Abstract
Leishmaniasis is a neglected disease that affects more than 12 million people, with a limited therapy. Plant-derived natural products represent a useful source of anti-protozoan prototypes. In this work, four derivatives were prepared from neolignans isolated from the Brazilian plant Nectandra leucantha, and their effects against intracellular amastigotes of Leishmania (L.) infantum evaluated in vitro. IC50 values between 6 and 35 µM were observed and in silico predictions suggested good oral bioavailability, no PAINS similarities, and ADMET risks typical of lipophilic compounds. The most selective (SI > 32) compound was chosen for lethal action and immunomodulatory studies. This compound caused a transient depolarization of the plasma membrane potential and induced an imbalance of intracellular Ca2+, possibly resulting in a mitochondrial impairment and leading to a strong depolarization of the membrane potential and decrease of ATP levels. The derivative also interfered with the cell cycle of Leishmania, inducing a programmed cell death-like mechanism and affecting DNA replication. Further immunomodulatory studies demonstrated that the compound eliminates amastigotes via an independent activation of the host cell, with decrease levels of IL-10, TNF and MCP-1. Additionally, this derivative caused no hemolytic effects in murine erythrocytes and could be considered promising for future lead studies.
Introduction
Leishmaniasis, a neglected tropical disease caused by protozoan parasites of the Leishmania genus, affects more than 12 million people worldwide. Currently, this disease is present in 98 countries and it is estimated that 60,000 new cases occur every year in Latin America alone1. Human visceral leishmaniasis (VL) is the most severe clinical form of the disease, affecting internal organs such as the spleen, liver, bone marrow and lymph nodes. Usually, VL is fatal within two years without treatment, and the number of deaths ranges from 20,000 to 50,000 people annually1,2. The VL chemotherapeutic arsenal comprises just three main drugs (pentavalent antimonials, amphotericin B and miltefosine), which exhibit several limitations, including long administration regimens, hospitalization, high costs and severe adverse effects3. The search for new drugs therefore remains a necessity, especially for developing countries.
In this context, natural products are excellent prototypes for the synthesis of potent antiparasitic derivatives3,4, and indeed around 50% of all FDA-approved drugs are based in some form on natural product scaffolds5. In the search for such natural leads against VL, we previously described the anti-L. (L.) donovani activity of neolignans isolated from Nectandra leucantha Nees & Mart6. Among these, dehydrodieugenol B (Fig. 1) was the most selective, and also presented a promising immunomodulatory effect for visceral leishmaniasis. Recently, we described the anti-Trypanosoma cruzi activity of dehydrodieugenol B and its natural methylated derivative, and an in silico analysis revealed a promising safety profile for these compounds7,8. In continuation of this work, we here report the preparation and biological evaluation of four new semi-synthetic derivatives (1–4) of dehydrodieugenol B and methyl dehydrodieugenol B. The anti-L. (L.) infantum activity of compounds 1–4 was determined, and physicochemical properties were studied via an in silico approach. Finally, insight into the mechanism of lethal action of the most potent compound 2 was obtained using spectrofluorimetric assays, flow cytometry, and transmission electron microscopy, and its immunomodulatory potential in macrophages was also investigated.
Figure 1.
Chemical structures of natural products dehydrodieugenol B, methyl dehydrodieugenol B and semi-synthetic compounds 1–4.
Results
In vitro anti-L. (L.) infantum activity and mammalian toxicity
The activity of the four semi-synthetic derivatives against intracellular amastigotes of L. (L.) infantum was evaluated by light microscopy counting. The studied compounds were effective with 50% inhibitory concentration (IC50) values between 6.1 and 35.9 μM (Table 1). In the promastigote assays, the activity was evaluated using the MTT method and the results showed that all four compounds killed 100% of the parasites at the highest concentration. The IC50 values for the promastigote forms ranged from 29 to 105.4 μM. Miltefosine was used as standard and presented IC50 values in intracellular amastigotes and promastigotes of 6.5 and 5.1 μM, respectively. Compounds 1 and 3 showed cytotoxicity at the tested concentrations, with 50% cytotoxic concentration (CC50) values of 75.0 and 57.7 µM, respectively. Considering the relationship between activity against intracellular amastigotes, and cytotoxicity in NCTC cells, it is possible to calculate the selectivity index (SI) of the compounds, which ranged from 2.1 to >32.8. Compound 2 was found to be the most potent and selective compound, and was therefore selected for lethal action studies. A second IC50 value for compound 2 against promastigotes was evaluated (190 µM) after 2 h of incubation, and used for following mechanism of action assays.
Table 1.
Antileishmanial activity and mammalian cytotoxicity of the semi-synthetic compounds 1–4.
| Compound | IC50 (µM) ± SD | CC50 (µM) ± SD NCTC | SI | |
|---|---|---|---|---|
| Promastigote | Amastigote | |||
| 1 | 93.3 ± 8.4 | 35.9 ± 6.6 | 75.0 ± 13.8 | 2.1 |
| 2 | 105.4 ± 9.4 | 6.1 ± 1.2 | >200 | >32.8 |
| 3 | 29.6 ± 2.3 | 21.7 ± 1.9 | 57.7 ± 1.1 | 2.7 |
| 4 | 29.0 ± 0.2 | 34.5 ± 11.4 | >200 | >5.8 |
| Miltefosine | 5.1 ± 0.6 | 6.5 ± 3.0 | 119.7 ± 4.2 | 18.4 |
IC50: 50% Inhibitory Concentration; CC50: 50% Cytotoxic Concentration; SI: Selectivity Index, given by the ratio between CC50 in NCTC cells and IC50 in intracellular amastigotes; SD: Standard Deviation.
In silico analysis
Semisynthetic analogs 1–4 were evaluated in silico using two web-based platforms, FAF-Drugs4 and ADMETlab, to identify their potential for pharmacokinetic or toxicologic risks or liabilities (Table 2). All four semi-synthetic derivatives are predicted to have good oral bioavailability based on twelve physicochemical descriptors for oral drugs. None of the compounds contain structural similarities to pan-assay interference compounds (PAINs). The compounds are predicted to be non-mutagenic by the AMES test and non-inducers of phospholipidosis. Toxicity alerts associated with high lipophilicity were predicted and include: hERG, DILI, and human hepatoxocity. Structural modifications that reduce lipophilicity can eliminate or reduce these toxicities. As such, these toxicity alerts are not major at this stage. Compounds 1 and 2 were predicted to inhibit cytochrome P450 enzymes. Application of the Lilly Med Chem rules identified the phenolic ester of compound 1 as a risk. It is predicted to metabolize in vivo to yield a phenol. Overall, the in silico predictions indicate that compounds 1–4 represent a promising, orally bioavailable scaffold with no major risks predicted.
Table 2.
In silico prediction of druglike properties.
| Properties | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| MW | 382.5 | 420.5 | 330.4 | 340.4 |
| LogP* | 5.76 | 7.29 | 5.72 | 5.38 |
| LogP | 5.27 | 6.98 | 5.11 | 5.57 |
| TPSA** | 54 | 36.9 | 47.9 | 36.9 |
| Solubility** | low | low | low | low |
| Permeability (Caco-2) | moderate | moderate | moderate | moderate |
| Pgp inhibitor | yes | yes | yes | yes |
| Pgp substrate | yes | yes | yes | yes |
| HIA (>/=30%) | yes | yes | yes | yes |
| Oral bioavailability | ≥30% F | ≥20% F | ≥30% | ≥30% |
| Protein binding | moderate | moderate | moderate | moderate |
| Distribution (VD) | low | good | good | good |
| CYP3A4 inhibitor | yes | yes | no | no |
| CYP2C9 inhibitor | no | yes | no | no |
| CYP3A4 substrate | yes | yes | yes | yes |
| CYP2C9 substrate | yes | yes | yes | yes |
| Clearance | low | low | low | low |
| hERG | yes | yes | yes | yes |
| Hepatotoxicity | yes | no | yes | yes |
| AMES | no | no | no | no |
| DILI | yes | yes | yes | yes |
| Phospholipidosis* | no | no | no | no |
| Lilly Med Chem Rules* | phenolic ester | pass | pass | pass |
| PAINs* | no | no | no | no |
ADMETlab was used to predict all values except: *Predicted using FAF-drugs4 and **Predicted using both ADMETlab and FAF-drugs4.
Hemolytic activity
Hemolytic activity was evaluated by a colorimetric assay using BALB/c mice erythrocytes. Even after 2 h of treatment with compound 2, no hemolytic activity could be detected in the range between 1.6 to 200 μM (data not shown).
Mechanism of action studies
Plasma membrane integrity
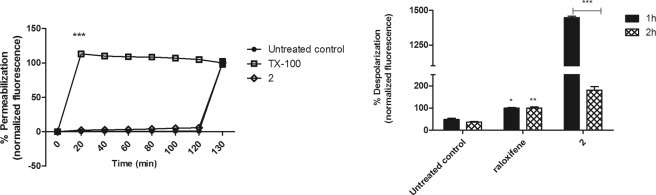
Damages in the plasma membrane permeability of L. (L.) infantum promastigotes were assessed using the fluorophore Sytox Green. No changes in the fluorescence levels were observed after treatment with compound 2, when compared to the untreated parasites (Fig. 2A). Therefore, the compound showed no influence on the plasma membrane permeability during 120 min of treatment. Triton X-100 was used to obtain the maximum permeabilization.
Figure 2.
Evaluation of plasma membrane permeabilization and potential in L. (L.) infantum promastigotes treated with compound 2 (190 μM). (A) The entrance of SYTOX Green dye was monitored spectrofluorimetrically (excitation 485 nm and emission 520 nm) every 20 min. Untreated promastigotes and treated with TX-100 (0.5%) were used to achieve minimal and maximal permeabilization, respectively. Fluorescence is reported as percentage relative to time 0 min (0%) and 120 min (100%). At 120 min, the addition of 0.5% TX-100 in all samples is represented. (B) DiSBAC2(3) dye fluorescence was measured by flow cytometry (excitation 488 nm and emission 574 nm) after 1 and 2 h of incubation. Untreated promastigotes and treated with raloxifene (60 μM) were used to achieve minimal and maximal depolarization, respectively. Fluorescence is reported as percentage relative to promastigotes treated with raloxifene (100%). A representative experiment is shown. *p < 0.05, **p < 0.01 e ***p < 0.0001.
Plasma membrane electric potential
By flow cytometry analysis using the probe DiSBAC2(3), changes in the Δψp of L. (L.) infantum promastigotes were investigated. According to membrane depolarization, dye fluorescence increases can be verified. At both treatment times (1 and 2 h), compound 2 induced a significant (p < 0.0001) depolarization of the potential when compared with untreated parasites (Fig. 2B). In addition, it was observed that the depolarization caused by compound 2 changed after 2 h, decreasing the fluorescence levels in a time-dependent manner. Raloxifene was used as a positive control and caused increased levels of fluorescence.
Mitochondrial membrane electric potential
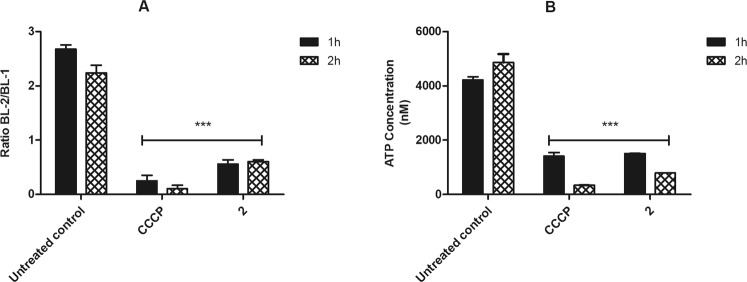
The Δψm was monitored using the fluorophore JC-1, using the ratio between BL-2/BL-1 channels in flow cytometry. According to the membrane depolarization, J-aggregates formation decreases (BL-2 fluorescence) and the monomers (BL-1 fluorescence) increases, leading to a decrease in the BL-2/BL-1 ratio. The effect of compound 2 in L. (L.) infantum promastigotes induced a significant (p < 0.0001) depolarization of the mitochondrial membrane potential after 1 and 2 h of incubation, when compared to untreated parasites (Fig. 3A). These results were similar to that obtained with the CCCP (positive control), a known mitochondrial uncoupler.
Figure 3.
Evaluation of mitochondrial membrane potential in L. (L.) infantum promastigotes treated with compound 2 (190 μM). (A) JC-1 dye fluorescence was measured by flow cytometry (excitation 488 nm and emission 530/574 nm) after 1 and 2 h of incubation. Untreated promastigotes and treated with CCCP (100 μM) were used to achieve minimal and maximal depolarization, respectively. Fluorescence is reported as the ratio between the emission channels BL2/BL1. (B) Evaluation of ATP concentration. ATP was measure spectrofluorimetrically after 1 and 2 h of incubation. Untreated promastigotes and treated with CCCP (25 μM) were employ to achieve minimal and maximal depolarization, respectively. Results are expressed in nM. A representative experiment is shown. ***p < 0.0001.
ATP levels
The ATP content in L. (L.) infantum promastigotes was evaluated using a bioluminescence assay. The treatment with compound 2 for 1 and 2 h resulted in a dose-dependent decrease of ATP concentration (p < 0.0001), when compared to untreated parasites (Fig. 3B). Additionally, these results present a similar ATP profile to those obtained with CCCP treatment, which was used as a positive control.
Reactive oxygen species (ROS)
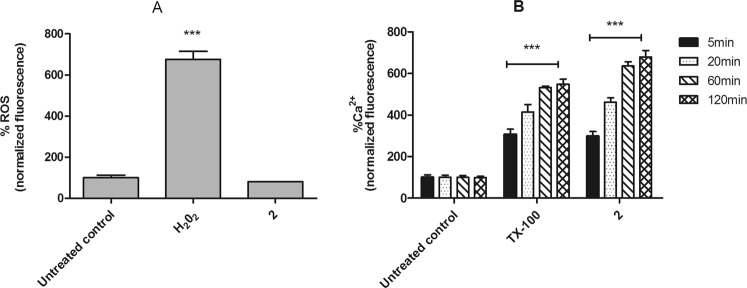
The levels of ROS were determined using the fluorophore H2DCFDA. The L. (L.) infantum promastigotes treated with the compound 2, showed similar ROS levels to those untreated parasites. H2O2 was used to obtain the maximum levels of reactive oxygen species (Fig. 4A).
Figure 4.
Evaluation of reactive oxygen species (ROS) and intracellular calcium levels in L. (L.) infantum promastigotes treated with compound 2 (190 μM). (A) H2DCFDA dye fluorescence was measured by spectrofluorimetrically (excitation 485 nm and emission 520 nm) after 2 h of incubation. Untreated promastigotes and treated with H2O2 (400 μM) were used to achieve minimal and maximal depolarization, respectively. (B) Fura-2 AM dye fluorescence was measured spectrofluorimetrically (excitation 360 nm and emission 500 nm) after 5, 20, 60 and 120 min of incubation. Untreated promastigotes and treated with TX-100 (0.5%) were used to achieve minimal and maximal depolarization, respectively. Fluorescence is reported as percentage relative to untreated promastigotes (100%). A representative experiment is shown. ***p < 0.0001.
Intracellular calcium (Ca2+)
The cytosolic Ca2+ levels were investigated in L. (L.) infantum promastigotes, by the changes in the florescence intensity of Fura-2 AM dye. The incubation with compound 2 induced a fast up-regulation of calcium levels, in a time-dependent manner. After five minutes of treatment, Ca2+ levels were significantly (p < 0.0001) higher than those obtained in the untreated parasites. Triton X-100 was used as a positive control (Fig. 4B).
Cell cycle analysis
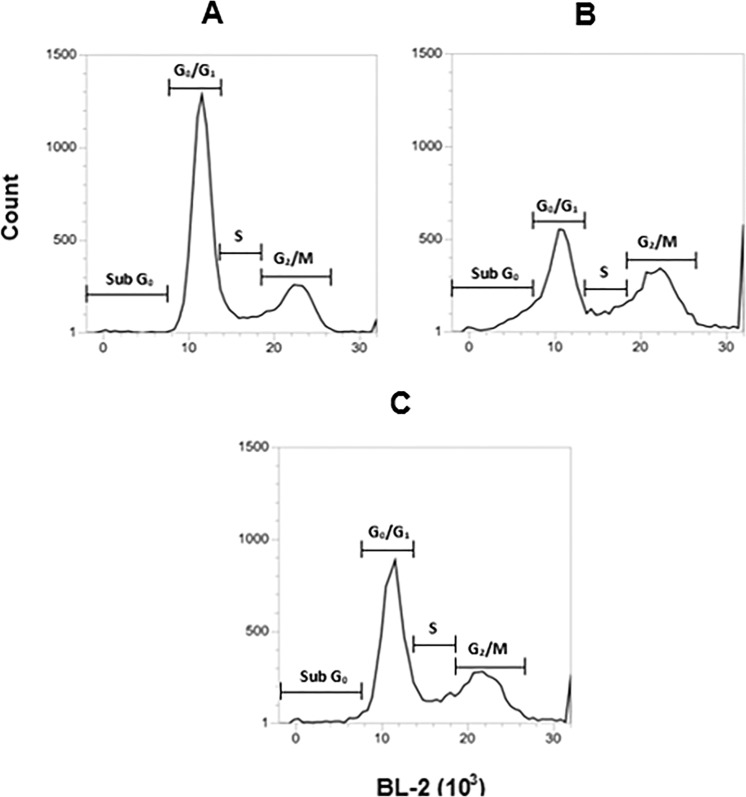
Cell cycle progression was evaluated in L. (L.) infantum promastigotes, using the fluorophore propidium iodide. By flow cytometry analysis, it was possible to observe an increase of BL-2 channel fluorescence, corresponding to an increased DNA content. According to the obtained results, treatment with compound 2 for 24 h resulted in significant changes (p < 0.05) in all phases of the cell cycle, when compared to untreated parasites (Fig. 5). Treatment with compound 2 clearly induced the decrease of G0/G1 cells percentage and increase of Sub G0, S and G2/M phases, a similar effect to that observed for the positive control, miltefosine (Table 3).
Figure 5.
Evaluation of cell cycle progression in L. (L.) infantum promastigotes treated with compound 2 (190 μM) by flow cytometry (488 nm excitation and 574 nm emission). Propidium iodide fluorescence was arranged in histograms. Untreated promastigotes and treated with miltefosine (25 μM) were employing to achieve minimal and maximal depolarization, respectively. A representative experiment is shown. (A) Untreated control, (B) miltefosine, and (C) compound 2. Sub G0: represent cells with fragmented DNA, cell death; G0/G1: diploid cells (2N); S: DNA replication; G2/M: cells with duplicate DNA content.
Table 3.
Cell cycle analysis in L. (L.) infantum promastigotes treated with compound 2.
| Groups | Proportion of promastigotes in different stages of cell cycle (% ± SD) | |||
|---|---|---|---|---|
| Sub G0 | G0/G1 | S | M/G2 | |
| Untreated control | 1.3 ± 0.1 | 67.5 ± 1.9 | 9.2 ± 0.3 | 20.9 ± 2.1 |
| 2 | 3.0 ± 0.2** | 52.3 ± 1.1** | 13.7 ± 0.7** | 26.4 ± 0.1* |
| Miltefosine | 10.1 ± 0.2*** | 36.4 ± 1.3*** | 12.3 ± 0.1* | 31.4 ± 0.5** |
Sub G0: fragmented DNA, cell death; G0/G1: diploid cells (2N); S: DNA replication; G2/M: cells with duplicated DNA content; SD: Standard deviation. *p < 0.05, **p < 0.01 and ***p < 0.001.
Ultrastructural studies
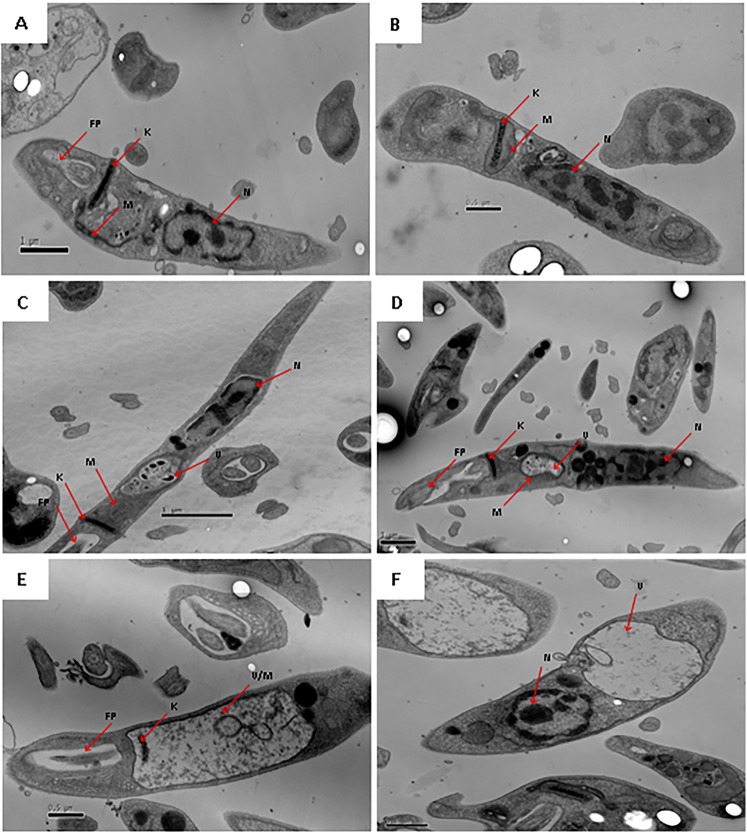
Using transmission electron microscopy, ultrastructural alterations of L. (L.) infantum promastigotes were investigated. Untreated cells demonstrated a normal morphology of cytoplasmic organelles and plasma membrane (Fig. 6A). At the initial time of incubation (30 min) with compound 2, the mitochondria begins to swell (Fig. 6B). During the incubation period ranging from 1 to 2 h, it was possible to observe an autophagic vacuole formation, and the presence of lipid droplets aggregated around the nucleus (Fig. 6C,D). At 4 and 6 h, there was an intense swelling of the mitochondria with severe loss of cristae and matrix and concentric membranous structures inside this organelle (Fig. 6E,F). Despite significant alterations in the mitochondria, the plasma membrane, kinetoplast DNA and flagellar pocket remained preserved, as well as the nucleus.
Figure 6.
Evaluation of ultrastructural changes in L. (L.) infantum promastigotes treated with compound 2 (190 μM), by transmission electron microscopy. Representative images are shown. (A) Untreated control, (B) 30 min, (C) 1 h, (D) 2 h, (E) 4 h and (F) 6 h of incubation with compound 2. K: kinetoplast; N: nucleus; M: mitochondria; V: vacuole and FP: flagellar pocket.
Immunomodulatory studies
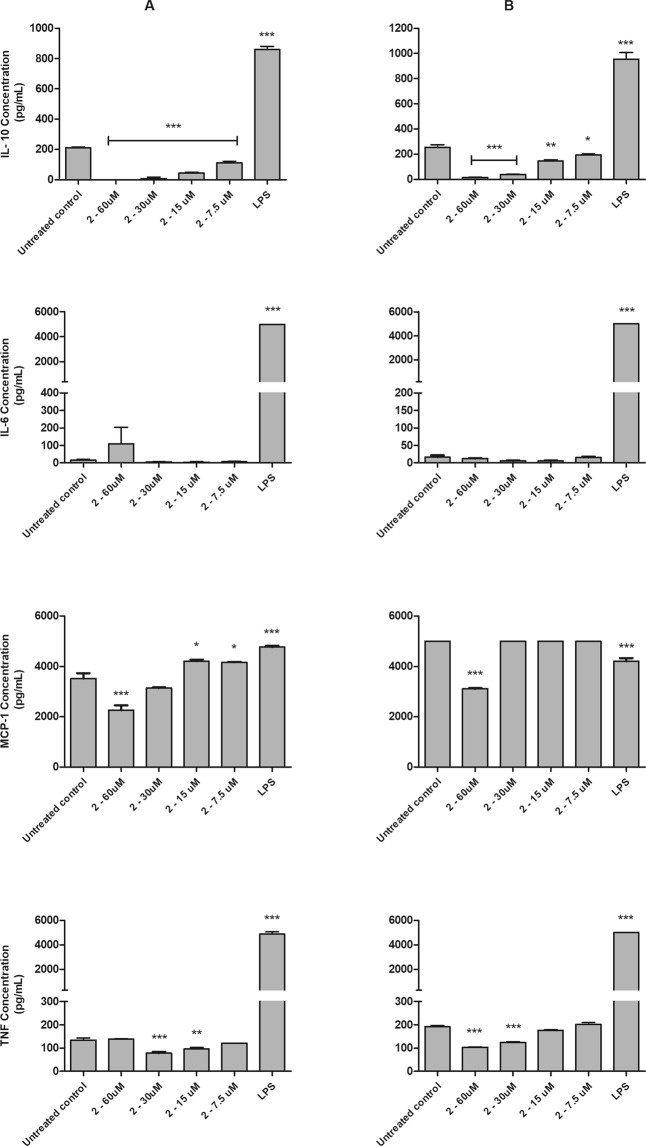
Using flow cytometry analysis, the cytokine profile of L. (L.) infantum-infected macrophages was determined in the presence of compound 2 (Fig. 7). Uninfected macrophages were also used for comparison. This compound induced a significant (p < 0.05) concentration-dependent decrease in the IL-10 levels of both macrophage groups, when compared to the untreated macrophages. No changes were observed in the IL-6 profile. The compound significantly (p < 0.05) reduced the TNF production of infected and uninfected macrophages at 30 and 15 μM and 60 and 30 μM, respectively. In addition, MCP-1 data demonstrated that, at an elevated concentration of 60 μM, compound 2 was able to significantly (p < 0.0001) decrease this chemokine in both macrophage groups. Conversely, at lower concentrations, the compound induced an increase of MCP-1. In the uninfected macrophages, this data was significant only at 15 and 7.5 μM, when compared to the untreated macrophages. LPS was used as positive control and increased the amount of all studied cytokines.
Figure 7.
Evaluation of cytokines profile (IL-10, IL-6, MCP-1 and TNF) in bone marrow-derived macrophages treated with compound 2 (60 a 7.5 µM) and untreated. Cytokines were measure with CBA Mouse Inflammation Kit by flow cytometry after 48 h of incubation. Untreated and LPS (50 µg/mL) treated bone marrow-derived macrophages were used to achieve minimal and maximal cytokine levels, respectively. Results are expressed in pg/mL. A representative experiment is shown. (A) bone marrow-derived macrophages (B) bone marrow-derived macrophages infected with L. (L.) infantum. *p < 0.05, **p < 0.01 e ***p < 0.0001.
Nitric oxide (NO) levels
The NO concentrations in bone marrow-derived macrophages was determined in the culture supernatant by the colorimetric Griess assay. Both untreated and treated macrophages with compound 2 (60 to 7.5 μM) did not produced detectable levels of NO, after 48 h of incubation (data not shown). LPS was used as positive control and increased the amount of NO in the studied time.
Discussion
A number of plant-derived secondary metabolites have been reported to show excellent antiparasitic potential against Leishmania parasites, including alkaloids, phenylpropanoids, saponins, flavonoids, lignoids, naphthoquinones, and iridoids9. In the present study, four novel derivatives of dehydrodieugenol B and methyl dehydrodieugenol B were synthesized and demonstrated activity against extracellular and intracellular forms of L. (L.) infantum. Compound 2 was identified as the most promising of this set, as it eliminated 100% of the amastigotes at the highest tested concentration, without affecting macrophage viability; it presented a selectivity index approximately 3 times higher than that of the dehydrodieugenol B. As compound 2 showed no toxicity to murine fibroblasts, its hemolytic activity was also evaluated in erythrocytes and the compound showed no hemolytic activity to the highest tested concentrations.
In contrast, derivatives 1 and 3 displayed significant toxicity profiles, which is likely due to the phenol motif present in these compounds. However, the maintained bioactivity of derivatives 2 and 4 shows that modification of this problematic motif is well-tolerated, providing opportunities for further development. In addition, modification of the allylic sidechains of the natural products is also possible without significant detriment to bioactivity. In light of these results, compound 2 was selected for mechanism of action studies.
Leishmania promastigotes were 17-fold more susceptible to compound 2 than intracellular amastigotes. Differential drug susceptibilities between promastigotes and amastigotes have been observed in literature, with10 or without host cell activation11. Besides the host cell influences, this effect could also be ascribed to the distinct metabolisms. An untargeted metabolomic study identified substantial differences between the two life stages. Compared to promastigotes, amastigotes showed decreased pools of metabolites and amino acids of the polyamine biosynthesis, alterations in the phospholipids and increased sterols. Additionally, amastigotes showed a decrease in ATP levels, kDNA mini-circles, RNA and proteins and also demonstrated lower capacity of biosynthesis, with a reduced metabolism12. Another comparative study of promastigotes metabolome of three Leishmania species, also confirmed the large differences in the extent of amino acid use and metabolism13. These metabolic differences can result in different drug susceptibilities between extracellular and intracellular forms of Leishmania, but other mechanisms related to host cells can influence the drug efficacy. In our assay, although no macrophage activation was observed, the incubation with compound 2 could have altered the intracellular transport/abundance of metabolites of the host cell, and consequently, might have affected the Leishmania survival in the intracellular milieu. Additionally, macrophages can also metabolize drugs, resulting in metabolites with enhanced or reduced activity/toxicity14. Due to this capacity, small chemical motifs can be coupled to drugs or compounds, aiming to increase the activity inside the macrophages15. Considering the several features that may influence the activity of compounds in the Leishmania-intracellular assay, additional studies will be mandatory to evaluate the action of these neolignan derivatives.
In silico approaches are extremely valuable for profiling new hit compounds, prioritizing experimental studies, and early risk identification. According to the selected filters, it was possible to predict some ADMET characteristics, as well as undesirable chemical groups16. The results obtained by the in silico analysis showed that the semi-synthetic derivatives present acceptable oral availability, no potential to induce phospholipidosis, are non-mutagenic, and do not resemble PAINS, corroborating previous studies with the prototype dehydrodieugenol B6. Risks associated with high lipophilicity were identified. The four new neolignans are predicted to exhibit hERG inhibition and drug induced liver injury (DILI). Three of the four are predicted to exhibit human hepatotoxicity. Compound 1 contains a phenolic ester, which may metabolize in vivo to a phenol-containing compound and, like any experimental agent, therefore requires further structural optimization. The in silico results enabled prioritization of follow-up studies and suggest that reducing lipophilicity, and replacement of the phenolic ester, should improve the safety of compound 1.
Mechanism of action studies provide vital information in the drug development process and also in the search for new biochemical targets17. The plasma membrane regulates the transport of nutrients, ions and pH homeostasis; due to its differential chemical composition and its essential role in parasite survival, the study of plasma membrane effects becomes indispensable when investigating new hits3. In previous studies, plasma membrane permeabilization was verified in Trypanosoma cruzi parasites treated with other neolignans such as dihydrodieugenol B8 and 1-[(7S)-hydroxy-8-propenyl]-3-[3′-methoxy-1′-(8′-propenyl)-phenoxy]-4,5-methoxybenzene7. In the present work, the transmission electron microscopy data demonstrated no alteration of the L. (L.) infantum promastigote plasma membrane in the presence of 2, corroborating the spectrofluorimetric study and confirming that neither pore-forming nor permeabilization activities are present.
Variations of the plasma membrane electric potential are extremely harmful to the parasite, affecting metabolite transportation and reducing the acquisition of essential nutrients18. The results obtained in this study demonstrate that compound 2 caused an intense depolarization of L. (L.) infantum promastigote plasma membrane potential, with a biological tendency for polarization as the incubation time increases. In this context, it is possible to suggest that compound 2 induced a reversible depolarization in Leishmania due to its penetration into the cell.
Unlike mammalian cells, trypanosomatids present single mitochondria that are essential for survival, making this organelle a target for new chemotherapeutics19. In previous research, neolignans eupomatenoid-520 and 1-[(7S)-hydroxy-8-propenyl]-3-[3′-methoxy-1′-(8′-propenyl)-phenoxy]-4,5-methoxybenzene8 were found to induce depolarization of the mitochondrial membrane in L. (L.) amazonensis and Trypanosoma cruzi parasites, respectively. In the present study, it was possible to verify an intense depolarization induced by compound 2. In addition, transmission electron microscopy studies have confirmed that compound 2 caused an intense swelling of mitochondria, with loss of cristae and matrix at later incubation times. Due to the early mitochondrial changes, this organelle might be a possible target of compound 2 in Leishmania.
Adenosine triphosphate (ATP) is a universal mediator of metabolism and signaling, being produced through the oxidative phosphorylation21. Depolarization of the mitochondrial membrane potential induces the collapse of the respiratory chain and lower ATP levels could generate a breakdown in the parasite metabolism, leading to cell death22,23. The present study also demonstrated a time dependent decrease in ATP levels in promastigotes treated with compound 2. Mitochondria is also the largest source of reactive oxygen species (ROS)24; in excess, these species can cause irreversible cellular damages21. In the present study, promastigotes treated with compound 2 showed no alteration of ROS, suggesting that antioxidant metabolism can regulate these levels.
Calcium ions (Ca2+) are important in the regulation of several signaling pathways, and are essential for trypanosomatid survival25. The intramitochondrial Ca2+ concentration is responsible for several key-enzymes activation; an exacerbated increase of its concentration induces the formation of high conductance channels across the mitochondrial membranes, leading to the electrical potential dissipation26,27. In the present study, a time-dependent increase of calcium levels was observed in the treated parasites, suggesting that mitochondrial membrane depolarization may be ascribed to this effect.
The use of chemotherapeutic agents that target the cell division mechanism can cause serious cellular disorders, leading to the parasite death or proliferation inhibition. Promastigotes treated with compound 2 resulted in an increased number of cells in Sub G0 phase, indicating that the DNA content is fragmented. Apoptosis-like programmed cell death was reported in protozoans28, including Leishmania parasites treated with miltefosine29, but the increase in cells in Sub G0 after treatment with compound 2 was modest and could not be considered apoptosis. Additionally, the increased number of parasites in the S and G2/M phases and the decrease of G0/G1 cells suggests that compound 2 may impair the DNA replication mechanism, and consequently cause mitosis.
In addition to direct effects on the parasite, drugs can also activate host cell defenses, contributing to disease control30. In the present study, compound 2 demonstrated no capacity to stimulate host cells, suggesting a lethal mechanism independent of NO activation. Other neolignans as licarin A31 and dehydrodieugenol B6, also showed an anti-Leishmania effect without NO upregulation.
Cytokines play different roles during infection by Leishmania parasites32. The present results showed that compound 2 reduced IL-10 production in a concentration-dependent manner in Leishmania-infected and uninfected macrophages. However, IL-6 levels remained unchanged. Studies with dehydrodieugenol B also demonstrated a down-regulation of IL-10 levels in macrophages infected by L. (L.) donovani6. Considering that the decrease of IL-10 levels is a positive aspect for the disease control, the observed effect could contribute to an improved efficacy.
Decreased levels of MCP-1 and TNF was also observed in macrophages treated with compound 2. In Leishmania-infected macrophages, treatment with MCP-1 induced pro-inflammatory cytokines and increased nitric oxide levels with reduced parasitic loads33,34. TNF is essential for the parasite growth control, and increased levels of this cytokine contribute to macrophage activation35. In the present study, the decrease of MCP-1 and TNF suggest a direct lethal effect of the compound towards the intracellular amastigotes, independent of host cell activation.
Conclusion
In this work, four semi-synthetic neolignan derivatives were found to exhibit promising activity against the intracellular forms of L. (L.) infantum. Investigations into the mechanism of action of the most promising derivative (2) suggested an impairment of mitochondria and cell division machinery, and an antileishmanial efficacy that is independent of host cell activation. These results suggest that compound 2 may be a prototype for future optimization studies, and work towards this end is underway in our groups.
Methods
General experimental procedures
Silica gel (Merck, 230–400 mesh) and Sephadex LH-20 (Sigma-Aldrich) were used for column chromatography. For all extraction and chromatography procedures, analytical grade solvents (Merck) were used. IR spectra were obtained on a Shimadzu IR Prestige-21 spectrophotometer. 1H and 13C NMR spectra were recorded at 300 and 75 MHz respectively on a Bruker Ultrashield 300 Avance III spectrometer, and at 400 and 100 MHz respectively on a Bruker AVIIIHD 400 nanobay spectrometer. CDCl3 (Aldrich) was used as the solvent, with TMS as reference. HRESIMS spectra were measured on a Bruker Daltonics MicroTOF QII spectrometer.
Plant material
Information concerning the source, identification and voucher number of the botanical material have been reported previously36.
Extraction and isolation of dehydrodieugenol B and methyl dehydrodieugenol B
Similarly to the already described procedure8, the n-hexane extract from twigs of N. leucantha (10 g) was initially subjected to column chromatography on silica gel, eluted with an increasing proportion of EtOAc in n-hexane to afford 75 fractions (150 mL each), which were pooled in eight groups (A to H). Group C (1.3 g) was composed of pure methyl dihydrodieugenol B. Group E (1.5 g) was subjected to further CC on Sephadex LH-20 (h = 52 cm), eluting with MeOH and yielding 40 fractions, which were pooled in three groups (E1 to E3). Dihydrodieugenol B was obtained in pure form from group E2 (1.2 g).
Dehydrodieugenol B, IR (film) νmax 3435, 2950, 2851, 1643, 1512, 1461, 1379, 1161, 976, 912, 831, 724, 590 cm−1; 1H NMR (CDCl3, 300 MHz) δ 6.89 (1H, d, J = 8.1 Hz, H-5′), 6.79 (1H, d, J = 2.0 Hz, H-2′), 6.70 (1H, dd, J = 8.1 and 2.0 Hz, H-6′), 6.49 (1H, d, J = 1.8 Hz, H-2), 6.40 (1H, d, J = 1.8 Hz, H-6), 5.84–6.01 (2H, m, H-8/H-8′), 5.00–5.13 (4H, m, H-9/H-9′), 3.89 (3H, s, 5-OCH3), 3.86 (3H, s, 3′-OCH3), 3.36 (2H, d, J = 6.6 Hz, H-7′), 3.24 (2H, d, J = 6.6 Hz, H-7); 13C NMR (CDCl3, 75 MHz) δ 150.4 (C-3′), 147.8 (C-5), 144.4 (C-3), 144.2 (C-4′), 137.4 (C-8′), 137.2 (C-8), 136.4 (C-1′), 135.2 (C-4), 131.0 (C-1), 120.8 (C-6′), 119.5 (C-5′), 116.0 (C-9′), 115.7 (C-9), 112.9 (C-2′), 111.8 (C-6), 107.3 (C-2), 56.2 (5-OCH3), 55.9 (3′-OCH3), 40.0 (C-7), 39.9 (C-7′); HRESIMS m/z 327.1595 [M + H]+ (calc. for C20H23O4, 327.1596).
Methyl dehydrodieugenol B, IR (film) νmax 2955, 2850, 1642, 1510, 1460, 1384, 1163, 978, 915, 832, 724, 593 cm−1; 1H NMR (CDCl3, 300 MHz) δ 6.81 (1H, d, J = 8.1 Hz, H-5′), 6.69 (1H, dd J = 8.1 and 2.0 Hz, H-6′), 6.79 (1H, d, J = 2.0 Hz, H-2′), 6.48 (1H, d, J = 1.8 Hz, H-2), 6.27 (1H, d, J = 1.8 Hz, H-6), 5.83–6.03 (2H, m, H-8/H-8′), 5.00–5.13 (4H, m, H-9/H-9′), 3.87 (6H, s, 4-OCH3/3′-OCH3), 3.83 (3H, s, 5-OCH3), 3.37 (2H, d, J = 6.6 Hz, H-7′), 3.24 (2H, d, J = 6.6 Hz, H-7); 13C NMR (CDCl3, 75 MHz) δ 153.5 (C-3′), 150.6 (2C, C-5/C-4′), 144.1 (C-3), 138.1 (C-4), 137.4 (C-8), 137.1 (C-8′), 136.0 (C-1′), 135.5 (C-1), 120.7 (C-6′), 119.4 (C-5′), 115.9 (2C, C-9/C-9′), 113.1 (C-2′), 111.4 (C-6), 107.3 (C-2), 61.0 (4-OCH3), 56.1 (5-OCH3), 56.0 (3′-OCH3), 40.1 (C-7′), 40.0 (C-7); HRESIMS m/z 341.1753 [M + H]+ (calc. for C21H25O4, 341.1753).
Preparation of semi-synthetic compounds
The compounds are shown in Fig. 1.
1-Allyl-3-(1′-allyl-3′-methoxyphenoxy)-5-methoxyphenyl-4-propionate (1)
To dehydrodieugenol B (24 mg, 0.071 mmol) in CH2Cl2 (0.12 mL) at 0 °C was added Et3N (19 µL, 0.14 mmol, 2.0 equiv.), 4-dimethylaminopyridine (DMAP) (0.8 mg, 7.0 µmol, 0.1 equiv.), and propionyl chloride (6.2 µL, 0.071 mmol, 1.0 equiv.). The reaction mixture was warmed to room temperature, and stirred for 3 h. The mixture was then concentrated, and the resulting residue was dissolved in water (1 mL) and extracted with EtOAc (4 × 1 mL). The combined organic phases were dried (MgSO4), filtered and concentrated. The crude product was purified by flash chromatography (silica gel, petroleum ether/EtOAc 8:2 eluent) to afford 1 (17.5 mg, 0.046 mmol, 64%) as a pale yellow oil. IR (film) νmax 2938, 1766, 1638, 1506, 1453, 1338, 1183, 1128, 914, 882, 752 cm−1; 1H NMR (CDCl3, 400 MHz) δ 6.85 (1H, d, J = 8.1 Hz, H-5′), 6.78 (1H, d, J = 1.8 Hz, H-2′), 6.69 (1H, dd, J = 8.1 and 1.8 Hz, H-6′), 6.50 (1H, d, J = 1.8 Hz, H-2), 6.29 (1H, d, J = 1.8 Hz, H-6), 5.83–6.02 (2H, m, H-8/H-8′), 5.02–5.12 (4H, m, H-9/H-9′), 3.81 (6H, s, 3′-OCH3/5-OCH3), 3.36 (2H, d, J = 6.7 Hz, H-7), 3.26 (2H, d, J = 6.7 Hz, H-7′), 2.53 (2H, q, J = 7.5 Hz, CH2CH3), 1.18 (3H, t, J = 7.5 Hz, CH2CH3); 13C NMR (CDCl3, 100 MHz) δ 172.1 (C=O), 152.4 (C-3′), 150.8 (C-5), 150.1 (C-4′), 143.7 (C-3), 138.2 (C-4), 137.3 (C-8), 136.8 (C-8′), 136.5 (C-1′), 128.7 (C-1), 120.9 (C-6′), 120.3 (C-5′), 116.2 (C-9), 115.9 (C-9′), 113.1 (C-2′), 110.9 (C-6), 106.9 (C-2), 56.2 (5-OCH3), 56.1 (3′-OCH3), 40.2 (C-7′), 40.0 (C-7), 27.1 (CH2CH3), 9.2 (CH2CH3); HRESIMS m/z 405.1669 [M + Na]+ (calc. for C23H26O5Na 405.1672).
1-Propyl-3-(1′-propyl-3′-methoxyphenoxy)-5-methoxy-4-benzoyloxybenzene (2)
To a suspension of NaH (3.6 mg, 60% in mineral oil, 0.090 mmol, 1.5 equiv.) in DMF (0.3 mL) at 0 °C was added compound 3 (20 mg, 0.060 mmol). Benzyl bromide (11 µL, 0.10 mmol, 1.67 mmol) was added, and the mixture was stirred at 0 °C for 3 h. After this period, the reaction was quenched by addition of NH4Cl (1 mL, aq., sat.) and extracted with EtOAc (3 × 1 mL). The combined organic extracts were dried over MgSO4 and concentrated. The crude product was purified by flash chromatography (silica gel, petroleum ether/EtOAc 8:2 eluent) to afford 2 (19.9 mg, 0.048 mmol, 80%) as a pale yellow oil. IR (film) νmax 2957, 2870, 1585, 1507, 1452, 1376, 1212, 1154, 1098, 995, 825, 731, 666 cm−1; 1H NMR (CDCl3, 400 MHz) δ 7.39–7.26 (5H, m, ArH), 6.77 (1H, d, J = 1.9 Hz, H-2′), 6.74 (1H, d, J = 8.1 Hz, H-5′), 6.66 (1H, dd, J = 8.1 and 1.9 Hz, H-6′), 6.48 (1H, d, J = 1.9 Hz, H-2), 6.33 (1H, d, J = 1.9 Hz, H-6), 5.04 (2H, s, ArCH2O), 3.83 (3H, s, 3′-OCH3), 3.80 (3H, s, H-5′), 2.56 (2H, t, J = 7.5 Hz, H-7′), 2.45 (2H, t, J = 7.5 Hz, H-7), 1.66 (2H, sext., J = 7.5 Hz, H-8′), 1.55 (2H, sext, J = 7.5 Hz, H-8), 0.96 (3H, t, J = 7.5 Hz, H-9′), 0.89 (3H, t, J = 7.5 Hz, H-9); 13C NMR (CDCl3, 100 MHz) δ 153.7 (C-3′), 150.4 (C-5), 150.1 (C-4′), 144.2 (C-3), 138.4 (C-4), 138.4 (Ar), 138.0 (C-1′), 136.7 (C-1), 128.3 (Ar), 128.0 (Ar), 127.5 (Ar), 120.6 (C-6′), 118.7 (C-5′), 112.9 (C-2′), 111.7 (C-6), 107.5 (C-2), 75.0 (OCH2Ar), 56.1 (5-OCH3), 55.9 (3′-OCH3), 38.1 (C-7′), 37.9 (C-7), 24.7 (C-8′), 24.5 (C-8), 13.9 (C-9′), 13.8 (C-9); HRESIMS m/z 443.2191 [M + Na]+ (calc. for C27H32O4Na 443.2193).
1-Propyl-3-(1′-propyl-3′-methoxyphenoxy)-4,5-dimethoxybenzene (3)
To dehydrodieugenol B (100 mg, 0.30 mmol) in EtOH (5 mL) was added 10% Pd/C (10 mg, 0.090 mmol). The reaction flask was purged with hydrogen, and the reaction was stirred for 1 hour. The solution was filtered through Celite and the filtrate was concentrated to give 3 (99 mg, 0.29 mmol, 99%) as a yellow oil, which was of sufficient purity not to require further purification. IR (film) νmax 3433, 2951, 2850, 1643, 1460, 1373, 1160, 911, 830, 725, 592 cm−1; 1H NMR (CDCl3, 400 MHz,) δ 6.87 (1H, d, J = 8.1 Hz, H-5′), 6.79 (1H, d, J = 1.9 Hz, H-2′), 6.69 (1H, dd, J = 8.1 and 1.9 Hz, H-6′), 6.48 (1H, d, J = 1.9 Hz, H-2), 6.40 (1H, d, J = 1.9 Hz, H-6), 5.85 (1H, s, OH), 3.89 (3H, s, 3′-OCH3), 3.87 (3H, s, 5-OCH3), 2.56 (2H, t, J = 7.5 Hz, H-7), 2.45 (2H, t, J = 7.5 Hz, H-7′), 1.65 (2H, sext, J = 7.5 Hz, H-8), 1.55 (2H, sext, J = 7.5 Hz, H-8′), 0.95 (3H, t, J = 7.5 Hz, H-9), 0.89 (3H, t, J = 7.3 Hz, H-9′); 13C NMR (CDCl3, 100 MHz) δ 150.2 (C-3′), 147.6 (C-5), 144.3 (C-3), 143.9 (C-4′), 139.1 (C-1′), 134.8 (C-4), 133.8 (C-1), 120.7 (C-6′), 119.3 (C-5′), 112.8 (C-2′), 111.7 (C-6), 107.1 (C-2), 56.2 (5-OCH3), 56.0 (3′-OCH3), 37.9 (2 C, C-7/7′), 24.7 (C-8), 24.7 (C-8′), 13.9 (C-9), 13.7 (C-9′); HRESIMS m/z 353.1720 [M + Na] + (calc. for C20H26O4Na 353.1723).
1-Prop-7-enyl-3-(1′-prop-7′-enyl-3′-methoxyphenoxy)-4,5-dimethoxybenzene (4)
A mixture of methyl dehydrodieugenol B (50 mg, 0.146 mmol) and solid KOH (81 mg, 1.46 mmol) in ethylene glycol (0.2 mL) was heated at 150 °C (bath temperature) for 16 hours. The reaction was cooled to room temperature, then diluted with H2O and extracted with EtOAc (4 × 1 mL). The combined organic extracts were dried over MgSO4 and concentrated. The crude product was purified by flash chromatography (silica gel, petroleum ether/EtOAc 8:2 eluent) to afford 4 (49 mg, 0.144 mmol, 98%) as a pale yellow oil. IR (film) νmax 2936, 1576, 1504, 1463, 1416, 1337, 1154, 1088, 960, 784, 665, 646 cm−1; 1H NMR (CDCl3, 400 MHz) δ 6.96 (1H, d, J = 1.9 Hz, H-2′), 6.83 (2H, m, H-5′/H-6′), 6.64 (1H, d, J = 1.9 Hz, H-2), 6.41 (1H, d, J = 1.9 Hz, H-6), 6.37 (1H, dd, J = 15.7 and 1.9 Hz, H-7), 6.14–6.24 (2H, m, H-7′/H-8′), 6.00–6.10 (1H, m, H-8), 3.89 (3H, s, 5-OCH3), 3.87 (3H, s, 3´-OCH3), 3.85 (3H, s, 4-OCH3), 1.88 (3H, dd, J = 6.6 and 1.5 Hz, H-9′), 1.81 (3H, dd, J = 6.5 and 1.5 Hz, H-9); 13C NMR (CDCl3, 100 MHz) δ 153.6 (C-3′), 150.7 (C-5), 150.7 (C-4′), 144.7 (C-3), 138.8 (C-4), 134.3 (C-1′), 133.6 (C-1), 130.5 (C-7), 130.4 (C-7′), 125.5 (C-8), 125.1 (C-8′), 119.6 (C-6′), 118.6 (C-5′), 109.8 (C-2′), 108.9 (C-6), 104.5 (C-2), 61.1 (4-OCH3), 56.1 (5-OCH3), 56.0 (3′-OCH3), 18.4 (C-9), 18.3 (C-9′); HRESIMS m/z 363.1563 [M + Na]+ (calc. for C21H24O4Na 363.1567).
Animals
Male golden hamsters (Mesocricetus auratus, 120 g) and female BALB/c mice (20 g) were obtained from the animal breeding facility at the Adolfo Lutz Institute-SP, Brazil. The animals were maintained in sterilized cages under a controlled environment, receiving water and food ad libitum. All procedures performed were previously approved by the Animal Care and Use Committee from Instituto Adolfo Lutz – Secretary of Health of Sao Paulo State (Project Number CTC 21H/2015, CEUA 04/2016) in agreement with the Guide for the Care and Use of Laboratory Animals from the National Academy of Sciences. The Animal Care and Use Committee was composed by the following members: Raquel dos Anjos Fazioli (Coordinator), Alcina Maria Liserre (Vice-Coordinator), Carmen Silvia Kira, Cristina da Silva Meira Strejevich, José Eduardo de Raeffray Barbosa, Mariana Sequetin Cunha, Roberta Morozetti Blanco, Roberto Colozza Hoffmann and Rodrigo Albergaria Réssio.
Parasites and mammalian cell maintenance
L. (L.) infantum (MHOM/BR/1972/LD) promastigotes were maintained in M-199 medium (Sigma-Aldrich) supplemented with 10% fetal bovine serum (FBS, Gibco), 0.25% hemin (Sigma-Aldrich), and 5% human urine at 24 °C. Amastigotes were obtained from the spleen of golden hamsters previously infected and purified by differential centrifugation37. Peritoneal macrophages were collected by washing the peritoneal cavity of BALB/c mice with RPMI-1640 medium (Sigma-Aldrich) supplemented with 10% FBS, and were maintained at 37 °C in a 5% CO2 humidified incubator. Murine fibroblasts NCTC (clone L929, ATCC) were maintained in RPMI-1640 supplemented with 10% FBS at 37 °C in a 5% CO2 humidified incubator. Bone marrow-derived macrophages were isolated from long bones (femurs and tibias) of BALB/c mice and maintained for approximately seven days at 37 °C in a 5% CO2 humidified incubator38.
Evaluation of in vitro anti-L. (L.) infantum activity
Amastigotes
Peritoneal macrophages (1 × 105 cell/well) in 16-well slide chambers (NUNC) were infected with amastigotes at a ratio of 10:1 (amastigotes/macrophage) and treated with compounds (60 to 10 µM) for 96 h. Stained slides (Giemsa) were counted using light microscopy and IC50 determined by the infection index39. Miltefosine was used as standard and untreated cells as a negative control.
Promastigotes
Promastigotes (1 × 106 parasites/well) in 96-well plates were incubated with the four compounds (150 to 1.2 μM) for 96 h at 24 °C. The parasite viability was determined using the MTT colorimetric method40. Miltefosine was used as standard, with untreated cells as a negative control. A parallel promastigote activity assay was performed for 2 h with compound 2 (200 to 1.56 μM) for mechanism of action studies. After this period of incubation with compound 2, the parasites were washed twice with M-199 medium and the parasite viability was determined using the MTT colorimetric method for 4 h incubation at 24 °C.
Evaluation of in vitro mammalian toxicity
Fibroblast NCTC cells clone 929 (6 × 104 cells/well) in 96-well plates were incubated with the compounds up to 200 μM in a 5% CO2 humidified incubator at 37 °C. CC50 was determined by the MTT colorimetric method40. The selectivity index was determined using the following equation: CC50 against NCTC cells/IC50 against amastigotes
In silico physical-chemical properties, ADMET and PAINS analysis
Pharmacokinetic and toxicological risks were predicted in silico using two web based servers FAF-Drugs441 and ADMTETlab42, each server is a suite of predictive models. The FAF-Drugs4 suite includes models for the prediction of physiochemical properties, solubility, oral bioavailability, drug likeness, phospholipidosis, PAINs compounds, and Lilly Med Chem Rules16. The Lilly MedChem Rules consist of 275 descriptors developed by Lilly using experimental data collected over 18 years. The rules were developed to identify compounds that may interfere with biological assays such as promiscuous, fluorescent, or unstable compounds43. ADMETlab predictions are based on a databank of over 288k entries from DrugBank and the literature, and include solubility (LogS), permeability (Caco-2), efflux transporter (Pgp) inhibition or substrate, human intestinal absorption (HIA), bioavailability (%F), plasma protein binding (PBP), volume of distribution (VD), cytochrome P450 isoform inhibition or substrate, elimination half-life (T1/2), clearance (CL), hERG inhibition, human hepatotoxicity, AMES mutagenicity, and drug induced liver injury (DILI).
Hemolytic activity
Erythrocytes were collected from BALB/c mice, seeded at a 3% suspension in 96-well plates U-shape microplate and incubated with compound 2 (200 to 1.6 μM) in PBS 1× (Sigma-Aldrich), for 2 h at 24 °C. The hemolytic activity was determined in the cell supernatant by optical density reading at 570 nm (FilterMax F5 Multi-Mode Microplate Reader, Molecular Devices). Maximum hemolysis was obtained using ultrapure distilled water and untreated erythrocytes were used as negative control44.
Mechanism of lethal action assessment
Determination of the plasma membrane integrity
Promastigotes (2 × 106 parasites/well) were incubated in 96-well black polystyrene microplates with 1 µM of Sytox Green (Molecular Probes) in HANKS’ balanced salt solution (Sigma-Aldrich) supplemented with 10 mM D-Glucose (Sigma-Aldrich, HBSS + Glu) at 24 °C for 15 min in the dark45. Compound 2 (190 µM) was added and the fluorescence was measured every 20 min for up to 2 h, using a fluorimetric microplate reader (FilterMax F5 Multi-Mode, Molecular Devices) with excitation and emission wavelengths of 485 and 520 nm, respectively. Maximum permeabilization was obtained using 0.5% Triton X-100 and untreated parasites were used as negative control46.
Determination of the plasma membrane electric potential
Promastigotes (2 × 106 parasites/well) were treated with compound 2 (190 µM) for 1 and 2 h in HBSS + Glu at 24 °C. DiSBAC2(3) (Molecular Probes) were added (0.2 µM) and the parasites were incubated by 5 min47. The fluorescence was measure using Attune NxT flow cytometer (Thermo Fisher Scientific) with excitation and emission wavelengths of 488 and 574 nm (BL-2), respectively. Raloxifene (60 μM) was used as positive control and untreated parasites were used as negative control48. Unstained parasites were used to set background fluorescence.
Mitochondrial membrane electric potential analysis
Promastigotes (2 × 106 parasites/well) were treated for 1 and 2 h with compound 2 (190 µM) in HBSS + Glu at 24 °C. JC-1 dye (Molecular Probes) was added at a final concentration of 10 μM. The parasites were incubated in the dark for 20 min and washed to eliminate the non-internalized dye. The fluorescence was measure using Attune NxT flow cytometer (Thermo Fisher Scientific) with excitation filter wavelengths of 488 nm and emission of 530 (BL-1) and 574 nm (BL-2). The mitochondrial membrane potential was determined using BL-2/BL-1 ratio49. Maximum depolarization was obtained in the presence of CCCP (100 μM) and untreated parasites were used as negative control. Unstained parasites were used to set background fluorescence.
Measurement of ATP levels
Promastigotes (2 × 106 parasites/well) were treated with compound 2 (190 µM) in HBSS + Glu for 1 and 2 h at 24 °C. Untreated parasites and treated with CCCP (25 µM) were included as negative and positive controls, respectively. The promastigotes were lysed using 0.5% Triton X-100 and mixed with a standard reaction buffer (ATP Determination Kit, Molecular Probes) containing DTT (1 mM), luciferin (0.5 mM) and firefly luciferase (1.25 µg/mL)50. Luminescence intensity was measured using a luminometer (FilterMax F5 Multi-Mode, Molecular Devices) and the amount of ATP was calculated from an ATP standard curve.
Measurement of reactive oxygen species (ROS) generation
Promastigotes (2 × 106 parasites/well) were seeded in 96-well black polystyrene microplates and treated with compound 2 (190 µM) for 2 h in HBSS + Glu at 24 °C. Then, H2DCFDA (Molecular Probes) were added (5 µM) and after 15 min of incubation, the fluorescence was measure using a fluorimetric microplate reader (FilterMax F5 Multi-Mode, Molecular Devices) with excitation and emission wavelengths of 485 and 520 nm, respectively49. H2O2 (400 μM) was used as positive control and untreated parasites were used as negative control.
Measurement of intracellular calcium levels (Ca2+)
Promastigotes (2 × 106 parasites/well) were pretreated with 5 µM of Fura-2 AM (Molecular Probes) in PBS 1x, for 40 min at 24 °C in the dark. The parasites were washed and treated with compound 2 (190 µM). The fluorescence was measured at 5, 20, 60 and 120 min, using a fluorimetric microplate reader (FilterMax F5 Multi-Mode, Molecular Devices) with excitation and emission wavelengths of 360 and 500 nm, respectively51. Maximum levels of calcium were obtained using 0.5% Triton X-100 and untreated parasites were used as negative control.
Cell cycle analysis
Promastigotes (2 × 106 parasites/well) in mid-log phase were incubated with compound 2 (190 µM) in M-199 medium for 24 h at 24 °C. Parasites were washed and fixed in 70% ice-cold ethanol overnight at −20 °C. After a further wash with PBS 1x, the promastigotes were ressuspended in propidium iodide (10 µg/mL, Molecular Probes) and RNase A (20 µg/mL, Molecular Probes) for 30 min in the dark at room temperature. The fluorescence intensity was analyses using Attune NxT flow cytometer (Thermo Fisher Scientific) with excitation filter wavelengths of 488 nm and emission of 574 nm (BL-2)47. Maximum change in the cell cycle was obtained in the presence of miltefosine (25 μM) and untreated parasites were used as negative control51. Unstained parasites were used to set background fluorescence.
Ultrastructural analysis by transmission electron microscopy (TEM)
Promastigotes (2 × 107 parasites/well) were treated with compound 2 (300 µM) in M-199 medium for 30 min, 1, 2, 4 and 6 h at 24 °C. Then, the parasites were washed, fixed in 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.3), postfixed in 1% osmium tetroxide. The parasites were dehydrated with acetone series and embedded in Epon resin. Ultrathin sections were stained with uranyl acetate and lead citrate52. The material was analyzed under transmission electron microscopy (JEOL JEM-1011). Untreated parasites were used as negative control.
Cytokine level quantification
Bone marrow-derived macrophages (5 × 105 cells/well) in 24-well plates were infected with amastigotes at a ratio of 10:1 (amastigotes/macrophage) and kept at 37 °C in a 5% CO2 humidified incubator. Cell were treated with compound 2 (60 to 7.5 µM) for 48 h and the supernatant was collected and cytokine quantification was achieved using the CBA Mouse Inflammation Kit (BD Biosciences) according to the manufacturer’s instructions. The fluorescence was measured using BD LSRFortessa flow cytometer (BD Biosciences) and the data analysis was performed using the software FCAP Array (v.3). LPS (50 μg/mL) was used as positive control and untreated parasites were used as negative control.
Nitric oxide evaluation
The nitric oxide (NO) content was quantified in the supernatants collected from bone marrow-derived macrophages treated for 48 h (compound 2–60 to 7.5 µM). The samples were analyzed by the Griess method using a microplate reader at 570 nm (FilterMax F5 Multi-Mode- Molecular Devices)53. The amount of NO was obtained from a standard curve prepared with NaNO2. Maximum nitric oxide production was obtained in the presence of LPS (25 μg/mL) and untreated parasites were used as negative control54.
Statistical analysis
The determination of the CC50 and IC50 values was obtained from sigmoid dose-response curves. The statistical significance (p value) between the samples was evaluated through the One-way ANOVA method using the Tukey’s Multiple Comparison test. All analyzes were performed using Graph Pad Prism 5.0 software. The samples were tested in duplicate and the assays were repeated at least twice.
Acknowledgements
This work was funded by grants and fellowships provided from São Paulo State Research Foundation (FAPESP 2018/10279-6, 2018/07885-1, 2015/50075-2), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Oxford SPRINT-FAPESP Fund. and EPSRC (EP/M019195/1) for additional support. We also thank Conselho Nacional de Pesquisa e Desenvolvimento scientific research award to A.G.T. and J.H.G.L.
Author Contributions
M.A. contributed to the bioactivity tests, mechanism of action studies and manuscript preparation. T.A.C.S. assisted with the mechanism of action studies. F.S.S. and E.A.A. prepared the semi-synthetic compounds. J.H.G.L. contributed to the isolation and identification of the Nectandra leucanta neolignans. D.M.J. conducted the in silico analysis. A.J.G.J. assisted with the immunomodulatory tests. N.N.T. preformed the transmission electron microscopy. A.G.T. proposed the idea and prepared the manuscript. All authors read, revised and approved the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Edward A. Anderson, Email: edward.anderson@chem.ox.ac.uk
Andre G. Tempone, Email: andre.tempone@ial.sp.gov.br
References
- 1.World Health Organization. Integrating neglected tropical diseases into global health and development: fourth WHO report on neglected tropical diseases (2017).
- 2.van Griensven J, Diro E. Visceral leishmaniasis. Infectious Disease Clinics of North America. 2012;26:309–322. doi: 10.1016/j.idc.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Tiwari N, Gedda MR, Tiwari VK, Singh SP, Singh RK. Limitations of current therapeutic options, possible drug targets and scope of natural products in control of leishmaniasis. Mini-Reviews in Medicinal Chemistry. 2017;18:26–41. doi: 10.2174/1389557517666170425105129. [DOI] [PubMed] [Google Scholar]
- 4.Rahman K, et al. In vitro biological screening of a critically endangered medicinal plant, Atropa acuminata Royle Ex Lindl of north western Himalaya. Scientific Reports. 2018;8:1–10. doi: 10.1038/s41598-017-17765-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Newman DJ, Cragg GM. Natural products as sources of new drugs from 1981 to 2014. Journal of Natural Products. 2016;79:629–661. doi: 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]
- 6.Costa-Silva TA, et al. Immunomodulatory and antileishmanial activity of phenylpropanoid dimers isolated from Nectandra leucantha. Journal of Natural Products. 2015;78:653–657. doi: 10.1021/np500809a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grecco SS, et al. Antitrypanosomal activity and evaluation of the mechanism of action of dehydrodieugenol isolated from Nectandra leucantha (Lauraceae) and its methylated derivative against Trypanosoma cruzi. Phytomedicine. 2017;24:62–67. doi: 10.1016/j.phymed.2016.11.015. [DOI] [PubMed] [Google Scholar]
- 8.Grecco SS, et al. Neolignans from leaves of Nectandra leucantha (Lauraceae) display in vitro antitrypanosomal activity via plasma membrane and mitochondrial damages. Chemico-Biological Interactions. 2017;277:55–61. doi: 10.1016/j.cbi.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 9.Lago, J. H. G. & Tempone, A. G. In Drug Discovery for Leishmaniasis 179–198 (Royal Society of Chemistry, 2018).
- 10.Wulsten IF, et al. Investigation of the anti-Leishmania (Leishmania) infantum activity of some natural sesquiterpene lactones. Molecules. 2017;22:1–12. doi: 10.3390/molecules22050685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martins LF, et al. Analogues of marine guanidine alkaloids are in vitro effective against Trypanosoma cruzi and selectively eliminate Leishmania (L.) infantum intracellular amastigotes. Journal of Natural Products. 2016;79:2202–2210. doi: 10.1021/acs.jnatprod.6b00256. [DOI] [PubMed] [Google Scholar]
- 12.Jara M, et al. Macromolecular biosynthetic parameters and metabolic profile in different life stages of Leishmania braziliensis: Amastigotes as a functionally less active stage. PLoS One. 2017;12:1–22. doi: 10.1371/journal.pone.0180532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Westrop GD, et al. Metabolomic analyses of Leishmania reveal multiple species differences and large differences in amino acid metabolism. PLoS One. 2015;10:1–29. doi: 10.1371/journal.pone.0136891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wickramasinghe SN. Evidence of drug metabolism by macrophages: possible role of macrophages in the pathogenesis of drug-induced tissue damage and in the activation of environmental procarcinogens. Clinical & Laboratory Haematology. 1987;9:271–280. doi: 10.1111/j.1365-2257.1987.tb00091.x. [DOI] [PubMed] [Google Scholar]
- 15.Ji X, et al. Click and release: bioorthogonal approaches to “on-demand” activation of prodrugs. Chemical Society Reviews. 2019;48:1077–1094. doi: 10.1039/C8CS00395E. [DOI] [PubMed] [Google Scholar]
- 16.Lagorce D, Bouslama L, Becot J, Miteva MA, Villoutreix BO. FAF-Drugs4: free ADME-tox filtering computations for chemical biology and early stages drug discovery. Bioinformatics. 2017;33:3658–3660. doi: 10.1093/bioinformatics/btx491. [DOI] [PubMed] [Google Scholar]
- 17.Schenone M, Dančík V, Wagner BK, Clemons PA. Target identification and mechanism of action in chemical biology and drug discovery. Nature Chemical Biology. 2013;9:232–240. doi: 10.1038/nchembio.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morth JP, et al. A structural overview of the plasma membrane Na+,K+-ATPase and H+-ATPase ion pumps. Nature Reviews Molecular Cell Biology. 2011;12:60–70. doi: 10.1038/nrm3031. [DOI] [PubMed] [Google Scholar]
- 19.Vannier-Santos M, Martiny A, Souza W. Cell biology of Leishmania spp.: invading and evading. Current Pharmaceutical Design. 2002;8:297–318. doi: 10.2174/1381612023396230. [DOI] [PubMed] [Google Scholar]
- 20.Vendrametto MC, et al. Evaluation of antileishmanial activity of eupomatenoid-5, a aompound isolated from leaves of Piper regnellii var. pallescens. Parasitology International. 2010;59:154–158. doi: 10.1016/j.parint.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 21.Menna-Barreto, R. F. S. & Castro, S. L. The double-edged sword in pathogenic trypanosomatids: the pivotal role of mitochondria in oxidative stress and bioenergetics. BioMed Research International2014 (2014). [DOI] [PMC free article] [PubMed]
- 22.Joshi DC, Bakowska JC. Determination of mitochondrial membrane potential and reactive oxygen species in live rat cortical neurons. Journal of Visualized Experiments. 2011;51:2–5. doi: 10.3791/2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manzano JI, Carvalho L, Pérez-Victoria JM, Castanys S, Gamarro F. Increased glycolytic ATP synthesis is associated with tafenoquine resistance in Leishmania major. Antimicrobial Agents and Chemotherapy. 2011;55:1045–1052. doi: 10.1128/AAC.01545-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boonstra J, Post JA. Molecular events associated with reactive oxygen species and cell cycle progression in mammalian cells. Gene. 2004;337:1–13. doi: 10.1016/j.gene.2004.04.032. [DOI] [PubMed] [Google Scholar]
- 25.Docampo R, Huang G. Calcium signaling in trypanosomatid parasites. Cell Calcium. 2015;57:194–202. doi: 10.1016/j.ceca.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: the calcium-apoptosis link. Nature Reviews Molecular Cell Biology. 2003;4:552–565. doi: 10.1038/nrm1150. [DOI] [PubMed] [Google Scholar]
- 27.Benaim G, Garcia CRS. Targeting calcium homeostasis as the therapy of chagas’ disease and leishmaniasis - a review. Tropical Biomedicine. 2011;28:471–481. [PubMed] [Google Scholar]
- 28.Kaczanowski S, Sajid M, Reece SE. Evolution of apoptosis-like programmed cell death in unicellular protozoan parasites. Parasites and Vectors. 2011;4:2–9. doi: 10.1186/1756-3305-4-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paris C, Loiseau PM, Bories C, Breard J. Miltefosine induces apoptosis-like death in Leishmania donovani promastigotes. Antimicrobial Agents and Chemotherapy. 2004;48:852–859. doi: 10.1128/AAC.48.3.852-859.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iniesta V, et al. Arginase I induction in macrophages, triggered by Th2-type cytokines, supports the growth of intracellular Leishmania parasites. Parasite Immunology. 2002;24:113–118. doi: 10.1046/j.1365-3024.2002.00444.x. [DOI] [PubMed] [Google Scholar]
- 31.Néris PLN, et al. Neolignan licarin A presents effect against Leishmania (Leishmania) major associated with immunomodulation in vitro. Experimental Parasitology. 2013;135:307–313. doi: 10.1016/j.exppara.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 32.Morais, C. G. V. et al. The dialogue of the host-parasite relationship: Leishmania spp. and Trypanosoma cruzi infection. BioMed Research International2015 (2015). [DOI] [PMC free article] [PubMed]
- 33.Brandonisio O, et al. Macrophage chemotactic protein-1 and macrophage inflammatory protein-1α induce nitric oxide release and enhance parasite killing in Leishmania Infantum-infected human macrophages. Clinical and Experimental Medicine. 2002;2:125–129. doi: 10.1007/s102380200017. [DOI] [PubMed] [Google Scholar]
- 34.Valencia-Pacheco G, et al. In situ cytokines (IL-4, IL-10, IL-12, IFN-γ) and chemokines (MCP-1, MIP-1α) gene expression in human Leishmania (Leishmania) mexicana infection. Cytokine. 2014;69:56–61. doi: 10.1016/j.cyto.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 35.Saporito L, Giammanco GM, Grazia S, Colomba C. Visceral leishmaniasis: host-parasite interactions and clinical presentation in the immunocompetent and in the immunocompromised host. International Journal of Infectious Diseases. 2013;17:1–5. doi: 10.1016/j.ijid.2012.12.024. [DOI] [PubMed] [Google Scholar]
- 36.Grecco SDS, et al. Chemical composition and in vitro cytotoxic effects of the essential oil from Nectandra leucantha leaves. Pharmaceutical Biology. 2015;53:133–137. doi: 10.3109/13880209.2014.912238. [DOI] [PubMed] [Google Scholar]
- 37.Stauber LA, Franchino EM, Grun J. An eight-day method for screening compounds against Leishmania donovani in the golden hamster. The Journal of Protozoology. 1958;5:269–273. doi: 10.1111/j.1550-7408.1958.tb02565.x. [DOI] [Google Scholar]
- 38.Weischenfeldt, J. & Porse, B. Bone marrow-derived macrophages (BMM): isolation and applications. Cold Spring Harbor Protocols3 (2008). [DOI] [PubMed]
- 39.Reimão JQ, Colombo FA, Pereira-Chioccola VL, Tempone AG. In vitro and experimental therapeutic studies of the calcium channel blocker bepridil: detection of viable Leishmania (L.) chagasi by real-time PCR. Experimental Parasitology. 2011;128:111–115. doi: 10.1016/j.exppara.2011.02.021. [DOI] [PubMed] [Google Scholar]
- 40.Tada H, Shiho O, Kuroshima K, Koyama M, Tsukamoto K. An improved colorimetric assay for interleukin 2. Journal of Immunological Methods. 1986;93:157–165. doi: 10.1016/0022-1759(86)90183-3. [DOI] [PubMed] [Google Scholar]
- 41.FAF-Drugs4. Available at, http://fafdrugs4.mti.univ-paris-diderot.fr/ (Accessed: 13th August 2018).
- 42.ADMTETlab. Available at, http://admet.scbdd.com (Accessed: 13th August 2018).
- 43.Bruns RF, Watson IA. Rules for identifying potentially reactive or promiscuous compounds. Journal of Medicinal Chemistry. 2012;55:9763–9772. doi: 10.1021/jm301008n. [DOI] [PubMed] [Google Scholar]
- 44.Rea, A. et al. Soulamarin isolated from Calophyllum brasiliense (Clusiaceae) induces plasma membrane permeabilization of Trypanosoma cruzi and mytochondrial dysfunction. PLoS Neglected Tropical Diseases7 (2013). [DOI] [PMC free article] [PubMed]
- 45.Chicharro C, Granata C, Lozano R, Andreu D, Rivas L. N-terminal fatty acid substitution increases the leishmanicidal activity of CA(1-7)M(2-9), a cecropin-melittin hybrid peptide. Antimicrobial Agents and Chemotherapy. 2001;45:2441–2449. doi: 10.1128/AAC.45.9.2441-2449.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kulkarni MM, McMaster WR, Kamysz W, McGwire BS. Antimicrobial peptide-induced apoptotic death of Leishmania results from calcium-dependent, caspase-independent mitochondrial toxicity. Journal of Biological Chemistry. 2009;284:15496–15504. doi: 10.1074/jbc.M809079200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Luque-Ortega JR, Rivas L. Characterization of the leishmanicidal activity of antimicrobial peptides. Methods in Molecular Biology. 2010;329:393–420. doi: 10.1007/978-1-60761-594-1_25. [DOI] [PubMed] [Google Scholar]
- 48.Reimão Juliana Q., Miguel Danilo C., Taniwaki Noemi N., Trinconi Cristiana T., Yokoyama-Yasunaka Jenicer K. U., Uliana Silvia R. B. Antileishmanial Activity of the Estrogen Receptor Modulator Raloxifene. PLoS Neglected Tropical Diseases. 2014;8(5):e2842. doi: 10.1371/journal.pntd.0002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mukherjee SB, Das M, Sudhandiran G, Shaha C. Increase in cytosolic Ca2+ levels through the activation of non-selective cation channels induced by oxidative stress causes mitochondrial depolarization leading to apoptosis-like death in Leishmania donovani Promastigotes. Journal of Biological Chemistry. 2002;277:24717–24727. doi: 10.1074/jbc.M201961200. [DOI] [PubMed] [Google Scholar]
- 50.Dolai S, Yadav RK, Pal S, Adak S. Overexpression of mitochondrial Leishmania major ascorbate peroxidase enhances tolerance to oxidative stress-induced programmed cell death and protein damage. Eukaryotic Cell. 2009;8:1721–1731. doi: 10.1128/EC.00198-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sen N, et al. Camptothecin-induced imbalance in intracellular cation homeostasis regulates programmed cell death in unicellular hemoflagellate Leishmania donovani. Journal of Biological Chemistry. 2004;279:52366–52375. doi: 10.1074/jbc.M406705200. [DOI] [PubMed] [Google Scholar]
- 52.Duarte MIS, Mariano ON, Hirata Takakura CF, Everson D. A Fast method for processing biologic material for electron microscopic diagnosis in infectious disease. Ultrastructural Pathology. 1992;16:475–482. doi: 10.3109/01913129209057832. [DOI] [PubMed] [Google Scholar]
- 53.Green SJ, Meltzer MS, Hibbs JB, Nacy CA. Activated macrophages destroy intracellular Leishmania major amastigotes by an L-arginine-dependent killing mechanism. Journal of immunology. 1990;144:278–83. [PubMed] [Google Scholar]
- 54.Choi IS, et al. Kaempferol inhibits P. intermedia lipopolysaccharide-induced production of nitric oxide through translational regulation in murine macrophages: critical role of heme oxygenase-1-mediated ROS reduction. Journal of Periodontology. 2013;84:545–555. doi: 10.1902/jop.2012.120180. [DOI] [PubMed] [Google Scholar]