Abstract
the present study the was done to evaluate chemopreventive and chemotherapeutic anti-tumor potential of some Egyptian plant extract (moringa, graviola, ginger garden cress and artemisinin) against 7,12-dimethylbenz(a)anthracene (DMBA)-induced mammary carcinogenesis in Swiss albino mice. chemopreventive and chemotherapeutic evaluation was assessed by monitoring the tumor incidence and tumor volume as well as by analyzing the status of (a) biochemical markers (maspin, survivin, livin, caveolin-1, osteopontin and Fucosyltransferase 4 gene expressions), oxidative stress related profile including; total antioxidant capacity (TAC), glutathione reductase (GR) activity, glutathione-s-transferase (GST) activity assay, superoxide dismutase (SOD) activity, catalase (CAT) activity and lipid peroxidation (MDA), renal and hepatic toxicity markers (urea, creatinine, alanine transaminase (alt) activity, aspartate aminotransferase (ast) activity, alkaline phosphatase (ALP) Activity and γ-Glutamyltransferase (GGT) activity also study of (b) biophysical markers (trace and heavy metals (lead (Pb), cadmium (Cd), chromium (Cr), nickel (Ni), iron (Fe), selenium (Se), copper (Cu) and zinc (Zn)), dielectric properties and body water distribution) finally (c) histopathological examination oral administration of increasing dose of moringa, graviola, ginger garden cress and artemisinin extracts, respectively significantly prevented the tumor incidence and tumor volume as well as brought back the status of the above mentioned biochemical and biophysical variables. Histopathological changes also confirmed the formation of tumor tubules and neovascularization after the treatment. Overall, these results suggest that treatment with moringa, graviola, ginger garden cress and artemisinin extracts provided antioxidant defense with strong chemopreventive and chemotherapeutic activity against DMBA-induced mammary tumors.
Keywords: 7,12-dimethylbenz(a)anthracene (DMBA); Chemopreventive; Chemotherapeutic; Plant extract
Highlights
-
•
Egyptian medicinal plant extracts provided potential antioxidant defense system with strong chemopreventive and chemotherapeutic activity against cancer.
-
•
Overall biochemical and biophysical study of anti-tumor potential of some Egyptian plant extract (moringa, graviola, ginger garden cress and artemisinin).
-
•
Promising nontoxic, safe chemopreventive and chemotherapeutic treatment to overcome limitation and side effect of conventional cancer treatment methods.
1. Introduction
Breast cancer is the most common female malignancy accounting for 22.9% and 37.7% of all female cancers worldwide and in Egypt, respectively. Breast cancer in Egypt carries an unfavorable prognosis with 29% mortality and 1: 3.7 incidences to mortality ratio [1]. Breast cancer accounts for 16% of all cancer deaths among women globally, according to the report by the World Health Organization (WHO). It is the most common solid tumor diagnosed in women. Although the incidence of breast cancer increases with age, certain lifestyle and environmental factors play an important role on breast cancer risk. In Egypt, breast cancer is the most common cancer among women, representing 18.9% of total cancer cases (35.5% in women and 2.2% in men) among the Egypt National Cancer Institute (NCI) series of 10,556 patients during the year 2002–2003 [2].
Breast cancer and cancer related diseases have been treated using surgery, chemotherapy, and radiation therapy, or a combination of these. But despite these therapeutic options, cancer remains associated with high mortality. This is basically due to difficulties in early diagnosis, exorbitant cost of treatment, with the often late presentation of breast cancer that generally characterizes cancer diagnosis among Egypt and other African women [[3], [4], [5]].Owing to these several shortcomings, there is a need for better therapeutic options which will increase the chances of survival of breast cancer patients with minimal or no side effects of treatment [6].
Tamoxifen (TAM) is widely used as a first-line endocrine therapy for breast cancer patients and as a chemoprophylactic agent for women at high risk of developing this disease. TAM is a nonsteroidal-selective ER modulator that has been shown to reduce the risk of invasive (and noninvasive) breast cancer in women. In addition, TAM also reduces the occurrence of ER+ invasive cancers by 69% and noninvasive cancers by 50% with no difference in the occurrence of ER− invasive cancer [7,8].Doxorubicin one of the best characterized agent of chemotherapeutic drugs for the treatment of breast cancer. Doxorubicin is an anthracycline antibiotic, which remains an important agent in many chemotherapy regimens. Although doxorubicin is currently considered to be one of the most effective agents in the treatment of breast cancer, resistance leads to an unsuccessful outcome in many patients [9].
Phytochemical prevention for severe health problems has recently gained scientific recognition worldwide. Studies on the pharmacological mechanisms and search for chemical structures of herbal extracts responsible for anticancer activity caught great interest [[10], [11], [12]].
Moringa (Moringa oleifera) (M. oleifera) or drumstick is a member of Moringaceae, it has long been known as a food plant in Thai cuisine and as an ingredient of Indian traditional medicine [13,14].The leaves contain nutrients especially essential amino acids, vitamins, minerals and β-carotene. For this reason, it is used as an alternative source for nutritional supplements and growth promoters in some countries [15].Apart from nutritional benefits, M. oleifera is reported to be used for the treatment of rheumatism, ascites, infection, hiccough influenza and internal abscess [14]. It also demonstrated potent antiproliferative activity and apoptosis inducing capacity on tumor (KB) cell line [16], and it also increased the cytotoxicity of chemotherapy on pancreatic cancer cells [17].
Graviola (Annona muricata) commonly called soursop is a small erect evergreen tropical fruit tree plant belonging to the family Annonaceae, growing 5–6 m in height. It is one of the easily found plants used traditionally in treating cancer. The leaf decoction is usually taken to lessen the symptoms of cancer [11,12].the Plant extract showed promising selective inhibitory effect for tumorigenicity and metastasis of cancer cells in vitro and in vivo through altering cell metabolism [18,19].
Ginger (Zingiber officinale) as perennial herbs belonging to the family Zingiberaceae have been widely used as spices, condiments and herbal medicine for treatment of cold, fever, headache, nausea and digestive problems [20]. Ginger and its general compounds such as gingerols, shogaols, paradols and zingerone exert immuno-modulatory, antiapoptotic, anti-tumourigenic, anti-inflammatory, antihyperglycaemic, anti-hyperlipidaemic, antioxidant and anti-emetic activities [20]. Ginger leaves have also been used for food flavouring and traditional medicine [21]. Past pharmacological studies of ginger were confined to rhizomes.
Garden cress (Lepidium sativum, [L. sativum]), Cruciferae or Brassicaceae vegetable, which are used in folk remedies, have many activities including thermogenic, depurative, rubefacient, tonic, aphrodisiac, abortive, ophthalmic, diuretic, and contraceptive [22,23]. They are useful as poultices for sprains and in leprosy, ophthalopathy, leucorrhoea, scurvy, seminal weakness, bronchial asthma, cough, and hemorrhoids [22]. L. sativum extract are recommended in the treatment of various ailments, but in therapeutic doses because of their known toxicity if used in high doses, although there is no scientific evidence. L. sativum also contains plant phytosterols and their derivatives, which have been shown [24] to possess antioxidant potential, anti-inflammatory activity, and to protect against some illnesses and cancers. Phenolic compounds, most importantly the flavonoids, may protect the human body from oxidative stress that may lead to cancer, aging, and cardiovascular diseases [24,25].
Artemisinin (Artemisia annua) is a plant, found throughout the world, with known medicinal properties. In recent years its function as an antimalarial agent has been further investigated, as well as any other medicinal properties it may possess. Special attention has been paid to determining the most potent form in which the plant has shown the plant may potentially kill cancer cells and behave as an antagonistic agent for estrogen receptors in breast cancer. Research has shown artemisinin demonstrates anti-cancer potential even for cell lines that are drug and radiation resistant [26]. Cancer cells typically uptake larger amounts of iron than healthy cells in order to proliferate. Artemisinin reacts with iron to form free radicals which cause cell death. The increased iron uptake of cancer cells leaves them susceptible to the free radicals artemisinin creates. Artemisinin has also been found to suppress vascular endothelial growth factor C in lung cancer, increase calcium levels and activate p38 in lung cells, and block estrogen receptors in breast cancer [27].
The aim of this work was to study the chemopreventive and chemotherapeutic effect of moringa, graviola, ginger, cress and artemisinin leaves extract compared to tamoxifen and doxorubicin drugs in 7,12-dimethylbenz(a)anthracene (DMBA)-induced cell proliferation in the breast tissues of female albino mice. to fulfill this aim the following was done:
2. Materials
2.1. Animals
Female Swiss albino mice weighting 20–25 gm of 8–10 weeks of age were divided into two groups. The experimental groups received different concentrations of moringa, graviola, ginger, cress and artemisinin with respect to the LD50. Mice were treated with increasing doses of moringa, graviola, ginger, cress and artemisinin. Uses of experimental animals in the study protocol were carried out in accordance with the ethical guidelines of the Medical Research Institute, Alexandria University (Appendix 2, Guiding Principles for Biomedical Research Involving Animals, 2011). Group A: 10 mice treated with distilled water only as a control group. Group B: 330 mice treated with 20 mg/kg/week of DMBA. This group was subdivided into nine sub-groups; sub group B-1: 10 mice treated with 20 mg/kg/week of DMBA only and were not receive any treatment.
2.2. For chemopreventive study
Sub group B-2: 50 Mice treated with 200 mg/kg/day of moringa, graviola, ginger, cress and artemisinin after DMBA administration on zero day, sub group B-3: 50 mice treated with 100 mg/kg/day of moringa, graviola, ginger, cress and artemisinin after DMBA administration on zero day, Sub Group B-4: 50 mice treated with 50 mg/kg/day of moringa, graviola, ginger, cress and artemisinin after DMBA administration on zero day and sub group B-5: 10 mice treated with 5 mg/kg/day of tamoxifen after DMBA administration on zero day.
2.3. For chemotherapeutic study
Sub group B-6: 50 mice treated with 200 mg/kg/day of moringa, graviola, ginger, cress and artemisinin after DMBA administration, sub group B-7: 50 mice treated with 100 mg/kg/day of moringa, graviola, ginger, cress and artemisinin after DMBA administration, Sub Group B-8: 50 mice treated with 50 mg/kg/day of moringa, graviola, ginger, cress and artemisinin after DMBA administration and sub group B-9: 10 mice treated with 5 mg/kg/day of doxorubicin after DMBA administration.
3. Methods
For evaluation of the treatment effects to all studied groups the following investigations were done:
3.1. Tumor growth/inhibition evaluation
During treatment session, tumor growth was examined regularly every day. Length and width of tumors were measured with a slide caliper and tumor volume (in mm3) was calculated by the use of the following equation. TV (mm3) = 22/7x4/3x (length/2) x (width/2)2. Two weeks after the treatment, the mice were sacrificed and the tumors were dissected out, weighed (in grams).
3.2. Dielectric properties measurements
Measurements of the dielectric properties of the tumor tissue and its surrounding to monitor the efficiency of the different treatment modality. Dielectric measurements were performed on untreated (animal bearing tumor), (50 mg, 100 mg, 200 mg moringa, graviola, ginger, cress and artemisinin), tamoxifen and doxorubicin treated tumor samples. The uncertainty in the measured data was determined as standard deviation and found to be less than 2%. Scanning the frequency of the applied frequency from 1 kHz up to 100 kHz was performed while recording the variation of both capacitance (C) and resistance (R). The conductivity was calculated as a reciprocal of R. These values were used to calculate the relative and imaginary permittivity (ε′, ε'') and conductivities (σ′, σ'') of the tumor samples. The corresponding complex diagrams (Cole-Cole) between (ε′-ε'') and (σ′- σ'') were constructed.
3.3. Toxic and trace metals analysis
Analysis of the studied heavy metals and trace elements [lead (Pb), cadmium (Cd), chromium (Cr), nickel (Ni), iron (Fe), selenium (Se), copper (Cu) and zinc (Zn)] by Atomic Absorption.
3.4. Body water distribution measurements
Measurements of intra, extra and total body water distribution.
3.5. Biochemical investigations
Blood sample (2.5 ml of venous blood) was withdrawn from all mice group. This blood samples were allowed to clot thoroughly for 20 min then centrifuged at 3000×g for 20 min for separating serum for biochemical examinations. All biochemical analysis was done on Indiko Plus Auto-analyzer.
3.6. Oxidative stress and antioxidant status
Lipid peroxidation (MDA) assay kit (BioVision Catalog #K739-100), total antioxidant capacity (TAC) assay kit (BioVision Catalog #K274-100), glutathione reductase (GR) activity assay kit (BioVision Catalog #K761-100), glutathione-s-transferase (GST) activity assay kit (BioVision Catalog #K263-100), superoxide dismutase (SOD) activity assay kit (BioVision Catalog #K335-100), Catalase (CAT) activity assay kit (BioVision Catalog #K773-100), were used according to the manufacturer's instructions.
3.7. Kidney and liver function tests
Urea (Sigma Catalog # MAK179), creatinine (Sigma Catalog # MAK080), Alanine Transaminase (ALT) Activity Assay Kit (Sigma Catalog # MAK052), Aspartate Aminotransferase (AST) Activity Assay Kit (Sigma Catalog #MAK055), alkaline phosphatase (ALP) Activity Assay Kit (Sigma Catalog #MAK089) and γ-Glutamyltransferase (GGT) Activity Assay Kit (Sigma Catalog #MAK089), were used according to the manufacturer's instructions.
3.8. Maspin, survivin, livin, caveolin-1, osteopontin and Fucosyltransferase 4 gene expressions
Molecular detection of maspin, survivin, livin, caveolin-1, osteopontin and Fucosyltransferase 4 mRNA gene expressions in excised tumor via RT-PCR: RNA was extracted from the Erich tumor of mice using QIAamp RNA tissue kit, was purchased from QIAGEN, USA according to the manufacturer's instructions. Preparation of Full-Length First strand cDNA from RNA template using RevertAid ™ First cDNA Strand Synthesis Kit. Reverse transcription reaction was carried out in a 20 μl reaction mixture by using RevertAid ™ First cDNA Strand Synthesis Kit #K1621,#1622, was purchased from MBI Fermentas, Lithuania according to manufacturer's instruction. For amplification; to each PCR tube the following were added 5 μl (0.25 μg) Template maspin, survivin, livin, caveolin-1, osteopontin and Fucosyltransferase 4- cDNAs, 10 μl Taq TM Green PCR Master Mix (2X) {dNTPs [0.4 mM of each dATP, dCTP, dGTP, dTTP], 0.05u/μl Taq DNA polymerase and reaction buffer} #k1081, was purchased from MBI Fermentas, Lithuania, (1.5 μl maspin forward (f) primer: 5̛-TGAGCTTCCTGCATTGGGAG-3̛, 1.5 μl maspin reverse primers (r): 5̛-CCCGCCAGCATCATAGCTTA-3̛, 1.5 μl survivin (f) primer: 5̛-TGAGCTTCCTGCATTGGGAG-3̛, 1.5 μl survivin (r) primers: 5̛-CCCGCCAGCATCATAGCTTA-3̛, 1.5 μl livin (f) primer: 5̛-GTCCCTGCCTCTGGGTAC-3̛, 1.5 μl livin (r) primers: 5̛-CAGGGAGCCCACTCTGCA-3̛, 1.5 μl caveolin-1 (f) primer: 5̛- TGAGCTTCCTGCATTGGGAG-3̛, 1.5 μl caveolin-1 (r) primer: 5̛-CCCGCCAGCATAGCTTA-3̛, 1.5 μl osteopontin (f) primer: 5̛-CTT TCACTC CAATCGTCCCTA C-3̛, 1.5 μl osteopontin (r) primers: 5̛-GCTCTC TTTGGAATGCTCAAGT -3̛, 1.5 μl Fucosyltransferase 4 (f) primer: 5̛-TTGCAGCCTGCGCTTCAACATCAG-3̛, 1.5 μl Fucosyltransferase 4 (r) primer: 5̛ -ACTCAGCTGGTGGTAGTAACGGAC-3̛ and deionized-RNase free water to final volume 20 μl. The reaction mixtures were gently vortexed, briefly centrifuged to collection all drops to the bottom of the tubes, then were placed in the thermal cycler (Little Genius, Bioer Co), The PCR mixture was subjected to 35 amplification cycles. PCR thermal profile was as follow: pre-denaturation (94 °C, 2min), followed by 35 cycles of denaturation (94 °C, 1min), annealing (52 °C, 1min), and extension (72 °C, 1min), with a final extension (72 °C, 7min). To verify the successful preparation of mRNA and as positive controls, samples were detected for the presence of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA. (fp): 5̛-AGGCCGGTGCTGAGTATGTC-3̛, (rp): 5̛-TGCCTGCTTCACCACCTTCT-3̛. Reaction tubes containing no cDNA control template and without cDNA sample addition were included as negative controls for each PCR reaction. For detection; Amplicons were analyzed with 2% (wt/vol) ethidium bromide stained agarose gel. The bands were visualized on a 302 nm UV transilluminator (BIO-RAD, USA). The gel was examined for bands of 199bp, 254bp, 191bp, 198bp, 305bp, 343bp and 530bp as determined by the molecular weight marker (Gene Ruler TM 100bp DNA marker #SM0323, was purchased from Fermentas, Lithuania) runs at the same time and then photographed using a digital camera.
3.9. Histopathological examination
Small pieces of Ehrlich tumor tissue of the experimental groups were processed and examined by haematoxylin and eosin (H&E) method as follows; small pieces of Ehrlich Tumor tissues were fixed at 10% formaldehyde, dehydrated in ascending grades using alcohol, embedded in paraffin to produce paraffin block, the blocks were cut into 3–4 μm thick sections and floated in water bath, cleaned with xylene, rehydrated in descending grades of alcohol, stained with haematoxylin and eosin stain, cleaned again ethylene and covered by covering slides, thus the slides were prepared to be examined by light microscopy.
4. Results
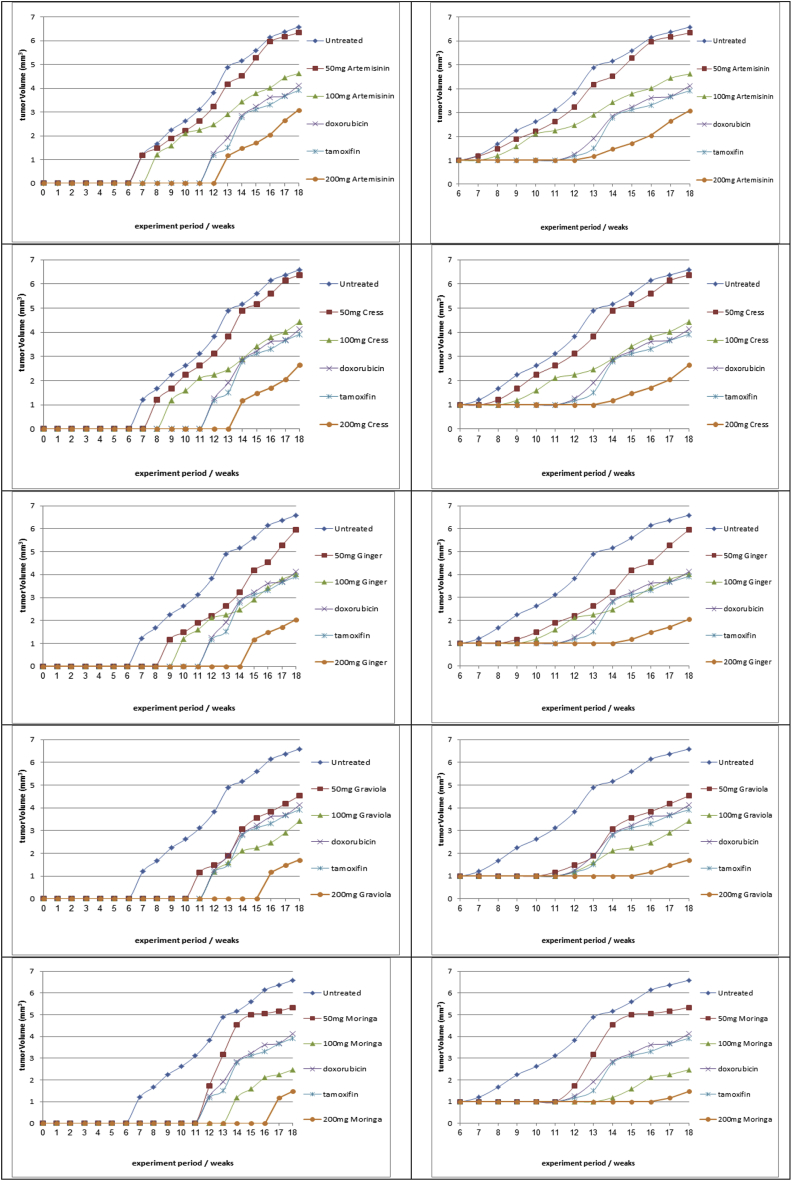
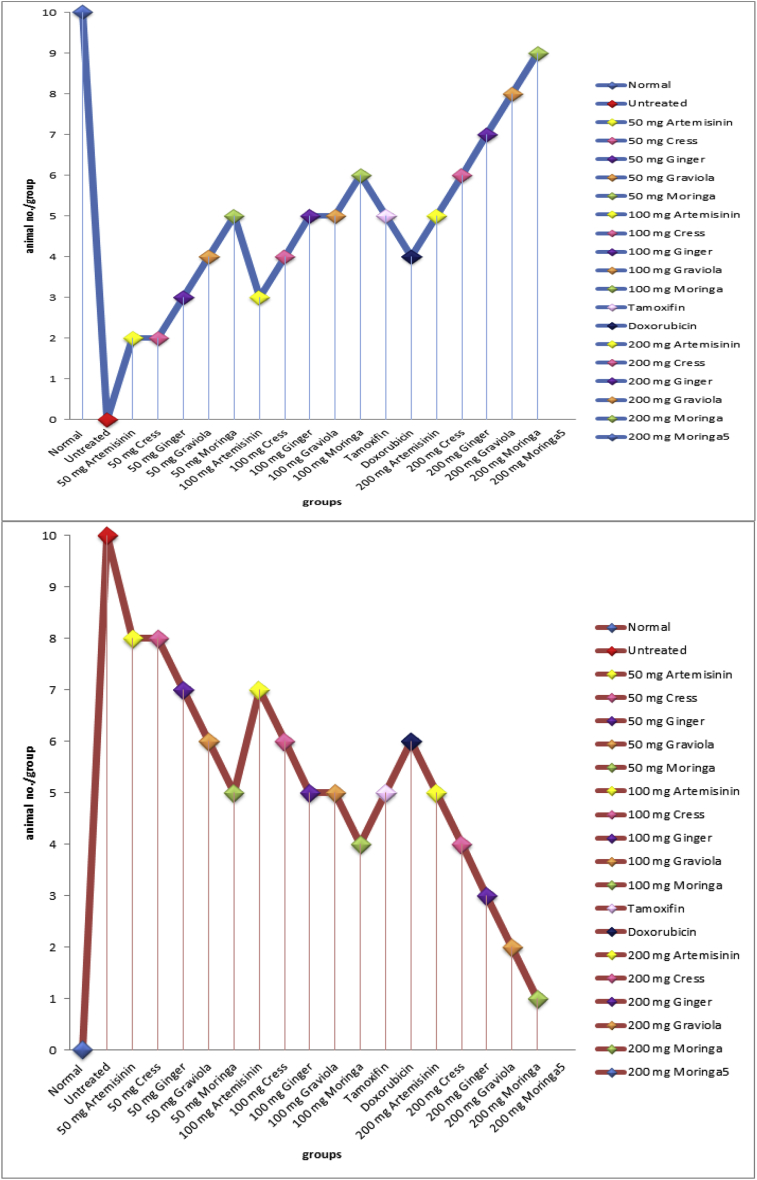
In the present study it was observed that 100% tumor incidence in mice treated with DMBA alone. Pretreatment upon administration of tamoxifen, doxorubicin and moringa, graviola, ginger, cress and artemisinin different doses (50, 100, 200 mg moringa/kg body weight) to DMBA-treated mice reduce the tumor incidence (survival and mortality rates) Fig. (1a,b).
Fig. 1.
aEffect of (moringa, graviola, ginger, cress and artemisinin; different doses), tamoxifen and doxorubicin on tumor volume (mm3).
b: Effect of (moringa, graviola, ginger, cress and artemisinin; different doses), tamoxifen and doxorubicin on study animals survival and death rate.
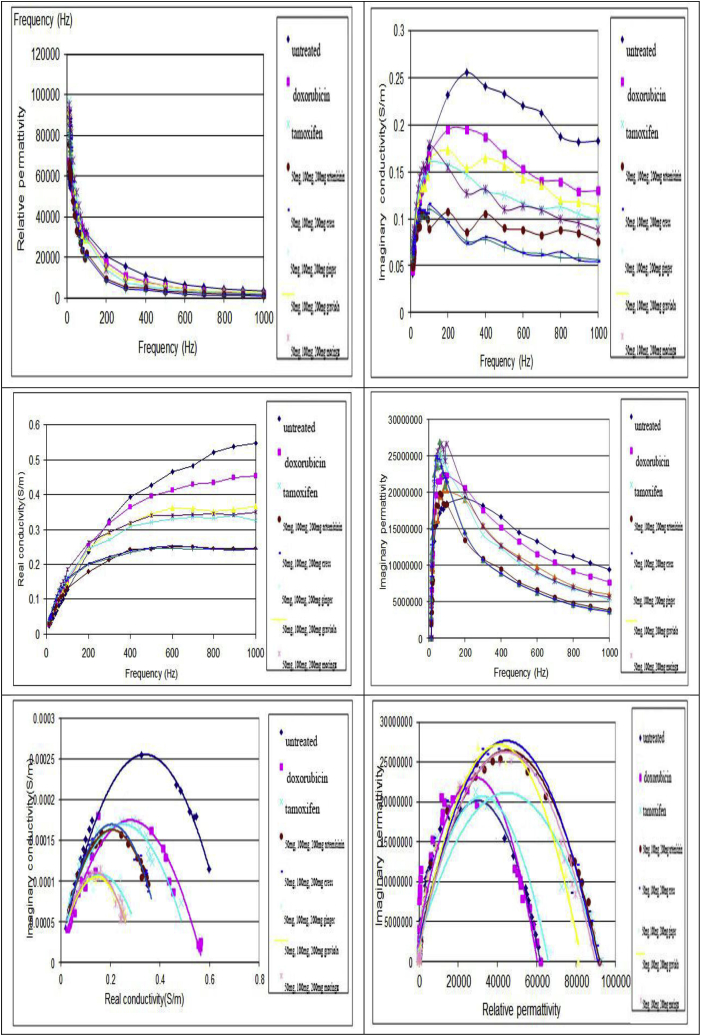
c: Effect of (moringa, graviola, ginger, cress and artemisinin; different doses), tamoxifen and doxorubicin on dielectric properties.
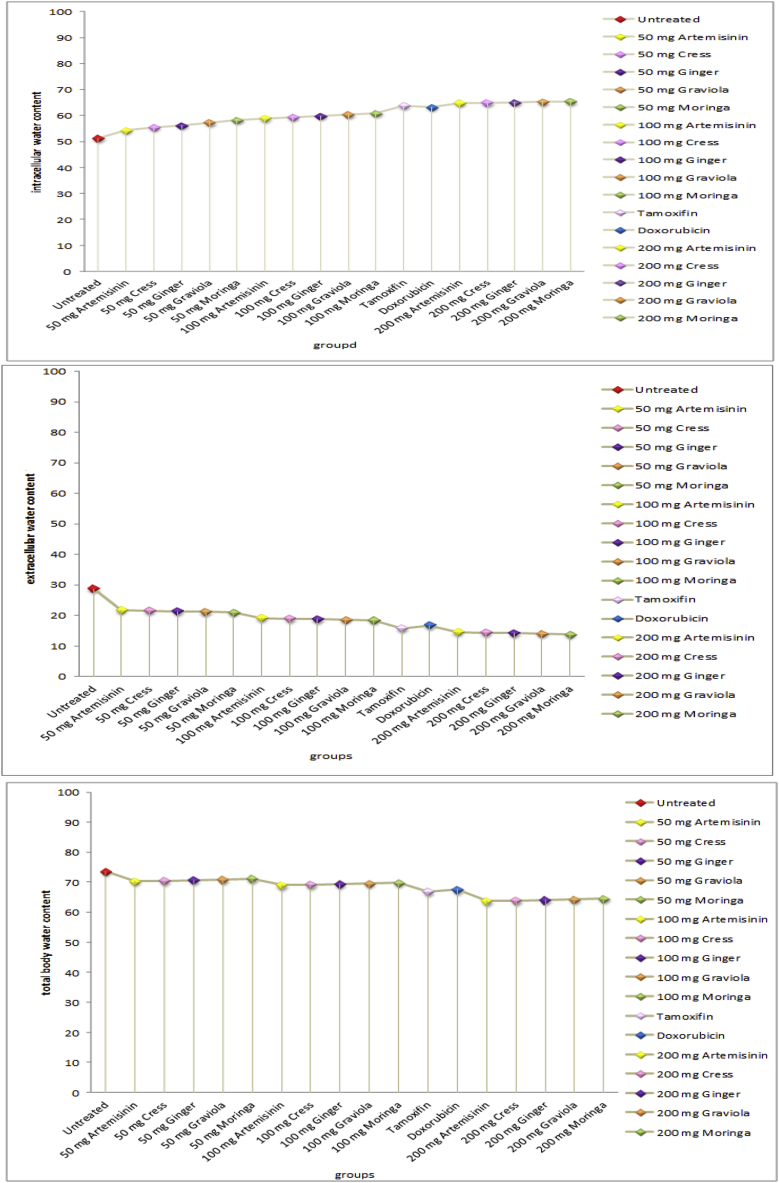
d: Effect of (moringa, graviola, ginger, cress and artemisinin; different doses), tamoxifen and doxorubicin on boy water distribution (%).
4.1. Effects of treatment on tumor volume and mass
The relationships between tumor volumes and treatment period for various treatment modalities treated with (50 mg, 100 mg, 200 mg; moringa, graviola, ginger, cress and artemisinin either simultaneously or post-treated), tamoxifen and doxorubicin; are presented in Fig. (1a). Results obtained indicated that 50 mg moringa, graviola, ginger, cress and artemisinin treatment modality has little effect on the tumor volume. Increasing dose of 100 mg moringa, graviola, ginger, cress and artemisinin become more effective on tumor cells and tumor volume than using of tamoxifen and doxorubicin treatment modality. 200 mg moringa, graviola, ginger, cress and artemisinin treatment modality showed the highest effect on tumor cells and reduction of tumor volume.
4.2. Effects of treatment on dielectric parameters
-
a.
permittivity and conductivity with change in frequency
Fig. (1c) show set of the frequency dependence of measured permittivity and conductivity of tumor untreated group and tumor of mice treated with (50 mg, 100 mg, 200 mg; moringa, graviola, ginger, cress and artemisinin either simultaneously or post-treated), tamoxifen and doxorubicin. Generally, with increasing conc./frequency the relative permittivity and imaginary conductivity both of them decreases, while the real conductivity increases. Imaginary tumor permittivity increases sharply at the beginning of the applied conc./frequency and then decreases exponentially with increasing the applied frequency. All treated groups show lower permittivity and conductivity than that of untreated group.
-
b.
Complex permittivity and conductivity diagrams
Complex permittivity and conductivity diagrams of tumor tissue taken from untreated and (50 mg, 100 mg, 200 mg; moringa, graviola, ginger, cress and artemisinin either simultaneously or post-treated), tamoxifen and doxorubicin treated groups are shown in Fig. (1c). As seen in these diagrams the degree of depressed center and the maximum values of the real and imaginary conductivity and permittivity depend on the treatment modality.
4.3. Effects of treatment on body water distribution
Fig. (1d) show Statistical analysis of the total body water percentage to body weight (WTBW %), extracellular water percentage to total body water (WECW %) and intracellular water percentage to total body water (WICW %) values of untreated and (50 mg, 100 mg, 200 mg; moringa, graviola, ginger, cress and artemisinin either simultaneously or post-treated), tamoxifen and doxorubicin treated groups. Generally, with increasing conc. extracellular and total body water both of them decreases, while the intracellular body water increases. All treated groups show lower extracellular and total body water and increased intracellular body water content than that of untreated group.
4.4. Effects of treatment on trace elements levels
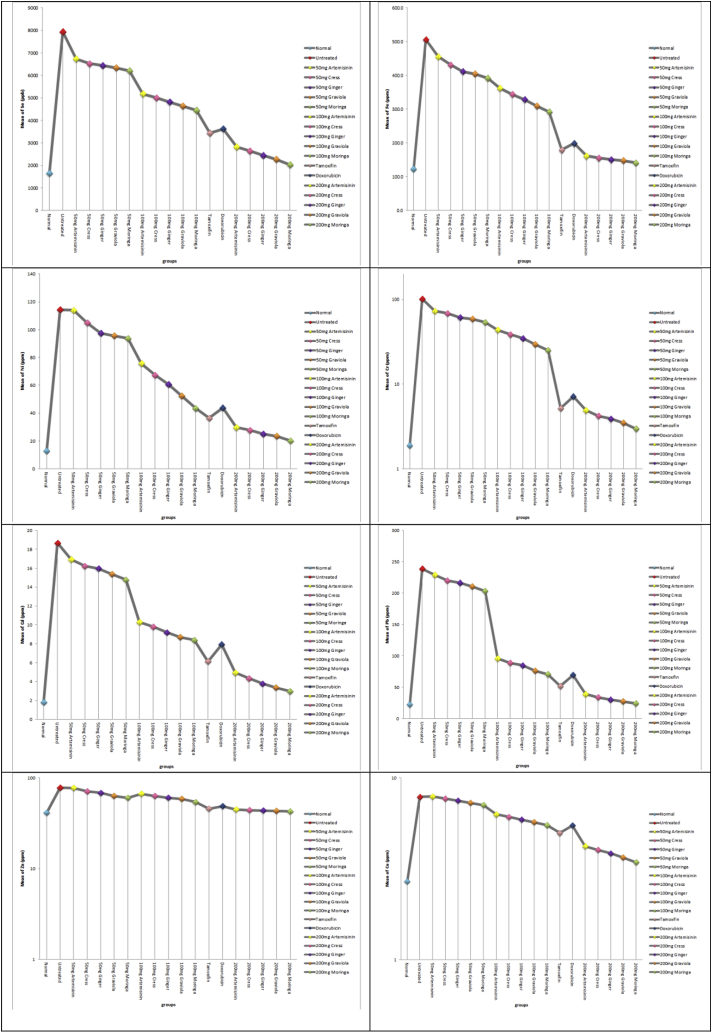
In the current study, the concentrations of eight heavy metals (selenium, iron, nickel chromium, cadmium, lead, zinc and copper) were estimated in the tissue samples of all mice studied groups; it was apparent that a statistically significant elevation of lead, cadmium, chromium, nickel and iron concentrations was detected in breast tissue of mice having malignant breast tumors in comparison to control group. On the other hand, there is a significant decrease in the concentrations of eight heavy metals in the animals administered moringa, graviola, ginger, cress and artemisinin either simultaneously or post-treated and carcinogen when compared with animals administered carcinogen only Fig. (2a).
Fig. 2.
a: concentrations of eight heavy metals (selenium, iron, nickel chromium, cadmium, lead, zinc and copper) in all studied groups.
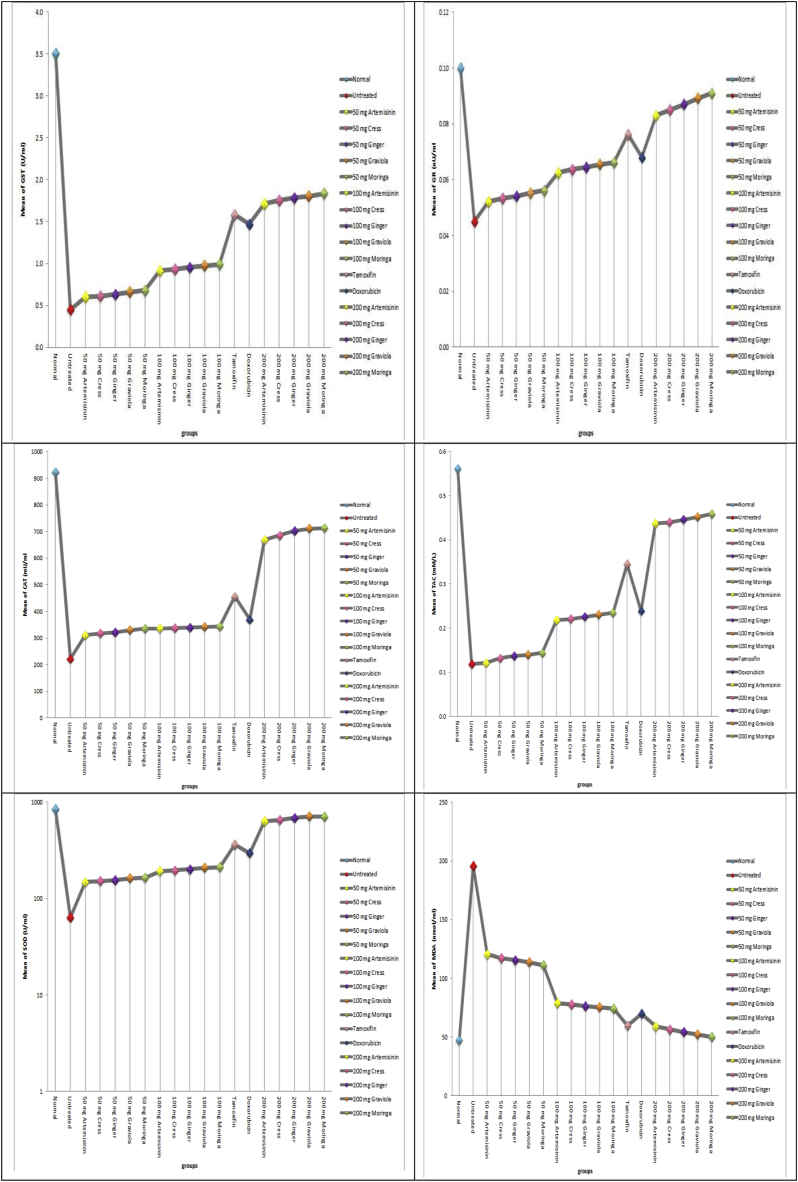
b: activities of oxidative stress related parameters; (SOD, CAT, GR, GST antioxidants, TAC) and MDA concentrations in all studied groups.
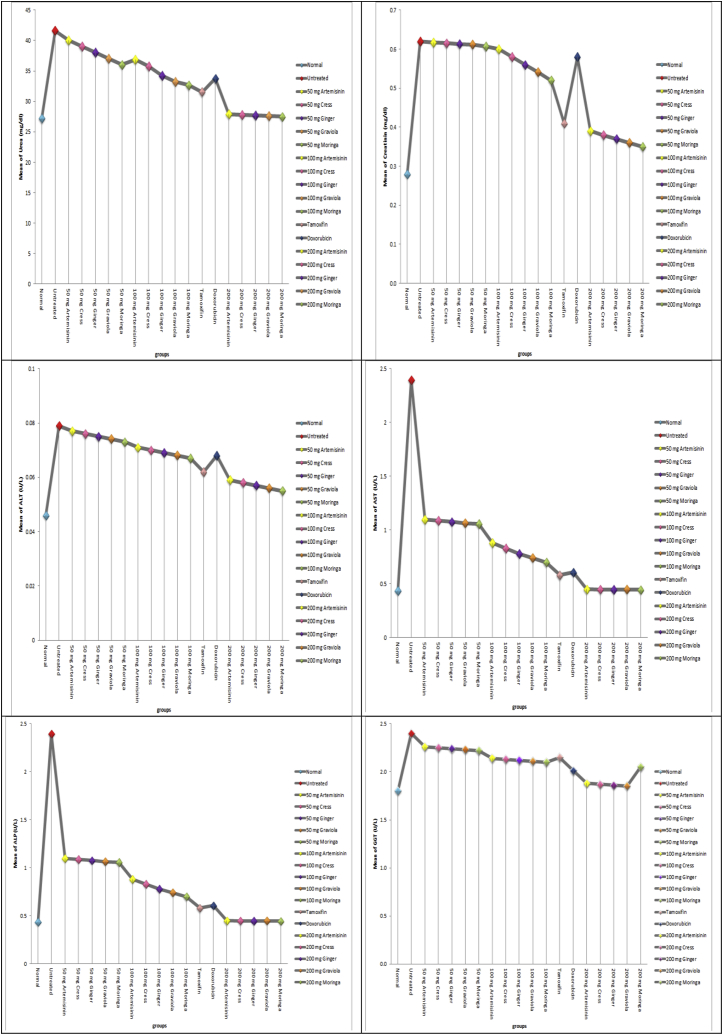
c: concentrations of renal and hepatic function tests (urea, creatinine, ALT, AST, ALP and GGT) in all studied groups.
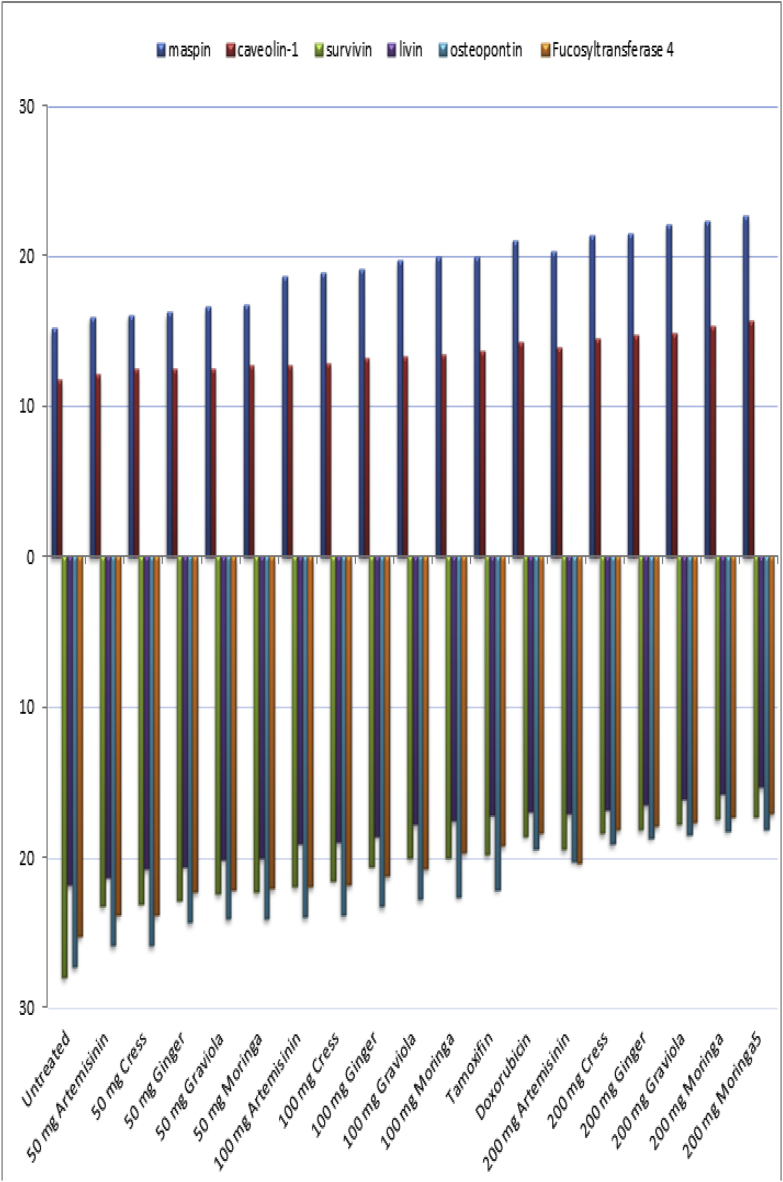
d: maspin, survivin, livin, caveolin-1, osteopontin and Fucosyltransferase 4 mRNAs gene expressions in all studied groups.
4.5. Effects of treatment on oxidative stress related parameters
In our study, the increase in lipid peroxidation was reported during DMBA induced carcinogenesis. All groups treated with DMBA, have a significant increase in the levels of MDA as compared with normal group animals. Animals in groups received moringa, graviola, ginger, cress and artemisinin either simultaneously or post-treated exhibited significantly low levels of MDA, when compared with animals treated with DMBA only.
In the present study, the cancer bearing mice showed decreased activities of antioxidants (SOD, CAT, GR, GST and TAC) in comparison with normal animals. On the other hand, there is a significant increase in the enzymatic and non-enzymatic antioxidant guard in the animals administered moringa, graviola, ginger, cress and artemisinin either simultaneously or post-treated and carcinogen when compared with animals administered carcinogen only Fig. (2b).
4.6. Effects of treatment on kidney and liver function tests
The biomarkers of renal function, creatinine and urea, were considered in this study. In this study, DMBA caused a significant increase in the serum urea and creatinine levels. On the other hand it was observed that moringa, graviola, ginger, cress and artemisinin either simultaneously or post-treated ameliorated the levels of serum creatinine and urea which is an indication of renal protection. This also confirms the protective role of moringa, graviola, ginger, cress and artemisinin against DMBA-induced renal toxicity. Also the biomarkers of hepatic function, ALT, AST and GGT, were considered in this study. In this study, DMBA caused a significant increase in the serum activities of ALT, AST and GGT. On the other hand pre- and cotreatment with moringa, graviola, ginger, cress and artemisinin protected against increase in serum levels of ALT, AST, and GGT, which is an indication of hepatoprotection by moringa, graviola, ginger, cress and artemisinin against DMBA-induced hepatotoxicity Fig. (2c).
4.7. Effects of treatment on maspin, survivin, livin, caveolin-1, osteopontin and Fucosyltransferase 4 gene expressions
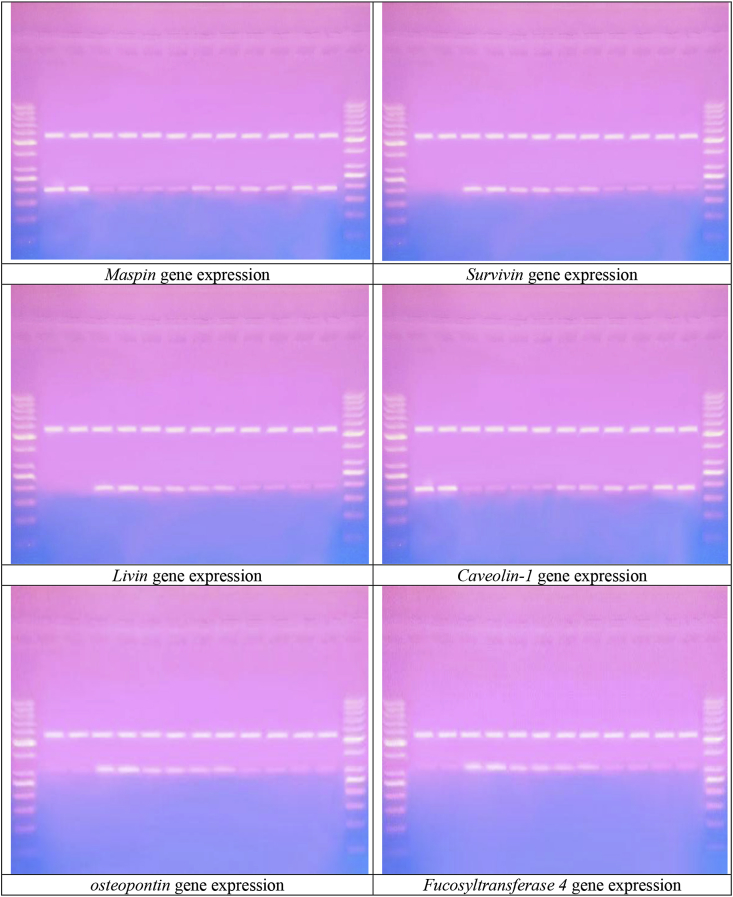
Amplification of maspin, survivin, livin, caveolin-1, osteopontin and Fucosyltransferase 4 gene expressions in breast tissues of all studied groups using RT-PCR is shown in Fig. (3a,b). PCR products were separated on 2% agarose gel electrophoresis. Products for maspin, survivin, livin, caveolin-1, osteopontin, Fucosyltransferase 4 and GADPH gene expressions were at 199bp, 254bp, 191bp, 198bp, 305bp, 343bp and 530bp respectively. All samples were positive to GADPH gene expression, ontheotherhand lanes, while with different intensity positive bands of maspin, survivin, livin, caveolin-1, osteopontin and Fucosyltransferase 4 gene expressions in DMBA cancerous untreated group, DMBA cancerous group treated with moringa, graviola, ginger, cress and artemisinin.
Fig. 3.
Ethidium bromide stained agarose gel electrophoresis of maspin, survivin, livin, caveolin-1, osteopontin and Fucosyltransferase 4 mRNAs gene expressions in all studied groups.
4.8. Effects of treatment on histological structural changes
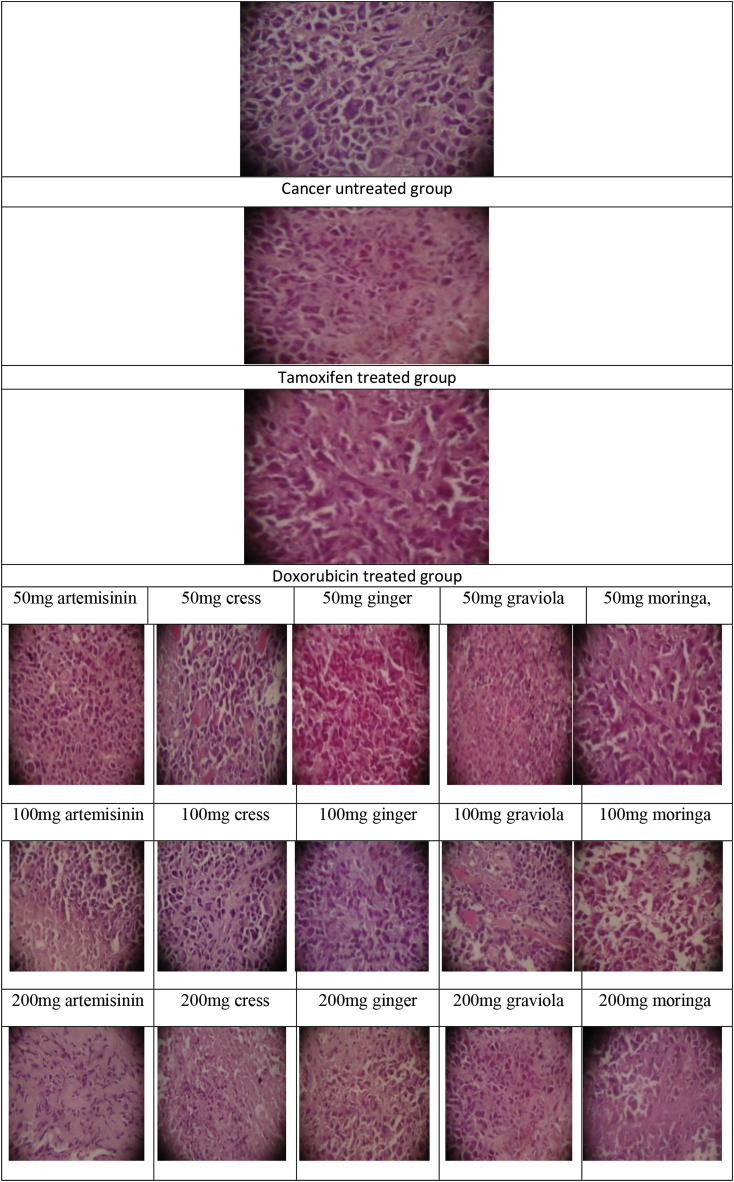
The histological evaluation revealed that all tumors from the cancerous control group were highly malignant cells and the tumors showed 5–10% necrosis. Tumors excised from animals receiving moringa, graviola, ginger, cress and artemisinin extract (100 and 200 mg/kg body weight), significant areas of necrosis were present (65, 85% respectively) compared to (50 mg/kg body weight) treated group (50%) while in case of tamoxifen and doxorubicin treated tumors, foci of necrosis areas (65–75%) were distinctly appeared Fig. (4).
Fig. 4.
The histological evaluation of (moringa, graviola, ginger, cress and artemisinin; different doses), tamoxifen and doxorubicin groups.
5. Discussion
Carcinogenesis, caused by physical, chemical, or viral mechanisms, is a multistage process of coordinated acquisition of favorable genetic lesions and complex interactions between tumor and host tissues that ultimately leads to an aggressive metastatic phenotype [28].
It has been suggested that the uncompromised generation of free radicals and reactive oxygen species (ROS) may be responsible for disturbing the antioxidant status and ultimately leading to oxidative stress and paving the way to carcinogenesis [29]. Oxidative stress is associated with damage to a wide range of macromolecular species including lipids, proteins, and nucleic acids thereby producing major interrelated derangements of cellular metabolism including peroxidation of lipids [30]. Lipid peroxidation plays an important role in carcinogenesis [31] and may lead to the formation of several toxic products, such as MDA and 4-hydroxynonenal. These products can attack cellular targets, thereby inducing carcinogenicity [32].
Breast tissue may be a major target for the toxicological effects of a variety of lipophilic carcinogens such as polycyclic aromatic hydrocarbon (PAH), if such compounds are not biotransformed to hydrophilic metabolites that are easily excretable [33].
Numerous studies have shown that 7,12-dimethylbenz(a)anthracene (DMBA), a member of the PAH family, can be used to induce experimental breast carcinomas in experimental animals and that this process involves disruption of tissue redox balance; in turn, this suggests that biochemical and pathophysiological disturbances may result from oxidative damage [34,35]. Under normal physiological conditions, any free radicals generated in subcellular compartments would subsequently be scavenged by antioxidant defense systems of the corresponding cells [36]. However, such protective mechanisms can be broken easily by chemicals, such as DMBA, which disrupt the pro-oxidant–antioxidant balance, leading to cellular anomalies. Furthermore, owing to their high content of polyunsaturated fatty acids, cellular membranes are highly susceptible to lipid peroxidation, and adverse alterations of the cell membrane can result in a pathological outcome [37].
Metabolic activation of DMBA produces radical cations, free radicals and oxygenated metabolites [38]. In turn, the ensuing oxidative stress produces deleterious effects by initiating lipid peroxidation [39]. Overall, therefore, DMBA can induce substantial oxidative damage in various bodily organs (in particular liver and breast), a property which has made DMBA a suitable and useful agent for generating in vivo models of breast cancer [40,41].
The onset of tumor promotion is marked by numerous biochemical alterations [42]. Since the pathological development of tumors in mankind takes a long time to pass through the preneoplastic and premalignant stages to actually become malignant. Therefore, the opportunity to reverse the development of tumor is always present. Resultantly, in recent years extensive research on cancer diminishes and prevention is being encouraged. The chemotherapeutic strategies encompass the use of agents with certain effects that diminish the carcinogenic process.
In recent years, using MDA as a marker of oxidative stress, there has been a growing interest in studying the role played by lipid peroxidation in cancer progression. MDA is low molecular weight aldehyde that can be produced from free radical attack on polyunsaturated fatty acids [43,44].
The probable reason for the elevated level of serum lipid peroxide in breast carcinoma may be due to defective antioxidant system which leads to the accumulation of lipid peroxides in cancer tissue which are released into the blood stream [45]. MDA constitutes a highly cytotoxic major aldehyde final peroxyl radical product of lipid peroxidation. It is claimed to be an inhibitor to protective enzymes. Hence, it could have both mutagenic and carcinogenic effects [46].
In our study, the increase in lipid peroxidation was reported during DMBA induced carcinogenesis. All groups treated with DMBA, have a significant increase in the levels of MDA as compared with normal group animals. The inhibition of peroxidation by moringa, graviola, ginger, cress and artemisinin, with ability descending, respectively is mainly attributed to the scavenging of the reactive free radicals involved in the peroxidation [47].Animals in groups received moringa, graviola, ginger, cress and artemisinin, with ability descending, respectively either simultaneously or post-treated exhibited significantly low levels of MDA, when compared with animals treated with DMBA only. This verifies the antilipid peroxidative role of moringa, graviola, ginger, cress and artemisinin, with ability descending, respectively by its ability to scavenge free radical generation.
For the purpose of preventing cellular damage induced by ROS, there is a lot of antioxidative defense system. The anti-oxidative defense system may scavenge ROS that play an important role in the initiation of lipid peroxidation and, therefore, play a protective role in cancer development [48]. This defense system operates through enzymatic (including SOD, GPx, GST and CAT), and nonenzymatic components (mainly GSH) [49,50]. SOD is the primary step of defense mechanism in the antioxidant system against the oxidative stress, as it dismutates the highly toxic superoxide anions (O2−) to O2 and H2O2. Gpx and catalase can scavange H2O2 and convert it into harmless byproducts, thereby providing protection against ROS [51].
Also, GPx has a high potency in scavenging reactive free radicals in response to oxidative stress and detoxifies peroxides and hydroperoxides that lead to the oxidation of GSH [52]. Furthermore, GST catalyzes the conjugation of the thiol functional groups of GSH to electrophilic xenobiotics, leading to elimination or conversion of xenobiotic-GSH conjugate [53]. In such reaction, the GSH is oxidized into GSSG, which can be reduced to GSH by GR with the consumption of NADPH [54]. GSH is the most important nonenzyme antioxidant in mammalian cells [55]. GSH is said to be involved in many cellular processes including the detoxification of endogenous and exogenous compounds and efficiently protects cells against deleterious effects of oxidative stress by scavenging free radicals, removing H2O2, and suppressing lipid peroxidation [56].
In the present study, the cancer bearing mice showed decreased activities of antioxidants (SOD, CAT, GR, GST and TAC) in comparison with normal animals. Our data are consistent with previous findings [57,58]. Pradeep et al. [59] reported that such subsequent decrease in the antioxidant defense is due to the decreased expression of these antioxidants during mammary gland damage. On the other hand, there is a significant increase in the enzymatic and non-enzymatic antioxidant guard in the animals administered moringa, graviola, ginger, cress and artemisinin and carcinogen when compared with animals administered carcinogen only. This increase is due to the ability of moringa, graviola, ginger, cress and artemisinin, with ability descending, respectively to prevent the formation of free radicals, enhance the endogenous antioxidant activity beyond its free radical scavenging property and the reduction of breast lipoperoxide formation [60].
The increase in the activities of the antioxidant enzymes in the moringa, graviola, ginger, cress and artemisinin, with ability descending, respectively treated mice compared to mice treated only with DMBA indicates its effective antioxidant activity owing to the presence of alkaloids, amino acids, flavonoids, glycosides, phytosterols, saponins, tannins and triterpinoids in moringa, graviola, ginger, cress and artemisinin extracts [[61], [62], [63], [64], [65], [66]]. All the above mentioned results suggest the protective role of graviola extracts, which could be due to the antioxidative effect of flavonoids [[61], [62], [63], [64], [65], [66], [67], [68], [69], [70]] present in such plant, which act as strong superoxide radical and singlet oxygen quenchers.
In this study, a statistically significant negative correlation between plasma means levels of MDA and antioxidant activities. The high MDA level could be explained by defect in the antioxidant system with accumulation of lipid peroxides in cancer tissue as stated by Kumaraguruparan et al. [71] Furthermore, Sener et al. [72] reported statistically significant lower total antioxidant capacity with significantly higher serum MDA levels in breast cancer group compared to treated and control group.
Trace elements and toxic heavy metals have critical roles in cancer biology. A large number of epidemiological studies indicate a close association between heavy metals and development of breast cancer such as lead (Pb), Copper (Cu), zinc (Zn), arsenic (As), cadmium (Cd), and nickel (Ni), which are found naturally in the environment [73]. In the current study, the concentrations of eight heavy metals (selenium, iron, nickel chromium, cadmium, lead, zinc and copper) were estimated in the tissue samples of all mice studied groups; it was apparent that a statistically significant elevation of lead, cadmium, chromium, nickel and iron concentrations was detected in breast tissue of mice having malignant breast tumors in comparison to control group. The present results are run in parallel with those obtained by previous studies [[74], [75], [76]].
Metals can be carcinogenic in various forms including free ions, metal complexes, or particles as well as soluble metal compounds. In general, metal carcinogenicity and genotoxicity are based on three main mechanisms, namely, oxidative stress, DNA repair modulation, and disturbances of signal transduction pathways [77]. Interestingly, some trace metals are claimed to be carcinogenic and capable of inducing a toxic effect through the formation of ROS and acting as cofactors in the oxidative damage of biological macromolecules and DNA. However, their exact role in carcinogenesis is still unclear [78].
Siddiqui et al. [79] analyzed blood, tumor tissue and breast adipose tissue from tumor free sections of mammary tissue with malignant and benign breast tumors. The Blood and tissues lead was significantly higher in malignant cases than in those of control. Most observed mechanisms of Pb carcinogenicity involve direct DNA damage, oxidative DNA damage through ROS generation, clastogenicity, endocrine disrupters, or inhibition of DNA synthesis or repair [80].
The increased levels of Cd, Cr and Ni in cancerous breast tissue samples and their suggested role in tumor development could be related to their disruption of the oxidative balance, production of oxidative DNA damage and inhibition of DNA repair [81]. Additionally, they functions also as an important class of endocrine disrupters [80]. Cadmium affects cell proliferation, differentiation, apoptosis and signal transduction by enhancement of protein phosphorylation and activation of transcription and translation factors [82]. Moreover, the demand for increased blood supply for a growing tumor provides a basis for the accumulation of many elements [78].
Metals such as iron and zinc were involved in breast tumorigenesis and their suggested role in tumor development could be related to their action as enzymatic co-factors involved in carcinogenesis [83]. In addition, zinc belongs to the group of oxidant metals causing disruption of the oxidative balance. Iron can also promote carcinogenesis by causing tissue damage as it acts as a catalyst in the conversion of hydrogen peroxide to free radical ions that attack cellular membranes, breaks DNA strands, inactivate enzymes and initiate lipid peroxidation [84].
Disruption of metal ion homeostasis may lead to oxidative stress, a state where increased formation of ROS overwhelms body antioxidant protection and subsequently induces DNA damage, lipid peroxidation, protein modification and carcinogenesis [78]. Our study revealed marked disruption in the oxidative stress markers as evidenced by a significant decrease in the superoxide dismutase, glutathione-s-transferase, glutathione reductase, catalase and total antioxidant capacity levels in breast cancer group versus treated and control group (p < 0.001). These finding run in parallel, with those obtained by other studies [[85], [86], [87]].
In the present study showed a statistically significant positive correlation between levels of lead, cadmium and iron in breast tissue and median MDA in plasma while a statistically significant negative correlation between levels of lead, cadmium and iron in breast tissue and antioxidant activities. This may be explained by the fact that elevation of these metals could lead to formation of free radicals or other reactive oxygen species. Valko et al. [88] stated that disruption of metal homeostasis and defect in the antioxidant system may lead to uncontrolled metal-mediated formation of deleterious free radicals participating in the modifications to DNA bases and enhanced lipid peroxidation with subsequent formation of MDA.
Creatinine and urea are metabolic products which are removed from circulation by the kidney to prevent their accumulation. Increase in serum level of these substances is regarded as an indication of loss of renal function [89,90]. Data from this study suggest that alkylating agents caused a loss of renal function and this is consistent with previous reports [91,92]. The biomarkers of renal function, creatinine and urea, were considered in this study. We observed that moringa, graviola, ginger, cress and artemisinin, with ability descending, respectively ameliorated the levels of serum creatinine and urea which is an indication of renal protection. This also confirms the protective role of moringa, graviola, ginger, cress and artemisinin, with ability descending, respectively against DMBA-induced renal toxicity. The liver is an organ involved in the biotransformation of drugs and other hepatotoxicants. The serum level of bilirubin and activities of the liver enzymes, ALT, AST, ALP, and GGT, are considered reliable indices of hepatotoxicity [93,94]. Increase in serum ALT and AST may have resulted from leakage from damaged hepatocytes (hepatocellular injury) [95]. Bilirubin is found in the liver, bile, intestines, and the reticuloendothelial cells of the spleen while ALP and GGT are associated with the cell membrane [96]. Serum bilirubin and activities of ALP and GGT are found to increase in conditions associated with hepatobiliary injury (decrease in hepatic clearance of bilirubin) and overproduction or leakage of ALP and GGT [96]. The biomarkers of hepatic function, ALT, AST and GGT, were considered in this study. In this study, DMBA caused a significant increase in the serum activities of ALT, AST and GGT. ALT and AST are primarily located in the cytoplasm and mitochondria of hepatocytes [97]. In this study, pre- and cotreatment with moringa, graviola, ginger, cress and artemisinin, with ability descending, respectively protected against increase in serum levels of ALT, AST, and GGT, which is an indication of hepatoprotection by moringa, graviola, ginger, cress and artemisinin, with ability descending, respectively. This also confirms the protective role of moringa, graviola, ginger, cress and artemisinin, with ability descending, respectively against DMBA-induced hepatotoxicity.
Monitoring the efficacy of the different treatment modalities by measuring dielectric properties of tumor tissue; the idea of classifying and evaluating the cancer treatment by measuring and comparing electrical properties of untreated and treated tumor tissue is a new idea in fact. In the present thesis, we address the issue of detecting changes in dielectric properties of the untreated and treated tumor tissue exposed to different treatment modalities. Experimental evidence strongly suggests that the measurements of the changes in dielectric properties of the tumor tissue after (50 mg, 100 mg, 200 mg; moringa, graviola, ginger, cress and artemisinin), tamoxifen and doxorubicin treatment can be used to quantify and predict the individual response of tumor tissue to different modalities of treatment. Equipped with a quantitative method that allows monitoring of tumor response at the earliest moment after treatment, the clinician will be able to customize and modify the treatment regimen in order to achieve the desired clinical results.
Dielectric properties of biological cells and tissues are very remarkable. They typically display extremely high dielectric constants at low frequencies, falling off in more or less distinct steps as the applied frequency is increased. Their frequency dependence permits identification and investigation of a number of completely different underlying mechanisms, and hence, dielectric studies of biomaterials have long been important in electrophysiology and biophysics. The dielectric behavior of biological tissues depends ultimately on the properties of the cells and molecules comprising it, thus interpretation of the dielectric information can therefore provide us with useful information on the cellular and molecular level. The electrical conductivity and permittivity of cancerous tissue has been found to be greater than the electrical conductivity and permittivity of normal tissues [98]. Because cancerous cells demonstrate greater permittivity, which is the ability to resist the formation of an electrical field they will resonate differently from normal cells. In turn the electrical conductivity and permittivity of biological materials will vary characteristically depending on the frequency applied [99].
To demonstrate the link between (50 mg, 100 mg, 200 mg; moringa, graviola, ginger, cress and artemisinin), tamoxifen and doxorubicin and dielectric properties of tumor tissue, we measured the permittivity and conductivity of untreated and treated tumor at multiple frequencies. The untreated tumor has highest permittivity and conductivity than treated tumor with different treatment modalities (Fig. 2). According to the research of Cure 1995, Gabriel et al., 1996 [100,101] cancer cells have higher intracellular sodium, higher content of unstructured water, lower intracellular potassium, magnesium and calcium concentrations, and more negative charges on their cell surface. These abnormalities result in cancer cells having lower transmembrane potentials than normal cells and altered membrane permeability. These cell membrane changes interfere with the flow of oxygen and nutrients into the cells and impair aerobic metabolism causing cancer cells to rely more on anaerobic metabolism for energy production. Anaerobic metabolism, excessive sodium concentrations, low transmembrane potential and pH alterations in turn create intracellular conditions more conducive to cellular mitosis and consequently lead to high permittivity and conductivity than normal tissue. The data of Joines et al. (1994) [102] at 0.9 GHz indicated that relative permittivity of malignant breast tumors exceeds that of normal breast tissue by 3.8:1, and conductivity by 6.4: 1. Also, the data of Chaudhary et al. (1984) [103] up to 3 GHz indicated corresponding malignant tumor–to-normal breast tissue ratios of 5: 1 and 4.7: 1.
In general, the relative permittivity of the tumor exposed to different treatment modalities 50 mg moringa, graviola, ginger, cress and artemisinin, 100 mg moringa, graviola, ginger, cress and artemisinin, doxorubicin and 200 mg moringa, graviola, ginger, cress and artemisinin respectively are lower than untreated group (Fig. 2). Decrease in relative permittivity may be referred to the decrease in tumor volume, inhibition in tumor growth rate and tumor mass as previously discussed. Additional factors may be involved in decreasing relative permittivity in treated tumors, changing the cell membrane potential, changing in the concentration of sodium and potassium inside and outside the cell, release of excessive water due to cell membrane ruptured.
The variation of imaginary permittivity (ε") with the applied frequency showed a sharp rise with low frequency at about (0.1 kHz) then suffered decline after that exponentially with increasing frequency (Fig. 2). Since the imaginary permittivity characterizes the dissipation of energy of the electrical oscillation of the dielectric subjected to the alternating electric field. Then the decrease in the permittivity in case of the studied parameters indicate that the dipoles formed as a result of the applied frequencies are unable to respond to the field which results in decrease in imaginary permittivity.
According to Pekar (1997) [104], “Every biological process is also an electric process” and “health and sickness are related to the bio-electric currents in our body”. The electrical conductivity of a tissue depends on both the physico-chemical bulk properties, i.e., properties of tissue fluids and solids and the microstructural properties, i.e., the geometry of microscopic compartments. In the present study, real conductivity (σ′) increased as the applied frequency increased (Fig. 2) while imaginary conductivity decreased in untreated and treated tumor (Fig. 2). Real and imaginary conductivity of tumor tissue treated with 50 mg moringa, graviola, ginger, cress and artemisinin, 100 mg moringa, graviola, ginger, cress and artemisinin, tamoxifen, doxorubicin and 200 mg moringa, graviola, ginger, cress and artemisinin respectively decreased than untreated tumor as shown in previous figures. The conductivity in treated tumor tissues may be affected by variations in: temperature, oxygen levels, free radical activity, mineral concentrations in intracellular and extracellular fluid, the types of minerals present in intracellular and extracellular fluids, pH (both intracellular and extracellular), level of hydration (cell water content and extracellular water content), the ratio of structural/unstructural water inside of the cell, membrane lipid/sterol composition, the amount of negative charges present on the surface of cell membranes, and the presence of chemical electrophilic toxins within the cell [105].
The complex diagram between the relative and imaginary permittivity, real and imaginary conductivity in (50 mg, 100 mg, 200 mg; moringa, graviola, ginger, cress and artemisinin) and doxorubicin follow the Cole-Cole semi-circle diagram (Fig. 2). This means that 50 mg moringa, graviola, ginger, cress and artemisinin, 100 mg moringa, graviola, ginger, cress and artemisinin, tamoxifen, doxorubicin and 200 mg moringa, graviola, ginger, cress and artemisinin respectively enhanced the release of oxygen level, vascular damage and cell necrosis and apoptosis [[106], [107], [108], [109]] which leads to inhibited and suppressed tumor growth and consequentially decreased conductivity and enhanced permittivity of treated tumor tissue. Correlation between the tumor growth and dielectric parameters (Real conductivity and Relative permittivity increment) estimated from the complex diagram (Fig. 2) is a new and good idea. The linear equations derived from the tumor volume growth, tumor mass inhibition and the dielectric parameters data. Values of the correlation coefficients of the equations indicate that, there is a very strong relation between tumor volume growth, mass inhibition ratio and each of the two explanatory variables real conductivity and relative permittivity. From this equation we are able to clearly distinguish changes in tumor growth and mass inhibition ratio when measuring the electrical properties of the tumor tissue.
The measurement of body fluid volumes, extracellular water (ECW), intracellular one (ICW) and their sum, total body water (TBW) is important in many pathologies [110]. Assessment of body fluid distribution would be useful to evaluate nutritional status, renal function and cardiovascular function [111].
The body water contents results [the total body water (TBW) percentage was of body weight, the intracellular water (ICW) percentage of the total body water and the extracellular water (ECW) percentage of the total body water] of normal mice (control) in present study were in-agreement with results reported by John R De Palma et al. (2001) [112] and Marieb et al. (2010) [113] in which total body water (TBW) percentage 60% of body weight, about 2/3 of (TBW) is intracellular water and 1/3 of (TBW) is extracellular water.
Extracellular Water (ECW) includes water in plasma, tissue spaces and lymph, and is commonly referred to as edema. The ECW also includes all water which is stored in the body fat. Higher levels of ECW are associated with poorer health [110,111].
When the renal function is impaired in the kidneys, an immediate physiological consequence is an excessive accumulation of liquid in the rest of the body, and consequently edema is formed. Edema is considered by the physician a common indicator of renal failure. During renal failure the amount of interstitial fluid increases, causing extracellular edema. The consequent interstitial swelling modifies the electrical properties of the tissue. These significant changes may indicate that there is a reduced activity of the ion pumps, leading to changes in ion distribution between inter- and extracellular spaces [114].
The results obtained from the present study indicate a significant decrease in both total body water and extracellular water percentage while a significant increase in intracellular water percentage of treated with 50 mg moringa, graviola, ginger, cress and artemisinin, 100 mg moringa, graviola, ginger, cress and artemisinin, tamoxifen, doxorubicin and 200 mg moringa, graviola, ginger, cress and artemisinin respectively compared to untreated group. That significant decrease in both total body water and extracellular water percentage may be due to restoring the normal renal function upon treatment [111].
The results obtained from the present study indicate that increase in intracellular water percentage in untreated group may due to lack of energy production, and cell membrane damage (toxicity, oxidative damage, lack of essential fatty acids) can all contribute to increase in intracellular water [111]. The decrease in total body water and extracellular water while increase in intracellular water as the dose of moringa, graviola, ginger, cress and artemisinin increased, indicating the degree of change in body water content seems to be dose-dependent.
In the present study molecular study of maspin, survivin, livin, caveolin-1, osteopontin and Fucosyltransferase 4 gene expressions as a molecular diagnostic and prognostic markers for breast cancer revealed that there was a significantly positive correlation between dose of moringa, graviola, ginger, cress and artemisinin and maspin and caveolin-1gene expressions in treated groups while a negative correlation between maspin and caveolin-1gene expressions and cancer progression in untreated cancerous group. Maspin and caveolin-1gene expression significantly higher in mice groups treated moringa, graviola, ginger, cress and artemisinin extract (100 and 200 mg/kg body weight) than those treated with (50 mg/kg body weight) while the lowest expression was among untreated cancerous group. Ontheotherhand, there was a significantly negative correlation between dose of moringa, graviola, ginger, cress and artemisinin and survivin, livin, osteopontin and Fucosyltransferase 4 gene expressions in treated groups while a positive correlation between survivin, livin, osteopontin and Fucosyltransferase 4 gene expressions and cancer progression in untreated cancerous group. Survivin, livin, osteopontin and Fucosyltransferase 4 gene expression significantly lower in mice groups treated moringa, graviola, ginger, cress and artemisinin extract (100 and 200 mg/kg body weight) than those treated with (50 mg/kg body weight) while the highest expression was among untreated cancerous group. The present results further support that molecular detection of maspin, survivin, livin, caveolin-1, osteopontin and Fucosyltransferase 4 gene expressions using RT-PCR could be used as a diagnostic and prognostic predictor of breast cancer and was in agreement with other studies done by other authors [[115], [116], [117], [118], [119], [120]].
The data in the present study showed that during the whole course of experiment the histopathological changes were synchronized with the biochemical changes. Histological evaluation revealed that all tumors from the cancerous control group were highly malignant cells and none of the tumors showed necrosis. Tumors excised from animals receiving moringa, graviola, ginger, cress and artemisinin extract (100 and 200 mg/kg body weight), significant areas of necrosis were present compared to (50 mg/kg body weight) moringa, graviola, ginger, cress and artemisinin treated group while in case of tamoxifen doxorubicin treated tumors, foci of necrosis areas were present which were distinctly appeared. According to current results there was an agreement with other studies done by other authors [35,39,40,72].
6. Conclusion
From the present study it could be concluded that moringa, graviola, ginger, cress and artemisinin showed promising chemopreventive and chemotherapeutic results for prevention and treatment of cancer compared to results obtained with tamoxifen and doxorubicin as conventional drugs.
7. Recommendations
From the present study it could be recommended that use of moringa, graviola, ginger, cress and artemisinin as prevention and/or therapeutic option for cancer treatment with increasing duration of treatment course.
Conflicts of interest
Author declare that there is no conflict of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbrep.2019.100637.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.King R.J. Natural history: the life of a cancer. In: King R.J., Robins M.W., editors. Cancer Biology. third ed. Longman Essex (pub.); 2006. pp. 9–31. (chapter 1) [Google Scholar]
- 2.Abeloff M.D., Wolff A.C., Weber B.L., Abeloff M.D. Cancer of the breast. In: Armitage J.O., Lichter A.S., editors. Clinical Oncology. fourth ed. Elsevier (pub.; Philadelphia, Pa: 2008. pp. 1875–1943. [Google Scholar]
- 3.Fleming I.D. Breast. In: Edge S.B., Byrd D.R., Compton C.C., Fritz A.G., Greene F.L., Trotti A., editors. AJCC Cancer Staging Manual. seventh ed. vol. 7. Springer (pub.; New York: 2010. pp. 347–369. [Google Scholar]
- 4.Alexandria Cancer Registry Annual Report . Medical Research Institute, Alexandria University; 2010. Alexandria, Egypt. [Google Scholar]
- 5.Beral V. Breast cancer and hormone-replacement therapy in the million women study. Lancet. 2003;362:419–427. doi: 10.1016/s0140-6736(03)14065-2. [DOI] [PubMed] [Google Scholar]
- 6.Burstein H.J., Harris J.R., Morrow M. Malignant tumors of the breast. In: DeVita V.T., Lawrence T.S., Rosenberg S.A., editors. DeVita, Hellman, and Rosenberg's Cancer: Principles and Practice of Oncology. eighth ed. Lippincott Williams & Wilkins (pub.; Philadelphia, Pa: 2008. pp. 1606–1654. [Google Scholar]
- 7.Khalkhali-Ellis Z., Christian A.L., Kirschmann D.A., Edwards E.M., Rezaie-Thompson M. Regulating the tumor suppressor gene maspin in breast cancer cells: a potential mechanism for the anticancer properties of tamoxifen. Clin. Cancer Res. 2004;10:449–454. doi: 10.1158/1078-0432.ccr-1002-03. [DOI] [PubMed] [Google Scholar]
- 8.Mansour A., Daba A., Baddour N., El-Saadani M., Aleem E. Schizophyllan inhibits the development of mammary and hepatic carcinomas induced by 7,12 dimethylbenz (α) anthracene and decreases cell proliferation: comparison with tamoxifen. J. Cancer Res. Clin. Oncol. 2012;138:1579–1596. doi: 10.1007/s00432-012-1224-0. [DOI] [PubMed] [Google Scholar]
- 9.Smith L., Watson M.B., O'Kane S.L., Drew P.J., Lind M.J., Cawkwell L. The analysis of doxorubicin resistance in human breast cancer cells using antibody microarrays. Mol. Canc. Therapeut. 2006;5:2115–2120. doi: 10.1158/1535-7163.MCT-06-0190. [DOI] [PubMed] [Google Scholar]
- 10.Ojeswi B.K., Khoobchandani M., Hazra D.K., Srivastava M.M. In vitro anti-breast cancer efficacy of two indigenous plants on human cancer cell line MCF-7. Natl. Acad. Sci. Lett. 2009;32:105–109. [Google Scholar]
- 11.Arthur F.K.N., Woode E., Terlabi E.O., Larbie C. Evaluation of acute and subchronic toxicity of Annona muricata (Linn.) aqueous extract in animals. Eur. J. Exp. Biol. 2011;1:115–124. [Google Scholar]
- 12.Mao J.J., Palmer C.S., Healy K.E., Desai K., Amsterdam J. Complementary and alternative medicine use among cancer survivors: a population-based study. J Cancer Surviv. 2011;5:8–17. doi: 10.1007/s11764-010-0153-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olson M.E. Combining data from DNA sequences and morphology for a phylogeny of Moringaceae (Brassicales) Syst. Bot. 2002;27:55–73. [Google Scholar]
- 14.Mishra G., Singh P., Verma R., Kumar S., Srivastav S., Jha K.K., Khosa R.L. Traditional uses, phytochemistry and pharmacological properties of Moringa oleifera plant: an overview. DerPharmacia Lettre. 2011;3:141–164. [Google Scholar]
- 15.Sharma N., Gupta P.C., Rao ChV. Nutrient content, mineral, content and antioxidant activity of Amaranthus viridis and Moringa oleifera leaves. Res. J. Med. Plant. 2012;6:253–259. [Google Scholar]
- 16.Sreelatha S., Jeyachitra A., Padma P.R. Antiproliferation and induction of apoptosis by Moringa oleifera leaf extract on human cancer cells. Food Chem. Toxicol. 2011;49:1270–1275. doi: 10.1016/j.fct.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 17.Berkovich L., Earon G., Ron I., Rimmon A., Vexler A., Lev-Ari S. Moringa oleifera aqueous leaf extract down-regulates nuclear factor kappaB and increases cytotoxic effect of chemotherapy in pancreatic cancer cells. BMC Complement Altern. Med. 2013;13:212–219. doi: 10.1186/1472-6882-13-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dai Y., Hogan S., Schmelz E.M., Ju Y.H., Canning C., Zhou K. Selective growth inhibition of human breast cancer cells by soursop fruit extract in vitro and in vivo involving downregulation of EGFR expression. Nut cancer. 2011;63:795–801. doi: 10.1080/01635581.2011.563027. [DOI] [PubMed] [Google Scholar]
- 19.Torres M.P., Rachagani S., Purohit V., Pandey P., Joshi S., Moore E.D., Johansson S.L., Singh P.K., Ganti A.K., Batra S.K. Graviola: a novel promising natural-derived drug that inhibits tumorigenicity and metastasis of pancreatic cancer cells in vitro and in vivo through altering cell metabolism. Cancer let. 2012;323:29–40. doi: 10.1016/j.canlet.2012.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rasmussen P. Ginger-Zingiber officinale roscoe, Zingiberaceae. J Prim Health Care. 2011;3:235–236. [PubMed] [Google Scholar]
- 21.Eric Chan W.C., Lim Y.Y., Wong S.K. Antioxidant properties of ginger leaves: an overview. Free Radic. Res. 2011;1:6–16. [Google Scholar]
- 22.Gokavi S.S., Malleshi N.G., Guo M. Chemical composition of garden cress (Lepidium sativum) seeds and its fractions and use of bran as a functional ingredient. Plant Foods Hum. Nutr. (Dordr.) 2005;59:105–111. doi: 10.1007/s11130-004-4308-4. [DOI] [PubMed] [Google Scholar]
- 23.Dugasani S.L., Balijepalli M.K., Mallikarjuna R.P. Growth inhibition and induction of apoptosis in estrogen receptor-positive and negative human breast carcinoma cells by Adenocalymma alliaceum flowers. Curr. Trends Biotechnol. Pharm. 2009;3:1–12. [Google Scholar]
- 24.Conforti F., Ioele G., Statti G.A., Marrelli M., Ragno G., Menichini F. Antiproliferative activity against human tumor cell lines and toxicity test on Mediterranean dietary plants. Food Chem. Toxicol. 2008;46:3325–3332. doi: 10.1016/j.fct.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 25.Hudaib M., Mohammad M., Bustanji Y., Tayyem R., Yousef M., Abuirjeie M., Aburjai T. Ethnopharmacological survey of medicinal plants in Jordan, Mujib nature reserve and surrounding area. J. Ethnopharmacol. 2008;120:63–71. doi: 10.1016/j.jep.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 26.Chekhun V.F., Lukianova N.Y., Borikun T.V., Zadvorny T.V., Mokhir A. Artemisinin modulating effect on human breast cancer cell lines with different sensitivity to cytostatics. Exp. Oncol. 2017;39:25–29. [PubMed] [Google Scholar]
- 27.Singh N.P., Lai H.C., Park J., Gerhardt T.E., Kim B.J., Wang S., Sasaki T. Effects of artemisinin dimers on rat breast cancer cells in vitro and in vivo. Anticancer Res. 2011;31:4111–4114. [PubMed] [Google Scholar]
- 28.Hanahan D., Weinberg R.A. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 29.Gey K.F. Prospects for the prevention of free radical disease, regarding cancer and cardiovascular disease. Br. Med. Bull. 1993;49:679–699. doi: 10.1093/oxfordjournals.bmb.a072640. [DOI] [PubMed] [Google Scholar]
- 30.Revathi R., Manju V. The effects of Umbelliferone on lipid peroxidation and antioxidant status in diethylnitrosamine induced hepatocellular carcinoma. Journal of Acute Medicine. 2013;3:73–82. [Google Scholar]
- 31.Banakar M.C., Paramasivan S.K., Chattopadhyay M.B., Datta S., Chakraborty P., Chatterjee M. 1alpha, 25- dihydroxyvitamin D3 prevents DNA damage and restores antioxidant enzymes in rat hepatocarcinogenesis induced by diethylnitrosamine and promoted by phenobarbital. World J. Gastroenterol. 2004;10(Suppl 9):1268–1275. doi: 10.3748/wjg.v10.i9.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Zwart L.L., Meerman J.H., Commandeur J.N., Vermeulen N.P. Biomarkers of free radical damage applications in experimental animals and in humans. Free Radic. Biol. Med. 1999;26:202–226. doi: 10.1016/s0891-5849(98)00196-8. [DOI] [PubMed] [Google Scholar]
- 33.Rieder C.R., Parsons R.B., Fitch N.J.S., Williams A.C. Human brain cytochrome P4501B1: immunohistochemical localization in human temporal lobe and induction by dimethylbenz(a)anthracene in astrocytoma cell line (MOG-G-CCM) Neurosci. Lett. 2000;278:177–180. doi: 10.1016/s0304-3940(99)00932-5. [DOI] [PubMed] [Google Scholar]
- 34.Giri U., Sharma S.D., Abdulla M., Athar M. Evidence that in situ generated reactive oxygen species act as a potent stage I tumor promoter in mouse skin. Biochem. Biophys. Res. Commun. 1995;209:698–705. doi: 10.1006/bbrc.1995.1555. [DOI] [PubMed] [Google Scholar]
- 35.Henry L., Narendra P.S. Oral artemisinin prevents and delays the development of 7,12-dimethylbenz[a]anthracene (DMBA)-induced breast cancer in the rat. Cancer Lett. 2006;231:43–44. doi: 10.1016/j.canlet.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 36.Salet C., Moreno G. Photosensitization of mitochondria. Molecular and cellular aspects. J. Photochem. Photobiol., B. 1990;5:133–150. doi: 10.1016/1011-1344(90)80002-f. [DOI] [PubMed] [Google Scholar]
- 37.Arulkumaran S., Ramprasath V.R., Shanthi P., Sachdanandam P. Restorative effect of Kalpaamruthaa, an indigenous preparation, on oxidative damage in breast gland mitochondrial fraction in experimental mammary carcinoma. Mol. Cell. Biochem. 2006;291:77–82. doi: 10.1007/s11010-006-9199-2. [DOI] [PubMed] [Google Scholar]
- 38.Cavalieri E., Roth R., Rogan E. Mechanisms of tumor initiation by polycyclic aromatic hydrocarbons. Carcinogenesis. 1978;3:273–287. [Google Scholar]
- 39.Choi E.J. Antioxidative effects of hesperetin against 7,12 dimethylbenz(a)anthracene-induced oxidative stress in mice. Life Sci. 2008;82:1059–1064. doi: 10.1016/j.lfs.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 40.Benakanakere I., Besch-Williford C., Carroll C.E., Hyder S.M. Synthetic progestins differentially promote or prevent 7,12-DMBAinduced mammary tumors in Sprague-Dawley rats. Cancer Prev. Res. 2010;3:1157–1167. doi: 10.1158/1940-6207.CAPR-10-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vinothini G., Murugan R.S., Nagini S. Evaluation of molecular markers in a rat model of mammary carcinogenesis. Oncol. Res. 2009;17:483–493. doi: 10.3727/096504009789735459. [DOI] [PubMed] [Google Scholar]
- 42.Boutwell R.K. The function and mechanism of promoters of carcinogenesis. CRC Crit. Rev. Toxicol. 1974;2:419–443. doi: 10.3109/10408447309025704. [DOI] [PubMed] [Google Scholar]
- 43.Gupta R.K., Patel A.K., Kumari R., Chugh S., Shrivastav C., Mehra S. Interactions between oxidative stress, lipid profile and antioxidants in breast cancer: a case control study. Asian Pac. J. Cancer Prev. APJCP. 2012;13:6295–6298. doi: 10.7314/apjcp.2012.13.12.6295. [DOI] [PubMed] [Google Scholar]
- 44.Rao C.S.S., Kumari D.S. Changes in plasma lipid peroxidation and the antioxidant system in women with breast cancer. Int. J. Basic Appl. Sci. 2012;1:429–438. [Google Scholar]
- 45.Kumaraguruparan R., Subapriya R., Viswanathan P., Nagini S. Tissue lipid peroxidation and antioxidant status in patients with adenocarcinoma of the breast. Clin. Chim. Acta. 2002;325:165–170. doi: 10.1016/s0009-8981(02)00292-9. [DOI] [PubMed] [Google Scholar]
- 46.Ziech D., Franco R., Georgakilas A.G., Georgakila S., Malamou-Mitsi V., Schoneveld O. The role of reactive oxygen species and oxidative stress in environmental carcinogenesis and biomarker development. Chem. Biol. Interact. 2010;188:334–339. doi: 10.1016/j.cbi.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 47.Arthur F.K.N., Woode E., Terlabi E.O., Larbie C. Evaluation of acute and subchronic toxicity of Annona muricata (Linn.) aqueous extract in animals. Eur. J. Exp. Biol. 2011;1:115–124. [Google Scholar]
- 48.LÓpez-Lázaro M. Anticancer and carcinogenic properties of curcumin: considerations for its clinical development as a cancer chemo preventive and chemotherapeutic agent. Mol. Nutr. Food Res. 2008;52:103–127. doi: 10.1002/mnfr.200700238. [DOI] [PubMed] [Google Scholar]
- 49.Zhang C., Zeng T., Zhao X., Yu L., Zhu Z., Xie K. Protective effects of garlic oil on hepatocarcinoma induced by Nnitrosodiethylamine in rats. Int. J. Biol. Sci. 2012;8:363–374. doi: 10.7150/ijbs.3796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen B., Ning M., Yang G. Effect of paeonol on antioxidant and immune regulatory activity in hepatocellular carcinoma rats. Molecules. 2012;17:4672–4683. doi: 10.3390/molecules17044672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vásquez-Garzón V.R., Arellanes-Robledo J., García-Román R., Aparicio-Rautista D.I., Villa-Treviño S. Inhibition of reactive oxygen species and pre-neoplastic lesions by quercetin through an antioxidant defense mechanism. Free Radic. Res. 2009;43:128–137. doi: 10.1080/10715760802626535. [DOI] [PubMed] [Google Scholar]
- 52.Usunomena U., Ademuyiwa A.J., Tinuade O.O., Uduenevwo F.E., Martin O., Okolie N.P. N-nitrosodimethylamine (NDMA), liver function enzymes, renal function parameters and oxidative stress parameters: a Review. Br. J. Pharmacol. Toxicol. 2012;3:165–176. [Google Scholar]
- 53.Rao G.M., Rao C.V., Pushpangadan P., Shirwaikar A. Hepatoprotective effects of rubiadin, a major constituent of Rubia cordifolia Linn. J. Ethnopharmacol. 2006;103:484–490. doi: 10.1016/j.jep.2005.08.073. [DOI] [PubMed] [Google Scholar]
- 54.Revathi R., Manju V. The effects of Umbelliferone on lipid peroxidation and antioxidant status in diethylnitrosamine induced hepatocellular carcinoma. Journal of Acute Medicine. 2013;3:73–82. [Google Scholar]
- 55.Wu G., Fang Y.Z., Yang S., Lupton J.R., Turner N.D. Glutathione metabolism and its implications for health. J. Nutr. 2004;134:489–492. doi: 10.1093/jn/134.3.489. [DOI] [PubMed] [Google Scholar]
- 56.Blair I.A. Endogenous glutathione adducts. Curr. Drug Metabol. 2006;7:853–872. doi: 10.2174/138920006779010601. [DOI] [PubMed] [Google Scholar]
- 57.Ghosh D., Choudhury S.T., Ghosh S., Mandal A.K., Sarkar S., Ghosh A. Nano capsulated curcumin: oral chemopreventive formulation against diethylnitrosamine induced hepatocellular carcinoma in rat. Chem. Biol. Interact. 2012;195:206–214. doi: 10.1016/j.cbi.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 58.Rajeshkumar N.V., Kuttan R. Inhibition of N-nitroso diethylamine induced hepatocarcinogenesis by Picroliv. J. Exp. Clin. Cancer Res. 2000;19:459–465. [PubMed] [Google Scholar]
- 59.Pradeep K., Mohen C.V., Gobian K., Karthikeyan S. Silymarin modulates the oxidant-antioxidant imbalance during diethylnitrosamine induced oxidative stress in rats. Eur. J. Pharmacol. 2007;560:110–116. doi: 10.1016/j.ejphar.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 60.Bruck R., Ashkenazi M., Weiss S., Goldiner I., Shapiro H., Aeed H. Prevention of liver cirrhosis in rats by curcumin. Liver Int. 2007;27:373–383. doi: 10.1111/j.1478-3231.2007.01453.x. [DOI] [PubMed] [Google Scholar]
- 61.Ren W., Qiao Z., Wang H., Zhu L., Zhang L. Flavonoids: promising anticancer agents. Med. Res. Rev. 2003;23:519–534. doi: 10.1002/med.10033. [DOI] [PubMed] [Google Scholar]
- 62.Bemis D.L., Capodice J.L., Gorroochurn P., Katz A.E., Buttyananti R. Anti-prostate cancer activity of a s-carboline alkaloid enriched extract from Rauwolfia vomitoria. Int. J. Oncol. 2006;29:1065–1073. [PubMed] [Google Scholar]
- 63.Anne A., Grippo K.C., Ben R., Bill J., Gurley C. Analysis of flavonoid phytoestrogens in botanical and ephedra-containing dietary supplements. Ann. Pharmacother. 2007;41:1375–1382. doi: 10.1345/aph.1H497. [DOI] [PubMed] [Google Scholar]
- 64.Jiang J., Evodiamine Hu C. A novel anti-cancer alkaloid from Evodia rutaecarpa. Molecules. 2009;14:1852–1859. doi: 10.3390/molecules14051852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kabashima H., Miura N., Shimizu M., Shinoda W., Wang X., Wang Z. Preventive impact of alkaloids with anti-cancer effect extracted from natural herb and the derivatives. WebmedCentral. 2010;1:1–19. [Google Scholar]
- 66.Thoppil R.J., Bishayee A. Terpenoids as potential chemopreventive and therapeutic agents in liver cancer. World J. Hepatol. 2011;3:228–249. doi: 10.4254/wjh.v3.i9.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kuno T., Tsukamoto T., Hara A., Tanaka T. Cancer chemoprevention through the induction of apoptosis by natural compounds. J. Biophys. Chem. 2012;3:156–173. [Google Scholar]
- 68.Haghiac M., Walle T. Quercetin induces necrosis and apoptosis in SCC-9 oral cancer cells. Nutr. Canc. 2005;53:220–231. doi: 10.1207/s15327914nc5302_11. [DOI] [PubMed] [Google Scholar]
- 69.Priyadarsini R.V., Murugan R.S., Maitreyi S., Ramalingam K., Karunagaran D., Nagini S. The flavonoid quercetin induces cell cycle arrest and mitochondria-mediated apoptosis in human cervical cancer (HeLa) cells through p53 induction and NF-jB inhibition. Eur. J. Pharmacol. 2010;649:84–91. doi: 10.1016/j.ejphar.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 70.Bishayee K., Ghosh S., Mukherjee A., Sadhukhan R., Mondal J.K., Bukhsh A.R. Quercetin induces cytochrome-c release and ROS accumulation to promote apoptosis and arrest the cell cycle in G2/M, in cervical carcinoma: signal cascade and drug–DNA interaction. Cell Prolif. 2013;46:153–163. doi: 10.1111/cpr.12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kumaraguruparan R., Subapriya R., Kabalimoorthy J., Nagini S. Antioxidant profile in the circulation of patients with fibroadenoma and adenocarcinoma of the breast. Clin. Biochem. 2002;35:275–279. doi: 10.1016/s0009-9120(02)00310-7. [DOI] [PubMed] [Google Scholar]
- 72.Sener D.E., Gönenç A., Akinci M., Torun M. Lipid peroxidation and total antioxidant status in patients with breast cancer. Cell Biochem. Funct. 2007;25:377–382. doi: 10.1002/cbf.1308. [DOI] [PubMed] [Google Scholar]
- 73.Florea A.M., Büsselberg D. Metals and breast cancer: risk factors or healing agents? J. Toxicol. 2011;2011:159619. doi: 10.1155/2011/159619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pasha Q., Malik S.A., Shah M.H. Statistical analysis of trace metals in the plasma of cancer patients versus controls. J. Hazard Mater. 2008;153:1215–1221. doi: 10.1016/j.jhazmat.2007.09.115. [DOI] [PubMed] [Google Scholar]
- 75.da Silva M.P., Zucchi O.L., Ribeiro-Silva A., Poletti M.E. Discriminant analysis of trace elements in normal, benign and malignant breast tissues measured by total reflection X-ray fluorescence. Spectrochim. Acta, Part B. 2009;64:587–592. [Google Scholar]
- 76.Romanowicz-Makowska H., Forma E., BryÅ› M., Krajewska W.M., Smolarz B. Concentration of cadmium, nickel and aluminium in female breast cancer. Pol. J. Pathol. 2011;62:257–261. [PubMed] [Google Scholar]
- 77.Valko M., Rhodes C.J., Moncol J., Izakovic M., Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006;160:1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 78.Jomova K., Valko M. Advances in metal-induced oxidative stress and human disease. Toxicology. 2011;283:65–87. doi: 10.1016/j.tox.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 79.Siddiqui M.K., Jyoti Singh S., Mehrotra P.K., Singh K., Sarangi R. Comparison of some trace elements concentration in blood, tumor free breast and tumor tissues of women with benign and malignant breast lesions: an Indian study. Environ. Int. 2006;32:630–637. doi: 10.1016/j.envint.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 80.Martin M.B., Reiter R., Pham T., Avellanet Y.R., Camara J. Estrogenlike activity of metals in MCF-7 breast cancer cells. Endocrinology. 2003;144:2425–2436. doi: 10.1210/en.2002-221054. [DOI] [PubMed] [Google Scholar]
- 81.Leonard S.S., Bower J.J., Shi X. Metal-induced toxicity, carcinogenesis, mechanisms and cellular responses. Mol. Cell. Biochem. 2004;255:3–10. doi: 10.1023/b:mcbi.0000007255.72746.a6. [DOI] [PubMed] [Google Scholar]
- 82.Siewit C.L., Gengler B., Vegas E., Puckett R., Louie M.C. Cadmium promotes breast cancer cell proliferation by potentiating the interaction between ERalpha and c-Jun. Mol. Endocrinol. 2010;24:981–992. doi: 10.1210/me.2009-0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Geraki K., Farquharson M.J., Bradley D.A. Concentrations of Fe, Cu and Zn in breast tissue: a synchrotron XRF study. Phys. Med. Biol. 2002;47:2327–2339. doi: 10.1088/0031-9155/47/13/310. [DOI] [PubMed] [Google Scholar]
- 84.Konemann S., Bolling T., Matzkies F., Willich N., Kisters K. Iron and iron-related parameters in oncology. Trace Elem. Electrolytes. 2005;22:142–149. [Google Scholar]
- 85.Kumaraguruparan R., Subapriya R., Kabalimoorthy J., Nagini S. Antioxidant profile in the circulation of patients with fibroadenoma and adenocarcinoma of the breast. Clin. Biochem. 2002;35:275–279. doi: 10.1016/s0009-9120(02)00310-7. [DOI] [PubMed] [Google Scholar]
- 86.Kasapovic J., Pejic S., Todorovic A., Stojiljkovic V., Pajovic S.B. Antioxidant status and lipid peroxidation in the blood of breast cancer patients of different ages. Cell Biochem. Funct. 2008;26:723–730. doi: 10.1002/cbf.1499. [DOI] [PubMed] [Google Scholar]
- 87.Yeh C.C., Hou M.F., Tsai S.M., Lin S.K., Hsiao J.K., Huang J.C. Superoxide anion radical, lipid peroxides and antioxidant status in the blood of patients with breast cancer. Clin. Chim. Acta. 2005;361:104–111. doi: 10.1016/j.cccn.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 88.Valko M., Leibfritz D., Moncol J., Cronin M.T., Mazur M., Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 89.Han W.K., Bonventre J.V. Biologic markers for the early detection of acute kidney injury. Curr. Opin. Crit. Care. 2004;10:476–482. doi: 10.1097/01.ccx.0000145095.90327.f2. [DOI] [PubMed] [Google Scholar]
- 90.George G.S., Wakasi M.E., Egoro E. Creatinine and urea levels as critical markers in end-stage renal failure. Res. Rev.: Journal of Medical and Health Sciences. 2014;3:41–44. [Google Scholar]
- 91.Paliwal R., Sharma V., Pracheta, Sharma S., Yadav S., Sharma S.H. Antinephrotoxic effect of administration ofMoringa oleifera Lam. in amelioration of DMBA-induced renal carcinogenesis in Swiss albino mice. Biol. Med. 2011;3:27–35. [Google Scholar]
- 92.Sharma V., Paliwal R., Janmeda P., Sharma S.H. The reno-protective efficacy of Moringa oleifera pods on xenobiotic enzymes and antioxidant status against7,12-dimethylbenz[a]anthracene exposed mice. J. Chin. Integr. Med. 2012;10:1171–1178. doi: 10.3736/jcim20121015. [DOI] [PubMed] [Google Scholar]
- 93.Boone L., Meyer D., Cusick P., Ennulat D., Bolliger A.P., Everds N. Selection and interpretation of clinical pathology indicators of hepatic injury in preclinical studies. Vet. Clin. Pathol. 2005;34:182–188. doi: 10.1111/j.1939-165x.2005.tb00041.x. [DOI] [PubMed] [Google Scholar]
- 94.Singh A., Bhat T.K., Sharma O.M. Clinical biochemistry of hepatotoxicity. J. Clin. Toxicol. 2011;4:1–19. [Google Scholar]
- 95.Ozer J., Ratner M., Shaw M., Bailey W., Schomaker S. The current state of serumbiomarkers of hepatotoxicity. Toxicology. 2008;245:194–205. doi: 10.1016/j.tox.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 96.Ramaiah S.K. A toxicologist guide to the diagnostic interpretation of hepatic biochemical parameters. Food Chem. Toxicol. 2007;45:1551–1557. doi: 10.1016/j.fct.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 97.Amacher D.E. A toxicologist's guide to biomarkers of hepatic response. Hum. Exp. Toxicol. 2002;21:253–262. doi: 10.1191/0960327102ht247oa. [DOI] [PubMed] [Google Scholar]
- 98.Foster K., Schepps J. Dielectric properties of tumor and normal tissues at radio through microwave frequencies. J. Microw. Power. 1981;16:107–119. doi: 10.1080/16070658.1981.11689230. [DOI] [PubMed] [Google Scholar]
- 99.Scharfetter H. Technical University Graz; November 1999. Structural Modeling for Impedance-based non-invasive Diagnostic Methods. Graz: Thesis for the Habilitation at the Faculty of Electrical Engineering. [Google Scholar]
- 100.Cure J. Paper Presented at the Second International Congress of Electrochemical Treatment of Cancer. Jupiter, Florida. October 1995. On the electrical characteristics of cancer. [Google Scholar]
- 101.Gabriel C., Gabriel S., Corthout E. The dielectric properties of biological tissues: I. Literature survey. Phys. Med. Biol. 1996;41:2231–2249. doi: 10.1088/0031-9155/41/11/001. [DOI] [PubMed] [Google Scholar]
- 102.Joines W., Dhenxing Y., Jirtle R. The measured electrical properties of normal and malignant human tissues from 50 to 900 MHz. Med. Phys. 1994;21:547–550. doi: 10.1118/1.597312. [DOI] [PubMed] [Google Scholar]
- 103.Ghaudhary S., Mishra R., Swarup A. Dielectric properties of normal and malignant human breast tissues at radiowave and microwave frequencies. Indian J. Biochem. Biophys. 1984;21:76–79. [PubMed] [Google Scholar]
- 104.Pekar R. Verlag Wilhelm Maudrich; Munich, Germany: 1997. Percutaneous Bio-Electrotherapy of Cancerous Tumors: A Documentation of Basic Principles and Experiences with Bio-Electrotherapy. [Google Scholar]
- 105.Haltiwanger S. 2003. The Electrical Properties of Cancer Cells [Internet]http://www.royalrife.com/haltiwanger1.pdf available from: [Google Scholar]
- 106.Kolarova H., Bajgar R., Tomankova K., Krestýn E., Dolezal L., Hálek J. In vitro study of reactive oxygen species production during photodynamic therapy in ultrasound-pretreated cancer cell. Physiol. Res. 2007;56:S27–S32. doi: 10.33549/physiolres.931298. [DOI] [PubMed] [Google Scholar]
- 107.Liu Q., Wang Pan, Meng Li. Apoptosis of Ehrlich ascites tumor cells by sonochemical activated hematoporphyrin. Acta Zool. Sin. 2003;49:620–628. [Google Scholar]
- 108.Liu Q., Liu S., Qi H., Wang P., Tang W., Zhang K. Preliminary study on the mechanism of apoptosis in Ehrlich ascites tumor cells by sonochemical-activated hematoporphyrin. Acta Zool. Sin. 2005;51:1073–1079. [Google Scholar]
- 109.Miyoshi N., Takeshita T., Misik V., Riesz P. Monomerization of photosensitizers by ultrasound irradiation in surfactant micellar soultions. Ultrason. Sonochem. 2001;8:367–371. doi: 10.1016/s1350-4177(00)00079-1. [DOI] [PubMed] [Google Scholar]
- 110.Jaffrin M.Y., Morel H. Body fluid volumes measurements by impedance: a review of bioimpedance spectroscopy (BIS) and bioimpedance analysis (BIA) methods. Med. Eng. Phys. 2008;30:1257–1269. doi: 10.1016/j.medengphy.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 111.Drobot J., Riddle S. The American center for biological medicine; 2013. Bio Impedance Analysis (BIA) [Google Scholar]
- 112.De Palma J.R., Pittard J.D. Hemodialysis, Inc; 2001. Body Water-Body Weight.http://www.hemodialysis-inc.com/articles/bodywater.pdf [Internet] Available from: [Google Scholar]
- 113.Marieb E.N., Hoehn K. Human Anatomy & Physiology. eighth ed. Benjamin Cummings; San Francisco, California: 2010. Fluid, Electrolyte, and acid-base balance. [Google Scholar]
- 114.Moustafa M., EL-Khatib A.M., Madi N.K., Rezk S., Elhaj A.M. Effects of x-ray irradiation on body water, and electrical properties of rat tissues. Rom. J. Biophys. 2012;22:51–64. [Google Scholar]
- 115.Jiang N., Meng Y., Zhang S., Mensah-Osman E., Sheng S. Maspin sensitizes breast carcinoma cells to induced apoptosis. Oncogene. 2002;21:4089–4098. doi: 10.1038/sj.onc.1205507. [DOI] [PubMed] [Google Scholar]
- 116.Ma W.H., Liu Y.C., Xue M.L., Zheng Z., Ge Y.L. Downregulation of survivin expression exerts antitumoral effects on mouse breast cancer cells in vitro and in vivo. Oncology Letters. 2016;11:159–167. doi: 10.3892/ol.2015.3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang X., Shen P., Coleman M., Zou W., Loggie B.W., Smith L.M. Caveolin-1 down-regulation activates estrogen receptor alpha expression and leads to 17‚-estradiolstimulated mammary tumorigenesis. Anticancer Res. 2005;25:369–376. [PubMed] [Google Scholar]
- 118.Wang L., Zhang Q., Liu B., Han M., Shan B. Challenge and promise: roles for Livin in progression and therapy of cancer. Mol. Canc. Therapeut. 2008;7:3661–3669. doi: 10.1158/1535-7163.MCT-08-0480. [DOI] [PubMed] [Google Scholar]
- 119.Hedley B.D., Welch D.R., Allan A.L., Al-Katib W., Dales D.W., Postenka C.O., Casey G., Macdonald I.C., Chambers A.F. Down regulation of osteopontin contributes to metastasis suppression by breast cancer metastasis suppressor. Int. J. Cancer. 2008;123:526–534. doi: 10.1002/ijc.23542. [DOI] [PubMed] [Google Scholar]
- 120.Yang X., Zhang Z., Jia S., Liu Y., Wang X., Yan Q. Overexpression of fucosyltransferase IV in A431 cell line increases cell proliferation. Int. J. Biochem. Cell Biol. 2007;39:1722–1730. doi: 10.1016/j.biocel.2007.04.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.