Abstract
Background:
The preclinical literature identifies the ventral striatum (VS) as a key player in drug-conditioned responses, guiding hypotheses examining neural substrates involved in human drug cue reactivity, including the study of sex differences. Men show a replicable response that includes the VS, while women’s responses have been weaker and variable. New evidence suggests that the hormonal milieu modulates women’s responses to drug cues in the dorsal striatum (DS), specifically, in the putamen. Here we tested the hypothesis that the hormonal milieu affects neural responses to smoking cues (SCs) in the putamen in women cigarette smokers.
Methods:
We re-examined our three previous neuroimaging studies of the influence of sex and menstrual cycle (MC) phase effects on SC neuroactivity, incorporating the DS as a region of interest.
Results:
As previously shown, men exhibited increased ventral medial prefrontal cortex (vmPFC) and VS/V pallidum responses, and women showed increased vmPFC responses that were greater in women during the follicular phase (high estradiol), compared to the luteal phase (high progesterone). Reducing the statistical threshold within luteal phase women revealed select deactivation of the putamen.
Conclusions:
These preliminary findings shed light upon factors that may modulate drug cue reactivity in women, specifically the influence of hormones on DS responses. Emerging literature suggests that manipulating the hormonal milieu may open a fundamental window into sex-specific treatment targets. More rigorous study of the brain substrates involved in drug cue reactivity and other reward-related behavior that may be influenced by sex and the hormonal milieu is imperative.
Keywords: Cigarette Smoking, Sex Differences, Estradiol, Progesterone Striatum, fMRI
1. Introduction
Smoking a cigarette and the cues associated with smoking, such as the smell of a lit cigarette, the feel of the smoke entering the lungs, or the sight of a favored brand of cigarettes, are rewarding to individuals who smoke. Exposure to smoking-related cues (SCs), days, months or even years after quitting smoking can lead to SC-induced craving and relapse (Sinha and Li, 2007). As such, the addiction field has studied SC-induced craving extensively, including the study of individual variability in the effects of SC exposure on relevant endpoints, such as craving and physiological and neurophysiological responses. Given its potential relevance to relapse and treatment strategies, variability conferred by sex differences has been one such area of study.
One method of examining SC-induced responses is through the use of neuroimaging during SC exposure. Our group has previously reported on sex differences in response to SC exposure using perfusion functional magnetic resonance imaging (fMRI). We reported that both men and women with nicotine use disorder (NUD) show increased responses to SCs compared to non-SCs, with men exhibiting increased ventral medial prefrontal cortex and ventral striatum/ventral pallidum responses, and women showing increased ventral medial prefrontal cortex responses (Wetherill et al., 2013). Subsequently, using the same methodology in a new cohort, we observed similar results within men who showed greater responses to SCs in the ventral medial prefrontal cortex, ventral striatum/ventral pallidum, the ventral anterior insula, and the parahippocampus (Dumais et al., 2017). These results are in agreement with our original study of SC reactivity independent of withdrawal (Franklin et al., 2007). However, in the Dumais et al. study, we did not observe a SC-elicited brain response in women, which is inconsistent with our prior study and other studies showing heightened SC-induced brain reactivity in women (Janes et al., 2010, 2009; Wetherill et al., 2013).
As our group has long been interested in the effects of hormonal fluctuations on SC-induced craving and relapse (Franklin et al., 2015, 2008; Franklin and Allen, 2009; Wetherill et al., 2014), we hypothesize that the variability in SC response in women may be related to the influence of gonadal sex hormones (i.e., estradiol and progesterone) that fluctuate over the course of the natural MC. Given that estradiol enhances rewarding behavior and progesterone may protect against it (Becker and Hu, 2008; Fattore et al., 2008), we hypothesize that hormonal variation among participants in our two prior studies (Dumais et al., 2017; Wetherill et al., 2013) masked our ability to replicate or see strong reward-related effects in women. In our studies and those of others (Janes et al., 2010, 2009), the composition of the hormonal status of the female participants was heterogenous, consisting of women who were naturally cycling and in different phases of their MC, taking exogenous hormones (i.e., birth control), or were peri- or post-menopausal. Such heterogeneity could result in reduced ability to see a SC-induced reward response and/or cause inconsistencies across studies.
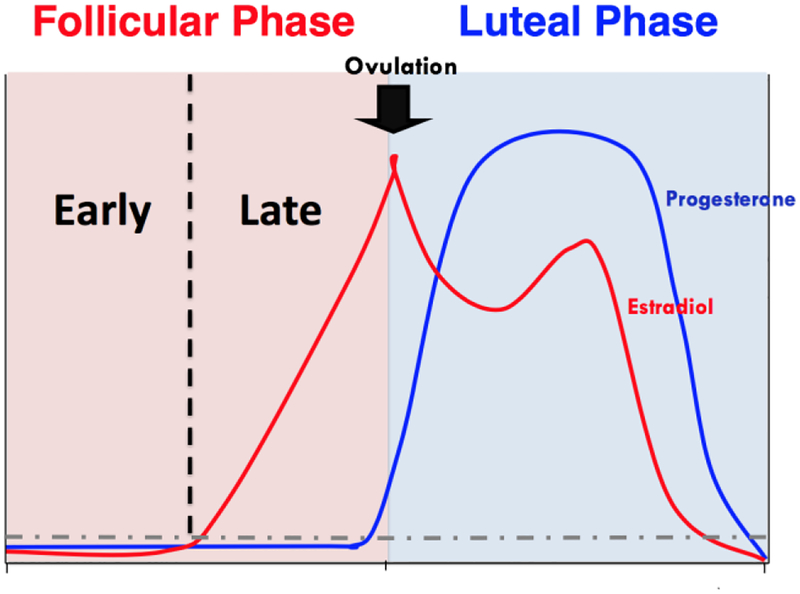
To probe our hypothesis of hormonal status effects on SC reactivity in women, we conducted another study comprising only women who were premenopausal and naturally cycling. The first half of the MC, the follicular phase, is dominated by a rapid increase in the level of estradiol, whereas the second half of the MC, the luteal phase, begins following ovulation and is dominated by progesterone (See Figure 1). For this study, women were grouped according to MC phase and participated in procedures consistent with those of the first two sex differences studies (Dumais et al., 2017; Franklin et al., 2015; Wetherill et al., 2013). Women in the follicular phase (FPs) showed increased neural responses to SCs compared to non-SCs in the ventral medial prefrontal cortex, whereas responses within luteal phase women (LPs) were nonexistent. Furthermore, FPs showed SC-elicited craving (i.e., greater craving following SC exposure) that was correlated with neural response in the ventral anterior insula, a known craving substrate (Droutman et al., 2015), whereas LPs did not report SC-elicited craving or show correlations (Franklin et al., 2015). These findings were somewhat consistent with our hypothesis that FPs would show greater SC-elicited responses; however, FPs did not show greater effects in the ventral striatum (i.e., the nucleus accumbens), a region clearly established within the preclinical literature as a critical reward substrate within the dopaminergic mesolimbic pathway (Corrigall et al., 1994; Laviolette et al., 2002). These preliminary findings were not without limitations. First, this study was retrospective; women were not recruited and randomized by MC phase, potentially resulting in selection bias. Second, MC phase was used as a crude proxy of hormonal status, without knowledge of whether ovulation actually occurred in the participants (Harlow and Ephross, 1995). And third, as depicted in Figure 1, hormonal variability exists in both of the major MC phases, diminishing the ability to observe the potential rewarding effects of high levels of estradiol (Di Paolo et al., 1985; Fattore et al., 2008; Lynch et al., 2002; Thompson and Moss, 1994) or protective effects of high levels of progesterone (Becker and Hu, 2008; DeVito et al., 2014; Schiller et al., 2012; Sofuoglu et al., 2001). Although it was not within our means to address the limitations of these novel and intriguing findings, recent advancements within the field revealed a fourth limitation that we could address.
Figure 1.
Diagram depicting the fluctuations of estradiol and progesterone across an idealized human menstrual cycle
Our original a priori hypothesis of the brain regions involved in SC reactivity was based on a large body of literature conducted over several decades that was generally conducted in males (Childress et al., 1999; Galvan et al., 2005; Robinson and Berridge, 1993; Wilson et al., 2005; Wise, 1988). Our regions of interest (ROIs) included the ventral striatum and other interconnected limbic circuitry; however, recent work by Cummings and colleagues (2014) suggests that we overlooked a key region that may be especially important in women. Specifically, Cummings et al. showed that 1) in female rats, estradiol escalates drug-related behavior including drug-induced sensitization of stereotypy and locomotion with no effect on male rats; and 2) estradiol enhances dopamine release in the dorsal but not ventral striatum in female rats with no effects in male rats in either ventral or dorsal striatum. The findings from this carefully conducted study suggest that the dorsal striatum, specifically the putamen, is a key player in drug reward in females and that it is heavily modulated by the hormonal milieu (Cummings et al., 2014). Contemporaneously, a human positron emission tomography (PET) study was published with findings that aligned well with Cummings et al. (2014). Cosgrove and colleagues (2014) showed that when cigarette smoking individuals smoke a cigarette during PET imaging, dopamine is released within the ventral striatum of male smokers, while in women, dopamine release is more rapid and is observed only in the putamen (Cosgrove et al., 2014). These two recent papers are not the first studies to observe an effect of conditioned drug cues in the dorsal striatum (Boileau et al., 2007; Redish et al., 2008; Volkow et al., 2006), yet the majority of studies consistently point to the ventral striatum. Findings from these two recent studies begin to tease apart the influence of sex and hormones on individual variability in responses and led us to test the hypothesis that SCs will preferentially activate the putamen in women and that the response will be modulated by the hormonal milieu. Thus, the current study addresses the fourth limitation of our three earlier studies by re-analyzing the previously acquired data and including the putamen as an a priori ROI to explore a potential mechanistic basis for the variable and weak responses we have observed previously in SC reactivity within women. In conjunction with the emerging literature described above, this exploration may aid in identifying previously unexplored treatment targets for women who are vulnerable to cue-induced relapse by providing a framework upon which to build future study.
2. Methods
The study participants and detailed methods are described in their respective published works (Dumais et al., 2017; Franklin et al., 2015; Wetherill et al., 2013). Briefly, sated cigarette-dependent individuals underwent pseudo-continuous arterial spin-labeled (pCASL) perfusion fMRI during exposure to audio/visual clips consisting of highly appetitive SCs and carefully matched non-SCs. The SC video included individuals differing in race, age, and sex, who were smoking and using language explicitly designed to induce appetitive desire for a cigarette. The non-SC video was similar in content; however, the video did not portray cigarette smoking or smoking reminders.
Analyses were conducted in SPM (The FIL Methods Group, 2016) using an arterial spin labeling (ASL) data processing toolbox (Wang et al., 2005) for pCASL perfusion data analyses, as described previously (Dumais et al., 2017; Franklin et al., 2015, 2007; Wetherill et al., 2013). Contrasts between SC versus non-SC sets were defined in the general linear model to assess the voxel by voxel CBF difference for each subject. Using the corresponding parametric maps of the contrast, random effects analysis was employed to test for a main effect of condition (SC versus non-SC) in each sex or group, with a statistical parametric map of the t-statistic at each voxel for population inference within the ROI mask.
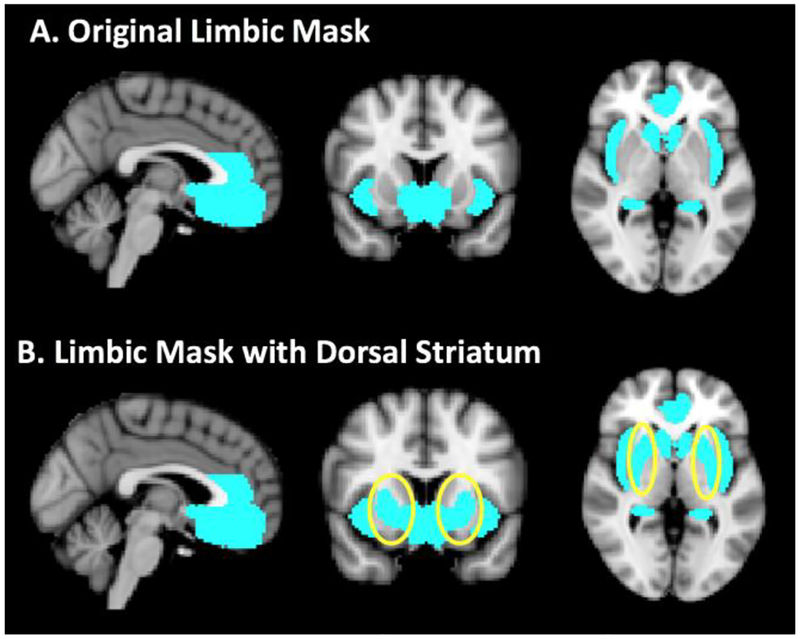
Based on a priori hypotheses generated from our previous studies on SC-reactivity among NUDs (Dumais et al., 2017; Franklin et al., 2015, 2007; Wetherill et al., 2013), the ROI mask included the ventral medial prefrontal cortex, ventral striatum/ventral pallidum, hippocampus, extended amygdala (i.e., amygdala; bed nucleus of stria terminalis), supra- and sub-genual anterior cingulate cortex, and insula. For the current retrospective re-analyses, the dorsal striatum was added to the mask (See Figure 2). The ROI mask was created using the Harvard–Oxford probabilistic anatomical atlas provided with FMRIB Software Library (FSL) (Smith et al., 2004). Significance level was set at p < 0.005, cluster size ≥ 20.
Figure 2.
Shown in A. are sagittal, coronal and axial images of the original limbic mask used in Wetherill et al, 2013, Dumais et al, 2017 and Franklin et al., 2015 which can be viewed in its entirety at http://franklinbrainimaging.com/mj%2010_13/Mask_axial.html. B. depicts the mask used in the current study that includes the dorsal striatum (circled).
3. Results
Data Set 1:
Wetherill et al., The impact of sex on brain responses to smoking cues: A perfusion fMRI study (Wetherill et al., 2013). Data Set 1 remained unchanged by the addition of the dorsal striatum to the ROI mask. Both men and women showed increased responses to SCs compared to non-SCs, with men exhibiting increased ventral medial prefrontal cortex and ventral striatal/ventral pallidum responses and women showing increased ventral medial prefrontal cortex responses.
Data Set 2:
Dumais et al., Multi-site exploration of sex differences in brain reactivity to smoking cues: Consensus across sites and methodologies (Dumais et al., 2017). Data Set 2 remained unchanged by the addition of the putamen to the ROI mask. Men had greater brain responses to SCs relative to non-SCs in the ventral medial prefrontal cortex, ventral striatum/pallidum, ventral anterior insula, and the parahippocampus. In women, there were no regions that showed greater responses to SCs relative to non-SCs.
Data Set 3:
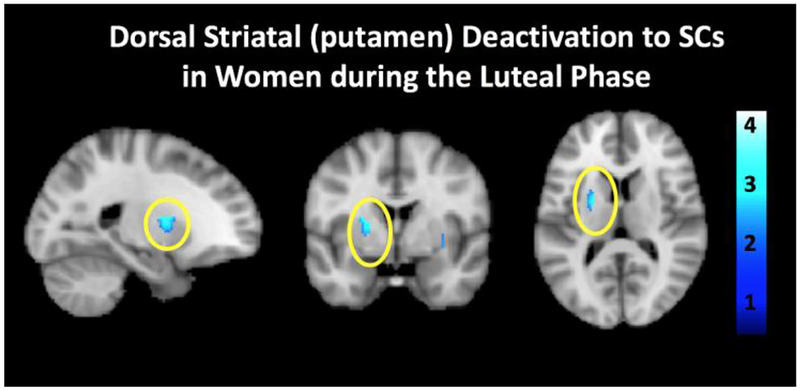
Franklin et al., Influence of menstrual cycle phase on neural and craving responses to appetitive smoking cues in naturally cycling females (Franklin et al., 2015). FPs showed increased neural responses to SCs compared to non-SCs in the ventral medial prefrontal cortex, while LPs had no response in any ROI. Notably, reducing the threshold to p < 0.05 revealed reduced responses to SC compared to nonSCs selectively in the left putamen in LPs (t = 2.77; see Figure 3). Although this finding does not pass the stringent correction that is warranted for neuroimaging studies, it is reported here as fundamental hypothesis-generating information for the field.
Figure 3.
Sagittal, coronal and axial images showing select deactivation of the putamen in luteal phase females (n=15) during exposure to SCs compared to non-SCs (t(12) = 2.77, p = 0.05).
4. Discussion
The purpose of this retrospective study was to re-examine our three previous neuroimaging studies of the influence of sex and/or MC phase differences on SC neuroactivity, incorporating the dorsal striatum into our a priori ROI mask. Our goal was to explore a potential mechanistic basis for the variable and weak responses we have observed in SC reactivity in women, providing a framework upon which to build future study. Based on an emerging literature (Cosgrove et al., 2014; Cummings et al., 2014), we tested the hypothesis that the hormonal milieu will affect the neural response to SCs in the putamen of women. In reanalyzing data from our two previous studies that examined the effects of sex on dorsal striatal neuroactivity, previous results remained unchanged. In the third study, wherein MC phase was used as a proxy for hormonal status in naturally cycling women, reducing the statistical threshold within women who were exposed to SCs during the luteal phase, when progesterone is high, revealed select deactivation of the putamen. These results provide a hint that our previous underwhelming results in women are a result of hormonal variation in our samples and suggest that hormonal influences may exert effects in other studies of drug cue reactivity.
Several lines of evidence throughout the preclinical and clinical literature show that estradiol escalates drug reward-related behavior while progesterone may be protective (Di Paolo et al., 1985; Dluzen and Anderson, 1997; Thompson and Moss, 1994). For example, within the animal literature, estradiol contributes to faster acquisition of drug-seeking behavior, escalation of drug consumption and accelerated drug-primed and drug-cue induced reinstatement, an animal model of relapse (Lynch et al., 2002). In particular, in rats trained to self-administer nicotine, levels of responding on a progressive ratio schedule were inversely correlated with progesterone and positively correlated with the estradiol to progesterone ratio (Lynch, 2008). The human literature on the effects of the hormonal milieu on addiction processes is less clear, as studies are limited by factors such as heavy reliance upon self-report, not randomizing by phase, use of arbitrary phase classifications and/or lack of hormonal verification. However, more recent studies are controlling for such factors (Fattore et al., 2008; Mello, 2010; Sofuoglu et al., 2001). For example, Sofuoglu and colleagues (2001) have demonstrated that exogenous administration of progesterone decreases the positive subjective effects of cigarette smoking and craving (Sofuoglu et al., 2001). Collectively the above-mentioned studies, and others (DeVito et al., 2014; Schiller et al., 2012; Sofuoglu et al., 2002), suggest that estradiol may increase the relative rewarding value of SCs while progesterone may protect against such effects.
It is important to note that this re-examination of previously acquired data is still flawed. Although we have expanded our prior examinations to include the putamen, the other limitations of the original studies remain. As noted previously, all three prior studies were retrospective, potentially introducing selection bias. Second, in both of the sex differences studies, the female group consisted of naturally cycling women at varying time points in their MC, women taking exogenous hormones, and peri- and post-menopausal women. Thus, if the hormonal milieu does exert an influence over SC neuroactivity, it could have been masked by the inclusion of women of variable hormonal status.
The MC study provided some confirmation that hormones exert an effect on SC reactivity. Naturally, cycling women exposed to SCs during the follicular phase showed increases in SC-induced neural responses that correlated with SC-elicited craving, while women in the luteal phase did not. However, given its focus on differences modulated by MC phase, this study was also limited. As previously mentioned, MC phase is a crude proxy of hormonal status. Indeed, approximately one-third of all MCs are anovulatory, and as such, the predicted monthly fluctuations in gonadal hormones are disrupted (Harlow and Ephross, 1995). Furthermore, and most importantly, hormonal variability exists within both the follicular and luteal phases. The follicular phase is characterized by low levels of both estradiol and progesterone in the early part of the phase, and rising levels of estradiol in the later stages of the phase. The luteal phase begins immediately after ovulation and ends at menses, and is characterized by high levels of progesterone in conjunction with a smaller estradiol peak, followed by sharp decreases in both hormones towards the end of the phase (See Figure 1). Thus, the vastly different hormonal milieu that exists within the major MC phases diminishes the ability to observe potential rewarding effects of high levels of estradiol or protective effects of high levels of progesterone. Although this re-analysis does not unequivocally illuminate the dorsal striatum as a key player in women’s drug cue reactivity, it suggests that it could be and that further study is needed while attending to sex and hormonal status.
Using a crude measure of hormonal variability, we observed a progesterone-mediated reduction of dorsal striatal brain activity during SC exposure, albeit weak. Recent findings implicate this region as an important substrate of drug motivation in females, and that it is modulated by the hormonal milieu (Cosgrove et al., 2014; Cummings et al., 2014). The majority of drug addiction research has been thus far conducted mostly in males, and has clearly identified the ventral striatum as the final common pathway of all drugs of abuse, albeit a few studies have revealed a role for the dorsal striatum (Boileau et al., 2007; Redish et al., 2008; Volkow et al., 2006). Perhaps the dorsal striatum, modulated by the hormonal milieu, plays a larger role in females. Thus, we consider these preliminary findings valuable as they contribute to a growing literature that may open a fundamental window into sex-specific treatment targets by providing a framework upon which to build future study. Given the plausible importance of the influence of hormone variation on reward-related behavior and its implications on relapse and sex-specific treatment strategies, more rigorous study of the regions involved in SC reactivity and other reward-related behavior that may be influenced by sex and the hormonal milieu is imperative.
Highlights.
The putamen may play a key role in drug cue reactivity in women.
In women, dorsal striatal activity may be modulated by the hormonal milieu.
Rigorous study of the role of hormones and the dorsal striatum is imperative.
Acknowledgements
None.
Role of Funding Source
This work was supported by the National Institute for Drug Abuse [R01 DA040670]
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
No conflicts declared.
References
- Becker JB, Hu M, 2008. Sex differences in drug abuse. Front. Neuroendocrinol 29, 36–47. 10.1016/j.yfrne.2007.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boileau I, Dagher A, Leyton M, Welfeld K, Booij L, Diksic M, Benkelfat C, 2007. Conditioned dopamine release in humans: a positron emission tomography [11C]raclopride study with amphetamine. J. Neurosci 27, 3998–4003. 10.1523/JNEUROSCI.4370-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O’Brien CP, 1999. Limbic activation during cue-induced cocaine craving. Am. J. Psychiatry 156, 11–18. 10.1176/ajp.156.1.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM, Adamson KL, 1994. Self-administered nicotine activates the mesolimbic dopamine system through the ventral tegmental area. Brain Res 653, 278–284. [DOI] [PubMed] [Google Scholar]
- Cosgrove KP, Wang S, Kim S-J, McGovern E, Nabulsi N, Gao H, Labaree D, Tagare HD, Sullivan JM, Morris ED, 2014. Sex differences in the brain’s dopamine signature of cigarette smoking. J. Neurosci 34, 16851–16855. 10.1523/JNEUROSCI.3661-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings JA, Jagannathan L, Jackson LR, Becker JB, 2014. Sex differences in the effects of estradiol in the nucleus accumbens and striatum on the response to cocaine: Neurochemistry and behavior. Drug Alcohol Depend 135, 22–28. 10.1016/j.drugalcdep.2013.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVito EE, Herman AI, Waters AJ, Valentine GW, Sofuoglu M, 2014. Subjective, physiological, and cognitive responses to intravenous nicotine: Effects of sex and menstrual cycle phase. Neuropsychopharmacology 39, 1431–1440. 10.1038/npp.2013.339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Paolo T, Rouillard C, Bédard P, 1985. 17 beta-Estradiol at a physiological dose acutely increases dopamine turnover in rat brain. Eur. J. Pharmacol 117, 197–203. [DOI] [PubMed] [Google Scholar]
- Dluzen DE, Anderson LI, 1997. Estrogen differentially modulates nicotine-evoked dopamine release from the striatum of male and female rats. Neurosci. Lett 230, 140–142. 10.1016/S0304-3940(97)00487-4 [DOI] [PubMed] [Google Scholar]
- Droutman V, Read SJ, Bechara A, 2015. Revisiting the role of the insula in addiction. Trends Cogn. Sci 19, 414–420. 10.1016/j.tics.2015.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumais KM, Franklin TR, Jagannathan K, Hager N, Gawrysiak M, Betts J, Farmer S, Guthier E, Pater H, Janes AC, Wetherill RR, 2017. Multi-site exploration of sex differences in brain reactivity to smoking cues: Consensus across sites and methodologies. Drug Alcohol Depend 178, 469–476. 10.1016/j.drugalcdep.2017.05.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattore L, Altea S, Fratta W, 2008. Sex differences in drug addiction: a review of animal and human studies. Womens Health (Lond) 4, 51–65. 10.2217/17455057.4.1.51 [DOI] [PubMed] [Google Scholar]
- Franklin TR, Allen SS, 2009. Influence of menstrual cycle phase on smoking cessation treatment outcome: A hypothesis regarding the discordant findings in the literature. Addiction 104, 1941–1942. 10.1111/j.1360-0443.2009.02758.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TR, Ehrman R, Lynch KG, Harper D, Sciortino N, O’Brien CP, Childress AR, 2008. Menstrual cycle phase at quit date predicts smoking status in an NRT treatment trial: A retrospective analysis. J. Womens Health (Larchmt) 17, 287–292. 10.1089/jwh.2007.0423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TR, Jagannathan K, Wetherill RR, Johnson B, Kelly S, Langguth J, Mumma J, Childress AR, 2015. Influence of menstrual cycle phase on neural and craving responses to appetitive smoking cues in naturally cycling females. Nicotine Tob. Res 17, 390–397. 10.1093/ntr/ntu183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TR, Wang Z, Wang J, Sciortino N, Harper D, Li Y, Ehrman R, Kampman K, O’Brien CP, Detre JA, Childress AR, 2007. Limbic activation to cigarette smoking cues independent of nicotine withdrawal: A perfusion fMRI study. Neuropsychopharmacology 32, 2301–2309. 10.1038/sj.npp.1301371 [DOI] [PubMed] [Google Scholar]
- Galvan A, Hare TA, Davidson M, Spicer J, Glover G, Casey BJ, 2005. The role of ventral frontostriatal circuitry in reward-based learning in humans. J. Neurosci 25, 8650–8656. 10.1523/JNEUROSCI.2431-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow SD, Ephross SA, 1995. Epidemiology of menstruation and its relevance to women’s health. Epidemiol. Rev 17, 265–286. [DOI] [PubMed] [Google Scholar]
- Janes AC, Frederick B, deB Richardt S, Burbridge C, Merlo-Pich E, Renshaw PF, Evins AE, Fava M, Kaufman MJ, 2009. Brain fMRI reactivity 6to smoking-related images before and during extended smoking abstinence. Exp. Clin. Psychopharmacol 17, 365–373. 10.1037/a0017797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes AC, Pizzagalli DA, Richardt S, deB Frederick B, Chuzi S, Pachas G, Culhane MA, Holmes AJ, Fava M, Evins AE, Kaufman MJ, 2010. Brain reactivity to smoking cues prior to smoking cessation predicts ability to maintain tobacco abstinence. Biol. Psychiatry 67, 722–729. 10.1016/j.biopsych.2009.12.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviolette SR, Alexson TO, van der Kooy D, 2002. Lesions of the tegmental pedunculopontine nucleus block the rewarding effects and reveal the aversive effects of nicotine in the ventral tegmental area. J. Neurosci 22, 8653–8660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, 2008. Acquisition and maintenance of cocaine self-administration in adolescent rats: effects of sex and gonadal hormones. Psychopharmacology (Berl.) 197, 237–246. 10.1007/s00213-007-1028-0 [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Carroll ME, 2002. Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology (Berl.) 164, 121–137. 10.1007/s00213-002-1183-2 [DOI] [PubMed] [Google Scholar]
- Mello NK, 2010. Hormones, nicotine, and cocaine: clinical studies. Horm. Behav 58, 57–71. 10.1016/j.yhbeh.2009.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redish AD, Jensen S, Johnson A, 2008. A unified framework for addiction: Vulnerabilities in the decision process. Behav. Brain. Sci 31, 415–437; discussion 437–487. 10.1017/S0140525X0800472X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC, 1993. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res. Brain Res. Rev 18, 247–291. [DOI] [PubMed] [Google Scholar]
- Schiller CE, Saladin ME, Gray KM, Hartwell KJ, Carpenter MJ, 2012. Association between ovarian hormones and smoking behavior in women. Exp. Clin. Psychopharmacol 20, 251–257. 10.1037/a0027759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Li CSR, 2007. Imaging stress- and cue-induced drug and alcohol craving: Association with relapse and clinical implications. Drug Alcohol Rev 26, 25–31. 10.1080/09595230601036960 [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM, 2004. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23 Suppl 1, S208–219. 10.1016/j.neuroimage.2004.07.051 [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Babb DA, Hatsukami DK, 2001. Progesterone treatment during the early follicular phase of the menstrual cycle: Effects on smoking behavior in women. Pharmacol. Biochem. Behav 69, 299–304. [DOI] [PubMed] [Google Scholar]
- The FIL Methods Group, 2016. Statistical Parametric Mapping 12. Functional Imaging Laboratory Wellcome Trust Centre for Neuroimaging Institute of Neurology, UCL 12 Queen Square, London WC1N 3BG, UK https://www.fil.ion.ucl.ac.uk/spm/software/spm12/
- Thompson TL, Moss RL, 1994. Estrogen regulation of dopamine release in the nucleus accumbens: genomic- and nongenomic-mediated effects. J. Neurochem 62, 1750–1756. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Telang F, Fowler JS, Logan J, Childress A-R, Jayne M, Ma Y, Wong C, 2006. Cocaine cues and dopamine in dorsal striatum: Mechanism of craving in cocaine addiction. J. Neurosci 26, 6583–6588. 10.1523/JNEUROSCI.1544-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Zhang Y, Wolf RL, Roc AC, Alsop DC, Detre JA, 2005. Amplitude-modulated continuous arterial spin-labeling 3.0-T perfusion MR imaging with a single coil: Feasibility study. Radiology 235, 218–228. 10.1148/radiol.2351031663 [DOI] [PubMed] [Google Scholar]
- Wetherill RR, Jagannathan K, Shin J, Franklin TR, 2014. Sex differences in resting state neural networks of nicotine-dependent cigarette smokers. Addict. Behav 39, 789–792. 10.1016/j.addbeh.2014.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherill RR, Young KA, Jagannathan K, Shin J, O’Brien CP, Childress AR, Franklin TR, 2013. The impact of sex on brain responses to smoking cues: A perfusion fMRI study. Biol. Sex Differ 4, 9 10.1186/2042-6410-4-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SJ, Sayette MA, Delgado MR, Fiez JA, 2005. Instructed smoking expectancy modulates cue-elicited neural activity: A preliminary study. Nicotine Tob. Res 7, 637–645. 10.1080/14622200500185520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, 1988. The neurobiology of craving: Implications for the understanding and treatment of addiction. J. Abnorm. Psychol 97, 118–132. [DOI] [PubMed] [Google Scholar]