044–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.McGregor BA, Antoni MH, Boyers A, Alferi SM, Blomberg BB, Carver CS. Cognitive-behavioral stress management increases benefit finding and immune function among women with early-stage breast cancer. Journal of Psychosomatic Research. 2004; 56: 1–8. [DOI] [PubMed] [Google Scholar]
- 109.Antoni MH, Lechner S, Diaz A, Vargas S, Holley H, Phillips K, McGregor BA, & Carver CS, & Blomberg B Cognitive behavioral stress management effects on psychosocial and physiological adaptation in women undergoing treatment for breast cancer. Brain, Behavior and Immunity. 2009; 23: 159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sephton S, Sapolsky RM, Kraemer HC, Spiegel D. Early mortality in metastatic breast cancer patients with absent of abnormal diurnal cortisol rhythms. J Nat Cancer Inst. 2000; 92: 994–1000. [DOI] [PubMed] [Google Scholar]
- 111.Sephton S, Lush E, Dedert E, Floyd A, Rebholz W, Dhabhar F, Spiegel D & Salmon P Diurnal cortisol rhythm as a predictor of lung cancer survival. Brain, Behavior Immun. 2013; 30 suppl: S163–70. [DOI] [PubMed] [Google Scholar]
- 112.Stark J, Avitsur R, Padgett D, Campbell K, Beck F & Sheridan J Social stress induces glucocorticoid resistance in macrophages. American Journal of Physiological Regulation Integrative Comparative Physiology. 2001; 280: R1799–1805. [DOI] [PubMed] [Google Scholar]
- 113.Miller GE, Chen E, Sze J, Marin T, Arevalo JM, Doll R, et al. A functional genomic fingerprint of chronic stress in humans: blunted glucocorticoid and increased NF-kappaB signaling. Biol Psychiatry. 2008;64: 266–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Miller GE, Chen E, Fok AK, Walker H, Lim A, Nicholls EF, et al. Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling. PNAS U S A. 2009;106: 14716–14721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Oh PJ, Shin SR, Ahn HS & Kim HJ Meta-analysis of psychosocial interventions on survival time in patients with cancer. Psychology and Health. 2016; 31: 396–419. [DOI] [PubMed] [Google Scholar]
- 116.Andersen BL, Yang HC, Farrar WB, et al. Psychologic intervention improves survival for breast cancer patients: A randomized clinical trial. Cancer. 2008; 113: 3450–3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Thornton L, Andersen B, Scholer T & Carson W A psychological intervention reduces inflammatory markers by alleviating depressive symptoms: secondary analysis of a randomized controlled trial. Psychosomatic Medicine. 2009; 71: 715–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Thornton L, Andersen B & Carson W Immune, endocrine, and behavioral precursors to breast cancer recurrence: a case-control analysis. Cancer Immunol. Immunother. 2008; 57: 1471–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Andersen BL., Thornton L, Shapiro C, Farrar W, Mundy B, Yang H & Carson W Biobehavioral, immune and health benefits following recurrence for psychological intervention participants. Clinical Cancer Research. 2010; 16: 3270–3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Stagl J, Lechner S, Carver C, Bouchard L, Gudenkauf L, Jutagir D, Diaz A, Yu Q, Blomberg B, Ironson G, Gluck S, & Antoni MH A Randomized Controlled Trial of Cognitive-Behavioral Stress Management in Breast Cancer: Survival and Recurrence at 11-year Follow-up. Breast Cancer Research and Treatment. 2015;154 (2): 319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Stagl JM, Bouchard LC, Lechner SC, Blomberg BB, Gudenkauf LM, Jutagir DR, Glück S, DerHagopian RP, Carver CS, Antoni MH. Long-term psychological benefits of cognitive-behavioral stress management for women with breast cancer: 11-year follow-up of a randomized controlled trial. Cancer. 2015; 121 (11): 1873–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sloan EK, Pricemen S, Cox BF, Yu S, Pimentel M, Tangkanangnukul V, Arevalo J, Morizono K, Karanikolas B, Wu L, Sood A & Cole SW The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res. 2010; 70: 7042–7052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kwak T, Drews-Elger K, Ergonul A, Miller PC, Braley A, Hwang GH, Zhao D, Besser A, Yamamoto Y, Yamamoto H, El-Ashry D, Slingerland JM, Lippman ME, Hudson BI. Targeting of RAGE-ligand signaling impairs breast cancer cell invasion and metastasis. Oncogene. 2017; 36 (11):1559–1572. [DOI] [PubMed] [Google Scholar]
- 124.Antoni MH, Bouchard LC, Jacobs JM, Lechner SC, Jutagir DR, Gudenkauf LM, Carver CS, Lutgendorf S, Cole SW, Lippman M, & Blomberg BB Stress management, leukocyte transcriptional changes and breast cancer recurrence in a randomized trial: An exploratory analysis. Psychoneuroendocrinology. 2016; 74: 269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Knight JM, Rizzo JD, Logan B, Wang T, Arevalo J, Ma J, Cole SW Low socioeconomic status, adverse gene expression profiles, and clinical outcomes in hematopoietic stem cell transplant recipients. Clin. Cancer Res. 2015; 22: 69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Andersen B, Shelby R, Golden-Kruetz D RCT of a psychological intervention for persons with cancer. I. Mechanisms of change. J. Consult Clinical Psychol 2007; 75: 927–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Phillips KM, Antoni MH, Carver CS, Lechner SC, Penedo FJ, McCullough ME, Glück S, DerHagopian R, & Blomberg BB Stress management skills and reductions in serum cortisol across the year after surgery for non-metastatic breast cancer. Cognitive Therapy and Research. 2011; 35: 595–600. [Google Scholar]
- 128.Gudenkauf L, Antoni MH, Stagl J, Lechner S, Jutagir D, Bouchard L, Blomberg B, Gluck S, Dehrogopian R, Torres-Salichs & Carver CS Brief Cognitive-Behavioral and Relaxation Training Interventions for Breast Cancer: A Randomized Controlled Trial. Journal of Consulting and Clinical Psychology. 2015; 83 (4): 677–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Taub C, Fisher H, Nahin E, Blomberg B, Lippman M, Hudson B, Diaz A, Lechner S, Carver CS & Antoni MH Brief Stress Management Interventions Reduce RAGE- Associated s100A8A9 levels in Breast Cancer Patients. Paper presented at Society of Behavioral Medicine Annual Meeting, New Orleans, LA 2018, April. [Google Scholar]
- 130.Banbury A, Nancarrow S, Dart J, Gray L, Parkinson L Telehealth interventions delivering home-based support group videoconferencing: Systematic review. Journal of Medical Internet Research. 2018; 20: e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Stanton A, Thompson E, Crespi C, Link J, & Waisman J Project connect online: Randomized trial of an internet-based program to chronical the cancer experience and facilitate communication. Journal of Clinical Oncology. 2013; 31: 3411–3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Antoni M, Frasca D, Diaz A, Fisher H, Taub C, Czaja S, Dolores P, Lechner S, Carver C, Lippman M, Blomberg B. Video-conferenced stress management and relaxation training for older women with breast cancer: VSMART. Presented at the Sylvester Comprehensive Cancer Center Retreat 2017, May. [Google Scholar]
- 133.Glasner A, Avraham R, Rosenne E, et al. Improving survival rates in two models of spontaneous postoperative metastasis in mice by combined administration of a beta-adrenergic antagonist and a cyclooxygenase-2 inhibitor. J Immunol. 2010;184: 2449–2457. [DOI] [PubMed] [Google Scholar]
- 134.Sorski L, Melamed R, Matzner P, Lavon H, Shaashua L, Rosenne E, et al. Reducing liver metastases of colon cancer in the context of extensive and minor surgeries through beta-adrenoceptors blockade and COX2 inhibition. Brain Behav Immun. 2016;58:91–8. doi: 10.1016/j.bbi.2016.05.017. PubMed PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Horowitz M, Neeman E, Sharon E & Ben- Eliyahu S Exploiting the critical perioperative period to improve long-term cancer outcomes. Nat. Rev. Clin. Oncol 2015; 12: 213–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Shaashua L, Shabat-Simon M, Haldar R, Matzer P, Zmora O, Shabtai M, Sharon E, Allweis T, Barshack I, Hayman L, Arevelo J, Ma J, Horowitz M, Cole SW & Ben-Eliyahu S Perioperative COX-2 and β-adrenergic blockade improves metastatic biomarkers in breast cancer patients in a phase-II randomized trial. Clin Canc Res. 2017; 23: 4651–4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lechner SC, Ennis-Whitehead N, Vargas S, Annane DW, Robertson BR, Carver CS, Kobetz-Kerman E, & Antoni MH Does a community-based stress management intervention affect psychological adaptation among underserved black breast cancer survivors? The Journal of the National Cancer Institute Monograph. 2014; 50: 315–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Penedo FJ, Traeger L, Dahn J, Molton I, Schneiderman N, & Antoni MH Cognitive Behavioral Stress Management Intervention Improves Quality of Life in Spanish Monolingual Hispanic Men Treated for Localized Prostate Cancer: Effects of a Randomized Controlled Trial. International Journal of Behavioral Medicine. 2007;14: 164–172. [DOI] [PubMed] [Google Scholar]
- 139.Cooksley C, Avritscher E, Berkele B, Rolston K, Geraci J & Elting L Epidemiology and outcomes of serious influenza-related infections in the cancer population. Cancer. 2005; 104; 618–628. [DOI] [PubMed] [Google Scholar]
- 140.Fagundes CP, Gillie B, Derry H, Bennett J & Kiecolt-Glaser JK Resilience and immune function in older adults. Annual Review of Gerontology and Geriatrics. 2012; 32: 29–47. [Google Scholar]
- 141.Steel J, Geller D, Gamblin T, Olek M & Carr B Depression, immunity, and survival in patients with hepatobilliary carcinoma. Journal of Clinical Oncology. 2007; 25: 2397–2405. [DOI] [PubMed] [Google Scholar]
- 142.El-Jawarhi A, Chen Y, Brazauskas R, He N, Lee S, Knight J et al. Impact of pre-transplant depression on outcomes of allogeneic and autologous hematopoietic stem cell transplantation. Cancer. 2017; 123: 1828–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kuchler T, Bestmann B, Rappa S et al. Impact of psychotherapeutic support for patients with gastrointestinal cancer undergoing surgery: 10-year survival results of a randomized trial. J Clin Oncol. 2007; 25: 2702–2708. [DOI] [PubMed] [Google Scholar]
- 144.Cohen L, Parker O, Vence L, Savary C, Kentor D, Pettaway C, Babaian R, Pisters L, Miles B, Wei Q, Wiltz L, Patel T & Radvanyi L. Presurgical stress management improves postoperative immune function in men with prostate cancer undergoing radical prostatectomy. Psychosomatic Medicine. 2011; 73: 218–225. [DOI] [PubMed] [Google Scholar]
- 145.Wang A, Bouchard L, Gudenkauf L, Jutagir D, Fisher H, Jacobs J, Blomberg B, Lechner S, Carver CS, & Antoni MH. Differential Psychological Effects of Cognitive-Behavioral Stress Management among Breast Cancer Patients with High and Low Initial Cancer-Specific Distress. J Psychosom Res, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Schneider S, Moyer A, Knapp-Oliver S, Sohl S, Cannella D, Targhetta V. Pre-intervention distress moderates the efficacy of psychosocial treatment for cancer patients: a meta-analysis. Journal of Behavioral Medicine. 2010;33(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]