Summary
Regulatory T cells (Tregs) modulate the magnitude of immune responses and possess therapeutic potential in an array of immune diseases. Statins reduce the activation and proliferation of conventional T cells (Tcons), and they seem to up‐regulate the frequency and function of Tregs. However, there is a lack of simultaneous evaluation of the in‐vitro effect of statins on the functional profile of Tregs versus Tcons. Herein, magnetically purified Tcons and Tregs were stimulated with CD3/CD28/interleukin (IL)‐2 in the presence of atorvastatin (ATV) at 1 or 10 µM. The suppressive function of Tregs, the expression of markers associated with Treg function, activation levels, cytokine production and calcium flux in both subpopulations were assessed by flow cytometry. ATV had no cytotoxic effect on T cells at the concentrations used. Interestingly, 10 µM ATV hampered the suppressive capacity of Tregs. Moreover, this higher concentration reduced the expression of forkhead box protein 3 (FoxP3), cytotoxic T lymphocyte antigen (CTLA‐4) and programmed death 1 (PD‐1). In Tcons, ATV at 10 µM decreased PD‐1 and CD45RO expression. The expression of CD25, CD69, CD95, CD38, CD62L, CCR7 and perforin was not affected in both subpopulations or at any ATV concentrations. Remarkably, 10 µM ATV increased the percentage of tumour necrosis factor (TNF)‐α‐producing Tregs. Although there was a reduction of calcium flux in Tcons and Tregs, it was only significant in 10 µM ATV‐treated Tcons. These results suggested that 10 µM ATV affects the cellular functions of both populations; however, this concentration particularly affected several aspects of Treg biology: its suppressive function, cytokine production and expression of Treg‐specific markers.
Keywords: activation, Ca2+ flux, FoxP3, statin, Regulatory T cell
Introduction
CD4+ regulatory T cells (Tregs) that express the forkhead box protein 3 (FoxP3) transcription factor can control the magnitude of immune responses under different clinical conditions, including autoimmune diseases, transplants, atherosclerosis, allergic diseases and colitis 1, 2, 3, 4, 5. Therefore, Tregs have become a topic of growing interest due to their therapeutic benefit in the modulation of immune responses. Their suppressive action is largely mediated by cell–cell interactions, soluble factor delivery and functions associated with cytolysis and metabolic disruption 6, 7, and these functions can be potentiated or induced by different drugs such as statins. Statins are drugs with potent immunomodulatory actions that inhibit the 3‐hydroxy‐3‐methylglutaryl‐CoA reductase (HMGCR) that converts HMGC into mevalonate, a precursor of the biosynthesis of cholesterol and non‐steroidal isoprenoid compounds. The immunomodulatory actions of statins have been associated mainly with a reduction of isoprenoid compounds that are essential for the post‐translational modifications of proteins participating in several cellular processes, such as survival, proliferation, differentiation and migration 8.
Natural and synthetic statins reduce the level of cellular activation as determined by the expression of CD69 and CD25 molecules, the proliferation of conventional T cells (Tcons) and cell cycle arrest 9, 10, 11, 12, 13, 14. Contradictory results regarding the effect of statins on the production of interferon (IFN)‐γ, tumour necrosis factor (TNF)‐α, interleukin (IL)‐2, IL‐4 and IL‐5 by Tcons have been associated with the type of statins, incubation time in culture and/or the pre‐existing inflammatory condition in which they were evaluated 10, 11, 12, 13, 14. Moreover, it is unclear if statins can modify early events downstream of T cell receptor (TCR) activation, such as calcium (Ca2+) mobilization 9, 13. Interestingly, statins seem to expand the Treg population under both steady‐state and inflammatory conditions as well as modulate their phenotypes, suppressive functions and migration patterns both in vivo and in vitro 15, 16, 17, 18, 19, 20, 21. The statin‐induced Treg expansion is primarily attributed to the conversion of forkhead box protein 3 (FoxP3)– T cells into FoxP3+ T cells through several mechanisms: (i) by negatively modulating the expression of mothers against decapentaplegic homologue 6 (Smad6) and Smad7 proteins responsible for inhibiting the transforming growth factor‐β (TGF‐β) signalling pathway 22; (ii) by reducing the phosphorylation of protein kinase B (PKB), mammalian target of rapamycin (mTOR) and extracellular signal‐regulated kinases, which are involved in Treg induction 15; (iii) by increasing the expression of TGF‐β and IL‐10 23 and reducing the IL‐6/signal transducer and activator of transcription 3 (STAT‐3) signalling pathway 24, promoting a higher Treg/T helper type 17 (Th)17 ratio; and (iv) by inducing the transcription factor Krüppel‐like factor 2 (KLF2), which promotes Treg accumulation in secondary lymphoid organs 25, 26.
Atorvastatin (ATV) is a second‐generation statin that reduces blood levels of low‐density lipoprotein cholesterol, even at very low therapeutic doses, compared with other available statins 27. Moreover, because ATV up‐regulates the Treg population, we wanted to evaluate whether ATV can differentially modulate several aspects of the biology of Tregs and Tcons, such as their activation status, suppressive functions, cytokine production and Ca2+ mobilization in a dose‐dependent manner. Thus, we determined the effector function and Ca2+ efflux of Tcons and Tregs isolated by electromagnetic sorting and activated in the absence or presence of either 1 or 10 µM ATV.
Materials and methods
Isolation of T cell populations and cell culture
Peripheral blood mononuclear cells (PBMCs) were isolated by gradient centrifugation using Histopaque‐1077 (Sigma‐Aldrich, St Louis, MO, USA) from healthy individuals recruited at the Universidad de Antioquia, Colombia. Informed consent was obtained from all donors and the research was approved by the Bioethical Board for Human Research from the Universidad de Antioquia. Fresh untouched CD4+ T cells were purified by negative selection with a CD4+ T Cell Isolation Kit II (Miltenyi Biotec, Bergisch Gladbach, Germany) followed by staining with anti‐CD25‐phycoerythrin‐cyanin 5 (PE‐Cy5) (clone M‐A251; BD Biosciences, San Jose, CA, USA) and anti‐CD127‐PE (clone eBioRDR5; eBioscience, San Diego, CA, USA) monoclonal antibodies. CD25highCD127low/– Treg populations and CD25–CD127+ Tcons were isolated in a MOFLO XDP high‐speed cell sorter using the single mode (Beckman Coulter, Fort Collins, CO, USA). The purity of the sorted cell populations ranged from 90 to 98%. The efficiency of this procedure was approximately 90%.
The isolated T cells were seed in U‐bottomed 96‐well plates to a density of 0·1–2 × 105 cells/well and cultured in RPMI‐1640 medium (Sigma‐Aldrich) supplemented with 10% heat‐inactivated fetal bovine serum (FBS). Cultures were incubated at 37˚C in 5% CO2 and 95% relative humidity. Cells were stimulated for 48 h with 2·5 µg/ml plate‐bound anti‐CD3 monoclonal antibody (mAb) (clone OKT3; Ancell, Bayport, MN, USA) and 1·5 µg/ml soluble CD28 (clone ANC28.1/5D10; Ancell) in the presence of 20 IU/ml of human recombinant IL‐2 (National Institutes of Health AIDS Research and Reference Reagent Program, Bethesda, MD, USA). Stimulus conditions were set at 48 h as Treg cells acquire the highest suppressive capacity at this time, as has been reported by other authors 28.
Atorvastatin treatment
ATV was provided by Biogen Laboratory, Bogotá, Colombia. It was diluted in dimethyl sulphoxide (DMSO; Carlo Erba, Rome, Italy) at a stock concentration of 100 mM. ATV was added to cultures for 48 h at different concentrations (0·5, 1, 2, 5 or 10 μM) that have been widely used to evaluate statin effects on several cellular subpopulations 12, 21. The highest DMSO concentration used in the experiments was 0·01%, which is below the concentrations causing any toxic effects on the cultures, as has been reported previously 29. Thus, the T cell subpopulations (Tcon and Treg) were cultured in the following conditions: unstimulated (or so‐called ‘basal condition’), stimulated and stimulated and treated with different ATV concentrations.
Viability assay
To assess viability, CD4+ T cells were harvested after 48 h incubation under the previously mentioned conditions and washed twice with phosphate buffered saline (PBS). They were then stained with 700 nM 3,3‐dihexyloxacarbocyanine iodide (DIOC‐6; Invitrogen Life Technologies, Paisley, UK) and 1 µg/mL 7‐aminoactinomycin D (7‐AAD; Invitrogen) for 20 min at room temperature in the dark. The cells were analysed on a LSR Fortessa flow cytometer (BD Biosciences).
Suppression assay
CD25–CD127+ T cells were labelled with carboxyfluorescein succinimidyl ester (CFSE) at 1·25 μM (Molecular Probes, Eugene, OR, USA). CD25highCD127low/– Tregs were treated under the conditions mentioned above for 48 h. Cells were then washed twice with PBS and co‐cultured in 96‐well round‐bottomed plates at a 1:1 ratio in the presence of anti‐CD3/CD28 plus IL‐2 for 72 h. The proliferation of CD4+ T cells was detected as the dilution of CFSE by flow cytometry using FlowJo software version 9.7.6 (TreeStar, Inc., Ashland, OR, USA). The percentage of suppression was calculated as follows: [100 − (% proliferation of Tcons in the presence of Tregs/% proliferation of Tcons alone) × 100].
Flow cytometry
The phenotype, activation status and cytokine production of both Tcons and Tregs in the presence or absence of ATV was assessed by flow cytometry using anti‐human mAbs against surface and intracellular molecules (Supporting information, Table S1). In some cultures, the cells were restimulated with 50 ng/ml phorbol myristate acetate (PMA) (Sigma‐Aldrich) plus 1 μg/ml ionomycin (Sigma‐Aldrich) in the presence of 10 μg/ml brefeldin A (Sigma‐Aldrich) for 5 h to detect cytokines. Antibodies against surface molecules were incubated for 20 min at 4°C. For intracellular staining the cells were fixed/permeabilized according to the manufacturer’s instructions using the FoxP3 staining kit (eBiosciences) and incubated with specific antibodies for 30 min. The samples were acquired on an LSR Fortessa flow cytometer (Becton‐Dickinson, Franklin Lakes, NJ, USA) with FACSDiva software version 6.1.3 (BD Biosciences) and the data were analysed using FlowJo software version 9.7.6 (TreeStar). In the design of initial experiments, fluorescence minus one controls were used to distinguish positive from negative populations and set the gates. Relevant biological controls, such as unstimulated and stimulated cells, were used to determine the positive populations and the dynamic of antibody fluorescence in further experiments, as previously suggested 30. Doublets and dead cells were excluded based on the forward/side light‐scatter profiles and the absence of fluorescence in the live/dead viability staining (Thermo Fisher Scientific, Karlsruhe, Germany).
Measurement of intracellular Ca2+ concentration by flow cytometry
Tcon and Treg cells were stimulated with (anti‐CD3/anti‐CD28/IL‐2) in the presence or absence of ATV for 48 h. The cells were then washed with PBS and ~0·6 × 106 cells were loaded with 4 μg/mL Fluo 3‐AM (Invitrogen) and incubated for 60 min at 30ºC in the presence of 0·02% pluronic F‐127 in Hanks’s buffered saline solution (HBSS) containing 1 mM CaCl2, 1 mM MgCl2 and 1% FBS. The loaded cells were washed and resuspended in PBS. Anti‐CD3 at 5 μg/ml was added to the loaded cells and baseline fluorescence was recorded during the first 60 s using a FACSCanto II flow cytometer; cross‐linking of CD3 was then performed by adding 10 μg/ml of mouse immunoglobulin (Ig)G and the resulting calcium release was recorded for approximately 15 min. The parameters of the kinetics of calcium fluxes were the areas under the curve (AUC), the maximum values (Max), the values of the slopes and the time required to reach the maximum value (Tmax) of Ca2+ were calculated through FlowJo software version 9.7.6 (TreeStar).
Statistical analysis
The results are expressed as mean ± standard error of the mean (s.e.m.). Normal distribution was assessed by Shapiro–Wilk normality test and comparisons between the groups were performed using one‐way analysis of variance (anova) or general linear model (GLM) anova for repeated measurements, followed by Dunnett’s post‐hoc test. Correlations between groups were determined by Spearman’s rank correlation. Statistical analyses were performed with Prism version 8.0 (GraphPad Software, La Jolla, CA, USA). P‐values < 0·05 were considered statistically significant.
Results
High ATV concentration blocks Treg suppressive function
To determine the effect of ATV on the suppressive function of Tregs, we evaluated the proliferation of Tcons cultured with previously preactivated Tregs using anti‐CD3/anti‐CD28/IL‐2 for 48 h in the presence or absence of different concentrations of ATV. While suppressive function was maintained at 0·5–1 μM ATV, 2–10 μM ATV blocked suppressive function (Fig. 1a,b). To rule out the possibility that the loss of suppressive activities was due to increased cell kinetics, DIOC6 or annexin‐V staining was performed. We observed that, similarly to lower ATV concentrations, more than 80% of CD4+ T cells remained viable at 48 and 72 h of TCR activation in the presence of the highest ATV concentration (10 µM) (Supporting information, Fig. S1a,c). Moreover, both Tcons and Tregs remained viable at 10 µM ATV as visualized by a live/dead fixable stain, suggesting that ATV concentrations used in this study were non‐toxic (Supporting information, Fig. S1b,c).
Figure 1.
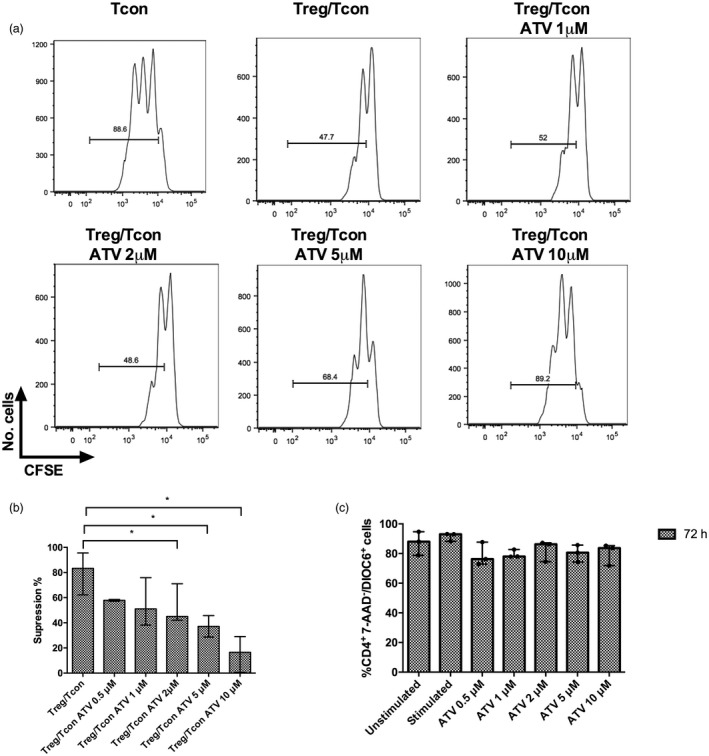
Ten µM atorvastatin (ATV) reduces the suppressive function of regulatory T cells (Tregs). Tregs from healthy donors were preactivated with anti‐CD3, anti‐CD28 and interleukin (IL)‐2 in the absence or presence of the indicated ATV concentrations for 48 h. They were then washed and mixed in a 1:1 ratio with conventional T cells (Tcons) labelled with carboxyfluorescein succinimidyl ester (CFSE) and activated for 72 h. The CFSE dilution was measured by flow cytometry. Representative histograms of four independent experiments are shown. (a) Percentage suppression of responder cell proliferation is shown for each condition (n = 4); (b) untouched total CD4+ T cells were isolated through magnetic separation from the peripheral blood mononuclear cells (PBMCs) of healthy donors and cultured in the conditions mentioned above. Viable cells were determined as (7‐aminoactinomycin D (7‐AAD)– and 3,3‐dihexyloxacarbocyanine iodide (DIOC)‐6+ cells (n = 3 donors) and (c) statistical significance determined by one‐way analysis of variance (anova) and Dunnett’s post‐hoc test (b: co‐cultures +2 μm ATV *P = 0·039, co‐cultures +5 μm ATV *P = 0·022 and co‐cultures +10 μm ATV *P = 0·029 compared with untreated co‐cultures).
High ATV concentrations decrease FoxP3, CTLA‐4 and programmed death 1 (PD‐1) expression on Tregs
We then examined if ATV could modulate the expression of molecules associated with Treg function. ATV at the highest concentration reduced the mean fluorescence intensity (MFI) of FoxP3 and CTLA‐4 to 42 and 20%, respectively, compared with stimulated cultures without ATV treatment (P = 0·018 and P = 0·033, respectively, Fig. 2a,b). Expression of PD1 was also reduced in the presence of 10 μM ATV by 38% compared with control cultures (P = 0·018, Fig. 2c).
Figure 2.
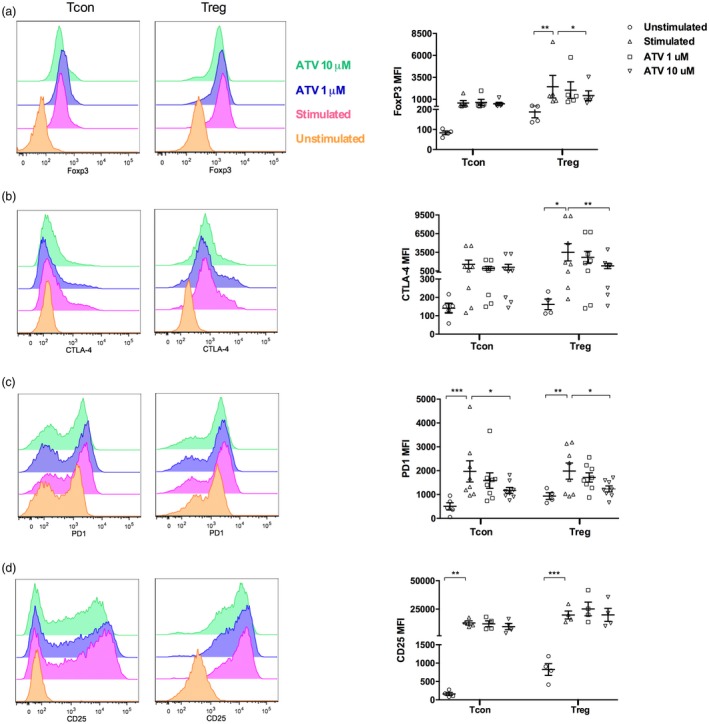
Ten μM atorvastatin (ATV) reduces the mean fluorescence intensity (MFI) of forkhead box protein 3 (FoxP3), cytotoxic T lymphocyte antigen (CTLA‐4) and PD‐1 on regulatory T cells (Tregs). Histogram of representative flow cytometry analysis and MFI analysis of FoxP3 (n = 5 donors) (a), CTLA‐4 (n = 8 donors) (b), programmed death 1 (PD‐1) (n = 8 donors) (c) and CD25 (n = 4 donors) (d) on conventional T cells (Tcons) and Tregs in basal conditions and after activation with anti‐CD3, anti‐CD28 and interleukin (IL)‐2 in the absence or presence of the indicated ATV concentrations for 48 h. Statistical analyses were performed using the general linear model (GLM) analysis of variance (anova) and Dunnett’s post‐hoc tests. Means and standard errors of the mean (s.e.m.) are indicated. *P < 0·05, **P < 0·01 and ***P < 0·001. Each dot represents one individual in the graph.
On Tcons, ATV treatment did not change FoxP3 expression (Fig. 2a) or CTLA‐4 expression patterns compared with stimulated cultures without treatment (Fig. 2b). However, 10 µM ATV significantly reduced expression of PD‐1 by 40% compared with cultures without treatment (P = 0·025, Fig. 2c). No significant differences were observed in CD25 and perforin expression in Tregs and Tcons after ATV treatment (Fig. 2d and Supporting information, Fig. S2).
Ten μM ATV reduced CD45RO expression in Tcons but did not change homing and activation markers on cell subpopulations
As ATV can alter the activation status of different cell subpopulations, we then determined how ATV affects the expression of several surface molecules on both subpopulations. Remarkably, only the highest ATV concentration decreased the expression of CD45RO on Tcons by 17% compared with untreated stimulated cultures (P = 0·037 Fig. 3a). In contrast, CD95, CD69 and CD38 expression was not modified by any ATV concentration on Tcons or Tregs compared with control cultures (Fig. 3b–d). In addition, we wanted to determine whether ATV changed the circulation pattern of these cell subsets. However, no significant differences in CD62L, CCR7 and CCR5 expression were observed after treatment with statins in both subpopulations (Supporting information, Fig. S3a–c).
Figure 3.

Ten μM atorvastatin (ATV) reduced CD45RO expression only in conventional T cells (Tcons), but did not change activation markers on cell subpopulations. Representative flow cytometry histogram and mean fluorescence intensity (MFI) analysis of CD45RO (n = 5 donors) (a), CD95 (n = 3 donors) (b), CD69 (n = 8 donors) (c) and CD38 (n = 5 donors) molecules (d) in Tcons and regulatory T cells (Tregs) under basal conditions and after activation with anti‐CD3, anti‐CD28 and interleukin (IL)‐2 in the absence or presence of the indicated ATV concentrations for 48 h. Statistical analyses were performed using general linear model (GLM) analysis of variance (anova) and Dunnett’s post‐hoc tests. Means and standard errors of the mean (s.e.m.) are presented. *P < 0·05, **P < 0·01 and ***P < 0·001. Each dot represents one individual in the graph.
Tregs treated with 10 µM ATV produce more TNF‐α
Functional plasticity is a feature of whole T cell subpopulations, including Tregs that can gain the inflammatory functions of Th1, Th2 or Th17 cells influenced by the cytokine environment. Unlike Tcons, we observed a lower frequency of Tregs producing IL‐2, IFN‐γ and TNF‐α cytokines after anti‐CD3/anti‐CD28 stimulation. The presence of 10 μM ATV reduced the frequency of IFN‐γ‐producing Tregs to approximately 76% compared with untreated stimulated cultures (P = 0·033, Fig. 4a). Interestingly, the high concentration of ATV increased the percentage of TNF‐α‐producing Tregs from 12 to 25% (P = 0·026, Fig. 4b). Interestingly, we observed a positive correlation between TNF‐α‐producing Tregs and the MFI of CTLA‐4 on Tregs (r = 0·553, P = 0·007, Supporting information, Fig. S4a) but not with the MFIs of FoxP3 or PD‐1 on Tregs (Supporting information, Fig. S4b,c). On the contrary, ATV treatment did not modify the production of IL‐2, IL‐10 and IL‐4 in Tcons and Tregs (Fig. 4c,d and Supporting information, Fig. S5).
Figure 4.
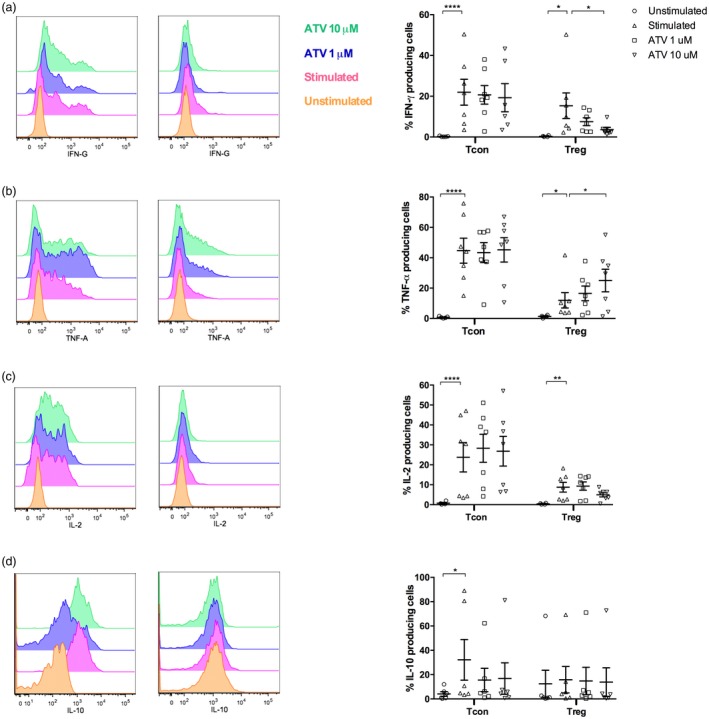
Regulatory T cells (Tregs) treated with 10 µM atorvastatin (ATV) produce more tumour necrosis factor (TNF)‐α than untreated‐Tregs. Conventional T cells (Tcons) and Tregs were isolated by cell sorting and stimulated with anti‐CD3, anti‐CD28 and IL‐2 in the absence or presence of the indicated ATV concentrations for 48 h. Cells were restimulated with phorbol myristate acetate (PMA)/ionomycin in the presence of brefeldin A for 5 h. Then, they were stained with monoclonal antibodies to detect the percentage of cells expressing interferon (IFN)‐γ (n = 7 donors) (a), cells expressing TNF‐α (n = 7 donors) (b), cells expressing interleukin (IL)‐2 (n = 7 donors) (c) and cells expressing IL‐10 (n = 6 donors) (d) by flow cytometry. Statistical analyses of percentages were performed using general linear model (GLM) analysis of variance (anova) and Dunnett’s post‐hoc tests. Means and standard errors of the mean (s.e.m.) are indicated. *P < 0·05, **P < 0·01 and ****P < 0·0001. Left panels show representative flow cytometry histograms, and each dot on the right graphs represents one individual.
Higher ATV concentrations impaired Ca2+ flux in Tcons
To determine the influence of ATV on early events downstream of TCR activation, we evaluated Ca2+ mobilization elicited by CD3 on both cell populations by flow cytometry (Fig. 5a). In general, in response to CD3, we observed a higher Ca2+ mobilization in Tcons compared with Tregs, corroborating previous work 31. When the Tcons were treated with ATV, there was a marked reduction in the AUC from 161 133 (without ATV) to 135 581 (P = 0·606) and to 105 447 (P = 0·020) with 1 µM and 10 µM ATV, respectively. In the case of Tregs, the effect of ATV was less evident. ATV induced a negligible increase in the AUC from 121 108 to 124 604 and 126 099 at 1 and 10 µM ATV, respectively, but there was also a delay in the times to achieve the maximal peaks (Fig. 5b). The effect of ATV on Tcons was also evident, probably being dose‐dependent regarding the maximum values (Max), as ATV decreased these values from 381 to 260 (P = 0·750) and 175 (P = 0·011) at 1 and 10 µM ATV, respectively (Fig. 5a,b).
Figure 5.
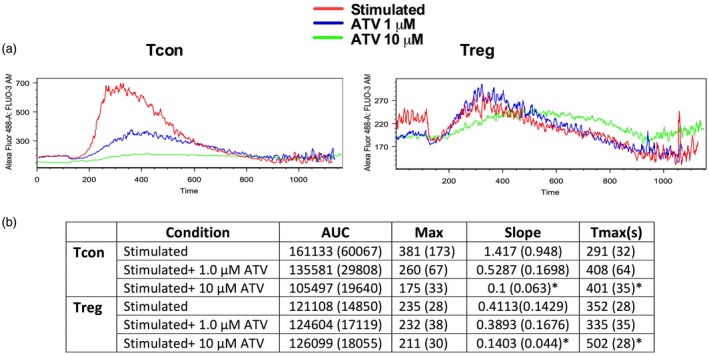
Ten μM atorvastatin (ATV) impairs Ca2+ flux in conventional T cells (Tcons). Representative histograms of Ca2+ flux in Tcons and regulatory T cells (Tregs) isolated and activated with anti‐CD3, anti‐CD28 and interleukin (IL)‐2 in the presence or absence of different ATV concentrations for 48 h. Cross‐linking of the CD3 monoclonal antibody (mAb) was performed through immunoglobulin (Ig)G (a). Area under the curve (AUC), Max value, slope and Tmax (b). Statistical analyses were performed using the general linear model (GLM) analysis of variance (anova) and Dunnett’s post‐hoc tests. Means and standard errors of the mean (s.e.m.) are indicated (n = 3 donors); *P < 0·05.
Discussion
In recent years, statins have been proposed as positive regulators of Tregs. Here we found that pretreatment of Tregs with 0·5–1 μM ATV did not modify suppressive function, and while at the highest ATV concentrations (2–10 μM), Tregs lost their suppressive function and became TNF‐α‐producing Tregs.
Early reports suggested that statins up‐regulate Treg suppressive function 15, 23, 32; however, subsequent studies demonstrated contradictory results that could be due to differences in assays or other present technical issues. For instance, in several in‐vitro experiments, regardless of the concentration used, statins were added directly into Treg/Tcon co‐cultures 16; this could lead to an over‐estimation of the Treg suppressive function because of statin‐induced anti‐proliferative effects on Tcons 14, an event that we also witnessed (Supporting information, Fig. S6). Although statins can also affect the proliferative response of Tregs 17, 33, further studies are necessary to determine if Tregs are less susceptible to statin‐induced anti‐proliferative activities. In other studies, human Tregs were obtained as CD4+CD25+ T cells, which might not represent true Tregs; therefore, activated Tcons could proliferate in the presence of strong stimulation, masking the suppression activity of Tregs 15, 34. This could be problematic, especially when the proliferative response is evaluated through [3H]‐thymidine incorporation, which does not particularly distinguish the proliferating cell population 35. Similarly, contradictory results could be derived from the type of statin used, considering the lipophilic or hydrophilic nature of these drugs, that could facilitate or block its spread across the cell membrane 36. Moreover, others have demonstrated higher Treg suppressive capacity in the presence of high concentrations of statins in vitro (25 µM) 32; however, these concentrations could affect also cell viability 37. Although we used supratherapeutic ATV concentrations (1 μM), this did not affect Treg suppressive function, which was similar to the effect of 0·001 μM ATV 37, a similar concentration to that found in individuals taking low‐dose statins 38. Similar to our findings, pretreatment with simvastatin at 2 μM or ATV or lovastatin at 5 μM impaired Treg suppressive capability in mice, as evaluated by the [3H]‐thymidine assay 33, highlighting the relevance of the mevalonate pathway in co‐ordinating Treg functions.
We found that sorted Tregs showed an activated phenotype, as has been reported previously, with higher expression of FoxP3, CD25, and PD‐1 compared with their Tcon counterparts. However, this feature was lost when they were cultured with the highest ATV concentration as a reduction of these molecules was observed. In line with these findings, previous reports have observed a down‐regulation of functional markers such as CTLA‐4, the inducible co‐stimulator (ICOS) and the glucocorticoid‐induced TNFR‐related protein (GITR) on murine Tregs pretreated with ATV, lovastatin or simvastatin at concentrations ≥ 2 μM 33, 39 as well as FoxP3 expression on CD4+CD25+ cells treated in vitro with 10 µM ATV 37, highlighting the relevance of the mevalonate pathway co‐ordinating Treg functions. Accordingly, the loss of FoxP3 in response to 10 μM ATV could be associated with reduced expression of CTLA‐4 and PD‐1 and with the inhibition of Treg suppressive function, as it has been previously suggested 40, 41. Interestingly, these effects were partially observed on Tcons, as only the expression of PD‐1 along with CD45RO was reduced by 10 μΜ ATV. Moreover, activation and homing markers such as CD25, CD69, CD95, CD38, CD62L and CCR7 were not modified by ATV on Tcons or Tregs. Our results are partially supported by others; for instance, CD45RO expression has been previously shown to be reduced after ATV treatment 11; however, several authors 9, 11, 12 have demonstrated a reduction in CD25 expression after ATV treatment, whereas it remained unchanged in our experiments. Similarly, the ATV effects on CD69 expression has been controversial 9, 11, 12, possibly because to differences in stimuli, activation time and experimental conditions. In general, these findings contrasted with our previous results that demonstrated higher expression levels of functional markers on Tregs in individuals on temporary treatment with statins 17. Such a difference could be explained because therapeutic doses are not equivalent to the doses used in vitro or is probably because of transitory regulation. As our study was limited to in‐vitro assays, these results could not be directly extrapolated to individuals on statin therapy. Thus, more studies are necessary to understand the dynamics of Tcon and Treg activation through clinical trials that allow evaluating the effects of long‐term statin use on these cell subsets.
In addition to phenotypical changes, ATV induced alterations on the Ca2+ influx, as 10 μM ATV reduced maximal peak (Max) and total mobilized Ca2+ (AUC) elicited by CD3 on Tcons. Although both ATV concentrations delayed the time required to reach the peak Ca2+ concentration (slope and Tmax) on Tcons, there was no statistical significance. Altogether, these results suggested that Tcons are more susceptible than Tregs to the effect of ATV. Probably, this lower effect on Tregs could be due to their TCR‐induced attenuated Ca2+ mobilization compared with Tcons 31. Furthermore, this lower Ca2+ mobilization on Tcons could be related to lower proliferation. It is noteworthy to mention that although 10 μM ATV reduced Ca2+ mobilization, it did not affect cytokine production by Tcons as Ca2+ activates nuclear factor of activated T cells (NFAT), which is essential to cytokine production 42. In contrast, ATV‐treated Tregs become TNF‐α‐producing cells, which could be attributed to lower FoxP3 expression that, in turn, down‐regulated CTLA‐4 expression, allowing for inflammatory phenotypes by producing Th1 cytokines 43, 44.
Taken together, our results suggested that 10 μM ATV disturbs the function of Tregs, diminishing their ‘regulator’ phenotype via decreasing the expression of surface molecules and suppressive function and inducing the acquisition of an inflammatory profile (TNF‐α‐producing Tregs). This concentration also affected Tcon function by reducing their proliferation, delaying Ca2+ mobilization and loss of PD‐1 expression, suggesting that ATV differentially affected the function of both cell populations. Moreover, our findings may explain why some individuals taking statins over a prolonged time at high doses are at higher risk to develop autoimmune diseases 45. Although our results are interesting, the low sample size in our study does not allow us to generalize our conclusions, and thus more studies are needed to determine the effect of low concentrations of ATV that simulate the plasmatic concentrations reached in individuals taking low therapeutic doses of statins on Treg function and the role of the mevalonate pathway on the biology of Tregs.
Disclosures
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Supporting information
Fig. S1. Untouched total CD4+ T cells were isolated through magnetic separation from PBMCs of healthy donors and cultured during 48 h under TCR activation as it was mentioned on material and methods. Viable cells were determined as 7‐AAD‐ and DIOC‐6+ cells (n = 3 donors) (a). Representative flow cytometry plot showing live and dead isolated T cells (Tcon and Treg) by using Live/dead fixable cell stain (b). Percentage analysis of live cells in subsets of T con and Treg (c). No statistical significant differences were found (n = 4).
Fig. S2. Mean fluorescence intensity (MFI) analysis of perforin (n = 3 donors) on Tcons and Tregs in basal conditions and after activation with anti‐CD3, anti‐CD28 and IL‐2 in the absence or presence of the indicated concentrations of ATV for 48 h. No significant differences were found between groups.
Fig. S3. Representative flow cytometry histogram and MFI analysis of CD62L (n = 8 donors) (a), CCR7 molecules (n = 3 donors) (b), and CCR5 (n = 4 donors) in Tcons and Tregs in basal conditions and after activation with anti‐CD3, anti‐CD28 and IL‐2 in the absence or presence of the indicated concentrations of ATV for 48 h. Statistical analyses were performed using the GLM ANOVA, Dunnett’s post‐hoc tests. Mean and SEM. ****P < 0·0001, ***P < 0·0002. Each dot represents one individual in the graph.
Fig. S4. Correlation of TNF‐a‐producing Tregs percentage with CTLA‐4 MFI (a), FoxP3 MFI (b), and PD1 MFI (c) in Treg cells. Spearman’s rank correlation coefficients (r) and p values (P) are indicated.
Fig. S5. Tcons and Tregs were isolated by cell sorting and stimulated with anti‐CD3, anti‐CD28 and IL‐2 in the absence or presence of the indicated concentrations of ATV for 48 h. Cells were re‐stimulated with PMA ionomycin in the presence of brefeldin A for 5 h. Then, they were stained with monoclonal antibodies to detect the percentage of cells expressing IL‐4 (n = 7 donors). No significant differences were found between groups.
Fig. S6. Untouched total CD4+ T cells were isolated through magnetic separation fromÐBMCs of healthy donors and they were labeled with CFSE at 1·25 μM. Cells were washed twice with PBS and put in co‐cultured in 96‐well round‐bottom plates at a ratio 1:1 in the presence of CD3/CD28/IL‐2 for 72 hours and different atorvastatin (ATV) concentrations. Proliferation of CD4+ T cells was detected as the dilution of CFSE on flow cytometry and Index division (ID) was calculated using FlowJo software. (n = 3 donors). Statistical analyses were performed using One‐Way ANOVA, Dunnett's post hoc test. Mean and SEM. *P = 0·011, **P = 0·001.
Table S1. Antibodies used for flow cytometry.
Acknowledgements
We thank all the volunteers involved in this study. This work was supported by grants 111551928730, 207‐2010 from the Departamento Administrativo de Ciencia, Tecnología e Innovación (COLCIENCIAS) and the Universidad de Antioquia UdeA (Sostenibilidad and CODI acta 624 de 2012) for its financial support. The authors wish to thank Anne‐Lise Haenni from the Université Paris‐Diderot for her positive revision and English proofreading.
References
- 1. Mor A, Planer D, Luboshits G et al Role of naturally occurring CD4+ CD25+ regulatory T cells in experimental atherosclerosis. Arterioscler Thromb Vasc Biol 2007;27:893–900. [DOI] [PubMed] [Google Scholar]
- 2. Kingsley CI, Karim M, Bushell AR, Wood KJ. CD25+CD4+ regulatory T cells prevent graft rejection: CTLA‐4‐ and IL‐10‐dependent immunoregulation of alloresponses. J Immunol 2002;168:1080–6. [DOI] [PubMed] [Google Scholar]
- 3. Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self‐tolerance maintained by activated T cells expressing IL‐2 receptor alpha‐chains (CD25). Breakdown of a single mechanism of self‐tolerance causes various autoimmune diseases. J Immunol 1995;155:1151–64. [PubMed] [Google Scholar]
- 4. Ling EM, Smith T, Nguyen XD et al Relation of CD4+CD25+ regulatory T‐cell suppression of allergen‐driven T‐cell activation to atopic status and expression of allergic disease. Lancet 2004;363:608–15. [DOI] [PubMed] [Google Scholar]
- 5. Mottet C, Uhlig HH, Powrie F. Cutting edge: cure of colitis by CD4+CD25+ regulatory T cells. J Immunol 2003;170:3939–43. [DOI] [PubMed] [Google Scholar]
- 6. Sakaguchi S. Regulatory T cells: key controllers of immunologic self‐tolerance. Cell 2000;101:455–8. [DOI] [PubMed] [Google Scholar]
- 7. Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell 2008;133:775–87. [DOI] [PubMed] [Google Scholar]
- 8. Greenwood J, Steinman L, Zamvil SS. Statin therapy and autoimmune disease: from protein prenylation to immunomodulation. Nat Rev Immunol 2006;6:358–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ghittoni R, Patrussi L, Pirozzi K et al Simvastatin inhibits T‐cell activation by selectively impairing the function of Ras superfamily GTPases. FASEB J 2005;19:605–7. [DOI] [PubMed] [Google Scholar]
- 10. Azor MH, dos Santos JC, Futata EA et al Statin effects on regulatory and proinflammatory factors in chronic idiopathic urticaria. Clin Exp Immunol 2011;166:291–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brinkkoetter PT, Gottmann U, Schulte J, van der Woude FJ, Braun C, Yard BA. Atorvastatin interferes with activation of human CD4(+) T cells via inhibition of small guanosine triphosphatase (GTPase) activity and caspase‐independent apoptosis. Clin Exp Immunol 2006;146:524–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Blank N, Schiller M, Krienke S et al Atorvastatin inhibits T cell activation through 3‐hydroxy‐3‐methylglutaryl coenzyme A reductase without decreasing cholesterol synthesis. J Immunol 2007;179:3613–21. [DOI] [PubMed] [Google Scholar]
- 13. Zhao N, Dong Q, Qian C et al Lovastatin blocks Kv1.3 channel in human T cells: a new mechanism to explain its immunomodulatory properties. Sci Rep 2015;5:17381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dunn SE, Youssef S, Goldstein MJ et al Isoprenoids determine Th1/Th2 fate in pathogenic T cells, providing a mechanism of modulation of autoimmunity by atorvastatin. J Exp Med 2006;203:401–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tang TT, Song Y, Ding YJ et al Atorvastatin upregulates regulatory T cells and reduces clinical disease activity in patients with rheumatoid arthritis. J Lipid Res 2011;52:1023–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mausner‐Fainberg K, Luboshits G, Mor A et al The effect of HMG‐CoA reductase inhibitors on naturally occurring CD4+CD25+ T cells. Atherosclerosis 2008;197:829–39. [DOI] [PubMed] [Google Scholar]
- 17. Rodríguez‐Perea AL, Montoya CJ, Olek S, Chougnet CA, Velilla PA. Statins increase the frequency of circulating CD4+ FOXP3+ regulatory T cells in healthy individuals. J Immunol Res 2015;2015:762506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mira E, Leon B, Barber DF et al Statins induce regulatory T cell recruitment via a CCL1 dependent pathway. J Immunol 2008;181:3524–34. [DOI] [PubMed] [Google Scholar]
- 19. Kagami S, Owada T, Kanari H et al Protein geranylgeranylation regulates the balance between Th17 cells and Foxp3+ regulatory T cells. Int Immunol 2009;21:679–89. [DOI] [PubMed] [Google Scholar]
- 20. Zhang X, Jin J, Peng X, Ramgolam VS, Markovic‐Plese S. Simvastatin inhibits IL‐17 secretion by targeting multiple IL‐17‐regulatory cytokines and by inhibiting the expression of IL‐17 transcription factor RORC in CD4+ lymphocytes. J Immunol 2008;180:6988–96. [DOI] [PubMed] [Google Scholar]
- 21. Jameel A, Ooi KG, Jeffs NR, Galatowicz G, Lightman SL, Calder VL. Statin modulation of human T‐cell proliferation, IL‐1β and IL‐17 production, and IFN‐γ T cell expression: synergy with conventional immunosuppressive agents. Int J Inflam 2013;2013:434586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim YC, Kim KK, Shevach EM. Simvastatin induces Foxp3 T regulatory cells by modulation of transforming growth factor‐beta signal transduction. Immunology 2010;130:484–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Meng X, Zhang K, Li J et al Statins induce the accumulation of regulatory T cells in atherosclerotic plaque. Mol Med 2012;18:598–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jougasaki M, Ichiki T, Takenoshita Y, Setoguchi M. Statins suppress interleukin‐6‐induced monocyte chemo‐attractant protein‐1 by inhibiting Janus kinase/signal transducers and activators of transcription pathways in human vascular endothelial cells. Br J Pharmacol 2010;159:1294–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pabbisetty SK, Rabacal W, Maseda D et al KLF2 is a rate‐limiting transcription factor that can be targeted to enhance regulatory T‐cell production. Proc Natl Acad Sci USA 2014;111:9579–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pabbisetty SK, Rabacal W, Volanakis EJ et al Peripheral tolerance can be modified by altering KLF2‐regulated Treg migration. Proc Natl Acad Sci USA 2016;113:E4662–E4670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Law MR, Wald NJ, Rudnicka AR. Quantifying effect of statins on low density lipoprotein cholesterol, ischaemic heart disease, and stroke: systematic review and meta‐analysis. BMJ 2003;326:1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chellappa S, Lieske NV, Hagness M, Line PD, Taskén K, Aandahl EM. Human regulatory T cells control TCR signaling and susceptibility to suppression in CD4+ T cells. J Leukoc Biol 2016; 100:5–16. [DOI] [PubMed] [Google Scholar]
- 29. de Abreu Costa L, Henrique Fernandes Ottoni M, Dos Santos MG et al Dimethyl sulfoxide (DMSO) decreases cell proliferation and TNF‐α, IFN‐γ, and IL‐2 cytokines production in cultures of peripheral blood lymphocytes. Molecules 2017;22:1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mahnke YD, Roederer M. Optimizing a multicolor immunophenotyping assay. Clin Lab Med 2007;27:469–85, v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gavin MA, Clarke SR, Negrou E, Gallegos A, Rudensky A. Homeostasis and anergy of CD4(+)CD25(+) suppressor T cells in vivo . Nat Immunol 2002;3:33–41. [DOI] [PubMed] [Google Scholar]
- 32. Zhao X, Liu Y, Zhong Y et al Atorvastatin improves inflammatory response in atherosclerosis by upregulating the expression of GARP. Mediators Inflamm 2015;2015:841472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zeng H, Yang K, Cloer C, Neale G, Vogel P, Chi H. mTORC1 couples immune signals and metabolic programming to establish T(reg)‐cell function. Nature 2013;499:485–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Moon BI, Kim TH, Seoh JY. Functional modulation of regulatory T cells by IL‐2. PLOS ONE 2015;10:e0141864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang L, Manirarora JN, Wei CH. Evaluation of immunosuppressive function of regulatory T cells using a novel in vitro cytotoxicity assay. Cell Biosci 2014;4:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sirtori CR. The pharmacology of statins. Pharmacol Res. 2014;88:3–11. [DOI] [PubMed] [Google Scholar]
- 37. Guasti L, Maresca AM, Schembri L et al Relationship between regulatory T cells subsets and lipid profile in dyslipidemic patients: a longitudinal study during atorvastatin treatment. BMC Cardiovasc Disord 2016;16:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lins RL, Matthys KE, Verpooten GA et al Pharmacokinetics of atorvastatin and its metabolites after single and multiple dosing in hypercholesterolaemic haemodialysis patients. Nephrol Dial Transplant 2003;18:967–76. [DOI] [PubMed] [Google Scholar]
- 39. Mao R, Xiao W, Liu H et al Systematic evaluation of 640 FDA drugs for their effect on CD4⁺Foxp3⁺ regulatory T cells using a novel cell‐based high throughput screening assay. Biochem Pharmacol 2013;85:1513–24. [DOI] [PubMed] [Google Scholar]
- 40. Sharma MD, Shinde R, McGaha TL et al The PTEN pathway in Tregs is a critical driver of the suppressive tumor microenvironment. Sci Adv 2015;1:e1500845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Le Mercier I, Lines JL, Noelle RJ. Beyond CTLA‐4 and PD‐1, the generation Z of negative checkpoint regulators. Front Immunol 2015;6:418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Feske S. Calcium signalling in lymphocyte activation and disease. Nat Rev Immunol 2007;7:690–702. [DOI] [PubMed] [Google Scholar]
- 43. Williams LM, Rudensky AY. Maintenance of the Foxp3‐dependent developmental program in mature regulatory T cells requires continued expression of Foxp3. Nat Immunol 2007;8:277–84. [DOI] [PubMed] [Google Scholar]
- 44. Fessler J, Raicht A, Husic R et al Novel senescent regulatory T‐cell subset with impaired suppressive function in rheumatoid arthritis. Front Immunol 2017;8:300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. de Jong HJ, Klungel OH, van Dijk L et al Use of statins is associated with an increased risk of rheumatoid arthritis. Ann Rheum Dis 2012;71:648–54. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Untouched total CD4+ T cells were isolated through magnetic separation from PBMCs of healthy donors and cultured during 48 h under TCR activation as it was mentioned on material and methods. Viable cells were determined as 7‐AAD‐ and DIOC‐6+ cells (n = 3 donors) (a). Representative flow cytometry plot showing live and dead isolated T cells (Tcon and Treg) by using Live/dead fixable cell stain (b). Percentage analysis of live cells in subsets of T con and Treg (c). No statistical significant differences were found (n = 4).
Fig. S2. Mean fluorescence intensity (MFI) analysis of perforin (n = 3 donors) on Tcons and Tregs in basal conditions and after activation with anti‐CD3, anti‐CD28 and IL‐2 in the absence or presence of the indicated concentrations of ATV for 48 h. No significant differences were found between groups.
Fig. S3. Representative flow cytometry histogram and MFI analysis of CD62L (n = 8 donors) (a), CCR7 molecules (n = 3 donors) (b), and CCR5 (n = 4 donors) in Tcons and Tregs in basal conditions and after activation with anti‐CD3, anti‐CD28 and IL‐2 in the absence or presence of the indicated concentrations of ATV for 48 h. Statistical analyses were performed using the GLM ANOVA, Dunnett’s post‐hoc tests. Mean and SEM. ****P < 0·0001, ***P < 0·0002. Each dot represents one individual in the graph.
Fig. S4. Correlation of TNF‐a‐producing Tregs percentage with CTLA‐4 MFI (a), FoxP3 MFI (b), and PD1 MFI (c) in Treg cells. Spearman’s rank correlation coefficients (r) and p values (P) are indicated.
Fig. S5. Tcons and Tregs were isolated by cell sorting and stimulated with anti‐CD3, anti‐CD28 and IL‐2 in the absence or presence of the indicated concentrations of ATV for 48 h. Cells were re‐stimulated with PMA ionomycin in the presence of brefeldin A for 5 h. Then, they were stained with monoclonal antibodies to detect the percentage of cells expressing IL‐4 (n = 7 donors). No significant differences were found between groups.
Fig. S6. Untouched total CD4+ T cells were isolated through magnetic separation fromÐBMCs of healthy donors and they were labeled with CFSE at 1·25 μM. Cells were washed twice with PBS and put in co‐cultured in 96‐well round‐bottom plates at a ratio 1:1 in the presence of CD3/CD28/IL‐2 for 72 hours and different atorvastatin (ATV) concentrations. Proliferation of CD4+ T cells was detected as the dilution of CFSE on flow cytometry and Index division (ID) was calculated using FlowJo software. (n = 3 donors). Statistical analyses were performed using One‐Way ANOVA, Dunnett's post hoc test. Mean and SEM. *P = 0·011, **P = 0·001.
Table S1. Antibodies used for flow cytometry.


