Abstract
We report a case of non–small-cell lung cancer (NSCLC) to small-cell lung cancer (SCLC) transformation after epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI) treatment. The patient was a man who diagnosed with EGFR-mutant advanced NSCLC. After he was introduced afatinib, his tumor had been reduced by the treatment. However, plasma pro–gastrin-releasing peptide (ProGRP) became higher with disease progression, and SCLC was detected at the second biopsy. It is suggested that elevation of plasma ProGRP level before EGFR-TKI therapy is useful for predicting EGFR-mutant NSCLC to SCLC transformation.
Keywords: Lung cancer, Epidermal growth factor receptor tyrosine kinase inhibitor, Small-cell lung cancer, Pro–gastrin-releasing peptide
1. Introduction
Recently, epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI) has a good antitumor effect in non–small-cell lung cancer (NSCLC) patients with common epidermal growth factor receptor mutation [1]. However, in many cases, their disease acquire resistance to EGFR-TKI treatment in approximately 1 year [2]. Some resistance mechanisms were reported in previous report. The presence of T790M mutation as the secondary mutation is the most common resistance mechanism of EGFR-TKI treatment [3]. Amplification of MET, or HER2 is known as a major mechanism of resistance of EGFR-TKI treatment by bypass signaling activation [[4], [5], [6]]. Meanwhile, NSCLC to small-cell lung carcinoma (SCLC) transformation has been reported as another mechanism [[7], [8], [9]]. Here, we report that we succeeded in early prediction of NSCLC to SCLC transformation after EGFR-TKI treatment by monitoring plasma pro–gastrin-releasing peptide (ProGRP).
2. Case report
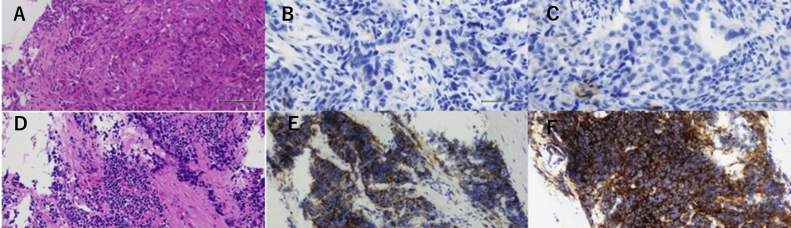
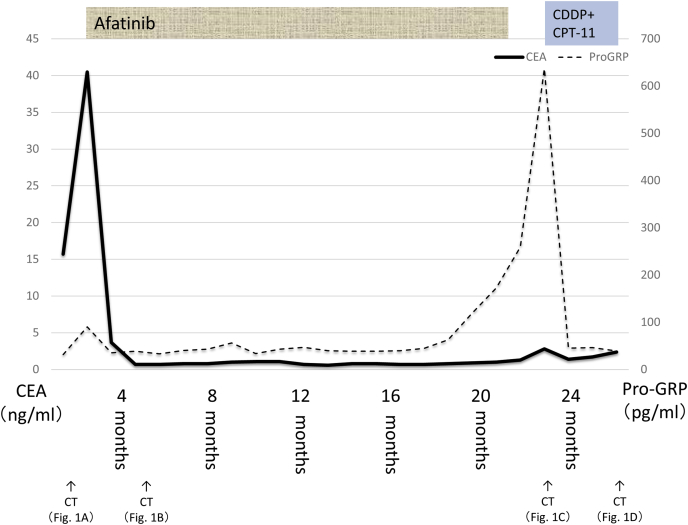
A 47-year-old male heavy smoker (30 pack-years) presented to our hospital because of hemosputum. Right hilar invasive lesion and multiple mediastinal and cervical lymphadenopathy revealed by Computed tomography (Fig. 1A). Serum carcinoembryonic antigen (CEA) level were elevated higher than the reference values (40.5 ng/mL) and mild elevation of ProGRP (103 pg/ml) were shown at the same timing [10,11]. Transbronchial lung biopsy was performed to primary lesion for diagnosis by transbronchoscopy. This biopsy sample showed tubular type of adenocarcinoma, diffusely positive for thyroid transcription factor-1 and focally positive for cytokeratin 5/6 by immunohistochemistry. On the contrary, CD56, synaptophysin, and chromogranin A which known for neuroendocrine marker were negative at biopsy sample (Fig. 2A, B, C). The biopsy sample was genotyped, and EGFR exon 19 deletion (E746_A750del) was detected by polymerase chain reaction invader method. After the patient received head MRI and FDG PET/CT, multiple brain and bone metastasis was reveled and he was diagnosed with stage IV lung adenocarcinoma (cT3N2M1b, LYM, OSS, BRA).
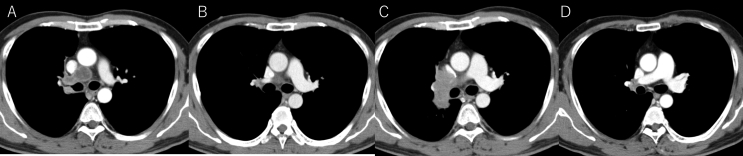
Fig. 1.
Chest CT of primary lesion and mediastinal lymph node findings. A: Pretreatment primary tumor. B: Primary tumor after four months from EGFR-TKI treatment started. C: Regrowth primary tumor after EGFR-TKI treatment. D: Primary tumor after two months from chemotherapy (CDDP+CPT-11) started.
Fig. 2.
Pathological findings at biopsy specimen. A: Pretreatment tumor (Hematoxylin and eosin stain) B: Pretreatment tumor (Synaptophysin) C: Pretreatment tumor (Chromogranin A) D: Rebiopsy tumor after EGFR-TKI treatment (Hematoxylin and eosin stain) E: Rebiopsy tumor after EGFR-TKI treatment (Synaptphysin) F: Rebiopsy tumor after EGFR-TKI treatment (Chromogranin A).
Afatnib (40mg once daily) was introduced to our case as EGFR-TKI in November X years. His best tumor response was assessed partial response (Fig. 1B), and both tumor markers were reduced after treatment started (Fig. 3). CEA and ProGRP had followed once a month and Chest CT of whole body conducted once eight weeks for following treatment effect. Plasma ProGRP level was elevated (639 pg/mL) 22 months later from afatinib started. Recurrence of primary lesion was revealed in chest CT without recurrence of mediastinal lymph nodes, although serum CEA level stayed in the normal range (Fig. 1C). We suspected tumor relapse due to SCLC transformation from adenocarcinoma and transbronchial lung biopsy was performed for diagnosis at the same primary lesion in first biopsy. Malignant small cells with high nuclear cytoplasmic ratio were observed in the biopsy sample, and synaptophysin and chromogranin A were positive in the cells (Fig. 2D, E, F). The biopsy sample were genotyped by polymerase chain reaction invader method and EGFR exon 19 deletion (E746_A750del) was detected again and Threonine790Methionine (T790M) was not detected. He was introduced standard chemotherapy (cisplatin and irinotecan) for advanced small cell lung cancer treatment in Japan. After 3 months, chest CT revealed tumor reduction (cisplatin and irinotecan) for SCLC [12]. Best response of standard chemotherapy was assessed partial response (Fig. 1D).
Fig. 3.
Clinical course.
3. Discussion
NSCLC to SCLC transformation after EGFR-TKI treatment is rare. Previous case series have revealed the frequency of transformation to SCLC as resistance to EGFR-TKI to be 5%–14% [6], and the usefulness of ProGRP and NSE for detecting NSCLC to SCLC transformation has been reported [13]. However, no study has reported success with detected NSCLC to SCLC transformation by monitoring plasma ProGRP level for EGFR-TKI treatment. We succeeded in early prediction of NSCLC transformation to SCLC by monitoring plasma ProGRP level.
Plasma ProGRP level is useful for diagnosing SCLC, with a sensitivity of 64.9% and specificity of 96.5% [14]. Kudo et al. reported that high plasma ProGRP level is useful to expect neuroendocrine differentiation components of NSCLC [15]. Previous case series reported that 2%–10% of de novo NSCLC combined SCLC [16,17]. These reports suggest that the SCLC component became dominant at the NSCLC in our case after EGFR-TKI treatment and mild elevation of plasma ProGRP of a patient in our study before initiated on afatinib may reflect neuroendocrine component of NSCLC. Our study may suggest that plasma ProGRP elevation is presented in patients with NSCLC before initiation of EGFR-TKIs. Plasma ProGRP level before EGFR-TKIs therapy may be useful to expect NSCLC to transformation. However, first biopsy sample were not presented neuroendocrine component. We considered tumor heterogeneity and small sample size affected on this result.
It is debatable argument whether plasma ProGRP was useful or not into consideration of cost-effectiveness. Generally, when tumors recurrence with EGFR mutation was shown while first or second generation EGFR-TKIs therapy, rebiopsy was conducted for decision of treatment sequence. Because the AURA3 trial showed that osimertinib is superior to platinum doublet chemotherapy in patients with T790M-positive NSCLC with acquired resistance to first- or second-generation EGFR-TKIs, osimertinib is considered as first treatment option for patients with T790M-positive NSCLC [18]. Therefore, SCLC transformation from NSCLC were detected by pathological examine of rebiopsy sample after tumors recurrence without monitoring ProGRP. However, past research reported success rate of rebiopsy were only 79.5% [19]. Moreover, SCLC is most aggressive diseases in lung cancer [20]. Therefore, we believed plasma ProGRP is useful for detecting SCLC transformation from NSCLC with EGFR mutation if plasma ProGRP elevation before first EGFR-TKIs therapy was presented like our case.
There are some limitations in our article. Plasma ProGRP level has physiological validation in human body. For example, renal dysfunction causes a false positive of high plasma ProGRP level for diagnosing SCLC [13]. Therefore, our case's plasma ProGRP level before EGFR-TKI treatment should be pointed false positive before especially EGFR-TKI treatment. However, we considered our case's plasma ProGRP level was unlikely to be false positive for two reasons. One reason, he had been estimated glomerular filtration rate was usually about 60 ml/min/1.73 m2 under EGFR-TKI treatment. Other reason, our case's plasma ProGRP level was evaluated mild elevation twice before EGFR-TKI treatment. Therefore, there is the possibility of the second primary cancer as small cell lung cancer after afatinib started. However, we believe our case presented NSCLC transformation to SCLC transformation because of recurrence of primary lesion at the same site. This is a single case report. Therefore, integration of similar cases will be needed.
4. Conclusion
Plasma ProGRP elevation in NSCLC with EGFR mutation may reflect SCLC components of NSCLC before EGFR-TKIs therapy. Plasma ProGRP level should be considered an early predictive marker of EGFR-mutant NSCLC to SCLC transformation.
Conflicts of interest
All authors declare no conflicts of interest associated with this manuscript.
Disclosure statements
The authors have no conflict of interest to declare. Appropriate written informed consent was obtained for publication of this case report and the accompanying images.
Acknowledgment
This research received no funding. We are grateful to to Dr. Hatori affiliates department of pathology, Chiba Hokusoh Hospital, Nippon Medical School for helpful discussions.
References
- 1.Wu Y.L., Zhou C., Hu C.P. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small cell lung cancer harbouring EGFR mutations (Lux-Lung 6): an open-label, randomized phase 3 trial. Lancet Oncol. 2014;15:213–222. doi: 10.1016/S1470-2045(13)70604-1. [DOI] [PubMed] [Google Scholar]
- 2.Mok T.S., Wu Y.L., Thongprasert S. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N. Engl. J. Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 3.Zhou W., Ercan D., Chen L. Novel mutant-selective EGFRkinase inhibitors against EGFR T790M. Nature. 2009;462:1070–1074. doi: 10.1038/nature08622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Engelman J.A., Jänne P.A. Mechanisms of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small cell lung cancer. Clin. Cancer Res. 2008;14:2895–2899. doi: 10.1158/1078-0432.CCR-07-2248. [DOI] [PubMed] [Google Scholar]
- 5.Floriana M., Carminia M.D.C., Morena F. Mechanisms of resistance to EGFR-targeted drugs: lung cancer. ESMO Open. 2016;1 doi: 10.1136/esmoopen-2016-000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wen-Zhao Z., Qing Z., Yi-Long W. The resistance mechanisms and treatment strategies for EGFR-mutant advanced non-small-cell lung cancer. Oncotarget. 2017;8:71358–71370. doi: 10.18632/oncotarget.20311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oser M.G., Niederst M.J., Sequist L.V., Engelman J.A. Transformation from non-small-cell lung cancer to small-cell lung cancer: molecular drivers and cells origin. Lancet Oncol. 2015;16:165–172. doi: 10.1016/S1470-2045(14)71180-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sequist L.V., Waltman B.A., Dias-Santagata D. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci. Transl. Med. 2011;3(75) doi: 10.1126/scitranslmed.3002003. 75ra26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu H.A., Arcila M.E., Rekhtman N. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin. Cancer Res. 2013;19:2240–2247. doi: 10.1158/1078-0432.CCR-12-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grunnet M., Sorensen J.B. Carcinoembryonic antigen (CEA) as tumor marker in lung cancer. Lung Cancer. 2012;76:138–143. doi: 10.1016/j.lungcan.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 11.Molina R., Filella X., Augé J.M. ProGRP: a new biomarker for small cell lung cancer. Clin. Biochem. 2004;37:505–511. doi: 10.1016/j.clinbiochem.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 12.Noda K., Nishiwaki Y., Kawahara M. Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. N. Engl. J. Med. 2002;346:85–91. doi: 10.1056/NEJMoa003034. [DOI] [PubMed] [Google Scholar]
- 13.Watanabe S., Sone T., Matsui T. Transformation to small-cell lung cancer following treatment with EGFR tyrosine kinase inhibitors in a patient with lung adenocarcinoma. Lung Cancer. 2013;82:370–372. doi: 10.1016/j.lungcan.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 14.Shibayama T., Ueoka H., Nishi K. Complementary roles of pro-gastrin-releasing peptide (ProGRP) and neuron specific enolase (NSE) in diagnosis and prognosis of small-cell lung cancer (SCLC) Lung Cancer. 2001;32:61–69. doi: 10.1016/s0169-5002(00)00205-1. [DOI] [PubMed] [Google Scholar]
- 15.Kudo K., Ohyanagi F., Horiike A. Clinicopathological findings of non-small cell lung cancer with high serum progastrin-releasing peptide concentration. Lung Cancer. 2011;74:401–404. doi: 10.1016/j.lungcan.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 16.Adelstein D.J., Tomashefski J.F., Jr., Snow N.J., Horrigan T.P., Hines J.D. Mixed small cell and non-small cell lung cancer. Chest. 1986;89:699–704. doi: 10.1378/chest.89.5.699. [DOI] [PubMed] [Google Scholar]
- 17.Mangum M.D., Greco F.A., Hainsworth J.D., Hande K.R., Johnson D.H. Combined small-cell and non-small-cell lung cancer. J. Clin. Oncol. 1989;7:607–612. doi: 10.1200/JCO.1989.7.5.607. [DOI] [PubMed] [Google Scholar]
- 18.Mok T.S., Wu Y.L., Ahn M.J., Garassino M.C., Kim H.R., Ramalingam S.S. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N. Engl. J. Med. 2017;376(7):629–640. doi: 10.1056/NEJMoa1612674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nosaki K., Satouchi M., Kurata Re-biopsy status among non-small cell lung cancer patients in Japan: a retrospective study. Lung Cancer. 2016;101:1–8. doi: 10.1016/j.lungcan.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 20.Abidin A., Garassino M., Califano R., Harle A., Blackhall F. Targeted therapies in small cell lung cancer: a review. Ther. Adv. Med. Oncol. 2010;2(1):25–37. doi: 10.1177/1758834009356014. [DOI] [PMC free article] [PubMed] [Google Scholar]