Abstract
Background and Purpose
We investigated the inhibitory effect and associated molecular mechanisms of tolvaptan on angiotensin II (AngII)‐induced aldosterone production in vitro and in vivo.
Experimental Approach
In vitro, H295R human adrenocarcinoma cells were incubated with 1 μmol·L−1 arginine vasopressin (AVP) or dDAVP, or tolvaptan (0.1, 1, and 3 μmol·L−1) in the presence and absence of 100 nmol·L−1 of AngII. In vivo, Sprague–Dawley rats were treated with tolvaptan 0.05% in the diet for 6 days in the presence and absence of 200 pmol·min−1 AngII.
Key Results
Tolvaptan suppressed AngII‐induced aldosterone production in a dose‐dependent manner in H295R cells, whereas neither AVP nor dDAVP in the presence or absence of AngII altered aldosterone production, suggesting the vasopressin V2 receptor was not involved in the inhibitory effect of tolvaptan on aldosterone synthesis. In addition, tolvaptan inhibited the AngII‐induced increase in aldosterone synthase (CYP11B2) protein levels without suppressing CYP11B2 mRNA expression. Notably, tolvaptan increased the levels of unfolded protein response (UPR) marker DDIT3 and eIF2α phosphorylation (a UPR‐induced event), which could block the translation of CYP11B2 mRNA into protein and thereby inhibit aldosterone production. In vivo, tolvaptan significantly inhibited AngII‐induced increases in serum and adrenal aldosterone levels and CYP11B2 protein levels. This anti‐aldosterone effect was associated with a reduction in the elevated systolic and diastolic BP.
Conclusions and Implications
Tolvaptan inhibited AngII‐stimulated aldosterone production via a V2 receptor‐independent pathway, which can counteract or even surpass its potential activating effect of diuresis‐induced aldosterone secretion in certain aldosterone‐mediated pathological conditions.
Abbreviations
- AngII
angiotensin II
- AVP
arginine vasopressin
- Cont
control
- DBP
diastolic BP
- dDAVP
1‐deamino‐8‐d‐arginine vasopressin
- DDIT3
DNA damage‐induced transcript 3
- eIF2α
eukaryotic initiation factor 2α
- HF
heart failure
- RAAS
renin–angiotensin–aldosterone system
- SBP
systolic BP
- V2 receptor
vasopressin V2 receptor
What is already known
The final step of aldosterone production in the adrenal gland is catalysed by CYP11B2.
Tolvaptan therapy avoids stimulation of aldosterone production despite its strong diuretic effect in HF.
What this study adds
Tolvaptan suppresses adrenal aldosterone production in response to AngII via an AVP–V2 receptor‐independent mechanism.
Tolvaptan attenuated AngII‐induced CYP11B2 protein synthesis through regulation of posttranscriptional processes.
What is the clinical significance
Tolvaptan might have a protective role against deleterious effects of excess aldosterone produced in certain pathological conditions.
1. INTRODUCTION
Aldosterone, a major mineralocorticoid, the synthesis and secretion of which is stimulated by angiotensin II (AngII), is normally under the tight regulation of the renin–angiotensin–aldosterone system (RAAS). Aldosterone plays an important role in the pathophysiology of hypertension, left ventricular hypertrophy, and heart failure (HF). It induces sodium and fluid retention, myocardial fibrosis, vascular stiffening, endothelial dysfunction, catecholamine release, and cardiac arrhythmias, all of which lead to the worsening of HF. Indeed, mineralocorticoid receptor (MR) antagonists effectively reduce BP in hypertensive individuals (Souza, Muxfeldt, Fiszman, & Salles, 2010) and improve morbidity and mortality in HF patients even if they are already on angiotensin‐converting enzyme inhibitors or AngII‐receptor blockers (Zannad et al., 2011; Miller, 2007). Despite their proven clinical efficacy, MR antagonists induce a broad range of side effects including hyperkalaemia and worsening renal function (Grune et al., 2018). Again, conventional diuretics have been widely used for treating HF, but they activate the RAAS and adversely affect renal function (Dohi & Ito, 2014).
Tolvaptan , a selective vasopressin V2 receptor antagonist, has been approved by the U.S. Food and Drug Administration in 2009 for the treatment of euvolaemic and hypervolaemic hyponatraemia associated with HF and in Japan for volume overload in HF patients since 2010. Tolvaptan promotes aquaresis by inhibiting the binding of vasopressin to the V2 receptor, a process that normally reduces free water clearance via cAMP‐stimulated aquaporin‐2 translocation into the apical plasma membrane of the principal cells in the collecting duct of the kidney (Tamma et al., 2017). Several experimental and clinical trials have shown the effectiveness of tolvaptan for improving symptoms and decreasing body fluids in HF patients. Importantly, several experimental (Miyazaki, Fujiki, Yamamura, Nakamura, & Mori, 2007) and clinical studies (Boerrigter et al., 2006; Jujo et al., 2016) have reported that tolvaptan therapy avoids RAAS activation despite its strong diuretic effect in HF. A recent experimental study in our laboratory has demonstrated that chronic tolvaptan therapy suppressed plasma aldosterone level in a rat model of pulmonary arterial hypertension (Goto et al., 2016). However, the precise effect of tolvaptan on the RAAS, especially under pathological conditions in which the adrenals produce more aldosterone in response to AngII, is not established.
Aldosterone synthesis in the adrenal zona glomerulosa cells is firmly regulated by the selective expression of the enzyme aldosterone synthase encoded by CYP11B2 (Hattangady, Olala, Bollag, & Rainey, 2012). Several studies have reported the expression of ectopic V2 receptors in the adrenal glands (Lee et al., 2005; Louiset et al., 2008). Moreover, recent experimental studies have strongly supported the existence of an extrarenal V2 receptor (Juul, Bichet, Nielsen, & Norgaard, 2014), including in transformed epithelial cells (North, 2000) and a number of human tumour cell lines (Iannucci et al., 2011; Noh et al., 2009; Wang et al., 2012). Considering the above, tolvaptan might have direct effects on adrenal aldosterone production via V2 receptor antagonism. Therefore, we investigated the potential of tolvaptan to affect the regulation of adrenal aldosterone synthesis and studied the molecular mechanisms using aldosterone‐producing H295R human adrenocarcinoma cells and an AngII‐induced hyperaldosterone rat model.
2. METHODS
2.1. In vitro studies
2.1.1. Cell culture
The human adrenocortical carcinoma cell line NCI‐H295R (Cat# CRL‐2128, RRID:CVCL_0458; Rainey, Bird, & Mason, 1994) was obtained from the ATCC (American Type Culture Collection, Manassas, VA) and cultured in DMEM/F12 (1:1) medium supplemented with 2.5% Nu‐serum, 1% ITS+ premix supplement (insulin, 6.25 mg·ml−1; transferrin, 6.25 mg·ml−1; selenium, 6.25 ng·ml−1; and linoleic acid, 5.35 mg·ml−1), and antibiotics (penicillin and streptomycin). The cells were maintained at 37°C in a humidified atmosphere containing air and carbon dioxide (95%/5%, vol/vol). The H295R cell lines provide a good in vitro system for the analysis of the human adrenal steroidogenic pathway at the level of hormone production and gene expression (Oskarsson, Ulleras, Plant, Hinson, & Goldfarb, 2006).
2.1.2. RNA isolation, cDNA synthesis, and real‐time quantitative PCR
Total RNA was extracted from H295R cells using the RNeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer's protocol. Purity and integrity of the RNA were checked spectroscopically using DS‐11 Nanopad (DeNovix®, Wilmington, DE, USA). From each sample, an equal amount of RNA was reverse transcribed to obtain the cDNA template using the SuperScript II RT (Invitrogen) following the manufacturer's protocol. Quantitative real‐time PCR was performed using the 7300 Real Time PCR Systems (Applied Biosystems). Amplification was performed with the following time course: preincubation at 95°C for 10 min, 40–50 cycles of 95°C for 10 s, 60°C for 5 s, and 72°C for 15 s; using a 20 μl total volume consisting of cDNA, Eagle Taq Master Mix (Roche Molecular Systems, Inc), primer‐probe mix, and GAPDH. Water instead of cDNA was used as the negative control (Cont). The mRNA levels were normalized to the levels of GAPDH housekeeping gene.
The TaqMan probe sets for human CYP11B2 (Hs01597732_m1) and GAPDH (Hs99999905_m1) were purchased from Applied Biosystems. The relative expression values of mRNA were measured by the 2−ΔΔCt method (Livak & Schmittgen, 2001). Statistical analysis was then performed by calculating the Ct (cycle‐to‐threshold) values obtained. ΔCt, correction for loading Cont, is equal to Cttarget − Ctreference, where reference is the Ct value for GAPDH; ΔΔCt is calculated as ΔCttreated − ΔCtcontrol.
2.2. In vivo studies
2.2.1. Animals
Male Sprague–Dawley (SD) rats (RGD Cat# 12910483, RRID:RGD_12910483), aged 4 weeks (145–155 g), purchased from Japan SLC, Shizuoka, Japan, and were housed in single cages (four rats per cage) with wood‐derived bedding material in a specific pathogen‐free facility. The animals were allowed free access to tap water and standard rat chow under controlled temperature (21 ± 1°C) and humidity (55 ± 2%) with a 12:12‐hr light–dark cycle. All experimental protocols for the present study were approved by the Mie University Board Committee for Animal Care and Use (approval number, 29‐2) and conducted strictly in accordance to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (8th Edition, Institute for Laboratory Animal Research).
As adrenal aldosterone synthesis is tightly regulated by AngII, we adopted an AngII‐infused SD rat model to elucidate the effects of tolvaptan in order to minimize the HF‐related confounding factors including neurohormonal activation and cardiorenal haemodynamic alterations. Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny, Browne, Cuthill, Emerson, & Altman, 2010) and with the recommendations made by the British Journal of Pharmacology.
2.2.2. Experimental protocol
Protocol 1: Dose–response analysis of AngII
For the preliminary study, the rats were divided into four groups (n = 5 for each) for a 6‐day treatment with AngII at different doses (0, 20, 60, and 200 pmol·min−1) dissolved in normal saline. After acclimatization for 1 week, the rats were anaesthetized using isoflurane (induction 5%, maintenance 2.0–2.5% mixed with 100% oxygen), and blood was collected from the tail vein at baseline. One week later, the rats were again anaesthetized with i.p. injection of ketamine (50 mg·kg−1) and xylazine (10 mg·kg−1) for implantation of a miniosmotic pump (model 2001; Alzet Durect™ Corp., Cupertino, CA 95014) to infuse the indicated doses of AngII s.c. on the dorsum of the neck. BP was measured by a programmable sphygmomanometer (BP‐98AW, Softron Corp., Tokyo, Japan) using the tail‐cuff method at two time points: (a) the day before pump implantation and (b) the day before dissection. At the end of the treatment, the rats were re‐anaesthetized by continuous isoflurane inhalation, and tail blood was collected in a serum collection tube. Then a longitudinal incision of the abdominal wall was performed, and the rats were killed by direct cardiac puncture. The adrenal glands were removed immediately, frozen in liquid nitrogen, and stored at −80°C until further analysis for CYP11B2 protein‐level measurement by Western blotting.
Protocol 2: Investigation of the effect of tolvaptan on the responses induced by AngII
In Protocol 2, the rats were divided into four groups (n = 8 each) and randomized to receive normal saline as vehicle Cont group, AngII treatment group, vehicle and tolvaptan treatment group, and AngII and tolvaptan treatment (AngII + tolvaptan) group (Figure S1). After 1‐week of acclimatization, rats were placed individually into the metabolic cages for 24 hr for baseline measurement of urine parameters and then underwent BP measurement and blood sample collection. After a 1‐week recovery period, the rats in the AngII and AngII + tolvaptan groups were surgically implanted with a miniosmotic pump to infuse 200 pmol·min−1 of AngII. The dose of 200 pmol·min−1 AngII has been selected on the basis of our findings from Protocol 1. Minipumps containing vehicle alone were implanted into the rats in the control and tolvaptan groups. In the tolvaptan and AngII + tolvaptan groups, 0.05% tolvaptan in the diet was started 24 hr after the initiation of AngII infusion, because the pumping of AngII started approximately 24 hr after implantation (Lu et al., 2015). This dose of tolvaptan was chosen from our recent (Goto et al., 2016) and previous (Morooka et al., 2012) studies. Then the rats of all four groups were individually placed into the metabolic cages, where they had free access to food and water, for the assessment of fluid and food intake and urine volume for 24 hr. After 6 days of treatment, the rats underwent BP measurement. Finally, under continuous isoflurane anaesthesia, blood was collected from the tail vein, and the adrenal glands were removed. Blood samples were centrifuged at 1,431× g for 20 min at 4°C to obtain the blood serum. Aliquots of urine and serum samples were kept at −20°C and − 80°C, respectively, until further analysis. The adrenal glands were kept at −80°C until further analysis for adrenal aldosterone‐level and CYP11B2 protein‐level measurement. No animal losses occurred throughout the study period, and all animals were used for all of the analyses, which were performed by investigators blinded to group assignment. To facilitate this, the samples were recorded by a technician (R. I., in the Acknowledgements) during collection.
2.2.3. Aldosterone production analysis
Aldosterone from H295R cells
H295R cells were cultured in 12‐well plates until about 80% confluence. The medium was then replaced with fresh reduced serum medium (0.2% Nu‐serum) and incubated for an additional 24 hr (serum starvation). Then the culture supernatants were replaced with the reduced serum experimental medium containing vehicle (0.1% DMSO) and AngII with and without tolvaptan. In some experiments, arginine vasopressin (AVP) and desmopressin (dDAVP) were used in the presence and absence of AngII. After 24 hr of incubation, the supernatants were collected and stored at −80°C.
Aldosterone from rat adrenal glands
The whole rat adrenal glands were individually homogenized in ice‐cold RIPA buffer containing 50 mmol·L−1 Tris–HCl, pH 7.4, and with 1 mmol·L−1 NaF, 150 mmol·L−1 NaCl, 10 mmol·L−1 Na4P2O7, 1 mmol·L−1 Na3VO4, 0.25% sodium deoxycholate, 1 mmol·L−1 EDTA, 1% Nonidet P‐40, 1 mmol·L−1 DTT, and 1× protease inhibitor mixture (Roche). The homogenates obtained were centrifuged at 1,610× g for 15 min at 4°C. The supernatants were collected and stored at −80°C.
Aldosterone levels in the supernatants from both H295R cells and rat adrenals were then measured by submitting them to a clinical laboratory testing service (SRL, Tokyo, Japan). Aldosterone levels were normalized to the total protein content of the respective sample, which was measured by performing the bicinchoninic acid (BCA) protein assay using a BCA kit (Pierce Chemical, Rockford, IL, USA).
2.2.4. Blood serum and urine measurements
Serum and urine electrolytes, urea nitrogen, creatinine, osmolality, serum aldosterone, and urine total protein were measured by a clinical laboratory testing service (SRL, Tokyo, Japan). Electrolyte clearance (E‐Cosm), electrolyte‐free water clearance (E‐CH2O), osmolar clearance (C‐Cosm), and free water clearance (C‐CH2O) were calculated as follows (Veeraveedu et al., 2008):
where UNa is the urinary sodium concentration, UK is the urinary potassium concentration, UV is the urine volume, SNa is the serum sodium concentration, Uosm is the urinary osmolality, and Sosm is the serum osmolality.
2.2.5. Western blotting for proteins from H295R cells and rat adrenal glands
All Western blotting procedures and analysis comply and adhere with the recommendations on goals and practicalities of immunoblotting in pharmacology (Alexander et al., 2018).
Proteins from H295R cells
H295R cells incubated for 20–24 hr in 0.1% low‐serum medium were treated with the appropriate agents as indicated. Cells were solubilized in ice‐cold RIPA buffer and 1× protease inhibitor mixture (Roche). The cell lysates were centrifuged, and the supernatants were mixed with 2× SDS sample buffer and then boiled at 97°C for 5 min. The proteins were separated through 12.5% SDS‐PAGE gels and electrophoretically transferred to PVDF membrane (EMD Millipore, Darmstadt, Germany). The membranes were blocked using 5% non‐fat dry milk for 1 hr and then incubated in 1% non‐fat dry milk containing human‐specific CYP11B2 antibody (mouse, 1:1,000 dilution), phospho‐ERK and ERK antibodies (rabbit, 1:1,000 dilution), DDIT3/GADD153/CHOP antibody (mouse, 1:1,000 dilution), (AVPR V2) V2 receptor antibody (rabbit, 1:500 dilution), and phospho‐eIF2α (Ser51) and eIF2α antibodies (rabbit, 1:1,000 dilution) overnight at 4°C with constant rocking. The membranes were further incubated for 1 hr at room temperature in 1% non‐fat dry milk that contained HRP‐conjugated secondary antibodies and then extensively washed in Tris‐buffered saline containing 0.1% Tween 20. The membranes were stripped and re‐incubated with anti‐β‐actin antibody (rabbit, 1:10,000 dilution) for normalization. Chemiluminescence was performed for visualization using the Amersham ECL advance Western blotting detection reagent (GE Healthcare Life Sciences, UK). The protein bands were obtained using ImageQuant LAS 4000 (Fuji, Tokyo, Japan), and the signal densities were quantified by ImageJ software (ImageJ, RRID:SCR_003070; National Institutes of Health).
Proteins from rat adrenal glands
The whole rat adrenal glands were individually homogenized in ice‐cold RIPA buffer and 1× protease inhibitor mixture (Roche). The homogenates obtained were sonicated and centrifuged at 1,610× g for 15 min at 4°C. The supernatant was collected, and total protein concentration was measured using a BCA Protein Assay (Pierce Chemical, Rockford, IL, USA). To the supernatant was added 2× SDS sample buffer (1:1) and then boiled at 97°C for 5 min. The samples were then separated on SDS‐PAGE (12.5% gel) and transferred to PVDF membrane. The membrane was then blocked for 1 hr and then probed with a rat‐specific CYP11B2 antibody (rabbit, 1:500 dilution) in 1% non‐fat dry milk, overnight at 4°C. This was further probed with HRP‐conjugated secondary antibody for 1 hr at room temperature and then washed in Tris‐buffered saline containing 0.1% Tween 20. The membrane was stripped and re‐incubated with anti‐β‐actin antibody (rabbit, 1:10,000 dilution) for normalization. The visualization and quantification of bands were performed as mentioned above for H295R cells.
2.3. Statistical analysis
The data and statistical analysis comply with the recommendations of the British Journal of Pharmacology on experimental design and analysis in pharmacology.
All data are represented as mean ± SD and % change (or fold mean where appropriate) versus control. Statistical analyses were performed with one‐way ANOVA, followed by Bonferroni's post hoc test for multiple comparisons. For the in vivo experiments, repeated measures ANOVA were performed to analyse haemodynamic, serum, and urine parameters when appropriate. The post hoc tests were run only if F achieved P < 0.05 and there was no significant variance inhomogeneity. In each experiment, n represents the number of separate experiments (in vitro) and the number of rats (in vivo). Technical replicates were used to ensure the reliability of single values. A P value <0.05 was considered as statistically significant. The SPSS version 13.0 (SPSS, RRID:SCR_002865) was used for all statistical tests.
2.4. Materials
AngII was purchased from Sigma‐Aldrich (St. Louis, MO, USA), AVP and 1‐deamino‐8‐d‐arginine vasopressin (dDAVP) from Tocris Bioscience (IO Centre, Bristol, UK), and tolvaptan from Wako Pure Chemical (Osaka, Japan). DMEM/F12 (1:1) medium was purchased from Gibco (Invitrogen Life Technologies, Carlsbad, CA, USA). Nu‐serum was obtained from BD Biosciences (Bedford, MA, USA). Penicillin–streptomycin was purchased from Sigma‐Aldrich. ITS+ Premix Universal Culture Supplement was purchased from Discover Labware Inc. (Bedford, MA, USA). Trypsin–EDTA (0.25%) was obtained from Life Technologies. Anti‐CYP11B2, clone 41‐17B, mouse monoclonal IgG1k antibody (Cat# MABS1251, lot# 2782057, RRID:AB_2783793) was purchased from EMD Millipore (Temecula, CA, USA) and was used only for the time course analysis of CYP11B2 protein levels by AngII in H295R cells. The human‐specific mouse anti‐CYP11B2 (Cat# CYP11B2, RRID:AB_2650562) and the rat‐specific rabbit anti‐CYP11B2 (Cat# 2066, RRID:AB_2716717) antibodies were generous gifts from Dr. Celso E. Gomez‐Sanchez (University of Mississippi Medical Center, Jackson, MS). The rabbit anti‐phospho‐ERK (Cat# 9101, lot# 29, RRID:AB_331646) and total ERK (Cat# 9102, lot# 26, RRID:AB_330744) and anti‐phospho‐eIF2α (Ser51; Cat# 9721, lot# 15, RRID:AB_330951) and total eIF2α (Cat# 9722, lot# 15, RRID:AB_2230924) antibodies were obtained from Cell Signaling Technology (Danvers, MA, USA). The mouse anti‐DDIT3/GADD153/CHOP (Cat# sc‐56107, sample lot# E0918, RRID:AB_783507) antibody was obtained from Santa Cruz Biotechnology (Dallas, TX, USA). The rabbit anti‐(AVPR V2) V2 receptor (Cat# ab108145, lot# GR68616‐6, RRID:AB_10892181) antibody was purchased from Abcam (Cambridge, MA, USA). The rabbit anti‐β‐actin (Cat# A5441, RRID:AB_476744) antibody was obtained from Sigma. The HRP‐conjugated donkey anti‐rabbit (Cat# NA934, RRID:AB_772206) and sheep anti‐mouse (Cat# NA9310‐1 ml, RRID:AB_772193) secondary antibodies were obtained from GE Healthcare UK Ltd.
2.5. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander, Christopoulos, et al., 2017; Alexander, Fabbro, et al., 2017; Alexander, Kelly, et al., 2017).
3. RESULTS
3.1. In vitro results
3.1.1. Tolvaptan inhibited AngII‐induced aldosterone production in H295R cells
AngII stimulated aldosterone production in a concentration‐dependent manner in H295R cells (Figure 1a), and this was consistent with results reported previously (Romero, Plonczynski, Vergara, Gomez‐Sanchez, & Gomez‐Sanchez, 2004). Tolvaptan at 0.1, 1, and 3 μmol·L−1 effectively and dose‐dependently inhibited AngII‐induced aldosterone production (Figure 1b). To analyse the responses of V2 receptor agonists in adrenal steroidogenesis, we treated the H295R cells with 1 μmol·L−1 AVP or dDAVP in the presence and absence of AngII (100 nmol·L−1); both treatments had no effect on aldosterone production (Figure 1c), suggesting an AVP–V2 receptor‐independent effect of tolvaptan in the reduction of AngII‐induced aldosterone levels.
Figure 1.
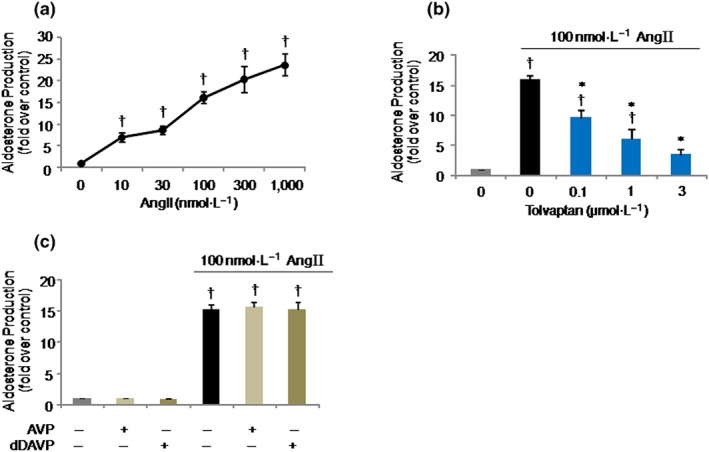
Effects of tolvaptan and arginine vasopressin (AVP)/1‐deamino‐8‐d‐arginine vasopressin (dDAVP) on angiotensin II (AngII)‐induced aldosterone production in H295R cells. (a) Dose‐dependency of AngII on aldosterone production. Cells were stimulated with AngII at the indicated doses for 24 hr. (b) Effect of tolvaptan on AngII‐induced aldosterone production. Cells were pretreated with tolvaptan at the indicated doses for 30 min and then stimulated with 100 nmol·L−1 AngII for 24 hr. (c) Effect of 1 μmol·L−1 AVP or dDAVP on aldosterone production with and without 100 nmol·L−1 AngII for 24 hr. Results are shown as mean ± SD (n = 5 separate experiments performed in duplicate). † P ˂ 0.05 versus Cont; *P ˂ 0.05 versus AngII
3.1.2. Effect of tolvaptan on AngII‐induced CYP11B2 mRNA and protein expression in H295R cells
CYP11B2 mRNA expression was significantly increased by the stimulation with 100 nmol·L−1 AngII, and the maximum level was observed at 12 hr (Figure 2a). This result was in agreement with those reported previously (Felizola et al., 2014; Romero et al., 2004). There was a tendency for AngII‐induced CYP11B2 mRNA expression to be increased by tolvaptan at all the indicated doses, but this was not significant (Figure 2b). Even 24 hr after the treatment with tolvaptan (1 μmol·L−1), no significant effect on AngII‐induced CYP11B2 mRNA expression was observed (Figure S2).
Figure 2.
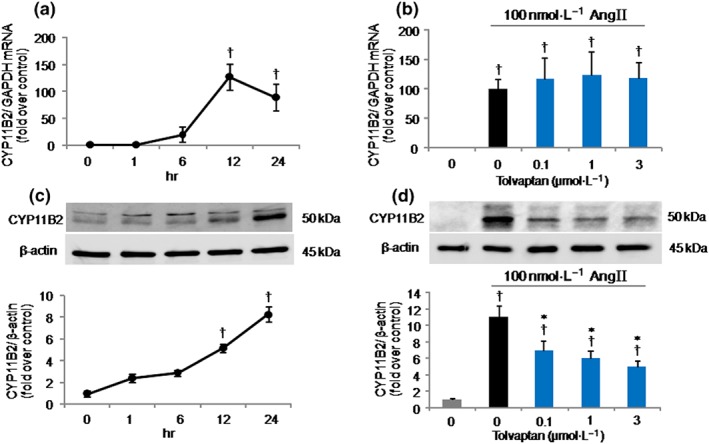
Effects of tolvaptan on CYP11B2 mRNA and protein expressions in H295R cells stimulated with angiotensin II (AngII). (a) Time course of the increase in CYP11B2 mRNA expression by AngII. Cells were treated with 100 nmol·L−1 AngII for the indicated periods, and CYP11B2 mRNA expression was measured by quantitative real‐time PCR. (b) Effect of tolvaptan on AngII‐induced CYP11B2 mRNA expression. Cells were pretreated with tolvaptan at the indicated doses for 30 min and then stimulated with AngII for 12 hr. (c) Time course of the increase in CYP11B2 protein levels induced by AngII. Cells were incubated with 100 nmol·L−1 AngII for the indicated periods, and CYP11B2 protein level was analysed by Western blotting (antibody used: Millipore). Upper, representative immunoblots; lower, relative quantification of protein expression. (d) Effect of tolvaptan on AngII‐induced CYP11B2 protein levels. Cells were pretreated with tolvaptan at the indicated doses for 30 min and then stimulated by 100 nmol·L−1 AngII for 24 hr. CYP11B2 protein level was analysed by Western blotting (antibody used: Dr Celso E. Gomez‐Sanchez). Upper, representative immunoblots; lower, relative quantification of protein expression (n = 5 separate experiments performed in duplicate). Results are shown as mean ± SD. † P ˂ 0.05 versus Cont; *P ˂ 0.05 versus AngII
As shown in Figure 2c, 100 nmol·L−1 AngII increased CYP11B2 protein levels in a time‐dependent manner up to 24 hr. Addition of tolvaptan at 0.1, 1, and 3 μmol·L−1 to the AngII‐treated cells significantly suppressed CYP11B2 protein levels stimulated by AngII in a dose‐dependent fashion (Figure 2d). The inhibitory effect of tolvaptan on AngII‐induced aldosterone production, therefore, can simply be explained by its ability to inhibit AngII‐induced CYP11B2 protein synthesis. We considered whether toxic effects of tolvaptan, if any, were responsible for the reduction of protein levels obtained in our experiment. However, the trypan blue cell viability assay (Figure S3a) and the MTT test (Figure S3b) results showed that tolvaptan did not affect the total number of viable cells and consequently the proliferation of H295R cells, suggesting no cytotoxic effect of tolvaptan. The results that tolvaptan inhibited AngII‐induced CYP11B2 protein levels without affecting its mRNA expression levels indicate that tolvaptan might have an inhibitory role in the translational processes requiring CYP11B2 protein synthesis, which can be largely responsible for the inhibition of aldosterone production.
3.1.3. Effect of tolvaptan on AngII‐induced ERK and eIF2α phosphorylation and DDIT3 protein expression in H295R cells
To figure out the possible mechanisms by which tolvaptan inhibits aldosterone production, we analysed the AngII‐response pathway in adrenal steroidogenesis. Using Western blot analysis, we assessed the effect of tolvaptan on the phosphorylation levels of ERK, a downstream molecule of the AngII signalling cascade (Nakamura, Felizola, Satoh, Fukaya, & Sasano, 2014; Spat & Hunyady, 2004). Cells were treated by tolvaptan (0, 0.1, 1, and 3 μmol·L−1) for 30 min and then stimulated with 100 nmol·L−1 AngII for 5 min. As shown in Figure 3a, AngII stimulation increased the phosphorylation level of ERK, and this level was not affected by tolvaptan treatment. We also checked for a longer treatment period (24 hr) with tolvaptan, but the result remained the same as with the shorter (30 min) incubation period (data not shown). These data indicate that the effect of tolvaptan to inhibit aldosterone production is not mediated through the ERK pathway.
Figure 3.
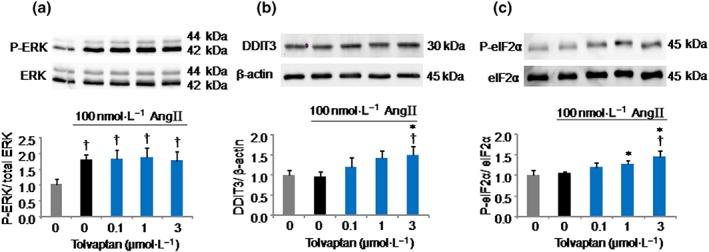
Effects of tolvaptan on angiotensin II (AngII)‐induced ERK and eIF2α phosphorylation and DDIT3 protein expression in H295R cells. ERK phosphorylation (a). Cells were pretreated with tolvaptan at the indicated doses for 30 min and then stimulated with 100 nmol·L−1 AngII for 5 min. The phosphorylation level of ERK was analysed by Western blotting. Upper, representative immunoblots; lower, relative quantification of protein expression. (b) Effect of tolvaptan on DDIT3 protein expression levels. Cells were pretreated with tolvaptan at the indicated doses for 30 min and then stimulated with 100 nmol·L−1 AngII for 24 hr. DDIT3 protein level was analysed by Western blotting. Upper, representative immunoblots; lower, relative quantification of protein expression. eIF2α phosphorylation (c). Cells were pretreated with tolvaptan at the indicated doses for 30 min and then stimulated with 100 nmol·L−1 AngII for 24 hr. The phosphorylation level of eIF2α was analysed by Western blotting. Upper, representative immunoblots; lower, relative quantification of protein expression. Results are shown as mean ± SD (n = 5 separate experiments) and expressed as fold over control. † P ˂ 0.05 versus Cont; *P ˂ 0.05 versus AngII
To test the potential regulatory effect of tolvaptan on the protein translation process of CYP11B2, we verified the effect of tolvaptan on DDIT3 protein expression and eukaryotic initiation factor 2α (eIF2α) phosphorylation by Western blot analysis. DDIT3 (also known as G1 arrest and DNA damage 153) encodes the protein C/EBP homologous protein (CHOP) and is a transcription factor known to be induced by endoplasmic reticulum (ER) stress. Induction of the ER stress activates multiple pathways to initiate the unfolded protein response (UPR), which triggers eIF2α phosphorylation (a UPR‐induced event that inhibits global protein translation) in an attempt to ameliorate the ER protein load (Pan et al., 2014). As shown in Figure 3b, the DDIT3 protein levels were significantly increased by tolvaptan only at high concentration, whereas the lower concentrations of tolvaptan and AngII had no significant effect on it. tolvaptan also significantly increased the expression levels of phospho‐eIF2α (Figure 3c), although AngII had no effect on eIF2α phosphorylation. These results suggest that tolvaptan could have attenuated CYP11B2 translation and subsequently inhibited the aldosterone biosynthesis, likely in part by promoting the UPR and subsequent inhibition of global protein translation initiation via eIF2α phosphorylation.
3.2. In vivo results
3.2.1. Effect of AngII on aldosterone and CYP11B2 protein levels as well as BP in rats
In Protocol 1, serum aldosterone levels (Figure 4a) and systolic BP (SBP; Figure 4c) showed no differences among the groups at baseline. Although SBP was significantly elevated by AngII treatment at 20 pmol·min−1 and further increased dose dependently (Figure 4c), significant elevations of serum aldosterone levels (Figure 4a) and adrenal CYP11B2 protein expression (Figure 4b) with AngII infusion were observed only at 200 pmol·min−1 AngII. Therefore, the dose of 200 pmol·min−1 AngII was used in Protocol 2.
Figure 4.
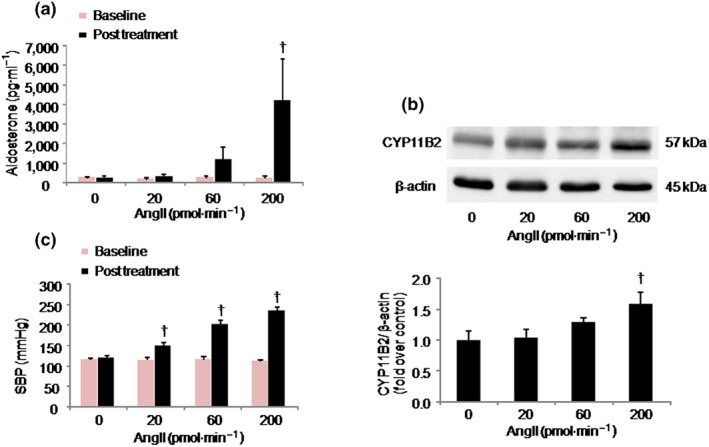
Effects of angiotensin II (AngII) on aldosterone production, CYP11B2 protein expression, and systolic BP (SBP) in Sprague–Dawley rats. Rats were treated with AngII at the indicated doses. Results are shown as mean ± SD (n = 5 rats per group). (a) Dose‐dependency of effect of AngII on aldosterone production. † P ˂ 0.05 versus all other AngII‐treated groups. (b) CYP11B2 protein levels were analysed by Western blotting in whole adrenal tissue homogenates. Upper, representative immunoblots; lower, relative quantification of protein expression. † P ˂ 0.05 versus AngII 0 and 20 pmol·min−1. (c) Dose dependency of effect of AngII on SBP elevation. † P ˂ 0.05 for AngII versus corresponding baseline
3.2.2. Effect of tolvaptan on the responses induced by AngII in rats
In Protocol 2, there were no significant differences in the metabolic, serum, and urine parameters at baseline among the four groups (Cont, AngII, tolvaptan, and AngII + tolvaptan; data not shown). The effects of tolvaptan on these parameters in rats treated with or without AngII are shown in Table 1. The AngII group had greater urine volume and water intake than the Cont group, and the tolvaptan and AngII + tolvaptan groups showed greater increases in these parameters with the highest results being seen in the tolvaptan group during the first 24 hr. Serum urea nitrogen and creatinine levels were higher in the AngII and AngII + tolvaptan groups compared with the Cont and tolvaptan groups. Serum sodium and chloride levels were significantly lower only in the AngII group, whereas serum potassium levels were similar in the four groups. Serum osmolality was higher in the AngII + tolvaptan group than in all other groups. A 24‐hr total urinary protein was greater in the AngII and AngII + tolvaptan groups than in the Cont and tolvaptan groups and the highest in the AngII group. Urine osmolality was lower, and E‐CH2O and C‐CH2O were higher in the tolvaptan and AngII + tolvaptan groups than in the Cont and AngII groups. There were no significant differences in E‐Cosm and C‐Cosm among the four groups.
Table 1.
Metabolic, serum, and urine parameters in rats with or without treatment with tolvaptan for 6 days in the absence or presence of AngII stimulation
| Parameters | Cont | AngII | Tolv | AngII + Tolv |
|---|---|---|---|---|
| Metabolic | ||||
| Body weight (g) | 180 ± 14.7 | 164 ± 6.4 | 205.9 ± 20.4† | 198.3 ± 21.3* |
| Urine volume (ml·day−1) | 9.15 ± 0.9 | 31.6 ± 9† | 118.3 ± 6.5† | 84.7 ± 13†, * |
| Food intake (g·day−1) | 16.9 ± 1.5 | 11.1 ± 2.7† | 15.75 ± 1.2 | 12.85 ± 3.44† |
| Water intake (ml·day−1) | 22.9 ± 2.3 | 40.6 ± 8.5† | 142 ± 13.1† | 99 ± 20.5†, * |
| Serum | ||||
| Urea nitrogen (mmol·L−1) | 5.4 ± 1.1 | 11.5 ± 3.2† | 5.7 ± 0.6 | 9.2 ± 2.7† |
| Creatinine (μmol·L−1) | 24.1 ± 4.1 | 34.7 ± 5.3† | 29.9 ± 2.4 | 32.7 ± 5.2† |
| Na (mmol·L−1) | 140.1 ± 1.7 | 134.6 ± 4.7† | 140.6 ± 2.5 | 139.6 ± 3.95* |
| Cl (mmol·L−1) | 106.3 ± 2.4 | 97.6 ± 3.8† | 107 ± 2.88 | 103.4 ± 3.1* |
| K (mmol·L−1) | 6.9 ± 0.37 | 7.66 ± 0.8 | 6.6 ± 0.67 | 6.9 ± 0.77 |
| Osmolality (mOsm·kg−1 H2O) | 294.6 ± 3.2 | 295 ± 4.73 | 297 ± 3.42 | 303.9 ± 4†,* |
| Urine | ||||
| Total protein (g·L−1) | 0.11 ± 0.06 | 0.97 ± 0.56† | 0.08 ± 0.05 | 0.24 ± 0.18* |
| Urea nitrogen (mmol·L−1) | 775.3 ± 162.9 | 133.5 ± 51.0† | 57.8 ± 27.0† | 91.2 ± 41.5† |
| Creatinine (μmol·L−1) | 4291.9 ± 780.8 | 997.4 ± 431.2† | 460.9 ± 220.8† | 583.4 ± 162.8† |
| Na (mmol·L−1) | 162.7 ± 12.8 | 24.1 ± 9.7† | 21.1 ± 7.1† | 13.6 ± 3.3† |
| Cl (mmol·L−1) | 200.9 ± 18.8 | 48.9 ± 23.3† | 25.6 ± 12.5† | 19 ± 3.2† , * |
| K (mmol·L−1) | 323.5 ± 41.3 | 60.8 ± 32.5† | 30.2 ± 16.7† | 31.4 ± 9.6† |
| Osmolality (mOsm·kg−1 H2O) | 1905 ± 369.7 | 601 ± 381† | 211.4 ± 125.9† | 228 ± 44† , * |
| E‐Cosm (ml·day−1) | 31.9 ± 2.4 | 20 ± 10.7 | 43.4 ± 20.8 | 27.68 ± 7.24 |
| E‐CH2O (ml·day−1) | −22.8 ± 2.4 | 11.6 ± 12† | 74.9 ± 20.9† | 57 ± 11.7†, * |
| C‐Cosm (ml·day−1) | 58.9 ± 11.5 | 59 ± 34.6 | 84 ± 49.9 | 65.1 ± 13.6 |
| C‐CH2O (ml·day−1) | −49.7 ± 11.4 | −27.4 ± 36.2 | 34.3 ± 49.7† | 19.6 ± 12.9† , * |
Note. Results are shown as mean ± SD (n = 8 rats per group). Cont: control; AngII: angiotensin II; Tolv: tolvaptan; E‐Cosm: electrolyte clearance; E‐CH2O: electrolyte‐free water clearance; C‐Cosm: osmolar clearance; C‐CH2O: free water clearance.
P ˂ 0.05 versus Cont group.
P ˂ 0.05 versus AngII group.
AngII stimulated serum aldosterone and adrenal CYP11B2 protein levels as well as adrenal aldosterone production 10.44 ± 5.37‐fold (P < 0.05), 2.32 ± 0.41‐fold (P < 0.05), and 5.44 ± 2.34‐fold (P < 0.05), respectively, compared with the Cont group. tolvaptan treatment significantly reduced serum aldosterone levels (% inhibition was 42.70 ± 16.16% vs. AngII, P < 0.05), adrenal CYP11B2 protein levels (% inhibition was 32.33 ± 10.29% vs. AngII, P < 0.05), and adrenal aldosterone levels (% inhibition was 60.45 ± 24.32% vs. AngII, P < 0.05) induced by AngII (Figure 5a–c, respectively), whereas tolvaptan given to the normal rats did not have a significant effect on these parameters compared with the Cont group (Figure 5a–c). Histological analysis of the treated rat adrenal tissues stained with haematoxylin and eosin showed no appreciable changes in the treatment groups compared with the Cont group (data not shown).
Figure 5.
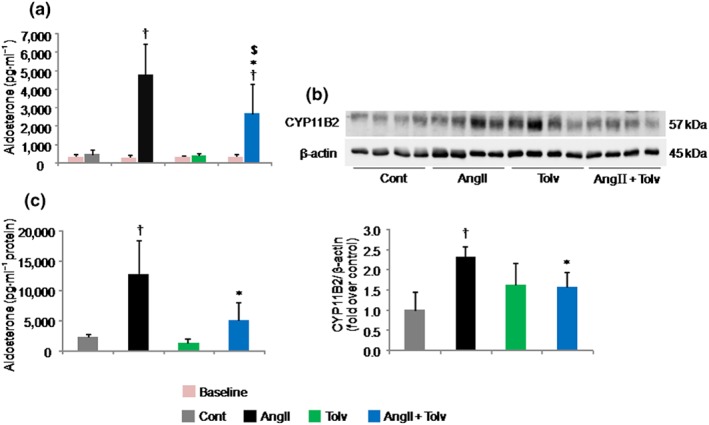
Effects of tolvaptan (Tolv) on serum aldosterone and adrenal CYP11B2 protein levels as well as adrenal aldosterone production in Sprague–Dawley rats treated with angiotensin II (AngII). Rats were treated with tolvaptan in the presence and absence of 200 pmol·min−1 AngII. Results are shown as mean ± SD (n = 8 rats per group). Effects of tolvaptan on AngII‐induced serum aldosterone (a) and CYP11B2 protein levels (b, upper, representative immunoblots; lower, relative quantification of protein expression) and adrenal aldosterone production (c). † P ˂ 0.05 versus Cont group; *P ˂ 0.05 versus AngII group; $ P ˂ 0.05 versus tolvaptan group
Baseline BP (Figure 6a,b) and heart rate (Figure 6c) among the four groups were similar. AngII infusion markedly elevated SBP (from Cont group 114 ± 6 mmHg to AngII group 248 ± 21 mmHg, P < 0.05) and diastolic BP (DBP; from Cont group 85 ± 6 mmHg to AngII group 188 ± 28 mmHg, P < 0.05). In contrast, SBP (196 ± 20 mmHg, P < 0.05 vs. AngII; Figure 6a) and DBP (144 ± 25 mmHg, P < 0.05 vs. AngII; Figure 6b) in the AngII + tolvaptan group were significantly lower than those in the AngII group but were higher than those in Cont and tolvaptan groups. None of the treatments affected the heart rate (Figure 6c).
Figure 6.
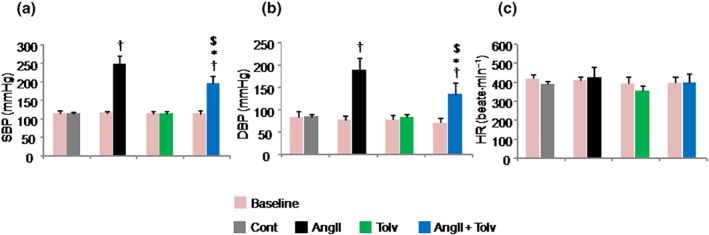
Effects of tolvaptan (Tolv) on BP and heart rate (HR) in Sprague–Dawley rats treated with angiotensin II (AngII). Rats were treated with tolvaptan in the presence and absence of 200 pmol·min−1 AngII. Results are shown as mean ± SD (n = 8 rats per group). Effects of tolvaptan on AngII‐induced systolic BP (SBP; a), diastolic BP (DBP; b), and HR (c). † P ˂ 0.05 versus Cont group; *P ˂ 0.05 versus AngII group; $ P ˂ 0.05 versus Tolv group
4. DISCUSSION
The present study firstly demonstrated that tolvaptan significantly suppressed AngII‐induced aldosterone production both in vitro in H295R human adrenocarcinoma cells and in vivo in rats, which was mediated by inhibiting AngII‐induced CYP11B2 protein production via V2 receptor‐independent pathway.
Aldosterone is one of the major drivers of cardiovascular diseases including HF. Several experimental (Miyazaki et al., 2007) and clinical studies (Boerrigter et al., 2006; Jujo et al., 2016) have reported that tolvaptan therapy avoids RAAS activation despite its strong diuretic effect in HF. Importantly, a recent experimental study performed in our laboratory has demonstrated that chronic tolvaptan therapy suppressed plasma aldosterone levels in a rat model of pulmonary arterial hypertension (Goto et al., 2016). These results indicate that tolvaptan might have the potential to suppress aldosterone synthesis independently of the cardiorenal haemodynamic condition.
In our study, aldosterone production was not influenced by the V2 receptor agonists AVP and dDAVP, an active synthetic analogue of the naturally occurring neurohypophyseal hormone AVP, regardless of AngII stimulation. Moreover, to clarify the specificity of V2 receptor signalling, we intended to examine the existence of V2 receptor, if any, in H295R cells. However, the Ct value of V2 receptor mRNA was undetected by quantitative real‐time PCR, and the V2 receptor protein specific bands by Western blotting were extremely low or even not identified in H295R cells (Figure S4). On the basis of the result of the present study, we speculated that the effect of tolvaptan on the inhibition of AngII‐induced aldosterone production might have been mediated by an AVP–V2 receptor‐independent pathway. In addition, tolvaptan had no effects on AngII‐induced ERK phosphorylation, a downstream molecule of the AngII signalling cascade, indicating that tolvaptan inhibited aldosterone production stimulated by AngII in an ERK‐independent pathway.
The underlying molecular mechanisms of tolvaptan in the inhibition of AngII‐induced aldosterone synthesis were further investigated using the H295R cell line. The inhibition of AngII‐induced CYP11B2 protein levels by tolvaptan occurred without affecting its mRNA expression, suggesting that tolvaptan posttranscriptionally controls CYP11B2 protein synthesis. Taking this into account, we hypothesized that the suppressive effects of tolvaptan on AngII‐induced CYP11B2 protein and aldosterone levels might be a result of the ability of tolvaptan to attenuate CYP11B2 mRNA translation and consequently a reduction in aldosterone production. Importantly, an earlier study reported that pioglitazone inhibited CYP11B2 protein levels and aldosterone production in AngII‐treated HAC15 human adrenocortical carcinoma cells, a clone of NCI H295R cells, likely by blocking global protein translation initiation through DDIT3 (CHOP) and phospho‐eIF2α (Pan et al., 2014). The phosphorylation of eIF2α at serine 51 is the best characterized mechanism for the regulation of mRNA translation. The eIF2α phosphorylation inhibits global protein synthesis and is generally considered a rate‐limiting step in mRNA translation (Kimball, 1999; Wek, 2018). In the present study, tolvaptan indeed significantly increased DDIT3 (the UPR marker) protein expression levels and the phosphorylation levels of eIF2α (a UPR‐induced event that inhibits global protein translation). These results suggest that tolvaptan attenuated CYP11B2 translation and subsequently inhibited aldosterone production, likely in part by interrupting the global protein translation initiation through DDIT3 and eIF2α activation. On the other hand, a previous study suggested that CYP11B2 enzyme activity can be posttranslationally controlled by protein phosphorylation (Lisurek & Bernhardt, 2004). Therefore, besides translation inhibition, we cannot ignore the possibility of some of the complex processes of posttranslational modifications including methylation, O‐linked glycosylation, and ubiquitination, affecting protein synthesis (Bober, Murray, Parker, & Van Eyk, 2018). Further investigations are needed to explore the possible involvement of the aforementioned processes.
Consistent with the results obtained from in vitro experiments, tolvaptan suppressed AngII‐induced aldosterone production and CYP11B2 protein expression levels in SD rat model. In the early phase (first 24 hr) in rats, AngII enhanced diuresis and promoted proteinuria secondary to increased glomerular capillary pressure and glomerular injury. The addition of tolvaptan to the AngII‐treated rats further increased the urine volume with increases in free water clearance. Tolvaptan reduced the urine protein, presumably due to BP reduction in the renal arteries. Importantly, the serum potassium levels in all groups remained unchanged. These results are in agreement with previous findings (Morooka et al., 2012; Yamazaki et al., 2012). As aldosterone production is regulated primarily by AngII and potassium levels, this result suggests that tolvaptan suppressed the AngII‐induced CYP11B2 protein and serum and adrenal aldosterone levels independently of the serum potassium concentration.
Although previous clinical studies have reported that tolvaptan maintains or only mildly reduces BP in HF (Matsue et al., 2016; Suzuki et al., 2013; Watanabe et al., 2012), we demonstrated that tolvaptan administered in the diet to the AngII‐treated rats significantly attenuated AngII‐induced increases in SBP and DBP, whereas the normal rats remained unaffected. As tolvaptan therapy led to body weight gain due to increased water intake despite its strong diuretic effect in both the presence and the absence of AngII, BP‐lowering effect of tolvaptan could be mediated through adrenal aldosterone production inhibition but not through intravenous volume depletion. Indeed, tolvaptan reduced both BP and circulating aldosterone level only in the presence of AngII in our experimental model. These results were consistent with previous studies, which revealed that BP elevation to exogenous AngII is associated with an increase in circulating aldosterone level (Fielitz et al., 2012; Neves, Amiri, Virdis, Diep, & Schiffrin, 2005; Neves, Virdis, & Schiffrin, 2003) in rats.
A very recent study has demonstrated that AngII–salt‐induced MR–epithelial sodium channel (ENaC)–AngII type 1 (AT1) receptor signalling in the brain increases circulating aldosterone levels and thereby contributes to severe hypertension (Lu et al., 2018). On the other hand, tolvaptan reduced ENaC abundance (Miranda, Lee, Chou, & Knepper, 2014). Thus, one possible explanation for the effect of tolvaptan on the inhibition of AngII‐induced aldosterone production would be the disruption of this pathway; this needs further investigation, as tolvaptan could prevent AngII–aldosterone‐mediated hypertension by suppressing the ENaC activity. Considering the above, we presumed that tolvaptan prevented AngII‐induced increase in BP possibly via its inhibitive effect on AngII‐mediated aldosterone production in our experimental model.
Our study has several limitations, such as (a) we have focused only on the adrenal aldosterone production, although its synthesis has also been reported in minor quantities in other organs, such as blood vessels and the heart in certain pathological conditions (Lemarie, Paradis, & Schiffrin, 2008; Yamamoto et al., 2002). (b) We did not analyse the cardiac function and/or cardioprotective effect, if any, of tolvaptan. (c) we did not compare the effect of tolvaptan on electrolyte disturbance, renal haemodynamics, and neurohormonal activation with other diuretics. The main strengths of this study include (a) preparation of positive Cont to validate the exact molecular size of human CYP11B2 for Western blotting (Figure S5) and (b) the results of the in vitro study are complemented by in vivo study.
In conclusion, our study demonstrates that tolvaptan inhibited AngII‐stimulated aldosterone production in cultured H295R cells and in vivo in rats. The anti‐aldosterone effect of tolvaptan occurred as a result of inhibition of CYP11B2 protein synthesis in response to AngII. Interestingly, tolvaptan suppressed the AngII‐induced CYP11B2 protein levels without affecting its mRNA expression. Thus, we conclude that the effect of tolvaptan could have been mediated by inhibiting the AngII‐induced increases in CYP11B2 protein, at least in part through a posttranscriptional mechanism.
AUTHOR CONTRIBUTIONS
A.Y. designed the studies and conducted all the experiments, analysed and interpreted the data, and wrote the manuscript. K.D., R.O., and M.I. contributed to the conception and design, acquisition of the data, updating the article by critical revision for important intellectual content, and final approval of the version to be published. K.K. contributed to the preparation of positive control and to the critical revision of the article for important intellectual content. All authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
CONFLICT OF INTEREST
M.I. received departmental research grant support equal to or more than 1,000,000 yen from Bristol‐Myers Squibb K.K., MSD K.K., Shionogi & Co., Ltd., Otsuka Pharma Inc., and Takeda Pharmaceutical Company Limited in 2017. M.I. received lecture fees equal to or more than 500,000 yen from Daiichi Sankyo Company Limited, Bayer Holding Ltd., and Takeda Pharmaceutical Company Limited in 2017. K.D. received lecture fees equal to or more than 500,000 yen from Otsuka Pharma Inc. in 2017. The other authors declared no conflicts of interest relevant to this article.
DECLARATION OF TRANSPARENCY AND SCIENTIFIC RIGOUR
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research as stated in the BJP guidelines for Design & Analysis, Immunoblotting and Immunochemistry, and Animal Experimentation and as recommended by funding agencies, publishers, and other organizations engaged with supporting research.
Supporting information
Data S1. Supporting information
Data S2. Supporting information
Data S3. Supporting information
Data S4. Supporting information
Data S5. Supporting information
Data S6. Supporting information
ACKNOWLEDGEMENTS
We thank Dr Celso E. Gomez‐Sanchez for the kind gift of the human‐specific mouse anti‐CYP11B2 and the rat‐specific rabbit anti‐CYP11B2 monoclonal antibodies. We also thank Dr Katsunori Uchida, Department of Oncologic Pathology, Mie University Graduate School of Medicine, for his valuable comment on the slides for histological analysis of rat adrenal glands. We are grateful to Ms Rie Ito and Ms Hiromi Nishii for their technical assistance.
Ali Y, Dohi K, Okamoto R, Katayama K, Ito M. Novel molecular mechanisms in the inhibition of adrenal aldosterone synthesis: Action of tolvaptan via vasopressin V2 receptor‐independent pathway. Br J Pharmacol. 2019;176:1315–1327. 10.1111/bph.14630
REFERENCES
- Alexander, S. P. H. , Christopoulos, A. , Davenport, A. P. , Kelly, E. , Marrion, N. V. , Peters, J. A. , … CGTP Collaborators (2017). The Concise Guide to PHARMACOLOGY 2017/18: G protein‐coupled receptors. British Journal of Pharmacology, 174, S17–S129. 10.1111/bph.13878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , … CGTP Collaborators (2017). The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. British Journal of Pharmacology, 174, S272–S359. 10.1111/bph.13877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , Harding, S. D. , … CGTP Collaborators (2017). The Concise Guide to PHARMACOLOGY 2017/18: Overview. British Journal of Pharmacology, 174, S1–S16. 10.1111/bph.13882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Roberts, R. E. , Broughton, B. R. S. , Sobey, C. G. , George, C. H. , Stanford, S. C. , … Ahluwalia, A. (2018). Goals and practicalities of immunoblotting and immunohistochemistry: A guide for submission to the British Journal of Pharmacology. British Journal of Pharmacology, 175, 407–411. 10.1111/bph.14112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bober, F. J. , Murray, C. I. , Parker, S. J. , & Van Eyk, J. E. (2018). Precision profiling of the cardiovascular post‐translationally modified proteome. Circulation Research, 122, 1221–1237. 10.1161/CIRCRESAHA.118.310966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boerrigter, L. C. , Smith, W. B. , Boerrigter, G. , Ouyang, J. , Zimmer, C. A. , Orlandi, C. , & Burnett, J. C. Jr. (2006). Vasopressin‐2‐receptor antagonism augments water excretion without changes in renal hemodynamics or sodium and potassium excretion in human heart failure. American Journal of Physiology. Renal Physiology, 290, F273–F278. 10.1152/ajprenal.00195.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohi, K. , & Ito, M. (2014). Novel diuretic strategies for the treatment of heart failure in Japan. Circulation Journal, 78, 1816–1823. 10.1253/circj.CJ-14-0592 [DOI] [PubMed] [Google Scholar]
- Felizola, S. J. A. , Nakamura, Y. , Ono, Y. , Kitamura, K. , Kikuchi, K. , Onodera, Y. , … Sasano, H. (2014). PCP4: A regulator of aldosterone synthesis in human adrenocortical tissues. Journal of Molecular Endocrinology, 52, 159–167. 10.1530/JME-13-0248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fielitz, H. M. , Lau, M. , Jöhren, O. , Stellmacher, F. , Schwaninger, M. , & Raasch, W. (2012). Blood pressure response to angiotensin II is enhanced in obese Zucker rats and is attributed to an aldosterone‐dependent mechanism. British Journal of Pharmacology, 166, 2417–2429. 10.1111/j.1476-5381.2012.01953.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto, I. , Dohi, K. , Ogihara, Y. , Okamoto, R. , Yamada, N. , Mitani, Y. , & Ito, M. (2016). Detrimental impact of vasopressin V2 receptor antagonism in a SU5416/hypoxia/normoxia‐exposed rat model of pulmonary arterial hypertension. Circulation Journal, 80, 989–997. 10.1253/circj.CJ-15-1175 [DOI] [PubMed] [Google Scholar]
- Grune, J. , Beyhoff, N. , Smeir, E. , Chudek, R. , Blumrich, A. , Ban, Z. , … Kintscher, U. (2018). Selective mineralocorticoid receptor cofactor modulation as molecular basis for finerenone's antifibrotic activity. Hypertension, 71, 599–608. 10.1161/HYPERTENSIONAHA.117.10360 [DOI] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: Updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Research, 46, D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattangady, N. G. , Olala, L. O. , Bollag, W. B. , & Rainey, W. E. (2012). Acute and chronic regulation of aldosterone production. Molecular and Cellular Endocrinology, 350, 151–162. 10.1016/j.mce.2011.07.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannucci, N. B. , Ripoll, G. V. , Garona, J. , Cascone, O. , Ciccia, G. N. , Gomez, D. E. , & Alonso, D. F. (2011). Antiproliferative effect of 1‐deamino‐8‐d‐arginine vasopressin analogs on human breast cancer cells. Future Medicinal Chemistry, 3, 1987–1993. 10.4155/fmc.11.152 [DOI] [PubMed] [Google Scholar]
- Jujo, K. , Saito, K. , Ishida, I. , Furuki, Y. , Kim, A. , Suzuki, Y. , … Hagiwara, N. (2016). Randomized pilot trial comparing tolvaptan with furosemide on renal and neurohumoral effects in acute heart failure. ESC Heart Failure, 3, 177–188. 10.1002/ehf2.12088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juul, K. V. , Bichet, D. G. , Nielsen, S. , & Norgaard, J. P. (2014). The physiological and pathophysiological functions of renal and extrarenal vasopressin V2 receptors. American Journal of Physiology. Renal Physiology, 306, 931–940. [DOI] [PubMed] [Google Scholar]
- Kilkenny, C. , Browne, W. , Cuthill, I. C. , Emerson, M. , & Altman, D. G. (2010). Animal research: Reporting in vivo experiments: The ARRIVE guidelines. British Journal of Pharmacology, 160, 1577–1579. 10.1111/j.1476-5381.2010.00872.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimball, S. R. (1999). Eukaryotic initiation factor eIF2. The International Journal of Biochemistry & Cell Biology, 31, 25–29. 10.1016/S1357-2725(98)00128-9 [DOI] [PubMed] [Google Scholar]
- Lee, S. , Hwang, R. , Lee, J. , Rhee, Y. , Kim, D. J. , Chung, U. , & Lim, S. K. (2005). Ectopic expression of vasopressin V1b and V2 receptors in the adrenal glands of familial ACTH‐independent macronodular adrenal hyperplasia. Clinical Endocrinology, 63, 625–630. 10.1111/j.1365-2265.2005.02387.x [DOI] [PubMed] [Google Scholar]
- Lemarie, C. A. , Paradis, P. , & Schiffrin, E. L. (2008). New insights on signaling cascades induced by cross‐talk between angiotensin II and aldosterone. Journal of Molecular Medicine, 86, 673–678. 10.1007/s00109-008-0323-5 [DOI] [PubMed] [Google Scholar]
- Lisurek, M. , & Bernhardt, R. (2004). Modulation of aldosterone and cortisol synthesis on the molecular level. Molecular and Cellular Endocrinology, 215, 149–159. 10.1016/j.mce.2003.11.008 [DOI] [PubMed] [Google Scholar]
- Livak, K. J. , & Schmittgen, T. D. (2001). Analysis of relative gene expression data using real‐time quantitative PCR and the 2−ΔΔC T method. Methods, 25, 402–408. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Louiset, E. , Contesse, V. , Groussin, L. , Cartier, D. , Duparc, C. , Perraudin, V. , … Lefebvre, H. (2008). Expression of vasopressin receptors in ACTH‐independent macronodular bilateral adrenal hyperplasia causing Cushing's syndrome: Molecular, immunohistochemical and pharmacological correlates. Journal of Endocrinology, 196, 1–9. 10.1677/JOE-07-0413 [DOI] [PubMed] [Google Scholar]
- Lu, H. , Howatt, A. , Balakrishnan, A. , Moorleghen, J. , Rateri, L. , Cassis, A. , & Daugherty, A. (2015). Subcutaneous angiotensin II infusion using osmotic pumps induces aortic aneurysms in mice. Journal of Visualized Experiments, (103), e53191 10.3791/53191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, J. , Wang, H. , Ahmad, M. , Jahromi, M. K. , Blaustein, M. P. , Hamlyn, J. M. , & Leenen, F. H. H. (2018). Central and peripheral slow‐pressor mechanisms contributing to Angiotensin II‐salt hypertension in rats. Cardiovascular Research, 114, 233–246. 10.1093/cvr/cvx214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsue, Y. , Suzuki, M. , Torii, S. , Yamaguchi, S. , Fukamizu, S. , Ono, Y. , … Goldsmith, S. R. (2016). Clinical effectiveness of tolvaptan in patients with acute heart failure and renal dysfunction. Journal of Cardiac Failure, 22, 423–432. 10.1016/j.cardfail.2016.02.007 [DOI] [PubMed] [Google Scholar]
- Miller, A. B. (2007). Aldosterone antagonism in heart failure. Vascular Health and Risk Management, 5, 605–609. [PMC free article] [PubMed] [Google Scholar]
- Miranda, C. A. , Lee, J. W. , Chou, C. L. , & Knepper, M. A. (2014). Tolvaptan as a tool in renal physiology. American Journal of Physiology. Renal Physiology, 306, 359–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki, T. , Fujiki, H. , Yamamura, Y. , Nakamura, S. , & Mori, T. (2007). Tolvaptan, an orally active vasopressin V2‐receptor antagonist, pharmacology and clinical trials. Cardiovascular Drug Reviews, 25, 1–13. 10.1111/j.1527-3466.2007.00001.x [DOI] [PubMed] [Google Scholar]
- Morooka, H. , Iwanaga, Y. , Tamaki, Y. , Takase, T. , Akahoshi, Y. , Nakano, Y. , … Miyazaki, S. (2012). Chronic administration of oral vasopressin type 2 receptor antagonist tolvaptan exerts both myocardial and renal protective effects in rats with hypertensive heart failure. Circulation. Heart Failure, 5, 484–492. 10.1161/CIRCHEARTFAILURE.111.965392 [DOI] [PubMed] [Google Scholar]
- Nakamura, Y. , Felizola, S. J. A. , Satoh, F. , Fukaya, S. K. , & Sasano, H. (2014). Dissecting the molecular pathways of primary aldosteronism. Pathology International, 64, 482–489. 10.1111/pin.12200 [DOI] [PubMed] [Google Scholar]
- Neves, M. F. , Amiri, F. , Virdis, A. , Diep, Q. N. , & Schiffrin, E. L. (2005). Role of aldosterone in angiotensin II‐induced cardiac and aortic inflammation, fibrosis, and hypertrophy. Canadian Journal of Physiology and Pharmacology, 83, 999–1006. 10.1139/y05-068 [DOI] [PubMed] [Google Scholar]
- Neves, M. F. , Virdis, A. , & Schiffrin, E. L. (2003). Resistance artery mechanics and composition in angiotensin II‐infused rats: Effects of aldosterone antagonism. Journal of Hypertension, 21, 189–198. 10.1097/00004872-200301000-00029 [DOI] [PubMed] [Google Scholar]
- Noh, J. M. , Park, W. , Huh, S. J. , Cho, E. Y. , Choi, Y. , Lee, J. H. , & Bae, D. S. (2009). Correlation between tumor volume response to radiotherapy and expression of biological markers in patients with cervical squamous cell carcinoma. Journal of Gynecologic Oncology, 20, 215–220. 10.3802/jgo.2009.20.4.215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- North, W. G. (2000). Gene regulation of vasopressin and vasopressin receptors in cancer. Experimental Physiology, 85, 27–40. [DOI] [PubMed] [Google Scholar]
- Oskarsson, A. , Ulleras, E. , Plant, K. E. , Hinson, J. P. , & Goldfarb, P. S. (2006). Steroidogenic gene expression in H295R cells and the human adrenal gland: Adrenotoxic effects of lindane in vitro. Journal of Applied Toxicology, 26, 484–492. 10.1002/jat.1166 [DOI] [PubMed] [Google Scholar]
- Pan, Z. , Xie, D. , Choudhary, V. , Seremwe, M. , Tsai, Y. , Olala, L. , … Bollag, W. B. (2014). The effect of pioglitazone on aldosterone and cortisol production in HAC15 human adrenocortical carcinoma cells. Molecular and Cellular Endocrinology, 394, 119–128. 10.1016/j.mce.2014.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainey, W. E. , Bird, I. M. , & Mason, J. I. (1994). The NCI‐H295 cell line: A pluripotent model for human adrenocortical studies. Molecular and Cellular Endocrinology, 100, 45–50. 10.1016/0303-7207(94)90277-1 [DOI] [PubMed] [Google Scholar]
- Romero, D. G. , Plonczynski, M. , Vergara, G. R. , Gomez‐Sanchez, E. P. , & Gomez‐Sanchez, C. E. (2004). Angiotensin II early regulated genes in H295R human adrenocortical cells. Physiological Genomics, 19, 106–116. 10.1152/physiolgenomics.00097.2004 [DOI] [PubMed] [Google Scholar]
- Souza, F. , Muxfeldt, E. , Fiszman, R. , & Salles, G. (2010). Efficacy of spironolactone therapy in patients with true resistant hypertension. Hypertension, 55, 147–152. 10.1161/HYPERTENSIONAHA.109.140988 [DOI] [PubMed] [Google Scholar]
- Spat, A. , & Hunyady, L. (2004). Control of aldosterone secretion: A model for convergence in cellular signaling pathways. Physiological Reviews, 84, 489–539. 10.1152/physrev.00030.2003 [DOI] [PubMed] [Google Scholar]
- Suzuki, S. , Yoshihisa, A. , Yamaki, T. , Sugimoto, K. , Kunii, H. , Nakazato, K. , … on behalf of the AVCMA investigators (2013). Acute heart failure volume control multicenter randomized (AVCMA) trial: Comparison of tolvaptan and carperitide. Journal of Clinical Pharmacology, 53, 1277–1285. 10.1002/jcph.197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamma, G. , Mise, A. D. , Ranieri, M. , Geller, A. , Tamma, R. , Zallone, A. , & Valenti, G. (2017). The V2 receptor antagonist tolvaptan raises cytosolic calcium and prevents AQP2 trafficking and function: An in vitro and in vivo assessment. Journal of Cellular and Molecular Medicine, 20, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeraveedu, P. T. , Watanabe, K. , Ma, M. , Palaniyandi, S. S. , Yamaguchi, K. , Kodama, M. , & Aizawa, Y. (2008). Effects of V2‐receptor antagonist tolvaptan and the loop diuretic furosemide in rats with heart failure. Biochemical Pharmacology, 75, 1322–1330. 10.1016/j.bcp.2007.11.011 [DOI] [PubMed] [Google Scholar]
- Wang, F. , Tang, K. , Yen, Y. , Ho, D. M. , Yang, A. , Huang, C. I. , … Won, J. G. (2012). Plasma corticotrophin response to desmopressin in patients with Cushing's disease correlates with the expression of vasopressin receptor 2, but not with that of vasopressin receptor 1 or 3, in their pituitary tumours. Clinical Endocrinology, 76, 253–263. 10.1111/j.1365-2265.2011.04179.x [DOI] [PubMed] [Google Scholar]
- Watanabe, K. , Dohi, K. , Sugimoto, T. , Yamada, T. , Sato, Y. , Ichikawa, K. , … Ito, M. (2012). Short‐term effects of low‐dose tolvaptan on hemodynamic parameters in patients with chronic heart failure. Journal of Cardiology, 60, 462–469. 10.1016/j.jjcc.2012.09.002 [DOI] [PubMed] [Google Scholar]
- Wek, R. C. (2018). Role of eIF2α kinases in translational control and adaptation to cellular stress. Cold Spring Harbor Perspectives in Biology, 10, a032995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto, N. , Yasue, H. , Mizuno, Y. , Yoshimura, M. , Fujii, H. , Nakayama, M. , … Ogawa, H. (2002). Aldosterone is produced from ventricles in patients with essential hypertension. Hypertension, 39, 958–962. 10.1161/01.HYP.0000015905.27598.E9 [DOI] [PubMed] [Google Scholar]
- Yamazaki, T. , Izumi, Y. , Nakamura, Y. , Yamashita, N. , Fujiki, H. , Osada‐Oka, M. , … Yoshiyama, M. (2012). Tolvaptan improves left ventricular dysfunction after myocardial infarction in rats. Circulation. Heart Failure, 5, 794–802. 10.1161/CIRCHEARTFAILURE.112.968750 [DOI] [PubMed] [Google Scholar]
- Zannad, F. , McMurray, J. V. , Krum, H. , Veldhuisen, D. J. , Swednerg, K. , Shi, H. , … EMPHASIS‐HF Study Group (2011). Eplerenone in patients with systolic heart failure and mild symptoms. The New England Journal of Medicine, 364, 11–21. 10.1056/NEJMoa1009492 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting information
Data S2. Supporting information
Data S3. Supporting information
Data S4. Supporting information
Data S5. Supporting information
Data S6. Supporting information


