Abstract
The diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae), is one of the main pests of Brassica crops worldwide. Management of P. xylostella is particularly challenging, as different field populations have readily acquired resistance to a wide range of insecticides, including Bacillus thuringiensis (Bt) toxins. In this study, a novel strain of P. xyllostela (Fuzhou-R2Ad) with 120-fold resistance to Bt Cry2Ad was selected in the laboratory, after screening for 66 generations from the susceptible strain Fuzhou-S. In the absence of Bt Cry2Ad toxin, the Fuzhou-R2Ad had significantly lower fitness as compared to the susceptible strain, which might be related to induced genetic changes to Bt toxins. We used several models to measure the dominance levels of insecticide resistance among different strains and found an incompletely recessive inheritance pattern of the Fuzhou-R2Ad resistance, which might be controlled by multiple genes. This study constitutes the first report of laboratory-acquired resistance to Cry2Ad toxin in P. xylostella. Our work presents further insights into the mechanism of Bt resistance and has immediate implications for the integrated pest management of P. xylostella globally.
Introduction
The diamondback moth (DBM), Plutella xylostella (L.) (Lepidoptera: Plutellidae), is one of the world’s most destructive pests of Brassica crops, and causes an estimated cost of US$4-5 billion annually in direct damage and pest management globally1,2. Although there are multiple tactics for DBM management, chemically-synthesized insecticides remain the most common and widely-used approach. As insecticide-based management has caused substantial resistance problems in DBM3–7, biological pesticides are increasingly promoted as sustainable and environmentally-friendly alternatives. More specifically, the use of Bacillus thuringiensis, a soil-dwelling bacterium, offers durable and effective pest control without negative side effects on humans, vertebrates and most beneficial organisms8,9. This also has led to the development of genetically-modified (GM) crops, using Bt genes that biosynthesize the toxic crystalline (Cry) protein. However, given the ability of DBM to rapidly develop resistance to insecticides, there is significant concern that this pest could equally inherit and sustain resistance to Bt toxins.
A lot of research has been conducted on the genetic basis of insect resistance to Bt toxins10–14. The work has shown that a high level of resistance is primarily conferred through one or several autosomal genes, which are either recessive or incompletely recessive6,15,16. In contrast, the relatively low resistance is acquired through dominant inheritance mechanisms17,18. Four different models have been defined for insecticide resistance and dominance, based on phenotypic traits. First, a DLC model was applied for insecticide resistance, centred on LC50 values of dose-mortality curves19–21. Next, Roush and McKenzie developed an effective dominance DML model by assessing mortality at a particular dose of a given insecticide22. Third, the relative fitness of dominance DWT was calculated based upon the fitness of particular genotypes in insecticide-treated areas23,24. Last, a general formula has been proposed for dominance levels in relation to insecticide resistance25. Overall, dominance levels can be calculated for different traits, including insect fitness in insecticide-treated or untreated area DWNT. Although the dominance level could be estimated by DLC, DML, DWT and DWNT, it may still be varied by environmental influences, genetic information and the selection of an insecticide resistance allele. Using DLC and DML models, it has been shown that the resistance in Cry1Ac-selected strains was incompletely recessive in a field-derived population of DBM6. Pereira et al. has demonstrated a recessive inheritance of Cry1F resistance in European corn borer Ostrinia nubilalis, which was indicated by a dominance level DLC less than 0.1116. However, to our knowledge, there are no published studies that utilize the various models, especially DWNT, to fully evaluate the degree of the dominance.
In the present study, we evaluate, for the first time, the inheritance properties of a laboratory DBM strain with high resistance to Bt Cry2Ad, by comparing dominance of insecticide resistance between the susceptible strain, the positive and negative cross of the resistant strain and the backcross. Furthermore, we investigate levels of dominance and inheritance of resistance to Cry2Ad toxin in the hybrid, resistant and susceptible strains without selection pressure. Additionally, we estimate whether inheritance of Bt Cry2Ad resistance in P. xylostella is controlled by a single-gene or multiple genes. The results of this research have direct implication for resistance management of DBM to Cry2Ad, and can provide further information to advance the effective control of DBM globally.
Materials and Methods
Cry Toxin
Cry2Ad toxin was obtained from a Bt strain, BRC-HZP10, which was supplied by the Key Laboratory of Biopesticide and Chemical Biology, Fujian Agriculture and Forestry University (Fuzhou, China). The purity of the extracted Cry2Ad protein reached 88.34%26. Prior to its use in the experiments, Cry2Ad toxin was prepared in 0.2% Triton X-100.
Insect strains
A susceptible strain of P. xylostella, Fuzhou-S, was collected in 2004 from fields of cabbage (Brassica oleracea var. capitata) in Fuzhou (Fujian, China; 26.08°N, 119.28°E). Whole-genome sequencing was applied to characterize the full genomic mapping27. The Fuzhou-S strain has been kept for over 150 generations under greenhouse conditions without exposure to insecticides, with individuals reared on potted radish seedlings (Raphanus sativus L. var. sativus) under the condition of 25° ± 1 °C, 65 ± 5% RH and 16 L:8D photoperiod.
A resistant strain was derived from the Fuzhou-S strain, by exposing the 3rd instar larvae of DBM to R. sativus leaves treated with Cry2Ad toxin. Fresh and untreated R. sativus leaves were dipped into the Cry2Ad toxin protein solution at LC75 concentration for 10 s, and excess solution was wiped off with filter paper. After 48 h, the surviving larvae were then selected, allowed to pupate and chosen for production of further progeny28. Similar to the Fuzhou-S strain, the resistant Fuzhou-R2Ad strain has been maintained for about 70 generations in the laboratory without any exposure to insecticides except for Cry2Ad.
Bioassay
Following the procedures as outlined above, R. sativus leaves (ca. 10 mm diameter) were treated with five gradient concentrations of Cry2Ad solution. After drying, leaves were fed to the 3rd-instar P. xylostella larvae that had previously been starved in clear plastic cups (78 mm (top) and 51 mm (bottom) in diameter, 82 mm height) for 2 h29–31. Each concentration was tested for a batch of 12 DBM larvae, and the experiments were independently repeated three times with 10 leaves in each replicate. In a control group, larvae were fed with leaf disks (ca. 10 mm diameter) that had been treated with distilled water containing 0.2% Triton X-100.
The treated larvae were then transferred to a climate chamber at 25° ± 1 °C, 65 ± 5% RH, and a 16 L:8D cycle. After 48 h, fresh untreated R. sativus leaves were added. Mortality of larvae was recorded after 72 h, and a toxicity regression curve was developed to estimate the value of LC50 with 95% confidence intervals.
Hybridization
After pupation, each pupa was transferred individually into a collection tube for further eclosion. Emerged adults were sexed, and used for production of a F1 generation through reciprocal mass crosses. For one cross, 30 Fuzhou-R2Ad females were allowed to mate with 30 Fuzhou-S males in one laying cage (100 mm diameter and 80 mm height). For a second cross, 30 Fuzhou-S females were paired with 30 Fuzhou-R2Ad males32, larvae from the two parental colonies were defined as F1 (Fuzhou-R2Ad♀ × Fuzhou-S♂) and F1’ (Fuzhou-R2Ad♂ × Fuzhou-S♀), and subject to the above bioassays. Subsequently, F2 progeny was obtained through single-pair crosses between F1 progeny, and a backcross (BC) was produced by pairing a F1 hybrid with the Fuzhou-S strain (F1 × Fuzhou-S). Lastly, 20 susceptible adults (i.e., 10 females and 10 males) were mixed with 20 resistant adults (10:10 sex ratio) for a pooled hybrid (R × S). Dominance of Cry2Ad toxin resistance in F1, F1’ and BC hybrids were determined based on the probit analysis (visualised by slopes of log dose–probit line (LD-P line)), LC50 value and corresponding 95% confidence limits.
Fitness tests
Newly-hatched larvae from Fuzhou-S, Fuzhou-R2Ad, F1 and F1’ hybrid populations were randomly chosen, and individualized on potted turnip sprouts (ca. 40 mm diameter). On a daily basis, development of P. xylostella was monitored and the relevant biological parameters, including mortality, pupation rate, eclosion rate, and adult sex ratio, were recorded. Single-pair crosses of P. xylostella adults were conducted in 60 mm Petri dishes lined with moist filter paper, and mated females were allowed to lay eggs on the moist filter paper. Mated females were fed with 10% honey solution, and fecundity of each strain was recorded until all moths died.
Eggs were individually collected and incubated in Petri dishes, and egg eclosion rates were computed. Net population growth rate (R0) was determined, defined as the ratio of new larvae (Nn+1) to the initial number (Nn). The relative fitness of the resistant strain was calculated by:
Data analysis
For each bioassay, LD-P line, LC50 value, 95% confidence limits and the relative standard deviation were assessed. Two LC50 values are considered to be significantly different (P < 0.05) if their 95% confidence intervals do not overlap33.
Based on the LC50, the resistance ratio was defined as the ratio between the LC50 value of Fuzhou-R2Ad, F1 or BC and that of the susceptible strain (i.e., Fuzhou-S). Degree of dominance (D) at LC50 was calculated by:
where LCR, LCRS and LCS represent lethal concentrations for resistant homozygotes, heterozygotes, and susceptible homozygotes, respectively. The value of D ranges from −1 to 1, representing a complete recessive towards an absolute dominance. Furthermore, DLC, was calculated by:
which is equal to (D + 1)/234. Hence, the DLC value varies between 0 (recessive resistance) and 1 (dominant resistance).
We equally applied the DWT model to evaluate relative fitness of dominance under Bt insecticide selection. DWT was calculated by:
where WTSS, WTRS and WTRR represent the relative fitness at a specific insecticide concentration for susceptible homozygotes, heterozygotes, and resistant homozygotes, respectively. If susceptible and resistant strains are considered as homozygous genotypes, DWT will be taken as h23,35. In a similar fashion as DLC, the h value ranges from 0 to 1 (i.e., from completely recessive to completely dominant resistance).
Another approach was used to assess dominance. For instance, DWNT value was calculated by:
where WNTSS, WNTRS and WNTRR represent relative fitness in the absence of insecticide for susceptible homozygotes, heterozygotes, and resistant homozygotes, respectively25. When the DWNT value is 0.5, resistance is called co-dominant. DWNT values ranging from 0 to 0.5 demonstrate partial recessive, while DWNT values between 0.5 to 1 refer to partial dominance.
To test the genetic mode of inheritance, the expected mortality (E) of BC and F2 under a certain concentration of insecticide was estimated according to Georghiou’s method36.
in which W1, W2, W3 represent the actual mortality of Fuzhou-S, Fuzhou-R2Ad, and F1, respectively, for a given dose of insecticide. Chi-square test was employed to compare observed and expected mortality of BC and F237. All of the above analyses, including one-way ANOVA with post-hoc Tukey’s honestly significant difference, were performed by using data processing system (DPS) V9.01, while figures were developed using Prism Graphpad 6.
Results
Cry2Ad resistance ratio
The resistance to Cry2Ad developed slow, and increased 1.04 times at the 12th generation as compared to the susceptible strain (Table 1). Resistance gradually increased over subsequent generations and by generation 37 a 8.70-fold increase was observed over the susceptible strain. In the 66th generation, the relative resistance ratio was 120.59 (Table 1).
Table 1.
Resistance ratio of P. xylostella to Cry2Ad over multiple generation selection as compared to the susceptible Fuzhou-S strain.
| Generation | number of insects tested | Slope ± SE | LC50 (95% fiducial limits) (ng/mL) | RR* | P (df = 3) |
|---|---|---|---|---|---|
| 0 | 216 | 4.34 ± 0.50 | 6.65(5.58–8.28) | 1.00 | 0.8812 |
| 12 | 216 | 1.78 ± 0.26 | 6.92(4.83–9.11) | 1.04 | 0.9964 |
| 16 | 216 | 4.54 ± 0.38 | 32.35(26.43–37.92) | 4.86 | 0.6909 |
| 27 | 216 | 1.68 ± 0.27 | 51.53(32.94–70.37) | 7.76 | 0.9999 |
| 37 | 216 | 2.35 ± 0.32 | 57.79(41.96–73.00) | 8.70 | 0.9998 |
| 41 | 216 | 2.13 ± 0.28 | 120.20(96.79–157.82) | 18.10 | 0.9973 |
| 52 | 216 | 2.35 ± 0.32 | 154.45(123.84–200.47) | 23.26 | 0.9058 |
| 66 | 216 | 1.26 ± 0.31 | 800.73(372.94–6142.62) | 120.59 | 0.9633 |
*RR (resistance ratio) is calculated as LC50 (Fuzhou-R2Ad, F1 or BC)/LC50 (Fuzhou-S). LC50(Fuzhou-S) is expressed as 6.65 ng/mL. Each LC50 value represents the average of 8 independent measurements.
Biological fitness parameters
In the Fuzhou-S strain, survival rates (% ± standard error) of the 1st, 2nd, 3rd and 4th instar larvae were 90.30 ± 0.50, 57.80 ± 0.77, 93.23 ± 1.63, and 91.00 ± 1.59, respectively (Table 2). For the resistant Fuzhou-R2Ad strain, corresponding survival rates (%) were 67.25 ± 0.59, 58.71 ± 0.19, 100.00 ± 0.12 and 74.90 ± 1.97, respectively. Survival rates of 1st and 4th instar larvae of the Fuzhou-R2Ad strain were significantly lower than those of the Fuzhou-S strain, and the relative fitness (DWT) of the Fuzhou-R2Ad strain was 0.29.
Table 2.
Population growth parameters of different P. xylostella strains.
| Biological characteristics | Fuzhou-S | Fuzhou-R2Ad | F1 | F1' | F value | P |
|---|---|---|---|---|---|---|
| Initial amount of eggs | 140 | 186 | 118 | 87 | ||
| Egg hatch (%) | 80.72 ± 1.22bB | 87.08 ± 0.36aA | 82.26 ± 0.57bAB | 74.57 ± 0.97cC | 36.82 | 0.0001 |
| Survival rate 1st instar (%) | 90.30 ± 0.5aA | 67.25 ± 0.59cC | 82.82 ± 0.89bB | 85.01 ± 1.27bAB | 127.19 | 0.0001 |
| Survival rate 2nd instar (%) | 57.80 ± 0.77cC | 58.71 ± 0.19cC | 97.57 ± 1.22aA | 85.74 ± 1.01bB | 498.22 | 0.0001 |
| Survival rate 3rd instar (%) | 93.23 ± 1.63bB | 100.00 ± 0.12aA | 96.30 ± 0.15abAB | 100.00 ± 1.03aA | 15.99 | 0.0010 |
| Survival rate 4th instar (%) | 91.00 ± 1.59aA | 74.90 ± 1.97cC | 84.22 ± 0.54bAB | 76.59 ± 1.05cBC | 28.22 | 0.0001 |
| Number of pupae | 17.00 ± 0.67abAB | 16.00 ± 1.00abAB | 21.00 ± 1.00aA | 12.00 ± 2.08bB | 8.02 | 0.0085 |
| Pupation rate (%) | 33.42 ± 1.62cC | 25.77 ± 1.02cC | 53.71 ± 1.47bB | 83.98 ± 2.46aA | 227.33 | 0.0001 |
| Adult number | 14 ± 0.67abAB | 12 ± 0.58bAB | 18.00 ± 1.00aA | 10 ± 1.53bB | 11.49 | 0.0029 |
| Emergence rate (%) | 86.11 ± 3.87aA | 75.18 ± 2.43aA | 86.42 ± 0.72aA | 83.99 ± 2.46aA | 4.06 | 0.0502 |
| Sexual ratio (female:male) | 1.17aA | 1.00aA | 1.31aA | 1.50aA | ||
| Fecundity/female | 102 ± 3.67abAB | 91 ± 5.29bAB | 129 ± 7.21aA | 82 ± 8.97bB | 9.65 | 0.0049 |
| Number of offspring eggs | 1414 | 546 | 1062 | 574 | — | — |
| R0 | 10.10 | 2.93 | 9.00 | 6.60 | — | — |
| Relative fitness | 1.00* | 0.29 | 0.89 | 0.65 |
According to one-way with post-hoc Tukey’s honestly significant difference, the same superscript letter following the numbers between rows of a given column indicates no significant difference between the strains at P > 0.05. The different upper and lower case letters stand for the significance with P < 0.01, and P < 0.05, respectively.
*Relative fitness of the susceptible Fuzhou-S strain is defined as 1.
Other fitness parameters, such as egg hatch rate, survival rate of the 2nd-instar larvae, pupation rate, and female fecundity were significantly higher in F1 hybrid compared to F1’. And the relative fitness values of the positive cross F1 and negative cross F1’ were 0.89 and 0.65, respectively.
Inheritance properties
All experimental strains proved susceptible to Cry2Ad, and no significant difference was recorded in LC50 values between F1 and F1’ strains (Table 3). In the pooled hybrid (R × S), the LC50 value was significantly lower than that of Fuzhou-R2Ad strain. Also, the overlap in 95% confidence limits of LC50 between F1 and F1’ strains confirmed that Cry2Ad resistance was autosomally inherited, without maternal effects and sex linkage.
Table 3.
Susceptibility to Cry2Ad toxin in a susceptible strain (Fuzhou-S), resistant strain (Fuzhou-R2Ad), and different reciprocal crosses of the P. xylostella strains.
| Strain or cross | Number of insects tested | Slope ± SE | LC50 (95% fiducial limits) (ng/mL) | RR* | P (df = 3) |
|---|---|---|---|---|---|
| Fuzhou-S | 216 | 1.44 ± 0.25 | 9.84 (6.98–13.61) | 1.00 | 0.8874 |
| Fuzhou-R2Ad | 216 | 1.26 ± 0.31 | 800.73 (372.94–6142.62) | 81.37 | 0.9633 |
| F1 (Fuzhou-R2Ad♀ × Fuzhou-S♂) | 216 | 1.39 ± 0.22 | 230.27 (155.81–457.35) | 23.40 | 0.9737 |
| F1’ (Fuzhou-R2Ad♂ × Fuzhou-S♀) | 216 | 1.15 ± 0.25 | 116.91 (77.44–187.60) | 11.88 | 0.8206 |
| R × S (pooled) | 432 | 1.27 ± 0.23 | 173.59 (116.62–322.47) | 17.64 | — |
| S × F1 (F1♀ × S♂) | 216 | 0.83 ± 0.24 | 297.84 (160.45–1591.57) | 30.27 | 0.9696 |
| F2 (F1 × F1) | 216 | 1.14 ± 0.25 | 77.71 (53.83–107.38) | 7.90 | 0.8943 |
Resistance ratio is presented by LC50 of a given strain or cross divided by LC50 of the susceptible Fuzhou-S strain.
Estimation of dominance
Upon testing five different Cry2Ad toxin concentrations, LC50 values for F1 and F1’ progenies yielded DF1 = −0.73, DF1′ = −0.44, DLC-F1 = 0.13, DLC-F1′ = 0.28. The effective dominance (h) varied between 0.33 up to 0.71, and negatively correlated with the Cry2Ad protein concentration (Table 4). Based on the relative DBM fitness (Table 2), the respective fitness values of F1 and F1’ in insecticide-treated areas DWNT were 0.15 and 0.49. Hence, D, DLC, and DWNT parameters indicate that the genes conferring resistance to Cry2Ad in DBM selected strain was incompletely recessive. However, when subject to Cry2Ad at 25.32-202.60 μg/ml, DBM larvae had relatively high h values (0.56–0.71), suggesting an incomplete dominant inheritance of the Cry2Ad resistance.
Table 4.
Effective dominance (h) of resistance to Cry2Ad in different strains of P. xylostella, as compared to Fuzhou-R2Ad.
| Concentration of Cry2Ad (ng/ml) | Strain or cross | Survival (%) | Fitness | h |
|---|---|---|---|---|
| 25.32 | Fuzhou-S | 27.80 ± 1.66 | 0.28 | |
| Fuzhou-R2Ad | 97.22 ± 1.60 | 1.00 | ||
| F1(Fuzhou-R2Ad♀ × Fuzhou-S♂) | 77.14 ± 1.19 | 0.79 | 0.71 | |
| 50.65 | Fuzhou-S | 15.30 ± 1.48 | 0.16 | |
| Fuzhou-R2Ad | 94.44 ± 0.00 | 1.00 | ||
| F1(Fuzhou-R2Ad♀ × Fuzhou-S♂) | 65.71 ± 2.83 | 0.69 | 0.64 | |
| 101.30 | Fuzhou-S | 7.30 ± 1.42 | 0.08 | |
| Fuzhou-R2Ad | 86.11 ± 2.78 | 1.00 | ||
| F1(Fuzhou-R2Ad♀ × Fuzhou-S♂) | 51.43 ± 2.89 | 0.60 | 0.56 | |
| 202.60 | Fuzhou-S | 3.00 ± 1.52 | 0.04 | |
| Fuzhou-R2Ad | 75.00 ± 2.78 | 1.00 | ||
| F1(Fuzhou-R2Ad♀ × Fuzhou-S♂) | 45.71 ± 2.03 | 0.61 | 0.59 | |
| 405.21 | Fuzhou-S | 1.01 ± 0.71 | 0.01 | |
| Fuzhou-R2Ad | 66.67 ± 3.21 | 1.00 | ||
| F1(Fuzhou-R2Ad♀ × Fuzhou-S♂) | 22.86 ± 1.28 | 0.34 | 0.33 |
Mortality (%) is calibrated before fitness calculation, and it is calculated as (WRR − WRS)/(WRR − WSS), where WRR, WRS, and WSS represent fitness values at a specific toxin concentration.
Genetic mode of inheritance
LD-P lines and expected values were distinguishable for both BC and F2 crosses (Figs 1 and 2). A plateau was not reached neither after the 50% mortality of BC progeny nor at 25% or 75% mortality levels of F2 hybrids. Chi-square analysis showed that the resistance heredity in experimental DBM strains may be controlled by multiple genes (Tables 5 and 6).
Figure 1.

The slopes of log dose–probit lines (LD-P lines) for BC and the expected LD-P line of BC progeny (EBC). Expected mortality at concentration x ng/ml is calculated as 0.5 × (mortality of F1 at x ng/ml + mortality of Fuzhou-S at x ng/ml), obtained from regression lines of parental strains.
Figure 2.
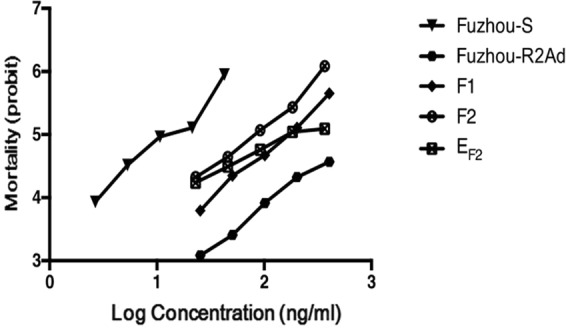
LD-P lines for susceptible (Fuzhou-S) and resistant parents (Fuzhou-R2Ad), F1, F2 and expected LD-P line of F2 progeny. Expected mortality at concentration x ng/ml is calculated as 0.25 × (Fuzhou-S mortality + Fuzhou-R2Ad mortality + F1 mortality), obtained from regression lines of parental strains.
Table 5.
Observed and expected mortality of the BC strain of P. xylostella treated with Cry2Ad, as evaluated with a Chi square test (χ2).
| Concentration of Cry2Ad (ng/ml) | Observed | Expected | χ2 | P | ||
|---|---|---|---|---|---|---|
| Dead | Alive | Dead | Alive | |||
| 22.93 | 7 | 29 | 28 | 44 | 3.30 | 0.0692 |
| 45.86 | 8 | 28 | 36 | 36 | 6.56 | 0.0104 |
| 91.72 | 12 | 24 | 43 | 29 | 5.67 | 0.0172 |
| 183.44 | 16 | 20 | 51 | 21 | 6.02 | 0.0141 |
| 366.88 | 19 | 17 | 58 | 14 | 7.74 | 0.0054 |
| ∑χ2 | 29.29 | |||||
The single gene conferring Cry2Ad resistance is defined as the Chi-square hypothesis.
Table 6.
Observed and expected mortality of F2 strain of P. xylostella treated with Cry2Ad.
| Concentration of Cry2Ad (ng/ml) | Observed | Expected | χ2 | P | ||
|---|---|---|---|---|---|---|
| Dead | Alive | Dead | Alive | |||
| 22.93 | 9 | 27 | 16 | 56 | 0.01 | 0.9357 |
| 45.86 | 13 | 23 | 22 | 50 | 0.13 | 0.5663 |
| 91.72 | 19 | 17 | 29 | 43 | 1.05 | 0.2222 |
| 183.44 | 24 | 12 | 37 | 35 | 1.70 | 0.1923 |
| 366.88 | 31 | 5 | 46 | 26 | 4.76 | 0.0131 |
| ∑χ2 | 7.65 | |||||
Discussion
A thorough understanding of pesticide resistance development in P. xylostella is crucial for an effective and sustainable management of this globally-important pest. Past research has shown that the development of Bt resistance depends on the particular Bt strain and the type of Bt toxin38. Induced by Bt subspecies kurstaki, the resistance ratio of P. xylostella strain NO was 30 times3. Another P. xylostella strain NO-95 selected with high resistance to Bt subspecies kurstaki has very low resistance to Bt subspecies aizawai5. In 2014, a Cry1Ie susceptible Ostrinia furnacalis strain of ACB-BtS was found to have cross resistance to Cry1Ab, Cry1Ac and Cry1F toxins39. Other work has shown that a given Bt toxin produced by the same Bt species may exhibit different impacts on a DBM strains/populations, due to the differential modes of action of the Bt toxins40,41. In this study, we determine that DBM resistance development to the Bt Cry2Ad toxin is possible, after laboratory-based screening for 5 years and 66 generations. The resulting Fuzhou-R2Ad resistant strain had 120.59 times higher levels of resistance than the susceptible Fuzhou-S strain.
When unexposed to Bt Cry2Ad toxin, the Fuzhou-R2Ad has significantly lower fitness as compared to the susceptible strain. Similar findings has been made with DBM populations in Hawaii, where Dipel 2X® (a wettable powder formulation of B. thuringiensis subsp. kurstaki strain HD-l) resistant strain NO-QA exhibited reduced survival, egg hatching and mating rates42. Such reduction in fitness is possibly related to induced genetic changes to Bt toxins, which may remain even in the absence of selection pressure24,43. Hence, it is possible that effective DBM pest control can still be attained for resistant populations by discontinuing Bt Cry2Ad applications.
Inheritance of Bt resistance in the diamondback moth is considered to occur autosomally14,28,44, and similar inheritance models have been recorded for the Asian corn borer Ostrinia furnacalis38, the southern house mosquito Culex quinquefasciatus45, and the cotton bollworm Helicoverpa armigera46,47. As one notable exception, Malaysian populations of P. xylostella exhibited maternal effects on Cry1Ac resistance development3. In the current research, we detect susceptibility to Cry2Ad in all experimental strains or crosses, and confirm this to be autosomal resistance to Cry2Ad, without maternal effects or sex linkage (Table 3).
Our work also show that the resistance inheritance to Cry2Ad toxin in DBM strains is incompletely recessive. This is clearly shown by the following parameters: DF1 values of 0.73 and 0.13, DLC values of 0.44 and 0.28, DWNT values of 0.15 and 0.49 for F1 and F1’ respectively. D, DLC and DWNT values indicate that resistance to Cry2Ad in the Fuzhou strains of P. xylostella is partially recessive. Secondly, the effective dominance is negatively regulated by concentrations of the Bt toxin48,49, namely an incomplete recessivity of resistance at a high Cry2Ad level and an incomplete dominance at low concentrations of Cry2Ad protein. However, when DBM populations are treated with a low dose of toxin, the reduced selection pressure may cause bias because of the increased survival rate in the susceptible strain.
Our work constitutes the first report of Cry2Ad resistance in P. xylostella, sheds light upon Bt resistance development, and could guide further pest management interventions against a globally-relevant lepidopteran pest. Caution needs to be taken when extrapolating our findings, as our research is conducted under highly-artificial conditions with laboratory-reared individuals. Hence, one could still encounter an incompletely coincident resistance to Cry2Ad due to variations in DBM field populations50. Further, we postulate that resistant heredity in local diamondback moth populations is conferred by multiple genes (Figs 1 and 2; Table 5). All of the above provide fundamental insights into the mechanism and evolution of Bt resistance, according to the neo-Darwinian theory51. Further investigation of Bt resistance genes through molecular biology approaches, including molecular marker selection, would be a great help for the genetic manipulation of the diamondback moth. Moreover, the knowledge obtained from this research could boost the effectiveness of pest management interventions and enable sustainable DBM control globally.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 31230061 and No. 31320103922), Fujian Education and Scientific Research Project for Young and Middle-Aged Teachers (No. JA15176), and Haixia Postdoctoral Exchange Program (No. 201806) .
Author Contributions
guarantor of integrity of the entire study: Jinying Liao, Shijun You, Minsheng You study concepts: Jinying Liao, Shijun You, Minsheng You study design: Jinying Liao, Minsheng You definition of intellectual content: Jinying Liao, Shijun You, Minsheng You literature research: Jinying Liao experimental studies: Jinying Liao, Yiqun Xue, Guangjing Xiao, Shuting Huang data acquisition: Jinying Liao, Yiqun Xue, Guangjing Xiao, Shuting Huang data analysis: Jinying Liao, Yiqun Xue, Kris A.G. Wyckhuys statistical analysis: Jinying Liao, Yiqun Xue, Kris A.G. Wyckhuys manuscript preparation: Jinying Liao, Yiqun Xue, Shijun You manuscript editing: Jinying Liao, Miao Xie, Kris A.G. Wyckhuys, Shijun You, Minsheng You manuscript review and revision: Jinying Liao, Miao Xie, Kris A.G. Wyckhuys, Shijun You, Minsheng You.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Shijun You, Email: sjyou@fafu.edu.cn.
Minsheng You, Email: msyou@fafu.edu.cn.
References
- 1.Zalucki MP, et al. Estimating the economic cost of one of the world’s major insect pests, Plutella xylostella (Lepidoptera: Plutellidae): just how long is a piece of string? J Econ Entomol. 2012;105(4):1115–29. doi: 10.1603/EC12107. [DOI] [PubMed] [Google Scholar]
- 2.Furlong MJ, Wright DJ, Dosdall LM. Diamondback moth ecology and management: problems, progress, and prospects. Annu Rev Entomol. 2013;58:517–41. doi: 10.1146/annurev-ento-120811-153605. [DOI] [PubMed] [Google Scholar]
- 3.Tabashnik BE, Cushing NL, Finson N, Johnson MW. Field Development of Resistance to Bacillus thuringiensis in Diamondback Moth (Lepidoptera: Plutellidae) Journal of Economic Entomology. 1990;83(5):1671–6. doi: 10.1093/jee/83.5.1671. [DOI] [Google Scholar]
- 4.Shelton AM, et al. Resistance of Diamondback Moth (Lepidoptera: Plutellidae) to Bacillus thuringiensis Subspecies in the Field. Journal of Economic Entomology. 1993;86(3):697–705. doi: 10.1093/jee/86.3.697. [DOI] [Google Scholar]
- 5.Liu Y-B, Tabashnik BE, Pusztai-Carey M. Field-Evolved Resistance to Bacillus thuringiensis Toxin Cry1C in Diamondback Moth (Lepidoptera: Plutellidae) Journal of Economic Entomology. 1996;89(4):798–804. doi: 10.1093/jee/89.4.798. [DOI] [Google Scholar]
- 6.Sayyed AH, Schuler TH, Wright DJ. Inheritance of resistance to Bt canola in a field-derived population of Plutella xylostella. Pest Manag Sci. 2003;59(11):1197–202. doi: 10.1002/ps.754. [DOI] [PubMed] [Google Scholar]
- 7.Shelton AM, et al. Assessment of insecticide resistance after the outbreak of diamondback moth (Lepidoptera: Plutellidae) in California in 1997. J Econ Entomol. 2000;93(3):931–6. doi: 10.1603/0022-0493-93.3.931. [DOI] [PubMed] [Google Scholar]
- 8.Flexner JL, Lighthart B, Croft BA. The effects of microbial pesticides on non-target, beneficial arthropods. Agriculture, Ecosystems & Environment. 1986;16(3):203–54. doi: 10.1016/0167-8809(86)90005-8. [DOI] [Google Scholar]
- 9.ISAAA, Global Status of Commercialized Biotech/GM Crops. Ithaca: The International Service for the Acquisition of Agri-biotech Applications; Report No.: 978-1-892456-66-4 (2016).
- 10.Council NR, Pesticide Resistance: Strategies and Tactics for Management. Washington, DC: The National Academies Press 484 p (1986).
- 11.Heckel DG. The complex genetic basis of resistance to Bacillus thuringiensis toxin in insects. Biocontrol Science and Technology. 1994;4(4):405–17. doi: 10.1080/09583159409355351. [DOI] [Google Scholar]
- 12.Tabashnik BE, et al. Global variation in the genetic and biochemical basis of diamondback moth resistance to Bacillus thuringiensis. Proc Natl Acad Sci USA. 1997;94(24):12780–5. doi: 10.1073/pnas.94.24.12780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferre J, Van Rie J. Biochemistry and genetics of insect resistance to Bacillus thuringiensis. Annu Rev Entomol. 2002;47:501–33. doi: 10.1146/annurev.ento.47.091201.145234. [DOI] [PubMed] [Google Scholar]
- 14.Tabashnik, B. E., Finson, N., Schwartz, J. M., Caprio, M. A. & Johnson, M. W. and editors. Diamondback moth resistance to Bacillus thuringiensis in Hawaii. Diamondback moth and other crucifer pests: proceedings of the Second International Workshop, Tainan, Taiwan (1990).
- 15.Sayyed AH, Raymond B, Ibiza-Palacios MS, Escriche B, Wright DJ. Genetic and biochemical characterization of field-evolved resistance to Bacillus thuringiensis toxin Cry1Ac in the diamondback moth, Plutella xylostella. Appl Environ Microbiol. 2004;70(12):7010–7. doi: 10.1128/AEM.70.12.7010-7017.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pereira EJ, Storer NP, Siegfried BD. Inheritance of Cry1F resistance in laboratory-selected European corn borer and its survival on transgenic corn expressing the Cry1F toxin. Bull Entomol Res. 2008;98(6):621–9. doi: 10.1017/S0007485308005920. [DOI] [PubMed] [Google Scholar]
- 17.Tang JD, Gilboa S, Roush RT, Shelton AM. Inheritance, stability, and lack-of-fitness costs of field-selected resistance to Bacillus thuringiensis in diamondback moth (Lepidoptera: Plutellidae) from Florida. Journal of Economic Entomology. 1997;90(3):732–41. doi: 10.1093/jee/90.3.732. [DOI] [Google Scholar]
- 18.Gould F, et al. Broad-spectrum resistance to Bacillus thuringiensis toxins in Heliothis virescens. Proc Natl Acad Sci USA. 1992;89(17):7986–90. doi: 10.1073/pnas.89.17.7986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bourguet D, et al. Variation of dominance of newly arisen adaptive genes. Genetics. 1997;147(3):1225–34. doi: 10.1093/genetics/147.3.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bourguet D, Prout M, Raymond M. Dominance of insecticide resistance presents a plastic response. Genetics. 1996;143(1):407–16. doi: 10.1093/genetics/143.1.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bourguet D, Raymond M. The molecular basis of dominance relationships: the case of some recent adaptive genes. Journal of Evolutionary Biology. 1998;11(1):103–22. doi: 10.1007/s000360050068. [DOI] [Google Scholar]
- 22.Roush RT, McKenzie JA. Ecological genetics of insecticide and acaricide resistance. Annu Rev Entomol. 1987;32:361–80. doi: 10.1146/annurev.en.32.010187.002045. [DOI] [PubMed] [Google Scholar]
- 23.Mallet J, Porter P. Preventing insect adaptation to insect-resistant crops: are seed mixtures or refugia the best strategy? Proceedings of the Royal Society of London B: Biological Sciences. 1992;250(1328):165–9. doi: 10.1098/rspb.1992.0145. [DOI] [Google Scholar]
- 24.Tabashnik BE. Evolution of resistance to Bacillus thuringiensis. Annual review of entomology. 1994;39(1):47–79. doi: 10.1146/annurev.en.39.010194.000403. [DOI] [Google Scholar]
- 25.Bourguet D, Genissel A, Raymond M. Insecticide resistance and dominance levels. J Econ Entomol. 2000;93(6):1588–95. doi: 10.1603/0022-0493-93.6.1588. [DOI] [PubMed] [Google Scholar]
- 26.Liao JY, Gao YQ, Wu QY, Zhu YC, You MS. Purification of the insecticidal Cry2Ad protein from a Bt-isolated BRC-HZP10 strain and toxin assay to the diamondback moth, Plutella xylostella (L.) Genet Mol Res. 2015;14(3):7661–70. doi: 10.4238/2015.July.13.11. [DOI] [PubMed] [Google Scholar]
- 27.You M, et al. A heterozygous moth genome provides insights into herbivory and detoxification. Nat Genet. 2013;45(2):220–5. doi: 10.1038/ng.2524. [DOI] [PubMed] [Google Scholar]
- 28.Liu Y, Tabashnik BE. Inheritance of Resistance to the Bacillus thuringiensis Toxin Cry1C in the Diamondback Moth. Appl Environ Microbiol. 1997;63(6):2218–23. doi: 10.1128/aem.63.6.2218-2223.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tabashnik BE. Plant secondary compounds as oviposition deterrents for cabbage butterfly,Pieris rapae (Lepidoptera: Pieridae) J Chem Ecol. 1987;13(2):309–16. doi: 10.1007/BF01025890. [DOI] [PubMed] [Google Scholar]
- 30.Tabashnik BE, Finson N, Johnson MW, Moar WJ. Resistance to Toxins from Bacillus thuringiensis subsp. kurstaki Causes Minimal Cross-Resistance to B. thuringiensis subsp. aizawai in the Diamondback Moth (Lepidoptera: Plutellidae) Appl Environ Microbiol. 1993;59(5):1332–5. doi: 10.1128/aem.59.5.1332-1335.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Székács A, Darvas B, Ishaaya I, Palli SR, Horowitz AR. Advanced Technologies for Managing Insect Pests. Dordrecht. Netherlands: Springer; 2013. Comparative aspects of Cry toxin usage in insect control; pp. 195–230. [Google Scholar]
- 32.Mittal A, Kalia V, Singh DK, Gujar GT. Inheritance of resistance to Bacillus thuringiensis toxin Cry1Ab in the diamondback moth, Plutella xylostella. Biopestic. Int. 2008;4(2):110–120. [Google Scholar]
- 33.Sun J, Liang P, Gao X. Inheritance of resistance to a new non-steroidal ecdysone agonist, fufenozide, in the diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae) Pest Manag Sci. 2010;66(4):406–11. doi: 10.1002/ps.1891. [DOI] [PubMed] [Google Scholar]
- 34.Liu YB, Tabashnik BE, Dennehy TJ, Patin AL, Bartlett AC. Development time and resistance to Bt crops. Nature. 1999;400(6744):519. doi: 10.1038/22919. [DOI] [PubMed] [Google Scholar]
- 35.Tabashnik BE. Delaying insect adaptation to transgenic plants: seed mixtures and refugia reconsidered. P Roy Soc London series B: Biolog Sciences. 1994;255(1342):7–12. doi: 10.1098/rspb.1994.0002. [DOI] [Google Scholar]
- 36.Georghiou GP. Genetic studies on insecticide resistance. Adv Pest Control Res. 1965;6(1):171–230. [PubMed] [Google Scholar]
- 37.Sokal, R. & Rohlf, F. J. The principles and practice of statistic in biological research. WH Creman, San Francisco 262–5 (1981).
- 38.Zhang T, et al. Inheritance patterns, dominance and cross-resistance of Cry1Ab- and Cry1Ac-selected Ostrinia furnacalis (Guenee) Toxins (Basel) 2014;6(9):2694–707. doi: 10.3390/toxins6092694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liang GM, et al. Changes of inheritance mode and fitness in Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae) along with its resistance evolution to Cry1Ac toxin. J Invertebr Pathol. 2008;97(2):142–9. doi: 10.1016/j.jip.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 40.Wu Y, Vassal JM, Royer M, Pieretti I. A single linkage group confers dominant resistance to Bacillus thuringiensis δ-endotoxin Cry1Ac in Helicoverpa armigera. J Applied. Entomol. 2009;133(5):375–80. [Google Scholar]
- 41.Groeters FR, Tabashnik BE, Finson N, Johnson MW. Resistance to Bacillus thuringiensis Affects Mating Success of the Diamondback Moth (Lepidoptera: Plutellidae) J Econ Entomol. 1993;86(4):1035–9. doi: 10.1093/jee/86.4.1035. [DOI] [Google Scholar]
- 42.Groeters FR, Tabashnik BE, Finson N, Johnson MW. Fitness Costs of Resistance to Bacillus Thuringiensis in the Diamondback Moth (Plutella Xylostella) Evolution. 1994;48(1):197–201. doi: 10.1111/j.1558-5646.1994.tb01306.x. [DOI] [PubMed] [Google Scholar]
- 43.Alves AP, Spencer TA, Tabashnik BE, Siegfried BD. Inheritance of resistance to the Cry1Ab Bacillus thuringiensis toxin in Ostrinia nubilalis (Lepidoptera: Crambidae) J Econ Entomol. 2006;99(2):494–501. doi: 10.1093/jee/99.2.494. [DOI] [PubMed] [Google Scholar]
- 44.Hama H, Suzuki K, Tanaka H. Inheritance and Stability of Resistance to Bacillus thuringiensis Formulations of the Diamondback Moth, Plutella xylostella (LINNAEUS) (Lepidoptera: Yponomeutidae) Applied Entomology and Zoology. 1992;27(3):355–62. doi: 10.1303/aez.27.355. [DOI] [Google Scholar]
- 45.Wirth MC, Walton WE, Federici BA. Inheritance, stability, and dominance of Cry resistance in Culex quinquefasciatus (Diptera: Culicidae) selected with the three Cry toxins of Bacillus thuringiensis subsp. israelensis. Journal of medical entomology. 2012;49(4):886–94. doi: 10.1603/ME11192. [DOI] [PubMed] [Google Scholar]
- 46.Kranthi KR, et al. Inheritance of resistance in Indian Helicoverpa armigera (Hübner) to Cry1Ac toxin of Bacillus thuringiensis. Crop Protection. 2006;25(2):119–24. doi: 10.1016/j.cropro.2005.03.011. [DOI] [Google Scholar]
- 47.Mahon RJ, Olsen KM, Garsia KA, Young SR. Resistance to Bacillus thuringiensis toxin Cry2Ab in a strain of Helicoverpa armigera (Lepidoptera: Noctuidae) in Australia. J Econ Entomol. 2007;100(3):894–902. doi: 10.1603/0022-0493(2007)100[894:RTBTTC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 48.Tan SY, et al. Comparative binding of Cry1Ab and Cry1F Bacillus thuringiensis toxins to brush border membrane proteins from Ostrinia nubilalis, Ostrinia furnacalis and Diatraea saccharalis (Lepidoptera: Crambidae) midgut tissue. J Invertebr Pathol. 2013;114(3):234–40. doi: 10.1016/j.jip.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 49.Devriendt M, Martouret D. Lack of resistance in the diamondback moth Plutella maculipennis [Lep.:Hyponomeutidae] to Bacillus thuringiensis. Entomophaga. 1976;21(2):189–199. doi: 10.1007/BF02371905. [DOI] [Google Scholar]
- 50.Krieg, A & Langenbruch, G. Susceptibility of arthropod species to Bacillus thuringiensis. In Microbial Control of Pests and Plant Diseases, ed. Burges, H. D. pp. 837–898. New York: Academic. 949 pp (1981).
- 51.Lande R. The minimum number of genes contributing to quantitative variation between and within populations. Genetics. 1981;99(3–4):541–53. doi: 10.1093/genetics/99.3-4.541. [DOI] [PMC free article] [PubMed] [Google Scholar]


