Abstract
Larval-derived nutritional reserves are essential in shaping insects' adult fitness. Early larval instars of many Lepidopteran species are often sessile, and the conditions experienced by these larvae are often highly dependent on the mother's oviposition choice. Later larval stages are more mobile and therefore can choose their food whenever alternatives are available. We tested how feeding on a drought-exposed host plant impacts life history in an insect herbivore, and whether the observed responses depended on developmental stage. We used drought to alter host plant quality of the ribwort plantain, Plantago lanceolata, and assessed whether host plant preference of postdiapause larvae and adult females increased their own or their offspring's performance, respectively, in the Glanville fritillary butterfly, Melitaea cinxia. Larval response to drought-exposed host plants varied with developmental stage: early larval stages (prediapause) had decreased survival and body mass on drought-exposed plants, while later larval stages (postdiapause) developed faster, weighed more and had a higher growth rate on the drought-exposed plants. Postdiapause larvae also showed a preference for drought-exposed host plants, i.e. those that increased their performance, but only when fed on well-watered host plants. Adult females, on the other hand, showed an oviposition preference for well-watered plants, hence matching the performance of their prediapause but not their postdiapause offspring. Our results highlight how variation in environmental conditions generates stage-specific responses in insects. Individuals fine-tune their own or their offspring's diet by behavioural adjustments when variation in host plant quality is available.
Keywords: butterfly, developmental stress, drought stress, food choice, oviposition choice, preference–performance hypothesis
Highlights
-
•
Responses to drought-exposed host plants are stage dependent.
-
•
Drought-exposed host plants reduced survival and body mass of prediapause larvae.
-
•
Postdiapause larvae grow faster and become larger on drought-exposed host plants.
-
•
Postdiapause larvae prefer host plants that increase their performance.
-
•
Mothers prefer plants that increase their young but not old offspring performance.
Nutrition has an essential role in the development and maturation of individuals, and the conditions experienced and resources acquired during development translate to resource allocation among key life history traits throughout an individual's life (Clissold and Simpson, 2015, Nestel et al., 2016). This is also the case in many insects, where a sufficient amount of nutrients needs to be acquired during development, and then utilized during larval, pupal and adult stages (Nestel et al., 2016). The energetic demands and nutritional requirements may, however, differ depending on the organism's life stage. Amino acids, for example, are utilized at larval stages for growth and development, whereas in adults proteins are allocated to reproduction (i.e. egg/sperm production) and body maintenance (i.e. life span; Barrett et al., 2009, Rodrigues et al., 2015, Simpson et al., 2002). For herbivorous insects, the primary and secondary metabolites, as well as specific nutrients such as nitrogen, are acquired from the host plants the individuals feed on (Machovsky-Capuska et al., 2016, Nestel et al., 2016). Alterations in the essential metabolites and in interactions among nutrients (i.e. nutritional balance) have been shown to impact life history traits across life stages, for example reproductive performance, life span and immunology (e.g. Cotter et al., 2011, Jensen et al., 2015, Lee et al., 2008, Maklakov et al., 2008, Runagall-McNaull et al., 2015, Simpson et al., 2015). The nutritional value (i.e. quality) of the host plant can, however, also vary greatly within species, owing to differences in genetic background, plant ontogeny, biotic stressors and environmental conditions experienced by the host plant (Atkinson and Urwin, 2012, Awmack and Leather, 2002, Rodrigues et al., 2015).
In Lepidoptera, the larvae, especially during the early stages, are often sessile, and hence the mother's choice of a host plant determines the conditions the offspring experience, consequently impacting their development and survival (Jaumann and Snell-Rood, 2017, Rausher, 1979). Accordingly, the preference–performance hypothesis predicts that females should prefer to lay eggs on host plant species that increase their offspring's performance and fitness. Most studies on the preference–performance hypothesis have focused on the female's choice of host plant species (Gripenberg, Mayhew, Parnell, & Roslin, 2010). The few studies that have assessed female preference for individuals within a host plant species often show positive correlation with the offspring's performance (Heisswolf et al., 2005, Wise and Weinberg, 2002). Examples of a mismatch between the mother's preference and offspring's performance also exist, but mainly in relation to between-species preference–performance associations (e.g. Gamberale Stille et al., 2014, Karolewski et al., 2017). In cases where the host plant requirements differ between the developmental stages of the larvae, conflicts may arise. However, the later developmental stages are likely to be less dependent on the maternal choice of the host plant, as individuals at this stage are often more mobile and can potentially choose a diet that best matches their own performance. Generally, the evolution between the female's oviposition preference and offspring's performance is impacted by host plant variation within a landscape as well as changing environmental conditions, all of which modify plant–insect interactions (Thompson, 1988).
An environmental condition likely to impact both host plants and herbivores feeding on them is drought. As drought, in general, has been predicted to increase in frequency in the future (Bale et al., 2002, Morecroft et al., 2002), understanding how it will impact the life history of individuals and potentially the performance of populations, communities and ecosystems is of crucial importance. Drought stress responses in plants are complex, and include accumulation of carbohydrates, soluble proteins and free amino acids, together with alterations in the levels of secondary metabolites (Chaves et al., 2003, Farooq et al., 2009). These responses also depend on the magnitude and the duration of the stress. Under mild and high levels of drought stress, photosynthesis has generally been shown to decline, with the concentration of nitrogen and sugars increasing and concentration of secondary metabolites decreasing in the plant tissue (Gutbrodt et al., 2011, Huberty and Denno, 2004, Mattson and Haack, 1987). Plants also control water status immediately by closing the stomata and by inhibiting leaf growth to protect them from prolonged water loss (Chaves et al., 2003). All these changes in the host plant in response to exposure to water limitation are likely to have consequences also for the performance of organisms utilizing them, with potential impact on their foraging behaviour and decisions (Lenhart et al., 2015, Veteli et al., 2002). Yet studies on the effects of host plant drought on plant–herbivore interactions are still limited (but see Bauerfeind and Fischer, 2013, Jamieson et al., 2012, Lenhart et al., 2015).
Here, we used the Glanville fritillary butterfly, Melitaea cinxia, and one of its host plant species, Plantago lanceolata, to assess the effects of drought-exposed host plants on life history variation and behaviour on this insect herbivore. In Finland, the butterfly exists at its northern range limit and is found in dry meadows on rocky outcrops and pastures (Ojanen, Nieminen, Meyke, Pöyry, & Hanski, 2013). In these habitats, females tend to prefer warm microclimatic conditions for oviposition, exposing their offspring to the risk of feeding on host plants that may at times experience even severe drought (M. Saastamoinen, personal observation). Reduced precipitation over summer is also known to be the major driver of decreased population growth at the metapopulation level (Kahilainen, van Nouhuys, Schulz, & Saastamoinen, 2018). We were specifically interested in (1) the responses of the larvae feeding on host plants that had been exposed to relatively long-lasting drought stress accounting for the potential differences in response between the developmental stage of the larvae (pre- versus postdiapause), (2) whether the developmental responses translate to carryover effects on adult fitness-related traits, and (3) whether postdiapause larvae or adult females show a preference for a host plant type that correlates with their own or their offspring performance, respectively.
Methods
Study System
In Finland, the Glanville fritillary is present only in the Åland Islands archipelago, southwest Finland, where it persists as a classic metapopulation, with a high turnover of local populations (Hanski, 1999). The ca. 4000 suitable habitat patches for the butterfly in Åland are defined by the presence of at least one of the host plant species, P. lanceolata or Veronica spicata (Ojanen et al., 2013). Changes in population dynamics and size are greatly influenced by environmental conditions, such as temperature and precipitation, which are partially driven by their impact on host plant quality, for example desiccation during drought periods (Kahilainen et al., 2018, Tack et al., 2015). During early summer females eclose and mate, after which they locate suitable host plants in which to oviposit. Females lay several clutches of 100–200 eggs each (Saastamoinen, 2007). The first-instar larvae are sessile and feed gregariously mostly on the host plant on which their mother oviposited them. At the beginning of autumn, the larvae spin a silk web in which they overwinter as a group. Larger families and those with heavier larvae have higher overwinter survival (Kuussaari and Singer, 2017, Saastamoinen et al., 2013). In spring, the larvae initiate feeding on newly grown host plants. At this stage, the larvae grow the most (Saastamoinen et al., 2013), become solitary and can move over longer distances (i.e. tens of metres) in search of food (Kuussaari, van Nouhuys, Hellmann, & Singer, 2004).
Ethical Note
The species used in the assays is not considered endangered and is not protected; thus, no federal permits were required to perform research on these insects. The individuals derived from a laboratory stock and all procedures used delicate and round-end tweezers that minimized any stress on the individuals during the experimental manipulation. We ensured that they were kept in large containers and cages to avoid additional stress due to growing. We adhered to the ASAB/ABS guidelines for the treatment of animals.
Plant Material Used for the Experiments
All plants were grown from field-collected seeds originating from eight different local populations across the main island of Åland. Once the plants reached 10 cm shoot length, they were potted singly using fertilized soil 15%:25%:25%, peat:perlite:sand, with a neutral pH. Plants were reared individually in well-watered 0.7-litre pots in designed blocks in common garden conditions (26:18 °C; 15:9 h light:dark). Each block contained both types of host plants and their location in the greenhouse was randomized throughout the experiment. Once the plants were full size we initiated the treatments: the well-watered, turgid, plants received 50 ml of water every third day, whereas the drought-exposed host plants received only 20 ml of water every third day. As P. lanceolata in the Åland islands occurs on open pastures and dry meadows we ensured that in the well-watered treatment the soil dried out fully before the next watering event took place (based on a pilot experiment, data not shown). However, to ensure significant changes in the plants, the drought treatments were started 12 weeks before the feeding treatments were initiated. At this point the plants presented some phenotypical differences between treatments: well-watered plants seemed to have more, thinner and lighter green leaves than the drought-exposed plants (A.L. Salgado, personal observation). A total of 193 well-watered and 218 drought-exposed plants were used in the experiments. For all experiments described below, the larvae were provided with either ‘well-watered’ or ‘drought-exposed’ P. lanceolata leaves, randomly picked from each treatment group on a daily basis.
Larval Rearing and Experimental Set-Ups
For logistic reasons (i.e. seed collection can only be done in late autumn) the experiment was initiated after the larval diapause was broken. Hence the traits were measured at the postdiapause, pupal, adult and prediapause stages (second generation), over the life cycle of the individuals.
Postdiapause Larval Rearing and Food Plant Choice Test
For the experiment, we reared in total 185 larvae from 13 laboratory-generated families. For logistic reasons, the larvae from each family were woken up from diapause in three different sets (12, 19 and 26 April 2017). The larvae were reared in family groups in mesh-covered containers with filter paper and fed ad libitum with leaves of well-watered host plants until they reached the final, seventh instar (28:15 °C; 12:12 h light:dark). Owing to their dark colour and preference for warm microclimatic conditions in the field, the larvae often experience temperatures much higher than the ambient conditions (up to 35 °C; Kallioniemi & Hanski, 2011). The humidity conditions within the room ranged between 40 and 60% (day:night). However, to ensure higher relative humidity (80%) within the containers we sprayed additional water on the filter paper. At the beginning of the seventh instar, individuals were randomly assigned to feed on either well-watered (N = 92) or drought-exposed (N = 93) host plants (see above), ensuring that individuals from different families were spread across treatments. From this stage onwards, larvae were kept individually in 100 ml transparent containers; they received the assigned diet and were otherwise reared as described above.
For the postdiapause food plant choice trials, we marked six equally spaced numbers on paper discs placed on 150 mm petri dishes. On each number, we placed a leaf piece 1.2 cm in diameter, from either the well-watered or drought-exposed treatment, using a drop of water to stick and flatten the discs. The type of leaf used (well-watered versus drought-exposed) was randomly alternated between odd and even numbers in each trial. The leaves used in the assay were collected from the plants in the morning before each trial. We used a cork borer to punch the leaves, and kept the leaf discs fresh by maintaining them inside a cold box with ice packs before each trial. For each trial, a single larva was set in the middle of the petri dish. Thirty minutes later, the test was terminated, the larva was placed back in its container and feeding was continued ad libitum with the assigned host plant. After each food choice test, the leaf discs were pasted onto white paper and scanned for further analysis of the area consumed. We used Fiji 2.0 software (Schindelin et al., 2012) to measure the leaf area left of every disc. To obtain the area eaten of each leaf disc, we subtracted the area left from the initial area (1.13 cm2). For each individual tested, the food plant choice trials were carried out three times, on days 2, 3 and 4 of the seventh instar. Prior to the test, each larva was deprived of food the night before. All trials were run between 1000 and 1300 hours at 28 °C. Finally, all individuals were weighed at pupation (resolution of 0.01 mg on an XS105 analytical balance, Mettler Toledo, Greifensee, Switzerland).
Host Plant Choice and Larval Rearing
Once pupae eclosed, individuals were sexed and placed in cylindrical cages (29 × 13 cm3) with no more than 25 individuals per cage. Matings took place when the individuals were on average 2 days old (range 1–7 days). To ensure a successful number of matings, each mating cage contained a 2:1 male-biased sex ratio. All males used for the mating were from the well-watered host plant treatment. They were obtained by rearing an extra set of larvae (N = 101) with well-watered host plant leaves; they came from the same families as above and were otherwise identically treated. Inbreeding was avoided by ensuring that individuals from the same family were not placed in the same mating cage. Matings occurred between 0900 and 1600 hours at a constant temperature of 26 °C. After mating, females were placed into oviposition cages (60 × 60 cm and 50 cm high) covered with nylon net; males were killed. All individuals were provided with fresh 20% honey:80% water every day and dead individuals were recorded.
We used a total of 66 mated females for testing oviposition host plant choice. For each trial, two well-watered and two drought-exposed host plants were placed within the oviposition cages, 20 cm apart (the host plant location was alternated in each trial). The plants were chosen to appear similar phenotypically, with a comparable amount of leaves and height. Each female was kept in the oviposition cage for 24 h, after which the host plants were inspected for egg clutches. As females rarely lay eggs on 2 consecutive days, and as the number of oviposition cages was limited, we removed the females from the oviposition cage once they had laid eggs and placed them together with other mated females for 24 h before their next oviposition trial. The oviposition choice of each female was assessed three times after which they were placed into a 2-litre netted cage with a well-watered host plant to assess female lifetime egg production and life span.
The egg clutches from each female were maintained under common garden conditions (28:15 °C; 12:12 h light:dark; 80% relative humidity) on petri dishes (100 mm). For each clutch, we counted eggs at day 4 after oviposition and larvae in each clutch once they hatched. To assess the performance of prediapause larvae on the two types of diet, we used the eggs from the first clutch of each female (N = 49). Forty larvae per clutch were split between the host plant diets (i.e. 20 larvae in each treatment group): well-watered and drought-exposed. The larvae were kept in petri dishes containing a filter paper, and new leaves from the treatment plants were added ad libitum each day. As before, water was sprayed each day on the filter paper to ensure higher humidity and old leaves were removed only after all larvae had moved to a fresh leaf. The prediapause larvae spin a silken web throughout development, which helps them to maintain optimal microclimate conditions, and we carefully avoided destroying these webs during the experiment. We measured the prediapause development time, weight and survival at diapause.
Statistical Analyses
The statistical analyses were performed in R software (R Core Team, 2015) using lme4, lmerTest, MASS and glmmTMB packages with the corresponding lmer, glmer and glmmTMB functions to fit the models (Bates et al., 2015, Brooks et al., 2017, Kuznetsova et al., 2017, Venables and Ripley, 2002). We constructed a full model for each response variable with second-order interactions included. The models were validated by checking overdispersion (when needed), plotting the residuals against the fitted values, and verified for normality by plotting the residuals against each fixed and random factor in the model. We selected models with the lowest Akaike information criterion (AIC) values and greater Akaike weights (Zuur & Ieno, 2016) and kept models within the AIC difference less than two from the best model.
Generalized linear mixed models (GLMM) were used in all analyses. Appendix Table A1 shows details of the error distributions, link functions, fixed and random factors for all analyses. To test for the effects of host plant treatment on postdiapause larval performance, we assessed development time, pupal weight, growth rate and survival to adulthood. Development time was calculated as the number of days each larva took from the seventh instar until pupation. The growth rate (g/day), from the seventh instar until pupation, was estimated by following the formula from Radford (1967). In the models for development time, pupal weight and growth rate, host plant treatment and sex were included as fixed factors and family as a random factor. In the model for survival to adulthood (1/0), we used seventh-instar weight instead of sex as a covariate, as the sex of the nonsurvivors was unknown. To assess whether the effect of developmental host plant treatment carried over to female reproductive performance, we assessed mating success (yes/no), oviposition success (yes/no), clutch size, lifetime egg production, hatching success (the proportion of eggs laid that hatched) and life span. For clutch size and hatching success, clutch rank (the order in which the mother oviposited the clutches) was additionally included as a fixed factor. To assess the effects of host plant treatment on the performance of the prediapause larvae, we assessed development time, weight at diapause and survival to diapause (number of larvae that reached diapause). We included offspring host plant treatment and maternal host plant treatment as fixed factors, and family and female ID nested within a family as random factors. We removed 75 larvae that skipped diapause from the analyses (in Finland skipping diapause is not an option for larvae in the wild). We also assessed with a chi-square test whether host plant treatment influenced the likelihood of larvae skipping diapause. All tested models are presented in Appendix Tables A2 and A3. Chi-square values and P values of all retained models are presented in Appendix Tables A4 and A5.
We used the difference in the leaf area eaten between the well-watered and the drought-exposed discs of host plants to determine the food preference of postdiapause larvae. This was tested using a GLMM with a Gaussian distribution where host plant treatment, trial number (1–3) and sex were included as fixed factors and family and larval ID as random factors. We also included second-order interactions in this model. We used statistical comparisons (multcomp R package; Hothorn, Bretz, & Westfall, 2008) to identify the effects of the fixed factors and a pairwise contrast to determine how means differed from each other. In the analysis of the oviposition host plant choice, we used a chi-square test for probabilities of oviposition on well-watered and drought-exposed plants (package stats, R Core Team, 2015). We fitted GLMM models with a binomial distribution (logit link function) to determine whether the oviposition host plant choice was influenced by host plant treatment and trial (1 and 2). Female ID was included as a random factor, and the second-order interaction was also added. Finally, we tested whether the host plant treatment of the female, the host plant choice or the trial influenced the clutch size. Here, we used a negative binomial distribution with log link function, with family and female ID nested within a family as random factors.
Results
As the effect and significance of the fixed factors did not change in the alternative models (i.e. those within ΔAIC < 2), we present here only the results from the best models (i.e. those with the lowest AIC, Appendix Tables A1 and A2). Details of all models and results of the alternative models are presented in Appendix Tables A4 and A5. We present results in the order that the experiments were carried out.
Postdiapause Performance
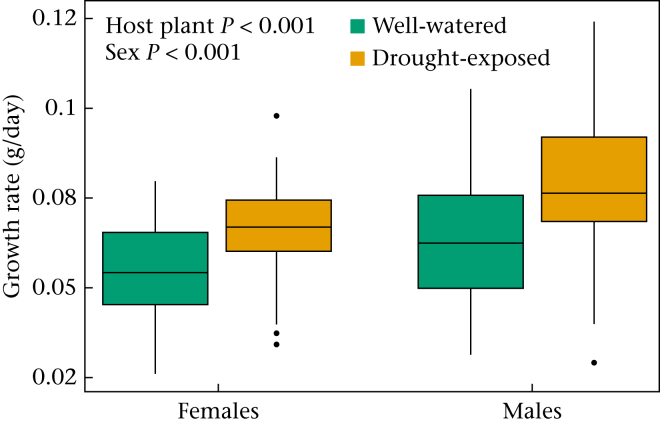
Postdiapause larvae feeding on drought-exposed P. lanceolata host plant leaves developed faster, weighed more as pupae and consequently had a faster growth rate than larvae feeding on well-watered host plants (Table 1, Fig. 1). In general, males developed faster, weighed less as pupae and had a faster growth than females (Table 2). The seventh, and final, instar weight did not influence survival (Table 1).
Table 1.
Results of the best models for postdiapause life history traits and food preference
| Traits | Factors | df | X2 | P |
|---|---|---|---|---|
| Larval performance | ||||
| Development time | Host plant treatment | 1 | 8.4 | 0.004 |
| Sex | 1 | 7.3 | 0.007 | |
| Pupal weight | Host plant treatment | 1 | 17.0 | <0.001 |
| Sex | 1 | 96.2 | <0.001 | |
| Growth rate | Host plant treatment | 1 | 29.5 | <0.001 |
| Sex | 1 | 17.0 | <0.001 | |
| Survival to adulthood | Weight | 1 | 0.3 | 0.609 |
| Host plant preference | ||||
| Host plant area eaten | Host plant treatment | 1 | 1.8 | 0.180 |
| Trial | 2 | 3.7 | 0.157 | |
| Host plant treatment*trial | 2 | 6.8 | 0.033 | |
Figure 1.
Effect of well-watered and drought-exposed host plant treatment on the growth rate of female and male postdiapause larvae. The box plots show the median and 25th and 75th percentiles; the whiskers indicate the values within 1.5 times the interquartile range and the circles are outliers.
Table 2.
Comparison of postdiapause larval and resulting female adult life history traits of individuals exposed to well-watered and drought-exposed host plants during their development
| Life history traits | Sex | Well-watered |
Drought-exposed |
||
|---|---|---|---|---|---|
| Mean (±SE) | N | Mean (±SE) | N | ||
| Postdiapause larvae | |||||
| Development time (days) | Female | 15.0 (±0.3) | 43 | 13.7 (±0.2) | 47 |
| Male | 13.8 (±0.5) | 39 | 11.7 (±0.3) | 37 | |
| Pupal weight (mg) | Female | 159.1 (±2.7) | 43 | 170.0 (±2.5) | 47 |
| Male | 135.6 (±2.7) | 40 | 143.7 (± 2.9) | 37 | |
| Growth rate (g/day) | Female | 0.05 (±0.002) | 43 | 0.07 (±0.002) | 47 |
| Male | 0.06 (±0.003) | 39 | 0.08 (±0.003) | 37 | |
| Adults | |||||
| Clutch size | Female | 130.9 (±9.5) | 51 | 141.0 (±8.8) | 53 |
| Lifetime egg production | Female | 267.1 (±24.7) | 25 | 311.5 (±38.0) | 24 |
| Hatching success (%) | Female | 87.7 (±7.8) | 51 | 91.3 (±7.1) | 53 |
| Life span (days) | Female | 14.8 (±1.0) | 28 | 16.8 (±1.2) | 30 |
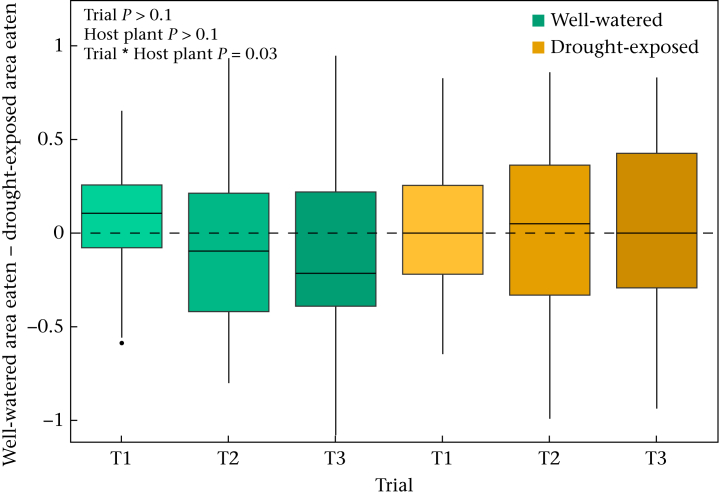
In the analyses to assess food plant choice in the postdiapause larvae, we found a significant interaction between host plant treatment and trial (Table 1), indicating that only larvae fed with well-watered host plants developed a food preference towards drought-exposed leaf discs over time (i.e. trial number; Fig. 2). Based on a post hoc comparison it is evident that there was a clear food preference only in the last trial between the two treatment groups (t = −2.46; P = 0.01; see Appendix Table A6 for the multiple comparisons). The host plant treatment and the trial alone did not explain differences in the area eaten by the larvae (Table 1).
Figure 2.
Diet preference of postdiapause larvae in the food choice test trial. Larvae were fed with host plants from either well-watered or drought-exposed host plants. Each individual was tested for 3 consecutive days (trials). The box plots show the median and 25th and 75th percentiles; the whiskers indicate the values within 1.5 times the interquartile range and the circle is an outlier. Dashed line indicates no preference.
Adult and Prediapause Performance
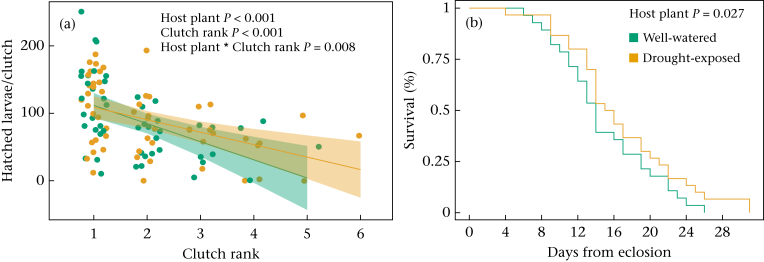
The mating success (81%) and oviposition success (74%) were not influenced by the host plant treatment or by pupal weight (Table 3). Clutch size and lifetime egg production were not affected by the developmental treatment, whereas the hatching success of the eggs was influenced by the host plant treatment, the clutch rank and their interaction (Table 3). Females that were fed with well-watered host plants during their development had eggs with lower hatching success, especially in the later clutches compared with females fed with drought-exposed host plants during their development (Fig. 3a). In general, the number of eggs deposited as well as their hatching success decreased with clutch rank. Females that had been fed with well-watered host plants during their development also had poorer survival than females that had fed on drought-exposed host plants (Fig. 3b). Pupal weight did not influence lifetime egg production (Table 3).
Table 3.
Results of best models for adult life history, prediapause life history and adults' oviposition preference test
| Traits | Factors | df | X2 | P |
|---|---|---|---|---|
| Adults | ||||
| Mating success | Pupal weight | 1 | 2.5 | 0.118 |
| Oviposition success | Host plant treatment | 1 | 0.9 | 0.332 |
| Clutch size | Host plant treatment | 1 | 1.7 | 0.187 |
| Clutch rank | 1 | 16.21 | <0.001 | |
| Lifetime egg production | Pupal weight | 1 | 3.11 | 0.077 |
| Hatching success | Host plant treatment | 1 | 133.361 | <0.001 |
| Clutch rank | 1 | 2407.161 | <0.001 | |
| Host plant treatment*clutch rank | 1 | 7.0991 | 0.008 | |
| Life span | Host plant treatment | 1 | 4.91 | 0.027 |
| Adults' oviposition preference | ||||
| Host plant choice | Host plant treatment | 1 | 0.3 | 0.589 |
| Clutch size | Trial | 1 | 6.41 | 0.011 |
| Prediapause larvae | ||||
| Development time | Host plant treatment | 1 | 0.6 | 0.43 |
| Weight at diapause | Host plant treatment | 1 | 6.4 | 0.012 |
| Survival to diapause | Host plant treatment | 1 | 4.5 | 0.034 |
| Maternal host plant treatment | 1 | 0.2 | 0.662 | |
| Host plant treatment*maternal host plant treatment | 1 | 11.45 | <0.001 | |
Likelihood ratio test.
Figure 3.
Adult life history trait responses to well-watered and drought-exposed host plant treatments. (a) Hatching success of eggs (regression line with 95% confidence intervals) and (b) female survival rate.
Females showed an oviposition preference for well-watered host plants: 76% of the host plants females laid eggs on were from the well-watered group ( = 24.511, P < 0.001). The oviposition choice was not explained by the female's own developmental host plant treatment. More eggs, in general, were laid in the first trial of each female (Table 3).
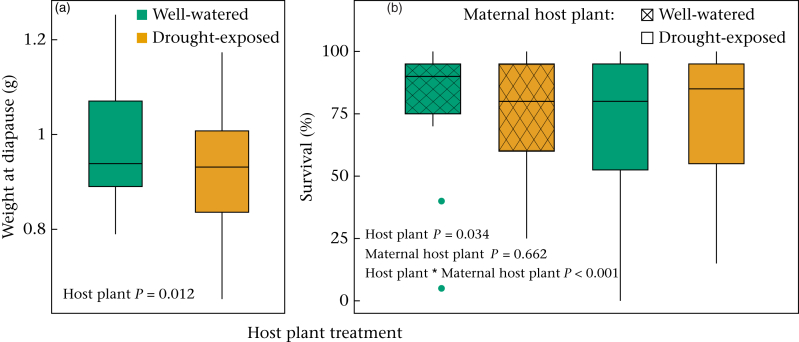
Prediapause larval development time was not influenced by the type of food on which the larvae were fed (Table 3). Prediapause larvae feeding on drought-exposed host plants did, however, weigh less at diapause (Fig. 4a), and had 33% lower survival rate to diapause than larvae fed with well-watered host plants (Table 3). Additionally, a significant interaction between prediapause host plant treatment and the maternal host plant treatment on the survival of the larvae indicated that the prediapause larvae from mothers that had been feeding on drought-exposed plants during their development showed a milder impact of the drought-exposed treatment (Fig. 4b). Maternal developmental host plant treatment alone did not influence prediapause offspring survival (Table 3).
Figure 4.
Effects of well-watered and drought-exposed host plant treatments on (a) weight at diapause and (b) survival rate of prediapause larvae. The box plots show the median and 25th and 75th percentiles; the whiskers indicate the values within 1.5 times the interquartile range and the circles are outliers.
Discussion
Nutritional and environmental conditions experienced during development can greatly impact the performance and fitness of individuals (Boggs, 2009, Lee et al., 2012, Monaghan, 2008, Nestel et al., 2016). As exposure to drought has been predicted to increase in intensity and frequency in the future (Bale et al., 2002, Morecroft et al., 2002), and precipitation is known to impact insect herbivores' population growth rates due to their effects on host plants (Kahilainen et al., 2018), we were explicitly interested here in how drought affects the performance of the herbivore larvae via the changes it induces in the host plants it feeds on. We were further interested in whether individuals can behaviourally adjust their own or their offspring's performance by choosing host plants that match their performance. Our results demonstrate that host plant drought greatly impacts life history variation and performance and that the responses depend on the developmental stage. The prediapause larval performance was reduced when feeding on drought-exposed host plants, whereas the effects were opposite at the postdiapause stage. The positive effect of feeding on drought-exposed host plants during the postdiapause stage even carried over to the adult stage as hatching success of the eggs and the life span of adult females were increased in females that had been feeding on drought-exposed host plants during their development. Additionally, we found behavioural adjustments at both the postdiapause and adult stages of the butterfly that increased their own and their young offspring's performance, respectively.
Responses to Drought-Exposed Host Plants are Stage Dependent
Plants respond to drought events by changes in morphology, physiology and chemistry (Showler & Moran, 2003). Plant exposure to stress may be beneficial to the herbivore feeding on them as, at least in some plants, stressful conditions have been shown to reduce the plants' availability of resources involved in herbivore resistance. Stressful conditions have been shown to increase the concentration of nutrients and/or decrease levels of secondary metabolites in the host plants (Jamieson et al., 2012, Mattson and Haack, 1987), resulting in increased performance, such as faster development time and increased body mass, of insects feeding on them (Gutbrodt et al., 2011). We found that even though the postdiapause larvae grew faster and the pupae weighed more when feeding on drought-exposed host plants, the prediapause larvae feeding on drought-exposed host plants were lighter at diapause and even had reduced survival. As the plants' responses to the treatment were not measured specifically, we can only speculate on the underlying mechanisms causing the observed differences. One may simply be the water status of the host plants. Even though plants that are exposed to drought often respond rapidly by closing stomata, increasing water uptake and reducing water loss, the prolonged exposure to drought in our experiment may have generated changes in the water profile of the host plants, thus increasing levels of nitrogen available for the herbivores and producing more nutritional plants to feed on, like those described above (Farooq et al., 2009, White, 1984). Differences in the response between the life stages may imply that the nutrition or nutritional balance differs between the developmental stages and that some of these core compounds were modified by the drought exposure in the plants. Consistent developmental stage-dependent responses have been shown in regard to secondary metabolites (M. cinxia; Saastamoinen, van Nouhuys, Nieminen, O'Hara, & Suomi, 2007) and proteins (Manduca sexta; Petersen, Woods, & Kingsolver, 2000). In Papilio cresphontes, final-instar larvae are less efficient at converting ingested food than the penultimate instar feeding on the same host plants (Scriber & Dowell, 2017). It is also possible that the underlying reasons for the stage-dependent responses involve not different nutritional requirements but more physical responses of the plants to the drought that may affect small and large larvae differently. In response to drought, plants often produce more sclerophyllous leaves (i.e. small, thick and tough; Chaves et al., 2003) which may be harder for young prediapause larvae to break and chew compared with the larger postdiapause larvae, preventing the former from reaching the nutrients that would otherwise be available to them. Physical defences, such as leaf toughness, are known to impact herbivore performance significantly, and they are indeed often the most important plant defences to deter herbivory (Massey & Hartley, 2009). The drought exposure of the host plants in the present study was relatively harsh and long (i.e. 12 weeks), giving the plants enough time to show these types of physical or chemical responses. Thus, milder drought exposure may alter the host plants differently and, consequently, the life history responses of the herbivore may also differ. Future work on the metabolite profiles and the physical responses of the host plant to different durations of drought are hence required to shed more light on the underlying mechanisms of the results obtained here.
Whatever the underlying mechanisms, the positive effects of feeding on drought-exposed host plants observed in the postdiapause larvae were significant and carried over even to adult performance. Even though the number of eggs was not affected by the developmental conditions, the hatching success of the eggs laid and the adult life span of individuals fed with drought-exposed host plants during the postdiapause stage was increased compared to those fed with well-watered host plants. The increased hatching success of eggs in females that had been fed with drought-exposed host plants was more pronounced at an older age further supporting changes in adult senescence. These results are consistent with several other studies in Lepidoptera that have shown the importance of larval-derived reserves for life span (Boggs, 2002, Boggs and Freeman, 2005, Swanson et al., 2016). In the Glanville fritillary shortage of food during the postdiapause stage has also been shown to reduce fecundity or life span depending on the severity of the treatment (Saastamoinen et al., 2013). Developmental conditions experienced by the mothers may also impact the performance of the offspring (i.e. transgenerational effects; Woestmann & Saastamoinen, 2016), and such effects are presumed to be adaptive when the parents match their offspring phenotype to the environment the offspring will experience (Mousseau & Fox, 1998). We observed that the negative impact of drought-exposed host plants was milder in the prediapause larvae whose mother had also experienced drought-exposed host plants during their own development.
Preference–Performance in Larvae and Adults
The preference–performance hypothesis predicts that females will choose to oviposit their eggs on host plants that will increase the growth, survival and reproduction of their offspring (Gripenberg et al., 2010). Generally, variation in host plant use follows the abundance of the potential host species and their characteristics (age, size, primary and secondary metabolite concentrations and/or physical properties). Theory predicts that whenever there is a conflict between the life stages, the mothers should favour oviposition on host plants that are best for eggs and early stage offspring, even if the survival of later stages is better in other host plants (Thompson, 1988). Consistently, we found a strong preference for females to deposit eggs on well-watered host plants over drought-exposed host plants, which increased the performance of the prediapause but not the postdiapause larvae. The behavioural choice test experiment with the postdiapause larvae further showed that the larger larvae are themselves able to choose among the leaves exposed to different treatments.
Interestingly, the choosiness of individual postdiapause larvae towards the host plant that increased their own performance (i.e. drought-exposed host plant) became apparent only when the larvae had been fed with the host plant that generally resulted in reduced performance (i.e. well-watered host plant). This result further suggests that the well-watered host plants were lacking some nutrients that were important for the postdiapause larvae or that the nutrients were unbalanced and that only the larvae missing these nutrient/compounds chose the ‘better quality diet’. This is consistent with studies that have shown that nutritional needs alter individual behaviour to feed on a diet with appropriate nutrients and/or to regulate their nutritional intake (Behmer, 2009, del Campo et al., 2009, Jones and Agrawal, 2017). The behavioural choosiness observed in the present study increased with the exposure that individuals acquired over time (i.e. with successive trials), suggesting some form of sensitization (whereby with repeated exposure to a specific stimulus the individuals increase their response towards it; Jones and Agrawal, 2017, Papaj and Prokopy, 1989). Similar sensitization (a form of nonassociative learning) has been observed, for example, in the larvae of M. sexta: after repeated feeding on solanaceous host plants the individuals become facultative specialists and stopped accepting other host plants (Anderson & Anton, 2014). Learning in insects generally improves foraging efficiency by decreasing the incidence of random food sampling, and consequently has a positive effect on fitness (Behmer, 2009, Jones and Agrawal, 2017).
Conclusions
Our work shows variation in response to changes in host plant quality along the life cycle of an insect herbivore, mediated by host plant responses to drought. These responses affected the development, fitness and survival, and even the behaviour, of the individuals. Very few studies so far have considered life history responses at different developmental stages within host plant species that vary in quality (but see Petersen et al., 2000, Rodrigues et al., 2015, Saastamoinen et al., 2007). Our results on the increased performance of postdiapause larvae on drought-exposed plants suggest that the general preference of the Glanville fritillary for sites with warmer microclimatic conditions may be due not solely to warmer thermal conditions but also, at least partially, to the changes in host plant quality. Future studies should aim to identify the underlying mechanisms that cause stage-dependent variation in the responses, as possibilities include both nutritional and physical modifications in the plants due to drought stress.
Acknowledgments
We thank Brandon Meter, Carolina Sánchez Carpena, Samuli Hapalainen and Suvi Ikonen for their assistance during the experiments. We acknowledge Aapo Kahilainen, Dimitri Stucki, Elena Rosa, Luisa Woestmann, Michelle Dileo, Elina Numminen, Rose Thorogood and two anonymous referees for their help with data analyses and comments on the manuscript. This work was supported by the European Research Council (Independent starting grant no. 637412 ‘META-STRESS’ to M.S.) and by Lammi Biological Station grant (to A.S.). We declare that we have no conflict of interest.
MS. number: 18-00479R
Appendix.
Table A1.
Model description including the error distribution, link function, fixed factors and random factors for each of the full models
| Life history traits | Distribution | Link function | Fixed factors | Random factors | |||
|---|---|---|---|---|---|---|---|
| Postdiapause larvae | Poisson | Log | Treatment | Sex | Family | ||
| Development time (days) | Gaussian | Identity | Treatment | Sex | Family | ||
| Pupal weight (mg) | Gaussian | Identity | Treatment | Sex | Family | ||
| Growth rate (g/day) | Binomial | Logit | Treatment | 7th instar weight | Family | ||
| Survival to adulthood | |||||||
| Adults | |||||||
| Mating success | Binomial | Logit | Treatment | Pupal weight | Family | ||
| Oviposition success | Binomial | Logit | Treatment | Pupal weight | Family | ||
| Clutch size (eggs/clutch) | Negative binomial | Log | Treatment | Clutch rank | Family | ||
| Lifetime egg production | Negative binomial | Log | Treatment | Pupal weight | Family | ||
| Hatching success | Binomial | Logit | Treatment | Clutch rank | Family | ||
| Life span (days) | Poisson | Log | Treatment | Pupal weight | Family | ||
| Prediapause larvae | |||||||
| Development time (days) | Gaussian | Identity | Treatment | Maternal treatment | Family | Female ID/Family | |
| Weight at diapause (mg) | Gaussian | Identity | Treatment | Maternal treatment | Family | Female ID/Family | |
| Survival to diapause | Binomial | Logit | Treatment | Maternal treatment | Family | Female ID/Family | |
| Postdiapause food preference | |||||||
| Host plant area eaten | Gaussian | Identity | Treatment | Trial | Sex | Family | Larval ID/Family |
| Adult's oviposition choice | |||||||
| Host plant choice | Binomial | Logit | Treatment | Trial | Maternal treatment | Family | Female ID/Family |
| Clutch size | Negative binomial | Log | Treatment | Trial | |||
Table A2.
List of all models tested for each life history trait
| Life history traits | Model | Fixed effects | Random effects | df | AIC | ΔAIC | Akaike weights |
|---|---|---|---|---|---|---|---|
| Postdiapause larvae | |||||||
| Developmental time | 1 | Host plant treatment*sex | Family | 5 | 796.794 | 1.324 | 0.311 |
| 2 | Host plant treatment+ sex | Family | 4 | 795.471 | 0 | 0.642 | |
| 3 | Host plant treatment | Family | 3 | 800.841 | 5.37 | 0.042 | |
| 4 | Sex | Family | 3 | 801.866 | 6.395 | 0.025 | |
| Pupal weight | 5 | Host plant treatment*sex | Family | 6 | −95.821 | 5.9 | 0.049 |
| 6 | Host plant treatment+ sex | Family | 5 | −99.963 | 0 | 0.94 | |
| 7 | Host plant treatment | Family | 4 | −31.549 | 68.414 | 0 | |
| 8 | Sex | Family | 4 | −91.301 | 8.663 | 0.011 | |
| Growth rate | 9 | Host plant treatment*sex | Family | 6 | −846.931 | 10.089 | 0.006 |
| 10 | Host plant treatment+ sex | Family | 5 | −857.02 | 0 | 0.883 | |
| 11 | Host plant treatment | Family | 4 | −841.89 | 4.152 | 0.111 | |
| 12 | Sex | Family | 4 | −841.89 | 15.129 | 0 | |
| Survival to adulthood | 13 | Host plant treatment*weight | Family | 5 | 318.561 | 3.812 | 0.062 |
| 14 | Host plant treatment+ weight | Family | 4 | 316.75 | 2 | 0.154 | |
| 15 | Host plant treatment | Family | 3 | 315.011 | 0.262 | 0.366 | |
| 16 | Weight | Family | 3 | 314.75 | 0 | 0.418 | |
| Adults | |||||||
| Mating success | 17 | Host plant treatment*pupal weight | Family | 5 | 81.665 | 0.589 | 0.29 |
| 18 | Host plant treatment+ pupal weight | Family | 4 | 82.361 | 1.286 | 0.204 | |
| 19 | Host plant treatment | Family | 3 | 83.477 | 2.401 | 0.117 | |
| 20 | Pupal weight | Family | 3 | 81.076 | 0 | 0.389 | |
| Oviposition success | 21 | Host plant treatment*pupal weight | Family | 5 | 81.446 | 1.106 | 0.219 |
| 22 | Host plant treatment+ pupal weight | Family | 4 | 82.1 | 1.76 | 0.158 | |
| 23 | Host plant treatment | Family | 3 | 80.34 | 0 | 0.381 | |
| 24 | Pupal weight | Family | 3 | 81.248 | 0.908 | 0.242 | |
| Clutch size | 25 | Host plant treatment*clutch rank | Family | 6 | 1165.556 | 1.191 | 0.355 |
| 26 | Host plant treatment+ clutch rank | Family | 5 | 1164.365 | 0 | 0.645 | |
| 27 | Host plant treatment | Family | 3 | 3778.379 | 2614.014 | 0 | |
| 28 | Clutch rank | Family | 3 | 3172.298 | 2007.933 | 0 | |
| Lifetime egg production | 29 | Host plant treatment*pupal weight | Family | 6 | 634.41 | 3.656 | 0.084 |
| 30 | Host plant treatment+ pupal weight | Family | 5 | 632.588 | 1.835 | 0.209 | |
| 31 | Host plant treatment | Family | 4 | 632.834 | 2.081 | 0.185 | |
| 32 | Pupal weight | Family | 4 | 630.754 | 0 | 0.523 | |
| Hatching success | 33 | Host plant treatment*clutch rank | Family | 5 | 5336.942 | 0 | 0.928 |
| 34 | Host plant treatment+ clutch rank | Family | 4 | 5342.04 | 5.099 | 0.072 | |
| 35 | Host plant treatment | Family | 3 | 7747.201 | 2410.259 | 0 | |
| 36 | Clutch rank | Family | 3 | 5473.4 | 136.458 | 0 | |
| Life span | 37 | Host plant treatment*pupal weight | Family | 5 | 392.207 | 2.785 | 0.135 |
| 38 | Host plant treatment+ pupal weight | Family | 4 | 391.109 | 1.687 | 0.233 | |
| 39 | Host plant treatment | Family | 3 | 389.422 | 0 | 0.542 | |
| 40 | Pupal weight | Family | 3 | 393.012 | 3.59 | 0.09 | |
| Prediapause larvae | |||||||
| Development time | 41 | Host plant treatment*maternal host plant treatment | female ID/family | 7 | 383.4 | 2.487 | 0.11 |
| 42 | Host plant treatment+ maternal host plant treatment | female ID/family | 6 | 383.011 | 2.098 | 0.134 | |
| 43 | Host plant treatment | female ID/family | 5 | 380.913 | 0 | 0.382 | |
| 44 | Maternal host plant treatment | female ID/family | 5 | 380.956 | 0.043 | 0.374 | |
| Weight at diapause | 45 | Host plant treatment*maternal host plant treatment | female ID/family | 7 | −109.229 | 10.473 | 0.004 |
| 46 | Host plant treatment+ maternal host plant treatment | female ID/family | 6 | −115.184 | 4.517 | 0.077 | |
| 47 | Host plant treatment | female ID/family | 5 | −119.702 | 0 | 0.734 | |
| 48 | Maternal host plant treatment | female ID/family | 5 | −116.949 | 2.752 | 0.185 | |
| Survival to diapause | 49 | Host plant treatment*maternal host plant treatment | female ID/family | 6 | 568.818 | 0 | 0.966 |
| 50 | Host plant treatment+ maternal host plant treatment | female ID/family | 5 | 578.122 | 9.304 | 0.009 | |
| 51 | Host plant treatment | female ID/family | 4 | 576.315 | 7.497 | 0.023 | |
| 52 | Maternal host plant treatment | female ID/family | 4 | 580.794 | 11.976 | 0.002 | |
The best model for each trait after Akaike information criterion (AIC) analysis is marked in bold.
Table A3.
List of all models tested on host plant preference
| Type of choice | Model | Fixed effects | Random effects | df | AIC | ΔAIC | Akaike weights | |
|---|---|---|---|---|---|---|---|---|
| Postdiapause food choice | ||||||||
| Difference in area of host plant eaten | 1 | Host plant treatment*trial*sex | Larva ID/family | 15 | 594.572 | 27.323 | 0 | |
| 2 | Host plant treatment*trial + sex | Larva ID/family | 10 | 573.664 | 6.415 | 0.032 | ||
| 3 | Host plant treatment + trial + sex | Larva ID/family | 8 | 570.147 | 2.899 | 0.184 | ||
| 4 | Host plant treatment*trial | Larva ID/family | 9 | 567.249 | 0 | 0.784 | ||
| Adults' oviposition choice | ||||||||
| Host plant type | 5 | Host plant treatment*trial | Female ID/family | 6 | 159.116 | 3.959 | 0.058 | |
| 6 | Host plant treatment + trial | Female ID/family | 5 | 157.152 | 1.994 | 0.155 | ||
| 7 | Host plant treatment | Female ID/family | 4 | 155.157 | 0 | 0.421 | ||
| 8 | Trial | Female ID/family | 4 | 155.446 | 0.289 | 0.365 | ||
| Clutch size | 9 | Host plant treatment*Maternal host plant treatment*trial | Family | 10 | 898.079 | 9.84 | 0.004 | |
| 10 | Host plant treatment*Maternal host plant treatment + trial | Family | 7 | 892.972 | 4.732 | 0.049 | ||
| 11 | Host plant treatment + Maternal host plant treatment + trial | Family | 6 | 891.534 | 3.294 | 0.101 | ||
| 12 | Host plant treatment*Maternal host plant treatment | Family | 6 | 897.634 | 9.395 | 0.005 | ||
| 13 | Maternal host plant treatment*trial | Family | 6 | 891.95 | 3.71 | 0.082 | ||
| 14 | Host plant treatment*Maternal host plant treatment | Family | 5 | 895.889 | 7.649 | 0.011 | ||
| 15 | Maternal host plant treatment + trial | Family | 5 | 889.956 | 1.717 | 0.223 | ||
| 16 | Trial | Family | 4 | 888.24 | 0 | 0.525 | ||
The best model after Akaike information criterion (AIC) analysis marked in bold.
Table A4.
Results of all top models for the life history traits (i.e. those that presented AIC value differences less than two units from the best model)
| Life history traits | Model | AIC | Factors | df | X2 | P | Variance estimates | SD |
|---|---|---|---|---|---|---|---|---|
| Postdiapause larvae | ||||||||
| Development time | 2 | 795.471 | Host plant treatment | 1 | 8.384 | 0.004 | ||
| Sex | 1 | 7.335 | 0.007 | |||||
| Family | 0 | 0 | ||||||
| 1 | 796.794 | Host plant treatment | 1 | 8.366 | 0.004 | |||
| Sex | 1 | 7.312 | 0.007 | |||||
| Host plant treatment*sex | 1 | 0.676 | 0.411 | |||||
| Family | 0 | 0 | ||||||
| Pupal weight | 6 | −99.963 | Host plant treatment | 1 | 17.034 | <0.001 | ||
| Sex | 1 | 96.203 | <0.001 | |||||
| Family | 0.004 | 0.066 | ||||||
| Residual | 0.025 | 0.159 | ||||||
| Growth rate | 10 | −857.020 | Host plant treatment | 1 | 29.540 | <0.001 | ||
| Sex | 1 | 17.034 | <0.001 | |||||
| Family | <0.001 | 0.001 | ||||||
| Residual | <0.001 | 0.016 | ||||||
| Survival to adulthood | 16 | 314.750 | Weight | 1 | 0.261 | 0.609 | ||
| Family | 0 | 0 | ||||||
| 15 | 315.011 | Host plant treatment | 1 | 0 | 0.995 | |||
| Family | 0 | 0 | ||||||
| 14 | 316.750 | Host plant treatment | 1 | 0 | 0.995 | |||
| Weight | 1 | 0.261 | 0.609 | |||||
| Family | 0 | 0 | ||||||
| Adults | ||||||||
| Mating success | 20 | 81.076 | Pupal weight | 1 | 2.449 | 0.118 | ||
| Family | 0 | 0 | ||||||
| 17 | 81.665 | Host plant treatment | 1 | 0.308 | 0.579 | |||
| Pupal weight | 1 | 2.205 | 0.138 | |||||
| Host plant treatment*pupal weight | 1 | 2.381 | 0.123 | |||||
| Family | <0.001 | <0.001 | ||||||
| 18 | 82.361 | Host plant treatment | 1 | 0.699 | 0.403 | |||
| Pupal weight | 1 | 2.99 | 0.084 | |||||
| Family | 0 | 0 | ||||||
| Oviposition success | 23 | 80.34 | Host plant treatment | 1 | 0.942 | 0.332 | ||
| Family | <0.001 | <0.001 | ||||||
| 24 | 81.248 | Pupal weight | 1 | 0.058 | 0.809 | |||
| Family | 0 | 0 | ||||||
| 21 | 81.446 | Host plant treatment | 1 | 0.824 | 0.364 | |||
| Pupal weight | 1 | 0.241 | 0.624 | |||||
| Host plant treatment*pupal weight | 1 | 2.536 | 0.111 | |||||
| Family | 0 | 0 | ||||||
| 22 | 82.1 | Host plant treatment | 1 | 1.116 | 0.291 | |||
| Pupal weight | 1 | 0.24 | 0.624 | |||||
| Family | 0 | 0 | ||||||
| Clutch size | 26 | 1164.365 | Host plant treatment | 1 | 1.7441 | 0.187 | ||
| Clutch rank | 1 | 16.2271 | <0.001 | |||||
| Family | 0.006 | 0.0804 | ||||||
| 25 | 1165.556 | Host plant treatment | 1 | 1.7441 | 0.187 | |||
| Clutch rank | 1 | 16.2271 | <0.001 | |||||
| Host plant treatment*clutch rank | 1 | 0.8091 | 0.368 | |||||
| Family | 0.005 | 0.067 | ||||||
| Lifetime egg production | 32 | 630.754 | Pupal weight | 1 | 3.1271 | 0.077 | ||
| Family | <0.001 | <0.001 | ||||||
| 30 | 632.588 | Host plant treatment | 1 | 0.1651 | 0.684 | |||
| Pupal weight | 1 | 2.2461 | 0.134 | |||||
| Family | <0.001 | <0.001 | ||||||
| Hatching success | 33 | 5336.942 | Host plant treatment | 1 | 133.3601 | <0.001 | ||
| Clutch rank | 1 | 2407.1601 | <0.001 | |||||
| Host plant treatment*clutch rank | 1 | 7.0991 | 0.008 | |||||
| Family | 0.450 | 0.671 | ||||||
| Life span | 39 | 389.422 | Host plant treatment | 1 | 4.9011 | 0.027 | ||
| Family | 0.017 | 0.133 | ||||||
| 38 | 391.109 | Host plant treatment | 1 | 3.9031 | 0.048 | |||
| Pupal weight | 1 | 0.3131 | 0.576 | |||||
| Family | 0.017 | 0.132 | ||||||
| Prediapause larvae | ||||||||
| Development time | 43 | 380.913 | Host plant treatment | 1 | 0.622 | 0.43 | ||
| Female ID | 0.674 | 0.821 | ||||||
| Family | 0 | 0 | ||||||
| Residuals | 1.985 | 1.409 | ||||||
| 44 | 380.956 | Maternal host plant treatment | 1 | 0.048 | 0.827 | |||
| Female ID | 0.715 | 0.846 | ||||||
| Family | 0 | 0 | ||||||
| Residuals | 1.969 | 1.403 | ||||||
| Weight at diapause | 47 | −119.702 | Host plant treatment | 1 | 6.356 | 0.012 | ||
| Female ID | 0.002 | 0.047 | ||||||
| Family | 0.001 | 0.033 | ||||||
| Residuals | 0.011 | 0.106 | ||||||
| Survival to diapause | 49 | 568.818 | Host plant treatment | 1 | 4.480 | 0.034 | ||
| Maternal host plant treatment | 1 | 0.190 | 0.662 | |||||
| Host plant treatment*maternal host plant treatment | 1 | 11.479 | <0.001 | |||||
| Female ID | 1.393 | 1.180 | ||||||
| Family | 0.035 | 0.187 | ||||||
The models are presented starting from the lowest AIC value.
Likelihood ratio test.
Table A5.
Results of all top models for the choice tests (i.e: those that presented AIC value differences less than two units from the best model)
| Preference | Model | AIC values | Factors | df | X2 | P | Variance estimates | SD |
|---|---|---|---|---|---|---|---|---|
| Postdiapause | ||||||||
| Host plant area eaten | 4 | 567.249 | Host plant treatment | 1 | 1.798 | 0.180 | ||
| Trial | 2 | 3.709 | 0.157 | |||||
| Host plant treatment*trial | 2 | 6.834 | 0.033 | |||||
| Larva ID | 0.003 | 0.056 | ||||||
| Family | 0 | 0 | ||||||
| Residuals | 0.164 | 0.405 | ||||||
| Adults' oviposition | ||||||||
| Host plant choice | 7 | 155.157 | Host plant treatment | 1 | 0.292 | 0.589 | ||
| Female | <0 | <0 | ||||||
| Family | 0 | 0 | ||||||
| 8 | 155.446 | Trial | 1 | 0.003 | 0.958 | |||
| Female ID | 0 | 0 | ||||||
| Family | 0 | 0 | ||||||
| 6 | 157.152 | Host plant treatment | 1 | 0.294 | 0.587 | |||
| Trial | 1 | 0.006 | 0.941 | |||||
| Female | 0 | 0 | ||||||
| Family | 0 | 0 | ||||||
| Clutch size | 16 | 888.240 | Trial | 1 | 6.4361 | 0.011 | ||
| Family | 0.003 | 0.055 | ||||||
| 15 | 889.956 | Maternal host plant treatment | 1 | 0.2831 | 0.595 | |||
| Trial | 1 | 6.3361 | 0.012 | |||||
| Family | 0.002 | 0.046 | ||||||
The models are presented starting from the lowest AIC value.
Likelihood ratio test.
Table A6.
Multiple comparisons of means in the post hoc analysis for the food choice test of the postdiapause larvae
| Group | Estimate | SE | Z | P |
|---|---|---|---|---|
| Well-watered 1 – Well-watered 2 | 0.156 | 0.063 | 2.493 | 0.067 |
| Well-watered 1 – Well-watered 3 | 0.187 | 0.063 | 2.997 | 0.015 |
| Well-watered 2 – Well-watered 3 | 0.031 | 0.063 | 0.504 | 0.983 |
| Drought-exposed 1 – Drought-exposed 2 | −0.020 | 0.063 | −0.327 | 0.997 |
| Drought-exposed 1 – Drought-exposed 3 | −0.030 | 0.063 | −0.484 | 0.985 |
| Drought-exposed 2 – Drought-exposed 3 | −0.010 | 0.063 | −0.157 | 0.999 |
The number after the host plant treatment refers to the trial.
References
- Anderson P., Anton S. Experience-based modulation of behavioural responses to plant volatiles and other sensory cues in insect herbivores. Plant, Cell & Environment. 2014;37(8):1826–1835. doi: 10.1111/pce.12342. [DOI] [PubMed] [Google Scholar]
- Atkinson N.J., Urwin P.E. The interaction of plant biotic and abiotic stresses: From genes to the field. Journal of Experimental Botany. 2012;63(10):3523–3543. doi: 10.1093/jxb/ers100. [DOI] [PubMed] [Google Scholar]
- Awmack C.S., Leather S.R. Host plant quality and fecundity in herbivorous insects. Annual Review of Entomology. 2002;47(1):817–844. doi: 10.1146/annurev.ento.47.091201.145300. [DOI] [PubMed] [Google Scholar]
- Bale J.S., Masters G.J., Hodkinson I.D., Awmack C., Bezemer T.M., Brown V.K. Herbivory in global climate change research: Direct effects of rising temperature on insect herbivores. Global Change Biology. 2002;8(1):1–16. [Google Scholar]
- Barrett E.L., Hunt J., Moore A.J., Moore P.J. Separate and combined effects of nutrition during juvenile and sexual development on female life-history trajectories: The thrifty phenotype in a cockroach. Proceedings of the Royal Society B: Biological Sciences. 2009;276(1671):3257–3264. doi: 10.1098/rspb.2009.0725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D., Mächler M., Bolker B., Walker S. Fitting linear mixed-effects models using lme4. Journal of Statistical Software. 2015;67(1):1–48. [Google Scholar]
- Bauerfeind S.S., Fischer K. Increased temperature reduces herbivore host-plant quality. Global Change Biology. 2013;19(11):3272–3282. doi: 10.1111/gcb.12297. [DOI] [PubMed] [Google Scholar]
- Behmer S.T. Insect herbivore nutrient regulation. Annual Review of Entomology. 2009;54:165–187. doi: 10.1146/annurev.ento.54.110807.090537. [DOI] [PubMed] [Google Scholar]
- Boggs C.L. Environmental variation, life histories, and allocation. In: Boggs C.L., Watt W.B., Ehrlich P.R., editors. Evolution and ecology: Taking flight: Butterflies as model systems. University of Chicago Press; Chicago, IL: 2002. pp. 185–206. [Google Scholar]
- Boggs C.L. Understanding insect life histories and senescence through a resource allocation lens. Functional Ecology. 2009;23(1):27–37. [Google Scholar]
- Boggs C.L., Freeman K.D. Larval food limitation in butterflies: Effects on adult resource allocation and fitness. Oecologia. 2005;144(3):353–361. doi: 10.1007/s00442-005-0076-6. [DOI] [PubMed] [Google Scholar]
- Brooks M.E., Kristensen K., van Benthem K.J., Magnusson A., Berg C.W., Nielsen A. bioRxiv; 2017. Modeling zero-inflated count data with glmmTMB; p. 132753. [Google Scholar]
- del Campo M.L., Miles C.I., Caillaud M.C. Effects of experience on the physiology of taste discrimination in insects. In: Newland P.L., Cobb M., Marion-Poll F., editors. Insect taste. Taylor & Francis; New York, NY: 2009. pp. 219–256. [Google Scholar]
- Chaves M.M., Maroco J.P., Pereira J.S. Understanding plant responses to drought—from genes to the whole plant. Functional Plant Biology. 2003;30(3):239–264. doi: 10.1071/FP02076. [DOI] [PubMed] [Google Scholar]
- Clissold F.J., Simpson S.J. Temperature, food quality and life history traits of herbivorous insects. Current Opinion in Insect Science. 2015;11:63–70. doi: 10.1016/j.cois.2015.10.011. [DOI] [PubMed] [Google Scholar]
- Cotter S.C., Simpson S.J., Raubenheimer D., Wilson K. Macronutrient balance mediates trade-offs between immune function and life history traits. Functional Ecology. 2011;25(1):186–198. [Google Scholar]
- Farooq M., Wahid A., Kobayashi N., Fujita D., Basra S.M.A. Plant drought stress: Effects, mechanisms and management. In: Lichtfouse E., Navarrete M., Debaeke P., Véronique S., Alberola C., editors. Sustainable agriculture. Springer Science & Business Media; Dordrecht, Netherlands: 2009. pp. 185–212. [Google Scholar]
- Gamberale Stille G., Söderlind L., Janz N., Nylin S. Host plant choice in the comma butterfly–larval choosiness may ameliorate effects of indiscriminate oviposition. Insect Science. 2014;21(4):499–506. doi: 10.1111/1744-7917.12059. [DOI] [PubMed] [Google Scholar]
- Gripenberg S., Mayhew P.J., Parnell M., Roslin T. A meta-analysis of preference–performance relationships in phytophagous insects. Ecology Letters. 2010;13(3):383–393. doi: 10.1111/j.1461-0248.2009.01433.x. [DOI] [PubMed] [Google Scholar]
- Gutbrodt B., Mody K., Dorn S. Drought changes plant chemistry and causes contrasting responses in lepidopteran herbivores. Oikos. 2011;120(11):1732–1740. [Google Scholar]
- Hanski I. Habitat connectivity, habitat continuity, and metapopulations in dynamic landscapes. Oikos. 1999:209–219. [Google Scholar]
- Heisswolf A., Obermaier E., Poethke H.J. Selection of large host plants for oviposition by a monophagous leaf beetle: Nutritional quality or enemy-free space? Ecological Entomology. 2005;30(3):299–306. [Google Scholar]
- Hothorn T., Bretz F., Westfall P. Simultaneous inference in general parametric models. Biometrical Journal. 2008;50(3):346–363. doi: 10.1002/bimj.200810425. [DOI] [PubMed] [Google Scholar]
- Huberty A.F., Denno R.F. Plant water stress and its consequences for herbivorous insects: A new synthesis. Ecology. 2004;85(5):1383–1398. [Google Scholar]
- Jamieson M.A., Trowbridge A.M., Raffa K.F., Lindroth R.L. Consequences of climate warming and altered precipitation patterns for plant-insect and multitrophic interactions. Plant Physiology. 2012;160(4):1719–1727. doi: 10.1104/pp.112.206524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaumann S., Snell-Rood E.C. Trade-offs between fecundity and choosiness in ovipositing butterflies. Animal Behaviour. 2017;123:433–440. [Google Scholar]
- Jensen K., McClure C., Priest N.K., Hunt J. Sex-specific effects of protein and carbohydrate intake on reproduction but not lifespan in Drosophila melanogaster. Aging Cell. 2015;14(4):605–615. doi: 10.1111/acel.12333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P.L., Agrawal A.A. Learning in insect pollinators and herbivores. Annual Review of Entomology. 2017;62:53–71. doi: 10.1146/annurev-ento-031616-034903. [DOI] [PubMed] [Google Scholar]
- Kahilainen A., van Nouhuys S., Schulz T., Saastamoinen M. Metapopulation dynamics in a changing climate: Increasing spatial synchrony in weather conditions drives metapopulation synchrony of a butterfly inhabiting a fragmented landscape. Global Change Biology. 2018;24(9):4316–4329. doi: 10.1111/gcb.14280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallioniemi E., Hanski I. Interactive effects of Pgi genotype and temperature on larval growth and survival in the Glanville fritillary butterfly. Functional Ecology. 2011;25(5):1032–1039. [Google Scholar]
- Karolewski P., Łukowski A., Walczak U., Baraniak E., Mucha J., Giertych M.J. Larval food affects oviposition preference, female fecundity and offspring survival in Yponomeuta evonymellus. Ecological Entomology. 2017;42(5):657–667. [Google Scholar]
- Kuussaari M., van Nouhuys S., Hellmann J.J., Singer M.C. Larval biology of checkerspots. In: Ehrlich P.R., Hanski I., editors. On the wings of checkerspots: A model system for population biology. Oxford University Press; Oxford, U.K.: 2004. pp. 138–160. [Google Scholar]
- Kuussaari M., Singer M.C. Group size and survival in eggs and larvae of the social butterfly Melitaea cinxia. Annales Zoologici Fennici. 2017;54:213–223. [Google Scholar]
- Kuznetsova A., Brockhoff P.B., Christensen R.H.B. lmerTest package: tests in linear mixed effects models. Journal of Statistical Software. 2017;82(13):1–26. [Google Scholar]
- Lee K.P., Kwon S.T., Roh C. Caterpillars use developmental plasticity and diet choice to overcome the early life experience of nutritional imbalance. Animal Behaviour. 2012;84(4):785–793. [Google Scholar]
- Lee K.P., Simpson S.J., Clissold F.J., Brooks R., Ballard J.W.O., Taylor P.W. Lifespan and reproduction in Drosophila: New insights from nutritional geometry. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(7):2498–2503. doi: 10.1073/pnas.0710787105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenhart P.A., Eubanks M.D., Behmer S.T. Water stress in grasslands: Dynamic responses of plants and insect herbivores. Oikos. 2015;124(3):381–390. [Google Scholar]
- Machovsky-Capuska G.E., Senior A.M., Simpson S.J., Raubenheimer D. The multidimensional nutritional niche. Trends in Ecology & Evolution. 2016;31(5):355–365. doi: 10.1016/j.tree.2016.02.009. [DOI] [PubMed] [Google Scholar]
- Maklakov A.A., Simpson S.J., Zajitschek F., Hall M.D., Dessmann J., Clissold F. Sex-specific fitness effects of nutrient intake on reproduction and lifespan. Current Biology. 2008;18(14):1062–1066. doi: 10.1016/j.cub.2008.06.059. [DOI] [PubMed] [Google Scholar]
- Massey F.P., Hartley S.E. Physical defences wear you down: Progressive and irreversible impacts of silica on insect herbivores. Journal of Animal Ecology. 2009;78(1):281–291. doi: 10.1111/j.1365-2656.2008.01472.x. [DOI] [PubMed] [Google Scholar]
- Mattson W.J., Haack R.A. The role of drought in outbreaks of plant-eating insects. BioScience. 1987;37(2):110–118. [Google Scholar]
- Monaghan P. Early growth conditions, phenotypic development and environmental change. Philosophical Transactions of the Royal Society B: Biological Sciences. 2008;363(1497):1635–1645. doi: 10.1098/rstb.2007.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morecroft M.D., Bealey C.E., Howells O., Rennie S., Woiwod I.P. Effects of drought on contrasting insect and plant species in the UK in the mid-1990s. Global Ecology and Biogeography. 2002;11(1):7–22. [Google Scholar]
- Mousseau T.A., Fox C.W. The adaptive significance of maternal effects. Trends in Ecology & Evolution. 1998;13(10):403–407. doi: 10.1016/s0169-5347(98)01472-4. [DOI] [PubMed] [Google Scholar]
- Nestel D., Papadopoulos N.T., Pascacio-Villafán C., Righini N., Altuzar-Molina A.R., Aluja M. Resource allocation and compensation during development in holometabolous insects. Journal of Insect Physiology. 2016;95:78–88. doi: 10.1016/j.jinsphys.2016.09.010. [DOI] [PubMed] [Google Scholar]
- Ojanen S.P., Nieminen M., Meyke E., Pöyry J., Hanski I. Long-term metapopulation study of the Glanville fritillary butterfly (Melitaea cinxia): Survey methods, data management, and long-term population trends. Ecology and Evolution. 2013;3(11):3713–3737. doi: 10.1002/ece3.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaj D.R., Prokopy R.J. Ecological and evolutionary aspects of learning in phytophagous insects. Annual Review of Entomology. 1989;34(1):315–350. [Google Scholar]
- Petersen C.R., Woods H.A., Kingsolver J.O.E.L.G. Stage-specific effects of temperature and dietary protein on growth and survival of Manduca sexta caterpillars. Physiological Entomology. 2000;25(1):35–40. [Google Scholar]
- R Core Team R. R Foundation for Statistical Computing; Vienna, Austria: 2015. R: A language and environment for statistical computing.https://www.R-project.org/ [Google Scholar]
- Radford P.J. Growth analysis formulae-their use and abuse. Crop Science. 1967;7(3):171–175. [Google Scholar]
- Rausher M.D. Larval habitat suitability and oviposition preference in three related butterflies. Ecology. 1979;60(3):503–511. [Google Scholar]
- Rodrigues M.A., Martins N.E., Balancé L.F., Broom L.N., Dias A.J., Fernandes A.S.D. Drosophila melanogaster larvae make nutritional choices that minimize developmental time. Journal of Insect Physiology. 2015;81:69–80. doi: 10.1016/j.jinsphys.2015.07.002. [DOI] [PubMed] [Google Scholar]
- Runagall-McNaull A., Bonduriansky R., Crean A.J. Dietary protein and lifespan across the metamorphic boundary: Protein-restricted larvae develop into short-lived adults. Scientific Reports. 2015;5:11783. doi: 10.1038/srep11783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saastamoinen M. Life-history, genotypic, and environmental correlates of clutch size in the Glanville fritillary butterfly. Ecological Entomology. 2007;32(2):235–242. [Google Scholar]
- Saastamoinen M., Hirai N., van Nouhuys S. Direct and trans-generational responses to food deprivation during development in the Glanville fritillary butterfly. Oecologia. 2013;171(1):93–104. doi: 10.1007/s00442-012-2412-y. [DOI] [PubMed] [Google Scholar]
- Saastamoinen M., van Nouhuys S., Nieminen M., O'Hara B., Suomi J. Development and survival of a specialist herbivore, Melitaea cinxia, on host plants producing high and low concentrations of iridoid glycosides. Annales Zoologici Fennici. 2007;44:70–80. [Google Scholar]
- Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T. Fiji: An open-source platform for biological-image analysis. Nature Methods. 2012;9(7):676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scriber J.M., Dowell R.V. Host plant suitability and a test of the feeding specialization hypothesis using Papilio cresphontes (Lepidoptera: Papilionidae) Great Lakes Entomologist. 2017;24(1):27–37. [Google Scholar]
- Showler A.T., Moran P.J. Effects of drought stressed cotton, Gossypium hirsutum L., on beet armyworm, Spodoptera exigua (Hübner) oviposition, and larval feeding preferences and growth. Journal of Chemical Ecology. 2003;29(9):1997–2011. doi: 10.1023/a:1025626200254. [DOI] [PubMed] [Google Scholar]
- Simpson S.J., Clissold F.J., Lihoreau M., Ponton F., Wilder S.M., Raubenheimer D. Recent advances in the integrative nutrition of arthropods. Annual Review of Entomology. 2015;60:293–311. doi: 10.1146/annurev-ento-010814-020917. [DOI] [PubMed] [Google Scholar]
- Simpson S.J., Raubenheimer D., Behmer S.T., Whitworth A., Wright G.A. A comparison of nutritional regulation in solitarious-and gregarious-phase nymphs of the desert locust Schistocerca gregaria. Journal of Experimental Biology. 2002;205(1):121–129. doi: 10.1242/jeb.205.1.121. [DOI] [PubMed] [Google Scholar]
- Swanson E.M., Espeset A., Mikati I., Bolduc I., Kulhanek R., White W.A. Nutrition shapes life-history evolution across species. Proceedings of the Royal Society B: Biological Sciences. 2016;283(1834):20152764. doi: 10.1098/rspb.2015.2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tack A.J., Mononen T., Hanski I. Increasing frequency of low summer precipitation synchronizes dynamics and compromises metapopulation stability in the Glanville fritillary butterfly. Proceedings of the Royal Society B: Biological Sciences. 2015;282(1806):20150173. doi: 10.1098/rspb.2015.0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J.N. Evolutionary ecology of the relationship between oviposition preference and performance of offspring in phytophagous insects. Entomologia Experimentalis et Applicata. 1988;47(1):3–14. [Google Scholar]
- Venables W.R., Ripley B.D. Springer; New York, NY: 2002. Modern applied statistics with S. [Google Scholar]
- Veteli T.O., Kuokkanen K., Julkunen-Tiitto R., Roininen H., Tahvanainen J. Effects of elevated CO2 and temperature on plant growth and herbivore defensive chemistry. Global Change Biology. 2002;8(12):1240–1252. [Google Scholar]
- White T.T. The abundance of invertebrate herbivores in relation to the availability of nitrogen in stressed food plants. Oecologia. 1984;63(1):90–105. doi: 10.1007/BF00379790. [DOI] [PubMed] [Google Scholar]
- Wise M.J., Weinberg A.M. Prior flea beetle herbivory affects oviposition preference and larval performance of a potato beetle on their shared host plant. Ecological Entomology. 2002;27(1):115–122. [Google Scholar]
- Woestmann L., Saastamoinen M. The importance of trans-generational effects in Lepidoptera. Current Zoology. 2016;62(5):489–499. doi: 10.1093/cz/zow029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuur A.F., Ieno E.N. A protocol for conducting and presenting results of regression-type analyses. Methods in Ecology and Evolution. 2016;7(6):636–645. [Google Scholar]