Abstract
Introduction
Evidence of human papillomavirus (HPV) vaccine impact on anogenital warts (AGWs) by race or urbanicity in the US is lacking. We evaluated HPV vaccine impact in Tennessee by assessing AGW trends among Tennessee Medicaid (TennCare) enrollees aged 15–39 years from 2006-2014.
Methods
Persons with incident AGWs were identified using diagnosis/pharmacy codes from TennCare billing claims. We calculated sex-specific annual AGW incidence by age group, race, and urbanicity; estimated annual percent changes (APCs) using log-linear models; and performed pairwise comparisons by race and urbanicity.
Results
AGW incidence decreased among females aged 15–19 (APC = −10.6; P < 0.01) and 20–24 years (APC = −3.9; P = 0.02). Overall trends were similar between Whites and Blacks, and between those living in metropolitan statistical areas (MSAs) and non-MSAs. Rates among males aged 15–19 years began decreasing after 2010. Among enrollees aged 25–39 years, rates increased or were stable.
Conclusions
Following introduction of the HPV vaccine in 2006, AGWs decreased among age groups most likely to be vaccinated. The change in trend among young males after 2010 suggests early herd effects. Our findings indicate vaccine effects and support the importance of improving adherence to current vaccination recommendations for preventing AGWs and other HPV-related diseases.
Keywords: Trend, HPV vaccination, Impact, Anogenital warts
1. Introduction
Human papillomavirus (HPV) is the most common sexually transmitted infection in the United States (US) [1], and the cause of anogenital warts (AGWs) and most cervical, anal, vaginal, oropharyngeal, vulvar, and penile cancers [2,3]. HPV vaccination has the potential to prevent the majority of these adverse health outcomes; however, in 2016, HPV vaccine ≥1-dose coverage and up-to-date (UTD) coverage (all recommended doses depending on age and spacing of doses) among US adolescents aged 13–17 years were only 60.4% and 43.4%, respectively [4]. Tennessee ranked 37th for ≥1 dose and 39th for UTD (55% and 36%, respectively) [4]. Lower coverage has also been documented in rural areas compared to urban areas [4]. Racial and ethnic disparities in HPV vaccination were observed initially, but these gaps closed over time [4,5].
Because AGWs typically appear three weeks to eight months after HPV infection [6,7], the evaluation of HPV vaccine impact on AGWs may be evident earlier than other HPV-related outcomes such as precancerous lesions and cancers, which can take years or even decades to manifest [8]. Over 40 HPV types can infect genital tissue [9]; however, 90% of AGWs are caused by HPV types 6 and 11 [10]. The quadrivalent HPV vaccine was first licensed in the US in mid-2006 for females and late-2009 for males, while the 9-valent HPV vaccine was approved in late-2014 for both males and females [11,12]. Both vaccines target HPV types 6 and 11, in addition to oncogenic types [10,11].
Despite recommendations by the Advisory Committee on Immunization Practices for routine HPV vaccination among US adolescent females and males, HPV vaccination coverage in the US has consistently lagged behind other developed countries [13]. Previous studies report declines in the incidence and prevalence of AGWs since the introduction of the HPV vaccine in both the US and other countries, suggesting both direct and indirect effects of female HPV vaccination [[14], [15], [16]]. Evidence of the vaccine's impact in US states with low vaccination coverage, such as Tennessee, is lacking. Further, we are unaware of previous studies examining vaccine impact on AGWs by race or urbanicity. Thus, we aimed to evaluate HPV vaccine impact in Tennessee, a state with low vaccination coverage, by assessing trends in AGW incidence among low-income persons aged 15–39 years enrolled in the Tennessee Medicaid program during the HPV vaccine post-licensure era (2006–2014).
2. Methods
2.1. Data source/study population
We used data from TennCare, Tennessee's Medicaid program, which included medical and pharmaceutical codes from billing claims, and patient demographic information on sex, age, race/ethnicity, and residential address. The analytic sample included 799,122 TennCare enrollees aged 15–39 years with at least 12 months of consecutive enrollment from January 2006 through December 2014. More recent data were not included because there was a major change in billing codes in 2015, which could affect the interpretation of trends.
Demographic characteristics included in the analysis were sex (female and male), age group (15–19, 20–24, 25–29, and 30–39 years), race/ethnicity (White, Black, Hispanic, other/unknown), Tennessee region [17] (West, Middle, East), and urbanicity [18] (metropolitan statistical area [MSA] and non-MSA). To preserve confidentiality of the study participants, the analysis file did not contain protected health information. The primary investigator received a password protected database in which all study variables were pre-classified into categories determined by the study protocol. As public health surveillance for HPV vaccine evaluation, this activity was considered exempt (not human subject research) by the Centers for Disease Control and Prevention, Vanderbilt, and Tennessee Department of Health Institutional Review Boards, and was reviewed and approved by the Division of TennCare.
2.2. Definition of incident anogenital warts
We used three types of codes to identify healthcare encounters for AGWs: International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes [19], Current Procedural Terminology (CPT) codes [20], and national drug codes (NDCs) [21]. We identified persons with AGWs by one of the following criteria: 1) a specific ICD-9-CM diagnosis code for condyloma acuminatum, or 2) a genital-specific CPT code for destruction or excision procedures within seven days before or after a less specific ICD-9-CM diagnosis code for viral warts, or 3) a genital-specific CPT code for destruction or excision procedures within seven days before or after a NDC for AGW medication (Table A1). We defined incident cases as persons who were at least 12 months AGW-free prior to meeting the AGW case definition. We considered using a five-year washout period; however, that would have reduced our sample by greater than 50%. In addition, only 9% of those labelled as incident cases with the 12-month criteria had evidence of prior AGWs when requiring five years of prior enrollment (prevalent, rather than incident cases.)
Table 1.
Demographic characteristics of enrollees aged 15–39 years, TennCare 2006–2014.
| Characteristic | Overall N = 2,765,564 PY % |
2006 n = 303,825 | 2007 n = 303,612 % |
2008 n = 289,205 % |
2009 n = 288,402 % |
2010 n = 303,210 % |
2011 n = 311,224 % |
2012 n = 308,918 % |
2013 n = 309,378 % |
2014 n = 347,790 % |
|---|---|---|---|---|---|---|---|---|---|---|
| Sex | ||||||||||
| Female | 66.3 | 65.4 | 64.4 | 64.4 | 64.9 | 67.3 | 67.3 | 67.7 | 67.6 | 67.2 |
| Male | 33.7 | 34.6 | 35.7 | 35.6 | 35.1 | 32.8 | 32.7 | 32.3 | 32.4 | 32.8 |
| Age Group, Years | ||||||||||
| 15–19 | 36.4 | 35.7 | 35.3 | 36.9 | 37.9 | 38.3 | 37.0 | 35.7 | 35.6 | 35.2 |
| 20–24 | 19.6 | 20.9 | 22.6 | 20.8 | 19.4 | 19.6 | 18.8 | 18.2 | 17.7 | 18.4 |
| 25–29 | 16.6 | 16.3 | 15.9 | 16.3 | 16.5 | 15.9 | 16.6 | 17.1 | 17.2 | 17.1 |
| 30–39 | 27.5 | 27.1 | 26.2 | 25.9 | 26.2 | 26.3 | 27.6 | 29.0 | 29.5 | 29.3 |
| Race/Ethnicity | ||||||||||
| White | 50.0 | 54.8 | 53.9 | 52.3 | 50.6 | 50.2 | 48.8 | 47.6 | 46.5 | 46.5 |
| Black | 31.0 | 34.1 | 34.5 | 34.0 | 32.8 | 30.6 | 29.5 | 29.1 | 28.3 | 27.2 |
| Hispanic | 1.4 | 1.0 | 1.0 | 1.1 | 1.2 | 1.4 | 1.4 | 1.5 | 1.7 | 1.9 |
| Other/Unknown | 17.6 | 10.2 | 10.5 | 12.6 | 15.5 | 17.9 | 20.2 | 21.8 | 23.6 | 24.4 |
| Region | ||||||||||
| West | 33.5 | 34.6 | 34.6 | 34.5 | 34.3 | 33.2 | 32.7 | 32.8 | 32.9 | 32.0 |
| Middle | 32.0 | 30.4 | 30.9 | 31.1 | 31.3 | 32.2 | 32.6 | 32.8 | 32.9 | 33.5 |
| East | 34.5 | 35.0 | 34.6 | 34.4 | 34.4 | 34.7 | 34.7 | 34.3 | 34.2 | 34.5 |
| Urbanicitya | ||||||||||
| MSA | 73.8 | 73.4 | 73.4 | 73.7 | 74.0 | 73.7 | 74.1 | 74.3 | 74.2 | 73.7 |
| Non-MSA | 26.2 | 26.6 | 26.6 | 26.3 | 26.0 | 26.3 | 25.9 | 25.7 | 25.9 | 26.3 |
MSA= Metropolitan Statistical Area; PY= Person-Years.
Urbanicity was categorized by the enrollee's county of residence using MSA definitions and boundaries set by the US Census Bureau, which classifies MSAs as counties associated with at least one urbanized area that has a population of at least 50,000 persons.
2.3. Statistical analysis
Person-time for each year was estimated by counting the total number of TennCare enrollees aged 15–39 years with at least 12 months of continuous enrollment on July 1st of each calendar year by sex, age group, race, and urbanicity. In the primary analysis, we calculated annual incidence of AGWs per 1000 person-years (PY) among females and males by age group from January 2006 through December 2014. In the secondary analysis, we assessed age-group-specific AGW incidence among females and males stratified by race and urbanicity. To avoid small cell sizes in the secondary analyses, we dichotomized age into 15–24 years and 25–39 years, and limited race to White and Black.
Annual percent changes (APCs) and average annual percent changes (AAPCs) in AGW incidence by sex, age group, race, and urbanicity were estimated using permutation tests with Poisson variance in the Joinpoint Desktop Software version 4.5.0.1 (National Cancer Institute, Bethesda, MD) [22]. Annual percent changes were the β-coefficients of each trend segment detected by the best fit log-linear model with the fewest inflection years (i.e. maximum of one joinpoint). Average annual percent changes over the entire study period (2006–2014) were weighted averages of the annual percent changes of trend segments before and after the detected inflection year. The AAPC was only reported if joinpoints were detected; otherwise, only the APC was reported. In the secondary analysis, we conducted pairwise comparisons of White vs. Black and MSA vs. non-MSA enrollees, stratified by sex and age group to assess differences in AGW trends between these subgroups. P-values less than 0.05 were considered statistically significant for all comparisons.
3. Results
3.1. Descriptive characteristics
A total of 799,122 TennCare enrollees aged 15–39 years contributed to more than 2.7 million PY of data over the nine-year study period (2006–2014) (Table 1). The distribution of demographic characteristics among the cohort was stable across calendar years except for race/ethnicity, for which the Other/Unknown category progressively increased from 10.2% in 2006 to 24.4% in 2014. Of all TennCare enrollees aged 15–39 years, 66.3% were female, 50.0% were White, and 73.8% lived in a MSA. Among all identified AGW incident cases, 92% were identified by the specific ICD-9-CM diagnosis code for condyloma acuminatum (result not shown).
3.2. Trends in females
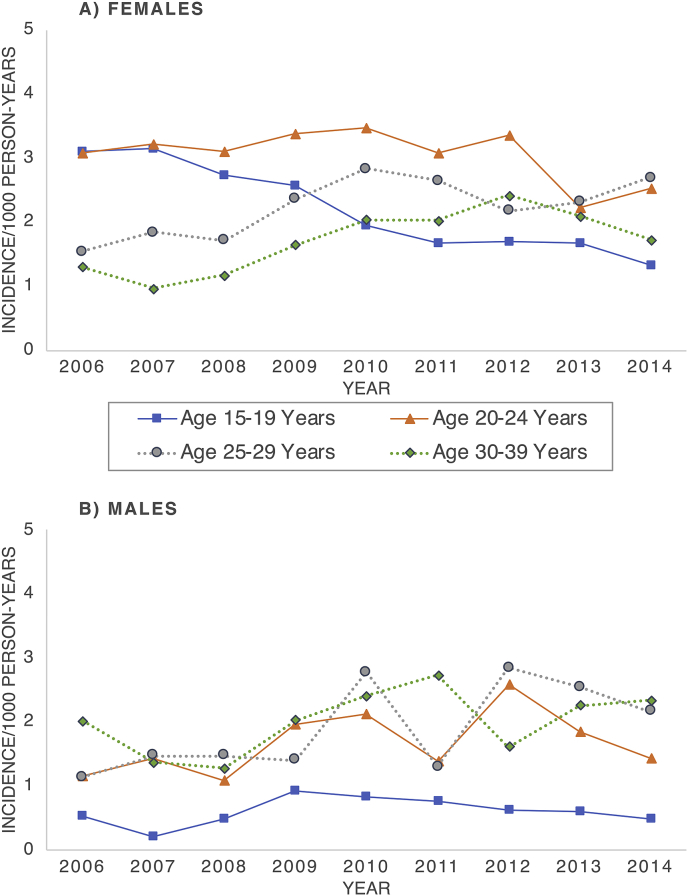
Among females, overall AGW incidence from 2006 through 2014 was highest for those aged 20–24 years (3.1/1000 PY) (Table A2). Incidence significantly decreased among females aged 15–19 years from 3.1/1000 PY in 2006 to 1.3/1000 PY in 2014 (APC = −10.6; P < 0.01). Among females aged 20–24 years, the trend changed in 2011 and declined from 3.1/1000 PY in 2011 to 2.5/1000 PY in 2014 (APC = −12.8; P = 0.03), yielding an overall AAPC of −3.9 (P = 0.02) (Table 2, Fig. 1). Conversely, from 2006 to 2014, AGW incidence significantly increased among females aged 25–29 years from 1.5/1000 PY to 2.7/1000 PY (APC = 5.2, P = 0.04). Among females aged 30–39 years, AGW incidence significantly increased from 2006 to 2012 (APC = 14.9; P = 0.02), and then decreased from 2012 to 2014; however, the decline from 2012 to 2014, and the overall trend were not statistically significant.
Table 2.
Sex-specific trends in anogenital wart incidence among enrollees aged 15–39 years by age group, TennCare 2006–2014.
| Age Group, Years | Inflection Year | Time Period | Annual Percent Changea (95% CI) | APC p-value | Average Annual Percent Changeb (95% CI) | AAPC p-value |
|---|---|---|---|---|---|---|
| Female | ||||||
| 15–19 | – | 2006–2014 | −10.6 (−12.6, −8.5) | < 0.01 | – | – |
| 20–24 | 2011 | 2006–2011 | 1.9 (−1.9, 5.8) | 0.25 | −3.9 (−7.1, −0.6) | 0.02 |
| 2011–2014 | −12.8 (−22.0, −2.54) | 0.03 | ||||
| 25–29 | – | 2006–2014 | 5.2 (0.3, 10.3) | 0.04 | – | – |
| 30–39 | 2012 | 2006–2012 | 14.9 (4.2, 26.8) | 0.02 | 6.5 (−4.7, 18.9) | 0.27 |
| 2012–2014 | −15.4 (−51.3, 47.0) | 0.45 | ||||
| Male | ||||||
| 15–19 | 2010 | 2006–2010 | 27.0 (−9.2, 77.7) | 0.12 | 4.4 (−11.4, 22.9) | 0.61 |
| 2010–2014 | −14.3 (−37.7, 17.9) | 0.25 | ||||
| 20–24 | – | 2006–2014 | 5.9 (−0.4, 12.6) | 0.06 | – | – |
| 25–29 | – | 2006–2014 | 10.0 (5.7, 14.6) | < 0.01 | – | – |
| 30–39 | – | 2006–2014 | 4.1 (−3.1, 11.9) | 0.23 | – | – |
AAPC = Average annual percent change; APC = Annual Percent Change.
Boldface indicates statistical significance (p < 0.05).
Annual percent changes were determined by the β-coefficient of the best fit log-linear model using a permutation test and Poisson variance; The AAPC was only reported if inflection years were detected; otherwise, only the APC was reported.
Average annual percent changes from 2006-2014 are weighted averages of the annual percent changes of all time periods.
Fig. 1.
Annual incidence of anogenital warts among A) female and B) male enrollees aged 15–39 years by age group, TennCare 2006–2014.
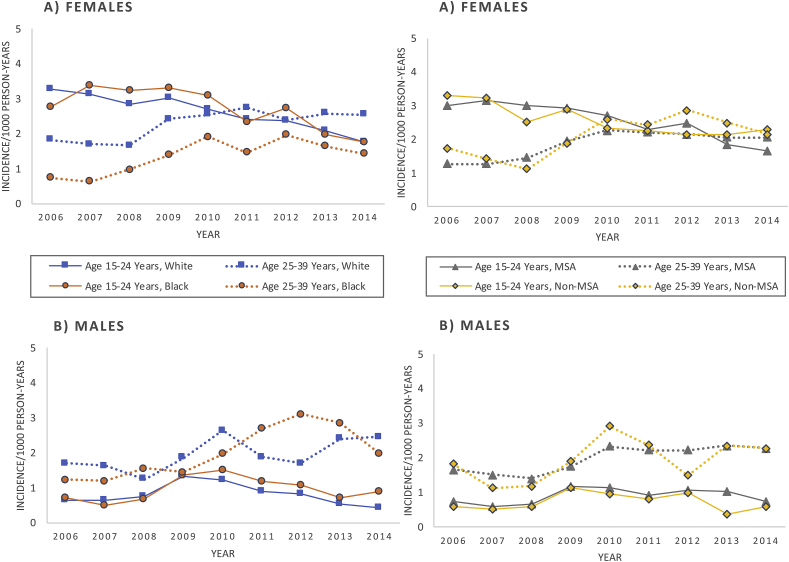
In race-stratified analyses among females, trends in AGWs by age group were similar to those in unstratified analyses in that both Whites and Blacks exhibited decreasing incidence between 2006 and 2014 among the younger age group (15–24 years) and increasing incidence among the older age group (25–39 years) (Fig. 2, Table A.3, Table A.4). For those aged 15–24 years, overall AGW trends were similar among White and Black females (APC among Whites = −6.5; P < 0.01, AAPC among Blacks = −6.1; P = 0.01) (pairwise comparison P = 0.423, results not shown). However, based on the detected inflection year, the downward trend for young Black females was delayed until 2009 (APC from 2009-2014 = −11.5; P = 0.01), and Black females exhibited steeper declines compared to White females during 2009–2014 (Fig. 2, Table A.4). For females aged 25–39 years, incidence rates were higher among older White females (2.3/1000 PY overall) than older Black females (1.4/1000 PY overall) across the entire study period; however, the increase in incidence was more than double and significantly higher for older Blacks (AAPC = 13.0; P = 0.01) than older Whites (APC = 5.4; P = 0.01) (pairwise comparison P = <0.001, results not shown).
Fig. 2.
Annual incidence of anogenital warts among A) female and B) male enrollees aged 15–39 years by age group, race, and urbanicity, TennCare 2006–2014.
MSA = Metropolitan Statistical Area.
In urbanicity-stratified analyses among females, both subgroups (MSA and non-MSA) displayed decreasing incidence for the younger age group (15–24 years) and increasing incidence for the older age group (25–39 years); however, the increasing trend among older females was not significant in non-MSAs (Fig. 2, Table A.3, Table A.4). Based on the pairwise comparison among young females aged 15–24 years, trends were similar (i.e., not statistically significantly different) between those living in MSAs and non-MSAs, (P = 0.813, results not shown), with significant decreases from 2006 to 2012 (APC among MSA = −5.1; P = 0.02, APC among non-MSA = −7.5; P < 0.01) and no significant trends from 2012 to 2014 (Table A.4).
3.3. Trends in males
Among males, AGW incidence was highest for those aged 30–39 years (2.0/1000 PY overall) (Table A2). Incidence among males aged 15–19 years shifted in 2010 from an increasing trend (APC from 2006 to 2010 = 27.0; P = 0.12) to a decreasing trend (APC from 2010 to 2014 = −14.3; P = 0.25), although neither segment was significant (Table 2, Fig. 1). Incidence increased for all other male age groups (i.e., had positive APCs) from 2006 to 2014, but was only significant for males aged 25–29 years (APC = 10.0; P < 0.01).
In race-stratified analyses among males, trends in AGW incidence by age group were more evident than in unstratified analyses (Fig. 2, Table A.3). Specifically, among young White males aged 15–24 years, AGW incidence shifted in 2010 from a significantly increasing trend (APC from 2006 to 2010 = 19.4; P = 0.02) to a significantly decreasing trend (APC from 2010 to 2014 = −31.8; P = 0.01). A similar segmented pattern of shifting in 2010 from an increasing to decreasing trend was observed for Black males aged 15–24 years, but the APCs for each time period were not statistically significant (Table A.4). Among males aged 25–39 years, annual increases in incidence were significantly higher for Blacks (APC = 15.9; P < 0.01) than Whites (APC = 11.9; P < 0.01) (pairwise comparison P < 0.01, results not shown).
In urbanicity-stratified analyses among males, no significant trend was observed for the overall period of 2006–2014 for young males aged 15–24 years living in either a MSA or non-MSA. Specifically, among young males living in a non-MSA, AGW incidence shifted in 2010 from an increasing trend (APC from 2006 to 2010 = 22.9; P = 0.06) to a decreasing trend (APC from 2010 to 2014 = −18.7; P = 0.07), although neither was significant (Fig. 2, Table A.3, Table A.4). Based on the pairwise comparison between males aged 25–39 years, the increasing annual percent changes in AGW incidence were similar between those living in a MSA vs. non-MSA (pairwise comparison P = 0.96, results not shown), but the trend was only significant among those living in a MSA (APC = 5.9; P = 0.01) (Table A.4).
4. Discussion
Following the licensure of the quadrivalent HPV vaccine in the US in mid-2006 for females and late-2009 for males [11,12], we observed decreasing trends in AGW incidence among Tennessee Medicaid enrollees most likely to be vaccinated (i.e. age groups for which the HPV vaccine is recommended), with the largest declines among females aged 15–19 years. Among males aged 15–19 years, we observed a shift from increasing to decreasing incidence after 2010 shortly before the vaccine was recommended for males in 2011. Among older enrollees aged 25–39 years, AGWs either increased or remained stable over time. Similar age group-specific patterns (i.e. decreasing trends among younger enrollees and stable or increasing trends among older enrollees) were observed after stratifying by race and urbanicity. Our results suggest substantial HPV vaccine impact, even among a population with relatively low vaccination coverage (55% ≥ 1 dose and 36% UTD) [4].
Decreasing trends among younger enrollees were expected given that HPV vaccination coverage has increased considerably since the Advisory Committee on Immunization Practices released recommendations for routine HPV vaccination for adolescents aged 11–12 years in 2006 for females and 2011 for males, with catch-up vaccination through age 26 and 21 years, respectively [23]. In Tennessee, HPV vaccine ≥1-dose coverage among adolescents aged 13–17 years increased from 29.6% in 2008 to 47.8% in 2014 for females and from 20.3% in 2012 to 30.5% in 2014 for males, rising to 55.3% by 2016 for females and males combined [4,5,24]. The increasing HPV vaccination coverage coupled with our observed declines in AGWs among young females in the vaccine era suggest vaccine impact for preventing AGWs. These results complement a recent Tennessee study that reported declines in cervical intraepithelial neoplasia grade 2 or greater among young females aged 18–24 years, but not in older females during the same time period [25]. Further, our findings corroborate previous studies in the US [[26], [27], [28], [29]] and other countries [14,[30], [31], [32]] which have reported declines in AGWs among adolescents and young adults since the introduction of the HPV vaccine.
Previous studies have reported declines in AGWs among young males in the US, likely due to indirect effects from female vaccination since male vaccination was introduced later and coverage was initially low [26,28]. Similarly, we observed decreasing incidence among young males, including White, Black, and non-MSA subgroups. Decreases began in 2010 and the segmented trends were only statistically significant among Whites. The change in trend in 2010 suggests indirect effects of female vaccination; however, some direct effects could have also contributed to declines through 2014.
We observed similar trends in AGW incidence by urbanicity, even though in 2016, HPV vaccine 1-dose and UTD coverage among Tennessee adolescents aged 13–17 years was 45.6% and 25.2% for those living in non-MSAs, respectively, compared to 68.5% and 45.6% among those living in central city MSAs, respectively [4]. State-level HPV vaccine coverage rates disaggregated by MSA are not available for previous years to know if this geographic disparity has existed over time. There was a lack of racial differences in trends of AGW incidence. Racial disparities in completing all recommended HPV vaccine doses were observed in the first several years after vaccine approval at the national level; however, we have no such data for Tennessee. In Tennessee, UTD coverage was higher for Black (44.9%) than White (31.1%) adolescents in 2016 [5]. A previous study conducted in Australia also reported similar patterns in genital warts by urbanicity and socioeconomic status [32].
We expected to observe no significant decreases in AGWs among older enrollees due to vaccine-ineligibility and low vaccine effectiveness due to prior exposure to the virus. Previous studies have also found low HPV vaccination rates for females and males aged 18–26 years [33]. The increases in AGWs in older age groups might be explained by background secular trends in the unvaccinated population, such as changes in behavior or increased reporting or recognition of AGWs, as has previously been suggested in other populations [34,35].
Our results are comparable to other populations with low vaccination coverage, as one systematic review that assessed patterns in AGWs within the first four years post-vaccine implementation in countries with low female vaccination coverage (<50%) found significant declines in AGW diagnoses among females aged 15–19 years [16]. Since we examined AGW incidence eight years post-vaccine implementation, we were also able to observe significant declines among females aged 20–24 years. However, declines in AGWs were less pronounced in Tennessee compared to populations with higher vaccination coverage. In Australia, AGWs among women under 21 years of age declined 92% [36] within four years of implementing a fully funded, national HPV vaccination program for females aged 12–13 years in 2007, with catch-up through age 26 years. By 2011, HPV vaccine coverage had already reached 83.2% for ≥1 dose and 73.0% for all recommended doses among Australian females aged 12–13 years [37]. Results from Australia demonstrate the potential impact of high population-level HPV vaccine coverage for preventing AGWs.
Our study has some limitations. It is possible the observed trends were due in part to unmeasured confounders that are major risk factors for AGWs, such as age at sexual debut and numbers of lifetime or recent sexual partners—information that is unavailable in administrative data. Additionally, because our incident case definition included a washout period of 12 months, some of the identified cases could have been prevalent rather than incident cases. When we restricted our analysis to those with five years of enrollment, we found that ∼9% of those labelled as incident cases had evidence of prior AGWs. We ultimately decided on a one-year washout period since a longer period would have reduced power, decreasing sample size by over 50%, and because most symptomatic AGWs have a short latent period (three weeks to eight months after HPV infection [6,7]). Another limitation was that by the end of the study period, the “Other/Unknown” race/ethnicity category had increased to nearly a quarter of enrollees aged 15–39 years, which raises concern about changes in population demographics. Because race/ethnicity is self-reported, the increase could be attributable to social shifts in racial and ethnic self-identification. According to a report by the Centers for Medicare and Medicaid Services [38], in 2017, over a third of US enrollees aged 18–64 years did not self-identify in a single racial group. Lastly, we were unable to include more recent data (2015 and beyond) because the US implemented a major administrative coding transition from ICD-9-CM to ICD-10-CM on October 1, 2015. ICD-10-CM codes have fundamental structural differences from ICD-9-CM and conversions between coding schemes can either be direct or approximate (e.g. one ICD-9-CM code is mapped to several ICD-10-CM codes or vice versa), which could affect the interpretation of AGW trends across coding eras. Further work is warranted to assess whether there are differences in discriminant ability for identifying AGW cases using administrative algorithms in the ICD-9-CM era compared to the ICD-10-CM era. Substantial changes in discrimination between the pre-and post-ICD-10-CM transition would require the application of weights or adjustments to account for these differences.
Our study has notable strengths. Our study represents the first assessment of race- and urbanicity-stratified trends in AGW incidence during an HPV vaccine era in the US. In addition, we were able to enumerate the entire population of TennCare enrollees aged 15–39 years. Thus, the reported age group- and sex-specific incidence rates reflect true AGW trends among Tennessee Medicaid enrollees. Because adolescents insured by Medicaid tend to have higher rates of HPV vaccination than the general public due to eligibility for the Vaccines for Children program [39]. The impact of HPV vaccination on AGWs may be more evident among Medicaid populations compared to the general population.
5. Conclusions
Significantly decreasing trends in AGWs among young females in age groups most likely to be vaccinated compared to increasing/stable trends among older enrollees suggests HPV vaccine impact on AGW incidence in Tennessee, a state with relatively low HPV vaccination coverage. Thus, working to improve vaccination coverage may further increase impact. We expect that increased impact of HPV vaccination on AGWs will occur as vaccine-eligible groups age into older cohorts and as HPV vaccination rates continue to increase in Tennessee. These results indicate the importance of continued adherence to current vaccination recommendations for preventing AGWs and other HPV-related diseases, including HPV-associated cancers.
Declaration of interest
This work was supported by the Emerging Infections Cooperative Agreement No. 5U01C10003 from the Centers for Disease Control and Prevention, and grant No. TL1TR002244 from the National Center for Advancing Translational Sciences, National Institutes of Health. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention nor the National Institutes of Health.
Individual statements generated by the ICMJE forms for disclosure of potential conflicts of interest
Ms. Shing reports grants from National Center for Advancing Translational Sciences during the conduct of the study. Dr. Hull has nothing to disclose. Dr. Zhu reports grants from Centers for Disease Control and Prevention during the conduct of the study. Dr. Gargano has nothing to disclose. Dr. Markowitz has nothing to disclose. Ms. Cleveland has nothing to disclose. Ms. Pemmaraju has nothing to disclose. Dr. Park has nothing to disclose. Ms. Whitney has nothing to disclose. Mr. Mitchel has nothing to disclose. Dr. Griffin reports grants from Centers for Disease Control and Prevention during the conduct of the study.
Acknowledgements
The authors thank the Tennessee Division of TennCare of the Department of Finance and Administration for providing the data, and Dr. Alicia Beeghly-Fadiel, Vanderbilt University Medical Center, for providing careful review and helpful comments.
Appendix A.
Table A1.
Indicators used to identify cases of anogenital warts, TennCare 2006–2014.
| Code | Description |
|---|---|
| ICD-9-CM | |
| 0.78.11 | Diagnosis code for condyloma acuminatum (i.e., anogenital warts) |
| 078.1 | Diagnosis code for viral warts due to human papillomavirus |
| 078.10 | Diagnosis code for viral warts, unspecified |
| 078.19 | Diagnosis code for other specified viral warts |
| CPT | |
| 46900–46942 | Destruction procedures on the anus |
| 46200–46320 | Excision procedures on the anus |
| 54050–54065 | Destruction procedures on the penis |
| 54100–54164 | Excision procedures on the penis |
| 56501–56515 | Destruction procedures on the vulva, perineum, and introitus |
| 56605–56740 | Excision procedures on the vulva, perineum, and introitus |
| 57061–57065 | Destruction procedure on the vagina |
| 57100–57135 | Excision procedures on the vagina |
| 57522 | Excision procedures on the cervix uteri |
| NDC | |
| 10337045003, 10337045015 | Prescription for sinecatechins |
| 00023611803, 00574061105, 00591320413, 49808012335, 52544004513, 52544004613, 55515010101, 55515010201, 68682092635 | Prescription for podofilox |
| 00395228191, 00574060115, 10106289800, 38245066822, 38779004604, 49452548002, 51552001630, 51927122000, 51927167300 | Prescription for podophyllum resin |
| 00089061012, 00093612619, 00093612664, 00115147623, 00115147659, 00168043224, 00168043227, 00781715209, 29336061012, 29336061024, 29336071028, 35356001012, 45802007662, 45802036853, 45802036862, 51672414506, 51672414508, 54569489400, 54868455400, 54868617900, 60505050105, 64380077300, 64380077302, 64380077319, 68462053670, 99207026012, 99207027028, 99207027175, 99207027675 | Prescription for imiquimod |
| 00406292411, 10106041700, 10106041704, 10481300801, 17317058404, 38779010005, 51552046902, 51927124300 | Prescription for trichloroacetic acid |
CPT= Current Procedural Terminology; ICD-9-CM= International Classification of Diseases, Ninth Revision, Clinical Modification; NDC= National drug codes.
Table A2.
Sex-specific incidencea of anogenital warts among enrollees aged 15–39 years by age group, TennCare 2006–2014.
| Age Group, Years | Overall IR | 2006 IR | 2007 IR | 2008 IR | 2009 IR | 2010 IR | 2011 IR | 2012 IR | 2013 IR | 2014 IR |
|---|---|---|---|---|---|---|---|---|---|---|
| Female | ||||||||||
| 15–19 | 2.2 | 3.1 | 3.1 | 2.7 | 2.6 | 2.0 | 1.7 | 1.7 | 1.7 | 1.3 |
| 20–24 | 3.0 | 3.1 | 3.2 | 3.1 | 3.4 | 3.5 | 3.1 | 3.3 | 2.2 | 2.5 |
| 25–29 | 2.3 | 1.5 | 1.8 | 1.7 | 2.4 | 2.8 | 2.6 | 2.2 | 2.3 | 2.7 |
| 30–39 | 1.7 | 1.3 | 1.0 | 1.2 | 1.7 | 2.0 | 2.0 | 2.4 | 2.1 | 1.7 |
| Male | ||||||||||
| 15–19 | 0.6 | 0.5 | 0.2 | 0.5 | 0.9 | 0.8 | 0.8 | 0.6 | 0.6 | 0.5 |
| 20–24 | 1.6 | 1.2 | 1.4 | 1.1 | 2.0 | 2.1 | 1.4 | 2.6 | 1.8 | 1.4 |
| 25–29 | 1.9 | 1.1 | 1.5 | 1.5 | 1.4 | 2.8 | 1.3 | 2.8 | 2.5 | 2.2 |
| 30–39 | 2.0 | 2.0 | 1.4 | 1.3 | 2.0 | 2.4 | 2.7 | 1.6 | 2.3 | 2.3 |
IR= Incidence Rate.
aIncidence rates are per 1000 person-years.
Table A3.
Sex-specific incidencea of anogenital warts among enrollees aged 15–39 years by age group, race and urbanicity, TennCare 2006–2014.
| Characteristic | Age Group, Years | Overall IR | 2006 IR | 2007 IR | 2008 IR | 2009 IR | 2010 IR | 2011 IR | 2012 IR | 2013 IR | 2014 IR |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Female | |||||||||||
| Race | |||||||||||
| White | 15–24 | 2.7 | 3.3 | 3.1 | 2.8 | 3.0 | 2.7 | 2.4 | 2.4 | 2.1 | 1.8 |
| 25–39 | 2.3 | 1.8 | 1.7 | 1.7 | 2.4 | 2.5 | 2.8 | 2.4 | 2.6 | 2.5 | |
| Black | 15–24 | 2.8 | 2.8 | 3.4 | 3.2 | 3.3 | 3.1 | 2.4 | 2.7 | 2.0 | 1.8 |
| 25–39 | 1.4 | 0.8 | 0.6 | 1.0 | 1.4 | 1.9 | 1.5 | 2.0 | 1.7 | 1.4 | |
| Urbanicityb | |||||||||||
| MSA | 15–24 | 2.6 | 3.0 | 3.2 | 3.0 | 2.9 | 2.7 | 2.3 | 2.5 | 1.8 | 1.7 |
| 25–39 | 1.9 | 1.3 | 1.3 | 1.5 | 2.0 | 2.3 | 2.2 | 2.2 | 2.1 | 2.1 | |
| Non-MSA | 15–24 | 2.6 | 3.3 | 3.3 | 2.5 | 2.9 | 2.3 | 2.3 | 2.1 | 2.2 | 2.3 |
| 25–39 | 2.1 | 1.8 | 1.4 | 1.1 | 1.9 | 2.6 | 2.4 | 2.9 | 2.5 | 2.2 | |
| Male | |||||||||||
| Race | |||||||||||
| White | 15–24 | 0.8 | 0.7 | 0.6 | 0.8 | 1.3 | 1.2 | 0.9 | 0.8 | 0.6 | 0.4 |
| 25–39 | 1.9 | 1.7 | 1.6 | 1.3 | 1.9 | 2.6 | 1.9 | 1.7 | 2.4 | 2.5 | |
| Black | 15–24 | 0.9 | 0.7 | 0.5 | 0.7 | 1.4 | 1.5 | 1.2 | 1.1 | 0.7 | 0.9 |
| 25–39 | 1.9 | 1.2 | 1.2 | 1.6 | 1.5 | 2.0 | 2.7 | 3.1 | 2.9 | 2.0 | |
| Urbanicityb | |||||||||||
| MSA | 15–24 | 0.9 | 0.7 | 0.6 | 0.7 | 1.2 | 1.2 | 0.9 | 1.1 | 1.1 | 0.8 |
| 25–39 | 2.0 | 1.7 | 1.5 | 1.4 | 1.8 | 2.3 | 2.2 | 2.2 | 2.4 | 2.3 | |
| Non-MSA | 15–24 | 0.7 | 0.6 | 0.5 | 0.6 | 1.2 | 1.0 | 0.8 | 1.0 | 0.4 | 0.6 |
| 25–39 | 1.9 | 1.8 | 1.1 | 1.2 | 1.9 | 2.9 | 2.4 | 1.5 | 2.4 | 2.3 | |
IR= Incidence Rate; MSA = Metropolitan Statistical Area.
aIncidence rates are per 1000 person-years.
bUrbanicity was categorized by the enrollee's county of residence using MSA definitions and boundaries set by the US Census Bureau, which classifies MSAs as counties associated with at least one urbanized area that has a population of at least 50,000 persons.
Table A4.
Sex-specific trends in anogenital wart incidence among enrollees aged 15–39 years by age group, race, and urbanicity, TennCare 2006–2014.
| Characteristicss | Age Group, Years | Inflection Year | Time Period | Annual Percent Changea (95% CI) | APC p-Value | Average Annual Percent Changeb (95% CI) | AAPC p-Value |
|---|---|---|---|---|---|---|---|
| Female | |||||||
| Race | |||||||
| White | 15–24 | – | 2006–2014 | −6.5 (−8.0, −5.0) | < 0.01 | – | – |
| 25–39 | – | 2006–2014 | 5.4 (1.6, 9.3) | 0.01 | – | – | |
| Black | 15–24 | 2009 | 2006–2009 | 3.4 (−9.4, 18.1) | 0.52 | −6.1 (−10.2, −1.9) | 0.01 |
| 2009–2014 | −11.5 (−16.6, −6.0) | 0.01 | |||||
| 25–39 | 2010 | 2006–2010 | 32.4 (7.1, 63.7) | 0.02 | 13.0 (2.9, 24.1) | 0.01 | |
| 2010–2014 | −3.5 (−17.8, 13.3) | 0.57 | |||||
| Urbanicityc | |||||||
| MSA | 15–24 | 2012 | 2006–2012 | −5.1 (−8.5, −1.5) | 0.02 | −8.5 (−15.0, −1.5) | 0.02 |
| 2012–2014 | −18.0 (−45.4, 22.9) | 0.25 | |||||
| 25–39 | 2010 | 2006–2010 | 18.1 (8.6, 28.3) | 0.01 | 7.5 (3.6, 11.6) | < 0.01 | |
| 2010–2014 | −2.1 (−8.1, 4.4) | 0.41 | |||||
| Non-MSA | 15–24 | 2012 | 2006–2012 | −7.5 (−9.4, −5.5) | < 0.01 | −4.7 (−9.6, 0.4) | 0.07 |
| 2012–2014 | 4.1 (−22.2, 39.2) | 0.72 | |||||
| 25–39 | – | 2006–2014 | 6.6 (−0.5, 14.2) | 0.07 | – | – | |
| Male | |||||||
| Race | |||||||
| White | 15–24 | 2010 | 2006–2010 | 19.4 (4.3, 36.7) | 0.02 | −9.8 (−16.8, −2.1) | 0.01 |
| 2010–2014 | −31.8 (−43.5 -17.8) | 0.01 | |||||
| 25–39 | – | 2006–2014 | 11.9 (6.7, 17.4) | < 0.01 | – | – | |
| Black | 15–24 | 2010 | 2006–2010 | 29.5 (−2.5, 71.9) | 0.07 | 1.2 (−12.8, 17.6) | 0.87 |
| 2010–2014 | −20.8 (−42.3, 8.6) | 0.11 | |||||
| 25–39 | – | 2006–2014 | 15.9 (8.6, 23.6) | < 0.01 | – | – | |
| Urbanicityc | |||||||
| MSA | 15–24 | – | 2006–2014 | 4.1 (−3.1, 11.7) | 0.23 | – | – |
| 25–39 | – | 2006–2014 | 5.9 (2.2, 9.7) | 0.01 | – | – | |
| Non-MSA | 15–24 | 2010 | 2006–2010 | 22.9 (−1.2, 52.9) | 0.06 | −0.01 (−10.7, 12.0) | 1.00 |
| 2010–2014 | −18.7 (−35.7, 2.9) | 0.07 | |||||
| 25–39 | – | 2006–2014 | 5.6 (−2.9, 14.8) | 0.17 | – | – | |
AAPC = Average annual percent change; APC = Annual Percent Change; MSA = Metropolitan Statistical Area.
Boldface indicates statistical significance (p < 0.05).
aAnnual percent changes were determined by the β-coefficient of the best fit log-linear model using a permutation test and Poisson variance; The AAPC was only reported if inflection years were detected; otherwise, only the APC was reported.
bAverage annual percent changes from 2006-2014 are weighted averages of the annual percent changes of all time periods.
cUrbanicity was categorized by the enrollee's county of residence using MSA definitions and boundaries set by the US Census Bureau, which classifies MSAs as counties associated with at least one urbanized area that has a population of at least 50,000 persons.
References
- 1.Satterwhite C.L., Torrone E., Meites E., Dunne E.F., Mahajan R., Ocfemia M.C.B., Su J., Xu F., Weinstock H. Sexually transmitted infections among US women and men: prevalence and incidence estimates, 2008. Sex. Transm. Dis. 2013;40:187–193. doi: 10.1097/OLQ.0b013e318286bb53. [DOI] [PubMed] [Google Scholar]
- 2.Braaten K.P., Laufer M.R. Human papillomavirus (HPV), HPV-related disease, and the HPV vaccine. Rev. Obstet. Gynecol. 2008;1:2–10. [PMC free article] [PubMed] [Google Scholar]
- 3.Saraiya M., Unger E.R., Thompson T.D., Lynch C.F., Hernandez B.Y., Lyu C.W., Steinau M., Watson M., Wilkinson E.J., Hopenhayn C., Copeland G., Cozen W., Peters E.S., Huang Y., Saber M.S., Altekruse S., Goodman M.T. HPV Typing of Cancers Workgroup, US assessment of HPV types in cancers: implications for current and 9-valent HPV vaccines. J. Natl. Cancer Inst. 2015;107:djv086. doi: 10.1093/jnci/djv086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walker T.Y., Elam-Evans L.D., Singleton J.A., Yankey D., Markowitz L.E., Fredua B., Williams C.L., Meyer S.A., Stokley S. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 Years - United States, 2016. MMWR Morb. Mortal. Wkly. Rep. 2017;66:874–882. doi: 10.15585/mmwr.mm6633a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reagan-Steiner S., Yankey D., Jeyarajah J., Elam-Evans L.D., Curtis C.R., MacNeil J., Markowitz L.E., Singleton J.A. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years - United States, 2015. MMWR Morb. Mortal. Wkly. Rep. 2016;65:850–858. doi: 10.15585/mmwr.mm6533a4. [DOI] [PubMed] [Google Scholar]
- 6.Oriel J.D. Natural history of genital warts. Br. J. Vener. Dis. 1971;47:1–13. doi: 10.1136/sti.47.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garland S.M., Steben M., Sings H.L., James M., Lu S., Railkar R., Barr E., Haupt R.M., Joura E.A. Natural history of genital warts: analysis of the placebo arm of 2 randomized phase III trials of a quadrivalent human papillomavirus (types 6, 11, 16, and 18) vaccine. J. Infect. Dis. 2009;199:805–814. doi: 10.1086/597071. [DOI] [PubMed] [Google Scholar]
- 8.Ylitalo N., Josefsson A., Melbye M., Sörensen P., Frisch M., Andersen P.K., Sparén P., Gustafsson M., Magnusson P., Pontén J., Gyllensten U., Adami H.O. A prospective study showing long-term infection with human papillomavirus 16 before the development of cervical carcinoma in situ. Cancer Res. 2000;60:6027–6032. [PubMed] [Google Scholar]
- 9.Burd E.M. Human papillomavirus and cervical cancer. Clin. Microbiol. Rev. 2003;16:1–17. doi: 10.1128/CMR.16.1.1-17.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yanofsky V.R., Patel R.V., Goldenberg G. Genital warts: a comprehensive review. J. Clin. Aesthet. Dermatol. 2012;5:25–36. [PMC free article] [PubMed] [Google Scholar]
- 11.Petrosky E., Bocchini J.A., Hariri S., Chesson H., Curtis C.R., Saraiya M., Unger E.R., Markowitz L.E., Centers for Disease Control and Prevention (CDC) Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the advisory committee on immunization practices. MMWR Morb. Mortal. Wkly. Rep. 2015;64:300–304. [PMC free article] [PubMed] [Google Scholar]
- 12.Markowitz L.E., Dunne E.F., Saraiya M., Chesson H.W., Curtis C.R., Gee J., Bocchini J.A., Jr., Unger E.R. Human papillomavirus vaccination: recommendations of the advisory committee on immunization practices (ACIP) Morb. Mortal. Wkly. Rep. Recomm. Rep. 2014;63:1–30. [PubMed] [Google Scholar]
- 13.Bruni L., Diaz M., Barrionuevo-Rosas L., Herrero R., Bray F., Bosch F.X., de Sanjosé S., Castellsagué X. Global estimates of human papillomavirus vaccination coverage by region and income level: a pooled analysis. Lancet Glob. Health. 2016;4 doi: 10.1016/S2214-109X(16)30099-7. e453–e463. [DOI] [PubMed] [Google Scholar]
- 14.Garland S.M., Kjaer S.K., Muñoz N., Block S.L., Brown D.R., DiNubile M.J., Lindsay B.R., Kuter B.J., Perez G., Dominiak-Felden G., Saah A.J., Drury R., Das R., Velicer C. Impact and effectiveness of the quadrivalent human papillomavirus vaccine: a systematic review of 10 years of real-world experience. Clin. Infect. Dis. 2016;63:519–527. doi: 10.1093/cid/ciw354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee L., Garland S.M. vol. 6. 2017. (Human Papillomavirus Vaccination: the Population Impact). F1000Research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drolet M., Bénard É., Boily M.-C., Ali H., Baandrup L., Bauer H., Beddows S., Brisson J., Brotherton J.M.L., Cummings T., Donovan B., Fairley C.K., Flagg E.W., Johnson A.M., Kahn J.A., Kavanagh K., Kjaer S.K., Kliewer E.V., Lemieux-Mellouki P., Markowitz L., Mboup A., Mesher D., Niccolai L., Oliphant J., Pollock K.G., Soldan K., Sonnenberg P., Tabrizi S.N., Tanton C., Brisson M. Population-level impact and herd effects following human papillomavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect. Dis. 2015;15:565–580. doi: 10.1016/S1473-3099(14)71073-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tenn Code Ann. § 4-1-201 (Lexis Advance through 2017 Regular Session (Chapter 493)), (n.d).
- 18.US Census Bureau Geography . 2010. Geographic Terms and Concepts - Core Based Statistical Areas and Related Statistical Areas.https://www.census.gov/geo/reference/gtc/gtc_cbsa.html (n.d) [Google Scholar]
- 19.ICD - ICD-9-CM - International Classification of Diseases Ninth Revision, Clinical Modification. https://www.cdc.gov/nchs/icd/icd9cm.htm (n.d.)
- 20.CPT® (Current Procedural Terminology) https://www.ama-assn.org/practice-management/cpt-current-procedural-terminology American Medical Association, (n.d.)
- 21.Center for Drug Evaluation and Research Drug Approvals and Databases - National Drug Code Directory. https://www.fda.gov/Drugs/InformationOnDrugs/ucm142438.htm (n.d.)
- 22.Average Annual Percent Change (AAPC) Joinpoint Help System 4.5.0.1. https://surveillance.cancer.gov/help/joinpoint/setting-parameters/method-and-parameters-tab/average-annual-percent-change-aapc (n.d.)
- 23.Meites E. Use of a 2-dose schedule for human papillomavirus vaccination — updated recommendations of the advisory committee on immunization practices. MMWR Morb. Mortal. Wkly. Rep. 2016;65 doi: 10.15585/mmwr.mm6549a5. [DOI] [PubMed] [Google Scholar]
- 24.Reagan-Steiner S., Yankey D., Jeyarajah J., Elam-Evans L.D., Singleton J.A., Curtis C.R., MacNeil J., Markowitz L.E., Stokley S. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years - United States, 2014. MMWR Morb. Mortal. Wkly. Rep. 2015;64:784–792. doi: 10.15585/mmwr.mm6429a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oakley F., Desouki M.M., Pemmaraju M., Gargano J.M., Markowitz L.E., Steinau M., Unger E.R., Zhu Y., Fadare O., Griffin M.R. Trends in high-grade cervical cancer precursors in the human papillomavirus vaccine era. Am. J. Prev. Med. 2018;55:19–25. doi: 10.1016/j.amepre.2018.03.015. [DOI] [PubMed] [Google Scholar]
- 26.Flagg E.W., Torrone E.A. Declines in anogenital warts among age groups most likely to be impacted by human papillomavirus vaccination, United States, 2006-2014. Am. J. Public Health. 2017 doi: 10.2105/AJPH.2017.304119. e1–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Flagg E.W., Schwartz R., Weinstock H. Prevalence of anogenital warts among participants in private health plans in the United States, 2003-2010: potential impact of human papillomavirus vaccination. Am. J. Public Health. 2013;103:1428–1435. doi: 10.2105/AJPH.2012.301182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bauer H.M., Wright G., Chow J. Evidence of human papillomavirus vaccine effectiveness in reducing genital warts: an analysis of California public family planning administrative claims data, 2007-2010. Am. J. Public Health. 2012;102:833–835. doi: 10.2105/AJPH.2011.300465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perkins R.B., Legler A., Hanchate A. Trends in male and female genital warts among adolescents in a safety-net health care system 2004–2013: correlation with introduction of female and male human papillomavirus vaccination. Sex. Transm. Dis. 2015;42:665–668. doi: 10.1097/OLQ.0000000000000369. [DOI] [PubMed] [Google Scholar]
- 30.Dominiak-Felden G., Gobbo C., Simondon F. Evaluating the early benefit of quadrivalent HPV vaccine on genital warts in Belgium: a cohort study. PLoS One. 2015;10 doi: 10.1371/journal.pone.0132404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Donovan B., Franklin N., Guy R., Grulich A.E., Regan D.G., Ali H., Wand H., Fairley C.K. Quadrivalent human papillomavirus vaccination and trends in genital warts in Australia: analysis of national sentinel surveillance data. Lancet Infect. Dis. 2011;11:39–44. doi: 10.1016/S1473-3099(10)70225-5. [DOI] [PubMed] [Google Scholar]
- 32.Smith M.A., Liu B., McIntyre P., Menzies R., Dey A., Canfell K. Trends in genital warts by socioeconomic status after the introduction of the national HPV vaccination program in Australia: analysis of national hospital data. BMC Infect. Dis. 2016;16 doi: 10.1186/s12879-016-1347-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu P.-J., Rodriguez-Lainz A., O'Halloran A., Greby S., Williams W.W. Adult vaccination disparities among foreign-born populations in the U.S., 2012. Am. J. Prev. Med. 2014;47:722–733. doi: 10.1016/j.amepre.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kjær S.K., Trung Nam T., Sparen P., Tryggvadottir L., Munk C., Dasbach E., Liaw K.-L., Nygård J., Nygård M. The burden of genital warts: a study of nearly 70,000 women from the general female population in the 4 Nordic countries. J. Infect. Dis. 2007;196:1447–1454. doi: 10.1086/522863. [DOI] [PubMed] [Google Scholar]
- 35.Gravitt P.E., Rositch A.F., Silver M.I., Marks M.A., Chang K., Burke A.E., Viscidi R.P. A cohort effect of the sexual revolution may be masking an increase in human papillomavirus detection at menopause in the United States. J. Infect. Dis. 2013;207:272–280. doi: 10.1093/infdis/jis660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ali H., Donovan B., Wand H., Read T.R.H., Regan D.G., Grulich A.E., Fairley C.K., Guy R.J. Genital warts in young Australians five years into national human papillomavirus vaccination programme: national surveillance data. BMJ. 2013;346:f2032. doi: 10.1136/bmj.f2032. [DOI] [PubMed] [Google Scholar]
- 37.Barbaro B., Brotherton J.M.L. Assessing HPV vaccine coverage in Australia by geography and socioeconomic status: are we protecting those most at risk? Aust. N. Z. J. Public Health. 2014;38:419–423. doi: 10.1111/1753-6405.12218. [DOI] [PubMed] [Google Scholar]
- 38.Guerino P., James C. Centers for Medicare and Medicaid Services, Office of Minority Health; 2017. Detailed Race, Ethnicity, and Language Preference in the Health Insurance Marketplaces. 2017. [Google Scholar]
- 39.Lindley M.C., Smith P.J., Rodewald L.E. Vaccination coverage among U.S. adolescents aged 13–17 years eligible for the Vaccines for Children Program, 2009. Publ. Health Rep. 2011;126:124–134. doi: 10.1177/00333549111260S214. [DOI] [PMC free article] [PubMed] [Google Scholar]