Abstract
Background and Purpose
The NaV1.7 channel is highly expressed in dorsal root ganglia of the sensory nervous system and plays a central role in the pain signalling process. We investigated a library prepared from original venoms of 117 different animals to identify new selective inhibitors of this target.
Experimental Approach
We used high throughput screening of a large venom collection using automated patch‐clamp experiments on human voltage‐gated sodium channel subtypes and then in vitro and in vivo electrophysiological experiments to characterize the active peptides that have been purified, sequenced, and chemically synthesized. Analgesic effects were evaluated in vivo in mice models.
Key Results
We identified cyriotoxin‐1a (CyrTx‐1a), a novel peptide isolated from Cyriopagopus schioedtei spider venom, as a candidate for further characterization. This 33 amino acids toxin belongs to the inhibitor cystine knot structural family and inhibits hNaV1.1–1.3 and 1.6–1.7 channels in the low nanomolar range, compared to the micromolar range for hNaV1.4–1.5 and 1.8 channels. CyrTx‐1a was 920 times more efficient at inhibiting tetrodotoxin (TTX)‐sensitive than TTX‐resistant sodium currents recorded from adult mouse dorsal root ganglia neurons and in vivo electrophysiological experiments showed that CyrTx‐1a was approximately 170 times less efficient than huwentoxin‐IV at altering mouse skeletal neuromuscular excitability properties. CyrTx‐1a exhibited an analgesic effect in mice by increasing reaction time in the hot‐plate assay.
Conclusions and Implications
The pharmacological profile of CyrTx‐1a paves the way for further molecular engineering aimed to optimize the potential antinociceptive properties of this peptide.
Abbreviations
- CMAP
compound muscle action potential
- CyrTx‐1a
cyriotoxin‐1a
- DRG
dorsal root ganglia
- HwTx‐IV
huwentoxin‐IV
- ICK
inhibitor cystine knot
- NaSpTx
NaV channel spider toxin
- TTX
tetrodotoxin
- TTX‐R
resistant to tetrodotoxin
- TTX‐S
sensitive to tetrodotoxin
- U2OS cell line
human bone osteosarcoma epithelial cell line
1. INTRODUCTION
Sensory neurons express many transmembrane proteins that are therapeutic target candidates for the treatment of pain. In particular, inhibitors of ion channels (such as voltage‐gated sodium [NaV] and calcium [CaV] channels, transient receptor potential channels, acid‐sensing ion channels, piezo proteins, and ionotropic P2X receptors), as well as potassium channel (KV) enhancers, are being investigated as potential analgesics (Bennett & Woods, 2014; Waxman & Zamponi, 2014). NaV channels include nine subtypes (NaV1.1–1.9), each of them having different functions due to specific expression patterns and/or singular biophysical properties (Catterall, Goldin, & Waxman, 2005; de Lera Ruiz & Kraus, 2015). During this last decade, attention has been given to several NaV channel subtypes (NaV1.1, 1.3, and 1.6–1.9) as potential analgesic targets (Cardoso et al., 2017).
Among these NaV channels, the NaV1.7 channel seems to be one of the most interesting target to treat chronic debilitating pain (Vetter et al., 2017). Indeed, this subtype is highly expressed in the sensory nervous system, principally in small and large dorsal root ganglia (DRG) neurons, the anatomical support of pain signalling from the skin and organs to the spinal cord (Dib‐Hajj, Yang, Black, & Waxman, 2013). Furthermore, a multitude of genetic mutations of the NaV1.7 protein are linked to painless or painful phenotypes (de Lera Ruiz & Kraus, 2015; Vetter et al., 2017). Moreover, it is well established that low MW compounds that target NaV channels, such as tetrodotoxin (TTX), attenuate chronic and debilitating pain in humans (Hagen et al., 2017). However, pronounced side effects have been described, such as nausea, dizziness, oral numbness, and tingling, due to a lack of selectivity (Hagen et al., 2017). The current challenge is thus to identify a new therapeutic class of analgesic molecules that blocks the NaV1.7 channel with high selectivity compared to other NaV channels, particularly the NaV1.5 and the NaV1.6 and 1.4 channels, because of cardiac and neuromuscular safety issues, respectively.
Several peptide toxins from animal venoms (spiders, scorpions, cone snails, sea anemones, centipedes) have been reported to block or modulate NaV channel function (Israel, Tay, Deuis, & Vetter, 2017). Some of them, mainly isolated from spider venoms, showed promising selectivity for NaV1.7 channels (Vetter et al., 2017). Their sequence is mostly composed of 30–35 amino acids, including three disulfide bridges with an inhibitor cystine knot (ICK) motif. These positively charged toxins are gating‐modifier peptides that bind to the receptor sites 3 (on Domain IV) and/or 4 (on Domain II) of NaV channels, inducing variable pharmacological effects in in vitro tests and in vivo pain models (Saez et al., 2010). For instance, huwentoxin‐IV (HwTx‐IV) and the tarantula toxin GpTx‐1, two well‐characterized NaV1.7 channel‐blocking spider toxins with high selectivity over NaV1.4 and NaV1.5 channels, have analgesic properties in animal models. However, they also produce strong side effects such as inactivity, paralysis, and death in standard pain tests in rodents, due to the lack of selectivity over the NaV1.6 channels (Deuis et al., 2016; Gonçalves, Boukaiba, et al., 2018). Other spider toxins, such as protoxin‐II, exhibit a 100‐fold higher potency on NaV1.7, compared with all NaV channels, except for NaV1.6 channels. Interestingly, the in vivo safety margin of this toxin is increased for the mutant peptide JNJ 63955918, due to an improved selectivity against NaV1.1, 1.2, and 1.6 channels (Flinspach et al., 2017). Very recently, the Jingzhaotoxin‐V analogue AM‐8145 was reported to display a more than 100‐fold higher potency on NaV1.7 compared to all NaV channels (Moyer et al., 2018).
The present work reports the identification, structural characterization, and pharmacological profile of the first toxin (CyrTx‐1a) isolated from the venom of Cyriopagopus schioedtei spider, using a high throughput electrophysiological screening assay on NaV channels. This ICK toxin is shown to possess nanomolar range affinity for NaV1.1–1.3, 1.6, and 1.7 channels and micromolar affinity for other channel subtypes and to exhibit analgesic effects in rodent pain models. It represents an interesting lead for new analogues designed to exhibit a better therapeutic window.
2. METHODS
2.1. Isolation and purification of CyrTx‐1a
A library was prepared from 117 different animal venoms by crude venom fractionation using an analytical RP‐HPLC C18 column (XBridge™ BEH 130, 3.5 μm and 4.6 mm ID × 250 mm L column) attached to an Agilent 1260 HPLC (Agilent Technologies). Primary fractions were first evaluated in a functional screening assay on an engineered HEK‐293 cell line (RRID:CVCL_0045) overexpressing human (h) NaV1.7 and hNaV1.5 channels using the IonWorks Quattro platform (Molecular Devices, USA). For instance, the C. schioedtei spider venom, one of the venoms of interest, was separated into fractions that contained between 5 and 15 peptides each at an estimated concentration of 0.5 μg·μl−1. Active fractions were finally subfractionated using cation exchange chromatography with a TOSOH Bioscience column (TSK gel SP‐STAT, 7 μm, 4.6 mm ID × 10 cm L, TOSOH Bioscience, Germany) onto an Agilent 1260 HPLC (Agilent Technologies) to individualize the compounds. The purified compounds were screened on HEK‐293 cells overexpressing hNaV1.7, hNaV1.2, hNaV1.5, and hNaV1.6 channels, using the QPatch HTX automated electrophysiology platform (Sophion BioScience, Denmark), leading to the identification of CyrTx‐1a as one of the peptides of interest.
2.2. Amino acid sequencing of CyrTx‐1a
The peptide amino acid sequence was determined by de novo MS/MS sequencing and Edman degradation. The purified venom peptide obtained after successive RP‐HPLC and cation exchange chromatography, from a starting material of 2 mg, was resuspended in 100 mM ammonium bicarbonate (pH 8), reduced with 17 mM tris(2‐carboxyethyl)phosphine hydrochloride (incubated at 55°C for 1 hr) and alkylated with 24 mM iodoacetamide (incubated at room temperature in the dark for 1 hr) prior to enzyme digestion. The reduced/alkylated venom peptide was digested by using trypsin or V8 proteases. The enzyme was added at a 1:10 ratio (enzyme/peptide, w/w) and incubated overnight at 37°C before LC–MS analyses.
A Waters Q‐TOF Xevo G2S mass spectrometer equipped with an Acquity UHPLC system and Lockspray source was used for the acquisition of the LC–ESI–MS and LC–ESI–MS/MS data and the amino acid sequence determination based on Edman degradation was performed using an Applied Biosystems gas‐phase sequencer model 492 (s/n: 9510287 J). These protocols are detailed in the Supporting Information.
2.3. Chemical synthesis and folding of CyrTx‐1a
CyrTx‐1a was assembled stepwise using 2‐chlorotrityl chloride resin (substitution rate of 1.6 mmol·g−1) by solid‐phase fmoc chemistry on a Symphony Synthesizer (Protein Technologies Inc.). Amino acid coupling reaction was 15 min (repeated three times to increase the coupling yield). After resin cleavage and deprotection with 92.5% (vol) TFA, 2.5% H2O, and scavengers (1,3‐dimethoxybenzene [2.5%] and triisopropylsilane [2.5%]), the peptide was purified to homogeneity by C18 RP‐HPLC on a Jupiter Proteo column (Phenomenex, 4 μm, 21.2 mm ID × 250 mm L) using an Agilent Technologies preparative HPLC (1260 Infinity). Finally, CyrTx‐1a was folded/oxidized in 50 mM Tris–HCl, pH 8.3 during 72 hr. The resulting oxidized CyrTx‐1a with its three disulfide bridges was purified to homogeneity (>99% purity according to the integration of the purified chromatogram peak at 214 nm) using RP‐HPLC with the Jupiter Proteo column. The molecular mass of CyrTx‐1a was determined by LC–ESI–QTOF MS. The absence of contaminant masses attested to the purity of synthetic CyrTx‐1a.
2.4. 3D structure of CyrTx‐1a
The structure of CyrTx‐1a was determined by high‐resolution NMR spectroscopy in aqueous solution (10% D2O) of 6 mg·peptide ml−1 in 50 mM phosphate buffer at pH 5.0 and a temperature of 305°K. Data were obtained on a Bruker Avance 700 MHz using standard 2D spectra. Resonances were assigned with 2D spectra including DQF‐COSY, TOCSY, NOESY, 1H‐13C‐HSQC, and 1H‐15N‐HSQC. For conformational analysis, NOE‐based distance restraints were obtained from a NOESY spectrum with 200 ms mixing time. Four hundred thirty‐one distance restraints were used in a simulated annealing protocol starting from a linear, extended structure including 71 intra‐residual distances, 126 sequential distances, 69 medium distances (two to four amino acids apart), and 165 long range distances (>4 amino acids apart). Calculations were performed with the software package SYBYL version 2.1.1. All energy calculations were based on AMBER7 F99 force field. Distance restraints for non‐separated methylene protons and methyl groups were used with pseudoatom correction: 0.9 Å were added to the upper bound for methylene groups, 1.0 Å was added for methyl groups. Twenty structures were obtained which converged well. The rmsd over all backbone atoms was 0.465 ± 0.285 Å.
2.5. Toxins used for functional assays
Lyophilized synthetic CyrTx‐1a (molecular mass of 3578.68, purity rate > 97%), lyophilized synthetic HwTx‐IV, molecular mass of 4106.811, purity rate > 97%; Smartox Biotechnology, Saint‐Egrève, France), and TTX citrate (molecular mass of 319.27, purity rate > 98%; Sigma‐Aldrich, Saint‐Quentin Fallavier, France) were dissolved in PBS (1×) solution to give stock solutions of 6.8, 6.1, and 2.85 mM, respectively. Successive dilutions were then performed in the different standard physiological media, prior to experiments.
2.6. Cell lines used for functional assays
Generation of inducible cell lines was achieved using the Flp‐In® T‐Rex® or Jump‐In® T‐Rex® expression system (Invitrogen, USA). For this purpose, cDNAs encoding for hNaV1.5 (NM_000335), hNaV1.2 (NM_021007.2), hCaV3.1 (NM_018896.4), and hCaV3.2 (NM_021098.2) were cloned into the Flp‐In® T‐Rex® expression vector and subsequently transfected into HEK‐293 or CHO cell line (RRID:CVCL_0213), using the FuGENE® transfection reagent (Promega, France). The cDNA encoding for hKV7.1 (NM_000218.2) was cloned into the Jump‐In® T‐Rex® expression vector and subsequently transfected into human bone osteosarcoma epithelial (U2OS) cell line (RRID:CVCL_0042), using the FuGENE® transfection reagent. Recombinant HEK‐293 cell lines stably overexpressing hNaV1.7, 1.1, and 1.8 channels were purchased from Eurofins (St. Charles, MO, USA), those for stably overexpressing hNaV1.6 channels from ChanTest (Cleveland, OH, USA), and those stably overexpressing hNaV1.3 and 1.4 channels from SB Drug Discovery (UK). Cells overexpressing hNaV1.7 channels were cultured in suspension in FreestyleTM293 (Gibco, Thermo Fisher Scientific, Villebon‐sur‐Yvette, France). Those overexpressing hNaV1.2 and 1.5 channels were cultured in DMEM with GlutaMAX™ supplement (Gibco), those overexpressing hNaV1.1, 1.6, and 1.8 channels were cultured in DMEM/F12 with GlutaMAX™ supplement (Gibco), while those overexpressing hNaV1.3 and 1.4 channels were cultured in minimum essential medium (Sigma). In‐house U2OS‐Jump‐In‐T‐REx cells were kept in culture in McCoy's 5A medium with GlutaMAX™ supplement (Gibco). CHO cells heterologously overexpressing hCaV1.2/β2/α2δ1 (ChanTest), hCaV3.1 and hCaV3.2 channels were cultured in Ham's F12 nutrient mix with GlutaMAX™ medium (Gibco). Those overexpressing hKir2.1 channels (ChanTest) were cultured in DMEM/F‐12 Glutamax (Gibco), and those overexpressing hKV11.1 channels (B'SYS GmbH, Switzerland), encoded by the human ether‐a‐go‐go‐related gene, were cultured in DMEM/F12 nutrient mixture Ham's medium (Sigma). All culture media contained FBS (10%, Gibco) and selected antibiotics and additives, as recommended by the manufacturer. Cells were grown in flasks, under standard conditions (37°C, air supplemented with 5% CO2), and sub‐cultured/passaged every 3 to 4 days using Accutase® (Sigma) or TrypLE Select (Gibco) as enzymatic dissociation to detach the cells. At least 12 to 24 hr prior to experiments, doxycycline (BD Biosciences) was added to induce target expression when needed.
2.7. Animals and primary culture of DRG neurons used for functional assays
All animal care and experimental procedures in this study complied with the guidelines established by the French Council on animal care “Guide for the Care and Use of Laboratory Animals” (EEC86/609 Council Directive—Decree 2001‐131), and the experimental protocols were approved on November 27, 2015, by the French General Directorate for Research and Innovation (project APAFIS#2671‐2015110915123958v3 authorized to E. B.). Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny et al., 2010) and with the recommendations made by the British Journal of Pharmacology. The experiments were performed using 113 (80 for pain sensitivity assays, 29 for neuromuscular system assay and four for primary cultures of DRG neurons) adult female Swiss mice (Mus musculus, 10–12 weeks of age and 28–32 g body weight; catalogue # APB:8115) purchased from Janvier Elevage (Le Genest‐Saint‐Isle, France). The provider health reports indicated that the animals were free of known viral, bacterial, and parasitic pathogens. The choice of adult Swiss mice was guided by the fact that these outbred animals are more sensitive to morphine than inbred ones (Capasso, Di Giannuario, Loizzo, Pieretti, & Sorrentino, 1994). In addition, taking into account, first, that a clear majority of patients with pain has been reported to be women (Mogil, 2012) and, second, the 3Rs for more ethical use of animals in testing, only female mice were included in our study. The animals were acclimatized for at least 48 hr before experiments at the CEA animal facility. They were housed in a 12‐hr light/dark cycle and controlled temperature room, four to a cage containing bedding and a cardboard tube for environmental enrichment and were allowed free access to water and food. This study was experimentally designed to have equal group sizes of at least 10 animals per group for pain sensitivity assays and at least five animals per group for neuromuscular system assay, with intravenous injection as an exclusion criterion. In addition, randomization and blinding (the experimenter being blind to treatment group) were undertaken in all animal experiments.
After anaesthesia, with 2.0–2.5% isoflurane inhalation, and killing, by cervical vertebrae dislocation, the DRG were dissected from intervertebral foramina of the vertebrate column and enzymically dissociated, as reported previously (Gonçalves, Boukaiba, et al., 2018). The neurons were cultured under standard conditions (37°C, 95% air and 5% CO2) on 12‐mm glass coverslips placed in a 24‐well plate coated with 100 μg·ml−1 of murin laminin and 10 μg·ml−1 of poly‐d‐lysine (Sigma‐Aldrich). The culture medium was composed of a Neurobasal A medium (Gibco) added with Dulbecco's PBS (1×) without CaCl2 and MgCl2 (1.68%; Gibco), BSA (16.83 μg·ml−1; Sigma‐Aldrich), corticosterone (214.85 nM; Sigma‐Aldrich), T3 hormone (56.06 nM; Sigma‐Aldrich), horse serum (5%; Gibco), penicillin/streptomycin (47.64 U·ml−1; Gibco), nerve growth factor (83.33 ng·ml−1; Sigma‐Aldrich), N2 supplement (3.18×; Gibco), and l‐glutamine (1.90 mM; Sigma‐Aldrich). One day later, cytosine β‐d‐arabinofuranoside (2 μM; Sigma‐Aldrich) was added to the medium to inhibit astrocyte proliferation. Experiments were carried out within 2 to 6 days after neuron dissociation.
2.8. Electrophysiological recordings
Automated and manual patch‐clamp recordings were performed on cell lines and DRG neurons as described in the Supporting Information. In vivo recordings from the neuromuscular system of anaesthetized mice were performed by using a minimally invasive electrophysiological method and the Qtrac© software (Prof. H. Bostock, Institute of Neurology, London, UK), as detailed previously (Gonçalves, Boukaiba, et al., 2018). By means of a digital‐to‐analogue converter, this software allowed delivering the stimulation sequences to be performed and, in return, recording (at a sampling frequency of 10 kHz) and analysing the compound muscle action potential (CMAP) collected from the stimulated muscle. After being weighed, a given mouse was placed in an anaesthesia‐induction chamber in which a mixture of oxygen (0.4 L·min−1), air (0.2 L·min−1), and isoflurane (AErrane®, Baxter S.A., Lessines, Belgique; 2.0–2.5%) was diffused. When the mouse was anaesthetised, it was transferred to a heating pad to maintain body temperature throughout the experiments (35.99 ± 0.03°C, as determined in 29 mice using a rectal probe). The animal's muzzle was positioned at the level of a mask where the anaesthetic gas mixture was conveyed to keep the animal anaesthetised. If necessary, the percentage of isoflurane was adjusted to maintain the depth of the anaesthesia. Electrical stimulations were delivered to the caudal motor nerve (at the base of the tail) by two stimulators (A395, World Precision Instruments, Sarasota, FL, USA) via two surface electrodes, and the CMAP was recorded using fine needle electrodes inserted into the tail muscle and connected to an amplifier (Disa EMG 14C13) and then to a hum bug (Quest Scientific). Intramuscular injections (4‐μl maximal volume) of PBS solution without (to test for any effect of the vehicle) or with various concentrations of CyrTx‐1a or TTX were administered at the base of the tail (between stimulation and ground electrodes) with a 10 μl micro‐syringe. The toxin and/or vehicle effects on selected excitability parameters, such as the excitability threshold and CMAP amplitude continuously recorded over time, were assessed by online recordings initiated ≈5 min before a given injection. The duration of CyrTx‐1a and TTX effects and the identification of the toxin underlying mechanism(s) of action were investigated by performing five different excitability tests (stimulus–response, strength–duration, and current–threshold relationships, as well as threshold electrotonus and recovery cycle; detailed in Cerles et al., 2017), before and from 30 min to 12 hr after a given injection. More than 30 parameters, providing complementary information on ion channels, receptors, and pumps, as well as on the passive membrane properties of the neuromuscular system (Kiernan & Bostock, 2000; Krishnan, Lin, Park, & Kiernan, 2008), were determined and analysed from these five excitability tests.
2.9. Heat and tactile pain sensitivity of mice in vivo
Prior to hot‐plate testing, each mouse underwent a 30‐min acclimation to the experimental laboratory environment (in its home cage). Then, mice were either not injected or received an intraplantar injection of 5–10 μl of PBS or toxin solution in each hind paw under low anaesthesia achieved by means of isoflurane (AErrane®, Baxter S.A.) inhalation. After 60 min rest in its home cage, the mouse was put on the hot‐plate set at the temperature of 55.0 ± 0.2°C. The measured parameter as the first pain‐related manifestation was the latency (in seconds) for the animal either to shake one of its two hind limbs or to jump. This latency, considered as a painful response to heat, was recorded simultaneously by two observers (one being blind to treatment group) with a timer integrated into the set‐up. A maximal cut‐off time of 30 s was used to prevent tissue damage.
Tactile sensitivity was assessed using an automated plantar von Frey apparatus (Dynamic Plantar Aesthesiometer 37450, Ugo Basile, Comerio, Italy). Prior to testing, each mouse was placed on a mesh grid, surrounded by a clear Plexiglas barrier with a top cover and left to calm down for 30 min without probing. After the settling phase, the mouse was motionless allowing for either of its hind limbs to be touched by a flexible plastic fibre of a fixed diameter. The fibre was pressed through the mesh grid against the plantar surface at a right angle, and the force of application increased slowly (at the determined rate of 1.67 g·s−1). The force intensity (in g) at which the animal removed its hind limb was recorded with a timer integrated into the set‐up as the mean of at least four tests. A cut‐off automatically occurred if the animal did not remove its hind limb when the point at which the greatest pre‐set force was met, to prevent tissue damage. The force intensity was determined every 5 min during 30–45 min before and 15 min after intraplantar injection of 5 μl of PBS or toxin solution in each hind limb, under mild anaesthesia with isoflurane.
2.10. Data and statistical analyses
The data and statistical analysis comply with the recommendations of the British Journal of Pharmacology on experimental design and analysis in pharmacology. Sigmoid nonlinear regressions through data points (correlation coefficient = r 2) were used to calculate theoretical concentration–response curves, according to the Hill equation (GraphPad Prism version 5 [RRID:SCR_002798] or QPatch assay software): Rt/Rc = 1/[1 + ([toxin]/IC50) n H], where Rt/Rc is the response recorded in the presence of a given toxin (Rt) and expressed as percentage of the value obtained in absence of toxin (Rc), [toxin] is the toxin concentration, IC50 is the toxin concentration necessary to inhibit 50% of the response, and n H is the Hill number. The conductance (g) was calculated from the peak current amplitude (I) according to the following equation: g = I/(V T – V Na), where V T is the test‐pulse voltage and V Na is the equilibrium potential of Na ions. The Boltzmann equation (GraphPad Prism version 5 software) was used to calculate the theoretical conductance–voltage curves corresponding to data point fit: g/g max = 1 – [1/(1 + exp((V T – V T50%)/k g))], where g/g max is the conductance expressed as percentage of the maximal conductance (g max) calculated at strongly positive test‐pulses, V T50% is the test‐pulse voltage corresponding to 50% maximal conductance, and k g is the slope of the curve. The Boltzmann equation was also used to calculate the theoretical steady‐state inactivation–voltage curves corresponding to data point fit: I/I max = 1/[1 + exp((V P – V P50%)/k h)], where I/I max is the peak current amplitude expressed as percentage of the maximal amplitude (I max) recorded in response to strongly negative pre‐pulses (V P), V P50% is the pre‐pulse voltage corresponding to 50% maximal peak amplitude of current, and k h is the slope of the curve. The evaluation of current kinetics was performed by calculating the time to peak (tp) and the time constant of the current inactivation (τh). The time to peak was defined as the time between test‐pulse triggering and the peak current, and the time constant of the current inactivation was calculated according to the following equation, assuming a mono‐exponential decay as a function of time: I (t) = I (0) e(−t/τh).
Data are expressed as means ± SDs of n different experimental biological samples. The statistical comparison of values was carried out using (a) the parametric two‐tailed Student's t test (either paired samples for comparison within a single population or unpaired samples for comparison between two independent populations) or (b) the one‐way ANOVA (for comparison between the means of three or more independent populations) followed, if F was significant and if no variance inhomogeneity occurred, by post hoc pairwise t tests with Bonferroni correction. Differences were considered to be statistically significant at P < 0.05.
2.11. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org/, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander et al., 2017).
3. RESULTS
3.1. Isolation, purification, and de novo amino acid sequencing of CyrTx‐1a
A primary high throughput screening of the Smartox venom collection was performed on automated patch‐clamp Ionworks Quattro platform using HEK‐293 cells overexpressing hNaV1.7 channels (Figure 1a). Following successful priming and sealing steps, a 10‐pulse train protocol from −120 to −10 mV at 10 Hz was elicited, bringing hNaV1.7 channels from closed to open configuration (Figure 1b,c). The so‐called pre‐scan performed in the presence of extracellular buffer was used as a control signal. An average peak current from pulse 1 of 880 ± 280 pA (n = 29 plates) was elicited. A similar average amplitude was measured from pulse 10 on the same recording (no current rundown was observed under our conditions). In control experiments using the same protocol, no inhibitory effect of 0.1% BSA was found. Conversely, addition of 1 μM TTX produced a full block of the elicited currents (n = 928 wells from 29 plates). This expected pharmacology was used as the internal positive control (data not shown).
Figure 1.
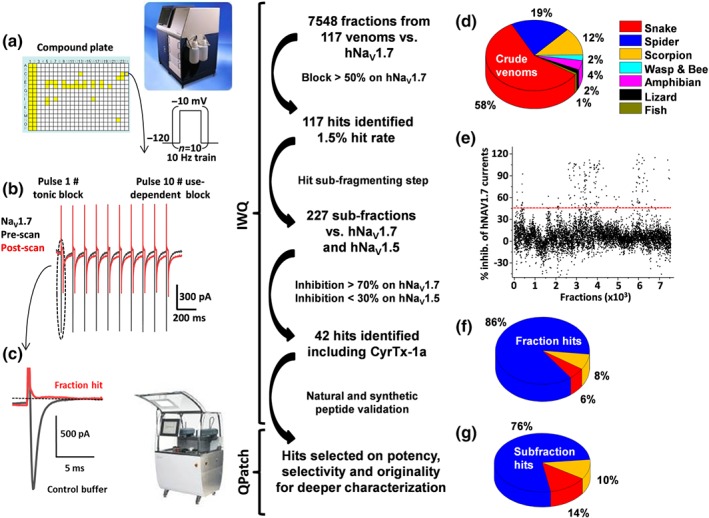
Screening flowchart from crude venom fraction to isolated peptide identification. (a) The collection of 117 venoms was prepared into 7,548 fractions individually added to 384‐well plates (Columns 1 and 2 are 1 μM TTX full block and Columns 3 and 4 are maximal current obtained in extracellular buffer) for testing versus hNaV1.7 channels on the Ionworks Quattro (IWQ, Molecular Devices). The train protocol described as an insert was applied before and after 10‐min incubation of cells with the fraction containing the toxin of interest. Traces of pre‐ and post‐scan are shown in full (b). Also, tonic block obtained on hNaV1.7 channels has been enlarged (c). Source of the screened crude venom is shown in (d). Percentage of inhibition for all tested fractions was plotted. As shown in (e), a threshold was set at 50% block (red line). From the venom library tested, only 8% hits on the hNaV1.7 channels came from scorpion venom, 6% from snake while the majority (86%) was derived from spider venoms (f). (g) Hits were sub‐fractioned and tested against hNaV1.7 and hNaV1.5 channels to identify most promising hits to be further characterized using whole‐cell automated patch‐clamp assays (QPatch)
As mentioned in Figure 1, a total of 7,548 fractions coming from 117 crude venoms were tested from a large variety of species including snake, spider, scorpion, wasp, bee, amphibians, lizard, and fish (see Figure 1d). The 384‐well screening plates were prepared in such a way that individual fractions were tested at an average concentration of 0.5 μg·μl−1 (500 ng of dry mass suspended in water). An overview of the percentage of inhibition obtained for each fraction before and after application is given in Figure 1e. Note that 97 fractions were discarded because of a negative impact on the sealing process or because they disrupted seals over time. Also, 26 samples which elicited large sodium current increases were removed from the analysis. From the primary screening, 117 fractions were flagged based on their potency versus recombinant NaV1.7channels, then selected for sub‐fractionation and compound isolation using cation exchange chromatography (Figure 1f). Following this process, 227 sub‐fractions from three species (snake, spider, and scorpion; Figure 1g) were prepared and used at a final estimated amount of 100 ng per well. These sub‐fractions were tested in our functional automated patch‐clamp assays with hNaV1.7, but also hNaV1.5 channels (in conventional closed to open configuration protocols), as a first‐line selectivity assay. From the 42 hits highlighted at this stage, 14 were discarded because of strong effects on hNaV1.5 channels.
Figure 2a,b illustrates the screening process for the spider venom C. schioedtei from primary fraction selection to individual purified compound selection by secondary screening. Using the IonWorks Quattro automated patch‐clamp system, a peptide was selected for its potent blocking effect on hNaV1.7 channels at 2.8 μM (98.9 ± 1.2% block, n = 6 wells from three plates) while fully sparing hNaV1.5 channels (4.1 ± 1.2% inhibition, n = 6 wells from three plates; Figure 2b). Following selection through the multi‐step chromatographic approach, a new peptide was identified using combined orthogonal reversed‐phase and ion exchange techniques (Figure 2c,d). The molecular mass value of 3578.68 Da for this peptide, as determined by LC–ESI–QTOF MS, indicates that the peptide should be amenable to chemical synthesis (inset in Figure 2d).
Figure 2.
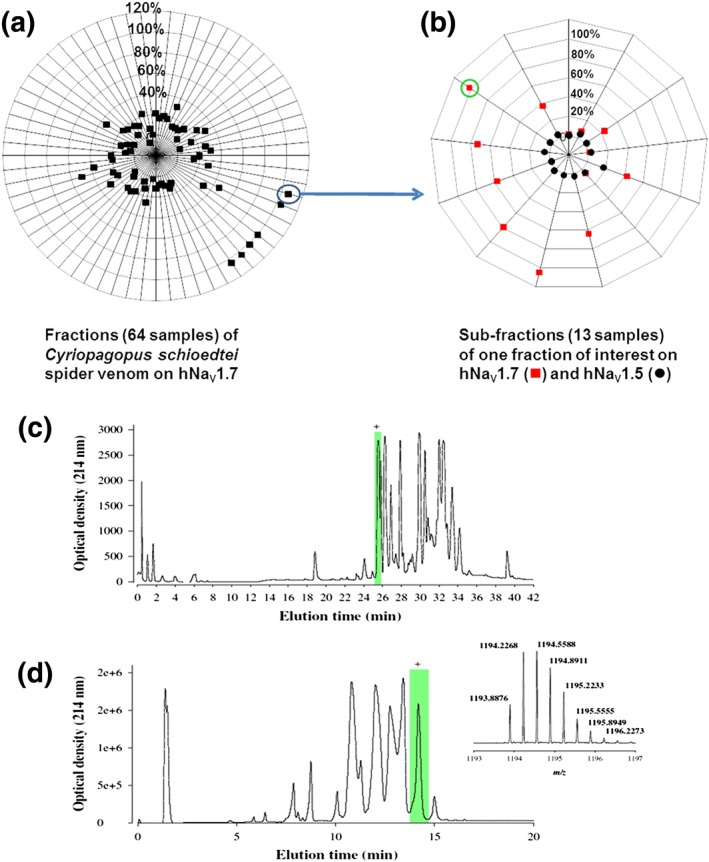
Flowchart for hNaV1.7 channel hit peptide identification. (a) Percentage of inhibition of peak hNaV1.7 elicited current by application of 0.05 μg of each of the 64 fractions obtained from Cyriopagopus schioedtei spider venom. (b) Each fraction of interest was then separated again to isolate one peptide per well. Sub‐fractions were tested again in our automated patch‐clamp Quattro assay. At this stage, inhibition of hNaV1.7 and hNaV1.5 channels was investigated. (c) Fractionation of the crude venom from C. schioedtei by reversed‐phase chromatography and detection by UV at 214 nm. The fraction containing the peptide of interest is highlighted in green. (d) Cation exchange sub‐fractionation of the primary fraction highlighted in (c). The inset illustrates the MS of m/z 1193.8947 [M + 3H]3+
As this peptide belongs to a species that has not been genotyped, its sequence was determined by de novo sequencing using MS analyses. Hence, the purified peptide was reduced using tris(2‐carboxyethyl)phosphine hydrochloride and alkylated with iodoacetamide. The alterations in molecular mass from 3578.7 to 3926.7 Da indicate that the peptide should contain six cysteine residues and hence three disulfide bridges if one takes into account the loss of 1 Da upon reduction of disulfide bridges and the addition of 57.02 Da upon alkylation on each cysteine residue. Samples of the reduced/alkylated toxin were then digested overnight with either trypsin or V8 proteases. The digests were next analysed by LC–ESI–MS(/MS) for de novo sequencing. Table S1 provides the list of fragments detected and sequenced after trypsin and Glu‐C digestion. While full sequence coverage of the toxin was obtained by MS, a complementary characterization was performed using Edman degradation, especially for the precise determination of the isobaric leucine and isoleucine residues. The final sequence is reported in Figure 3a with a single post‐translational modification identified as C‐terminal amidation of the peptide.
Figure 3.
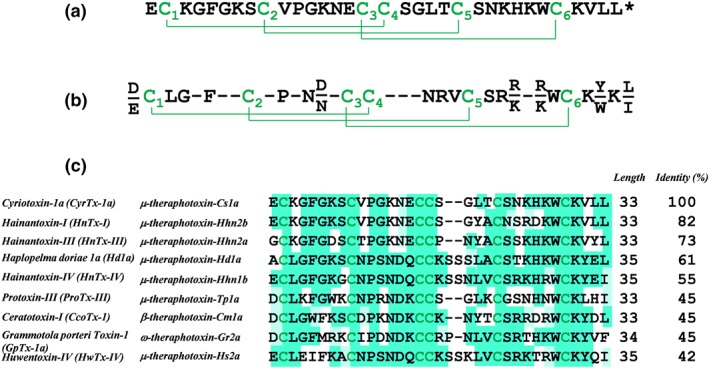
Primary structure, disulfide bridge alignment, and sequence homologies of CyrTx‐1a. (a) Primary structure of CyrTx‐1a. Asterisk denotes amidation. Disulfide bridging, as defined by homology, occurs according to the C1–C4, C2–C5, and C3–C6 pattern. (b) Consensus sequence of the NaSpTx family 1, adapted from Klint et al. (2012). (c) Comparison of amino acid sequences between CyrTx‐1a and the nine most similar analgesic toxins from NaSpTx family 1. Sequence alignment performed with Clustal Omega (version 1.2.4 from Emboss programs, EBlosum62 matrix for two pair alignment). The green shading highlights the percentage of identity (Jalview program according to EBlosum62 matrix)
The peptide, composed of 33 amino acids (3578.68 Da), was identified as μ‐theraphotoxin‐Cs1a or cyriotoxin‐1a (CyrTx‐1a). It is the first toxin described so far from the crude venom of C. schioedtei spider, known as the Malaysian earth tiger tarantula, classified in the Ornithoctoninae subfamily. CyrTx‐1a contains the ICK architectural motif previously reported in toxins from the same theraphosid spider family, the NaV channel spider toxin (NaSpTx) family 1 (Figure 3b). Furthermore, comparison of amino acid sequences between CyrTx‐1a and the nine most similar toxins (of 33–35 amino acids) with analgesic properties from the NaSpTx family 1 revealed that the peptide shares 82% of identity with hainantoxin‐I and μ‐theraphotoxin‐Hhn2b, and 73% of identity with hainantoxin‐III and μ‐theraphotoxin‐Hhn2a (Figure 3c). In contrast, the well‐known potent analgesic peptides ω‐theraphotoxin‐Gr2a (GpTx‐1) and μ‐theraphotoxin‐Hh2a (HwTx‐IV) share only 45% and 42% of identity, respectively, with CyrTx‐1a.
3.2. Chemical synthesis and in vitro folding of CyrTx‐1a
CyrTx‐1a was chemically synthesized using solid‐phase Fmoc chemistry. Figure S1a,b illustrates the HPLC profiles of the crude and purified synthesized peptide, respectively. MS data established that the purified reduced peptide had the expected mass with m/z value of 1195.9097 [M + 3H]3+. Finally, the reduced CyrTx‐1a peptide was oxidized to produce oxidized/folded CyrTx‐1a along with its three disulfide bridges (Figure S1c). The yield of oxidation was 13%, indicating good formation of the secondary structures and easy disulfide bridge connectivity during oxidation. The experimental molecular mass of the synthetic peptide (inset in Figure S1c, 1193.8956 [M + 3H]3+) was in close agreement with the theoretical mass (1193.8918). To confirm that the synthetic CyrTx‐1a was indeed identical to its native counterpart, both peptides were mixed at equal concentrations and run simultaneously onto analytical RP‐HPLC. As a single major peak was detected, we conclude that the two peptides co‐elute, hence demonstrating identical retention times on the C18 column (Figure S1d).
3.3. 3D structure of CyrTx‐1a
The 3D solution structure of CyrTx‐1a was determined by 2D homonuclear 1H‐NMR spectroscopy. Spectra were recorded at 305°K where the amide resonances show a good dispersion. One signal set of sharp and well‐dispersed resonances is indicative of a single structure in solution (Figure 4a). NMR‐derived interproton distances were used for structure calculations with a molecular dynamic‐based protocol. An ensemble of 20 conformations was obtained, containing a well‐determined backbone conformation of an ICK motif (Figure 4b; PDB: 6GFT). The 1H‐chemical shifts of CyrTx‐1a in H2O/D2O highlight the high precision and stereochemical quality of the ensemble of CyrTx‐1a structures (Table S2). The entire structure has an electrical dipole moment with a larger positive pole, likely to be important for CyrTx‐1a binding to NaV channels (Figure 4c). Such a motif has also been found for other NaV1.7 channel inhibitory peptides such as HnTx‐IV, ProTx‐III, and HwTx‐IV (Figure 4d).
Figure 4.
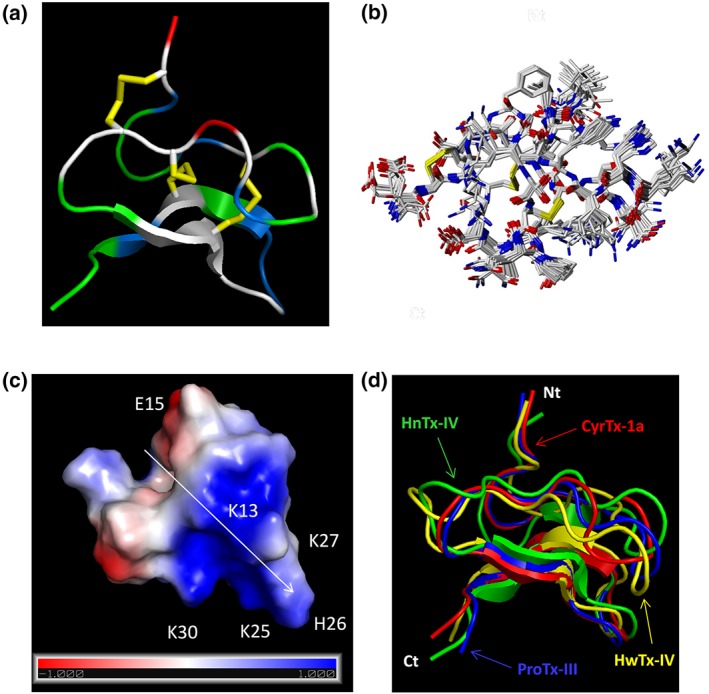
Representation of CyrTx‐1a identified by PyMOL. (a) Representation of the backbone peptide folding of CyrTx‐1a determined by 1H 2D NMR method. The structure topology is composed of double stranded antiparallel β sheet. The three disulfide bonds are C2–C17, C9–C22, and C16–C29 (in yellow). Hydrophobic residues are coloured in green, and basic and acidic residues are coloured in blue and red, respectively. The other polar residues are coloured in white. (b) Superposition of 20 structures derived from a 6‐ns restrained MD simulation (all heavy atoms are shown). All backbone atoms of residues two to 31 were used for fitting. Structures were sampled in 300 ps intervals and energy minimized. The rmsd over all backbone atoms (including residues one to 33) is 0.465 Å with an SD of 0.157 Å. Considering all heavy atoms, the rmsd is 1.072 Ǻ with an SD of 0.285 Ǻ (PDB: 6GFT). (c) Electrostatic charged surface representation of CyrTx‐1a. The molecule is rendered as a surface coloured according to the electrostatic potential. As indicated in the coloured legend, an excess of negative and positive charges near the surface are represented in red (−1,000) and blue (1,000), respectively, while fairly neutral potentials are represented in white. The entire structure has a clear dipole potential with E1 and E15 forming a negative zone while K3, K7, K13, K25, H26, K27, and K30 form a positive zone. (d) Superposition of backbone peptide folding of CyrTx‐1a and three other toxins of the NaSpTx family 1 previously described to possess analgesic effects (PDB entries of HnTx‐IV: 1NIY, ProTx‐III: 2MXM, and HwTx‐IV: 1MB6)
3.4. Effects of CyrTx‐1a on hNaV, hCaV, hKV, and hKir channels overexpressed in cell lines
Whole‐cell automated patch‐clamp (QPatch HTX) experiments performed on HEK‐293 cells overexpressing hNaV1.1–1.8 channels revealed that 1 μM of synthetic CyrTx‐1a was effective to block hNaV1.1–1.2–1.3–1.6–1.7 currents while hNaV1.4–1.5–1.8 currents were unaffected (Figure 5a). The following increasing order for IC50 values was obtained from the concentration–response curves of CyrTx‐1a effects on currents flowing through the different channels (Figure 5b): hNaV1.1 (72.0 ± 10.0 nM, n = 5) ≈ hNaV1.2 (75.5 ± 4.3 nM, n = 8) ≈ hNaV1.6 (115.0 ± 7.5 nM, n = 5) ≈ hNaV1.7 (129.5 ± 2.1 nM, n = 7) > hNaV1.3 (306.6 ± 15.2 nM, n = 8) > > hNaV1.4 (7.7 ± 0.2 μM, n = 7) for TTX‐sensitive (TTX‐S) subtypes, and hNaV1.5 (>10 μM, n = 5) = hNaV1.8 (>10 μM, n = 8) for TTX‐resistant (TTX‐R) subtypes. Additionally, the peptide had very low affinity for hCaV1.2, 3.1 and 3.2 and hKV7.1 and 11.1 and hKir2.1 channels overexpressed in CHO, HEK‐293, and U2OS cells, since 10 μM of toxin had no marked effect on currents flowing through these six channels (Figure S2).
Figure 5.
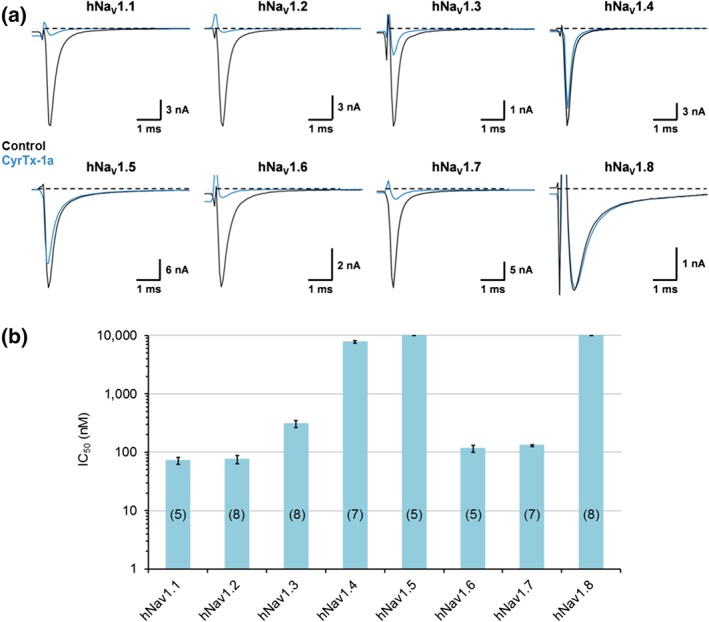
Effects of CyrTx‐1a on HEK‐293 cells overexpressing hNaV1.1–1.8 channels, using whole‐cell automated patch‐clamp. (a) Representative traces of sodium currents flowing through hNaV1.1–1.8 channels, recorded before (control) and after exposure to 1 μM CyrTx‐1a. (b) Histograms of IC50 values obtained from the concentration–response curves of CyrTx‐1a effects on HEK‐293 cells overexpressing hNaV1.1–1.8 channels. Each value represents the mean ± SD of data obtained from n cells (numbers in parentheses). Mean value ± SD of n H was 1.0 ± 0.3
Further investigation, using whole‐cell manual patch‐clamp, provided IC50 values of 52.7 nM from the concentration–response curves of CyrTx‐1a effects on currents flowing through hNaV1.7 channels overexpressed in HEK‐293 cells (Figure 6a,b). This CyrTx‐1a‐induced blocking action occurred without any change in steady‐state inactivation‐ and conductance‐voltage relationships of hNaV1.7 channels (Figure 6c and Table S3).
Figure 6.
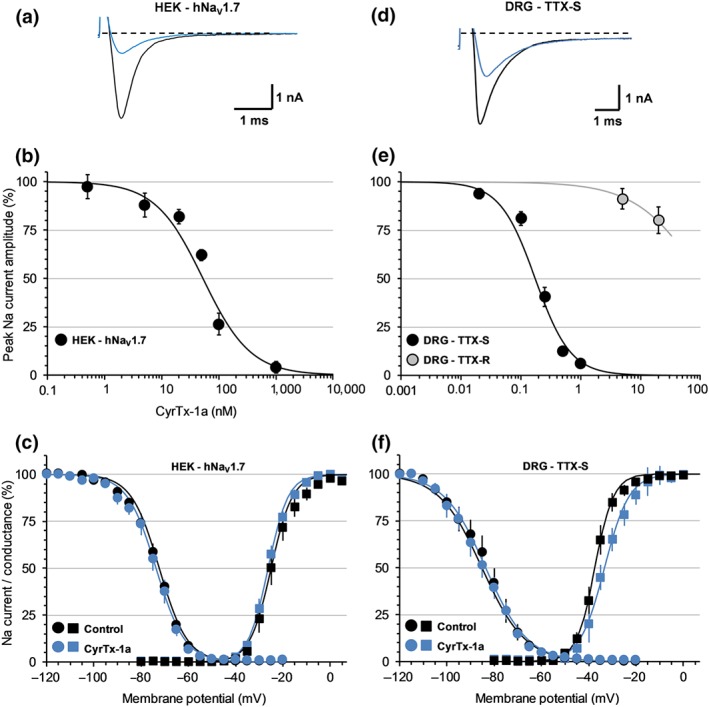
Effects of CyrTx‐1a on HEK‐293 cells overexpressing hNaV1.7 channels (a–c) and on TTX‐S and TTX‐R sodium currents of adult mouse DRG neurons (d–f), using whole‐cell manual patch‐clamp. Representative traces of sodium currents flowing through hNaV1.7 channels (a) and of TTX‐S sodium currents of DRG neurons (d), recorded before (black) and after (blue) exposure to 100 and 250 nM CyrTx‐1a, respectively. Concentration–response curves of CyrTx‐1a effects on hNaV1.7 channel current (b) and on TTX‐S and TTX‐R sodium currents of DRG neurons (e). Each value, expressed as percentage of that obtained before toxin application, represents the mean ± SD of data obtained from five HEK‐293 cells and five DRG neurons from four different cell cultures. IC50 and n H values were, respectively, 52.7 nM and 1.0 for hNaV1.7 current (r 2 = 0.954), 0.17 μM and 1.5 for TTX‐S current (r 2 = 0.961), and 156 μM and 0.7 for TTX‐R current (r 2 = 1.000). Steady‐state inactivation‐ (circles) and conductance‐ (squares) voltage relationships for HEK‐293 cells overexpressing hNaV1.7 channels (c) and for neurons having TTX‐S current (f), before and after exposure to 50 nM and 0.25–0.5 μM CyrTx‐1a, respectively. Each value represents the mean ± SD of data obtained from five HEK‐293 cells and eight DRG neurons from four different cell cultures and is expressed as percentage of either maximal peak amplitude of current at strongly negative pre‐pulse voltages or maximal conductance calculated at strongly positive test voltages. The theoretical curves correspond to data point fits with the mean V P50%, k h, V T50%, and kg values indicated in Table S3
3.5. Effects of CyrTx‐1a and TTX on adult mouse DRG neurons
Before evaluating the effects of CyrTx‐1a on the sodium currents of DRG neurons, the sensitivity of these currents to 100 nM TTX was first determined. Under this condition, two types of neurons were recorded. The first type (76%, i.e., 16/21 neurons) had only TTX‐S current, which was blocked by the toxin to 4.5 ± 3.0% of initial peak amplitude values within 1 min. The effects of CyrTx‐1a (from 0.02 to 1 μM) on these neurons were evaluated by expressing the current peak amplitude recorded in the presence of the peptide relatively to its initial value determined after washing‐out TTX with a toxin‐free solution for 8–10 min. The second type of neurons (24%, i.e., 5/21 neurons) had a mixed TTX‐S and TTX‐R current, which was decreased by the toxin to around 55% of initial peak amplitude values. The effects of CyrTx‐1a (from 5 to 20 μM) on these neurons were evaluated by expressing the current peak amplitude recorded in the presence of the peptide relatively to its initial value determined in the presence of 100 nM TTX. It is worth noting that relatively large neurons (of more than 25‐pF membrane capacitance) were patched, which explains the high percent of TTX‐S and low percent of TTX‐R cell recordings. However, the membrane capacitance of neurons having TTX‐S current was statistically smaller than that of neurons having TTX‐R current, that is, 24.9 ± 8.7 pF (n = 16) and 31.9 ± 7.9 pF (n = 5), respectively.
Exposing neurons to standard physiological solutions containing various CyrTx‐1a concentrations, using a fast solution application system, produced a decrease of sodium current amplitude (Figure 6d) which was dependent on peptide concentration and duration of exposure and on the current sensitivity to TTX. In particular, the concentration–response curves of CyrTx‐1a effects on the peak amplitude of TTX‐S and TTX‐R currents revealed IC50 values of 0.17 and 156 μM, respectively (Figure 6e). The peptide was thus approximately 920 times more efficient to inhibit TTX‐S than TTX‐R sodium currents of adult mouse DRG neurons. The blocking effects of CyrTx‐1a on the peak amplitude of TTX‐S current were stationary 5 and 1.5 min after the application of 0.02 and 1 μM of peptide, respectively. Those on the peak amplitude of the TTX‐R current were stationary 4 min after the application of 20 μM of peptide. The CyrTx‐1a effects on the TTX‐R current, not very noticeable, were not further studied, in contrast to those on the TTX‐S current.
The peak amplitude of TTX‐S current, which was 6 ± 2% of initial values after exposure to 1 μM CyrTx‐1a returned to 32 ± 5% of initial values (n = 5) by exposing neurons to a peptide‐free solution for 12–15 min, indicating that the effect of CyrTx‐1a was, at least, partly reversible. The analyses of activation and inactivation kinetics of TTX‐S sodium current in the absence and in the presence of 0.25 and 0.5 μM CyrTx‐1a showed that the peptide did not affect these kinetics, as the time to peak (tp) and the time constant of the current decay (τh) were not significantly modified (Table S4). Similarly, CyrTx‐1a (0.25–0.5 μM) did not produce any alteration of steady‐state inactivation‐ and conductance‐voltage relationships for neurons exhibiting TTX‐S current (Figure 6f and Table S3).
3.6. Effects of CyrTx‐1a on tactile and heat sensitivity of mice in vivo—Comparison with HwTx‐IV
Tactile and heat sensitivity testing in mice was performed by intraplantar injection of 102 nmol·kg−1 of CyrTx‐1a or 49 nmol·kg−1 of HwTx‐IV. A first attempt to evaluate the antinociceptive effect of the two toxins was made using an automated von Frey assay. Tactile sensitivity testing showed that the force intensity at which the mice, injected with 102 nmol·kg−1 of CyrTx‐1a or 49 nmol·kg−1 of HwTx‐IV, removed their hind limb in response to fibre pressure, that is, 8.6 ± 1.1 g (n = 8) and 8.5 ± 1.4 g (n = 8), respectively, had tendency (P < 0.17) to increase compared to animals injected with PBS, that is, 7.0 ± 1.7 g (n = 19; Figure 7a). However, these effects were not significant. Thus, the antinociceptive effect of the two toxins was further investigated using a hot‐plate assay. A significant increase in the treated‐mouse reaction time to heat, that is, the latency either to shake one of the two hind limbs or to jump, was observed compared to animals injected with PBS (Figure 7b). In particular, the reaction time was increased by 1.83 times for mice injected with CyrTx‐1a (17.2 ± 1.7 s, n = 13) and by 1.64 times for animals injected with HwTx‐IV (15.4 ± 1.9 s, n = 13), compared with animals injected with PBS (9.4 ± 0.6 s, n = 12). The injection itself did not have any effects as there was no difference in the reaction time or force intensity between mice injected with PBS and non‐injected animals (Figure 7).
Figure 7.
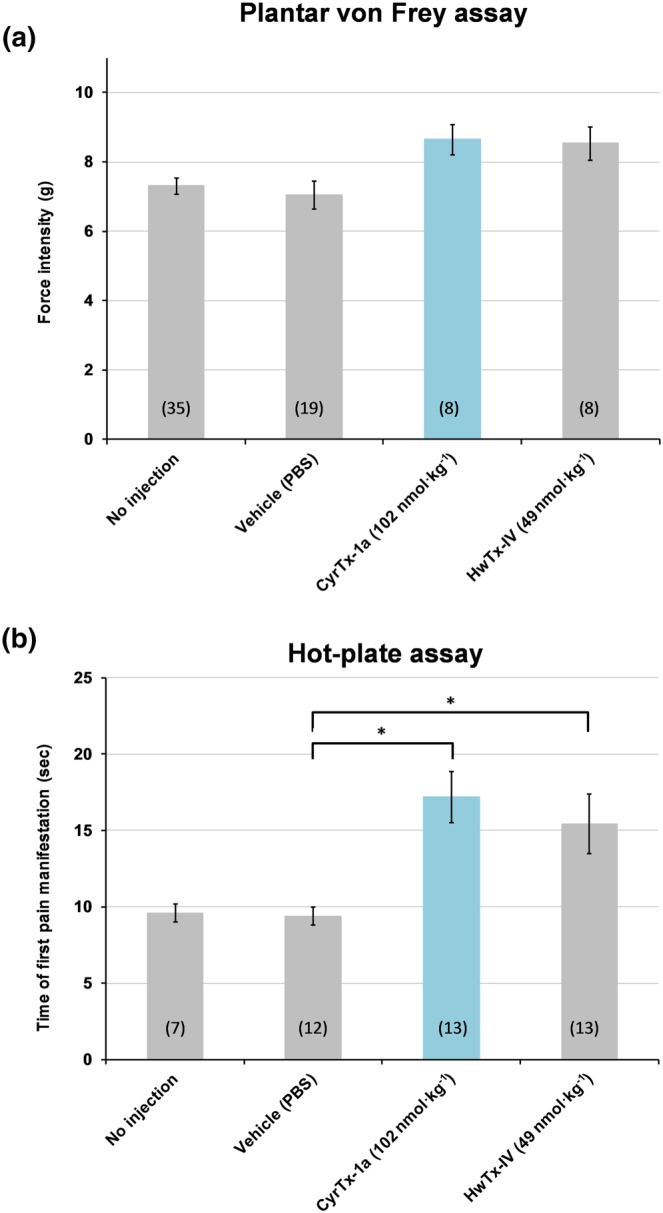
Effects of CyrTx‐1a on tactile and heat sensitivity of mice in vivo. (a) The tactile sensitivity of mice was assessed using an automated plantar von Frey apparatus, by determining the force intensity at which the animals removed their hind limb submitted to an increasing fibre pressure. The same mice were tested before (no injection) and 15 min after intraplantar injection in each hind limb of 5 μl of PBS, CyrTx‐1a (i.e., 102 nmol·kg−1) or HwTx‐IV (i.e., 49 nmol·kg−1). (b) The heat sensitivity of mice was assessed using a hot‐plate set at 55.0 ± 0.2°C, by determining the latency for the animals either to shake one of their two hind limbs or to jump. Three groups of mice were tested 60 min after intraplantar injection in each hind limb of 5 μl of PBS, CyrTx‐1a (i.e., 102 nmol·kg−1) or HwTx‐IV (i.e., 49 nmol·kg−1), while another group of animals was tested without any injection. (a) and (b) Means ± SD of data obtained from n mice (numbers in parentheses) under each condition. * P < 0.05, significantly different as indicated
3.7. Effects of CyrTx‐1a, compared to HwTx‐IV, on the mouse neuromuscular system in vivo
Online recordings revealed that the major effect of intramuscular injections of PBS solutions containing various concentrations of either CyrTx‐1a (from 0.3 to 448.7 nmol·kg−1 mouse) or HwTx‐IV (from 4.1 pmol·kg−1 to 41.4 nmol·kg−1 mouse) to anaesthetized mice was a marked decrease of CMAP amplitude. This is exemplified in Figure 8a for CMAP recordings performed before and between 10 and 15 min after injections of 29.4 nmol kg−1 of CyrTx‐1a and 41.4 nmol kg−1 of HwTx‐IV. The maximal CMAP amplitude measured 30 min after injections of PBS solution alone, and compared to values before injections, was not significantly affected, that is, 97.8 ± 2.3% (n = 8 mice), indicating that injections of the toxin vehicle had no effect on the maximal CMAP amplitude and that no marked run‐down of the response occurred.
Figure 8.
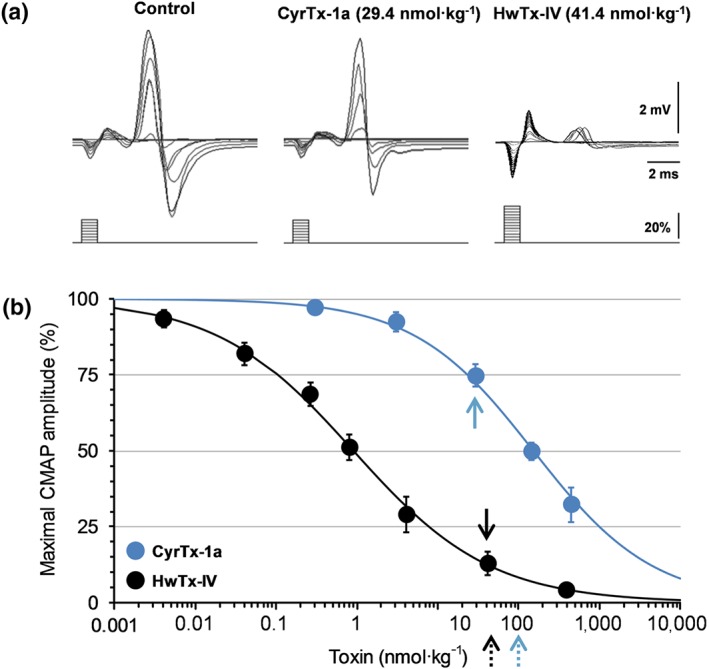
Effects of CyrTx‐1a and HwTx‐IV on the CMAP recorded in vivo from mouse tail muscle in response to caudal motor nerve stimulation. (a) Superimposed traces of CMAP following increasing intensities of stimulation (scheme), before (control), and after CyrTx‐1a (29.4 nmol·kg−1, full blue arrow in (b)) or HwTx‐IV (41.4 nmol·kg−1, full black arrow in (b)) injection. (b) Concentration–response curves of the effects of CyrTx‐1a and HwTx‐IV on the maximal CMAP amplitude. Each value, expressed as percentage of that obtained before injection, represents the mean ± SD of data obtained from four (CyrTx‐1a) and five (HwTx‐IV) mice. IC50 and n H values were, respectively, 152.3 nmol·kg−1 and 0.6 for CyrTx‐1a (r 2 = 0.992) and 0.9 nmol·kg−1 and 0.5 for HwTx‐IV (r 2 = 0.998). The dashed arrows indicate the toxin concentrations used for hot‐plate and von Frey assays
The toxin blocking effect was quantified by establishing the concentration–response curves and determining the IC50 values. As shown in Figure 8b, the concentration–response curves for CyrTx‐1a and HwTx‐IV revealed IC50 values of 152.3 and 0.9 nmol·kg−1 mouse, respectively. The five different excitability tests (stimulus–response, strength–duration, and current–threshold relationships, as well as threshold electrotonus and recovery cycle) were performed together before and 30 min after intramuscular injections of PBS solution containing CyrTx‐1a (448.7 nmol·kg−1 mouse), and the derived neuromuscular excitability parameters were determined (Figure S3 and Table S5). With the exception of decreased maximal CMAP amplitude and increased stimulus intensity required to generate a 50% maximal amplitude CMAP (stimulus–response relationship), analysis of strength–duration relationship, threshold electrotonus, current–threshold relationship, and recovery cycle did not reveal other CyrTx‐1a effects. The CyrTx‐1a‐induced effects were completely reversed within 12 hr after peptide injections.
3.8. In vivo toxicity of CyrTx‐1a, compared to HwTx‐IV, in mice
No deaths followed the intraplantar injection of 102 nmol kg−1 of CyrTx‐1a and 49 nmol kg−1 of HwTx‐IV in mice. In contrast, intramuscular injection of 144.4 nmol kg−1 of CyrTx‐1a (a concentration which produced an inhibition of ~50% of maximal CMAP amplitude), at the tail base, caused death of 50% (2/4) of animals within 1 hr. By comparison, a similar in vivo toxicity (60% of animals [3/5] died within 1 hr) was observed following injection of an approximatively 3.5 times lower HwTx‐IV concentration, that is, 41.4 nmol·kg−1. These results strongly suggest a lower in vivo toxicity of CyrTx‐1a, compared to HwTx‐IV, following intramuscular injections of toxins at the base of mouse tail.
4. DISCUSSION
This study was undertaken to identify a peptide with antinociceptive properties, among the Smartox venom collection. The strategy consisted of (a) a high throughput screening of 117 different venoms using automated patch‐clamp platforms on cells overexpressing the antinociceptive target hNaV1.7 and the cardiac hNaV1.5 channels, (b) the isolation and identification of a new peptide, CyrTx‐1a, from the C. schioedtei venom, (c) its structure characterization and chemical synthesis, and (d) the evaluation of the synthetic peptide functional properties using multiscale (from individual cell to in vivo) approaches.
With the identification of a large number of hits from our primary screening campaigns, a triage was performed based on potency, in both tonic‐ and use‐dependent current inhibition protocols. The use of venoms from different species (snakes, spiders, scorpions, wasps, bees, amphibians, lizards, and fishes) provided interesting information with regard to the target on which these libraries were screened on. The most striking observation was that spider venoms contain by far the greatest number of compounds active on hNaV1.7 channels with a hit rate (for the primary screening) that was seven times higher than scorpion venoms and up to 40‐fold higher than snake venoms. These data point to the impressive specialization of spider venoms for targeting NaV channels. Based on this screening procedure, we focused our attention on CyrTx‐1a which was identified in the screening and highlighted for progression in our flowchart for more extensive in vitro and in vivo investigation.
The isolation of CyrTx‐1a followed a two‐step purification procedure using a double in vitro‐guided assay (block of hNaV1.7 channels and inactivity on the cardiac safety‐compromising hNaV1.5 channels). Once the activity of the purified compound was confirmed by patch‐clamp, the toxin entered the phase of sequence deconvolution. Any doubts on Leu or Ile residues were solved by Edman sequencing. The peptide was then synthesized and properly folded according to mass determination and coelution properties, a sine qua non condition for in vitro characterization and in vivo evaluation.
Despite a high sequence identity with HnTx‐I (82%), CyrTx‐1a shares more pharmacological properties with less similar toxins, such as HwTx‐IV (42% identity) and GpTx‐1 (45% identity). This is likely to be mainly due to the presence of Asn23 instead of Ser23 in the HnTx‐I sequence that excludes any NaV1.7 channel activity (Klint, Chin, & Mobli, 2015), while a high potency for NaV1.7 channels associated with a good selectivity against NaV1.5 and NaV1.4 channels is due to the conservation of highly functional residues (Murray et al., 2015; Xiao et al., 2008). Indeed, CyrTx‐1a possesses several highly conserved and crucial amino acids, known to govern the NaV channel activity, such as the Phe5, Pro11, Leu20, Ser23, His26, and more importantly the Trp28 and Lys30 residues (Minassian et al., 2013; Murray et al., 2016; Shcherbatko et al., 2016). In addition, its sequence includes a hydrophobic patch (Gly4, Gly6, and Val31) that has been described to reinforce the inhibitory potency of ICK toxins at NaV1.7 channels (Agwa, Huang, Craik, Henriques, & Schroeder, 2017). Due to these similarities, CyrTx‐1a may share the same binding site on TTX‐sensitive NaV channels as the one determined by mutational analysis and in silico docking for HnTx‐IV, HwTx‐IV, and GpTx‐1a (Cai et al., 2015; Minassian et al., 2013; Murray et al., 2016). Indeed, positively charged amino acids Lys25, His26, Lys27, and Lys30, surrounded by hydrophobic Phe5 and Trp28 clustered on one toxin face may be involved in interactions with negatively charged Glu753, Glu811, Asp816, and Glu818 or aliphatic residues (Met750) located in S1–S2 and S3–S4 loops of DII domain of TTX‐sensitive NaV channels (Klint et al., 2014; Li et al., 2004; Liu et al., 2012; Xiao et al., 2008; Xiao, Blumenthal, Jackson, Liang, & Cummins, 2010).
The first step to evaluate CyrTx‐1a functional properties was to study the effects of the synthetic peptide on cells overexpressing hNaV1.1–1.8 channels, using patch‐clamp techniques. This study allowed (a) to test whether the potent blocking effect of the synthetic peptide, compared to the native molecule, was conserved on hNaV1.7 channels and (b) to reinforce the evaluation of its selectivity profile on the various NaV channels.
Synthetic (1 μM) and native (0.57 μM) CyrTx‐1a produced 89.1 ± 4.1% (n = 14 wells from seven plates) and 98.8 ± 0.7% (n = 6 wells from three plates) inhibition of hNaV1.7 channels, respectively, indicating that the two peptides were similarly, and highly, potent in interacting with this channel. The mean IC50 values of CyrTx‐1a interaction with hNaV1.7 channels, obtained from automated and manual patch‐clamp experiments, were 129.5 and 52.7 nM, respectively. From this point of view and compared to toxins belonging to the NaSpTx family 1 such as HnTx‐I, HnTx‐III, Hd1a, HnTx‐IV, ProTx‐III, Cm1a, GpTx‐1, and HwTx‐IV previously reported to interact with this subtype, CyrTx‐1a is thus among the most efficient peptides (Cardoso et al., 2015; Klint et al., 2014; Klint et al., 2015; Liu et al., 2012; Liu et al., 2013; Murray et al., 2015; Murray et al., 2016; Shcherbatko et al., 2016; Xiao et al., 2008). In addition to hNaV1.7 channels, CyrTx‐1a was also shown to be highly potent to block the TTX‐S hNaV1.1, 1.2, 1.3, and 1.6 channels with the following increasing order for mean IC50 values (between approximately 75 and 300 nM): hNaV1.1 ≈ hNaV1.2 ≈ hNaV1.6 ≈ hNaV1.7 > hNaV1.3. The recent discovery that NaV1.1 and 1.3 channels are involved in pain pathways (Cardoso & Lewis, 2018; Chen et al., 2014; Osteen et al., 2016) and that the NaV1.2 channel is only located in the CNS (de Lera Ruiz & Kraus, 2015) does not impair further development of CyrTx‐1a as a potential antinociceptive agent to access only peripheral NaV channel subtypes. In agreement, no central side effect was detected when the toxin was locally injected to mice for studying its action on neuromuscular system and on heat and tactile sensitivity, in vivo. In addition, the analgesic property of CyrTx‐1a seems not to be associated with inhibition of hCaV3.1 and hCaV3.2, two channels known to be involved in pain process (Choi, Yu, Hwang, & Llinas, 2016; Sekiguchi, Tsubota, & Kawabata, 2018). Moreover, the synthetic peptide has low, at best micromolar, affinities for hNaV1.5, hCaV1.2, hKV7.1, hKV11.1, and hKir2.1 channels, well‐known targets in cardiac safety (Crumb, Vicente, Johannesen, & Strauss, 2016). However, although fully sparing the skeletal muscle hNaV1.4 channel, CyrTx‐1a also targets the peripheral nerve hNaV1.6 channel. This may represent a limitation for the in vivo efficacy of the toxin, despite the fact that this channel has been reported to be is up‐regulated in various peripheral pain pathways (Gonçalves, Benoit, Partiseti, & Servent, 2018). The development of analogues with improved hNaV channel selectivity will thus be required.
The second step to evaluate CyrTx‐1a functional properties was to study the peptide effects on TTX‐S and TTX‐R sodium currents of adult mouse DRG neurons, including mainly the TTX‐S NaV1.1, 1.6, and 1.7 channels and the TTX‐R NaV1.8 and 1.9 channels (Rush, Cummins, & Waxman, 2007). This study was motivated by the well‐known physiological importance of DRG neurons in pain signalling. As expected, the preferential blocking effect of TTX‐S NaV channels by CyrTx‐1a was confirmed on mouse DRG neurons since the peptide was 920 times more efficient to inhibit the peak amplitude of TTX‐S than TTX‐R sodium currents recorded from these neurons. These results are consistent with previous observations on adult rodent DRG neurons showing that other potential antinociceptive toxins, such as HnTx‐IV, GpTx‐1, and HwTx‐IV, inhibit TTX‐S current without markedly affecting TTX‐R current (Liu et al., 2003; Murray et al., 2015; Peng, Shu, Liu, & Liang, 2002). Most of these peptides, including CyrTx‐1a, inhibit hNaV17 and/or TTX‐S currents without any significant modification of activation and inactivation kinetics and/or voltage dependence. From a general point of view, CyrTx‐1a was therefore more efficient to block TTX‐S than TTX‐R channels overexpressed in HEK‐293 cells, as well as TTX‐S than TTX‐R currents of DRG neurons. These results may suggest that the toxin interacted with the TTX receptor binding site, or the other way around, of the NaV channel protein. However, this hypothesis is not further supported taking into account that the affinity of CyrTx‐1a for the TTX‐S hNaV1.4 channel was relatively low (mean IC50 of ≈8 μM).
The third step to evaluate CyrTx‐1a functional properties, and to go deeper in the antinociceptive appraisal of the peptide, was to study its effects on heat and tactile sensitivity of mice in vivo, using hot‐plate and von Frey assays, respectively. Following intraplantar injections, the peptide (102 nmol·kg−1) was as efficient as HwTx‐IV (49 nmol·kg−1) in increasing the time to first pain manifestation of animals to nociceptive heat, while non‐significant change was detected in the force intensity at which the mice, injected with CyrTx‐1a, HwTx‐IV or PBS, removed their hind limb in response to fibre pressure. Similar results were obtained from mice lacking the NaV1.7 channels (global NaV1.7 knockout animals) which were reported to be insensitive to thermal pain while the tactile sensitivity measured with von Frey testing was unchanged (Gingras et al., 2014). These results highlight the more pronounced involvement of the NaV1.7 channels in heat than tactile sensitivity. The fact that CyrTx‐1a also targets the NaV1.6 channel with high affinity, a subtype located in motor axons innervating skeletal muscles (Caldwell, Schaller, Lasher, Peles, & Levinson, 2000), could limit the safe use of this peptide as an antinociceptive agent. Experiments were thus also conducted to test CyrTx‐1a effects on the mouse neuromuscular system in vivo. These effects mainly consisted of CMAP inhibition, as shown in the present work and previously reported for HwTx‐IV (Gonçalves, Boukaiba, et al., 2018). These results strongly suggest that the two peptides produce a marked decrease of the density of functional “transient” NaV channels. Besides these effects, CyrTx‐1a, as HwTx‐IV (Gonçalves, Boukaiba, et al., 2018), did not modify other excitability parameters, indicating that the peptide does not affect the density of other functional ion channels, receptors, and pumps, nor the passive membrane properties of the neuromuscular system (Kiernan & Bostock, 2000; Krishnan et al., 2008). CyrTx‐1a was approximately 170 times less efficient than HwTx‐IV to inhibit CMAP. Assuming that both toxins have also similar affinity on the mouse NaV1.6 channels, we infer that the accessibility to this subtype, located mainly at the nodes of Ranvier of motor myelinated axons (Caldwell et al., 2000), is somehow more limited for CyrTx‐1a than for HwTx‐IV.
In conclusion, the present results highlight that CyrTx‐1a purified from C. schioedtei spider venom is a new toxin interacting with hNaV1.7 channels associated with an antinociceptive effect. Further structure–activity relationships and engineering studies will be necessary to improve the NaV channel selectivity profile and analgesic potency of CyrTx‐1a. In particular, it is likely that synthetic modified homologues, associated with molecular dynamics simulation using CyrTx‐1a and NaV channels, will reinforce the potential use of the peptide as a lead molecule for the potential development of novel pain therapeutic agents.
AUTHOR CONTRIBUTIONS
L.L. performed experiments and J.M.C. analysed the data for the venom fraction screenings. S.C. and L.J. performed experiments and M.D.W. and R.Bé. analysed the data for the peptide purification, sequencing, and synthesis experiments. T.C.G., S.F., R.Bo., B.S., A.B., and M.P. performed and analysed experiments done on recombinant ion channel cell lines. T.C.G. and E.B. performed and analysed experiments done on DRG neurons and mice. M.K. and G.H. determined 3D structure. L.B. and S.H. aided in the preparation of the manuscript. T.C.G., E.B., M.P., M.D.W., and D.S. wrote the manuscript and supervised the study. All authors approved the manuscript.
CONFLICT OF INTEREST
The authors L.B., A.B., R.B., J.M.C., S.F., T.C.G., G.H., S.H., M.K., L.L., B.S., and M.P. declare the following competing interest: current or former employees of Sanofi.
DECLARATION OF TRANSPARENCY AND SCIENTIFIC RIGOUR
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research as stated in the BJP guidelines for Design & Analysis, and Animal Experimentation, and as recommended by funding agencies, publishers and other organisations engaged with supporting research.
Supporting information
Data S1
Tables and additional figures illustrating the fragments sequenced by LC‐MS/MS analyses of CyrTx‐1a digests, chemical synthesis, refolding and 1H‐chemical shifts of CyrTx‐1a, detailed in vitro and in vivo electrophysiological parameters, and a supplementary Experimental Section. This material is available free of charge via the Internet at http://pubs.acs.org.
Table S1. Fragments sequenced by LC‐MS/MS analyses of the CyrTx‐1a digests. Symbol *indicates C‐terminal amidation, and all cysteine residues were detected as carbamidomethyl derivates with the addition of 57 Da.
Table S2. 1H‐chemical shifts of CyrTx‐1a in H2O/D2O, 50 mM phosphate buffer, pH 5.0 at 305°K (concentration: 6 mg/mL)*.
Table S3. Parameters of steady‐state inactivation‐ (VP50% and kh) and conductance‐ (VT50% and kg) voltage relationships for HEK‐293 cells overexpressing the hNaV1.7 channel subtype (means ± S.D. of 5 cells) and mouse DRG neurons displaying endogenous TTX‐S sodium current (means ± S.D. of 8 neurons).
Table S4. Kinetic parameters of activation (tp) and inactivation (τh) of endogenous TTX‐S sodium current recorded from DRG neurons under the indicated conditions (means ± S.D. of 8 cells).
Table S5. Comparison of neuromuscular excitability parameters (means ± S.D.) from mouse tail muscle recordings before toxin injections (control, n= 29 mice) and ~30 min after injections of CyrTx‐1a (448.7 nmol/kg mouse, n = 4 mice).
Figure S1. Chemical synthesis and refolding of CyrTx‐1a. (a) Crude CyrTx‐1a synthesis as revealed by a C18 reversed‐phase chromatography. (b) Crude folded/oxidized CyrTx‐1a. (c) Purified folded/oxidized CyrTx‐1a illustrating the purity of the synthetic compound. Inset: illustrates the MS of synthetic CyrTx‐1a of m/z 1193.8556 [M+3H]3+. (d) C18 coelution profile of natural CyrTx‐1a (4 μg) mixed with synthetic CyrTx‐1a (4 μg). The presence of a single uniform peak demonstrates the identity of both compounds. The two contaminating peaks preceding the major peak represent contaminants from the natural peptide.
Figure S2. Effects of CyrTx‐1a on HEK‐293, CHO and U2OS cells overexpressing hCaV1.2, 3.1 and 3.2, hKV7.1 and 11.1 and hKir2.1 channel subtypes, using whole‐cell automated patch‐clamp. Histograms of unblocked current, expressed as percentage of control. Each value represents the mean ± S.D. of data obtained from n cells (numbers in parentheses). *: P<0.05 versus control.
Figure S3. Superimposed excitability curves obtained in vivo by stimulating the mouse caudal motor nerve and recording the CMAP from tail muscle before (black circles, n = 29 mice) and ~30 min after injections of CyrTx‐1a (448.7 nmol/kg mouse, blue circles, n = 4 mice). Data are represented as means ± S.D. (a) stimulus‐response relationships [absolute (a1) and relative (a2) CMAP amplitudes], (b) strength‐duration relationship, (c) threshold electrotonus, (d) currentthreshold relationship, and (e) recovery cycle. In a1, arrows indicate stimulus currents for 50% maximal response.
ACKNOWLEDGEMENTS
This research was funded by a collaborative grant (#153114) between Sanofi Research & Development (Chilly‐Mazarin, France) and the French Alternative Energies and Atomic Energy Commission (CEA, Gif‐sur‐Yvette, France). M.D.W. acknowledges financial support from the French Agence Nationale de la Recherche (Grant ANR‐11‐LABX‐0015). T.C.G. was supported by a doctoral CIFRE fellowship from Sanofi. The authors wish to thank Dr. Muriel AMAR (CEA de Saclay, Gif‐sur‐Yvette, France) for her help in the calculation of kinetic parameters of sodium current activation and inactivation and Dr. Isabel LEFEVRE (Sanofi R&D, Chilly‐Mazarin, France) for her critical reading of the manuscript.
Gonçalves TC, Benoit E, Kurz M, et al. From identification to functional characterization of cyriotoxin‐1a, an antinociceptive toxin from the spider Cyriopagopus schioedtei . Br J Pharmacol. 2019;176:1298–1314. 10.1111/bph.14628
Contributor Information
Denis Servent, Email: denis.servent@cea.fr.
Michel Partiseti, Email: michel.partiseti@sanofi.com.
REFERENCES
- Agwa, A. J. , Huang, Y. H. , Craik, D. J. , Henriques, S. T. , & Schroeder, C. I. (2017). Lengths of the C‐terminus and interconnecting loops impact stability of spider‐derived gating modifier toxins. Toxins, 9, 248–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. , Striessnig, J. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , … CGTP Collaborators (2017). The concise guide to pharmacology 2017/18: Voltage‐gated ion channels. British Journal of Pharmacology, 174, S160–S194. 10.1111/bph.13884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett, D. L. , & Woods, C. G. (2014). Painful and painless channelopathies. Lancet Neurology, 13, 587–599. 10.1016/S1474-4422(14)70024-9 [DOI] [PubMed] [Google Scholar]
- Cai, T. , Luo, J. , Meng, E. , Ding, J. , Liang, S. , Wang, S. , & Liu, Z. (2015). Mapping the interaction site for the tarantula toxin hainantoxin‐IV (β‐TRTX‐Hn2a) in the voltage sensor module of domain II of voltage‐gated sodium channels. Peptides, 68, 148–156. 10.1016/j.peptides.2014.09.005 [DOI] [PubMed] [Google Scholar]
- Caldwell, J. H. , Schaller, K. L. , Lasher, R. S. , Peles, E. , & Levinson, S. R. (2000). Sodium channel Na(v)1.6 is localized at nodes of ranvier, dendrites, and synapses. Proceedings of the National Academy of Sciences of the United States of America, 97, 5616–5620. 10.1073/pnas.090034797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capasso, A. , Di Giannuario, A. , Loizzo, A. , Pieretti, S. , & Sorrentino, L. (1994). Dexamethasone influence on morphine‐induced analgesia in outbred Swiss and inbred DBA/2J and C57BL/6 mice. Progress in Neuro‐Psychopharmacology & Biological Psychiatry, 18, 779–792. 10.1016/0278-5846(94)90084-1 [DOI] [PubMed] [Google Scholar]
- Cardoso, F. C. , Dekan, Z. , Rosengren, K. J. , Erickson, A. , Vetter, I. , Deuis, J. R. , … Lewis, R. J. (2015). Identification and characterization of ProTx‐III [mu‐TRTX‐Tp1a], a new voltage‐gated sodium channel inhibitor from venom of the tarantula Thrixopelma pruriens. Molecular Pharmacology, 88, 291–303. 10.1124/mol.115.098178 [DOI] [PubMed] [Google Scholar]
- Cardoso, F. C. , Dekan, Z. , Smith, J. J. , Deuis, J. R. , Vetter, I. , Herzig, V. , … Lewis, R. J. (2017). Modulatory features of the novel spider toxin mu‐TRTX‐Df1a isolated from the venom of the spider Davus fasciatus. British Journal of Pharmacology, 174, 2528–2544. 10.1111/bph.13865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso, F. C. , & Lewis, R. J. (2018). Sodium channels and pain: From toxins to therapies. British Journal of Pharmacology, 175, 2138–2157. 10.1111/bph.13962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall, W. A. , Goldin, A. L. , & Waxman, S. G. (2005). International Union of Pharmacology. XLVII. Nomenclature and structure‐function relationships of voltage‐gated sodium channels. Pharmacological Reviews, 57, 397–409. 10.1124/pr.57.4.4 [DOI] [PubMed] [Google Scholar]
- Cerles, O. , Benoit, E. , Chereau, C. , Chouzenoux, S. , Morin, F. , Guillaumot, M. A. , … Nicco, C. (2017). Niclosamide inhibits oxaliplatin neurotoxicity while improving colorectal cancer therapeutic response. Molecular Cancer Therapeutics, 16, 300–311. 10.1158/1535-7163.MCT-16-0326 [DOI] [PubMed] [Google Scholar]
- Chen, H. P. , Zhou, W. , Kang, L. M. , Yan, H. , Zhang, L. , Xu, B. H. , & Cai, W. H. (2014). Intrathecal miR‐96 inhibits NaV1.3 expression and alleviates neuropathic pain in rat following chronic construction injury. Neurochemical Research, 39, 76–83. 10.1007/s11064-013-1192-z [DOI] [PubMed] [Google Scholar]
- Choi, S. , Yu, E. , Hwang, E. , & Llinas, R. R. (2016). Pathophysiological implication of CaV3.1 T‐type Ca2+ channels in trigeminal neuropathic pain. Proceedings of the National Academy of Sciences of the United States of America, 113, 2270–2275. 10.1073/pnas.1600418113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crumb, W. J. Jr. , Vicente, J. , Johannesen, L. , & Strauss, D. G. (2016). An evaluation of 30 clinical drugs against the comprehensive in vitro proarrhythmia assay (CiPA) proposed ion channel panel. Journal Of Pharmacological And Toxicological Methods, 81, 251–262. 10.1016/j.vascn.2016.03.009 [DOI] [PubMed] [Google Scholar]
- Deuis, J. R. , Wingerd, J. S. , Winter, Z. , Durek, T. , Dekan, Z. , Sousa, S. R. , … Vetter, I. (2016). Analgesic effects of GpTx‐1, PF‐04856264 and CNV1014802 in a mouse model of NaV1.7‐mediated pain. Toxins, 8, 78–87. 10.3390/toxins8030078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dib‐Hajj, S. D. , Yang, Y. , Black, J. A. , & Waxman, S. G. (2013). The NaV1.7 sodium channel: From molecule to man. Nature Reviews. Neuroscience, 14, 49–62. 10.1038/nrn3404 [DOI] [PubMed] [Google Scholar]
- Flinspach, M. , Xu, Q. , Piekarz, A. D. , Fellows, R. , Hagan, R. , Gibbs, A. , … Wickenden, A. D. (2017). Insensitivity to pain induced by a potent selective closed‐state NaV1.7 inhibitor. Scientific Reports, 7, 39662 10.1038/srep39662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingras, J. , Smith, S. , Matson, D. J. , Johnson, D. , Nye, K. , Couture, L. , … McDonough, S. I. (2014). Global NaV1.7 knockout mice recapitulate the phenotype of human congenital indifference to pain. PLoS ONE, 9, e105895 10.1371/journal.pone.0105895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves, T. C. , Benoit, E. , Partiseti, M. , & Servent, D. (2018). The NaV1.7 channel subtype as an antinociceptive target for spider toxins in adult dorsal root ganglia neurons. Frontiers in Pharmacology, 9, 1000 10.3389/fphar.2018.01000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves, T. C. , Boukaiba, R. , Molgo, J. , Amar, M. , Partiseti, M. , Servent, D. , & Benoit, E. (2018). Direct evidence for high affinity blockade of NaV1.6 channel subtype by huwentoxin‐IV spider peptide, using multiscale functional approaches. Neuropharmacology, 133, 404–414. 10.1016/j.neuropharm.2018.02.016 [DOI] [PubMed] [Google Scholar]
- Hagen, N. A. , Cantin, L. , Constant, J. , Haller, T. , Blaise, G. , Ong‐Lam, M. , … Lapointe, B. (2017). Tetrodotoxin for moderate to severe cancer‐related pain: A multicentre, randomized, double‐blind, placebo‐controlled, parallel‐design trial. Pain Research & Management, 2017(7212713), 1–7. 10.1155/2017/7212713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR (2018). The IUPHAR/BPS Guide to pharmacology in 2018: Updates and expansion to encompass the new guide to immunopharmacology. Nucleic Acids Research, 46, D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel, M. R. , Tay, B. , Deuis, J. R. , & Vetter, I. (2017). Sodium channels and venom peptide pharmacology. Advances in Pharmacology, 79, 67–116. 10.1016/bs.apha.2017.01.004 [DOI] [PubMed] [Google Scholar]
- Kiernan, M. C. , & Bostock, H. (2000). Effects of membrane polarization and ischaemia on the excitability properties of human motor axons. Brain, 123, 2542–2551. 10.1093/brain/123.12.2542 [DOI] [PubMed] [Google Scholar]
- Kilkenny, C. , Browne, W. , Cuthill, I. C. , Emerson, M. , & Altman, D. G. (2010). Animal research: Reporting in vivo experiments: The ARRIVE guidelines. British Journal of Pharmacology, 160, 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klint, J. K. , Berecki, G. , Durek, T. , Mobli, M. , Knapp, O. , King, G. F. , … Rash, L. D. (2014). Isolation, synthesis and characterization of omega‐TRTX‐Cc1a, a novel tarantula venom peptide that selectively targets L‐type Cav channels. Biochemical Pharmacology, 89, 276–286. 10.1016/j.bcp.2014.02.008 [DOI] [PubMed] [Google Scholar]
- Klint, J. K. , Chin, Y. K. Y. , & Mobli, M. (2015). Rational engineering defines a molecular switch that is essential for activity of spider‐venom peptides against the analgesics target NaV1.7. Molecular Pharmacology, 88, 1002–1010. 10.1124/mol.115.100784 [DOI] [PubMed] [Google Scholar]
- Klint, J. K. , Senff, S. , Rupasinghe, D. B. , Er, S. Y. , Herzig, V. , Nicholson, G. M. , & King, G. F. (2012). Spider‐venom peptides that target voltage‐gated sodium channels: pharmacological tools and potential therapeutic leads. Toxicon, 60, 478–491. [DOI] [PubMed] [Google Scholar]
- Krishnan, A. V. , Lin, C. S. Y. , Park, S. B. , & Kiernan, M. C. (2008). Assessment of nerve excitability in toxic and metabolic neuropathies. Journal of the Peripheral Nervous System, 13, 7–26. 10.1111/j.1529-8027.2008.00155.x [DOI] [PubMed] [Google Scholar]
- de Lera Ruiz, M. , & Kraus, R. L. (2015). Voltage‐gated sodium channels: Structure, function, pharmacology, and clinical indications. Journal of Medicinal Chemistry, 58, 7093–7118. 10.1021/jm501981g [DOI] [PubMed] [Google Scholar]
- Li, D. , Xiao, Y. , Xu, X. , Xiong, X. , Lu, S. , Liu, Z. , … Liang, S. (2004). Structure–activity relationships of hainantoxin‐IV and structure determination of active and inactive sodium channel blockers. The Journal of Biological Chemistry, 279, 37734–37740. 10.1074/jbc.M405765200 [DOI] [PubMed] [Google Scholar]
- Liu, Y. , Li, D. , Wu, Z. , Li, J. , Nie, D. , Xiang, Y. , & Liu, Z. (2012). A positively charged surface patch is important for hainantoxin‐IV binding to voltage‐gated sodium channels. Journal of Peptide Science, 18, 643–649. 10.1002/psc.2451 [DOI] [PubMed] [Google Scholar]
- Liu, Z. , Cai, T. , Zhu, Q. , Deng, M. , Li, J. , Zhou, X. , … Liang, S. (2013). Structure and function of hainantoxin‐III, a selective antagonist of neuronal tetrodotoxin‐sensitive voltage‐gated sodium channels isolated from the Chinese bird spider Ornithoctonus hainana. The Journal of Biological Chemistry, 288, 20392–20403. 10.1074/jbc.M112.426627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Z. , Dai, J. , Chen, Z. , Hu, W. , Xiao, Y. , & Liang, S. (2003). Isolation and characterization of hainantoxin‐IV, a novel antagonist of tetrodotoxin‐sensitive sodium channels from the Chinese bird spider Selenocosmia hainana. Cellular and Molecular Life Sciences, 60, 972–978. 10.1007/s00018-003-2354-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minassian, N. A. , Gibbs, A. , Shih, A. Y. , Liu, Y. , Neff, R. A. , Sutton, S. W. , … Wickenden, A. D. (2013). Analysis of the structural and molecular basis of voltage‐sensitive sodium channel inhibition by the spider toxin huwentoxin‐IV (mu‐TRTX‐Hh2a). The Journal of Biological Chemistry, 288, 22707–22720. 10.1074/jbc.M113.461392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogil, J. S. (2012). Sex differences in pain and pain inhibition: Multiple explanations of a controversial phenomenon. Nature Reviews. Neuroscience, 13, 859–866. 10.1038/nrn3360 [DOI] [PubMed] [Google Scholar]
- Moyer, B. D. , Murray, J. K. , Ligutti, J. , Andrews, K. , Favreau, P. , Jordan, J. B. , … Miranda, L. P. (2018). Pharmacological characterization of potent and selective NaV1.7 inhibitors engineered from Chilobrachys jingzhao tarantula venom peptide JzTx‐V. PLoS ONE, 13, e0196791 10.1371/journal.pone.0196791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray, J. K. , Ligutti, J. , Liu, D. , Zou, A. , Poppe, L. , Li, H. , … Miranda, L. P. (2015). Engineering potent and selective analogues of GpTx‐1, a tarantula venom peptide antagonist of the NaV1.7 sodium channel. Journal of Medicinal Chemistry, 58, 2299–2314. 10.1021/jm501765v [DOI] [PubMed] [Google Scholar]
- Murray, J. K. , Long, J. , Zou, A. , Ligutti, J. , Andrews, K. L. , Poppe, L. , … Miranda, L. P. (2016). Single residue substitutions that confer voltage‐gated sodium ion channel subtype selectivity in the NaV1.7 inhibitory peptide GpTx‐1. Journal of Medicinal Chemistry, 59, 2704–2717. 10.1021/acs.jmedchem.5b01947 [DOI] [PubMed] [Google Scholar]
- Osteen, J. D. , Herzig, V. , Gilchrist, J. , Emrick, J. J. , Zhang, C. , Wang, X. , … Julius, D. (2016). Selective spider toxins reveal a role for the NaV1.1 channel in mechanical pain. Nature, 534, 494–499. 10.1038/nature17976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, K. , Shu, Q. , Liu, Z. , & Liang, S. (2002). Function and solution structure of huwentoxin‐IV, a potent neuronal tetrodotoxin (TTX)‐sensitive sodium channel antagonist from Chinese bird spider Selenocosmia huwena. The Journal of Biological Chemistry, 277, 47564–47571. 10.1074/jbc.M204063200 [DOI] [PubMed] [Google Scholar]
- Rush, A. M. , Cummins, T. R. , & Waxman, S. G. (2007). Multiple sodium channels and their roles in electrogenesis within dorsal root ganglion neurons. The Journal of Physiology, 579, 1–14. 10.1113/jphysiol.2006.121483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saez, N. J. , Senff, S. , Jensen, J. E. , Er, S. Y. , Herzig, V. , Rash, L. D. , & King, G. F. (2010). Spider‐venom peptides as therapeutics. Toxins, 2, 2851–2871. 10.3390/toxins2122851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiguchi, F. , Tsubota, M. , & Kawabata, A. (2018). Involvement of voltage‐gated calcium channels in inflammation and inflammatory pain. Biological & Pharmaceutical Bulletin, 41, 1127–1134. 10.1248/bpb.b18-00054 [DOI] [PubMed] [Google Scholar]
- Shcherbatko, A. , Rossi, A. , Foletti, D. , Zhu, G. , Bogin, O. , Galindo Casas, M. , … Strop, P. (2016). Engineering highly potent and selective microproteins against NaV1.7 sodium channel for treatment of pain. The Journal of Biological Chemistry, 291, 13974–13986. 10.1074/jbc.M116.725978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter, I. , Deuis, J. R. , Mueller, A. , Israel, M. R. , Starobova, H. , Zhang, A. , … Mobli, M. (2017). NaV1.7 as a pain target—From gene to pharmacology. Pharmacology & Therapeutics, 172, 73–100. 10.1016/j.pharmthera.2016.11.015 [DOI] [PubMed] [Google Scholar]
- Waxman, S. G. , & Zamponi, G. W. (2014). Regulating excitability of peripheral afferents: Emerging ion channel targets. Nature Neuroscience, 17, 153–163. 10.1038/nn.3602 [DOI] [PubMed] [Google Scholar]
- Xiao, Y. , Bingham, J. P. , Zhu, W. , Moczydlowski, E. , Liang, S. , & Cummins, T. R. (2008). Tarantula huwentoxin‐IV inhibits neuronal sodium channels by binding to receptor site 4 and trapping the domain II voltage sensor in the closed configuration. The Journal of Biological Chemistry, 283, 27300–27313. 10.1074/jbc.M708447200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao, Y. , Blumenthal, K. , Jackson, J. O. 2nd , Liang, S. , & Cummins, T. R. (2010). The tarantula toxins ProTx‐II and huwentoxin‐IV differentially interact with human NaV1.7 voltage sensors to inhibit channel activation and inactivation. Molecular Pharmacology, 78, 1124–1134. 10.1124/mol.110.066332 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Tables and additional figures illustrating the fragments sequenced by LC‐MS/MS analyses of CyrTx‐1a digests, chemical synthesis, refolding and 1H‐chemical shifts of CyrTx‐1a, detailed in vitro and in vivo electrophysiological parameters, and a supplementary Experimental Section. This material is available free of charge via the Internet at http://pubs.acs.org.
Table S1. Fragments sequenced by LC‐MS/MS analyses of the CyrTx‐1a digests. Symbol *indicates C‐terminal amidation, and all cysteine residues were detected as carbamidomethyl derivates with the addition of 57 Da.
Table S2. 1H‐chemical shifts of CyrTx‐1a in H2O/D2O, 50 mM phosphate buffer, pH 5.0 at 305°K (concentration: 6 mg/mL)*.
Table S3. Parameters of steady‐state inactivation‐ (VP50% and kh) and conductance‐ (VT50% and kg) voltage relationships for HEK‐293 cells overexpressing the hNaV1.7 channel subtype (means ± S.D. of 5 cells) and mouse DRG neurons displaying endogenous TTX‐S sodium current (means ± S.D. of 8 neurons).
Table S4. Kinetic parameters of activation (tp) and inactivation (τh) of endogenous TTX‐S sodium current recorded from DRG neurons under the indicated conditions (means ± S.D. of 8 cells).
Table S5. Comparison of neuromuscular excitability parameters (means ± S.D.) from mouse tail muscle recordings before toxin injections (control, n= 29 mice) and ~30 min after injections of CyrTx‐1a (448.7 nmol/kg mouse, n = 4 mice).
Figure S1. Chemical synthesis and refolding of CyrTx‐1a. (a) Crude CyrTx‐1a synthesis as revealed by a C18 reversed‐phase chromatography. (b) Crude folded/oxidized CyrTx‐1a. (c) Purified folded/oxidized CyrTx‐1a illustrating the purity of the synthetic compound. Inset: illustrates the MS of synthetic CyrTx‐1a of m/z 1193.8556 [M+3H]3+. (d) C18 coelution profile of natural CyrTx‐1a (4 μg) mixed with synthetic CyrTx‐1a (4 μg). The presence of a single uniform peak demonstrates the identity of both compounds. The two contaminating peaks preceding the major peak represent contaminants from the natural peptide.
Figure S2. Effects of CyrTx‐1a on HEK‐293, CHO and U2OS cells overexpressing hCaV1.2, 3.1 and 3.2, hKV7.1 and 11.1 and hKir2.1 channel subtypes, using whole‐cell automated patch‐clamp. Histograms of unblocked current, expressed as percentage of control. Each value represents the mean ± S.D. of data obtained from n cells (numbers in parentheses). *: P<0.05 versus control.
Figure S3. Superimposed excitability curves obtained in vivo by stimulating the mouse caudal motor nerve and recording the CMAP from tail muscle before (black circles, n = 29 mice) and ~30 min after injections of CyrTx‐1a (448.7 nmol/kg mouse, blue circles, n = 4 mice). Data are represented as means ± S.D. (a) stimulus‐response relationships [absolute (a1) and relative (a2) CMAP amplitudes], (b) strength‐duration relationship, (c) threshold electrotonus, (d) currentthreshold relationship, and (e) recovery cycle. In a1, arrows indicate stimulus currents for 50% maximal response.


