Abstract
Proteins that act as global transcriptional regulators play key roles in bacterial adaptation to new niches. These proteins recognize multiple DNA sites across the bacterial genome by different mechanisms. Enterococcus faecalis is able to survive in various niches of the human host, either as a commensal or as a leading cause of serious infections. Nonetheless, the regulatory pathways involved in its adaptive responses remain poorly understood. We reported previously that the MafR protein of E. faecalis causes genome-wide changes in the transcriptome. Here we demonstrate that MafR functions as a transcription activator. In vivo, MafR increased the activity of the P12294 and P11486 promoters and also the transcription levels of the two genes controlled by those promoters. These genes are predicted to encode a calcium-transporting P-type ATPase and a QueT transporter family protein, respectively. Thus, MafR could have a regulatory role in calcium homeostasis and queuosine synthesis. Furthermore, MafR recognized in vitro specific DNA sites that overlap the −35 element of each target promoter. The MafR binding sites exhibit a low sequence identity, suggesting that MafR uses a shape readout mechanism to achieve DNA-binding specificity.
Introduction
Global transcriptional regulators play crucial roles during bacterial adaptation to specific niches. They activate and/or repress the transcription of multiple genes and, therefore, make possible to rapidly adjust the gene expression pattern to new environmental situations. Enterococcus faecalis is usually found as a harmless commensal in the human gastrointestinal tract. However, this Gram-positive bacterium is able to colonize other niches of the human host and cause a variety of life-threatening infections, such as urinary tract infections, endocarditis or bacteraemia1–3. Despite the pathogenic potential of E. faecalis, our understanding of the regulatory circuits involved in its adaptive responses is still very limited.
The MafR protein (482 amino acids) of E. faecalis is highly conserved among the strains whose genomes have been totally or partially sequenced4. Genome-wide microarray assays showed that MafR is involved in global regulation of gene expression5. In such experiments, the transcriptional profiles of strains OG1RF (wild-type) and OG1RF∆mafR (mafR deletion mutant) were compared, demonstrating that MafR activates, directly or indirectly, the expression of at least 87 genes. Many of them are organized in operons and encode proteins involved in the utilization of carbon sources (e.g. mannitol, glycerol, gluconate, maltose and citrate). Furthermore, compared to OG1RF, the OG1RF∆mafR strain was shown to induce a lower degree of inflammation in the peritoneal cavity of mice. Because of these findings, we proposed that MafR could facilitate the growth of E. faecalis in particular human niches and, consequently, could contribute to its potential virulence5.
Different protein-DNA recognition mechanisms have been characterized. In some cases, proteins recognize a sequence-dependent DNA shape (shape readout mechanism) rather than the unique chemical signatures of the DNA bases (base readout mechanism)6,7. MafR is a new member of the Mga/AtxA family of global transcriptional regulators4,5. This family includes AtxA from Bacillus anthracis, MgaSpn from Streptococcus pneumoniae, and Mga from S. pyogenes. Like these three regulatory proteins8, MafR has two putative helix-turn-helix DNA-binding motifs within the N-terminal region, the so-called HTH_Mga (residues 11–69) and Mga (residues 76–164) motifs5. In the Mga regulator, both motifs were found to be required for DNA-binding and transcriptional activation9,10. In vitro protein-DNA interaction studies have shown that MafR binds to linear double-stranded DNAs with little or no sequence specificity. Furthermore, MafR was able to generate multimeric complexes on linear double-stranded DNAs4. Similar DNA-binding properties have been described for the pneumococcal MgaSpn regulator. MgaSpn has a preference for AT-rich DNA sites, as well as a high affinity for a naturally occurring curved DNA11–13. On DNA fragments that contain the promoter of the mafR gene (Pma promoter), MafR recognizes a potentially curved DNA region, which is located upstream of the promoter (positions −69 to −104)4. We hypothesized that MafR, and most likely the regulators of the Mga/AtxA family, recognizes structural features in its target DNAs rather than specific nucleotide sequences4. Nevertheless, verification of this hypothesis requires the identification of additional MafR binding sites across the bacterial genome.
A further DNA microarray assay using an OG1RF∆mafR derivative that overproduces MafR (plasmid-encoded MafR) allowed us to identify two new potential MafR target genes: OG1RF_12294 and OG1RF_11486. In the presence of plasmid-encoded MafR, the highest increase in gene expression corresponded to both genes (our unpublished results). In this manuscript, we addressed the validation of such a finding by in vivo and in vitro approaches. Gene OG1RF_12294 encodes a putative phosphorylated intermediate-type ATPase (P-type ATPase) transporter, which could contribute to maintain calcium homeostasis. Gene OG1RF_11486 encodes a putative QueT transporter family protein, which could be involved in uptake of a queuosine biosynthetic intermediate. Here we demonstrate that MafR activates directly the transcription of both genes by binding to a specific DNA site overlapping the core promoter. Such sites exhibit a low sequence identity. This study shows, for the first time, that MafR functions as a transcription activator. Moreover, it supports that MafR might recognize particular DNA shapes.
Results
Transcription of mafR under laboratory conditions
The genome of the E. faecalis strain OG1RF has been totally sequenced (GenBank CP002621.1)14. By quantitative RT-PCR (qRT-PCR) assays and using the comparative CT method15, we determined the relative expression of the regulatory mafR gene (locus_tag OG1RF_12293) in cells grown under laboratory conditions (Brain Heart Infusion (BHI) broth, 37 °C, without aeration) to both logarithmic and stationary phases. Transcription of mafR was found to be higher at logarithmic phase. Compared to stationary phase, the fold change (log2FC) in mafR expression was ∼4. Therefore, all the experiments shown in this work were performed at the logarithmic growth phase.
Gene OG1RF_12294 encodes a putative P-type ATPase cation transporter
P-type ATPases constitute a large superfamily of cation and lipid pumps that use ATP hydrolysis for energy. They are integral, multispanning membrane proteins that are found in bacteria and in a number of eukaryotic plasma membranes and organelles16. The enterococcal OG1RF_12294 gene, which is adjacent to mafR (Fig. 1A), encodes a putative P-type ATPase cation transporter. Such a gene has been annotated as pmr1 (GeneID: 12289043) because it encodes a protein (850 amino acids) that has sequence similarity (∼52%) to eukaryotic PMR1 (plasma membrane ATPase related) P-type ATPases (Supplementary Table S1). Some PMR1-type pumps are able to transport calcium, as well as manganese, into the Golgi apparatus17–19.
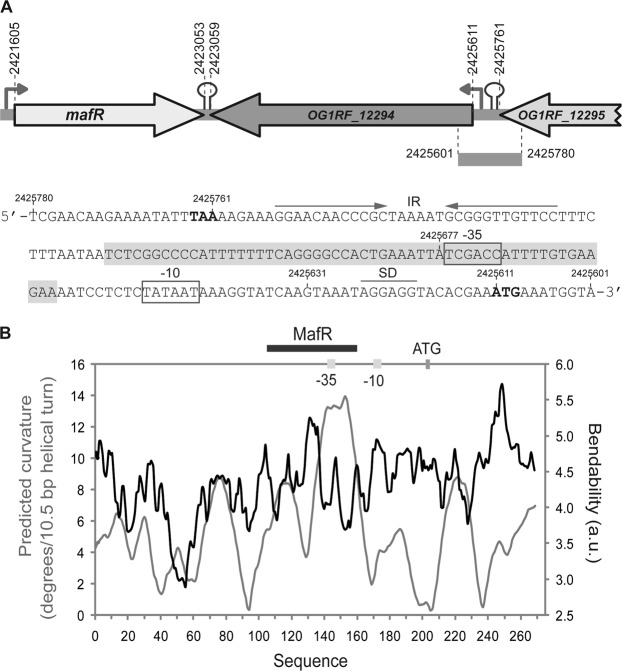
Figure 1.
Relevant features of the P12294 promoter region. (A) Genetic organization of the chromosome region that contains OG1RF_12294. Coordinates of the translation start and stop codons are indicated. Stem-loop elements represent potential transcriptional terminators. Arrows upstream of the genes represent promoters. The nucleotide sequence of the region spanning coordinates 2425780 to 2425601 is shown. The stop codon (TAA) of OG1RF_12295 and the start codon (ATG) of OG1RF_12294 are indicated in boldface letters. IR: inverted-repeat. SD: Shine-Dalgarno sequence. The main sequence elements (−35 box and −10 box) of the P12294 promoter are indicated. The MafR binding site defined in this work is shown (shadowed box). Genes OG1RF_12294 and OG1RF_12295 correspond to genes EF3014 and EF3015 in E. faecalis strain V583. (B) Bendability/curvature propensity plot of the region spanning coordinates 2425817 to 2425548. The location of the P12294 core promoter, the start codon of OG1RF_12294 and the MafR binding site are indicated.
In addition to OG1RF_12294, the OG1RF genome encodes two putative calcium-transporting ATPases: OG1RF_10600 and OG1RF_11602 (Supplementary Table S2). Using the BLASTP protein sequence alignment program20, we found that OG1RF_12294 has sequence similarity (∼53–56%) to both ATPases (Supplementary Table S1). Furthermore, OG1RF_12294 has sequence similarity (∼53–56%) to several prokaryotic proteins characterized as calcium P-type ATPases (Supplementary Table S1)21–25. Thus, protein OG1RF_12294 might contribute to maintain calcium homeostasis in enterococcal cells.
MafR influences positively the transcription of OG1RF_12294
To analyse whether MafR regulates the expression of the OG1RF_12294 gene, we determined its relative expression in OG1RF (wild-type) and OG1RFΔmafR (deletion mutant) by qRT-PCR. The log2FC in OG1RF_12294 expression due to the presence of MafR was ∼3, indicating that MafR has a positive effect on the transcription of such a gene. This conclusion was further confirmed by increasing the intracellular level of MafR. Specifically, we determined the relative expression of OG1RF_12294 in two strains: OG1RFΔmafR harbouring pDLF (absence of MafR) and OG1RFΔmafR harbouring pDLFmafR (plasmid-encoded MafR). In addition, we determined the relative expression of the OG1RF_10600 and OG1RF_11602 genes, which encode putative calcium-transporting ATPases (Supplementary Table S1). In the presence of plasmid-encoded MafR, only transcription of OG1RF_12294 was increased (log2FC ∼4). Thus, MafR influences positively and specifically the transcription of the OG1RF_12294 gene.
MafR activates the P12294 promoter in vivo
In the OG1RF genome14, the ATG codon at coordinate 2425611 is likely the translation start site of the OG1RF_12294 gene (Fig. 1A). It is preceded by a putative ribosome binding site sequence (AGGAGG). Upstream of such a sequence there is a putative promoter (here named P12294) that has a canonical −10 element (TATAAT) but lacks a potential −35 element (consensus TTGACA) at the optimal length of 17 nucleotides. Nevertheless, there is a near-consensus −35 element (TCGACC) at the suboptimal spacer length of 22 nucleotides. These features suggested that promoter P12294 could be recognized by a σ factor similar to the Escherichia coli σ70 and that its activity could be enhanced by regulatory proteins. Sequence analysis of the region located between the TAA stop codon of the OG1RF_12295 gene (coordinate 2425761) and the P12294 promoter revealed the existence of an inverted-repeat (IR) that may function as a Rho-independent transcriptional terminator (Fig. 1A).
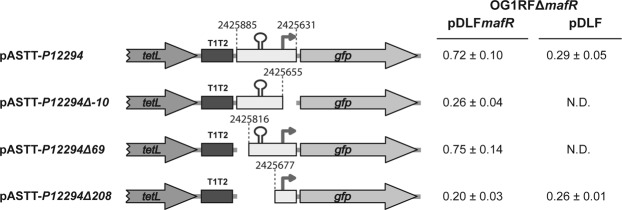
To characterize the P12294 promoter, a 255-bp DNA fragment (coordinates 2425885 to 2425631) (Fig. 2) was inserted into the pASTT promoter-probe vector, which is based on the gfp reporter gene. The recombinant plasmid (pASTT-P12294) was first introduced into OG1RF and OG1RFΔmafR. In these strains, the expression of gfp (0.32 ± 0.02 and 0.26 ± 0.04 units, respectively) was similar to the basal level (OG1RF harbouring pASTT; 0.38 ± 0.02 units). Different results were obtained when pASTT-P12294 was introduced into OG1RFΔmafR harbouring either pDLF or pDLFmafR (plasmid-encoded MafR) (Fig. 2). The expression of gfp was ∼2.5-fold higher in the presence of plasmid-encoded MafR. This result indicated that the 255-bp DNA fragment contains a MafR-dependent promoter activity. Removal of the −10 element of the P12294 promoter resulted in loss of such an activity (plasmid pASTT-P12294Δ-10). A further deletion analysis allowed us to conclude that the 186-bp region between coordinates 2425816 and 2425631 contains both the P12294 promoter and the site required for its activation by MafR (plasmids pASTT-P12294Δ69 and pASTT-P12294Δ208) (Fig. 2).
Figure 2.
Effect of plasmid-encoded MafR on the activity of the P12294 promoter. Four regions from the OG1RF chromosome were inserted independently into the SacI site of pASTT. The coordinates of such regions are indicated. Gene tetL: tetracycline resistance determinant. Gene gfp: green fluorescent protein. The T1T2 box represents the tandem transcriptional terminators T1 and T2 of the Escherichia coli rrnB rRNA operon. The stem-loop element represents the inverted-repeat located upstream of the P12294 promoter (see Fig. 1A). The arrow represents the canonical −10 element of the P12294 promoter. The intensity of fluorescence (arbitrary units) corresponds to 0.8 ml of culture (OD650 = 0.4). In each case, three independent cultures were analysed. N.D.: non-determined.
MafR binds to the P12294 promoter region in vitro
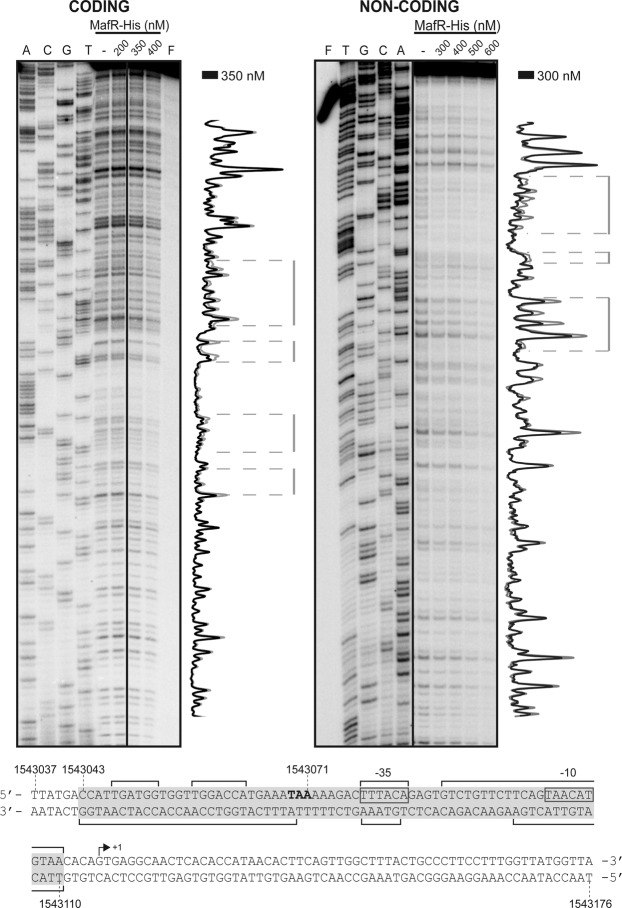
To investigate whether MafR activates directly the expression of the OG1RF_12294 gene, we performed DNase I footprinting experiments. We used a His-tagged MafR protein (MafR-His) and a 270-bp DNA fragment (coordinates 2425817 to 2425548). This fragment contains the P12294 promoter and the site required for its activation by MafR in vivo (Fig. 2). The presence of a His-tag at the C-terminal end of MafR does not affect its DNA-binding properties4. The 270-bp DNA fragment was radioactively labelled either at the 5′-end of the coding strand or at the 5′-end of the non-coding strand (Fig. 3). On the coding strand and at 100 nM of MafR-His, protections against DNase I digestion were observed within the region spanning coordinates 2425708 and 2425658. On the non-coding strand and at 125 nM of MafR-His, diminished cleavages were observed between coordinates 2425712 and 2425686. Thus, MafR-His recognizes a site overlapping the −35 element of the P12294 promoter (Fig. 3). This result allowed us to conclude that MafR activates directly the transcription of the OG1RF_12294 gene.
Figure 3.
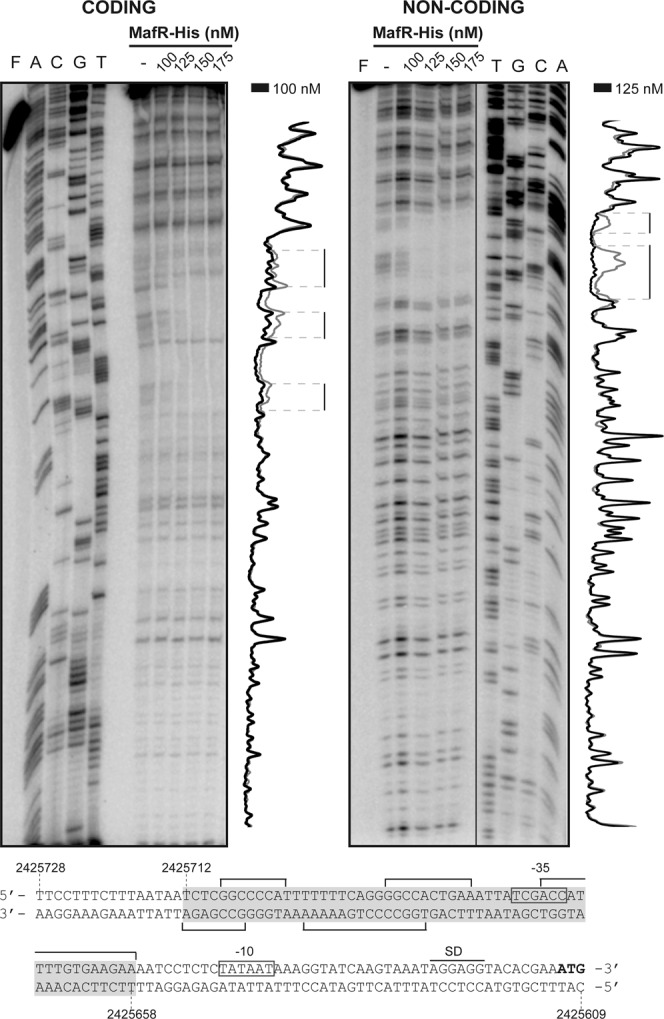
DNase I footprints of complexes formed by MafR-His on the 270-bp DNA fragment that contains the P12294 promoter. 32P-labelled DNA (2 nM) was incubated with the indicated concentrations of MafR-His and then it was digested with DNase I. Non-digested DNA (F) and dideoxy-mediated chain termination sequencing reactions (A, C, G, T) were run in the same gel. All the lanes displayed came from the same gel (delineation with dividing lines). Densitometer scans corresponding to free DNA (grey line) and DNA with protein (black line) are shown. The nucleotide sequence of the region spanning coordinates 2425728 to 2425609 is shown. The −35 and −10 boxes of the P12294 promoter are indicated. SD: Shine-Dalgarno sequence. Brackets indicate regions protected against DNase I digestion. The site recognized by MafR-His (coordinates 2425712-2425658) is indicated with a grey box.
Figure 1B shows the bendability/curvature propensity plot of the 270-bp DNA fragment according to the bend.it program26. The profile contains an intrinsic curvature of high magnitude (~13 degrees per helical turn), which is adjacent to the MafR binding site. In addition, the site recognized by MafR contains a region of potential bendability (~5.2 units).
Gene OG1RF_11486 encodes a putative QueT transporter family protein
Energy-coupling factor (ECF) transporters are a family of ATP-binding cassette (ABC) transporters that are responsible for the uptake of essential micronutrients in prokaryotes. They consist of a membrane-embedded S-component that provides substrate specificity and a three-subunit ECF module that couples ATP hydrolysis to transport. In the so-called group II ECF transporters, different S-components share the same ECF module. Furthermore, the S-component genes are not located in the same operon as the genes for the ECF module27–29.
The enterococcal OG1RF_11486 gene encodes a putative QueT transporter family protein (GenBank AEA94173.1). Proteins identical to OG1RF_11486 (173 residues) are encoded by Mycobacterium abscessus (CPW17925.1), Listeria monocytogenes (CWW42654.1; 172 up to 173 residues are identical) and S. agalactiae (KLL29182.1). In the two former bacteria, the corresponding protein has been annotated as queuosine precursor ECF transporter S-component QueT. Therefore, protein OG1RF_11486 could be involved in the uptake of a queuosine biosynthetic intermediate. Using the BLASTP program20, we found that the OG1RF genome encodes an additional QueT transporter family protein (OG1RF_12031; 168 residues; AEA94718.1). It has 55% of similarity to the OG1RF_11486 protein.
MafR activates the P11486 promoter in vivo
By qRT-PCR assays, we found that MafR has a positive effect on the transcription of OG1RF_11486. Compared to strain OG1RFΔmafR, the relative expression of OG1RF_11486 was slightly higher in strain OG1RF (log2FC ∼0.9). Moreover, the relative expression of OG1RF_11486 was higher in strain OG1RFΔmafR harbouring pDLFmafR (plasmid-encoded MafR) than in strain OG1RFΔmafR harbouring pDLF (log2FC ∼2.4).
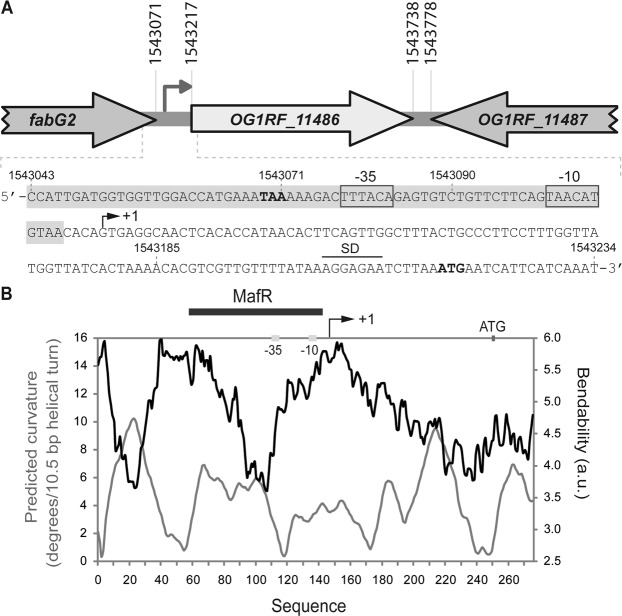
The BPROM program (Softberry, Inc.) predicts a promoter sequence (named P11486 herein) upstream of the OG1RF_11486 gene. The −35 (TTTACA) and −10 (TAACAT) elements of this promoter are separated by 17 nucleotides (Fig. 4A). By primer extension using total RNA from OG1RF cells, we demonstrated that the P11486 promoter is functional in vivo (Fig. 5). Oligonucleotide R11486-D was used as primer (Table 1). A cDNA product of 130 nucleotides was detected, indicating that transcription of OG1RF_11486 starts at coordinate 1543115 (Fig. 4A).
Figure 4.
Relevant features of the P11486 promoter region. (A) Genetic organization of the chromosome region that contains OG1RF_11486. Coordinates of the translation start and stop codons are indicated. The arrow upstream of the OG1RF_11486 gene represents its promoter. The nucleotide sequence of the region spanning coordinates 1543043 to 1543234 is shown. The stop codon (TAA) of fabG2 and the start codon (ATG) of OG1RF_11486 are indicated in boldface letters. SD: Shine-Dalgarno sequence. The transcription start site (+1 position) of the OG1RF_11486 gene, and the main sequence elements (−35 box and −10 box) of the P11486 promoter identified in this work are indicated. The MafR binding site defined in this work is shown (shadowed box). Genes OG1RF_11486 and OG1RF_11487 correspond to genes EF1774 and EF1775 in E. faecalis strain V583. (B) Bendability/curvature propensity plot of the region spanning coordinates 1542969 to 1543243. The location of the P11486 core promoter, the start codon of OG1RF_11486 and the MafR binding site are indicated.
Figure 5.
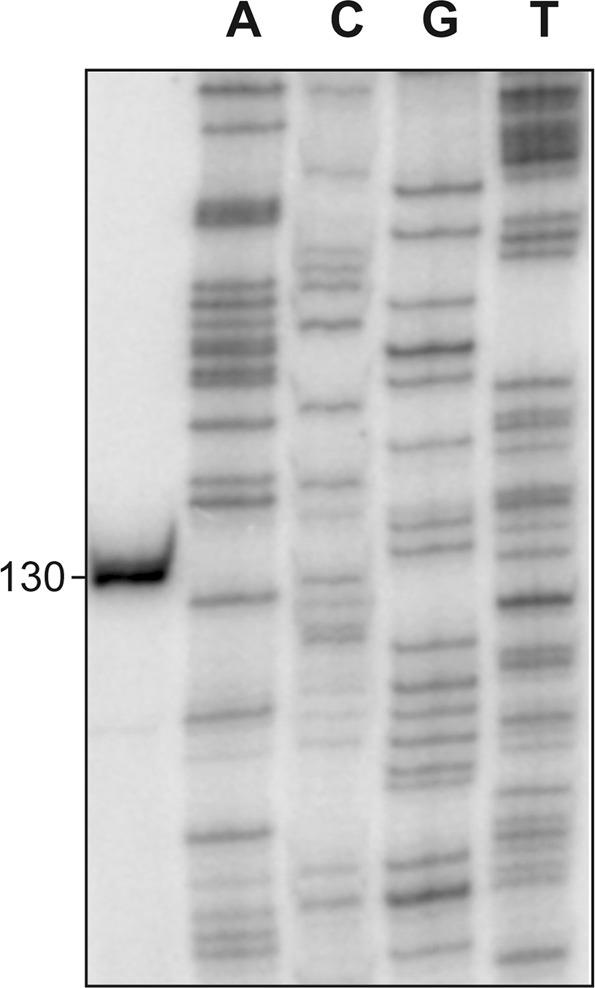
Transcription initiation site of the OG1RF_11486 gene. Primer extension reactions were carried out using total RNA from OG1RF cells. Oligonucleotide R11486-D (coordinates 1543222-1543243) was used as primer. The size (in nucleotides) of the cDNA product is indicated on the left of the gel. Dideoxi-mediated chain termination sequencing reactions were used as DNA size markers (lanes A, C, G, T).
Table 1.
Oligonucleotides used in this work.
| Name | Sequence (5′ to 3′)(a) |
|---|---|
| FmafR-q | ACTTTATCAACCGTCCTTGG |
| RmafR-q | GTTTCGCCATAGACATTATC |
| Fzwf-q | CGGTCAAGGGTTCAATACAACT |
| Rzwf-q | CCAAGATTGGGCAACTTCGTCCCA |
| F12294-q | TCCTCTACCGTTGACACCTG |
| R12294-q | TGCCTTCGTTGACATCTCTTG |
| FrecA-q | GCAACGAAATGGTGGAACAG |
| RrecA-q | AAGGCATCGGCAATCTCTAAG |
| F10600-q | GCGTAGAAGAGTCAGCACTA |
| R10600-q | GCCATTCACAACGGTACAGC |
| F11602-q | CAACACCTCATTAGCGAAAC |
| R11602-q | GTCAATCATACCGACTAAACCA |
| F11486-q | TGGTTACCGCTTTGTATGTTG |
| R11486-q | CCCTAACGTAATGGACCAGAT |
| F12294 | GAAACAGCGTTGAGCTCTTCTAGTGAC |
| R12294 | CATTTCGTGTACCTCCGAGCTCCTTGATACCT |
| F12294Δ69 | GTAAAAATGGTGAAAGAGCTCATGTCAAAGCGT |
| F12294Δ208 | CAGGGGCCACTGAGCTCATCGACCATT |
| R12294Δ-10 | CCTTTATTATAGAGCTCATTTTCTTCACA |
| F11486 | ACACCCATGAACGGAGCTCATTTTGTA |
| R11486 | ATAAAACAACGAGCTCTTTTAGTGATAACC |
| F11486Δ66 | GGGCCGTTGAGCTCAGCCACAGGAAGTA |
| F11486Δ145 | GGCACAGTTATGAGCTCTGATGGTGGT |
| F11486Δ169 | GTTGGACCATGAGCTCAAAAGACTTTACA |
| F11486Δ188 | GACTTTACAGAGCTCCTGTTCTTCAGTA |
| F12294-D | GATGTCAAAGCGTTAATTGGCA |
| R12294-D | GACCCGTTTGCTTCGTCTTAGT |
| F11486-D | GCCACAGGAAGTAGCAAAACT |
| R11486-D | GGTTTGTGGATTTGATGAATGA |
(a)Restriction sites are underlined, and the base changes that generate restriction sites are in bold.
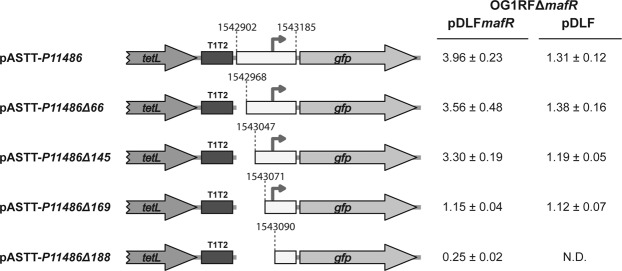
To further characterize the P11486 promoter, we constructed several transcriptional fusions (Fig. 6). A 284-bp DNA fragment (coordinates 1542902 to 1543185) was inserted into pASTT. The recombinant plasmid (pASTT-P11486) was first introduced into OG1RF and OG1RFΔmafR. In both strains, gfp expression (1.48 ± 0.10 and 1.51 ± 0.16 units, respectively) was ∼4-fold higher than the basal level (OG1RF harbouring pASTT). This result indicated that the 284-bp DNA fragment has promoter activity, however, the chromosomal copy of mafR is not sufficient to activate such a promoter located on pASTT (multicopy plasmid). Next, we introduced pASTT-P11486 into OG1RFΔmafR harbouring pDLFmafR (plasmid-encoded MafR). In this strain, gfp expression was ∼3-fold higher than in the control strain (OG1RFΔmafR harbouring pDLF) (Fig. 6). Similar results were obtained with plasmids pASTT-P11486Δ66 and pASTT-P11486Δ145, which allowed us to conclude that the 139-bp region between coordinates 1543047 and 1543185 contains both the P11486 promoter and the site required for its activation by MafR. A further deletion analysis showed that sequences between coordinates 1543047 and 1543071 (plasmid pASTT-P11486Δ169) are needed for MafR-mediated activation of the P11486 promoter but not for promoter activity. Moreover, deletion of the region that spans coordinates 1543071 and 1543090 (plasmid pASTT-P11486Δ188) removes the −35 element of the P11486 promoter and, consequently, reduces the expression of gfp to basal levels (Fig. 6).
Figure 6.
Effect of plasmid-encoded MafR on the activity of the P11486 promoter. Five regions from the OG1RF chromosome were inserted independently into the SacI site of pASTT. The coordinates of such regions are indicated. Gene tetL: tetracycline resistance determinant. Gene gfp: green fluorescent protein. The T1T2 box represents the tandem transcriptional terminators T1 and T2 of the Escherichia coli rrnB rRNA operon. The arrow represents the −35 element of the P11486 promoter. The intensity of fluorescence (arbitrary units) corresponds to 0.8 ml of culture (OD650 = 0.4). In each case, three independent cultures were analysed. N.D.: non-determined.
MafR binds to the P11486 promoter region in vitro
By DNase I footprinting assays, we analysed whether MafR-His binds to the P11486 promoter region (Fig. 7). We used a 275-bp DNA fragment (coordinates 1542969 to 1543243), which contains both the P11486 promoter and the site required for its activation by MafR in vivo (Fig. 6). On the coding strand and at 350 nM of MafR-His, changes in DNase I sensitivity (diminished cleavages) were observed within the region spanning coordinates 1543047 and 1543110. On the non-coding strand and at 300 nM of MafR-His, diminished cleavages were observed between coordinates 1543043 and 1543110. On both strands and at 400 nM of MafR-His, regions protected against DNase I digestion were observed along the DNA fragment, which is consistent with the ability of MafR-His to generate multimeric complexes4. Therefore, MafR-His recognizes preferentially a DNA site overlapping the P11486 core promoter. Such a DNA site includes sequences needed for MafR-mediated activation of the P11486 promoter in vivo (Fig. 6). According to the bendability/curvature propensity plot of the 275-bp DNA fragment, the MafR binding site contains regions of potential bendability (Fig. 4B).
Figure 7.
DNase I footprints of complexes formed by MafR-His on the 275-bp DNA fragment that contains the P11486 promoter. 32P-labelled DNA (4 nM) was incubated with the indicated concentrations of MafR-His and then it was digested with DNase I. Non-digested DNA (F) and dideoxy-mediated chain termination sequencing reactions (A, C, G, T) were run in the same gel. All the lanes displayed came from the same gel (delineation with dividing lines). Densitometer scans corresponding to free DNA (grey line) and DNA with protein (black line) are shown. The nucleotide sequence of the region spanning coordinates 1543037 to 1543176 is shown. The transcription initiation site (+1 position) of OG1RF_11486 is shown. The −35 and −10 elements of the P11486 promoter are indicated. Brackets indicate regions protected against DNase I digestion. The site recognized by MafR-His (coordinates 1543043-1543110) is indicated with a grey box.
Discussion
Gene regulation plays a key role during bacterial adaptation to environmental fluctuations. The ability of enterococci to metabolize numerous carbohydrates enables them to colonize diverse environments1. Our previous work showed that MafR activates, directly or indirectly, the transcription of numerous genes on a genome-wide scale. Many of such genes encode proteins involved in transport or metabolism of carbon sources5. Now, by qRT-PCR, transcriptional fusions and DNase I footprinting experiments, we have demonstrated that MafR functions as a transcription activator. It activates directly the transcription of the OG1RF_12294 and OG1RF_11486 genes. Gene OG1RF_12294 encodes a protein that has sequence similarity to several eukaryotic and prokaryotic proteins characterized as calcium P-type ATPases (Supplementary Table S1). This finding suggests that MafR could have a regulatory role in maintaining cellular calcium homeostasis. Calcium ions are known to affect different physiological processes in prokaryotic organisms, such as division, secretion, transport, and stress response30. Gene OG1RF_11486 encodes a putative ECF transporter S-component, likely involved in the uptake of a queuosine precursor. Thus, MafR could have an additional regulatory role in the biosynthesis of queuosine, a modified nucleoside found at the wobble position of particular transfer RNAs31. There is evidence that queuosine contributes to the efficiency of protein synthesis. In Shigella flexneri, the intracellular concentration of the virulence-related transcriptional regulator VirF is reduced in the absence of queuosine32. Moreover, it has been reported that the lack of queuosine affects the growth of some bacteria under stress conditions33,34.
Bacteria use a variety of mechanisms to activate transcription from specific promoters. Genetic and biochemical studies have shown that some proteins stimulate transcription by binding to a specific DNA site either upstream of or overlapping the core promoter35. By DNase I footprinting experiments, we have found that MafR recognizes a site overlapping the P12294 core promoter, as well as a site overlapping the P11486 core promoter (this work). These results suggest that MafR might enhance the efficiency of both promoters by recruitment of RNA polymerase through direct interactions with the sigma factor. In addition, MafR might induce conformational changes in the target promoters, as it has been described for some transcription activators35. Transcriptional activation from specific promoters has also been reported for other members of the Mga/AtxA family. The pneumococcal MgaSpn regulator stimulates transcription of a four-gene operon (spr1623-spr1626) by binding to a specific DNA site upstream of the promoter (positions −60 to −99)12. Regarding the Mga regulator from S. pyogenes, the position of its DNA-binding site with respect to the start of transcription varies among the promoters tested. Nevertheless, the majority of the promoters contain an Mga binding site located around position −54, thereby overlapping the −35 element of the promoter8.
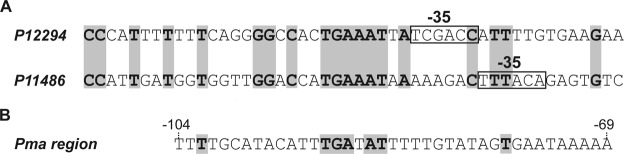
Simple protein-DNA recognition mechanisms do not exist36. Based on the structures of various protein-DNA complexes, Rohs et al. proposed that particular proteins use likely a combination of readout mechanisms: base readout and shape readout6. The DNA sites recognized by MafR on the P12294 and P11486 promoters have a low sequence identity: they share the GG(C/A)C(A/C)(C/A)TGAAAT(T/A)A sequence element (Fig. 8A). Moreover, both MafR binding sites contain regions of potential bendability (Figs 1B and 4B). We have also shown that MafR recognizes a DNA site upstream of the Pma promoter (positions −69 to −104)4. The function of this interaction remains unknown. Such a MafR binding site is adjacent to the peak of a potential intrinsic curvature4 and shares a short DNA sequence motif (TGATAT) with the two MafR binding sites identified in this work (Fig. 8B). Therefore, MafR does not seem to recognize a specific nucleotide sequence. Several findings suggest that recognition of particular DNA shapes could be a characteristic of the global regulators that constitute the Mga/AtxA family. MgaSpn from S. pneumoniae recognizes a DNA site upstream of the P1623B promoter (positions −60 to −99), as well as a DNA site overlapping the Pmga promoter (positions −23 to +21)12. The former interaction enhances the efficiency of the promoter11, whereas the function of the latter remains unknown. Such MgaSpn binding sites have a low sequence identity and, according to predictions, they contain an intrinsic curvature flanked by regions of bendability12. Furthermore, MgaSpn was shown to have a preference for AT-rich DNA regions13. Concerning Mga from S. pyogenes, several DNA-binding sites have been identified. These sites exhibit a low sequence identity (13.4%)37, although a consensus Mga binding sequence was initially proposed38. In the case of AtxA from B. anthracis, in vitro protein-DNA interaction studies have not been reported. Nevertheless, sequence similarities are not apparent in its target promoter regions, and some of them are intrinsically curved39.
Figure 8.
DNA sites recognized by MafR. (A) Nucleotide sequence alignment of the DNA sites recognized by MafR on the P12294 and P11486 promoter regions. Identical nucleotides are highlighted in grey boxes. (B) Nucleotide sequence of the DNA site recognized by MafR on the Pma promoter region (positions −69 to −104)4. Nucleotides shared with the MafR binding sites shown in (A) are highlighted in grey boxes.
In conclusion, our study shows for the first time that MafR is a transcription activator. It stimulates transcription from the P12294 and P11486 promoters in vivo. Moreover, MafR binds in vitro to a specific DNA site that overlaps the −35 element of each promoter. The two MafR binding sites have a low sequence identity but share a six-base pair motif. We propose that MafR would recognize intrinsic DNA structural features rather than particular DNA sequences on its target DNAs.
Materials and Methods
Oligonucleotides, bacterial strains, and plasmids
Oligonucleotides used in this work are listed in Table 1. E. faecalis strains OG1RF14 and OG1RFΔmafR5 were used. Plasmids pDLF (expression vector) and pDLFmafR were described5. These plasmids carry a kanamycin resistance gene. Plasmid pASTT (D. García-Rincón, V. Solano-Collado and A. Bravo, unpublished results) is based on the pAST promoter-probe vector40, which carries a tetracycline resistance gene. Plasmid pASTT carries the TrsiV transcriptional terminator40 downstream of the gfp reporter gene. The following pASTT-derivatives were constructed in this work. In all cases, a region of the OG1RF chromosome was amplified by PCR using the indicated primers, digested with SacI, and inserted into pASTT: pASTT-P12294 (primers F12294 and R12294, 260-bp restriction fragment), pASTT-P12294Δ-10 (primers F12294 and R12294Δ-10, 236-bp restriction fragment), pASTT-P12294Δ69 (primers F12294Δ69 and R12294, 192-bp restriction fragment), pASTT-P12294Δ208 (primers F12294Δ208 and R12294, 53-bp restriction fragment), pASTT-P11486 (primers F11486 and R11486, 290-bp restriction fragment), pASTT-P11486Δ66 (primers F11486Δ66 and R11486, 224-bp restriction fragment), pASTT-P11486Δ145 (primers F11486Δ145 and R11486, 145-bp restriction fragment), pASTT-P11486Δ169 (primers F11486Δ169 and R11486, 121-bp restriction fragment), pASTT-P11486Δ188 (primers F11486Δ188 and R11486, 102-bp restriction fragment).
Growth and transformation of bacteria
E. faecalis was grown in BHI medium, which was supplemented with tetracycline (4 μg/ml) and/or with kanamycin (250 μg/ml) when strains carrying plasmids were used. Experiments were performed at 37 °C without aeration. The protocol used to transform E. faecalis by electroporation was described41.
DNA and RNA isolation
Genomic DNA was prepared using the Bacterial Genomic Isolation Kit (Norgen Biotek Corporation). Plasmid DNA was prepared using the High Pure Plasmid Isolation Kit (Roche Applied Science) as described5. Total RNA was isolated using the RNeasy mini Kit (QIAGEN). In general, bacteria were grown to an optical density at 650 nm (OD650) of 0.4 (logarithmic growth phase). For stationary phase, bacteria were grown to an OD650 of 0.8 and then incubated for two hours at the same temperature. Then, cultures were processed as reported5. The integrity of rRNAs was analysed by agarose gel electrophoresis. RNA concentration was determined using a NanoDrop ND-2000 Spectrophotometer.
Polymerase chain reaction (PCR)
The Phusion High-Fidelity DNA polymerase (Thermo Scientific) and the Phusion HF buffer were used. Reaction mixtures (50 μl) contained 5–30 ng of template DNA, 20 pmol of each primer, 200 μM each deoxynucleoside triphosphate (dNTP), and one unit of DNA polymerase. PCR conditions were reported40. To amplify the 270-bp DNA fragment (promoter P12294) used in footprinting experiments, the Phusion GC buffer was used. In this case, reaction mixtures were supplemented with 7% DMSO and the annealing step was performed at 59 °C. PCR products were purified with the QIAquick PCR purification kit (QIAGEN).
Quantitative RT-PCR (qRT-PCR)
For cDNA synthesis with random primers, the iScript Select cDNA Synthesis kit (Bio-Rad) was used as described5. Quantitative PCRs were performed using the iQ SYBR Green Supermix (Bio-Rad) and a iCycler Thermal Cycler (Bio-Rad) as reported5. Forward (Fgene-q) and reverse (Rgene-q) primers used in the quantitative PCRs are listed in Table 1. Relative quantification of gene expression was performed using the comparative CT method15 as described5. Except for gene mafR, the internal control gene was recA (OG1RF_12439; recombination protein RecA). In the case of mafR, the internal control gene was zwf (OG1RF_10737; glucose-6-phosphate 1-dehydrogenase) because its expression level was similar at the logarithmic and stationary growth phases.
Primer extension
Oligonucleotide R11486-D was radioactively labelled at the 5′-end using [γ-32P]-ATP (PerkinElmer) and T4 polynucleotide kinase (New England Biolabs) as reported12. Primer extension reactions (20 μl) contained 1.2 pmol of 32P-labelled oligonucleotide and 5 μg of total RNA isolated from strain OG1RF. The ThermoScript Reverse Transcriptase enzyme (Invitrogen) was used. Reactions were incubated at 55 °C for 45 min. After heating at 85 °C for 5 min, samples were ethanol precipitated and dissolved in loading buffer (80% formamide, 1 mM EDTA, 10 mM NaOH, 0.1% bromophenol blue, 0.1% xylene cyanol). cDNA products were analysed by sequencing gel (8 M urea, 6% polyacrylamide) electrophoresis. Dideoxy-mediated chain termination sequencing reactions were run in the same gel. Labelled products were visualized using a Fujifilm Image Analyser FLA-3000.
Fluorescence assays
Plasmid-carrying cells were grown to an OD650 of 0.4 (logarithmic phase). Then, different volumes of culture (0.4 to 1 ml) were centrifuged, and cells were resuspended in 200 μl of phosphate-buffered saline (PBS). In each case, three independent cultures were analysed. Fluorescence intensity was measured using a Thermo Scientific Varioskan Flash instrument (excitation at 488 nm and emission at 515 nm). The fluorescence corresponding to 200 μl of PBS buffer without cells was ~0.03 arbitrary units.
Purification of MafR-His
The procedure to overproduce and purify a His-tagged MafROG1RF protein (herein MafR-His) was reported4. MafR-His carries the Leu-Glu-6xHis peptide (His-tag) fused to its C terminus. Protein concentration was determined using a NanoDrop ND-2000 Spectrophotometer (Thermo Scientific).
DNase I footprinting assays
Oligonucleotides were 32P-labelled at the 5′-end as described12. 32P-labelled oligonucleotides were used for PCR amplification to obtain double-stranded DNA fragments labelled at either the coding or the non-coding strand. Two regions of the OG1RF chromosome were amplified: a 270-bp region (coordinates 2425817-2425548) using the F12294-D and R12294-D oligonucleotides, and a 275-bp region (coordinates 1542969-1543243) using the F11486-D and R11486-D oligonucleotides. Binding reactions (8 μl) contained 30 mM Tris-HCl, pH 7.6, 1 mM DTT, 1 mg/ml BSA, 1.25% glycerol, 0.25 mM EDTA, 50 mM NaCl, 10 mM MgCl2, 1 mM CaCl2, 2–4 nM 32P-labelled DNA and different concentrations of MafR-His (100 to 600 nM). Reaction mixtures were incubated at room temperature for 20 min. Then, 0.015 units of DNase I (Roche Applied Science) was added and the reaction proceeded for 5 min at the same temperature. DNase I digestion was stopped by adding 1 μl of 250 mM EDTA. Then, 4 μl of loading buffer (80% formamide, 1 mM EDTA, 10 mM NaOH, 0.1% bromophenol blue and 0.1% xylene cyanol) was added. Samples were heated at 95 °C for 5 min and loaded onto sequencing gels (6% polyacrylamide, 8 M urea). Dideoxy-mediated chain termination sequencing reactions were run in the same gel. Labelled products were visualized using a Fujifilm Image Analyser FLA-3000. The intensity of the bands was quantified using the Quantity One software (Bio-Rad).
In silico prediction of intrinsic curvature
The bendability/curvature propensity plots were calculated with the bend.it server26 (http://hydra.icgeb.trieste.it/dna/bend_it.html) as described previously12.
Supplementary information
Acknowledgements
Thanks are due to Dr. Virtu Solano-Collado and Daniel García-Rincón for providing the pASTT plasmid, and to Verónica Navarro for her excellent technical assistance. This work was supported by grants BIO2016-76412-C2-2-R (AEI/FEDER, UE) and BIO2015-69085-REDC from the Spanish Ministry of Economy and Competitiveness.
Author Contributions
S.R.-C. and A.M.-B. performed experiments. S.R.-C., M.E. and A.B. designed the study and wrote the manuscript. All authors read and approved the final manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-42484-4.
References
- 1.Ramsey, M., Hartke, A. & Huycke, M. The Physiology and Metabolism of Enterococci. In: Gilmore MS, Clewell DB, Ike Y, et al. editors. Enterococci: From Commensals to Leading Causes of Drug Resistant Infection (Internet). Boston, Massachusetts Eye and Ear Infirmary, 1–43 (2014).
- 2.Kim S, Covington A, Pamer EG. The intestinal microbiota: antibiotics, colonization resistance, and enteric pathogens. Immunol. Rev. 2017;279:90–105. doi: 10.1111/imr.12563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beganovic M, et al. A review of combination antimicrobial therapy for Enterococcus faecalis bloodstream infections and infective endocarditis. Clin. Infect. Dis. 2018;67:303–309. doi: 10.1093/cid/ciy064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruiz-Cruz S, Moreno-Blanco A, Espinosa M, Bravo A. DNA-binding properties of MafR, a global regulator of Enterococcus faecalis. FEBS Lett. 2018;592:1412–1425. doi: 10.1002/1873-3468.13032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruiz-Cruz S, Espinosa M, Goldmann O, Bravo A. Global regulation of gene expression by the MafR protein of Enterococcus faecalis. Front. Microbiol. 2016;6:1521. doi: 10.3389/fmicb.2015.01521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rohs R, et al. Origins of specificity in protein-DNA recognition. Annu. Rev. Biochem. 2010;79:233–269. doi: 10.1146/annurev-biochem-060408-091030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abe N, et al. Deconvolving the recognition of DNA shape from sequence. Cell. 2015;161:307–318. doi: 10.1016/j.cell.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hondorp ER, McIver KS. The Mga virulence regulon: infection where the grass is greener. Mol. Microbiol. 2007;66:1056–1065. doi: 10.1111/j.1365-2958.2007.06006.x. [DOI] [PubMed] [Google Scholar]
- 9.McIver KS, Myles RL. Two DNA-binding domains of Mga are required for virulence gene activation in the group A streptococcus. Mol. Microbiol. 2002;43:1591–1601. doi: 10.1046/j.1365-2958.2002.02849.x. [DOI] [PubMed] [Google Scholar]
- 10.Vahling CM, McIver KS. Domains required for transcriptional activation show conservation in the Mga family of virulence gene regulators. J. Bacteriol. 2006;188:863–873. doi: 10.1128/JB.188.3.863-873.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Solano-Collado V, Espinosa M, Bravo A. Activator role of the pneumococcal Mga-like virulence transcriptional regulator. J. Bacteriol. 2012;194:4197–4207. doi: 10.1128/JB.00536-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Solano-Collado V, Lurz R, Espinosa M, Bravo A. The pneumococcal MgaSpn virulence transcriptional regulator generates multimeric complexes on linear double-stranded DNA. Nucleic Acids Res. 2013;41:6975–6991. doi: 10.1093/nar/gkt445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Solano-Collado V, Hüttener M, Espinosa M, Juárez A, Bravo A. MgaSpn and H-NS: Two unrelated global regulators with similar DNA-binding properties. Front. Mol. Biosci. 2016;3:60. doi: 10.3389/fmolb.2016.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bourgogne A, et al. Large scale variation in Enterococcus faecalis illustrated by the genome analysis of strain OG1RF. Genome Biol. 2008;9:R110. doi: 10.1186/gb-2008-9-7-r110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 16.Palmgren MG, Nissen P. P-Type ATPases. Annu. Rev. Biophys. 2011;40:243–266. doi: 10.1146/annurev.biophys.093008.131331. [DOI] [PubMed] [Google Scholar]
- 17.Rudolph HK, et al. The yeast secretory pathway is perturbed by mutations in PMR1, a member of a Ca2+ ATPase family. Cell. 1989;58:133–145. doi: 10.1016/0092-8674(89)90410-8. [DOI] [PubMed] [Google Scholar]
- 18.Sorin A, Rosas G, Rao R. PMR1, a Ca2+ -ATPase in yeast Golgi, has properties distinct from sarco/endoplasmic reticulum and plasma membrane calcium pumps. J. Biol. Chem. 1997;272:9895–9901. doi: 10.1074/jbc.272.15.9895. [DOI] [PubMed] [Google Scholar]
- 19.Van Baelen K, Vanoevelen J, Missiaen L, Raeymaekers L, Wuytack F. The Golgi PMR1 P-type ATPase of Caenorhabditis elegans: Identification of the gene and demonstration of calcium and manganse transport. J. Biol. Chem. 2001;276:10683–10691. doi: 10.1074/jbc.M010553200. [DOI] [PubMed] [Google Scholar]
- 20.Altschul SF, et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raeymaekers L, Wuytack EY, Willems I, Michiels CW, Wuytack F. Expression of a P-type Ca2+ -transport ATPase in Bacillus subtilis during sporulation. Cell Calcium. 2002;32:93–103. doi: 10.1016/S0143-4160(02)00125-2. [DOI] [PubMed] [Google Scholar]
- 22.Rosch JW, Sublett J, Gao G, Wang Y-D, Tuomanen EI. Calcium efflux is essential for bacterial survival in the eukaryotic host. Mol. Microbiol. 2008;70:435–444. doi: 10.1111/j.1365-2958.2008.06425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Faxén K, et al. Characterization of a Listeria monocytogenes Ca2+ pump: a SERCA-type ATPase with only one Ca2+ -binding site. J. Biol. Chem. 2011;286:1609–1617. doi: 10.1074/jbc.M110.176784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geisler M, Richter J, Schumann J. Molecular cloning of a P-type ATPase gene from the cyanobacterium Synechocystis sp. PCC 6803. Homology to eukaryotic Ca2+ -ATPases. J. Mol. Biol. 1993;234:1284–1289. doi: 10.1006/jmbi.1993.1684. [DOI] [PubMed] [Google Scholar]
- 25.Hein KL, Nissen P, Morth JP. Purification, crystallization and preliminary crystallographic studies of a PacL homologue from Listeria monocytogenes. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2012;68:424–427. doi: 10.1107/S1744309112004046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vlahovicek K, Kaján L, Pongor S. DNA analysis servers: plot.it, bend.it, model.it and IS. Nucleic Acids Res. 2003;31:3686–3687. doi: 10.1093/nar/gkg559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodionov DA, et al. A novel class of modular transporters for vitamins in prokaryotes. J. Bacteriol. 2009;191:42–51. doi: 10.1128/JB.01208-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Slotboom DJ. Structural and mechanistic insights into prokaryotic energy-coupling factor transporters. Nat. Rev. Microbiol. 2014;12:79–87. doi: 10.1038/nrmicro3175. [DOI] [PubMed] [Google Scholar]
- 29.Majsnerowska M, Ter Beek J, Stanek WK, Duurkens RH, Slotboom DJ. Competition between different S-components for the shared energy coupling factor module in energy coupling factor transporters. Biochemistry. 2015;54:4763–4766. doi: 10.1021/acs.biochem.5b00609. [DOI] [PubMed] [Google Scholar]
- 30.Domínguez DC, Guragain M, Patrauchan M. Calcium binding proteins and calcium signaling in prokaryotes. Cell Calcium. 2015;57:151–165. doi: 10.1016/j.ceca.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 31.Hutinet G, Swarjo MA, de Crécy-Lagard V. Deazaguanine derivatives, examples of crosstalk between RNA and DNA modification pathways. RNA Biol. 2017;14:1175–1184. doi: 10.1080/15476286.2016.1265200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Durand JMB, Dagberg B, Uhlin BE, Björk GR. Transfer RNA modification, temperature and DNA superhelicity have a common target in the regulatory network of the virulence of Shigella flexneri: the expression of the virF gene. Mol. Microbiol. 2000;35:924–935. doi: 10.1046/j.1365-2958.2000.01767.x. [DOI] [PubMed] [Google Scholar]
- 33.Noguchi S, Nishimura Y, Hirota Y, Nishimura S. Isolation and characterization of an Escherichia coli mutant lacking tRNA-guanine transglycosylase. Function and biosynthesis of queuosine in tRNA. J. Biol. Chem. 1982;257:6544–6550. [PubMed] [Google Scholar]
- 34.Thibessard A, et al. Identification of Streptococcus thermophilus CNRZ368 genes involved in defense against superoxide stress. Appl. Environ. Microbiol. 2004;70:2220–2229. doi: 10.1128/AEM.70.4.2220-2229.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Browning DF, Busby SJW. Local and global regulation of transcription initiation in bacteria. Nat. Rev. Microbiol. 2016;14:638–650. doi: 10.1038/nrmicro.2016.103. [DOI] [PubMed] [Google Scholar]
- 36.Siggers T, Gordân R. Protein-DNA binding: complexities and multi-protein codes. Nucleic Acids Res. 2014;42:2099–2111. doi: 10.1093/nar/gkt1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hause LL, McIver KS. Nucleotides critical for the interaction of the Streptococcus pyogenes Mga virulence regulator with Mga-regulated promoter sequences. J. Bacteriol. 2012;194:4904–4919. doi: 10.1128/JB.00809-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McIver KS, Heath AS, Green BD, Scott JR. Specific binding of the activator Mga to promoter sequences of the emm and scpA genes in the group A streptococcus. J. Bacteriol. 1995;177:6619–6624. doi: 10.1128/jb.177.22.6619-6624.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hadjifrangiskou M, Koehler TM. Intrinsic curvature associated with the coordinately regulated anthrax toxin gene promoters. Microbiology. 2008;154:2501–2512. doi: 10.1099/mic.0.2007/016162-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ruiz-Cruz S, Solano-Collado V, Espinosa M, Bravo A. Novel plasmid-based genetic tools for the study of promoters and terminators in Streptococcus pneumoniae and Enterococcus faecalis. J. Microbiol. Methods. 2010;83:156–163. doi: 10.1016/j.mimet.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 41.Shepard BD, Gilmore MS. Electroporation and efficient transformation of Enterococcus faecalis grown in high concentrations of glycine. Methods Mol. Biol. 1995;47:217–226. doi: 10.1385/0-89603-310-4:217. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.