Abstract
Background and Purpose
Endocannabinoids are critically involved in brain reward functions, mediated by activation of CB1 receptors, reflecting their high density in the brain. However, the recent discovery of CB2 receptors in the brain, particularly in the midbrain dopamine neurons, has challenged this view and inspired us to re‐examine the roles of both CB1 and CB2 receptors in the effects of cannabis.
Experimental Approach
In the present study, we used the electrical intracranial self‐stimulation paradigm to evaluate the effects of various cannabinoid drugs on brain reward in laboratory rats and the roles of CB1 and CB2 receptors activation in brain reward function(s).
Key Results
Two mixed CB1 / CB2 receptor agonists, Δ9‐tetrahydrocannabinol (Δ9‐THC) and WIN55,212‐2, produced biphasic effects—mild enhancement of brain‐stimulation reward (BSR) at low doses but inhibition at higher doses. Pretreatment with a CB1 receptor antagonist (AM251) attenuated the low dose‐enhanced BSR, while a CB2 receptor antagonist (AM630) attenuated high dose‐inhibited BSR. To confirm these opposing effects, rats were treated with selective CB1 and CB2 receptor agonists. These compounds produced significant BSR enhancement and inhibition, respectively.
Conclusions and Implications
CB1 receptor activation produced reinforcing effects, whereas CB2 receptor activation was aversive. The subjective effects of cannabis depend on the balance of these opposing effects. These findings not only explain previous conflicting results in animal models of addiction but also explain why cannabis can be either rewarding or aversive in humans, as expression of CB1 and CB2 receptors may differ in the brains of different subjects.
Abbreviations
- ACEA
arachidonyl‐2′‐chloroethylamide
- BSR
brain‐stimulation reward
- ICSS
intracranial self‐stimulation
- JWH 133
(6aR,10aR)‐3‐(1,1‐Dimethylbutyl)‐6a,7,10,10a‐tetrahydro‐6,6,9‐trimethyl‐6H‐dibenzo[b,d]pyran
- NAc
nucleus accumbens
- WIN55,212‐2
(R)‐(+)‐[2,3‐Dihydro‐5‐methyl‐3‐(4‐morpholinylmethyl)pyrrolo[1,2,3‐de]‐1,4‐benzoxazin‐6‐yl]‐1‐naphthalenylmethanone
- Δ9‐THC
Δ9‐tetrahydrocannabinol
What is already known
Cannabis can produce both positive and negative effects in different subjects or at different times.
The neurobehavioural effects of cannabinoids are generally thought to be mediated by activation of CB1 receptors.
What this study adds
In rats, CB1 receptor activation produces reinforcing effects, whereas CB2 receptor activation is aversive.
These differential effects may explain the previous conflicting results of Δ9‐THC treatment in animals.
What is the clinical significance
The subjective effects of cannabis may depend on the balance of opposing CB1 and CB2 receptor effects.
1. INTRODUCTION
Marijuana or cannabis has now been legalized in many states of the United States, although it is still unclear whether cannabis is entirely safe (Schulden, Thomas, & Compton, 2009). In humans, cannabis produces “paradoxical” effects that are often diametrically opposed. For instance, cannabis is well known for its ability to produce euphoria, pleasure, and relaxation (Fattore, Fadda, Spano, Pistis, & Fratta, 2008; Maldonado, Valverde, & Berrendero, 2006; Parsons & Hurd, 2015). However, not all users enjoy cannabis, and some experience dysphoria, anxiety, and depression after its use (D'Souza et al., 2004; Raft, Gregg, Ghia, & Harris, 1977). Even in the same person, cannabis may produce positive effects at one time but negative effects at another (Farris, Zvolensky, Boden, & Bonn‐Miller, 2014; Gregg, Small, Moore, Raft, & Toomey, 1976). Similar paradoxical effects of Δ9‐tetrahydrocannabinol (Δ9‐THC, the major psychoactive component of cannabis; Gaoni & Mechoulam, 1971) have been found in non‐human primates. Specifically, Δ9‐THC is self‐administered by squirrel monkeys (Justinova, Tanda, Redhi, & Goldberg, 2003; Tanda, Munzar, & Goldberg, 2000), suggesting that it has rewarding effects, but it is not self‐administered in rhesus monkeys (John, Martin, & Nader, 2017; Mansbach, Nicholson, Martin, & Balster, 1994). In rodents (laboratory rats and mice), Δ9‐THC or other cannabinoid compounds can be rewarding, ineffective or aversive (Panagis, Vlachou, & Nomikos, 2008; Vlachou & Panagis, 2014). For example, Δ9‐THC has been reported to facilitate electrical intracranial brain‐stimulation reward (BSR; Gardner et al., 1988; Katsidoni, Kastellakis, & Panagis, 2013; Lepore, Liu, Savage, Matalon, & Gardner, 1996), while other groups and/or studies found depression of BSR (Kwilasz & Negus, 2012; Negus & Miller, 2014; Vlachou, Nomikos, Stephens, & Panagis, 2007; Wiebelhaus et al., 2015). Conflicting findings have also been reported in studies using conditioned place preference and intravenous self‐administration (Panagis, Vlachou, & Nomikos, 2008; Vlachou & Panagis, 2014). The neurobiological mechanisms underlying such paradoxical effects are poorly understood.
With the identification of cannabinoid CB1 and CB2 receptors as the major targets of cannabinoids (Matsuda, Lolait, Brownstein, Young, & Bonner, 1990; Munro, Thomas, & Abu‐Shaar, 1993) and the finding that CB1 receptors are highly expressed in the CNS and CB2 receptors are expressed predominantly in peripheral tissues, it has generally been thought that the neurobehavioural and psychotropic effects of cannabinoids are mediated by activation of CB 1 receptors not CB 2 receptors (Mackie, 2005). This hypothesis is supported by electrophysiological and neurochemical evidence demonstrating that activation of CB1 receptors on GABAergic neurons may increase midbrain dopaminergic neuron activity in the ventral tegmental area (VTA) by dopamine neuron disinhibition (Lupica & Riegel, 2005; Szabo, Siemes, & Wallmichrath, 2002) and that Δ9‐THC increases dopamine release in the nucleus accumbens (NAc) as assessed by in vivo microdialysis in rats (Chen, Paredes, Lowinson, & Gardner, 1991; Tanda, Pontieri, & Di Chiara, 1997; although cf. Castaneda, Moss, Oddie, & Whishaw, 1991). However, there is no direct behavioural evidence in vivo demonstrating whether a CB1 receptor‐dependent mechanism underlies cannabis reward. Moreover, we have recently reported that activation of CB1 receptors in glutamatergic neurons by Δ9‐THC produces aversive effects (Han et al., 2017).
In addition to CB1 receptors, growing evidence indicates that CB2 receptors are also expressed in the brain although the level is much lower than CB1 receptors in healthy subjects (Onaivi et al., 2006, 2008). Immunohistochemistry and in situ hybridization assays detect CB2 receptor‐immunostaining or CB2 receptor mRNA in various brain regions (Aracil‐Fernandez et al., 2012; Ashton, Friberg, Darlington, & Smith, 2006; Baek, Zheng, Darlington, & Smith, 2008; Brusco, Tagliaferro, Saez, & Onaivi, 2008; Gong et al., 2006; Liu et al., 2009, 2017; Schmidt, Schafer, Striggow, Frohlich, & Striggow, 2012; Stempel et al., 2016; Van Sickle et al., 2005; Zhang et al., 2019). Notably, CB2 receptors were recently identified in VTA dopaminergic neurons (Zhang et al., 2014, 2017, 2019) and dopaminergic terminals in the NAc (Foster et al., 2016), two critical brain regions involved in drug reward and addiction. Activation of CB2 receptors in both brain regions inhibits VTA dopaminergic neuron activity and NAc dopamine release (Xi et al., 2011; Zhang et al., 2014, 2017). In addition, overexpression of brain CB2 receptors inhibits cocaine self‐administration and cocaine‐enhanced locomotion in mice (Aracil‐Fernandez et al., 2012). These findings suggest that CB2 receptors may also be involved in cannabis reward or aversion.
In the present study, we used the electrical intracranial self‐stimulation (ICSS) paradigm to evaluate the effects of various cannabinoid ligands on ICSS and explored the roles of CB1 and CB2 receptors in these actions. Specifically, we used a wide range of doses of Δ9‐THC, as well as the synthetic mixed CB1/CB2 receptor agonist WIN55,212‐2 and then extended the findings using individual selective CB1 and CB2 receptor agonists, as well as pretreatment with selective antagonists.
2. METHODS
Animals
All animal care and experimental procedures outlined in the animal research protocol were approved by the Animal Care and Use Committee of the National Institute on Drug Abuse of the U.S. National Institutes of Health under approved animal use protocol 07‐BNRB‐47 and were carried out in compliance with applicable U.S. Federal and Maryland state laws and regulations. Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny et al., 2010) and with the recommendations made by the British Journal of Pharmacology. Adult male Long–Evans rats (Charles River Laboratories, Raleigh, NC; RGD Cat# 2308852, RRID:RGD_2308852), 300–325 g at the time of surgery, were used. Animals were housed individually post‐surgery in a climate‐controlled environment (70~74°F, humanity 40–50%, reverse 12 h light/dark cycle) with food (TestDiet, St. Louis, MO, USA) and water freely available with the exception of the time spent each day in the test chambers.
2.1. Surgery
Under 60 mg·kg−1 sodium pentobarbital anaesthesia, rats were surgically implanted with a unilateral monopolar stainless steel stimulating electrode (Plastics One, Roanoke, VA) targeted at the medial forebrain bundle at the level of the lateral hypothalamus (stereotaxic coordinates from bregma: AP + 2.5 mm, ML + 1.7 mm, and DV − 8.4 mm). A wire wrapped around a jeweller's screw implanted in the skull and connected to a mini‐pin in the electrical connector at the top of the electrode was used to accommodate return electrical current. The electrodes were cemented to the skull with acrylic resin cement. Each animal was kept warm and under observation until all effects of the anaesthetic had dissipated. Rats were monitored closely and allowed a minimum of 7 days to recover, prior to the start of experiments.
2.2. ICSS apparatus
All training and testing occurred in standard operant chambers (MED Associates, Georgia, VT), each of which contained a retractable wall‐mounted lever and a cue light immediately above the lever. The operant chambers were enclosed in ventilated, sound‐attenuating cabinets. Depression of the operant lever activated a brain stimulator.
2.3. General ICSS procedure
The general BSR procedures were as reported previously (Pak et al., 2006; Spiller et al., 2008; Xi et al., 2007, 2008). Briefly, rats were allowed to self‐train to lever press for BSR. Each press on the operant lever resulted in a 500‐ms train of 0.1‐ms rectangular cathodal pulses through the electrode, followed by a 500‐ms “timeout” in which further presses did not produce brain stimulation. The initial stimulation parameters were 72 Hz and 200 mA. If the animal did not learn to lever press, the stimulation intensity was increased daily by 50 mA until the animal learned to press (45–60 responses per 30 s) or a maximum of 800 mA was reached. Animals (three of 50 rats) that did not lever press at 800 mA or in which the stimulation produced unwanted effects (e.g., head or body movements or vocalization) were removed from the experiment.
2.4. Rate‐frequency ICSS procedure
Following establishment of lever pressing for BSR, animals were presented with a series of 16 different pulse frequencies, ranging from 141 to 25 Hz in descending order. At each pulse frequency, animals responded for two 30‐s time periods, with the mean number of lever responses recorded as the response rate. Between frequencies, the lever retracted for 5 s. Animals were run for three sessions per day; within each session, animals were run twice on the full range of stimulation frequency over a 40‐min trial. The first session was a “warm up,” the second session was the baseline session, and the third session was the test session. The BSR threshold (θ 0) was defined as the minimum frequency at which the animal responded for rewarding stimulation. Y max was defined as the maximal rate of lever responding. The BSR threshold (θ 0) and Y max were mathematically derived for each baseline run and each test session run by analysing each rate‐frequency BSR function generated by a given animal over a given descending series of pulse frequencies using best‐fit mathematical algorithms as reported previously (Spiller et al., 2009; Xi, Gilbert, et al., 2006; Xi, Newman, et al., 2006).
2.5. Testing the effects of Δ9‐THC, WIN55,212‐2, AM251, AM630, ACEA, or JWH 133 on BSR
Once a baseline value was achieved (<10% variation over five continuous days), the rats were randomly divided into five experimental groups and treated with different test compounds (Table 1) to assess the effects of Δ9‐THC, cocaine, WIN55,212‐2, AM251, AM630, ACEA, or JWH 133 on BSR. All animals were injected, between the baseline and test BSR sessions with an i.p. injection of sterile water, 0.5% Tween‐80 or tocrisolve vehicle (i.e., the 0 mg·kg−1 dose in each group) or one of various doses of test compounds. Thirty minutes after test compound injection, the test sessions began. After each test, animals received an additional 5–7 days of BSR re‐stabilization until a new baseline θ 0 was established. The order of testing of various drug doses was counterbalanced according to a Latin square design. To monitor potential drug effects on motor behaviour, the maximum rate of lever pressing (Y max) was measured, and any treatment that altered this significantly in either direction was eliminated from the study (see also Section 2.8).
Table 1.
Experimental groups and the drug treatments in each group of rats
| Group # | Test drugs | Treatment (mg·kg−1)a |
|---|---|---|
| 1 | Δ9‐THC (n = 14) | Δ9‐THC (0, 0.3, 1, 3, 5), (AM251 + THC), (AM630 + THC) |
| 2 | WIN55,212‐2 (n = 11) | WIN (0, 0.3, 1, 3), (AM251 + WIN), (AM630 + WIN) |
| 3 | ACEA (n = 8) | ACEA (0, 0.1, 0.3, 1), (AM251 + ACEA), (AM630 + ACEA) |
| 4 | JWH 133 (n = 7) | JWH (0, 3, 10, 20), (AM251 + JWH), (AM630 + JWH), Δ9‐THC (1) |
| 5 | Cocaine (n = 7) | Cocaine (0, 3), Δ9‐THC (1), AM251 (0, 1, 3), AM630 (0, 1, 3, 10) |
The order of testing for the various drug doses in each group was counterbalanced according to a Latin square design.
2.6. Testing the effects of AM251 or AM630 pretreatment on drug‐enhanced or drug‐inhibited BSR
For pretreatment studies, rats were injected, between the baseline and test BSR sessions, with 0.5% Tween‐80 vehicle, AM251 (3 mg·kg−1), or AM630 (3 mg·kg−1) 10 min prior to the second drug injection. Then, a dose of the second drug (Δ9‐THC, WIN55,212‐2, ACEA, or JWH 133) was administered 30 min before the test session began. After each test, animals received an additional 3–7 days of BSR re‐stabilization until a new baseline θ 0 was established.
2.7. Locomotor activity
Four additional groups of rats (n = 8 each) were used to evaluate the locomotor effects of cannabinoid compounds. These additional drug‐naive rats were placed in locomotor detection chambers (Accuscan, Columbus, OH) and habituated for 1 hr. Each group then randomly received one dose of Δ9‐THC (0, 1, 3, or 5 mg·kg−1 i.p.), WIN55,212‐2 (0, 0.3, 1, or 3 mg·kg−1 i.p.), JWH 133 (0, 10, 20 mg·kg−1), or ACEA (0, 0.3, 1, 3 mg·kg−1). The Δ9‐THC and WIN55,212‐2 groups of rats were also used to observe the effects of AM251 (3 mg·kg−1 i.p.) or AM630 (3 mg·kg−1 i.p.) on open‐field locomotion. Following injection, locomotor activity was recorded for 2 hr in 10‐min intervals. Each animal was tested three to five times with different drug doses in a counterbalanced manner. The time interval was 1–3 days between each test. Distance counts per 10 min bin (cm) were used to evaluate the effects of each cannabinoid compound on locomotion.
2.8. Data and statistical analysis
The data and statistical analysis comply with the recommendations of the British Journal of Pharmacology on experimental design and analysis in pharmacology. Experiments showing biphasic effects on BSR by Δ9‐THC and WIN55,212‐2 were performed in two independent groups of rats, with seven to 14 animals per group as shown in figure legends. All other experiments were performed once, with seven to 14 rats per treatment. Though the experimenter was not blinded to the animals' identity and treatment condition during data collection, the data were blinded during analyses. No data points were excluded from the analysis in any experiment. Data were checked for normality using the Shapiro–Wilk method and for equal variance by the Brown–Forsythe method. Statistical significance was determined using paired two‐tailed t tests when comparing two groups, and one‐way ANOVAs for repeated measures when comparing multiple groups, using SigmaPlot. For significant results by one‐way ANOVA, all pairwise multiple comparisons were made using the Holm–Sidak method. A P value of less than 0.05 was considered significant.
2.9. Materials
Δ9‐THC and cocaine (provided by the National Institute on Drug Abuse, Intramural Research Program, Baltimore, MD) were dissolved in sterile 0.5% Tween‐80 (Sigma‐Aldrich) and saline, respectively. WIN55,212‐2, AM251, AM630, and ACEA (Tocris) were dissolved in sterile 0.5% Tween‐80. JWH 133 (Tocris) was dissolved in Tocrisolve™ (Tocris Bioscience brand of Bio‐Techne Corporation, Minneapolis, MN).
2.10. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander, Christopoulos et al., 2017).
3. RESULTS
3.1. Mixed CB1 receptor/ CB2 receptor agonists have biphasic effects on BSR
Systemic administration of a wide range of doses of Δ9‐THC produced biphasic effects (Figure 1a,b). A low dose of Δ9‐THC (1.0 mg·kg−1) significantly enhanced BSR (i.e., reduced the minimum frequency at which the animal responded for rewarding stimulation) by 7–9%, while the highest dose tested (5.0 mg·kg−1) significantly inhibited BSR by about 9%. No dose of Δ9‐THC affected the maximal operant response (Y max; Figure 1a), suggesting no significant sedation or locomotor impairment by Δ9‐THC administration (see also Section 2.8).
Figure 1.
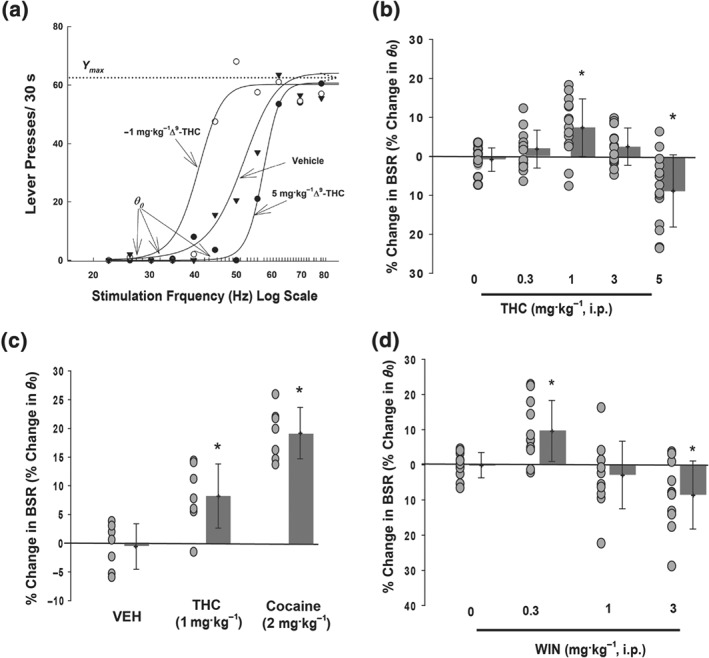
Biphasic effects of Δ9‐THC or WIN55,212‐2 on electrical BSR —low doses enhance, whereas high doses inhibit BSR. (a) Representative stimulation‐response curves, indicating that a low dose of Δ9‐THC (1 mg·kg−1 i.p.) shifted the stimulation‐response curve to the left and decreased the stimulation threshold (θ 0) value, while a higher dose of Δ9‐THC (5 mg·kg−1 i.p.) significantly shifted the curve to the right and increased the stimulation threshold (θ 0). Δ9‐THC did not affect maximal operant responses (Y max level) at any dose tested. (b) Summary of all Δ9‐THC doses tested, with both rewarding and aversive effects apparent as percentage enhancement or inhibition of θ 0 (n = 14, one‐way ANOVA for repeated measures, F 4, 52 = 11.9). (c) The rewarding effects of low dose Δ9‐THC are only about half of those produced by 2 mg·kg−1 cocaine (n = 7, one‐way ANOVA for repeated measures, F 2, 12 = 23.6 ). (d) The synthetic full CB1/CB2 receptor agonist WIN55,212‐2 had similar effects to Δ9‐THC, wherein a low dose of WIN55,212‐2 (0.3 mg·kg−1) enhanced brain reward, and the highest dose tested (3 mg·kg−1) inhibited brain reward (n = 11, F 3, 30 = 9.3). For all panels, individual data points are shown as black circles, with bars indicating group means ± SD shown to the right. *P < 0.05, significantly different from vehicle treatment groups
Because there has been controversy over the effect of Δ9‐THC on reward, we treated a separate group of rats with the enhancing dose of Δ9‐THC as well as a low dose of the appetitive drug, cocaine, for comparison (Figure 1c). The facilitating effect of cocaine was roughly twice as much as that of Δ9‐THC, even at the dose of 2 mg·kg−1, which is a moderately low dose compared to the doses of cocaine used to produce reward ICSS in other recent studies (Bauer, Banks, & Negus, 2014; Yang et al., 2017). The 1 mg·kg−1 dose of Δ9‐THC significantly facilitated ICSS in this second independent cohort of rats, relative to vehicle treatment (difference of means = 8.8%).
Similar to Δ9‐THC, systemic administration of the synthetic high affinity CB1/CB2 receptor agonist WIN55,212‐2 (Compton, Gold, Ward, Balster, & Martin, 1992) produced biphasic effects (Figure 1d), with similar percent shifts. We also found that doses of 5 mg·kg−1 i.p. and above of WIN55,212‐2 produced a sedative effect in the rats as assessed by a decrease in the maximum frequency of lever pressing (Y max). Those animals were excluded from the study in order to differentiate treatment effects on reward from potentially confounding effects on motor function.
3.2. Effects of Δ9‐THC or WIN55,212‐2 on open‐field locomotion
To further determine the potential involvement of locomotor effects, we observed the effects of the same doses of Δ9‐THC or WIN55,212‐2 on open‐field locomotion in rats. Δ9‐THC produced a trend towards reduction (not significant) in open‐field locomotion (Figure 2a,b). However, systemic administration of WIN55,212‐2 produced a significant, dose‐dependent reduction in basal levels of locomotor activity (Figure 2c,d), suggesting locomotor depression or sedation. Post‐hoc individual group comparisons revealed a significant reduction in locomotion only after 3 mg·kg−1 WIN55,212‐2 administration (Figure 2d).
Figure 2.
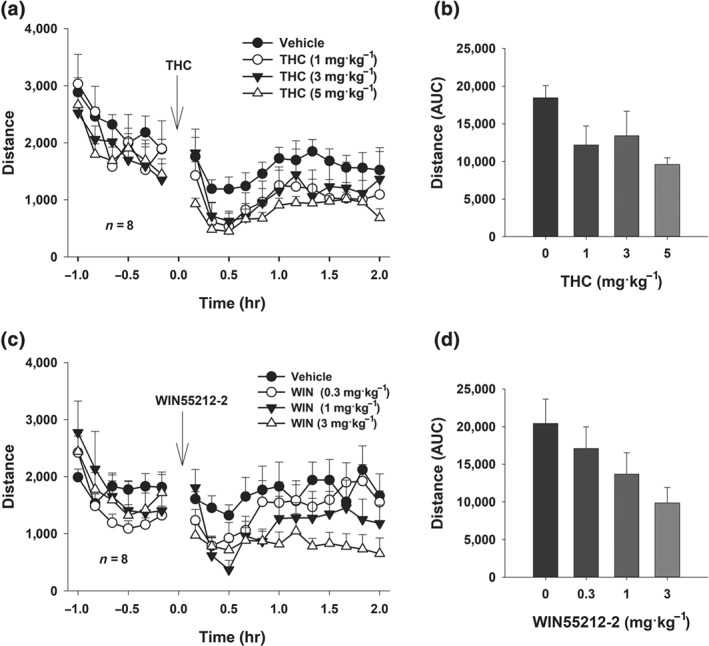
High doses of WIN55,212‐2 decrease spontaneous locomotion. (a, b) The time course and AUC measurements after systemic administration of different doses of Δ9‐THC or vehicle in the open‐field test revealed a significant time main effect (two‐way ANOVA for repeated measures, F11, 77 = 5.93), but no significant Δ9‐THC treatment main effect (F 3, 21 = 2.30) or Treatment × Time interaction (F 33, 231 = 0.39). (c, d) In contrast, WIN55,212‐2 administration produced a more pronounced impairment of spontaneous movement in the open‐field test (two‐way ANOVA for repeated measures revealed a significant Time main effect (F 11,77 = 6.35), treatment main effect (F 3,21 = 7.08), and Treatment X Time interaction (F 33,231 = 1.97)), shown here by the time course for individual doses (c) and the AUC summary data (d)
3.3. Effects of selective CB1 or CB2 receptor antagonists on Δ9‐THC‐ or WIN‐altered BSR
In order to understand the nature of the biphasic effects produced by these mixed CB1/CB2 receptor agonists, we next pretreated Δ9‐THC or WIN55,212‐2 with the selective CB1 or CB2 receptor antagonists AM251 and AM630, respectively. We first confirmed that neither AM251 nor AM630 itself altered BSR or Y max levels at the doses tested (AM251, 1 and 3 mg·kg−1 i.p. or AM630, 1, 3, and 10 mg·kg−1 i.p.; Figure 3).
Figure 3.
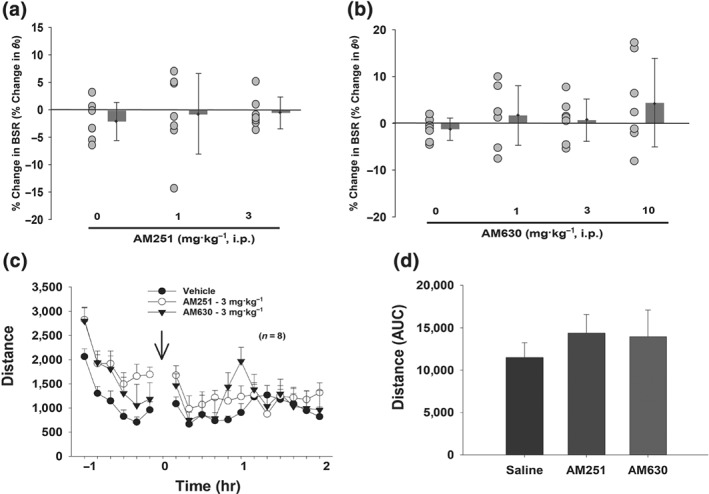
Neither AM251 nor AM630 produced a significant alteration in BSR (a, b) or open‐field locomotion as assessed by the time course of locomotion (c) or the AUC after each drug administration (d) at any dose tested
We then selected doses in the middle of the ranges that have been previously shown to be effective in antagonizing CB1 receptors (3 mg·kg−1 of AM251; Xi, Gilbert, et al., 2006) and CB2 receptors (3 mg·kg−1 of AM630; Rahn et al., 2014). Pretreatment with AM251 10 min prior to a 1.0 mg·kg−1 Δ9‐THC injection moderately attenuated the Δ9‐THC‐enhanced BSR, with a change in BSR facilitation from about 7.4% after the Vehicle + Δ9‐THC treatment to 2.1% after the AM251 + Δ9‐THC treatment (Figure 4a, left panel), although this change was not statistically significant. Given the low level of Δ9‐THC‐enhancement in this group of rats, this result may have been due to a floor effect. However, compared to the vehicle control group, 1 mg·kg−1 Δ9‐THC‐enhanced BSR was clearly blocked by AM251 (Figure 1b vs. Figure 4a). In contrast to Δ9‐THC, AM251 significantly blocked the 0.3 mg·kg−1 WIN‐enhanced BSR (Figure 4b, left panel), from mean value of 10.5% to 1.2%. AM251 pretreatment had no effect on the inhibition of BSR by the 5.0 mg·kg−1 dose of Δ9‐THC (Figure 4a, right panel) or the 3 mg·kg−1 dose of WIN55,212‐2 (Figure 4b, right panel). These data suggest that the enhancement of BSR by low doses of CB1/ CB2 receptor agonists is driven by action on the CB1 receptor.
Figure 4.
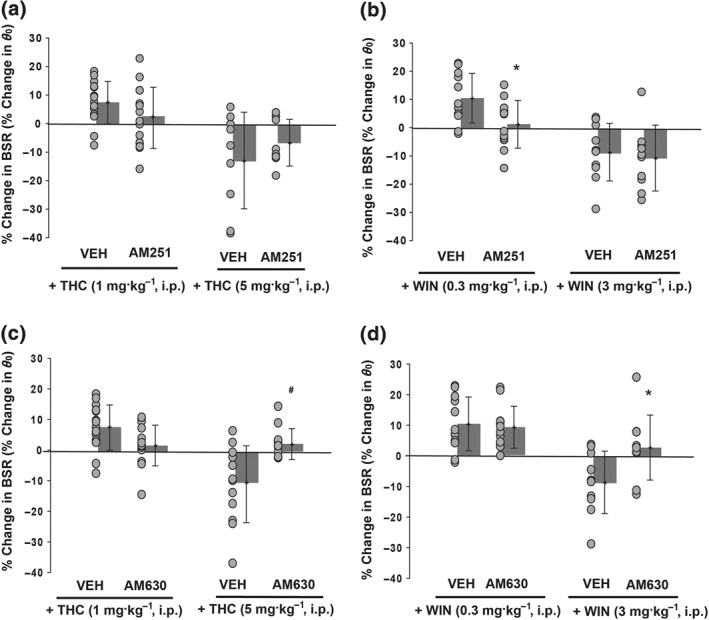
Pretreatment with AM251, a selective CB1 receptor antagonist, blocks low dose WIN55,212‐2‐induced enhancement of BSR but not high dose‐induced BSR inhibition. (a) Pretreatment with AM251 (3 mg·kg−1 i.p., 10 min prior to Δ9‐THC) appeared to attenuate Δ9‐THC‐mediated enhancement and inhibition of BSR although paired t tests did not reveal significant differences. (b) Pretreatment with the same dose of AM251 significantly blocked WIN‐enhanced BSR (n = 12) but not high dose WIN‐induced inhibition of BSR (n = 10, paired t test). (c, d) Pretreatment with AM630, a selective CB2 receptor antagonist, blocks high dose Δ9‐THC‐ or WIN55,212‐2‐mediated BSR inhibition. When AM630 (3 mg·kg−1 i.p.) was injected 10 min prior to Δ9‐THC (c) or WIN55,212‐2 treatment (d), the high dose BSR inhibition was significantly attenuated (5 mg·kg−1 Δ9‐THC, n = 12, paired t test 3 mg·kg−1 WIN55,212‐2, n = 10). For all panels, individual data points are shown as black circles, with bars indicating group means ± SD shown to the right. *P < 0.05, significantly different from vehicle + WIN treatment; #P < 0.05, significantly different from vehicle + Δ9‐THC group
To assess the inhibitory effects on ICSS of high dose Δ9‐THC or WIN55,212‐2, we pretreated the animals with the selective CB2 antagonist AM630 (3 mg·kg−1 i.p., 10 min prior). The inhibition produced by both 5.0 mg·kg−1 Δ9‐THC (Figure 4c) and 3 mg·kg−1 WIN55,212‐2 (Figure 4d) was attenuated to baseline levels by this pretreatment, with a 13% and 10% shift, respectively, compared to when the rats were pretreated with vehicle. AM630 did not alter the low dose facilitation of ICSS by either compound. Taken together, these results suggest that the biphasic effects of Δ9‐THC or WIN55,212‐2 result from differential CB1 or CB2 receptor‐mediated effects.
3.4. CB1 receptor activation is rewarding, whereas CB2 receptor activation is dysphorogenic
To further investigate our hypothesis that it is the actions on different cannabinoid receptor subtypes that drive Δ9‐THC's and WIN55,212‐2's biphasic effects on BSR in rats, we used selective agonists in different groups of rats. First, we treated rats with the highly selective CB1 receptor agonist ACEA (Figure 5a, 0.1, 0.3, 1.0 mg·kg−1) and found that this treatment produced only a monophasic enhancement of BSR, which was significantly attenuated by pretreatment with AM251 (3 mg·kg−1) but not by the CB2 receptor antagonist AM630 (Figure 5b). Consistent with these data, the selective CB2 receptor agonist, JWH 133, increased BSR threshold at 20 mg·kg−1 (Figure 5c) by about 14% from baseline. This inhibition was attenuated by AM630 (Figure 5d) but not AM251, confirming the CB2 receptor specificity of this effect.
Figure 5.
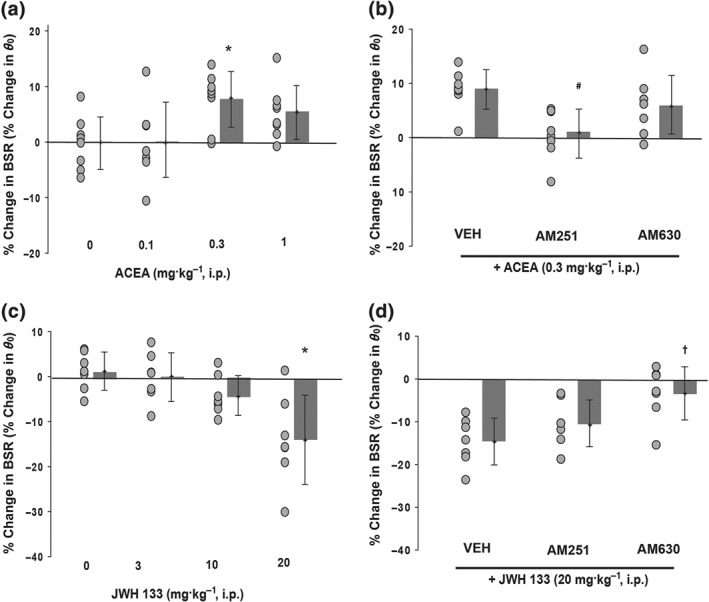
Effects of selective CB1 or CB2 receptor agonists on electrical brain‐stimulation reward. (a) ACEA, a selective CB1 receptor agonist, produced a significant enhancement in BSR (one‐way ANOVA for repeated measures, F 3, 21 = 4.6, n = 8). (b) Pretreatment with AM251 (3 mg·kg−1) but not AM630 (3 mg·kg−1) blocked ACEA‐enhanced BSR (n = 8, one‐way ANOVA for repeated measures, F 2, 14 = 11.8). (c) JWH 133, a selective CB2 receptor agonist, dose‐dependently inhibited BSR (n = 7, F 3, 27 = 12.5). (d) Pretreatment with AM630 (3 mg·kg−1) but not AM251 (3 mg·kg−1) blocked 20 mg·kg−1 JWH 133‐induced BSR inhibition (n = 7, F 2, 12 = 9.5). For all panels, individual data points are shown as black circles, with bars indicating group means ± SD shown to the right. *P < 0.05, significantly different from vehicle; #P < 0.05, significantly different from vehicle + ACEA treatment; †P < 0.05, significantly different from VEH + JWH 133 treatment
3.5. Effects of ACEA and JWH 133 on open‐field locomotion
Finally, we observed the effects of ACEA and JWH 133 on open‐field locomotion. We found that systemic administration of the same doses of ACEA that enhanced BSR had no effect (Figure 6a,b), while JWH 133 produced a dose‐dependent reduction, on open‐field locomotion (Figure 6c,d). Post hoc individual group comparisons revealed a significant reduction in locomotion after 20 mg·kg−1 JWH 133 administration (Figure 6d).
Figure 6.
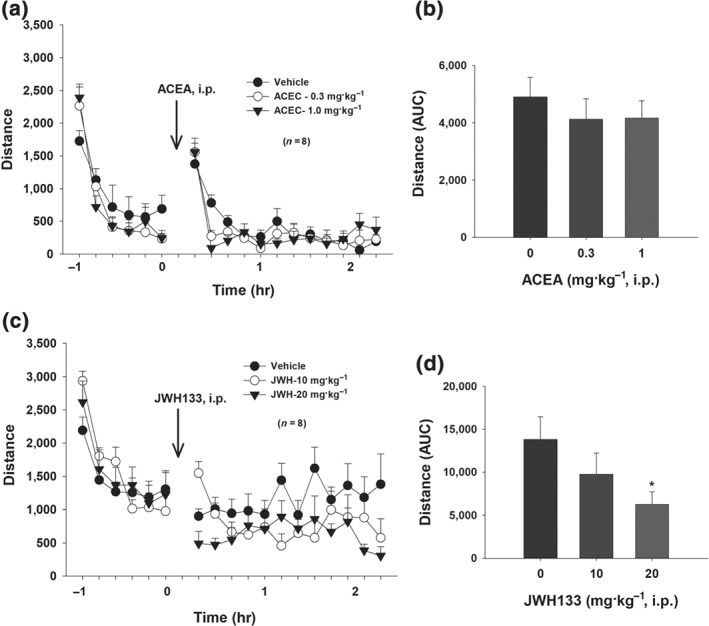
Effects of ACEA and JWH 133 on open‐field locomotion. (a) Time courses of basal levels of locomotor activity after systemic administration of different doses of ACEA or vehicle, illustrating that ACEA did not significantly alter open‐field locomotor activity. A two‐way ANOVA for repeated measures over time and drug dose revealed a significant time main effect (F 11, 77 = 5.93, P < 0.001) but no significant ACEA treatment main effect (F 3, 21 = 2.230) or Treatment × Time interaction (F 33, 231 = 0.38). (b) The AUC data from (a) after ACEA administration. A one‐way ANOVA for repeated measures over Δ9‐THC dose failed to show a significant treatment main effect (F 3, 21 = 2.30). (c) Time course of the effects of JWH 133 on open‐field locomotion. A two‐way ANOVA for repeated measures over time and drug dose revealed a significant treatment main effect (F 2,14 = 6.74), time main effect (F 11, 77 = 1.68), and Treatment × Time interaction (F 22,154 = 2.86). (d) AUC data from (c) after JWH 133 administration. A one‐way ANOVA for repeated measures over dose revealed a significant JWH 133 treatment main effect (F 2,14 = 6.74). *P < 0.05, significantly different from vehicle group
4. DISCUSSION
The major findings in the present study are that systemic administration of Δ9‐THC or the synthetic cannabinoid agonist WIN55,212‐2 produced dose‐dependent biphasic effects —lower doses enhanced, while high doses inhibited BSR, as assessed by electrical ICSS. The selective CB1 receptor agonist, ACEA, produced a BSR‐enhancing effect, while the selective CB2 receptor agonist, JWH 133, produced a dose‐dependent inhibition of BSR. The BSR‐enhancing effect produced by low doses of Δ9‐THC, WIN55,212‐2, or ACEA was blocked selectively by the CB1 receptor selective antagonist, AM251, while the inhibition of BSR produced by high doses of Δ9‐THC or WIN55,212‐2 or by JWH 133 was blocked by the selective CB2 receptor antagonist AM630. Together, these data suggest that brain cannabinoid CB1 and CB2 receptors modulate brain reward function in opposite directions, that is, CB1 receptor activation‐producing enhancement and CB2 receptor activation‐producing inhibition of BSR.
It is well known that cannabis can be rewarding or aversive in both humans and experimental animals (Panagis, Vlachou, & Nomikos, 2008; Vlachou & Panagis, 2014). ICSS is a commonly used behavioural paradigm to study brain reward functions (Bauco & Wise, 1997; Peng et al., 2010; Wise, 1996). In this model, animals press a lever to deliver brief electrical pulses to a discrete brain region such as the VTA of the midbrain or the middle forebrain bundle via an implanted electrode. Most drugs of abuse such as cocaine, heroin, or nicotine lower the stimulation threshold for electrical BSR, indicating enhanced BSR and implying a summation between the BSR and the drug reward (Bauco & Wise, 1997; Peng et al., 2010). However, the effects of cannabinoids on BSR have been controversial. In some studies, Δ9‐THC produced a significant reduction in the electrical stimulation threshold in rats (Gardner et al., 1988; Lepore, Liu, Savage, Matalon, & Gardner, 1996), suggesting enhanced BSR. However, in other studies, Δ9‐THC or other cannabinoid agonists either had no effect on electrical BSR (Vlachou et al., 2007) or produced a reduction in electrical BSR (i.e., aversion) in rats (Katsidoni, Kastellakis, & Panagis, 2013; Vlachou, Nomikos, & Panagis, 2005, 2006). An important finding in the present study is that the hedonic effects of cannabis or cannabinoids depend on a drug dose—lower doses are rewarding, while higher doses are aversive. This may in part explain the previous conflicting findings regarding cannabis actions in humans and experimental animals.
We note that our results on the enhancing effects of low dose Δ9‐THC differ from some previous studies showing Δ9‐THC‐induced inhibition (Kwilasz & Negus, 2012; Negus & Miller, 2014; Vlachou et al, 2007; Wiebelhaus et al., 2015). This could be related to smaller sample sizes used in these studies. For example, Vlachou and colleagues tested all Δ9‐THC doses (0, 0.5, 1 or 2 mg·kg−1 i.p.) using only five animals (Vlachou et al., 2007). The power analysis performed for the present study suggests at least n = 7 are needed to detect an 8% change in BSR with a power of 0.79 (α = 0.05). Assuming that the Vlachou et al. study had similar low levels of variability between rats as in the present study, a sample size of five would be underpowered to detect an 8% change.
Nevertheless, because of the negative findings in those previous studies, we repeated the low dose Δ9‐THC treatment in three independent groups of rats (see Table 1, n = 7–14). We found the same moderate but significant enhancement in all groups with low dose Δ9‐THC treatment. This is further supported by a similar level of enhancement after treatment with the CB1 receptor agonist, ACEA. Our results also fit well with observations in other animal models of drug reward, in which CB1 receptor agonists increase the motivational and reinforcing effects of alcohol, nicotine, and opiates, whereas diminished CB1 receptor signalling diminishes the rewarding effects of these drugs (Parsons & Hurd, 2015). In all of the figures presented in the present study, we show individual data points for each comparison to allow for greater future reproducibility.
Another important finding in the present study is that different receptor mechanisms may underlie cannabis reward versus aversion. This is supported by several lines of evidence. First, the selective CB1 receptor agonist, ACEA, enhanced electrical BSR, an effect that was blocked by the selective CB1 receptor antagonist AM251 but not by the selective CB2 receptor antagonist, AM630. Second, the selective CB2 receptor agonist, JWH 133, dose‐dependently inhibited electrical BSR, an effect that was selectively blocked by AM630 not by AM251. Third, the BSR‐enhancing effect produced by low doses of WIN55,212‐2 was also blocked by AM251 not AM630, while the BSR‐suppressing effect produced by higher doses of Δ9‐THC or WIN55,212‐2 was blocked by AM630 not AM251. We note that the BSR‐enhancing effect produced by low dose Δ9‐THC appeared to be reduced by both AM251 and AM630 (Figure 4a,c). This may be related to the fact that (a) Δ9‐THC‐enhanced BSR is moderate (~7%) and marginally significant and (b) brain levels of CB2 receptors are much lower than those of CB1 receptors. Thus, AM630 may also bind to brain CB2 receptors to affect Δ9‐THC‐enhanced BSR to a certain extent. Compared to Δ9‐THC, WIN55,212‐2 produced more potent biphasic effects on BSR, which were blocked by AM251 and AM630, respectively. WIN55,212‐2 also produced more potent locomotor reduction than Δ9‐THC. The mechanisms underlying the different pharmacological efficacies or potencies of Δ9‐THC and WIN55,212‐2 on BSR and locomotion are unclear. They may be related to different receptor binding profiles—Δ9‐THC may act as a CB1/CB2 receptor partial agonist, while WIN55,212‐2 may act as a CB1/CB2 receptor full agonist (Paronis, Nikas, Shukla, & Makriyannis, 2012; Pertwee, 2010; Tai & Fantegrossi, 2017). Whatever the mechanisms, the present findings with both Δ9‐THC and WIN55,212‐2 suggest that activation of CB1 receptors is rewarding, while activation of CB2 receptors is aversive. This means that the final subjective effect of cannabis depends on the balance of two opposite actions on brain reward function. Individual differences in brain CB1 receptor and CB2 receptor expression may in part explain why cannabis is rewarding in some subjects but aversive in others. These findings may also relate to our previous reports that both CB1 receptor antagonists and CB2 receptor agonists produce inhibitory effects on cocaine self‐administration and reinstatement of drug‐seeking behaviour (Xi, Gilbert, et al., 2006; Xi et al., 2008, 2011; Zhang et al., 2014, 2015). Similarly, overexpression of CB2 receptors in the brain inhibits cocaine self‐administration and attenuates cocaine‐induced locomotor sensitization (Aracil‐Fernandez et al., 2012). Consistent with these findings, several recent reports indicate that CB1 receptors and CB2 receptors may play opposing roles in modulating cocaine's action, e.g., CB2 receptor agonism exerting behavioural effects similar to those of CB1 receptor antagonism on acquisition and expression of cocaine‐induced conditioned place preference, cocaine‐induced locomotion, cocaine‐induced c‐Fos expression and MAPK expression (Delis et al., 2017; Garcia‐Cabrerizo & Garcia‐Fuster, 2016). Such differential CB1 versus CB2 receptor effects may also partially explain some of the difficulty in parsing the neurological effects of cannabis use (Filbey et al., 2014).
We note that, while WIN55,212‐2 or JWH 133 did produce a significant reduction in open‐field locomotion in a dose‐dependent manner, neither Δ9‐THC nor ACEA produced such effects, suggesting possible involvement of locomotor suppression in high dose WIN55,212‐2‐ or high dose JWH 133‐inhibited BSR. Although we cannot completely exclude it, such a possibility could be low since WIN55,212‐2 or JWH 133, at the same high doses, did not alter maximal operant lever responses (Y max). In addition, we have previously reported that JWH 133, at the same high doses (10 and 20 mg·kg−1) did not alter cocaine self‐administration under fixed ratio 1 schedule of reinforcement but produced an increase in break point (maximal lever response to receive a drug infusion) for cocaine self‐administration under progressive ratio schedule of reinforcement in rats (Zhang et al., 2015). These findings suggest that in the presence of drug or BSR, animals still worked very hard to get the reward and displayed high motivation to overcome drug‐induced locomotor inhibition or sedation to get reward.
The cellular mechanisms underlying CB1 receptor‐mediated reward are not fully understood. We have recently reported that CB1 receptor mRNA is expressed in VTA GABAergic and glutamatergic neurons in mice (Han et al., 2017), which may project to VTA dopaminergic neurons and modulate dopaminergic neuron activity (Lupica, Riegel, & Hoffman, 2004). CB1 receptor‐mediated inhibition of VTA GABAergic neurons may disinhibit dopaminergic neuron activity, producing reward‐enhancing effects, while CB1 receptor inhibition of VTA glutamatergic neurons may be aversive by decreasing VTA glutamate release and thereby decreasing dopaminergic neuronal activity (Han et al., 2017). We have hypothesized that the hedonic effect of CB1 receptor activation may depend on the net effect of these two opposing actions (Han et al., 2017). In the present study, we found that activation of CB1 receptors was rewarding in rats. This would be congruent with a supposition that more CB1 receptors are expressed in VTA GABAergic neurons or GABAergic afferents or that CB1 receptor‐mediated GABAergic disinhibition of VTA dopaminergic neurons is dominant when animals are exposed to low loses of cannabis or Δ9‐THC. Conversely, at high doses, cannabis or Δ9‐THC may activate CB1 receptors on VTA glutamatergic neurons or glutamatergic afferents, producing aversive or reward‐depressing effects.
In addition to CB1 receptors, CB2 receptors are found in VTA dopaminergic neurons in both rats and mice (Zhang et al., 2014, 2017). Given that activation of CB2 receptors inhibits dopaminergic neuronal activity in the VTA, dopamine release in the NAc (Foster et al., 2016; Xi et al., 2011; Zhang et al., 2014, 2017), and dopamine‐related behaviours such as intravenous cocaine self‐administration, cocaine‐induced conditioned place preference, and cocaine‐induced hyperactivity (Delis et al., 2017; Liu et al., 2017; Xi et al., 2011), we believe that the aversive or reward‐depressing effects produced by the selective CB2 receptor agonist, JWH 133, or high doses of Δ9‐THC or WIN55,212‐2 are mediated at least in part by direct activation of CB2 receptors on VTA dopaminergic neurons.
In conclusion, CB1 receptor activation produces reinforcing effects, whereas CB2 receptor activation is aversive. These opposing effects may not only explain the conflicting findings in previous ICSS studies but also explain why cannabis is rewarding or aversive in different subjects under different circumstances.
AUTHOR CONTRIBUTIONS
K.S., E.L.G., and Z.‐X.X. designed the experiments. K.S., G.‐H.B., E.G., and H.Y. conducted the experiments. K.S., G.‐H.B., E.G., and Z.‐X.X. analysed the data and made the figures. K.S. and Z.‐X.X. wrote the manuscript. E.L.G. revised the manuscript.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
DECLARATION OF TRANSPARENCY AND SCIENTIFIC RIGOUR
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research as stated in the BJP guidelines for Design & Analysis, and Animal Experimentation, and as recommended by funding agencies, publishers and other organisations engaged with supporting research.
ACKNOWLEDGEMENT
This work was supported by the Intramural Research Program (IRP) at the National Institute on Drug Abuse (NIDA) National Institutes of Health (NIH), U.S. Public Health Service.
Spiller KJ, Bi G, He Y, Galaj E, Gardner EL, Xi Z‐X. Cannabinoid CB1 and CB2 receptor mechanisms underlie cannabis reward and aversion in rats. Br J Pharmacol. 2019;176:1268–1281. 10.1111/bph.14625
This article has been contributed to by US Government employees and their work is in the public domain in the USA.
Contributor Information
Krista J. Spiller, Email: spillerk@upenn.edu.
Zheng‐Xiong Xi, Email: zxi@intra.nida.nih.gov.
REFERENCES
- Alexander, S. P. H. , Christopoulos, A. , Davenport, A. P. , Kelly, E. , Marrion, N. V. , Peters, J. A. , … Collaborators, C. G. T. P. (2017). The Concise Guide to PHARMACOLOGY 2017/18: G protein‐coupled receptors. British Journal of Pharmacology, 174, S17–S129. 10.1111/bph.13878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aracil‐Fernandez, A. , Trigo, J. M. , Garcia‐Gutierrez, M. S. , Ortega‐Alvaro, A. , Ternianov, A. , Navarro, D. , … Manzanares, J. (2012). Decreased cocaine motor sensitization and self‐administration in mice overexpressing cannabinoid CB2 receptors. Neuropsychopharmacology, 37, 1749–1763. 10.1038/npp.2012.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton, J. C. , Friberg, D. , Darlington, C. L. , & Smith, P. F. (2006). Expression of the cannabinoid CB2 receptor in the rat cerebellum: An immunohistochemical study. Neuroscience Letters, 396, 113–116. 10.1016/j.neulet.2005.11.038 [DOI] [PubMed] [Google Scholar]
- Baek, J. H. , Zheng, Y. , Darlington, C. L. , & Smith, P. F. (2008). Cannabinoid CB2 receptor expression in the rat brainstem cochlear and vestibular nuclei. Acta Oto‐Laryngologica, 128, 961–967. 10.1080/00016480701796944 [DOI] [PubMed] [Google Scholar]
- Bauco, P. , & Wise, R. A. (1997). Synergistic effects of cocaine with lateral hypothalamic brain stimulation reward: Lack of tolerance or sensitization. The Journal of Pharmacology and Experimental Therapeutics, 283, 1160–1167. [PubMed] [Google Scholar]
- Bauer, C. T. , Banks, M. L. , & Negus, S. S. (2014). The effect of chronic amphetamine treatment on cocaine‐induced facilitation of intracranial self‐stimulation in rats. Psychopharmacology, 231, 2461–2470. 10.1007/s00213-013-3405-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brusco, A. , Tagliaferro, P. , Saez, T. , & Onaivi, E. S. (2008). Postsynaptic localization of CB2 cannabinoid receptors in the rat hippocampus. Synapse, 62, 944–949. 10.1002/syn.20569 [DOI] [PubMed] [Google Scholar]
- Castaneda, E. , Moss, D. E. , Oddie, S. D. , & Whishaw, I. Q. (1991). THC does not affect striatal dopamine release: Microdialysis in freely moving rats. Pharmacology, Biochemistry, and Behavior, 40, 587–591. 10.1016/0091-3057(91)90367-B [DOI] [PubMed] [Google Scholar]
- Chen, J. P. , Paredes, W. , Lowinson, J. H. , & Gardner, E. L. (1991). Strain‐specific facilitation of dopamine efflux by Δ9‐tetrahydrocannabinol in the nucleus accumbens of rat: An in vivo microdialysis study. Neuroscience Letters, 129, 136–180. 10.1016/0304-3940(91)90739-G [DOI] [PubMed] [Google Scholar]
- Compton, D. R. , Gold, L. H. , Ward, S. J. , Balster, R. L. , & Martin, B. R. (1992). Aminoalkylindole analogs: Cannabimimetic activity of a class of compounds structurally distinct from Δ9‐tetrahydrocannabinol. The Journal of Pharmacology and Experimental Therapeutics, 263, 1118–1126. [PubMed] [Google Scholar]
- Delis, F. , Polissidis, A. , Poulia, N. , Justinova, Z. , Nomikos, G. G. , Goldberg, S. R. , & Antoniou, K. (2017). Attenuation of cocaine‐induced conditioned place preference and motor activity via cannabinoid CB2 receptor agonism and CB1 receptor antagonism in rats. The International Journal of Neuropsychopharmacology, 20, 269–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza, D. C. , Perry, E. , MacDougall, L. , Ammerman, Y. , Cooper, T. , Wu, Y. T. , … Krystal, J. H. (2004). The psychotomimetic effects of intravenous Δ9‐tetrahydrocannabinol in healthy individuals: Implications for psychosis. Neuropsychopharmacology, 29, 1558–1572. 10.1038/sj.npp.1300496 [DOI] [PubMed] [Google Scholar]
- Farris, S. G. , Zvolensky, M. J. , Boden, M. T. , & Bonn‐Miller, M. O. (2014). Cannabis use expectancies mediate the relation between depressive symptoms and cannabis use among cannabis‐dependent veterans. Journal of Addiction Medicine, 8, 130–136. 10.1097/ADM.0000000000000010 [DOI] [PubMed] [Google Scholar]
- Fattore, L. , Fadda, P. , Spano, M. S. , Pistis, M. , & Fratta, W. (2008). Neurobiological mechanisms of cannabinoid addiction. Molecular and Cellular Endocrinology, 286, S97–S107. 10.1016/j.mce.2008.02.006 [DOI] [PubMed] [Google Scholar]
- Filbey, F. M. , Aslan, S. , Calhoun, V. D. , Spence, J. S. , Damaraju, E. , Caprihan, A. , & Segall, J. (2014). Long‐term effects of marijuana use on the brain. Proceedings of the National Academy of Sciences of the United States of America, 111, 16913–16918. 10.1073/pnas.1415297111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster, D. J. , Wilson, J. M. , Remke, D. H. , Mahmood, M. S. , Uddin, M. J. , Wess, J. , … Conn, P. J. (2016). Antipsychotic‐like effects of M4 positive allosteric modulators are mediated by CB2 receptor‐dependent inhibition of dopamine release. Neuron, 91, 1244–1252. 10.1016/j.neuron.2016.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaoni, Y. , & Mechoulam, R. (1971). The isolation and structure of Δ1‐tetrahydrocannabinol and other neutral cannabinoids from hashish. Journal of the American Chemical Society, 93, 217–224. 10.1021/ja00730a036 [DOI] [PubMed] [Google Scholar]
- Garcia‐Cabrerizo, R. , & Garcia‐Fuster, M. J. (2016). Opposite regulation of cannabinoid CB1 and CB2 receptors in the prefrontal cortex of rats treated with cocaine during adolescence. Neuroscience Letters, 615, 60–65. 10.1016/j.neulet.2016.01.018 [DOI] [PubMed] [Google Scholar]
- Gardner, E. L. , Paredes, W. , Smith, D. , Donner, A. , Milling, C. , Cohen, D. , & Morrison, D. (1988). Facilitation of brain stimulation reward by Δ9‐tetrahydrocannabinol. Psychopharmacology, 96, 142–144. 10.1007/BF02431546 [DOI] [PubMed] [Google Scholar]
- Gong, J. P. , Onaivi, E. S. , Ishiguro, H. , Liu, Q. R. , Tagliaferro, P. A. , Brusco, A. , & Uhl, G. R. (2006). Cannabinoid CB2 receptors: Immunohistochemical localization in rat brain. Brain Research, 1071, 10–23. 10.1016/j.brainres.2005.11.035 [DOI] [PubMed] [Google Scholar]
- Gregg, J. M. , Small, E. W. , Moore, R. , Raft, D. , & Toomey, T. C. (1976). Emotional response to intravenous Δ1‐tetrahydrocannabinol during oral surgery. Journal of Oral Surgery, 34, 301–313. [PubMed] [Google Scholar]
- Han, X. , He, Y. , Bi, G. H. , Zhang, H. Y. , Song, R. , Liu, Q. R. , … Xi, Z. X. (2017). CB1 receptor activation on VgluT2‐expressing glutamatergic neurons underlies Δ9‐tetrahydrocannabinol (Δ9‐THC)‐induced aversive effects in mice. Scientific Reports, 7, 12315 10.1038/s41598-017-12399-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR (2018). The IUPHAR/BPS guide to pharmacology in 2018: Updates and expansion to encompass the new guide to immunopharmacology. Nucl Acids Res, 46, D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- John, W. S. , Martin, T. J. , & Nader, M. A. (2017). Behavioral determinants of cannabinoid self‐administration in old world monkeys. Neuropsychopharmacology, 42, 1522–1530. 10.1038/npp.2017.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justinova, Z. , Tanda, G. , Redhi, G. H. , & Goldberg, S. R. (2003). Self‐administration of Δ9‐tetrahydrocannabinol (THC) by drug naive squirrel monkeys. Psychopharmacology, 169, 135–140. 10.1007/s00213-003-1484-0 [DOI] [PubMed] [Google Scholar]
- Katsidoni, V. , Kastellakis, A. , & Panagis, G. (2013). Biphasic effects of Δ9‐tetrahydrocannabinol on brain stimulation reward and motor activity. The International Journal of Neuropsychopharmacology, 16, 2273–2284. 10.1017/S1461145713000709 [DOI] [PubMed] [Google Scholar]
- Kilkenny, C. , Browne, W. , Cuthill, I. C. , Emerson, M. , & Altman, D. G. (2010). Animal research: Reporting in vivo experiments: The ARRIVE guidelines. British Journal of Pharmacology, 160, 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwilasz, A. J. , & Negus, S. S. (2012). Dissociable effects of the cannabinoid receptor agonists Δ9‐tetrahydrocannabinol and CP55940 on pain‐stimulated versus pain‐depressed behavior in rats. The Journal of Pharmacology and Experimental Therapeutics, 343, 389–400. 10.1124/jpet.112.197780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepore, M. , Liu, X. , Savage, V. , Matalon, D. , & Gardner, E. L. (1996). Genetic differences in Δ9‐tetrahydrocannabinol‐induced facilitation of brain stimulation reward as measured by a rate‐frequency curve‐shift electrical brain stimulation paradigm in three different rat strains. Life Sciences, 58, PL365–PL372. 10.1016/0024-3205(96)00237-8 [DOI] [PubMed] [Google Scholar]
- Liu, Q. R. , Canseco‐Alba, A. , Zhang, H. Y. , Tagliaferro, P. , Chung, M. , Dennis, E. , … Onaivi, E. S. (2017). Cannabinoid type 2 receptors in dopamine neurons inhibits psychomotor behaviors, alters anxiety, depression and alcohol preference. Scientific Reports, 7, 17410 10.1038/s41598-017-17796-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Q. R. , Pan, C. H. , Hishimoto, A. , Li, C. Y. , Xi, Z. X. , Llorente‐Berzal, A. , … Uhl, G. R. (2009). Species differences in cannabinoid receptor 2 (CNR2 gene): Identification of novel human and rodent CB2 isoforms, differential tissue expression and regulation by cannabinoid receptor ligands. Genes, Brain, and Behavior, 8, 519–530. 10.1111/j.1601-183X.2009.00498.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupica, C. R. , & Riegel, A. C. (2005). Endocannabinoid release from midbrain dopamine neurons: A potential substrate for cannabinoid receptor antagonist treatment of addiction. Neuropharmacology, 48, 1105–1116. 10.1016/j.neuropharm.2005.03.016 [DOI] [PubMed] [Google Scholar]
- Lupica, C. R. , Riegel, A. C. , & Hoffman, A. F. (2004). Marijuana and cannabinoid regulation of brain reward circuits. British Journal of Pharmacology, 143, 227–234. 10.1038/sj.bjp.0705931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie, K. (2005). Distribution of cannabinoid receptors in the central and peripheral nervous system. Handbook of Experimental Pharmacology, 168, 299–325. 10.1007/3-540-26573-2_10 [DOI] [PubMed] [Google Scholar]
- Maldonado, R. , Valverde, O. , & Berrendero, F. (2006). Involvement of the endocannabinoid system in drug addiction. Trends in Neurosciences, 29, 225–232. 10.1016/j.tins.2006.01.008 [DOI] [PubMed] [Google Scholar]
- Mansbach, R. S. , Nicholson, K. L. , Martin, B. R. , & Balster, R. L. (1994). Failure of Δ9‐tetrahydrocannabinol and CP 55,940 to maintain intravenous self‐administration under a fixed‐interval schedule in rhesus monkeys. Behavioural Pharmacology, 5, 219–225. 10.1097/00008877-199404000-00014 [DOI] [PubMed] [Google Scholar]
- Matsuda, L. A. , Lolait, S. J. , Brownstein, M. J. , Young, A. C. , & Bonner, T. I. (1990). Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature, 346, 561–564. 10.1038/346561a0 [DOI] [PubMed] [Google Scholar]
- Munro, S. , Thomas, K. L. , & Abu‐Shaar, M. (1993). Molecular characterization of a peripheral receptor for cannabinoids. Nature, 365, 61–65. 10.1038/365061a0 [DOI] [PubMed] [Google Scholar]
- Negus, S. S. , & Miller, L. L. (2014). Intracranial self‐stimulation to evaluate abuse potential of drugs. Pharmacological Reviews, 66, 869–917. 10.1124/pr.112.007419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onaivi, E. S. , Ishiguro, H. , Gong, J. P. , Patel, S. , Meozzi, P. A. , Myers, L. , … Uhl, G. R. (2008). Functional expression of brain neuronal CB2 cannabinoid receptors are involved in the effects of drugs of abuse and in depression. Annals of the New York Academy of Sciences, 1139, 434–449. 10.1196/annals.1432.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onaivi, E. S. , Ishiguro, H. , Gong, J. P. , Patel, S. , Perchuk, A. , Meozzi, P. A. , … Uhl, G. R. (2006). Discovery of the presence and functional expression of cannabinoid CB2 receptors in brain. Annals of the New York Academy of Sciences, 1074, 514–536. 10.1196/annals.1369.052 [DOI] [PubMed] [Google Scholar]
- Pak, A. C. , Ashby, C. R. Jr. , Heidbreder, C. A. , Pilla, M. , Gilbert, J. , Xi, Z. X. , & Gardner, E. L. (2006). The selective dopamine D3 receptor antagonist SB‐277011A reduces nicotine‐enhanced brain reward and nicotine‐paired environmental cue functions. The International Journal of Neuropsychopharmacology, 9, 585–602. 10.1017/S1461145706006560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panagis, G. , Vlachou, S. , & Nomikos, G. G. (2008). Behavioral pharmacology of cannabinoids with a focus on preclinical models for studying reinforcing and dependence‐producing properties. Current Drug Abuse Reviews, 1, 350–374. 10.2174/1874473710801030350 [DOI] [PubMed] [Google Scholar]
- Paronis, C. A. , Nikas, S. P. , Shukla, V. G. , & Makriyannis, A. (2012). Δ9‐Tetrahydrocannabinol acts as a partial agonist/antagonist in mice. Behavioural Pharmacology, 23, 802–805. 10.1097/FBP.0b013e32835a7c4d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons, L. H. , & Hurd, Y. L. (2015). Endocannabinoid signalling in reward and addiction. Nature Reviews Neuroscience, 16, 579–594. 10.1038/nrn4004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, X. Q. , Xi, Z. X. , Li, X. , Spiller, K. , Li, J. , Chun, L. , … Gardner, E. L. (2010). Is slow‐onset long‐acting monoamine transport blockade to cocaine as methadone is to heroin? Implication for anti‐addiction medications. Neuropsychopharmacology, 35, 2564–2578. 10.1038/npp.2010.133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee, R. G. (2010). Receptors and channels targeted by synthetic cannabinoid receptor agonists and antagonists. Current Medicinal Chemistry, 17, 1360–1381. 10.2174/092986710790980050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raft, D. , Gregg, J. , Ghia, J. , & Harris, L. (1977). Effects of intravenous tetrahydrocannabinol on experimental and surgical pain. Psychological correlates of the analgesic response. Clinical Pharmacology & Therapeutics, 21, 26–33. 10.1002/cpt197721126 [DOI] [PubMed] [Google Scholar]
- Rahn, E. J. , Deng, L. , Thakur, G. A. , Vemuri, K. , Zvonok, A. M. , Lai, Y. Y. , … Hohmann, A. G. (2014). Prophylactic cannabinoid administration blocks the development of paclitaxel‐induced neuropathic nociception during analgesic treatment and following cessation of drug delivery. Molecular Pain, 10, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt, W. , Schafer, F. , Striggow, V. , Frohlich, K. , & Striggow, F. (2012). Cannabinoid receptor subtypes 1 and 2 mediate long‐lasting neuroprotection and improve motor behavior deficits after transient focal cerebral ischemia. Neuroscience, 227, 313–326. 10.1016/j.neuroscience.2012.09.080 [DOI] [PubMed] [Google Scholar]
- Schulden, J. D. , Thomas, Y. F. , & Compton, W. M. (2009). Substance abuse in the United States: Findings from recent epidemiologic studies. Current Psychiatry Reports, 11, 353–359. 10.1007/s11920-009-0053-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiller, K. , Xi, Z. X. , Li, X. , Ashby, C. R. Jr. , Callahan, P. M. , Tehim, A. , & Gardner, E. L. (2009). Varenicline attenuates nicotine‐enhanced brain‐stimulation reward by activation of α4β2 nicotinic receptors in rats. Neuropharmacology, 57, 60–66. 10.1016/j.neuropharm.2009.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiller, K. , Xi, Z. X. , Peng, X. Q. , Newman, A. H. , Ashby, C. R. Jr. , Heidbreder, C. , … Gardner, E. L. (2008). The selective dopamine D3 receptor antagonists SB‐277011A and NGB 2904 and the putative partial D3 receptor agonist BP‐897 attenuate methamphetamine‐enhanced brain stimulation reward in rats. Psychopharmacology, 196, 533–542. 10.1007/s00213-007-0986-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stempel, A. V. , Stumpf, A. , Zhang, H. Y. , Ozdogan, T. , Pannasch, U. , Theis, A. K. , … Schmitz, D. (2016). Cannabinoid type 2 receptors mediate a cell type‐specific plasticity in the hippocampus. Neuron, 90, 795–809. 10.1016/j.neuron.2016.03.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo, B. , Siemes, S. , & Wallmichrath, I. (2002). Inhibition of GABAergic neurotransmission in the ventral tegmental area by cannabinoids. The European Journal of Neuroscience, 15, 2057–2061. 10.1046/j.1460-9568.2002.02041.x [DOI] [PubMed] [Google Scholar]
- Tai, S. , & Fantegrossi, W. E. (2017). Pharmacological and toxicological effects of synthetic cannabinoids and their metabolites. Current Topics in Behavioral Neurosciences, 32, 249–262. 10.1007/7854_2016_60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanda, G. , Munzar, P. , & Goldberg, S. R. (2000). Self‐administration behavior is maintained by the psychoactive ingredient of marijuana in squirrel monkeys. Nature Neuroscience, 3, 1073–1074. 10.1038/80577 [DOI] [PubMed] [Google Scholar]
- Tanda, G. , Pontieri, F. E. , & Di Chiara, G. (1997). Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common mu1 opioid receptor mechanism. Science, 276, 2048–2050. 10.1126/science.276.5321.2048 [DOI] [PubMed] [Google Scholar]
- Van Sickle, M. D. , Duncan, M. , Kingsley, P. J. , Mouihate, A. , Urbani, P. , Mackie, K. , … Sharkey, K. A. (2005). Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science, 310, 329–332. 10.1126/science.1115740 [DOI] [PubMed] [Google Scholar]
- Vlachou, S. , Nomikos, G. G. , & Panagis, G. (2005). CB1 cannabinoid receptor agonists increase intracranial self‐stimulation thresholds in the rat. Psychopharmacology, 179, 498–508. 10.1007/s00213-004-2050-0 [DOI] [PubMed] [Google Scholar]
- Vlachou, S. , Nomikos, G. G. , & Panagis, G. (2006). Effects of endocannabinoid neurotransmission modulators on brain stimulation reward. Psychopharmacology, 188, 293–305. 10.1007/s00213-006-0506-0 [DOI] [PubMed] [Google Scholar]
- Vlachou, S. , Nomikos, G. G. , Stephens, D. N. , & Panagis, G. (2007). Lack of evidence for appetitive effects of Δ9‐tetrahydrocannabinol in the intracranial self‐stimulation and conditioned place preference procedures in rodents. Behavioural Pharmacology, 18, 311–319. 10.1097/FBP.0b013e3282186cf2 [DOI] [PubMed] [Google Scholar]
- Vlachou, S. , & Panagis, G. (2014). Regulation of brain reward by the endocannabinoid system: A critical review of behavioral studies in animals. Current Pharmaceutical Design, 20, 2072–2088. 10.2174/13816128113199990433 [DOI] [PubMed] [Google Scholar]
- Wiebelhaus, J. M. , Grim, T. W. , Owens, R. A. , Lazenka, M. F. , Sim‐Selley, L. J. , Abdullah, R. A. , … Lichtman, A. H. (2015). Δ9‐tetrahydrocannabinol and endocannabinoid degradative enzyme inhibitors attenuate intracranial self‐stimulation in mice. The Journal of Pharmacology and Experimental Therapeutics, 352, 195–207. 10.1124/jpet.114.218677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise, R. A. (1996). Addictive drugs and brain stimulation reward. Annual Review of Neuroscience, 19, 319–340. 10.1146/annurev.ne.19.030196.001535 [DOI] [PubMed] [Google Scholar]
- Xi, Z. X. , Gilbert, J. G. , Peng, X. Q. , Pak, A. C. , Li, X. , & Gardner, E. L. (2006). Cannabinoid CB1 receptor antagonist AM251 inhibits cocaine‐primed relapse in rats: Role of glutamate in the nucleus accumbens. The Journal of Neuroscience, 26, 8531–8536. 10.1523/JNEUROSCI.0726-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi, Z. X. , Newman, A. H. , Gilbert, J. G. , Pak, A. C. , Peng, X. Q. , Ashby, C. R. Jr. , … Gardner, E. L. (2006). The novel dopamine D3 receptor antagonist NGB 2904 inhibits cocaine's rewarding effects and cocaine‐induced reinstatement of drug‐seeking behavior in rats. Neuropsychopharmacology, 31, 1393–1405. 10.1038/sj.npp.1300912 [DOI] [PubMed] [Google Scholar]
- Xi, Z. X. , Peng, X. Q. , Li, X. , Song, R. , Zhang, H. Y. , Liu, Q. R. , … Gardner, E. L. (2011). Brain cannabinoid CB receptors modulate cocaine's actions in mice. Nature Neuroscience, 14, 1160–1166. 10.1038/nn.2874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi, Z. X. , Spiller, K. , Pak, A. C. , Gilbert, J. , Dillon, C. , Li, X. , … Gardner, E. L. (2008). Cannabinoid CB1 receptor antagonists attenuate cocaine's rewarding effects: Experiments with self‐administration and brain‐stimulation reward in rats. Neuropsychopharmacology, 33, 1735–1745. 10.1038/sj.npp.1301552 [DOI] [PubMed] [Google Scholar]
- Xi, Z. X. , Yang, Z. , Li, S. J. , Li, X. , Dillon, C. , Peng, X. Q. , … Gardner, E. L. (2007). Levo‐tetrahydropalmatine inhibits cocaine's rewarding effects: Experiments with self‐administration and brain‐stimulation reward in rats. Neuropharmacology, 53, 771–782. 10.1016/j.neuropharm.2007.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, H. J. , Zhang, H. Y. , Bi, G. H. , He, Y. , Gao, J. T. , & Xi, Z. X. (2017). Deletion of type 2 metabotropic glutamate receptor decreases sensitivity to cocaine reward in rats. Cell Reports, 20, 319–332. 10.1016/j.celrep.2017.06.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H. Y. , Bi, G. H. , Li, X. , Li, J. , Qu, H. , Zhang, S. J. , … Liu, Q. R. (2015). Species differences in cannabinoid receptor 2 and receptor responses to cocaine self‐administration in mice and rats. Neuropsychopharmacology, 40, 1037–1051. 10.1038/npp.2014.297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H. Y. , Gao, M. , Liu, Q. R. , Bi, G. H. , Li, X. , Yang, H. J. , … Xi, Z. X. (2014). Cannabinoid CB2 receptors modulate midbrain dopamine neuronal activity and dopamine‐related behavior in mice. Proceedings of the National Academy of Sciences of the United States of America, 111, E5007–E5015. 10.1073/pnas.1413210111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H. Y. , Gao, M. , Shen, H. , Bi, G. H. , Yang, H. J. , Liu, Q. R. , … Xi, Z. X. (2017). Expression of functional cannabinoid CB2 receptor in VTA dopamine neurons in rats. Addiction Biology, 22, 752–765. 10.1111/adb.12367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H. Y. , Shen, H. , Jordan, C. J. , Liu, Q. R. , Gardner, E. L. , Bonci, A. , & Xi, Z. X. (2019). CB2 receptor antibody signal specificity: Correlations with the use of partial CB2‐knockout mice and anti‐rat CB2 receptor antibodies. Acta Pharmacologica Sinica, 40, 398–409. [DOI] [PMC free article] [PubMed] [Google Scholar]


