Abstract
Novel therapies to override chemo-radiation resistance in prostate cancer (PCa) are needed. Prostate cancer sphere-forming cells (PCSCs) (also termed prostate cancer stem-like cells) likely participate in tumor progression and recurrence, and they are important therapeutic targets. We established PCSC-enriched spheres by culturing human (DU145) and murine (TRAMP-C2) PCa cells in growth factor-defined serum-free medium, and we characterized stem-like properties of clonogenicity and tumorigenicity. The efficacy of two different oncolytic herpes simplex viruses (oHSVs) (G47Δ and MG18L) in PCSCs was tested alone and in combination with radiation; chemotherapy; and inhibitors of phosphoinositide 3-kinase (PI3K), Wnt, and NOTCH in vitro; and, G47Δ was tested with the PI3K inhibitor BKM120 in a PCSC-derived tumor model in vivo. PCSCs were more tumorigenic than serum-cultured parental cells. Human and murine PCSCs were sensitive to oHSV and BKM120 killing in vitro, while the combination was synergistic. oHSV combined with radiation, docetaxel, Wnt, or NOTCH inhibitors was not. In athymic mice bearing DU145 PCSC-derived tumors, the combination of intra-tumoral G47Δ and systemic BKM120 induced complete regression of tumors in 2 of 7 animals, and it exhibited superior anti-tumor activity compared to either monotherapy alone, with no detectable toxicity. oHSV synergizes with BKM120 in killing PCSCs in vitro, and the combination markedly inhibits tumor growth, even inducing regression in vivo.
Keywords: prostate cancer, oncolytic virus, herpes virus, G47Δ, MG18L, prostate cancer stem-like cells, PI3K inhibitor, BKM120, TrampC2, DU145
Introduction
It is estimated that there were almost 1.3 million new cases of prostate cancer and 359,000 associated deaths worldwide in 2018, ranking as the second most frequent cancer and the fifth leading cause of cancer death in men.1 Radical prostatectomy, hormone therapy, chemotherapy, and radiotherapy are effective in the initial phase of the disease, but prostate cancer (PCa) can eventually progress to invasive, drug- and castration-resistant metastatic disease.2 The cancer stem cell hypothesis posits that a subpopulation of cancer cells drives tumor growth and metastasis and is more resistant to chemo-radiotherapy than differentiated daughter cells.3 Prostate cancer sphere-forming (or stem-like) cells (PCSCs) have been isolated from human PCa biopsies and cell lines, and they self-renew in vitro.4, 5 PCSCs are thought to be important in PCa progression, heterogeneity, recurrence, and resistance to therapy.5 We define PCSCs physiologically as cells that can proliferate and self-renew in vitro as spheroids in the absence of serum and initiate tumors in vivo with increased efficiency.
Oncolytic herpes simplex viruses (oHSVs) are genetically engineered HSV that can selectively replicate in and kill cancer cells, amplifying themselves and spreading within tumors, but sparing normal tissue.6 oHSV has been safely used in human subjects with a variety of cancers,6 including PCa.7 Here we used 2 genetically distinct oHSVs: G47Δ, with deletions of both copies of the γ34.5 gene, a lacZ insertion inactivating the UL39 gene, and a deletion within the α47 gene;8 and MG18L, with a US3 deletion and an inactivating LacZ insertion in UL39.9 The safety of oHSVs for cancer therapy has been demonstrated in numerous clinical trials, and one oHSV, talimogene laherparepvec (Imlygic), is approved for the treatment of advanced melanoma.6 Our previous work demonstrated target specificity and replication competence of oHSV in human PCa cell lines and tumor specimens.10, 11, 12 We herein demonstrate the efficacy of oHSV in PCSCs and PCSC-derived tumors.
oHSV can interact synergistically with other therapeutic modalities.13, 14 We combined oHSV with chemotherapy, radiotherapy, and cancer stem cell pathway inhibitors to identify potentially synergistic interactions. We found that oHSV synergizes with the phosphoinositide 3-kinase (PI3K) pathway inhibitor BKM120 (Buparlisib) in killing PCSCs in vitro and in vivo. BKM120 is a potent and highly specific oral pan-class I PI3K inhibitor that does not inhibit mechanistic target of rapamycin (mTOR) and Vps34 kinases.15 However, in clinical trial, BKM120 was not effective against PCa.16
Results
PCSCs Have High Self-Renewal Potentiality In Vitro
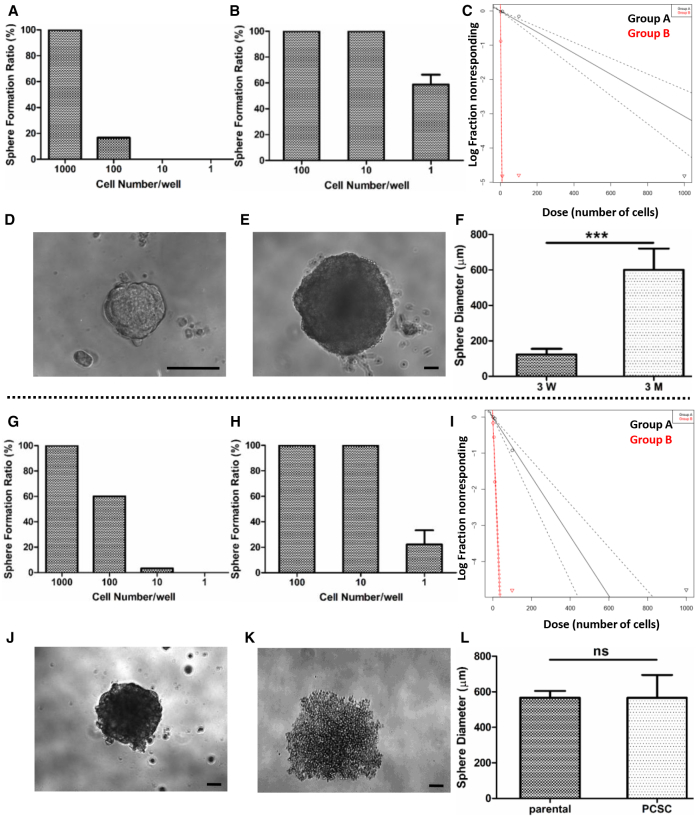
We cultured human DU145 prostate cancer cells in growth factor-defined (epidermal growth factor [EGF] and fibroblast growth factor [FGF]-2) serum-free (GFDSF) medium for 3 weeks. When 100 cells/well were plated, 17% of wells had spheres, while no spheres were observed at 10 cells/well (Figure 1A). DU145 cells cultured in GFDSF medium for 3 months (PCSCs) had higher self-renewal potential, so that 58% of wells plated with 1 cell/well had spheres (Figure 1B).
Figure 1.
PCSCs Have Self-Renewal Potential In Vitro
(A) Sphere formation assay in 3-week DU145 sphere-cultured cells (n = 60 wells). (B) Sphere formation assay in 3-month DU145 sphere-cultured cells. For 1 cell/well assay, experiment was repeated 4 times. (C) Extreme limiting dilution analysis (ELDA) comparing 3-week DU145 sphere cultures (group A, black) and 3-month DU145 sphere cultures (group B, red) (p < 0.001). (D) Morphology of 3-week DU145 sphere plated at 1,000 cells/well. (E) Morphology of 3-month DU145 sphere plated at 1 cell/well. (F) Comparison of sphere diameters between 3-week DU145 spheres (1,000 cells/well) and 3-month DU145 spheres (1 cell/well). n = 3, mean ± SD, ***p < 0.0001. (G) Sphere formation assay of TRAMP-C2 parental cells (n = 60 wells). (H) Sphere formation assay of TRAMP-C2 sphere-cultured cells. For 1 cell/well assay, experiment was repeated 3 times. (I) ELDA comparing TRAMP-C2 parental (group A, black) and TRAMP-C2 sphere-cultured cells (group B, red) (p < 0.001). (J) Morphology of sphere in 100 cells/well of TRAMP-C2 parental. (K) Morphology of sphere in 1 cell/well of TRAMP-C2 sphere culture. (L) Comparison of sphere diameters between TRAMP-C2 parental (100 cells/well) and TRAMP-C2 sphere cultures (1 cell/well). There is no statistical difference. Scale bars, 100 μm.
Extreme limiting dilution analysis (ELDA) comparing 3-week to 3-month DU145 spheres demonstrated that 3-week spheres were estimated to have a 1/325 repopulating cell frequency, while 3-month-cultured DU145 spheres were estimated to have a 1/1.7 repopulating cell frequency (Figure 1C). The 3-month-cultured spheres when plated at 1 cell/well were larger than any of the parental 3-week spheres (Figures 1D–1F), suggesting greater proliferation. When mouse parental TRAMP-C2 (serum-cultured) PCa cells were assayed for sphere formation, 3.3% of wells had spheres in the 10 cells/well group (Figure 1G). In contrast, TRAMP-C2 cells grown in GFDSF medium for 3 months (PCSCs) had spheres in 22% of wells at 1 cell/well (Figure 1H). ELDA comparing TRAMP-C2 parental cells with sphere-cultured cells indicated that TRAMP-C2 parental cells had a 1/121 repopulating cell frequency, while sphere-cultured cells had a 1/3.8 repopulating cell frequency (Figure 1I). Sphere diameters for TRAMP-C2 sphere culture cells and parental cells were similar (Figures 1J–1L).
Radiation and Docetaxel Sensitivity of PCSCs and Parental Cells In Vitro
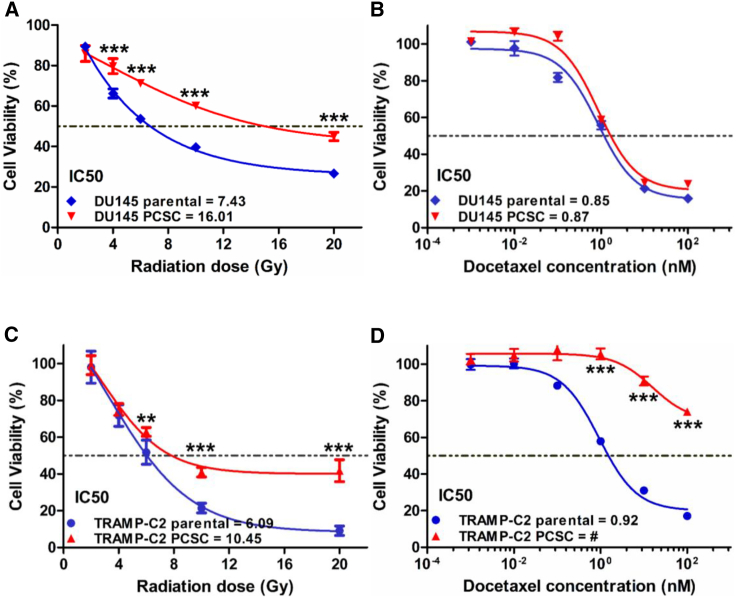
Cancer stem-like cells have been shown to exhibit resistance to radiotherapy and chemotherapy.2, 17 We examined whether PCSCs have a differential response to these modalities compared with parental cells. Spheres and parental cells were treated with various doses of radiation or docetaxel in vitro. Both DU145 and TRAMP-C2 PCSCs were more radiotherapy resistant than parental cells (Figures 2A and 2C). DU145 PCSCs had similar sensitivity to docetaxel as parental cells (Figure 2B), while TRAMP-C2 PCSCs were much more docetaxel resistant than parental cells (Figure 2D).
Figure 2.
DU145 and TRAMP-C2 PCSCs Are Less Sensitive to Radiotherapy Than Parental Cells
Cell viability of DU145 cells after irradiation (A) and docetaxel (B) and TRAMP-C2 cells after irradiation (C) and docetaxel (D). Viability was normalized to no treatment. #no IC50. Data are shown as mean ± SD. **p < 0.01, ***p < 0.001.
PCSCs Are More Tumorigenic Than Parental Cells
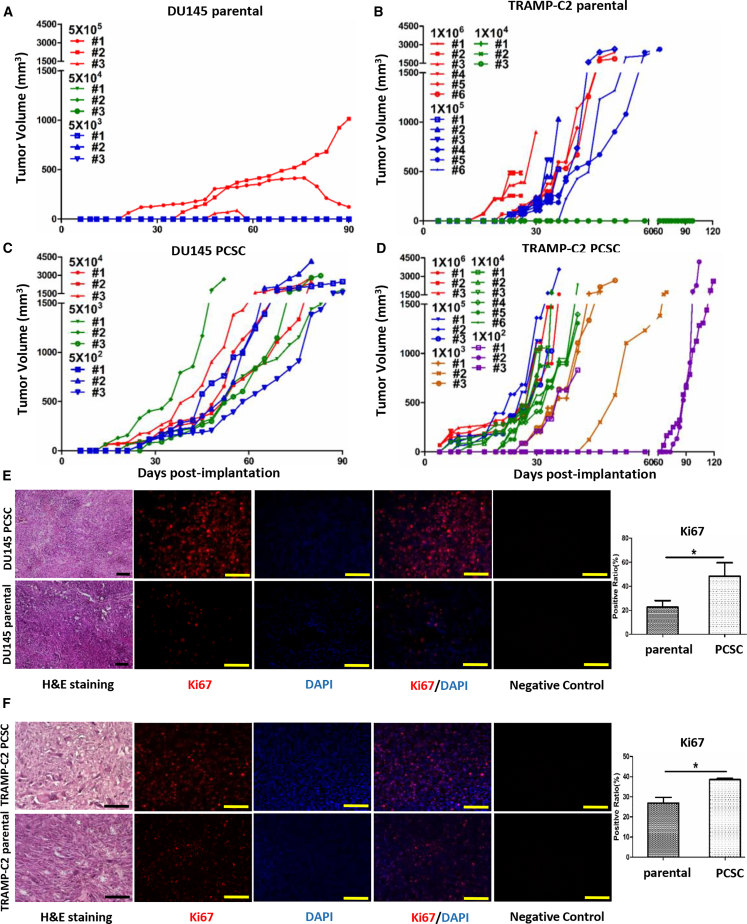
We compared the tumorigenicity of PCSCs after subcutaneous implantation. It took at least 5 × 105 implanted DU145 parental cells before tumors were observed in nude mice, with two of three tumors regressing after initiation (Figure 3A). In contrast, DU145 PCSCs formed tumors with only 5 × 102 cells in all three mice tested (Figure 3C). Greater than 1 × 104 TRAMP-C2 parental cells were required to form tumors in syngeneic C57BL/6 mice (Figure 3B), while only 1 × 102 PCSCs formed tumors in all three mice (Figure 3D). Thus, PCSCs have a 1,000-fold increased tumorigenic potential in vivo relative to their parental counterparts.
Figure 3.
PCSCs Are More Tumorigenic In Vivo
Tumor growth after subcutaneous implantation of DU145 parental cells (A), TRAMP-C2 parental cells (B), DU145 PCSCs (C), and TRAMP-C2 PCSCs (D). Number of cells implanted and mouse number are indicated. DU145 subcutaneous tumors (E) and TRAMP-C2 subcutaneous tumors (F) stained with H&E (left). Immunofluorescent staining of Ki67 (red) and DAPI (blue) (middle) and quantitation of Ki67 expression (right) are also shown. n = 3, mean ± SD, *p < 0.05. Scale bars, 100 μm.
Histologic evaluation was performed on tumors from selected animals when the tumor diameter reached 15 mm. With H&E staining, there was no difference in the morphology between TRAMP-C2 or DU145 PCSC-derived tumors and their parental cell-derived tumors (Figures 3E and 3F). However, the percentage of Ki67-positive cells was higher in both the TRAMP-C2 and the DU145 PCSC-derived tumors compared to their parental counterparts (Figures 3E and 3F).
PCSCs Are Sensitive to oHSV In Vitro
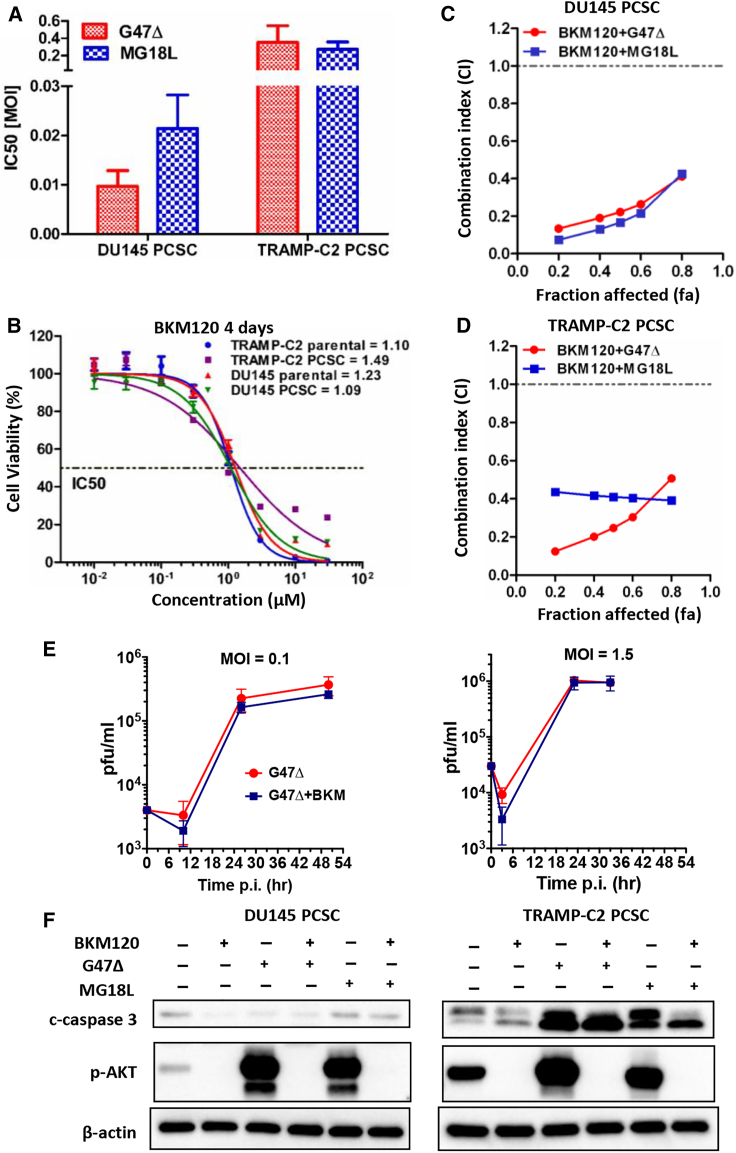
We previously showed that oHSV kills PCa cell lines and selectively infected PCa cells in human organotypic cultures.10, 18, 19 Therefore, we determined whether PCSCs were sensitive to oHSV. We first used G47Δ-mCherry to evaluate virus spread in infected DU145 spheres with and without heparin at 24, 48, and 72 h after infection. Heparin inhibited oHSV infection and spread; mCherry expression only increased in the absence of heparin (Figure S1A). The presence of heparin in PCSC media inhibited oHSV killing of DU145 PCSCs (Figures S1B and S1C). However, in heparin-free medium, DU145 PCSCs were very sensitive to killing by G47Δ and MG18L, with half-maximum inhibitory concentration (IC50) values of 0.009 and 0.021 MOI, respectively (Figures S1D, 4A, and S2A). Thereafter, all virus infections were done in heparin-free medium. TRAMP-C2 PCSCs were less but similarly sensitive to both viruses, like most mouse cancer cells, with IC50 values of 0.35 and 0.27 MOI, respectively (Figures 4A and S2B).
Figure 4.
oHSV and BKM120 Synergize in Killing PCSCs In Vitro
(A) IC50 values for oHSV (G47Δ or MG18L) in DU145 or TRAMP-C2 PCSCs, from 3 experiments. Mean ± SD. (B) BKM120 dose-response curves for DU145 and TRAMP-C2 PCSCs and parental cells, with IC50 values listed. (C and D) Chou-Talalay synergy analysis for oHSV combined with BKM120 in DU145 (C) and TRAMP-C2 PCSCs (D). Combination index (CI) < 1, CI = 1, and CI > 1 indicate synergistic, additive, and antagonistic, respectively. (E) G47Δ virus (left, MOI = 0.1; right, MOI = 1.5) growth curve in DU145 PCSCs with or without BKM120 (0.2 μM). (F) Representative western blot of BKM120- and oHSV-treated DU145 (left) and TRAMP-C2 (right) PCSCs. Experimental conditions were as follows: BKM120, 10 μM; G47Δ, 1 MOI; and MG18L, 1 MOI. All assays were in the absence of heparin.
oHSV Synergizes with PI3K Inhibitor BKM120 to Kill PCSCs In Vitro
Several therapeutic agents were tested in combination with oHSV. oHSV combined with chemotherapy (docetaxel or cisplatin) (Figure S3A) or radiation (Figure S3B) did not exhibit combination effects in vitro. DU145 and TRAMP-C2 PCSCs were not very sensitive to molecularly targeted inhibitors of the NOTCH (gamma-secretase inhibitor [GSI]) and Wnt (ICG001) pathways, which was not increased with oHSV (G47Δ and MG18L) (Figures S3C and S3D). In contrast, both DU145 and TRAMP-C2 PCSCs and their parental cells were sensitive to PI3K pathway inhibition with BKM120 (Figure 4B). Moreover, combining BKM120 with either G47Δ or MG18L was synergistic in DU145 and TRAMP-C2 PCSCs (Figures 4C and 4D). The addition of BKM120 at 0.2 μM (∼IC10) to DU145 PCSCs did not increase the ability of G47Δ to replicate at either of two MOIs (0.1 and 1.5) (Figure 4E). Western blot demonstrated oHSV-induced phosphorylation of AKT in both DU145 and TRAMP-C2 PCSCs, which was inhibited by BKM120 (Figure 4F). In TRAMP-C2, oHSV induced cleavage of caspase-3, indicative of apoptosis, which was not increased by BKM120 (Figure 4F).
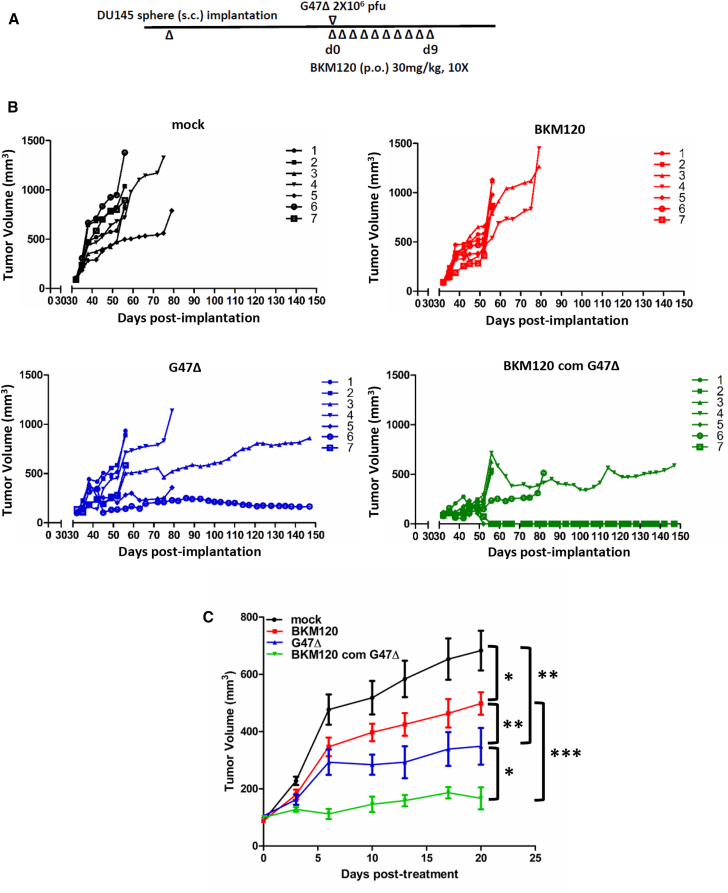
Combination of G47Δ with PI3K Inhibitor BKM120 In Vivo
We tested the efficacy of monotherapy and combination therapy in subcutaneous DU145 PCSC-derived tumors. The schema is illustrated in (Figure 5A). We used G47Δ because it is already in clinical trial for primary prostate cancer and glioblastoma. Thus, we felt that data using G47Δ could relatively rapidly progress to clinical trial. In contrast, MG18L is a laboratory construct with no clinical efficacy or safety demonstrated thus far. Combination treatment (G47Δ and BKM120) induced complete, durable (3-month) tumor regression in 2 of 7 animals, and it reduced tumor growth in the others (Figure 5B). Both G47Δ and BKM120 monotherapy delayed tumor growth, but the combination was significantly better than either monotherapy (Figures 5B and 5C). No toxicity due to monotherapy or combination therapy was observed. We documented normal histopathology and stable weight (Figures S4A and S4B). There was no fatty degeneration of liver, pulmonary or splenic congestion, cardiac muscle cell hypertrophy, or glomerular atrophy in either the single-treatment or combination group (Figure S4A).
Figure 5.
oHSV and BKM120 Combination Therapy In Vivo
(A) Schematic illustrating the dosing and scheduling of treatment regimens in mice bearing subcutaneous DU145 PCSC-derived tumors. (B) Tumor volumes indicated for individual mice from each treatment group. (C) Mean DU145 PCSC tumor growth of 4 treatment groups ±SEM from (B). Comparisons between the indicated groups from days 13–20 (except between mock and BKM120, only on day 20); *p < 0.05, **p < 0.005, ***p < 0.0001.
Discussion
There is increasing evidence that initiation, growth, recurrence, and metastasis of cancers are related to the behavior of cancer stem-like cells, including in PCa.3, 5 Putative stem-like cells have been isolated from PCa biopsies and the DU145 cell line.4, 20, 21 Serum-free medium can enrich the growth of cancer stem-like cells, and the sphere formation assay is a classic method for the analysis of their self-renewal ability.3 We used EF20 medium, commonly used for neural stem cells, to enrich PCSCs from human DU145 and mouse TRAMP-C2 cell lines. Both demonstrated high self-renewal potential and the ability to form spheres from even 1 cell/well. PCSC tumors formed with as few as 5 × 102 DU145 PCSCs and 1 × 102 TRAMP-C2 PCSCs, in contrast to the parental serum-cultured cells that required at least 1 × 105 cells. Both DU145 and TRAMP-C2 PCSCs demonstrated increased resistance to radiotherapy. Thus, we believe that PCSCs provide a better model to test therapeutic strategies.
oHSV kills tumors by oncolytic mechanisms, which are different from standard anticancer therapies. In previous studies, we and others demonstrated that oHSV was effective in human PCa cell lines,10 organotypic cultures,18 and xenografts;14, 22, 23 in mouse PCa subcutaneous, orthotopic, and metastatic tumors;19, 24, 25 and in spontaneously arising tumors in transgenic mice.26 Here we found that PCSCs in vitro were very sensitive to two oHSVs with different genetic alterations, MG18L and G47Δ. G47Δ recently completed a phase I clinical trial in Japan for castration-resistant PCa, where it was well tolerated without attributable serious adverse events.7 oHSV talimogene laherparepvec (T-Vec), similar to G47Δ except expressing GM-CSF, was recently approved by the U.S. Food and Drug Administration (FDA) and European Medicines Agency (EMA) for the treatment of advanced melanoma.6
oHSV interacts beneficially with many pharmacological agents in killing cancer cells in vitro,13 including PCa.11, 12 Therefore, we tested the combination of chemotherapy, radiotherapy, and oncogenic pathway inhibitors with oHSV to kill PCSCs. Only BKM120 synergized with oHSV. BKM120 neither increased nor decreased the replication ability of G47Δ. However, it did block the phosphorylated protein kinase B (p-AKT) increase induced by the virus. Additional studies into its mechanism are needed and should include isoform-specific studies, as BKM120 is an oral pan-class I PI3K inhibitor that targets all four catalytic isoforms (p110α, β, δ, and γ).16 PI3K-signaling pathways are critical for PCa stem-like cell maintenance, and targeting PI3K signaling may be beneficial in PCa treatment by eliminating PCSCs.27 Targeting PI3K with BKM120 inhibited PI3K signaling in the PCSCs, and it was reported to have anti-proliferative, pro-apoptotic, and anti-angiogenic effects.15 The combination of a single intra-tumoral G47Δ injection and systemic BKM120 was effective in inhibiting DU145 PCSC-derived tumor growth and inducing complete regression in some tumors. Treatment-related serious adverse events of BKM120 in prostate clinical trials included fatigue, hyperglycemia, anemia, diarrhea, weight loss, and rash.16, 28 We noted no skin changes, weight losses, or organ histopathology in our xenograft study. Thus, oHSV combined with BKM120 was not only effective but safe and well tolerated. While two recent clinical trials of BKM120 in metastatic castration-resistant PCa in combination with AR inhibitors did not find any clinical benefit,16, 28 the synergy with oHSV suggests that this combination warrants clinical evaluation.
Materials and Methods
Cells, Viruses, and Reagents
DU145 cells (ATCC) were grown in Eagle’s minimum essential medium with 1.5 g/L sodium bicarbonate, non-essential amino acids, L-glutamine, and sodium pyruvate supplemented with 10% fetal bovine serum (FBS). TRAMP-C2 cells, obtained from N. Greenberg (Fred Hutchinson Cancer Research Center, Seattle WA), were cultured in DMEM with 4.5 g/L glucose supplemented with 5% FBS, 5% Nu-serum IV, 10−8 mol/L dihydrotestosterone, 5 μg/mL insulin, and 25 μg/mL penicillin-streptomycin. Cells were cultured at 37°C and 5% CO2. Construction of G47Δ, G47Δ-mCherry, and MG18L has been described.8, 9, 29 Compounds used in this study were as follows: Docetaxel Injection (Sandoz), BKM120 (Selleck, dissolved in DMSO), Wnt pathway inhibitor ICG-001 (MedChem Express, dissolved in DMSO), and GSI (PF-03084014, Selleck, dissolved in DMSO).
PCSCs
PCSCs were cultured in EF20 medium composed of Neurobasal medium (Invitrogen) supplemented with 3 mM L-Glutamine (Mediatech), 1 × B27 supplement (Invitrogen), 0.5 × N2 supplement (Invitrogen), 2 μg/mL heparin (Sigma), 20 ng/mL human EGF (R&D Systems), 20 ng/mL human FGF-2 (PeproTech), and 0.5 × penicillin G-streptomycin sulfate-amphotericin B complex (Mediatech). For passaging, spheres were centrifuged, treated with Accutase (Innovative Cell Technologies) at 37°C for 5 min, dissociated by pipetting, washed with PBS, and resuspended in EF20 medium.
Cell Susceptibility Assays and Chou-Talalay Analysis
DU145 and TRAMP-C2 parental cells were seeded into 96-well plates at 2,000 and 1,500 cells/well, respectively. DU145 and TRAMP-C2 PCSCs were seeded into 96-well plates at 5,000 cells/well for the 4-day assay or 3,000 and 2,500 cells/well, respectively, for the 6-day assay. Cells were treated with radiation (2, 4, 6, 10, and 20 Gy) or docetaxel (0.001, 0.01, 0.1, 1, 10, and 100 nM) in vitro. G47Δ at an MOI and the indicated compound concentrations were added alone or in combination. 5-(3-Carboxymethoxyphenyl)-2-(4,5-dimethylthiazoly)-3-(4-sulfophenyl)tetrazolium, inner salt (MTS) assays (Promega) were performed according to the manufacturer’s instructions, and dose-response curves were plotted (non-linear fit; GraphPad Prism). For Chou-Talalay analysis, experiments were carried out as described.30 Combination index (CI) < 1, CI = 1, and CI > 1 indicate synergistic, additive, and antagonistic interactions, respectively. All experiments were repeated at least three times.
Virus Yield Assay
DU145 PCSCs were dissociated and the following day infected with G47Δ (MOI = 0.1 or 1.5) for 30 min to 1 h. For high MOI, virus inoculum was removed and vehicle or BKM120 was added to 0.2 μM. Infected cells were incubated for the indicated times, and cells and media were harvested (in triplicate), freeze-thawed and sonicated, and titered on Vero cells.
Sphere Formation Assay
Cells were dissociated and passed through a 70-μm filter to produce single-cell suspensions, counted, and diluted to the indicated cell number in 100 μL media/well in a 96-well plate. Then 14 days later, spheres with diameters ≥50 μm in each well were microscopically counted.
Immunoblots
Cells (500,000/well) were treated as indicated. Cell pellets were lysed 24 h later in radioimmunoprecipitation assay (RIPA) buffer (Boston Bioproducts, Worcester, MA) plus a cocktail of protease and phosphatase inhibitors (Roche). 18 μg protein was separated by 10% SDS-PAGE and transferred to polyvinylidene fluoride (PVDF) membranes by electroblotting. After blocking with 5% nonfat milk in TBS-Tween 20, membranes were incubated at 4°C overnight with the following antibodies: anti-p-AKT (Ser 473), anti-cleaved-caspase-3, and β-actin (Cell Signaling Technology, Danvers, MA).
In Vivo Studies
For xenograft tumorigenicity studies, DU145 parental and PCSCs were implanted subcutaneously in 6- to 7-week-old athymic male mice (NCI). TRAMP-C2 parental and PCSCs were implanted subcutaneously in 6- to 7-week-old male C57BL/6 mice (NCI). Tumor volume (mm3) = a2 × b × 0.52, where a and b are the shortest and longest diameters, respectively. Tumors measuring at least 5 mm in diameter were considered a positive take. For efficacy studies, DU145 PCSCs (5 × 104 in 100 μL) were subcutaneously implanted in male athymic mice. On day 32, randomly grouped mice (N = 7/group) were intra-tumorally injected with G47Δ (2 × 106 plaque-forming units [PFU]) or virus buffer (PBS with 10% glycerol), and/or BKM120 was initiated (30 mg/kg/day, gavage, dissolved in 0.5% methylcellulose, daily for 10 days). Tumor specimens and organs were harvested when tumors reached 15-mm diameter, fixed in 4% paraformaldehyde, and embedded in paraffin. Sections were stained with H&E. All in vivo procedures were approved by the Institutional Animal Care and Use Committee at Massachusetts General Hospital.
Statistics
Unpaired Student’s t test (two tailed) was used to analyze significance between two treatment groups. p values less than 0.05 were considered statistically significant (GraphPad Prism 5). For ELDA, data were uploaded into http://bioinf.wehi.edu.au/software/elda/ and results plotted and analyzed.
Author Contributions
L.W. was involved with the conception and performance of experiments, statistical analysis, and writing the manuscript. J.N. assisted with some of the experiments. H.W. designed and supervised some experiments. S.W. and C.W. reviewed histology and analyzed data. M.R.H. performed virus yield assays and assisted with animal studies. S.D.R. and R.L.M. were involved with the conception and design of experiments, supervised, analyzed data, and participated in manuscript preparation. All authors reviewed and edited the manuscript.
Conflicts of Interest
The authors declare no competing interests. R.L.M. and S.D.R. are co-inventors on patents relating to oHSV, owned and managed by Georgetown University and Massachusetts General Hospital, for which royalties have been received.
Acknowledgments
These studies were supported in part by a grant from NIH to R.L.M. (R01CA102139).
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.omto.2019.03.008.
Supplemental Information
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Attard G., Parker C., Eeles R.A., Schröder F., Tomlins S.A., Tannock I., Drake C.G., de Bono J.S. Prostate cancer. Lancet. 2016;387:70–82. doi: 10.1016/S0140-6736(14)61947-4. [DOI] [PubMed] [Google Scholar]
- 3.Batlle E., Clevers H. Cancer stem cells revisited. Nat. Med. 2017;23:1124–1134. doi: 10.1038/nm.4409. [DOI] [PubMed] [Google Scholar]
- 4.Collins A.T., Berry P.A., Hyde C., Stower M.J., Maitland N.J. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65:10946–10951. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- 5.Rybak A.P., Bristow R.G., Kapoor A. Prostate cancer stem cells: deciphering the origins and pathways involved in prostate tumorigenesis and aggression. Oncotarget. 2015;6:1900–1919. doi: 10.18632/oncotarget.2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bommareddy P.K., Peters C., Saha D., Rabkin S.D., Kaufman H.L. Oncolytic Herpes Simplex Viruses as a Paradigm for the Treatment of Cancer. Annu. Rev. Cancer Biol. 2018;2:155–173. [Google Scholar]
- 7.Taguchi S., Fukuhara H., Homma Y., Todo T. Current status of clinical trials assessing oncolytic virus therapy for urological cancers. Int. J. Urol. 2017;24:342–351. doi: 10.1111/iju.13325. [DOI] [PubMed] [Google Scholar]
- 8.Todo T., Martuza R.L., Rabkin S.D., Johnson P.A. Oncolytic herpes simplex virus vector with enhanced MHC class I presentation and tumor cell killing. Proc. Natl. Acad. Sci. USA. 2001;98:6396–6401. doi: 10.1073/pnas.101136398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanai R., Wakimoto H., Martuza R.L., Rabkin S.D. A novel oncolytic herpes simplex virus that synergizes with phosphoinositide 3-kinase/Akt pathway inhibitors to target glioblastoma stem cells. Clin. Cancer Res. 2011;17:3686–3696. doi: 10.1158/1078-0432.CCR-10-3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walker J.R., McGeagh K.G., Sundaresan P., Jorgensen T.J., Rabkin S.D., Martuza R.L. Local and systemic therapy of human prostate adenocarcinoma with the conditionally replicating herpes simplex virus vector G207. Hum. Gene Ther. 1999;10:2237–2243. doi: 10.1089/10430349950017211. [DOI] [PubMed] [Google Scholar]
- 11.Passer B.J., Castelo-Branco P., Buhrman J.S., Varghese S., Rabkin S.D., Martuza R.L. Oncolytic herpes simplex virus vectors and taxanes synergize to promote killing of prostate cancer cells. Cancer Gene Ther. 2009;16:551–560. doi: 10.1038/cgt.2009.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Passer B.J., Cheema T., Wu S., Wu C.L., Rabkin S.D., Martuza R.L. Combination of vinblastine and oncolytic herpes simplex virus vector expressing IL-12 therapy increases antitumor and antiangiogenic effects in prostate cancer models. Cancer Gene Ther. 2013;20:17–24. doi: 10.1038/cgt.2012.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanai R., Wakimoto H., Cheema T., Rabkin S.D. Oncolytic herpes simplex virus vectors and chemotherapy: are combinatorial strategies more effective for cancer? Future Oncol. 2010;6:619–634. doi: 10.2217/fon.10.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fukuhara H., Martuza R.L., Rabkin S.D., Ito Y., Todo T. Oncolytic herpes simplex virus vector g47delta in combination with androgen ablation for the treatment of human prostate adenocarcinoma. Clin. Cancer Res. 2005;11:7886–7890. doi: 10.1158/1078-0432.CCR-05-1090. [DOI] [PubMed] [Google Scholar]
- 15.Maira S.M., Pecchi S., Huang A., Burger M., Knapp M., Sterker D., Schnell C., Guthy D., Nagel T., Wiesmann M. Identification and characterization of NVP-BKM120, an orally available pan-class I PI3-kinase inhibitor. Mol. Cancer Ther. 2012;11:317–328. doi: 10.1158/1535-7163.MCT-11-0474. [DOI] [PubMed] [Google Scholar]
- 16.Armstrong A.J., Halabi S., Healy P., Alumkal J.J., Winters C., Kephart J., Bitting R.L., Hobbs C., Soleau C.F., Beer T.M. Phase II trial of the PI3 kinase inhibitor buparlisib (BKM-120) with or without enzalutamide in men with metastatic castration resistant prostate cancer. Eur. J. Cancer. 2017;81:228–236. doi: 10.1016/j.ejca.2017.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baumann M., Krause M., Hill R. Exploring the role of cancer stem cells in radioresistance. Nat. Rev. Cancer. 2008;8:545–554. doi: 10.1038/nrc2419. [DOI] [PubMed] [Google Scholar]
- 18.Passer B.J., Wu C.L., Wu S., Rabkin S.D., Martuza R.L. Analysis of genetically engineered oncolytic herpes simplex viruses in human prostate cancer organotypic cultures. Gene Ther. 2009;16:1477–1482. doi: 10.1038/gt.2009.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Varghese S., Rabkin S.D., Liu R., Nielsen P.G., Ipe T., Martuza R.L. Enhanced therapeutic efficacy of IL-12, but not GM-CSF, expressing oncolytic herpes simplex virus for transgenic mouse derived prostate cancers. Cancer Gene Ther. 2006;13:253–265. doi: 10.1038/sj.cgt.7700900. [DOI] [PubMed] [Google Scholar]
- 20.Wei C., Guomin W., Yujun L., Ruizhe Q. Cancer stem-like cells in human prostate carcinoma cells DU145: the seeds of the cell line? Cancer Biol. Ther. 2007;6:763–768. doi: 10.4161/cbt.6.5.3996. [DOI] [PubMed] [Google Scholar]
- 21.Rybak A.P., He L., Kapoor A., Cutz J.C., Tang D. Characterization of sphere-propagating cells with stem-like properties from DU145 prostate cancer cells. Biochim. Biophys. Acta. 2011;1813:683–694. doi: 10.1016/j.bbamcr.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 22.Taneja S., MacGregor J., Markus S., Ha S., Mohr I. Enhanced antitumor efficacy of a herpes simplex virus mutant isolated by genetic selection in cancer cells. Proc. Natl. Acad. Sci. USA. 2001;98:8804–8808. doi: 10.1073/pnas.161011798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakamori M., Fu X., Pettaway C.A., Zhang X. Potent antitumor activity after systemic delivery of a doubly fusogenic oncolytic herpes simplex virus against metastatic prostate cancer. Prostate. 2004;60:53–60. doi: 10.1002/pros.20056. [DOI] [PubMed] [Google Scholar]
- 24.Lardizabal J., Ding J., Delwar Z., Rennie P.S., Jia W. A TRAMP-derived orthotopic prostate syngeneic (TOPS) cancer model for investigating anti-tumor treatments. Prostate. 2018;78:457–468. doi: 10.1002/pros.23490. [DOI] [PubMed] [Google Scholar]
- 25.Varghese S., Rabkin S.D., Nielsen P.G., Wang W., Martuza R.L. Systemic oncolytic herpes virus therapy of poorly immunogenic prostate cancer metastatic to lung. Clin. Cancer Res. 2006;12:2919–2927. doi: 10.1158/1078-0432.CCR-05-1187. [DOI] [PubMed] [Google Scholar]
- 26.Varghese S., Rabkin S.D., Nielsen G.P., MacGarvey U., Liu R., Martuza R.L. Systemic therapy of spontaneous prostate cancer in transgenic mice with oncolytic herpes simplex viruses. Cancer Res. 2007;67:9371–9379. doi: 10.1158/0008-5472.CAN-07-0674. [DOI] [PubMed] [Google Scholar]
- 27.Dubrovska A., Kim S., Salamone R.J., Walker J.R., Maira S.M., García-Echeverría C., Schultz P.G., Reddy V.A. The role of PTEN/Akt/PI3K signaling in the maintenance and viability of prostate cancer stem-like cell populations. Proc. Natl. Acad. Sci. USA. 2009;106:268–273. doi: 10.1073/pnas.0810956106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Massard C., Chi K.N., Castellano D., de Bono J., Gravis G., Dirix L., Machiels J.P., Mita A., Mellado B., Turri S. Phase Ib dose-finding study of abiraterone acetate plus buparlisib (BKM120) or dactolisib (BEZ235) in patients with castration-resistant prostate cancer. Eur. J. Cancer. 2017;76:36–44. doi: 10.1016/j.ejca.2017.01.024. [DOI] [PubMed] [Google Scholar]
- 29.Sgubin D., Wakimoto H., Kanai R., Rabkin S.D., Martuza R.L. Oncolytic herpes simplex virus counteracts the hypoxia-induced modulation of glioblastoma stem-like cells. Stem Cells Transl. Med. 2012;1:322–332. doi: 10.5966/sctm.2011-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aghi M., Rabkin S., Martuza R.L. Effect of chemotherapy-induced DNA repair on oncolytic herpes simplex viral replication. J. Natl. Cancer Inst. 2006;98:38–50. doi: 10.1093/jnci/djj003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.