Abstract
Exposure to food toxins generate multiple adverse health effects. Heavy metals, antibiotics residue, mycotoxins, pesticides and some food additives are examples of the most important food toxins. The common mechanism of toxicity and carcinogenicity effects of food toxins is the generation of oxidative stress that leads to DNA damages. Moreover, based on epidemiologic evidence unhealthy eating habits and food toxicities are associated with cancers occurrence. Therefore, application of bioactive food additives as harmless or safe components in food industry is expensive. Nigella sativa L. is a broadly used herb-drug for various diseases all over the world and has been used as preservative and food additive. Based on various studies N. sativa has shown various pharmacological activities including therapeutic efficacy against different human diseases and antioxidant anti-inflammatory effects against environmental toxins. N. sativa decreases the adverse health effects induced by mentioned food toxins via modulating the action of antioxidant enzymes such as glutathione peroxidase (GPx), glutathione-S-transferase catalase and act as reactive oxygen species (ROS) scavengers in different organs. Besides, N. sativa and thymoquinone (TQ) have protective effects on food products through removal and inhibition of various toxic compounds. Therefore, in the present review we will describe all protective effects of N. sativa and its main constituents, TQ, against food induced toxicities.
Keywords: Nigella sativa, Thymoquinone, Oxidative stress, Food protectant, Food toxicity
Introduction
One of the basic human need is achieving safe and nutritious food with the advent of the modern industrial world. Due to current methods in food technology, food products can become contaminated by biological and chemical agents that have the potential to cause side effects on human health. Human diet contains thousands of different chemical agents which only a few of them have nutritional value. Recently, public concerns result in more demand for systematic food safety assessment and efforts to produce healthy and improved food. However, food toxicity may result from chemicals existence that is normal components of plant and animal foodstuffs or enters foods as natural contaminants before of manipulation by human. The US Food and Drug Administration (FDA) has classified the relative significance of health hazards related to food including microbiological contamination, incorrect eating habits, environmental contamination, natural toxic components, pesticide residues, food additives.1 Various studies have been done to reduce toxicity and presently, the pharmaceutical and food industries are in exploration of novel composites that are multi-functional, bioactive and harmless.
Nigella sativa L. is one of the most admired medicinal herb seeds in traditional medicines. The seeds are the most commonly used parts of the plant in the traditional medicine. Seeds have pungent and bitter taste with considerable amount of oil inside its united follicles, which mostly are utilized as a food preservative and spice and the most often used therapeutic agent is its oil. The seeds are very pleasant with hot, strong, peppery taste and are usually consumed in cooking curries, pastries and Mediterranean cheeses. Also, it can be added to coffee, tea, and bread, canned foods, wine and vinegar.2 The black seed oil contains more than 30% fixed oil and 0.4%-0.45% (w/w) volatile oil such as thymoquinone (TQ) (4%–24%) and 46% of monoterpenes such as α-pinene and r-cymene. Ghosheh et al identified 4 main compounds of BS oil using high performance liquid chromatography (HPLC) method including TQ, dithymoquinone, thymohydroquinone, and thymol.3 The popularity of N. sativa was due to the ideological belief in the herb as a remedy for various diseases. N. sativa and its active constituents, TQ, (Figure 1) have been investigated for its biological effects and remedy potential owing to its broad spectrum of activities including antimicrobial, anticancer, immunomodulation, analgesic, anti-inflammatory, spasmolytic, hepatoprotective, renal protective, gastro-protective, antidiabetic, antioxidant and bronchodilator.4 In addition, various researchers demonstrated that N. sativa and TQ have very low side effects and toxicity. Therefore, in this article, food protective effects of N. sativa and its main constituents through inhibition of various toxic compounds which have been generated in food processing steps like heavy metals, antibiotics, mycotoxins, pesticides and food additives both in vitro and in vivo experimental models are overviewed. Also, the effects of TQ on inhibition of food toxicity induced in different organs including brain, liver, kidney, blood, genome and reproductive tract (Figure 2) have been discussed.
Figure 1.
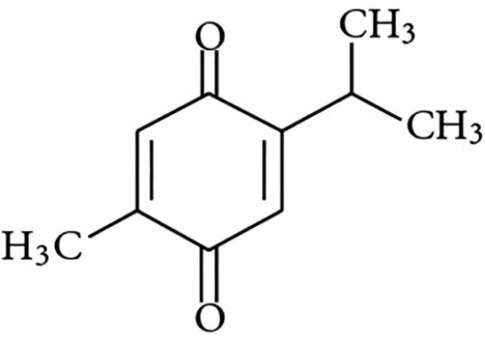
Chemical structure of thymoquinone.
Figure 2.
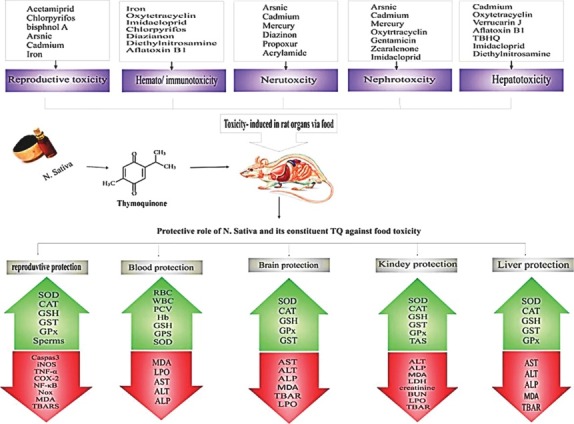
Schematic illustration of Nigella sativa its main constituent, TQ, protective effect against food induced toxicities in brain, liver, kidney, blood, genome and reproductive organs.
Protective roles of Nigella sativa and its constituent thymoquinone against heavy metals toxicity
Heavy metals such as arsenic (As), aluminum (Al), chromium (Cr), lead (Pb), nickel (Ni), cadmium (Cd), mercury (Hg), etc contribute lots of environmental and health problems based on their toxicity effects. Toxic metals can be exposed to human and environment through numerous ways such as air, food, water, waste, and industries and the accumulation of their ions led to serious environmental and health hazards. There are various methods to remove or reduce the toxicity effect of these heavy metals based on their types. Physical, biological and chemical methods are widely used in the treatment and removal of organic pollutant. Herbal treatment is one of the well-known and reliable methods to decrease chronic and acute toxic effects of organic pollutants like heavy metals in animal and human organs. Meanwhile, medicinal plant like N. sativa (black seed) is a widely used as an antidotal and protective agent due to its effective constituent, TQ. There are various reports on its biological activities and protective effects in different organs and tissues including brain, genome, liver, kidney, lung, etc. In this section of the review article, several studies in scientific databases that evaluate the protective effects of N. sativa and its main components against natural and chemical-induced toxicities are demonstrated. At the end of this section, the schematic description of N. sativa and TQ against toxicities induced by some heavy metals is shown in Figure 3. Besides, Table 1 has shown the effect of N. sativa and TQ against heavy metals toxicity.
Figure 3.
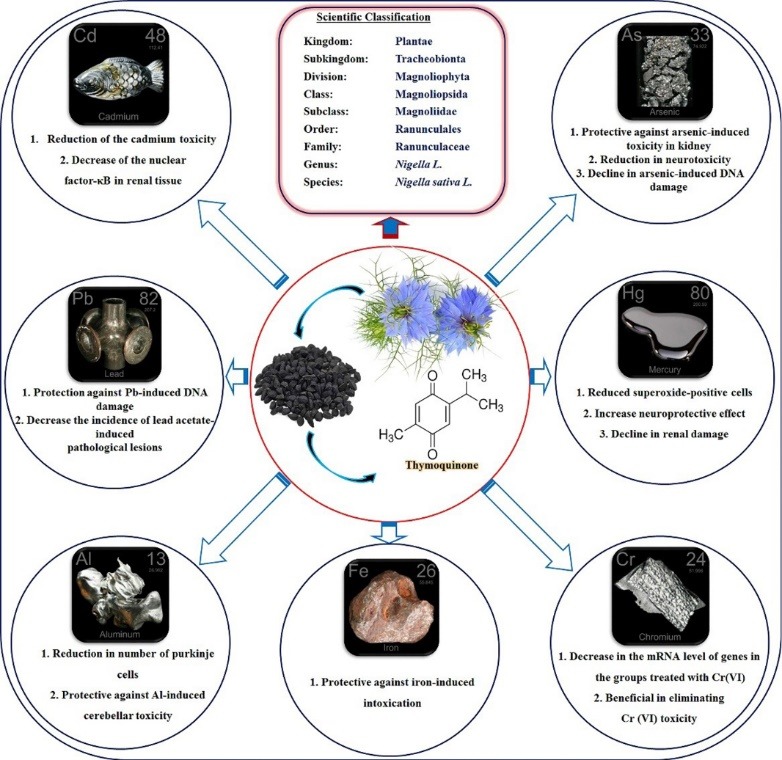
Schematic description of Nigella sativa and TQ against toxicities induced by some heavy metals.
Table 1. Protective effects of Nigella sativa and its constituent thymoquinone against some heavy metals in different tissues .
| Food Toxins | Food Source | Targets | Treatment Conditions | Main Findings | Ref. |
| Arsenic | Rice, vegetables, seafood | Neurotoxicity | In vivo, rat, TQ: 10 µM | The neurotoxicity decrease was due to antioxidant potential of TQ | 5 |
| Testicular Toxicity | In vivo, rat, TQ: 10 mg/kg/d | TQ could protect against arsenic-induced testicular injury. | 6 | ||
| Renal Failure | In vivo, rat, TQ: 10 mg/kg/d | TQ plays a protective role against arsenic-induced toxicity in the kidney. | 7 | ||
| Neurotoxicity | In vivo, rat, TQ: 10 mg/kg/d | TQ can reduce the neurotoxic effect of arsenate. | 8 | ||
| Cadmium | Shellfish, mushroom , grains | Hepatotoxicity | In vivo, mice, TQ: 10 µM | TQ protected against cadmium-induced through its antioxidant oxidative stress. | 9 |
| Mercury | Fish | Nephrotoxicity | In vivo, rat, TQ, 10 mg/kg/d | Antioxidant effect of TQ prevents acute renal failure due to mercury toxicity. | 10 |
| - | - | Nephrotoxicity | In vivo, rat, TQ (5 mg/kg/d, per os); | Protective effect of TQ against Pb-induced renal antioxidant capacity impairment | 11 |
| Neurotoxicity | In vivo, rat, TQ:20 mg/kg b.w | TQ decreased the incidence of lead acetate-induced pathological lesions. | 12 | ||
| Aluminum (AL chloride) | Air, but not in its metallic form, in the food industry, drinking water, pharmaceutica and cooking utensils. | Cerebellar toxicity | In vivo, rat, NS oil:(1 ml/kg) orally | N. sativa may play a protective role against Al-induced cerebellar toxicity in humans. | 13 |
| Iron | Water, iron supplement | Hematotoxicity | In vivo, Rabbit N. Sativa and Oriental Spices | N. sativa can be effective for health protection and health enhancement of organism. | 14 |
| Reduce toxicity | In vivo, rat, TQ: 0.005M | TQ can inhibit the toxicity of some metals. | 15 | ||
| Chromium | Leather tanning, electroplating and stainless steel industries | Genotoxicity | In vivo, Tilapia and Zebrafish crude extract Nile of N. sativa:0.5 mg/kg | Inhibition may be mediated through antioxidant activity and inhibition of Cr(VI) enzymatic activation. | 16 |
| Reduce toxicity | In vivo. Rat, TQ: 500 µM | TQ can be beneficial for eliminating Cr(VI) toxicity | 17 |
Protective roles against arsenic toxicity
As is an example of the earliest poisons known to human and widely dispersed in its various forms in the atmosphere.18 It can contaminated environment via natural weathering of geological forms and human factors as well as mining manufacturing, wash-off, burning of fossil fuels, agricultural and incineration.19 An exposure to a high-dose of As may result in severe adverse reactions like diarrhea, pain, vomiting, dehydration and weakness.18 Pigmentation changes, liver disease, anemia, and gastrointestinal symptoms, hyperkeratosis, Bowen’s disease, squamous and basal cell carcinoma, diabetes mellitus and Blackfoot disease are chronic symptoms of As exposure.20,21 Since As leads in formation constitution of reactive oxygen species (ROS) and induces lipid peroxidation (LPO), ROS-mediated oxidative damage is a common denominator of As pathogenesis that in turn produce active oxygen and nitrile molecule.22 TQ can play a considerable mitigatory role against various toxicities that induced with heavy metal such as As.23 For example, Firdaus et al have shown that in brain preparations of male Wistar rats, significant reduction in the As-induced neurotoxicity has been observed upon pre-treatment with TQ.5 Also, decrease in As-induced DNA damage upon pre-treatment with TQ was approved through comet assay.19 Protective role of TQ against As-induced toxicity in kidney have been demonstrated by Sener at el. They concluded that TQ can potentially be used as a remedial agent.7 Similarly, Fouad et al have reported that TQ not only decrease the As-induced expression of inducible nitric oxide (NO) synthase and caspase-3 but also considerably increased the level of serum testosterone and glutathione (GSH) and considerably diminished malondialdehyde (MDA) and NO levels caused from As administration in the testicular tissue.6 More recently TQ was reported to ameliorate the neurotoxic effect of As and through its antioxidant mechanism suppress the induced oxidative stress in the nervous system.8
Protective roles against cadmium toxicity
Cd as naturally happening metal is extensively used in industries. Natural and anthropogenic activities can contaminate environment with Cd and stays intact for long periods of time (half-life is about 10-30 years). It can enter into the food chain via consumption of contaminated plants, fish, and animals. Food is a main pathway for human exposure to Cd (90%).24 Cd can be accumulated in some foods like shellfish/mollusks, animal offal, and certain mushroom and cereals in high concentration. Several side effects including kidney and bone damage upon chronic exposure to Cd was reported.25 The mechanisms of Cd toxicity include induction of oxidative, inflammatory response, endoplasmic reticulum stress, genotoxicity, and interfere with the functions of essential metal (specially zinc).26 According to the study of Zafeer et al, treatment with CdCl2 (5 mM) caused a considerable increase in protein carbonyl and reduction of GSH content.9 However, the histopathological studies of rat’s kidney showed that TQ noticeably decreased the Cd toxicity and protected against kidney histological damage. Also immunohistochemical analysis showed that TQ meaningfully decreased the Cd-induced over expression of nuclear factor-kB in renal tissue.27 Sayed et al have been shown that long-term treatment with low doses of Cd changed the antioxidant enzymatic profile and induced oxidative and spermatological damages.28 In addition, histopathological changes in testes, epididymis and accessory glands have been observed. However, the co-treatment with TQ exhibited favorable effect in all aforementioned parameters. It has been concluded that testes protection effect of TQ against the detrimental was due to its antioxidant and anti-inflammatory activities. Besides, free radical scavenging activity may be another protective effect of TQ.29
Protective roles against Mercury toxicity
Hg and its organic and inorganic species are toxic compounds (the most toxic form is methylmercury [MeHg]) and human can exposure to mercury via consumption of food like fish.30 Half-life of Hg species are nearly 1–3 months, and can be eliminated mainly as MeHg in urine and as iHg (inorganic mercury) in faeces.30 Due to the volatile characteristics of Hg species, the inhalation of Hg vapor and its compounds have adverse effects on the nervous, gastrointestinal and immune systems, lungs and renal tissues. In addition, it can be changed into neurotoxic and teratogenic agents.31 TQ nephroprotective effect against inflammation and oxidative stress has been reported as well. For example, Fouda et al, revealed that the number of superoxide-positive cells decreased up to 70% upon treatment with TQ and proposed it as a protection method from mercury chloride–induced kidney failure.10 Another study confirmed that TQ improves the renal proliferative reaction and decreased histological injury like renal cell apoptosis and proliferative responses owing to Hg exposure in rats.32
Protective roles against Lead toxicity
Pb is non-essential heavy metal with high toxicity potential. Pb in cereal and dairy products are of specific concern and are carefully checked by international organizations because these products are introduced in infants and children diets as the first solid food.33 It has several clinical adverse functions and the most important effect of it is on the oxidative stress mechanism, whereas antioxidants including GSH decrease ROS induced cell damage.34 According to current guidelines delivered by the Centers for Disease Control and Prevention (CDC), 10 μg/dL of blood Pb levels can be hazardous and proper treatment should be considered.35 TQ can be a promising substitute for the conventional therapeutic drugs due to its high pharmacological activity and low systemic toxicity.36 In this context, Mabrouk and Cheikh reported that supplementation with TQ significantly protected rat kidney against Pb-induced renal impairment.11 Another study showed that co-treatment of TQ with Pb acetate noticeably diminished the occurrence of Pb acetate-induced pathological injuries.12 In this context, Mahrous et al reported that TQ significantly reduced the harmful effect of Pb acetate in male rats through reducing DNA damage and alterations in the gene expression, levels of MDA and protein carbonyl (PC) and also increasing GSH levels.37
Protective roles against aluminum toxicity
Al exists in air, water, and many foods but not in its metallic form. It is used in the food additive, medicines and cooking utensils.38 Neurotoxicity occurrence via treatment with Al was related to its high levels in brain and neurofibrillary tangles (NFT) that are known as symptom of Alzheimer’s disease.39 N. sativa and TQ have a protective effect against Al-induced cerebellar toxicity in animals. Kamal and Kamal reported that administration of Al in the rat cerebellum significantly reduced the number of Purkinje cells and induced some damages such as cytoplasmic vacuolation, dilatation of Golgi cisternae, and mitochondria with dilated cristae in Purkinje cells and administration of N. sativa with Al showed a noticeable protection against these alterations.13
Protective roles against iron toxicity
Fe as the fourth most abundant element by weight constitutes the main part of earth crust. The Fe ions involve in biological systems through oxidation-reduction process of Fe2+ to Fe3+ or reverse and they are part of various chemical substances with a very important role in various organisms.14 Although the most common causes of anemia in human is deficiency in Fe level, high concentrations of Fe become toxic for cells and it can result in various side effects as well as arising risk of cancer, heart diseases, and other disorders. Protective effects of N. sativa in Fe intoxication experiment has been carried out. According to the study of Ahmadi et al, N. sativa can be effective for health protection and improvement of rabbit’s organism, even with administration of high doses of Fe for a short period of time.14 Similarly, Kishwaret al demonstrated that TQ can successfully form complexes with trace metals such as Fe(III), Cr(VI), Cu(II), V(IV) and Co(II) in pH range of 3.00-4.00 as chelating agent in case of toxicity of aforementioned metals.15
Protective roles against chromium toxicity
Cr is well known essential trace element for human metabolism that plays role in preserving of glucose, protein, and fat normal metabolism for the human. Cr as a seventh most abundant metal in the earth crust can pollute environment.40 The major Cr contamination can be through the leather tanning, electroplating, and stainless steel industries.41 DNA damage including the formation of DNA adducts and alteration in DNA replication and transcription owing to Cr administration has been confirmed by various studies. In addition, toxic, genotoxic, mutagenic and carcinogenic properties on human and animals has been reported.42 Several research studies have shown the TQ pharmacological activities and its other beneficial effects. Kishwar et al had reported that Cr(VI) toxicity can be eliminated through complex formation between the TQ and Cr(VI) or by reduction of Cr(VI) to Cr(III).17 Another study had demonstrated that treated with Cr (VI) caused a considerable raise in liver and brain mRNA level of cytochrome as CYP1A2, CYP3A, and CYP2E1 compared to the control group. On the other word, co-treatment with Cr(VI) and N. sativa oil or TQ showed that mRNA level of genes as compared to treatment with Cr(VI) alone reduced considerably and also has been shown a considerable amelioration in the histological images.16
Protective effects of Nigella sativa and thymoquinone against antibiotics residue toxicity
The employ of veterinary drugs that prevent the growth of microorganisms as feed additives in food-producing animals has capacity to produce residues in animal derived products (meat different, milk and dairy products, eggs and honey) and can generate a potential health hazard for consumers including hypersensitivity reactions, antimicrobial drug resistance, toxicity, mutagenicity, teratogenicity, and carcinogenicity.43 In addition, various studies have shown that antibiotics can lead to mitochondrial dysfunction and oxidative stress in mammalian cells.44 It has been reported that TQ reduces oxidative damage induced by a different free radical producing agents including oxytetracyclin (OTC) and gentamicin (GEM). In this section, the effects of TQ against some antibiotics residue toxicity are discussed. Table 2 shows the effect of N. sativa and its constituent TQ against antibiotics residue toxicity.
Table 2. Protective effects of Nigella sativa and its constituent thymoquinone against some antibiotics residue toxicity in different tissues .
| Food Toxins | Food Source | Targets | Treatment Conditions | Main Findings | Ref. |
| OTC | Milk - meat | Hematotoxicity | In vivo, Pigeons black seed: level of 2.5% with OTC | Black seed completely blocked the elicited effects by OTC. | 45 |
| hepato-renal toxicity |
In vivo, rabbits N. sativa oil and ascorbic acid |
Protective role of NSO against the toxic effects of OTC by their free radical scavenging and strong antioxidant activities | 46 | ||
| Nephrotoxicity |
In vivo rats N. sativa oil: 0.5, 1.0 or 2.0 mL/kg/d |
N. sativa may be useful in ameliorating signs of GEM nephrotoxicity in rats | 47 | ||
| GEM | Milk - meat | Nephrotoxicity |
In vivo rats N. sativa: 0.2 -0.4 mL/kg |
N. sativa prevents the toxic effects of GEM on the biochemical and histopathological parameters. | 48 |
|
In vivo, rabbit, Nigella oil: 2 mL and vitamin C: 250 mg |
Decreasing oxidative stress and ability to prevent the energy decline in kidney tissues. | 49 |
Abbreviations: OTC, oxytetracyclin; GEM, gentamicin; NSO, Nigella sativa oil.
Protective effects on oxytetracyclin toxicity
OTC as one of the tetracyclines antibiotics is used extensively not only in human but also in veterinaries and meat industry. It also is used as feed additive or in drinking water to preserve ideal animal health for food production.50 For veterinary animals like poultries, cows, pigs and sheep, it is used to treat pneumonia, enteritis, septicemia, endometritis, metritis mastitis and other secondary bacterial infections.50 Excessive use of antibiotics in the treatment of dairy cows may result in antibiotic remaining in the milk and consumption of milk with high levels of OTC by humans leads to side effects including allergic responses, increase of bacterial resistance, the development of teratogenicity risk in the first trimester of pregnancy, the color change of the primary and permanent teeth.50 A recent study has reported that administration of OTC to pigeons meaningfully reduced total leukocyte and lymphocyte counts, raised lymphocyte, heterophil ratio, lysosomal enzyme activity and diminished reticuloendothelial system utility compared to controls. Co-administration of black seed with OTC perfectly inhabited the effects provoked by OTC and made immunostimulant effects in pigeons.45 In a similar study, Abdel-Daim reported that OTC leads in considerable modifications in serum biochemical renal-hepato hurt markers, and significantly inhibited the tissue antioxidant biomarkers and renal-hepatolipid peroxidation in animal treated with it. However, combination of N. sativa oil (NSO) with OTC protects animal against OTC induced serum and tissue biochemical revisions. Moreover, NSO has been hepatoprotective and antioxidant attributes and it has been shown that the toxic effects of OTC can be protected by NSO via their free radical scavenging and strong antioxidant activities.46
Protective effects on Gentamicin toxicity
GEM is an effective antibiotic against gram-negative bacteria that cause infection in human and animals. It is used for the treatment of mastitis in the dairy cow and often appears in raw milk.51 Nephrotoxicity and renal failure are main side effects of its remedial doses. Some studies showed that GEM caused proximal tubular injury, glomerular, tubular necrosis, interstitial nephritis and desquamation of the tubular epithelial cells in rat’s kidney. N. sativa increased GSH and total antioxidant status (TAS) in renal cortex and improved the histological and biochemical effect of GEM. A similar study has shown administration of N. sativa and GEM compared to the group treated with just GEM caused significant reductions in MDA level and nitric oxide production and rises superoxide dismutase (SOD) and glutathione peroxidase (GPx) activities. In addition, N. sativa improves induced oxidative stress.48 Moreover, Saleem et al have been evaluated the synergistic nephroprotective effects of NSO and vitamin C and reported that they reduced levels of nephrotoxicity indicators like; serum creatinine, blood urea nitrogen (BUN), and antioxidant activity compared to GEM treated rabbits.49 A recent study has shown that GEM could significantly raise the levels of creatinine, BUN, thiobarbituric acid-reactive substances (TBARS) and total nitrate/nitrite (NOx) and contrarily significantly reduced the levels of GSH, catalase (CAT), ATP in renal tissues. Moreover, TQ inhibited not only the increase of TBARS, BUN, creatinine and NOx but also the decrease of CAT, GPx, GSH, and ATP in GEM-treated rats compared to untreated group. In addition, histopathological inspection of renal tissues showed that TQ supplementation can inhibit the increase of GEM-induced nephrotoxicity in rats.
Protective roles of Nigella sativa and its constituent against pesticides toxicity
Pesticides are produced to control or remove pests (weeds, insects, or other organisms that interfere with human activity) but they can also be toxic (poisonous) to plants and animals as well as humans. Pesticides toxicity is dose dependent and can be either acute or chronic including oral, dermal or inhalation poisoning, carcinogenic, teratogenic, mutagenic and reproductive toxicity.43 Numerous plant natural products are accessible for reduction of toxicity effects of pesticides in the environment and human. NSO or its active ingredient TQ, can decrease toxicity of pesticides. For example, Mostafalou et al. have confirmed protective role of TQ against Malathion induced disruption in isolated pancreatic islets of dog.52 In this section, several studies, which investigated protective effects of TQ against pesticides induced toxicities are introduced as follows. In addition, Table 3 shows the effect of N. sativa and TQ against pesticides toxicity.
Table 3. Protective effects of Nigella sativa and its constituents against various pesticides toxicity in different tissues .
| Food toxins | Food Source | Targets | Treatment Conditions | Main Findings | Ref. |
| ACMP | Leafy vegetables, citrus fruits, pome fruits, grapes, cotton, Cole crops, cucumber, potato, tomato, eggplant cotton, corn, almonds and fruit trees including oranges, bananas, and apples | Reproductive toxicity | In vivo, rat, NSO: 1 mL/kg/bw) | NSO protects against reproductive toxicity of ACMP. | 53,54 |
| CPS | Reproductive toxicity, hormonal alterations, and oxidative damage | In vivo, rat, NSO: (1 mL/kg/d) | NSO can improve semen picture and moderate CPS-induced reproductive toxicity. | 55 | |
| IC | Immunological and histological changes | In vivo, rat, TQ: 1 mg/kg/d | TQ has been able to improve toxicity due to decreasing oxidative. | 56 | |
| Oxidative stress (blood, liver, kidney, and heart) | In vivo, mice, TQ: 10 mg/kg/d | TQ protect mice against IC-induced oxidative stress. | 57 | ||
| DI | Hematotoxicity, genotoxicity, immunotoxicity | In vivo, rat, TQ: 2.5, 5, 10 mg/kg/d | Metabolism of the TQ antioxidant properties caused reduction in toxicity. | 58 | |
| Brain damage |
In vivo, rat, TQ: 10 mg/kg/d |
The intoxication decrease was due to antioxidant potential of TQ | 59 | ||
| PPr | Brain regions | In vivo, rat NSO: 1 mL/kg/bw/d | NSO significantly reduces PPr-induced oxidative stress in rat brain regions via a free radicals scavenging mechanism | 60 |
Abbreviations: ACMP, acetamiprid; NSO, Nigella sativa oil; CPS, chlorpyrifos; TQ, thymoquinone; IC, imidacloprid; DI, diazinon; PPr, propoxur.
Protective roles against acetamiprid toxicity
Acetamiprid (ACMP) is an odorless organic neonicotinoid insecticide and has systemic effect on control of sucking insects on crops including leafy vegetables, citrus fruits, pome fruits, grapes, cotton, Cole crops, cucumber, potato, tomato, eggplant, Japanese radish and ornamental plants.61 ACMP did not show any carcinogen effects on human while it has a low chronic and acute toxicity in animals with no sign of carcinogenicity, mutagenicity or neurotoxicity.62 A recent study has indicated ACMP as a cause of sexual dysfunction in males that may be associated with the problem of decreasing fertility.63 ACMP can lead to reproductive toxicity in male rats by a decrease in body weight gain, relative weights of reproductive organs (testis, epididymis and seminal vesicle), spermatids number, sperm count, sperm motility, and testosterone levels. It also has induced histopathological changes including tubular atrophy, disorganization, and degenerative aspect of the seminal epithelium in some seminiferous tubules marked by spermatogenesis perturbation and poor sperm and presence of sloughing cell debris in their lumens. It is revealed that NSO co-administration modulated ACMP-induced reproductive side effects. This protective role may be due to antioxidant effects and the ability to reduce TBARS levels.53 Additionally, a similar study has reported that ACMP meaningfully reduced the body weight gain and the total weights of reproductive organs (epididymis, testes, and seminal vesicles). Besides, the failure in spermatids number, sperm count, sperm motility and testosterone level as well as the increased TBARS level and dead sperm were the least significant modifications in semen characteristics of ACMP group. Treatment with NSO alone may significantly incite the enhancement of spermatids number, spermatogenesis, and the weight of seminal vesicles. On the other hand, the co-administration of NSO along with ACMP can more efficiently modulate the ACMP-induced harmful effects on reproductive organs weights, semen quality, testosterone, and TBARS levels. Obviously, the protective role of NSO against ACMP- induced reproductive toxicity may be due to its antioxidant properties and capability to decrease TBARS level.54
Protective roles against chlorpyrifos toxicity
Chlorpyrifos (CPS) is an organophosphate pesticide that applied for destroying of pests like worms and control of insects in agricultural, residential and commercial settings.64 WHO introduced CPS as a relatively hazardous substance to humans and reported that exposure to CPS during pregnancy can damage the mental development of fetus.65 Recently, the studies have reported that CPS can induce side effects on male rat reproductive system by a decrease in sperm count and production, body weight, sexual hormones, and relative weight of reproductive organs accompanied by the increase in dead and abnormal sperms. All altered semen parameters normalized in rats treated with CPS during treatment with NSO and its protective effect can be because of its antioxidant capacity.55
Protective roles against imidacloprid toxicity
IC as a member of the neonicotinoid insecticide class is highly effective on different insects.66 Prevention of termite damage, pest control for gardens and turf, fleas control in treated domestic pets and protection of trees from boring insects are among other examples of this pesticides application. It can be considered as an “unlikely” carcinogen and a weakly mutagenic by the United States. Environmental Protection Agency (EPA) (group E).67 Recent studies have shown that IC significantly increases the total leukocyte counts, (especially IgGs), the hemagglutination of antibodies, MDA, alanine transaminase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), and total immunoglobulins (Igs) in comparison with the untreated control group. TQ could decrease the toxicity of IC by declining oxidative stress and improving immune efficiency.56 Similarly, Ince et al demonstrated that administration of TQ reduced IC-induced oxidative stress, LPO, and activities of the antioxidant enzymes. Besides, TQ had protective effect on the IC-induced oxidative stress and histopathological alterations in tissues of mice by improving antioxidant protection mechanisms.57
Protective roles against diazinon toxicity
Diazinon (DI) as a non-systemic organophosphate insecticide can be utilized for the control of agricultural, and domestic pests. The acute and chronic toxicity of DI has been approved in humans and animals.68 Recent studies indicated that acute and chronic toxicity of DI induces oxidative stress and leads to the production of free radicals. The production of ROS induces oxidization in various biomolecules such as membrane lipids, proteins and nucleic acids and alters the activity of antioxidants enzymes or ROS scavenging enzymes in animals.69 Danaei et al have shown that DI reduced red blood cells (RBCs), white blood cells (WBCs), hemoglobin, hematocrit, platelets, butyrl- and acetylcholinesterase function and interferon gamma and enhanced the micronucleus index of interleukin 10 and interleukin 4 in comparison with the control group. Co-treatment with TQ and DI decreased hematotoxicity and immunotoxicity but did not inhibit genotoxicity very well. Also, it has showed that TQ had not considerable effect on genotoxicity but it reduced the hematological toxicity, immunotoxicity, butyrl- and acetylcholinesterase activity in DI treated rats.58 Additionally, TQ reduces nitrous oxide and significantly increases SOD in damaged brain of rats that induced with DI.59 The successful protective effect of TQ on DI toxicity can be related to the antioxidant properties of its main component.
Protective roles against propoxur toxicity
Propoxur (PPr) is a carbamate insecticide with long residual effect used against forestry, household pests, turf, and fleas. PPr exerts neurotoxic effects through the massive reversible inhibition of acetylcholine esterase (AChE). Consequently, the health risk of human is high due to the exposure by frequent low doses of these materials. The exact mechanism of PPr-induced toxicity is not being completely defined. Involvement of ROS may be a probable mechanism of PPr-induced toxicity. PPr treatment considerably raised the levels of protein carbonyl content (PCC), LPO and oxidized glutathione (GSSG) in brain areas while levels of GSH and the activities of CAT, SOD, GSH-Px, GST (glutathione-S-transferase) and AChE were remarkably reduced. Administration of PPr with NSO repaired such biochemical factors to the control levels except for GST activity. NSO meaningfully decreased oxidative stress induced in rat brain areas through a free radicals scavenging mechanism that emphasized its antioxidant activity.60
Protective roles of Nigella sativa and thymoquinone against food process toxicants
Food processing and preservation have played important roles in achieving food sufficiency for the human being.70 Food processing and cooking can generate toxic compounds in food as a naturally-occurring constituent or form as the result of handling or processing such as N-Nitrosamines, Acrylamide, polycyclic aromatic hydrocarbons (PAHs), phenolic compounds, heterocyclic aromatic amines (PhIP), lipid polymerisation products resulting from deep-fat frying, lipid oxidation products, Maillard-browning products, ethylcarbamate and furan.70,71 Also, Bisphenol A (BPA) can produce during food packaging.72 A number of studies have reported that all food process toxicants can induce toxicity in animals and humans.73 TQ has displayed different properties in modern pharmacology and scientific findings and can reduce toxicity effect of food process toxicants.73 The present part was undertaken to overview the protective effect of TQ on some well-known food process toxicants. Table 4 shows the effect of N. sativa and TQ against food processing toxicants.
Table 4. Protective effects of Nigella sativa and its constituents against some food process toxicants in different tissues .
| Food toxins | Food source | Targets | Treatment Conditions | Main Findings | Ref. |
| DENA | Fried foods, cosmetic products, tobacco smoke, cheddar cheese, and pesticides. | Erythrocyte fragility | In vivo, rat TQ:4 mg/kg/5 days/p.o. | - | 74 |
| Hepatic carcinogenesis | In vivo, rat TQ: (4 mg/kg/d) | TQ decreases oxidative stress and preserves both the activity and mRNA expression of antioxidant enzymes | 75 | ||
| ACR | Making paper, dyes, plastics, and in treating drinking water and wastewater. caulk, food packaging, | Neurotoxicity |
In vivo, rat TQ: 2.5, 5, 10 mg/kg IP |
Neuroprotective effect of TQ in this model due to the antioxidant activity | 76 |
| Vitamin E: 200 mg/kg/d orally TQ:5 mg/kg/d, ip | The neurotoxicity decrease was due to antioxidant potential of TQ | 77 | |||
| B[a]P | Coal tar, tobacco smoke, and grilled meats | Forestomach carcinogenesis | In vivo, mice 0.01% of TQ in drinking water | Detoxification processes of TQ may be through its antioxidant and anti-inflammatory activities. | 78 |
| Mutagenic effect in murine bone marrow cells | In vivo, mice 0.01% of TQ in drinking water | TQ can inhibit the cytotoxic effects of exposure to the carcinogen B[a]P in initial phases. | 73 | ||
| Genotoxicity | In vitro, cultured human lymphocytes TQ: TQ ranging from 0.625, 1.25, 2.5, 5, 10 µM | TQ can reduce DNA damage. | 79 | ||
| BPA | Baby bottles, and beverage containers | Reproductive system |
In vivo, mice TQ: 10 mg/kg/d |
TQ ameliorates these toxic effects | 80 |
| Hepatotoxicity | In vivo, rat, TQ: 10 mg/kg/d | TQ reduces elevated levels of hepatic biomarkers and decreases lipid peroxidation | 81 |
Abbreviations: DENA, diethylnitrosamine; TQ, thymoquinone; ACR, acrylamide; B[a]P, banzo[a]pyren; BPA, Bisphenol A.
Protective roles against diethylnitrosamine toxicity
Diethylnitrosamine (DENA) as a chemical toxin can be generated in fried foods, tobacco smoke, cheddar cheese, pesticides and cosmetic products. DENA is well-known as a carcinogenic and in addition to hepatocarcinogenic effect, it can lead to different gastrointestinal cancers like gastric cancers, esophagus.82 A study has shown that DENA significantly increased ALP, total bilirubin, TBARS, ALT, total NOx, GSH, GST, GPx and CAT activity in hepatic tissues and led to severe histopathological alterations in hepatic tissue. Moreover, TQ supplementation could be ameliorated biochemical and histopathological alterations induced by DENA to the same value of control case. DENA induced initiation of liver carcinogenesis through reduced mRNA expression of GPx, CAT and GST. TQ inhibited the development of DENA-mediated primary hepatic cancer by declining oxidative stress and protected both the function and expression of antioxidant enzymes at mRNA level.75 Also in a recent study by Amin et al, enhancements in erythrocyte, hematocrit, and hemoglobin count have been seen in DENA-induced rats in which the effect of TQ on these markers was not statistically significant.74
Protective roles against acrylamide toxicity
Acrylamide (ACR) has wide usage in different industries such as fabricating paper, dyes, and plastics, and in treating drinking water and wastewater. There are low amounts of ACR in several products like caulk, food packaging and some adhesives. ACR is found in cigarette smoke as well.83 ACR can also form in some starchy foods during high-temperature cooking as well as roasting, frying, and baking. The U.S. government agencies considered ACR as a potential occupational carcinogen and it is categorized as a Group 2A carcinogen by the International Agency for Research on Cancer (IARC).84 It also forms in food during process heating. A critical role of oxidative stress in ACR-induced neurotoxicity in both in vitro and in vivo models has been demonstrated. Protective effects of TQ against ACR induced neurotoxicity in experimental animal models have been reported. Mehri et al reported that TQ supplementation significantly decreased ACR induced gait abnormalities in Wistar rats.76 Also in a similar study by El-Aaron combined treatment of ACR with TQ or vitamin E reduced the neurotoxicity.77
Protective roles against benzo[a]pyrene toxicity
Banzo[a]pyren (B[a]P)46 is a PAH. Coal tar, tobacco smoke and many foods, particularly grilled meats may contain ubiquitous compound. Its diol epoxide metabolites (more commonly known as BPDE) result in mutations and eventually cancer due to interaction with DNA. It is classified as a Group 1 carcinogen by the IARC.85 Treatment with TQ alone indicated a significant inhibition of the enzyme activities of liver GST and DT-diaphorase. Moreover, administration of TQ in combination with B[a]P resulted in normal enzyme actions and significant reduction in liver lipid peroxides.78 Previous studies investigated that daily intake of TQ significantly decreased the frequencies of chromosomal aberrations and damaged cells compared to the clastogenic activity of B[a]P alone in mouse bone marrow cells.73 The results of studies suggested the importance of TQ as a natural dietetic supplement for counteracting the cytotoxic effects of the mutagen B[a]P in primary phases. Co-administrated with TQ and B[a]P moderated LPO and GSH levels in normal rat liver.78 The possible chemoprotective effect of TQ against B[a]P-induced chromosomal aberrations (CAs) in mouse bone marrow cells was described. Daily intake of TQ significantly decreased the frequencies of CAs and injured cells related to the clastogenic activity of group treated with B[a]P alone.73 Hussein reported that TQ treatment was able to mitigate the induced lung cancer by B[a]P through enhancing the levels of serum carcinoembryonic antigen (CEA), haptoglobin (HPT), adenosine deaminase (ADA) and gamma glutamyltransferase (ɤ GT) as well as increasing the expression of caspase 3 gene, DNA fragmentation, cycloxygenase-2 (COX-2) and L-malondialdehyde (L-MDA) in lung tissues.86 It could be concluded that TQ may be effective in decreasing lung cancer by its radical scavenging activity, anti-inflammatory effect, regenerating endogenous antioxidant mechanisms, decreasing caspase-3 gene, and DNA damage in lung tissues.
Protective roles against bisphenol A toxicity
Bisphenol A (BPA) is a broadly used compound in resin and plastic industry and is the main constituent of plastic baby bottles, food containers and children’s toys.87 Exposure of BPA led to various health problems including cardiovascular disease, neurobehavioral disorders, metabolic disorders, abnormalities of the reproductive system, and cancer.88 A recent study has shown that administration of BPA significantly decreased seminiferous tubules diameter and epithelial height with impaired spermatogenesis, plasma testosterone, levels of luteinizing hormone (LH) and follicle-stimulating hormone (FSH), androgen receptors. Administration of TQ with BPA led to improvement of these side effects.80 BPA has a wide range of side effects and can cause oxidative stress in hepatic cells.89 Decrease in hepatocytes viability and their mitochondrial action have shown in the rats treated with BPA.90 Administration of TQ greatly normalized suppressed enzymatic and non-enzymatic antioxidants such as GSH, GPx, GST, SOD, and CAT. It also reduced elevated levels of hepatic biomarkers and decreased LPO.81
Protective roles of Nigella sativa and thymoquinone against mycotoxins toxicity
Mycotoxins like aflatoxins, ochratoxins, patulin, trichothecenes, zearalenone, fumonisins, tremorgenic toxins, and ergot alkaloids are secondary metabolites of fungus which not only have harmful effects on living creatures (humans, animals), and economic losses (in crops) but also cause in humans and animals death and illness.91,92 Consumption of food that contaminated via mycotoxin cause both short-term and long-term toxicity in human and animals. Mycotoxins, especially aflatoxins (AFB1) have acute toxicity and chronic carcinogenicity, teratogenicity, hepatotoxicity, mutagenicity, nephrotoxicity and immunosuppressive effects. The human disease acquired by eating the different agricultural commodities infected by mycotoxins including oilseeds, tree nuts, cereals, spices and dairy products.91,92 N. sativa has been considered as a natural medicine to treat various poisoning or disorder in liver and kidney function induced by mycotoxins. The anti-yeast activity of TQ was evaluated by various researchers against some dairy spoilage yeast species.93 This section was undertaken to evaluate the TQ protective effects against mycotoxins-induced acute and chronic toxicity. Table 5 shows the efficacy of N. sativa and TQ against mycotoxins toxicity.
Table 5. Nigella sativa and its constituent thymoquinone against some mycotoxins toxicity in different tissues .
| Food Toxins | Food Source | Targets | Treatment Conditions | Main Findings | Ref. |
| ZEN | Corn, wheat, barley, oats, sorghum, and sesame, cereals, peanuts, dried vine fruits, cocoa beans, feedstuff, green coffee beans, wine grapes, poultry feeds and beer. | Nephrotoxicity |
In vivo, mice TQ: (10 mg/kg b.w) |
TQ can enhance kidney cells detoxification of ZEN mycotoxin. | 94,95 |
| Ochratoxin A | Toxicity in Liver and kidney |
In vivo, rat NSO: (0.3 mL/d) |
NSO can reduce the toxic effect of OTA on liver and kidney tissue. | 96 | |
| VJ | Hepatotoxicity |
In vivo, rat NSO:800 mg/kg b.w |
NSO leads to adsorption of the toxic substance. | 97 | |
| toxicity | In vivo, mice TQ: 4.5, 9 and 18 mg/kg | TQ can increase resistance to oxidative stress and reduce LPO. | 98 | ||
| AFB1 | Hematological and biochemical changes |
In vivo, rat NSO: 5 mg /kg/body wt. Syzygium aromaticum oil: 5 mg /kg/body |
NSO was found to be more effective in restoring the parameters that were altered by AFB1 in rats. | 99 |
Abbreviatopns: ZEN, zearalenone; LPO, lipid peroxidation; OTA, ochratoxin A; VJ, verrucarin J; AFB1, aflatoxin B1; NSO, Nigella sativa oil.
Protective roles against zearalenone toxicity
Zearalenone (ZEN) is an example of mycotoxin made by Fusarium species with estrogenic and anabolic activity. It is one of the most extensively dispersed Fusarium mycotoxins that is observed at high occurrence in many important corps likes corn, wheat, barley, oats, sorghum and sesame and infected human and animal upon their consumptions.100 Nephrotoxicity of ZEN was evaluated in mice by given single and repeated doses of ZEN mycotoxin via the oral route. To overcome renal toxicity of ZEN, BUN and alpha-fetoprotein (AFP) concentration change was evaluated. The attained results showed that BUN and AFP concentration were remarkably decreased when mice treated with combination of TQ and ZEN in comparison with mice treated with ZEN alone. In addition, a highly significant increase in the blood creatinine and pyruvate kinase isoenzyme tumor M2 concentration was observed in TQ and ZEN treated group (T4) compared to control group. There was no significant difference in TAS concentration between T4 and control group while a highly significant reduction was detected between T4 and T3 groups. It should be mentioned that histological changes of mice kidney coincided with biochemical changes.94
Protective roles against ochratoxin A toxicity
Ochratoxin A (OTA) is a naturally occurring mycotoxin generated by Aspergillus,101 which has been found in a variety of foodstuffs such as cereals, peanuts, dried vine fruits, cocoa beans, feedstuff, green coffee beans, wine grapes, poultry feeds and beer.102 OTA is classified by IARC of WHO as a probably carcinogenic agent to humans.103 It is carcinogenic, teratogenic, nephrotoxic, hepatotoxic agent to animals and possibly to humans.104 Alhussaini and AL-Yahya have examined the histological alterations in the kidney and liver tissues of rats treated with OTA and OTA + NSO. Significant histological alterations have revealed in the liver and kidney tissues of rats treated with OTA. In liver tissue, it led to vacuolar deterioration and necrosis of the liver cells, central vein dilation and sinusoidal, bile duct replication, congestion of portal vein and dilation. Also cellular infiltration, slight rise in the collagen fibers, reduction in carbohydrates and protein were detected. In the kidney tissue, OTA resulted in necrosis in the nuclei, tubular epithelial cells deterioration, decline in the carbohydrates and protein and fibrous tissue replication. However, NSO significantly decreased the severity of injuries induced by OTA. Also administration of NSO with OTA considerably decreased the toxic effect of OTA on liver and kidney tissue of rats.96
Protective roles against verrucarin J toxicity
Verrucarin J (VJ) belongs to trichothecene family of mycotoxins that generated in different cereals such as wheat, oats or maize by several species of Trichothecium, Fusarium, Trichoderma, Myrothecium, Cephalosporium.105 Some of molds that generate trichothecene mycotoxins like Stachybotrys chartarum can growth in wet environments and causes health problems among residents of the building.106 VJ also can infect foods of animals and humans. A recent study has reported that VJ can raise levels of SOD, TBARs and 5-nucleotidase in the renal and blood tissue of the rats. Treatment of hepatic tissue with VJ reduced levels of zinc and glucose in blood, which in turn decreased the GSH and glucose-6-phosphate dehydrogenase level and this problem can be ameliorated by NSO. It has been found that treatment of rats with NSO may improve the harmful effects of toxin and the results suggested the utility of NSO as an antidote and antioxidant protectant against VJ in rats.107
Protective roles against aflatoxin B1 toxicity
Aflatoxin B1 (AFB1) is an aflatoxin created by Aspergillus parasiticus and Aspergillus flavus. AFB1 is commonly found in a variety of foods such as peanut, a promise of cotton, corn and other seeds and animals feed.108 It is an extremely potent carcinogen with a TD50 of 0.0032 mg/kg/d in animal models and it seems that rats are much more sensitive than other animals like monkey.109 AFB1 is the most toxic aflatoxin in hepatocellular carcinoma (HCC). Similarly in animals, AFB1 has been revealed to be mutagenic, teratogeni and exhibit immunosuppressive properties.110 TQ showed a protective effect against AFB1-induced hepatotoxicity in mice by decrease of liver hurt indicators including AST, ALT, and ALP and also via inhibiting degradation and necrosis of liver tissue. MDA which is a marker of LPO was increased in AFB1-intoxicated mice in liver, while pretreatment with TQ significantly prevented MDA production. Histopathological effects such as inflammation, necrosis, disruption of hepatocytes, hyperplasia of Kupffer cells, and infiltration of mononuclear cells and increased in size of hepatocytes were measured as signs of toxicity. TQ reduced the number of inflammatory cells and ameliorated the histopathological changes.98 In a similar study, it has been shown that treatment with NSO and Syzygium aromaticum oil have protective efficacy against aflatoxin contamination in rats and NSO was found more efficient in rats protection and it significantly improved the biochemical parameters affected by aflatoxin.99
Protective roles of Nigella sativa and TQ against food additives toxicity
Food additives are natural or synthetic constituents added to foods to carry out specific technological functions. There are around 3000 food additives that allowed for use in food industry and are divided by 6 main parts including preservatives, nutritional additives, coloring, flavoring and texturing agents. Also, other various agents which can be mentioned are enzymes, chelating agents, antifoaming agents, surface finishing agents.111-114 Some studies have shown the sub-chronic, chronic and acute toxicity of various food additives in human and animals. TQ possess beneficial effects against toxicity that can occur via food additives.115 In this part the protective effects of N. sativa and its main component against toxicity of food additives has been discussed. Table 6 shows the effect of N. sativa and its active ingredient TQ against food additives toxicity.
Table 6. Protective effects of Nigella sativa and its constituents against toxicity of 2 food additives in different tissues .
| Food Toxins | Food Source | Targets | Treatment Conditions | Main Findings | Ref. |
| TBHQ | Vegetable oils, numerous edible animal fats, and meat products | Hepatotoxicity | In vivo, rat TQ: 1 mM | TQ protects the liver enzymes leakage. | 116 |
| Carrageenan | Thickening, gelling and stabilizing abilities | Inflammatory | In vivo, rat TQ: 500 mg/kg body weight | TQ inhibits carrageenan-induced paw edema in a dose-dependent manner. | 117 |
| Inflammatory | In vivo, rat extract of N. sativa: 250, 500 mg/kg body weight | N. sativa inhibits inflammation and pain. | 118 |
Abbreviations: TBHQ, Tert-butylhydroquinone.
Protective roles against Tert-butylhydroquinone toxicity
TBHQ is an effective preservative, which has been used as an antioxidant for unsaturated vegetable oils, many eatable animal fats and meat products at concentrations less than 0.02%.119 TBHQ in high doses has some adverse effects on animal model including DNA damage and stomach tumors and in addition to leakage of cytosolic enzymes. It can also be hepatotoxic agent which in case of exposure stimulates rapid oxidation of intracellular GSH and pyridine nucleotides.120 TQ not only could increase viability of hepatocytes in TBHQ treated rat but also decrease the leakage of ALT and AST.116
Protective roles against Carrageenan toxicity
Carrageenan as a sulphated linear polysaccharide of D-galactose and 3,6-anhydro-D-galactose can be attained from certain red seaweeds of the Rhodophyceae class. Carrageenans are generally used in food industry owing to their exceptional physical functional properties like thickening, gelling, emulsifying and stabilizing abilities. They also have been utilized for improvement of the texture of cottage cheese, control of the final texture in most dairy desserts and pudding, and as stabilizers for production of sausages and also in the meat-processing industry. Extract of black seed was studied for its anti-inflammatory activity in carrageenan-induced rat paw edema model. Dose-dependent and remarkable anti-inflammatory properties were reported. Therefore, extract of black seed could inhibit carrageenan-induced inflammation in rats.118 A similar study reported that N. sativa reserved carrageenan-induced paw edema in a dose-dependent manner as well.117
Conclusion
Recently, some of the natural herbs and their bioactive components have been used in several studies with the purpose of toxicity prevention in different tissues induced by different chemical and natural toxins especially toxins in food due to daily intake. The accessibility and cost benefit properties and less toxic effects of natural plant constituents compared with synthetic products make them an ideal candidate for inhibition of food and chemical toxicity. This review summarized several in vivo and in vitro studies in order to realize the role of N. sativa and its bioactive component, TQ, in inhibition of food toxins related toxicities in different tissues. Heavy metals, antibiotics residue, food processing toxicants, mycotoxins and food additives are examples of natural and chemical toxic agents in foods that can be prevented by N. sativa and TQ. N. sativa and its bioactive components could protect different tissues against food toxins through numerous mechanisms as well as free radical scavenging, anti-inflammatory, antioxidant, amelioration in the disturbed levels of biochemical indicators, modulation of antioxidant defense systems, prevention of apoptosis and controlling effects on genes expression, and different signaling pathways. N. sativa has also ability to protect different organs and tissues such as kidney, liver, gastrointestinal, lung, heart, blood, brain, and reproductive system against toxins. In conclusion, based on the present review, N. sativa has a wide spectrum of protective activities against food toxicants induced toxicities. Therefore, N. sativa can be introduced as a supplement in the daily diet of individuals for inhibition of food toxins side effects. Also since there are not enough clinical trial studies on human, further investigations are required to determine the efficacy of natural products as a protective agent in human intoxication.
Ethical Issues
Not applicable
Conflict of Interest
The authors declare that they have no conflict of interests.
Acknowledgments
The authors gratefully acknowledge the financial support of this study by the Tabriz University of Medical Sciences, Tabriz, Iran.
References
- 1. Williams P. Food toxicity and safety. In: Mann J, Truswell A, eds. Essentials of Human Nutrition. Oxford: Oxford University Press; 2012:415.
- 2.Hassanien MF. Nutritional value, functional properties and nutraceutical applications of black cumin (Nigella sativa l) oilseeds: An overview. Nat Prod Indian J. 2006;2(1):23–34. [Google Scholar]
- 3.Ghosheh OA, Houdi AA, Crooks PA. High performance liquid chromatographic analysis of the pharmacologically active quinones and related compounds in the oil of the black seed (Nigella sativa L) J Pharm Biomed Anal. 1999;19(5):757–62. doi: 10.1016/s0731-7085(98)00300-8. [DOI] [PubMed] [Google Scholar]
- 4.Gilani AH, Aziz N, Khurram IM, Chaudhary KS, Iqbal A. Bronchodilator, spasmolytic and calcium antagonist activities of Nigella sativa seeds (Kalonji): A traditional herbal product with multiple medicinal uses. J Pak Med Assoc. 2001;51(3):115–20. [PubMed] [Google Scholar]
- 5.Firdaus F, Zafeer MF, Anis E, Fatima M, Hossain MM, Afzal M. Antioxidant potential of thymoquinone against arsenic mediated neurotoxicity. Free Radicals and Antioxidants. 2016;6(1):115–23. doi: 10.5530/fra.2016.1.14. [DOI] [Google Scholar]
- 6.Fouad AA, Albuali WH, Jresat I. Protective effect of thymoquinone against arsenic-induced testicular toxicity in rats. Kuala Lumpur, Malaysia: International Conference on Pharmacology and Pharmaceutical Medicine (ICPPM); 2014. 8 [Google Scholar]
- 7.Sener U, Uygur R, Aktas C, Uygur E, Erboga M, Balkas G. et al. Protective effects of thymoquinone against apoptosis and oxidative stress by arsenic in rat kidney. Ren Fail. 2016;38(1):117–23. doi: 10.3109/0886022x.2015.1103601. [DOI] [PubMed] [Google Scholar]
- 8.Kassab RB, El-Hennamy RE. The role of thymoquinone as a potent antioxidant in ameliorating the neurotoxic effect of sodium arsenate in female rat. Egypt J Basic Appl Sci. 2017;4(3):160–7. doi: 10.1016/j.ejbas.2017.07.002. [DOI] [Google Scholar]
- 9.Zafeer MF, Waseem M, Chaudhary S, Parvez S. Cadmium-induced hepatotoxicity and its abrogation by thymoquinone. J Biochem Mol Toxicol. 2012;26(5):199–205. doi: 10.1002/jbt.21402. [DOI] [PubMed] [Google Scholar]
- 10.Fouda AM, Daba MH, Dahab GM, Sharaf El-Din OA. Thymoquinone ameliorates renal oxidative damage and proliferative response induced by mercuric chloride in rats. Basic Clin Pharmacol Toxicol. 2008;103(2):109–18. doi: 10.1111/j.1742-7843.2008.00260.x. [DOI] [PubMed] [Google Scholar]
- 11.Mabrouk A, Ben Cheikh H. Thymoquinone ameliorates lead-induced suppression of the antioxidant system in rat kidneys. Libyan J Med. 2016;11:31018. doi: 10.3402/ljm.v11.31018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Radad K, Hassanein K, Al-Shraim M, Moldzio R, Rausch WD. Thymoquinone ameliorates lead-induced brain damage in Sprague Dawley rats. Exp Toxicol Pathol. 2014;66(1):13–7. doi: 10.1016/j.etp.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 13.Kamal I, Kamal H. Effects of aluminum on rat cerebellar cortex and the possible protective role of Nigella sativa: A light and electron microscopic study. Egypt J Histol. 2013;36(4):979–90. doi: 10.1097/01.EHX.0000440828.86537.e7. [DOI] [Google Scholar]
- 14.Ahmadi M, Dronca D, Dodi G, Milovanov C, Gavojdian D, Dumitru C. et al. Nigella sativa and oriental spices with protective role in iron intoxication: In vivo experiments on rabbits. Bulletin UASVM Animal Science and Biotechnologies. 2016;73(2):192–6. doi: 10.15835/buasvmcn-asb:12232. [DOI] [Google Scholar]
- 15.Kishwar F, Haq Q, Anwar H. Use of active ingredient of Nigella sativa to reduce toxicity of some trace elements (Fe (III), Cr (Vi), Cu (II), V (Iv) And Co (II)) FUUAST J Biol. 2012;2(2):95–101. [Google Scholar]
- 16.Khalil W, Abdel-Gawad F, Belattar N, Senator A, Abdel-Wahhab M. Protective effects of Nigella sativa extract against chromiumvi-induced genotoxicity in Nile tilapia (Oreochromis niloticus) and Zebrafish (Danio rerio) Glob Vet. 2011;7(3):283–93. [Google Scholar]
- 17.Kishwar F, Mahmood T, Mahmood I, Anwar A, Perween R, Mustafa S. Complexation of active ingredient thymoquinone of Nigella sativa (black seed) with chromium (VI) FUUAST J Biol. 2016;6(1):65–72. [Google Scholar]
- 18.Vahidnia A, van der Voet GB, de Wolff FA. Arsenic neurotoxicity--a review. Hum Exp Toxicol. 2007;26(10):823–32. doi: 10.1177/0960327107084539. [DOI] [PubMed] [Google Scholar]
- 19.Firdaus F, Zafeer MF, Anis E, Fatima M, Hossain MM, Afzal M. Antioxidant potential of thymoquinone against arsenic mediated neurotoxicity. Free Rad Antiox. 2016;6(1):115–23. doi: 10.5530/fra.2016.1.14. [DOI] [Google Scholar]
- 20.Sattar A, Xie S, Hafeez MA, Wang X, Hussain HI, Iqbal Z. et al. Metabolism and toxicity of arsenicals in mammals. Environ Toxicol Pharmacol. 2016;48:214–24. doi: 10.1016/j.etap.2016.10.020. [DOI] [PubMed] [Google Scholar]
- 21.Chen CJ, Wang SL, Chiou JM, Tseng CH, Chiou HY, Hsueh YM. et al. Arsenic and diabetes and hypertension in human populations: a review. Toxicol Appl Pharmacol. 2007;222(3):298–304. doi: 10.1016/j.taap.2006.12.032. [DOI] [PubMed] [Google Scholar]
- 22.Ma N, Sasoh M, Kawanishi S, Sugiura H, Piao F. Protection effect of taurine on nitrosative stress in the mice brain with chronic exposure to arsenic. J Biomed Sci. 2010;17 Suppl 1:S7. doi: 10.1186/1423-0127-17-s1-s7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khattab MM, Nagi MN. Thymoquinone supplementation attenuates hypertension and renal damage in nitric oxide deficient hypertensive rats. Phytother Res. 2007;21(5):410–4. doi: 10.1002/ptr.2083. [DOI] [PubMed] [Google Scholar]
- 24.Kim SH, Lim YW, Park KS, Yang JY. Relation of rice intake and biomarkers of cadmium for general population in Korea. J Trace Elem Med Biol. 2017;43:209–16. doi: 10.1016/j.jtemb.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 25. Zang Y, Bolger PM. Toxic metals: Cadmium A2 - Motarjemi, Yasmine. Encyclopedia of Food Safety. Waltham: Academic Press; 2014. p. 346-8.
- 26.Filipic M. Mechanisms of cadmium induced genomic instability. Mutat Res. 2012;733(1-2):69–77. doi: 10.1016/j.mrfmmm.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 27.Al-Attar AM, Elnaggar MHR, Almalki EA. Protective effect of some plant oils on diazinon induced hepatorenal toxicity in male rats. Saudi J Biol Sci. 2017;24(6):1162–71. doi: 10.1016/j.sjbs.2016.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sayed MM, Hassanein KMA, Senosy W. Protective effects of thymoquinone and l-cysteine on cadmium-induced reproductive toxicity in rats. Toxicol Rep. 2014;1:612–20. doi: 10.1016/j.toxrep.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagi MN, Mansour MA. Protective effect of thymoquinone against doxorubicin-induced cardiotoxicity in rats: A possible mechanism of protection. Pharmacol Res. 2000;41(3):283–9. doi: 10.1006/phrs.1999.0585. [DOI] [PubMed] [Google Scholar]
- 30.Guerin T, Chekri R, Chafey C, Testu C, Hulin M, Noel L. Mercury in foods from the first French total diet study on infants and toddlers. Food Chem. 2018;239:920–5. doi: 10.1016/j.foodchem.2017.07.039. [DOI] [PubMed] [Google Scholar]
- 31.Ferreira SLC, Lemos VA, Silva LOB, Queiroz AFS, Souza AS, da Silva EGP. et al. Analytical strategies of sample preparation for the determination of mercury in food matrices--a review. Microchem J. 2015;121:227–36. doi: 10.1016/j.microc.2015.02.012. [DOI] [Google Scholar]
- 32.Moura FA, de Andrade KQ, dos Santos JC, Araujo OR, Goulart MO. Antioxidant therapy for treatment of inflammatory bowel disease: Does it work? Redox Biol. 2015;6:617–39. doi: 10.1016/j.redox.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.European Food Safety Authority (EFSA. Scientific opinion on lead dietary exposure in the European population. EFSA Journal. 2012;10(7):2831. doi: 10.2903/j.efsa.2012.2831. [DOI] [Google Scholar]
- 34.Iyer S, Sengupta C, Velumani A. Lead toxicity: An overview of prevalence in Indians. Clin Chim Acta. 2015;451(Pt B):161–4. doi: 10.1016/j.cca.2015.09.023. [DOI] [PubMed] [Google Scholar]
- 35.O’Connor D, Hou D, Ye J, Zhang Y, Ok YS, Song Y. et al. Lead-based paint remains a major public health concern: A critical review of global production, trade, use, exposure, health risk, and implications. Environ Int. 2018;121:85–101. doi: 10.1016/j.envint.2018.08.052. [DOI] [PubMed] [Google Scholar]
- 36.Darakhshan S, Bidmeshki Pour A, Hosseinzadeh Colagar A, Sisakhtnezhad S. Thymoquinone and its therapeutic potentials. Pharmacol Res. 2015;95-96:138–58. doi: 10.1016/j.phrs.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 37.Mahrous KF, Alakilli SY, Khalil WK. The protective effect of thymoquinone against lead acetate induced DNA damage and alterations in tumor initiation genes. Asian J Pharm Clin Res. 2015;8(5):302–8. [Google Scholar]
- 38.You J, Song Z. Determination of picomole concentrations of aluminum (III) in human saliva and urine by a luminol-carboxymethyl chitosan chemiluminescence system. Instrum Sci Technol. 2013;41(5):524–34. doi: 10.1080/10739149.2013.796561. [DOI] [Google Scholar]
- 39.Nehru B, Anand P. Oxidative damage following chronic aluminium exposure in adult and pup rat brains. J Trace Elem Med Biol. 2005;19(2-3):203–8. doi: 10.1016/j.jtemb.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 40.Krzysik M, Grajeta H, Prescha A. Chromium content in selected convenience and fast foods in Poland. Food Chem. 2008;107(1):208–12. doi: 10.1016/j.foodchem.2007.08.006. [DOI] [Google Scholar]
- 41.Diwan H, Ahmad A, Iqbal M. Characterization of chromium toxicity in food crops and their role in phytoremediation. J Bioremediat Biodegrad. 2012;3:159. doi: 10.4172/2155-6199.1000159. [DOI] [Google Scholar]
- 42.Mount DR, Hockett JR. Use of toxicity identification evaluation methods to characterize, identify, and confirm hexavalent chromium toxicity in an industrial effluent. Water Res. 2000;34(4):1379–85. doi: 10.1016/S0043-1354(99)00271-7. [DOI] [Google Scholar]
- 43.Darwish WS, Eldaly EA, El-Abbasy MT, Ikenaka Y, Nakayama S, Ishizuka M. Antibiotic residues in food: The African scenario. Jpn J Vet Res. 2013;61 Suppl:S13–22. [PubMed] [Google Scholar]
- 44.Kalghatgi S, Spina CS, Costello JC, Liesa M, Morones-Ramirez JR, Slomovic S. et al. Bactericidal antibiotics induce mitochondrial dysfunction and oxidative damage in mammalian cells. Sci Transl Med. 2013;5(192):192ra85. doi: 10.1126/scitranslmed.3006055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Al-Ankari AS. Immunomodulating effects of black seed and oxytetracycline in pigeons. Immunopharmacol Immunotoxicol. 2005;27(3):515–20. doi: 10.1080/08923970500242327. [DOI] [PubMed] [Google Scholar]
- 46.Abdel-Daim MM, Ghazy EW. Effects of Nigella sativa oil and ascorbic acid against oxytetracycline-induced hepato-renal toxicity in rabbits. Iran J Basic Med Sci. 2015;18(3):221–7. [PMC free article] [PubMed] [Google Scholar]
- 47.Ali BH. The effect of Nigella sativa oil on gentamicin nephrotoxicity in rats. Am J Chin Med. 2004;32(1):49–55. doi: 10.1142/s0192415x04001710. [DOI] [PubMed] [Google Scholar]
- 48.Yaman I, Balikci E. Protective effects of Nigella sativa against gentamicin-induced nephrotoxicity in rats. Exp Toxicol Pathol. 2010;62(2):183–90. doi: 10.1016/j.etp.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 49.Saleem U, Ahmad B, Rehman K, Mahmood S, Alam M, Erum A. Nephro-protective effect of vitamin C and Nigella sativa oil on gentamicin associated nephrotoxicity in rabbits. Pak J Pharm Sci. 2012;25(4):727–30. [PubMed] [Google Scholar]
- 50.Kaale E, Chambuso M, Kitwala J. Analysis of residual oxytetracycline in fresh milk using polymer reversed-phase column. Food Chem. 2008;107(3):1289–93. doi: 10.1016/j.foodchem.2007.08.075. [DOI] [Google Scholar]
- 51.Heller DN, Clark SB, Righter HF. Confirmation of gentamicin and neomycin in milk by weak cation-exchange extraction and electrospray ionization/ion trap tandem mass spectrometry. J Mass Spectrom. 2000;35(1):39–49. doi: 10.1002/(sici)1096-9888(200001)35:1<39::aidjms911>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 52.Mostafalou S, Nili-Ahmadabadi A, Pourkhalili N, Banani A, Pakzad M, Hassani S. et al. Protective effect of thymoquinone against malathion induced disruption in isolated pancreatic islets of dog. Res Pharm Sci. 2012;7(5):S68. [Google Scholar]
- 53.Mosbah R, Yousef MI, Chettoum A. Nigella sativa oil protects against reproductive toxicity of acetamiprid insecticide in male rats. Toxicol Lett. 2013;221:S218. doi: 10.1016/j.toxlet.2013.05.652. [DOI] [Google Scholar]
- 54.Mosbah R, Djerrou Z, Mantovani A. Protective effect of Nigella sativa oil against acetamiprid induced reproductive toxicity in male rats. Drug Chem Toxicol. 2018;41(2):206–12. doi: 10.1080/01480545.2017.1337127. [DOI] [PubMed] [Google Scholar]
- 55.Mosbah R, Yousef MI, Maranghi F, Mantovani A. Protective role of Nigella sativa oil against reproductive toxicity, hormonal alterations, and oxidative damage induced by chlorpyrifos in male rats. Toxicol Ind Health. 2016;32(7):1266–77. doi: 10.1177/0748233714554675. [DOI] [PubMed] [Google Scholar]
- 56.Mohany M, El-Feki M, Refaat I, Garraud O, Badr G. Thymoquinone ameliorates the immunological and histological changes induced by exposure to imidacloprid insecticide. J Toxicol Sci. 2012;37(1):1–11. doi: 10.2131/jts.37.1. [DOI] [PubMed] [Google Scholar]
- 57.Ince S, Kucukkurt I, Demirel HH, Turkmen R, Zemheri F, Akbel E. The role of thymoquinone as antioxidant protection on oxidative stress induced by imidacloprid in male and female swiss albino mice. Toxicol Environ Chem. 2013;95(2):318–29. doi: 10.1080/02772248.2013.764672. [DOI] [Google Scholar]
- 58.Danaei GH, Karami M. Protective effect of thymoquinone against diazinon-induced hematotoxicity, genotoxicity and immunotoxicity in rats. Environ Toxicol Pharmacol. 2017;55:217–22. doi: 10.1016/j.etap.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 59.Beydilli H, Yilmaz N, Cetin ES, Topal Y, Topal H, Sozen H. et al. The effects of thymoquinone on nitric oxide and superoxide dismutase levels in a rat model of diazinon-induced brain damage. Stud Ethno-Med. 2015;9(2):191–5. doi: 10.1080/09735070.2015.11905434. [DOI] [Google Scholar]
- 60.Mohamadin AM, Sheikh B, Abd El-Aal AA, Elberry AA, Al-Abbasi FA. Protective effects of Nigella sativa oil on propoxur-induced toxicity and oxidative stress in rat brain regions. Pestic Biochem Physiol. 2010;98(1):128–34. doi: 10.1016/j.pestbp.2010.05.011. [DOI] [Google Scholar]
- 61.Pastor-Belda M, Garrido I, Campillo N, Vinas P, Hellin P, Flores P. et al. Determination of spirocyclic tetronic/tetramic acid derivatives and neonicotinoid insecticides in fruits and vegetables by liquid chromatography and mass spectrometry after dispersive liquid-liquid microextraction. Food Chem. 2016;202:389–95. doi: 10.1016/j.foodchem.2016.01.143. [DOI] [PubMed] [Google Scholar]
- 62.Pratheeshkumar N, Chandran M. Method validation, dissipation kinetics and processing factor for acetamiprid residues in cardamom (Elettaria cardamomum L Maton) Pestic Res J. 2015;27(1):96–103. [Google Scholar]
- 63.Kaur RP, Gupta V, Christopher AF, Bansal P. Potential pathways of pesticide action on erectile function – a contributory factor in male infertility. Asian Pac J Reprod. 2015;4(4):322–30. doi: 10.1016/j.apjr.2015.07.012. [DOI] [Google Scholar]
- 64.Racke KD. Environmental fate of chlorpyrifos. Rev Environ Contam Toxicol. 1993;131:1–150. doi: 10.1007/978-1-4612-4362-5_1. [DOI] [PubMed] [Google Scholar]
- 65.Zama D, Meraihi Z, Tebibel S, Benayssa W, Benayache F, Benayache S. et al. Chlorpyrifos-induced oxidative stress and tissue damage in the liver, kidney, brain and fetus in pregnant rats: The protective role of the butanolic extract of Paronychia argentea L. Indian J Pharmacol. 2007;39(3):145–50. doi: 10.4103/0253-7613.33434. [DOI] [Google Scholar]
- 66.Tomizawa M, Casida JE. Neonicotinoid insecticide toxicology: Mechanisms of selective action. Annu Rev Pharmacol Toxicol. 2005;45:247–68. doi: 10.1146/annurev.pharmtox.45.120403.095930. [DOI] [PubMed] [Google Scholar]
- 67.Fenner-Crisp PA, Maciorowski AF, Timm GE. The endocrine disruptor screening program developed by the US Environmental Protection Agency. Ecotoxicology. 2000;9(1-2):85–91. doi: 10.1023/a:1008972330318. [DOI] [Google Scholar]
- 68.Gokcimen A, Gulle K, Demirin H, Bayram D, Kocak A, Altuntas I. Effects of diazinon at different doses on rat liver and pancreas tissues. Pestic Biochem Physiol. 2007;87(2):103–8. doi: 10.1016/j.pestbp.2006.06.011. [DOI] [Google Scholar]
- 69.Ogutcu A, Uzunhisarcikli M, Kalender S, Durak D, Bayrakdar F, Kalender Y. The effects of organophosphate insecticide diazinon on malondialdehyde levels and myocardial cells in rat heart tissue and protective role of vitamin E. Pestic Biochem Physiol. 2006;86(2):93–8. doi: 10.1016/j.pestbp.2006.01.010. [DOI] [Google Scholar]
- 70. Lineback DR, Stadler RH. Introduction to food process toxicants. Process-induced food toxicants: Occurrence, Formation, Mitigation, and Health Risks. John Wiley & Sons, Inc; 2008. p. 1-19.
- 71.Gray JI, Morton ID. Some toxic compounds produced in food by cooking and processing. J Hum Nutr. 1981;35(1):5–23. doi: 10.3109/09637488109143481. [DOI] [PubMed] [Google Scholar]
- 72.Fasano E, Bono-Blay F, Cirillo T, Montuori P, Lacorte S. Migration of phthalates, alkylphenols, bisphenol A and di (2-ethylhexyl) adipate from food packaging. Food Control. 2012;27(1):132–8. doi: 10.1016/j.foodcont.2012.03.005. [DOI] [Google Scholar]
- 73.Badary OA, Abd-Ellah MF, El-Mahdy MA, Salama SA, Hamada FM. Anticlastogenic activity of thymoquinone against benzo(a)pyrene in mice. Food Chem Toxicol. 2007;45(1):88–92. doi: 10.1016/j.fct.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 74.Amin HAM, Arihan O, Ragbetli MC. Effect of thymoquinone administration on erythrocyte fragility in diethylnitrosamine administered rats. J Cell Biotechnol. 2017;3(1):1–7. doi: 10.3233/JCB-179008. [DOI] [Google Scholar]
- 75.Sayed-Ahmed MM, Aleisa AM, Al-Rejaie SS, Al-Yahya AA, Al-Shabanah OA, Hafez MM. et al. Thymoquinone attenuates diethylnitrosamine induction of hepatic carcinogenesis through antioxidant signaling. Oxid Med Cell Longev. 2010;3(4):254–61. doi: 10.4161/oxim.3.4.12714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mehri S, Shahi M, Razavi BM, Hassani FV, Hosseinzadeh H. Neuroprotective effect of thymoquinone in acrylamide-induced neurotoxicity in Wistar rats. Iran J Basic Med Sci. 2014;17(12):1007–11. [PMC free article] [PubMed] [Google Scholar]
- 77.El-Haroun H. Comparative study of the possible protective effect of thymoquinone (black seeds) when compared with vitamin E on acrylamide-induced neurotoxicity in the adult guinea pig cerebellar cortex. Egypt J Histol. 2016;39(2):203–15. [Google Scholar]
- 78.Badary OA, Al-Shabanah OA, Nagi MN, Al-Rikabi AC, Elmazar MM. Inhibition of benzo(a)pyrene-induced forestomach carcinogenesis in mice by thymoquinone. Eur J Cancer Prev. 1999;8(5):435–40. doi: 10.1097/00008469-199910000-00009. [DOI] [PubMed] [Google Scholar]
- 79.Fouda AM, Daba MH, Yousef Ahmed AR. Antigenotoxic effects of thymoquinone against benzo [a] pyrene and mitomycin C-induced genotoxicity in cultured human lymphocytes. Research in Immunology: An International Journal. 2014;2014:535279. doi: 10.5171/2014.535279. [DOI] [Google Scholar]
- 80.Fahmy A. The effect of bisphenol a on the testis of adult male albino rats and the possible protective effect of thymoquinone: A histological and immunohistochemical study. J Cell Tissue Res. 2017;17(1):6021–34. [Google Scholar]
- 81.Abdel-Wahab WM. Thymoquinone attenuates toxicity and oxidative stress induced by bisphenol a in liver of male rats. Pak J Biol Sci. 2014;17(11):1152–60. doi: 10.3923/pjbs.2014.1152.1160. [DOI] [PubMed] [Google Scholar]
- 82.Stauffer UG. [Tooth changes caused by tetracycline in the fetus, infant and child] Schweiz Med Wochenschr. 1967;97(9):291–3. [PubMed] [Google Scholar]
- 83.Mottram DS, Wedzicha BL, Dodson AT. Acrylamide is formed in the Maillard reaction. Nature. 2002;419(6906):448–9. doi: 10.1038/419448a. [DOI] [PubMed] [Google Scholar]
- 84. Hudson NL, Dotson GS. NIOSH skin notation (SK) profile: Acrylic acid [CAS No. 79-10-7]. DHHS publication; 2017.
- 85. International Agency for Research on Cancer (IARC). Benzo[a]pyrene. In: Chemical Agents and Related Occupations: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, No. 100F. Lyon (FR): International Agency for Research on Cancer; 2012. [PMC free article] [PubMed]
- 86.Hussein SA, Abdel-Aal SA, Khalaf HA. Chemopreventive effect of thymoquinone on benzo (a) pyrene-induced lung cancer in male swiss albino mice. Benha Veterinary Medical Journal (BVMJ) 2014;27(2):330–40. [Google Scholar]
- 87.Lorber M, Schecter A, Paepke O, Shropshire W, Christensen K, Birnbaum L. Exposure assessment of adult intake of bisphenol a (BPA) with emphasis on canned food dietary exposures. Environ Int. 2015;77:55–62. doi: 10.1016/j.envint.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Staples CA, Dorn PB, Klecka GM, O’Block ST, Harris LR. A review of the environmental fate, effects, and exposures of bisphenol a. Chemosphere. 1998;36(10):2149–73. doi: 10.1016/s0045-6535(97)10133-3. [DOI] [PubMed] [Google Scholar]
- 89.Hassan ZK, Elobeid MA, Virk P, Omer SA, ElAmin M, Daghestani MH. et al. Bisphenol a induces hepatotoxicity through oxidative stress in rat model. Oxid Med Cell Longev. 2012;2012:194829. doi: 10.1155/2012/194829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nakagawa Y, Tayama S. Metabolism and cytotoxicity of bisphenol a and other bisphenols in isolated rat hepatocytes. Arch Toxicol. 2000;74(2):99–105. doi: 10.1007/s002040050659. [DOI] [PubMed] [Google Scholar]
- 91.Zain ME. Impact of mycotoxins on humans and animals. J Saudi Chem Soc. 2011;15(2):129–44. doi: 10.1016/j.jscs.2010.06.006. [DOI] [Google Scholar]
- 92.Bennett JW, Klich M. Mycotoxins. Clin Microbiol Rev. 2003;16(3):497–516. doi: 10.1128/cmr.16.3.497-516.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Halamova K, Kokoska L, Flesar J, Sklenickova O, Svobodova B, Marsik P. In vitro antifungal effect of black cumin seed quinones against dairy spoilage yeasts at different acidity levels. J Food Prot. 2010;73(12):2291–5. doi: 10.4315/0362-028x-73.12.2291. [DOI] [PubMed] [Google Scholar]
- 94.EL-Sawi NM, Al-Seeni MN, Younes SHH, AL-Jahdali SM, Ali SS. Biochemical and histological studies on the effect of zearalenone mycotoxin and thymoquinone on male mice kidney. J Am Sci. 2012;8(10):378–88. [Google Scholar]
- 95.Ouanes Z, Abid S, Ayed I, Anane R, Mobio T, Creppy EE. et al. Induction of micronuclei by zearalenone in vero monkey kidney cells and in bone marrow cells of mice: protective effect of vitamin E. Mutat Res Genet Toxicol Environ Mutagen. 2003;538(1):63–70. doi: 10.1016/S1383-5718(03)00093-7. [DOI] [PubMed] [Google Scholar]
- 96.Alhussaini MS, AL-Yahya AA. Protective role of Nigella sativa oil on ochratoxin a toxicity in liver and kidney of male albino rats: Histological and histochemical studies. Wulfenia. 2014;21(4):59–77. [Google Scholar]
- 97.El-Sawi NM, Gashlan HM. Effect of Nigella sativa oil and activated charcoal as antioxidant on Verrucarin J induced hepatotoxicity in male rats. J Appl Anim Res. 2010;37(2):285–8. doi: 10.1080/09712119.2010.9707143. [DOI] [Google Scholar]
- 98.Nili-Ahmadabadi A, Tavakoli F, Hasanzadeh G, Rahimi H, Sabzevari O. Protective effect of pretreatment with thymoquinone against aflatoxin b(1) induced liver toxicity in mice. Daru. 2011;19(4):282–7. [PMC free article] [PubMed] [Google Scholar]
- 99.Abdel-Wahhab MA, Aly SE. Antioxidant property of Nigella sativa (black cumin) and Syzygium aromaticum (clove) in rats during aflatoxicosis. J Appl Toxicol. 2005;25(3):218–23. doi: 10.1002/jat.1057. [DOI] [PubMed] [Google Scholar]
- 100.Mirocha CJ, Pathre SV, Schauerhamer B, Christensen CM. Natural occurrence of fusarium toxins in feedstuff. Appl Environ Microbiol. 1976;32(4):553–6. doi: 10.1128/aem.32.4.553-556.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Baudrimont I, Sostaric B, Yenot C, Betbeder AM, Dano-Djedje S, Sanni A. et al. Aspartame prevents the karyomegaly induced by ochratoxin a in rat kidney. Arch Toxicol. 2001;75(3):176–83. doi: 10.1007/s002040100229. [DOI] [PubMed] [Google Scholar]
- 102.Magnoli C, Astoreca A, Ponsone ML, Fernandez-Juri MG, Barberis C, Dalcero AM. Ochratoxin A and Aspergillus section Nigri in peanut seeds at different months of storage in Córdoba, Argentina. Int J Food Microbiol. 2007;119(3):213–8. doi: 10.1016/j.ijfoodmicro.2007.07.056. [DOI] [PubMed] [Google Scholar]
- 103. International Agency for Research on Cancer (IARC). IARC monographs on the evaluation of carcinogenic risk to humans: Some naturally occurring substances; food items and constituents, heterocyclic aromatic amines and mycotoxins. Lyon, France: IARC; 1993.
- 104.el Khoury A, Atoui A. Ochratoxin A: General overview and actual molecular status. Toxins (Basel) 2010;2(4):461–93. doi: 10.3390/toxins2040461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rocha O, Ansari K, Doohan FM. Effects of trichothecene mycotoxins on eukaryotic cells: A review. Food Addit Contam. 2005;22(4):369–78. doi: 10.1080/02652030500058403. [DOI] [PubMed] [Google Scholar]
- 106.Jarvis BB, Miller JD. Mycotoxins as harmful indoor air contaminants. Appl Microbiol Biotechnol. 2005;66(4):367–72. doi: 10.1007/s00253-004-1753-9. [DOI] [PubMed] [Google Scholar]
- 107.Tavakkoli A, Ahmadi A, Razavi BM, Hosseinzadeh H. Black seed (Nigella sativa) and its constituent thymoquinone as an antidote or a protective agent against natural or chemical toxicities. Iran J Pharm Res. 2017;16(Suppl):2–23. [PMC free article] [PubMed] [Google Scholar]
- 108. Diaz DE. The Mycotoxin Blue. UK: Nottingham University Press; 2005.
- 109. Gold L, Slone T, Manley N, Garfinkel G, Ames B. Summary table by chemical of carcinogenicity results in CPDB on 1485 chemicals. 2005. http://potency.berkeley.edu/pdfs/ChemicalTable.pdf.
- 110.Meissonnier GM, Pinton P, Laffitte J, Cossalter AM, Gong YY, Wild CP. et al. Immunotoxicity of aflatoxin B1: Impairment of the cell-mediated response to vaccine antigen and modulation of cytokine expression. Toxicol Appl Pharmacol. 2008;231(2):142–9. doi: 10.1016/j.taap.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 111.Mohammadzadeh-Aghdash H, Ezzati Nazhad Dolatabadi J, Dehghan P, Panahi-Azar V, Barzegar A. Multi-spectroscopic and molecular modeling studies of bovine serum albumin interaction with sodium acetate food additive. Food Chem. 2017;228:265–9. doi: 10.1016/j.foodchem.2017.01.149. [DOI] [PubMed] [Google Scholar]
- 112.Sohrabi Y, Panahi-Azar V, Barzegar A, Ezzati Nazhad Dolatabadi J, Dehghan P. Spectroscopic, thermodynamic and molecular docking studies of bovine serum albumin interaction with ascorbyl palmitate food additive. Bioimpacts. 2017;7(4):241–6. doi: 10.15171/bi.2017.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hamishehkar H, Khani S, Kashanian S, Ezzati Nazhad Dolatabadi J, Eskandani M. Geno- and cytotoxicity of propyl gallate food additive. Drug Chem Toxicol. 2014;37(3):241–6. doi: 10.3109/01480545.2013.838776. [DOI] [PubMed] [Google Scholar]
- 114.Mohammadzadeh-Aghdash H, Sohrabi Y, Mohammadi A, Shanehbandi D, Dehghan P, Ezzati Nazhad Dolatabadi J. Safety assessment of sodium acetate, sodium diacetate and potassium sorbate food additives. Food Chem. 2018;257:211–5. doi: 10.1016/j.foodchem.2018.03.020. [DOI] [PubMed] [Google Scholar]
- 115.Badary OA, Taha RA, Gamal el-Din AM, Abdel-Wahab MH. Thymoquinone is a potent superoxide anion scavenger. Drug Chem Toxicol. 2003;26(2):87–98. doi: 10.1081/dct-120020404. [DOI] [PubMed] [Google Scholar]
- 116.Daba MH, Abdel-Rahman MS. Hepatoprotective activity of thymoquinone in isolated rat hepatocytes. Toxicol Lett. 1998;95(1):23–9. doi: 10.1016/s0378-4274(98)00012-5. [DOI] [PubMed] [Google Scholar]
- 117.Al-Ghamdi MS. The anti-inflammatory, analgesic and antipyretic activity of Nigella sativa. J Ethnopharmacol. 2001;76(1):45–8. doi: 10.1016/s0378-8741(01)00216-1. [DOI] [PubMed] [Google Scholar]
- 118.Parveen S, Bachar SC, Begum ZA. Effect of Nigella sativa Linn (Ranunculaceae) ground seed extract on carrageenan induced inflammation in rats. Ibrahim Med Coll J. 2011;5(1):22–4. [Google Scholar]
- 119.Kashanian S, Dolatabadi JEN. DNA binding studies of 2-tert-butylhydroquinone (TBHQ) food additive. Food Chem. 2009;116(3):743–7. doi: 10.1016/j.foodchem.2009.03.027. [DOI] [Google Scholar]
- 120.Eskandani M, Hamishehkar H, Ezzati Nazhad Dolatabadi J. Cytotoxicity and DNA damage properties of tert-butylhydroquinone (TBHQ) food additive. Food Chem. 2014;153:315–20. doi: 10.1016/j.foodchem.2013.12.087. [DOI] [PubMed] [Google Scholar]


