Abstract
Due to its Ca2+ buffering capacity, the mitochondrion is one of the most important intracellular organelles in regulating Ca2+ dynamic oscillation. Mitochondrial calcium uniporter (MCU) is the primary mediator of Ca2+ influx into mitochondria, manipulating cell energy metabolism, ROS production, and programmed cell death, all of which are critical for carcinogenesis. The understanding of the uniporter complex was significantly boosted by recent groundbreaking discoveries that identified the uniporter pore‐forming subunit MCU and its regulatory molecules, including MCU‐dominant negative β subunit (MCUb), essential MCU regulator (EMRE), MCU regulator 1 (MCUR1), mitochondrial calcium uptake (MICU) 1, MICU2, and MICU3. These provide the means and molecular platform to investigate MCU complex (uniplex)‐mediated impaired Ca2+ signalling in physiology and pathology. This review aims to summarize the progress of the understanding regulatory mechanisms of uniplex, roles of uniplex‐mediated Ca2+ signalling in cancer, and potential pharmacological inhibitors of MCU.
Abbreviations
- [Ca2+]c
cytoplasm Ca2+
- [Ca2+]m
mitochondrial matrix Ca2+
- ΔΨ(m)
mitochondrial transmembrane potential
- +AuNPs
positively charged gold nanoparticles
- CaMKII
Ca2+/calmodulin‐dependent protein kinase II
- cl‐CD95L
cleaved CD95L
- COXs
cytochrome c oxidases
- CytC
cytochrome c
- EMRE
essential MCU regulator
- ER
endoplasmic reticulum
- HCC
hepatocellular carcinoma
- HCX
H+/Ca2+ exchanger
- HINT2
histidine triad nucleotide‐binding 2
- hMCU
human MCU
- IMM
inner mitochondrial membrane
- IMS
intermembrane space
- IP3R
inositol trisphosphate receptor
- MCU
mitochondrial calcium uniporter
- MCUb
MCU‐dominant negative beta subunit
- MCUR1
MCU regulator 1
- MICU
mitochondrial calcium uptake
- mROS
mitochondrial ROS
- Mfn2
mitofusin‐2
- mPTP
mitochondrial permeability transition pore
- NCX
Na+/Ca2+ exchanger
- PM
plasma membrane
- PRMT1
protein arginine methyl transferase 1
- Pyk2
proline‐rich TK 2
- RIPK1
receptor‐interacting protein kinase 1
- RuR
ruthenium red
- TCA
tricarboxylic acid
- TMD
transmembrane domain
- TNBCs
triple‐negative breast cancers
1. INTRODUCTION
Mitochondria play critical roles in cell physiology, including bioenergetics, cell cycle, autophagy, and apoptosis (Jouaville, Pinton, Bastianutto, Rutter, & Rizzuto, 1999; Kroemer & Reed, 2000; Strzyz, 2018; Westermann, 2010). The second messenger, Ca2+, is essential for carcinogenesis and tumour development and, therefore, is a promising target for cancer treatment (Cui et al., 2018; Cui, Merritt, Fu, & Pan, 2017; Monteith, McAndrew, Faddy, & Roberts‐Thomson, 2007). Since it was discovered that mitochondria can take in and store large amounts of Ca2+, it has been well established that most mitochondrial functions are dependent on Ca2+ (Deluca & Engstrom, 1961; Drago, Pizzo, & Pozzan, 2011; Hajnoczky et al., 2006; Jouaville et al., 1999; Zeng et al., 2018). Together with the sarcoplasmic/endoplasmic reticulum (SR/ER) and Ca2+ channels/pumps located in plasma membrane (PM), mitochondria are vital for the fine tuning of Ca2+ oscillation signalling (Figure 1a). Upon Ca2+ influx from extra cytoplasm or its release from the SR/ER, mitochondria translocate to the areas close to either the PM or SR/ER. Through this process, mitochondria regulate the activities of corresponding Ca2+ channels, and either propagate Ca2+ oscillation‐encoded signals or restrict Ca2+ signalling to specific cellular domains (Clapham, 2007; Glitsch, Bakowski, & Parekh, 2002; Hoth, Button, & Lewis, 2000; Rizzuto et al., 1998; Rizzuto, Brini, Murgia, & Pozzan, 1993; Rizzuto, De Stefani, Raffaello, & Mammucari, 2012; Szabadkai & Duchen, 2008).
Figure 1.
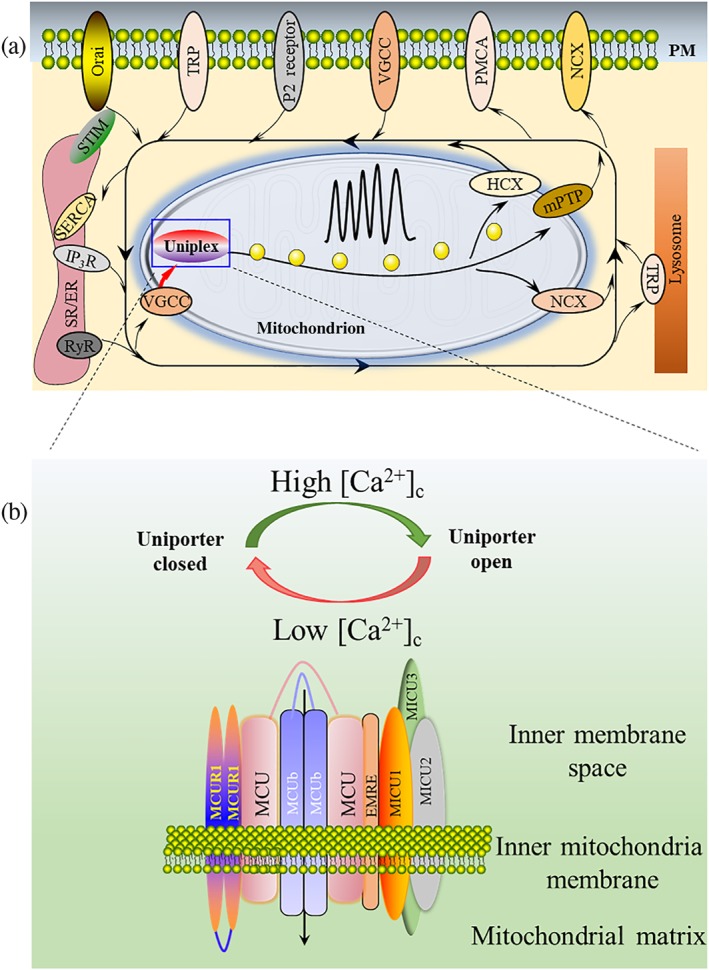
Uniplex components and uniplex‐mediated mitochondrial Ca2+ uptake in the global Ca2+ dynamic. (a) The dynamics of the intracellular Ca2+ oscillation are finely regulated by the mitochondria, SR/ER, lysosome, and channels located on the PM. When Ca2+ is transported from the extracellular space or released from intracellular Ca2+ stores, mitochondria will move close to the corresponding channel to take up the elevated [Ca2+]c, thus propagate or restrict Ca2+ signals to specific cellular domains. Ca2+ influx into mitochondria is mainly mediated by the uniplex. (b) Uniplex is composed of the pore‐forming subunit MCU, MCUb, EMRE, MCUR1, MICU1, MICU2, and MICU3. All these molecules localize at the inner mitochondrial membrane. MICU family protein or EMRE molecule contains only single TMD. Both MCU and MCUb contain two TMDs, while both N‐ and C‐terminals face the mitochondrial matrix. With two TMDs, N‐ and C‐terminals of MCUR1 face the mitochondria inner membrane space
There is a growing body of evidence suggesting that the mitochondrial calcium uniporter (MCU) is the primary transporter to mediate Ca2+ influx into mitochondria (Figure 1a; De Stefani, Raffaello, Teardo, Szabo, & Rizzuto, 2011; Gunter, Buntinas, Sparagna, Eliseev, & Gunter, 2000). The MCU resides in the inner mitochondrial membrane (IMM) and is a highly selective Ca2+ uniporter (Baughman et al., 2011; Chaudhuri, Sancak, Mootha, & Clapham, 2013; Kirichok, Krapivinsky, & Clapham, 2004). Under resting conditions, mitochondrial matrix Ca2+ ([Ca2+]m) level is similar to that of cytoplasm (~100 nM), and the MCU is closed, despite the driving force of the mitochondrial transmembrane potential (ΔΨ(m)) produced by the respiratory chain. However, once cytoplasmic Ca2+ ([Ca2+]c) is rapidly elevated (>300–500 nM), the MCU will be activated promptly and transport Ca2+ into mitochondria, even against the Ca2+ concentration gradient (Chaudhuri et al., 2013; Kamer, Grabarek, & Mootha, 2017; Marchi & Pinton, 2014). The MCU is universally expressed in different organs and tissues, but its tissue‐specific activities are various (Fieni, Lee, Jan, & Kirichok, 2012). The MCU and its regulatory molecules, including the MCU‐dominant negative beta subunit (MCUb), essential MCU regulator (EMRE), MCU regulator 1 (MCUR1), mitochondrial calcium uptake (MICU) 1, MICU2, and MICU3, form a large complex to manipulate its activities (Lambert, Luongo, Shah, & Elrod, 2016; Mallilankaraman, Cardenas et al., 2012; Patron, Granatiero, Espino, Rizzuto, & De Stefani, 2019; Perocchi et al., 2010; Plovanich et al., 2013; Sancak et al., 2013).
A respiratory chain‐generated proton gradient across the IMM is required for mitochondrial Ca2+ uptake. The primary functions of [Ca2+]m include modulation of tricarboxylic acid (TCA) cycle and oxidative phosphorylation via stimulating Ca2+‐sensitive dehydrogenases, resulting in increased NADH oxidase activity, ATP, and ROS production. Thus, it forms a positive feedback regulatory loop for the calcium influx into mitochondria induced by the respiratory chain‐IMM proton gradient (Cárdenas et al., 2010; Jouaville et al., 1999; Nicholls, 2005; Rizzuto et al., 2012). Silencing the MCU or MICU1 attenuates Ca2+‐dependent activation of the TCA cycle and NAD(P)H oxidase (Perocchi et al., 2010; Tosatto et al., 2016). [Ca2+]m signalling goes far beyond the general stimulation of cellular energetics (Figure 2). An elevated [Ca2+]m increases mitochondrial ROS (mROS) production, which promotes the oxidation of membrane phospholipids and proteins. Ca2+ binding to the β subunit of F‐type ATP synthase activates the mitochondrial permeability transition pore (mPTP), leading to a reduction in the ΔΨ(m) and the release of pro‐apoptotic factors such as cytochrome c (CytC) into the cytoplasm, which finally activate the mitochondria‐dependent apoptotic signalling pathway. [Ca2+]m‐activated calpain can also directly stimulate caspases, thus promoting apoptosis independent of CytC release (C. Giorgi et al., 2012; V. Giorgio et al., 2017; Kim et al., 2013; Mattson & Chan, 2003; Schinzel et al., 2005). Besides apoptosis, it is well accepted that [Ca2+]m also regulates necrosis, autophagy, and necroptosis (Gomez‐Suaga, Paillusson, & Miller, 2017; Rasola & Bernardi, 2011).
Figure 2.
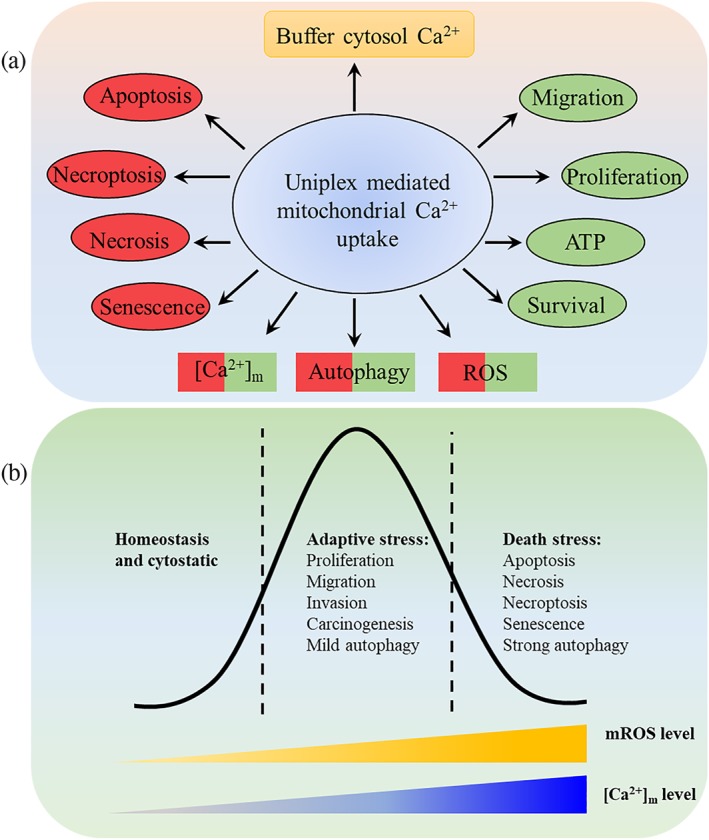
Functions of uniplex‐mediated mitochondrial Ca2+ uptake. (a) Effect of uniplex‐mediated Ca2+ signalling. It buffers the elevated [Ca2+]c, thus transduces or restricts calcium oscillation coded signalling. [Ca2+]m binding to specific proteins directly induces biochemical events, including cell migration, proliferation, survival, ATP production, autophagy, ROS, apoptosis, necrosis, necroptosis, and senescence. Autophagy, [Ca2+]m, and ROS are multifunctional events or factors. (b) Dual roles of [Ca2+]m and mROS. [Ca2+]m and mROS are required for normal cell functions and signalling. Under moderate stress, the increased Ca2+ and ROS could enhance the corresponding signalling pathway for adapting to the elevated requirement of ATP, proliferation, and autophagy. Once this promoted adaptive stress is out of control, it may lead to tumourigenesis and cell invasion. However, once [Ca2+]m and mROS accumulation exceed the threshold, death‐related events, such as apoptosis and strong autophagy, will be induced. Manipulation of these processes can regulate the fate
It is well known that mitochondria are critical for tumour initiation and development, while the reprogramming of mitochondrial metabolism is currently considered to be an emerging hallmark of cancer (Boroughs & Deberardinis, 2015; Hanahan & Weinberg, 2011; Wallace, 2012; Weinberg et al., 2010; Weinberg & Chandel, 2015). Ca2+‐dependent ROS production and Ca2+ itself are versatile and are the main effectors of mitochondria‐mediated cancer signalling (Figure 2). A reduction in [Ca2+]m in cancer inhibits mitochondrial apoptotic signals and thus favours cancer cells resistant to apoptotic stimuli. The mROS are commonly deregulated in cancers and are viewed as vital molecular effectors for cancer progression (Klimova & Chandel, 2008; Porporato, Filigheddu, Pedro, Kroemer, & Galluzzi, 2017; Ren et al., 2017; Tosatto et al., 2016). Therefore, uniplex‐mediated mitochondrial Ca2+ signalling is a promising to target for cancer chemotherapy.
2. UNIPLEX COMPONENTS AND REGULATORY MECHANISMS
2.1. Mitochondrial calcium uniporter
In 2011, by using integrative genomics, Baughman et al. first reported a novel gene CCDC109A that encodes the pore‐forming subunit of uniplex (Baughman et al., 2011; De Stefani et al., 2011). The ubiquitously expressed MCU is a ~35 kDa protein containing two transmembrane domains (TMDs) that are linked by a highly conserved sequence (Figure 1b). This conserved sequence faces the intermembrane space (IMS), while both the N‐ and C‐terminal regions of MCU protrude into the mitochondria matrix (Baughman et al., 2011). The channel formed by MCU is sensitive to ruthenium red (RuR), RU360, and lanthanides (Cao, Wang, Cui, Su, & Chou, 2017; Crompton, Heid, Baschera, & Carafoli, 1979; De Stefani et al., 2011). The high affinity of MCU binding to Ca2+ (K D < 2 nM) relies on its WDXXEP motif located between TM1 and TM2 (X denotes hydrophobic amino acids, WDIMEP in humans). This motif forms the narrowest region of the pore and is the only region lined by acidic amino acids. The Asp residues of these motifs form a mouth with a radius of ~2.2 Å on the side of the pore‐facing IMS, while Glu residues form a second ring with a radius of <1 Å (Baradaran, Wang, Siliciano, & Long, 2018; Kirichok et al., 2004; Yoo et al., 2018). The first X residue in the DXXE motif (Ile262 in human MCU, hMCUI262) is insensitive to mutation, since both the hMCUI262V and hMCUI262A have full activity. The second X residue (Met263 in hMCU) is sensitive to mutation, since hMCUM263A displays reduced activity compared to the wild type. The various effects of the mutant X residues on MCU activities are probably due to the first X residue that has no protein contacts in the structure, while the second X residue can interact with TM1 (Baradaran et al., 2018). The replacement of the DXXE motif with QXXQ in mouse MCU abolishes MCU activity in lipid bilayer membranes. In HeLa cells, the overexpressed hMCUQIMQ acts as a dominant negative regulator, suggesting that the active MCU channel works as an oligomer (Baradaran et al., 2018; De Stefani et al., 2011; Yoo et al., 2018). The hMCUS259A retains Ca2+ transport ability but has diminished sensitivity to RuR (Chaudhuri et al., 2013).
MCU activities are also affected by phosphorylation. Under phenylephrine treatment, the proline‐rich TK 2 (Pyk2) will translocate to mitochondria and subsequently binds to and phosphorylates MCU in an mROS‐dependent manner. This promotes MCU oligomerization and enhances mitochondrial Ca2+ uptake, leading to MCU‐dependent mROS generation and forms a positive feedback loop between ROS accumulation and MCU activation. The MCU‐mediated mitochondrial Ca2+ uptake enhanced by Pyk2 also results in [Ca2+]m overload and cell apoptosis. The phosphorylation site of hMCU by Pyk2 is most likely Y289; this has not yet been proved. Other mechanisms have also been proposed for the phosphorylation of MCU, and Pyk2 may also phosphorylate other uniplex components (O‐Uchi et al., 2014). The multifunctional Ca2+/calmodulin‐dependent protein kinase II (CaMKII) has also been demonstrated to interact with MCU and increase MCU activities. The CaMKII‐dependent MCU activities could be significantly reduced by Ru360 or S57A/S92A mutation in MCU (Joiner et al., 2012). The hMCUC97 is sensitive to ROS and will undergo S‐glutathionylation, resulting in the formation of a MCU higher order oligomer and an elevated [Ca2+]m uptake rate (Dong et al., 2017). The N‐terminal matrix domain of hMCU contains a β‐grasp‐like fold with a cluster constituted by negatively charged residues. These residues bind to Ca2+ or Mg2+ and destabilize and shift the self‐association equilibrium of the domain towards a monomer, which markedly decreases MCU activity (Lee et al., 2016).
2.2. MCU‐dominant negative beta subunit
MCUb protein shares 50% sequence similarity with MCU and also has two highly conserved putative TMDs. It contains several amino acid substitutions (key replacements of R252W and E257V in human) in the critical pore‐forming region. MCUb directly interacts with MCU (Figure 1b) and exerts a dominant negative effect on MCU (Raffaello et al., 2013; Tomar et al., 2016). Once MCUb presents in uniplex, MCU activity will be reduced (Figure 3a). The silencing of MCUb markedly increases histamine‐induced mitochondrial Ca2+ uptake, without affecting the protein expression of MCU. Unlike MCU, MCUb is expressed only in vertebrates (Raffaello et al., 2013). Even in vertebrates, the ratio of MCU/MCUb is highly variable (from 3:1 to 40:1), indicating that the functions of uniplex‐mediated Ca2+ signals are tissue‐specific (Raffaello et al., 2013; Tomar et al., 2016).
Figure 3.
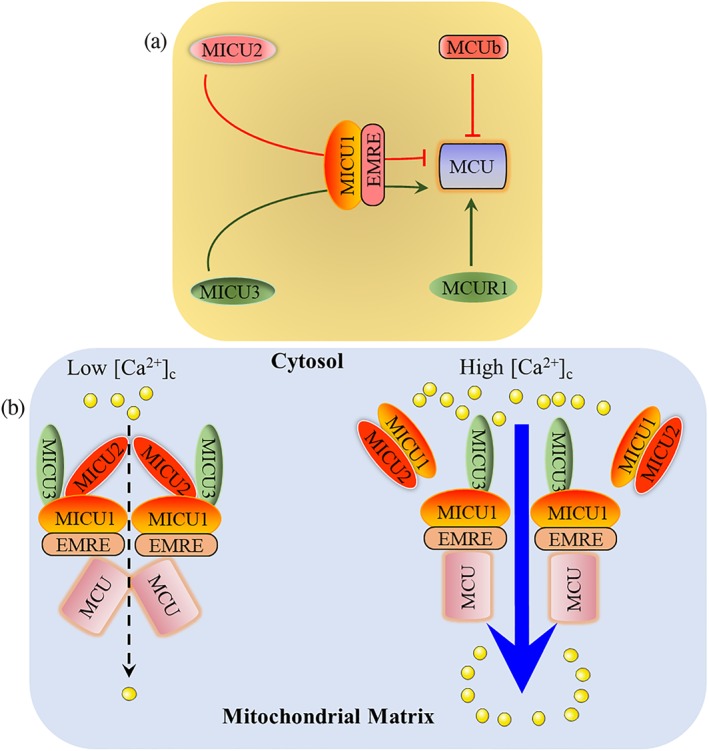
Regulatory mechanisms of uniplex. (a) MCUR1 and MCUb directly bind to MCU, which exerts a positive and dominant negative effect on MCU, respectively. MICU2 is a gatekeeper and MICU3 is an enhancer of MCU, both of which require MICU1‐EMRE heterodimer as a bridge. (b) EMRE directly binds to MICU1 and MCU. At low [Ca2+]c levels, both MICU2 and MICU3 bind to MICU1, and MICU2 inhibits MCU activation. When [Ca2+]c increases and exceeds the Ca2+ threshold set by MICU2, the MICU1–MICU2 dimer will dissociate from the uniplex, and MICU2 will lose its inhibitory function; MICU3 enhances MCU activity to transfer Ca2+ from the cytosol into the mitochondrial matrix
2.3. MCU regulator 1
MCUR1 (CCDC90A), a ~40 kDa protein with two TMDs localized to IMM (Figure 1b), has been identified as another regulatory molecule of MCU. It has been predicted that both N‐ and C‐terminal regions of MCUR1 face the cytosolic side, exposed to the IMS (Mallilankaraman, Cardenas, et al., 2012). At first, it was proposed that the ubiquitously expressed MCUR1 is required for MCU activity and physically interacts with MCU (Figures 1b and 3). Although MCUR1 knockdown does not affect the expression and localization of MCU, it reduces the basal [Ca2+]m level and attenuates mitochondrial Ca2+ uptake (Mallilankaraman, Cardenas, et al., 2012; Tomar et al., 2016). Ablation of MCUR1 can disrupt oxidative phosphorylation, decrease cellular ATP, and activate AMP kinase‐dependent pro‐survival autophagy. When exogenous MCUR1 is increased, the enhanced mitochondrial Ca2+ uptake can be inhibited by Ru360. Thus, MCUR1 is required for mitochondrial Ca2+ uptake and is a critical component of the uniplex (Mallilankaraman, Cardenas, et al., 2012).
However, in 2015, Paupe et al. reported that a fraction of MCUR1 consistently locates outside the IMM, indicating a potential unknown function of MCUR1. They proposed that MCUR1 primarily functions as a CytC oxidases (COXs) assembly factor but not a regulator of MCU. Inhibition of MCUR1 specifically results in COXs assembly defect. This leads to an impaired respiratory chain and reduced ΔΨ(m), which are the real reasons that MCUR1 affects mitochondrial Ca2+ uptake (Paupe, Prudent, Dassa, Rendon, & Shoubridge, 2015). Interestingly, not long after these findings, using immune‐affinity binding assays and bimolecular fluorescence complementation, Tomar et al. demonstrated that MCUR1 could directly bind to MCU and EMRE (Tomar et al., 2016). It has been clearly proven that MCUR1 and EMRE can co‐localize in the IMM, and a deficiency in MCUR1 considerably decreases the molecular size of the uniplex. The highly conserved regions of the coiled‐coil domain in both the MCU and MCUR1 are required for the formation of their heterooligomeric complex. MCUR1 functions as a scaffold factor for the uniplex assembly (Tomar et al., 2016). Deletion of either MCU or MCUR1 does not affect ΔΨ(m) in cardiomyocytes, endothelial cells, and fibroblasts, which is contrary to Paupe's conclusion that MCUR1 is a COX assembly factor that affects the ΔΨ(m) (Paupe et al., 2015; Tomar et al., 2016; Vais et al., 2015). It has also been reported that MCUR1 is not essential for mitochondrial Ca2+ uptake and only serves as a regulator to set the Ca2+ threshold for the mitochondrial permeability transition. Inhibition of MCUR1 expression increases the Ca2+ threshold for inducing mPTP transition, thus decreasing mitochondrial cell death induced by Ca2+ overload (Chaudhuri, Artiga, Abiria, & Clapham, 2016). Therefore, the current findings regarding the actions of MCUR1 on MCU are still controversial and need to be further elucidated.
2.4. MICU family proteins
MICU1 was identified by scientists 1 year preceding MCU. MICU1 is a ~50 kDa protein localized at the IMM and characterized by a single putative TMD (Figure 1b), which makes it unlikely that MICU1 functions as a Ca2+ channel. Initially, it was reported that silencing MICU1 neither disrupts mitochondrial respiration nor affects ΔΨ(m), whereas it abolishes mitochondrial Ca2+ uptake. Two canonical EF‐hands of MICU1 are required for MCU‐mediated Ca2+ influx into mitochondria (Perocchi et al., 2010). Based on the characteristic of the EF‐hand‐sensitive Ca2+ concentration, it was later proposed that MICU1 sets the Ca2+ threshold for MCU activation, which means MICU1 works as a vital gatekeeper of MCU and inhibits MCU activity to maintain a normal [Ca2+]m level under resting conditions.
Knockdown of MICU1 leads to a constitutive MCU‐mediated Ca2+ transfer into mitochondria, inducing the accumulation of mROS and sensitizing cells to apoptotic stimulation (Mallilankaraman, Doonan, et al., 2012). MICU1 also imparts MCU selectivity to discriminate between Ca2+ and Mn2+. Although both Mn2+ and Ca2+ can bind to MICU1, only Ca2+ is capable of inducing a conformation change in MICU1 and activating MCU, further supporting the negative function of MICU1 on MCU (Kamer et al., 2018).
Scientists further showed that MICU1 enhances MCU activity under the elevated [Ca2+]c condition. A lack of MICU1 not only induces a continuous elevation of [Ca2+]m under resting conditions but also reduces MCU‐mediated Ca2+ influx into mitochondria while fast [Ca2+]c increases (de la Fuente, Matesanz‐Isabel, Fonteriz, Montero, & Alvarez, 2014; Mallilankaraman, Doonan, et al., 2012). In the planar lipid membrane, MICU1 exerts a stimulatory effect on MCU activity even at low concentrations of Ca2+or in the absence of Ca2+. However, in intact cells, MICU1 exerts an inhibitory effect on MCU activity under lower [Ca2+]c levels (Mallilankaraman, Doonan, et al., 2012). These interesting results suggest that MICU1 itself is not enough as a gatekeeper, and other molecules are required for maintaining its actions. Now it has been demonstrated that the action of MICU1 as a gatekeeper or enhancer of MCU depends on MICU2 and MICU3, respectively (Figure 3).
By using bioinformatics, Plovanich et al. identified MICU2 (EFHA1) and MICU3 (EFHA2) as paralogous genes to MICU1, which also have mitochondrial localization targeting sequences at their N‐terminals. MICU2 has 41% sequence similarity to MICU1, while MICU3 has 34% sequence similarity to MICU1 and 47% sequence similarity to MICU2 (Csordas et al., 2013; Patron et al., 2019; Plovanich et al., 2013). The expression patterns of the MICU family proteins are different. MICU1 is broadly expressed in most of the tissues; MICU2 is expressed primarily in visceral organs, whereas MICU3 is mostly in skeletal muscles and the CNS (Pagliarini et al., 2008; Plovanich et al., 2013). This suggests that the MCU‐mediated Ca2+ currents regulated by MICU molecules are tissue‐ or cell‐specific.
MICU2 interacts with MICU1 to increase the [Ca2+]c threshold for MCU activation; thus, MICU2 has the ability to inhibit MCU activity under low [Ca2+]c conditions (Payne, Hoff, Roskowski, & Foskett, 2017). It has been reported that MICU2 rather than MICU1 might act as the gatekeeper of MCU (Figure 3). MICU2 has a conserved domain architecture like MICU1, and is found to reside within mitochondria with high‐content microscopy and MitoCarta (an inventory of human and mouse genes encoding proteins with strong support of mitochondrial localization). MICU3 also has the targeting sequence to support its mitochondrial localization, and has been shown to localize in mitochondria (Patron et al., 2019; Plovanich et al., 2013), although it does not achieve the score needed to be listed in MitoCarta (Patron et al., 2019; Plovanich et al., 2013).
Once MICU1 matures and localizes to the IMM, it will bind to MCU, this enables MICU1 to bind to Mia40. Following MICU1 interacting with oxidoreductase Mia40, MICU1 and MICU2 form a heterodimer (Figure 3b) by a disulfide bond. The binding of MICU1 to MCU only occurs under low Ca2+ or no Ca2+ conditions. High Ca2+ levels will result in the dissociation of the MICU1–MICU2 dimer from the uniplex. However, this hypothesis is still controversial because MICU1 has been shown to cooperatively activate MCU at high Ca2+ concentrations (Csordas et al., 2013; Petrungaro et al., 2015). This may result from the remnant MICU1 proteins in the uniplex binding to MICU3 (Figure 3b). The dimerization of MICU1 and MICU2 would stabilize both proteins, without affecting their mRNA level (Patron et al., 2014; Petrungaro et al., 2015; Plovanich et al., 2013). MCU was reported to physically interact with and stabilize MICU1 and MICU2. A later study demonstrated that this interaction is mediated by EMRE (Figures 1b and Figure 3; Plovanich et al., 2013; Sancak et al., 2013).
The overexpression of MICU3 causes a 10‐fold increase in [Ca2+]m transients. However, MICU1 overexpression only induces a threefold increase. MICU3EFmut still retains a certain ability to enhance MCU, but its efficiency is less than the wild type. The silencing of MICU1 also abolishes the cooperative function of MICU3 on MCU, indicating that MICU1 is a common platform for both MICU2 and MICU3. MICU3 also binds to MICU1 via a disulfide bond, in which C465 of MICU1 and C515 of MICU3 are required (Patron et al., 2019). The protein arginine methyl transferase 1 (PRMT1) is able to methylate R455 of hMICU1, thus reduces MICU1 sensitivity to Ca2+ and increases the threshold of [Ca2+]c for activation of MCU. However, methylation of MICU1 by PRMT1 enables MICU1 to bind to uncoupling protein 2 (UCP2), which rescues the decreased MCU activity (Madreiter‐Sokolowski et al., 2016). Whether MICU2 can be methylated by PRMT1 is not yet known.
MICU1, MICU2, and MICU3 exert various effects on MCU, although they belong to the same family (Figure 3). MICU2 is a gatekeeper of MCU, while MICU3 mainly functions as an enhancer of MCU activation. The diverse functions of the MICU family proteins maintain normal [Ca2+]m levels under the resting conditions and enable prompt activation of MCU to mediate rapid mitochondrial Ca2+ uptake.
2.5. Essential MCU regulator
Sancak et al. identified another regulatory protein of MCU, called essential MCU regulator (EMRE, previously known as C22orf32) in 2013 (Sancak et al., 2013). EMRE is a ~10 kDa metazoan‐specific protein with a single TMD, located at IMM (Figure 1b). By use of a protease sensitivity biochemistry experiment, it was shown that the C‐terminal of EMRE faces the mitochondrial matrix and contains a motif similar to the Ca2+ binding “Ca2+ bowl” and “Ca2+ clasp” to sense Ca2+. However, due to the small size of EMRE's extra‐membrane regions, it is not suitable for standard protease digestion assays. Tsai's group used directed mass‐tagging and MCU‐EMRE fusion construction demonstrated that EMRE exposes its N‐terminal region to the matrix and C‐terminal to IMS (Tsai et al., 2016).
Overexpression of MCU in cells lacking EMRE fails to restore MCU activity. Interestingly, depletion of MCU can decrease EMRE abundance without affecting its mRNA level, indicating that MCU is able to stabilize EMRE protein post‐transcriptionally. Depletion of EMRE considerably impairs MCU‐mediated mitochondrial Ca2+ uptake, despite the normal expression and oligomerization of MCU (Sancak et al., 2013). The interaction of MCU and EMRE is mediated by the transmembrane helices from both proteins. Loss of EMRE or MICU1 results in a similar decrease in molecular size of the uniplex to ~300 kDa, suggesting that EMRE may serve as a bridge between MCU and MICU1 (Figures 1b and 3), which has been demonstrated by an immunoprecipitation assay (Sancak et al., 2013). When EMRE is depleted, the interaction between MICU1 and MICU2 does not change, and MCU oligomerization and interaction with MCUb are also normal, whereas the association of MCU with the MICU1–MICU2 dimer is significantly decreased (Sancak et al., 2013).
3. REPROGRAMMING OF UNIPLEX‐MEDIATED CALCIUM SIGNALLING IN CANCER
Metabolic reprogramming is essential for the increased activity of energetic and biosynthetic pathways in cancer. The TCA cycle‐dependent production of ATP and metabolic intermediates are required for the synthesis of fatty acids and nucleotides (Boroughs & Deberardinis, 2015). [Ca2+]m overload is commonly considered as a trigger for the initiation of cell death. Reduced mitochondrial Ca2+ uptake has been demonstrated to reduce O2 consumption and ATP level, thus activate AMP‐activated protein kinase‐dependent pro‐survival macro‐autophagy (Cárdenas et al., 2010). Down‐regulation or inhibition of MCU increases proliferation and renders cells resistant to apoptosis by diminishing Ca2+ entry into mitochondria. Oncogenes or tumour suppressors can promote cell survival or induce death by modifying uniplex activity (Garcia‐Prieto et al., 2013; Marchi et al., 2013). Here, we summarize the data available on the deregulation of uniplex‐mediated Ca2+ signalling in cancer (Table 1).
Table 1.
Alterations of uniplex in cancer
| Cancer type | [Ca2+]m alteration | Uniplex alteration | Upstream modulator and alteration | Reference | |
|---|---|---|---|---|---|
| Pancreatic cancer | Decreased | MICU1 and MICU2 increased; EMRE decreased | HINT2, decreased | Chen et al. (2017) | |
| Breast cancer | Increased | MCU increased and MCUb decreased | miR‐340, decreased | Curry, Peters, Kenny, Roberts‐Thomson, and Monteith (2013), Fouque et al. (2016), Tang et al. (2015), Tosatto et al. (2016), and Yu et al. (2017) | |
| Colon cancer | Decreased | MCU decreased | MiR‐25 Increased | Marchi et al. (2013) | |
| Prostate cancer | Decreased | MCU decreased | miR‐25, increased | Marchi et al. (2013) | |
| Ovarian cancer | — | MICU1 mRNA increased | Unknown | Sancak et al. (2013) | |
| HCC | Increased | MCU, MCUR1, and MICU2 increased; alteration of MICU1 was controversial | Mfn2, decreased | Ren et al. (2017, 2018) and Wang et al. (2015) | |
| Multiple myeloma | Bortezomib‐sensitive cells | Resting: increased, compared with resistant cells; after bortezomib treatment: increased ~10 fold | Increased by bortezomib treatment | — | Song et al. (2013) |
| Bortezomib‐resistant cells | Resting: Decreased, compared with sensitive cells; after bortezomib treatment: increased ~2 fold | No change with bortezomib treatment | — | ||
3.1. Pancreatic cancer
The histidine triad nucleotide‐binding 2 (HINT2) protein is an adenosine phosphoramidase localized in mitochondria. Decrease of HINT2 expression is closely associated with metastasis of pancreatic cancer lymph nodes, pathological grade, and poor prognosis. Forced overexpression of HINT2 in pancreatic cancer cells results in almost threefold elevation of [Ca2+]m and induction of apoptosis. These processes can be prevented by RuR, suggesting that the uniplex is involved. Coupled with the elevated expression of exogenous HINT2 in pancreatic cancer cells, MICU1 and MICU2 proteins are decreased, whereas EMRE is increased (Chen et al., 2017). However, the expression patterns and the roles of MICU1, MICU2, EMRE, and other uniplex components in pancreatic cancer, as well as the mechanisms responsible for the effects of HINT2 on MICU1, MICU2, and EMRE remain unclear.
3.2. Cervical carcinoma
Currently, there have been a few reports on the role of the uniplex in the pathobiology of cervical cancer, primarily obtained using HeLa cells. Knockdown of MICU1 in HeLa cells results in an elevated [Ca2+]m, while ΔΨ(m) and the histamine‐induced mitochondrial Ca2+ uptake seem to be unaffected. Although MICU1 knockdown does not affect the proliferation of HeLa cells, it considerably enhances ceramide‐induced cell death, which is attributed to the [Ca2+]m‐dependent chronic elevation of mROS (Csordas et al., 2013; Mallilankaraman, Doonan, et al., 2012). After silencing MCU, most of the mitochondrial parameters, such as morphology and ΔΨ(m), remain unchanged. Ablation of EMRE or MCU causes a decrease in Ca2+ influx into mitochondria, while cell proliferation is not affected (Sancak et al., 2013).
3.3. Ovarian cancer
Ca2+‐dependent permeabilization of the outer mitochondrial membrane is a key event in the mitochondrial intrinsic apoptotic pathway (Deniaud et al., 2008). MICU1 mRNA level is increased in ovarian cancer cells, per mitochondrion and per cell (Table 1). Compared to normal cells, ovarian cancer cells are highly resistant to cytotoxicity induced by positively charged gold nanoparticles (+AuNPs). +AuNPs can substantially increase [Ca2+]c levels. A2780 and OV202 ovarian cancer cells display at least a threefold higher expression of MICU1 than normal ovarian surface epithelial cells. Ovarian cancer cells exposed to +AuNPs display a rapid increase in [Ca2+]c and a chronic sustained increase in MCU‐mediated mitochondrial Ca2+ uptake. Silencing MICU1 promotes +AuNPs‐induced mitochondrial membrane depolarization and mitochondrial‐dependent apoptosis in ovarian cancer cells, coupled with reduced mitochondrial Ca2+ uptake. These results suggest that an up‐regulation of MICU1 can enhance mitochondrial ability to buffer [Ca2+]c elevations induced by +AuNPs, thus prevent cells from [Ca2+]c‐dependent apoptosis (Arvizo et al., 2013).
3.4. Multiple myeloma
Multiple myeloma is a haematological malignancy with a high risk of relapse and death. The proteasome inhibitor bortezomib is a first‐line chemotherapeutic agent for clinical treatment of multiple myeloma by inducing apoptosis. Intrinsic and acquired drug resistances are obstacles encountered when bortezomib is applied for treating multiple myeloma patients. Bortezomib treatment induces ER Ca2+ release, activation of a store‐operated ion, Orai channel, and mitochondrial Ca2+ uptake. [Ca2+]m deregulation induced by bortezomib treatment precedes caspase activation, which can be prevented by RuR and Ru360 (Rizzuto et al., 2012). Under bortezomib treatment, MCU level increases almost 15‐ to 20‐fold in the cells sensitive to bortezomib, while few changes occur in the cells resistant to bortezomib, although the resistant cells have higher MCU expression levels under the resting conditions (Table 1) (Song et al., 2013). When the transfer of Ca2+ from ER to mitochondria is inhibited, AMPK ‐dependent autophagy will be activated, and the growth of xenografted melanoma cells will be reduced by nearly 60–70% (Boroughs & Deberardinis, 2015). This indicates that the uniplex is essential for bortezomib‐induced apoptosis. It may be a promising strategy to combine uniplex‐targeted agents with bortezomib to overcome chemotherapy resistance in multiple myeloma treatment.
3.5. Breast cancer
Analysis of the Oncomine cancer microarray database and qPCR results from clinical breast cancer samples reveals that breast cancer cells display high levels of MCU mRNA (Curry et al., 2013; Tang et al., 2015). Analysis of the Cancer Genome Atlas breast cancer dataset shows that the increased MCU and reduced MCUb mRNA, but not MICU molecules and EMRE, are positively correlated with the growth of triple‐negative breast cancers (TNBCs) and lymph node metastasis (Table 1; Tosatto et al., 2016). Orais have been demonstrated to mediate migration and metastasis of breast tumour cells (Yang, Zhang, & Huang, 2009). RNAi or pharmacological inhibition of MCU activity significantly reduces Orais’ activity, migration, and viability of breast cancer cells in vitro. MCU−/− basal‐like MDA‐MB‐231 breast cancer cells consistently display significantly reduced lymph node infiltration and lung metastasis in vivo. Knockdown of MCU decreases NADH and ROS levels while increasing the NADPH/NADH ratio. Transcriptions of HIF‐1α and its target genes are significantly reduced by MCU knockdown (Tang & Wang et al., 2015; Tosatto et al., 2016). Knockdown of MCU in MDA‐MB‐231 cancer cells does not affect proliferation and [Ca2+]c levels but markedly enhances the Ca2+ ionophore ionomycin‐induced cell death that is independent of caspase. This results from alterations in mitochondria‐associated membrane Ca2+ signals and the elimination of the [Ca2+]m‐induced autophagic survival pathway (Curry et al., 2013).
Fouqué et al. demonstrated that the cleaved CD95L (cl‐CD95L)‐induced Ca2+ transfer from ER to mitochondria in TNBC cells is dependent on a Bcl‐xL/VDAC1/MCU complex. Overexpression of cl‐CD95L results in poor prognosis and a higher metastatic ability of TNBCs. Treating cells with Ru360 inhibits CD95‐mediated mitochondrial Ca2+ influx and cell migration (Fouque et al., 2016). Compared to normal tumour‐adjacent tissues and ductal carcinoma tissues, the breast cancers with distant metastases and/or lymph node infiltration display an increased expression of MCU protein. Also, the expression of MCU protein in the highly migratory and invasive ZR‐75‐30 and MDA‐MB‐231 breast cancer cells is higher than that in BT‐474 and MCF‐7 cells with poorer invasive ability (Table 1). Exogenous MCU markedly increases the migratory and invasive ability of MCF‐7 cells, which are sensitive to the inhibitory action of Ru360. It is known that the Warburg effect contributes to cell metabolism and motility, while an up‐regulation of MCU enhances the Warburg effect in breast cancer cells. Yu et al. demonstrated that miR‐340 directly interacts with MCU mRNA and inhibits MCU‐mediated mitochondrial Ca2+ influx, the Warburg effect, and breast cancer cells motility (Table 1). Nude mice injected with MCF‐7 cells with overexpressed MCU or down‐regulated miR‐340 developed more metastatic lesions in their lungs (Yu et al., 2017).
3.6. Prostate cancer and colon cancer
Prostate cancer is one of the widely studied cancers that commonly exhibit impaired Ca2+ signals (Prevarskaya, Skryma, Bidaux, Flourakis, & Shuba, 2007; Thebault et al., 2006; Vanden Abeele et al., 2002). It has been demonstrated that the Na+/Ca2+ exchanger (NCX)‐specific inhibitor, CGP‐37157, markedly enhances TNF‐related apoptosis‐inducing ligand (TRAIL)‐induced apoptosis in prostate cancer cells by causing [Ca2+]m overload. Although the MCU inhibitor, Ru360, failed to attenuate TRAIL‐induced cell death, MCU agonists are showing promise in enhancing this action, which is still worthy of further study (Kaddour‐Djebbar et al., 2006). MCU is down‐regulated in prostate cancer and colon cancer (Table 1; Marchi et al., 2013). MiR‐25 with 22 nucleotides is highly expressed in these cancers. MiR‐25 has the potential to decrease mitochondrial Ca2+ uptake by selectively inhibiting MCU expression, resulting in resistance of cancer cells to apoptotic challenges. Anti‐miR‐25 in PC‐3 prostate cancer cells and HCT116 colon cancer cells can increase the [Ca2+]m level and re‐sensitize cells to apoptosis. MiR‐92a, miR‐363, and miR‐1 exert a similar effect on MCU expression and Ca2+ signalling (Marchi et al., 2013; Marchi & Pinton, 2013; Zaglia et al., 2017). These results reveal how cancer cell survival is favoured by the down‐regulation of MCU and suggest that activation of MCU is a potential target for cancer therapy.
Receptor‐interacting protein kinase 1 (RIPK1) plays a central role in cancers via apoptosis and necroptosis. It has been shown that expression of RIPK1 positively correlates with 3 years mortality in colorectal cancer patients. Zeng et al. demonstrated that MICU1 is decreased, and MCU is significantly up‐regulated in colorectal cancer tissues (Table 1), and these changes correlate with RIPK1 levels. Co‐immunoprecipitation results further revealed a physical interaction between RIPK1 and MCU, which was enhanced in colorectal cancer. Silencing RIPK1 decreased MCU protein levels, whereas an overexpression of RIPK1 increased [Ca2+]m level in an MCU‐dependent manner thus modulating colorectal cancer energy metabolism (Zeng et al., 2018).
3.7. Hepatocellular carcinoma
Based on the bioinformatic analysis of public microarray mRNA expression data sets, MCU is found to be increased and MICU1 decreased at both mRNA and protein levels in hepatocellular carcinoma (HCC) tissues. Furthermore, MCU is markedly higher and MICU1 is lower in the metastatic HCC tissues than in the primary HCC tissues (Table 1). The expression of these two deregulated molecules is closely correlated with the risk of recurrence and death in HCC patients. Highly metastatic HCC cells display higher basal levels of [Ca2+]m than HCC cells with low metastatic ability or normal hepatocyte HL‐7702 cells. Ca2+ influx to mitochondria is reduced by MCU knockdown or MICU1 overexpression, which is dissimilar to effects observed in ovarian cancer, suggesting that MCU‐mediated Ca2+ signalling may be tissue‐specific (Arvizo et al., 2013; Ren et al., 2017). With pro‐apoptotic and anti‐proliferative effects, mitofusin‐2 (Mfn2) plays a critical role in mitochondrial fusion and contributes to the maintenance of mitochondrial function. Expression of Mfn2 is reduced in HCC and correlates with poor prognosis. When exogenous Mfn2 is introduced into HCC HepG2 cells, [Ca2+]m levels and apoptotic cells will be increased, which can be inhibited by Ru360. The introduced Mfn2 can reduce the originally up‐regulated MICU1 and MICU2 proteins in HCC (Wang et al., 2015). How Mfn2 affects MICU1 and MICU2 expression has not yet been elucidated; thus, the lack of conformity of MICU1 expression in HCC is still in need of a careful validation. Silencing of MCU increases the ratio of NAD+/NADH both in the cellular and mitochondrial compartments, the deacetylase activity of SIRT3, and enzymatic activity of SOD2, while decreasing ATP production and the acetylated SOD2 (Ac‐SOD2) level. By inhibiting the NAD+/SIRT3/SOD2 signalling pathway, MCU‐mediated mitochondrial Ca2+ uptake promotes mROS accumulation and subsequently activates ROS/JNK signalling to facilitate HCC metastasis in vitro and in vivo (Ren et al., 2017).
Expression of MCUR1 is also increased in HCC (Table 1), which is related to poorer overall survival and recurrence‐free survival. Knockdown of MCUR1 arrests cells in G1 phase, decreases HCC cell growth and colony‐formation, while increasing cell apoptosis. This leads to markedly reduced mitochondrial Ca2+ uptake, basal [Ca2+]m, and mROS production. As a well‐known tumour suppressor, p53 displays a negative correlation with MCUR1 levels. Elimination of ROS inhibits MCUR1‐dependent activation of the Akt/MDM2 pathway, while it increases p53 expression (Ren et al., 2018). The controversial observations regarding the levels and functions of [Ca2+]m in HCC cells probably result from the dual roles of both [Ca2+]m and mROS (Figure 2), highlighting the complexity of [Ca2+]m signalling.
Increasing evidence indicates that the uniplex manipulates proliferative, survival and metastatic signals, and death‐related pathways in cancer. Uniplex can serve as a promising target for cancer therapy. Since the uniplex is the primary channel mediating Ca2+ entrance into mitochondria, the altered Ca2+ current will be easily remolded by potential uniplex‐modulatory compounds and result in non‐resistant drugs. The limitations are that regulatory mechanisms of uniplex are still not completely clear and even remain controversial. Alterations of the MCU are tissue‐ and tumour‐specific. Currently, only MCU inhibitors are available but not agonists.
4. PHARMACOLOGICAL INHIBITORS OF THE MCU
Previously, the uniplex modulators were limited to lanthanides and ruthenium‐related compounds, which had been shown to inhibit the development of various cancers (Anghileri, 1975; Cao et al., 2017; Kirichok et al., 2004; Moore, 1971). Ru360 and RuR (Table 2) have been shown to directly bind to the solvent‐accessible Asp ring formed by the DXXE motif in MCU (Cao et al., 2017). The tetracycline‐derived minocycline and doxycycline (Table 2), but not tetracycline itself and other tetracycline‐derived compounds, exert inhibitory effects on MCU. They can suppress mitochondrial Ca2+ uptake, [Ca2+]m overload‐induced mPTP open and cell death, similarly to that of Ru360 (Schwartz et al., 2013). The promising anti‐cancer potential of minocycline and doxycycline has been shown in numerous studies on a variety of cancers (Ali, Alfarouk, Reshkin, & Ibrahim, 2017; Garrido‐Mesa, Zarzuelo, & Gálvez, 2013). Whether the inhibitory functions of these two compounds on MCU are related to their anti‐cancer effect and the underlying mechanisms of these compounds inhibiting MCU are still in need of further investigation.
Table 2.
Pharmacological inhibitors of uniplex
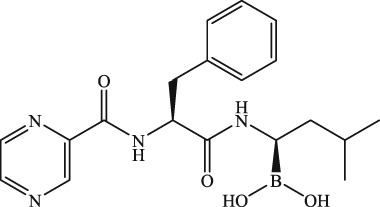
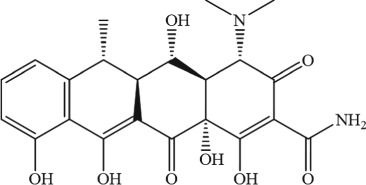
| Compound | Molecular formula | Skeletal formula | Formula weight | Target | IC50 | Reference | |
|---|---|---|---|---|---|---|---|
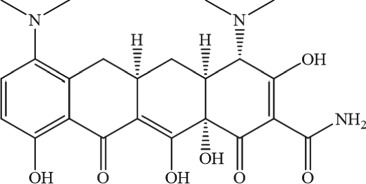
| Bortezomib | C19H25BN4O4 |
|
384.24 | — | — | Landowski, Megli, Nullmeyer, Lynch, and Dorr (2005) | |
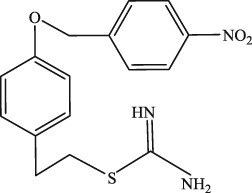
| KB‐R7943 | C16H17N3O3S.CH3SO3H |
|
427.49 | — | ~5.5 μM | Santo‐Domingo et al. (2007) | |
| DS16570511 | C30H25Cl2N3O4 |
|
562.44 | — | ~7 μM | Kon et al. (2017 | |
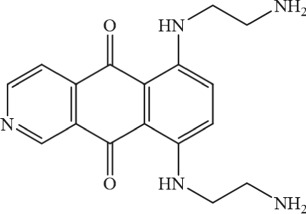
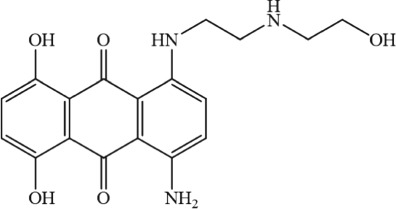
| Mitoxantrone analogues | Pixantrone | C17H19N5O2 |
|
325.37 | Asp residue in DXXE motif of MCU | ~15 μM | Arduino et al. (2017) |
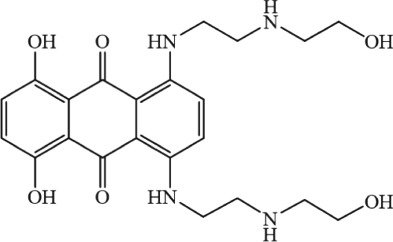
| Mitoxantrone | C22H28N4O6 |
|
444.48 | ~8.3 μM | |||
| Mitoxantrone impurity A | C18H19N3O5 |
|
357.36 | ~36 μM | |||
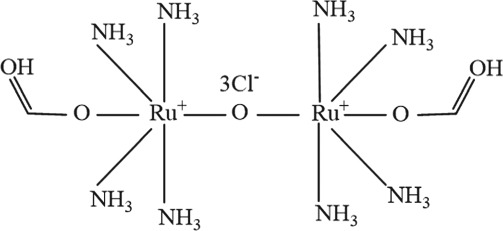
| Ruthenium red analogues | Ru360 | C2H26N8O5Ru2Cl3 |
|
550.78 | Asp residue in DXXE motif of MCU | 2 nM | Fouque et al. (2016) and Yu et al. (2017) |
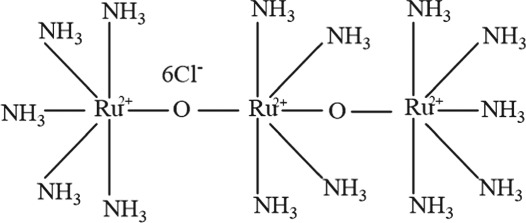
| Ruthenium red | H42N14O2Ru3Cl6 |
|
786.35 | DXXE motif of MCU | 9 nM | Fouque et al. (2016) and Yu et al. (2017) | |
| Lanthanides | La3+, Gd3+, and Pr3+ | — | — | — | — | — | Crompton et al. (1979) |
| Tetracycline analogues | Minocycline | C23H27N3O7 |
|
457.48 | — | — | Schwartz et al. (2013) |
| Doxycycline | C22H24N2O8 |
|
444.43 | — | — | Schwartz et al. (2013) | |
The molecular structures were generated from ChemDraw15.0 programme.
The meaningful molecular discoveries of uniplex components will definitely provide the platform and possibility for developing specific inhibitors or agonists for cancer chemotherapy. By using high‐throughput screening, Kon et al. identified DS16570511 (Table 2) as a cell‐permeable inhibitor of MCU. Without disruption of the ΔΨ(m), DS16570511 can inhibit serum‐induced mitochondrial Ca2+ influx in HEK293A cells with an IC50 ≈ 7 μM, but the underlying mechanisms are still unclear (Kon et al., 2017). The thiourea derivative, KB‐R7943 (Table 2), was originally developed as a selective inhibitor of the NCX located at PM. KB‐R7943 has also been shown to be a potent inhibitor of MCU without modifying ΔΨ(m) in cervical carcinoma HeLa cells (Santo‐Domingo et al., 2007). By inhibiting MCU, KB‐R7943 further reduces the fast initial ER Ca2+ release via Ca2+‐inhibition feedback on the inositol trisphosphate receptor (IP3R). As it has no detectable PM NCX activity, KB‐R7943 shows promise as a specific MCU inhibitor in treatment of these cancers (Furman, Cook, Kasir, & Rahamimoff, 1993; Iwamoto, Watano, & Shigekawa, 1996; Santo‐Domingo et al., 2007). But Brustovetsky et al. reported that KB‐R7943 did not directly affect MCU in their research. They postulated that KB‐R7943 reduced the activity of complex I in the respiratory chain and the energy required for Ca2+ transport, which are responsible for the decreased mitochondrial Ca2+ uptake (Brustovetsky et al., 2011; Clerc & Polster, 2012). Currently, the findings on the effect of KB‐R7943 on MCU‐mediated mitochondrial Ca2+ uptake are contraversial and in need of further studies.
There are challenges associated with high‐throughput screening to develop MCU modulators. As an intracellular protein complex, uniplex's activities are dependent on the alterations of [Ca2+]c and its driving force is dependent on the respiratory chain. These features of uniplex make the traditional high‐throughput screening prone to getting false‐positive hits, such as modulators of the respiratory chain, TCA cycle, or other molecules involved in intracellular Ca2+‐signalling networks. Many false‐positive hits could be eliminated by introducing hMCU and EMRE into yeast Saccharomyces cerevisiae, which is deficient in endogenous MCU and with delicately designed bioenergetic characteristics. Utilizing this system, Arduino et al. identified mitoxantrone as a selective and specific inhibitor of MCU (Arduino et al., 2017). In traditional cancer chemotherapeutics, mitoxantrone, is a well‐known agent with DNA intercalating activity to inhibit DNA topoisomerase II and cell proliferation. Mitoxantrone has been widely applied in the clinic for several cancers, such as non‐Hodgkin's lymphomas and acute myeloid leukaemia. Mitoxantrone has a concentration‐dependent inhibitory effect on MCU. In HEK293 cells, 10 μM mitoxantrone significantly inhibits (~85%) MCU‐mediated Ca2+ currents via its charged side arms binding to acidic residues in the selectivity filter of MCU (Arduino et al., 2017).
There is no difference in reduction of viability in MDA‐MB‐231 breast cancer cell and PLB‐985 peripheral blood acute myeloid leukaemia cell, regardless of MCU expression level. Certain MCU‐dependent pro‐oncogenic events are thought to be more sensitive to mitoxantrone, such as the increased metastatic abilities of MCF‐7 cells and HCC cells, as well as the MCU‐dependent chemotherapy resistance in multiple myeloma cells (Arduino et al., 2017; Ren et al., 2017; Song et al., 2013; Yu et al., 2017). If this hypothesis is true, as double‐targeting agents, mitoxantrone and its effective analogues should be more potent in treating specific cancers.
Recently, the cryo‐EM/crystal structures of full length MCU were revealed by several groups. By using an N‐terminal domain (NTD) dimer‐of‐dimers assembly manner, which is similar to that of ionotropic glutamate receptors, MCU forms the channel. The interactions between the Tyr residue (Y268 in hMCU) with nearby transmembrane helices facilitate a rotamer switch of one pair of tyrosines to control Ca2+ flow through the pore. This is probably how the phosphorylation of the NTD or divalent cation binding to the NTD manipulate MCU function. In the selectivity filter, unlike the large superfamily of cation channels that share loop‐like secondary structures, it is formed by α‐helices that coordinate Ca2+ with rings of acidic amino acids in MCU. They also indicate potential sites of channel regulation by other molecules within the mitochondrial matrix, besides the above listed MCU accessory proteins located at the IMM (Baradaran et al., 2018; Fan et al., 2018; Yoo et al., 2018). In Metarhizium acridum MCU, a conserved interaction network among Trp332, Glu336, and Pro337 stabilizes closely spaced carboxylate groups of the Glu336 ring, which might contribute to the high‐affinity binding and high selectivity of Ca2+ (Fan et al., 2018). Although higher resolution is needed for further validation of the structure and functional mechanism of MCU, this approach will definitely accelerate the development of structure‐based computer‐aided drug design, such as the identification of novel molecules, hit‐to‐lead optimization of affinity, and selectivity.
5. CONCLUDING REMARKS
The discoveries of MCU and its regulatory molecules open a new era in study of the uniplex‐mediated mitochondrial Ca2+ homeostasis in cancer. It is now possible to search the specific modulators of MCU based on molecular structure and to create transgenic animals by genetic tools. Studies have revealed that the regulation of MCU‐dependent mitochondrial Ca2+ homeostasis may be context‐ and tissue‐specific. Some questions regarding the regulatory mechanisms still remain to be answered. For example, why uniplex needs dual regulators (MCUb and MICU2) as negative regulators or gatekeeper proteins? The post‐translational regulation mechanisms of MCU, especially its regulatory proteins, are still mysterious. Currently, investigations into the alterations of uniplex‐mediated [Ca2+]m signalling in cancer are still at the nascent stage, and more studies are urgently needed. The range of candidate agents available for targeting the uniplex for cancer chemotherapy is still limited, especially with regard to MCU agonists and modulators of MCU regulatory proteins. Once these problems are solved, they will definitely shed light on the contribution of uniplex‐mediated Ca2+ signalling in carcinogenesis and improve the clinical chemotherapeutic outcomes in cancer patients.
5.1. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (Alexander, Fabbro et al., 2017; Alexander, Kelly, Marrion, Peters, Faccenda, Harding, Pawson, Sharman, Southan, Buneman et al., 2017; Alexander, Kelly, Marrion, Peters, Faccenda, Harding, Pawson, Sharman, Southan, Davies et al., 2017a,b; Alexander, Peters et al., 2017).
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
ACKNOWLEDGEMENTS
This work is supported by the National Natural Science Foundation of China (81873459 and U1804166), the support project for the Disciplinary Group of Psychology and Neuroscience, Xinxiang Medical University (2016PN‐KFKT‐04), Henan Key Laboratory of Neurorestoratology (HNSJXF‐2018‐005), and the start‐up funds of Xinxiang Medical University (505284).
Cui C, Yang J, Fu L, Wang M, Wang X. Progress in understanding mitochondrial calcium uniporter complex‐mediated calcium signalling: A potential target for cancer treatment. Br J Pharmacol. 2019;176:1190–1205. 10.1111/bph.14632
REFERENCES
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , … CGTP Collaborators . (2017). The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. British Journal of Pharmacology, 174(S1), S272–S359. 10.1111/bph.13877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , Harding, S. D. , … CGTP Collaborators . (2017). The Concise Guide to PHARMACOLOGY 2017/18: Overview. British Journal of Pharmacology, 174(S1), S1–S16. 10.1111/bph.13882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , Harding, S. D. , … CGTP Collaborators . (2017a). The Concise Guide to PHARMACOLOGY 2017/18: Transporters. British Journal of Pharmacology, 174(S1), S360–S446. 10.1111/bph.13883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , Harding, S. D. , … CGTP Collaborators . (2017b). The Concise Guide to PHARMACOLOGY 2017/18: Other ion channels. British Journal of Pharmacology, 174(S1), S195–S207. 10.1111/bph.13881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Peters, J. A. , Kelly, E. , Marrion, N. V. , Faccenda, E. , Harding, S. D. , … CGTP Collaborators . (2017). The Concise Guide to PHARMACOLOGY 2017/18: Ligand‐gated ion channels. British Journal of Pharmacology, 174(S1), S130–S159. 10.1111/bph.13879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali, I. , Alfarouk, K. O. , Reshkin, S. J. , & Ibrahim, M. E. (2017). Doxycycline as potential anti‐cancer agent. Anti‐Cancer Agents in Medicinal Chemistry, 17, 1617–1623. 10.2174/1871520617666170213111951 [DOI] [PubMed] [Google Scholar]
- Anghileri, L. J. (1975). The in vivo inhibition of tumor growth by ruthenium red: Its relationship with the metabolism of calcium in the tumor. Zeitschrift Fur Krebsforschung Und Klinische Onkologie Cancer Research and Clinical Oncology, 83, 213–217. 10.1007/BF00304090 [DOI] [PubMed] [Google Scholar]
- Arduino, D. M. , Wettmarshausen, J. , Vais, H. , Navas‐Navarro, P. , Cheng, Y. , Leimpek, A. , … Perocchi, F. (2017). Systematic identification of MCU modulators by orthogonal interspecies chemical screening. Molecular Cell, 67, 711–723.e717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvizo, R. R. , Moyano, D. F. , Saha, S. , Thompson, M. A. , Bhattacharya, R. , Rotello, V. M. , … Mukherjee, P. (2013). Probing novel roles of the mitochondrial uniporter in ovarian cancer cells using nanoparticles. The Journal of Biological Chemistry, 288, 17610–17618. 10.1074/jbc.M112.435206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baradaran, R. , Wang, C. , Siliciano, A. F. , & Long, S. B. (2018). Cryo‐EM structures of fungal and metazoan mitochondrial calcium uniporters. Nature, 559, 580–584. 10.1038/s41586-018-0331-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baughman, J. M. , Perocchi, F. , Girgis, H. S. , Plovanich, M. , Belcher‐Timme, C. A. , Sancak, Y. , … Mootha, V. K. (2011). Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature, 476, 341–345. 10.1038/nature10234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boroughs, L. K. , & DeBerardinis, R. J. (2015). Metabolic pathways promoting cancer cell survival and growth. Nature Cell Biology, 17, 351–359. 10.1038/ncb3124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brustovetsky, T. , Brittain, M. K. , Sheets, P. L. , Cummins, T. R. , Pinelis, V. , & Brustovetsky, N. (2011). KB‐R7943, an inhibitor of the reverse Na+/Ca2+ exchanger, blocks N‐methyl‐d‐aspartate receptor and inhibits mitochondrial complex I. British Journal of Pharmacology, 162, 255–270. 10.1111/j.1476-5381.2010.01054.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, C. , Wang, S. , Cui, T. , Su, X. C. , & Chou, J. J. (2017). Ion and inhibitor binding of the double‐ring ion selectivity filter of the mitochondrial calcium uniporter. Proceedings of the National Academy of Sciences of the United States of America, 114, E2846–e2851. 10.1073/pnas.1620316114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cárdenas, C. , Miller, R. A. , Smith, I. , Bui, T. , Molgó, J. , Müller, M. , … Foskett, J. K. (2010). Essential regulation of cell bioenergetics by constitutive InsP(3) receptor Ca2+ transfer to mitochondria. Cell, 142, 270–283. 10.1016/j.cell.2010.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri, D. , Artiga, D. J. , Abiria, S. A. , & Clapham, D. E. (2016). Mitochondrial calcium uniporter regulator 1 (MCUR1) regulates the calcium threshold for the mitochondrial permeability transition. Proceedings of the National Academy of Sciences of the United States of America, 113, E1872–E1880. 10.1073/pnas.1602264113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri, D. , Sancak, Y. , Mootha, V. K. , & Clapham, D. E. (2013). MCU encodes the pore conducting mitochondrial calcium currents. eLife, 2, e00704 10.7554/eLife.00704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, L. , Sun, Q. , Zhou, D. , Song, W. , Yang, Q. , Ju, B. , … Wang, W. (2017). HINT2 triggers mitochondrial Ca influx by regulating the mitochondrial Ca uniporter (MCU) complex and enhances gemcitabine apoptotic effect in pancreatic cancer. Cancer Letters, 411, 106–116. 10.1016/j.canlet.2017.09.020 [DOI] [PubMed] [Google Scholar]
- Clapham, D. E. (2007). Calcium signaling. Cell, 131, 1047–1058. 10.1016/j.cell.2007.11.028 [DOI] [PubMed] [Google Scholar]
- Clerc, P. , & Polster, B. M. (2012). Investigation of mitochondrial dysfunction by sequential microplate‐based respiration measurements from intact and permeabilized neurons. PLoS ONE, 7, e34465 10.1371/journal.pone.0034465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crompton, M. , Heid, I. , Baschera, C. , & Carafoli, E. (1979). The resolution of calcium fluxes in heart and liver mitochondria using the lanthanide series. FEBS Letters, 104, 352–354. 10.1016/0014-5793(79)80850-9 [DOI] [PubMed] [Google Scholar]
- Csordas, G. , Golenar, T. , Seifert, E. L. , Kamer, K. J. , Sancak, Y. , Perocchi, F. , … Hajnóczky, G. (2013). MICU1 controls both the threshold and cooperative activation of the mitochondrial Ca2+ uniporter. Cell Metabolism, 17, 976–987. 10.1016/j.cmet.2013.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui, C. , Chang, Y. , Zhang, X. , Choi, S. , Tran, H. , Penmetsa, K. V. , … Pan, Z. (2018). Targeting Orai1‐mediated store‐operated calcium entry by RP4010 for anti‐tumor activity in esophagus squamous cell carcinoma. Cancer Letters, 432, 169–179. 10.1016/j.canlet.2018.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui, C. , Merritt, R. , Fu, L. , & Pan, Z. (2017). Targeting calcium signaling in cancer therapy. Acta Pharmaceutica Sinica B, 7, 3–17. 10.1016/j.apsb.2016.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry, M. C. , Peters, A. A. , Kenny, P. A. , Roberts‐Thomson, S. J. , & Monteith, G. R. (2013). Mitochondrial calcium uniporter silencing potentiates caspase‐independent cell death in MDA‐MB‐231 breast cancer cells. Biochemical and Biophysical Research Communications, 434, 695–700. 10.1016/j.bbrc.2013.04.015 [DOI] [PubMed] [Google Scholar]
- De Stefani, D. , Raffaello, A. , Teardo, E. , Szabo, I. , & Rizzuto, R. (2011). A forty‐kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature, 476, 336–340. 10.1038/nature10230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deluca, H. F. , & Engstrom, G. W. (1961). Calcium uptake by rat kidney mitochondria. Proceedings of the National Academy of Sciences of the United States of America, 47, 1744–1750. 10.1073/pnas.47.11.1744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deniaud, A. , Sharaf el dein, O. , Maillier, E. , Poncet, D. , Kroemer, G. , Lemaire, C. , & Brenner, C. (2008). Endoplasmic reticulum stress induces calcium‐dependent permeability transition, mitochondrial outer membrane permeabilization and apoptosis. Oncogene, 27, 285–299. 10.1038/sj.onc.1210638 [DOI] [PubMed] [Google Scholar]
- Dong, Z. , Shanmughapriya, S. , Tomar, D. , Siddiqui, N. , Lynch, S. , Nemani, N. , … Madesh, M. (2017). Mitochondrial Ca2+ uniporter is a mitochondrial luminal redox sensor that augments MCU channel activity. Molecular Cell, 65, 1014–1028.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drago, I. , Pizzo, P. , & Pozzan, T. (2011). After half a century mitochondrial calcium in‐ and efflux machineries reveal themselves. The EMBO Journal, 30, 4119–4125. 10.1038/emboj.2011.337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan, C. , Fan, M. , Orlando, B. J. , Fastman, N. M. , Zhang, J. , Xu, Y. , … Feng, L. (2018). X‐ray and cryo‐EM structures of the mitochondrial calcium uniporter. Nature, 559, 575–579. 10.1038/s41586-018-0330-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fieni, F. , Lee, S. B. , Jan, Y. N. , & Kirichok, Y. (2012). Activity of the mitochondrial calcium uniporter varies greatly between tissues. Nature Communications, 3, 1317 10.1038/ncomms2325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouque, A. , Lepvrier, E. , Debure, L. , Gouriou, Y. , Malleter, M. , Delcroix, V. , … Legembre, P. (2016). The apoptotic members CD95, BclxL, and Bcl‐2 cooperate to promote cell migration by inducing Ca2+ flux from the endoplasmic reticulum to mitochondria. Cell Death and Differentiation, 23, 1702–1716. 10.1038/cdd.2016.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman, I. , Cook, O. , Kasir, J. , & Rahamimoff, H. (1993). Cloning of two isoforms of the rat brain Na+Ca2+ exchanger gene and their functional expression in HeLa cells. FEBS Letters, 319, 105–109. 10.1016/0014-5793(93)80046-W [DOI] [PubMed] [Google Scholar]
- Garcia‐Prieto, C. , Riaz Ahmed, K. B. , Chen, Z. , Zhou, Y. , Hammoudi, N. , Kang, Y. , … Huang, P. (2013). Effective killing of leukemia cells by the natural product OSW‐1 through disruption of cellular calcium homeostasis. The Journal of Biological Chemistry, 288, 3240–3250. 10.1074/jbc.M112.384776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido‐Mesa, N. , Zarzuelo, A. , & Gálvez, J. (2013). Minocycline: Far beyond an antibiotic. British Journal of Pharmacology, 169, 337–352. 10.1111/bph.12139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgi, C. , Baldassari, F. , Bononi, A. , Bonora, M. , De Marchi, E. , Marchi, S. , … Pinton, P. (2012). Mitochondrial Ca2+ and apoptosis. Cell Calcium, 52, 36–43. 10.1016/j.ceca.2012.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgio, V. , Burchell, V. , Schiavone, M. , Bassot, C. , Minervini, G. , Petronilli, V. , … Bernardi, P. (2017). Ca2+ binding to F‐ATP synthase beta subunit triggers the mitochondrial permeability transition. EMBO Reports, 18, 1065–1076. 10.15252/embr.201643354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glitsch, M. D. , Bakowski, D. , & Parekh, A. B. (2002). Store‐operated Ca2+ entry depends on mitochondrial Ca2+ uptake. The EMBO Journal, 21, 6744–6754. 10.1093/emboj/cdf675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez‐Suaga, P. , Paillusson, S. , & Miller, C. C. J. (2017). ER‐mitochondria signaling regulates autophagy. Autophagy, 13, 1250–1251. 10.1080/15548627.2017.1317913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunter, T. E. , Buntinas, L. , Sparagna, G. , Eliseev, R. , & Gunter, K. (2000). Mitochondrial calcium transport: Mechanisms and functions. Cell Calcium, 28, 285–296. 10.1054/ceca.2000.0168 [DOI] [PubMed] [Google Scholar]
- Hajnoczky, G. , Csordas, G. , Das, S. , Garcia‐Perez, C. , Saotome, M. , Sinha Roy, S. , & Yi, M. (2006). Mitochondrial calcium signalling and cell death: Approaches for assessing the role of mitochondrial Ca2+ uptake in apoptosis. Cell Calcium, 40, 553–560. 10.1016/j.ceca.2006.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan, D. , & Weinberg, R. A. (2011). Hallmarks of cancer: The next generation. Cell, 144, 646–674. 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Ireland S, Gray AJG, Bruce L, Alexander SPH, Anderton S, Bryant C, Davenport AP, Doerig C, Fabbro D, Levi‐Schaffer F, Spedding M, Davies JA, NC‐IUPHAR (2018). The IUPHAR/BPS guide to pharmacology in 2018: Updates and expansion to encompass the new guide to immunopharmacology. Nucleic Acids Research 46: D1091‐D1106, DOI: 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoth, M. , Button, D. C. , & Lewis, R. S. (2000). Mitochondrial control of calcium‐channel gating: A mechanism for sustained signaling and transcriptional activation in T lymphocytes. Proceedings of the National Academy of Sciences of the United States of America, 97, 10607–10612. 10.1073/pnas.180143997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto, T. , Watano, T. , & Shigekawa, M. (1996). A novel isothiourea derivative selectively inhibits the reverse mode of Na+/Ca2+ exchange in cells expressing NCX1. The Journal of Biological Chemistry, 271, 22391–22397. 10.1074/jbc.271.37.22391 [DOI] [PubMed] [Google Scholar]
- Joiner, M.‐l A. , Koval, O. M. , Li, J. , He, B. J. , Allamargot, C. , Gao, Z. , … Anderson, M. E. (2012). CaMKII determines mitochondrial stress responses in heart. Nature, 491, 269–273. 10.1038/nature11444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouaville, L. S. , Pinton, P. , Bastianutto, C. , Rutter, G. A. , & Rizzuto, R. (1999). Regulation of mitochondrial ATP synthesis by calcium: Evidence for a long‐term metabolic priming. Proceedings of the National Academy of Sciences of the United States of America, 96, 13807–13812. 10.1073/pnas.96.24.13807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaddour‐Djebbar, I. , Lakshmikanthan, V. , Shirley, R. , Ma, Y. , Lewis, R. , & Kumar, M. (2006). Therapeutic advantage of combining calcium channel blockers and TRAIL in prostate cancer. Molecular Cancer Therapeutics, 5, 1958–1966. 10.1158/1535-7163.MCT-06-0011 [DOI] [PubMed] [Google Scholar]
- Kamer, K. , Grabarek, Z. , & Mootha, V. (2017). High‐affinity cooperative Ca binding by MICU1‐MICU2 serves as an on‐off switch for the uniporter. EMBO Reports, 18, 1397–1411. 10.15252/embr.201643748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamer, K. , Sancak, Y. , Fomina, Y. , Meisel, J. , Chaudhuri, D. , Grabarek, Z. , & Mootha, V. K. (2018). MICU1 imparts the mitochondrial uniporter with the ability to discriminate between Ca and Mn. Proceedings of the National Academy of Sciences of the United States of America. 115(34), E7960–E7969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, K.‐Y. , Cho, H.‐J. , Yu, S.‐N. , Kim, S.‐H. , Yu, H.‐S. , Park, Y.‐M. , … Ahn, S. C. (2013). Interplay of reactive oxygen species, intracellular Ca2+ and mitochondrial homeostasis in the apoptosis of prostate cancer cells by deoxypodophyllotoxin. Journal of Cellular Biochemistry, 114, 1124–1134. 10.1002/jcb.24455 [DOI] [PubMed] [Google Scholar]
- Kirichok, Y. , Krapivinsky, G. , & Clapham, D. E. (2004). The mitochondrial calcium uniporter is a highly selective ion channel. Nature, 427, 360–364. 10.1038/nature02246 [DOI] [PubMed] [Google Scholar]
- Klimova, T. , & Chandel, N. S. (2008). Mitochondrial complex III regulates hypoxic activation of HIF. Cell Death and Differentiation, 15, 660–666. 10.1038/sj.cdd.4402307 [DOI] [PubMed] [Google Scholar]
- Kon, N. , Murakoshi, M. , Isobe, A. , Kagechika, K. , Miyoshi, N. , & Nagayama, T. (2017). DS16570511 is a small‐molecule inhibitor of the mitochondrial calcium uniporter. Cell Death Discovery, 3, 17045 10.1038/cddiscovery.2017.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer, G. , & Reed, J. C. (2000). Mitochondrial control of cell death. Nature Medicine, 6, 513–519. 10.1038/74994 [DOI] [PubMed] [Google Scholar]
- de la Fuente, S. , Matesanz‐Isabel, J. , Fonteriz, R. I. , Montero, M. , & Alvarez, J. (2014). Dynamics of mitochondrial Ca2+ uptake in MICU1‐knockdown cells. The Biochemical Journal, 458, 33–40. 10.1042/BJ20131025 [DOI] [PubMed] [Google Scholar]
- Lambert, J. , Luongo, T. , Shah, N. , & Elrod, J. (2016). MCUb (CCDC109B) modulates mitochondrial calcium uptake dynamics and susceptibility to calcium overload and permeability transition. The FASEB Journal, 30, 1224.1212–1224.1212. [Google Scholar]
- Landowski, T. H. , Megli, C. J. , Nullmeyer, K. D. , Lynch, R. M. , & Dorr, R. T. (2005). Mitochondrial‐mediated disregulation of Ca2+ is a critical determinant of Velcade (PS‐341/bortezomib) cytotoxicity in myeloma cell lines. Cancer Research, 65, 3828–3836. 10.1158/0008-5472.CAN-04-3684 [DOI] [PubMed] [Google Scholar]
- Lee, S. K. , Shanmughapriya, S. , Mok Mac, C. Y. , Dong, Z. , Tomar, D. , Carvalho, E. , … Stathopulos, P. B. (2016). Structural insights into mitochondrial calcium uniporter regulation by divalent cations. Cell Chemical Biology, 23, 1157–1169. 10.1016/j.chembiol.2016.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madreiter‐Sokolowski, C. T. , Klec, C. , Parichatikanond, W. , Stryeck, S. , Gottschalk, B. , Pulido, S. , … Graier, W. F. (2016). PRMT1‐mediated methylation of MICU1 determines the UCP2/3 dependency of mitochondrial Ca2+ uptake in immortalized cells. Nature Communications, 7, 12897 10.1038/ncomms12897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallilankaraman, K. , Cardenas, C. , Doonan, P. J. , Chandramoorthy, H. C. , Irrinki, K. M. , Golenar, T. , … Madesh, M. (2012). MCUR1 is an essential component of mitochondrial Ca2+ uptake that regulates cellular metabolism. Nature Cell Biology, 14, 1336–1343. 10.1038/ncb2622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallilankaraman, K. , Doonan, P. , Cardenas, C. , Chandramoorthy, H. C. , Muller, M. , Miller, R. , … Madesh, M. (2012). MICU1 is an essential gatekeeper for MCU‐mediated mitochondrial Ca2+ uptake that regulates cell survival. Cell, 151, 630–644. 10.1016/j.cell.2012.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchi, S. , Lupini, L. , Patergnani, S. , Rimessi, A. , Missiroli, S. , Bonora, M. , … Pinton, P. (2013). Downregulation of the mitochondrial calcium uniporter by cancer‐related miR‐25. Current Biology, 23, 58–63. 10.1016/j.cub.2012.11.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchi, S. , & Pinton, P. (2013). Mitochondrial calcium uniporter, MiRNA and cancer: Live and let die. Communicative & Integrative Biology, 6, e23818 10.4161/cib.23818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchi, S. , & Pinton, P. (2014). The mitochondrial calcium uniporter complex: Molecular components, structure and physiopathological implications. The Journal of Physiology, 592, 829–839. 10.1113/jphysiol.2013.268235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson, M. P. , & Chan, S. L. (2003). Calcium orchestrates apoptosis. Nature Cell Biology, 5, 1041–1043. 10.1038/ncb1203-1041 [DOI] [PubMed] [Google Scholar]
- Monteith, G. R. , McAndrew, D. , Faddy, H. M. , & Roberts‐Thomson, S. J. (2007). Calcium and cancer: Targeting Ca2+ transport. Nature Reviews. Cancer, 7, 519–530. 10.1038/nrc2171 [DOI] [PubMed] [Google Scholar]
- Moore, C. L. (1971). Specific inhibition of mitochondrial Ca++ transport by ruthenium red. Biochemical and Biophysical Research Communications, 42, 298–305. 10.1016/0006-291X(71)90102-1 [DOI] [PubMed] [Google Scholar]
- Nicholls, D. G. (2005). Mitochondria and calcium signaling. Cell Calcium, 38, 311–317. 10.1016/j.ceca.2005.06.011 [DOI] [PubMed] [Google Scholar]
- O‐Uchi, J. , Jhun, B. , Xu, S. , Hurst, S. , Raffaello, A. , Liu, X. , … Sheu, S.‐S. (2014). Adrenergic signaling regulates mitochondrial Ca2+ uptake through Pyk2‐dependent tyrosine phosphorylation of the mitochondrial Ca2+ uniporter. Antioxidants & Redox Signaling, 21, 863–879. 10.1089/ars.2013.5394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliarini, D. J. , Calvo, S. E. , Chang, B. , Sheth, S. A. , Vafai, S. B. , Ong, S. E. , … Mootha, V. K. (2008). A mitochondrial protein compendium elucidates complex I disease biology. Cell, 134, 112–123. 10.1016/j.cell.2008.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patron, M. , Checchetto, V. , Raffaello, A. , Teardo, E. , Vecellio Reane, D. , Mantoan, M. , … Rizzuto, R. (2014). MICU1 and MICU2 finely tune the mitochondrial Ca2+ uniporter by exerting opposite effects on MCU activity. Molecular Cell, 53, 726–737. 10.1016/j.molcel.2014.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patron, M. , Granatiero, V. , Espino, J. , Rizzuto, R. , & De Stefani, D. (2019). MICU3 is a tissue‐specific enhancer of mitochondrial calcium uptake. Cell Death and Differentiation, 26(1), 179–195. 10.1038/s41418-018-0113-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paupe, V. , Prudent, J. , Dassa, E. P. , Rendon, O. Z. , & Shoubridge, E. A. (2015). CCDC90A (MCUR1) is a cytochrome c oxidase assembly factor and not a regulator of the mitochondrial calcium uniporter. Cell Metabolism, 21, 109–116. 10.1016/j.cmet.2014.12.004 [DOI] [PubMed] [Google Scholar]
- Payne, R. , Hoff, H. , Roskowski, A. , & Foskett, J. K. (2017). MICU2 restricts spatial crosstalk between InsP3R and MCU channels by regulating threshold and gain of MICU1‐mediated inhibition and activation of MCU. Cell Reports, 21, 3141–3154. 10.1016/j.celrep.2017.11.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perocchi, F. , Gohil, V. M. , Girgis, H. S. , Bao, X. R. , McCombs, J. E. , Palmer, A. E. , & Mootha, V. K. (2010). MICU1 encodes a mitochondrial EF hand protein required for Ca2+ uptake. Nature, 467, 291–296. 10.1038/nature09358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrungaro, C. , Zimmermann, K. M. , Kuttner, V. , Fischer, M. , Dengjel, J. , Bogeski, I. , & Riemer, J. (2015). The Ca2+‐dependent release of the Mia40‐induced MICU1‐MICU2 dimer from MCU regulates mitochondrial Ca2+ uptake. Cell Metabolism, 22, 721–733. 10.1016/j.cmet.2015.08.019 [DOI] [PubMed] [Google Scholar]
- Plovanich, M. , Bogorad, R. L. , Sancak, Y. , Kamer, K. J. , Strittmatter, L. , Li, A. A. , … Mootha, V. K. (2013). MICU2, a paralog of MICU1, resides within the mitochondrial uniporter complex to regulate calcium handling. PLoS ONE, 8, e55785 10.1371/journal.pone.0055785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porporato, P. E. , Filigheddu, N. , Pedro, J. M. B.‐S. , Kroemer, G. , & Galluzzi, L. (2017). Mitochondrial metabolism and cancer. Cell Research, 28, 265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevarskaya, N. , Skryma, R. , Bidaux, G. , Flourakis, M. , & Shuba, Y. (2007). Ion channels in death and differentiation of prostate cancer cells. Cell Death and Differentiation, 14, 1295–1304. 10.1038/sj.cdd.4402162 [DOI] [PubMed] [Google Scholar]
- Raffaello, A. , De Stefani, D. , Sabbadin, D. , Teardo, E. , Merli, G. , Picard, A. , … Rizzuto, R. (2013). The mitochondrial calcium uniporter is a multimer that can include a dominant‐negative pore‐forming subunit. The EMBO Journal, 32, 2362–2376. 10.1038/emboj.2013.157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasola, A. , & Bernardi, P. (2011). Mitochondrial permeability transition in Ca2+‐dependent apoptosis and necrosis. Cell Calcium, 50, 222–233. 10.1016/j.ceca.2011.04.007 [DOI] [PubMed] [Google Scholar]
- Ren, T. , Wang, J. , Zhang, H. , Yuan, P. , Zhu, J. , Wu, Y. , … Xing, J. (2018). MCUR1‐mediated mitochondrial calcium signaling facilitates cell survival of hepatocellular carcinoma via reactive oxygen species‐dependent P53 degradation. Antioxidants & Redox Signaling, 28, 1120–1136. 10.1089/ars.2017.6990 [DOI] [PubMed] [Google Scholar]
- Ren, T. , Zhang, H. , Wang, J. , Zhu, J. , Jin, M. , Wu, Y. , … Xing, J. (2017). MCU‐dependent mitochondrial Ca2+ inhibits NAD+/SIRT3/SOD2 pathway to promote ROS production and metastasis of HCC cells. Oncogene, 36, 5897–5909. 10.1038/onc.2017.167 [DOI] [PubMed] [Google Scholar]
- Rizzuto, R. , Brini, M. , Murgia, M. , & Pozzan, T. (1993). Microdomains with high Ca2+ close to IP3‐sensitive channels that are sensed by neighboring mitochondria. Science, 262, 744–747. 10.1126/science.8235595 [DOI] [PubMed] [Google Scholar]
- Rizzuto, R. , De Stefani, D. , Raffaello, A. , & Mammucari, C. (2012). Mitochondria as sensors and regulators of calcium signalling. Nature Reviews Molecular Cell Biology, 13, 566–578. 10.1038/nrm3412 [DOI] [PubMed] [Google Scholar]
- Rizzuto, R. , Pinton, P. , Carrington, W. , Fay, F. S. , Fogarty, K. E. , Lifshitz, L. M. , … Pozzan, T. (1998). Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science, 280, 1763–1766. 10.1126/science.280.5370.1763 [DOI] [PubMed] [Google Scholar]
- Sancak, Y. , Markhard, A. L. , Kitami, T. , Kovacs‐Bogdan, E. , Kamer, K. J. , Udeshi, N. D. , … Mootha, V. K. (2013). EMRE is an essential component of the mitochondrial calcium uniporter complex. Science, 342, 1379–1382. 10.1126/science.1242993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santo‐Domingo, J. , Vay, L. , Hernandez‐Sanmiguel, E. , Lobaton, C. D. , Moreno, A. , Montero, M. , & Alvarez, J. (2007). The plasma membrane Na+/Ca2+ exchange inhibitor KB‐R7943 is also a potent inhibitor of the mitochondrial Ca2+ uniporter. British Journal of Pharmacology, 151, 647–654. 10.1038/sj.bjp.0707260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinzel, A. C. , Takeuchi, O. , Huang, Z. , Fisher, J. K. , Zhou, Z. , Rubens, J. , … Korsmeyer, S. J. (2005). Cyclophilin D is a component of mitochondrial permeability transition and mediates neuronal cell death after focal cerebral ischemia. Proceedings of the National Academy of Sciences of the United States of America, 102, 12005–12010. 10.1073/pnas.0505294102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz, J. , Holmuhamedov, E. , Zhang, X. , Lovelace, G. L. , Smith, C. D. , & Lemasters, J. J. (2013). Minocycline and doxycycline, but not other tetracycline‐derived compounds, protect liver cells from chemical hypoxia and ischemia/reperfusion injury by inhibition of the mitochondrial calcium uniporter. Toxicology and Applied Pharmacology, 273, 172–179. 10.1016/j.taap.2013.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, I. S. , Kim, H. K. , Lee, S. R. , Jeong, S. H. , Kim, N. , Ko, K. S. , … Han, J. (2013). Mitochondrial modulation decreases the bortezomib‐resistance in multiple myeloma cells. International Journal of Cancer, 133, 1357–1367. 10.1002/ijc.28149 [DOI] [PubMed] [Google Scholar]
- Strzyz, P. (2018). Autophagy: Mitochondria encaged. Nature Reviews Molecular Cell Biology, 19, 212 10.1038/nrm.2018.16 [DOI] [PubMed] [Google Scholar]
- Szabadkai, G. , & Duchen, M. (2008). Mitochondria: The hub of cellular Ca2+ signaling. Physiology (Bethesda, Md), 23, 84–94. [DOI] [PubMed] [Google Scholar]
- Tang, S. , Wang, X. , Shen, Q. , Yang, X. , Yu, C. , Cai, C. , … Zou, F. (2015). Mitochondrial Ca2+ uniporter is critical for store‐operated Ca2+ entry‐dependent breast cancer cell migration. Biochemical and Biophysical Research Communications, 458, 186–193. 10.1016/j.bbrc.2015.01.092 [DOI] [PubMed] [Google Scholar]
- Thebault, S. , Flourakis, M. , Vanoverberghe, K. , Vandermoere, F. , Roudbaraki, M. , Lehen'kyi, V. , … Prevarskaya, N. (2006). Differential role of transient receptor potential channels in Ca2+ entry and proliferation of prostate cancer epithelial cells. Cancer Research, 66, 2038–2047. 10.1158/0008-5472.CAN-05-0376 [DOI] [PubMed] [Google Scholar]
- Tomar, D. , Dong, Z. , Shanmughapriya, S. , Koch, D. A. , Thomas, T. , Hoffman, N. E. , … Madesh, M. (2016). MCUR1 is a scaffold factor for the MCU complex function and promotes mitochondrial bioenergetics. Cell Reports, 15, 1673–1685. 10.1016/j.celrep.2016.04.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosatto, A. , Sommaggio, R. , Kummerow, C. , Bentham, R. B. , Blacker, T. S. , Berecz, T. , … Mammucari, C. (2016). The mitochondrial calcium uniporter regulates breast cancer progression via HIF‐1α. EMBO Molecular Medicine, 8, 569–585. 10.15252/emmm.201606255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai, M. F. , Phillips, C. B. , Ranaghan, M. , Tsai, C. W. , Wu, Y. , Willliams, C. , & Miller, C. (2016). Dual functions of a small regulatory subunit in the mitochondrial calcium uniporter complex. eLife, 5 10.7554/eLife.15545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vais, H. , Tanis, J. E. , Muller, M. , Payne, R. , Mallilankaraman, K. , & Foskett, J. K. (2015). MCUR1, CCDC90A, is a regulator of the mitochondrial calcium uniporter. Cell Metabolism, 22, 533–535. 10.1016/j.cmet.2015.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanden Abeele, F. , Skryma, R. , Shuba, Y. , Van Coppenolle, F. , Slomianny, C. , Roudbaraki, M. , … Prevarskaya, N. (2002). Bcl‐2‐dependent modulation of Ca2+ homeostasis and store‐operated channels in prostate cancer cells. Cancer Cell, 1, 169–179. 10.1016/S1535-6108(02)00034-X [DOI] [PubMed] [Google Scholar]
- Wallace, D. C. (2012). Mitochondria and cancer. Nature Reviews. Cancer, 12, 685–698. 10.1038/nrc3365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, W. , Xie, Q. , Zhou, X. , Yao, J. , Zhu, X. , Huang, P. , … Zheng, S. (2015). Mitofusin‐2 triggers mitochondria Ca2+ influx from the endoplasmic reticulum to induce apoptosis in hepatocellular carcinoma cells. Cancer Letters, 358, 47–58. 10.1016/j.canlet.2014.12.025 [DOI] [PubMed] [Google Scholar]
- Weinberg, F. , Hamanaka, R. , Wheaton, W. W. , Weinberg, S. , Joseph, J. , Lopez, M. , … Chandel, N. S. (2010). Mitochondrial metabolism and ROS generation are essential for Kras‐mediated tumorigenicity. Proceedings of the National Academy of Sciences of the United States of America, 107, 8788–8793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg, S. E. , & Chandel, N. S. (2015). Targeting mitochondria metabolism for cancer therapy. Nature Chemical Biology, 11, 9–15. 10.1038/nchembio.1712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermann, B. (2010). Mitochondrial fusion and fission in cell life and death. Nature Reviews. Molecular Cell Biology, 11, 872–884. 10.1038/nrm3013 [DOI] [PubMed] [Google Scholar]
- Yang, S. , Zhang, J. J. , & Huang, X. Y. (2009). Orai1 and STIM1 are critical for breast tumor cell migration and metastasis. Cancer Cell, 15, 124–134. 10.1016/j.ccr.2008.12.019 [DOI] [PubMed] [Google Scholar]
- Yoo, J. , Wu, M. , Yin, Y. , Herzik, M. A. Jr. , Lander, G. C. , & Lee, S. Y. (2018). Cryo‐EM structure of a mitochondrial calcium uniporter. Science, 361(6401), 506–511. 10.1126/science.aar4056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, C. , Wang, Y. , Peng, J. , Shen, Q. , Chen, M. , Tang, W. , … Zou, F. (2017). Mitochondrial calcium uniporter as a target of microRNA‐340 and promoter of metastasis via enhancing the Warburg effect. Oncotarget, 8, 83831–83844. 10.18632/oncotarget.19747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaglia, T. , Ceriotti, P. , Campo, A. , Borile, G. , Armani, A. , Carullo, P. , … Mongillo, M. (2017). Content of mitochondrial calcium uniporter (MCU) in cardiomyocytes is regulated by microRNA‐1 in physiologic and pathologic hypertrophy. Proceedings of the National Academy of Sciences of the United States of America, 114, E9006–e9015. 10.1073/pnas.1708772114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng, F. , Chen, X. , Cui, W. , Wen, W. , Lu, F. , Sun, X. , … Zhang, X. (2018). RIPK1 binds MCU to mediate induction of mitochondrial Ca2+ uptake and promotes colorectal oncogenesis. Cancer Research, 78(11), 2876–2885. 10.1158/0008-5472.CAN-17-3082 [DOI] [PubMed] [Google Scholar]