Abstract
Background and Purpose
Inflammasome‐mediated pyroptosis is an important neuronal cell death mechanism. Previous studies reported that activation of melanocortin MC4 receptor exerted neuroprotection in several neurological diseases. Here, we have investigated the role of MC4 receptor activation with RO27‐3225 in suppressing neuronal pyroptosis after experimental intracerebral haemorrhage (ICH) and the underlying mechanism.
Experimental Approach
One hundred and sixty‐nine male CD1 mice were used. ICH was induced by injection of bacterial collagenase into the right‐side basal ganglia. RO27‐3225, a selective agonist of MC4 receptor, was injected intraperitoneally at 1 hr after ICH. To elucidate the underlying mechanism, we used the specific MC4 receptor antagonist HS024 and NQDI‐1, a specific inhibitor of the apoptosis signalling‐regulating kinase 1 (ASK1). Neurological tests, Western blot, Fluoro‐Jade C, TUNEL, and immunofluorescence staining were conducted.
Key Results
Expression of MC4 receptor and the NOD‐like receptor family, pyrin domain containing 1 (NLRP1) inflammasome in brain were increased after ICH. RO27‐3225 treatment decreased neuronal pyroptosis and neurobehavioural deficits at 24 and 72 hr after ICH. RO27‐3225 reduced the expression of p‐ASK1, p‐JNK, p‐p38 MAPK, NLRP1 inflammasome, cleaved caspase‐1, and IL‐1β after ICH. HS024 pretreatment prevented the effects of RO27‐3225. Similar to RO27‐3225, NQDI‐1 alone improved neurological functions and down‐regulated ASK1/JNK/p38MAPK expression after ICH.
Conclusions and Implications
RO27‐3225 suppressed NLRP1‐dependent neuronal pyroptosis and improved neurological function, possibly mediated by activation of MC4 receptor and inhibition of ASK1/JNK/p38 MAPK signalling pathways, after experimental ICH in mice. The MC4 receptor may be a promising therapeutic target for the management of ICH.
Abbreviations
- ASK1
apoptosis signalling‐regulating kinase 1
- FJC
Fluoro‐Jade C
- ICH
intracerebral haemorrhage
- MSH
melanocyte‐stimulating hormones
- NLRP1
NOD‐like receptor (NLR) family, pyrin domain containing 1
What is already known
Activation of melanocortin MC4 receptor with RO27‐3225 exerts neuroprotective effects in several neurological diseases.
What this study adds
RO27‐3225 attenuates NOD‐like receptor family, pyrin domain containing 1‐dependent neuronal pyroptosis after intracerebral haemorrhage in mice.
What is the clinical significance
MC4 receptor activation may provide a promising therapeutic strategy for treating intracerebral haemorrhage patients.
1. INTRODUCTION
Intracerebral haemorrhage (ICH) remains a devastating subtype of stroke with high mortality and morbidity, accounting for approximately 10–15% of all stroke cases (Wang, Zhou, et al., 2018; Zhou, Wang, Wang, Anne Stetler, & Yang, 2014). The initial haemorrhagic volume and the haematoma expansion are the two key factors of primary brain injury following ICH, even though early haematoma removal and prevention of haematoma expansion have not yielded conclusive benefits in clinical trials (Mendelow et al., 2005; Zhou et al., 2014). Neuroinflammation on the other hand plays a critical role in the pathogenesis of ICH‐induced secondary brain injury, leading to brain oedema formation, blood–brain barrier disruption, and neuronal cell death (Nadeau et al., 2018; Wilkinson et al., 2018). The molecular mechanisms of neuronal cell death, specifically associated with post‐ICH inflammation, are complex and poorly understood.
Inflammasome‐mediated neuronal programmed cell death, namely, pyroptosis, has recently been shown as an important mechanism of neuronal cell death, in several neurological diseases (Fann, Lee, Manzanero, Chunduri, et al., 2013; Fann, Lee, Manzanero, Tang, et al., 2013; Lin et al., 2016; Tan et al., 2014; Tomura, de Rivero Vaccari, Keane, Bramlett, & Dietrich, 2012). The NOD‐like receptor (NLR) family, pyrin domain containing 1 (NLRP1) inflammasome is the first member to be characterized among the NLR family and mostly expressed in neurons and glial cells (Abulafia et al., 2009; Tan et al., 2014). The activation of NLRP1 inflammasome converts precursor caspase‐1 into cleaved caspase‐1 via proximity‐induced autoactivation (Fann, Lee, Manzanero, Chunduri, et al., 2013; Fann, Lee, Manzanero, Tang, et al., 2013; Martinon, Burns, & Tschopp, 2002; Tan et al., 2015; Zhu et al., 2015). Cleaved caspase‐1 further cleaves precursor IL‐1β into biologically active mature pro‐inflammatory IL‐1β (Chavarria‐Smith & Vance, 2015). Additionally, cleaved caspase‐1 is a specific enzyme for cell pyroptosis, which leads to cell membrane pore formation, rapid loss of membrane integrity, and release of pro‐inflammatory intracellular contents (Mortezaee, Khanlarkhani, Beyer, & Zendedel, 2018).
The inhibition of NLRP1 inflammasome reduced innate immune response and ameliorated neuronal pyroptotic cell death in animal models of temporal lobe epilepsy, Alzheimer's disease, traumatic brain, spinal cord injury, and ischaemic stroke (de Rivero Vaccari et al., 2009; Fann, Lee, Manzanero, Chunduri, et al., 2013; Fann, Lee, Manzanero, Tang, et al., 2013; Lin et al., 2016; Tan et al., 2015, 2014; Tomura et al., 2012). Thus, the anti‐inflammatory approach targeting the NLRP‐1 inflammasome‐mediated neuronal pyroptosis could provide a potential therapeutic strategy in the treatment of ICH.
Melanocortin peptides including adrenocorticotropic hormone and α‐, β‐, and γ‐melanocyte‐stimulating hormones (MSH) are all derived from precursor polypeptide pro‐opiomelanocortin and act through five subtypes of melanocortin receptors (Flores‐Bastias & Karahanian, 2018; Garfield, Lam, Marston, Przydzial, & Heisler, 2009). By binding with melanocortin MC4 receptor, the neuropeptide α‐MSH exerted anti‐inflammatory, anti‐apoptotic, and neuroprotective effects in the experimental settings of ischaemic renal failure, traumatic brain injury, and cerebral ischaemia (Bitto et al., 2012; Forslin Aronsson et al., 2006; Jo et al., 2001). The MC4 receptor is a seven‐transmembrane GPCR and primarily expressed in the CNS including hypothalamus, thalamus, hippocampus, cortex, basal ganglia, brainstem, and spinal cord (Tao, 2010). Pharmacological and genetic studies demonstrated the beneficial effects of MC4 receptor activation in neuroprotection, regulation of energy homeostasis, anti‐anxiety, cardiovascular protection, and other functions (Giuliani et al., 2006; Tao, 2010). In our previous study, we have showed that activation of MC4 receptor with the low MW, non‐peptide, agonist RO27‐3225 attenuated neuroinflammation in a mouse model of ICH (Chen et al., 2018). Specifically, activation of MC4 receptor significantly decreased phosphorylation of JNK and p38 MAPK (Chai, Li, Zhang, Wang, & Mulholland, 2009; Chen et al., 2018; Rodrigues, Almeida, & Gouveia, 2015; Spaccapelo et al., 2011).
MAPKs, consisting of ERK1/2, c‐JNK, and p38 MAPK, are involved in directing cellular responses to various stimuli, thus regulating cell proliferation and differentiation, stress responses, immune function, and cell death (Cai et al., 2011; Gan, Gao, Zhao, & Qi, 2016). The apoptosis signalling‐regulating kinase 1 (ASK1) is known as a MAP3K that activates the JNK/p38 MAPK signalling pathway (Barros‐Minones et al., 2013; Tobiume et al., 2001). Previous studies have shown that the JNK/p38 MAPK signalling pathway was activated following ICH, leading to the release of pro‐inflammatory mediators and neuronal cell death (Ni et al., 2015; Qi et al., 2017). Interestingly, the JNK/p38 MAPK signalling pathway promoted NLRP1 inflammasome activation in neurons following ischaemic stroke in vivo and in vitro (Fann et al., 2014, 2018).
In the present study, we hypothesized that MC4 receptor activation with RO27‐3225 would attenuate the neurological deficits and decrease NLRP1‐dependent neuronal pyroptosis in a mouse model of ICH and that those neuroprotective effects might be partly mediated by inhibiting the ASK1/JNK/p38 MAPK signalling pathway.
2. METHODS
2.1. Animals
All animal care and experimental protocols and procedures were approved by the Institutional Animal Care and Use Committee at Loma Linda University and were in compliance with the National Institutes of Health's Guide for the Care and Use of Laboratory Animals in Neuroscience Research. Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny, Browne, Cuthill, Emerson, & Altman, 2010) and with the recommendations made by the British Journal of Pharmacology. Adult male CD1 mice (8‐week‐old, weight 30–40 g; Charles River, IMSR Cat# CRL:22, RRID:IMSR_CRL:22) were housed in the designated animal care facility at Loma Linda University in a temperature (70‐72oF) and humidity (45‐50%) controlled environment, with a 12‐hr light/dark cycle. Mice were housed in standard disposable cages (Innocage) with corn cob bedding (Innovive, San Diego, CA). Each cage housed 5 mice with access to food and water ad libitum. Mice were held in the facility for a minimum of 3 days before ICH induction.
2.2. Experimental design
In this study, all mice were randomly assigned to the following four separate experiments (Figure 1).
Figure 1.
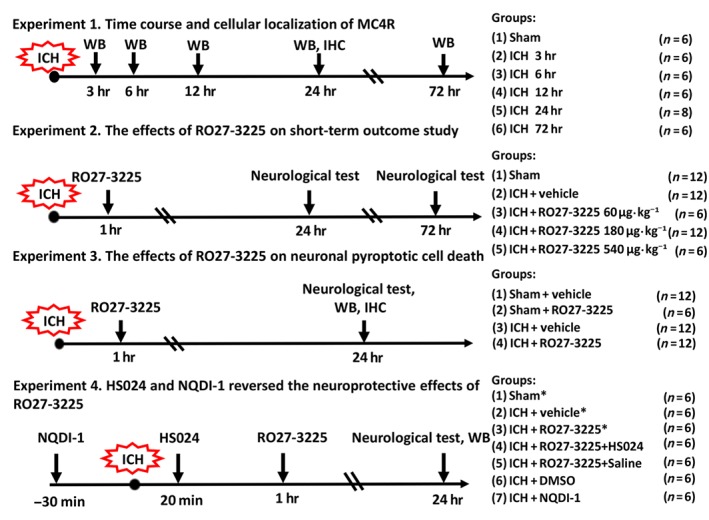
Experimental design and animal groups. The asterisk indicates samples shared with Experiment III. WB: western blot; IHC: immunohistochemistry
2.2.1. Experiment I: Time course of MC4 receptor and NLRP1
To determine the time course of the expression of endogenous MC4 receptor and NLRP1 after inducing ICH, Western blot analysis was performed to measure their protein levels in the ipsilateral/right hemisphere. Thirty‐six mice were randomly divided into six groups (n = 6 each group) as sham, 3, 6, 12, 24, and 72 hr after ICH induction.
In addition, the co‐localization of MC4 receptors in neurons was evaluated using double immunofluorescence labelling of MC4 receptors with the neuronal specific nuclear protein (NeuN) at 24 hr after ICH (n = 2).
2.2.2. Experiment II: RO27‐3225 treatment
Neurobehavioural tests were used to determine the optimal dosage of the MC4 receptor ‐selective agonist RO27‐3225 treatment at 24 hr after ICH; 30 mice were randomly assigned into five groups: sham (n = 6), ICH + vehicle (n = 6), ICH + RO27‐3225 60 μg·kg−1 (n = 6), ICH + RO27‐3225 180 μg·kg−1 (n = 6), and ICH + RO27‐3225 540 μg·kg−1 (n = 6).
As RO27‐3225 at 180 μg·kg−1 was the most effective dose to improve neurological functions at 24 hr after ICH, this optimal dosage was tested at 72 hr after ICH. Another 18 mice were randomly divided into three groups: sham (n = 6), ICH + vehicle (n = 6), and ICH + RO27‐3225 180 μg·kg−1 (n = 6).
2.2.3. Experiment III: Signalling pathways
To assess the effects of RO27‐3225 treatment on JNK/p38 MAPK/NLRP1/caspase 1/IL‐1β signalling pathway and neuronal pyroptotic cell death, Western blots, Fluoro‐Jade C (FJC) staining, co‐staining of TUNEL, or caspase‐1 with neuron marker NeuN and neurobehavioural tests were performed at 24 hr after ICH.
For Western blot measurement, 24 mice were randomly divided into four groups: sham + vehicle (n = 6), sham + RO27‐3225 180 μg·kg−1 (n = 6), ICH + vehicle (n = 6), and ICH + RO27‐3225 180 μg·kg−1 (n = 6). For assessing the neuronal cell death, additional 18 mice were randomly divided into three groups: sham (n = 6), ICH + vehicle (n = 6), and ICH + RO27‐3225 180 μg·kg−1 (n = 6).
2.2.4. Experiment IV: Neuronal pyroptosis
To explore the underlying mechanism of RO27‐3225 in suppressing neuronal pyroptosis after ICH, the following two interventions were used: (a) the specific MC4 receptor antagonist HS024 was administered by i.p. injection 20 min after ICH induction, followed by RO27‐3225 (180 μg·kg−1) treatment 1 hr after ICH, and (b) the specific ASK1 inhibitor NQDI‐1 alone was administered by i.c.v. injection 30 min before ICH induction. Neurobehavioural tests and Western blots were assessed at 24 hr after ICH.
Thirty mice were randomly divided into five groups: sham (n = 6), ICH + vehicle (n = 6), ICH + RO27‐3225 (n = 6), ICH + RO27‐3225 + HS024 (n = 6), and ICH + RO27‐3225 + saline (n = 6). An additional 18 mice were randomly divided into three groups: sham (n = 6), ICH + DMSO (n = 6), and ICH + NQDI‐1 (n = 6). The samples for the sham (n = 6), ICH + vehicle (n = 6), and ICH + RO27‐3225 (n = 6) groups were shared from Experiment III.
2.3. Model of ICH following collagenase injection
The ICH model was induced by intrastriatal injection of bacterial collagenase in mice, as previously described (Manaenko et al., 2018). Briefly, mice were anaesthetized with ketamine (100 mg·kg−1) and xylazine (10 mg·kg−1; 2:1, i.p.) and positioned prone in a stereotaxic head frame (Kopf Instruments, Tujunga, CA). An artificial tears ointment (Rugby, Livonia, MI) was used for keeping the eye moist during surgery. With a variable speed drill (Fine Scientific Tools, Foster City, CA), a 1‐mm burr hole was made 0.2 mm posterior and 2.2 lateral to the bregma. A 26‐gauge needle on a 10‐μl Hamilton syringe was inserted stereotactically 3.5 mm into the right deep cortex/basal ganglia at a rate of 1 mm·min−1. Bacterial collagenase type VII‐S (0.075 units; Sigma‐Aldrich, St. Louis, MO) dissolved in 0.5‐μl sterile PBS was infused into the brain at a rate of 0.167 μl·min−1 with an infusion pump (Stoelting, Harvard Apparatus, Holliston, MA). The needle was left in place for an additional 5 min after the injection to prevent possible leakage of the collagenase solution and withdrawn slowly at a rate of 1 mm·min−1. The cranial burr hole was sealed with bone wax, the scalp was sutured, and 0.4 ml of normal saline was injected subcutaneously to avoid postsurgical dehydration. Mice were allowed to recover fully under close observation. The sham operation was performed with needle insertion only.
2.4. Intracerebroventricular injection
Intracerebroventricular administration was performed as previously described (Iniaghe et al., 2015; Tong et al., 2017). Briefly, the 26‐gauge needle of a 10‐μl Hamilton syringe was inserted into the left lateral ventricle through a cranial burr hole at the following coordinates relative to bregma: 0.3 mm posterior, 1.0 lateral, and 2.3 mm deep. A microinfusion pump was used for i.c.v. administration at a rate of 0.667 μl·min−1. The needle was left in place for an additional 8 min after the end of infusion and then removed over 3 min. The burr hole was sealed with bone wax.
2.5. Drug administration
The selective MC4 receptor agonist RO27‐3225 was dissolved in saline and tested at three different doses (60, 180, and 540 μg·kg−1; Chen et al., 2018; Minutoli et al., 2011). It was administered by intraperitoneally (i.p.) at 1 hr after ICH. The specific MC4 receptor antagonist HS024 (130 μg·kg−1) was also dissolved in saline and administered by i.p. 20 min after ICH. Vehicle‐treated mice received an equal volume of saline (Bitto et al., 2011; Chen et al., 2018). NQDI‐1 (250 nmol per mouse;), a highly specific ASK1 inhibitor, was dissolved in DMSO (Sigma‐Aldrich) and then injected by i.c.v. into the left lateral ventricle 30 min prior to ICH induction (Hao et al., 2016). The control group was given the same volume of equally diluted DMSO by i.c.v. injection.
2.6. Neurological outcomes assessment
Neurological outcomes were assessed with modified Garcia test, forelimb placement test, and corner turn test by an independent researcher, blinded to the treatments, at 24 and 72 hr after ICH as previously described (Manaenko et al., 2018; Tong et al., 2017). Briefly, the modified Garcia test consists of seven individual items that evaluate spontaneous activity, axial sensation, vibrissae proprioception, symmetry of limb movement, lateral turning, forelimb walking, and climbing. Each subtest was given a score ranging from 0 to 3, with a composite maximum score of 21 (no neurological deficits). Forelimb placement test was used to assess the animals' responsiveness to vibrissae stimulation. Mice were positioned parallel to a table top and moved slowly up and down, allowing the vibrissae on one side of the head to brush along the table surface. The results were expressed as a percentage of the number of successful left forepaw placements out of 10 stimulations, normalized to the mean of sham performance. For the corner turn test, animals were allowed to advance into a 30° corner and exit by turning either to the left or to the right. Choice of turning was recorded for a total of 10 trials, and a score was calculated as number of left turns/all trials × 100.
2.7. Western blot analysis
Protein extraction and Western blotting were performed as previously reported (Enkhjargal et al., 2017). At 24 hr after ICH, mice were deeply anaesthetized with isoflurane and transcardially perfused with ice‐cold PBS, followed by removal of the brain and separation of two hemispheres. The ipsilateral/right brain hemispheres were homogenized in RIPA (Santa Cruz Biotechnology, Santa Cruz, CA) and centrifuged at 14,000 g at 4°C for 30 min. The supernatant was collected, and the protein concentration was measured using a detergent compatible assay (DC protein assay, Bio‐Rad laboratories, CA). Equal amounts of protein were loaded on SDS‐PAGE gels. After proteins were separated by electrophoresis and transferred to nitrocellulose membranes, the membranes were blocked and incubated at 4°C overnight with the following primary antibodies: rabbit anti‐ MC4 receptor (1:500, Abcam Cat# ab24233, RRID:AB_2250589), rabbit anti‐NLRP1 (1:500, Novus Cat# NBP1‐54899, RRID:AB_11010379), rabbit anti‐phosphorylated ASK1 (p‐ASK1, 1:1000, Cell Signaling Technology Cat# 3764, RRID:AB_2281595), rabbit anti‐ASK1 (1:1000, Cell Signaling Technology Cat# 8662, RRID:AB_11220434), rabbit anti‐phosphorylated JNK (p‐JNK, 1:1000, Abcam Cat# ab124956, RRID:AB_10973183), rabbit anti‐JNK (1:1000, Abcam Cat# ab179461, RRID:AB_2744672), mouse anti‐phosphorylated p38 (p‐p38, 1:300, Santa Cruz Biotechnology Cat# sc‐7973, RRID:AB_670359), mouse anti‐p38 (1:300, Santa Cruz Biotechnology Cat# sc‐7972, RRID:AB_628079), rabbit anti‐cleaved caspase‐1 (1:500, Novus Cat# NBP1‐76605, RRID:AB_11028149), rabbit anti‐IL‐1β (1:1000, Abcam Cat# ab9722, RRID:AB_308765), and goat anti‐β‐actin (1:5000, Santa Cruz Biotechnology Cat# sc‐1615, RRID:AB_630835). Appropriate secondary antibodies (1:3000, Santa Cruz Biotechnology; 1:5000, Abcam, Cambridge, MA) were selected to incubate with the membrane for 2 hr at room temperature. Immunoblots were probed with an ECL Prime Western Blotting Detection Reagent (Amersham Biosciences, Arlington Heights, PA) and visualized with an imaging system (Bio‐Rad, Versa Doc, model 4000), and analysed by ImageJ software (ImageJ, Version 1.4 RRID:SCR_003070). All image analysis was carried out without knowledge of the treatment groups (blinded).
2.8. Immunofluorescence staining
After mice were anaesthetized with isoflurane and intracardially perfused with ice‐cold PBS and 10% formalin, the whole brain was removed, fixed in 10% formalin at 4°C overnight, and dehydrated with 30% sucrose for 3 days in prior to snap‐freezing at −80°C. The brains were further sliced into coronal sections (10μm) using a cryostat (CM3050S; Leica Microsystems). Immunofluorescence staining was performed as described previously (Wang, Nowrangi, et al., 2018). Briefly, brain sections were incubated overnight at 4°C with primary antibodies including mouse anti‐NeuN (1:200, Abcam Cat# ab104224, RRID:AB_10711040), rabbit anti‐MC4 receptor (1:500, Abcam Cat# ab24233, RRID:AB_2250589), and rabbit anti‐cleaved caspase‐1 (1:200, Novus Biologicals, Littleton, CO). The sections were then incubated with the appropriate fluorescence dye‐conjugated secondary antibodies (1:200, Jackson Immunoresearch, West Grove, PA) in the dark room for 2 hr at room temperature. Finally, the sections were covered with DAPI (Vector Laboratories Inc.). The slides were visualized with a fluorescence microscope Leica DMi8 (Leica Microsystems, Germany) and analysed by Leica Application Suite software. All image analysis was carried out without knowledge of the treatment groups (blinded).
2.9. FJC staining
Degenerating neurons were evaluated by FJC staining with a modified FJC ready‐to‐dilute staining kit (Biosensis, USA) as previously reported (Xie et al., 2018; Xu et al., 2018). Briefly, the brain extraction and slicing procedure were the same as that described above for immunofluorescence staining. The slides were immersed in 1% sodium hydroxide solution for 5 min, followed by being rinsed in 70% ethanol for 2 min and subsequently transferred into distilled water for 2 min. After incubation with 0.06% potassium permanganate solution for 10 min, the slides were rinsed in distilled water for 2 min and incubated with 0.0001% solution of FJC (Millipore, USA) which was dissolved in 0.1% acetic acid for 10 min. The slides were rinsed with distilled water three times for 1 min each, dried in a slide incubator at 50°C for 5 min. After submerging in xylene for 5 min, the slides were cover slipped with DPX mounting media (Sigma‐Aldrich, USA). FJC‐positive neurons were manually counted in the peri‐haematomal regions of six sections per brain at ×200 magnification using ImageJ software (ImageJ, Version 1.4 RRID:SCR_003070). The data were averaged and expressed as positive cells·mm−2. All image analysis was carried out without knowledge of the treatment groups (blinded).
2.10. TUNEL staining
Double staining with TUNEL (red) and NeuN (green) were performed to quantify neuronal cell death at 24 hr after ICH. For TUNEL staining, in situ Cell Death Detection kit (Roche, USA) was used according to the manufacturer's instructions (Xu et al., 2018). The number of TUNEL‐positive neurons was counted manually in perihaematomal area of six sections per brain at ×200 magnification using ImageJ software (ImageJ, Version 1.4 RRID:SCR_003070). Data were expressed as the ratio of TUNEL‐positive neurons (%). All image analysis was carried out without knowledge of the treatment groups (blinded).
2.11. Data and statistical analysis
The data and statistical analysis in this study comply with the recommendations of the British Journal of Pharmacology on experimental design and analysis in pharmacology. All data are expressed as the mean ± SD. Statistical analysis was performed with GraphPad Prism (RRID:SCR_002798). One‐way ANOVA was followed by multiple comparisons between groups using Tukey's post hoc test; post hoc tests were run only if F achieved the necessary level of statistical significance (P < 0.05) and there was no significant variance inhomogeneity. Statistical significance was defined as P < 0.05.
2.12. Materials
RO27‐3225 was supplied by Sigma‐Aldrich, HS024 by Tocris, Minneapolis, MN and NQDI‐1 by Cayman Chemical, Ann Arbor, MI.
2.13. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander, Christopoulos et al., 2017; Alexander, Fabbro, et al., 2017a; Alexander, Fabbro, et al. 2017b).
3. RESULTS
3.1. Mortality and exclusion
A total of 169 mice were used: 36 mice were sham and 133 mice had ICH induced. The overall mortality of mice with ICH was 9.77% (13/133), and there were no deaths in the sham group. Four mice were excluded from this study due to no haemorrhage (Table S1).
3.2. Expression of MC4 receptor and NLRP1 inflammasomes increased in a time‐dependent manner after ICH
The expression of endogenous MC4 receptor and NLRP1 inflammasomes in the ipsilateral/right cerebral hemispheres was evaluated by Western blot analysis at 0 (sham), 3, 6, 12, 24, and 72 hr after ICH. The results showed that the expression of MC4 receptor was significantly increased at 6 hr, reached the peak at 24 hr, and decreased at 72 hr after ICH when compared with sham group (P < 0.05, Figure 2a). Expression of NLRP1 inflammasomes was significantly up‐regulated at 12 hr and peaked at 72 hr after ICH when compared with sham group (P < 0.05, Figure 2b). Double immunofluorescence staining revealed that the MC4 receptor co‐localized with neurons, and the number of MC4 receptor‐positive neurons in the perihaematomal area at 24 hr after ICH was significantly increased, compared with that in the sham group (Figure 2c).
Figure 2.
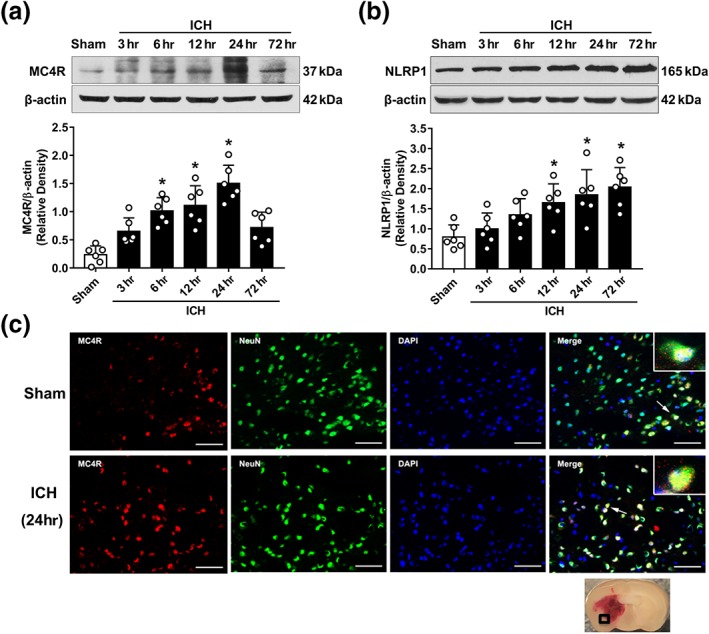
Endogenous expression of MC4 receptor and NLRP1 inflammasomes after ICH. (a) Representative western blot bands and quantitative analyses of the time‐dependent expression of MC4 receptor (MC4R) from the ipsilateral hemisphere after ICH. (b) Representative western blot bands and quantitative analyses of NLRP1 inflammasome time‐dependent expression from the ipsilateral hemisphere after ICH. Data shown are individual values with mean ± SD; n = 6. *P < 0.05, significantly different from sham; one‐way ANOVA, Tukey's test. (c) Representative microphotograph of double immunofluorescence staining showed the co‐localization of MC4 receptor (MC4R, red) with neurons (NeuN, green) in the perihaematomal area in sham and ICH (24 hr) animals. Nuclei were stained with DAPI (blue). Scale bar = 50 μm, n = 2
3.3. Administration of RO27‐3225 decreased neurobehavioural deficits at 24 and 72 hr after ICH
To determine the optimal dose of RO27‐3225, given i.p., three different doses were used to evaluate their efficacy on neurological outcomes at 24 hr after ICH. Mice in the ICH + vehicle group showed significantly worse neurobehavioural performances compared with the sham group in forelimb placement test (Figure 3a), corner turn test (Figure 3b), and modified Garcia test (Figure 3c). RO27‐3225 at dose of 180 μg·kg−1 significantly improved the neurological outcomes compared to the ICH + vehicle group (Figure 3a–c). To further confirm the efficacy of this optimal dose, neurobehavioural tests were also performed at 72 hr after ICH. Consistently, the ICH + RO27‐3225 (180 μg·kg−1) group exhibited significantly decreased neurobehavioural deficits, compared with ICH + vehicle group (Figure 3d–f) at 72 hr after ICH.
Figure 3.

The effects of RO27‐3225 (RO) on neurobehavioural outcomes at 24 and 72 hr after ICH. (a–c) Forelimb placement, corner turn test, and modified Garcia test at 24 hr after ICH. (d–f) Forelimb placement, corner turn test, and modified Garcia test at 72 hr after ICH. Data shown are individual values with mean ± SD, n = 6. *P < 0.05, significantly different from sham, # P < 0.05, significantly different from ICH + vehicle; one‐way ANOVA, Tukey's test
3.4. RO27‐3225 decreased the expression of phosphorylated JNK, phosphorylated p38 MAPK, NLRP1 inflammasome, cleaved caspase‐1, and IL‐1β at 24 hr after ICH
Neurobehavioural tests and Western blots were performed among sham + vehicle, sham + RO27‐3225, ICH + vehicle, and ICH + RO27‐3225 groups at 24 hr after ICH. The results showed that RO27‐3225 did not change the neurobehavioural performance (Figure 4a–c) and the expressions of phosphorylated JNK (p‐JNK), phosphorylated p38 MAPK (p‐p38 MAPK), NLRP1 inflammasome, cleaved caspase‐1, and IL‐1β (Figure 4d–i) in sham animals.
Figure 4.
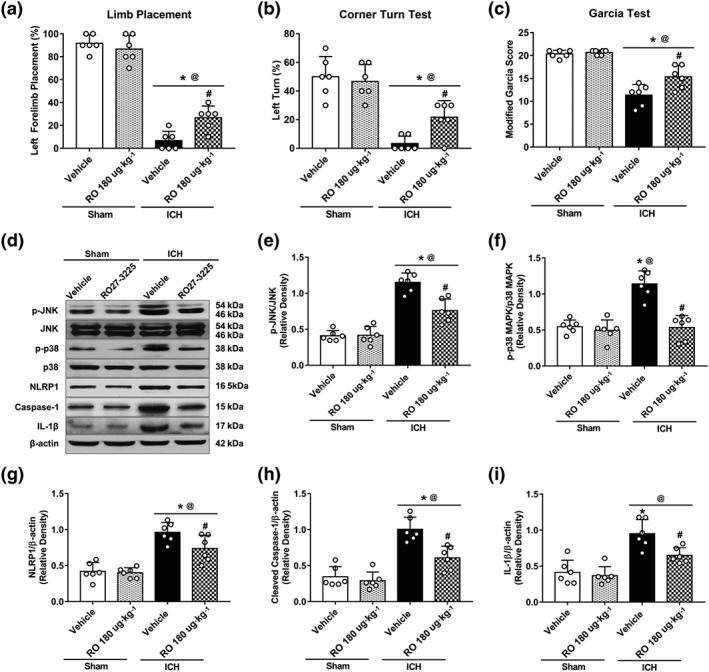
The effects of RO27‐3225 (RO) on neurobehavioural outcomes and JNK/p38 MAPK/NLRP1/caspase‐1/IL‐1β signalling at 24 hr after ICH. (a–c) Forelimb placement, corner turn test, and modified Garcia test at 24 hr after ICH. (d) Representative western blot bands. Quantitative analyses of (e) phosphorylated JNK, (f) phosphorylated p38 MAPK, (g) NLRP1 inflammasome, (h) cleaved caspase‐1, and (i) IL‐1β at 24 hr after ICH. Data shown are individual values with mean ± SD; n = 6. *P < 0.05, significantly different from sham + vehicle, @ P < 0.05, significantly different from sham + RO27‐3225, # P < 0.05, significantly different from ICH + vehicle; one‐way ANOVA, Tukey's test
In ICH mice, the neurological deficits were observed, and the expression of p‐JNK, p‐p38 MAPK, NLRP1 inflammasome, cleaved caspase‐1, and IL‐1β was markedly increased compared with the expression in the sham mice (Figure 4). RO27‐3225 treatment significantly improved the neurological outcomes (Figure 4a–c) and reduced the expression of p‐JNK, p‐p38 MAPK, NLRP1 inflammasome, cleaved caspase‐1, and IL‐1β when compared with vehicle‐treated ICH mice (Figure 4d–i).
3.5. RO27‐3225 attenuated neuronal pyroptotic cell death at 24 hr after ICH
FJC staining, neuronal marker NeuN co‐staining with TUNEL, or caspase‐1 were used to assess whether RO27‐3225 treatment reduced neuronal cell death including neuronal pyroptosis at 24 hr after ICH. The number of FJC‐positive neurons in the perihaematomal region at 24 hr after ICH was significantly increased in ICH + vehicle group when compared with the sham group. This number was significantly decreased in the ICH + RO27‐3225 group, compared with ICH + vehicle group (Figure 5a,b). In addition, TUNEL‐positive neurons in ICH + vehicle group were significantly increased in the perihaematomal region at 24 hr after ICH when compared with the sham group (Figure 5c,d). RO27‐3225 treatment significantly reduced the number of TUNEL‐positive neurons in the perihaematomal region at 24 hr after ICH when compared with ICH + vehicle group (Figure 5c,d). Specifically, double immunofluorescence staining showed that RO27‐3225 significantly reduced the ICH‐induced increases in the number of cleaved caspase‐1‐positive neurons in the perihaematomal region, suggesting its protection against neuronal pyroptosis (Figure 5e,f).
Figure 5.
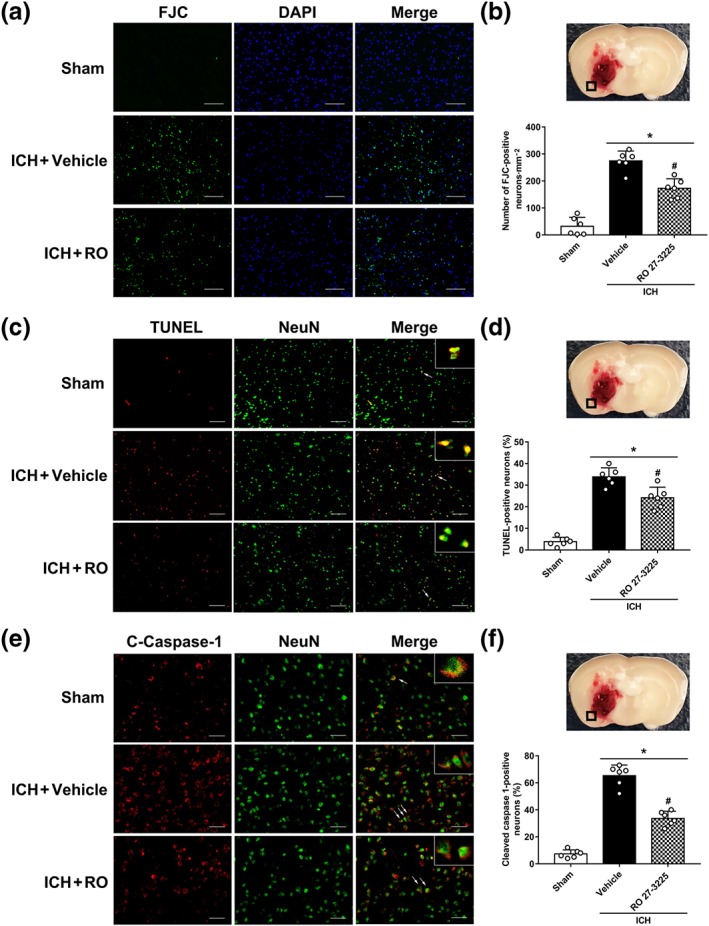
The effects of RO27‐3225 (RO) on neuronal degeneration and pyroptosis at 24 hr after ICH. (a, b) Representative microphotographs and quantitative analysis of Fluoro‐Jade C (FJC) positive degenerating neurons in the perihaematomal area. (c, d) Representative microphotographs and quantitative analysis of TUNEL‐positive neurons in the perihaematomal area. (e, f) Representative microphotographs and quantitative analysis of cleaved caspase‐1 (C‐caspase‐1)‐positive neurons in the perihaematomal area. Scale bar = 50 μm. Data shown are individual values with mean ± SD; n = 6. *P < 0.05, significantly different from sham, # P < 0.05, significantly different from ICH + vehicle; one‐way ANOVA, Tukey's test
3.6. The MC4 receptor antagonist HS024 reversed the neuroprotective effects of RO27‐3225 at 24 hr after ICH
Blockade of MC4 receptor with the selective antagonist HS024 markedly reversed the neurological improvements of ICH mice treated by RO27‐3225 in forelimb placement test (Figure 6a), corner turn test (Figure 6b), and modified Garcia test (Figure 6c) at 24 hr post‐ICH. Additionally, there was a significantly greater neurological deficit in ICH + RO27‐3225 + HS024 group than that in ICH + RO27‐3225 + saline group (Figure 6a–c). Consistent with these findings, HS024 intervention significantly increased the expression of p‐ASK1, p‐JNK, p‐p38 MAPK, NLRP1 inflammasome, cleaved caspase‐1, and IL‐1β, compared with ICH + RO27‐3225 + saline group (Figure 6d–j).
Figure 6.
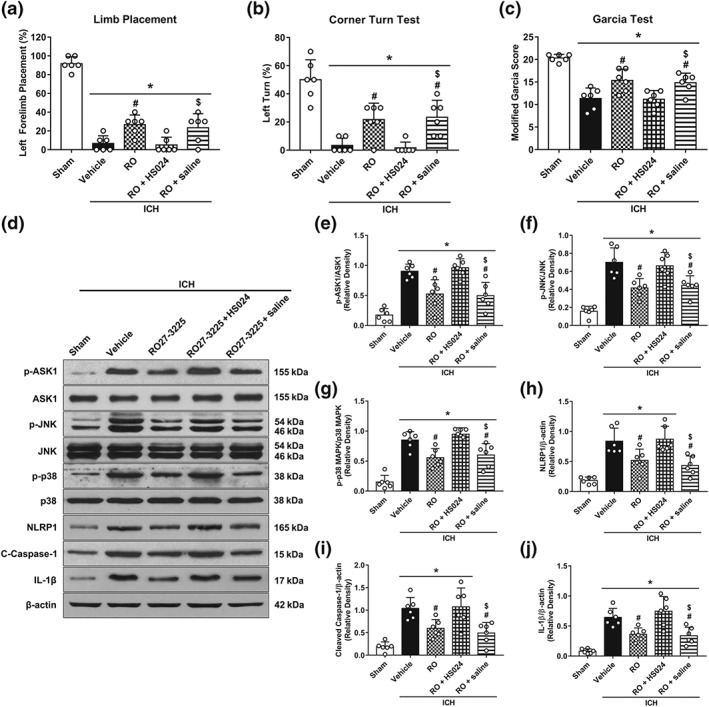
Pretreatment with the selective MC4 receptor antagonist HS024 abolished the effects of RO27‐3225 on neurobehavioural outcomes and ASK1/JNK/p‐38 MAPK/NLRP1/caspase‐1/IL‐1β signalling at 24 hr after ICH. (a–c) Forelimb placement, corner turn test, and modified Garcia test at 24 hr after ICH. (d) Representative western blot bands. Quantitative analyses of (e) phosphorylated ASK1, (f) phosphorylated JNK, (g) phosphorylated p38 MAPK, (h) NLRP1 inflammasome, (i) cleaved caspase‐1, and (j) IL‐1β at 24 hr after ICH. Data shown are individual values with mean ± SD; n = 6. *P < 0.05, significantly different from sham, # P < 0.05, significantly different from ICH + vehicle, $ P < 0.05, significantly different from ICH + RO27‐3225 + HS024; one‐way ANOVA, Tukey's test
3.7. ASK1 inhibitor NQDI‐1 decreased the expression of phosphorylated ASK1, phosphorylated JNK, phosphorylated p38 MAPK, and NLRP1 inflammasome at 24 hr after ICH
To investigate whether MC4 receptor activation down‐regulated JNK/p38 MAPK signalling via ASK1, NQDI‐1, as a specific inhibitor of ASK1, was administered i.c.v 30 min before ICH induction. The neurological outcomes and Western blot results showed that ASK1 inhibition prior to ICH induction resulted in effects, similar to those of RO27‐3225, given as a post‐treatment. Neurological performances in forelimb placement test (Figure 7a), corner turn test (Figure 7b), and modified Garcia test (Figure 7c) were better in ICH + NQDI‐1 group when compared with ICH + DMSO group at 24 hr post‐ICH. In addition, the expression of p‐ASK1, p‐JNK, p‐p38 MAPK, and NLRP1 inflammasome were significantly decreased in ICH + NQDI‐1 group when compared with ICH + DMSO group at 24 hr after ICH (Figure 7d–h).
Figure 7.
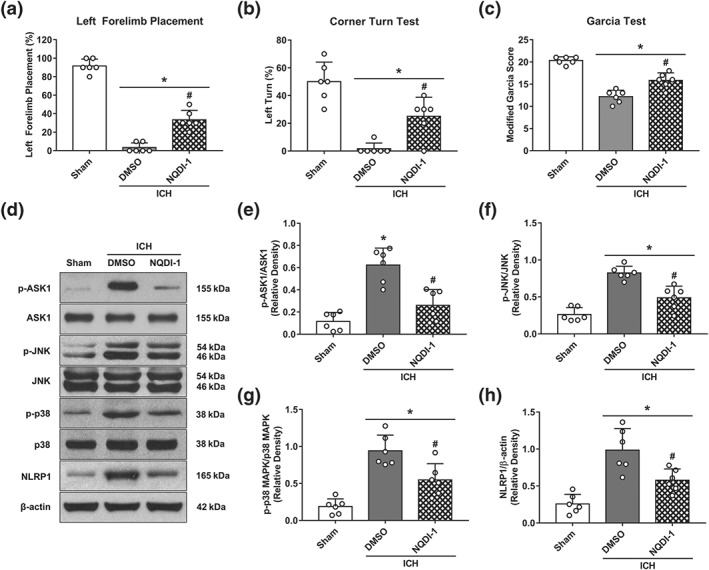
The effects of selective ASK1 inhibitor NQDI‐1 on the neurobehavioural outcomes and ASK1/JNK/p‐38 MAPK/NLRP1signaling at 24 hr after ICH. (a–c) Forelimb placement, corner turn test, and modified Garcia test at 24 hr after ICH. (d) Representative western blot bands. Quantitative analyses of (e) phosphorylated ASK1, (f) phosphorylated JNK, (g) phosphorylated p38 MAPK, and (h) NLRP1 inflammasome at 24 hr after ICH. Data shown are individual values with mean ± SD; n = 6. *P < 0.05, significantly different from sham; # P < 0.05, significantly different from ICH + vehicle; one‐way ANOVA, Tukey's test
Figure 8.
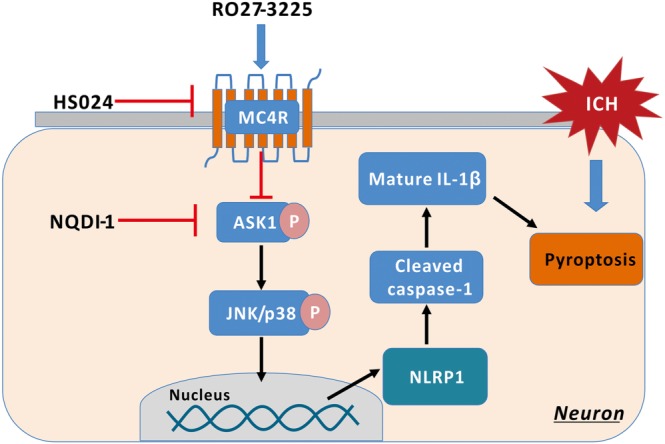
Scheme of the mechanism of the anti‐pyroptosis effects of RO27‐3225 after ICH. MC4R: MC4 receptor
4. DISCUSSION
In the present study, we explored the effects of MC4 receptor activation with RO27‐3225 on the neuronal pyroptosis in a mouse model of ICH and investigated the possible underlying mechanism(s)| involved. We found that the expression of MC4 receptor and NLRP1 inflammasomes was increased in a time‐dependent manner after ICH and that the MC4 receptor co‐localized with neurons. In addition, treatment with RO27‐3225 (180 μg·kg−1 at 1 hr after ICH) significantly improved neurological functions, attenuated pyroptotic neuronal death, and decreased expression of p‐ASK1, p‐JNK, p‐p38 MAPK, NLRP1 inflammasome, cleaved caspase‐1, and IL‐1β at 24 hr after ICH. Conversely, blockade of MC4 receptor with the selective antagonist HS024 abolished the neuroprotective effects of RO27‐3225. Furthermore, inhibition of ASK1 by NQDI‐1, given as a pretreatment alone, resulted in effects similar to those of RO27‐3225, given as a post‐treatment, including improvement of neurological outcomes and down‐regulation of p‐ASK1, p‐JNK, p‐p38 MAPK, and NLRP1 inflammasome expression after ICH. These observations indicated that activation of MC4 receptor with RO27‐3225 reduced NLRP1‐dependent neuronal pyroptosis after ICH, possibly mediated by inhibiting ASK1/JNK/p38 MAPK signalling pathways.
Unlike apoptosis and necrosis, pyroptosis is one type of programmed cell death that is highly inflammatory and exclusively mediated by cleaved caspase‐1 (Fann, Lee, Manzanero, Chunduri, et al., 2013; Fann, Lee, Manzanero, Tang, et al., 2013). Previous studies showed that NLRP1 inflammasomes regulated inflammatory caspase‐1 and consequent IL‐1β processing (Chavarria‐Smith & Vance, 2015; Tan et al., 2015). In addition, NLRP1 inflammasome promoted pyroptosis in the setting of autoimmune thyroiditis (Guo et al., 2018). Silencing NLRP1 inflammasomes attenuated neuronal pyroptosis in a mouse model of Alzheimer's disease (Tan et al., 2014). Furthermore, in a focal cerebral ischaemia–reperfusion stroke model, the expression of NLRP1 inflammasomes in ipsilateral brain tissue increased as early as 1 hr and was maintained at a higher level at 12, 24, and 72 hr after stroke (Fann, Lee, Manzanero, Chunduri, et al., 2013; Fann, Lee, Manzanero, Tang, et al., 2013). Similarly, we observed a time‐dependent up‐regulation of the endogenous expression of NLRP1 inflammasomes in the ipsilateral/right hemisphere after ICH, which started at 12 hr and peaked at 72 hr after ICH.
α‐MSH and other melanocortins belong to a family of endogenous neuropeptides derived from pro‐opiomelanocortin and, through binding to five subtypes of melanocortin receptors, these peptides exert a range of effects on the host, including anti‐inflammation, reduction of food intake, control of autonomic functions, and stimulation of exocrine secretions (Catania, 2008; Flores‐Bastias & Karahanian, 2018). The MC4 receptor is the predominant subtype of melanocortin receptors that is highly expressed in neurons, microglia, and astrocytes (Mountjoy, Mortrud, Low, Simerly, & Cone, 1994). Consistent with these earlier findings, expression of MC4 receptor, in our experiments, was co‐localized with neurons, and was significantly increased after ICH. The up‐regulation of MC4 receptor peaking at 24 hr after ICH may suggest its role as an endogenous protective response to stress and deleterious stimuli in the acute phase after ICH (Chen et al., 2018; Lasaga, Debeljuk, Durand, Scimonelli, & Caruso, 2008). There is increasing evidence that MC4 receptor activation resulted in strong protective effects in experimental ischaemic stroke, haemorrhagic shock, and myocardial ischaemia–reperfusion injury (Bazzani et al., 2001; Giuliani et al., 2006, 2007; Spaccapelo et al., 2011). By binding with its endogenous ligand α‐MSH, MC4 receptor exerted anti‐inflammatory effects via inhibiting the production and activity of pro‐inflammatory cytokines in animal models of ischaemic renal failure, cerebral ischaemia, and traumatic brain injury (Bitto et al., 2012; Forslin Aronsson et al., 2006; Jo et al., 2001).
RO27‐3225 is a novel‐selective agonist of MC4 receptor and is anti‐inflammatory and anti‐apoptotic in animal models of global cerebral ischaemia, severe pancreatitis and haemorrhagic shock (Giuliani et al., 2007; Minutoli et al., 2011; Spaccapelo et al., 2011). However, the effect of MC4 receptor activation on neuronal pyroptosis has not been elucidated. In this study, we found that post‐treatment with RO27‐3225 significantly improved neurological deficits and inhibited the expression of NLRP1 inflammasome, cleaved caspase‐1, and IL‐1β after ICH. Importantly, immunohistochemical assays showed that RO27‐3225 reduced the numbers of degenerating neurons, especially cleaved caspase‐1‐positive neurons after ICH, suggesting the suppression of NLRP1 inflammasome‐mediated pyroptosis. Therefore, the anti‐pyroptotic property of MC4 receptor activation with RO27‐3225 might have neuroprotective potentials against cerebrovascular diseases.
Previous studies indicated that activation of MC4 receptor significantly inhibited the JNK/p38 MAPK signalling pathway in vivo and in vitro (Chai et al., 2009; Lasaga et al., 2008; Spaccapelo et al., 2011). Our previous study had also demonstrated that the activation of MC4 receptor with RO27‐3225 attenuated neuroinflammation via inhibiting the JNK/p38 MAPK signalling pathway in a mouse model of ICH (Chen et al., 2018). Interestingly, ASK1 has been shown as one of the proteins upstream of the JNK/p38 MAPK cascade, required for sustaining JNK/p38 MAPK activation and apoptosis (Amos et al., 2018; Tobiume et al., 2001). Even though MC4 receptor and ASK1 have been reported to regulate JNK/p38 MAPK signalling, the interaction of MC4 receptor activation and ASK1 phosphorylation has not been investigated so far. In the current study, there was increased ASK1 phosphorylation and JNK/p38 MAPK signalling activity, 24 hr after ICH. These effects of ASK1 were significantly suppressed by the MC4 receptor agonist RO27‐3225. These results suggested that MC4R activation was most likely to be an upstream event that could subsequently inhibit the ASK1/JNK/p38 MAPK cascade, after ICH. In addition, the JNK/p38 MAPK signalling pathway regulates the expression of NLRP inflammasome proteins and precursors IL‐1β in peripheral immune cells, ischaemic primary cortical neurons, and brain tissue under ischaemic conditions (Burm et al., 2015; Fann et al., 2018; He et al., 2012). Consistent with these observations, our results showed that RO27‐3225 significantly decreased the expression of p‐ASK1, p‐JNK, p‐p38 MAPK, NLRP1 inflammasome, cleaved caspase‐1, and IL‐1β and improved the neurological outcomes after ICH induction.
To further validate this proposed underlying mechanism, we administered the selective MC4 receptor antagonist HS024, before RO27‐3225 treatment. HS024 abolished the effects of RO27‐3225 on neurological improvements and reversed suppression effects of RO27‐3225 on downstream proteins including ASK1/JNK/p38 MAPK/NLRP1 inflammasome/cleaved caspase‐1/IL‐1β. Similarly, when NQDI‐1 was given prior to ICH induction to inhibit ASK1, NQDI‐1 produced RO27‐3225‐like neuroprotection in our ICH model. Therefore, these observations indicate that a novel neuroprotective mechanism of MC4 receptor activation may suppress NLRP1‐dependent neuronal pyroptosis by inhibiting the ASK1/JNK/p38 MAPK signalling pathways after ICH.
There are some limitations in the present study. First, previous studies have reported that activation of MC4 receptor exerts multiple neuroprotective effects including blood–brain barrier preservation, anti‐apoptosis, anti‐inflammation, and synaptic plasticity (Chen et al., 2018; Shen, Fu, Cheng, Fu, & Ip, 2013; Spaccapelo et al., 2011). In this study, we focused on the anti‐pyroptotic effect of MC4 receptor after ICH. Therefore, further investigations are needed to elucidate the other neuroprotective mechanisms of MC4 receptor activation after ICH. Second, NLRP3 inflammasomes are mainly expressed in microglia, which can produce pro‐inflammatory cytokines to induce inflammatory events. As the NLRP1 inflammasome is mainly expressed in neurons, this inflammasome might be more closely linked to pyroptotic cell death (Tan et al., 2014, 2015), but we cannot rule out the possibility of NLRP3 signalling in neuronal pyroptosis. Lastly, ICH primarily occurs in the elderly population and is mostly accompanied by hypertension, vascular disorders, or anticoagulation (Zhao et al., 2018). Further studies are needed to validate the neuroprotection effects of MC4 receptor activation after experimental ICH in aged mice, with other systemic co‐morbidities.
In conclusion, the present study showed that MC4 receptor activation with RO27‐3225 suppressed NLRP1‐dependent neuronal pyroptosis and improved neurological functions, at least in part, by inhibiting the ASK1/JNK/p38 MAPK signalling pathway after experimental ICH in mice. Thus, MC4 receptor activation may provide a promising therapeutic strategy for management of patients with ICH.
AUTHOR CONTRIBUTIONS
S.P.C., Y.C.Z., P.S., and J.P.T. worked on the experimental design. S.P.C., Z.T.Y., J.Z., J.Y.Z., L.H.Z., and D.D. conducted the experiments, analysed the data, and drafted the manuscript. L.H. and P.S. worked on data interpretation and the manuscript preparation. J.H.Z., Y.X., and J.P.T. participated in the experimental design, data analysis and interpretation, and manuscript preparation.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
DECLARATION OF TRANSPARENCY AND SCIENTIFIC RIGOUR
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research as stated in the BJP guidelines for Design & Analysis, Immunoblotting and Immunochemistry, and Animal Experimentation, and as recommended by funding agencies, publishers and other organisations engaged with supporting research.
Supporting information
Table S1.
Summary of experimental groups and mortality rate in the study.
ACKNOWLEDGEMENTS
This study was supported partially by Grants NS091042 and NS082184 from the National Institutes of Health and grants from National Natural Science Foundation of China (81760234 and 81801176).
Chen S, Zuo Y, Huang L, et al. The MC4 receptor agonist RO27‐3225 inhibits NLRP1‐dependent neuronal pyroptosis via the ASK1/JNK/p38 MAPK pathway in a mouse model of intracerebral haemorrhage. Br J Pharmacol. 2019;176:1341–1356. 10.1111/bph.14639
Contributor Information
Ying Xia, Email: xiaying008@163.com.
Jiping Tang, Email: jtang@llu.edu.
REFERENCES
- Abulafia, D. P. , de Rivero Vaccari, J. P. , Lozano, J. D. , Lotocki, G. , Keane, R. W. , & Dietrich, W. D. (2009). Inhibition of the inflammasome complex reduces the inflammatory response after thromboembolic stroke in mice. Journal of Cerebral Blood Flow and Metabolism, 29, 534–544. 10.1038/jcbfm.2008.143 [DOI] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Christopoulos, A. , Davenport, A. P. , Kelly, E. , Marrion, N. V. , Peters, J. A. , … CGTP Collaborators . (2017). The Concise Guide to PHARMACOLOGY 2017/18: G protein‐coupled receptors. British Journal of Pharmacology, 174, S17–S129. 10.1111/bph.13878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , … CGTP Collaborators . (2017a). The Concise Guide to PHARMACOLOGY 2017/18: Catalytic receptors. British Journal of Pharmacology, 174, S225–S271. 10.1111/bph.13876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , … CGTP Collaborators . (2017b). The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. Br J Pharmacol, 174(Suppl 1), S272–S359. 10.1111/bph.13877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amos, L. A. , Ma, F. Y. , Tesch, G. H. , Liles, J. T. , Breckenridge, D. G. , Nikolic‐Paterson, D. J. , & Han, Y. (2018). ASK1 inhibitor treatment suppresses p38/JNK signalling with reduced kidney inflammation and fibrosis in rat crescentic glomerulonephritis. Journal of Cellular and Molecular Medicine, 22, 4522–4533. 10.1111/jcmm.13705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros‐Minones, L. , Orejana, L. , Goni‐Allo, B. , Suquia, V. , Hervias, I. , Aguirre, N. , & Puerta, E. (2013). Modulation of the ASK1‐MKK3/6‐p38/MAPK signalling pathway mediates sildenafil protection against chemical hypoxia caused by malonate. British Journal of Pharmacology, 168, 1820–1834. 10.1111/bph.12071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazzani, C. , Guarini, S. , Botticelli, A. R. , Zaffe, D. , Tomasi, A. , Bini, A. , … Bertolini, A. (2001). Protective effect of melanocortin peptides in rat myocardial ischemia. The Journal of Pharmacology and Experimental Therapeutics, 297, 1082–1087. [PubMed] [Google Scholar]
- Bitto, A. , Polito, F. , Altavilla, D. , Irrera, N. , Giuliani, D. , Ottani, A. , … Squadrito, F. (2011). Melanocortins protect against multiple organ dysfunction syndrome in mice. British Journal of Pharmacology, 162, 917–928. 10.1111/j.1476-5381.2010.01098.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitto, A. , Polito, F. , Irrera, N. , Calo, M. , Spaccapelo, L. , Marini, H. R. , … Altavilla, D. (2012). Protective effects of melanocortins on short‐term changes in a rat model of traumatic brain injury. Critical Care Medicine, 40, 945–951. 10.1097/CCM.0b013e318236efde [DOI] [PubMed] [Google Scholar]
- Burm, S. M. , Zuiderwijk‐Sick, E. A. , 't Jong, A. E. , van der Putten, C. , Veth, J. , Kondova, I. , & Bajramovic, J. J. (2015). Inflammasome‐induced IL‐1beta secretion in microglia is characterized by delayed kinetics and is only partially dependent on inflammatory caspases. The Journal of Neuroscience, 35, 678–687. 10.1523/JNEUROSCI.2510-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai, Y. , Cho, G. S. , Ju, C. , Wang, S. L. , Ryu, J. H. , Shin, C. Y. , … Kim, W. K. (2011). Activated microglia are less vulnerable to hemin toxicity due to nitric oxide‐dependent inhibition of JNK and p38 MAPK activation. Journal of Immunology, 187, 1314–1321. 10.4049/jimmunol.1002925 [DOI] [PubMed] [Google Scholar]
- Catania, A. (2008). Neuroprotective actions of melanocortins: A therapeutic opportunity. Trends in Neurosciences, 31, 353–360. 10.1016/j.tins.2008.04.002 [DOI] [PubMed] [Google Scholar]
- Chai, B. , Li, J. Y. , Zhang, W. , Wang, H. , & Mulholland, M. W. (2009). Melanocortin‐4 receptor activation inhibits c‐Jun N‐terminal kinase activity and promotes insulin signaling. Peptides, 30, 1098–1104. 10.1016/j.peptides.2009.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavarria‐Smith, J. , & Vance, R. E. (2015). The NLRP1 inflammasomes. Immunological Reviews, 265, 22–34. 10.1111/imr.12283 [DOI] [PubMed] [Google Scholar]
- Chen, S. , Zhao, L. , Sherchan, P. , Ding, Y. , Yu, J. , Nowrangi, D. , … Zhang, J. H. (2018). Activation of melanocortin receptor 4 with RO27‐3225 attenuates neuroinflammation through AMPK/JNK/p38 MAPK pathway after intracerebral hemorrhage in mice. Journal of Neuroinflammation, 15, 106 10.1186/s12974-018-1140-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enkhjargal, B. , Malaguit, J. , Ho, W. M. , Jiang, W. , Wan, W. , Wang, G. , … Zhang, J. H. (2017). Vitamin D attenuates cerebral artery remodeling through VDR/AMPK/eNOS dimer phosphorylation pathway after subarachnoid hemorrhage in rats. Journal of Cerebral Blood Flow and Metabolism, 39, 272–284. 10.1177/0271678X17726287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fann, D. Y. , Lee, S. Y. , Manzanero, S. , Chunduri, P. , Sobey, C. G. , & Arumugam, T. V. (2013). Pathogenesis of acute stroke and the role of inflammasomes. Ageing Research Reviews, 12, 941–966. 10.1016/j.arr.2013.09.004 [DOI] [PubMed] [Google Scholar]
- Fann, D. Y. , Lee, S. Y. , Manzanero, S. , Tang, S. C. , Gelderblom, M. , Chunduri, P. , … Arumugam, T. (2013). Intravenous immunoglobulin suppresses NLRP1 and NLRP3 inflammasome‐mediated neuronal death in ischemic stroke. Cell Death & Disease, 4, e790 10.1038/cddis.2013.326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fann, D. Y. , Lim, Y. A. , Cheng, Y. L. , Lok, K. Z. , Chunduri, P. , Baik, S. H. , … Arumugam, T. V. (2018). Evidence that NF‐κB and MAPK signaling promotes NLRP inflammasome activation in neurons following ischemic stroke. Molecular Neurobiology, 55, 1082–1096. 10.1007/s12035-017-0394-9 [DOI] [PubMed] [Google Scholar]
- Fann, D. Y. , Santro, T. , Manzanero, S. , Widiapradja, A. , Cheng, Y. L. , Lee, S. Y. , … Arumugam, T. V. (2014). Intermittent fasting attenuates inflammasome activity in ischemic stroke. Experimental Neurology, 257, 114–119. 10.1016/j.expneurol.2014.04.017 [DOI] [PubMed] [Google Scholar]
- Flores‐Bastias, O. , & Karahanian, E. (2018). Neuroinflammation produced by heavy alcohol intake is due to loops of interactions between Toll‐like 4 and TNF receptors, peroxisome proliferator‐activated receptors and the central melanocortin system: A novel hypothesis and new therapeutic avenues. Neuropharmacology, 128, 401–407. 10.1016/j.neuropharm.2017.11.003 [DOI] [PubMed] [Google Scholar]
- Forslin Aronsson, S. , Spulber, S. , Popescu, L. M. , Winblad, B. , Post, C. , Oprica, M. , & Schultzberg, M. (2006). α‐Melanocyte‐stimulating hormone is neuroprotective in rat global cerebral ischemia. Neuropeptides, 40, 65–75. 10.1016/j.npep.2005.10.006 [DOI] [PubMed] [Google Scholar]
- Gan, P. , Gao, Z. , Zhao, X. , & Qi, G. (2016). Surfactin inducing mitochondria‐dependent ROS to activate MAPKs, NF‐κB and inflammasomes in macrophages for adjuvant activity. Scientific Reports, 6, 39303 10.1038/srep39303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfield, A. S. , Lam, D. D. , Marston, O. J. , Przydzial, M. J. , & Heisler, L. K. (2009). Role of central melanocortin pathways in energy homeostasis. Trends in Endocrinology and Metabolism, 20, 203–215. 10.1016/j.tem.2009.02.002 [DOI] [PubMed] [Google Scholar]
- Giuliani, D. , Mioni, C. , Altavilla, D. , Leone, S. , Bazzani, C. , Minutoli, L. , … Guarini, S. (2006). Both early and delayed treatment with melanocortin 4 receptor‐stimulating melanocortins produces neuroprotection in cerebral ischemia. Endocrinology, 147, 1126–1135. 10.1210/en.2005-0692 [DOI] [PubMed] [Google Scholar]
- Giuliani, D. , Mioni, C. , Bazzani, C. , Zaffe, D. , Botticelli, A. R. , Capolongo, S. , … Guarini, S. (2007). Selective melanocortin MC4 receptor agonists reverse haemorrhagic shock and prevent multiple organ damage. British Journal of Pharmacology, 150, 595–603. 10.1038/sj.bjp.0707115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, Q. , Wu, Y. , Hou, Y. , Liu, Y. , Liu, T. , Zhang, H. , … Teng, W. (2018). Cytokine secretion and pyroptosis of thyroid follicular cells mediated by enhanced NLRP3, NLRP1, NLRC4, and AIM2 inflammasomes are associated with autoimmune thyroiditis. Frontiers in Immunology, 9, 1197 10.3389/fimmu.2018.01197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao, H. , Li, S. , Tang, H. , Liu, B. , Cai, Y. , Shi, C. , & Xiao, X. (2016). NQDI‐1, an inhibitor of ASK1 attenuates acute perinatal hypoxic‐ischemic cerebral injury by modulating cell death. Molecular Medicine Reports, 13, 4585–4592. 10.3892/mmr.2016.5123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR . (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: Updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Research, 46, D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, Q. , You, H. , Li, X. M. , Liu, T. H. , Wang, P. , & Wang, B. E. (2012). HMGB1 promotes the synthesis of pro‐IL‐1beta and pro‐IL‐18 by activation of p38 MAPK and NF‐κB through receptors for advanced glycation end‐products in macrophages. Asian Pacific Journal of Cancer Prevention, 13, 1365–1370. 10.7314/APJCP.2012.13.4.1365 [DOI] [PubMed] [Google Scholar]
- Iniaghe, L. O. , Krafft, P. R. , Klebe, D. W. , Omogbai, E. K. I. , Zhang, J. H. , & Tang, J. (2015). Dimethyl fumarate confers neuroprotection by casein kinase 2 phosphorylation of Nrf2 in murine intracerebral hemorrhage. Neurobiology of Disease, 82, 349–358. 10.1016/j.nbd.2015.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo, S. K. , Yun, S. Y. , Chang, K. H. , Cha, D. R. , Cho, W. Y. , Kim, H. K. , & Won, N. H. (2001). α‐MSH decreases apoptosis in ischaemic acute renal failure in rats: Possible mechanism of this beneficial effect. Nephrology, Dialysis, Transplantation, 16, 1583–1591. 10.1093/ndt/16.8.1583 [DOI] [PubMed] [Google Scholar]
- Kilkenny, C. , Browne, W. , Cuthill, I. C. , Emerson, M. , & Altman, D. G. (2010). Animal research: Reporting in vivo experiments: The ARRIVE guidelines. British Journal of Pharmacology, 160, 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasaga, M. , Debeljuk, L. , Durand, D. , Scimonelli, T. N. , & Caruso, C. (2008). Role of α‐melanocyte stimulating hormone and melanocortin 4 receptor in brain inflammation. Peptides, 29, 1825–1835. 10.1016/j.peptides.2008.06.009 [DOI] [PubMed] [Google Scholar]
- Lin, W. P. , Xiong, G. P. , Lin, Q. , Chen, X. W. , Zhang, L. Q. , Shi, J. X. , … Lin, J. H. (2016). Heme oxygenase‐1 promotes neuron survival through down‐regulation of neuronal NLRP1 expression after spinal cord injury. Journal of Neuroinflammation, 13, 52 10.1186/s12974-016-0521-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manaenko, A. , Yang, P. , Nowrangi, D. , Budbazar, E. , Hartman, R. E. , Obenaus, A. , … Tang, J. (2018). Inhibition of stress fiber formation preserves blood‐brain barrier after intracerebral hemorrhage in mice. Journal of Cerebral Blood Flow and Metabolism, 38, 87–102. 10.1177/0271678X16679169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinon, F. , Burns, K. , & Tschopp, J. (2002). The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL‐β. Molecular Cell, 10, 417–426. 10.1016/S1097-2765(02)00599-3 [DOI] [PubMed] [Google Scholar]
- Mendelow, A. D. , Gregson, B. A. , Fernandes, H. M. , Murray, G. D. , Teasdale, G. M. , Hope, D. T. , … STICH investigators . (2005). Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): A randomised trial. Lancet, 365, 387–397. 10.1016/S0140-6736(05)70233-6 [DOI] [PubMed] [Google Scholar]
- Minutoli, L. , Squadrito, F. , Nicotina, P. A. , Giuliani, D. , Ottani, A. , Polito, F. , … Altavilla, D. (2011). Melanocortin 4 receptor stimulation decreases pancreatitis severity in rats by activation of the cholinergic anti‐inflammatory pathway. Critical Care Medicine, 39, 1089–1096. 10.1097/CCM.0b013e318207ea80 [DOI] [PubMed] [Google Scholar]
- Mortezaee, K. , Khanlarkhani, N. , Beyer, C. , & Zendedel, A. (2018). Inflammasome: Its role in traumatic brain and spinal cord injury. Journal of Cellular Physiology, 233, 5160–5169. 10.1002/jcp.26287 [DOI] [PubMed] [Google Scholar]
- Mountjoy, K. G. , Mortrud, M. T. , Low, M. J. , Simerly, R. B. , & Cone, R. D. (1994). Localization of the melanocortin‐4 receptor (MC4‐R) in neuroendocrine and autonomic control circuits in the brain. Molecular Endocrinology, 8, 1298–1308. 10.1210/mend.8.10.7854347 [DOI] [PubMed] [Google Scholar]
- Nadeau, C. A. , Dietrich, K. , Wilkinson, C. M. , Crawford, A. M. , George, G. N. , Nichol, H. K. , & Colbourne, F. (2018). Prolonged blood–brain barrier injury occurs after experimental intracerebral hemorrhage and is not acutely associated with additional bleeding. Translational Stroke Research. 10.1007/s12975-018-0636-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni, W. , Okauchi, M. , Hatakeyama, T. , Gu, Y. , Keep, R. F. , Xi, G. , & Hua, Y. (2015). Deferoxamine reduces intracerebral hemorrhage‐induced white matter damage in aged rats. Experimental Neurology, 272, 128–134. 10.1016/j.expneurol.2015.02.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi, B. , Hu, L. , Zhu, L. , Shang, L. , Wang, X. , Liu, N. , … Fang, D. (2017). Metformin attenuates neurological deficit after intracerebral hemorrhage by inhibiting apoptosis, oxidative stress and neuroinflammation in rats. Neurocheml Res, 42, 2912–2920. 10.1007/s11064-017-2322-9 [DOI] [PubMed] [Google Scholar]
- de Rivero Vaccari, J. P. , Lotocki, G. , Alonso, O. F. , Bramlett, H. M. , Dietrich, W. D. , & Keane, R. W. (2009). Therapeutic neutralization of the NLRP1 inflammasome reduces the innate immune response and improves histopathology after traumatic brain injury. Journal of Cerebral Blood Flow and Metabolism, 29, 1251–1261. 10.1038/jcbfm.2009.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues, A. R. , Almeida, H. , & Gouveia, A. M. (2015). Intracellular signaling mechanisms of the melanocortin receptors: Current state of the art. Cellular and Molecular Life Sciences, 72, 1331–1345. 10.1007/s00018-014-1800-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen, Y. , Fu, W. Y. , Cheng, E. Y. , Fu, A. K. , & Ip, N. Y. (2013). Melanocortin‐4 receptor regulates hippocampal synaptic plasticity through a protein kinase A‐dependent mechanism. The Journal of Neuroscience, 33, 464–472. 10.1523/JNEUROSCI.3282-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaccapelo, L. , Bitto, A. , Galantucci, M. , Ottani, A. , Irrera, N. , Minutoli, L. , … Guarini, S. (2011). Melanocortin MC4 receptor agonists counteract late inflammatory and apoptotic responses and improve neuronal functionality after cerebral ischemia. European Journal of Pharmacology, 670, 479–486. 10.1016/j.ejphar.2011.09.015 [DOI] [PubMed] [Google Scholar]
- Tan, C. C. , Zhang, J. G. , Tan, M. S. , Chen, H. , Meng, D. W. , Jiang, T. , … Tan, L. (2015). NLRP1 inflammasome is activated in patients with medial temporal lobe epilepsy and contributes to neuronal pyroptosis in amygdala kindling‐induced rat model. Journal of Neuroinflammation, 12, 18 10.1186/s12974-014-0233-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan, M. S. , Tan, L. , Jiang, T. , Zhu, X. C. , Wang, H. F. , Jia, C. D. , & Yu, J. T. (2014). Amyloid‐β induces NLRP1‐dependent neuronal pyroptosis in models of Alzheimer's disease. Cell Death & Disease, 5, e1382 10.1038/cddis.2014.348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao, Y. X. (2010). The melanocortin‐4 receptor: Physiology, pharmacology, and pathophysiology. Endocrine Reviews, 31, 506–543. 10.1210/er.2009-0037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobiume, K. , Matsuzawa, A. , Takahashi, T. , Nishitoh, H. , Morita, K. , Takeda, K. , … Ichijo, H. (2001). ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Reports, 2, 222–228. 10.1093/embo-reports/kve046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomura, S. , de Rivero Vaccari, J. P. , Keane, R. W. , Bramlett, H. M. , & Dietrich, W. D. (2012). Effects of therapeutic hypothermia on inflammasome signaling after traumatic brain injury. Journal of Cerebral Blood Flow and Metabolism, 32, 1939–1947. 10.1038/jcbfm.2012.99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong, L. S. , Shao, A. W. , Ou, Y. B. , Guo, Z. N. , Manaenko, A. , Dixon, B. J. , … Zhang, J. H. (2017). Recombinant Gas6 augments Axl and facilitates immune restoration in an intracerebral hemorrhage mouse model. Journal of Cerebral Blood Flow and Metabolism, 37, 1971–1981. 10.1177/0271678X16658490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, T. , Nowrangi, D. , Yu, L. , Lu, T. , Tang, J. , Han, B. , … Zhang, J. H. (2018). Activation of dopamine D1 receptor decreased NLRP3‐mediated inflammation in intracerebral hemorrhage mice. Journal of Neuroinflammation, 15, 2 10.1186/s12974-017-1039-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Z. , Zhou, F. , Dou, Y. , Tian, X. , Liu, C. , Li, H. , … Chen, G. (2018). Melatonin alleviates intracerebral hemorrhage‐induced secondary brain injury in rats via suppressing apoptosis, inflammation, oxidative stress, DNA damage, and mitochondria injury. Translational Stroke Research, 9, 74–91. 10.1007/s12975-017-0559-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson, D. A. , Pandey, A. S. , Thompson, B. G. , Keep, R. F. , Hua, Y. , & Xi, G. (2018). Injury mechanisms in acute intracerebral hemorrhage. Neuropharmacology, 134, 240–248. 10.1016/j.neuropharm.2017.09.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, Z. , Enkhjargal, B. , Wu, L. , Zhou, K. , Sun, C. , Hu, X. , … Zhang, J. H. (2018). Exendin‐4 attenuates neuronal death via GLP‐1R/PI3K/Akt pathway in early brain injury after subarachnoid hemorrhage in rats. Neuropharmacology, 128, 142–151. 10.1016/j.neuropharm.2017.09.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, N. , Zhang, Y. , Doycheva, D. M. , Ding, Y. , Zhang, Y. , Tang, J. , … Zhang, J. H. (2018). Adiponectin attenuates neuronal apoptosis induced by hypoxia‐ischemia via the activation of AdipoR1/APPL1/LKB1/AMPK pathway in neonatal rats. Neuropharmacology, 133, 415–428. 10.1016/j.neuropharm.2018.02.024 [DOI] [PubMed] [Google Scholar]
- Zhao, L. , Chen, S. , Sherchan, P. , Ding, Y. , Zhao, W. , Guo, Z. , … Zhang, J. H. (2018). Recombinant CTRP9 administration attenuates neuroinflammation via activating adiponectin receptor 1 after intracerebral hemorrhage in mice. Journal of Neuroinflammation, 15, 215 10.1186/s12974-018-1256-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, Y. , Wang, Y. , Wang, J. , Anne Stetler, R. , & Yang, Q. W. (2014). Inflammation in intracerebral hemorrhage: From mechanisms to clinical translation. Progress in Neurobiology, 115, 25–44. 10.1016/j.pneurobio.2013.11.003 [DOI] [PubMed] [Google Scholar]
- Zhu, L. , Li, J. , Guo, L. , Yu, X. , Wu, D. , Luo, L. , … Zhang, D. (2015). Activation of NALP1 inflammasomes in rats with adjuvant arthritis; a novel therapeutic target of carboxyamidotriazole in a model of rheumatoid arthritis. British Journal of Pharmacology, 172, 3446–3459. 10.1111/bph.13138 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Summary of experimental groups and mortality rate in the study.


