Summary
Current therapies for inflammatory bowel diseases (IBD) are aimed at controlling the exacerbated response in the gut, but no treatment is fully effective for many refractory patients. Mesenchymal stromal cells (MSC) are multi‐potent cells with regulatory immunosuppressive activity that may control inflammatory diseases. In this study, we investigated the short‐ and especially the long‐term protective effects of MSC on experimental colitis. We show that MSC elicited protection to acute intestinal inflammation with gain of weight, improvement in the clinical disease score and expressive reduction in the mortality rate of treated mice. MSC changed the population of neutrophils, eosinophils and augmented the frequency of CD4 T lymphocytes in the gut‐draining lymph nodes, together with reduced accumulation of these cells in the colon intraepithelial compartment. Interestingly, there were increased levels of programmed death 1 (PD‐1) and glucocorticoid‐induced tumour necrosis factor receptor family‐related receptor (GITR) in the spleen regulatory T cells of mice that received MSC treatment, which also presented a reversal in the pattern of immune response in the gut, with diminished inflammatory, T helper type 1 (Th1) and Th17 profile, in contrast to augmented Th2 responses. Most strikingly, this balanced response elicited by a single administration of MSC during the acute colitis persisted long‐term, with restored goblet cells, eosinophils and maintenance of elevated gut interleukin (IL)‐4, besides increased CD4+CD25+PD‐1+ cells in the spleen and reduced Th17 response in mesenteric lymph nodes (MLN) of treated mice on day 60. Taken together, our findings provided a significant contribution to translational immunology by pointing human adipose tissue‐derived MSC as a novel therapeutic approach with long‐term beneficial regulatory effects in experimental colitis.
Keywords: inflammatory bowel diseases, long‐term remission, mesenchymal stromal cells, Th17 response
Introduction
Inflammatory bowel diseases (IBD), such as Crohn’s disease (CD) and ulcerative colitis (UC), are chronic inflammation of intestinal mucosa affecting millions of people. These diseases are characterized by abdominal pain, diarrhea, rectal bleeding and bowel obstruction or fistulae 1. Although IBD etiology is not yet fully understood, gut inflammation results from unregulated immune reactions in genetically susceptible individuals 2, 3, 4.
IBD response is driven by cytokines and leukocyte infiltrate in the gut 5, 6, 7, with histological features of crypt cell hyperplasia, interstitial edema and mucosa ulcerations 8. Most importantly, there is an imbalance between effector and regulatory T cell (Treg) responses in the gut that predispose to uncontrolled immunity against intestinal or microbiota antigens 9, 10, 11, 12. Accordingly, current available treatments are aimed at reducing inflammation in an attempt to avoid disease recurrence or to prolong clinical remission periods. However, many patients are refractory, so that surgery remains their only alternative 13.
The partial inefficacy of pharmacological interventions and the unresponsiveness of some subjects has spurred clinical interest in developing other approaches for effectively controlling IBD. Recently, transplantation of hematopoietic stem cells (HSCT) has become a therapeutic alternative for autoimmune or inflammatory diseases 14, 15. Preclinical studies have shown that the infusion of bone marrow cells (BMC) has successfully controlled experimental colitis 16, 17, with the pretransplant conditioning of immunosuppressive therapies such as cyclophosphamide or total body irradiation. The success of these therapies seem to be more correlated with immunosuppression than with transplantation per se, although prompt bone marrow reconstitution following immune ablation is essential to avoid receptor mortality 16, 17.
Despite the importance of immunosuppression for IBD treatment with HSCT, its aggressive feature has prompted the use of more safe approaches. In this sense, mesenchymal stromal cells (MSC) have been applied to treat intestinal inflammation, considering their immunomodulatory properties, with reduced harmful effects 14. The immunosuppressive capacity of MSC allows tolerance in transplantation, while their potential to induce regeneration of damaged tissues and cell differentiation make them an effective tool for treating IBD 18. Indeed, several clinical trials have shown short‐term positive results in more than 200 non‐responsive patients with refractory IBD, in which MSC promoted a complete clinical remission in approximately 40% and an overall response in approximately 60% (for review, see Grégoire et al. [19]). These effects involve Treg recruitment and T helper type 1 (Th1)/Th17 inhibition 19, 20, 21, 22, 23, 24, 25. Nevertheless, despite these well‐described short‐term properties, little is known about long‐term MSC effects on IBD. A few studies have showed that MSC injections persistently improved murine colitis by down‐regulating Th1 inflammation 25, suppressing T cells 26, inducing Treg differentiation 27 and up‐regulating Treg responses 28.
Taken together, in view of the known regulatory properties of MSC in the gut inflammatory responses, in this study we evaluated the persistent protective effects of an initial single dose of MSC infusion in the long‐term modulation of experimental colitis, especially in the balance of the Th1–Th17–Treg axis that controls gut homeostasis.
Materials and methods
Animals and experimental design
Colitis was induced in 6–8‐week‐old female BALB/c mice, which are the receptors for human MSC transplantation. Mice were maintained with food and water ad libitum in the animal housing facility of the Federal University of Triângulo Mineiro (UFTM), Brazil. The animals were divided into four groups (five animals/group), as follows: (1) the phosphate‐buffered saline (PBS group), containing the control animals (without colitis induction) that received intrarectal enema of PBS; (2) the ethanol group, containing control animals that received the drug vehicle (ethanol 50%) used to induce colitis; (3) the colitis group, containing sick animals in which colitis was induced by an enema of 2,4,6‐trinitrobenzene sulfonic acid (TNBS) diluted in ethanol 50%; and (4) the colitis + MSC group, containing sick animals with TNBS‐induced colitis treated with MSC derived from human adipose tissue. All experiments were performed in accordance with protocols approved by the UFTM Animal Care and Use Committee under the protocol number 106.
Induction of experimental colitis by TNBS
For colitis induction, mice were anesthetized with ketamine hydrochloride (100 mg/kg) and xylazine hydrochloride (10 mg/kg) followed by an intrarectal enema of TNBS (Sigma‐Aldrich, Deisenhofen, Germany) diluted in 50% ethanol at a dose of 175 mg/kg in a total volume of 120 μl. The solution was administered through a 3·5‐F catheter carefully inserted into the rectum until 4 cm proximal to the anal verge. To ensure TNBS distribution throughout the colon, mice were held in a vertical position for 2 min after the enema 16. Control animals received PBS or 50% ethanol using the same technique. As there were no significant differences between both groups, we chose to show only ethanol data as the control group in the figures. The results shown in this paper are representative of two to five independent experiments, with five animals/group at each time‐point evaluated, as stated in the figure legends. As the animals were killed on days 3, 7, 14 or 60, the samples were obtained from different mice at each time‐point evaluated, except for Fig. 1, which shows the total number of mice still alive throughout the clinical disease evaluation period.
Figure 1.
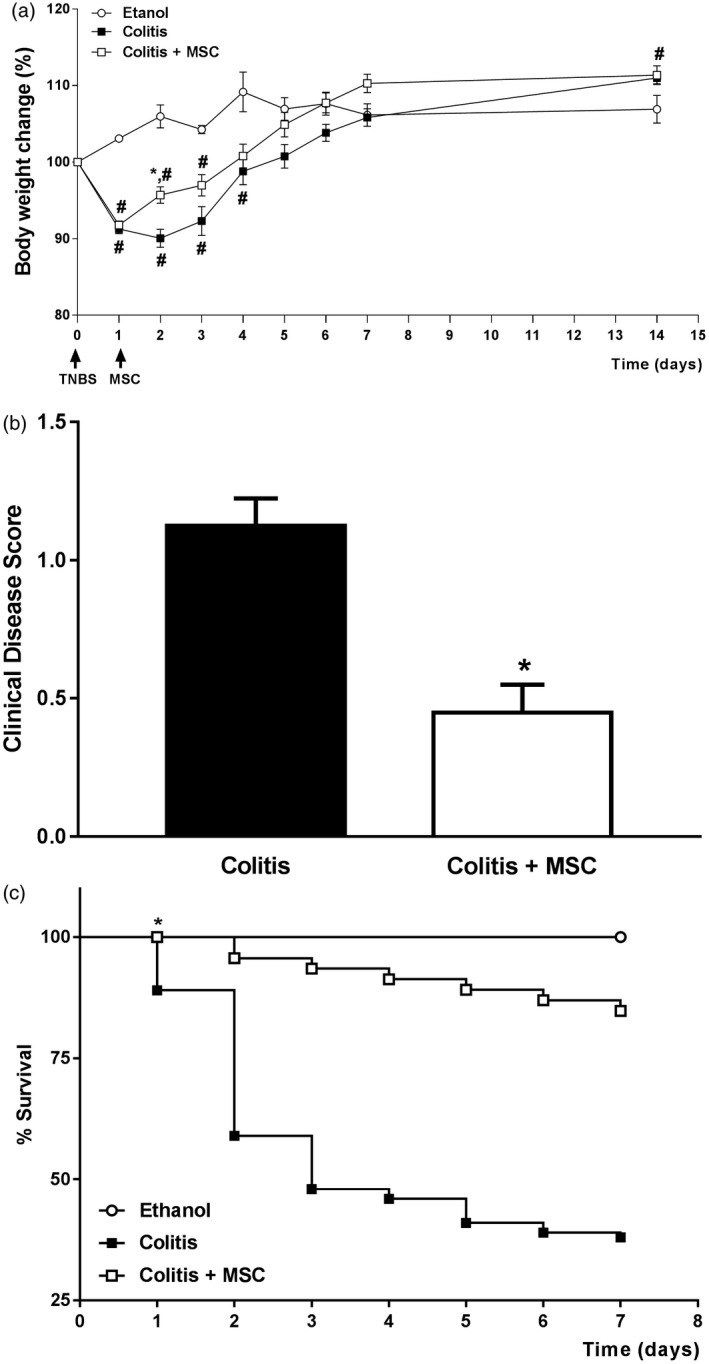
Mesenchymal stromal cells (MSC) treatment improves mice survival and clinical signs of experimental colitis. Mice received intrarectal enema of 2,4,6‐trinitrobenzene sulfonic acid (TNBS) for induction of colitis and were treated 24 h later with 1 × 106 human MSC cells. (a) Body mass change relative to the first day of colitis. (b) Sum of clinical disease score during the first 14 days, according to the presence of diarrhea, bleeding, blood plus diarrhea and severe weight loss (more than 5%). (c) Survival curve of colitis and MSC‐treated mice during the first 7 experimental days. Results are representative of five independent experiments, with five animals per group. Symbols represent significant differences (P < 0·05) from the respective ethanol (#) or colitis (*) groups.
MSC isolation, culture and characterization
Samples of adipose tissue were harvested from healthy donors at Clinics Hospital from UFMT (Brazil) for MSC isolation, culture and characterization, as described previously 23, 25. For the entire study we used adipose tissue from three non‐obese female subjects aged 25–55 years which were submitted to plastic surgery for aesthetic reasons. These donors were healthy and did not present any significant disease conditions. The collected adipose tissue was immersed in PBS containing antibiotic (penicillin G solid at 0·01 IU/ml plus streptomycin sulfate at 0·01 g/ml) and anti‐fungal (amphotericin B at 25 μg/ml) drugs for at least 4 h, and then incubated at 37oC with collagenase type I (0·3 mg/ml in PBS) (gibco brl, Grand Island, NY, USA) to digest the tissue. The cells released by collagenase treatment were carefully collected and centrifuged twice at 4°C for 10 min at 677 g with RPMI‐1640 (gibco BRL) containing 10% of fetal bovine serum (FBS) (gibco BRL). Afterwards, cells were resuspended in Dulbecco’s modified Eagle’s medium (DMEM) (gibco BRL) supplemented with 15% of FBS (Hyclone Thermo Scientific, South Logan, CA, USA) and antibiotic drugs (0·01 mg/ml of penicillin + streptomycin). The cells were counted with 0·2% Trypan blue exclusion in a Neubauer chamber, plated in 75‐cm2 bottles and cultured at 37oC with 5% of CO2. After 24 h, the non‐adherent cells and the culture medium were removed and 20 ml of fresh DMEM were added to the flasks. After the third passage, cell samples were separated for immunophenotyping, multi‐potential characterization and infusion in mice with experimentally induced colitis. For immunophenotypical characterization, monoclonal antibodies against the markers CD45, CD14, CD90, CD73, CD31, CD166, CD34 and CD13 were used. For differentiation into adipocytes, we used 15% of FBS containing DMEM supplemented with 10 mmol/l of dexamethasone (Decadron injection; Prodome, Campinas, SP, Brazil), 10 g/ml of insulin (Sigma‐Aldrich, St Louis, MO, USA) and 100 mmol/l of indomethacin (Sigma‐Aldrich, USA). To differentiate into osteocytes, 7·5% of FBS containing DMEM supplemented with 0·10 mmol/l of dexamethasone (Decadron injection; Prodome), 100 mmol/l of ascorbic acid and 10 mmol/l of β‐glycerolphosphate (React, Reagen, Colombo, Brazil) was used. All experiments with human adipose tissue were performed in accordance with protocols approved by the UFTM Human Committee under protocol number 1447.
Colitis treatment with MSC and sample collection
For determining the optimal cell concentration to treat colitis, MSC were injected by the intraperitoneal route 24 h after TNBS enema at various concentrations (0·25 × 106 cells, 1 × 106 cells or 2 × 106 cells per mouse; Supporting information, Fig. 1), as well as literature searching for similar studies with MSC infusion 23, 25. To avoid clumps, MSC were resuspended in an appropriate volume of saline solution supplemented with 1 mg/ml of a recombinant human DNase (Pulmozyme; Roche, South San Francisco, CA, USA) before infusion. The next experiments were all performed with 1 × 106 cells/mouse, according to the results from the dose–response preliminary studies (Supporting information). Colitis mice that did not receive MSC were injected with the same saline volume used to administer the cells in the treated group. Mice were then examined for the evaluation of colitis clinical score, weight and mortality, and the samples were collected for further assays performed at 3, 7, 14 or 60 days after colitis induction and treatments. The time‐point of TNBS enema was considered as day 0 in all experiments. For the evaluation of circulating leukocytes, the animal blood was collected by retro‐orbital puncture and cells were counted with 0·2% of Trypan blue exclusion in a Neubauer chamber. The differential counts were made in smears stained with Panotico LB (Laborclin, Pinhais, PR Brazil).
Clinical disease score evaluation
Mice with colitis treated or not with MSC transplantation were evaluated for the presence of clinical signs of disease, such as weight loss, diarrhea, rectal bleeding and bloody diarrhea, as well as for mortality during the first 7 days post‐induction. This evaluation used a criterion score in which each signal exhibited by the animal corresponded to one point. Finally, the sum of all values for each animal was calculated and plotted in the respective graphics 16.
Histopathological analysis and morphometry
Tissue samples were fixed in formaldehyde 10% in PBS for 24 h followed by dehydration in 70% ethanol. The samples were then processed and embedded in paraffin, cut into 5‐μm‐thick sections and stained with hematoxylin and eosin (H&E). The histological images (at ×20 or ×40 magnification) were captured by a common optic microscope connected to a digital camera (Evolution MP 5.0‐color; Media Cybernetic, Silver Spring, MD, USA). In each colon sample, a total area of 0·0093958 mm2 was evaluated to quantify the lamina propria infiltrate using Image J software (http://rsb.info.nih.gov/ij/), as described previously 29. The criteria used for evaluation of tissue infiltrate and diagnosis of inflammatory bowel disease were adapted from guidelines of the British Society of Gastroenterology, as described previously 30. Furthermore, another set of slides was stained with periodic acid‐Schiff (PAS) and the frequency of areas considered positive for this staining was quantified in the same total area described above in each colon sample evaluated. The results were depicted as percentage of area covered by goblet cells.
Tissue homogenization and cytokine quantification
For quantification of cytokines, colon segments were collected and resuspended in buffer containing protease inhibitors (cOmplete; Roche, South San Francisco, CA, USA). The cytokines presented in the homogenates were measured by enzyme‐linked immunosorbent assay (ELISA) (PharmingenTM BD, San Diego, CA, USA) or cytometric bead array (CBA) (PharmingenTM BD), according to the manufacturer’s instructions.
Eosinophil peroxidase (EPO) and myeloperoxidase (MPO) activity assays
For neutrophils, MPO activity was evaluated in the colon samples. The reactions were developed by addition of TMB (3,3′,5,5′‐tetramethylbenzidine (PharmingenTM BD) and the readings were performed in a spectrophotometer at 450 nm after reaction stopping with 4 mol/l of H2SO4. For eosinophils, EPO enzyme activity was evaluated and the reactions were developed with o‐phenylenediamine dihydrochloride (OPD; Sigma‐Aldrich, USA) diluted in Tris/HCl and H2O2 for 30 min at room temperature. The reaction was stopped with 4 mol/l of H2SO4 and the absorbance read at 492 nm. In both cases, results were expressed as optical density (nm) per gram of tissue 16.
Flow cytometry
Spleen, mesenteric lymph nodes and colon were collected for flow cytometry assays for quantification and phenotyping of inflammatory cells. Colon was removed and separated into fragments for assessing lamina propria and intraepithelial lymphocytes. Lamina propria tissue was cut into small pieces, which were immersed in PBS containing ethylenediamine tetraacetic acid (EDTA) (3 mmol/l) then placed on a magnetic stirrer for 20 min. The samples were centrifuged at 470 g for 10 min at 4°C, the supernatant was discarded and RPMI‐1640 containing FBS (1%), EDTA (1 mmol/l) and MgCl2 (1·5 mmol/l) were added to the pellet that was stirred again for 30 min followed by centrifugation at 470 g for 10 min at 4oC. The samples were vortexed for 2 min and incubated with liberase at 50 μg/ml (Roche, Mannheim, Germany) diluted in incomplete RPMI (with no FBS) medium for 60 min at 37oC. Cells extracted from tissues were washed twice, counted in a Neubauer chamber and then used in culture or immunophenotyping assays. For detection of intracellular cytokines, cells were cultured in RPMI FBS (10%) and stimulated with phorbol myristate acetate (PMA, 50 ng/ml) plus ionomycin (1 µg/ml) for 4 h at 37ºC in the presence of brefeldin. The selected antibodies against specific markers or isotype controls were conjugated with fluorochromes such as fluorescein isothiocyanate (FITC), Alexa Fluor 488, phycoerythrin (PE) or phycoerythrin/cyanin 5 (PerCy5) (BD PharmingenTM, San Diego, CA, USA). To obtain the percentage of positively stained cell populations, the samples were acquired in a fluorescence activated cell sorter (FACS)calibur (Becton Dickson®, San Jose, CA, USA) cytometer and analyzed in FlowJo version 7.6.5 software in accordance with the properties of size and granularity [forward‐ (FSC) and side‐scatter (SSC)], followed by dot‐plot or histogram evaluation.
Statistical analysis
Results were analyzed in GraphPad Prism version 7.0 by analysis of variance (anova) followed by Tukey’s post‐hoc test for parametric data. In the case of non‐Gaussian distribution (non‐parametric data), Mann–Whitney or Kruskal–Wallis tests followed by Dunn’s post‐hoc analysis were used. The Kaplan–Meyer log‐rank test was used to analyze the survival curve. P < 0·05 was considered statistically significant.
Results
MSC treatment improves clinical signs of colitis
First, our preliminary results confirmed the phenotypical characterization of human MSC, their potential in differentiating into osteocytes and adipocytes as well as their in‐vitro suppressive capacity, thus validating the subsequent studies (data not shown). Colitis was then induced in BALB/c mice, which were treated with 1 × 106 xenogeneic MSC/mice by intraperitoneal route 24 h afterwards. This dose was chosen based on literature findings and on preliminary studies performed with different numbers of MSC administration, as shown in Supporting information, Fig. S1. We show that MSC treatment elicited protective effects on acute intestinal inflammation, as treated sick mice gained weight during the first days after cell infusion when compared to the untreated animals (Fig. 1a). We also found a significant improvement in the clinical disease score (Fig. 1b), followed by an expressive reduction in the mortality rate exhibited by treated colitis mice (Fig. 1c).
MSC treatment reduces systemic and local inflammation in colitis
The peripheral changes induced by intestinal inflammation and the consequences of MSC treatment were evaluated by differential counts of leukocytes in mice blood. Our data show that intestinal inflammation led to an overall increase in the number of blood leukocytes, mainly neutrophils, as early as 3 days after TNBS enema. Such leukocytosis was transitorily reduced after MSC treatment and controlled within 7 days, followed by a reduction in the number of neutrophils and mononuclear cells at this time‐point. On day 14, no significant difference was observed among the groups (Table 1).
Table 1.
Total and differential systemic leukocyte counts in mice with colitis and post‐MSC treatment
| Groups | Total blood leukocytes (×104/ml) | Eosinophils (×104/ml) | Neutrophils (×104/ml) | Mononuclear cells (×104/ml) | |
|---|---|---|---|---|---|
| 3 days | Ethanol | 815 ± 334·54 | 17·82 ± 14·52 | 96·34 ± 21·66 | 700·84 ± 304·56 |
| Colitis | 1381·25 ± 368·77 | 38·06 ± 29·15 | 493·71 ± 197·29# | 846·14 ± 374·46 | |
| Colitis + MSC | 1450 ± 467·80 | 27·62 ± 22·09 | 511·17 ± 210·03# | 897·25 ± 440·68 | |
| 7 days | Ethanol | 1121·25 ± 227·46 | 25·15 ± 37·91 | 347·96 ± 141·89 | 745·46 ± 81·96 |
| Colitis | 1172·5 ± 475·93 | 36·31 ± 7·18 | 524·99 ± 229·17 | 611·2 ± 265·01 | |
| Colitis + MSC | 316·25 ± 128·35* | 7·96 ± 8·62 | 65·95 ± 33·95* | 242·34 ± 88·56# | |
| 14 days | Ethanol | 1123·75 ± 129·57 | 7·82 ± 10·18 | 195·99 ± 43·07 | 919·94 ± 124·05 |
| Colitis | 1425 ± 396·88 | 28·07 ± 24·38 | 428·67 ± 354·37 | 962 ± 256·96 | |
| Colitis + MSC | 1287·5 ± 387·59 | 29·19 ± 29·22 | 307·05 ± 103·94 | 949·15 ± 322·51 | |
Results are expressed as mean ± standard deviation. (*)Significantly different (P < 0·05) from the respective ethanol (#) or colitis (*) groups. MAC = mesenchymal stromal cells.
Histopathological analysis showed a significant increase in the number of inflammatory cells in the colon from untreated sick animals at day 3 when compared to ethanol‐infused mice (Fig. 2a). We also observed that goblet cells from both treated and untreated sick mice were decreased within 3 days when compared to ethanol animals, and at day 14 there was a complete restructuring of goblet cells in mice which received MSC, but not in the untreated sick group (Fig. 2b).
Figure 2.
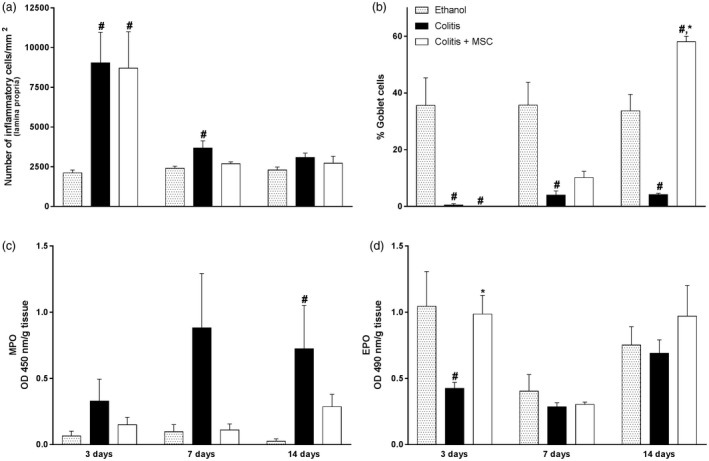
Colitis treatment with mesenchymal stromal cells (MSC) restores intestinal homeostasis and gut architecture. On days 3, 7 and 14, mice had their colon removed, processed for paraffin, stained with hematoxylin and eosin (H&E) or periodic acid‐Schiff (PAS) and evaluated for quantification of inflammatory cells in the lamina propria (a) or goblet cells (b) by morphometry. Neutrophils [myeloperoxidase, MPO)] (c) and eosinophils [eosinophil peroxidase (EPO)] (d) activities were determined by enzymatic assays in the colon of colitis and MSC‐treated mice throughout the experimental period. Results are representative of three independent experiments, with five animals/group, at each time‐point evaluated. Symbols represent significant differences (P < 0·05) from the respective ethanol (#) or colitis (*) groups.
In ethanol‐treated mice, the mucosal epithelial surface was similar to the PBS group (data not shown), despite a mild and focal increase in lamina propria cellularity, composed of a mixed infiltrate with predominance of mononuclear cells on days 3, 7 and 14. There was no accumulation of polymorphonuclear cells on crypts or intraepithelial lymphocytes. These ethanol mice did not present any abnormality, such as epithelial flattening, mucin depletion, focal loss of cells or erosion, except for a discrete infiltrate in submucosa, that was not different among the time‐points evaluated (Supporting information, Fig. S2, upper panel). Conversely, the TNBS‐colitis group presented large erosion areas with loss of surface epithelial and tubular glands, especially on the third day, along with deep ulcers that went beyond the muscularis mucosae. The lesion borders had irregular surface epithelia and dilated crypts, which accounted for abnormal tissue architecture. These structures were infiltrated with polymorphonuclear cells, thus characterizing cryptitis or crypt abscesses. There was epithelial flattening and mucin depletion. Furthermore, mixed and intense lamina propria inflammatory infiltrate were observed, especially in ulcer or erosion areas (Supporting information, Fig. S2, middle panel). Mice which had TNBS infusion and were treated with MSC presented similar histological alterations in the colon on the third day when compared to the non‐treated group, except for an apparently increased frequency of re‐epithelialization interleaved with lesion areas (Supporting information, Fig. S2, lower panel). On day 7, the TNBS‐colitis mice had some re‐epithelialization of the mucosa, interleaved with ulcers or erosion. The lesion borders still presented mucin depletion and lamina propria had edema, with fibroblasts and mixed leukocyte infiltrate of varied intensity. The submucosa also presented moderate to intense mixed inflammatory infiltrate (Supporting information, Fig. S2, middle panel). Conversely, mice treated with MSC maintained similar infiltrate, but with the absence of mucosal ulcers or erosion. Despite the presence of just a few crypts, the mucosal surface was re‐epithelialized (Supporting information, Fig. S2, lower panel). On day 14, we observed crypt formation with a few goblet cells in colitis mice, in contrast to the well‐structured crypts with columnar epithelium and increased number of goblet cells in the group treated with MSC. There was a mixed leukocyte accumulation on the lamina propria of TNBS mice with varied intensity and predominance of mononuclear cells, similar to that found in the MSC group. Furthermore, in the mice that received cellular therapy there was mucosal preservation in the areas apart from the lesions (Supporting information, Fig. S2). On day 60, the inflammatory infiltrate in the colon of TNBS‐treated mice was similar to the control ethanol, despite the treatment with MSC (data not shown). The most remarkable alteration at this time‐point concerned the reduced goblet cells in the group not treated with MSC, which were restored to normality in mice that received cell administration (Fig. 6).
Figure 6.
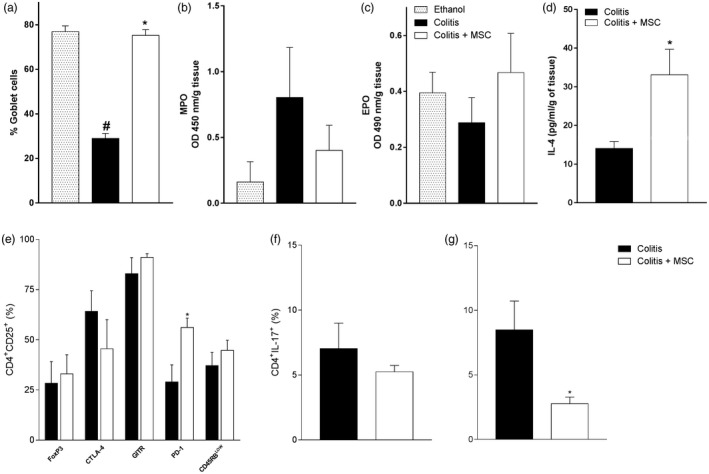
Long‐term effects of mesenchymal stromal cells (MSC) therapy. Mice received intrarectal 2,4,6‐trinitrobenzene sulfonic acid (TNBS) to induce colitis on day zero and were treated with a single dose of MSC after 24 h. The colon was removed on day 60 and processed for goblet cell counting (a), determination of myeloperoxidase (MPO) (b), (c) eosinophil peroxidase (EPO) activity and (d) IL‐4 quantification. In (e), evaluation of regulatory T lymphocytes in the spleen, (f) and (g) T helper type 17 (Th17) response on day 60 in the spleen and mesenteric lymph nodes (MLN), respectively, from treated or untreated sick mice. Results are representative of two to three independent experiments, with five animals/group, at each time‐point evaluated. Symbols represent significant differences (P < 0·05) from the respective ethanol (#) or colitis (*) groups.
Quantitative analysis of the accumulation of neutrophils and eosinophils in the inflamed gut and its relation to MSC treatment was performed by MPO and EPO assays, respectively. Overall, the results showed that mice undergoing intestinal inflammation had a higher accumulation of neutrophils in the colon when compared to the ethanol group, followed by a reduction after MSC treatment (Fig. 2c). Interestingly, while MPO activity tends to be reduced in treated sick animals throughout 3, 7 or 14 days when compared to the untreated sick group, EPO activity tends to be increased after MSC therapy, mainly on the first days after cellular infusion (day 3) (Fig. 2d).
MSC treatment changed lymphocyte frequency and induced Treg cell response
We observed a significant increase in the frequency of CD3+ cells in the gut‐draining lymph nodes of treated sick mice compared to the untreated group at day 7 (Fig. 3a) that could be related to the elevated frequency of CD3+CD4+ cells at days 7 and 14 (Fig. 3b). These changes were followed by a reduced frequency of CD3+CD4+ cells in intraepithelial T lymphocytes from treated colitis animals, mainly at day 14 (Fig. 3b). No significant differences in any immune compartments were found in the population of CD3+CD8+ T cells or CD3+CD49b+ cells [supposedly natural killer T cells (NK T)] or NK CD49b+ cells from spleen or mesenteric lymph nodes between untreated and treated colitis mice. Furthermore, there were no changes in CD3+γδ+ T cells from intraepithelial lymphocytes (data not shown). Nevertheless, by comparing specific markers of Treg cells, we found increased levels of programmed cell death 1 (PD‐1) in both CD4+CD25+ and CD4+CD25– cells of spleen, as well as augmented frequency of CD4+CD25– leukocytes expressing glucocorticoid‐induced tumour necrosis factor (TNF) receptor family‐related receptor (GITR) in the spleen of mice that received MSC treatment on day 14 (Fig. 3c). There were no significant changes in the regulatory markers of MLN on day 14 or in spleen and MLN on day 7 (data not shown).
Figure 3.
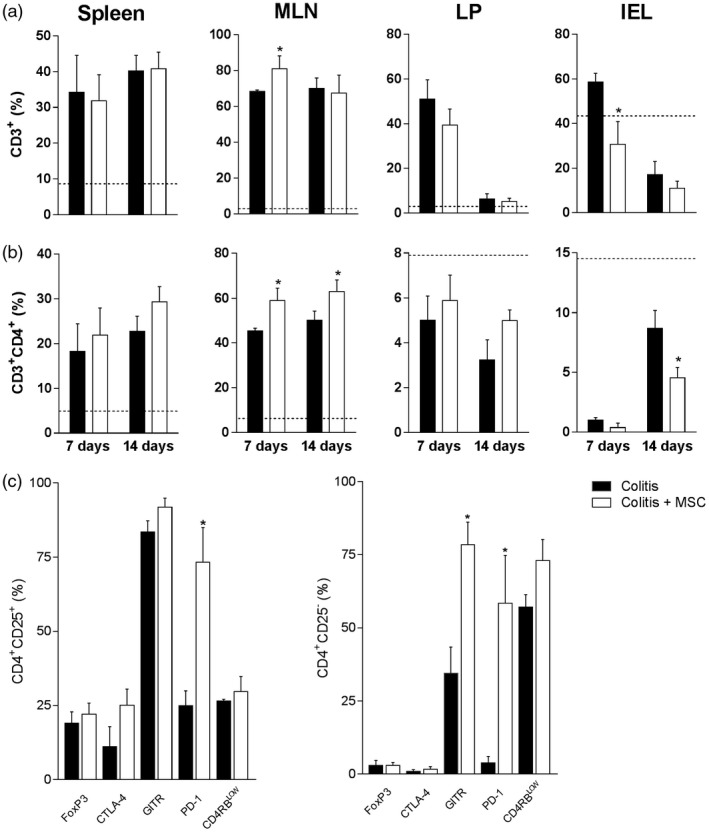
Mesenchymal stromal cells (MSC) treatment modifies inflammatory infiltrate in the gut mucosa and draining lymphoid organs. Leukocytes from the spleen, mesenteric lymph nodes (MLN), colon lamina propria (LP) and intraepithelial (IEL) compartments of colitis and MSC‐treated mice were isolated and stained with fluorochrome‐conjugated antibodies for flow cytometry on days 7 or 14. After sample acquisition, the frequency of CD3+ T cells (a), CD3+CD4+ (b) or regulatory T cells in the spleen (c, day 14) was determined after analysis by FlowJo software. Results are representative of two independent experiments, with five animals/group, at each time‐point evaluated. Dashed line: control animals treated with ethanol [2,4,6‐trinitrobenzene sulfonic acid (TNBS) (TNBS) vehicle] at day14. (*) Symbols represent significant differences (P < 0·05) from the respective colitis groups.
MSC treatment modulates uncontrolled production of inflammatory cytokines in colitis
A reduction of the proinflammatory cytokines IL‐1β and TNF‐α was observed in treated colitis animals within 3 days (Fig. 4a and 4b, respectively). At day 7, mice that received MSC exhibited elevated IL‐1β and TNF‐α levels, whereas TNF‐α levels remained increased within 14 days of treatment when compared to the colitis non‐treated group. Interestingly, the augmented TNF‐α levels at days 7 and 14 was not higher than the values observed at day 3; on the contrary, a clear reduction on this cytokine was observed throughout the experimental period (Fig. 4b). Among the proinflammatory cytokines from the Th1 pattern, IL‐12 was reduced in the treated colitis group after 14 days of MSC infusion (Fig. 4c). Otherwise, considering cytokines associated with the Th17 response, only IL‐23 and IL‐17 were detected at reduced levels at days 3 and 7, respectively, in treated colitis mice when compared to untreated sick group (Fig. 4e and 4f, respectively). IL‐6 levels did not alter significantly post‐treatment (Fig. 4d). Cytokines from the Th2 profile, including IL‐4 and IL‐5, exhibited higher production in treated colitis mice at days 3 and 14 when compared to the untreated sick group (Fig. 4g and 4h, respectively). These data pointed to a reversal of the immune inflammatory profile in intestinal inflammation after MSC treatment.
Figure 4.
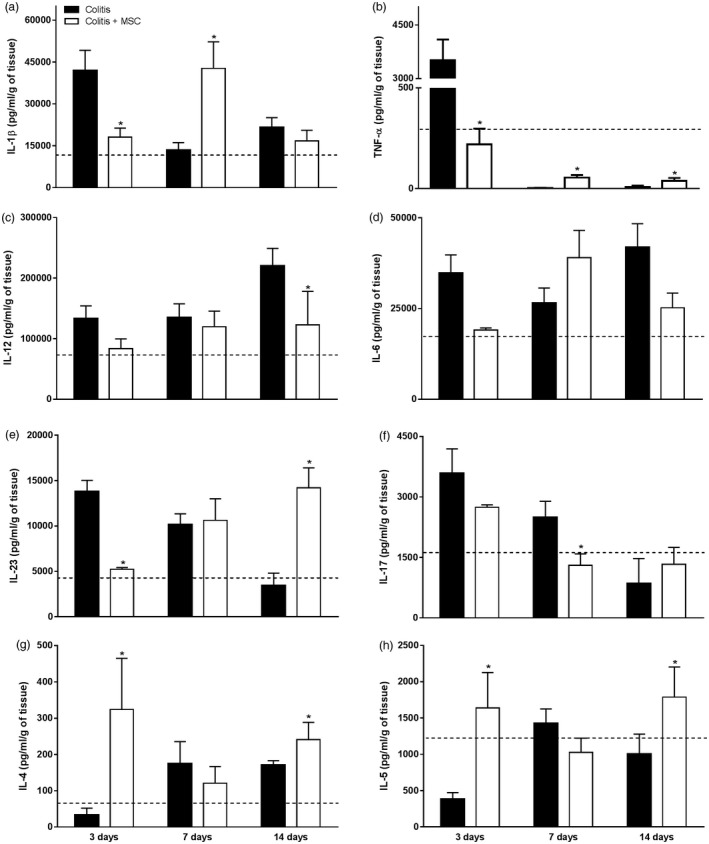
Evaluation of the cytokine profile in the colon of mice with colitis and treated with mesenchymal stromal cells (MSC). Colon fragments of mice receiving ethanol, 2,4,6‐trinitrobenzene sulfonic acid (TNBS) or treated with MSC were removed and homogenized in a solution containing protease inhibitors. The cytokines interleukin (IL)‐1β (a), IL‐12 (c), IL‐23 (e), IL‐17 (f), IL‐4 (g) and IL‐5 (h) were assessed by enzyme‐linked immunosorbent assay (ELISA). The presence of the cytokines tumour necrosis factor (TNF)‐α (b) and IL‐6 (d) was quantified by cytometric bead array (CBA) in samples acquired on the flow cytometer. The data were expressed as pg/ml per gram of tissue. Results are representative of two independent experiments, with five animals/group, at each time‐point evaluated. Dashed line: control animals treated with ethanol (TNBS vehicle) at day 14. (*) Symbols represent significant differences (P < 0·05) from the respective colitis groups.
MSC treatment reduces Th1 and Th17 response in colitis
The increase in regulatory markers previously described was followed by an altered functional profile of Th1 and Th17 cells, mainly by a reduced frequency of CD4+ T cells producing IL‐17 in spleen and MLN from treated mice at day 14 when compared to untreated sick mice (Fig. 5a), which was also observed at day 7 (data not shown). Furthermore, interferon (IFN)‐γ production by T CD4+ cells was reduced, especially in spleen from colitis animals that received MSC at day 14 (Fig. 5b). The simultaneous production of IL‐17 and IFN‐γ by CD4+ T cells was also decreased in spleen, but not in MLN, from treated sick mice when compared to untreated colitis animals at day 14 (Fig. 5c).
Figure 5.
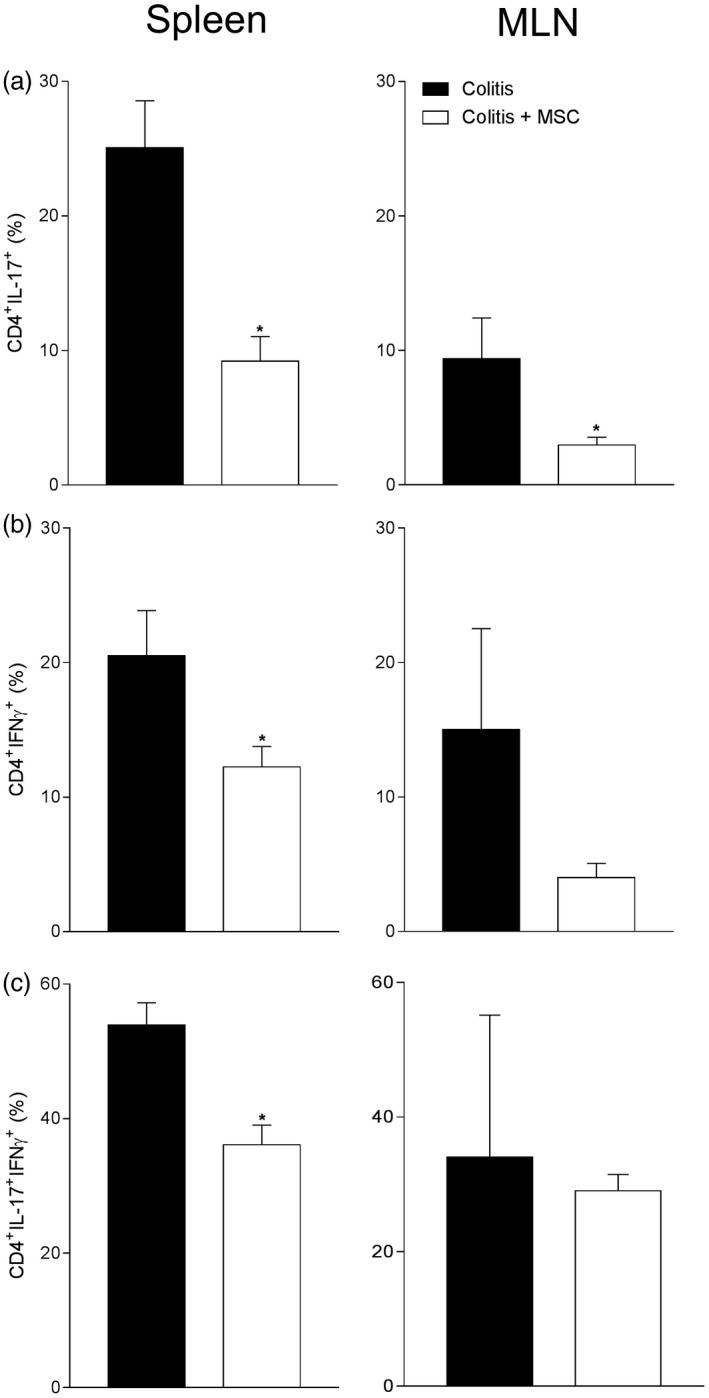
Quantification of interleukin (IL)‐17 and/or interferon (IFN)‐γ production by CD4 cells after 14 days of induction of colitis and/or treatment with mesenchymal stromal cells (MSC). The spleen and mesenteric lymph nodes (MLN) from mice with colitis treated or not with MSC were removed and macerated for extracting leukocytes. After 4 h of ex‐vivo re‐stimulating culture, cells were collected and stained with specific antibodies against CD4 [phycoerythrin/cyanin 5 (PE/Cy5)], IL‐17 (Alexa 488) and IFN‐γ (PE) for flow cytometric detection of CD4+ T lymphocytes producing IL‐17 (a), CD4+ T lymphocytes producing IFN‐γ (b) and CD4+ T lymphocytes double‐positive for IFN‐γ and IL‐17 (c). Results are representative of two independent experiments, with five animals/group, at each time‐point evaluated. (*) Symbols represent significant differences (P < 0·05) from the respective colitis groups.
Long‐term persistence of the therapeutic efficacy of MSC treatment
To investigate whether the immune modulatory effects assigned to MSC treatment could persist long‐term after transplantation, we evaluated the experimental intestinal inflammation induced by a single TNBS infusion during a chronic period of 60 days, as previous studies of the group showed that immunological changes persisted long‐term after breakdown of the mucosal tolerance by this hapten 17. Indeed, even after 60 days of treatment, the potential immune modulatory effects of MSC could still be observed. Histopathological analyses showed regular colon tissue architecture with the re‐establishment of goblet cell counts in treated colitis mice, while untreated animals were not able to restore normal mucus production in the gut (Fig. 6a). Regarding these local alterations, although EPO reached control levels at day 60 (Fig. 6c), neutrophil activity remained elevated at this time‐point in colitis mice, indicating that a chronic barrier dysfunction may still exist in mice not treated with MSC (Fig. 6b). In agreement with these results, IL‐4 levels were still elevated in mice that received cellular therapy, indicating the maintenance of the immunological shift of the local response (Fig. 6d).
Similarly to the acute colitis, the percentage of CD4+CD25+ cells in the spleen from treated sick mice was unchanged. However, a significant increase in the frequency of PD‐1 levels by this putative regulatory population persisted to 60 days post‐MSC treatment (Fig. 6e), pointing to an important role for this molecule in inflammation control. Interestingly, the production of IL‐17 by CD4+ cells from MLN was also constrained long‐term by an early injection of MSC, indicating that this proposed therapy may lead to chronic beneficial effects in treated animals presenting an initial acute intestinal inflammation (Fig. 6g).
Discussion
Human adipose tissue has been described as an ideal source of MSC, which are easily isolated and whose proliferative and immunological properties have been widely explored in several inflammatory diseases 18, 23, 24, 25, 31, 32, 33, 34, 35, 36. Therefore, we used MSC for treating experimental colitis and especially showed the long‐term effects of this immunomodulatory therapy, which is essential for chronic relapsing diseases such as IBD. Overall, MSC attenuate immune and inflammatory diseases by suppressing the mixed lymphocyte reaction, involving allogenic or autologous T cells by inhibiting autoimmune responses, by reducing infiltrate or proinflammatory cytokine production or by improving local tissue repair 18, 20, 32, 33, 34, 35, 36.
Our first result showed that MSC treatment promoted short‐term protective effects on acute experimental colitis, as treated sick animals exhibited lower weight loss followed by an improved clinical disease score and an increased survival rate. In agreement with the macroscopic findings, we observed that the colon from treated sick animals presented a higher frequency of re‐epithelized areas that were repaired earlier. Accordingly, these results corroborated previous studies that showed the short‐term protective efficacy of MSC on macroscopic and histopathological severity of inflammatory diseases, which has been correlated with the down‐regulation of Th1 inflammation 23, 24, 25, 26, 27, 28, 33, 37 and the suppression of collagen‐reactive T cell‐driven production of matrix degrading enzymes 38.
Leukocytosis was observed to be temporally reduced after MSC treatment at day 7, with a reduced number of neutrophils and mononuclear cells at this time‐point. As MSC suppress cell activation in vivo, this may be a way for the body to maintain homeostasis by inhibiting the activation of the immune system in different compartments. However, the molecular mechanism underlying this immunosuppressive effect of MSC is not yet completely understood 18, 31, 39.
IBD exhibit chronic inflammation with enhanced IFN‐γ production in intestinal lamina propria, which is followed by an excess of TNF‐α and neutrophil infiltration in CD 9, 40, 41, 42, 43, 44, 45, in contrast to the excess of IL‐4, IL‐5 and IL‐13 in UC 9, 40, 42. The experimental model of TNBS enema is immunologically similar to CD in humans 37. Thus, when we analyzed the inflammatory infiltrate of neutrophils by MPO and its correlation with MSC treatment, we observed that these cells were directly related to the colon mucosa inflammation, corroborating previous studies that showed accumulation of neutrophils in the inflammatory site and its reduction after MSC injection 23, 24, 25, 37. In addition, the apparent decrease of MPO and increased EPO levels after MSC treatment suggested that this therapy interferes in the activity of neutrophils and eosinophils at the site of disease, leading to a possible reversal of immune response to a Th2 profile. In this sense, the recruitment of eosinophils may be directly related to the regression of intestinal inflammation. Furthermore, our results showed that MSC treatment led to an overall reduction in the gut proinflammatory cytokines such as IL‐12, IL‐1β, TNF‐α, IL‐6, IL‐23 and IL‐17, especially during the first days of treatment, while IL‐4 and IL‐5 were kept at increased levels, confirming the results obtained for EPO. These findings indicated that MSC treatment can revert the local profile of immune response and down‐regulate cytokines responsible for the exacerbated inflammation of colon mucosa, in agreement with the reduction of IFN‐γ or IL‐17 production in the gut of treated mice 25, 26, 27, 28.
A relative increase in T cells from MLN was observed after 7 days of MSC treatment, which could be explained by the higher frequency of CD4+ T cells as effector or regulatory lymphocytes. However, the reduced accumulation of T lymphocytes in the intraepithelial compartment at day 14 after MSC infusion could be indicative of the presence of a potentially inflammatory or pathogenic population in non‐treated colitis. In general, our results partially corroborate previous studies which showed that the treatment of experimental IBD with bone marrow transplantation associated with immunosuppression leads to the reduction of CD4+ and CD8+ cells in the colon, which seemingly contributes to the improvement of intestinal inflammation and the regression of the disease 16, 17.
The regulation of intestinal inflammation depends on the presence and functionality of Treg in the gut, where these cells may act by inhibiting excessive inflammatory responses such as Th1 and Th17 45, 46. Here, we also showed that MSC treatment increased the frequency of Treg in contrast to a reduction in Th17 and Th1 responses. In agreement, MSC administration alleviates TNBS‐induced murine colitis by correcting Th1/Th17/Treg imbalances 47. Furthermore, MSC are able to induce the differentiation of Treg from effector T cells so that these new Treg are immunosuppressive 48, 49. Therefore, we believe that the colitis amelioration after MSC treatment may be correlated with the control of inflammatory responses upon direct or indirect modulation of the recipient’s Treg. Yamanishi et al. 50 have shown that the induction of forkhead box protein 3 (FoxP3)+CD4+CD25+ cells improves experimental colitis, corroborating our findings. Moreover, although we have not observed higher expression of FoxP3 in mice treated with MSC, the increase of other markers of Treg is in agreement with the hypothesis that infused MSC may have promoted immunoregulation and controlled the intestinal response in animals with colitis. Indeed, Treg can also express other phenotypical molecules, including CD45RBlow 51, CTLA‐4 52, 53, 54, PD‐1 55, 56, 57, 58 and GITR 59.
PD‐1 and its ligand PD‐L1 are highly expressed in Treg during colitis, where they act by limiting autoimmune response and attenuating inflammation. Indeed, the blockage of immune checkpoints by anti‐PD‐1 or anti‐PD‐L1 monoclonal antibodies, usually applied for reducing colorectal cancer malignancy, exacerbates gut inflammation during colitis 60. Interestingly, in this study we found that MSC treatment led to increased PD‐1 expression in Treg after short‐ and long‐term evaluation, which may contribute to the protective effects of MSC on gut inflammation. In fact, MSC present PD‐L1, which enhances Treg‐mediated immunossupression by affecting PD‐1 expression and the activation of PD‐1 downstream signaling 61. Conversely, GITR is constitutively expressed by Treg and upon exposure to alloantigens 62. The interaction of these surface molecules has been reported as important factor that attenuates the inflammatory response in autoimmune diseases 63. Accordingly, we show that MSC treatment led to an early increase in GITR expression, which may contribute to the protective effects of MSC on gut inflammation.
As discussed previously, our results indicate an important role for MSC in reducing the pathogenic Th17 response in experimental colitis, in agreement with Godoi et al. 16, who observed reduced levels of IL‐17 after bone marrow transplantation for the treatment of experimental intestinal inflammation. Th17 cells and the cytokine IL‐17 have been found at elevated levels in intestinal tissue and serum from patients with IBD, confirming the finding that this pattern of immune response contributes to the development of the disease 64. Interestingly, we also show that MSC infusion reduced the frequency of IL‐17/IFN‐γ double‐producing cells at spleen, which was followed by a decreased accumulation of neutrophils in the colon of treated mice. Taken together, these findings suggested that IL‐17/IFN‐γ double‐producing cells may also be a pathogenic population that is constrained by MSC treatment. Accordingly, IL‐17 recruits mononuclear cells and neutrophils to peripheral tissues for amplifying local inflammation 46, 65, 66, 67, 68, 69, 70. Human Th17 cells in IBD can express IFN‐γ, IL‐23R and IL‐12Rβ2 66. In turn, IL‐12 can reduce the expression of IL‐17 and retinoid‐related orphan receptor gamma t (RORγt) 66, 67, thus pointing to the interplay between Th17 and Th1 cell profiles in the pathogenesis of IBD. Indeed, both IFN‐γ and IL‐17 responses are prejudicial in CD 68.
Finally, our results showed that, even after a long period of time, MSC were able to keep the intestinal inflammation under control. This effect was observed in the histopathological results and was also represented by the maintenance of regulation and higher IL‐4 levels, together with a reduction of Th17 response after treatment. Therefore, although previous studies have already shown the relevance of MSC treatment in variable periods after cellular therapy 25, 26, 27, 28, our findings are pioneering in showing such long‐term effects of this modulatory single therapy in colitis, as described previously in other models. This durable effect of cellular therapy is of great relevance for IBD, as CD and UC are chronic disabling diseases usually treated with complex and prolonged treatments that require patient compliance, despite the unpleasant adverse effects of medications. Therefore, a single long‐term intervention (with MSC alone or even combined with biologicals or other drugs – to be tested) could encourage a higher engagement of the patients and then ameliorate their commitment to the therapy as well as the disease outcome. Furthermore, the patient’s stabilization for a longer time could also contribute to fewer relapse episodes, improved mucosal healing, inflammation control and reduced complications.
In conclusion, our findings confirm the long‐term protective efficacy assigned to MSC for treating IBD, thus supporting the use of MSC in the modulation of inflammatory and autoimmune diseases. Most importantly, we show, for the first time to our knowledge, that acute effects of MSC administration on correcting Th1/Th17/Treg imbalance persist chronically and may be essential to the long‐term maintenance of gut homeostasis in hosts treated with these cells (7). Finally, our results open new perspectives for more effective treatments for diseases of difficult control such as CD and UC, based on cellular therapy with human MSC.
Figure 7.
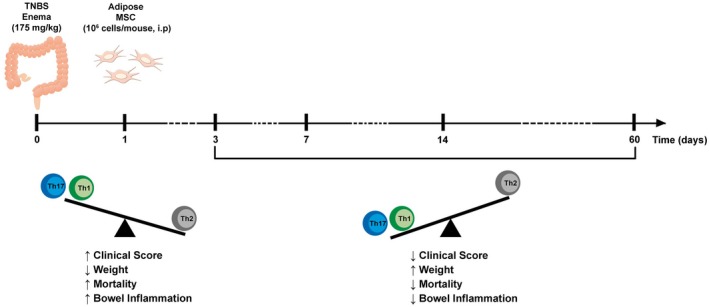
Summary of the long‐term effects of MSC on experimental colitis. TNBS (2,4,6‐trinitrobenzene sulfonic acid) enema triggered intestinal inflammation in mice by enhancing Th1 and Th17 response and reducing Th2 cells. An early and unique intraperitoneal (i.p.) administration of adipose tissue‐derived MSC (mesenchymal stromal cells) produced persistent protective effects on bowel inflammatory response by constraining inflammation and restoring Th1/Th17/Th2 balance.
Disclosure
The authors declare that they have no conflicts of interest.
Supporting information
Fig. S1. Dose‐response evaluation of body weight variation of mice treated with mesenchymal stromal cells (MSC). Mice received intrarectal enema of 2,4,6‐trinitrobenzene sulfonic acid (TNBS) for induction of colitis and were treated 24h later with 0.25, 1 or 2x106 human MSC cells by intraperitoneal route. The body mass change (in percentage) was evaluated in relation to the first day of colitis.
Fig. S2. Histopathological evaluation of colitis outcome in mice treated with mesenchymal stromal cells (MSC). Mice received intrarectal enema of 2,4,6‐trinitrobenzene sulfonic acid (TNBS) for induction of colitis and were treated 24h later with 1x106 human MSC cells by intraperitoneal route. The histological alterations in colon tissues were observed after haemaoxilin‐eosin staining of samples collected on days 3, 7, 14 and 60 post TNBS infusion and treatment. In the upper panel, image depicts control ethanol mice, on day 3. The middle panel shows images representative of TNBS colitis group on days 3, 7 and 14, while the lower panel depicts TNBS mice on the same days, treated with MSC infusion.
Acknowledgements
We are grateful to Professor Júlio César Voltarelli (in memoriam; Ribeirão Preto Medical School, University of São Paulo), who participated in the initial delineation of this work. We also would like to acknowledge the healthy patient donors of the adipose tissue, who participated in this study. This work was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, no. 573841/2008‐0; 310174/2016‐3; 311882/2013‐7 and 307915/2009‐3), the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), the Núcleo de Apoio à Pesquisa em Doenças Inflamatórias ‐ NAP‐DIN (NAP‐DIN, grant number 11.1.21625.01.0) (Brazil) and Fundação de Amaparo à Pesquisa do Estado de São Paulo (FAPESP, no. 2010/20162‐7). In addition, we thank Dr Viviani Nardini and Miss Rosane Bolzoni for their technician support.
References
- 1. Papadakis KA, Targan SR. Current theories on the causes of inflammatory bowel disease. Gastroenterol Clin North Am 1999;28:283–96. [DOI] [PubMed] [Google Scholar]
- 2. Fung KY, Putoczki T. In vivo models of inflammatory bowel disease and colitis‐associated cancer. Methods Mol Biol 2018;1725:3–13. [DOI] [PubMed] [Google Scholar]
- 3. Goethel A, Croitoru K, Philpott DJ. The interplay between microbes and the immune response in inflammatory bowel disease. J Physiol 2018;596:3869–82. 10.1113/JP275396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Watabe T, Nagaishi T, Tsugawa N et al B cell activation in the cecal patches during the development of an experimental colitis model. Biochem Biophys Res Commun 2018;496:367–73. [DOI] [PubMed] [Google Scholar]
- 5. McLean MH, Andrews C, Hanson ML et al Interleukin‐27 is a potential rescue therapy for acute sever colitis through interleukin‐10‐dependent, T‐cell‐independent attenuation of colonic mucosal innate immune responses. Inflamm Bowel Dis 2017;23:1983–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Seregin SS, Golovchenko N, Schaf B, Chen J, Eaton KA, Chen GY. NLRP6 function in inflammatory monocytes reduces susceptibility to chemically induced intestinal injury. Mucosal Immunol 2017;10:434–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Edison N, Belhanes‐Peled H, Eitan Y et al Indolent T‐cell lymphoproliferative disease of the gastrointestinal tract after treatment with adalimumab in resistant Crohn’s colitis. Hum Pathol 2016;57:45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rubio CA. Corrupted colonic crypts bordering regenerating mucosal ulcers in ulcerative colitis. In Vivo 2017;31:669–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Komine‐Aizawa S, Masuda H, Mazaki T, Shiono M, Hayakawa S, Takayama T. Plasma osteopontin predicts inflammatory bowel disease activities. Int Surg 2015;100:38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mohammadnia‐Afrouzi M, Hosseini AZ, Khalili A et al Altered microRNA expression and immunosuppressive cytokine production by regulatory T cells of ulcerative colitis patients. Immunol Invest 2016;45:63–74. [DOI] [PubMed] [Google Scholar]
- 11. Lubberts E, Koenders MI, van den Berg WB. The role of T‐cell interleukin‐17 in conduction destructive arthritis: lessons from animal models. Arthritis Res Ther 2005;7:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Iwakura Y, Ishigame H. The IL‐23/IL‐17 axis in inflammation. J Clin Invest 2006;116:1218–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pillai N, Dusheiko M, Burnand B, Pittet V. A systematic review of cost‐effectiveness studies comparing conventional, biological and surgical interventions for inflammatory bowel disease. PLOS ONE 2017;12:e0185500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ricart E. Current status of mesenchymal stem cell therapy and bone marrow transplantation in IBD. Dig Dis 2012;30:387–91. [DOI] [PubMed] [Google Scholar]
- 15. Voltarelli JC, Couri CE, Stracieri AB et al Autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA 2007;297:1568–76. [DOI] [PubMed] [Google Scholar]
- 16. Godoi DF, Cardoso CR, Ferraz DB, Provinciatto PR, Cunha FQ, Silva JS et al. Hematopoietic SCT modulates gut inflammation in experimental inflammatory bowel disease. Bone Marrow Transplant 2010;45:1562‐71. [DOI] [PubMed] [Google Scholar]
- 17. Godoi DF, Cardoso CR, Silva MJ et al Reappraisal of total body irradiation followed by bone marrow transplantation as a therapy for inflammatory bowel disease. Immunobiology 2013;218:317‐24. [DOI] [PubMed] [Google Scholar]
- 18. Zhang J, Li Y, Chen J et al Human bone marrow stromal cell treatment improves neurological functional recovery in EAE mice. Exp Neurol 2005;195:16–26. [DOI] [PubMed] [Google Scholar]
- 19. Grégoire C, Lechanteur C, Briquet A et al Review article: mesenchymal stromal cells therapy for inflammatory bowel diseases. Aliment Pharmacol Ther 2017;45:205–21. [DOI] [PubMed] [Google Scholar]
- 20. Le Blanc K, Pittenger M. Mesenchymal stem cells: progress toward promise. Cytotherapy 2005;7:36–45. [DOI] [PubMed] [Google Scholar]
- 21. Cui L, Yin S, Liu W, Zhang W, Cao Y. Expanded adipose‐derived stem cells suppress mixed lymphocyte reaction by secretion of prostaglandin E2. Tissue Eng 2007;13:1185–95. [DOI] [PubMed] [Google Scholar]
- 22. Ren G, Zhang L, Zhao X et al Mesenchymal stem cell‐mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell 2008;2:141–50. [DOI] [PubMed] [Google Scholar]
- 23. Gonzalez MA, Gonzalez‐Rey E, Rico L, Buscher D, Delgado M. Adipose‐derived mesenchymal stem cells alleviate experimental colitis by inhibiting inflammatory and autoimmune responses. Gastroenterology 2009;136:978–89. [DOI] [PubMed] [Google Scholar]
- 24. Gonzalez MA, Gonzalez‐Rey E, Rico L, Buscher D, Delgado M. Treatment of experimental arthritis by inducing immune tolerance with human adipose‐derived mesenchymal stem cells. Arthritis Rheum 2009;60:1006–19. [DOI] [PubMed] [Google Scholar]
- 25. Gonzalez‐Rey E, Per A, González MA, Rico L, Büscher D, Delgado M. Human adult stem cells derived from adipose tissue protect against experimental colitis and sepsis. Gut 2009;58:929–39. [DOI] [PubMed] [Google Scholar]
- 26. Parekkadan B, Fletcher AL, Li M et al Aire controls mesenchymal stem cell‐mediated suppression in chronic colitis. Mol Ther 2012;20:178–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tang R‐J, Shen S‐N, Zhao X‐Y et al Mesenchymal stem cells – regulated Treg cells suppress colitis‐associated colorectal cancer. Stem Cell Res Ther 2015;6:71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee HJ, Oh S‐H, Jang HW et al Long‐term effects of bone marrow‐derived mesenchymal stem cells in dextran sulfate sodium‐induced murine chronic colitis. Gut Liver 2016;10:412–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Alves VB, Basso PJ, Nardini V, Silva A, Chica JE, Cardoso CR. Dehydroepiandrosterone (DHEA) restrains intestinal inflammation by rendering leukocytes hyporesponsive and balancing colitogenic inflammatory responses. Immunobiology 2016;221:934–43. [DOI] [PubMed] [Google Scholar]
- 30. Jenkins D, Balsitis M, Gallivan S et al Guidelines for the initial biopsy diagnosis of suspected chronic idiopathic inflammatory bowel disease. The British Society of Gastroenterology Initiative. J Clin Pathol 1997;50:93–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Waterman RS, Tomchuck SL, Henkle SL, Betancourt AM. A new mesenchymal stem cells (MSC) paradigm: polarization into a pro‐inflammaotyr MSC1 or an immunossupressiveMSC2 phenotype. PLOS ONE 2010;5:e10088–e10101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Augello A, Tasso R, Negrini SM, Cancedda R, Pennesi G. Cell therapy using allogeneic bone marrow mesenchymal stem cells prevents tissue damage in collagen‐induced arthritis. Arthritis Rheum 2007;56:1175–86. [DOI] [PubMed] [Google Scholar]
- 33. Fiorina P, Jurewicz M, Augello A et al Immunomodulatory function of bone marrow‐derived mesenchymal stem cells in experimental autoimmune type 1 diabetes. J Immunol 2009;183:993–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhu J, Zhang J, Li Q, Du Y, Qiao B, Hu X. Transplanting of mesenchymal stem cells may affect proliferation and function of CD4(+) T cells in experimental autoimmune encephalomyelitis. Exp Clin Transplant 2012;10:492–500. [DOI] [PubMed] [Google Scholar]
- 35. Gu Z, Akiyama K, Ma X et al Transplantation of umbilical cord mesenchymal stem cells alleviates lupus nephritis in MRL/lpr mice. Lupus 2010;19:1502–14. [DOI] [PubMed] [Google Scholar]
- 36. Iseri K, Iyoda M, Ohtaki H et al Therapeutic effects and mechanism of conditioned media from human mesenchymal stem cells on anti‐GBM glomerulonephritis in WKY rats. Am J Physiol Renal Physiol 2016;310:F1182–F1191. [DOI] [PubMed] [Google Scholar]
- 37. Liang L, Dong C, Chen X et al Human umbilical cord mesenchymal stem cells ameliorate mice trinitrobenzene sulfonic acid (TNBS)‐induced colitis. Cell Transplant 2011;20:1395–408. [DOI] [PubMed] [Google Scholar]
- 38. Gonzalez‐Rey E, Gonzalez MA, Varela N et al Human adipose‐derived mesenchymal stem cells reduce inflammatory and T cell responses and induce regulatory T cells in vitro in rheumatoid arthritis. Ann Rheum Dis 2010;69:241–8. [DOI] [PubMed] [Google Scholar]
- 39. Casiraghi F, Azzollini N, Cassis P et al Pretransplant infusion of mesenchymal stem cells prolongs the survival of a semiallogeneic heart transplant though the generation of regulatory T cells. J Immunol 2008;181:3933–46. [DOI] [PubMed] [Google Scholar]
- 40. Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol 2003;3:521–33. [DOI] [PubMed] [Google Scholar]
- 41. Schirbel A, Fiocchi C. Inflammatory bowel disease: established and evolving considerations on its etiopathogenesis and therapy. J Dig Dis 2010;11:366–76. [DOI] [PubMed] [Google Scholar]
- 42. Gonçalves F da C, Schneider N, Pinto FO et al Intravenous vs intraperitoneal mesenchymal stem cells administration: what is the best route for treating experimental colitis? World Gastroenterol 2014;20:18228–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Aggarwal D, Limdi JK. Anti‐TNF therapy is associated with a reduction in radiation exposure in patients with Crohn’s disease. Eur J Gastroenterol Hepatol 2015;27:13–9. [DOI] [PubMed] [Google Scholar]
- 44. Jarry A, Cerf‐Bensussan N, Brousse N, Selz F, Guy‐Grand D. Subsets of CD3+ (T cell receptor alpha/beta or gamma/delta) and CD3‐ lymphocytes isolated from normal human gut epithelium display phenotypical features different from their counterparts in peripheral blood. Eur J Immunol 1990;20:1097–103. [DOI] [PubMed] [Google Scholar]
- 45. Strober W, Fuss IJ. Pro inflammatory cytokines in the pathogenesis of inflammatory bowel diseases. Gastroenterology 2011;140:1756–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shen W, Durum SK. Synergy of Il‐23 and Th17 cytokines: new light on inflammatory bowel disease. Neurochem Res 2010;35:940–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chao K, Zhang S, Qiu Y et al Human umbilical cord‐derived mesenchymal stem cells protect against experimental colitis via CD5(+) B regulatory cells. Stem Cell Res Ther 2016;7:109–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Engela AU, Hoogduijn MJ, Boer K et al Human adipose‐tissue derived mesenchymal stem cells induce functional de‐novo regulatory T cells with methylated FOXP3 gene DNA. Clin Exp Immunol 2013;173:343–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hu J, Zhang X, Zhou L, Zhang Y. Immunomodulatory properties of colonic mesenchymal stem cells. Immunol Lett 2013;156:23–9. [DOI] [PubMed] [Google Scholar]
- 50. Yamashita J, Iwamura C, Ito T et al Paraoxonase‐1 suppresses experimental colitis via the inhibition of IFN‐γ production from CD4 T cells. J Immunol 2013;191:949–60. [DOI] [PubMed] [Google Scholar]
- 51. Read S, Mauze S, Asseman C, Bean A, Coffman R, Powrie F. CD38+ CD45RB(low) CD4+ T cells: a population of T cells with immune regulatory activities in vitro . Eur J Immunol 1998;28:435–47. [DOI] [PubMed] [Google Scholar]
- 52. Kaufmann DE, Kavanagh DG, Pereyra F et al Upregulation of CTLA‐4 by HIV‐specific CD4+ T cells correlates with disease progression and defines a reversible immune dysfunction. Nat Immunol 2007;8:1246–54. [DOI] [PubMed] [Google Scholar]
- 53. Read S, Malmström V, Powrie F. Cytotoxic T lymphocyte‐associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med 2000;192:295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kingsley CI, Karim M, Bushell AR, Wood KJ. CD25+CD4+ regulatory T cells prevent graft rejection: CTLA‐1‐ and IL‐10‐dependent immunoregulation of alloresponses. J Immunol 2002;168:1080–6. [DOI] [PubMed] [Google Scholar]
- 55. Barber DL, Wherry EJ, Masopust D et al Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 2006;439:682–7. [DOI] [PubMed] [Google Scholar]
- 56. Velu V, Titanji K, Zhu B et al Enhancing SIV‐specific immunity in vivo by PD‐1 blockade. Nature 2009;458:206–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Augello A, Tasso R, Negrini SM et al Bone marrow mesenchymal progenitor cells inhibit lymphocyte proliferation by activation of the programmed death 1 pathway. Eur J Immunol 2005;35:1482–90. [DOI] [PubMed] [Google Scholar]
- 58. Sharma MD, Baban B, Chandler P et al Plasmacytoid dendritic cells from mouse tumor‐draining lymph nodes directly activate mature Tregs via indoleamine 2,3‐dioxygenase. J Clin Invest 2007;117:2570–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. McHugh RS, Whitters MJ, Piccirillo CA et al CD4(+)CD25(+) immunoregulatory T cells: gene expression analysis reveals a functional role for the glucocorticoid‐induced TNF receptor. Immunity 2002;16:311–23. [DOI] [PubMed] [Google Scholar]
- 60. Wang P‐F, Chen Y, Song S‐Y et al Immune‐related adverse events associated with anti‐PD‐1/PD‐L1 treatment for malignancies: a meta‐analysis. Front Pharmacol 2017;8:730–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Davies LC, Heldring N, Kadri N, Le Blanc K. Mesenchymal stromal cells secretion of programmed death‐1 ligands regulates T cell mediated immunosuppression. Stem Cells 2017;35:766–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Shimizu J, Yamazaki S, Takahashi T, Ishida Y, Sakaguchi S. Stimulation of CD25(+)CD4(+) regulatory T cells through GITR breaks immunological self‐tolerance. Nat Immunol 2002;3:135–42. [DOI] [PubMed] [Google Scholar]
- 63. Guo Y, Walsh AM, Canavan M et al Immune checkpoint inhibitor PD‐1 pathway is down‐regulated in synovium at various stages of rheumatoid arthritis disease progression. PLOS ONE 2018;13:e0192704‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Larussa T, Oliverio M, Suraci E et al Oleuropein decreases cyclooxygenase‐2 and interleukin‐17 expression and attenuates inflammatory damage in colonic samples from ulcerative colitis patients. Nutrients 2017;9:391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Elson CO, Cong Y, Weaver CT et al Monoclonal anti‐interleukin 23 reverses active colitis in a T cell‐mediated model in mice. Gastroenterology 2007;132:2359–70. [DOI] [PubMed] [Google Scholar]
- 66. Annunziato F, Cosmi L, Santarlasci V et al Phenotypic and functional features of human Th17 cells. J Exp Med 2007;204:1849–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL‐17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol 2007;25:821–52. [DOI] [PubMed] [Google Scholar]
- 68. Schmitt H, Billmeier U, Dieterich W et al Expansion of IL‐23 receptor bearing TNFR2+ T cells is associated with molecular resistance to anti‐TNF therapy in Crohn’s disease. Gut 2018. 10.1136/gutjnl-2017-315671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Holzer U, Reinhardt K, Lang P, Handgretinger R, Fischer N. Influence of a mutation in IFN‐γ receptor 2 (IFNGR2) in human cells on the generation of Th17 cells in memory T cells. Hum Immunol 2013;74:693–700. [DOI] [PubMed] [Google Scholar]
- 70. Zielinski CE, Mele F, Aschenbrenner D et al Pathogen‐induced human TH17 cells produce IFN‐γ or IL‐10 and are regulated by IL‐8β. Nature 2012;484:514–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Dose‐response evaluation of body weight variation of mice treated with mesenchymal stromal cells (MSC). Mice received intrarectal enema of 2,4,6‐trinitrobenzene sulfonic acid (TNBS) for induction of colitis and were treated 24h later with 0.25, 1 or 2x106 human MSC cells by intraperitoneal route. The body mass change (in percentage) was evaluated in relation to the first day of colitis.
Fig. S2. Histopathological evaluation of colitis outcome in mice treated with mesenchymal stromal cells (MSC). Mice received intrarectal enema of 2,4,6‐trinitrobenzene sulfonic acid (TNBS) for induction of colitis and were treated 24h later with 1x106 human MSC cells by intraperitoneal route. The histological alterations in colon tissues were observed after haemaoxilin‐eosin staining of samples collected on days 3, 7, 14 and 60 post TNBS infusion and treatment. In the upper panel, image depicts control ethanol mice, on day 3. The middle panel shows images representative of TNBS colitis group on days 3, 7 and 14, while the lower panel depicts TNBS mice on the same days, treated with MSC infusion.


