Abstract
To evaluate, by Magnetic Resonance Imaging, if there is a typical pattern or severity of PRES in transplanted children for hemoglobinopathy. Secondary point was to investigate the pattern and severity of PRES in children with thalassemia-THAL and sickle-cell disease-SCD after autologous hematopoietic stem cell transplantation (aHSCT). Finally, we evaluate the presence of atypical PRES presentation and the involved area of central nervous system. Two neuroradiologists analyzed retrospectively MRI of 21 transplanted children for THAL or SCD treated with CI, with neurological symptoms and signs of PRES. The Bartynski and Boardman classification has been used for PRES pattern while McKinney scale for PRES severity. Fisher Exact Probability test or Chi-square test were used to compare the categorical data. In the 21 transplanted children the PRES severity was typically mild (85.7%) without preferring radiological pattern at MRI. The analysis didn't show significant association between PRES pattern or PRES severity and previous hemoglobinopathy (THAL or SCD). No atypical PRES presentation has been found. PRES severity in transplanted children for hemoglobinopathy is typically mild. Notwithstanding children affected by SCD have a damage on the capillary endothelium, after aHSCT our data didn't show a different PRES severity and pattern than THAL children.
Keywords: PRES, aHSCT, MRI, Thalassemia, Sickle-cell disease, Hemoglobinopathy
1. Introduction
Hemoglobinopathies are hereditary diseases determining production defects of hemoglobin and can be divided into quantitative (e.g. thalassemia: THAL) or qualitative (e.g. sickle-cell disease: SCD) defects. THAL and SCD involved more than 330.00 newborns per year in the world; the World Health Organization has reported that hemoglobinopathies are a growing health problem in 71% of 229 countries, specially in low and middle-income countries [1], being the main causes of hereditary hemolytic anemias, and they lead to chronic complications with several implications on patient’s quality life and early death. Even though transfusion therapy is able to increase the life expectancy in these patients, hematopoietic stem cell transplantation (HSCT) is the only well-established curative treatment for THAL and SCD [2,3].
After HSCT, to avoid the Graft-versus-Host-Disease (GvHD), is necessary a prophylactic treatment with calcineurin inhibitors (CI) as cyclosporin and tacrolimus; however these drugs lead to several adverse effects such as neurotoxicity and Posterior Reversible Encephalopathy Syndrome (PRES).
The purpose of this study is to investigate, by magnetic resonance imaging (MRI), the severity and pattern of PRES in transplanted children for hemoglobinopathies. Secondary point is analyzed if transplanted children with SCD have particular radiological features or a more severe PRES than transplanted children previous affected by THAL when both of group underwent to aHSCT and CI therapy. As last we evaluated the most affected cerebral lobes and infratentorial structures (brainstem and cerebellum) and we performed a qualitatively analysis of MR images.
2. Material and methods
We enrolled in this retrospective neuroimaging study 21 pediatric patients in therapy with CI after HSCT for THAL or SCD, with clinical sign of PRES.
The study was approved by the “……. Review Board”. The parents of all patients provided a written informed consent in accordance with the Declaration of Helsinki and its later amendments. Moreover, National laws have been observed.
2.1. Patients
Of 281 transplants performed for THAL or SCD at the “Mediterrean Institute of Hematology” of University Hospital of Rome Tor Vergata 31 patients treated with CI for GVHD, have had the onset of neurological sign and symptoms highly suspected of PRES such as seizures, mental state alterations, headache, tremors and blurred vision. Diagnosis of PRES related to CI was made on:
-
1)
typical neurological symptoms (e.g. acute headache, seizures, mental and visual alterations);
-
2)
supra and infratentorial lesions suspicion for vasogenic edema at MR imaging;
-
3)
exclusion of other possible causes (e.g. infections, metabolic disorders, hemorrhagic diseases);
-
4)
resolution of neurological signs and symptoms with stop of CI administration.
Based on the abovementioned criteria, the MRI of 21 children, with clinical diagnosis of PRES, have been considered for the analysis. The study group was made up by 15 male and 6 females, with age between 4 and 16years (mean age: 9.5 years old). Of these 21 transplanted children 8 were affected by SCD and 13 by THAL.
All of 21 children had high values of blood pressure which required antihypertensive therapy.
The demographics features and the results of diagnostic imaging are summarized in Table 1.
Table 1.
Characteristics of transplanted children with respective PRES imaging pattern and severity at Magnetic Resonance Imaging at day 0 and at discharge.
| N° | Age | Sex | Haemoglobinopathy | Days from aHSCT to PRES | Pattern | Severity | MRI findings | MRI at discharge |
|---|---|---|---|---|---|---|---|---|
| 1 | 16 | F | Thalassemia | 13 | Superior Frontal Sulcus | Mild | T2 and T2-FLAIR hyperintensity in cortical-subcortical white matter of frontal lobes | No alterations |
| 2 | 5 | F | Thalassemia | 10 | Superior Frontal Sulcus | Mild | T2 and T2-FLAIR hyperintensity in cortical-subcortical white matter of frontal and parietal lobes | No alterations |
| 3 | 11 | M | Thalassemia | 11 | Holohemispheric Watershed | Mild | T2 and T2-FLAIR hyperintensity in cortical-subcortical white matter of frontal, parietal and occipital lobes | Slight hyperintensity on DWI at discharge |
| 4 | 7 | M | Thalassemia | 16 | Holohemispheric Watershed | Mild | T2 and T2-FLAIR hyperintensity in cortical-subcortical white matter of frontal, parietal and occipital lobes. Focal hypointensity on T2-FFE in the left parietal lobe. After administration of Gd-contrast agent diffuse subcortical and patchy enhancement | Laminar necrosis in left parietal lobe |
| 5 | 9 | F | Thalassemia | 11 | Dominant Parietal-Occipital | Mild | T2 and T2-FLAIR hyperintensity in subcortical white matter of the parietal and occipital lobes with slight hyperintensity on DWI | No alterations |
| 6 | 12 | M | Thalassemia | 115 | Holohemispheric Watershed | Moderate | T2 and T2-FLAIR hyperintensity in cortical-subcortical white matter of frontal, parietal and occipital lobes. T2 hyperintensity in cerebellum and left thalamus | No alterations |
| 7 | 3 | M | Thalassemia | 158 | Dominant Parietal-Occipital | Mild | T2 and T2-FLAIR hyperintensity in subcortical white matter of the parietal and occipital lobes | No alterations |
| 8 | 7 | M | Thalassemia | 13 | Holohemispheric Watershed | Mild | T2 and T2-FLAIR hyperintensity in cortical-subcortical white matter of the frontal, parietal and occipital lobes. DWI hyperintensity in cortical and subcortical white matter | No alterations |
| 9 | 11 | M | Thalassemia | 82 | Superior Frontal Sulcus | Mild | T2 and T2-FLAIR hyperintensity in cortical-subcortical white matter of the frontal and parietal lobes | No alterations |
| 10 | 7 | M | Thalassemia | 45 | Superior Frontal Sulcus | Mild | T2 and T2-FLAIR hyperintensity in subcortical white matter of the parietal lobes and in the left frontal lobe | No alterations |
| 11 | 4 | M | Thalassemia | 170 | Dominant Parietal-Occipital | Mild | T2 and T2-FLAIR hyperintensity in subcortical white matter of the parietal and occipital lobes | No alterations |
| 12 | 4 | M | Thalassemia | 208 | Holohemispheric Watershed | Mild | T2 and T2-FLAIR hyperintensity in subcortical white matter of the frontal, temporal and parietal lobes bilaterally, with less involvement of occipital lobes | No alterations |
| 13 | 5 | M | Thalassemia | 110 | Holohemispheric Watershed | Moderate | T2 and T2-FLAIR hyperintensity in subcortical white matter in the frontal, temporal, parietal and occipital lobes bilaterally. T2 and T2-FLAIR hyperintensity in the right thalamus | Several reductions of T2 hyperintensity with slight DWI alteration |
| 14 | 5 | M | Sickle-cell disease | 73 | Dominant Parietal-Occipital | Mild | T2 and T2-FLAIR hyperintensity in subcortical white matter in the occipital and parietal lobes | No alterations |
| 15 | 15 | F | Sickle-cell disease | 13 | Holohemispheric Watershed | Moderate | T2, T2-FLAIR and DWI hyperintensity in cortical-subcortical white matter of frontal, temporal, parietal and occipital lobes | No alterations |
| 16 | 16 | M | Sickle-cell disease | 7 | Dominant Parietal-Occipital | Mild | T2 and T2-FLAIR hyperintensity in subcortical white matter in the occipital and parietal lobes. T2 hyperintensity in the left internal capsule | Slight T2 alterations |
| 17 | 15 | M | Sickle-cell disease | 11 | Superior Frontal Sulcus | Mild | T2 and T2-FLAIR hyperintensity in subcortical white matter in the frontal, occipital and parietal lobes with slight T2 hyperintensity in temporal lobes | No alterations |
| 18 | 16 | M | Sickle-cell disease | 71 | Dominant Parietal-Occipital | Mild | T2 and T2-FLAIR hyperintensity in subcortical white matter in parietal lobes with less involvement of the occipital lobes | No alterations |
| 19 | 9 | F | Sickle-cell disease | 8 | Dominant Parietal-Occipital | Mild | T2 and T2-FLAIR hyperintensity in subcortical white matter in occipital lobes with less involvement of the parietal lobes | No alterations |
| 20 | 9 | F | Sickle-cell disease | 28 | Dominant Parietal-Occipital | Mild | T2 and T2-FLAIR hyperintensity in subcortical white matter in occipital lobes with DWI hyperintensity | No alterations |
| 21 | 12 | M | Sickle-cell disease | 69 | Superior Frontal Sulcus | Mild | T2 and T2-FLAIR hyperintensity in cortical-subcortical white matter of the frontal, parietal and occipital lobes | No alterations |
aHSCT: Autologous Haematopoietic Stem Cell Transplantation; FLAIR: FLuid Attenuated Inversion Recovery; DWI: Diffusion Weighted Imaging; FFE: Fast Field Echo; Gd: Gadolinium.
2.2. MR imaging protocol
The MRI was performed by 1.5 T MR scanner (Intera; Philips Medical System, Best, The Netherlands) or 3.0 MR scanner (Achieva; Philips Medical System, Best, The Netherlands) with the 8-channel head coil for the 21 children patients who arrived to emergency room of ….
The MR protocol included Diffusion Weighted Imaging-DWI (TE:89ms; TR: 3023 ms), an unenhanced T1 Turbo Spin Echo (TE:15ms; TR: 597 ms), a T2 Fluid Attenuated Inversion Recovery-FLAIR (TE:120ms; TR:6000ms) and a T2 Turbo Spin Echo (TE:110 ms; TR: 4868 ms) and T2 Fast Field Echo (TE:16ms; TR:724ms) all on axial plane, section thickness: 4 mm; slice gap: 1 mm and total scan time of about 15 min. Only in 12 patients has been acquired a T1 Turbo Spin Echo (TE: 10 ms; TR: 2000 ms) after administration of contrast media (Gadoterate Meglumine).
After 30 days the 21 patients performed a MR imaging before discharge.
2.3. Analysis of imaging
Image analysis was performed independently by two neuroradiologists (…), not blinded about patient’s clinical features, with at least 8 years of experience in neuroradiological field. The three-topographic patterns of Bartynski and Boardman have been used for classifying the PRES patterns [4] while the McKinney scale, based on T2-FLAIR extension, was used to classify the severity of PRES [5,6].
2.4. Statistical analysis
The data from this retrospective study were described as absolute frequencies and percentages for categorical variables and median for continuous variables. The association between categorical variables were evaluated with Fisher Exact Probability test or Chi-square test, because the total of our sample was small, N = 21. A p-value <0.05 was considered as statistically significant. Matlab version 9.3.0, Release 2017b (The MathWorks, Natick, MA, USA) was used for the analysis data.
3. Results
Between July 2004 and April 2016, of 281 transplanted children for haemoglobinopathies 31 (11%) developed PRES. 21 of these 31 transplanted children have been hospitalized at University Hospital of Rome Tor Vergata and underwent to MR examination. PRES was developed between 6 and 208 days from the aHSCT (mean value: 55days) in patients treated with CI.
The severity of PRES in the 21 transplanted children was mild for 18 patients (85.7%) and moderate in 3 patients (14.3%); none patient had a severe form of PRES (p-value = 0.002).
About the pattern of PRES, following the Bartynski and Boardman classification, 6 (28.6%) transplanted children showed the “Holohemispheric Watershed Pattern”, 6 (28.6%) the “Partial or Asymmetric Expression”, 5 (23.8%) had the “Dominant Parietal-Occipital Pattern” and 4 (19%) had the “Superior Frontal Sulcus Pattern” (Fig. 1). The pattern of PRES has been not significant statistically.
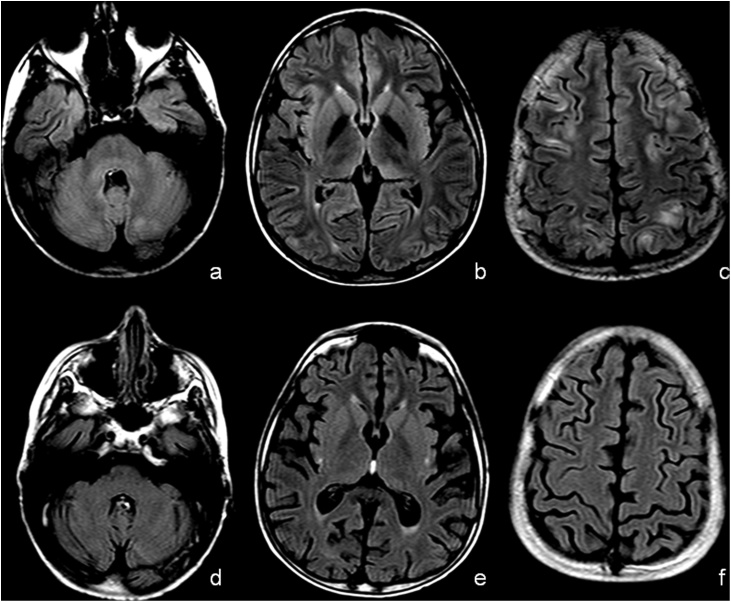
Fig. 1.
Mild PRES with Superior Frontal Sulcus pattern in 15 years-old male child previous affected by Sickle Cells Disease. T2-Fluid Attenuated Inversion Recovery (FLAIR) at basal ganglia level (a and c) and over the semioval centers (b and d). The MRI performed at day 0 (a and b) showed T2-FLAIR hyperintensity “U-fibers” of right occipital lobe and into iuxta-cortical white matter of left frontal lobe. At day 30 (c and d) MRI showed no signal abnormalities.
The parietal lobes were the most involved by T2 hyperintensity (16 patients) followed by the occipital (13 patients) and frontal lobes (12 patients). The temporal lobes have been less involved by T2 hyperintensity (4 patients). 2 children showed involvement of thalami and cerebellum (Fig. 2), a child showed hyperintensity in the internal capsule. None of them showed abnormal signal intensity in the brain stem. T2-hyperintensithy was typically localized at subcortical white matter (“U”-fibers). No midline shift or herniation has been detected.
Fig. 2.
Moderate PRES with Holohemispheric Watershed pattern in 12 years-old male child previous affected by Thalassemia. T2-Fluid Attenuated Inversion Recovery (FLAIR) at cerebellum (a and d), basal ganglia (b and e) and over the semioval centers (c and f). The MRI performed at day 0 (a, b and c) showed T2-FLAIR hyperintensity in cerebellum, slightly at pons, into left thalamus and at “U-fibers” in the frontal and parietal lobes. At day 28 (d, e and f) MRI showed no signal abnormalities.
By qualitative analysis of DWI in the 21 patients emerged that 9 showed restricted diffusion while 12 patients had no alterations. Also, in 11 patients we added to MR protocol T2-FFE on axial plane and T1 after administration of contrast medium on axial, sagittal and coronal plane; just 1 of these 11 patients showed T2 hypointensity in the T2-FFE with patchy enhancement on the T1 after contrast medium (Fig. 3, Fig. 4).
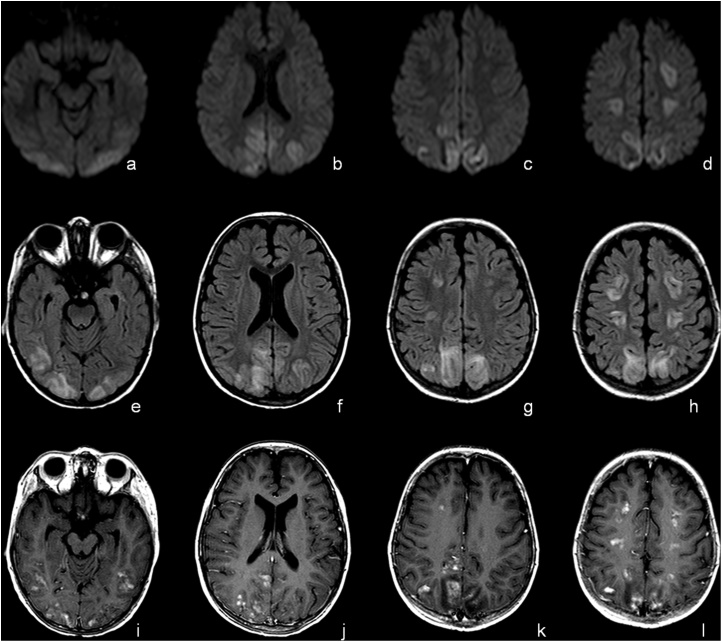
Fig. 3.
Mild PRES with Holohemispheric Watershed pattern in 7 years-old male child previous affected by Thalassemia. MRI at day 0. Diffusion Weighted Imaging (DWI), T2-FLAIR and T1 after administration of contrast media at mid-brain (a, e and i), lateral ventricles (b, f and j), into (c, g and k) and over (d, h and l) semioval centers. The exam showed T2-FLAIR hyperintensity in cortical-subcortical white matter of frontal, parietal and occipital lobes with alteration on Diffusion Weighted Imaging (pseudorestriction). Focal After administration of Gd-contrast agent diffuse subcortical and patchy enhancement has been detected.
Fig. 4.
MRI at day 32 of the same patients showed in Fig. 3. The MRI showed reduction of signal abnormalities on Diffusion Weighted Imaging (a, b, c, d) and T2-FLAIR (e, f, g, h) imaging. After administration of Gd-contrast agent no enhancement has been detected (i, j, k, l). T2-Fast Field Echo imaging (m, n, o, p) showed focal hypointensity at cortical-subcortical white matter of left parietal lobe (o) with moderate atrophy of left superior parietal lobule gyrus.
Moreover, the 21 patients have been divided following the previous haemoglobinopathy in THAL (13 patients) and SCD (8 patients) and we have evaluated the severity and pattern of PRES referring to the previous haemoglobinopathy (THAL and SCD): the results showed no significant association between the previous haemoglobinopathy and pattern or severity.
At last, 15 patients have had a short-term follow-up by MR which showed resolution of the T2-hyperintensity in 9 patients, while 5 patients have been yet T2-hyperintensity at hospital discharges and 1 patient had cortical laminar necrosis (pseudolaminar necrosis) with moderate atrophy in the parietal lobe: this result is in accordance with previous literature [7].
4. Discussion
The hemoglobinopathies are endemic disorders in Mediterranean area, Africa, Asia and Europe [8,9], with prevalence rate that varies for different countries and in different areas of the same country. The HSCT is the only well-established curative treatment [2,3] but after transplant is necessary a prophylactic treatment with CI (e.g. cyclosporin and tacrolimus) to avoid the GvHD; however these drugs lead to several adverse effects such as neurotoxicity and PRES. PRES, also known as Hypertensive Encephalopathy, was for the first time described by Hinckey in 1996 [10] and it is a hypertensive encephalopathy characterized by neurological symptoms as headache, visual disturbances, altered mental state, seizures and hypertension. Several conditions are associated with PRES as eclampsia, severe infection, prophylactic treatment for GvHD in transplanted patients, thrombotic microangiopathies and uremic syndrome [4]. Although PRES is more common in adults it may occur frequently in children with hemoglobinopathies who underwent HSCT [11,12] respect to other conditions [13,14].
PRES is characterized by a damage of endothelium due to acute hypertension with failure of central nervous system (CNS) autoregulation and disruption of blood-brain barrier which lead to vasogenic edema. PRES is more frequent in patients treated with Ciclosporin even though, in the last years, have been reported with higher frequency cases of PRES Tacrolimus-associated [15].
Although the physio-pathological mechanism of PRES is not fully understood, seems to be a straight connection between hypertension and immunosuppressive drugs in fact vasoconstriction, hypoperfusion and loss of cerebral self-regulation are the main hypothesis, maybe also related to the pharmacological treatment. Moreover, notwithstanding the low incidence of PRES, it determines remarkable mortality and morbidity [16].
It's well known the alterations and dysfunction of the capillary endothelium caused by SCD, mainly due to the polidistrectual vaso-occlusive crisis, hypoxia and chronic hemolysis [17] as well as the key role of capillary endothelium in pathophysiology of PRES [18].
Only few previous published studies have analyzed the correlation between radiological severity of PRES and its predisposing risk factors: Gaziev et al reported a more incidence of PRES in transplanted patients for SCD than THAL [19].
MR is the gold standard for diagnosis and follow-up of PRES notwithstanding it could be not performed in acute phase due to the neurological state of patients (e.g. altered mental status, seizures) therefore CT could be considered an excellent screening test for acute pathology [20].
At MR patients with PRES show T2-hyperintensity, referred to vasogenic edema, within parietal and occipital lobes with symmetrical distribution involving typically the cortical and subcortical “U”-fibers due to the own lower density; less common is the involvement of deep CNS and infratentorial structures. In according to Bartynski and Boardman classification there are four main imaging patterns:
-
1
holohemispheric at watershed zones pattern
-
2
superior frontal sulcus pattern
-
3
dominant parieto-occipital pattern
-
4
Partial or asymmetric distribution of previously listed pattern.
From the analysis of our entire population emerged that PRES severity, in transplanted children, is typically mild (85.7%) followed by moderate form (14.3%); none of our 21 transplanted children had a severe PRES. The study does not show significant statistical difference in PRES severity referring to the previous haemoglobinopathies (THAL or SCD): therefore, the previous damage on the CNS capillary endothelium, caused by drepanocytes, doesn’t seem influence the radiological severity of PRES. As corollary, we aren’t able to predict the radiological severity of cerebral involvement when symptoms of PRES arise in this population.
Regarding the three main radiological PRES patterns [4], the present study does not identify a specific pattern that significantly correlates with the previous hemoglobinopathy despite the holohemispheric watershed pattern has been more frequently found in THAL group (46%) contrary to SCD children where it was the least frequent diagnosed (13%). No partial or asymmetric distribution has been found in our children.
Morphological imaging of our entire young population showed that the parietal lobe was the most affected lobe (76%) by T2-hyperintensity, followed by occipital (62%) and frontal (57%) lobes; less affected by T2-hyperintensity has been the temporal lobe (19%). 2 transplanted children (9.5%), 1 SCD and 1 THAL, with moderate severity of PRES showed involvement of the cerebellum and thalamus with mild reduction of T2 hyperintensity at discharge maybe due to a slower recovery of deep CNS structures within vertebrobasilar system: the last result is in accord with Bartynski et al [4]. The T2 hyperintensities in pediatric transplanted children has higher incidence in the frontal and temporal lobes than adult patients: these findings agree with Donmez et al [21] who found frequently involvement of parietal and frontal lobes with almost the same incidence of T2 hyperintensities into frontal lobes.
Donmez reported an involvement of cerebellum in 48% of their population [21] while Siebert et al [22] had cerebellar involvement in more than one-third of their children. In our study court the cerebellum there has been just in 1 child (5%) while involvement of thalamus and/or internal capsule of our transplanted children there has been in 4 cases (20%).
The DWI restriction, atypical feature of PRES, was founded in 9 of 21 transplanted children (43%) and it is related to vasogenic edema which is the underlying mechanism of PRES resulting from hyperperfusion, failure of cerebral auto-regulation and blood-barrier disruption. This result is moderately greater than previously published reports in which the incidence of diffusion restriction ranges from 9.1 to 36.3% in pediatric population [[22], [23], [24]]: these data could be related to greater vulnerability of the blood-brain barrier in children associated to other risk factors (as SCD) and therefore could explain the most widespread of the T2 hyperintensity both in the white matter and in the thalami.
In our study DWI restriction was mainly localized at cortical and iuxta-cortical cerebral white matter. The qualitative analysis of apparent diffusion coefficient (ADC) showed increased of ADC which reflect the underlying vasogenic edema. The evaluation of ADC is considered to have, by Lamy et al, a prognostic value: higher ADC correlates with lesion reversibility while lower ADC values indicate with cerebral ischemia and correlate with poor clinical outcome [25].
From the analysis of T2-FFE and T1 after contrast medium just a child showed focal T2-hypointensity in the left parietal lobe, with diffuse and “patchy” occipital-parietal enhancement after contrast medium; this patient showed in the follow-up by MR of mild atrophy with pseudolaminar necrosis in the left parietal lobe. Surely, the presence of microbleeds, enhancement after administration of Gd-based contrast agent and restriction on DWI could be indicative of less good prognosis in these patients.
Our study is limited by the relatively small number of patients studied by MR and the single center experience; therefore, is necessary a multicenter study and a wide-group of patients with the same treatment schedule in order to confirm our results in particular the lack of association between radiological severity and type of hemoglobinopathies.
5. Conclusion
PRES should be taken into consideration in patients treated with calcineurin inhibitors after hematopoietic stem cell transplantation for treatment of hemoglobinopathies, such as THAL and SCD, with suggestive neurological symptoms. Although children previous affected by SCD had alterations and dysfunction of the capillary endothelium due to the drepanocytes, this previous damage on the endothelium didn’t seem to influence the severity of PRES after aHSCT and CI therapy. Moreover, the involvement of frontal lobes in quite common in these population.
Author contributions
-Study concept and design: S Marziali, J Gaziev, F Di Giuliano.
-Acquisition of data: E Picchi, V Ferrazzoli, V Da Ros.
-Analysis and interpretation of data: S Marziali, F Di Giuliano, S Minosse.
-Study supervision: F Garaci, R Floris, J Gaziev.
-Critical revision of manuscript for intellectual content: S Marziali, E Picchi. C.A. Pistolese.
Funding information
No fund has been obtained for this study.
Competing interests
The authors declare no competing interests.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
For this type of study formal consent is not required.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Disclosures
None.
References
- 1.Aguilar Martinez P. Haemoglobinopathies in Europe: health & migration policy perspectives. Orphanet J. Rare Dis. 2014;9:97. doi: 10.1186/1750-1172-9-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lucarelli G., Gaziev J. Advances in the allogeneic transplantation for thalassemia. Blood Rev. 2008;22:53–63. doi: 10.1016/j.blre.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Gaziev J. Optimal outcomes in young class 3 patients with thalassemia undergoing HLA-identical sibling bone marrow transplantation. Transplantation. 2016;100:925–932. doi: 10.1097/TP.0000000000000928. [DOI] [PubMed] [Google Scholar]
- 4.Bartynski W.S., Boardman J.F. Distinct imaging patterns and lesion distribution in posterior reversible encephalopathy syndrome. AJNR Am. J. Neuroradiol. 2007;28:1199–1206. doi: 10.3174/ajnr.A0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McKinney A.M. Posterior reversible encephalopathy syndrome: incidence of atypical regions of involvement and imaging findings. Am J Roentgenol. 2007;189:904–912. doi: 10.2214/AJR.07.2024. [DOI] [PubMed] [Google Scholar]
- 6.Casey S.O., Sampaio R.C., Michel E., Truwit C.L. Posterior reversible encephalopathy syndrome: utility of fluid-attenuated inversion recovery MR imaging in the detection of cortical and subcortical lesions. AJNR Am. J. Neuroradiol. 2000;21:1199–1206. [PMC free article] [PubMed] [Google Scholar]
- 7.Ollivier M. Neuroimaging features in posterior reversible encephalopathy syndrome: a pictorial review. J. Neurol. Sci. 2017;373:188–200. doi: 10.1016/j.jns.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 8.Modell B., Darlison M. Global epidemiology of haemoglobin disorders and derived service indicators. Bull. World Health Organ. 2008;86:480–487. doi: 10.2471/BLT.06.036673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gulbis B. Epidemiology of rare anaemias in Europe. Adv. Exp. Med. Biol. 2010;686:375–396. doi: 10.1007/978-90-481-9485-8_22. [DOI] [PubMed] [Google Scholar]
- 10.Hinchey J. Reversible posterior leukoencephalopathy syndrome. N. Engl. J. Med. 1996;334:494–500. doi: 10.1056/NEJM199602223340803. [DOI] [PubMed] [Google Scholar]
- 11.Erer B. Cs-A associated neurotoxicity and ineffective prophylaxis with clonazepam in patients transplanted for thalassemia major: analysis of risk factors. Bone Marrow Transplant. 1996;18:157–162. [PubMed] [Google Scholar]
- 12.Noè A. High incidence of severe cyclosporine neurotoxicity in children affected by haemoglobinopaties undergoing myeloablative haematopoietic stem cell transplantation: early diagnosis and prompt intervention ameliorates neurological outcome. Ital. J. Pediatr. 2010;36:14. doi: 10.1186/1824-7288-36-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wong R. Tacrolimus-associated posterior reversible encephalopathy syndrome after allogeneic haematopoietic stem cell transplantation. British Journal Hematology. 2003;122:128–134. doi: 10.1046/j.1365-2141.2003.04447.x. [DOI] [PubMed] [Google Scholar]
- 14.Zama D. Risk factor analysis of posterior reversible encephalopathy syndrome after allogeneic hematopoietic SCT in children. Bone Marrow Transplant. 2014;49:1538–1540. doi: 10.1038/bmt.2014.182. [DOI] [PubMed] [Google Scholar]
- 15.Hammerstrom A.E. Tacrolimus-associated posterior reversible encephalopathy syndrome in hematopoietic allogeneic stem cell transplantation. Am. J. Hematol. 2013;88:301–305. doi: 10.1002/ajh.23402. [DOI] [PubMed] [Google Scholar]
- 16.Sudulagunta S.R., Sodalagunta M.B., Kumbhat M., Settikere Nataraju A. Posterior reversible encephalopathy syndrome (PRES) Oxf. Med. Case Reports. 2017;3:43–46. doi: 10.1093/omcr/omx011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khademian Z., Speller-Brown B., Nouraie S.M., Minniti C.P. Reversible posterior leuko-encephalopathy in children with sickle cell disease. Pediatr. Blood Cancer. 2009;52:373–375. doi: 10.1002/pbc.21812. [DOI] [PubMed] [Google Scholar]
- 18.Fischer M., Schmutzhard E. Posterior reversible encephalopathy syndrome. J. Neurol. 2017;264:1608–1616. doi: 10.1007/s00415-016-8377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaziev J. Posterior reversible encephalopathy syndrome after hematopoietic cell transplantation in children with hemoglobinopathies. Biol. Blood Marrow Transplant. 2017;23:1531–1540. doi: 10.1016/j.bbmt.2017.05.033. [DOI] [PubMed] [Google Scholar]
- 20.Dandoy C.E. Clinical utility of computed tomography and magnetic resonance imaging for diagnosis of posterior reversible encephalopathy syndrome after stem cell transplantation in children and adolescents. Biol. Blood Marrow Transplant. 2015;21:2028–2032. doi: 10.1016/j.bbmt.2015.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Donmez F.Y., Guleryuz P., Agildere M. MRI findings in childhood PRES: what is different than the adults? Clin. Neuroradiol. 2016;26:209–213. doi: 10.1007/s00062-014-0350-2. [DOI] [PubMed] [Google Scholar]
- 22.Siebert E., Spors B., Bohner G., Endres M., Liman T.G. Posterior reversible encephalopathy syndrome in children: radiological and clinical findings – a retrospective analysis of a German tertiary care center. Eur. J. Paediatr. Neurol. 2013;17:169–175. doi: 10.1016/j.ejpn.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 23.Onder A.M. Posterior reversible encephalopathy syndrome in the pediatric renal population. Pediatr. Nephrol. 2007;22:1921–1929. doi: 10.1007/s00467-007-0578-z. [DOI] [PubMed] [Google Scholar]
- 24.Chen T.H. Posterior reversible encephalopathy syndrome in children: case series and systematic review. J. Child Neurol. 2013;28:1378–1386. doi: 10.1177/0883073813500714. [DOI] [PubMed] [Google Scholar]
- 25.Lamy C., Oppenheim C., Mas J.L. Posterior reversible encephalopathy syndrome. Handb. Clin. Neurol. 2014;121:1687–1701. doi: 10.1016/B978-0-7020-4088-7.00109-7. [DOI] [PubMed] [Google Scholar]