Abstract
Background
Neoadjuvant chemotherapy (NAC) is the standard approach for downstaging of locally advanced breast cancer and can improve breast conservation rates. A pathological complete response (pCR) after NAC associated with favorable long-term outcomes has been described. There is still a high locoregional recurrence (LRR) rate after NAC and the influence of age on LRR after NAC is unclear. This study analyzed the relationship between age and LRR after NAC.
Methods
Two hundred and sixty-three patients with invasive breast cancer who received NAC followed by mastectomy or breast conserving surgery (BCS) were enrolled. Concurrent weekly epirubicin and docetaxel was the NAC regimen.
Results
Twenty-nine patients (11%) achieved a pCR after NAC. In univariate analysis, age <50 years, luminal B (HER2 positive) subtype, HER2 overexpression subtype, and triple-negative subtype were factors to predict a pCR. In multivariate analysis, age <50 years, luminal B (HER2 positive) type, HER2 overexpression, and triple-negative subtype were the independent factors to predict a pCR. No patients in the pCR group developed LRR compared with 31 patients in the non-pCR group. Eleven patients (6.9%) in the younger group (age <50 years) developed LRR compared with 20 patients (19.4%) in the older group (age ≥50 years). In multivariate analysis, younger age (<50 years) was the only independent prognostic factor for a LRR-free survival.
Conclusion
Younger age can predict a pCR and is an independent prognostic factor for LRR in locally advanced breast cancer patients after NAC as concurrent epirubicin and docetaxel.
Keywords: Neoadjuvant chemotherapy, Pathological complete response, Breast cancer, Age
At a glance of commentary
Scientific background on the subject
Young age is an independent prognostic factor both in locoregional control and survival analysis in early breast cancer. There is a high locoregional recurrence rate after neoadjuvant chemotherapy for locally advanced breast cancer in meta-analysis, but lack of data in those pathological complete response achieved after chemotherapy.
What this study adds to the field
Our finding demonstrate age younger than 50, HER-2 overexpression and triple negative subtypes were the predicting factors of pathological complete response. Age younger than 50 is an independent prognostic factor for locoregional recurrence after concurrent epirubicin and docetaxel neoadjuvant chemotherapy in the study.
Neoadjuvant chemotherapy (NAC) is a systemic treatment for breast cancer that is administered prior to definitive surgical treatment. In locally advanced and large operable tumors, NAC may reduce the tumor size and achieve operability, or reduce the extent of the surgery. Furthermore, it checks the sensitivity of certain drugs to the tumor or determines which drugs will achieve the optimal response [1], [2], [3], [4], [5]. NAC can be used to determine which of the tumor subtypes has a favorable response, even in small size; this is the case for human epidermal growth factor receptor 2 (HER2)-positive and triple-negative breast cancer (TNBC) [6]. Tumors that are positive for hormone receptors (HR) and negative for HER-2 have a poor clinical and pathological response; therefore, surgery may be more preferable than NAC in such subtypes [7], [8], [9]. The Definition of a pathological complete response (pCR) is no residual invasive tumor in the surgical specimen after NAC and definitive surgery. HER-2-positive breast cancer is associated with a high pCR rate, especially if the NAC contained HER-2 directed therapy such as trastuzumab, which was also proved in the Investigation of Serial Studies to Predict Your Therapeutic Response with Imaging and Molecular Analysis (I-SPY 1) Trial [10], [11], [12]. The high pCR rate of HER-2-positive breast cancer is independent of the HR status [6], [10], [11]. A higher pCR rate of 27%–45% has been reported in TNBC, in contrast to <10% in HR-positive and HER-2-negative breast cancer [11], [12], [13], [14]. Across the entire TNBC population, patients who achieved a pCR have a similar prognosis to that of patients with other breast cancer subtypes. However, patients with TNBC with residual tumor at surgery have a higher risk of early distant disease recurrence [13], [15]. In a clinical trial, the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-18, comparing preoperative and postoperative chemotherapy, no statistically significant differences in overall survival or disease-free survival in long term follow up was observed between the two groups. For example, no patients with clinically node-positive disease who achieved a pCR at mastectomy (ypT0N0) could also get a 0% locoregional recurrence (LRR) at 10 years [16]. This trial also pointed out that younger patients may benefit from preoperative therapy. A large pooled meta-analysis from the Collaborative Trials in Neoadjuvant Breast Cancer (CTNeoBC) groups could not validate a pCR as a surrogate endpoint for improved event-free survival and overall survival for all subtypes of breast cancer [17]. The prognostic value is the greatest in aggressive tumor subtypes, such as triple negative breast cancer and HER2 positive receiving Trastuzumab.
Breast conservation after neoadjuvant chemotherapy results in acceptably low rates (5% at 5-year and 10% at 10-year) of local recurrence in appropriately selected patients, even with T3 or T4 disease at MD Anderson Cancer Center. A higher rate of local recurrence was predicted in advanced nodal involvement at diagnosis, residual tumor larger than 2 cm, multifocal residual disease, and lymphovascular space invasion [18]. Recent reports of 751 patients undergoing breast-conserving surgery (BCS) after NAC demonstrated that a pCR, clinical stage, and triple-negative subtypes were independent factors for locoregional control [19]. In 2016, a combined analysis of NSABP B-18 and B-27 reported that in patients treated with NAC, age, clinical tumor characteristics before NAC, and both pathologic nodal status/breast tumor response after NAC can be used to predict risk for LRR. Younger age (<50 years) was a significant predictor to LRR for BCS, but not for mastectomy [20]. Furthermore, the role of age on locoregional control has been rarely demonstrated in the literature. Among these previous studies, most patients were limited to a smaller tumor size (<5 cm) and clinically negative node status before NAC. Therefore, the emphasis on the impact of age factor in locally advanced breast cancer on locoregional control was unknown. This study aimed to analyze the relationship between age and LRR after NAC in locally advanced breast cancer.
Patients and methods
Patient selection
The study was a retrospective cohort study and therefore the requirement for patient consent was waived. The study was approved by our institutional review board and have therefore been performed in accordance with the ethical standards laid down in the 1964 declaration of Helsinki and all subsequent revisions. Patients with invasive breast cancer who received NAC and underwent mastectomy or BCS from 2002 to 2011 at the Linkou branch of Chang Gung Memorial Hospital were enrolled. Eligible patients met the following criteria: histologically proven unilateral invasive breast carcinoma, normal baseline blood count, normal serum creatinine, alanine aminotransferase, aspirate aminotransferase, alkaline phosphatase, and bilirubin levels, a negative pregnancy test, and a World Health Organization (WHO) performance status <2. Exclusion criteria were: inflammatory cancer and the initial presence of metastasis. Initial staging was determined using physical examination, mammography, ultrasonography of the breast and axillary lymph nodes, chest radiography, bone scan, and whole body computed tomography (CT) scan.
Treatment
All patients received 3 to 6 cycles of NAC and the regimen was weekly epirubicin/docetaxel. Epirubicin was administered at a dose of 45 mg/m2 via intravenous infusion on day 1 and day 8, while docetaxel was administered at a dose of 35 mg/m2 via intravenous infusion on day 1 and day 8 of a 3-week cycle. Dose modification was based on nadir blood counts and interval toxicity. Surgery (mastectomy or BCS) and axillary sentinel lymph node biopsy or axillary lymph node dissection were performed 2–4 weeks after NAC was completed. The indication for postoperative radiotherapy included all patients after BCS and the part of patients if receiving mastectomy with initial tumor size >5 cm or clinical N2 status. Patients received postoperative adjuvant therapy, including hormone therapy, chemotherapy and targeted therapy, according to their clinicopathological factors.
Analysis of estrogen receptor (ER), progesterone receptor (PgR), and HER-2/neu expression
ER, PgR, and HER-2/neu analysis was performed on pretreatment core needle biopsy specimens using immunohistochemical (IHC) staining techniques. For ER and PgR, positivity was defined as expression in >1% of tumor cells. For HER-2/neu, IHC staining with a score of 3+ (moderate to strong complete membrane staining observed in 10% of the tumor cells) or a positive fluorescence in situ hybridization (FISH) test if IHC staining with a score of 2+ (weak to moderate complete membrane staining in 10% of the tumor cells) was defined as positive. The molecular subtype was classified according to the 13th St Gallen International Breast Cancer Conference (2013) Expert Consensus [21].
Definition of response
The rates of objective response were evaluated according to the revised Response Evaluation Criteria in Solid Tumors (RECIST) guidelines (version 1.1). At least a 30% decrease in the sum of the diameters of the tumor was considered a partial response (PR). Progressive disease (PD) was defined as an increase of >20% in the sum of the diameters. Stable disease (SD) was defined as neither sufficient shrinkage to qualify for PR nor sufficient increase to qualify for PD. A pCR was defined as no residual invasive breast cancer in the histopathology specimen of the breast and axillary lymph nodes (ypT0/ypTisN0).
Statistical analysis
Numerical data were compared using a Student's t-test and presented as the mean + standard deviation (SD) and a two-tailed p value < 0.05 was considered significant. Pearson's chi-square test (χ2 test) was used to compare the differences in the proportions of categorical data. A log-rank test and the Kaplan–Meier method were used for survival analysis. For multivariate analysis of prognostic factors, Firth's penalized maximum likelihood logistic regression was used. The statistical software used for assessment was SPSS 17.0 for Windows (SPSS, Chicago, IL).
Results
We enrolled 263 consecutive patients with locally advanced breast cancer who were treated with NAC followed by surgery and adjuvant therapy. The median follow-up time was 54.6 months (range 9.2–126.9). The median age was 48 years (range 18–75). The mean initial tumor size was 6.01 cm (SD ± 3.41 cm) and clinically positive nodes were detected in 202 patients (76.8%). Among the patients, 62.7% had stage II disease and 37.3% had stage III disease respectively. The distribution of the 5 subtypes according to the final pathologic reports, was 8.7%, 36.5%, 19.0%, 18.6%, and 17.1% for luminal A, luminal B/HER-2 negative, luminal B/HER-2 positive, triple-negative, and HER-2 overexpression, respectively. Two hundred seventeenth patients (82.5%) completed at least 4 cycles of NAC. There was no dose reduction among the cycles and the treatment cycle was delayed for 1 week if hematological toxicity occurred. Most patients could tolerate it. The most common side effects were grade 1–2 nausea/vomiting, alopecia and leukopenia. Post-operative adjuvant chemotherapy regimens included anthracycline or taxane containing regimens. CEF (cyclophosphamide, epirubicin, 5-fluorouracil) was the most common adjuvant regimen in 230 patients (87.5%). No grade 4 cardiac toxicity was found after completion of NAC and adjuvant chemotherapy.
After NAC, 200 (76%) patients underwent mastectomy and 63 (24%) underwent BCS. Sixteen patients (6.1%) achieved a clinical complete response (CCR), 223 (84.8%) achieved a clinical partial response (PR), 19 (7.2%) had SD, and 5 (1.9%) had progressive disease (PD). Twenty-nine patients (11%) achieved a pathological complete response pCR. In comparison with patients with the older group (≥50 years old), the younger group (<50 years old) had fewer clinical positive lymph nodes (72.5% vs 83.5%, p = 0.039) and received more breast conserving surgery (33.1% vs 9.7%, p < 0.0001) [Table 1].
Table 1.
Comparison between patients with age <50 and age ≥50.
| Parameters | Age <50 (n = 160) | Age ≥50 (n = 103) | Total (n = 263) | p value |
|---|---|---|---|---|
| Initial tumor size (cm) | 0.852 | |||
| <5 | 82 (51.3) | 54 (52.4) | 136 (51.7) | |
| >5 | 78 (48.7) | 49 (47.6) | 127 (48.3) | |
| Clinical nodal status | 0.039 | |||
| Positive | 116 (72.5) | 86 (83.5) | 202 (76.8) | |
| Negative | 44 (27.5) | 17 (16.5) | 61 (23.2) | |
| Surgery type | <0.0001 | |||
| Mastectomy | 107 (66.9) | 93 (90.3) | 200 (76.0) | |
| BCS | 53 (33.1) | 10 (9.7) | 63 (24.0) | |
| ER | 0.755 | |||
| Negative | 56 (35.0) | 38 (36.9) | 94 (35.7) | |
| Positive | 104 (65.0) | 65 (63.1) | 169 (64.3) | |
| PgR | 0.538 | |||
| Negative | 73 (45.6) | 51 (49.5) | 124 (47.1) | |
| Positive | 87 (54.3) | 52 (50.5) | 139 (52.9) | |
| HER2 | 0.074 | |||
| Negative | 109 (68.1) | 59 (57.3) | 168 (63.9) | |
| Positive | 51 (31.9) | 44 (42.7) | 95 (36.1) | |
| Molecular subtype | 0.496 | |||
| Luminal A | 14 (8.7) | 9 (8.7) | 23 (8.7) | |
| Luminal B (HER-2 negative) | 63 (39.4) | 33 (32.0) | 96 (36.5) | |
| Luminal B (HER-2 positive) | 27 (16.9) | 23 (22.3) | 50 (19.0) | |
| HER-2 overexpression | 24 (15.0) | 21 (20.4) | 45 (17.1) | |
| Triple-negative | 32 (20.0) | 17 (16.5) | 49 (18.6) | |
| Clinical response | 0.069 | |||
| CR | 12 (7.5) | 4 (3.9) | 16 (6.1) | |
| PR | 139 (86.9) | 84 (81.6) | 223 (84.8) | |
| SD | 5 (3.1) | 9 (8.7) | 19 (7.2) | |
| PD | 4 (2.5) | 6 (5.8) | 5 (1.9) | |
| pCR | 0.031 | |||
| No | 137 (85.6) | 97 (94.2) | 234 (88.9) | |
| Yes | 23 (14.4) | 6 (5.8) | 29 (11.0) |
Numbers in parentheses are percentages.
Abbreviations: CR: complete response; ER: estrogen receptor; HER-2: human epidermal growth factor receptor 2; pCR: pathological complete response; PgR: progesterone receptor; PD: progressive disease; PR: partial response; SD: stable disease.
The mean initial tumor size was 6.01 cm. The average percentage of tumor size reduction (the median size measured pathologically after NAC/the median initial clinical size) was 47% in the luminal A group, 69% in the luminal B HER2+ group, 58% in the luminal B HER2-group, 84% in the HER-2 overexpression group, and 79% in the triple-negative group. A pCR was achieved in 20.4% triple-negative cases, 16.0% luminal B HER2+ cases, 15.6% HER-2 overexpression cases, 4.2% luminal B HER2-cases, and none in the luminal A group [Table 2].
Table 2.
Tumor response.
| Tumor size | All cases | Luminal A-like | Luminal B-like (HER2 negative) | Luminal B-like (HER2 positive) | HER2 overexpression | Triple negative |
|---|---|---|---|---|---|---|
| Pre-NAC echo size (cm), mean ± SD | 5.89 ± 3.27 | 5.83 ± 2.74 | 5.81 ± 3.14 | 5.45 ± 2.50 | 5.90 ± 2.83 | 6.65 ± 4.54 |
| Pathological size (cm), mean ± SD | 2.13 ± 2.08 | 3.31 ± 2.23 |
2.35 ± 2.12 | 1.96 ± 2.23 | 1.64 ± 1.68 | 1.83 ± 1.87 |
| Molecular subtype | Size reduction (%) Median (IQR) | Pairwise comparisonsb |
||||
| Luminal A-like | Luminal B-like (HER2 negative) | Luminal B-like (HER2 positive) | HER2 overexpression | Triple negative |
||
| Luminal A-like | 47.4 (32.8) | – | ||||
| Luminal B-like (HER2 negative) | 57.6 (53.7) | NS | – | |||
| Luminal B-like (HER2 positive) | 69.3 (47.0) | 0.020 | NS | – | ||
| HER2 positive (non-luminal) | 84.4 (46.0) | 0.001 | 0.034 | NS | – | |
| Triple negative (ductal) | 78.8 (56.8) | 0.006 | NS | NS | NS | – |
| p valuea | <0.001 | |||||
| Luminal A-like | Luminal B-like (HER2 negative) | Luminal B-like (HER2 positive) | HER2 overexpression | Triple negative |
||
| Pathological response | ||||||
| pCR | 29 (11.0%) | 0 | 4 (4.2%) | 8 (16.0%) | 7 (15.6%) | 10 (20.4%) |
| Non-pCR | 234 (89.0%) | 23 (100.0%) | 92 (95.8%) | 42 (84.0%) | 38 (84.4%) | 39 (79.6%) |
| p valuec | 0.007 | |||||
Abbreviations: HER-2: human epidermal growth factor receptor 2; pCR: pathological complete response; mean ± SD, mean ± standard deviation.
Kruskal-Wallis Test.
Dunn's pairwise tests were carried out for the five pairs of groups.
Pearson's Chi-square Test.
Younger age (p = 0.031), negative ER status (p = 0.006), negative PgR status (p = 0.004), luminal B HER2+ type, HER2 overexpression, and triple-negative subtype (p = 0.007) were predictors of a pCR in univariate analysis [Table 3]. There was no statistically significant difference in the use of radiotherapy in terms of locoregional control. Multivariate analysis showed younger age, luminal B HER2+, HER2 overexpression, and triple-negative subtype were predictive factors of a pCR [Table 4].
Table 3.
Comparison between patients with a pathologic complete response (pCR) and a non-pCR.
| Parameters | pCR (n = 29) | Non-pCR (n = 234) | p value |
|---|---|---|---|
| Age (yrs), mean + SD | 41.4 ± 11.4 | 48.9 ± 9.5 | <0.001 |
| <50 | 23 (79.3) | 137 (58.5) | 0.031 |
| ≥50 | 6 (20.7) | 97 (41.5) | |
| Initial tumor size (cm) | 5.6 + 3.3 | 6.1 + 3.4 | 0.540 |
| <5 | 15 (51.7) | 121 (52.6) | 0.928 |
| ≥5 | 14 (48.3) | 109 (47.4) | |
| Surgery type | 0.005 | ||
| Mastectomy | 16 (55.2) | 184 (78.6) | |
| BCS | 13 (44.8) | 50 (21.4) | |
| ER | 0.006 | ||
| Negative | 17 (58.6) | 77 (32.9) | |
| Positive | 12 (41.1) | 157 (67.1) | |
| PgR | 0.004 | ||
| Negative | 21 (72.4) | 103 (44.0) | |
| Positive | 8 (27.6) | 131 (56.0) | |
| HER2 | |||
| Negative | 14 (48.3) | 154 (65.8) | 0.064 |
| Positive | 15 (51.7) | 80 (34.2) | |
| Subtype | 0.007 | ||
| Luminal A | 0 | 23 (9.8) | |
| Luminal B (HER-2 negative) | 4 (13.8) | 92 (39.3) | |
| Luminal B (HER-2 positive) | 8 (27.6) | 42 (17.9) | |
| HER-2 overexpression | 7 (24.1) | 38 (16.2) | |
| Triple-negative | 10 (34.5) | 39 (16.7) | |
| Any recurrence | |||
| No | 24 (82.8) | 151 (64.5) | 0.050 |
| Yes | 5 (17.2) | 83 (35.5) | |
| Locoregional recurrence | 0.037 | ||
| No | 29 (100.0) | 203 (86.8) | |
| Yes | 0 | 31 (13.2) |
Numbers in parentheses are percentages.
Abbreviations: ER: estrogen receptor; HER-2: human epidermal growth factor receptor 2; mean + SD: mean + standard deviation; pCR: pathological complete response; PgR: progesterone receptor.
Table 4.
Firth's bias-reduced penalized-likelihood logistic regression to predict a pCR.
| Parameters | Odds ratio | 95% C.I. of odds ratio | p value |
|---|---|---|---|
| Age (yrs) | |||
| <50 | 2.894 | 1.207–7.898 | 0.016 |
| ≥50 | 1 | ||
| Subtype | |||
| Luminal A | 0.080 | 0.001–0.684 | 0.016 |
| Luminal B (HER-2 negative) | 0.177 | 0.049–0.547 | 0.002 |
| Luminal B (HER-2 positive) | 0.835 | 0.294–2.321 | 0.729 |
| HER-2 overexpression | 0.818 | 0.276–2.339 | 0.708 |
| Triple-negative | 1 |
Abbreviations: pCR: pathological complete response; HER-2: human epidermal growth factor receptor 2.
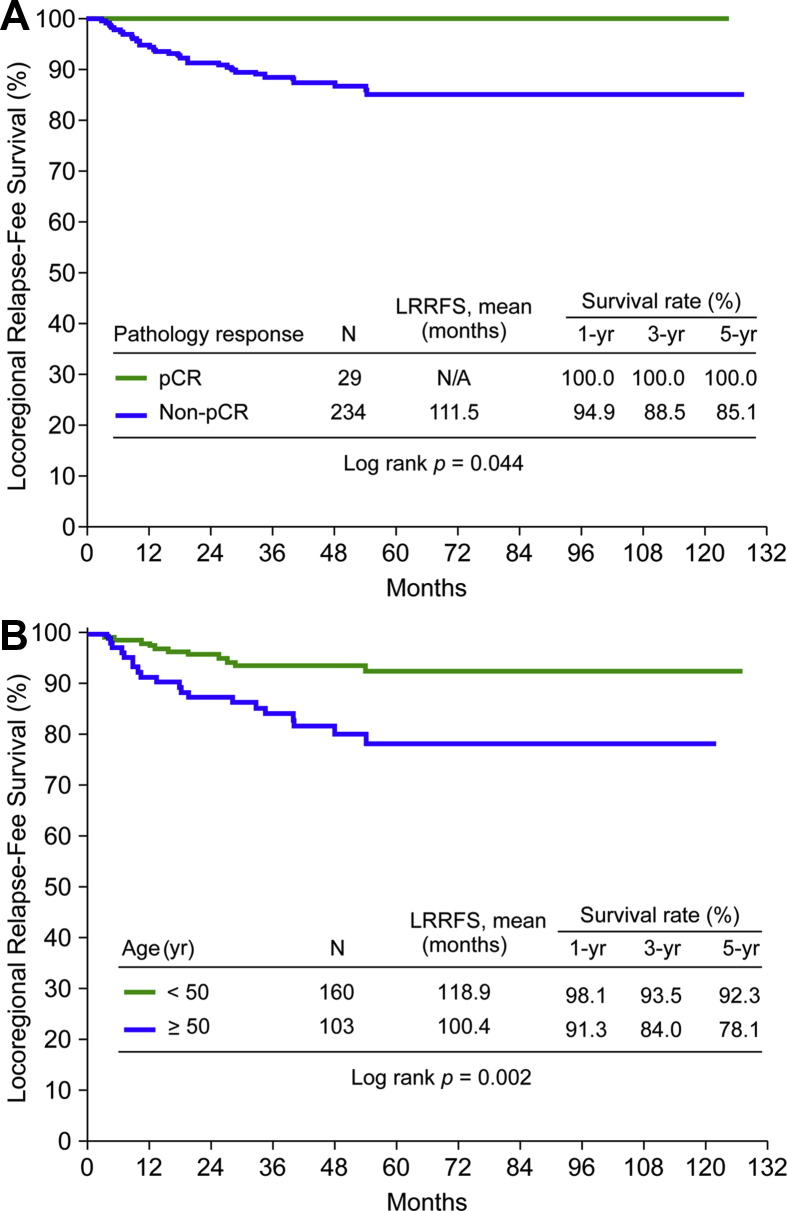
Further, 31 patients (11.8%) developed LRR while any disease recurrence (LRR with or without distant metastasis) happened in 88 patients (33.5%). Patients with a pCR had significantly a lower incidence of disease recurrence and LRR (p = 0.05 and 0.037, respectively). In univariate analysis of the locoregional relapse-free survival rate, the younger group (<50 years) had better outcomes, as did those who achieved a pCR [Table 5]. Other parameters, including ER, PgR, HER2 status, and types of surgery, did not influence LRR. Eleven patients (6.9%) in the younger group (<50 years) developed LRR compared with 20 patients (19.4%) in the older group (≥50 years) (p = 0.002). In multivariate analysis, younger age (<50 years) remained an independent factor favorable for LRR-free survival (p = 0.016) [Table 6]. The LRR-free survival rate in the pCR group and the non-pCR group is shown in Fig. 1A. The effect of age on LRR-free survival rate is demonstrated in Fig. 1B.
Table 5.
Locoregional relapse (LRR) free survival analysis (N = 263) (all patients).
| Parameters | N | N of events | Mean | 95% C.I. of mean | p value |
|---|---|---|---|---|---|
| Age (yrs) | 0.002 | ||||
| <50 | 160 | 11 (6.9) | 118.9 | 114.3–123.5 | |
| ≥50 | 103 | 20 (19.4) | 100.4 | 91.9–108.9 | |
| Initial tumor size (cm) | 0.640 | ||||
| <5 | 136 | 14 (10.3) | 114.9 | 108.9–120.9 | |
| ≥5 | 123 | 15 (12.2) | 109.4 | 103.0–115.9 | |
| Surgery type | 0.274 | ||||
| Mastectomy | 200 | 26 (13.0) | 111.2 | 105.7–116.6 | |
| BCS | 63 | 5 (7.9) | 117.8 | 110.3–125.5 | |
| ER | 0.843 | ||||
| Negative | 94 | 11 (11.7) | 113.0 | 105.2–120.7 | |
| Positive | 169 | 20 (11.8) | 110.9 | 105.5–116.2 | |
| PgR | 0.743 | ||||
| Negative | 124 | 15 (12.1) | 112.6 | 105.9–119.4 | |
| Positive | 139 | 16 (11.5) | 102.9 | 97.6–108.2 | |
| HER2 | 0.429 | ||||
| Negative | 168 | 18 (10.7) | 114.3 | 108.8–119.8 | |
| Positive | 95 | 13 (13.7) | 108.5 | 100.7–116.3 | |
| Subtype | 0.946 | ||||
| Luminal A | 23 | 2 (8.7) | 99.0 | 89.3–108.7 | |
| Luminal B (HER2 negative) | 96 | 11 (11.5) | 110.2 | 103.0–117.3 | |
| Luminal B (HER2 positive) | 50 | 7 (14) | 108.9 | 98.5–119.2 | |
| HER2 overexpression | 45 | 6 (13.3) | 106.6 | 95.3–117.9 | |
| Triple-negative | 49 | 5 (10.2) | 114.7 | 104.5–124.9 | |
| NAC cycles | 0.144 | ||||
| ≤4 | 256 | 29 (11.3) | 113.8 | 109.3–118.3 | |
| >4 | 7 | 2 (28.5) | 78.4 | 30.4–126.4 | |
| Pathology response | 0.044 | ||||
| pCR | 29 | 0 (0) | N/A | ||
| Non-pCR | 234 | 31 (13.2) | 111.5 | 106.4–116.5– |
N: number; Numbers in parentheses are percentages.
Abbreviations: BCS: breast-conserving surgery; CR: complete response; ER: estrogen receptor; HER-2: human epidermal growth factor receptor 2; NAC: neoadjuvant chemotherapy; pCR: pathological complete response; PgR: progesterone receptor.
Table 6.
Cox regression with Firth's penalized maximum likelihood for analysis the locoregional relapse (LRR) free survival (all patients).
| Parameters | Hazard ratio | 95% C.I. of hazard ratio | p value |
|---|---|---|---|
| Age (yrs) | |||
| <50 | 1 | ||
| ≥50 | 2.711 | 1.338–5.770 | 0.005 |
| Response | |||
| pCR | 1 | ||
| Non-pCR | 6.806 | 0.955–863.204 | 0.058 |
Abbreviation: pCR, pathological complete response.
Fig. 1.
(A) Locoregional relapse-free survival (LRRFS) rate in the pathological complete response (pCR) group compared with the non-pCR group. (B) Locoregional relapse-free survival rate according to age.
Discussion
Our study revealed that pCR after NAC provided better local control in all subtypes of breast cancer. This study was conducted to follow our previous phase II trial at a single center in 2009 [22], whereby we have concluded that weekly docetaxel and epirubicin were well-tolerated and a very high pCR rate was achieved in HER-2 overexpression subtypes. Furthermore, this regimen showed a high clinical response rate with good patient compliance, and also shortened the period between the initial diagnosis and definitive surgery. One of the aims of this study was to enroll patients with all different subtypes of the same indication to avoid patient bias. Trastuzumab was included as a neoadjuvant treatment in 2010 in our institution; however, we excluded patients receiving trastuzumab because of possible bias resulting from a different regimen. Previous reports from the NSABP B27 trial showed that anthracycline-based regimens with the addition of a taxane are associated with increased pCR rates [23]. Our overall pCR rate at 11% was consistent with that of Taucher's series at 12.4%, both with the same regimen of weekly combination of epirubicin and docetaxel [24]. Although trastuzumab was not added in ours and Yang's series, the 15.8% pCR rate in our HER-2 positive patients looked better than the 8% rate in Yang's series also without using trastuzumab. The 5-year LRR rate was 11% and 8%, respectively in ours and Yang's series [25].
Our series confirmed that luminal B HER2+, HER-2 overexpression, and TNBC were favorable subtypes to achieve a pCR. This result was compatible with that shown in most of previous reports [11], [12], [13], [14], [15], [17], [19]. The tumor size reduction rate ranged from 47 to 84% according to different subtypes [Table 2]. However, 200 (76%) patients still chose mastectomy in our study, which was relatively high compared with Western series. Worse disease-free survival rate was eventually observed in the mastectomy group (60.5%) than in the BCS group (85.7%); however, the choice of surgery types did not affect the LRR rate, 13% in mastectomy patients and 7.9% in BCS patients respectively (p = 0.274). The probable causes were a smaller tumor size and more negative lymph nodes in the BCS group, although they were not statistically significant. The BCS group also had a lower clinical stage in the I-SPY 1 trial in which the surgeon chose the most appropriate surgery [25]. Results from the combined analysis of NSABP B-18 and B-27 showed the 10-year cumulative incidence of LRR was 12.3% for mastectomy patients and 10.3% for lumpectomy plus breast radiotherapy patients. The study found that independent predictors of LRR in all patients were younger age, clinical status before NAC, and residual tumor status. The beneficial effect of younger age was greater in the BCS group than in the mastectomy group [20]. There was no significant difference in LRR between the BCS group and mastectomy group in I-SPY trial as did in our series. Importantly, higher rate of breast conservation would not increase the incidence of LRR, which was also confirmed by the NSABP B-18 and the European Organisation for Research and Treatment of Cancer (EORTC) studies [26].
Although CTNeoBC trials did not validate pCR as a surrogate endpoint for an improved event-free survival or overall survival [17], pCR is a suitable surrogate endpoint selectively for patients with the aggressive subtypes [6]. In our study, the LRR rate was 11.8% in all patients, but 0% in the pCR group. A pCR in our patients was achieved using only epirubicin and docetaxel. Yang et al. reported that 233 patients with stage II–III disease who were treated with NAC, mastectomy, and post-mastectomy radiotherapy at a median 62 months follow-up had an 8% LRR rate in 5 years. No LRR occurred in the pCR group versus a rate of 9% in the non-pCR group (p = 0.05) in Yang's report [27]. LRR occurred in 10.2% and 8.7%, respectively in patients with TNBC and luminal A subtypes, it was difficult to predict which of the 5 molecular subtypes would have a lower or higher LRR rate in our study. In contrast, we found that younger age was associated with better locoregional control after both mastectomy and BCS. This finding may be because of the higher pCR rate in our young patients, which lead to a favorable outcome. Another large analysis of the EORTC 10994/BIG 1-00 study of patients with large operable or locally advanced breast cancer receiving NAC showed no significant association of age with LRR, and patient population had a more advanced disease stage than that in NSABP B-18 and B-27 [28]. Therefore, the influence of age on LRR remained unclear in patients with locally advanced breast cancer after NAC. Without NAC, some other series demonstrated that younger age was a positive risk factor to predict local recurrence in breast cancer patients treated with BCS [29], [30], [31], [32]. The result was strongly consistent with meta-analysis of 17 prospective randomized control trials of BCS, with or without radiotherapy, which revealed that a younger age was associated with higher rates of 10-year LRR [33]. In a retrospective study of 1451 patients who were all younger than 40 years old, local tumor control was worse after BCS than after mastectomy [34]. In our series, most patients underwent mastectomy following NAC. Radosa et al. retrospectively reported that younger patients were more likely to present with a higher stage of disease; however, younger age was not an independent risk factor for local recurrence in patients with TNBC [35]. Therefore, age may not be a crucial factor for LRR among patients who presented at a similar clinical stage and all received chemotherapy. Moreover, Lin et al. reported that younger breast cancer patients (<50 years) in Taiwan are characterized by a high prevalence of luminal A subtype and low prevalence of histologic grade 3 tumor and basal-like subtype [36]. The same study group, collected data from Taiwan Cancer Database, concluded that younger patient (<50 years) in Taiwan is uniquely associated with a higher rate of stage 1 and ER-positive and PgR-positive, favorable pathological features and better outcomes than the older patients (≥50 years). These features are quite different from its Western counterparts. Difference in environmental, genetic, and ethnic factors may play a role in such discrepancy of clinicopathological features and outcome between Eastern and Western patients [37].
One of the limitations of this study is that it is a retrospective study from a single institution, which may result in selection bias. The relatively small sample size is another weakness. Another limitation of the study was the shorter follow-up time; however, it may not make a significant bias, as in an overview of the Early Breast Cancer Trialists’ Collaborative Group, around 80% of LRRs developed in the first 5 years in patients with node positive disease [38]. Despite these limitations, a strength of our study is that all patients received the same treatment regimen.
In conclusion, our findings suggested that younger age (<50 years) and the three molecular subtypes (luminal B/HER2-positive, HER-2 overexpression, and triple-negative) are highly predictive of a pCR. The younger patients (<50 years) is the only independent factor favorable for LRR-free survival in patients with breast cancer after NAC with concurrent epirubicin and docetaxel. Further larger prospective studies will be conducted to draw more definitive conclusions.
Funding
This study was supported by integrated research plan in Chang Gung Memorial Hospital at Linkou, Taiwan, study number CORPG1F0061 and CORPG1F0031.
Conflicts of interest
No any conflicts of interest to declare.
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.Gralow J.R., Burstein H.J., Wood W., Hortobagyi G.N., Gianni L., von Minckwitz G. Preoperative therapy in invasive breast cancer: pathologic assessment and systemic therapy issues in operable disease. J Clin Oncol. 2008;26:814–819. doi: 10.1200/JCO.2007.15.3510. [DOI] [PubMed] [Google Scholar]
- 2.Kaufmann M., Hortobagyi G.N., Goldhirsch A., Scholl S., Makris A., Valagussa P. Recommendations from an international expert panel on the use of neoadjuvant (primary) systemic treatment of operable breast cancer: an update. J Clin Oncol. 2006;24:1940–1949. doi: 10.1200/JCO.2005.02.6187. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz G.F., Hortobagyi G.N. Proceedings of the consensus conference on neoadjuvant chemotherapy in carcinoma of the breast, April 26-28, 2003, Philadelphia, Pennsylvania. Cancer. 2004;100:2512–2532. doi: 10.1002/cncr.20298. [DOI] [PubMed] [Google Scholar]
- 4.Shannon C., Smith I. Is there still a role for neoadjuvant therapy in breast cancer? Crit Rev Oncol Hematol. 2003;45:77–90. doi: 10.1016/s1040-8428(02)00091-4. [DOI] [PubMed] [Google Scholar]
- 5.King T.A., Morrow M. Surgical issues in patients with breast cancer receiving neoadjuvant chemotherapy. Nat Rev Clin Oncol. 2015;12:335–343. doi: 10.1038/nrclinonc.2015.63. [DOI] [PubMed] [Google Scholar]
- 6.von Minckwitz G., Untch M., Blohmer J.U., Costa S.D., Eidtmann H., Fasching P.A. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30:1796–1804. doi: 10.1200/JCO.2011.38.8595. [DOI] [PubMed] [Google Scholar]
- 7.Hayes D.F. Targeting adjuvant chemotherapy: a good idea that needs to be proven! J Clin Oncol. 2012;30:1264–1267. doi: 10.1200/JCO.2011.38.4529. [DOI] [PubMed] [Google Scholar]
- 8.Coates A.S., Colleoni M., Goldhirsch A. Is adjuvant chemotherapy useful for women with luminal a breast cancer? J Clin Oncol. 2012;30:1260–1263. doi: 10.1200/JCO.2011.37.7879. [DOI] [PubMed] [Google Scholar]
- 9.Schott A.F., Hayes D.F. Defining the benefits of neoadjuvant chemotherapy for breast cancer. J Clin Oncol. 2012;30:1747–1749. doi: 10.1200/JCO.2011.41.3161. [DOI] [PubMed] [Google Scholar]
- 10.Carey L.A., Dees E.C., Sawyer L., Gatti L., Moore D.T., Collichio F. The triple negative paradox: primary tumour chemosensitivity of breast cancer subtypes. Clin Cancer Res. 2007;13:2329–2334. doi: 10.1158/1078-0432.CCR-06-1109. [DOI] [PubMed] [Google Scholar]
- 11.Rouzier R., Perou C.M., Symmans W.F., Ibrahim N., Cristofanilli M., Anderson K. Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin Cancer Res. 2005;11:5678–5685. doi: 10.1158/1078-0432.CCR-04-2421. [DOI] [PubMed] [Google Scholar]
- 12.Esserman L.J., Berry D.A., DeMichele A., Carey L., Davis S.E., Buxton M. Pathologic complete response predicts recurrence-free survival more effectively by cancer subset: results from the I-SPY 1 TRIAL--CALGB 150007/150012, ACRIN 6657. J Clin Oncol. 2012;30:3242–3249. doi: 10.1200/JCO.2011.39.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Untch M., Fasching P.A., Konecny G.E., Hasmüller S., Lebeau A., Kreienberg R. Pathologic complete response after neoadjuvant chemotherapy plus trastuzumab predicts favorable survival in human epidermal growth factor receptor 2-overexpressing breast cancer: results from the TECHNO trial of the AGO and GBG study groups. J Clin Oncol. 2011;29:3351–3357. doi: 10.1200/JCO.2010.31.4930. [DOI] [PubMed] [Google Scholar]
- 14.Isakoff S.J. Triple-negative breast cancer: role of specific chemotherapy agents. Cancer J. 2010;16:53–61. doi: 10.1097/PPO.0b013e3181d24ff7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liedtke C., Mazouni C., Hess K.R., André F., Tordai A., Mejia J.A. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008;26:1275–1281. doi: 10.1200/JCO.2007.14.4147. [DOI] [PubMed] [Google Scholar]
- 16.Wolmark N., Wang J., Mamounas E., Bryant J., Fisher B. Preoperative chemotherapy in patients with operable breast cancer: nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J Natl Cancer Inst Monogr. 2001;2001:96–102. doi: 10.1093/oxfordjournals.jncimonographs.a003469. [DOI] [PubMed] [Google Scholar]
- 17.Cortazar P., Zhang L., Untch M., Mehta K., Costantino J.P., Wolmark N. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384:164–172. doi: 10.1016/S0140-6736(13)62422-8. [DOI] [PubMed] [Google Scholar]
- 18.Chen A.M., Meric-Bernstam F., Hunt K.K., Thames H.D., Oswald M.J., Outlaw E.D. Breast conservation after neoadjuvant chemotherapy: the MD Anderson cancer center experience. J Clin Oncol. 2004;22:2303–2312. doi: 10.1200/JCO.2004.09.062. [DOI] [PubMed] [Google Scholar]
- 19.Swisher S.K., Vila J., Tucker S.L., Bedrosian I., Shaitelman S.F., Litton J.K. Locoregional control according to breast cancer subtype and response to neoadjuvant chemotherapy in breast cancer patients undergoing breast-conserving therapy. Ann Surg Oncol. 2016;23:749–756. doi: 10.1245/s10434-015-4921-5. [DOI] [PubMed] [Google Scholar]
- 20.Mamounas E.P., Anderson S.J., Bear H.D., Julian T.B., Geyer C.E., Jr., Taghian A. Predictors of locoregional recurrence after neoadjuvant chemotherapy: results from combined analysis of national surgical adjuvant breast and Bowel Project B-18 and B-27. J Clin Oncol. 2012;30:3960–3966. doi: 10.1200/JCO.2011.40.8369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldhirsch A., Winer E.P., Coates A.S., Gelber R.D., Piccart-Gebhart M., Thürlimann B. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen international expert consensus on the primary therapy of early breast cancer 2013. Ann Oncol. 2013;24:2206–2223. doi: 10.1093/annonc/mdt303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen S.C., Chang H.K., Lin Y.C., Hsueh S., Cheung Y.C., Leung W.M. High pathologic complete response in HER 2-positive locally advanced breast cancer after primary systemic chemotherapy with weekly docetaxel and epirubicin. Jpn J Clin Oncol. 2008;38:99–105. doi: 10.1093/jjco/hym172. [DOI] [PubMed] [Google Scholar]
- 23.Bear H.D., Anderson S., Smith R.E., Geyer C.E., Jr., Mamounas E.P., Fisher B. Sequential preoperative or postoperative docetaxel added to preoperative doxorubicin plus cyclophosphamide for operable breast cancer: National Surgical Adjuvant Breast and Bowel Project protocol B-27. J Clin Oncol. 2006;24:2019–2027. doi: 10.1200/JCO.2005.04.1665. [DOI] [PubMed] [Google Scholar]
- 24.Taucher S., Rudas M., Mader R.M., Gnant M., Sporn E., Dubsky P. Influence of neoadjuvant therapy with epirubicin and docetaxel on the expression of HER-2/neu in patients with breast cancer. Breast Cancer Res Treat. 2003;82:207–213. doi: 10.1023/B:BREA.0000004378.15859.51. [DOI] [PubMed] [Google Scholar]
- 25.Cureton E.L., Yau C., Alvarado M.D., Krontiras H., Ollila D.W., Ewing C.A. Local recurrence rates are low in high-risk neoadjuvant breast cancer in the I-SPY 1 Trial (CALGB 150007/150012; ACRIN 6657) Ann Surg Oncol. 2014;21:2889–2896. doi: 10.1245/s10434-014-3721-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buchholz T.A., Mittendorf E.A., Hunt K.K. Surgical considerations after neoadjuvant chemotherapy: breast conservation therapy. J Natl Cancer Inst Monogr. 2015;2015:11–14. doi: 10.1093/jncimonographs/lgv020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang T.J., Morrow M., Modi S., Zhang Z., Krause K., Siu C. The effect of molecular subtype and residual disease on locoregional recurrence in breast cancer patients treated with neoadjuvant chemotherapy and postmastectomy radiation. Ann Surg Oncol. 2015;22:495–501. doi: 10.1245/s10434-015-4697-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gillon P., Touati N., Breton-Callu C., Slaets L., Cameron D., Bonnefoi H. Factors predictive of locoregional recurrence following neoadjuvant chemotherapy in patients with large operable or locally advanced breast cancer: an analysis of the EORTC 10994/BIG 1-00 study. Eur J Cancer. 2017;79:226–234. doi: 10.1016/j.ejca.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 29.Bosma S.C., van der Leij F., van Werkhoven E., Bartelink H., Wesseling J., Linn S. Very low local recurrence rates after breast-conserving therapy: analysis of 8485 patients treated over A 28-year period. Breast Cancer Res Treat. 2016;156:391–400. doi: 10.1007/s10549-016-3732-0. [DOI] [PubMed] [Google Scholar]
- 30.Courdi A., Doyen J., Gal J., Chamorey E. Local recurrence after breast cancer affects specific survival differently according to patient age. Oncology. 2010;79:349–354. doi: 10.1159/000323483. [DOI] [PubMed] [Google Scholar]
- 31.Vrieling C., Collette L., Fourquet A., Hoogenraad W.J., Horiot J.C., Jager J.J. Can patient-, treatment- and pathology-related characteristics explain the high local recurrence rate following breast conserving therapy in young patients? Eur J Cancer. 2003;39:932–944. doi: 10.1016/s0959-8049(03)00123-0. [DOI] [PubMed] [Google Scholar]
- 32.Miles R.C., Gullerud R.E., Lohse C.M., Jakub J.W., Degnim A.C., Boughey J.C. Local recurrence after breast-conserving surgery: multivariable analysis of risk factors and the impact of young age. Ann Surg Oncol. 2012;19:1153–1159. doi: 10.1245/s10434-011-2084-6. [DOI] [PubMed] [Google Scholar]
- 33.Early Breast Cancer Trialists' Collaborative Group (EBCTCG), Darby S., McGale P., Correa C., Taylor C., Arriagada R. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breastcancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;12:1707–1716. doi: 10.1016/S0140-6736(11)61629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van der Sangen M.J.C., van de Wiel F.M.M., Poortmans P.M.P., Tjan-Heijnen V.C.G., Nieuwenhuijzen G.A.P., Roumen R.M.H. Are breast conservation and mastectomy equally effective in the treatment of young women with early breast cancer? Long-term results of a population-based cohort of 1,451 patients aged ≤ 40 years. Breast Cancer Res Treat. 2011;127:207–215. doi: 10.1007/s10549-010-1110-x. [DOI] [PubMed] [Google Scholar]
- 35.Radosa J.C., Eaton A., Stempel M., Khander A., Liedtke C., Solomayer E.F. Evaluation of local and distant recurrence patterns in patients with triple-negative breast cancer according to age. Ann Surg Oncol. 2017;24:698–704. doi: 10.1245/s10434-016-5631-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin C.H., Liau J.Y., Lu Y.S., Huang C.S., Lee W.C., Kuo K.T. Molecular subtypes of breast cancer emerging in young women in Taiwan: evidence for more than just westernization as a reason for the disease in Asia. Cancer Epidemiol Biomarkers Prev. 2009;18:1807–1814. doi: 10.1158/1055-9965.EPI-09-0096. [DOI] [PubMed] [Google Scholar]
- 37.Lin C.H., Chuang P.Y., Chiang C.J., Lu Y.S., Cheng A.L., Kuo W.H. Distinct clinicopathological features and prognosis of emerging young-female breast cancer in an East Asian country: a nationwide cancer registry-based study. Oncologist. 2014;19:583–591. doi: 10.1634/theoncologist.2014-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clarke M., Collins R., Darby S., Davies C., Elphinstone P., Evans V. Early Breast Cancer Trialists' Collaborative Group (EBCTCG): effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]