Abstract
Objectives:
To determine the role of G128C and C218T variants in ABCC1 gene with the risk of developing colon cancer in Jeddah, Kingdom of Saudi Arabia.
Methods:
This case-control study was conducted on 51 colon cancer patients and 65 controls from King Abdulaziz University Hospital and King Abdullah Medical City in the period from January 2015 to April 2017, and was approved by the Unit of Biomedical Ethics (no: 261-15). Experiments were performed in the experimental biochemistry unit at King Fahd Medical Research Center. The genotype distributions and allele frequencies were determined by polymerase chain reaction-restriction fragments length polymorphism (PCR-RFLP) and DNA sequencing. A Chi-square test was used to determine allele and genotype distributions, odds ratio (OR), risk ratio (RR) and 95% confidence intervals (CI). P-values of <0.05 were considered statistically significant.
Results:
The results showed a novel association between heterozygous (CT) genotype for variant C218T and increased risk of colon cancer [OR=3.4, 95% CI (1.56-7.48), and RR=1.92, 95% CI (1.26-2.93), p=0.002]. These ratios were correlated with high-grade stages (III and IV). In contrast, for variant G128C, there was no significant association with the risk of developing colon cancer.
Conclusion:
The novel findings of the study revealed that the CT genotype of variant C218T in ABCC1 gene may increase the risk of developing colon cancer.
Colon cancer is one of the most common cancers worldwide and is characterized by a high mortality rate. In the Kingdom of Saudi Arabia (KSA), this type of cancer represents the first most common cancers among men and the third among women.1 Although the number of colon cancer patients in the KSA is considered low, compared to other countries, there has been progressive increase over the last 20 years.2 Genetic and environmental factors were found to increase the likelihood of developing colon cancer in KSA.3 Generally, the application of chemotherapy in cancer treatments is controlled by several factors, such as sensitivity to chemotherapeutic drugs used for treatment.4 One of the mechanisms that affect the individual’s sensitivity to drugs and subsequently increases the risk of developing cancer is the expression of drug transporters on the surface of cancer cells.5 Adenosine Triphosphate (ATP) binding cassette (ABC) transporters play an essential role in the development of many diseases, including cancers, through the mechanism of drug resistance. Adenosine Triphosphate binding cassette transporters contain 7 subgroups (ABCA to ABCG).5,6 Nine out of 13 members of the ABCC subfamily are involved in drug resistance.7 ABCC1 or MRP1 was the first identified member of the ABCC subfamily in a drug-resistant cell line (small cell lung cancer).8 The ABCC1 gene contains 31 exons, which are translated into 1,531 amino acids protein with a molecular weight of 190-kDa.9 The expression of genetic variants, either mutations or single nucleotide polymorphisms (SNPs), in the ABCC1 gene showed a high degree of variability among populations, which might affect the individuals’ responses to drugs significantly. Some of these data showed that genetic variability in ABCC1 gene could predict the toxicity in patients with breast cancer.10 Moreover, genetic mutations in ABCC1 gene, in conjugation with variants of another ABC transporter member, ABCB1, can increase the chance of developing lung cancer.11 However, fewer studies that correlate ABCC1 genetic variations with the development of colon cancer were performed. Therefore, more studies should be conducted to show the correlation between genetic variations in the ABCC1 gene and the risk of colon cancer or to confirm the preliminary observations. As far as we are aware, this is the first study that aims to determine the effect of 2 major SNPs (G128C and C218T) in the ABCC1 gene on the risk of colon cancer development in the KSA.
Methods
Subjects and samples
This case-control study was conducted on 116 participants, comprising colon cancer patients (n=51) and healthy controls (n=65) who visited the oncology centers and blood banks in King Abdullah Medical City and King Abdulaziz University Hospital (KAUH) in Jeddah in the period from January 2015 until April 2017. The study was approved by the Unit of Biomedical Ethics at the Faculty of Medicine in King Abdulaziz University (KAU), Jeddah, KSA (No:261-15). This study was conducted according to the principles of the Declarations of Helsinki in dealing with patients’ information, samples, and results. The inclusion criteria of the patients were as follows: all patients were from Saudi ethnicity, age ranged (30-80), recently diagnosed with histopathology to have colon cancer at any stage, agreed to participate and to give blood sample, and most importantly agreed to proceed for any future harmless investigations, if required. The exclusion criteria for the patients were mostly focused on excluding any metastatic cancer patients and involving only localized cancer in colon. Regarding the inclusion criteria for the controls, they were from Saudi origin matched by age and gender with the colon cancer patients, having no history of colon cancer or any other type of cancers, and they must be selected randomly from blood bank units, whereas, the most exclusion criterion was excluding any control under certain diet or taking any medications during sample collection period. After participants read and signed an informed consent form, 5ml whole blood sample was collected in an ethylenediaminetetraacetic acid (EDTA) tube. Genomic deoxyribonucleic acid (gDNA) was then extracted from the peripheral blood leukocytes using a QIAamp DNA Mini kit (QIAGEN, Hilden, Germany) according to the manufacturer’s instructions. The concentration and quality of each extracted gDNA sample was assessed by measuring the absorbance at 2 wavelengths (260 and 280nm) on a NanoDrop™ 2000/2000c Spectrophotometer. The practical work was carried out in the experimental biochemistry unit at King Fahd Medical Research Center at King Abdulaziz University, Jeddah, KSA.
Genotyping SNPs G128C and C218T in ABCC1 gene
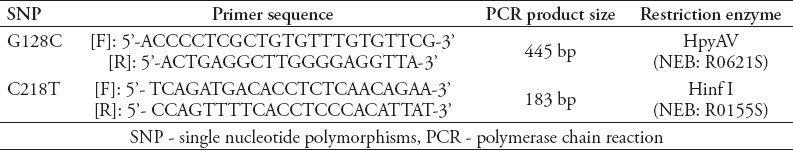
The several genotypes of SNPs G128C and C218T in the ABCC1 gene were determined using a polymerase chain reaction-restriction fragments length polymorphism (PCR-RFLP) assay. The genotypes then were confirmed by a DNA sequencing. The PCR primers and conditions that were used to amplify exon 2 were designed by Dr. Ann Yezerski (for SNP G128C) or, as described previously, by Yin et al12 (for SNP C218T) (Table 1). For a 25 µl PCR reaction, 1 µl genomic DNA template (50 ng), 12.5 µl USB® HotStart-IT® FideliTaq™ PCR Master Mix (2X) (Affymetrix/USB™, product number 71156), 9.5 µl RNase free water (Affymetrix/USB™, product number 7732-18-5), and 1 µl of each primer (0.2 µmol) were used. The PCR condition was as follows: initial denaturation at 95ºC for 5 min. This was followed by 25 cycles of denaturation at 95+C for 30 sec, annealing at 59+C for 30 sec (for G128C) or at 62+C for 30 sec (for C218T), and extension at 72+C for 1 min. A final extension was run at 72+C for 5 min before the PCR products were separated on 2% agarose gel and visualized after ethidium bromide staining. The genotype analysis was performed with RFLP and DNA-sequencing.
Table 1.
Polymerase chain reaction-restriction fragments length polymorphism conditions for SNPs G128C and C218T in ABCC1 gene.
Statistical analysis
A χ2 test in GraphPad Prism software version 7.00 (San Diego, California, USA) was used to compare the allele frequency, genotype distribution, odds ratio (OR), risk ratio (RR) and 95% confidence interval (CI) of each variant with what was expected for a population in the Hardy-Weinberg equilibrium model. The p-values of <0.05 were considered statistically significant.
Results
Genotype distribution and allele frequency of ABCC1 G128C in colon cancer and control subjects
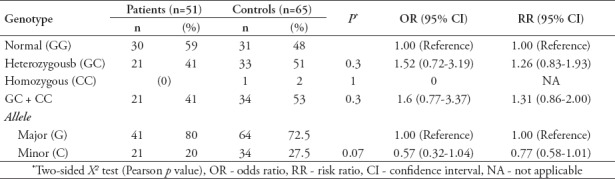
Polymerase chain reaction-restriction fragments length polymorphism and DNA-sequencing analyses showed that among the colon cancer patients (n=51), 59% (n=30) were normal (GG), 41% (n=21) were heterozygous (GC), and 0% (n=0) were homozygous (CC). Moreover, among the patients group, the G allele frequency was 80% and the C allele frequency was 20%. However, genotype analysis for the control group (n=65) showed that, 48% (n=31) were normal (GG), 51% (n=33) were heterozygous (GC), and 2% (n=1) were homozygous (CC). Among the control group, the frequency of G allele was 72.5% and the frequency of C allele was 27.5%. Although the comparison between patients and controls carrying the heterozygous genotype showed a slightly increased odds ratio [OR=1.5, 95% CI (0.72-3.19)], the difference was non-significant (p=0.30). Patients were within Hardy-Weinberg equilibrium whereas the controls were out of Hardy-Weinberg equilibrium. (For patients: χ2=3.55 (0.1<p<0.05) and for controls: χ2=6.05 (0.02<p<0.01); degree of freedom (DF)=1, according to MedCalc χ2 distribution table values) (Table 2).
Table 2.
Genotypic and allelic frequencies of ABCC1 gene G128C in patients and controls.
Genotype distribution and allele frequency of ABCC1 C218T in colon cancer and control subjects
Polymerase chain reaction-restriction fragments length polymorphism and DNA sequencing analyses showed that among the colon cancer patients (n=51), 60.8% (n=31) were normal (CC), 39.21% (n=20) were heterozygous (CT), and 0% (n=0) were homozygous (TT). The C allele frequency was 81% and the T allele frequency was 19%. However, genotype analysis for the control group (n=65) showed that 29.23% (n=19) were normal (CC), 64.62% (n=42) were heterozygous (CT), and 6.15% (n=4) were homozygous (TT). Regarding the allele frequency, the frequency of C allele among the control group was 61.5% and the T allele was 38.5%. Patients group distribution was within Hardy-Weinberg equilibrium (χ2=2.28, DF=1, 0.2<p<0.1), whereas, controls group was out Hardy-Weinberg equilibrium (χ2=8.6, DF=1, and 0.005<p<0.002) (Table 3).
Table 3.
Genotypic and allelic frequencies of ABCC1 gene C218T in patients and controls.
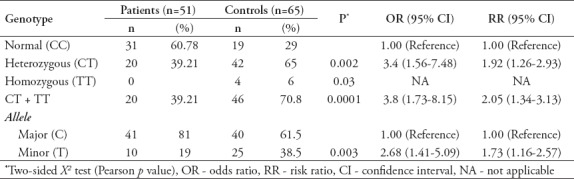
Interestingly, comparing patients and controls carrying heterozygous (CT) genotype with subjects carrying wild genotype (CC) showed a high degree of risk, as evidenced by the calculated OR and RR [OR=3.4, 95% CI (1.56-7.48), and RR=1.92, 95%CI (1.26-2.93), p=0.002]. Combining the heterozygous and homozygous (CT+TT) genotypes increased the risk more rapidly compared to the heterozygous (CT) or homozygous genotype alone (TT) [OR=3.8, 95%CI (1.73-8.15), and RR=2.05, 95% CI (1.34-3.13), p=0.0001]. These values indicated that the heterozygous (CT) genotype in ABCC1 SNP C218T might play an important role in increasing the risk of developing colon cancer (Table 3).
Contribution of genotype distributions of SNPs G128C and C218T in ABCC1 with clinical stages
In this research, the TNM staging system was used to classify the extent of the disease to the different classes (0-IV). Regarding the correlation among the 3 genotypes of SNP G128C with clinical stages of colon cancer, the genotypes in the (n=51, 100%) colon cancer patients were as follows: for stage I (T1 or T2, N0, M0), n=3, 5.88% were wild type (GG) and heterozygous (GC). For stage II (T3-4, N0, M0), n=5, 9.8% were wild type (GG) and n=4, 7.84% were heterozygous (GC). For stage III (T1-4, N1-4, M0), n=10, 19.61% were wild type (GG) and n=2, 3.92% were heterozygous (GC). Finally, for stage IV (Any T, any N, M1), n=12, 23.53% were wild (GG) and heterozygous (GC). None of the patients at any stage were classified as homozygous genotype (CC). Regarding SNP C218T, the (n=51, 100%) colon cancer patients were as follows: for stage I (T1 or T2, N0, M0), n=6, 11.76% were wild type (CC) and none of them were heterozygous (CT) or homozygous (TT). For stage II (T3-4, N0, M0), n=3, 5.88% were wild type (CC), n=6, 11.76% were heterozygous (CT), and none of them were homozygous (TT). For stage III (T1-4, N1-4, M0), n=8, 15.69% were wild type (CC), n=4, 7.84% were heterozygous (CT), and none of them were homozygous (TT). Finally, for stage IV (any T, any N, M1), n=14, 27.45% were wild (CC), n=10, 19.61% were heterozygous (CT), and none of them were homozygous (TT). As shown by the percentages, most of the patients who had heterozygous (GC) or (CT) genotype, were classified clinically in stages III and IV. Moreover, there was a remarkable increase in cases number as the clinical pathological features (prognosis) of the disease increased (stages III and IV), which might indicate that heterozygous genotype, particularly in SNP C218T, contributes to an increased risk of colon cancer development.
Discussion
Colon cancer is one of the most aggressive tumors in the world. According to the latest statistical incidence report by the International Agency for Research on Cancer (IARC), colon cancer is considered the third most common cancer in men and the second most common cancer in women.13 Although advanced diagnostic methods helped in understanding the biology of this cancer, it is still characterized by a high mortality rate. Understanding the personalized genetic map for each patient will help in developing the most appropriate treatment for its case. Drug transporters such as ABC transporters were found to alter the response of the patient to the treatment either positively or negatively according to the different factors that affect the expression and function of that transporter. Unlike other ABC transporters, the preliminary findings on the role of ABCC1 transporter in cancer drug resistance, patient survival rate, and other clinicopathologic features of cancer are insufficient, and therefore, need more studies to confirm this relation.4 The current study was the first study that evaluates the genotypes distribution and alleles frequency of 2 genetic variants (G128C and C218T) in ABCC1 gene in colon cancer patients and compared them to healthy control subjects, to determine their effects on the risk of colon cancer development.
Sequencing of the whole ABCC1 gene identified several genetic variants (mutations and SNPs). However, the exact effect of these variants is inconsistent, which makes it difficult to determine the exact role of these variants in cancer. The non-synonymous SNPs G128C and C218T have been studied in many diseases and to a lesser extent in cancer. Most of these studies, were interested in determining the SNPs’ role in drug resistance rather than their ability to increase the risk of the disease.12,14,15 In our study, regarding SNP G128C, we found that most of the patients were carrying the normal genotype (GG), followed by the heterozygous (GC) genotype, and none of them carried the homozygous genotype (CC). When the genotype distribution and allele frequency for this SNP were compared to the control group, the calculated odds ratio (OR) and risk ratio (RR) showed that this SNP is not significantly associated with increased risk of colon cancer development in our study. However, there was a borderline risk association, which might become significant with a higher number of samples. In contrast, heterozygous (CT) genotype in SNP C218T was found to increase the risk of colon cancer development 3 times over. In agreement with our finding, a study performed on blood samples of Australian patients with neuroblastoma, found that SNP G128C was not associated with patient outcome and expressed at a very low rate and therefore could not be used as a prognostic marker for neuroblastoma.16 Another study done on 540 breast cancer patients found also that SNPs G128C and C218T had no effect on ABCC1 expression, which indicates that the amino acid substitution that resulted from those 2 SNPs may not affect the function of the final protein.17 Moreover, Zhang et al18 found that SNPs G128C and C218T are not associated with drug resistance in Chinese lung cancer patients, whereas, subjects carrying homozygous (AA) genotype of SNP G2168A in the ABCC1 gene had a higher risk ratio (RR=3.4 folds) when compared to wild type subjects in the Chinese population.
In conclusion, the current study identified a novel relationship between heterozygous (CT) genotype of SNP C218T and increased risk of colon cancer in Saudi Arabia. Interestingly, this genotype is associated with the high-grade colon cancer stages (stages III and IV); therefore, it can be used as a prognostic marker for patients with advanced colon cancer. However, more investigations are needed to confirm this finding on a larger population and on human colon cancer tissues.
Study limitations
This study has a number of limitations, most importantly, the small number of population used. Therefore, we recommend to repeat the experiments on larger number of samples or on cancer tissues and using other techniques such as next generation sequencing or immunohistochemistry to reveal the expression of different genetic variants on the ABCC1 gene which might contribute to colon cancer tumorigenesis.
Acknowledgment
This article contains the results and findings of a research project that is funded by King Abdulaziz City for Science and Technology (KACST), Riyadh, Saudi Arabia. Grant No. LGP-36-15. We would like also to thank Scribendi (www.scribendi.com) for English language editing.
Footnotes
References
- 1.Saudi Cancer Registry. KSA:Saudi Cancer Registry. Saudi Arabia: 2017. Cancer incidence report (2014) pp. 1–82. https://nhic.gov.sa/eServices/Documents/2014.pdf . [Google Scholar]
- 2.Alsanea N, Abduljabbar AS, Alhomoud S, Ashari LH, Hibbert D, Bazarbashi S. Colorectal cancer in Saudi Arabia:incidence, survival, demographics and implications for national policies. Ann Saudi Med. 2015;35:196–202. doi: 10.5144/0256-4947.2015.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zubaidi A, AlSubaie N, AlHumaid A, Shaik S, AlKhayal K, AlObeed O. Public awareness of colorectal cancer in Saudi Arabia:A survey of 1070 participants in Riyadh. Saudi J Gastroenterol. 2015;21:78–83. doi: 10.4103/1319-3767.153819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Panczyk M. Pharmacogenetics research on chemotherapy resistance in colorectal cancer over the last 20 years. World J Gastroenterol. 2014;20:9775–9827. doi: 10.3748/wjg.v20.i29.9775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilkens S. Structure and mechanism of ABC transporters. F1000Prime Rep. 2015;7:14. doi: 10.12703/P7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mamo G, Pandi A. A review on ATP binding cassette (ABC) transporters. Int J Pharma Res Health Sci. 2017;5:1607–1615. [Google Scholar]
- 7.Robey RW, Pluchino KM, Hall MD, Fojo AT, Bates SE, Gottesman MM. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat Rev Cancer. 2018;18:452–464. doi: 10.1038/s41568-018-0005-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cole SPC, Bhardwaj G, Gerlach JH, Mackie JE, Grant CE, Almquist KC, et al. Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science. 1992;258:1650–1654. doi: 10.1126/science.1360704. [DOI] [PubMed] [Google Scholar]
- 9.Yin J, Zhang J. Multidrug resistance-associated protein 1 (MRP1/ABCC1) polymorphism:from discovery to clinical application. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2015;36:927–938. doi: 10.3969/j.issn.1672-7347.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vulsteke C, Lambrechts D, Dieudonné A, Hatse S, Brouwers B, van Brussel T, et al. Genetic variability in the multidrug resistance associated protein-1 (ABCC1/MRP1) predicts hematological toxicity in breast cancer patients receiving (neo-)adjuvant chemotherapy with 5-fluorouracil, epirubicin and cyclophosphamide (FEC) Ann Oncol. 2013;24:1513–1525. doi: 10.1093/annonc/mdt008. [DOI] [PubMed] [Google Scholar]
- 11.Wang H, Jin G, Wang H, Liu G, Qian J, Jin L, et al. Genetic susceptibility of lung cancer associated with common variants in the 30 untranslated regions of the adenosine triphosphate-binding cassette B1 (ABCB1) and ABCC1 candidate transporter genes for carcinogen export. Cancer. 2009;115:595–607. doi: 10.1002/cncr.24042. [DOI] [PubMed] [Google Scholar]
- 12.Yin J, Huang Q, Yang Y, Zhang J, Zhong M, Zhou M, et al. Characterization and analyses of multidrug resistance-associated protein 1 (MRP1/ABCC1) polymorphisms in Chinese population. Pharmacogenet Genomics. 2009;19:206–216. doi: 10.1097/FPC.0b013e328323f680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018:GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 14.Keppler D. Multidrug resistance proteins (MRPs, ABCCs):importance for pathophysiology and drug therapy. Handb Exp Pharmacol. 2011;201:299–323. doi: 10.1007/978-3-642-14541-4_8. [DOI] [PubMed] [Google Scholar]
- 15.Abdul-Ghani R, Serra V, Gyorffy B, Jürchott K, Solf A, Dietel M, et al. The PI3K inhibitor LY294002 blocks drug export from resistant colon carcinoma cells overexpressing MRP1. Oncogene. 2006;25:1743–1752. doi: 10.1038/sj.onc.1209201. [DOI] [PubMed] [Google Scholar]
- 16.Pajic M, Murray J, Marshall GM, Cole SP, Norris MD, Haber M. ABCC1 G2012T single nucleotide polymorphism is associated with patient outcome in primary neuroblastoma and altered stability of the ABCC1 gene transcript. Pharmacogenet Genomics. 2011;21:270–279. doi: 10.1097/FPC.0b013e328343dd5f. [DOI] [PubMed] [Google Scholar]
- 17.Kunická T, Václavíková R, Hlaváč V, Vrána D, Pecha V, Rauš K, et al. Non-coding polymorphisms in nucleotide binding domain 1 in ABCC1 gene associate with transcript level and survival of patients with breast cancer. PLoS One. 2014;9:e101740. doi: 10.1371/journal.pone.0101740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang W, Zhou HH, Liu Z, He YJ. Pharmacogenomics in China. In: Padmanabhan S, editor. Handbook of pharmacogenomics and stratified medicine. London: Academic press; 2014. pp. 999–1013. [Google Scholar]


