Abstract
Background & Purpose:
Post-stroke fatigue (PSF) is rife among stroke survivors and it exerts a detrimental toll on recovery from functional deficits. The burden of PSF is unknown in sub-Saharan Africa. We have assessed the prevalence, trajectory and predictors of PSF among 60 recent Ghanaian stroke patients.
Methods:
Study participants in this prospective cohort (recruited between January/2017 and June/2017) were stroke survivors, aged >18 years, with CT scan confirmed stroke of less than 1-month onset. PSF was assessed using the Fatigue Severity Scale (FSS) at enrollment, months 3, 6, and 9. Those with a score of ≥4 points on FSS were categorized as “fatigued”. A multivariate logistic regression analysis was performed to identify independent predictors of PSF at enrollment and at month 9.
Results:
Sixty-five percent (65%) of our sample were males with a mean age of 55.1 ± 12.7 years. In addition to all participants having hypertension, 85% had dyslipidemia and 25% had diabetes mellitus. Ischemic strokes comprised 76.6% of the study population. The prevalence of PSF was 58.9% at baseline and declined to 23.6% at month 9, p=0.0002. Diabetes mellitus was significantly associated with PSF at baseline with an adjusted odds ratio of 15.12 (95% CI: 1.70 – 134.30), p=0.01. However, at month 9, age ≥65 years, aOR of 7.02 (95% CI: 1.16 – 42.52); female sex, aOR of 8.52 (1.23 – 59.16) and depression, aOR of 8.86 (1.19 – 65.88) were independently associated with PSF.
Conclusion:
Approximately 6 out of 10 Ghanaian stroke survivors experience PSF within the first month of stroke onset. PSF persists in approximately 1 out of 4 stroke survivors at 10 months after the index stroke. Further studies to elucidate the underlying mechanisms for PSF are required and adequately powered interventional multi-center trials are eagerly awaited to provide solid evidence base for the clinical management of PSF.
Keywords: Post-stroke Fatigue, Ghana, Risk factors, Depression
INTRODUCTION
Post-stroke fatigue (PSF) is a major enduring and disabling complaint after stroke with detrimental impacts on quality of life, rehabilitation, work capacity, suicide risk, and all-cause mortality.1–7 PSF is variously described as a sense of early exhaustion with weariness, lack of energy and an aversion to effort8 involving physical, emotional and cognitive experiences9 and is usually not relieved by rest.10 The prevalence of PSF has varied between 23 and 75% from various reports.11 Factors associated with PSF include female sex, older age, presence of neurological deficits, sleep disturbances, use of medications, depression, cognitive dysfunction, pre-stroke fatigue, family dysfunction, and location of strokes.11–15 A meta-analysis of randomized controlled trials of interventions for PSF found insufficient evidence for five pharmacological interventions: fluoxetine, enerion, (−)-OSU6162, citicoline, and a combination of Chinese herbs, and two non-pharmacological interventions namely a fatigue education program and a mindfulness-based stress reduction program to prevent or treat PSF.16
The burden of post-stroke fatigue is unknown on the African continent. However, similar to other Low-and-Middle Income countries (LMICs) on the globe, the burden of stroke in sub-Saharan Africa (SSA) is rising 18–23 although recent estimates suggest higher lifetime risk of stroke and stroke related mortality in East and central Asia.24,25 Stroke in SSA characteristically affects a younger age group and is associated with significant post-stroke mortality and morbidity including depression, cognitive impairment, and social stigma.26–36 Post-stroke rehabilitation in the region is severely challenged due to limited availability of physiotherapists and the high cost of rehabilitation services.37–40 In view of the aforementioned features of stroke in SSA, we postulated that PSF may be prevalent in the region. We therefore sought to assess and describe the frequency, the trajectory and the factors associated with PSF among a prospective cohort of stroke survivors.
METHODS
Study design and setting:
This study is a secondary analysis of a cohort of 60 recent Ghanaian stroke survivors involved a pilot randomized trial assessing the feasibility of using a mobile health (mHealth) intervention under nurse guidance to improve blood pressure (BP) control.41 Our study was approved by the Committee on Human Research Publication and Ethics (CHRPE) of the School of Medical Sciences, Kwame Nkrumah University of Science and Technology, in Kumasi, Ghana, as well as the Institutional Review Board at the Medical University of South Carolina. The study was conducted at the Neurology Clinic of the Komfo Anokye Teaching Hospital, a tertiary medical center in Kumasi, Ghana. Kumasi is the second largest city in Ghana with an estimated population of 4 million inhabitants. The Neurology clinic was instituted in 2011 and currently runs once a week providing care for adults >16 years with neurologic disorders from 6 out of the 10 administrative regions of Ghana and serves an estimated population of 10 million.42 The protocol and baseline characteristics of study participants have been previously published elsewhere.41,43,44 Prospective assessments for PSF were conducted at baseline, months 3, 6 and 9.
Evaluation of study participants:
We collected demographic information including age, gender, educational and occupational status as well as location of residence. Vascular risk factor profile was assessed among stroke survivors based on self-report, use of relevant medications and review of medical records for evidence of hypertension, diabetes mellitus, dyslipidemia, atrial fibrillation or other cardiac disorders, and history of cigarette smoking and alcohol use. The following criteria were used to assess vascular risk factor status and post-stroke fatigue.
PSF was assessed using the Fatigue Severity Scale (FSS), one of the most widely used instruments in stroke studies45,46 with good validity and reliability.47,48 FSS evaluates the influence of fatigue on daily life and contains nine items, each with a score ranging from 1 (completely disagree) to 7 (completely agree) on a Likert scale. The average score of the nine item responses was calculated for each participant. Participants with a score of ≥4 points were categorized as “fatigued” with higher scores indicating more severe fatigue.
Participant weight was measured in kilograms using a scale with patient standing at the anatomical position on a scale, and height measured in centimeters using a stadiometer with patient standing at the anatomical position in front of the stadiometer. The weight and height measurements were used to calculate the body mass index (BMI) at enrollment.
Blood pressure was measured thrice on the upper left arm using a validated automatic sphygmomanometer, after at least 5 minutes of rest and the second and third readings were averaged for analysis. Hypertension was diagnosed if the patient was on antihypertensive medications over the last 15 consecutive days or if the patient had a systolic and/or diastolic blood pressure of ≥140 / 90 mmHg.
Participants were considered to have diabetes mellitus if they were on hypoglycemic medications or if their fasting blood glucose levels were > 126mg/dl and/or HbA1C >6.5%.
Dyslipidemia was defined as a high total cholesterol > 200mg/dl or LDL-cholesterol > 130mg/dl, triglyceride > 150mg/dl or HDL-cholesterol <40mg/dl for women and <50mg/dl for men or previous use of statin for dyslipidemia.
Current smoking status and alcohol intake status were ascertained from either the patient self-report or report from a reliable relative. A high alcohol intake was defined as ≥ 14 units per week for women, and ≥ 21 units per week for men.
Stroke type was defined radiologically into ischemic and hemorrhagic based on cranial CT scan done at onset of stroke symptoms for all study participants. Stroke severity was assessed using National Institute of Health Stroke Scale (NIHSS) 49, and functional status was assessed using the Barthels’ Activities of Daily Living.50 The Hamilton Depression Rating Scale51 was used to assess risk of depression. Data were obtained by two trained Research Assistants by reviewing medical charts, and interviewing stroke survivors and/or their proxies where applicable.
Statistical Analysis
Means and medians were compared using the Student’s t-test or Mann-Whitney’s U-test for paired comparisons. Proportions were compared using the Chi-squared test with Yates correction for proportions with subgroupings <5. Pearson’s correlation coefficient was calculated for two continuous variables. A multivariate logistic regression analysis was performed to identify independent predictors of PSF at enrollment and at month 9. The putative factors included in the multivariable logistic regression were carefully selected on the basis of literature review and empiric evidence from our data (significant associations observed in bivariate analyses). In all analysis, two-tailed p-values <0.05 were considered statistically significant with no adjustments for multiple comparisons. Descriptive statistics were used for trajectory of PSF. Statistical analysis was performed using SPSS version 19 and GraphPad Prism version 7.
RESULTS
Demographic and clinical characteristics of study participants:
We recruited 60 recent stroke survivors with hypertension within 1 month of onset of stroke with a mean age of 55.1 ± 12.7 years, of which 65% were males. Ischemic strokes constituted 76.6% of primary stroke types. In addition to all participants having hypertension, 85% had dyslipidemia and 25% had diabetes mellitus. (see STROBE statement).
Prevalence of PSF at baseline:
The mean averaged score on the Fatigue Severity Scale (FSS) at baseline was 4.2 ± 1.1. The distribution of FSS scores as depicted in Figure 1 shows a rightward shift towards more severe fatigue symptoms for the prospective cohort recruited within 1 month after stroke (Figure 1). The prevalence of PSF was 58.9% (95% CI: 45.9 – 70.8%). Table 1 shows a comparison of demographic and clinical characteristics of the study population according to Post-stroke Fatigue status. Demographic and clinical characteristics were comparable between the two groups with the following notable exceptions. First, there was a preponderance of diabetes mellitus among those with PSF at baseline of 38.9% compared with 4.2% among those without PSF, p=0.002. Secondly, the mean systolic blood pressure among participants with PSF of 137.3 ± 27.8 mmHg was significantly lower than 153.7 ± 21.8 mmHg among those without PSF, p = 0.02. Thirdly, statin therapy were more likely to have been initiated post-stroke among those without PSF, 87.5% versus 61.1% among those with PSF at baseline, p=0.03. (Table 1)
Figure 1.
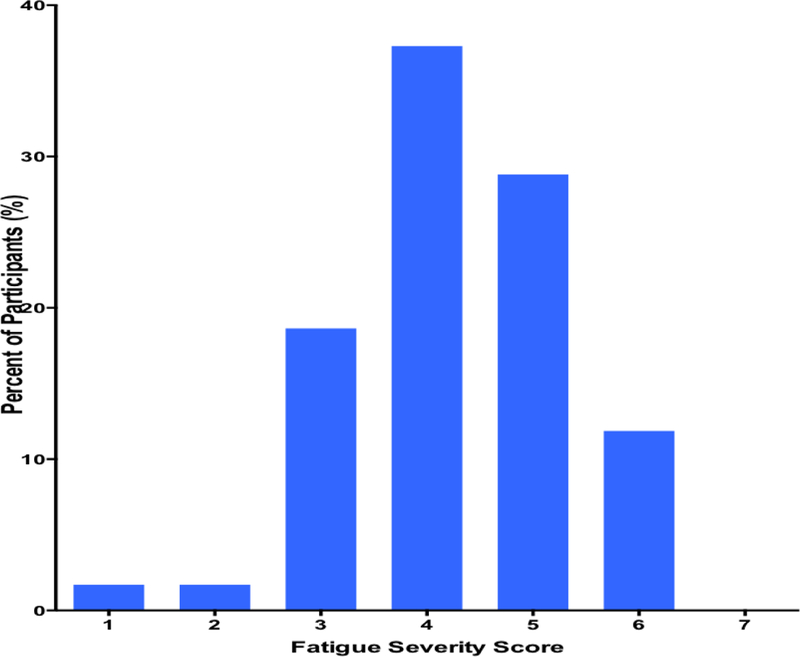
Distribution of Fatigue severity scores among Ghanaian stroke patients
Table 1.
Demographic and Clinical Characteristics of Study Participants according to Fatigue Severity Score
| Characteristic | Fatigue Severity Score <4 n = 24 | Fatigue Severity Score ≥4 n = 36 | Total n = 60 | P-value |
|---|---|---|---|---|
| Age, mean ± SD | 56.3 ± 13.5 | 54.3 ± 12.3 | 55.1 ± 12.7 | 0.56 |
| Females, n (%) | 8 (33.3) | 13 (36.1) | 21 (35.0) | 0.83 |
| Location of residence, n (%) | 0.29 | |||
| urban | 16 (66.7) | 19 (52.8) | 35 (58.3) | |
| semi-urban/rural | 8 (33.3) | 17 (47.2) | 25 (41.7) | |
| Educational status, n (%) | 0.07 | |||
| None | 0 (0.0) | 3 (8.3) | 3 (5.0) | |
| Primary | 8 (33.4) | 20 (55.6) | 28 (46.7) | |
| Secondary | 11 (45.8) | 7 (19.4) | 18 (30.0) | |
| Tertiary | 5 (20.8) | 6 (16.7) | 11 (18.3) | |
| Stroke type | 0.80 | |||
| Ischemic | 18 (75.0) | 28 (77.8) | 46 (76.7) | |
| Hemorrhagic | 6 (25.0) | 8 (22.2) | 14 (23.3) | |
| Diabetes Mellitus, n (%) | 1 (4.2) | 14 (38.9) | 15 (25.0) | 0.002** |
| Systolic BP, mean ± SD | 153.7 ± 21.8 | 137.3 ± 27.8 | 143.8 ± 26.6 | 0.02 |
| Diastolic BP, mean ± SD | 94.8 ± 13.4 | 87.7 ± 16.4 | 90.5 ± 15.5 | 0.08 |
| Waist circumference, mean ± SD | 93.3 ± 12.4 | 91.0 ± 11.6 | 91.9 ± 11.9 | 0.48 |
| Body Mass Index, mean ± SD | 27.7 ± 5.2 | 26.2 ± 5.6 | 26.8 ± 5.5 | 0.33 |
| Barthels Index, mean ± SD | 75.4 ± 22.6 | 78.3 ± 24.1 | 77.2 ± 23.4 | 0.64 |
| NIHSS Score, mean ± SD | 4.7 ± 2.8 | 4.6 ± 3.5 | 4.7 ± 3.2 | 0.94 |
| HDRS Score, mean ± SD | 10.7 ± 5.8 | 10.9 ± 5.7 | 10.9 ± 5.7 | 0.90 |
| # Antihypertensive medications, mean ± SD | 2.9 ± 1.0 | 2.4 ± 0.9 | 2.6 ± 1.0 | 0.09 |
| Methyldopa, n (%) | 4 (16.7) | 11 (30.6) | 15 (25.0) | 0.22 |
| Angiotensin Receptor Blocker, n (%) | 14 (58.3) | 19 (52.8) | 33 (55.0) | 0.67 |
| Fluoxetine, n (%) | 9 (37.5) | 16 (44.4) | 25 (41.7) | 0.59 |
| Statins, n (%) | 21 (87.5) | 22 (61.1) | 43 (71.7) | 0.03* |
| Antiplatelets, n (%) | 15 (62.5) | 16 (44.4) | 31 (51.7) | 0.17 |
| Multivitamins, n (%) | 3 (12.5) | 8 (22.2) | 11 (18.3) | 0.34 |
Trajectory of PSF:
Mean scores of FSS declined from 37.4 ± 9.3 at baseline, to 33.0 ± 8.0 at month 3, 33.8 ± 7.5 at month 6 and to 30.5 ± 9.8 at month 9, p = 0.0005. Scores on the Hamilton’s Depression Rating Scale (HDRS) declined significantly at the same time points (Figure 2A). The slope of decline in FSS scores over time among those with low risk score for depression, HDRS (0–7) at baseline compared with those with HDRS scores >7 at baseline was not significantly different (p-value=0.97, for comparison of two slopes by linear regression). However, at month 9, there was a significantly lower mean FSS score of 24.2 ± 5.4 among those with no depression risk at baseline compared with 32.4 ± 10.2, p=0.01 among those at high risk of depression at baseline (Figure 2B). The trajectory of FSS scores among those on fluoxetine was not significantly divergent from those not on fluoxetine (Figure 2C). The slopes of FSS scores over time among participants with PSF at baseline on fluoxetine compared with those not on fluoxetine were not significantly different, p-value=0.92, for comparison of two slopes by linear regression) (Figure 2D). Overall, the proportion of stroke survivors at baseline with PSF was 33/56 (58.9%) with a significant decline to 13/55 (23.6%) at month 9, p=0.0002. (Figure 3)
Figure 2. Trajectory of Post-stroke fatigue among Ghanaians.
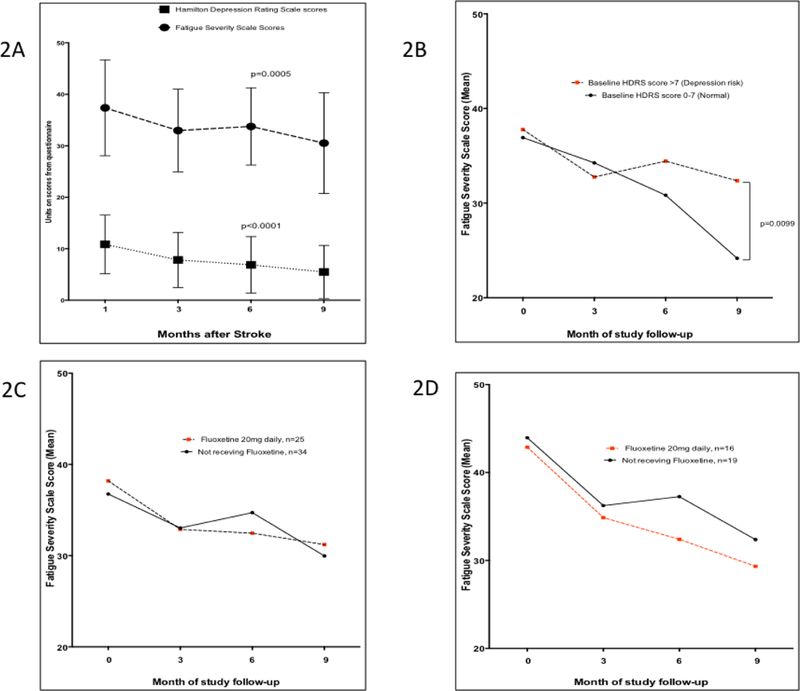
Fig. 2A: Trajectory of post-stroke fatigue scores in relation to Depression scores assessed using the Hamilton Depression Rating Scale (HDRS) over time. Fig. 2B. Trajectory of Fatigue severity scores over time according to baseline depression risk assessed using the Hamilton Depression Rating Scale (HDRS) cut-off of 0–7 (normal) versus >7 (risk of depression). Fig. 2C. Comparison of Trajectories of Fatigue scores among those prescribed Fluoxetine and those not on Fluoxetine at baseline. Fig. 2D. Comparison of trajectory of fatigue severity scores among a subset of patients with Post-stroke Fatigue prescribed Fluoxetine versus those not on Fluoxetine over time. Dots are means and bar represent standard deviations.
Figure 3.
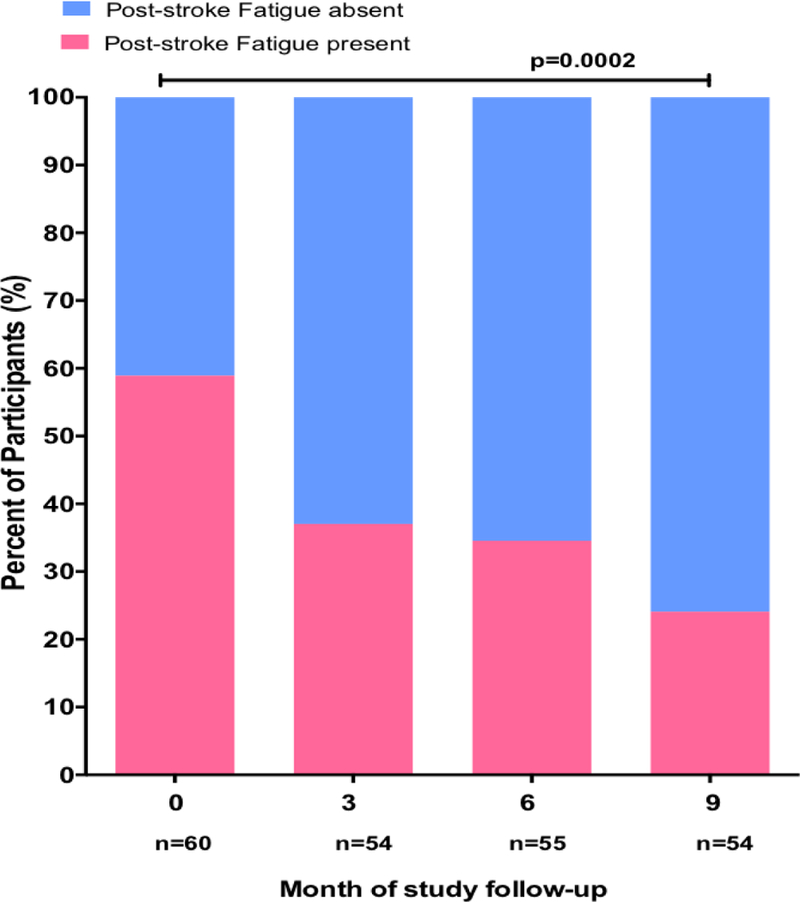
Proportion of study participants with Post-stroke fatigue at enrollment and during follow-up.
Factors associated with PSF at baseline:
In unadjusted analyses, having no or primary level education, having diabetes mellitus, systolic blood pressure at enrollment and use of statin were associated with risk of PSF. Upon adjustment in a multivariate analysis, only diabetes mellitus maintained a significant association with PSF with adjusted odds ratio of 15.12 (95% CI: 1.70 – 134.30), p=0.01, explaining 28% of the variance of the outcome variable. Having no/primary education had aOR of 3.35 (95% CI: 0.94 – 11.97), p=0.06 compared with secondary and tertiary educational attainment and use of statin, aOR of 0.25 (0.05 – 1.13), p=0.07 were not significantly associated with PSF upon adjustment for confounders. (Table 2)
Table 2:
Factors associated with Post-Stroke Fatigue at Baseline
| Predictor | Unadjusted OR (95% CI) | P-value | Adjusted OR (95% CI) | P-value |
|---|---|---|---|---|
| Age ≥65 years | 0.69 (0.21 – 2.25) | 0.54 | - | - |
| Female gender | 1.13 (0.38 − 3.35) | 0.83 | - | - |
| No/Primary education | 3.54 (1.19 − 10.50) | 0.03 | 3.35 (0.94 − 11.97) | 0.06 |
| Secondary education or higher | 1.00 | |||
| Urban residence | 0.59 (0.19 − 1.63) | 0.29 | - | - |
| Rural/semi-urban residence | 1.00 | |||
| Diabetes Mellitus | 14.64 (1.77 − 120.88) | 0.01 | 15.12 (1.70 − 134.30) | 0.01 |
| Body Mass Index, each unit higher | 0.95 (0.87 − 1.05) | 0.33 | - | - |
| Systolic Blood Pressure, each 10mmHg higher | 0.77 (0.61 − 0.97) | 0.02 | 0.97 (0.77 − 1.22) | 0.77 |
| Diastolic Blood Pressure, each 10mmHg higher | 0.74 (0.52 − 1.05) | 0.09 | - | - |
| NIHSS Score >4 | 1.18 (0.42 − 3.33) | 0.75 | - | - |
| Ischemic Stroke | 0.95 (0.30 − 3.00) | 0.93 | - | - |
| Statin use after stroke | 0.22 (0.06 − 0.89) | 0.03 | 0.25 (0.05 − 1.13) | 0.07 |
Factors associated with PSF at month 9 of follow-up:
Four factors were associated with persisting PSF at month 9 in unadjusted analysis, namely age ≥65 years, female gender, Hamilton depression rating score >7 at month 9, and stroke severity at month 9 measured using NIHSS. In adjusted analysis, factors which remained significantly associated with PSF at month 9 were, age ≥65 years, aOR of 7.02 (95% CI: 1.16 – 42.52); female gender, aOR of 8.52 (1.23 – 59.16) and depression, aOR of 8.86 (1.19 – 65.88). (Table 3)
Table 3:
Factors associated with Post-Stroke Fatigue at Month 9
| Predictor | Unadjusted OR (95% CI) | P-value | Adjusted OR (95% CI) | P-value |
|---|---|---|---|---|
| Age ≥65 years | 5.83 (1.50 – 22.71) | 0.01 | 7.02 (1.16 − 42.52) | 0.03 |
| Female gender | 4.00 (1.09 − 14.71) | 0.04 | 8.52 (1.23 − 59.16) | 0.03 |
| HDRS score at month 9 of >7 | 15.17(3.05–75.31) | 0.0009 | 8.86 (1.19 − 65.88) | 0.03 |
| NIHSS score at month 9 of >4 | 12.50 (2.05–76.15) | 0.006 | 5.44 (0.49 − 60.67) | 0.17 |
| Ischemic stroke | 6.00 (0.70 − 51.30) | 0.10 | - | - |
| Hemorrhagic stroke (referent) | 1.00 | |||
| Diabetes mellitus | 3.14 (0.84 − 11.72) | 0.09 | - | - |
| Body Mass Index, each unit higher | 1.06 (0.95 − 1.17) | 0.31 | ||
| Number of antihypertensive medications | 0.66 (0.33 − 1.30) | 0.23 | - | - |
| Fluoxetine use | - | - | ||
| Continuously for 9 months | 1.78 (0.32 − 9.85) | 0.51 | ||
| Started and stopped | 1.58 (0.29 − 8.62) | 0.60 | ||
| Started later | 2.85 (0.48 − 17.10) | 0.25 | ||
| Never used | 1.00 | |||
| Interaction between Fluoxetine use at month 9 and HDRS>7 | 8.67 (1.37 − 54.88) | 0.02 |
DISCUSSION
We have evaluated the burden, trajectory and factors associated with post-stroke fatigue among a sample of 60 stroke survivors in Ghana. At enrollment into the study, we found that approximately 60% of Ghanaian stroke survivors had PSF within one month of incident stroke onset. Among studies that have estimated the prevalence of PSF within 2 to 4 weeks after stroke, the prevalence of PSF in our cohort concurs with 57% from Norway52, 56% from Japan53, 52% from the Netherlands54 but is higher than 40% recorded in a Chinese cohort16 with acute ischemic stroke. Early onset PSF in our cohort was adversely associated with diabetes mellitus while having no or primary educational attainment and use of statins therapy after stroke were moderated into non-significance in adjusted analyses. Diabetes mellitus as a risk factor for PSF among stroke survivors has also been observed among Norwegians who had survived a stroke for at least 6 months.7
There were significant reductions in both the severity and frequency of PSF over the course of follow-up. This notwithstanding, almost a quarter of stroke survivors in the cohort still had post-stroke fatigue approximately 10 months after their incident strokes. Interestingly, the resolution of PSF over time occurred in tandem with regression of post-stroke depression emphasizing the well-established association between post-stroke depression and PSF.1,7,16,52 The downward trajectory of fatigue severity scores over the course of time was steeper among participants with low risk for depression and diverged from those with higher scores on the Hamilton depression rating scale at baseline (Figure 2A) with a significant difference at month 9 between the two groups. Consequently, depression at month 9 was significantly and independently associated with persisting PSF with an adjusted odds ratio of 8.86. It is noteworthy that although no specific interventions were specifically tested to treat PSF among stroke survivors in this study, study physicians prescribed fluoxetine, a selective serotonin reuptake inhibitor, for up to 40% of participants. However, the slopes of decline in FSS scores over time among those with PSF at baseline did not significantly differ by fluoxetine use. Activation of the kynurenine pathway in the acute phase of stroke has been linked with serotonin synthesis which has been shown to play key pathological roles in post-stroke depression and fatigue.55 Hence it has been postulated that treatment for depression with psychostimulants might be useful in the management of PSF. However, no beneficial effects for anti-depressants or psycho-stimulants have been demonstrated on PSF in RCTs performed to date largely due to insufficient power to detect significant differences in these studies.56,57 Perhaps the complex nature of PSF with its admixture of interacting biological, physical, psychological, and behavioral factors may lend it potentially amenable to a combination of interventions including cognitive behavioral and physical training therapies.
A limitation of our study is the relatively small sample size which resulted in wide confidence intervals for adjusted odds ratios. This was a secondary analysis of a pilot clinical trial data with no formal power calculations performed a priori to assess the predictors of PSF. Other key variables known to be associated with PSF such as pre-stroke fatigue, myocardial infarction, and family dysfunction were not assessed in the current study with potential for residual confounding due to these and other unmeasured covariates. We also cannot draw causal associations between PSF and the factors identified in the present study. In spite of these limitations, our study findings contribute to the weight of evidence accruing in support of the salience and burden of PSF globally and within the context of a resource-limited setting such as ours.
In conclusion, 6 in 10 Ghanaian stroke survivors experience PSF within a month of stroke onset with persistence of fatigue in about 1 in 4 at 10 months after incident stroke. Larger scale observational studies are required to elucidate the underlying mechanisms and potential overlaps between PSF and post-stroke depression with the need for adequately powered interventional multi-center trials eagerly awaited to provide solid evidence base for the clinical management of PSF.
Acknowledgements:
We are grateful to Nathaniel Adusei Mensah, Michael Ampofo and Raelle Tagge for help with data collection.
Funding: National Institute of Neurological Disorders & Stroke; R21 NS094033.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interests: None to declare
REFERENCES
- 1.Choi-Kwon S, Han SW, Kwon SU, Kim JS. Poststroke fatigue: characteristics and related factors. Cerebrovasc 2015; 19:84–90. [DOI] [PubMed] [Google Scholar]
- 2.Christensen D, Johnsen SP, Watt T, Harder I, Kirkevold M, et al. Dimensions of post-stroke fatigue: a two-year follow-up study. Cerebrovasc Dis 2008; 26:134–141. [DOI] [PubMed] [Google Scholar]
- 3.Naess H, Nyland HI, Thomassen L, Aarseth J, Myhr KM. Fatigue at long-term follow-up in young adults with cerebral infarction. Cerebrovasc Dis 2005;20:245–250. [DOI] [PubMed] [Google Scholar]
- 4.Duncan F, Wu S, Mead GE. Frequency and natural history of fatigue after stroke: a systematic review of longitudinal studies. J Psychosom Res 2012; 73:18–27. [DOI] [PubMed] [Google Scholar]
- 5.Andersen G, Christensen D, Kirkevold M, Johnsen SP. Post-stroke fatigue and return to work: a 2-year follow-up. Acta Neurol Scand 2012; 125:248–253. [DOI] [PubMed] [Google Scholar]
- 6.Tang WK, Lu JY, Mok V, Ungvari GS, Wong KS. Is fatigue associated with suicidality in stroke? Arch Phys Med Rehabil 2011;92:1336–1338. [DOI] [PubMed] [Google Scholar]
- 7.Naess H, Lunde L, Brogger J, Waje-Andreassen U. Fatigue among stroke patients on long-term follow-up. The Bergen Stroke Study. J Neurol Sci 2012;138–141. [DOI] [PubMed]
- 8.Staub F, Bougousslavsky J. Fatigue after stroke: a major but neglected issue. Cerebrovasc Dis 2001; 12:75–81. [DOI] [PubMed] [Google Scholar]
- 9.Annoni JM, Staub F, Bougousslavsky J, Brioschi A. Frequency, characterization and therapies of fatigue after stroke. Neurol Sci 2008; 29:S244–246. [DOI] [PubMed] [Google Scholar]
- 10.Jaracz K, Mielcarek L, Kozubski W. Clinical and psychological correlates of poststroke fatigue. Neurol Neurochir 2007;41:36–43. [PubMed] [Google Scholar]
- 11.Choi-Kwon S, Kim JS. Poststroke fatigue: an emerging, critical issue in stroke medicine. Int J Stroke 2011; 6:328–336. [DOI] [PubMed] [Google Scholar]
- 12.Glader EL, Stegmayr B, Asplund K. Poststroke fatigue: a 2-year follow-up study of stroke patients in Sweden. Stroke 2002; 33:1327–1333. [DOI] [PubMed] [Google Scholar]
- 13.Appelros P. Prevalence and predictors of pain and fatigue after stroke: a population-based study. Int J Rehabil Res 2006; 29:329–333. [DOI] [PubMed] [Google Scholar]
- 14.Tang WK, Liang HJ, Chen YK, Chu WC, Abrigo J, et al. Poststroke fatigue is associated with caudate infarcts. J Neurol Sci 2013;15:131–135. [DOI] [PubMed] [Google Scholar]
- 15.Hinkle JL, Becker KJ, Kim JS, Choi-Kwon S, Saban KL, McNair N, et al. Poststroke fatigue: emerging evidence and approaches to management: a scientific statement for healthcare professionals from the American Heart Association. Stroke 2017;48(7):e159–e170. [DOI] [PubMed] [Google Scholar]
- 16.Wang SS, Wang JJ, Wang PX, Chen R. Determinants of fatigue after first-ever ischemic stroke during acute phase. PLoS ONE 2014; 9(10): e110037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu S, Kutlubaev MA, Chun HY, Cowey E, Pollock A, Macleod MR, et al. Interventions for post-stroke fatigue. Cochrane Database Syst Rev 2015;(7): CD007030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.WHO. The top ten causes of death http://www.who.int/mediacentre/factsheets/fs310/en/ 2017;Available at: URL: http://www.who.int/mediacentre/factsheets/fs310/en/.
- 19.Seshadri S, Wolf PA. Lifetime risk of stroke and dementia: current concepts, and estimates from the Framingham Study. Lancet Neurol 2007. December;6(12):1106–14. [DOI] [PubMed] [Google Scholar]
- 20.Walker R, Whiting D, Unwin N, Mugusi F, Swai M. Stroke incidence in rural and urban Tanzania: a prospective, community-based study. Lancet Neurology 2010;9:786–92. [DOI] [PubMed] [Google Scholar]
- 21.Ezejimofor MC, Uthman OA, Maduka O, et al. Stroke survivors in Nigeria: A door-to-door prevalence survey from the Niger Delta region. J Neurol Sci 2017. January 15;372:262–9. [DOI] [PubMed] [Google Scholar]
- 22.Sarfo FS, Awuah DO, Nkyi C, Akassi J, Opare-Sem OK, Ovbiagele B. Recent patterns and predictors of neurological mortality among hospitalized patients in Central Ghana. J Neurol Sci 2016; 363:217–224. [DOI] [PubMed] [Google Scholar]
- 23.Sarfo FS, Akassi J, Awuah D, Adamu S, Nkyi C, Owolabi M, Ovbiagele B. Trends in Stroke admission & mortality rates from 1983 to 2013 in Central Ghana. J Neurol Sci 2015; 357(1–2):240–5. [DOI] [PubMed] [Google Scholar]
- 24.GBD 2016 Lifetime Risk of Stroke Collaborators, Feigin VL, Nguyen G, Cercy K, Johnson CO, Alam T, Parmar PG, et al. Global, regional, and country-specific lifetime risks of stroke, 1990 and 2016. N Engl J Med 2018;379(25):2429–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feigin VL, Norrving B, Mensah GA. Global burden of stroke. Circ Res 2017;120(3):439–448. [DOI] [PubMed] [Google Scholar]
- 26.Owolabi MO, Akarolo-Anthony S, Akinyemi R, et al. The burden of stroke in Africa: a glance at the present and a glimpse into the future. Cardiovasc J Afr 2015. March;26(2 Suppl 1):S27–S38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feigin VL, Roth GA, Naghavi M, et al. Global burden of stroke and risk factors in 188 countries, during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet Neurol 2016;15(9):913–924. [DOI] [PubMed] [Google Scholar]
- 28.O’Donnell MJ, Chin SL, Rangarajan S, et al. Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): a case-control study. Lancet 2016;388(10046):761–75. [DOI] [PubMed] [Google Scholar]
- 29.Owolabi M, Sarfo FS, Akinyemi R, Gebregziabher M, Akpa O, Akpalu A, et al. Dominant risk factors for stroke among West Africans: findings from the SIREN large multisite study. Lancet Global Health 2018;6(4): e436–e446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walker RW, Jusabani A, Aris E, et al. Post-stroke case fatality within an incident population in rural Tanzania. J Neurol Neurosurg Psychiatry 2011;82(9):1001–5. [DOI] [PubMed] [Google Scholar]
- 31.Danesi MA, Okubadejo NU, Ojini FI, Ojo OO. Incidence and 30-day case fatality rate of first-ever stroke in urban Nigeria: the prospective community based Epidemiology of Stroke in Lagos (EPISIL) phase II results. J Neurol Sci 2013; 331:43–7. [DOI] [PubMed] [Google Scholar]
- 32.Gomes J, Damasceno A, Carrilho C, et al. Determinants of early case-fatality among stroke patients in Maputo, Mozambique and impact of in-hospital complications. Int J Stroke 2013; 8 (Suppl. 100):69–75. [DOI] [PubMed] [Google Scholar]
- 33.Sarfo FS, Akassi J, Kyem G, Adamu S, Awuah D, Kantanks OS, et al. Long-term outcomes of Stroke in a Ghanaian Outpatient Clinic. J Stroke Cerebrovasc Dis 2018;27(3):1090–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sarfo FS, Jenkins C, Singh A, Owolabi M, Ojagbemi A, Adusei N, Saulson R, Ovbiagele B. Post-stroke depression in Ghana: characteristics and correlates. J Neurol Sci 2017;379:261–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sarfo FS, Akassi J, Adamu S, Obese V, Ovbiagele B. Burden and predictors of vascular cognitive impairment among long-term Ghanaian stroke survivors. J Stroke Cerebrovas Dis 2017;26(11):2553–2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sarfo FS, Nichols M, Qanungo S, Teklehaimanot A, Singh A, Mensah N, et al. Stroke-related stigma among West Africans: Patterns and predictors. J Neurol Sci 2017;375: 270–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sarfo FS, Adamu S, Awuah D, Sarfo-Kantanka O, Ovbiagele B. Potential role of tele-rehabilitation to address barriers to implementation of physical therapy among West African stroke survivors: A cross-sectional survey. J Neurol Sci 2017; 381:203–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sarfo FS, Ovbiagele B. Response by Sarfo and Ovbiagele to letter regarding “Potential role of tele-rehabilitation to address barriers to implementation of physical therapy among West African stroke survivors: A cross-sectional survey”. J Neurol Sci 2017;382:162–163. [DOI] [PubMed] [Google Scholar]
- 39.Sarfo FS, Ulasavets U, Opare-Sem OK, Ovbiagele B. Tele-rehabilitation after stroke: an updated systematic review of the literature. J Stroke Cerebrovasc Dis 2018; 27(9):2306–2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sarfo FS, Adusei N, Ampofo M, Kpeme FK, Ovbiagele B. Pilot trial of a tele-rehab intervention to improve outcomes after stroke in Ghana: a feasibility and user satisfaction study. J Neurol Sci 2018; 387:94–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sarfo FS, Treiber F, Jenkins C, Patel S, Gebregziabher M, Singh A, et al. Phone-based intervention under Nurse Guidance after Stroke (PINGS): study protocol for a randomized controlled trial. Trials 2016;17(1):436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sarfo FS, Akassi J, Badu E, Okorozo A, Ovbiagele B, Akpalu A. Profile of neurological disorders in an sdult neurology clinic in Kumasi, Ghana. eNeurologicalSci 2016;3:69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sarfo F, Treiber F, Gebregziabher M, Adamu S, Patel S, Nichols M, et al. PINGS (Phone-based Intervention under Nurse Guidance after Stroke): Interim results of a pilot randomized controlled trial. Stroke 2018; 49(1):236–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sarfo FS, Treiber F, Gebregziabher M, Adamu S, Nichols M, Singh A, et al. Phone-based intervention for blood pressure control among Ghanaian stroke survivors: a pilot randomized controlled trial. Int J Stroke 2018; November 22:1747493018816423. doi: 10.1177/1747493018816423. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 45.Naess H, Waje-Andreassen U, Thomassen L, Nyland H, Myhr KM. Health-related quality of life among young adults with ischemic stroke on long-term follow-up. Stroke 2006;1232–1236. [DOI] [PubMed]
- 46.Lerdal A, Bakken LN, Kouwenhoven SE, Pedersen G, Kirkevold M, et al. Poststroke fatigue- a review. J Pain Symptom Manage 38: 928–949. [DOI] [PubMed] [Google Scholar]
- 47.Lerdal A, Kottorp A. Psychometric properties of the Fatigue Severity Scale: Rasch analyses of individual responses in a Norwegian stroke cohort. Int J Nurs Stud 2011; 48:1258–1265. [DOI] [PubMed] [Google Scholar]
- 48.Valko PO, Bassetti CL, Bloch KE, Held U, Baumann CR. Validation of the fatigue severity scale in a Swiss cohort. Sleep 2008; 31:1601–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lyden PD, Lu M, Levine S, Brott TG, Broderick J. A Modified National Institutes of Health Stroke Scale for Use in Stroke Clinical Trials. Preliminary Reliability and Validity. Stroke 2001;32:1310–1317. [DOI] [PubMed] [Google Scholar]
- 50.Mahoney FI, Barthel DW. Functional evaluation: the Barthel Index. Md Med J 1965;14:61–65. [PubMed] [Google Scholar]
- 51.Hamilton M A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry 1960; 23: 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lerdal A, Bakken LN, Rasmussen EF, Beiermann C, Ryen S, et al. Physical impairment, depressive symptoms and pre-stroke fatigue are related to fatigue in the acute phase after stroke. Disabil Rehabil 2011; 33:334–342. [DOI] [PubMed] [Google Scholar]
- 53.Mutai H, Furukawa T, Houri A, Suzuki A, Hanihara T. Factors associated with multidimensional aspect of post-stroke fatigue in acute stroke period. Asian J Psychiatr 2017; 26:1–5. [DOI] [PubMed] [Google Scholar]
- 54.Schepers VP, Visser-Meily AM, Ketelaar M, Lindeman E. Poststroke fatigue: course and its relation to personal and stroke-related factors. Arch Phys Med Rehabil 2006;87: 184–188. [DOI] [PubMed] [Google Scholar]
- 55.Ormstad H, Verkerk R, Amthor K-F, Sandvik L. Activation of the kynurenine pathway in the acute phase of stroke and its role in fatigue and depression following stroke. Journal of Molecular Neuroscience 2014;54:1–7. [DOI] [PubMed] [Google Scholar]
- 56.Johansson B, Carlsson A, Carlsson ML, Karlsson M, Nilsson MKL, Nordquist-Brandt E. Placebo-controlled cross-over study of the monoaminergic stabiliser (−)-OSU6162 in mental fatigue following stroke or traumatic brain injury. Acta Neuropsychiatrica 2012;24:266–74. [DOI] [PubMed] [Google Scholar]
- 57.Karaiskos D, Tzavellas E, Spengos K, Vassilopoulou S, Paparrigopolous T. Duloxetine versus citalopram and sertraline in the treatment of poststroke depression, anxiety, and fatigue. Journal of Neuropsychiatry and Clinical Neurosciences 2012;24:349–53. [DOI] [PubMed] [Google Scholar]


