Abstract
Background
Intestinal ischemia reperfusion injury is a frequent clinical damage, in which the oxidative stress and inflammation play an important role. Interleukin-1 receptor antagonist (IL-1Ra) is a natural anti-inflammatory factor, however, its effect on intestinal ischemia reperfusion injury remains unclear.
Methods
The rat model of intestinal I/R was induced by occlusion (for 60 min) and reopening (for 60 min) of superior mesenteric artery. The rats were randomly divided into the following 5 groups: sham-operation(S), model (I/R),10 mg/kgIL-1Ra + I/R (C1),20 mg/kgIL-1Ra + I/R (C2), and30 mg/kgIL-1Ra + I/R (C3).
Results
In this study it was the first time to confirm that IL-1Ra had a significant protection against the intestinal ischemia reperfusion injury. IL-1Ra not only effectively inhibited the expression of inflammatory factors (such as IL-1β, IL-6 and TNF-α) and the activation of neutrophil in intestinal tissues, but also decreased the death of intestinal cells and the damages of intestinal tissues. Interestingly, besides anti-inflammation effect, it was also found that IL-1Ra possessed a significant inhibitory effect on the oxidative stress caused by ischemia/reperfusion injury. Furthermore, the expression of nuclear factor erythroid 2-related factor 2 (Nrf2) and hemeoxygenase-1 (HO-1), and the phosphorylation level of Nrf2 were greatly promoted by IL-1Ra. At the same time, IL-1Ra inhibited the mitogen-activated protein kinase (MAPKs) pathway.
Conclusion
IL-1Ra had the protective effect against intestinal ischemia reperfusion injury, its mechanism included anti-inflammation and anti-oxidative stress in which the Nrf2/HO-1 pathway played an important role. The above-mentioned results may extend the clinical application of IL-1Ra in the treatment of intestinal ischemia reperfusion injury.
Keywords: Interleukin 1 receptor antagonist (IL-1Ra), Intestinal ischemia reperfusion injury, Protective effect, Nrf2/HO-1, Clinical application
At a glance of commentary
Scientific background on the subject
Intestinal ischemia reperfusion(I/R) injury is a frequent clinical damage, and the pathophysiological mechanism is very complex. At present, there are not ideal drugs for this injury. Interleukin-1 receptor antagonist(IL-1Ra) is a natural anti-inflammatory factor, and has a good therapeutic effect on a variety of intestinal inflammatory diseases.
What this study adds to the field
Firstly, we confirm that IL-1Ra has a protection against the intestinal I/R injury. Secondly, IL-1Ra possess a inhibitory effect on the oxidative stress caused by intestinal I/R injury. Thirdly, Nrf2/HO-1 pathway plays an important role in intestinal I/R injury, which can be regulated by IL-1Ra.
Ischemia reperfusion (I/R) injury is that the normal functions of organs or tissues are not able to restore even if the bloodstream was recanalized after the interruption of their blood supply. It is known that I/R is an injury-aggravated pathophysiological phenomenon [1]. The small intestine is the most sensitive organ to I/R. In the case of hemorrhagic shock, trauma and so on, the ischemic injury of the small intestine is unavoidable, and intestinal I/R injury will occur after the relief from ischemic injury [2]. The pathophysiological mechanism of intestinal I/R injury is very complex, in which the injury of oxygen free radical, the adhesive infiltration of leukocyte, the release of inflammatory mediators, the disorder of energy metabolism and so on are involved [3], [4]. At present, the drugs of anti-energy deficiency [5], regulating macrophage activity [6], inhibiting oxygen free radical [3] and so on are used for the clinical treatment of intestinal I/R injury, but all these drugs are not ideal and the mortality of intestinal I/R hasn't been decreased.
Many researches show that inflammation plays a crucial role in the process of intestinal I/R injury. Interleukin-1(IL-1) is an important proinflammatory cytokine, which can up-regulate immunological function, accelerate the expression of other inflammatory cytokines, and induce the infiltration and proliferation of neutrophil etc. [7]. IL-1Ra is a natural anti-inflammatory factor, as a terminal anti-inflammatory factor, which can not only antagonize competitively the biological effects of IL-1, but also inhibit the effects of other inflammatory factors [8]. As we know, a great amount of oxygen free radical can be generated by intestinal I/R, which further aggravates the tissue injury. Previous studies demonstrated that IL-1Ra also possessed a certain capability to clear free radical and to intensify the endogenous anti-oxidative system, thus possessing a certain therapeutic effects on the I/R injury-induced oxidative stress of brain, ovary and other organs [9], [10]. However, it is unclear whether IL-1Ra has a certain preventive and therapeutic effect on intestinal I/R injury or not. Therefore, we applied the rat model of intestinal I/R injury to explore the possible role and mechanism of IL-1 Ra, which may provide new intervention and theoretical basis for the treatment of intestinal I/R injury.
Materials and methods
Reagents
The IL-1β (cat. no. ERC007), IL-6 (cat. no. ERC003), and TNF-α (cat. no. ERC102a) enzyme-linked immunosorbent assay (ELISA) kits specific for rat cytokines were obtained from Booster Biological Technology Co. (Beijing, China). The MPO (cat. no. A044), MDA (cat. no. A003-1), and SOD (cat. no. A001-2) Colorimetric Activity Assay kit were purchased from Nanjing Jianchang Bioengineering Institute (Nanjing, China). Antibodies to p-ERK (cat. no. 4370s), ERK (cat. no. 4695s), p-JNK (cat. no. 4668s), p-p38 (cat. no. 9211s), p38 (cat. no. 8690s), Bcl–xl (cat. no. 2764s), and cleaved-caspase-3 (cat. no. 9664s) were purchased from Cell signaling technology (Boston, USA). Antibodies to JNK (cat. no. ab32137), Nrf2 (cat. no. ab89443), HO-1 (cat. no. ab189491) were purchased from Abcam (Cambridge, USA). Antibodies to Bax (cat. no. YF031802R) were purchased from Epitomics (California, USA). Antibodies to Caspase-3 (cat. no. 19677-1-ap) were purchased from Proteintech Group (Rosemont, USA). Antibodies directed against β-Actin (cat. no. 01264/40133) were purchased from CWbiotech (Beijing, China), Antibodies to p-Nrf2(DF7519) were purchased from Affinity (Ancaster, USA). HRP-conjugated anti-rabbit (cat. no. 109525) or anti-mouse (cat. no. 131224) antibodies were purchased from ZSGB-BI (Beijing, China).
Preparation of IL-1Ra protein
The recombinant plasmid pBV220/IL-1Ra was transformed into E.coli BL21 (DE3) competent cells, and the resulted engineering bacteria were cultured, and induced to express the recombinant protein. Then the expressed protein IL-1Ra was purified by the anion- and cation-exchange chromatography [11].
Experimental animal model and drug administration
Male Sprague Dawley rats weighing between 245 and 265 g were purchased from Weitong-Lihua Laboratory Co., Ltd (Beijing, China). Rats were acclimatized for 2 weeks before the study. All rats received humane care and all experiments were carried out in accordance with the Guide for the Care and Use of Laboratory Animals published by the United States National Institute of Health (NIH). The protocols were approved by the Animal Ethics Committee of Institute of Military Cognitive and Brain Sciences, Academy of Military Medical Sciences (Beijing, China).
Rats fasted for 12 h before the experiment, but drinking water should be normally supplied. Then rats were anesthetized by peritoneal injection of 2.5% pentobarbital sodium. Afterwards, the abdomen was opened by cutting along with the epigastric median line to expose the superior mesenteric artery (SMA), which then was nipped at its root to interrupt blood supply for 1 h. And then the arterial nipper was taken off to permit reperfusion of the bloodstream for 1 h. The animals were euthanized after the completion of the experiment, and the small intestine was taken off at the 5 cm site distant from the ileocecal junction.
The experimental rats were randomly divided into the following 5 groups: sham-operation(S), model (I/R), 10 mg/kgIL-1Ra + I/R (C1), 20 mg/kg IL-1Ra + I/R (C2), and 30 mg/kg IL-1Ra + I/R (C3). For S group, only SMA was separated after opening the abdomen. The rats in I/R group, after nipping SMA for 1 h, reperfusion for 1 h. The procedures for C1, C2 and C3 group were the same to that in I/R group, and different dose of IL-1Ra was injected into the tail vein at the time-point of 10 min before relaxing the nippers.
Histologic examination
Samples from the intestinal were fixed in 4% paraformaldehyde and were treated with dehydration, clearing, embedding in paraffin. Then tissue was sectioned and stained by hematoxylin-eosin (HE). HE-stained sections were used to observe the pathological changes of the intestinal mucosa, and the pathological injury was assessed under a light microscope (Leica Microsystems GmbH, Wetzlar, Germany) at 400× magnification and scored by Chu's method [12], then the mean value should be used for statistics.
TUNEL staining
Paraformaldehyde-fixed intestinal tissues were embedded in paraffin, then cut into the tissue sections which was terminal deoxynucleotidyl transferase -mediated dUTP nick end labeling (TUNEL) (Vazyme Biotech Co. Nanjing, China). The tissue sections from each rat was stained by TUNEL method. The intestinal mucosal injury was assessed under a light microscope (Leica Microsystems GmbH, Wetzlar, Germany) at 200× magnification and we counted the positive cells with Image J software (NIH, BethSesda, MD, UA) to determine apoptosis.
Detection by Transmission Electron Microscopy (TEM)
The intestinal tissues were fixed by 2.5% glutaraldehyde solution and 1% osmic acid solution, then dehydrated with absolute ethyl alcohol, embedded with resin penetration, solidified, and cut into ultrathin sections, and finally observed and photographed by H-7650 transmission electron microscopy (Hitachi, Tokyo, Japan) at 12,000× magnification.
Measurement of IL-1β, IL-6 and TNF-α
The intestinal IL-1β, IL-6, and TNF-α levels were determined according to the instructions of the Kit and were measured by microplate reader (Biorad, California, USA) at 450 nm.
Measurement of MPO, SOD and MDA
The contents of MPO, SOD and MDA in intestine were measured by Spectrophotometry (Beckman, California, USA) for the operation proceeds to see the enclosed instructions within the kits. Then the optical density of MPO was recorded at 460 nm, MDA was determined at 532 nm, and SOD activity was measured at 500 nm. Finally, the contents of MPO, MDA, and SOD should be calculated.
Western blotting
According to the previously described methods [11], the isolated intestines were homogenized in ice-cold Radio-Immunoprecipitation Assay (RIPA) lysis buffer (cat. no. 01408/50132, CW biotech, Beijing, China) containing a protease inhibitor cocktail (cat. no. 13909100, Roche, Indianapolis, IN, USA) and a phosphatase inhibitor cocktail (cat. no. 18681300, Roche, Indianapolis, IN, USA) for extracting protein samples. The protein concentration was determined using the bicinchoninic acid (BCA, cat. no. CW0014S, CWbiotech, Beijing, China) method. The proteins were separated by SDS-PAGE (100 V for 80–120 min), and transferred to PVDF membrane (Millipore Corporation, Billerica, MA, USA) at 250 mA for 100 min. Then, the membranes were blocked with 5% dried defatted milk in Tris-Buffered Saline Tween-20 (TBST) for 1 h at room temperature, and then incubated with primary antibodies at 4 °C overnight. The following antibodies were used as primary antibodies: Bcl-xl (1:1000 dilution), Bax (1:1000 dilution), cleaved-caspase-3 (1:1000 dilution), Caspase-3 (1:1000 dilution), p-Nrf2(1:500 dilution), Nrf2(1:500 dilution), HO-1 (1:500 dilution), β-Actin (1:1000 dilution), p-ERK (1:1000 dilution), ERK (1:1000 dilution), p-JNK(1:1000 dilution), JNK(1:1000 dilution), p-p38 (1:1000 dilution), and p38 (1:1000 dilution). Following washing, the membranes were incubated with respective horseradish peroxidase-conjugated secondary antibodies (1:2500 dilution). Immunoreactive bands were visualized using the kit of chemiluminescent detection system (Millipore Corporation, Billerica, MA, USA). Densitometric analyses were performed using Image J software (NIH, BethSesda, MD, UA), and β-actin was used as an endogenous reference.
Statistic analysis
All the data were analyzed with the statistical software GraphPad Prism 5 (La Jolla, CA, USA) and presented as mean ± SD except for scores, which was presented as median. Non-parametric Kruskal–Wallis test was applied to compare the histological scores between the groups. Parametric data of two groups were analyzed using Student's test, and Welch's correction was applied for data with unequal variances. All the differences of other data between groups were assessed by one-way ANOVA analysis followed by Bonferroni's test for multiple comparisons.
Results
Preparation of Recombinant IL-1Ra
The purity of the prepared recombinant protein IL-1Ra was higher than 90% (Fig. 1) and could be used for the subsequent studies [11].
Fig. 1.
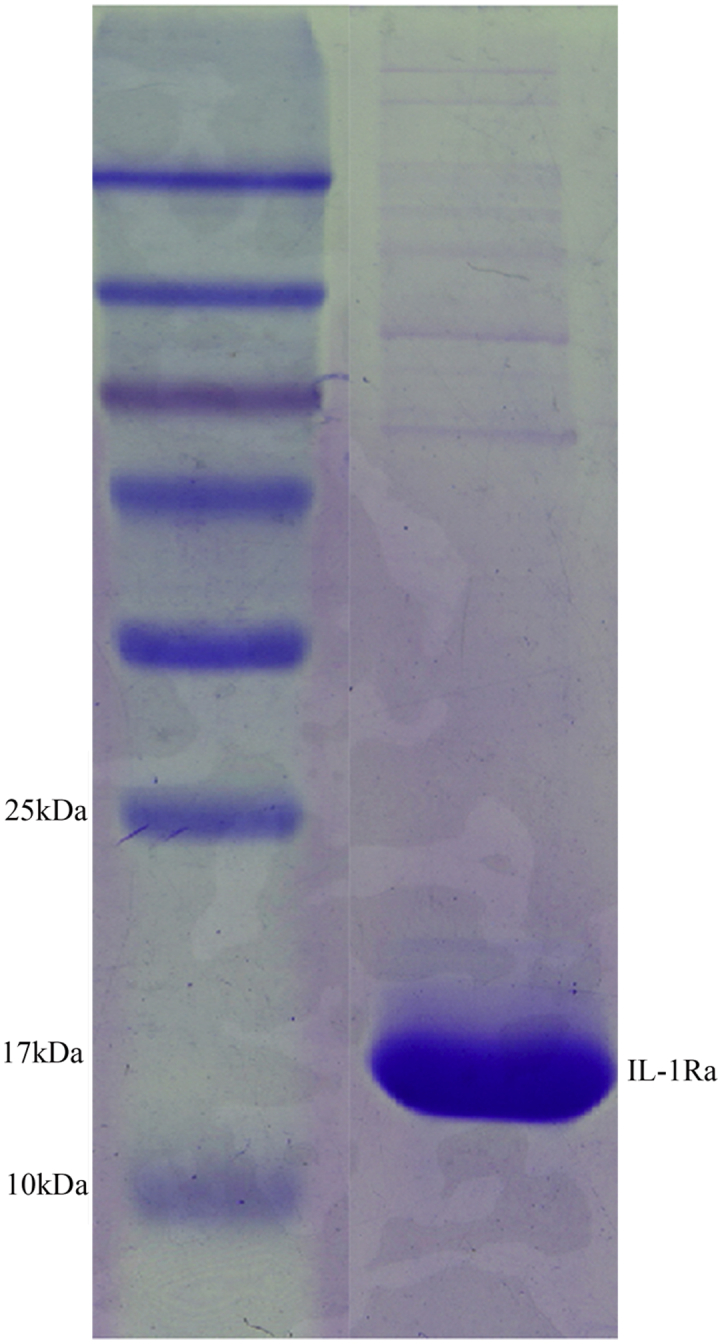
Preparation of IL-1Ra protein. Purified recombinant protein IL-1Ra.
Alleviation of the injury in intestinal tissue by IL-1RA after I/R
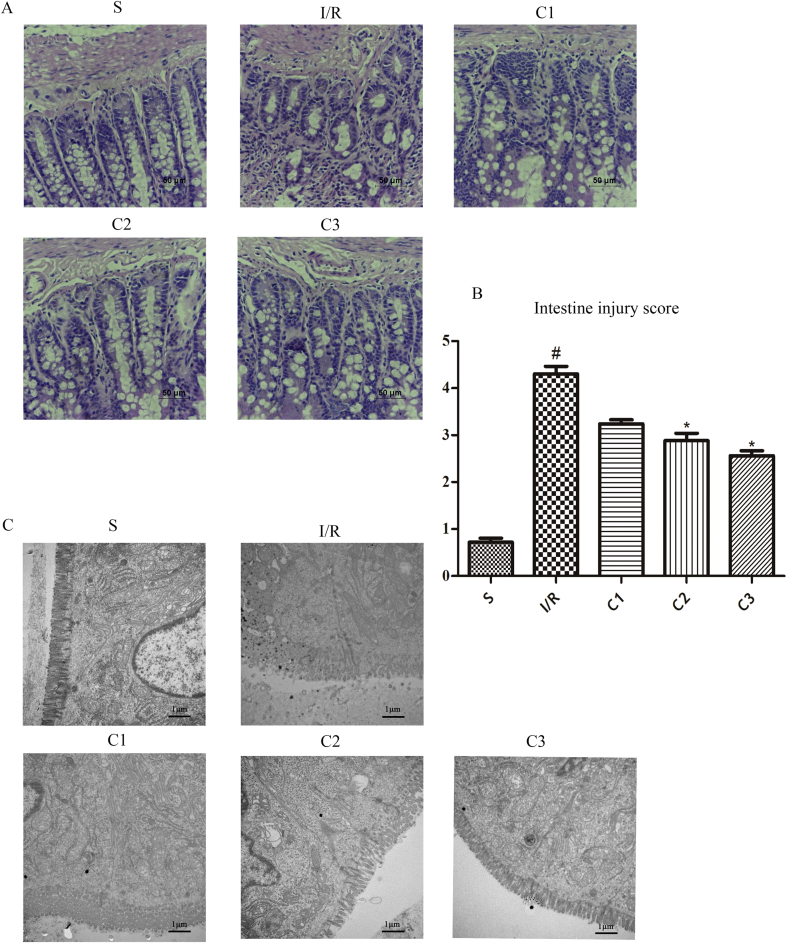
In order to confirm the effects of IL-1Ra on the I/R injury the intestinal tissues from the various group in this study were stained by HE staining (Fig. 2A), and the microstructure of intestinal tissue was observed by TEM(Fig. 2C). As a result, it was found that I/R could injure the intestinal tissue severely. In I/R group, both the epithelial cells and mucosa of the villous apex suffered from obvious abscission and disintegration of lamina propria, and the closely connected structure and glands suffered from conspicuous injury. However, in the groups treated by different doses of IL-1Ra, the injury of the intestinal mucosa in the rats was notably alleviated and only slight injury appeared in the mucous membrane, the structure of villous epithelium was basically integral, and no obvious injury of structure and gland could be observed. The results of histological scores agreed with the above-mentioned findings (Fig. 2B), and demonstrated that IL-1Ra possessed a definite protective effect on the I/R induced intestinal injury.
Fig. 2.
IL-1Ra protecting intestinal from I/R injury in rat. (A) The representative morphology of the HE-stained intestines. (B) Intestine injury score. (C) The representative micrograph of the intestines taken by transmission electron microscopy. Data were shown as the mean ± SD (n = 3). #p < 0.05 versus S; *p < 0.05 versus I/R.
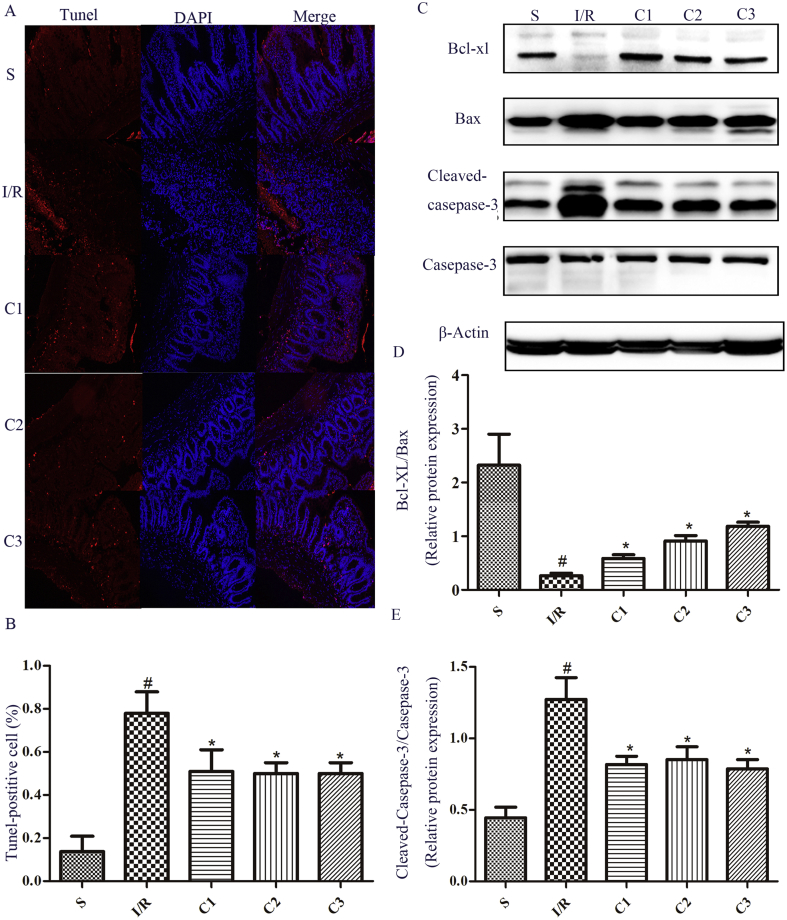
IL-1Ra decreased the intestinal cell apoptosis
Cell apoptosis is one of the main forms of intestinal tissue injury [13]. By TUNEL staining, cell apoptosis can be quantified. After treatment with IL-1Ra, the TUNEL-positive cell number in the injured tissues could be largely decreased (Fig. 3A and B). By a further detection, it was found that Bcl-xl protein expression was decreased significantly in I/R group compared to that of S group (Fig. 3C). Meanwhile, the protein contents of Bax and cleaved-caspase-3 (Fig. 3C) were significantly increased, further confirming that I/R could result in the apoptosis of intestinal cells. Compared to I/R group, the ratio of Bcl-xl/Bax (Fig. 3C and D) was increased in the group treated with IL-1Ra, and the protein level of cleaved-caspase-3 (Fig. 3C, E) was significantly decreased. This showed that IL-1Ra could decrease the intestinal cell apoptosis by regulating the expression of apoptosis-related proteins.
Fig. 3.
The effects of IL-1Ra on cell apoptosis after intestinal I/R. (A) Intestinal sections were stained with TUNEL (green) and DAPI (blue). Representative photographs of TUNEL-positive cells. (B) Quantitative analyses of TUNEL-positive cells. (C) The protein expression levels of Bcl-xl, Bax, Cleaved-caspase-3 and Caspase-3 were analyzed by Western blot. (D, E) The gray value of band was scanned by optical densitometry, the ratio value was statistically analyzed. Data were shown as the mean ± SD (n = 3). #p < 0.05 versus S; *p < 0.05 versus I/R.
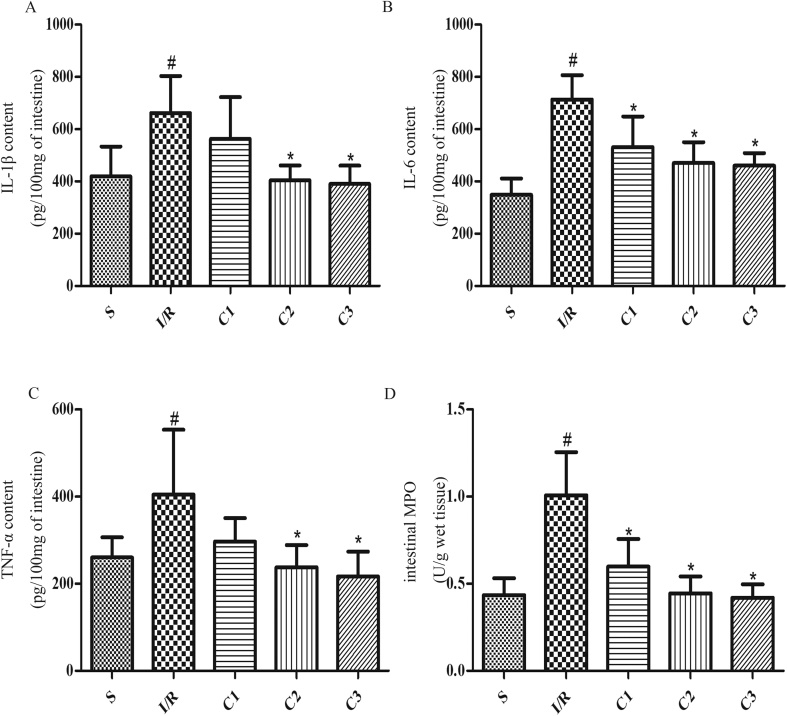
IL-1Ra inhibited inflammation in intestinal tissue
The great amount release of inflammatory mediators and high activation of neutrophils played a key role on the I/R injury. By detecting inflammatory cytokines such as IL-1β (Fig. 4A), IL-6 (Fig. 4B), TNF-α (Fig. 4C) and a neutrophil marker MPO (Fig. 4D) in intestinal tissues, it was found that IL-1Ra could effectively suppress the inflammatory response secondary to I/R.
Fig. 4.
IL-1Ra attenuates I/R-associated intestinal inflammatory response. The effects of IL-1Ra on (A) IL-1β, (B) IL-6, (C) TNF-αand (D) MPO. Data were shown as the mean ± SD (n = 7). #p < 0.05 versus S; *p < 0.05 versus I/R.
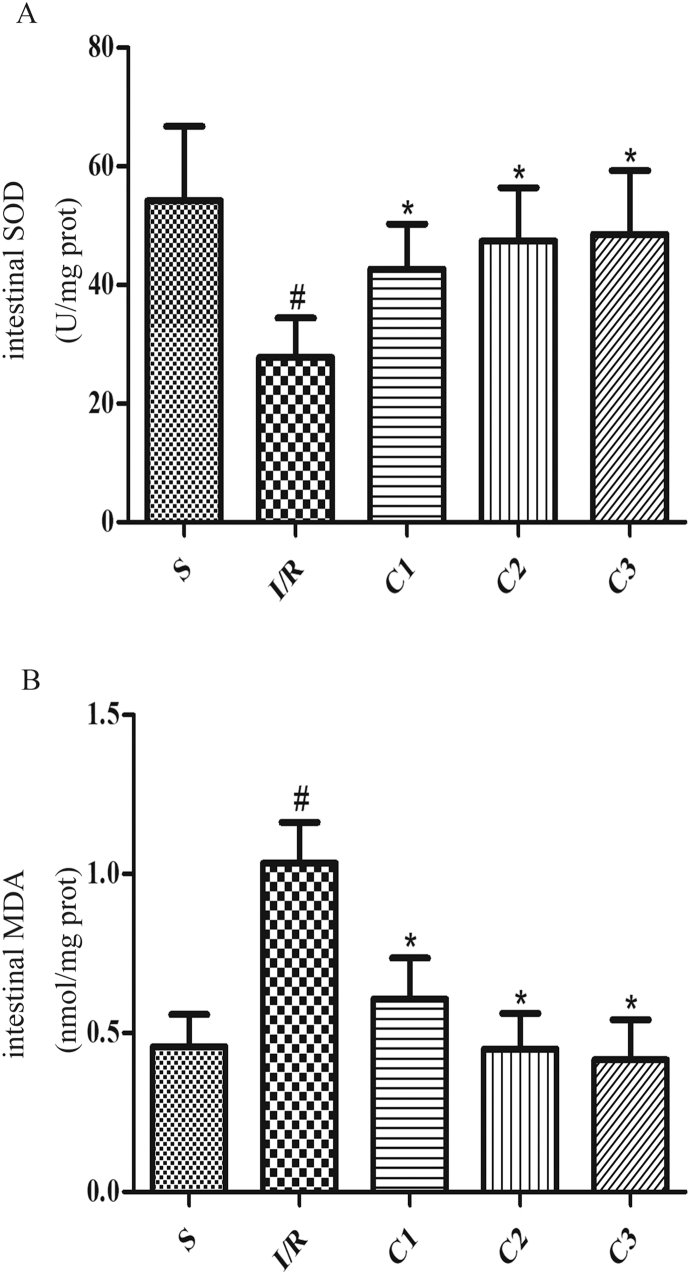
IL-1Ra inhibited oxidative stress in intestinal tissue
Besides, in comparison with I/R group, the activity of anti-oxidase SOD (Fig. 5A) was significantly increased in IL-1Ra pretreated group, meanwhile, the level of MDA (Fig. 5B), a product of lipid peroxidation, was significantly decreased. These results suggested that IL-1Ra may be effective both to anti-inflammatory and anti-oxidative stress.
Fig. 5.
IL-1Ra attenuates I/R-associated oxidative stress. The effects of IL-1Ra on (A) SOD and (B) MDA. Data were shown as the mean ± SD (n = 7). #p < 0.05 versus S; *p < 0.05 versus I/R.
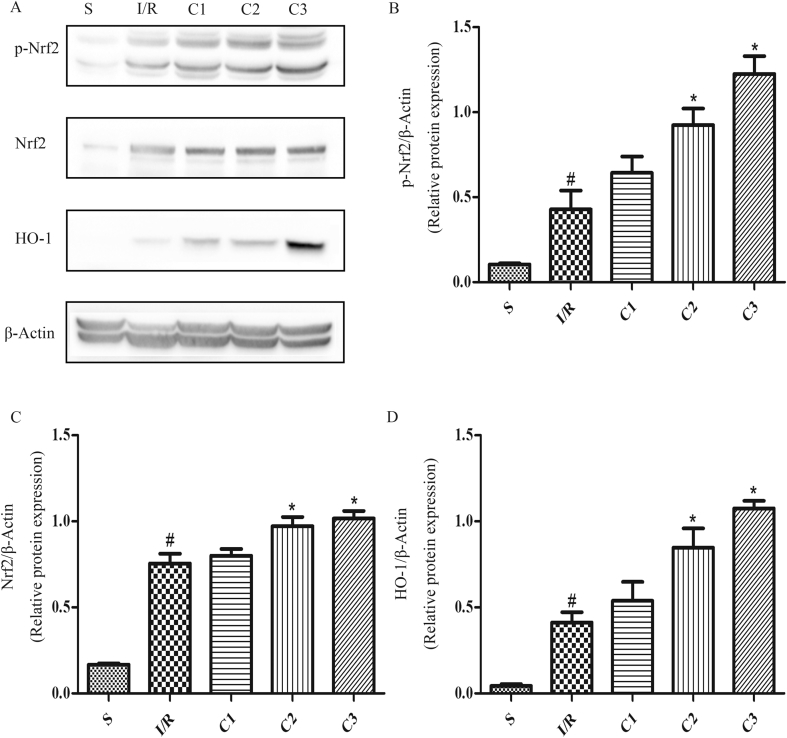
IL-1Ra activated Nrf2/HO-1 signal pathway
IL-1Ra displays its anti-inflammatory action by blocking many signal pathways activated by IL-1 (14–16), nevertheless, there hasn't been a clear report about the mechanism for anti-oxidative stress. In order to further explore the mechanism for IL-1Ra regulating oxidative stress, the changes of Nrf2/HO-1 signal pathway in intestinal tissues were detected, and it was found that the expression of Nrf2 (Fig.6A, C) and HO-1 (Fig. 6A, D), and p-Nrf2 level (Fig. 6A and B)in the IL-Ra-administered group were significantly higher than that in I/R group, illustrating that IL-1Ra could activate Nrf2/HO-1 signal pathway.
Fig. 6.
IL-1Ra activates Nrf2/HO-1 signaling in I/R intestine. (A) The protein expression levels of p-Nrf2, Nrf2 and HO-1. (B–D) The gray values of band were measured and calculated by optical densitometry, the ratio values were statistically analyzed. “p-” represented the phosphorylated form of protein. Data were shown as the mean ± SD (n = 3). #p < 0.05 versus S; *p < 0.05 versus I/R.
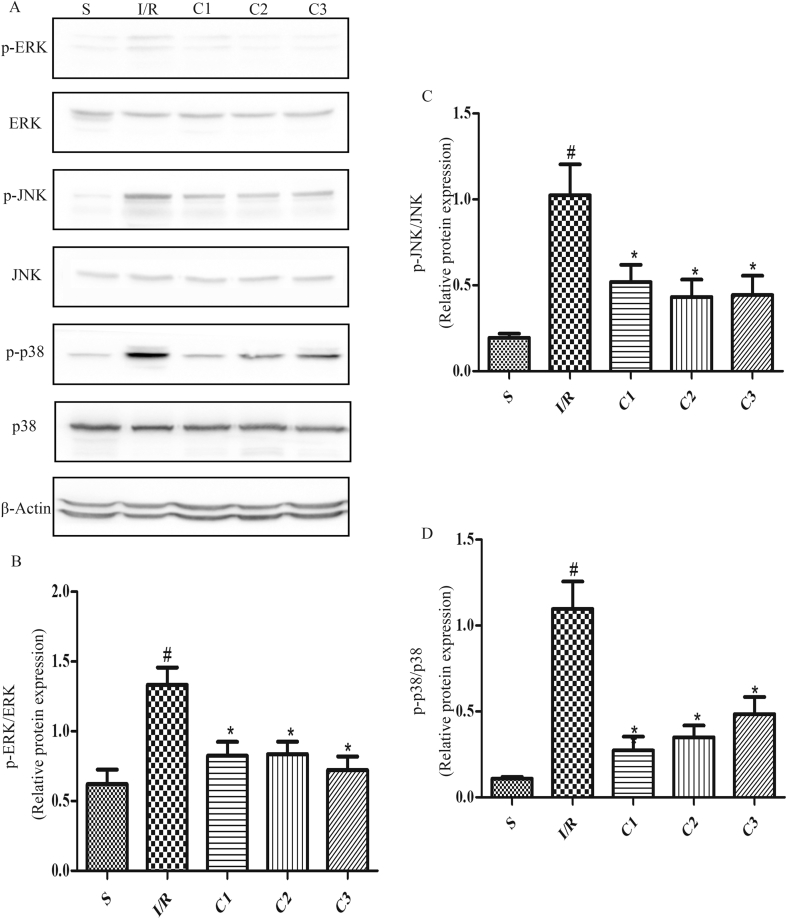
IL-1Ra inhibited MAPKs signal pathways
MAPKs are not only important anti-inflammatory signal pathways, but also have a crucial influence on the activity of Nrf2. Therefore, the levels of p-ERK (Fig. 7A and B), p-JNK (Fig. 7A, C) and p-p38 (Fig. 7A, D) were detected, and the results showed that the contents of p-ERK, p-JNK and p-p38 in I/R group were significantly higher than that in S group. And after the treatment with IL-1Ra, the levels of p-ERK, p-JNK and p-p38 were all decreased significantly, demonstrating that IL-1Ra could effectively inhibit the MAPKs signal pathways activated by I/R.
Fig. 7.
IL-1Ra suppression MAPKs signaling in I/R intestine. (A) The protein expression and phosphorylation levels of ERK, JNK and p38 were analyzed by Western blot. (B–D) The gray values of band were measured and calculated by optical densitometry, the ratio values were statistically analyzed. “p-” represented the phosphorylated form of protein. Data were shown as the mean ± SD (n = 3). #p < 0.05 versus S; *p < 0.05 versus I/R.
Discussion
Intestinal I/R injury is a common clinical disease with higher fatality, which not only damages the intestinal tissue, but also results in inflammatory response in the distal organs or whole body such as acute lung damage and the systematic syndrome of inflammatory response and so on [14], [15]. Because I/R can damage intestinal mucous barrier, and lead to the migration of bacteria and the release of inflammatory mediators, so the therapy against intestinal I/R damage has continuously been a hot issues. In our study, by setting up a rat model of intestinal I/R, it was found that IL-1Ra could effectively decrease the intestinal tissue damage and significantly decrease the cell apoptosis of the injured tissue.
The experimental results demonstrated that IL-1Ra, as an anti-inflammatory factor, had an antagonist effect on the secondary inflammatory reaction caused by intestinal I/R injury, which was related to its effective blockade of the intracellular inflammatory signal pathway activated by IL-1 [16], [17], [18]. A great amount of free radicals are generated during I/R, which can induce cell death, and this oxidative stress procedure interacts closely with inflammation. Our study found that IL-1Ra could restrain I/R-induced oxidative stress damage, it not only significantly increased the activity of antioxidant enzyme SOD in the damaged tissue, but also down-regulated the expression of the oxidative product MDA. The effect of anti-oxidative stress of IL-1Ra may provide a possibility to expand its clinical application.
Ntf2 is a key transcriptional factor in regulating the anti-oxidative stress response in cells, which regulates the expression of genes encoding HO-1 and other anti-oxidative enzymes, and plays an important role in the preventive mechanism of anti-oxidative stress [19]. In order to further explore the mechanism of IL-1Ra regulating oxidative stress, the changes of Nrf2/HO-1 signal pathway were monitored. In comparison with that in S group, the contents of Nrf2, HO-1 and p-Nrf2 in the intestinal tissue of I/R group were slightly increased, illustrating that the reactivity of the endogenous immune system in this period could be increased to antagonize the damage. However, this protective effect was not enough to antagonize the oxygen radical released by I/R in this period, thus the damage of the intestinal tissue was not significantly relieved. Treatment with IL-1Ra could further activate Nrf2/HO-1 signal pathway, then the levels of Nrf2 phosphorylation, the expression of Nrf2 and HO-1 were all raised significantly, and the damage of intestinal tissue was significantly reduced. The results indicated that IL-1Ra may display its effect of anti-oxidative stress through the activation of the Nrf2/HO-1 signal pathway.
The available researches confirm that MAPKs are not only one of the important signal pathways of mediating inflammatory reaction, but also involve in the regulation of Nrf2 transcription activity by inducing Nrf2 phosphorylation, although the above-mentioned regulation is only indirect. In different cells, after receiving various stimulation of external or chemical foreign matters, the results of MAPKs regulating Nrf2 may be different [20], [21], [22]. Our study showed that IL-1Ra could effectively inhibit MAPKs signal pathway activated by I/R damage, while further activating Nrf2/HO-1 signal pathway. Therefore, it was speculated that the action of IL-1Ra on Nrf2 may be closely related to the block of MAPKs signal pathway, but its mechanism is still to be further explored.
Conclusion
In summary, in this study it was for the first time to clarify that IL-1Ra possessed an obvious protective effect on intestinal I/R injury by anti-inflammatory, anti-oxidative stress and anti-apoptosis, in which both Nrf2/HO-1 and MAPKs pathways may be involved. The result indicated that IL-1Ra may be a new drug against intestinal I/R injury, and provided a basis for its clinical application.
Conflicts of interest
Authors declare no conflicts of interest.
Funding
This work was supported in part by grants from the National Science and Technology Major Project (no. 2012ZX09102301-017) and from the National Natural Science Foundation of China (no. 81770857), to D.G.X.
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.Huffmyer J., Raphael J. Physiology and pharmacology of myocardial preconditioning and postconditioning. Semin Cardiothorac Vasc Anesth. 2009;13:5–18. doi: 10.1177/1089253208330709. [DOI] [PubMed] [Google Scholar]
- 2.Kalogeris T., Baines C.P., Krenz M., Korthuis R.J. Ischemia/reperfusion. Compr Physiol. 2016;7:113–170. doi: 10.1002/cphy.c160006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woodman O.L., Long R., Pons S., Eychenne N., Berdeaux A., Morin D. The cardioprotectant 3′,4′-dihydroxyflavonol inhibits opening of the mitochondrial permeability transition pore after myocardial ischemia and reperfusion in rats. Pharmacol Res. 2014;81:26–33. doi: 10.1016/j.phrs.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Bertoni S., Arcaro V., Vivo V., Rapalli A., Tognolini M., Cantoni A.M. Suppression of inflammatory events associated to intestinal ischemia-reperfusion by 5-HT1A blockade in mice. Pharmacol Res. 2014;81:17–25. doi: 10.1016/j.phrs.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Slijper N., Sukhotnik I., Chemodanov E., Bashenko Y., Shaoul R., Coran A.G. Effect of simvastatin on intestinal recovery following gut ischemia-reperfusion injury in a rat. Pediatr Surg Int. 2010;26:105–110. doi: 10.1007/s00383-009-2508-6. [DOI] [PubMed] [Google Scholar]
- 6.Karhausen J., Qing M., Gibson A., Moeser A.J., Griefingholt H., Hale L.P. Intestinal mast cells mediate gut injury and systemic inflammation in a rat model of deep hypothermic circulatory arrest. Crit Care Med. 2013;41:e200–e210. doi: 10.1097/CCM.0b013e31827cac7a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vivo V., Zini I., Cantoni A.M., Grandi A., Tognolini M., Castelli R. Protection by the EPH-EPHRIN system against mesenteric ischemia-reperfusion injury. Shock. 2017;48:681–689. doi: 10.1097/SHK.0000000000000890. [DOI] [PubMed] [Google Scholar]
- 8.Lin Y.Z., Yao S.Y., Veach R.A., Torgerson T.R., Hawiger J., Lin Y.-Z. Inhibition of nuclear translocation of transcription factor NF-[IMAGE]B by a synthetic peptide containing a cell membrane-permeable motif and nuclear localization sequence. J Biol Chem. 1995;270:14255–14258. doi: 10.1074/jbc.270.24.14255. [DOI] [PubMed] [Google Scholar]
- 9.Pradillo J.M., Murray K.N., Coutts G.A., Moraga A., Oroz-Gonjar F., Boutin H. Reparative effects of interleukin-1 receptor antagonist in young and aged/Co-morbid rodents after cerebral ischemia. Brain Behav Immun. 2017;61:117–126. doi: 10.1016/j.bbi.2016.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nayki U.A., Nayki C., Cetin N., Cimen F.K., Coban A., Mammadov R. Effect of Kineret(R) on ovarian ischemia reperfusion injury in a rat model. J Obstet Gynaecol Res. 2016;42:1525–1533. doi: 10.1111/jog.13095. [DOI] [PubMed] [Google Scholar]
- 11.Zhang D.D., Zou M.J., Zhang Y.T., Fu W.L., Xu T., Wang J.X. A novel IL-1RA-PEP fusion protein with enhanced brain penetration ameliorates cerebral ischemia-reperfusion injury by inhibition of oxidative stress and neuroinflammation. Exp Neurol. 2017;297:1–13. doi: 10.1016/j.expneurol.2017.06.012. [DOI] [PubMed] [Google Scholar]
- 12.Chiu C.J. Intestinal mucosal lesion in low-flow States. Arch Surg. 1970;101:478–483. doi: 10.1001/archsurg.1970.01340280030009. [DOI] [PubMed] [Google Scholar]
- 13.Zu G., Guo J., Che N., Zhou T., Zhang X. Protective effects of ginsenoside Rg1 on intestinal ischemia/reperfusion injury-induced oxidative stress and apoptosis via activation of the Wnt/beta-catenin pathway. Sci Rep. 2016;6:38480. doi: 10.1038/srep38480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marshall J.C. The gut as a potential trigger of exercise-induced inflammatory responses. Can J Physiol Pharmacol. 1998;76:479–484. doi: 10.1139/cjpp-76-5-479. [DOI] [PubMed] [Google Scholar]
- 15.Grootjans J., Lenaerts K., Derikx J.P., Matthijsen R.A., de Bruïne A.P., van Bijnen A.A. Human intestinal ischemia-reperfusion–induced inflammation characterized : experiences from a new translational model. Am J Pathol. 2010;176:2283–2291. doi: 10.2353/ajpath.2010.091069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang X., Bi Z., Chu W., Wan Y. IL-1 receptor antagonist attenuates MAP kinase/AP-1 activation and MMP1 expression in UVA-irradiated human fibroblasts induced by culture medium from UVB-irradiated human skin keratinocytes. Int J Mol Med. 2005;16:1117–1124. [PubMed] [Google Scholar]
- 17.Dinarello C.A. The role of the interleukin-1-receptor antagonist in blocking inflammation mediated by interleukin-1. N Engl J Med. 2000;343:732–734. doi: 10.1056/NEJM200009073431011. [DOI] [PubMed] [Google Scholar]
- 18.Cogulu D., Onay H., Ozdemir Y., IA G., Ozkinay F., Kutukculer N. Associations of interleukin (IL)-1Î2, IL-1 receptor antagonist, and IL-10 with dental caries. J Oral Sci. 2015;57:31–36. doi: 10.2334/josnusd.57.31. [DOI] [PubMed] [Google Scholar]
- 19.Tebay L.E., Robertson H., Durant S.T., Vitale S.R., Penning T.M., Dinkovakostova A.T. Mechanisms of activation of the transcription factor Nrf2 by redox stressors, nutrient cues, and energy status and the pathways through which it attenuates degenerative disease. Free Radical Biol Med. 2015;88:108–146. doi: 10.1016/j.freeradbiomed.2015.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naidu S., Vijayan V., Santoso S., Kietzmann T., Immenschuh S. Inhibition and genetic deficiency of p38 MAPK up-regulates heme oxygenase-1 gene expression via Nrf2. J Immunol. 2009;182:7048–7057. doi: 10.4049/jimmunol.0900006. [DOI] [PubMed] [Google Scholar]
- 21.Jeayeng S., Wongkajornsilp A., Slominski A.T., Jirawatnotai S., Sampattavanich S., Panich U. Nrf2 in keratinocytes modulates UVB-induced DNA damage and apoptosis in melanocytes through MAPK signaling. Free Radic Biol Med. 2017;108:918–928. doi: 10.1016/j.freeradbiomed.2017.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen L., Teng H., Zhang K.Y., Skalickawoźniak K., Georgiev M.I., Xiao J. Agrimonolide and desmethylagrimonolide induced HO-1 expression in HepG2 cells through Nrf2-transduction and p38 inactivation. Front Pharmacol. 2017;7:513. doi: 10.3389/fphar.2016.00513. [DOI] [PMC free article] [PubMed] [Google Scholar]