Summary
Dysfunction of the immune regulatory system plays an important role in the pathogenesis of rheumatoid arthritis (RA). Vasoactive intestinal peptide (VIP) has multiple bioactivities. This study aims to investigate the role of VIP in the maintenance of the immune regulatory capacity of monocytes (Mos). Human peripheral blood samples were collected from RA patients and healthy control (HC) subjects. Mos and CD14+ CD71–CD73+CD25+ regulatory Mos (RegMos) were isolated from the blood samples and characterized by flow cytometry. A rat RA model was developed to test the role of VIP in the maintenance of the immune regulatory function of Mos. The results showed that RegMos of HC subjects had immune suppressive functions. RegMos of RA patients expressed less interleukin (IL)‐10 and showed an incompetent immune regulatory capacity. Serum levels of VIP were lower in RA patients, which were positively correlated with the expression of IL‐10 in RegMos. In‐vitro experiments showed that the IL‐10 mRNA decayed spontaneously in RegMos, which could be prevented by the presence of VIP in the culture. VIP suppressed the effects of tristetraprolin (TTP) on inducing IL‐10 mRNA decay in RegMos. Administration of VIP inhibited experimental RA in rats through restoring the IL‐10 expression in RegMos. RegMos have immune suppressive functions. VIP is required in maintaining IL‐10 expression in RegMos. The data suggest that VIP has translational potential in the treatment of immune disorders such as RA.
Keywords: inflammation, interleukin‐10, immune regulation, monocytes, rheumatoid arthritis
Introduction
Rheumatoid arthritis (RA) is a chronic immune disease of the joints. The causative factors of RA are not clear. It is accepted that aberrant immune responses cause lesions in the joints of RA patients 1. The overproduction of proinflammatory cytokines, such as interferon (IFN)‐γ, tumor necrosis factor (TNF)‐α and interleukin (IL)‐17, are associated with the pathogenesis of RA 1. The aberrant production of proinflammatory cytokines in the body reveals that the immune regulatory functions are impaired. Currently, the therapeutics of RA are not satisfactory 2. Therefore, to elucidate the underlying mechanism of the aberrant immune responses in RA may help us to understand more clearly the pathogenesis of RA and design novel and more effective remedies for the treatment of RA.
The immune regulatory system in the body consists of immune regulatory cells and immune regulatory mediators. The cellular part includes several cell types, such as regulatory T cells (Tregs), regulatory B cells (Bregs), tolerogenic dendritic cells (DCs) and tolerogenic monocytes (Mos), etc. 3, 4. Immune regulatory cells release specific mediators, such as transforming growth factor (TGF)‐β and interleukin (IL)‐10, to suppress other immune cell activities 5 to maintain immune responses in a proper range. Dysfunction of the immune regulatory system may result in immune inflammation in the body, such as inflammatory bowel disease, rheumatoid arthritis and allergic diseases 6, 7, 8. A lower frequency or/and dysfunction of Treg or Breg was found in RA patients 9, 10. However, the mechanism of immune regulation disruption in RA patient is not yet fully understood.
Published data indicate that vasoactive intestinal peptide (VIP) has immune regulatory functions and has inhibitory effects on immune inflammation 11. VIP can be produced by a variety of cells, including neurons, epithelial cells and immune cells 11. Multiple functions have been observed in VIP, such as regulating the tone of blood vessels, increasing gland secretion and modulating protein production 12. VIP can also regulate immune functions and suppresses inflammation such as arthritis 13; however, the underlying mechanism remains to be further investigated.
Monocytes (Mos) are a fraction of the leukocytes 4. By differentiating into regulatory macrophages and tolerogenic DCs, Mos contribute to immune regulatory activities. After exposure to the proper stimuli, Mos also express immune regulatory mediators, such as IL‐10, to be directly involved in immune regulation 4. Published data demonstrate that the immune regulatory system is dysfunctional in RA patients 9, 10. The functional status of regulatory Mos (RegMos) in RA patients remains to be further understood. Whether or not VIP regulates RegMos in RA patients is not clear. Therefore, in this study, we collected peripheral blood samples from RA patients. The immune regulatory capacity of RA RegMos was evaluated and the effects of VIP on recovering RegMo immune regulatory capability was investigated.
Materials and methods
Reagents
RNA interference (RNAi) kits of tristetraprolin (TTP), antibodies of IL‐10, TTP and VIP, were purchased from Santa Cruz Biotech (Santa Cruz, CA, USA). Enzyme‐linked immunosorbent assay (ELISA) kits of IL‐4, IL‐13, IFN‐γ, TNF, IL‐10 and VIP were purchased from R&D Systems (Minneapolis, MN, USA). AS101 [IL‐10 inhibitor; ammonium trichloro (1,2‐ethanediolato‐O,O')‐tellurate] was purchased from Bio‐Techne (Shanghai, China). Immune cell isolation kits (for CD3, CD4, CD14, CD16, CD25, CD71, CD73, respectively) were purchased from Miltenyi Biotech (San Diego, CA, USA). Fluorochrome‐labeled antibodies were purchased from BD Biosciences (Franklin Lakes, NJ, USA). Reagents and materials for reverse transcription–quantitative polymerase chain reaction (RT–qPCR) and Western blotting were purchased from Invitrogen (Carlsbad, CA, USA). IL‐10 protein, reagents and materials for immunoprecipitation (IP) were purchased from Sigma Aldrich (St Louis, MO, USA).
Human subjects
Patients with RA and healthy control (HC) subjects were recruited at the Affiliated Hospitals of Shenzhen University. The diagnosis and management of RA were carried out by our physicians. RA patients were at remission stage. All the RA patients did not receive RA‐related medication for at least 2 months before the recruitment. The demographic data of human subjects are presented in Table 1. Human subjects with any of the following conditions were excluded from this study: cancer, autoimmune disorders of other organs, allergic diseases, severe organ diseases or receiving treatment with corticosteroids or other immune suppressors. The experimental procedures were approved by the Human Ethics Committee at Shenzhen University. A written informed consent was obtained from each human subject.
Table 1.
Demographic data of human subjects
| Items | RA patients | HC subjects |
|---|---|---|
| Gender (M/F) | 10/10 | 10/10 |
| Age (mean ± s.d.; years) | 44·5 ± 6·5 | 41·4 ± 5·3 |
| RA history (years) | 6·6 ± 3·1 | n.a. |
| ESR (mm/h) | 38·3 ± 4·4* | 8·8 ± 2·1 |
| Rheumatoid factor positive | 20 (100%) | 0 |
| Serum IL‐4 (pg/ml) | 28·3 ± 4·2* | 5·5 ± 2·5 |
| Serum IL‐13 (pg/ml) | 55·2 ± 6·8* | 11·4 ± 3·2 |
| Serum IL‐17 (pg/ml) | 46·5 ± 6·7* | 12·5 ± 3·2 |
| Serum TNF (pg/ml) | 76·7 ± 8·6* | 12·2 ± 3·4 |
| Serum IFN‐γ (pg/ml) | 55·8 ± 7·6* | 8·8 ± 2·3 |
Data are presented as mean ± standard deviation (s.d.). RA = rheumatoid arthritis; ESR = erythrocyte sedimentation rate; HC = healthy control; IL = interleukin; TNF = tumor necrosis factor; IFN = interferon; M/F = male/female; n.a. = not available.
P < 0·01 (t‐test), compared with HC group.
Preparation of peripheral blood mononuclear cells (PBMCs)
Blood samples were collected from human subjects by an ulnar vein puncture. PBMCs were isolated from the samples by Ficoll gradient density centrifugation following published procedures 14.
Isolation of immune cells
Immune cells were isolated from PBMCs or spleen cells by magnetic cell sorting (MACS) with commercial reagent kits, following the manufacturer’s instructions. Briefly, to isolate CD4+CD25– T cells, the CD3+CD4+ cells were isolated first, then the CD25+ cells were selected from the CD3+CD4+ cells. The remaining cells were CD4+CD25– T cells. To isolate RegMos, CD14+CD73+CD25+ cells were selected first. CD71– cells were then selected from the CD14+CD73+CD25+ cells; the remaining cells were CD14+CD73+CD25+CD71– cells (Supporting information, Fig. S1). To isolate the CD14+CD16– Mos, CD14+ cells were selected first; the CD16+ cells were then selected from the CD14+ cells. The remaining cells were the CD14+CD16– Mos. Purity of isolated cells was assessed by flow cytometry. If the purity was less than 95%, MACS was repeated with the cells.
Cell culture
Cells were cultured in RPMI‐1640 medium supplemented with fetal calf serum (10%), penicillin (100 U/ml), streptomycin (0·1 mg/ml) and glutamine (2 mM). The medium was changed in 2–3 days. Cell viability was greater than 99%, as assessed with the Trypan blue exclusion assay.
Flow cytometry
Cells collected from relevant experiments were stained with fluorochrome‐labeled antibodies of interest or isotype IgG for 30 min at 4°C. In the case of intracellular staining, cells first were treated with BD Cytofix/CytopermTM (BD Biosciences), then stained with fluorochrome‐labeled antibodies, including CD71‐fluorescein isothiocyanate (FITC), CD14‐phycoerythrin (PE), CD73‐cyanin 5 (Cy5), CD25‐allophycocyanin (APC)‐Cy5 and IL‐10 Cy7 or isotype immunoglobulin (Ig)G at 4°C for 30 min. The cells were analyzed with a flow cytometer (FACSCanto II; BD Biosciences). Data were analyzed with the FlowJo software package (TreeStar, Inc., Ashland, OR, USA). Data from isotype IgG staining were used as gating references.
Assessment of RegMo immune suppressive function
CD3+CD4+CD25– T cells were isolated from PBMCs by MACS with commercial reagent kits, labeled with carboxyfluorescein succinimidyl ester (CFSE) and used as effector T cells (Teffs). Following published procedures 15, CD14+CD71–CD73+CD25+ Mos were isolated from PBMCs and used as regulatory Mos (RegMos). RegMos and Teffs were co‐cultured (without Transwells) at a ratio of 1 : 5. Non‐specific activators, phorbol myristate acetate (PMA) (50 ng/ml) and ionomycin (100 ng/ml) were added to the culture to activate both RegMos and Teffs. Three days later, the cells were collected from the culture by centrifugation, washed three times with PBS and analyzed by flow cytometry. Proliferation of Teff was calculated and used as an indicator of T cell immune response.
RT–qPCR
RNA was extracted from cells collected from relevant experiments with TRIzol reagents. Total RNA was reverse‐transcribed into cDNA with a reverse transcription kit following the manufacturer’s instructions. The samples were amplified in a qPCR device with SYBR Green Master Mix in the presence of relevant primers, including IL‐10 (GCCAAGCCTTGTCTGAGATG and AAGAAATCGATGACAGCGCC) and TTP (GACTGAGCTATGTCGGACCT and GGTTGTGGATGAAGTGGCAG). The results are presented as fold change against the housekeeping gene β‐actin (CATGGAATCCTGTGGCATCC and CACACAGAGTACTTGCGCTC).
Protein extraction
Total proteins were extracted from cells collected from relevant experiments. Briefly, cells were lysed with a lysis buffer for 30 min. Lysates were centrifuged at 10 000 g for 10 min. Supernatant was collected and used as cytosolic extracts. The pellets were lysed with a nuclear lysis buffer for 30 min. Lysates were centrifuged at 13 000 rpm for 10 min. Supernatant was collected and used as nuclear extracts. The procedures were carried out at 4°C.
Western blotting
Proteins were fractioned by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS‐PAGE) and transferred onto a polvinylidene fluoride (PVDF) membrane. The membrane was blocked with a buffer containing 5% skimmed milk, incubated with primary antibodies [including IL‐10 (kappa light chain), TTP (lambda light chain), VIP (mouse IgG2b kappa light chain, 1 : 500 diluted] of interest overnight at 4°C, followed by incubating with peroxidase‐labeled secondary antibodies [mouse IgGκ binding protein‐horseradish peroxidase (HRP), 1 : 3000; mouse IgGλ binding protein‐HRP, 1 : 3000; mouse IgGκ binding protein‐HRP, 1 : 3000] for 2 h at room temperature. The membrane was washed with Tris‐buffered saline containing 0·1% Tween 20 after each incubation. Immunoblots on the membrane were developed with enhanced chemiluminescence (Invitrogen) and photographed with an imaging device (UVI Image Station, Cambridge, UK).
Immunoprecipitation (IP)
Proteins were precleared by incubating with protein G agarose beads for 2 h. The beads were removed by centrifugation. Supernatant was incubated with antibodies of VIP and TTP (diluted 1 : 200) overnight. Immune complexes in the samples were precipitated by incubating with protein G agarose beads for 2 h, and then eluted with an eluting buffer. The proteins were analyzed by Western blotting.
Detection of TTP–IL‐10 mRNA complex
Mos were incubated with lipopolysaccharide (LPS) (100 ng/ml) for 48 h. To cross‐link protein and RNA, the Mos were irradiated with 0·15 J/cm2 of 365 nm UV light in a Stratalinker 2400 (Stratagene, La Jolla, CA, USA) and lysed with a lysis buffer. The lysates were then processed using the IP procedures. After elution with an eluting buffer, RNA was recovered from the samples with an RNA extracting reagent kit following the manufacturer’s instructions and analyzed by RT–qPCR. Protein was recovered from the samples and analyzed by Western blotting.
Rat arthritis model
Male Sprague‐Dawley rats (body weight approximately 200 g) were purchased from Guangdong Experimental Animal Center. Rats were maintained in a specific pathogen‐free facility with freely accessed food and water. Following published procedures 16 with modification, rats were injected subcutaneously (at the tail base) with a type‐II collagen (CII; 0·3 mg per rat) emulsion in 0·3 ml incomplete Freund’s adjuvant on day 0, and repeated on days 3 and 7, respectively. Intraknee articular injection with CII (0·1 mg in 0·1 ml saline per rat) was performed on days 14, 21 and 27, respectively. Rats were killed on day 28. Control rats were injected with saline. The experimental procedures were approved by the Animal Ethics Committee at Shenzhen University.
ELISA
Cytokine levels in the serum were analyzed by ELISA with commercial reagent kits (VIP, TNF, IFN‐γ, IL‐17 and IL‐10), following the manufacturer’s instructions.
Histology of articular joints
The articular joints were removed from rats immediately after killing and fixed with 4% formalin for 24 h. After decalcifying, the joints were paraffin‐embedded. Paraffin sections were prepared, stained with hematoxylin and eosin and observed with a light microscope.
Statistics
The difference between two groups was determined by Student’s t‐test. Analysis of variance (anova), followed by Dunnett’s t‐test or Student–Newman–Keuls test, was performed for multiple comparisons. Pearson’s correlation assay was performed to determine the correlation between two data sets when appropriate. P < 0·05 was considered statistically significant.
Results
RegMos are dysfunctional in RA patients
Inspired by published data that CD19+CD71–CD73+CD25+ B cells have immune suppressive functions and are designated as Bregs 15, we wondered if other cell types with this cytokine profile also have a similar function. Thus, we collected blood samples from HC subjects; PBMCs were isolated from the samples and analyzed by flow cytometry. CD14+CD71–CD73+CD25+ Mos were detected in PBMCs and designated as RegMos (Fig. 1). RegMos showed immune suppressive effects on effector T cell (Teff) proliferation (Fig. 2). RegMos were also found in the peripheral blood samples of RA patients. The frequency of peripheral RegMos was not significantly different between the HC and the RA groups (Fig. 1), while the immune suppressive effects on Teff proliferation were significantly weaker in the RA group than in the HC group (Fig. 2). The results indicate that the CD14+CD71–CD73+CD25+ RegMos have an immune suppressive function, which is impaired in RA RegMos (RegMos collected from RA patients), although RegMo frequency is not significantly different between RA patients and HC subjects.
Figure 1.
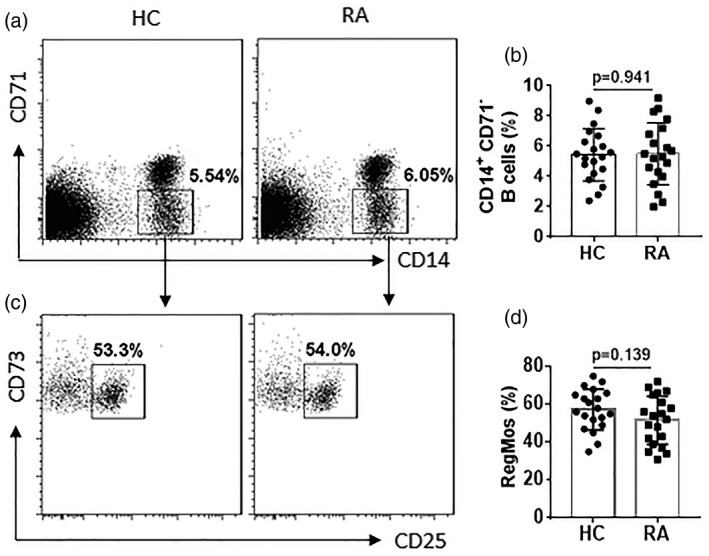
Assessment of peripheral regulatory monocytes (RegMos) of human subjects. Peripheral blood mononuclear cells (PBMCs) were prepared from blood samples of healthy control (HC) subjects (n = 20) and rheumatoid arthritis (RA) patients (n = 20) and analyzed by flow cytometry. (a) Gated dot‐plots show frequency of CD14+CD71– monocytes. (b) Bars show summarized frequency of CD14+CD71– monocytes. (c) Gated dot‐plots show frequency of RegMos. (d) Bars show summarized frequency of RegMos. The data of bars are presented as mean ± standard error of the mean (s.e.m.). Each dot inside bars presents data from an independent experiment.
Figure 2.
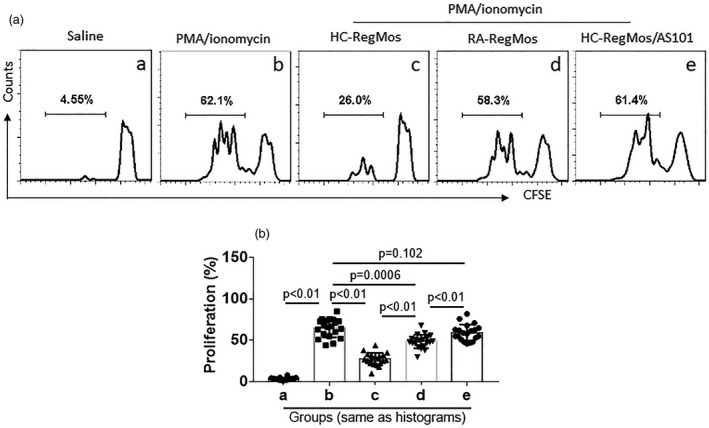
Rheumatoid arthritis (RA) regulatory monocytes (RegMos) show incompetent immune suppressive function. RegMos were prepared from peripheral blood mononuclear cells (PBMCs) of RA patients (n = 20) and healthy control (HC) subjects (n = 20). Effector CD4+CD25– T cells (Teffs) were prepared from PBMCs of HC subjects and labeled with carboxyfluorescein succinimidyl ester (CFSE). RegMos and Teffs were co‐cultured at a ratio of 1 : 5 with the treatment denoted above each subpanel of (a) for 3 days. (a) Gated histograms show frequency of proliferating Teffs. (b) Bars indicate summarized frequency of proliferating Teffs. AS101: 2·5 μg/ml AS101 in the culture. Data of bars are presented as mean ± standard error of the mean (s.e.m.). Each dot inside bars presents data from an independent experiment.
Peripheral RegMos express IL‐10 that is positively correlated with serum VIP
As IL‐10 is the immune regulatory mediator in Bregs 17, we wondered if IL‐10 was also the immune regulatory mediators in RegMos. To this end, we isolated RegMos from PBMCs by MACS and analyzed them by RT–qPCR and Western blotting. The results showed that HC RegMos expressed IL‐10, which was significantly lower in RA RegMos (Fig. 3a,b). Published data show that VIP has immune regulatory functions 18. Serum VIP levels were determined by ELISA. The results showed that VIP was detected in the serum that was significantly lower in RA patients than that in HC subjects (Fig. 3c). A positive correlation was detected between IL‐10 mRNA of RA RegMos and serum levels of VIP (Fig. 3d). The results demonstrate that RA RegMos have lower expression of IL‐10 and lower levels of serum VIP. The serum VIP is probably required in the maintenance of IL‐10 expression in RegMos.
Figure 3.
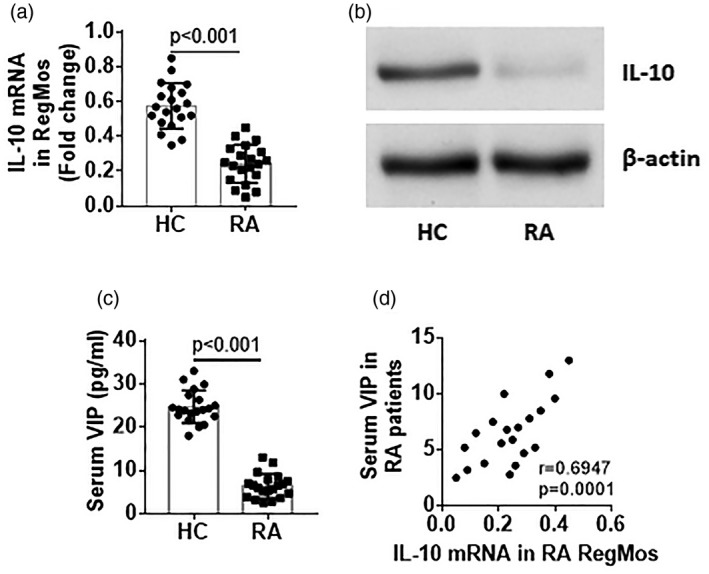
Expression of interleukin (IL)‐10 in regulatory monocytes (RegMos) is correlated with serum vasoactive intestinal peptide (VIP). RegMos were isolated from peripheral blood mononuclear cells (PBMCs) collected from healthy control (HC) subjects (n = 20) and rheumatoid arthritis (RA) patients (n = 20). RNA was extracted from the RegMos and analyzed by reverse transcription–quantitative polymerase chain reaction (RT–qPCR) and Western blotting. (a) Bars indicate IL‐10 mRNA levels in RegMos. B, proteins were extracted from the RegMos, pooled and analyzed by Western blotting. The immunoblots indicate protein levels of IL‐10 in RegMos (representing three independent experiments). (c) Bars indicate serum levels of VIP. (d) Scatter‐plots show a positive correlation between serum VIP and IL‐10 mRNA levels in RA RegMos. Data of bars are presented as mean ± standard error of the mean (s.e.m.). Each dot inside bars presents data from an independent experiment.
VIP prevents IL‐10 mRNA decay in RegMos
We then looked further into the low expression mechanism of IL‐10 in RA RegMos. Blood samples were collected from HC subjects. CD14+CD16– Mos were isolated from the samples and exposed to LPS in the culture. The results showed that exposure to LPS increased IL‐10 mRNA levels in Mos (Fig. 4a,b); the levels of mRNA in Mos decayed spontaneously in a time‐dependent manner. Addition of VIP to the culture prevented the IL‐10 mRNA decay (Fig. 4c). Together with the results of Fig. 3, the data demonstrate that IL‐10 mRNA can decay spontaneously in Mos and the presence of VIP can extend the life span of IL‐10 mRNA in Mos.
Figure 4.
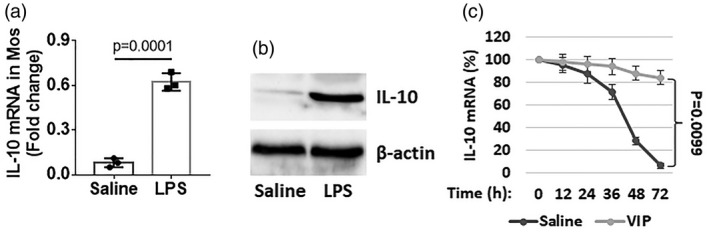
Vasoactive intestinal peptide (VIP) blocks interleukin (IL)‐10 mRNA decay in monocytes (Mos). (a,b) CD14+CD16– Mos were isolated from blood samples collected from healthy control (HC) subjects and exposed to lipopolysaccharide (LPS) (100 ng/ml) in the culture for 48 h. The cells were analyzed by reverse transcription–quantitative polymerase chain reaction (RT–qPCR) and Western blotting. Bars indicate IL‐10 mRNA levels (a); immunoblots indicate IL‐10 protein levels (b). (c) Mos were stimulated with LPS in the culture for 48 h. The cells were washed with fresh medium and recultured with fresh medium in the presence or absence of VIP (10 ng/ml). The cells were collected at indicated time‐points (on the x‐axis) and analyzed by RT–qPCR. Curves indicate IL‐10 mRNA in Mos. Data of bars are presented as mean ± standard error of the mean (s.e.m.). Each dot inside bars presents data from an independent experiment.
VIP counteracts effects of TTP on IL‐10 mRNA decay in Mos
The published data indicate that TTP can bind to IL‐10 mRNA to accelerate IL‐10 mRNA decay 19. We also observed that TTP expression was increased in Mos after exposure to LPS (Fig. 5a,b). The presence of VIP or knockdown of TTP abolished the IL‐10 mRNA decay in Mos (Fig. 5c,d). Further analysis showed that VIP bound TTP to form a complex (Fig. 5e); such a physical contact reduced the binding between TTP and IL‐10 mRNA (Fig. 5f,g). The results indicate that VIP prevents IL‐10 mRNA decay in Mos through interfering with the formation of a complex between TTP and IL‐10 mRNA.
Figure 5.
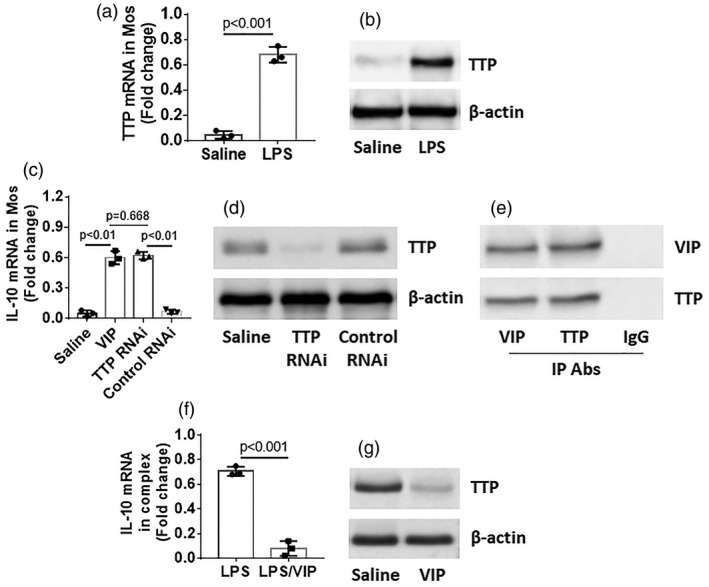
Vasoactive intestinal peptide (VIP) prevents tristetraprolin (TTP) from binding interleukin (IL)‐10 mRNA in monocytes (Mos). CD14+CD16– Mos were prepared from blood samples of healthy control (HC) subjects. (a–c) Mos were stimulated with lipopolysaccharide (LPS) for 30 min in the culture, washed and cultured with fresh medium for 48 h. The cells were analyzed by reverse transcription–quantitative polymerase chain reaction (RT–qPCR) and Western blotting. Bars indicate levels of tristetraprolin (TTP) mRNA (a); immunoblots indicate TTP protein levels (b). (c) A portion of the Mos were further treated with the procedures denoted on the x‐axis. Bars indicate IL‐10 mRNA levels in Mos. (d) TTP RNAi results in Mos. (e) Mos were treated with LPS and VIP in the culture for 48 h. The cells were analyzed by immunoprecipitation (IP). Immunoblots indicate a complex of VIP and TTP in Mos. (f) Mos were exposed to LPS or LPS/VIP in the culture for 48 h. The cells were analyzed by RT–qPCR and Western blotting. Bars indicate IL‐10 mRNA (f) and immunoblots indicate TTP protein in a complex in Mos (the β‐actin is from cytosolic extracts serving as a reference). Data of bars are presented as mean ± standard error of the mean (s.e.m.). Each dot inside bars presents data from an independent experiment.
VIP inhibits experimental RA through restoring immune suppressive function in RegMos
Finally, we tested the immune regulatory effects of VIP on experimental rheumatoid arthritis. Rats were sensitized and challenged with type II collagen (CII) with or without administration of VIP. Rats showed redness and swelling of the joint skin (not shown), articular tissue destruction and profound exudates in the articular cavity (Fig. 6a). Increases in serum levels of TNF‐α, IL‐17 and IFN‐γ and a decrease in serum IL‐10 in RA rats were observed (Fig. 6b–e). Although the total number of RegMos was comparable between RA rats and control rats, the frequency of IL‐10+ RegMos was markedly lower in the spleen of RA rats than that in control rats (Fig. 6f, Supporting information, Fig. S2). Levels of mRNA and protein of IL‐10 were lower in RegMos isolated from the spleen of RA rats than that in control rats (Fig. 6g,h). Administration of VIP significantly attenuated RA‐related pathological changes in the rats, which was abolished by the administration of anti‐CD14 antibodies to deplete CD14+ cells (Fig. 6; Supporting information, Fig. S2). The results demonstrate that administration of VIP inhibits RA‐like inflammation in rats through induction of RegMos.
Figure 6.
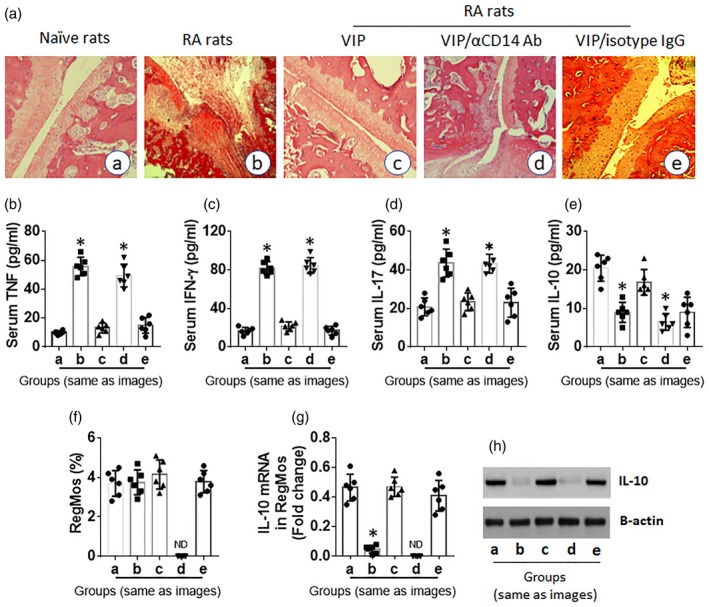
Vasoactive intestinal peptide (VIP) suppresses experimental arthritis through restoring interleukin (IL)‐10 expression in regulatory monocytes (RegMos). Rats (six per group) were treated with procedures denoted with each histology image. (a) Representative images show rat articular joint histology. (b) Bars show serum levels of tumor necrosis factor (TNF) (b), interferon (IFN)‐γ (c), IL‐17 (d) and IL‐10 (e). (f) Bars show frequency of RegMos in the rat spleen. (g) Bars show IL‐10 mRNA levels in isolated RegMos. (h) Immunoblots show IL‐10 protein levels in isolated RegMos. Data of bars are presented as mean ± standard error of the mean (s.e.m.). Each dot inside bars presents data from an independent experiment. *P < 0·01, compared with group (a). VIP: rats were peritoneally injected with VIP (0·166 mg/kg/day in 0·1 ml saline) 1 day before each administration of type II collagen (CII). αCD14 antibody [or isotype immunoglobulin (IgG)]: anti‐CD14 antibody (or isotype IgG; control); rats were peritoneally injected with αCD14 antibody on day 0 (0·5 mg/rat); n.d. = not detectable.
Discussion
The present data show that, similar to Bregs 15, the CD14+CD25+CD71–CD73+ RegMos can be detected in the peripheral blood system of both HC subjects and RA patients. Although the RegMo frequency is comparable between HC subjects and RA patients, the immune regulatory function of RegMos is impaired in the RA group. IL‐10 is an important mediator in the immune regulation that is expressed in tolerogenic DCs 20, type I Tregs 20 and Bregs 15. RA RegMos express less IL‐10, which is positively correlated with serum VIP levels. VIP prevents IL‐10 mRNA decay in RegMos by forming a complex with TTP. Administration of VIP inhibits experimental RA.
The data show that RegMos have an immune regulatory function. By expressing IL‐10, RegMos can inhibit Teff proliferation. Such activities were found in monocytes upon exposure to microbial products 21; Bregs can also suppress other immune cell activities upon correct stimulation. Our previous work showed that Bregs released TGF‐β in response to re‐exposure to endothelial cell‐derived exosomes, which suppressed Teff proliferation 22. Exposure to insulin‐like growth factor‐1 can induce Mos to express IL‐10 and have an immune suppressive function 4. Based on reported data that CD19+CD25+CD71–CD73+ B cells express IL‐10 and have an immune regulatory capacity 15, we found that CD14+CD25+CD71–CD73+ Mos isolated from HC subjects also express IL‐10 and have an immune suppressive function. Thus, our data demonstrate that this Mo fraction is a subtype of immune regulatory cells and designated as RegMos.
The published data indicate that IL‐10 is an important mediator in immune regulation 23, and also show that RegMo‐derived IL‐10 plays an important role in RegMo‐induced immune suppression on Teff proliferation. This feature of RegMos is similar to IL‐10‐producing Bregs and type 1 regulatory T cells (Tr1). The IL‐10‐producing Bregs are designated as B10 cells. B10 cells are CD19+CD5+IgMhiIgDloCD1dhi and play an important role in the maintenance of the homeostasis in the intestine 24. Tr1 cells can be induced by exposure to non‐pathogenic bacteria; this fraction of Tregs may also produce IFN‐γ and suppress Teff cells and cutaneous inflammation 25. The present data demonstrate that RegMos, the fraction of IL‐10‐producing regulatory cells, are functionally similar to B10 cells and Tr1 cells.
We found that, similar to the B cell response to LPS, Mos also expressed IL‐10 after stimulation by LPS. However, the expression of IL‐10 declined spontaneously in Mos after removal of LPS from the culture, a phenomenon designated ‘RNA decay’ 26. This may be the reason that the levels of IL‐10 in RegMos were significantly lower in RA patients than that in HC subjects. The positive correlation between serum VIP levels and IL‐10 mRNA in RegMos proposes a probability that serum VIP maintains the expression of IL‐10 in RegMos. It has been noted that VIP has an immune regulatory function; for example, VIP down‐regulates Langerhans’ cell function and the associated immune response 27, stimulates IL‐10 expression in macrophages to suppress inflammation 28, enhances myeloid‐derived suppressor cell immune suppressive function 29, regulates activities of Toll‐like receptors 30 and induces tolerogenic DCs 31. All these examples suggest that VIP has immune regulatory functions. The present data provide mechanistic evidence that VIP maintains the expression of immune regulatory mediator, IL‐10, to contribute to the activities of immune regulation 4 and directly fulfill the immune suppressive function.
RNA decay is a common phenomenon in the process of gene expression 26. The present data show IL‐10 mRNA decay in Mos that may be the factor causing the lower expression of IL‐10 in RA RegMos and dysfunction of immune regulatory machinery. RNA decay is a built‐in mechanism to maintain the genetic information at high fidelity 32. In line with published data, that TTP can bind to IL‐10 mRNA to accelerate IL‐10 mRNA decay 19, the present data show that TTP plays a critical role in causing IL‐10 mRNA decay in Mos; this can be counteracted by the presence of VIP in the culture, suggesting that VIP can block the activities of TTP and helps to maintain the expression of IL‐10 in RegMos.
Pathologically, RA is an immune disease. Previous studies have noted that the serum levels of proinflammatory cytokines, such as TNF‐α, IFN‐γ and IL‐17, were higher in RA patients. The present data also found that these proinflammatory cytokines were increased in the serum of both RA patients and RA rats. Immune activities in the body, including cytokine expression, are tightly regulated by the immune regulatory system. The aberrant increases in serum cytokines can be a sign of dysfunction of the immune regulatory system. The lower levels of IL‐10 in RA RegMos verified this inference. As the presence of VIP can block IL‐10 mRNA decay in Mos, we administered VIP in rats with RA. Indeed, VIP efficiently inhibited the RA‐like inflammation through restoring the expression of IL‐10 in RegMos.
Macrophages are the major inflammatory cells in RA and are the main contributor of the inflammatory cytokines in inflamed joints 33. Macrophages can be divided into two types, M1 and M2. M1 macrophages produce proinflammatory cytokines such as TNF‐α, IL‐1β, IL‐6 and IL‐12. M2 macrophages are also called ‘alternative activated macrophages’; they secrete anti‐inflammatory cytokines such as IL‐10 34. Because macrophages are developed from monocytes, whether or not the RegMos can develop into M2 macrophages and administration of VIP can modulate macrophage phenotypes remain to be investigated.
In summary, the present data indicate that the immune suppressive function is impaired in RA RegMos. Exposure to VIP can recover the immune regulatory function of RegMos through restoring IL‐10 expression. The administration of VIP has translational potential in the treatment of RA.
Disclosures
None to declare.
Author contributions
L. G., D. Y., G. U. W., H. J. N., S. D. H., S. S. L., T. Y. H., G. Y., Z. Q. L., H. Q. Y. and X. Z. S. performed experiments, analyzed data and reviewed the manuscript. P. C. Y. and Z. G. L. organized the study and supervised experiments. P. C. Y. designed the project and wrote the manuscript.
Supporting information
Fig. S1. Isolation of RegMos. Blood samples were collected from human subjects. Peripheral blood mononuclear cells (PBMCs) were isolated from the blood samples. RegMos were purified from PBMCs by magnetic cell sorting with commercial reagent kits following the manufacturer's instructions. (a), the gated dot plots show CD14+ CD25+ cells. (b), the gated histograms show CD73+ cells in CD14+ CD25+ cells in panel (a). (c), the gated histograms show CD71¯ cells in CD14+ CD25+ cells in panel (a). The data represent 3 independent experiments.
Fig. S2. Assessment of RegMos in the rat spleen. Rats were treated with the procedures denoted above panel A. Spleen cells were prepared from the rats and analyzed by flow cytometry. (a), gated dot plots indicate frequency of CD14+ CD71¯ Mos. (b), gated dot plots indicate frequency of RegMos. (c), gated histograms indicate frequency of IL‐10+ RegMos. Each group consists of 6 rats. αCD14 Ab (or isotype IgG): Anti‐CD14 antibody (or isotype IgG; control); rats were peritoneally injected with αCD14 Ab on day 0 (0·5 mg/rat).
Acknowledgement
This study was supported by grants from the National Nature and Science Foundation of China (81870706, 31570932, 81700888, 81701589) and the Shenzhen Science, Technology and Innovation Committee (JCYJ20170307162827158, KQTD20170331145453160).
Contributor Information
Z.‐G. Liu, Email: lzg@szu.edu.cn.
P.‐C. Yang, Email: pcy2356@szu.edu.cn.
References
- 1. Alam J, Jantan I, Bukhari SNA. Rheumatoid arthritis: Recent advances on its etiology, role of cytokines and pharmacotherapy. Biomed Pharmacother 2017;92:615–33. [DOI] [PubMed] [Google Scholar]
- 2. Reynolds G, Cooles FA, Isaacs JD, Hilkens CM. Emerging immunotherapies for rheumatoid arthritis. Hum Vaccin Immunother 2014;10:822–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sun M, He C, Cong Y, Liu Z. Regulatory immune cells in regulation of intestinal inflammatory response to microbiota. Mucosal Immunol 2015;8:969–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ge RT, Mo LH, Wu R et al Insulin‐like growth factor‐1 endues monocytes with immune suppressive ability to inhibit inflammation in the intestine. Sci Rep 2015;5:7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Palomares O, Martin‐Fontecha M, Lauener R et al Regulatory T cells and immune regulation of allergic diseases: Roles of IL‐10 and TGF‐beta. Genes Immun 2014;15:511–20. [DOI] [PubMed] [Google Scholar]
- 6. Pedros C, Duguet F, Saoudi A, Chabod M. Disrupted regulatory T cell homeostasis in inflammatory bowel diseases. World J Gastroenterol 2016;22:974–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cooles FA, Isaacs JD, Anderson AE. Treg cells in rheumatoid arthritis: An update. Curr Rheumatol Rep 2013;15:352. [DOI] [PubMed] [Google Scholar]
- 8. Terhune TD, Deth RC. A role for impaired regulatory T cell function in adverse responses to aluminum adjuvant‐containing vaccines in genetically susceptible individuals. Vaccine 2014;32:5149–55. [DOI] [PubMed] [Google Scholar]
- 9. Nie H, Zheng Y, Li R et al Phosphorylation of FOXP3 controls regulatory T cell function and is inhibited by TNF‐alpha in rheumatoid arthritis. Nat Med 2013;19:322–8. [DOI] [PubMed] [Google Scholar]
- 10. Salomon S, Guignant C, Morel P et al Th17 and CD24(hi)CD27(+) regulatory B lymphocytes are biomarkers of response to biologics in rheumatoid arthritis. Arthritis Res Ther 2017;19:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ganea D, Hooper KM, Kong W. The neuropeptide vasoactive intestinal peptide: Direct effects on immune cells and involvement in inflammatory and autoimmune diseases. Acta Physiol (Oxf) 2015;213:442–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Delgado M, Pozo D, Ganea D. The significance of vasoactive intestinal peptide in immunomodulation. Pharmacol Rev 2004;56:249–90. [DOI] [PubMed] [Google Scholar]
- 13. Villanueva‐Romero R, Gutierrez‐Canas I, Carrion M et al The anti‐inflammatory mediator, vasoactive intestinal peptide, modulates the differentiation and function of Th subsets in rheumatoid arthritis. J Immunol Res 2018;2018:6043710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ulmer AJ, Scholz W, Ernst M, Brandt E, Flad HD. Isolation and subfractionation of human peripheral blood mononuclear cells (PBMC) by density gradient centrifugation on Percoll. Immunobiology 1984;166:238–50. [DOI] [PubMed] [Google Scholar]
- 15. van de Veen W, Stanic B, Yaman G et al IgG4 production is confined to human IL‐10‐producing regulatory B cells that suppress antigen‐specific immune responses. J Allergy Clin Immunol 2013;131:1204–12. [DOI] [PubMed] [Google Scholar]
- 16. Correa MG, Sacchetti SB, Ribeiro FV et al Periodontitis increases rheumatic factor serum levels and citrullinated proteins in gingival tissues and alter cytokine balance in arthritic rats. PLOS ONE 2017;12:e0174442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tedder TF. B10 cells: A functionally defined regulatory B cell subset. J Immunol 2015;194:1395–401. [DOI] [PubMed] [Google Scholar]
- 18. Jiang W, Wang H, Li YS, Luo W. Role of vasoactive intestinal peptide in osteoarthritis. J Biomed Sci 2016;23:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stoecklin G, Tenenbaum SA, Mayo T et al Genome‐wide analysis identifies interleukin‐10 mRNA as target of tristetraprolin. J Biol Chem 2008;283:11689–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee HY, Kim J, Ryu JS, Park SJ. Trichomonas vaginalis alpha‐actinin 2 modulates host immune responses by inducing tolerogenic dendritic cells via IL‐10 production from regulatory T cells. Korean J Parasitol 2017;55:375–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. O’Regan NL, Steinfelder S, Venugopal G et al Brugia malayi microfilariae induce a regulatory monocyte/macrophage phenotype that suppresses innate and adaptive immune responses. PLOS Negl Trop Dis 2014;8:e3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Song J, Chen X, Wang M, Xing Y, Zheng Z, Hu S. Cardiac endothelial cell‐derived exosomes induce specific regulatory B cells. Sci Rep 2014;4:7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Saraiva M, O’Garra A. The regulation of IL‐10 production by immune cells. Nat Rev Immunol 2010;10:170–81. [DOI] [PubMed] [Google Scholar]
- 24. Noh G, Lee JH. Regulatory B cells and allergic diseases. Allergy Asthma Immunol Res 2011;3:168–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Volz T, Skabytska Y, Guenova E et al Nonpathogenic bacteria alleviating atopic dermatitis inflammation induce IL‐10‐producing dendritic cells and regulatory Tr1 cells. J Invest Dermatol 2014;134:96–104. [DOI] [PubMed] [Google Scholar]
- 26. Silva IJ, Saramago M, Dressaire C, Domingues S, Viegas SC, Arraiano CM. Importance and key events of prokaryotic RNA decay: The ultimate fate of an RNA molecule. Wiley Interdiscipl Rev RNA 2011;2:818–36. [DOI] [PubMed] [Google Scholar]
- 27. Kodali S, Ding W, Huang J, Seiffert K, Wagner JA, Granstein RD. Vasoactive intestinal peptide modulates Langerhans cell immune function. J Immunol 2004;173:6082–8. [DOI] [PubMed] [Google Scholar]
- 28. Delgado M, Munoz‐Elias EJ, Gomariz RP, Ganea D. Vasoactive intestinal peptide and pituitary adenylate cyclase‐activating polypeptide enhance IL‐10 production by murine macrophages: in vitro and in vivo studies. J Immunol 1999;162:1707–16. [PubMed] [Google Scholar]
- 29. Forghani P, Petersen CT, Waller EK. Activation of VIP signaling enhances immunosuppressive effect of MDSCs on CMV‐induced adaptive immunity. Oncotarget 2017;8:81873–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gomariz RP, Arranz A, Juarranz Y et al Regulation of TLR expression, a new perspective for the role of VIP in immunity. Peptides 2007;28:1825–32. [DOI] [PubMed] [Google Scholar]
- 31. Gonzalez‐Rey E, Chorny A, Fernandez‐Martin A, Ganea D, Delgado M. Vasoactive intestinal peptide generates human tolerogenic dendritic cells that induce CD4 and CD8 regulatory T cells. Blood 2006;107:3632–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ghosh S, Jacobson A. RNA decay modulates gene expression and controls its fidelity. Wiley Interdiscip Rev RNA 2010;1:351–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Udalova IA, Mantovani A, Feldmann M. Macrophage heterogeneity in the context of rheumatoid arthritis. Nat Rev Rheumatol 2016;12:472–85. [DOI] [PubMed] [Google Scholar]
- 34. Genin M, Clement F, Fattaccioli A, Raes M, Michiels C. M1 and M2 macrophages derived from THP‐1 cells differentially modulate the response of cancer cells to etoposide. BMC Cancer 2015;15:577. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Isolation of RegMos. Blood samples were collected from human subjects. Peripheral blood mononuclear cells (PBMCs) were isolated from the blood samples. RegMos were purified from PBMCs by magnetic cell sorting with commercial reagent kits following the manufacturer's instructions. (a), the gated dot plots show CD14+ CD25+ cells. (b), the gated histograms show CD73+ cells in CD14+ CD25+ cells in panel (a). (c), the gated histograms show CD71¯ cells in CD14+ CD25+ cells in panel (a). The data represent 3 independent experiments.
Fig. S2. Assessment of RegMos in the rat spleen. Rats were treated with the procedures denoted above panel A. Spleen cells were prepared from the rats and analyzed by flow cytometry. (a), gated dot plots indicate frequency of CD14+ CD71¯ Mos. (b), gated dot plots indicate frequency of RegMos. (c), gated histograms indicate frequency of IL‐10+ RegMos. Each group consists of 6 rats. αCD14 Ab (or isotype IgG): Anti‐CD14 antibody (or isotype IgG; control); rats were peritoneally injected with αCD14 Ab on day 0 (0·5 mg/rat).


