Abstract
Iron homeostasis in the yeast Saccharomyces cerevisiae is regulated at the transcriptional level by Aft1p, which activates the expression of its target genes in response to low-iron conditions. The yeast genome contains a paralog of AFT1, which has been designated AFT2. To establish whether AFT1 and AFT2 have overlapping functions, a mutant containing a double aft1Δaft2Δ deletion was generated. Growth assays established that the single aft2Δ strain exhibited no iron-dependent phenotype. However, the double-mutant aft1Δaft2Δ strain was more sensitive to low-iron growth conditions than the single-mutant aft1Δ strain. A mutant allele of AFT2 (AFT2-1up), or overexpression of the wild-type AFT2 gene, led to partial complementation of the respiratory-deficient phenotype of the aft1Δ strain. The AFT2-1up allele also increased the uptake of 59Fe in an aft1Δ strain. DNA microarrays were used to identify genes regulated by AFT2. Some of the AFT2-regulated genes are known to be regulated by Aft1p; however, AFT2-1up-dependent activation was independent of Aft1p. The kinetics of induction of two genes activated by the AFT2-1up allele are consistent with Aft2p acting as a direct transcriptional factor. Truncated forms of Aft1p and Aft2p bound to a DNA duplex containing the Aft1p binding site in vitro. The wild-type allele of AFT2 activated transcription in response to growth under low-iron conditions. Together, these data suggest that yeast has a second regulatory pathway for the iron regulon, with AFT1 and AFT2 playing partially redundant roles.
Keywords: metalloregulation‖iron homeostasis
Iron is an essential nutrient but it is toxic in the absence of effective homeostasis. Mechanisms have therefore evolved to ensure adequate but not excess levels of reactive intracellular iron. In the yeast Saccharomyces cerevisiae, transcriptional regulation of the iron regulon is mediated by the transcriptional activator Aft1p (1). The Aft1p regulon consists of many genes that are involved in the acquisition, compartmentalization, and utilization of iron. These include genes involved in iron uptake (FET3, FTR1, and FRE1,2), siderophore uptake (ARN1-4 and FIT1-3), iron transport across the vacuole membrane (FTH1), and iron-sulfur cluster formation (ISU1,2; refs. 1–6). Aft1p binds to a conserved promoter sequence in an iron-dependent manner and activates transcription under low-iron conditions (2). Mutants lacking a functional Aft1p grow poorly in iron-limiting conditions (1, 7). A Cys291Phe substitution within Aft1p results in derepressed transcriptional activation in iron-replete cells (1). Because Cys291 is part of a Cys-X-Cys motif, an attractive hypothesis is that Aft1p is capable of binding iron; however, the mechanism by which Aft1p senses iron levels is not known.
The S. cerevisiae genome has many duplicated chromosomal regions that account for up to 16% of the yeast proteome, and it has been proposed that these regions have arisen from an ancient duplication of the entire genome (8). AFT1 lies within such a region of chromosome VII, with its duplicate ORF YPL202c (designated AFT2), located on chromosome XVI (9). Characterization of paralogs that have arisen from the genome duplication has revealed differences in the extent to which genes within a pair have functionally diverged. The transcription factors Swi5p and Ace2p have nearly identical DNA-binding domains, yet they activate the transcription of different genes (10). Alternatively, there may be redundancy of function so that a clear phenotype is apparent only in a mutant lacking both paralogs, as in the case of the Pcl8p and Pcl10p cyclins (11).
Previous work relating to the function of AFT1 has suggested that there is an AFT1-independent pathway for iron regulation in S. cerevisiae (1, 7). We were interested to learn whether such a pathway involves AFT2. We have used a combination of phenotypic analysis, functional complementation, and analysis of global gene expression to identify the relationship between AFT1 and AFT2. We present evidence that AFT2 codes for a transcription factor that activates gene expression in response to low-iron conditions. Although Aft1p and Aft2p are functionally similar, and have overlapping functions, comparison of gene activation by both transcriptional factors suggests that they also have distinct functions.
Materials and Methods
Yeast Strains and Culture Conditions.
The following S. cerevisiae strains were purchased from Research Genetics (Huntsville, AL) and used in this study: BY4741 (MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0) as wild type; BY4741aft1Δ (MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 aft1∷kanMX4); BY4742aft1Δ (MATα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 aft1∷kanMX4); and BY4741aft2Δ (MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 aft2∷kanMX4). A haploid aft1Δaft2Δ strain (MATα his3Δ1 leu2Δ0 lys2Δ0 met15Δ0 ura3Δ0 aft1∷kanMX4 aft2∷kanMX4) was isolated after the mating of BY4741aft2Δ and BY4742aft1Δ, and its allele status was verified by using PCR and DNA sequencing. Cells were grown in either 1% yeast extract, 2% peptone medium (YP), complete-synthetic medium (CM), or, when appropriate, complete-synthetic medium lacking uracil [CM(−Ura)]. These media were supplemented with either 2% glucose, 2% raffinose, or 3% glycerol as indicated. For plate phenotypes, 10-fold serial dilutions of cells were spotted onto agar plates and grown at 30°C. For anaerobic growth, agar plates were incubated in a GasPak (Becton Dickinson) anaerobic chamber.
Vectors.
Yeast-genomic DNA from strain BY4741 was isolated and used as template for PCR to amplify a 2.6-kb fragment containing AFT2, with both upstream and downstream sequences, which was ligated into the BamHI/XbaI site of the YCp vector pRS416 (12) to create pAFT2. QuikChange (Stratagene) mutagenesis was used to generate an allele of AFT2 that codes for a Cys187Phe mutation by using pAFT2 as template to create pAFT2-1up. To place the AFT2-1up allele under the control of the GAL10 promoter, pAFT2-1up was used as template for PCR to amplify a 1.3-kb fragment containing AFT2-1up, which was ligated into the BamHI/ClaI site of GFP-pYeF2, a derivative of pYeF2 (13), to generate pGAL-AFT2-1up. Plasmid pAFT1-1up contains a 3-kb fragment, consisting of AFT1 with both upstream and downstream sequences, that originated from pCM16 (ref. 14; a kind gift from Enrique Herrero, Universitat de Lleida, Lleida, Spain), which was ligated into the XhoI/SacI site of pRS316 (12). Site-directed mutagenesis by using the pALTER mutagenesis system (Promega) was used to generate an allele of AFT1 that codes for a Cys291Phe mutation. To generate plasmids that express truncated forms of Aft1p (pAft1-313) and Aft2p (pAft2-214), PCR was used to amplify the 5′ ends of AFT1 and AFT2 containing the first 313 codons of Aft1p and the first 214 codons of Aft2p, which were then ligated into pET20 and pET3 (Novagen), respectively. All of the AFT1, AFT2, AFT1-1up, and AFT2-1up sequences were confirmed by DNA sequencing. All yeast transformations were performed by using the lithium acetate procedure. In the case of transformations using the aft1Δaft2Δ strain, cells were pregrown in yeast extract/peptone/dextrose (YPD) under nitrogen, and the agar plates were supplemented with FeCl2 (100 μM).
Electrophoretic Mobility-Shift Assay (EMSA).
The identification of specific protein-DNA complexes was analyzed as described with the following modifications (15). The DNA probe consisted of oligonucleotide 5′-ATCTTCAAAAGTGCACCCATTTGCAGGTGC-3′ and its reverse complement, which contains the Aft1p-binding site within the FET3 promoter.
Crude protein extracts were isolated from Escherichia coli cells (BL21-CodonPlus(DE3)-RIL from Stratagene) that had been transformed separately with pET3, pAft1-313, and pAft2-214. Cells were grown to mid-log phase at 37°C with ampicillin and chloramphenicol, protein expression was induced, and the cells were incubated for an additional 2 h before harvesting. The cells were pelleted and resuspended in 1/50th vol of lysis buffer (20 mM Tris⋅HCl, pH 7.5/1 mM DTT) and sonicated, and the cell debris was pelleted. The standard EMSA binding reaction consisted of equal volumes of an E. coli cell extract and the hybridization mix with the addition of poly(dI-dC)⋅poly(dI-dC) to a final concentration of 4 ng⋅μl−1. The binding reaction was incubated for 15 min and applied to a 6% polyacrylamide nondenaturing gel and electrophoresed by using Tris-borate-EDTA buffer.
mRNA Quantification by S1 Nuclease Analysis.
Total RNA was isolated from mid-log cells and was hybridized with 32P-labeled, single-stranded DNA oligonucleotides that were complementary to the candidate gene (between 50 and 69 nt) and the control gene CMD1 (40 nt). After digestion with S1 nuclease, the samples were electrophoresed through an 8% polyacrylamide/5 M urea gel.
Microarray Analysis.
Cells were harvested from 300 ml of culture at an A at 600 nm of 0.4. Total RNA was isolated by the hot phenol method. mRNA was isolated from total RNA by using the PolyATtract mRNA Isolation System IV kit from Promega following the manufacturer's instructions. Cy3-dUTP or Cy5-dUTP (Amersham Pharmacia) was incorporated during reverse transcription of the polyadenylated RNA. The fluorescently labeled product was recovered and hybridized to microarrays, which were washed and scanned as described (16).
59Fe Uptake Analysis.
Iron uptake was analyzed as described (17). Briefly, mid-log phase cells were grown in complete synthetic medium and harvested, and the cells were washed and resuspended in low-iron medium plus 1 mM ascorbate. 59Fe (0.5 μM) was added to the cells, which were then incubated at 30°C for 10 min. The cell cultures were then filtered through Whatman GF/C filters, which were then washed, and the level of 59Fe was measured. In some cases, the Fe(II) chelator bathophenanthroline disulfonate (BPS) was added to the cell cultures 4 h before the cells being harvested.
Results
S. cerevisiae Contains a Paralog of AFT1.
Analysis of the S. cerevisiae genome has revealed two similar regions of chromosomes VII and XVI that contain the paralogs AFT1 and AFT2 (8). The predicted product of AFT2 exhibits strong identity to Aft1p from residues 38 to 285 (39% identity; Fig. 1). The homologous region includes the N-terminal basic region of Aft1p, which is predicted to represent the Aft1p DNA-binding domain, and the region that contains the Cys-X-Cys motif that confers iron sensitivity. The two histidine-rich regions of the extreme N terminus and C terminus of Aft1p are not conserved in Aft2p. It has been proposed that these regions may be involved in iron binding (1). The high degree of identity within the N terminus of both proteins encourages the prediction that AFT2 codes for a DNA-binding protein.
Figure 1.
Sequence comparison of Aft1p and Aft2p. The amino acid sequences of Aft1p and Aft2p of S. cerevisiae were aligned by using the clustalw program. The conserved CXC motif is underlined and in bold (see text).
The Effect of Iron on the aft Mutants.
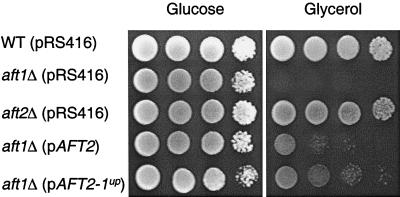
To determine whether AFT2 plays a role in iron homeostasis, the growth of strains with either a single deletion of AFT1 or AFT2, or the double deletion of both genes, was compared. The growth of a strain lacking AFT1 is impaired under low-iron conditions, and this defect is exacerbated when the medium contains a nonfermentable carbon source (7). As expected, the aft1Δ strain failed to grow on medium containing glycerol, whereas the aft2Δ strain grew equivalently to the wild-type strain (Fig. 2). The growth defect of the aft1Δ strain was partially reversed in an aft1Δ strain harboring low-copy plasmids containing either the wild-type AFT2 allele (pAFT2) or the AFT2-1up allele (pAFT2-1up). The AFT2-1up allele contains a mutation that codes for a Cys187Phe substitution, which corresponds to the mutation within the AFT1-1up allele that codes for a Cys291Phe substitution.
Figure 2.
AFT2 partially complements the aft1Δ mutant strain. Overnight cultures of each strain were grown in CM(−Ura) medium containing glucose. The cultures were washed, and 10-fold serial dilutions were spotted onto CM(−Ura) agar plates containing either glucose or glycerol, which were then incubated at 30°C.
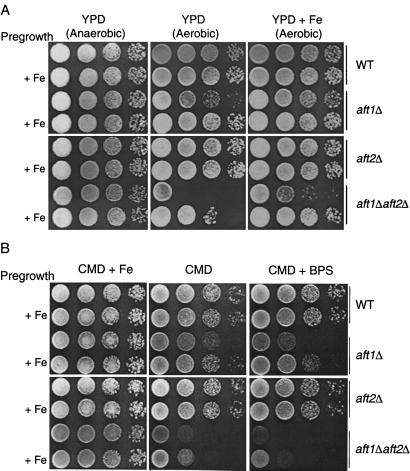
The growth defect of the aft1Δ strain is less pronounced on medium containing a fermentable carbon source, and it can be suppressed in three ways (Fig. 3A). The aft1Δ strain grows normally on complex YPD medium containing glucose, in the absence of oxygen. Alternatively, full aerobic growth can be attained by pregrowth of the aft1Δ strain in liquid medium supplemented with Fe(II) or by supplementing the agar with Fe(II) (Fig. 3A). The aft1Δaft2Δ strain exhibits a more exaggerated iron-deficient phenotype under aerobic conditions (Fig. 3A). This growth defect is reversed only by supplementing both the preculture and the agar with exogenous Fe(II). As in the case of the aft1Δ strain, the aft1Δaft2Δ strain grows fully under anaerobic conditions.
Figure 3.
The effect of iron on the growth of the aft mutant strains. Overnight cultures of the wild-type strain and each of the aft-mutant strains were grown in liquid CM(−Ura) with glucose medium. Each strain was grown in duplicate, with one of each pair being supplemented with FeCl2 (100 μM). Iron supplementation in the pregrowth medium is indicated by “+ Fe.” The cells were washed, and 10-fold serial dilutions were spotted onto (A) complex medium (YPD) agar plates with or without supplemented FeCl2 (100 μM) or (B) synthetic medium (CMD) agar plates with or without supplemental FeCl2 (100 μM) or BPS (10 μM). Plates were incubated at 30°C and, in the case of one YPD plate, under anaerobic conditions.
The exaggerated iron-deficient growth defect of the aft1Δaft2Δ strain relative to the aft1Δ strain is also apparent on minimal medium (Fig. 3B). The addition of the Fe(II) chelator BPS to the agar markedly attenuates the aerobic growth of the aft1Δaft2Δ strain even when it is pregrown in liquid medium supplemented with Fe(II). The addition of BPS to cultures is known to lower iron availability, resulting in Aft1p activation (1, 18). In contrast to growth on YPD, supplementation of the minimal medium agar with Fe(II) fully restores growth of the aft1Δaft2Δ strain. The growth defect of the aft1Δaft2Δ strain is also reversed when it is transformed with either pAFT2 or pAFT2-1up (data not shown).
The Effect of the AFT2-1up Allele on Gene Expression Within S. cerevisiae.
The exacerbated growth phenotype of the aft1Δaft2Δ strain and the observed sequence similarity between Aft1p and Aft2p are consistent with both proteins having partial overlapping functions. We hypothesized that Aft2p may be a transcriptional activator and that the AFT2-1up allele may result in the derepressed transcriptional activation of genes regulated by Aft2p. DNA microarray experiments were conducted to identify candidate target genes regulated by Aft2p. We compared the differential gene expression of the aft2Δ strain harboring either pAFT2-1up or a control vector. These microarray experiments were conducted at both the Stanford Microarray Facility and the Huntsman Cancer Institute Microarray Facility at the University of Utah. A number of genes were reproducibly elevated in their expression in the strain containing the AFT2-1up allele (Table 1). These include genes that are known to be regulated by Aft1p (FIT1, FIT3, FTR1, FTH1, FRE1, and TIS11) and by the zinc-responsive transcription factor Zap1p (ZRT1 and YOL154w; refs. 1, 4, and 19). Genes that are not known to be metalloregulated by either Aft1p or Zap1p were also identified (MRS4, UBC8, PRB1, and ECM4) (Caroline Philpott and Dave Eide, personal communications).
Table 1.
Genes activated by the AFT2-1up allele
| Target gene | Cy5/Cy3 ratio | Function |
|---|---|---|
| FIT3 | 8.8 | Cell wall protein |
| YOL154w | 8.8 | Zn-metalloprotease-like protein |
| ZRT1 | 6.2 | High affinity zinc transporter |
| FIT1 | 5.7 | Cell wall protein |
| ECM4 | 5.0 | Cell wall structure/biosynthesis |
| YOL083w | 4.2 | Unknown |
| UBC8 | 4.1 | Ubiquitin-conjugating enzyme |
| YJR078w | 3.6 | Similar to indoleamine 2,3-dioxygenase |
| MRS4 | 3.5 | Member of mitochondrial carrier family |
| TIS11 | 3.3 | Similar to mammalian TIS11 family |
| FTR1 | 3.3 | High affinity iron transporter |
| FTH1 | 3.2 | Vacuolar iron transporter |
| LAP4 | 3.0 | Vacuolar aminopeptidase I |
| PRB1 | 2.9 | Vacuolar protease B |
| GRX4 | 2.8 | Glutaredoxin |
| AHP1 | 2.7 | Alkyl hydroperoxide reductase |
| HCR1 | 2.6 | Putative component of eIF3 |
| FET5 | 2.6 | Vacuolar multicopper oxidase |
| PEP4 | 2.6 | Vacuolar proteinase A |
| YDL124w | 2.5 | Unknown |
| FRE1 | 2.2 | Cell surface iron reductase |
| SMF3 | 2.0 | Vacuolar iron transporter |
Transcripts of these genes were at least 2-fold more abundant in the strain containing the AFT2-1up allele compared with the control strain. The value shown is the Cy5/Cy3 ratio (which reflects the ratio of the transcripts' abundance in the two strains) measured in one experiment at the Stanford Microarray Facility. The same genes were identified in two similar experiments conducted at the University of Utah Microarray Facility.
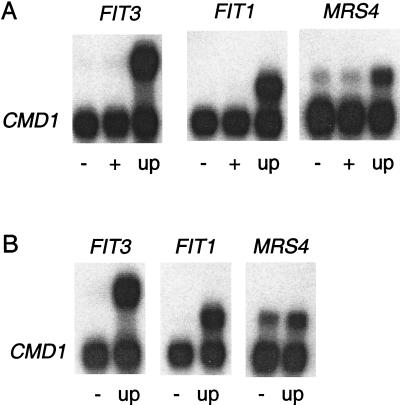
To confirm the microarray data, S1 analysis was performed to quantify the expression of selected AFT2-1up induced genes. The expression of FIT3, FIT1, and MRS4 was clearly activated in the aft2Δ strain containing pAFT2-1up but not in the control strain or the strain containing pAFT2 (Fig. 4A). The AFT2-1up allele also activated the expression of FIT3, FIT1, and MRS4 in an aft1Δ strain, demonstrating that the AFT2-1up allele activates transcription in an Aft1p-independent manner (Fig. 4B). The ZRT1 and YOL154w genes were induced in only a subset of the S1 experiments (data not shown). Activation of these two genes was not observed in a zap1Δ strain harboring pAFT2-1up, suggesting that their induction was the indirect result of fluctuating concentrations of intracellular zinc.
Figure 4.
S1 nuclease protection assays to quantify mRNA levels of genes activated by the AFT2-1up allele. RNA was isolated from (A) the aft2Δ strain and (B) the aft1Δ strain transformed with pRS416 (−), pAFT2 (+), and pAFT2-1up (up) and grown in CM(−Ura) with glucose medium to mid-log phase. The upper band for each sample is the specified gene, and the lower band the calmodulin loading control (CMD1).
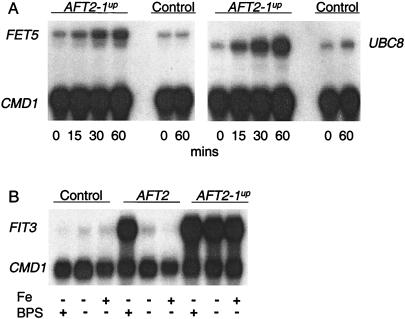
The AFT2-1up allele was placed under the control of a galactose-inducible promoter to test whether it directly regulates two of its target genes. The aft2Δ strain containing the GAL10/AFT2-1up construct was precultured in raffinose. Galactose was then added to induce the expression of AFT2-1up, which was maximal within 15 min (data not shown). Both FET5 and UBC8 were significantly induced within 15 min of the addition of galactose (Fig. 5A). No induction was observed in the aft2Δ strain lacking the GAL10/AFT2-1up construct. The coinduction of AFT2-1up, FET5, and UBC8 is consistent with Aft2p being a direct transcriptional regulator.
Figure 5.
Aft2p is a direct transcriptional activator that responds to low iron. S1 nuclease protection assays were carried out by using RNA isolated from (A) the aft2Δ strain transformed with either pGAL-AFT2-1up or pYeF2 (control), which were grown in CM(−Ura) with raffinose to mid-log phase, at which time 2% galactose was added to induce the transcription of the AFT2-1up allele. Cells were harvested at the specified times (B), the aft1Δaft2Δ strain was transformed with pRS416 (control), pAFT2, and pAFT2-1up, which were pregrown overnight with 10 μM FeCl2, harvested, washed, and resuspended in CMD(−Ura) medium with or without supplemental FeCl2 (100 μM) or BPS (100 μM) and then grown exponentially for 8 h. The Upper band for each sample is the specified gene, and the Lower band the calmodulin loading control (CMD1).
Aft1p is activated in iron-deficient cells and inhibited in iron-replete cells (1). To determine whether wild-type Aft2p is similarly iron regulated, the aft1Δaft2Δ strain containing either pAFT2 or pAFT2-1up was cultured under iron-deficient and iron-replete conditions (Fig. 5B). The AFT2-1up allele induced the expression of FIT3 in cells cultured in either BPS or Fe(II), demonstrating that the mutation in this allele results in derepressed activity (Fig. 5B). In contrast, the wild-type AFT2 allele induced the expression of FIT3 only in iron-deficient conditions. Therefore, Aft2p induces gene expression in iron-deficient cells.
Aft1p and Aft2p Bind to the Same Region of the FET3 Promoter.
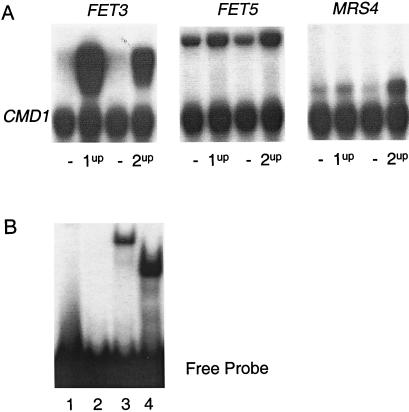
To determine whether the level of transcriptional activation by Aft1p and Aft2p is the same, the expression of FET3, FET5, and MRS4 was analyzed in an aft1Δaft2Δ strain containing either the AFT1-1up allele or the AFT2-1up allele (Fig. 6A). In the strain carrying the AFT1-1up allele, FET3 is more highly expressed than in the strain carrying the AFT2-1up allele. This pattern of expression is reversed with respect to MRS4, which is more highly expressed in the strain carrying the AFT2-1up allele. The level of activation of FET5 is the same in the strains containing either the AFT1-1up allele or the AFT2-1up allele. Therefore, the AFT1-1up and the AFT2-1up alleles differentially activate gene expression.
Figure 6.
Aft1p and Aft2p bind to the same promoter fragment in vitro but differentially regulate gene expression. (A) S1 nuclease protection assays to quantify mRNA levels of genes activated by the AFT1-1up and AFT2-1up alleles. RNA was isolated from the aft1Δaft2Δ strain transformed with a control plasmid (−) (pRS316 in the case of pAFT1-1up and pRS416 in the case of pAFT2-1up), pAFT1-1up (1up), and pAFT2-1up (2up) and grown in CMD(−Ura) medium to mid-log phase. The Upper band for each sample is the specified gene, and the Lower band the calmodulin loading control (CMD1). (B) Gel retardation assays were performed by using a 32P-labeled 30-bp duplex of the FET3 promoter as probe with no protein (lane 1), and lysates from E. coli containing pET3 (lane 2), pAft1-313 (lane 3), and pAft2-214 (lane 4).
To address whether Aft2p can bind to the same DNA site as Aft1p, EMSAs were performed using a 30-bp fragment that included the Aft1p-binding site within the FET3 promoter. The Aft1p consensus binding site is PyPuCACCCPu and was identified by DNase1 footprinting of the FET3 promoter and sequence comparison of Aft1p-regulated genes (2). Truncated forms of Aft1p (Aft1p313) and of Aft2p (Aft2p214) containing the conserved putative DNA-binding domains of each protein and the conserved Cys-X-Cys motif were used. A specific single complex was formed between the FET3 promoter fragment and lysate from E. coli cells that contained plasmids that separately expressed the truncated forms of Aft1p and Aft2p (Fig. 6B). No complex was formed by using lysate from control E. coli cells that did not express Aft1p313 or Aft2p214. Aft1p and Aft2p are therefore capable of binding to the same DNA fragment in vitro.
Iron Uptake by the aft Mutants.
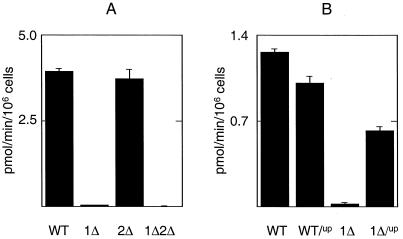
Iron uptake is markedly attenuated in a strain lacking a functional AFT1 (1). The observed AFT2-1up induction of genes involved in iron uptake (FET3, FTR1 and FRE1) suggested that iron uptake would be enhanced in cells containing AFT2-1up. To determine whether AFT2 stimulates iron uptake, the level of 59Fe uptake was quantified in all of the aft mutant strains. The level of 59Fe uptake was similar between the wild-type and the aft2Δ strain but was significantly reduced in the aft1Δ and the aft1Δaft2Δ strains (Fig. 7A). This result suggests that the chromosomally encoded Aft2p is not a significant factor in regulating iron uptake in exponential cells grown in synthetic glucose medium. However, expression of the AFT2-1up allele in the aft1Δ strain significantly increased 59Fe uptake (Fig. 7B). The 59Fe uptake data are therefore consistent with the growth phenotypes of each strain.
Figure 7.
Iron uptake in the aft mutants. The level of 59Fe uptake was analyzed in the following cultures. (A) The wild-type strain and the aft-deletion strains grown to mid-log phase in synthetic medium (CMD) with BPS (100 μM). (B) The wild-type strain and the aft1Δ strain containing either pRS416 (WT, 1Δ) or pAFT2-1up (WT/up, 1Δ/up) grown to mid-log phase in CMD(−Ura) medium.
Discussion
We found that Aft2p, like its paralog, Aft1p, is a functional transcriptional activator that responds to iron. A strain that lacks both Aft1p and Aft2p is more sensitive to growth in low-iron conditions than a strain lacking only Aft1p. An allele of AFT2 that contains a mutation analogous to the AFT1-1up mutation activates the transcription of a subset of the Aft1p regulon in an AFT1-independent manner. The expression profiles of two Aft2p target genes after the induction of the AFT2-1up allele is consistent with Aft2p directly regulating these genes. The wild-type AFT2 allele activates transcription of its target genes in response to treatment with the iron-chelator BPS. In addition, truncated forms of both Aft1p and Aft2p bind to the same DNA fragment in vitro, consistent with Aft2p being a direct transcriptional regulator. There are, however, differences in the extent to which Aft1p and Aft2p regulate the iron regulon. The aft1Δ strain and the aft2Δ strain exhibit different iron-deficient and respiratory-deficient phenotypes. The AFT2-1up allele only partially suppresses the respiratory-deficient phenotype of the aft1Δ strain and partially stimulates iron uptake in the aft1Δ strain. Furthermore, the AFT1-1up allele and the AFT2-1up allele differentially regulate gene expression.
Microarray experiments identified a subset of the Aft1 regulon as being activated by the AFT2-1up allele. Those genes that were not identified include FET3 and ARN1-4. However, S1 analysis clearly shows that the AFT2-1up allele is able to activate FET3 expression in the absence of Aft1p. The microarray experiments were carried out in an AFT1 wild-type strain, and S1 analysis showed that FET3 is expressed in the control strain to such a level that no differential expression was observed in the strain carrying the AFT2-1up allele (data not shown). This result implies that there are different thresholds of activation of genes within the iron regulon by Aft1p. Therefore, AFT2 may be capable of activating the entire Aft1p regulon in the absence of Aft1p. Identification of the extent to which the Aft1p and Aft2p regulons overlap will require more detailed analysis. It is clear however, that there is differential expression of FET3 and MRS4, depending on whether the AFT1-1up allele or the AFT2-1up allele is present. This result is consistent with both Aft1p and Aft2p having distinct effects on the cell and therefore distinct functions. Within 500 bp of the start codon of MRS4, there are AFT1-like binding sites that contain the conserved central CACCC motif but that differ in the flanking bases. Identification of the ability of Aft1p and Aft2p to bind these sites will provide insight into what determines Aft1p- or Aft2p-specific gene expression. During the drafting of this manuscript, Blaiseau et al. published work relating to AFT2 that shows that AFT2 activates the expression of FET3 in an iron-dependent manner (20). Furthermore, a double mutant lacking both functional AFT1 and AFT2 has a more severe iron-dependent phenotype and is more sensitive to oxidative stress than either of the single-mutant strains. Blaiseau et al. conclude that Aft2p is an iron-responsive transcriptional regulator. Our data relating to the phenotype of the aft-mutant strains is in agreement with Blaiseau et al., and our microarray data strengthens the conclusions that both groups have made.
Aft2p may mediate a regulatory program of the iron regulon different from that mediated by Aft1p. Microarray experiments analyzing global gene expression in S. cerevisiae in response to different stress conditions show that the expression of AFT2 is elevated during stationary phase, under nitrogen starvation conditions, and after treatment with the alkylating agent methyl methanesulfonate (21, 22). Therefore, there may be environmental conditions where one factor is the predominant regulator of the iron regulon. Alternatively, Aft1p and Aft2p may both be activated under iron-deficient conditions, but the mechanism by which each senses the levels of reactive iron may be different. The activity of the two mammalian translational iron regulators IRP1 and IRP2 is inhibited in iron-replete cells but through different mechanisms (23). If Aft2p is the predominant iron-responsive regulator of a group of genes, then it may be significant that many of the apparently Aft2p-specific genes identified by the microarray analysis are involved in vacuole function. Aft2p may play a special role in mobilizing internal iron stores. Yeast vacuoles are an important site of iron storage and reutilization using a transport system that involves Fet5p and Fth1p (6, 24), both of which were found to be induced by the AFT2-1up allele. The iron-deficient phenotype of the aft1Δaft2Δ strain was significantly alleviated when it was pregrown with supplemental iron. Similar observations were made with a fth1Δfet5Δ strain (6). Pregrowth of this strain in iron-deficient medium compromised the ability of that strain to make a growth transition from fermentative to respiratory metabolism, which was not alleviated by the addition of exogenous iron (6). Thus, a specialized role of AFT2 in regulating vacuolar iron storage, particularly under conditions of starvation, warrants further investigation.
Acknowledgments
We acknowledge the excellent technical assistance of the University of Utah Microarray Core Facility led by Dr. Brian Dalley and supported by the Huntsman Cancer Foundation. We thank Jerry Kaplan's group for assistance in radioiron uptake. This research was supported by a grant (CA 61286) from the National Cancer Institute, National Institutes of Health (to D.R.W.) and a microarray supplemental award (ES03817) from the National Institutes of Environmental Health Sciences (to D.R.W.). P.O.B. acknowledges support of HG00983 and the Howard Hughes Medical Institute. P.O.B. is an associate investigator of the Howard Hughes Medical Institute. We acknowledge support from the National Institutes of Health (5P30-CA 42014) to the Biotechnology Core Facility for DNA synthesis at the University of Utah.
Abbreviations
- BPS
bathophenanthroline disulfonate
- EMSA
electrophoretic mobility-shift assay
- YPD
yeast extract/peptone/dextrose
- CM
complete medium
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Yamaguchi-Iwai Y, Dancis A, Klausner R D. EMBO J. 1995;14:1231–1239. doi: 10.1002/j.1460-2075.1995.tb07106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yamaguchi-Iwai Y, Stearman R, Dancis A, Klausner R D. EMBO J. 1996;15:3377–3384. [PMC free article] [PubMed] [Google Scholar]
- 3.Yun C-W, Ferea T, Rashford J, Ardon O, Brown P O, Botstein D, Kaplan J, Philpott C C. J Biol Chem. 2000;275:10709–10715. doi: 10.1074/jbc.275.14.10709. [DOI] [PubMed] [Google Scholar]
- 4.Foury F, Talibi D. J Biol Chem. 2001;276:7762–7768. doi: 10.1074/jbc.M005804200. [DOI] [PubMed] [Google Scholar]
- 5.Garland S A, Hoff K, Vickery L E, Culotta V C. J Mol Biol. 1999;294:897–907. doi: 10.1006/jmbi.1999.3294. [DOI] [PubMed] [Google Scholar]
- 6.Urbanowski J L, Piper R C. J Biol Chem. 1999;274:38061–38070. doi: 10.1074/jbc.274.53.38061. [DOI] [PubMed] [Google Scholar]
- 7.Casas C, Aldea M, Espinet C, Gallego C, Gil R, Herrero E. Yeast. 1997;13:621–637. doi: 10.1002/(SICI)1097-0061(19970615)13:7<621::AID-YEA121>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 8.Seoighe C, Wolfe K H. Gene. 1999;238:253–261. doi: 10.1016/s0378-1119(99)00319-4. [DOI] [PubMed] [Google Scholar]
- 9.Wolfe K H, Shields D C. Nature (London) 1997;387:708–713. doi: 10.1038/42711. [DOI] [PubMed] [Google Scholar]
- 10.Dohrmann P R, Voth W P, Stillman D J. Mol Cell Biol. 1996;16:1746–1758. doi: 10.1128/mcb.16.4.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang D, Moffat J, Wilson W A, Moore L, Cheng C, Roach P J, Andrews B. Mol Cell Biol. 1998;18:3289–3299. doi: 10.1128/mcb.18.6.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sikorski R S, Hieter P. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jensen L T, Winge D R. EMBO J. 1998;17:5400–5408. doi: 10.1093/emboj/17.18.5400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gil R, Zueco J, Sentandreu R, Herrero E. Yeast. 1991;7:1–14. doi: 10.1002/yea.320070102. [DOI] [PubMed] [Google Scholar]
- 15.Bird A, Evans-Galea M V, Blankman E, Zhao H, Luo H, Winge D R, Eide D J. J Biol Chem. 2000;275:16160–16166. doi: 10.1074/jbc.M000664200. [DOI] [PubMed] [Google Scholar]
- 16.DeRisi J L, Iyer V R, Brown P O. Science. 1997;278:680–686. doi: 10.1126/science.278.5338.680. [DOI] [PubMed] [Google Scholar]
- 17.Eide D, Davis-Kaplan S, Jordan I, Sipe D, Kaplan J. J Biol Chem. 1992;267:20774–20781. [PubMed] [Google Scholar]
- 18.Hassett R F, Romeo A M, Kosman D J. J Biol Chem. 1998;273:7628–7636. doi: 10.1074/jbc.273.13.7628. [DOI] [PubMed] [Google Scholar]
- 19.Lyons T J, Gasch A P, Gaither L A, Botstein D, Brown P O, Eide D J. Proc Natl Acad Sci USA. 2000;97:7957–7962. doi: 10.1073/pnas.97.14.7957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blaiseau P-L, Lesuisse E, Camadro J-M. J Biol Chem. 2001;276:34221–34226. doi: 10.1074/jbc.M104987200. [DOI] [PubMed] [Google Scholar]
- 21.Jelinsky S A, Estep P, Church G M, Samson L D. Mol Cell Biol. 2000;20:8157–8167. doi: 10.1128/mcb.20.21.8157-8167.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gasch A P, Spellman P T, Kao C M, Carmel-Harel O, Eisen M B, Storz G, Botstein D, Brown P O. Mol Biol Cell. 2000;11:4241–4257. doi: 10.1091/mbc.11.12.4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo B, Brown F M, Phillips J D, Yu Y, Leibold E A. J Biol Chem. 1995;270:16529–16535. doi: 10.1074/jbc.270.28.16529. [DOI] [PubMed] [Google Scholar]
- 24.Li L, Chen O S, McVey Ward D, Kaplan J. J Biol Chem. 2001;276:29515–29519. doi: 10.1074/jbc.M103944200. [DOI] [PubMed] [Google Scholar]