Abstract
Objectives:
To describe and interpret local antibiograms from a single tertiary care center to monitor the trends of antimicrobial resistance (AMR) patterns and establish baseline data for further surveillance.
Methods:
We performed a retrospective descriptive review of antibiograms data between January 2010 and December 2015 from King Fahad Medical City, Riyadh, Kingdom of Saudi Arabia.
Results:
A total of 51,491 isolates were identified, and most were gram-negative (76.2%). Escherichia coli was the most frequently isolated organism (36.8%), followed by Coagulase-negative Staphylococcus (28.4%) and Staphylococcus aureus (27.5%). The detection of antibiotic-resistant organisms, especially extended-spectrum beta-lactamase-producing Escherichia coli (31%-41%), increased over time. The sensitivity of Streptococcus pneumoniae to penicillin improved from 66% to 100% (p<0.001). Gram-negative isolates had excellent overall susceptibility to amikacin, variable susceptibility to piperacillin-tazobactam and carbapenems, and declining susceptibility to ceftazidime, ciprofloxacin, and cefepime.
Conclusion:
Streptococcus pneumoniae susceptibility to penicillin significantly improved over time, which might be because of the introduction of the pneumococcal vaccine. Conversely, the upward trend in resistant gram-negative organisms is worrisome and warrants the implementation of antimicrobial stewardship programs.
Antimicrobial resistance (AMR) is becoming a global threat to public health. Antibiotic-resistant organisms kill millions of people each year and are expected to cost the world economy as much as $100 trillion by 2050 if no proactive strategies are taken.1 Thus, the AMR problem requires global commitment and action plans, as declared by the World Health Organization (WHO) in the AMR global report on surveillance in 2014. The General Assembly of the United Nations held a high-level meeting on September 2016, where the global leaders discussed this issue, and they committed to fighting together against AMR.2 Antimicrobial stewardship programs are one fundamental strategy to combat AMR and are becoming mandatory requirements for hospital accreditation. The first step in these programs is to establish local antibiograms which are a summary of the susceptibility profiles of the tested bacterial pathogens generated in tables over a period of time. Antibiograms aid physicians to optimally select empiric antimicrobial therapy according to local susceptibility; moreover, it helps to monitor resistance trends over time.3
Considering that King Fahad Medical City (KFMC) is a tertiary facility that deals with critical cases, knowledge of local susceptibility is essential to guide its physicians toward appropriate antimicrobial choices. Currently, the facility lacks organized local data that is readily available to clinicians. Our local AMR status is not yet well known, and the real magnitude of the problem is not yet determined.
We aimed to determine the trends of the antimicrobial susceptibility patterns (from January 2010 to December 2015), to compare it with local and international data. In addition, we aimed to perform a study that would serve as a baseline for further antimicrobial surveillance on a regular basis to assess the emergence of resistant organisms.
Our goal is to measure the susceptibility patterns of selected organisms such as Staphylococcus aureus (S. aureus), Methicillin-resistant S. aureus (MRSA), Streptococcus pneumoniae (S. pneumoinae), Escherichia coli (E. coli), Pseudomonas aeruginosa (P. aeruginosa), Klebsiella pneumoniae (K. pneumoniae), and Acinetobacter baumannii (A. baumannii). Moreover, we sought to determine the trends in terms of the number of isolates of extended-spectrum beta-lactamase producers (ESBLs) and carbapenemase producers, with special attention to S. pneumoinae, MRSA, and ESBLs, because the noted changes in their susceptibility patterns might affect the choice of empirical antibiotics.
Methods
King Fahad Medical City is a tertiary hospital in Riyadh, Saudi Arabia with 1000-bed capacity. It includes 4 hospitals (the main hospital, children’s specialized hospital, women’s specialized hospital, and rehabilitation hospital) and 4 specialized centers (the National Neurosciences Institute; Obesity, Endocrine & Metabolism Center; King Salman Heart Center; and Comprehensive Cancer Center). King Fahad Medical City receives referral cases from all over the kingdom. We performed a retrospective, descriptive review of KFMC antibiograms from all departments during the period of January 2010 up to December 2015. Ethical approval was obtained from the institutional review board at KFMC.
Blood, urine, cerebrospinal fluid, respiratory, and other specimens are routinely processed in the KFMC microbiology laboratory which is accredited by the College of American Pathologists (CAP). All positive specimens presented to the microbiology laboratory at KFMC between January 2010 and December 2015, except for isolates collected from MRSA surveillance or screening, were included in this study.
Microorganisms were identified to the species level using the Phoenix100 automated system, and then confirmed using the American Proficiency Institute (API) tests. Antimicrobial susceptibility testing was conducted using the automated system (Phoenix100) and confirmed by epsilometer test (E-test). Further confirmatory tests were performed for the multi-drug resistant organisms (MDRO) including Modified Hodge test for carbapenemase producer organisms, Cephalosporin/clavulanate combination disks for ESBLs and Vancomycin E-test for vancomycin-resistant enterococcus (VRE).
Testing and identification of the specimens were carried out according to the guidelines of the Clinical and Laboratory Standards Institute (CLSI) along with breakpoint interpretation of the tested antibiotics. Regular API validation tests were performed.
The details of the tested organisms were collected and quantitatively summarized as percentages, according to their susceptibility profiles to particular antibiotics to generate antibiograms. The data management was performed using Microsoft Excel 2010. Subsequently, the trend in susceptibility was calculated over a 5-year period using epi curves and bar graphs. Statistical analysis was carried out using Statistical Package for Social Science version 25.0 (IBM Corp., Armonk, NY, USA). P-values were calculated using chi-square analysis with significance level at 0.01.
Results
Isolates
A total of 51,491 isolates were collected over a 5-year period. Gram-negative isolates (76.2%, [39,213]) where far more common than gram-positive isolates (23.8%, [12,278]). Among the gram-negative isolates, E. coli was the most frequently detected pathogen (36.8%, [14,450]), followed by P. aeruginosa (24%, [9,521]) and K. pneumonia (18.8%, [7,410]). Other gram-negative isolates were convergent; A. baumannii (4.4%, [1,726]), Proteus mirabilis (4%, (1,629]), Enterobacter cloacae (3.0%, [1,267]), and Stenotrophomonas maltophilia (2%, [775]), whereas Serratia marcescens (1.7%, [655]) was the least frequently detected organism. The remaining gram-negative isolates collectively constituted 4.6%, (1,780) (Figure 1).
Figure 1.
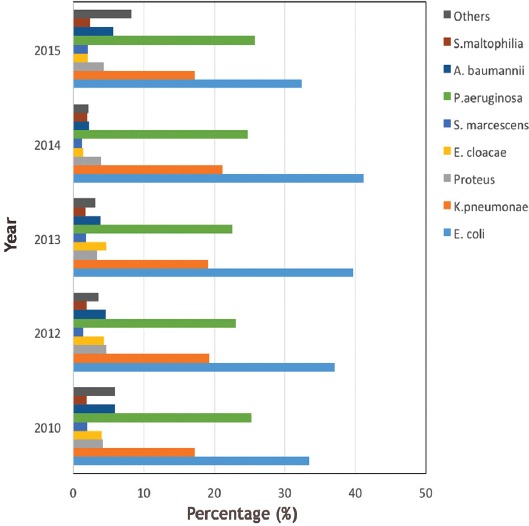
The percentages of the gram-negative isolates. S. maltophilia - Stenotrophomonas maltophilia, A. baumannii - Acinetobacter baumannii, P. aeruginosa - Pseudomonas aeruginosa, S. marcescens - Serratia marcescens, E. cloacae - Enterobacter cloacae, K. pneumoniae - Klebsiella pneumoniae, E. coli - Escherichia coli.
Among gram-positive isolates, coagulase-negative Staphylococci (28.4%, [3,491]) and S. aureus (27.5%, [3,382]) were the most frequently detected, followed by MRSA (17.5%, [2,139]) and Enterococcus (15.8%, [1,939]). On the other hand, vancomycin-resistant Enterococcus (1.8%, [225]) and S. pneumoniae (2.6%, [324]) were the least detected isolates. Streptococcus species other than S. pneumoniae accounted for 6.3% (778), with S. agalactiae predominating (595 isolates) (Figure 2).
Figure 2.
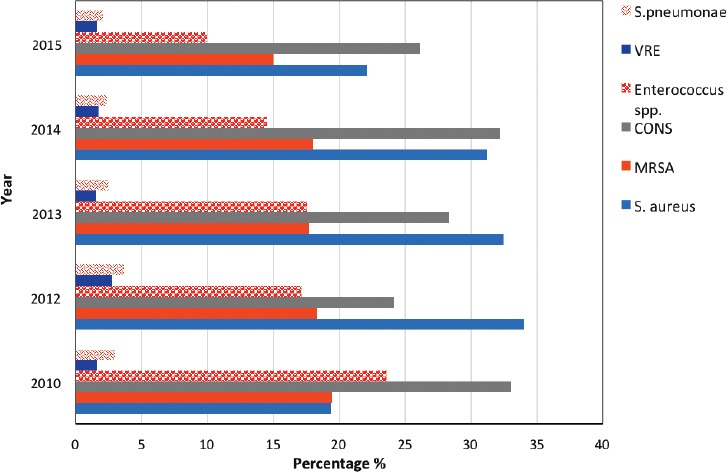
The percentages of the gram-positive isolates. S. pneumoniae - Streptococcus pneumoniae, MRSA - Methicillin-resistant S. aureus, S. aureus - Staphylococcus aureus, VRE - Vancomycin-resistant enterococci, CONS - coagulase negative staphylococcus
Among ESBLs, the total number of isolates of E. coli, K. pneumoniae, and Proteus mirabilis were 20.8%, (8,177), with the number gradually increasing over time (p<0.001) (Figure 3). Klebsiella pneumoniae carbapenemase-producer isolates constituted 0.28% (n=21), with the number of isolates gradually increasing over the study period (from one isolate in 2010 to 8 isolates in 2015).
Figure 3.
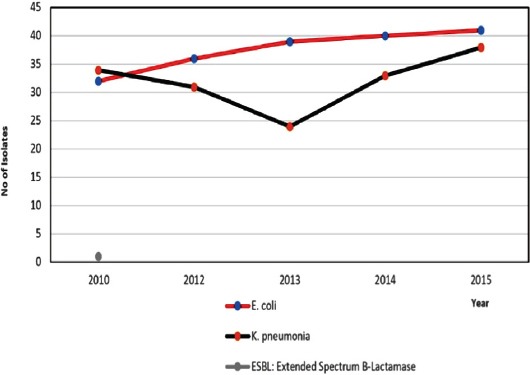
The Trend of the Extended-Spectrum Beta-Lactamase-producing isolates over the study period. E. coli - Escherichia coli, K. pneumoniae - Klebsiella pneumoniae
Susceptibility patterns
Staphylococcus aureus methicillin susceptible (MSSA) was 100% susceptible to oxacillin, cefazolin, and vancomycin, whereas low resistance rates were observed for clindamycin (94%) and trimethoprim/sulfamethoxazole (TMP-SMX) (87%-90%). Methicillin-resistant Staphylococcus aureus -susceptibility rate gradually improved to clindamycin (52%-70%, p<0.01), TMP-SMX (60%-68%, p=0.014), and erythromycin (52%-65%, p<0.01) over the study period. (Figure 4).
Figure 4.
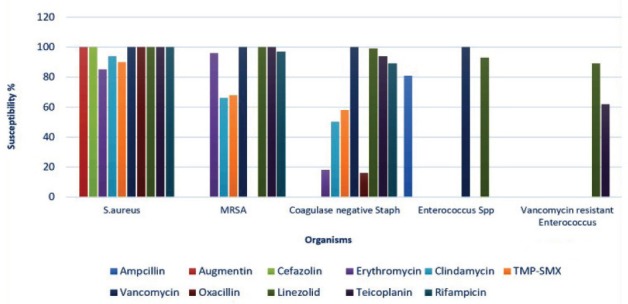
The mean antibiotic susceptibility of the gram-positive isolates. S. aureus - Staphylococcus aureus MRSA - Methicillin-resistant S. aureus, Enterococcus sp - Enterococcus species, TMP-SMX - Trimethoprim - Sulfamethoxazole
The susceptibility of S. pneumoniae to penicillin gradually improved from 66%-100%, which is statistically significant (p<0.001). The susceptibility of S. pneumoniae to ceftriaxone was 100% for 3 consecutive years (Figure 5).
Figure 5.
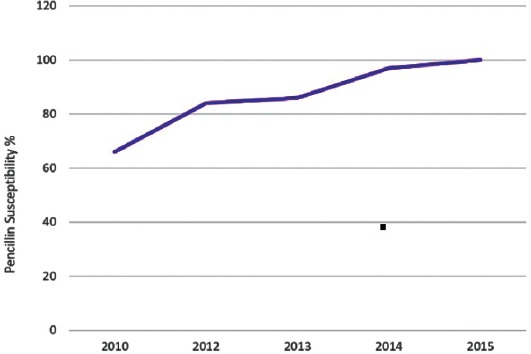
Streptococcus pneumoniae susceptibility to penicillin over a 5-year period.
Escherichia coli was 100% susceptible to meropenem, and its susceptibility to piperacillin-tazobactam and amikacin was excellent (90%-99%). Susceptibility to ciprofloxacin, ceftazidime, and cefepime gradually declined (ranged from 64% to 58%, p=0.01). Pseudomonas aeruginosa susceptibility for meropenem declined over the 5-year period from 82% to 72% (p<0.001). Its susceptibility to piperacillin-tazobactam was almost static (83%–85%, p=0.10). Susceptibility to ceftazidime ranged from 68% to 76 %,(p<0.01) and amikacin susceptibility remained the best all throughout the study period (89%-94%, p<0.01). For K. pneumoniae, carbapenem and amikacin showed the higher susceptibility pattern; for example, 92%-100% and 92%-97% respectively. Susceptibility rate to piperacillin-tazobactam and ciprofloxacin remained static (72%-86%), and resistance rate to cephalosporin gradually increased. Acinetobacter baumannii susceptibility to colistin was high (97%-99%), whereas susceptibility to tigecycline gradually declined from 65% to 39% (p<0.01). (Figure 6).
Figure 6.
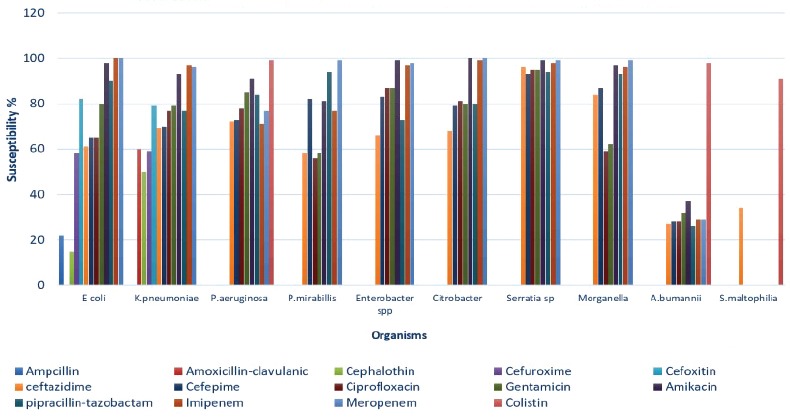
The mean antibiotic susceptibility of the gram-negative isolates. E. coli - Escherichia coli, K. pneumoniae - Klebsiella pneumoniae, P. aeruginosa - Pseudomonas aeruginosa, P. mirabilis - Proteus mirabilis, A. baumannii - Acinetobacter baumannii, S. maltophilia - Stenotrophomonas maltophilia
Discussion
The global action plan to fight AMR adopted by the WHO sets several objectives and recommendations to enhance antibiotic surveillance and research, to strengthen knowledge and improve the awareness regarding AMR, to optimize the use of antimicrobial agents, and to decrease the rate of infections.2 Our study is in line with these recommendations. Gram-negative isolates predominated in our results, with E. coli being the most frequent isolate, followed by P. aeruginosa and K. pneumoniae. Our results were close to what was reported by Amer et al,4 who reported an increasing in resistance rate of gram negative isolates. Our data are in agreement with a review of 45 published articles addressing the AMR prevalence in the gulf corporation countries over 2 decades. Escherichia coli, K. pneumoniae, and P. aeruginosa were the most prevalent agents; however, MRSA rate was higher in our present study at 17.5% compared to 5.4% in theirs.5
A recent survey conducted in a rehabilitation center showed similar results with regard to the most frequently detected organisms, but the susceptibility patterns varied. For example, in the previous study, Pseudomonas was most susceptible to ciprofloxacin and gentamicin, whereas in our study, amikacin and piperacillin-tazobactam had higher susceptibility rates.6 The noted reduction in ceftazidime and meropenem susceptibilities to gram-negative organisms, especially Pseudomonas, is likely because of selection pressure. On the other hand, piperacillin-tazobactam susceptibility remained static, and amikacin had the best susceptibility profile.
Isolates of vancomycin-resistant Enterococcus, carbapenemase-producing K. pneumoniae, and ESBLs tended to increase over the study period; this result is an alarming signal that strict policies for infection control transmission-based precautions and antimicrobial prescriptions need to be followed; moreover, these data need to be taken into consideration when selecting empirical treatment for critically ill patients.
Streptococcus pneumoniae is an important gram-positive organism that causes invasive diseases in children <5 years of age with substantial morbidity and mortality. Before 1967, S. pneumoinae was almost uniformly susceptible to penicillin. The emergence of resistance gradually increased worldwide over time.7 Alteration in the serotype distribution along with the susceptibility patterns has been observed after the introduction of the pneumococcal conjugate vaccines, particularly, post the 7-valent in 2000 and 13-valent in 2010. PCV7 was incorporated into the national immunization program in Saudi Arabia in 2009 followed by PCV13 in 2013. In the results of the present study, there was a significant and steady increase in penicillin susceptibility, with an increase from 66% in 2010 to 100% in 2015. Moreover, S. pneumoniae was the second least detected gram-positive organism at only 2.6%.
In a national prospective surveillance performed in 12 hospitals from 7 different regions in Saudi Arabia between 2007 and 2009, 78% isolates were found to be multidrug resistant. Penicillin susceptibility was observed in only 30% isolates, yet all isolates were sensitive to ceftriaxone.8 In another study published in 2015, recruited data between 2005 and 2010 to assess the antibiotic resistance and serotype differences before and after PCV7 conjugate vaccine administration showed that 66% isolates were resistant to penicillin and 62% were resistant to erythromycin. It was noted that penicillin susceptibility was 54.6% immediately after switching to PCV13.9,10 Same findings were noted in a study conducted at Twam Hospital in the United Arab Emirates over an 8-year period from 2004 to 2011.11
Unfortunately, there are no reports of recent surveillance data in Saudi Arabia to assess the antimicrobial susceptibility trends after PCV13 administration. However, a survey conducted in 4 Gulf and near East countries between 2011 and 2013 showed the penicillin susceptibility was variable, with >85% overall susceptibility and 100% ceftriaxone susceptibility in all countries.12 A similar study conducted in Kuwait to evaluate the impact of PCV7 and PCV13 over a 10-year period documented a drop in penicillin resistance from 67% to 46%.13 A systemic review and meta-analysis study published in 2017 analyzed 68 studies and surveillance data from 2000 to 2015 to define the serotypes causing IPD after PCV vaccination. They found a reduction in IPD caused by vaccine serotypes, but nonvaccine serotypes predominated as the cause of IPD (replacement disease).14
To examine the impact of PCV on antibiotic resistance, several studies have been conducted in different countries, and their results were encouraging.15-17 One study was conducted to compare susceptibility between pre-vaccination and post-vaccination eras (in the 2007–2009 and 2010–2014 periods) for S. pneumoniae isolates from patients with otitis media. They showed significant improvement in penicillin susceptibility, from 37% in the pre-PCV13 vaccination period to 51% in the transitional period and to 100% in the post-PCV13 vaccination period. Ceftriaxone susceptibility also improved from 95% to 100%.15
In line with these studies, our results showed improved penicillin susceptibility. This finding reflects the effect of the post-vaccination era, as it is highly documented that vaccination leads to a dramatic decrease in the nasopharyngeal carriage rate and IPD by vaccine serotypes in children, in addition to the development of herd immunity in adults.14 Moreover, these findings might lead to major changes in empirical antimicrobial selection when coverage of S. pneumoniae is needed. Physicians may reassess the needs for the previously used practice of broad-spectrum coverage to a narrower-spectrum antibiotic. This practice is noted in the recently published guideline for community-acquired pneumonia endorsed by the Saudi Pediatric Infectious Disease Society, when experts decided to choose ampicillin as initial therapy for treating uncomplicated pneumonia.18
The exact prevalence of MRSA, either community-acquired (CA-MRSA) or hospital-acquired (HA-MRSA) infections, in Saudi Arabia is unknown despite extensive studies. In one review, it was estimated to be 35.6% with great variations among regions. Another study reported an MRSA rate of 23.2% among S. aureus carriers.19,20 However, it is estimated by the Center of Disease Control’s report on MRSA tracking that 33% of people are typically colonized with Staphylococcus and 2 in 100 carry MRSA.21
In our present data, S. aureus was the second most common gram-positive isolate (27.5%), among which 17.5% isolates were MRSA (defined as oxacillin resistant S. aureus (MIC >4 in Mueller-Hinton agar). The calculated rate of MRSA among S. aureus was 39%. It is difficult to extrapolate the CA-MRSA rate based on antibiograms alone, because it is distinctively different from HA-MRSA both genetically and phenotypically. Infection control (IC) policies have a fundamental role in decreasing HA-MRSA rates, although the effectiveness of active surveillance in HA-MRSA prevention is still controversial.22-24 A recent study documented that early identification of MRSA using rapid diagnostic technologies (PCR) along with timely implementation of infection control strategies have a great financial impact.25
At KFMC, the IC department designed an MRSA-prevention program, which includes 2 components, preemptive screening of at-risk patients and implementation of strict transmission-based precautions for the tested positive patients. There was a reduction in HA-MRSA, from 0.17 case per 1000 to 0.03 case per 1000 between years 2007 to 2009.26 Wider surveillance is needed in the future to determine the rates of CA-MRSA and HA-MRSA, because it has a direct effect on the empirical therapy of skin and soft-tissue infections as well as osteoarticular infections.
The infection rates of ESBLs, such as E. coli, K. pneumoniae, and P. mirabilis are increasing. These infections are usually associated with longer hospital stays, increased costs, and worse outcomes if they are not anticipated early and treated, especially in neonates. Therefore, we aimed to assess their trends during the study period. The overall percentage of total ESBLs of the 3 most common isolates (E. coli, K. pneumoniae, and P. mirabilis) including bloodstream and urine infections, was estimated to be 20.8%, with a steadily increasing frequency over the years from 32% to 41% per year for E. coli.
In comparison, a systemic review and meta-analysis of bloodstream infections caused by ESBLs showed the overall percentage was 9%, with a 3% annual increase. The higher prevalence and mortality rates were observed in neonates. In Saudi Arabia, it was noted to range between 6% and 38.5% in different regions.27,28 The risk of increasing rates of ESBLs is significant and alarming; it needs to be considered in the empirical coverage of bloodstream and urinary tract infections, especially in critically ill patients.
In light of the above mentioned findings, particularly, with regard to S. pneumoniae susceptibility, lack of exact prevalence of certain organisms such as MRSA, and the rising trends of the MDRO, further research and multi-center surveillance studies are extremely needed to tackle the antimicrobial resistance.
Study limitations
This is a single-center study. The inclusion of duplicate isolates might affect the specificity of the antibiograms. Although antibiograms are essential in monitoring the trends of resistance, they cannot track AMR during therapy.
In conclusion, antimicrobial surveillance is an important tool in assessing the AMR burden. Nationwide surveillance is urgently needed to provide policymakers with essential information to guide proper action plans. The exclusion of duplicate isolates will improve the specificity of antibiograms. Unit-specific antibiograms and incorporating patient’s data are more beneficial in making informed decisions about optimal empirical treatment.
The observed improvement of S. pneumoniae susceptibility to penicillin is significant and supported by other findings in other studies; however, countrywide surveillance is warranted to assess the overall susceptibility patterns. These findings will affect the choice of empirical therapy in the future. On the contrary, the resistant gram-negative organisms are becoming a major threat that affects the quality of patient care and necessitates strict antimicrobial stewardship to track the resistance and optimize antibiotic usage.
Footnotes
References
- 1.O'Neill J. Antimicrobial resistance:tackling a crisis for the health and wealth of nations. The Review on Antimicrobial Resistance. 2014:20. [Google Scholar]
- 2.World Health Organization. Antimicrobial resistance:global report on surveillance. Geneva: World Health Organization; 2014. [Google Scholar]
- 3.Joshi S. Hospital antibiogram:a necessity. Indian J Med Microbiol. 2010;28:277–280. doi: 10.4103/0255-0857.71802. [DOI] [PubMed] [Google Scholar]
- 4.Al Johani SM, Akhter J, Balkhy H, El-Saed A, Younan M, Memish Z. Prevalence of antimicrobial resistance among gram-negative isolates in an adult intensive care unit at a tertiary care center in Saudi Arabia (2010-2014) Ann Saudi Med. 2010;30:364–369. doi: 10.4103/0256-4947.67073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aly M, Balkhy HH. The prevalence of antimicrobial resistance in clinical isolates from Gulf Corporation Council countries. Antimicrob Resist Infect Control. 2012;1:26. doi: 10.1186/2047-2994-1-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaudhry LA, Al-Tawfiq JA, Zamzami MM, Al-Ghamdi SA, Robert AA. Antimicrobial susceptibility patterns:a three-year surveillance study in a rehabilitation setting. Pan Afr Med J Pan. 2016;23:214. doi: 10.11604/pamj.2016.23.214.8474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tomasz A. Antibiotic resistance in Streptococcus pneumoniae. Clin Infect Dis. 1997;24:S85–S88. doi: 10.1093/clinids/24.supplement_1.s85. [DOI] [PubMed] [Google Scholar]
- 8.Almazrou Y, Shibl AM, Alkhlaif R, Pirçon JY, Anis S, Kandeil W, et al. Epidemiology of invasive pneumococcal disease in Saudi Arabian children younger than 5 years of age. J Epidemiol Glob Health. 2016;6:95–104. doi: 10.1016/j.jegh.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shibl AM, Memish ZA, Al-Kattan KM. Antibiotic resistance and serotype distribution of invasive pneumococcal diseases before and after introduction of pneumococcal conjugate vaccine in the Kingdom of Saudi Arabia (KSA) Vaccine. 2012;30:G32–G36. doi: 10.1016/j.vaccine.2012.07.030. [DOI] [PubMed] [Google Scholar]
- 10.Alharbi NS, Al-Barrak AM, Al-Moamary MS, Zeitouni MO, Idrees MM, Al-Ghobain MO, et al. The Saudi thoracic society pneumococcal vaccination guidelines-2016. Ann Thorac Med. 2016;11:93. doi: 10.4103/1817-1737.177470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tariq Z. Changes in susceptibility pattern of streptococcus pneumonia at Tawam Hopsital in Al Ain, United Arab Emirates durin (2004-2011) Pakistan Armed Forces Medical Journal. 2016;(66):14–21. [Google Scholar]
- 12.Jamsheer A, Rafay AM, Daoud Z, Morrissey I, Torumkuney D. Results from the survey of antibiotic resistance (SOAR) 2011-13 in the gulf states. 2016. J Antimicrob Chemother. 2016;71:i45–i61. doi: 10.1093/jac/dkw064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mokaddas E, Albert MJ. Serotype distribution and penicillin-non-susceptibility of Streptococcus pneumoniae causing invasive diseases in Kuwait:A 10-year study of impact of pneumococcal conjugate vaccines. Expert Rev Vaccines. 2016;15:1337–1345. doi: 10.1080/14760584.2016.1198698. [DOI] [PubMed] [Google Scholar]
- 14.Balsells E, Guillot L, Nair H, Kyaw MH. Serotype distribution of Streptococcus pneumoniae causing invasive disease in children in the post-PCV era:A systematic review and meta-analysis. PloS One. 2017;12:e0177113. doi: 10.1371/journal.pone.0177113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jabarin B, Pitaro J, Lazarovitch T, Gavriel H, Muallem-Kalmovich L, Eviatar E, et al. Decrease in pneumococcal otitis media cultures with concomitant increased antibiotic susceptibility in the pneumococcal conjugate vaccines era. Otol Neurotol. 2017;38:853–859. doi: 10.1097/MAO.0000000000001419. [DOI] [PubMed] [Google Scholar]
- 16.Angoulvant F, Cohen R, Doit C, Elbez A, Werner A, Béchet S, et al. Trends in antibiotic resistance of streptococcus pneumoniae and haemophilus influenzae isolated from nasopharyngeal flora in children with acute otitis media in France before and after 13 valent pneumococcal conjugate vaccine introduction. BMC Infect Dis. 2015;15:236. doi: 10.1186/s12879-015-0978-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Richter SS, Diekema DJ, Heilmann KP, Dohrn CL, Riahi F, Doern GV. Changes in pneumococcal serotypes and antimicrobial resistance after introduction of the 13-valent conjugate vaccine in the United States. Antimicrob Agents Chemother. 2014;58:6484–6489. doi: 10.1128/AAC.03344-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alzomora O, Alhajjarb S, Aljobairc F, Alenizid A, Alodyanie A, Alzahran M, et al. Management of community-acquired pneumonia in infants and children:Clinical practice guidelines endorsed by the Saudi Pediatric Infectious Diseases Society. International Journal of Pediatrics and Adolescent Medicine. 2017;(4):153–158. doi: 10.1016/j.ijpam.2017.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.AL Yousef SA, Mahmoud SY, Taha EM. Prevalence of Methicillin-Resistant Staphylococcus aureus in Saudi Arabia:systemic review and meta-analysis. African Journal of Clinical and Experimental Microbiology. 2013;14:146–154. [Google Scholar]
- 20.Alaklobia F, Aljobaira F, Alrashodb A, Alhababic R, Alshamrania M, Alaminb W, et al. The prevalence of community-associated methicillin-resistant Staphylococcus aureus among outpatient children in a tertiary hospital:A prospective observational study in Riyadh, Saudi Arabia. International Journal of Pediatrics and Adolescent Medicine. 2015;2:136–140. doi: 10.1016/j.ijpam.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention. [[Update 2017;Accessed 2018 May 30 May]];MRSA Tracking. Available from: https://www.cdc.gov/mrsa/tracking/index.html . [Google Scholar]
- 22.Lee BY, Bailey RR, Smith KJ, Muder RR, Strotmeyer ES, Lewis GJ, et al. Universal methicillin-resistant Staphylococcus aureus (MRSA) surveillance for adults at hospital admission:an economic model and analysis. Infect Control Hosp Epidemiol. 2010;31:598–606. doi: 10.1086/652524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lucet JC, Paoletti X, Lolom I, Paugam-Burtz C, Trouillet JL, Timsit JF, et al. Successful long-term program for controlling methicillin-resistant Staphylococcus aureus in intensive care units. Intensive Care Med. 2005;31:1051–1057. doi: 10.1007/s00134-005-2679-0. [DOI] [PubMed] [Google Scholar]
- 24.West TE, Guerry C, Hiott M, Morrow N, Ward K, Salgado CD. Effect of targeted surveillance for control of methicillin-resistant Staphylococcus aureus in a community hospital system. Infect Control Hosp Epidemiol. 2006;27:233–238. doi: 10.1086/500372. [DOI] [PubMed] [Google Scholar]
- 25.Hübner C, Hübner NO, Wegner C, Flessa S. Impact of different diagnostic technologies for MRSA admission screening in hospitals-a decision tree analysis. Antimicrob Resist Infect Control. 2015;4:50. doi: 10.1186/s13756-015-0093-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghazal SS, Hakawi AM, Demeter CV, Joseph MV, Mukahal MA. Intervention to reduce the incidence of healthcare-associated methicillin-resistant Staphylococcus aureus infection in a tertiary care hospital in Saudi Arabia. Infect Control Hosp Epidemiol. 2011;32:411–413. doi: 10.1086/659256. [DOI] [PubMed] [Google Scholar]
- 27.Flokas ME, Karanika S, Alevizakos M, Mylonakis E. Prevalence of ESBL-producing Enterobacteriaceae in pediatric bloodstream infections:a systematic review and meta-analysis. PloS One. 2017;12:e0171216. doi: 10.1371/journal.pone.0171216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zowawi HM, Balkhy HH, Walsh TR, Paterson DL. β-Lactamase production in key gram-negative pathogen isolates from the Arabian Peninsula. Clin Microbiol Rev. 2013;26:361–380. doi: 10.1128/CMR.00096-12. [DOI] [PMC free article] [PubMed] [Google Scholar]


