Summary
It is easy to argue that vaccine development represents humankind’s most important and successful endeavour, such is the impact that vaccination has had on human morbidity and mortality over the last 200 years. During this time the original method of Jenner and Pasteur, i.e. that of injecting live‐attenuated or inactivated pathogens, has been developed and supplemented with a wide range of alternative approaches which are now in clinical use or under development. These next‐generation technologies have been designed to produce a vaccine that has the effectiveness of the original live‐attenuated and inactivated vaccines, but without the associated risks and limitations. Indeed, the method of development has undoubtedly moved away from Pasteur’s three Is paradigm (isolate, inactivate, inject) towards an approach of rational design, made possible by improved knowledge of the pathogen–host interaction and the mechanisms of the immune system. These novel vaccines have explored methods for targeted delivery of antigenic material, as well as for the control of release profiles, so that dosing regimens can be matched to the time‐lines of immune system stimulation and the realities of health‐care delivery in dispersed populations. The methods by which vaccines are administered are also the subject of intense research in the hope that needle and syringe dosing, with all its associated issues regarding risk of injury, cross‐infection and patient compliance, can be replaced. This review provides a detailed overview of new vaccine vectors as well as information pertaining to the novel delivery platforms under development.
Keywords: delivery, novel, vaccine, vaccination
Introduction
Vaccines are arguably the most important medical technology developed to date, and have provided dramatic reductions in disease morbidity and mortality since Edward Jenner first tested his smallpox vaccine in 1798 1. The World Health Organization (WHO) estimates that vaccinations for diphtheria, tetanus, whooping cough and measles currently prevent 2–3 million deaths per year 2. Smallpox was once one of the most feared diseases, until the implementation of global vaccination programmes enabled it to be declared eradicated in 1979 3. This eradication cost approximately 100 million US dollars ($US), but is estimated to generate annual savings of 1.35 billion $US 4.
Although vaccines have been undeniably successful, improvements in vector production, delivery and ease of use would be of great benefit. Historically, vaccine development has been based on the ‘three Is’ paradigm of Louis Pasteur (isolate, inactivate, inject) 5. However, an improved understanding of immunology, pathology and microbiology is now helping vaccine development to adopt a more ‘rational design’ approach 5, 6. A large portion of these rationally designed vaccines consist of a ‘minimalist’ composition (i.e. they are subunit‐ or peptide‐based), and while this provides safety and cost‐of‐production benefits, they are typically less immunogenic 7. However, it is hoped that the optimization and combination of rational design approaches and the use of novel dosing and adjuvanting strategies can help to close this efficacy gap.
Delivery is an important issue, because the majority of vaccines used currently are still administered with a hypodermic needle, either intramuscularly (e.g. hepatitis B or inactivated poliomyelitis), subcutaneously (e.g. measles or yellow fever) or intradermally [e.g. bacillus Calmette–Guérin (BCG)]. The hypodermic needle is the mainstay of vaccine delivery technology because it provides a direct, low‐cost method of administration with instant validation that the dose has been delivered and an impressive efficacy profile defined over decades of use. However, the many drawbacks and limitations of needle and syringe delivery are beginning to make it look like a rather out‐dated approach. Prime among these limitations is the effect pain and fear of needles has on patient compliance and ultimately vaccination rates 8. In the United Kingdom and United States, infants may have received up to 23 prophylactic inoculations for 10 different pathogens by the age of 18 months 9. A further major concern, for developed but especially developing countries, is the spread of blood‐borne pathogens as a consequence of needle reuse or needle‐stick injuries 10, 11. In 2000, approximately 16 billion injections were administered, of which an alarming 40% were administered with reused equipment in the absence of sterilization 12. This led to an estimated 20·6 million new hepatitis B infections, 2 million new hepatitis C infections and 260 000 new HIV infections 12. In 2000, approximately half of all US physicians and 77% of nurses experienced needle‐stick injuries, which led to 16 000 hepatitis C, 66 000 hepatitis B and 1000 HIV infections 13, 14. It is clear that in the intervening 18 years improved training and working practices have reduced these levels, but a more recent report still showed that 14·9–69·4% of health‐care workers have reported needle‐stick injuries, with the wide range due to differences in practices between countries 15. This report also showed that needle‐stick injuries were responsible for 37–39% of global hepatitis B and C infections in health‐care workers 15. It is no surprise that when faced with this iatrogenicity, researchers have looked to develop alternative approaches that might allow vaccination without the use of a needle. A further limitation of the reliance on needles is the requirement it creates for the use of liquid formulations, which in many cases require expensive cold‐chain transport and storage 16.
This review begins by giving a broad overview of novel approaches in vaccine design and composition and describes how formulation approaches are improving delivery platforms (see below: ‘Novel vaccine designs’ and ‘Novel vaccine delivery platforms’). A further notable means of improving vaccine efficacy, the development and use of technologies for the improved administration of vaccines, is then covered in the later section (see below: ‘Vaccine administration routes and technologies’).
Novel vaccine designs
Virus‐like particles
Virus‐like particles (VLPs) are highly ordered, repetitive structures that contain a high density of viral capsid proteins. This high density of capsid proteins provides copious amounts of conformational viral epitopes, capable of eliciting strong immune responses 17. Crucially, VLPs are formed by the self‐assembly of viral capsid proteins in the absence of any of the infectious nucleic acids from the virus. Thus, they are a potentially safer alternative to the attenuated viruses commonly used for vaccination due to their absolute inability to replicate. VLPs have been shown to be capable of generating strong immune responses, even in the absence of an adjuvant 18.
Being simpler in composition, VLPs also allow faster production of vaccine than traditional methods, which is especially useful for treatment of highly mutating pathogens such as influenza. Traditional production of an influenza vaccine takes 9 months after a new annual strain has been sequenced, but VLP production takes only 3–12 weeks 19, 20. The first VLP vaccine to be brought to market was the vaccine for hepatitis B (Recombivax HB), in 1986, which consists of self‐assembled particles made from the virus capsid protein, hepatitis B surface antigen 21. Since then, VLP vaccines for human papillomavirus (HPV) (Gardasil) and hepatitis E (Hecolin) have also made it to market in 2006 and 2011, respectively 22, 23, with many more undergoing evaluation in clinical trials 24.
Conjugate vaccines
Vaccines that use either live‐attenuated or inactivated pathogens contain a wide array of different antigens, both polysaccharide‐ and protein‐based. However, only a small number of these may be required to induce protective immunity 25, 26. This logic has been further extended for proteins by the realization that each protein contains hundreds of possible immunogenic epitopes, not all of which are necessary. This has led to interest in peptide‐based vaccines 25. However, antigenic epitopes on a protein are not simply a sequence of amino acids, as the peptides used must mimic the conformation of the immunogenic epitope within the native protein. Computational modelling has provided a powerful tool for locating and mapping the conformation of immunogenic epitopes within proteins 27, 28.
Peptide‐ or polysaccharide‐based vaccines tend to be less immunogenic than when they are present on the surface of a pathogen, thus they require the inclusion of an adjuvant when being administered 7, 29. Another possibility is to conjugate the antigen to a second ‘helper’ protein or polysaccharide that is known to increase immunogenicity; however, this can lead to the immune response being redirected towards the helper molecule 26, 30, 31. Careful pairing and orientation of target and helper portions of the vaccine or spatial segregation of the two subunits by use of carrier systems, such as liposomes, are approaches to overcome this issue (Fig. 1) 32, 33. Peptides and polysaccharides are relatively cheap and simple to manufacture synthetically, which also removes the risk of contamination with infectious material, as is possible with traditional live‐attenuated or inactivated vaccines. Conjugate vaccines for haemophilus influenzae type B (Hib), pneumococcus (PCV), meningococcus (MenACWY) and malaria (Mosquirix) have been approved for use in humans. The RTS,S/AS01 (Mosquirix) vaccine, in particular, is a conjugate vaccine of a repeated region of the circumsporozoite protein from Plasmodium sporozoites conjugated to the hepatitis B surface antigen. This conjugate vaccine subsequently assembles into a VLP.
Figure 1.
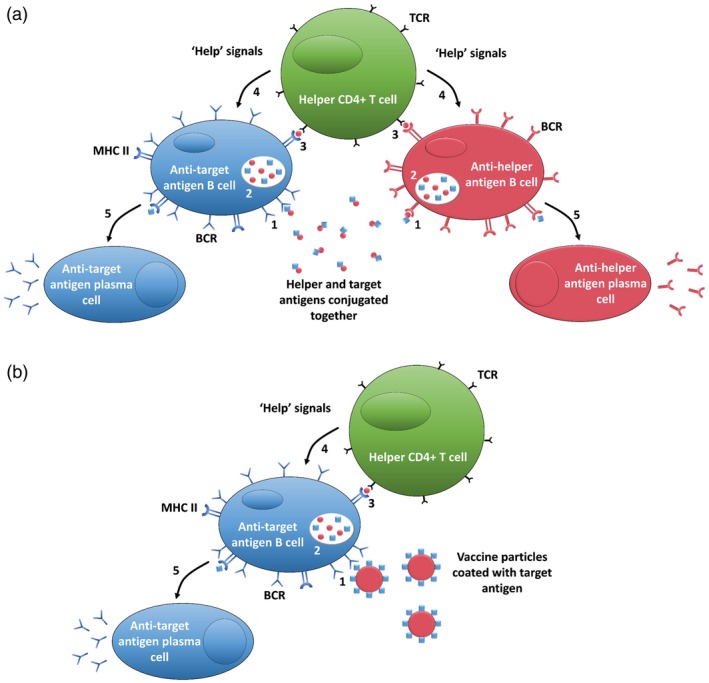
Mechanism of action for initiation of humoral immune responses to a target antigen aided by a secondary helper antigen when they are conjugated together (a) and spatially segregated by use of a liposome (b). (1) B cell with specific B cell receptor (BCR) for target/helper antigen binds to vaccine particle; (2) B cells engulf and digest vaccine particles; (3) vaccine particle antigens are presented on B cells class II major histocompatibility complexes (MHC II) for helper T cell recognition with specific T cell receptor (TCR) for presented antigen; (4) upon TCR binding, T cells produce ‘help’ signals to stimulate B cell differentiation; (5) B cells differentiate into plasma cells that secrete antibodies exclusively targeting antigen recognized by BCR in step 1.
Nucleic acid vaccines
Long‐term gene expression from plasmid DNA has been demonstrated to be achieved by intramuscular injection in mice 34. Such plasmids can be used to encode a viral antigen, which can lead to both humoral and cellular antigen‐specific immune responses 35. These studies led to a huge amount of research into DNA‐based vaccines against a multitude of diseases, such as influenza, human immunodeficiency virus (HIV) and lymphocytic choriomeningitis virus (LCMV) 36, 37, 38. Practically, DNA vaccines are more cost‐effective than protein, whole cell or viral vectors, as DNA can be synthesized by simple scalable chemistry or produced at scale in bacteria. However, the main drawback of DNA vaccines is their low immunogenicity due to the very low transfection rate they achieve. Non‐condensed plasmid is a highly distended and negatively charged structure, which is prone to degradation in the extracellular compartment and lacks a mechanism to achieve cell entry. Even if cell entry can be achieved, localization to and entry into the nucleus, which are required to achieve transcription, are extremely inefficient 39. To increase immunogenicity, plasmids can be designed to encode multiple antigens as well as other immunostimulatory molecules to induce an adjuvanted immune response 40. While no DNA vaccine has yet been licensed for use in humans, there have been several licensed for veterinary use, including West Nile (West‐Nile Innovator DNA) and salmon pancreas disease (Clynav) 41, 42.
The limitations of DNA vectors have resulted in RNA‐based vaccines gaining momentum in recent years 43. Like DNA‐based vaccines, they are low‐cost and can be manufactured rapidly on a large scale. However, their application has previously been restricted by the instability of RNA and inefficient in‐vivo delivery 43. Several methods of structural modification have been employed to increase the intracellular stability of the RNA molecules 44. Crucially, in contrast to DNA, RNA does not require targeting to and entry into the nucleus, so the main barrier RNA vaccines face is cell entry 45. This can be addressed by formulation with polycationic carrier molecules that can condense and protect the RNA and aid its rapid cellular uptake 46.
The main focus of RNA‐based vaccine development has been cancer, with numerous Phases I–III clinical trials in progress 43, 47. For infectious pathogens, two major types of RNA vaccine have been utilized: non‐replicating and self‐amplifying. Non‐replicating RNA vaccines are simpler and less expensive to manufacture, but may be limited in the duration and level of expression they can achieve. Self‐amplifying RNA systems can be based on sequences and principles borrowed from single‐positive strand RNA viruses, such as alphaviruses (Alphavax). These vectors encode the non‐structural genes and the immunogen, but no structural genes, so in theory can achieve a single replication cycle without the risk of infectious virus production. They therefore enable a small dose of vaccine to produce a large amount of antigen due to intracellular amplification of the antigen‐encoding RNA. Several clinical trials using RNA‐based vaccination have been undertaken for infectious pathogens such as HIV, rabies and zika 48, 49, 50. While RNA may currently seem to be the more attractive of the nucleotide‐based options, it should be noted that DNA potentially provides advantages in terms of coding capacity and the level and duration of immunogenic protein production. Should the delivery barrier faced by DNA be overcome, a resurgence in the interest in its use may follow. The recent development of scalable, cell‐free, enzyme‐driven DNA production technologies strengthens the case for the translatability of DNA vaccines 51.
Cellular vaccines
Due to the history of success of vaccination using live‐attenuated viruses, inactivated viruses or bacteria, attempts have been made to apply a similar approach to vaccinate against cancer. Attenuated tumour cells have been administered to induce an immune response against specific types of cancers. Two types of whole cell vaccines have been used: autologous and allogenic. Autologous cell vaccines have been tested on a variety of cancers, including lung, colorectal, melanoma, renal and prostate cancer 52, 53, 54, 55, 56. However, autologous cell vaccines are limited to only certain types and stages of tumours, as they require a sufficient amount of the patient’s tumour for preparation. In contrast, allogenic cell vaccines typically contain a combination of established human tumour cell lines and so, while not being patient‐specific, they do not have the production limitations of autologous cell vaccines. Many whole cell vaccines have been genetically modified to increase immune stimulation by inducing expression of cytokines, chemokines and co‐stimulatory molecules 55, 57, 58. To date, their clinical impact has been demonstrated by GVAX, a vaccine for the treatment of pancreatic cancer, currently in Phase II clinical trials 59.
Another kind of cellular‐based vaccination exploits a patient’s own immune cells, specifically their dendritic cells. Dendritic cell vaccines can be formulated by loading a patient’s autologous dendritic cells, that are simultaneously treated with immunoadjuvants with either tumour‐associated antigens or nucleic material encoding for tumour‐associated antigens ex vivo. The newly matured, antigen‐loaded dendritic cells are then readministered to the patient to induce anti‐tumour immunity. Dendritic cell vaccines have been tested against prostate, melanoma, renal and glioma tumours in clinical trials 60, 61, 62, 63. The first therapeutic cancer vaccine to receive Food and Drug Administration (FDA) approval was a dendritic cell‐based vaccine for prostate cancer, Sipuleucel‐T (Provenge) 64. This was on the basis of a Phase III trial in 2010, which found that patients receiving Sipuleucel‐T had a 4.1‐month median improvement in overall survival 65, 66. This approval was a great boon for this approach, but the development and approval rate of similar strategies has since been unremarkable. This may reflect factors relating to intellectual property or may be a consequence of the expense of the treatment ($93 000 for three infusions of the treatment). This vaccine regimen requires the isolation of peripheral blood mononuclear cells from the patient, followed by cell culture processing and reinfusion, each of which is a costly and laborious process. Indeed, while these cell‐based approaches are an interesting development, they do not obviously contribute to the continuum of the journey away from live and attenuated vaccines into an era of vaccines with lower complexity and cost of production that are more suited to the treatment of large populations within the confines of reducing health‐care resources.
From this section, it is clear that there is a range of novel vaccines with impressive immunological activity. It is notable that the majority of these have been designed and are being used under the presumption that conventional needle‐and‐syringe delivery offers the best route to their optimal efficacy. The following sections provide information on vaccine formulation delivery platforms and administration routes/technologies which may be worthy of consideration as a means to challenge this dogma and perhaps enhance vaccine utility further.
Novel vaccine delivery platforms
Liposomes
Liposomes are spherical vesicles with a lipid bilayer formed of biocompatible phospholipids. Their main use in vaccinology is either as a delivery vehicle or as an adjuvant 67, 68. A key advantage of liposomes is their plasticity and versatility; the choice of lipids and their formulation method allows control over their charge, size and location of antigen incorporation 69. Liposomes are often made up of four key components: a charged lipid, which affects how liposomes behave in vivo; a lipid‐linked polyethylene glycol (PEG), to increase in‐vivo stability; cholesterol, to increase structural stability; and a phospholipid, which supports the formation of a lipid bilayer. Cationic liposomes, unlike their anionic counterparts, are able to bypass the endosomal–lysomal route of degradation in cells 70. Furthermore, their net positive charge provides a means of condensing nucleic acid constructs (DNA or RNA) into discrete structures capable of achieving entry into target cells 70.
Antigens can be encapsulated within, conjugated to the surface of, or embedded within the lipid bilayer 33, 71. The location of antigen in liposomes influences the type of immune responses generated towards the vaccine. T cell responses are induced by both encapsulated and surface‐conjugated antigens, while B cell responses are exclusively induced by surface‐conjugated antigen 67. Incorporation of CD4 T cell helper epitopes can aid in generating a stronger antibody response to a B cell target antigen 33, 72, and complete spatial segregation of the two antigens by the liposomal bilayer minimizes the immune responses focus on the T cell epitope (Fig. 1) 33. The carrying capacity of liposomes allows immunostimulatory molecules, such as cytokines or Toll‐like receptor agonists, to be co‐delivered to target immune cells, thereby reducing systemic exposure to these adjuvant molecules 33, 67. Liposomes were first used as part of a vaccine for diphtheria toxin in 1974 73. Since then, liposome‐based vaccines for hepatitis A (Epaxal) and influenza (Inflexal V) have been approved for use in humans 74, 75.
Polymeric particles
Polymeric particles have been increasingly researched in the field of vaccine delivery due to their potentially advantageous biocompatibility and biodegradability 76. A wide range of both natural and synthetic polymers has been used to make particles for vaccine delivery, such as polysaccharides 77, poly(D,L‐lactic‐coglycolic acid) (PLGA) 78, poly(lactic acid) (PLA) 78 and poly(D,L‐lactide‐co‐glycolide) (PLG) 79. These particles are able to either entrap or adsorb antigen for delivery to specific cells or allow for sustained antigen release over time because of their slow biodegradation rate 80, 81. Polymeric particles are the main focus for development of a single‐dose delayed‐release vaccine that could replace the need for booster doses of many current vaccines 81. The antigen release profile of a PLGA particle can be modified from a couple of days to more than a year 82, 83. Many studies have also used polymeric particles for their ability to act as an adjuvant, rather than their antigen release profile. Advax, an insulin‐derived microparticle, has been used in clinical trials as an adjuvant for hepatitis B, influenza and insect‐sting allergy vaccines 84, 85, 86.
Inorganic particles
Many inorganic particle‐based vaccines have been studied, despite their low biodegradability. Their main advantage lies in how much control can be achieved over their synthesis 87, 88. Inorganic particles have been used as both adjuvants and antigen delivery vehicles in order to enhance an immune response 89. The four most commonly used types of inorganic particles deployed in vaccines are: gold, aluminium, calcium phosphate and silica. Structures made from pure carbon have also been investigated.
Gold particles are highly stable and can be easily synthesized in a variety of different shapes and sizes 87. Their surface is highly modifiable, making antigen conjugation straightforward in practice 90, 91. However, there is often limited control over the orientation of antigen, which can be suboptimal 92. Gold particles have been used as carriers in several clinical trials for a range of diseases, including melanoma, influenza and hepatitis B 93, 94, 95.
Aluminium is a commonly used adjuvant in vaccines 88, 96, 97, 98. This stimulated some studies into the conjugation of antigen to aluminium nanoparticles 97, 98. These studies showed that the aluminium particles are able to play the role of both carrier and adjuvant to stimulate the immune system, although it has also been shown that aluminium particles are capable of adsorbing antigen so tightly that antigen structure is altered, which reduces vaccine efficacy 98, 99.
Calcium phosphate particles are a promising candidate for vaccine applications as they are bioresorbable, non‐toxic, have adjuvanting properties and can easily be loaded with antigen 100. Previously, calcium phosphate was used as an adjuvant for a commercialized diphtheria, tetanus, pertussis and poliomyelitis vaccine, but was replaced by aluminium salts in the 1980s 101. It has been suggested that calcium phosphate should replace aluminium as an adjuvant in currently commercialized vaccines due to toxicity and side‐effect concerns with aluminium adjuvants 92, 101, 102.
Silica‐based particles are a popular form of inorganic particle in vaccine research, as they are biocompatible and their interactions with cells can be modified by altering their size and shape 103. Their surface can also be modified to allow for improved cellular targeting and cellular uptake 104, 105. Mesoporus silica particles have been shown to be effective antigen carriers for sustained antigen in vivo 106.
Carbon nanoparticles have also been studied extensively for drug and vaccine delivery 76. They can be synthesized into a variety of different nanotubes and mesoporous spheres 107, 108. Carbon nanotubes are potentially capable of carrying multiple antigens and are rapidly taken up by antigen‐presenting cells 109. Carbon nanoparticles have also been synthesized to be responsive to magnetic force and, with a model antigen attached, were used to track, target and manipulate dendritic cells 110. Carbon mesoporous spheres, encapsulating antigen, have been utilized as an oral vaccination method for bovine serum albumin as a model antigen 111.
Plant‐like material
Plant cells are an attractive vaccine delivery platform for oral administration because of the ease and low cost with which large populations could be vaccinated. Furthermore, their tough cell wall is able to protect intracellular material from harsh environments encountered within the stomach 112. Once within the gastrointestinal tract, the cell wall is then broken down by microbes and the intracellular material is released 112. Transgenic plant cells have been produced to express antigenic material for use in vaccination 113. These transgenic cells may then be able to deliver antigenic material to the intestines, where it is free to interact with the gut‐associated lymphoid tissue. Crops such as rice and maize have been utilized as expression vectors. These crops are staple foods in target vaccination areas, are inexpensive to produce and easy to grow in large quantities 114, 115. Plant cell‐based vaccines have been developed for a wide range of pathogens, including influenza, hepatitis B and anthrax 115, 116, 117. A potential limitation of this approach is how the complexity and diversity of the microbiome will impact upon the reliability of the response.
Similar to plant cells, single‐cellular algae also have a tough cell wall, making them another attractive delivery platform. Algae‐based vaccines have many advantages over plant cell‐based vaccines: they are easier to genetically modify, they can be grown in bioreactors and do not require large areas of land, seasonal conditions or extended durations to grow 118. Several pathogens have been targeted preclinically by algae‐based vaccines such as foot‐and‐mouth disease, malaria and staphylococcus 119, 120, 121.
Pollen grains are naturally occurring plant‐based materials that have also recently been investigated as a vaccine delivery platform 122. They consist of a tough outer shell that is used to protect the male gamete of the plant for pollination. The outer shell has been shown to be able to survive the harsh conditions of the stomach, thus making them a possible delivery platform for oral vaccination 123. Recently, it has been shown that Lycopodium clavatum (clubmoss) and Ambrosia elatior (ragweed) spores can be chemically cleaned to remove any native proteins present that risk being allergenic, before being refilled with proteins of interest for vaccination 122, 124. These studies demonstrated that systemic and mucosal antibody responses could be generated against the model antigen, ovalbumin, when encapsulated within the pollen grains. Questions remain concerning the complexity and scalability of this approach.
Infectious material
Bacteria and viruses can be genetically modified to produce antigenic material from another pathogen 125, 126. The bacterial or viral strains used for this method of vaccination are generally considered to be safe, either through natural lack of pathogenicity or through attenuation, but can still closely mimic a natural infection, and therefore can stimulate an immune response. However, the immune response generated is often dominated by a response against the carrier vector and not the desired target antigen 127.
Outer membrane vesicles
Outer membrane vesicles are naturally occurring, non‐replicating vesicles produced by Gram‐negative bacteria 128. They consist of bacterial phospholipids, lipopolysaccharides, outer membrane proteins and entrapped periplasmic components 128, 129. This gives them inherent immunostimulatory properties, as they naturally contain several pathogen‐associated molecular patterns on their surface, which makes them an adjuvanting particle. Antigen can be present either on the surface, inside the lumen of the vesicle or unbound in solution. Antigens within the lumen of the vesicles were believed to be hidden from B cell recognition. However, several groups have reported strong antibody responses to a luminal antigen 130, 131. One of the difficulties of using outer membrane vesicles to target an antigen non‐native to the producing bacteria is that the vesicles naturally contain many immunogenic components, which could lead to an immune response dominantly targeting the vesicle instead of the antigen of interest. To date, two outer membrane vesicle vaccines for meningitis B (Bexsero and Trumenba) have been licensed for use in humans 132, 133.
Immunostimulating complexes
Immunostimulating complexes (ISCOMs) are spherical cage‐like particles that are spontaneously formed by mixing phospholipids, cholesterol, saponin and protein antigens 134, 135. ISCOM formulation requires the use of amphipathic proteins which restricts the type of antigens that can be included in the complex 136. An alternative form of ISCOM is ISCOMATRIX, which is formulated without antigen 136. This approach allows for a more flexible application, as almost any antigen can potentially be mixed with the ISCOMATRIX adjuvant. ISCOMATRIX adjuvant has been used for both prophylactic and therapeutic vaccines in clinical trials 137, 138.
Emulsions
Emulsions are heterogeneous liquid systems commonly used in vaccines as adjuvants. Their simplest iteration is in the form of water‐in‐oil or oil‐in‐water, but they can be formulated in more complex multiple emulsion systems such as water‐in‐oil‐in‐water 139. The antigen release characteristics of an emulsion are determined by a range of factors such as droplet size, viscosity and the oil‐to‐water ratio 140. One emulsion frequently used in human vaccines today is an oil‐in‐water, squalene‐based emulsion, MF59, which has been included in the inactivated flu vaccine, Flaud, since 1997 140, 141.
Vaccine administration routes and technologies
Transdermal
Using a needle and syringe is a very effective method of introducing a substance to the body, as the barrier properties of the skin are very easily breached by a needle. However, use of needles and syringes has many disadvantages, such as pain, needle phobia, risk of needle‐stick injuries and transmission of infections, all of which lead to increased cost and poor patient compliance 8, 12. Therefore, there is a great need for alternative methods of vaccination that do not have these disadvantages. The skin houses a large number of immune cells indicating that the skin is a hub of immunological activity, and a target location for vaccine administration 142. Indeed, when compared to traditional vaccination methods using a needle and syringe, transdermal delivery has been shown to be capable of inducing improved immune responses 143.
Some studies have investigated the use of passive delivery methods to administer vaccines transdermally 144, 145. These efforts have focused on several drugs that are already licensed for transdermal administration, such as nicotine and testosterone 145. However, in order to be amenable to successful passive delivery, vaccine molecules must have a low molecular weight, be reasonably lipophilic and have a very high potency (as the percentage of dose delivered is so low) 145. The main drawback of passive delivery is the long lag time to induce a response 146; this has been demonstrated by one study that showed a prolonged exposure of 16 h to antigen on the skin was needed to induce a potent antigen‐specific response 144.
Arguably, the most explored method of transdermal delivery is in the use of microneedles, which consist of 10s to 1000s of pointed microsized projections fabricated onto a surface 147. There have been a number of different microneedle systems developed, including: solid 148, hollow 148, coated 149 and dissolving 150. Solid microneedles have been fabricated from a range of materials such as silicon, polymers, water‐soluble compounds, metals and ceramics 147 and can be used to permeabilize the skin before topical application of the vaccince 148. Hollow microneedles are similar to hypodermic needles in that they enable pressure‐driven injection of a liquid allowing control over injection rate, although they have also been used to deliver drug reservoirs without the use of a pressure force 147, 148. Coated microneedles use solid microneedles as vehicles to deliver drug or vaccine deposited on their surface into the dermal layers; this may be a quick method to administer the desired dose 147, 149. Dissolving microneedles are an ideal alternative to hypodermic needles, as they are designed to completely dissolve when inserted into the skin, therefore leaving no hazardous sharps waste 147, 150. Microneedles have been used for both transdermal and mucosal vaccination 147, 149. They can be self‐administered without the need for professional training, thus easing the burden on medical staff 147. It is theorized that as microneedles are so small, they are unable to penetrate deep enough into the skin to cause pain, therefore potentially increasing patient compliance 147. To date, no microneedle technology has been FDA‐approved for the delivery of a vaccine. Important remaining barriers to such translation include skin irritation, confirmation of dose delivered, scale‐up and compatibility of vaccines with the microneedle manufacture process. As microneedles contain many microscopic needles, they may not ultimately reduce the amount of medical sharps waste currently generated.
Electricity has also been used to facilitate the delivery of drugs and vaccines transdermally in two differing methods, iontophoresis and electroporation. Iontophoresis relies on the application of an electrical current to drive charged particles into the skin through electrostatic effects 151. Electroporation uses electrical pulses in the order of hundreds of volts for 10 µs–10 ms to temporarily disrupt cellular membranes 152. Due to short pulse length, electroporation largely relies upon compromising the stratum corneum to assist passive diffusion to the layers below 153, although the ability to disrupt target cell membranes also aids in the delivery of nucleic acid‐based vaccines 154. This restricts translatability when compared to a needle and syringe due to an increase in pain experienced by the patient 155, especially when used post‐injection of nucleic acid‐based vaccines. Iontophoresis, in contrast, is believed to have negligible effects on skin architecture over short treatment intervals 152. However, in 2016, Zecuity, a commercialized iontophoretic transdermal device, lost FDA approval after post‐marketing reports of application site reactions, including burns and scars in patients 156.
Sonophoresis is the use of ultrasound to improve transdermal drug and vaccine delivery 157. The main mechanism that drives the enhanced delivery of sonophoresis is cavitation 158. This process involves the application of focused ultrasound to achieve expansion and collapse of gas bubbles which, in turn, creates microstreaming and shockwaves 159. Sonophoresis has been used to either increase the permeability of the skin before topical application of the vaccine or as a method of concurrently applying both cavitation and vaccine, thereby actively pushing the vaccine particles into the skin 160, 161. The recent development of nano‐sized polymeric cavitation nuclei (nanocups), capable of sustaining and promoting cavitation activity, provides a means of sustaining such delivery over extended periods 161, 162. Indeed, when mixed with nanocups and exposed to ultrasound, the model antigen, ovalbumin, was delivered to depths of 500 µm and a specific anti‐ovalbumin antibody response was raised 161. The major limitation of sonophoresis is the inability to deliver comparable amounts of antigen to that of a needle and syringe.
Biolistics involves the use of high pressures to accelerate vaccines to velocities high enough to allow penetration of the stratum corneum and epidermal cell membranes 163. It can be used to deliver a jet of liquid or particulate vaccines 163. Jet injection of liquid vaccine was tested for smallpox vaccination 164 and has also been tested for measles, BCG and influenza 165, 166, 167, 168. In principle, nucleic acid‐based vaccines can be delivered by first coating them onto gold particles and propelling them into the skin using this approach 163. One study found that for successful delivery of particulate vaccine by biolistics, particles must be of a similar size, smaller than 70 µm, have a density above 1 g/ml and be able to maintain physical stability in the process 169. Work continues to improve the compatibility of vaccine to use in biolistic devices 170, 171, but issues remain regarding tissue damage and pain.
Transdermal delivery is an attractive goal because of the potentially easy and pain‐free access it provides to a rich immunological milieu. However, in addition to the unique limitations faced by each of the technologies described above, there is also a general challenge to overcome the huge inter‐ and intraperson hetrogeneity in stratum corneum thickness, hydration levels and hair‐follicle density. Furthermore, in order to depose needle and syringe as the preferred administration technology, all the approaches described above will also face the challenge of matching the impressive needle‐and‐syringe price point. It might be argued that patients in more economically developed countries may be prepared to pay a premium for more bespoke ‘pain‐free’ alternatives to needle and syringe. A suggestion supported by the increased cost of Flumist ($20 versus $15 172). However, it is clear that the ‘dollar‐per‐dose’ ideal for mass vaccination programmes in resource‐challenged countries will provide a substantial initial hurdle to widespread translation of these new approaches, especially as they also often involve bulky, complex equipment. It is difficult to compare the cost of these approaches to that of a needle and syringe in terms of vaccine delivery due to the lack of commercialization. However, in terms of drug delivery, the cost of a Zecuity patch, before the removal of its licence, was $300 per patch 173. It is therefore even more imperative that these new administration technologies show superiority in efficacy and safety.
Mucosal administration
The majority of pathogens invade via mucosal surfaces such as the respiratory, gastrointestinal or reproductive tracts. These surfaces come into direct contact with the air, water and food from our surrounding environment, giving a first point‐of‐contact for opportunistic pathogens. Despite this fact, only five pathogens currently have mucosal vaccines licensed for their treatment: cholera, typhoid, rotavirus, poliomyelitis and influenza. Conventional systemic vaccination procedures using a needle and syringe are generally considered unable to induce strong mucosal immune responses 174. However, delivery of vaccines across mucosal surfaces has the potential to stimulate such responses, providing neutralization of invading pathogens before they are able to cause a widespread infection. Mucosal vaccination has also been shown to be able to elicit systemic immunity comparable to vaccination with a needle and syringe 175. There are, however, many challenges to mucosal vaccination, from the harsh acidic environment of the stomach to the layer of mucus which coats all mucosal surfaces. Despite these challenges, there is a wide range of mucosal vaccination routes being explored, the two most common of which are oral and intranasal; others include ocular, intravaginal and intrarectal.
Oral vaccination is a preferred route for vaccination, as it is painless, safe, low‐cost and does not require trained personnel for administration. The oral route commonly involves swallowing the vaccine which then passes through to the gastointestinal tract. Alternatively, the vaccine can also achieve entry in the oral cavity, which has far less harsh conditions 149, although this method has not been explored in the same depth. In order to induce immunity through the oral gastrointestinal route, a relatively large amount of vaccine must be administered due to factors such as dilution while passing through the gastrointestinal tract, degradation within the stomach or failure to breach the epithelial tight junctions 176. One commonly employed method of reducing the severity of the conditions in the stomach is to include a basic substance such as sodium bicarbonate to neutralize the conditions 122. There are currently oral vaccines licensed for human use for cholera (Dukoral, Vaxchora and Shanchol), poliomyelitis (OPV), rotavirus (Rotarix, RotaTeq, Rotavac, Rotavin‐M1, Lanzhou lamb and Rotasiil) and typhoid (Vivotif) vaccination.
Intranasal vaccination is a popular choice for alternative vaccination methods, as it uses a site that is easily accessible and has the potential for self‐administration. The nasal cavity is also a highly vascularized region with a large surface area for antigen uptake. The nasal route, one of the main sites of pathogen entry, thus inducing a strong local mucosal immunity, is highly desirable. As nasal vaccination delivers the antigenic material directly to the targeted site, a relatively small dose is required when compared to alternative forms of vaccination 177. However, most antigens have very little affinity for the nasal epithelium and thus have a fast clearance rate 178. Therefore, many groups have undertaken research on improving this, resulting in a wealth of patented delivery methods 179. Currently the only licensed intranasal vaccines for use in humans is for influenza (Flumist), which consists of a live attenuated virus delivered by nasal spray.
Many pathogens are transmitted sexually via the genital tract, such as HIV, HPV and chlamydia. Therefore, intravaginal vaccination has been explored as an option to prevent sexually transmitted infections 180. This is a challenging method of generating an immune response as the immunological features of the female reproductive system alter dramatically in response to hormonal fluctuations during the menstrual cycle 181. One reason this method of vaccination has not been explored as extensively as others could be that it only has the ability to immunize females in a population in which, increasingly, it is being shown that it does not provide sufficient herd immunity, especially when considering the impact of immunocompromised females who are unable to receive the vaccinations 182, 183.
Intrarectal vaccination is another method of administration explored to prevent diseases such as enteric pathogens, sexually transmitted diseases and cancer 181, 184, 185, 186. In general, an immune response is more strongly induced at the site of vaccination and in nearby mucosal sites; as such, it is possible to generate both rectal and genital tract immunity in response to an intrarectal vaccination 187. This method of vaccination is not widely used as it has poor acceptability 176.
Conclusions
The field of vaccinology continues to advance at an impressive rate, with more effective and acceptable new vectors and approaches reaching clinical practice. The traditional ‘three Is’ model (isolate, inactivate, inject) of vaccine development is increasingly being phased out for a more rational design paradigm. Occurring alongside the progress of these new rationally designed vaccines is the development of improved and more patient acceptable delivery techniques to target and sustain the pain‐free administration of antigen more effectively. Enhancing methods for administering these vaccines is crucial in reducing the number of needle‐stick injuries and lowering the burden on medical staff by making vaccines more amenable to self‐administration. Unfortunately, new administration technologies (e.g. electroporation, sonophoresis, ionotophoresis) will never be able to match the dose level that can be delivered using a needle and syringe. However, it is hoped that by combining the new delivery methods with the raft of new more effective vaccines this limitation may be negated. Understanding the particular mechanism‐of‐action of new vaccines and vaccine delivery platforms and how their benefits can be enhanced and their limitations mitigated by matching to particular administration routes and technologies will be essential to this process. Vaccines are the world’s most effective, life‐saving, medical technology to date. The literature review presented here indicates that the range of complementary approaches and technologies emerging from research and clinical testing will allow the beneficial impact of vaccines to continue to grow. Potential barriers to the widespread uptake of these approaches will be the cost involved and the complexities of some of technology required, and so these aspects deserve careful consideration during the development phase alongside the scientific and technical challenges.
Acknowledgements
J. W. acknowledges funding from the University of Oxford, the EPSRC and BBSRC Centre for Doctoral Training in Synthetic Biology (grant EP/L016494/1) and the Defence Science and Technology Laboratory (DSTL) (grant DSTLX‐1000102376). R. C. is supported by the EPSRC under the Oxford Centre for Drug Delivery Devices OXCD3 (Grant EP/L024012/1).
Disclosures
The authors declare no competing interests.
OTHER ARTICLES PUBLISHED IN THIS REVIEW SERIES
Vaccines for emerging pathogens: from research to the clinic. Clinical and Experimental Immunology 2019, 196: 155–156.
Emerging viruses and current strategies for vaccine intervention. Clinical and Experimental Immunology 2019, 196: 157–166.
HLA‐E: exploiting pathogen‐host interactions for vaccine development. Clinical and Experimental Immunology 2019, 196: 167–177.
Novel multi‐component vaccine approaches for Burkholderia pseudomallei. Clinical and Experimental Immunology 2019, 196: 178–188.
Mucosal vaccines and technology. Clinical and Experimental Immunology 2019, 196: 205–214.
Vaccines for emerging pathogens: prospects for licensure. Clinical and Experimental Immunology 2019, doi: 10.1111/cei.13284
References
- 1. Riedel S. Edward Jenner and the history of smallpox and vaccination. Proc Baylor Univ Med Center 2005;18:21–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization . Immunization coverage. Available at: http://www.who.int/features/factfiles/immunization/en/ (accessed 26 June 2018).
- 3. World Health Organization . The global eradication of smallpox: final report of the Global Commission for the Certification of Smallpox Eradication, Geneva, December 1979. Geneva: Global Commission for the Certification of Smallpox Eradication and World Health Organization, 1980. [Google Scholar]
- 4. Barrett S. Eradication versus control: the economics of global infectious disease policies. Bull World Health Organ 2004; 82:683–8. [PMC free article] [PubMed] [Google Scholar]
- 5. Bragazzi NL, Gianfredi V, Villarini M, Rosselli R, Nasr A, Hussein A, Martini M, Behzadifar M. Vaccine meet big data: State‐of‐the‐art and future prospects. from the classical 3is (‘isolate‐inactivate‐inject’) vaccinology 1.0 to vaccinology 3.0, vaccinomics and beyond: A historical overview. Front Public Health 2018; 6:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gregorio ED, Rappuoli R. From empiricism to rational design: a personal perspective of the evolution of vaccine development. Nat Rev Immunol 2014; 14:505–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mamo T, Poland GA. Nanovaccinology: the next generation of vaccines meets 21st century materials science and engineering. Vaccine 2012; 30:6609–11. [DOI] [PubMed] [Google Scholar]
- 8. Nir Y, Paz A, Sabo E, Potasman I. Fear of injections in young adults: prevalence and associations. Am J Trop Med Hyg 2003; 68:341–4. [PubMed] [Google Scholar]
- 9. Centers for Disease Control and Prevention . Recommended immunization schedule for children and adolescents aged 18 years or younger, United States, 2018. Available at: https://www.cdc.gov/vaccines/schedules/hcp/imz/child-adolescent-shell.html (accessed: 10 October 2018).
- 10. Nir Y, Paz A, Sabo E, Potasman I. One needle, one syringe, only one time? A survey of physician and nurse knowledge, attitudes, and practices around injection safety. Am J Infect Control 2017; 45:1018–23. [DOI] [PubMed] [Google Scholar]
- 11. Drucker E, Alcabes PG, Marx PA. The injection century: massive unsterile injections and the emergence of human pathogens. Lancet 2001; 358:1989–92. [DOI] [PubMed] [Google Scholar]
- 12. Hauri AM, Armstrong GL, Hutin YJF. The global burden of disease attributable to contaminated injections given in health care settings. Int J STD AIDS 2004; 15:7–16. [DOI] [PubMed] [Google Scholar]
- 13. Rapiti R, Pruss‐Ustun A, Hutin Y. Sharps injuries – assessing the burden of disease from sharps injuries to health‐care workers at national and local levels. Environ Burden Dis Ser 2005; 11. [Google Scholar]
- 14. Prüss‐Üstün A, Rapiti E, Hutin Y. Estimation of the global burden of disease attributable to contaminated sharps injuries among health‐care workers. Am J Ind Med 2005; 48:482–90. [DOI] [PubMed] [Google Scholar]
- 15. Cooke CE, Stephens JM. Clinical, economic, and humanistic burden of needlestick injuries in healthcare workers. Med Devices (Auckland, NZ) 2017; 10:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kumru OS, Joshi SB, Smith DE, Middaugh CR, Prusik T, Volkin DB. Vaccine instability in the cold chain: mechanisms, analysis and formulation strategies. Biologicals 2014; 42:237–59. [DOI] [PubMed] [Google Scholar]
- 17. Akahata W, Yong Yang Z, Andersen H et al A vlp vaccine for epidemic Chikun‐Gunya virus protects non‐human primates against infection. Nat Med 2010; 16:334–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang LF, Zhou J, Chen S et al Hpv6b virus like particles are potent immunogens without adjuvant in man. Vaccine 2000; 18:1051–8. [DOI] [PubMed] [Google Scholar]
- 19. Kaiser J. A one‐size‐fits‐all flu vaccine? Science 2006; 312:380–2. [DOI] [PubMed] [Google Scholar]
- 20. López‐Macías C. Virus‐like particle (vlp)‐based vaccines for pandemic influenza. Human Vaccines and Immunotherapeutics 2012; 8:411–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McAleer WJ, Buynak EB, Maigetter RZ, Wampler DE, Miller WJ, Hilleman MR. Human hepatitis B vaccine from recombinant yeast. Nature 1984; 307:178–80. [DOI] [PubMed] [Google Scholar]
- 22. Siddiqui MAA, Perry CM. Human papillomavirus quadrivalent (types 6, 11, 16, 18) recombinant vaccine (gardasil R). Drugs 2006; 66:1263–71. [DOI] [PubMed] [Google Scholar]
- 23. Wedemeyer H, Pischke S. Hepatitis: hepatitis e vaccination – is hev 239 the breakthrough? Nat Rev Gastroenterol Hepatol 2011; 8:8–10. [DOI] [PubMed] [Google Scholar]
- 24. Huang X, Wang X, Zhang J, Xia N, Zhao Q. Escherichia coli‐derived virus‐like particles in vaccine development. NPJ Vaccines 2017; 2:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li W, Joshi MD, Singhania S, Ramsey KH, Murthy AK. Peptide vaccine: progress and challenges. Vaccines 2014; 2:515–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gonzalez‐Fernandez A, Faroa J, Fernandez C. Immune responses to polysaccharides: lessons from humans and mice. Vaccine 2007; 26:292–300. [DOI] [PubMed] [Google Scholar]
- 27. Droppa‐Almeida D, Franceschi E, Padilha FF. Immune‐informatic analysis and design of peptide vaccine from multi‐epitopes against corynebacterium pseudotuberculosis. Bioinform Biol Insights 2018; 12:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mirza MU, Rafique S, Ali A et al Towards peptide vaccines against zika virus: Immunoinformatics combined with molecular dynamics simulations to predict antigenic epitopes of zika viral proteins. Nat Sci Rep 2016; 6:37313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Perrie Y, Mohammed AR, Kirby DJ, McNeil SE, Bramwell VW. Vaccine adjuvant systems: enhancing the efficacy of sub‐unit protein antigens. Int J Pharm 2008; 364:272–80. [DOI] [PubMed] [Google Scholar]
- 30. Schutze MP, Leclerc C, Jolivet M, Audibert F, Chedid L. Carrier‐induced epitopic suppression, a major issue for future synthetic vaccines. J Immunol 1985; 135:2319–22. [PubMed] [Google Scholar]
- 31. Bröker M, Berti F, Schneider J, Vojtek I. Polysaccharide conjugate vaccine protein carriers as a ‘neglected valency’ – potential and limitations. Vaccine 2017; 35:3286–94. [DOI] [PubMed] [Google Scholar]
- 32. Kagan E, Ragupathi G, Yi SS et al Comparison of antigen constructs and carrier molecules for augmenting the immunogenicity of the monosaccharide epithelial cancer antigen tn. Cancer Immunol Immunother 2005; 54:424–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hills T, Jakeman PG, Carlisle RC, Klenerman P, Seymour LW, Cawood R. A rapid‐response humoral vaccine platform exploiting pre‐existing non‐cognate populations of anti‐vaccine or anti‐viral CD4+ T helper cells to confirm B cell activation. PLOS ONE 2016; 11:e0166383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wolff J, Malone R, Williams P et al Direct gene transfer into mouse muscle in vivo . Science 1990; 247:1465–8. [DOI] [PubMed] [Google Scholar]
- 35. Raz E, Carson DA, Parker SE et al Intradermal gene immunization: the possible role of DNA uptake in the induction of cellular immunity to viruses. Proc Natl Acad Sci USA 1994; 91:19–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ulmer JB, Donnelly JJ, Parker SE et al Heterologous protection against influenza by injection of DNA encoding a viral protein. Science 1993; 259. [DOI] [PubMed] [Google Scholar]
- 37. Wang B, Ugen KE, Srikantan V et al Gene inoculation generates immune responses against human immunodeficiency virus type 1. Proc Natl Acad Sci USA 1993; 90:1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Martins LP, Lau LL, Asano MS, Ahmed R. DNA vaccination against persistent viral infection. Am Soc Microbiol 1995; 69:2574–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zanta MA, Belguise‐Valladier P, Behr J‐P. Gene delivery: a single nuclear localization signal peptide is sufficient to carry DNA to the cell nucleus. Proc Natl Acad Sci USA 1999; 96:91–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Suschak JJ, Williams JA, Schmaljohn CS. Advancements in DNA vaccine vectors, non‐mechanical delivery methods, and molecular adjuvants to increase immunogenicity. Hum Vaccines Immunother 2017; 13:2837–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Redding L, Weiner DB. DNA vaccines in veterinary use. Exp Rev Vaccines 2009; 8:1251–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. European Food Safety Authority (EFSA) , Houston R, Moxon S, Nogu’e F, Papadopoulou N, Ramon M, Waigmann E. Assessment of the potential integration of the DNA plasmid vaccine clynav into the salmon genome. EFSA J 2017; 15:e04689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines – a new era in vaccinology. Nat Rev Drug Discov 2018; 17:261–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sahin U, Kariko K, Tureci O. mRNA‐based therapeutics – developing a new class of drugs. Nat Rev Drug Discov 2013; 13:759–80. [DOI] [PubMed] [Google Scholar]
- 45. Bettinger T, Carlisle RC, Read ML, Ogris M, Seymour LW. Peptide‐mediated RNA delivery: a novel approach for enhanced transfection of primary and post‐mitotic cells. Nucleic Acids Res 2001; 29:3882–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kauffman KJ, Webber MJ, Anderson DG. Materials for non‐viral intracellular delivery of messenger RNA therapeutics. J Controlled Release 2016; 240:227–234. SI: North America Part II. [DOI] [PubMed] [Google Scholar]
- 47. Grunwitz C, Kranz LM. mRNA cancer vaccines – messages that prevail. Curr Top Microbiol Immunol 2017; 405:145–64. [DOI] [PubMed] [Google Scholar]
- 48. Jacobson JM, Routy J‐P, Welles S et al Dendritic cell immunotherapy for HIV‐1 infection using autologous HIV‐1 RNA: a randomized, double‐blind, placebo controlled clinical trial. J Acquir Immune Defic Syndr 2016; 72:31–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Alberer M, Gnad‐Vogt U, Hong HS et al Safety and immunogenicity of a mRNA rabies vaccine in healthy adults: an open‐label, non‐randomised, prospective, first‐in‐human phase 1 clinical trial. Lancet 2017; 390:1511–20. [DOI] [PubMed] [Google Scholar]
- 50. Richner JM, Himansu S, Dowd KA et al Modified mRNA vaccines protect against zika virus infection. Cell 2017; 168:1114–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Walters AA, Kinnear E, Shattock RJ et al Comparative analysis of enzymatically produced novel linear DNA constructs with plasmids for use as DNA vaccines. Gene Ther 2014; 21:645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rüttinger D, van den Engel NK, Winter H, Schlemmer M et al Adjuvant therapeutic vaccination in patients with non‐small cell lung cancer made lymphopenic and reconstituted with autologous PBMC: first clinical experience and evidence of an immune response. J Trans Med 2007; 5:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Schirrmacher V, Fournier P, Schlag P. Autologous tumor cell vaccines for postoperative active‐specific immunotherapy of colorectal carcinoma: long‐term patient survival and mechanism of function. Exp Rev Vaccines 2014; 13:117–30. [DOI] [PubMed] [Google Scholar]
- 54. Méndez R, Ruiz‐Cabello F,Rodríguez T, Campo AD, Paschen A, Schadendorf D, Garrido F. Identification of different tumor escape mechanisms in several metastases from a melanoma patient undergoing immunotherapy. Cancer Immunol Immunother 2007; 56:88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Fishman M, Hunter TB, Soliman H et al Phase II trial of b7–1 (CD‐86) transduced, cultured autologous tumor cell vaccine plus subcutaneous interleukin‐2 for treatment of stage iv renal cell carcinoma. J Immunother 2008; 31:72–80. [DOI] [PubMed] [Google Scholar]
- 56. Berger M, Kreutz FT, Horst JL, Baldi AC, Koff WJ. Phase I study with an autologous tumor cell vaccine for locally advanced or metastatic prostate cancer. J Pharm Pharm Sci 2007; 10:144–52. [PubMed] [Google Scholar]
- 57. Simons JW, Jaffee EM, Weber CE et al Bioactivity of autogous irradiated renal cell carcinoma vaccines generated by ex vivo granulocyte‐macrophage colony‐stimulating factor gene transfer. Can Res 1997; 57:1537–46. [PMC free article] [PubMed] [Google Scholar]
- 58. Asada H, Kishida T, Hirai H et al Significant antitumor effects obtained by autologous tumor cell vaccine engineered to secrete interleukin (Il)‐12 and Il‐18 by means of the EBV/lipoplex. Mol Ther 2002; 5:609–16. [DOI] [PubMed] [Google Scholar]
- 59. Lee V, Rodriguez C, Shupe E‐M et al Phase ii study of GM‐CSF secreting allogeneic pancreatic cancer vaccine (GVAX) with PD‐1 blockade antibody and stereotactic body radiation therapy (SBRT) for locally advanced pancreas cancer (LAPC). J Clin Oncol 2017: Suppl 15. [Google Scholar]
- 60. Tryggestad A, Bigalke I, Axcrona K et al 21 – results from a first in man phase i/ii adjuvant dendritic cell vaccine study in high risk prostate cancer patients following radical surgery. Cytotherapy 2017; 19:S15. [Google Scholar]
- 61. Carreno BM, Magrini V, Becker‐Hapak M et al A dendritic cell vaccine increases the breadth and diversity of melanoma neoantigen‐specific T cells. Science 2015; 348:803–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Holtl L, Rieser C, Papesh C et al Cellular and humoral immune responses in patients with metastatic renal cell carcinoma after vaccination with antigen pulsed dendritic cells. J Urol 1999; 16:777–82. [PubMed] [Google Scholar]
- 63. Phuphanich S, Wheeler CJ, Rudnick JD et al Phase I trial of a multi‐epitope‐pulsed dendritic cell vaccine for patients with newly diagnosed glioblastoma. Cancer Immunol Immunother 2013; 62:125–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Plosker GL. Sipuleucel‐T in metastatic castration‐resistant prostate cancer. Drugs 2011; 71:101–8. [DOI] [PubMed] [Google Scholar]
- 65. Kantoff PW, Higano CS, Shore ND et al Sipuleucel‐t immunotherapy for castration‐resistant prostate cancer. N Engl J Med 2010; 363:411–22. [DOI] [PubMed] [Google Scholar]
- 66. Brower V. Approval of provenge seen as first step for cancer treatment vaccines. J Natl Cancer Inst 2010; 102:1108–10. [DOI] [PubMed] [Google Scholar]
- 67. Schwendener RA. Liposomes as vaccine delivery systems: a review of the recent advances. Therap Adv Vaccines 2014; 2:159–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Alving CR, Beck Z, Matyas GR, Rao M. Liposomal adjuvants for human vaccines. Expert Opin Drug Delivery 2016; 13:807–16. [DOI] [PubMed] [Google Scholar]
- 69. Watson DS, Endsley AN, Huang L. Design considerations for liposomal vaccines: influence of formulation parameters on antibody and cell‐mediated immune responses to liposome associated antigens. Vaccine 2012; 30:2256–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Simöes S, Filipe A, Faneca H et al Cationic liposomes for gene delivery. Exp Opin Drug Delivery 2005; 2:237–54. [DOI] [PubMed] [Google Scholar]
- 71. Matyas GR, Mayorov AV, Rice KC et al Liposomes containing monophosphoryl lipid A: a potent adjuvant system for inducing antibodies to heroin hapten analogs. Vaccine 2013; 31:2804–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Elbahnasawy MA, Donius LR, Reinherz EL, Kim M. Co‐delivery of a CD4 T cell helper epitope via covalent liposome attachment with a surface‐arrayed B cell target antigen fosters higher affinity antibody responses. Vaccine 2018; 36:6191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Allison AC, Gregoriadis G. Liposomes as immunological adjuvants. Nature 1974; 252:252. [DOI] [PubMed] [Google Scholar]
- 74. Bovier PA. Epaxal R: a virosomal vaccine to prevent hepatitis a infection. Exp Rev Vaccines 2014; 7:1141–50. [DOI] [PubMed] [Google Scholar]
- 75. Herzog C, Hartmann K, Künzi V et al Eleven years of inflexal R v – a virosomal adjuvanted influenza vaccine. Vaccine 2009; 27:4381–7. [DOI] [PubMed] [Google Scholar]
- 76. Zhao L, Seth A, Wibowo N et al Nanoparticle vaccines. Vaccine 2014; 32:327–37. [DOI] [PubMed] [Google Scholar]
- 77. Janes K, Calvo P, Alonso M. Polysaccharide colloidal particles as delivery systems for macromolecules. Adv Drug Delivery Rev 2001; 47:83–97. [DOI] [PubMed] [Google Scholar]
- 78. Fredriksen BN, Grip J. Plga/pla micro‐ and nanoparticle formulations serve as antigen depots and induce elevated humoral responses after immunization of Atlantic salmon (Salmo salar l.). Vaccine 2012; 30:656–67. [DOI] [PubMed] [Google Scholar]
- 79. Ali OA, Lewin SA, Dranoff G, Mooney DJ. Vaccines combined with immune checkpoint antibodies promote cytotoxic T‐cell activity and tumor eradication. Cancer Immunol Res 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Zeng Q, Li H, Jiang H et al Tailoring polymeric hybrid micelles with lymph node targeting ability to improve the potency of cancer vaccines. Biomaterials 2017; 122:105–13. [DOI] [PubMed] [Google Scholar]
- 81. Walters AA, Krastev C, Hill AV, Milicic A. Next generation vaccines: single‐dose encapsulated vaccines for improved global immunisation coverage and efficacy. J Pharm Pharmacol 2014; 67:400–8. [DOI] [PubMed] [Google Scholar]
- 82. Bailey BA, Desai K‐GH, Ochyl LJ, Ciotti SM, Moon JJ, Schwendeman SP. Self‐encapsulating poly(lactic‐co‐glycolic acid) (PLGA) microspheres for intranasal vaccine delivery. Mol Pharm 2017; 14:3228–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. O’Hagan DT, Rahman D, McGee JP et al Biodegradable microparticles as controlled release antigen delivery systems. Br Soc Immunol 1991; 73:239–42. [PMC free article] [PubMed] [Google Scholar]
- 84. Gordon D, Kelley P, Heinzel S, Cooper P, Petrovsky N. Immunogenicity and safety of AdvaxTM, a novel polysaccharide adjuvant based on delta inulin, when formulated with hepatitis B surface antigen: a randomized controlled Phase I study. Vaccine 2014; 32:6469–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Gordon DL, Sajkov D, Honda‐Okubo Y et al Human phase 1 trial of low‐dose inactivated seasonal influenza vaccine formulated with AdvaxTM delta inulin adjuvant. Vaccine 2016; 34:3780–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Heddle R, Russo P, Petrovsky N, Hanna R, Smith A. Immunotherapy – 2076. A controlled study of delta inulin‐adjuvanted honey bee venom immunotherapy. World Allergy Org J 2013; 6:158. [Google Scholar]
- 87. Niikura K, Matsunaga T, Suzuki T et al Gold nanoparticles as a vaccine platform: influence of size and shape on immunological responses in vitro and in vivo . ACS Nano 2013; 7:3926–38. [DOI] [PubMed] [Google Scholar]
- 88. Sun B, Ji Z, Liao Y‐P et al Engineering an effective immune adjuvant by designed control of shape and crystallinity of aluminum oxyhydroxide nanoparticles. ACS Nano 2013; 7:10834–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Smith JD, Morton LD, Ulery BD. Nanoparticles as synthetic vaccines. Curr Opin Biotechnol 2015; 34:217–24. [DOI] [PubMed] [Google Scholar]
- 90. Chen Y‐S, Hung Y‐C, Lin W‐H, Huang GS. Assessment of gold nanoparticles as a size‐dependent vaccine carrier for enhancing the antibody response against synthetic foot‐and‐mouth disease virus peptide. Nanotechnology 2010; 21:195101. [DOI] [PubMed] [Google Scholar]
- 91. Gregory AE, Judy BM, Qazi O et al A gold nanoparticle‐linked glycoconjugate vaccine against burkholderia mallei. Nanomedicine 2015; 11:447–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Fogarty JA, Swartz JR. The exciting potential of modular nanoparticles for rapid development of highly effective vaccines. Curr Opin Chem Eng 2018; 19:1–8. [Google Scholar]
- 93. Ginsberg BA, Gallardo HF, Rasalan TS et al Immunologic response to xenogeneic gp100 DNA in melanoma patients: comparison of particle mediated epidermal delivery with intramuscular injection. Clin Cancer Res 2010; 16:4057–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Jones S, Evans K, McElwaine‐Johnna H et al DNA vaccination protects against an influenza challenge in a double‐blind randomised placebo‐controlled Phase Ib clinical trial. Vaccine 2009; 27:2506–12. [DOI] [PubMed] [Google Scholar]
- 95. Roy MJ, Wu MS, Barr LJ et al Induction of antigen‐specific CD8+ T cells, T helper cells, and protective levels of antibody in humans by particle‐mediated administration of a hepatitis B virus DNA vaccine. Vaccine 2000; 19:764–78. [DOI] [PubMed] [Google Scholar]
- 96. Lindblad EB. Aluminium compounds for use in vaccines. Immunol Cell Biol 2004; 82:497–505. [DOI] [PubMed] [Google Scholar]
- 97. Maquieira A, Brun EM, Garcés‐García M, Puchades R. Aluminum oxide nanoparticles as carriers and adjuvants for eliciting antibodies from non‐immunogenic haptens. ACS Anal Chem 2012; 84:9340–8. [DOI] [PubMed] [Google Scholar]
- 98. Frey A, Mantis N, Kozlowski PA et al Immunization of mice with peptomers covalently coupled to aluminum oxide nanoparticles. Vaccine 1999; 17:3007–19. [DOI] [PubMed] [Google Scholar]
- 99. Fox CB, Kramer RM, Barnes VL, Dowling QM, Vedvick TS. Working together: interactions between vaccine antigens and adjuvants. Therapeutic Adv Vaccines. 2013; 1:7–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Lin Y, Wang X, Huang X, Zhang J, Xia N, Zhao Q. Calcium phosphate nanoparticles as a new generation vaccine adjuvant. Exp Rev Vaccines 2017; 16:895–906. [DOI] [PubMed] [Google Scholar]
- 101. Masson J‐D, Thibaudon M, Bélec L, Crépeaux G. Calcium phosphate: a substitute for aluminum adjuvants? Exp Rev Vaccines 2017; 16:289–99. [DOI] [PubMed] [Google Scholar]
- 102. Mitchell TC, Casella CR. No pain no gain? Adjuvant effects of alum and monophosphoryl lipid A in pertussis and HPV vaccines. Curr Opin Immunol 2017; 47:17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Niu Y, Popat A, Yu M, Karmakar S, Gu W, Yu C. Recent advances in the rational design of silica‐based nanoparticles for gene therapy. Therapeutic Deliv 2012; 3:1217–37. [PubMed] [Google Scholar]
- 104. Xia T, Kovochich M, Liong M et al Polyethyleneimine coating enhances the cellular uptake of mesoporous silica nanoparticles and allows safe delivery of siRNA and DNAconstructs. ACS Nano 2009; 3:3273–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Yu M, Jambhrunkar S, Thorn P, Chen J, Gu W, Yu C. Hyaluronic acid modified mesoporous silica nanoparticles for targeted drug delivery to CD44‐overexpressing cancer cells. Nanoscale 2013; 5:178–83. [DOI] [PubMed] [Google Scholar]
- 106. Kwon S, Singh RK, Perez RA, Neel EAA, Kim H‐W, Chrzanowski W. Silica‐based mesoporous nanoparticles for controlled drug delivery. J Tissue Eng 2013; 4:2041731413503357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Parra J, Abad‐Somovilla A, Mercader JV, Taton TA, Abad‐Fuentes A. Carbon nanotube‐protein carriers enhance size‐dependent self‐adjuvant antibody response to haptens. J Control Release 2013; 170:242–51. [DOI] [PubMed] [Google Scholar]
- 108. Li S, Pasc A, Fierro V, Celzard A. Hollow carbon spheres, synthesis and applications – a review. J Mater Chem A 2016; 4:12686–713. [Google Scholar]
- 109. Kim M‐G, Park JY, Shon Y, Kim G, Shim G, Oh Y‐K. Nanotechnology and vaccine development. Asian J Pharm Sci 2014; 9:227–35. [Google Scholar]
- 110. Schreiber HA, Prechl J, Jiang H et al Using carbon magnetic nanoparticles to target, track, and manipulate dendritic cells. J Immunol Meth 2010; 356:47–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Wang T, Zou M, Jiang H, Ji Z, Gao P, Cheng G. Synthesis of a novel kind of carbon nanoparticle with large mesopores and macropores and its application as an oral vaccine adjuvant. Eur J Pharm Sci 2011; 44:653–9. [DOI] [PubMed] [Google Scholar]
- 112. Kwon K‐C, Verma D, Singh ND, Herzog R, Daniell H. Oral delivery of human biopharmaceuticals, autoantigens and vaccine antigens bioencapsulated in plant cells. Adv Drug Delivery Rev 2013; 65:782–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Sala F, Rigano MM, Barbante A, Basso B, Walmsley AM, Castiglione S. Vaccine antigen production in transgenic plants: strategies, gene constructs and perspectives. Vaccine 2003;21:803–808. [DOI] [PubMed] [Google Scholar]
- 114. Azegami T, Itoh H, Kiyono H, Yuki Y. Novel transgenic rice‐based vaccines. Archi Immunol Therapiae Exp 2015; 63:87–99. [DOI] [PubMed] [Google Scholar]
- 115. Nahampun HN, Bosworth B, Cunnick J, Mogler M, Wang K. Expression of h3n2 nucleoprotein in maize seeds and immunogenicity in mice. Plant Cell Rep 2015; 34:969–80. [DOI] [PubMed] [Google Scholar]
- 116. Pniewski T. The twenty‐year story of a plant‐based vaccine against hepatitis B: stagnation or promising prospects? Int J Mol Sci 2013; 14:1978–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Koya V, Moayeri M, Leppla SH, Daniell H. Plant‐based vaccine: mice immunized with chloroplast‐derived anthrax protective antigen survive anthrax lethal toxin challenge. Infect Immun 2005; 73:8266–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Specht E, Mayfield S. Algae‐based oral recombinant vaccines. Front Microbiol 2014; 5:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Sun M, Qian K, Su N, Chang H, Liu J, Shen G. Foot‐and‐mouth disease virus vp1 protein fused with cholera toxin B subunit expressed in Chlamydomonas reinhardtii chloroplast. Biotech Lett 2003; 25:1087–92. [DOI] [PubMed] [Google Scholar]
- 120. Dauvillée D, Delhaye S, Gruyer S et al Engineering the chloroplast targeted malarial vaccine antigens in chlamydomonas starch granules. PLOS ONE 2010; 5:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Dreesen IA, Hamri GC‐E, Fussenegger M. Heat‐stable oral alga‐based vaccine protects mice from Staphylococcus aureus infection. J Biotechnol 2010; 145:273–80. [DOI] [PubMed] [Google Scholar]
- 122. Atwe SU, Ma Y, Gill HS. Pollen grains for oral vaccination. J Control Release 2014; 194:45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Roulston TH, Cane JH. Pollen nutritional content and digestibility for animals. Vienna: Springer, 2000:187–209. [Google Scholar]
- 124. Uddin MJ, Gill HS. Ragweed pollen as an oral vaccine delivery system: mechanistic insights. J Control Release 2017; 268:416–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Jong WS, Daleke‐Schermerhorn MH, Vikström D et al An autotransporter display platform for the development of multivalent recombinant bacterial vector vaccines. Microb Cell Fact 2014; 13:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Brault AC, Domi A, McDonald EM et al A zika vaccine targeting NS1 protein protects immunocompetent adult mice in a lethal challenge model. Nat Sci Rep 2017; 7:14769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Kotton CN, Lankowski AJ, Scott N et al Safety and immunogenicity of attenuated Salmonella enterica serovar typhimurium delivering an HIV‐1 gag antigen via the salmonella type III secretion system. Vaccine 2006; 24:6216–24. [DOI] [PubMed] [Google Scholar]
- 128. Gerritzen MJ, Martens DE, Wijffels RH, van der Pol L, Stork M. Bioengineering bacterial outer membrane vesicles as vaccine platform. Biotechnol Adv 2017; 35:565–74. [DOI] [PubMed] [Google Scholar]
- 129. Kulp A, Kuehn MJ. Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Annu Rev Microbiol 2010; 64:163–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Fantappiè L, de Santis M, Chiarot E et al Antibody‐mediated immunity induced by engineered Escherichia coli OMVS carrying heterologous antigens in their lumen. J Extracel Vesicles 2014; 3:24015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Bartolini E, Ianni E, Frigimelica E et al Recombinant outer membrane vesicles carrying Chlamydia muridarum HTRA induce antibodies that neutralize chlamydial infection in vitro . J Extracel Vesicles 2013; 2:20181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Carter NJ. Multicomponent meningococcal serogroup B vaccine (4cmenb; bexsero R): a review of its use in primary and booster vaccination. BioDrugs 2013; 27:263–74. [DOI] [PubMed] [Google Scholar]
- 133. Shirley M, Dhillon S. Bivalent rlp2086 vaccine (trumenba R): a review in active immunization against invasive meningococcal group B disease in individuals aged 10–25 years. BioDrugs 2015; 29:353–61. [DOI] [PubMed] [Google Scholar]
- 134. Morein B, Sundquist B,Höglund S, Dalsgaard K, Osterhaus A. ISCOM a novel structure for antigenic presentation of membrane proteins from enveloped viruses. Nature 1984;308:457–60. [DOI] [PubMed] [Google Scholar]
- 135. Sanders MT, Brown LE, Deliyannis G, Pearse MJ. ISCOMTM‐based vaccines: the second decade. ImmunolCell Biolo 2005; 83:119–128. [DOI] [PubMed] [Google Scholar]
- 136. Drane D, Gittleson C, Boyle J, Maraskovsky E. ISCOMATRIXTM adjuvant for prophylactic and therapeutic vaccines. Exp Rev Vaccines 2007; 6:761–72. [DOI] [PubMed] [Google Scholar]
- 137. Cebon JS, McArthur GA, Chen W et al Randomized, double‐blind Phase II trial of ny‐eso‐1 iscomatrix vaccine and iscomatrix adjuvant alone in patients with resected stage IIc, III, or IV malignant melanoma. J Clin Oncol 2014; 32(suppl 15):9050. [Google Scholar]
- 138. Manoff SB, George SL, Bett AJ et al Preclinical and clinical development of a dengue recombinant subunit vaccine. Vaccine 2015; 33:7126–7134. [DOI] [PubMed] [Google Scholar]
- 139. Khan AY, Talegaonkar S, Iqbal Z, Ahmed FJ, Khar RK. Multiple emulsions: an overview. Curr Drug Deliv 2006; 3:429–43. [DOI] [PubMed] [Google Scholar]
- 140. Saroja C, Lakshmi P, Bhaskaran S. Recent trends in vaccine delivery systems: a review. Int J Pharm Invest 2011; 1:64–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. O’Hagan DT, Ott GS, Nest GV, Rappuoli R, Giudice GD. The history of mf59 R adjuvant: a phoenix that arose from the ashes. Expert Review of Vaccines 2013; 12:13–30. [DOI] [PubMed] [Google Scholar]
- 142. Kim T‐G, Kim S, Lee M‐G. The origin of skin dendritic cell network and its role in psoriasis. Int J Mol Sci 2018; 19:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Kim Y‐C, Quan F‐S, Yoo D‐G, Compans RW, Kang S‐M, Prausnitz MR. Improved influenza vaccination in the skin using vaccine coated microneedles. Vaccine 2009; 27:6932–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Naito S, Maeyama JI, Mizukami T, Takahashi M, Hamaguchi I, Yamaguchi K. Transcutaneous immunization by merely prolonging the duration of antigen presence on the skin of mice induces a potent antigen‐specific antibody response even in the absence of an adjuvant. Vaccine 2007; 25:8762–70. [DOI] [PubMed] [Google Scholar]
- 145. Watkinson AC, Kearney M‐C, Quinn HL, Courtenay AJ, Donnelly RF. Future of the transdermal drug delivery market – have we barely touched the surface? Expert Opin Drug Deliv 2016; 13:523–32. [DOI] [PubMed] [Google Scholar]
- 146. Alkilani AZ, McCrudden MT, Donnelly RF. Transdermal drug delivery: innovative pharmaceutical developments based on disruption of the barrier properties of the stratum corneum. Pharmaceutics 2015; 7:438–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Kim Y‐C, Park J‐H, Prausnitz MR. Microneedles for drug and vaccine delivery. Adv Drug Delivery Rev 2012; 64:1547–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Siddhapura K, Harde H, Jain S. Immunostimulatory effect of tetanus toxoid loaded chitosan nanoparticles following microneedles assisted immunization. Nanomedicine 2016; 12:213–22. [DOI] [PubMed] [Google Scholar]
- 149. Ma Y, Tao W, Krebs SJ, Sutton WF, Haigwood NL, Gill HS. Vaccine delivery to the oral cavity using coated microneedles induces systemic and mucosal immunity. Pharm Res 2014; 31:2393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Liao J‐F, Lee J‐C, Lin C‐K, Wei K‐C, Chen P‐Y, Yang H‐W. Self‐assembly DNA polyplex vaccine inside dissolving microneedles for high‐potency intradermal vaccination. Theranostics 2017; 7:2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Kalia YN, Naik A, Garrison J, Guy RH. Iontophoretic drug delivery. Adv Drug Delivery Rev 2004; 56:619–658. [DOI] [PubMed] [Google Scholar]
- 152. Schoellhammer CM, Blankschtein D, Langer R. Skin permeabilization for transdermal drug delivery: recent advances and future prospects. Expert Opin Drug Deliv 2014; 11:393–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Zorec B, Becker S, Reberšek M, Miklavãiã D, Pavšelj N. Skin electroporation for transdermal drug delivery: the influence of the order of different square wave electric pulses. Int J Pharm 2013; 457:214–223. [DOI] [PubMed] [Google Scholar]
- 154. Sardesai NY, Weiner DB. Electroporation delivery of DNA vaccines: prospects for success. Curr Opin Immunol 2011; 23:421–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Weiland O, Ahlén G, Diepolder H et al Therapeutic DNA vaccination using in vivo electroporation followed by standard of care therapy in patients with genotype 1 chronic hepatitis C. Mol Ther 2013; 21:1796–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Teva Pharmaceuticals . Urgent – zecuity R (sumatriptan iontophoretic transdermal system) suspension of marketing. Available at: https://www.fda.gov/downloads/Drugs/DrugSafety/UCM506332.pdf (accessed 13 December 2018).
- 157. Ita K. Recent progress in transdermal sonophoresis. Pharm Dev Technol 2017; 22:458–66. [DOI] [PubMed] [Google Scholar]
- 158. Feiszthuber H, Bhatnagar S, Gyöngy M, Coussios C‐C. Cavitation‐enhanced delivery of insulin in agar and porcine models of human skin. Phys Med Biol 2015; 60:2421. [DOI] [PubMed] [Google Scholar]
- 159. Mo S, Coussios C‐C, Seymour L, Carlisle R. Ultrasound‐enhanced drug delivery for cancer. Expert OpinDrug Delive 2012; 9:1525–38. [DOI] [PubMed] [Google Scholar]
- 160. Tezel A, Paliwal S, Shen Z, Mitragotri S. Low‐frequency ultrasound as a transcutaneous immunization adjuvant. Vaccine 2005; 23:3800–7. [DOI] [PubMed] [Google Scholar]
- 161. Bhatnagar S, Kwan JJ, Shah AR, Coussios C‐C, Carlisle RC. Exploitation of sub‐micron cavitation nuclei to enhance ultrasound‐mediated transdermal transport and penetration of vaccines. J Control Release 2016; 238:22–30. [DOI] [PubMed] [Google Scholar]
- 162. Kwan JJ, Graham S, Myers R, Carlisle R, Stride E, Coussios CC. Ultrasound‐induced inertial cavitation from gas‐stabilizing nanoparticles. Phys Rev E 2015; 92:023019. [DOI] [PubMed] [Google Scholar]
- 163. Kim Y‐C. Skin vaccination methods: gene gun, jet injector, tattoo vaccine, and microneedle. Berlin, Heidelberg: Springer, 2017:485–499. [Google Scholar]
- 164. Millar JD, Roberto RR, Wulff H, Wenner HA, Henderson D. Smallpox vaccination by intradermal jet injection: 1. Introduction, background and results of pilot studies. Bull World Health Organ 1969; 41:749. [PMC free article] [PubMed] [Google Scholar]
- 165. Kok P, Kenya P, Ensering H. Measles immunization with further attenuated heat stable measles vaccine using five different methods of administration. Trans R Soc Trop Med Hyg 1983; 77:171–6. [DOI] [PubMed] [Google Scholar]
- 166. Parker V. Jet gun or syringe? A trial of alternative methods of BCG vaccination. Public Health 1984; 98:315–20. [DOI] [PubMed] [Google Scholar]
- 167. Jackson LA, Austin G, Chen RT et al Safety and immunogenicity of varying dosages of trivalent inactivated influenza vaccine administered by needle‐free jet injectors. Vaccine 2001; 19:4703–9. [DOI] [PubMed] [Google Scholar]
- 168. Weniger BG, Papania MJ. Alternative vaccine delivery methods In: Plotkin SA, Orenstein WA, Offit PA, eds. Vaccines, 5th edn Amsterdam: Elsevier, 2008:1357–92. [Google Scholar]
- 169. Maa Y‐F, Ameri M, Rigney R, Payne LG, Chen D. Spray‐coating for biopharmaceutical powder formulations: beyond the conventional scale and its application. Pharmaceut Res 2004; 21:515–23. [DOI] [PubMed] [Google Scholar]
- 170. Weissmueller NT, Schiffter HA, Carlisle RC, Rollier CS, Pollard AJ. Needle‐free dermal delivery of a diphtheria toxin crm197 mutant on potassium‐doped hydroxyapatite microparticles. Clin Vaccine Immunol 2015; 22:586–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Weissmueller NT, Marsay L, Schiffter HA et al. Alternative vaccine administration by powder injection: needle‐free dermal delivery of the glycoconjugate meningococcal group Y vaccine. PLOS ONE 2017; 12:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Centers for Disease Control (CDC) . CDC vaccine price list. Available at: https://www.cdc.gov/vaccines/programs/vfc/awardees/vaccine-management/price-list/index.html (accessed 21 December 2018).
- 173. Loder EW, Rayhill M, Burch RC. Safety problems with a transdermal patch for migraine: lessons from the development, approval, and marketing process. Headache 2018; 58:1639–57. [DOI] [PubMed] [Google Scholar]
- 174. Su F, Patel GB, Hu S, Chen W. Induction of mucosal immunity through systemic immunization: phantom or reality? Hum Vaccines Immunotherap 2016; 12:1070–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Sasaki S, Sumino K, Hamajima K et al Induction of systemic and mucosal immune responses to human immunodeficiency virus type 1 by a DNA vaccine formulated with qs‐21 saponin adjuvant via intramuscular and intranasal routes. J Virol 1998; 72:4931–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Shakya AK, Chowdhury MY, Tao W, Gill HS. Mucosal vaccine delivery: current state and a pediatric perspective. J Control Release 2016; 240:394–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Zaman M, Chandrudu S, Toth I. Strategies for intranasal delivery of vaccines. Drug Deliv Translat Res 2013; 3:100–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Velasquez LS, Shira S, Berta AN et al Intranasal delivery of norwalk virus‐like particles formulated in an in situ gelling, dry powder vaccine. Vaccine 2011; 29:5221–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Garg NK, Mangal S, Khambete H, Sharma PK, Tyagi RK. Mucosal delivery of vaccines: role of mucoadhesive/biodegradable polymers. Recent Pat Drug Deliv Formulat 2010; 4:114–28. [DOI] [PubMed] [Google Scholar]
- 180. Pavot V, Rochereau N, Genin C, Verrier B, Paul S. New insights in mucosal vaccine development. Vaccine 2012; 30:142–54. [DOI] [PubMed] [Google Scholar]
- 181. Kozlowski PA, Williams SB, Lynch RM, et al Differential induction of mucosal and systemic antibody responses in women after nasal, rectal, or vaginal immunization: influence of the menstrual cycle. J Immunol 2002; 169:566–74. [DOI] [PubMed] [Google Scholar]
- 182. Lehtinen M, Söderlund‐Strand A, Vanska S et al Impact of gender‐neutral or girls‐only vaccination against human papillomavirus – results of a community‐randomized clinical trial (I). Int J Cancer 2018; 142:949–58. [DOI] [PubMed] [Google Scholar]
- 183. Lehtinen M, Luostarinen T, Vanska S et al Gender‐neutral vaccination provides improved control of human papillomavirus types 18/31/33/35 through herd immunity: results of a community randomized trial (III). Int J Cancer 2018;143:2299–310. [DOI] [PubMed] [Google Scholar]
- 184. Parez N, Fourgeux C, Mohamed A et al Rectal immunization with rotavirus virus‐like particles induces systemic and mucosal humoral immune responses and protects mice against rotavirus infection. J Virol 2006; 80:1752–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Pais R, Omosun Y, He Q et al. Rectal administration of a chlamydial subunit vaccine protects against genital infection and upper reproductive tract pathology in mice. PLOS ONE 2017; 12:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Kim‐Schulze S, Kim HS, Wainstein A et al Intrarectal vaccination with recombinant vaccinia virus expressing carcinoembronic antigen induces mucosal and systemic immunity and prevents progression of colorectal cancer. J Immunol 2008; 181:8112–9. [DOI] [PubMed] [Google Scholar]
- 187. Lycke N. Recent progress in mucosal vaccine development: potential and limitations. Nat Rev Immunol 2012; 12:592–605. [DOI] [PubMed] [Google Scholar]


