Abstract
Hormones are messengers circulating in the body that interact with specific receptors on the cell membrane or inside the cells and regulate, at a distal site, the activities of specific target organs. The definition of hormone has evolved in the last years. Hormones are considered in the context of cell–cell communication and mechanisms of cellular signaling. The best-known mechanisms of this kind are chemical receptor-mediated events, the cell–cell direct interactions through synapses, and, more recently, the extracellular vesicle (EV) transfer between cells. Recently, it has been extensively demonstrated that EVs are used as a way of communication between cells and that they are transporters of specific messenger signals including non-coding RNAs (ncRNAs) such as microRNAs (miRNAs) and long non-coding RNAs (lncRNAs). Circulating ncRNAs in body fluids and extracellular fluid compartments may have endocrine hormone-like effects because they can act at a distance from secreting cells with widespread consequences within the recipient cells. Here, we discuss and report examples of the potential role of miRNAs and lncRNAs as mediator for intercellular communication with a hormone-like mechanism in cancer.
Keywords: non-coding RNAs, microRNAs, long non-coding RNAs, hormones, hormone-like action
1. Introduction
The term “hormone” was first introduced in 1905 by Starling, referring to the discovery of secretin [1]. A hormone is a chemical messenger (in general, a peptide or a steroid) produced by the endocrine glands and circulating in the body to regulate the activities of specific target organs at a distal site [2,3]. The mode of action of hormones requires an interaction of these chemical messengers with specific receptors located on the cell membrane or inside the cell. The binding hormone-receptor generates a signaling cascade that modifies cellular activity [4]. The definition of hormones is quite restrictive since not all hormones are originated from endocrine glands, with many of them acting locally via autocrine/paracrine regulation. Specialized cells in various other organs also secrete hormones in response to specific biochemical signals from a wide range of regulatory systems. Serum/calcium concentration, for instance, affects parathyroid hormone synthesis while serum glucose concentration affects insulin synthesis. In addition, since the outputs of the stomach and exocrine pancreas become the input of the small intestine, the small intestine itself secretes hormones to stimulate/inhibit the stomach and pancreas in accordance to how busy it is, in a regulated feedback known more generally as “diffuse endocrine system” [5]. In a broader view, hormones are considered in the cell–cell communication context and in mechanisms of cellular signaling [2,3]. The best-known mechanisms of this kind are chemical receptor-mediated events, the cell–cell direct interactions through synapses, and, more recently, the extracellular vesicle (EV) transfer between cells [6]. EVs are small membrane-enclosed structures produced by different mechanisms that can be secreted from almost all cell types [7,8] in a process evolutionary conserved from bacteria to humans [9]. Each cell type is able to turn on EV biogenesis depending on the physiological states and, also, the EV cargo components can be highly regulated [10]. EVs, such as exosomes and microvesicles, represent the way donor cells communicate with recipient cells and influence their gene expression [11]. In the last years, it has been extensively demonstrated that EVs are used as a way of communication between cells and that they are transporters of specific messenger signals. EVs are, in fact, enriched for specific proteins (as for example cytokines), lipids, messenger RNAs (mRNA), and non-coding RNAs (ncRNAs), such as microRNAs (miRNAs) and long non-coding RNAs (lncRNAs) [6,8,12]. The nature and abundance of EV content are related to the specific cell type, and are influenced by the physiopathological state of the donor cell [13]. The cell–cell communication mediated by RNAs included in EVs has been described for the first time by Valadi et al. in 2007: exosomes carried miRNAs and other RNAs from one cell to another and, when released in the target cell, were able to interact with the gene expression machinery to modify the gene expression profile of the recipient cell [6].
EVs result as an alternative mode of communication between neighboring and distant cells. Respect to conventional mechanisms of cell communication, EVs differ because of specific temporal and spatial properties and mostly because of the potentiality to group multiple signals together [14].
2. miRNAs and Their Hormone-Like Activity in Cancer
The advances in high-throughput sequencing technology and bioinformatics have revolutionized ncRNA discovery [15]. Mammalian genomes are highly transcribed and the majority of the transcripts do not code for proteins. However, this high rate of transcription is not done in an indiscriminate way: the cellular repertoire of ncRNAs includes small housekeeping RNAs (such as ribosomal RNAs (rRNAs) and transfer RNAs (tRNAs)), as well as miRNAs and lncRNAs [16].
miRNAs are a class of single-stranded ncRNAs that play a critical role in the negative regulation of gene expression at post-transcriptional level [17]. Thousands of miRNAs have been identified in all eukaryotes and, so far, the latest version of miRBase (release 22 March 2018) accounts for over 38,000 miRNA gene loci in 271 species. In animal cells, miRNAs pair, in a complementary manner, with the 3′UTR of target mRNAs, inhibiting their translation or inducing their degradation [17]. miRNAs are crucial regulators in a wide range of biological processes but they are also implicated in human diseases, including cancer [16,18,19,20]. There are several lines of evidence that miRNAs are involved in endocrinology. It has been demonstrated that miRNAs can regulate directly genes encoding hormones or other enzymes involved in hormone maturation and metabolism. miRNAs can also target hormone antagonists or receptors indirectly modifying the hormone-mediated cell signaling transmission [21,22] or could be regulated by hormones either at the level of miRNA transcription and processing [23,24,25]. For instance, miR-21 and miR-181-b1 genes are expressed after STAT3 induction, which is activated by interleukin 6 (IL-6) [26]. Moreover, miR-21 is repressed by thyroid hormone (TH) and this downregulation regulates GRHL3, a transcriptional inhibitor of type 3 iodothyronine deiodinase (D3) which, in turn, inactivates TH [27].
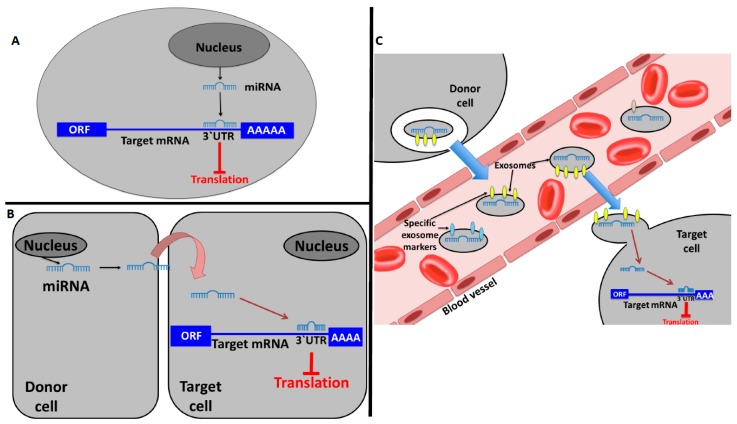
Recently, the role of miRNAs as mediators for intercellular communication with a hormone-like mechanism has also been established. miRNAs can work as autocrine, paracrine, and endocrine messengers. In fact, the classic mechanism of action of a miRNA is to be transcribed by a cell and induce local signaling on that same cell (autocrine signaling, Figure 1A). On the other hand, a miRNA can also transmit local signaling between nearby cells (paracrine signaling, Figure 1B) [8]. In cancer, the intercellular signaling mediated by miRNAs has been related to the tumor microenvironment (TME) setting or pre-metastatic niche induction [28,29,30]. An example of this kind of signaling in cancer is the one in which tumor-derived EVs containing miRNAs can directly modify tumor cell invasiveness and motility through modification of the TME [31,32]. Moreover, it has been observed that the ectopic expression of miR-409 in normal prostate fibroblasts conferred a cancer-associated stroma-like phenotype. The release of this miRNA via EVs was able to promote tumorigenesis and epithelial-to-mesenchymal transition (EMT) through repression of Ras suppressor 1 (RUS1) and stromal antigen 2 (STAG2), well-known tumor suppressors [33]. The discovery of miRNAs in extracellular fluids or loaded in EVs, such as exosomes, is the main evidence that miRNAs may act as paracrine and endocrine interactors [11,34]. EVs containing miRNAs can either work locally or distally via transport within the circulatory system. Moreover, miRNAs circulating within bodily fluids and extracellular fluid compartments may have an endocrine hormone-like effect because they can reach cells that are distant from the secreting cell, modifying their gene expression (Figure 1C) [34,35]. Once released, the EVs containing miRNAs can interact with a recipient cell, deliver its cargo to the cytosol, and modulate the phenotype of the target cell [36]. There is evidence that demonstrates that miRNAs in EVs can be taken up into neighboring or distant cells and modulate the function of those recipient cells in many physiological and pathological conditions [37,38,39]. For example, Le et al. demonstrated, in vitro and in vivo, that murine and human metastatic breast cancer cells release miR-200 family miRNAs to nonmetastatic cells via EVs. The transfer of these molecules altered gene expression in the recipient cells (which were lung cancer cells in the in vivo experiments) and promoted mesenchymal-to-epithelial transition [40]. Interestingly, the exosomal miRNA cargo occurs non-randomly and, also, the recipient cells are finely targeted, enforcing the idea of specific function for a single miRNA on a specific target [6,16]. miRNA species that are transported via EVs do not reflect the miRNA expression profiling of the donor cells [41,42] and, interestingly, several studies demonstrated that cancer patients have elevated levels of tumor-derived exosomes in plasma or serum compared with healthy controls [43,44,45]. The secretion of tumor-specific miRNAs via exosomes indicates the importance of this mechanism in influencing the surrounding microenvironment [34].
Figure 1.
MicroRNAs (miRNAs) working in a hormone-like fashion. (A) Autocrine communication: a miRNA produced by a cell binds to autocrine receptors of the same cell inducing a local signaling. (B) Paracrine communication: a miRNA produced by a cell transmit a local signaling between nearby cells. (C) Endocrine communication: an extracellular vesicle (EV)-embedded miRNA is the mediator of distant signaling.
Functional interactions between cancer cells and the TME are mediated by small molecules such as cytokines and growth factors [46]. In addition, cancer cells may also transfer important functional information through paracrine communication via EVs [47]. EV cargo may actually influence the stroma by activating molecular pathways that differ from those mediated by soluble factors [8]. Therefore, tumor-derived EVs can alter the physiology of surrounding cells and distant non-tumor cells to facilitate cancer dissemination and growth [31].
The TME (which includes extracellular matrix, cancer-associated fibroblasts, tumor-associated macrophages, immune cells, and others) plays a crucial role in all steps of carcinogenesis [48]. Several examples of this biological information transfer between malignant cells and TME components via EV-transported miRNAs have been reviewed in a recent work by Bayraktar et al. [8]. For instance, Baroni et al. [49] found that in cancer-associated fibroblasts from triple-negative breast cancer patients, miR-9 was upregulated when compared with normal fibroblasts. Moreover, miR-9 was released by tumor cells and transferred via exosomes to normal fibroblasts recipients which, as a consequence, overexpressed this miRNA and increased their motility. Therefore, high expression of miR-9 in fibroblasts affects breast cancer progression [49]. Another example was described in the work of Chen et al. in which it was found that miR-940, released in exosomes by ovarian cancer cells, targeted tumor-associated macrophages and promoted tumor growth via the CD206 and CD163 pathways [50].
The assumption that a miRNA might work as a hormone or with a hormone-like mechanism implicates the possibility of the existence of a protein receptor for miRNAs (defined as miRceptor by [34]) and miRNA–protein interaction. The first study demonstrating miRNA–protein binding was published in 2010 by Eiring et al., where the authors provided evidence of steric binding between miR-328 and hnRNPE2 in blast crisis of chronic myelogenous leukemia. This “decoy activity” of miRNA prevents hnRNPE2 binding to CEBPA mRNA, thus restoring C/EBPα expression that further and directly enhances miR-328 transcription [51].
In 2012, it was demonstrated that EVs containing miR-21 and miR-29a released by non-small cell lung cancer cell lines were targeting tumor-associated macrophages and, more specifically, the human toll-like receptor 8 (TLR8), triggering the downstream pathway. As a result, authors observed an increased secretion of IL-6 and tumor necrosis factor-α (TNFα) by tumor-associated macrophages, which determines a pro-tumoral inflammatory response promoting cancer growth and metastasis [52].
Patel and Gooderham observed that IL-6 triggers the IL-6R/STAT3 pathway and also increases miR-21 and miR-29b expression in colorectal cancer cells. The authors proposed a model in which these miRNAs are released via exosomes and reach immune cells, where they interact with the TLR8 miRceptor. This interaction may induce an increase of IL-6 in a feed-forward loop involving miRNA–miRceptor interactions which are responsible for the increased secretion of IL-6, a typical phenomenon in the colorectal cancer microenvironment [53]. Interestingly, a similar mechanism has also been found in neuroblastoma. Endovesicular miR-21, released by neuroblastoma cells, binds to TLR8 in surrounding tumor-associated macrophages, inducing in these cells the upregulation and the release in EVs of miR-155. Macrophage-derived EVs containing miR-155 are transferred back to neuroblastoma cells where miR-155 acts on its target, telomeric repeat-binding factor 1 (TERF1, a telomerase inhibitor). The silencing of TERF1 induces increased resistance to cisplatin in neuroblastoma cells [54].
miRNAs released by exosomes and working in a hormone-like fashion could also be an optimal therapeutic target in the case of tumor drug resistance [55]. Wei et al., for example, demonstrated the role of exosomal miR-221/222 in the resistance to tamoxifen in breast cancer cells [56]. In another study, it was demonstrated that cancer-associated fibroblasts released exosomes containing miR-21, miR-378e, and miR-143-3p, that were able to induce stemness and epithelial–mesenchymal transition phenotypes in breast cancer cell lines [57].
An intriguing aspect that could be bound to the hormone-like action of miRNAs has been raised by Zhang et al. in 2012 [58] and reinforced by Zhou et al. in 2015 [59]. In these works, researchers demonstrated the possibility that miRNAs derived from plants could potentially travel, through food, from plants to animals via the gastrointestinal tract and access host cellular targets, where they work as bioactive compounds able to influence recipients’ physiopathological conditions. The authors proposed that epithelial cells in the intestine could absorb plant-derived miRNAs contained in food and include them into EVs to protect them from degradation and facilitate their release into the blood stream. These “exogenous miRNAs” then seem to be able to reach organs and tissues via circulation and modulate gene expression. The evidence supporting this theory has been summarized in a recent review by Li et al. [60]. This sort of plant–animal communication, named cross-kingdom transmission, is still source of debate in the scientific community. In fact, there is a large amount of evidence contradicting this cross-kingdom communication hypothesis (also widely reviewed by [60]). The main concern is the mechanisms by which exogenous miRNAs can bypass and survive in the gastrointestinal tract, to enter the bloodstream and ultimately reach specific targets. This “exogenous post-transcriptional regulation” could be another factor influencing the development in special cases of diseases, such as cancer, inserting additional levels of complication into an already complicated scenario. If validated, this hypothesis may expand the current knowledge on dietary bioactive compounds and their biological actions once internalized in the organism [60,61].
3. Long Non-Coding RNAs Acting as Hormones
miRNAs are the most studied species of ncRNAs but, in the last years, the attention of researchers has also been focused on other ncRNAs whose functions are still not well described. A special mention should be made for lncRNAs, since their biological roles and mechanisms of action are not yet completely understood, especially in the context of carcinogenesis [62]. Assigning molecular, cellular, and physiological functions to lncRNAs is among the greatest challenges of the next decade, and there is now increased attention on their biological functions in hormonal signaling systems [63,64,65,66].
lncRNAs are defined as non-protein coding RNA transcripts larger than 200 nucleotides, but this definition is quite vague since a universal scheme does not exist [62,67]. The working definition for lncRNAs includes all RNA molecules longer than 200 nucleotides, having little coding potential, transcribed by PolII, capped, spliced, and polyadenylated [63]. The expression of lncRNAs is dependent on the cellular, tissue, and metabolic context. As a consequence, there are specific lncRNAs associated with specific cellular processes that may be inferred by their differential pattern of expression in tissues but also in different developmental time points or under specific stimuli [61,63,68]. It is a common belief that lncRNAs are mostly involved in transcriptional regulation and, therefore, reside principally in the nucleus. However, several lncRNAs act, or are even exclusively localized, within the cytoplasm by working as post-transcriptional regulators in interaction with miRNAs, mRNAs, or proteins [69,70,71,72]. Interestingly, the EV cargo may be enriched in lncRNAs [10,73,74], as observed in plasma exosomes of patients with castration-resistant prostate cancer [75] and in renal cancer [76]. The scenario is even more complicated due to a large number of lncRNAs that have been implicated in competing endogenous RNA (ceRNA) mechanisms. This is possible since lncRNAs can function as sponges, able to bind and reduce the targeted effects of miRNAs on mRNAs [77,78].
lncRNA have been recognized as having endocrine, paracrine, and autocrine regulatory functions in a way similar to the one already described for miRNAs [74]. In fact, they can have an autocrine hormone-like behavior since they can modulate cellular activity directly by controlling transcription. For example, they can interact with hormone-encoding genes or hormone antagonists/receptors, indirectly modifying the cell signaling transmission [79]. Steroid receptor RNA activator (SRA) was among the first lncRNA to be associated to hormone receptor pathways and acting with a hormone-like mechanism. SRA is expressed in tissues specifically targeted by steroid hormone, and it works as a co-activator of the steroid receptor to facilitate ligand-dependent transactivation [80]. Additionally, SRA can interact with co-repressors of nuclear receptors [81]. Different expression patterns of SRA have been observed in breast cancer cell lines [82], demonstrating that its hormone receptor-associated activity may be crucial in breast tumorigenesis.
Growth arrest-specific 5 (GAS5) is another interesting example of multifunctional lncRNAs. It works as a multiple nuclear receptor decoy, forming an RNA stem–loop structure that mimics nuclear receptor DNA response elements. For example, it interacts with glucocorticoid DNA binding domain working as a decoy for glucocorticoid receptor response element [83]. As a consequence, the glucocorticoid receptor is liberated from its sites of transcriptional activity. Therefore, the overexpression of GAS5 blocks cell growths and induces apoptosis in adherent human cell lines. On the other hand, its reduced expression has been observed in human breast cancer cell lines, indicating a possible involvement of this lncRNA in breast carcinogenesis [84].
lncRNAs can also travel via EVs to nearby or distant cells, where they can induce specific phenotypical changes in a paracrine and endocrine way [31]. The most interesting examples in cancer apply to drug resistance, angiogenesis promotion, and tumorigenesis induction [74].
The ability of tumor cells to disseminate the drug-resistant phenotype via exosomes has been recognized mainly through transferring of miRNAs and drug-efflux pumps [85]. However, there is substantial evidence supporting a role for lncRNAs embedded in exosomes in this mechanism. Expression levels of exosomal lncRNAs are greatly different from those of the donor cells, and there is evidence that lncRNAs are not randomly secreted in EVs [86,87].
The role of EVs and lncRNAs in tumor progression and aggressiveness has been demonstrated in several studies reviewed by Andaloussi et al. [55]. The lncRNA called metastasis-associated lung adenocarcinoma transcript (MALAT1) regulates alternative splicing and gene expression [88,89] contributing to lung cancer metastasis [90]. In addition, high levels of MALAT1 have been detected in serum exosomes from non-small cell lung cancer patients and connected with the promotion of cell proliferation and migration of this cancer [91].
Notably, Qu and collaborators demonstrated, in an elegant way, that lncRNA activated in renal cell carcinoma with sunitinib resistance (lncARSR) is correlated with poor response to sunitinib, a drug used for the treatment of advanced renal cell carcinoma. The resistance to the drug was directly induced by lncARSR that works as a ceRNA for miR-34/miR-449 to facilitate the expression of specific genes implicated in the sunitinib resistance. Most interestingly, the authors found that lncARSR is incorporated into exosomes and transmitted to sensitive cells for the dissemination of the resistance in a hormone-like fashion. The transmission of resistance is not only between tumor cells but also involves endothelial cells, implicating that the exosome-mediated communication is also between tumor and stromal cells [76]. The exosomal secretion of lncRNAs is highly selective and different between normal and cancer cells or between sensitive and resistant cells, therefore, identifying cellular molecules responsible for RNA secretion may help in finding a strategy to block this cell-specific mechanism [76].
Lang and collaborators found that glioma cells were enriched in POU class 3 homeobox 3 (POUF3) lncRNA. These cells were able to release POUF3 into the exosomes and target the surrounding normal tissue, inducing cell proliferation, migration, and angiogenesis in an in vivo model [92].
In the last years, another lncRNA, the colon cancer-associated transcript 2 (CCAT2), attracted the attention of researchers because of its dysregulation in cancer [65,66,93,94]. Notably, CCAT2 has been demonstrated to work in a hormone-like fashion [95]. Our group demonstrated an important role of CCAT2 in regulating MYC, miR-17-5p, and miR-20a [96]. Interestingly, CCAT2 interacts with these targets through TCF7L2 enhancing the WNT signaling activity. However, it has been demonstrated that CCAT2 is itself a WNT downstream target. Therefore, in colon cancer, there is a feedback loop mechanism between MYC, WNT, and CCAT2 [96]. Moreover, CCAT2 released in exosomes by glioma cells has also been found to be responsible of angiogenesis induction and apoptosis inhibition in endothelial cells [92]. The pro-angiogenesis phenotype of endothelial cells can be induced also by H19, another important lncRNA in carcinogenesis. Conigliaro et al. found that CD90+ liver cancer cells can reprogram endothelial cells by releasing H19-enriched exosomes [97].
4. Conclusions
In conclusion, there is an increasing interest in circulating ncRNAs as mediators of cell–cell communication and regulators of gene expression in recipient cells. The concept that an ncRNA might function as a hormone (i.e., mediating cells communication) is a challenge for the research community, and the current knowledge is still insufficient for clarifying this topic. Understanding the role of exogenous ncRNAs that could work as messengers in inter-individual and cross-species molecular communication is one of the next scientific targets for researchers. There is high potential for clinical applications not only as diagnostic or prognostic biomarkers but also as therapeutics [98]. Given the rapid and extensive progress made in the field of ncRNAs in the last decade, in the near future, researchers will be able to address these challenges.
Funding
B.P. was supported by a Fulbright Research Scholarships (year 2018). G.A.C. is the Felix L. Haas Endowed Professor in Basic Science. Work in G.A.C.’s laboratory is supported by National Institutes of Health (NIH/NCATS) grant UH3TR00943-01 through the NIH Common Fund, Office of Strategic Coordination (OSC), the NCI grants 1R01 CA182905-01 and 1R01CA222007-01A1, an NIGMS 1R01GM122775-01 grant, a U54 grant #CA096297/CA096300—UPR/MDACC Partnership for Excellence in Cancer Research 2016 Pilot Project, a Team DOD (CA160445P1) grant, a Chronic Lymphocytic Leukemia Moonshot Flagship project, a Sister Institution Network Fund (SINF) 2017 grant, and the Estate of C. G. Johnson, Jr.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Bayliss W.M., Starling E.H. On the relation of enterokinase to trypsin. J. Physiol. 1905;32:129–136. doi: 10.1113/jphysiol.1905.sp001069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Henderson J. Ernest Starling and ‘Hormones’: An historical commentary. J. Endocrinol. 2005;184:5–10. doi: 10.1677/joe.1.06000. [DOI] [PubMed] [Google Scholar]
- 3.Litwack G. Hormones and transport systems. Preface. Vitam. Horm. 2015;98:xvii–xviii. doi: 10.1016/S0083-6729(15)00025-4. [DOI] [PubMed] [Google Scholar]
- 4.White A. The interaction of enzymes and hormones. Pediatrics. 1960;26:476–481. [PubMed] [Google Scholar]
- 5.Ameri P., Ferone D. Diffuse Endocrine System, Neuroendocrine Tumors and Immunity: What’s New? Neuroendocrinology. 2012;95:267–276. doi: 10.1159/000334612. [DOI] [PubMed] [Google Scholar]
- 6.Valadi H., Ekstrom K., Bossios A., Sjostrand M., Lee J.J., Lotvall J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 7.Raposo G., Stoorvogel W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013;200:373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bayraktar R., Van Roosbroeck K., Calin G.A. Cell-to-cell communication: MicroRNAs as hormones. Mol. Oncol. 2017;11:1673–1686. doi: 10.1002/1878-0261.12144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Niel G., D’Angelo G., Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018;19:213–228. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- 10.Colombo M., Raposo G., Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 2014;30:255–289. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- 11.Cortez M.A., Bueso-Ramos C., Ferdin J., Lopez-Berestein G., Sood A.K., Calin G.A. MicroRNAs in body fluids--the mix of hormones and biomarkers. Nat. Rev. Clin. Oncol. 2011;8:467–477. doi: 10.1038/nrclinonc.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colombo M., Moita C., van Niel G., Kowal J., Vigneron J., Benaroch P., Manel N., Moita L.F., Thery C., Raposo G. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J. Cell Sci. 2013;126:5553–5565. doi: 10.1242/jcs.128868. [DOI] [PubMed] [Google Scholar]
- 13.Kalra H., Drummen G.P., Mathivanan S. Focus on Extracellular Vesicles: Introducing the Next Small Big Thing. Int. J. Mol. Sci. 2016;17:170. doi: 10.3390/ijms17020170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gangoda L., Boukouris S., Liem M., Kalra H., Mathivanan S. Extracellular vesicles including exosomes are mediators of signal transduction: Are they protective or pathogenic? Proteomics. 2015;15:260–271. doi: 10.1002/pmic.201400234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cieslik M., Chinnaiyan A.M. Cancer transcriptome profiling at the juncture of clinical translation. Nat. Rev. Genet. 2018;19:93–109. doi: 10.1038/nrg.2017.96. [DOI] [PubMed] [Google Scholar]
- 16.Anfossi S., Babayan A., Pantel K., Calin G.A. Clinical utility of circulating non-coding RNAs—An update. Nat. Rev. Clin. Oncol. 2018;15:541–563. doi: 10.1038/s41571-018-0035-x. [DOI] [PubMed] [Google Scholar]
- 17.Gebert L.F.R., MacRae I.J. Regulation of microRNA function in animals. Nat. Rev. Mol. Cell Biol. 2019;20:21–37. doi: 10.1038/s41580-018-0045-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Munker R., Calin G.A. MicroRNA profiling in cancer. Clin. Sci. (Lond.) 2011;121:141–158. doi: 10.1042/CS20110005. [DOI] [PubMed] [Google Scholar]
- 19.Sevignani C., Calin G.A., Nnadi S.C., Shimizu M., Davuluri R.V., Hyslop T., Demant P., Croce C.M., Siracusa L.D. MicroRNA genes are frequently located near mouse cancer susceptibility loci. Proc. Natl. Acad. Sci. USA. 2007;104:8017–8022. doi: 10.1073/pnas.0702177104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shah M.Y., Ferrajoli A., Sood A.K., Lopez-Berestein G., Calin G.A. microRNA Therapeutics in Cancer—An Emerging Concept. EBioMedicine. 2016;12:34–42. doi: 10.1016/j.ebiom.2016.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inui M., Martello G., Piccolo S. MicroRNA control of signal transduction. Nat. Rev. Mol. Cell Biol. 2010;11:252–263. doi: 10.1038/nrm2868. [DOI] [PubMed] [Google Scholar]
- 22.Wang H., Gou X., Jiang T., Ouyang J. The effects of microRNAs on glucocorticoid responsiveness. J. Cancer Res. Clin. Oncol. 2017;143:1005–1011. doi: 10.1007/s00432-017-2388-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abramov R., Fu G., Zhang Y., Peng C. Expression and regulation of miR-17a and miR-430b in zebrafish ovarian follicles. Gen. Comp. Endocrinol. 2013;188:309–315. doi: 10.1016/j.ygcen.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 24.Sen A., Prizant H., Light A., Biswas A., Hayes E., Lee H.J., Barad D., Gleicher N., Hammes S.R. Androgens regulate ovarian follicular development by increasing follicle stimulating hormone receptor and microRNA-125b expression. Proc. Natl. Acad. Sci. USA. 2014;111:3008–3013. doi: 10.1073/pnas.1318978111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McFall T., McKnight B., Rosati R., Kim S., Huang Y., Viola-Villegas N., Ratnam M. Progesterone receptor A promotes invasiveness and metastasis of luminal breast cancer by suppressing regulation of critical microRNAs by estrogen. J. Biol. Chem. 2018;293:1163–1177. doi: 10.1074/jbc.M117.812438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iliopoulos D., Jaeger S.A., Hirsch H.A., Bulyk M.L., Struhl K. STAT3 activation of miR-21 and miR-181b-1 via PTEN and CYLD are part of the epigenetic switch linking inflammation to cancer. Mol. Cell. 2010;39:493–506. doi: 10.1016/j.molcel.2010.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Di Girolamo D., Ambrosio R., De Stefano M.A., Mancino G., Porcelli T., Luongo C., Di Cicco E., Scalia G., Vecchio L.D., Colao A., et al. Reciprocal interplay between thyroid hormone and microRNA-21 regulates hedgehog pathway-driven skin tumorigenesis. J. Clin. Investig. 2016;126:2308–2320. doi: 10.1172/JCI84465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aucher A., Rudnicka D., Davis D.M. MicroRNAs Transfer from Human Macrophages to Hepato-Carcinoma Cells and Inhibit Proliferation. J. Immunol. 2013;191:6250–6260. doi: 10.4049/jimmunol.1301728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bovy N., Blomme B., Freres P., Dederen S., Nivelles O., Lion M., Carnet O., Martial J.A., Noel A., Thiry M., et al. Endothelial exosomes contribute to the antitumor response during breast cancer neoadjuvant chemotherapy via microRNA transfer. Oncotarget. 2015;6:10253–10266. doi: 10.18632/oncotarget.3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nishida-Aoki N., Ochiya T. Interactions between cancer cells and normal cells via miRNAs in extracellular vesicles. Cell. Mol. Life Sci. 2015;72:1849–1861. doi: 10.1007/s00018-014-1811-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tkach M., Thery C. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell. 2016;164:1226–1232. doi: 10.1016/j.cell.2016.01.043. [DOI] [PubMed] [Google Scholar]
- 32.Sung B.H., Ketova T., Hoshino D., Zijlstra A., Weaver A.M. Directional cell movement through tissues is controlled by exosome secretion. Nat. Commun. 2015;6:7164. doi: 10.1038/ncomms8164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Josson S., Gururajan M., Sung S.Y., Hu P., Shao C., Zhau H.E., Liu C., Lichterman J., Duan P., Li Q., et al. Stromal fibroblast-derived miR-409 promotes epithelial-to-mesenchymal transition and prostate tumorigenesis. Oncogene. 2015;34:2690–2699. doi: 10.1038/onc.2014.212. [DOI] [PubMed] [Google Scholar]
- 34.Fabbri M. MicroRNAs and miRceptors: A new mechanism of action for intercellular communication. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2018;373 doi: 10.1098/rstb.2016.0486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Y.J., Liu D.Q., Chen X., Li J., Li L.M., Bian Z., Sun F., Lu J.W., Yin Y.A., Cai X., et al. Secreted Monocytic miR-150 Enhances Targeted Endothelial Cell Migration. Mol. Cell. 2010;39:133–144. doi: 10.1016/j.molcel.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 36.Huang-Doran I., Zhang C.Y., Vidal-Puig A. Extracellular Vesicles: Novel Mediators of Cell Communication In Metabolic Disease. Trends Endocrinol. Metab. 2017;28:3–18. doi: 10.1016/j.tem.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 37.Ying W., Riopel M., Bandyopadhyay G., Dong Y., Birmingham A., Seo J.B., Ofrecio J.M., Wollam J., Hernandez-Carretero A., Fu W., et al. Adipose Tissue Macrophage-Derived Exosomal miRNAs Can Modulate In Vivo and In Vitro Insulin Sensitivity. Cell. 2017;171:372–384.e12. doi: 10.1016/j.cell.2017.08.035. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Y., Shi L., Mei H., Zhang J., Zhu Y., Han X., Zhu D. Inflamed macrophage microvesicles induce insulin resistance in human adipocytes. Nutr. Metab. (Lond.) 2015;12:21. doi: 10.1186/s12986-015-0016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Costa-Silva B., Aiello N.M., Ocean A.J., Singh S., Zhang H., Thakur B.K., Becker A., Hoshino A., Mark M.T., Molina H., et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat. Cell Biol. 2015;17:816–826. doi: 10.1038/ncb3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Le M.T.N., Hamar P., Guo C.Y., Basar E., Perdigao-Henriques R., Balaj L., Lieberman J. miR-200-containing extracellular vesicles promote breast cancer cell metastasis. J. Clin. Investig. 2014;124:5109–5128. doi: 10.1172/JCI75695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pigati L., Yaddanapudi S.C., Iyengar R., Kim D.J., Hearn S.A., Danforth D., Hastings M.L., Duelli D.M. Selective release of microRNA species from normal and malignant mammary epithelial cells. PLoS ONE. 2010;5:e13515. doi: 10.1371/journal.pone.0013515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anfossi S., Fu X., Nagvekar R., Calin G.A. MicroRNAs, Regulatory Messengers Inside and Outside Cancer Cells. Adv. Exp. Med. Biol. 2018;1056:87–108. doi: 10.1007/978-3-319-74470-4_6. [DOI] [PubMed] [Google Scholar]
- 43.Jin X.C., Chen Y.F., Chen H.B., Fei S.R., Chen D.D., Cai X.N., Liu L., Lin B.C., Su H.F., Zhao L.H., et al. Evaluation of Tumor-Derived Exosomal miRNA as Potential Diagnostic Biomarkers for Early-Stage Non-Small Cell Lung Cancer Using Next-Generation Sequencing. Clin. Cancer Res. 2017;23:5311–5319. doi: 10.1158/1078-0432.CCR-17-0577. [DOI] [PubMed] [Google Scholar]
- 44.Matsumura T., Sugimachi K., Iinuma H., Takahashi Y., Kurashige J., Sawada G., Ueda M., Uchi R., Ueo H., Takano Y., et al. Exosomal microRNA in serum is a novel biomarker of recurrence in human colorectal cancer. Br. J. Cancer. 2015;113:275–281. doi: 10.1038/bjc.2015.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sugimachi K., Matsumura T., Hirata H., Uchi R., Ueda M., Ueo H., Shinden Y., Iguchi T., Eguchi H., Shirabe K., et al. Identification of a bona fide microRNA biomarker in serum exosomes that predicts hepatocellular carcinoma recurrence after liver transplantation. Br. J. Cancer. 2015;112:532–538. doi: 10.1038/bjc.2014.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schoepp M., Strose A.J., Haier J. Dysregulation of miRNA Expression in Cancer Associated Fibroblasts (CAFs) and Its Consequences on the Tumor Microenvironment. Cancers. 2017;9:54. doi: 10.3390/cancers9060054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mulcahy L.A., Pink R.C., Carter D.R. Routes and mechanisms of extracellular vesicle uptake. J. Extracell. Vesicles. 2014;3 doi: 10.3402/jev.v3.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Berindan-Neagoe I., Calin G.A. Molecular pathways: MicroRNAs, cancer cells, and microenvironment. Clin. Cancer Res. 2014;20:6247–6253. doi: 10.1158/1078-0432.CCR-13-2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baroni S., Romero-Cordoba S., Plantamura I., Dugo M., D’Ippolito E., Cataldo A., Cosentino G., Angeloni V., Rossini A., Daidone M.G., et al. Exosome-mediated delivery of miR-9 induces cancer-associated fibroblast-like properties in human breast fibroblasts. Cell Death Dis. 2016;7:e2312. doi: 10.1038/cddis.2016.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen X., Ying X., Wang X., Wu X., Zhu Q. Exosomes derived from hypoxic epithelial ovarian cancer deliver microRNA-940 to induce macrophage M2 polarization. Oncol. Rep. 2017;38:522–528. doi: 10.3892/or.2017.5697. [DOI] [PubMed] [Google Scholar]
- 51.Eiring A.M., Harb J.G., Neviani P., Garton C., Oaks J.J., Spizzo R., Liu S., Schwind S., Santhanam R., Hickey C.J., et al. miR-328 functions as an RNA decoy to modulate hnRNP E2 regulation of mRNA translation in leukemic blasts. Cell. 2010;140:652–665. doi: 10.1016/j.cell.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fabbri M., Paone A., Calore F., Galli R., Gaudio E., Santhanam R., Lovat F., Fadda P., Mao C., Nuovo G.J., et al. MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proc. Natl. Acad. Sci. USA. 2012;109:E2110–E2116. doi: 10.1073/pnas.1209414109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Patel S.A., Gooderham N.J. IL6 Mediates Immune and Colorectal Cancer Cell Cross-talk via miR-21 and miR-29b. Mol. Cancer Res. 2015;13:1502–1508. doi: 10.1158/1541-7786.MCR-15-0147. [DOI] [PubMed] [Google Scholar]
- 54.Challagundla K.B., Wise P.M., Neviani P., Chava H., Murtadha M., Xu T., Kennedy R., Ivan C., Zhang X., Vannini I., et al. Exosome-mediated transfer of microRNAs within the tumor microenvironment and neuroblastoma resistance to chemotherapy. J. Natl. Cancer Inst. 2015;107 doi: 10.1093/jnci/djv135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.EL Andaloussi S., Mager I., Breakefield X.O., Wood M.J. Extracellular vesicles: Biology and emerging therapeutic opportunities. Nat. Rev. Drug Discov. 2013;12:347–357. doi: 10.1038/nrd3978. [DOI] [PubMed] [Google Scholar]
- 56.Wei Y., Lai X., Yu S., Chen S., Ma Y., Zhang Y., Li H., Zhu X., Yao L., Zhang J. Exosomal miR-221/222 enhances tamoxifen resistance in recipient ER-positive breast cancer cells. Breast Cancer Res. Treat. 2014;147:423–431. doi: 10.1007/s10549-014-3037-0. [DOI] [PubMed] [Google Scholar]
- 57.Donnarumma E., Fiore D., Nappa M., Roscigno G., Adamo A., Iaboni M., Russo V., Affinito A., Puoti I., Quintavalle C., et al. Cancer-associated fibroblasts release exosomal microRNAs that dictate an aggressive phenotype in breast cancer. Oncotarget. 2017;8:19592–19608. doi: 10.18632/oncotarget.14752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang L., Hou D., Chen X., Li D., Zhu L., Zhang Y., Li J., Bian Z., Liang X., Cai X., et al. Exogenous plant MIR168a specifically targets mammalian LDLRAP1: Evidence of cross-kingdom regulation by microRNA. Cell Res. 2012;22:107–126. doi: 10.1038/cr.2011.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou Z., Li X., Liu J., Dong L., Chen Q., Kong H., Zhang Q., Qi X., Hou D., Zhang L., et al. Honeysuckle-encoded atypical microRNA2911 directly targets influenza A viruses. Cell Res. 2015;25:39–49. doi: 10.1038/cr.2014.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li Z., Xu R., Li N. MicroRNAs from plants to animals, do they define a new messenger for communication? Nutr. Metab. (Lond.) 2018;15:68. doi: 10.1186/s12986-018-0305-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xie W., Weng A., Melzig M.F. MicroRNAs as New Bioactive Components in Medicinal Plants. Planta Med. 2016;82:1153–1162. doi: 10.1055/s-0042-108450. [DOI] [PubMed] [Google Scholar]
- 62.Wilusz J.E., Sunwoo H., Spector D.L. Long noncoding RNAs: Functional surprises from the RNA world. Genes Dev. 2009;23:1494–1504. doi: 10.1101/gad.1800909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sun M., Kraus W.L. From Discovery to Function: The Expanding Roles of Long Non-Coding RNAs in Physiology and Disease. Endocr. Rev. 2015 doi: 10.1210/er.2014-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ferdin J., Nishida N., Wu X., Nicoloso M.S., Shah M.Y., Devlin C., Ling H., Shimizu M., Kumar K., Cortez M.A., et al. HINCUTs in cancer: Hypoxia-induced noncoding ultraconserved transcripts. Cell Death Differ. 2013;20:1675–1687. doi: 10.1038/cdd.2013.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Redis R.S., Vela L.E., Lu W., Ferreira de Oliveira J., Ivan C., Rodriguez-Aguayo C., Adamoski D., Pasculli B., Taguchi A., Chen Y., et al. Allele-Specific Reprogramming of Cancer Metabolism by the Long Non-coding RNA CCAT2. Mol. Cell. 2016;61:640. doi: 10.1016/j.molcel.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 66.Shah M.Y., Ferracin M., Pileczki V., Chen B., Redis R., Fabris L., Zhang X., Ivan C., Shimizu M., Rodriguez-Aguayo C., et al. Cancer-associated rs6983267 SNP and its accompanying long noncoding RNA CCAT2 induce myeloid malignancies via unique SNP-specific RNA mutations. Genome Res. 2018;28:432–447. doi: 10.1101/gr.225128.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Djebali S., Davis C.A., Merkel A., Dobin A., Lassmann T., Mortazavi A., Tanzer A., Lagarde J., Lin W., Schlesinger F., et al. Landscape of transcription in human cells. Nature. 2012;489:101–108. doi: 10.1038/nature11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wu W., Wagner E.K., Hao Y., Rao X., Dai H., Han J., Chen J., Storniolo A.M., Liu Y., He C. Tissue-specific Co-expression of Long Non-coding and Coding RNAs Associated with Breast Cancer. Sci. Rep. 2016;6:32731. doi: 10.1038/srep32731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhao J., Ohsumi T.K., Kung J.T., Ogawa Y., Grau D.J., Sarma K., Song J.J., Kingston R.E., Borowsky M., Lee J.T. Genome-wide identification of polycomb-associated RNAs by RIP-seq. Mol. Cell. 2010;40:939–953. doi: 10.1016/j.molcel.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gong C., Maquat L.E. lncRNAs transactivate STAU1-mediated mRNA decay by duplexing with 3’ UTRs via Alu elements. Nature. 2011;470:284–288. doi: 10.1038/nature09701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang P., Xue Y., Han Y., Lin L., Wu C., Xu S., Jiang Z., Xu J., Liu Q., Cao X. The STAT3-binding long noncoding RNA lnc-DC controls human dendritic cell differentiation. Science. 2014;344:310–313. doi: 10.1126/science.1251456. [DOI] [PubMed] [Google Scholar]
- 72.Long Y., Wang X., Youmans D.T., Cech T.R. How do lncRNAs regulate transcription? Sci. Adv. 2017;3:eaao2110. doi: 10.1126/sciadv.aao2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yanez-Mo M., Siljander P.R., Andreu Z., Zavec A.B., Borras F.E., Buzas E.I., Buzas K., Casal E., Cappello F., Carvalho J., et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles. 2015;4:27066. doi: 10.3402/jev.v4.27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dragomir M., Chen B., Calin G.A. Exosomal lncRNAs as new players in cell-to-cell communication. Transl. Cancer Res. 2018;7:S243–S252. doi: 10.21037/tcr.2017.10.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huang X., Yuan T., Liang M., Du M., Xia S., Dittmar R., Wang D., See W., Costello B.A., Quevedo F., et al. Exosomal miR-1290 and miR-375 as prognostic markers in castration-resistant prostate cancer. Eur. Urol. 2015;67:33–41. doi: 10.1016/j.eururo.2014.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Qu L., Ding J., Chen C., Wu Z.J., Liu B., Gao Y., Chen W., Liu F., Sun W., Li X.F., et al. Exosome-Transmitted lncARSR Promotes Sunitinib Resistance in Renal Cancer by Acting as a Competing Endogenous RNA. Cancer Cell. 2016;29:653–668. doi: 10.1016/j.ccell.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 77.Cesana M., Cacchiarelli D., Legnini I., Santini T., Sthandier O., Chinappi M., Tramontano A., Bozzoni I. A long noncoding RNA controls muscle differentiation by functioning as a competing endogenous RNA. Cell. 2011;147:358–369. doi: 10.1016/j.cell.2011.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Salmena L., Poliseno L., Tay Y., Kats L., Pandolfi P.P. A ceRNA hypothesis: The Rosetta Stone of a hidden RNA language? Cell. 2011;146:353–358. doi: 10.1016/j.cell.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fatica A., Bozzoni I. Long non-coding RNAs: New players in cell differentiation and development. Nat. Rev. Genet. 2014;15:7–21. doi: 10.1038/nrg3606. [DOI] [PubMed] [Google Scholar]
- 80.Lanz R.B., McKenna N.J., Onate S.A., Albrecht U., Wong J., Tsai S.Y., Tsai M.J., O’Malley B.W. A steroid receptor coactivator, SRA, functions as an RNA and is present in an SRC-1 complex. Cell. 1999;97:17–27. doi: 10.1016/S0092-8674(00)80711-4. [DOI] [PubMed] [Google Scholar]
- 81.Hatchell E.C., Colley S.M., Beveridge D.J., Epis M.R., Stuart L.M., Giles K.M., Redfern A.D., Miles L.E., Barker A., MacDonald L.M., et al. SLIRP, a small SRA binding protein, is a nuclear receptor corepressor. Mol. Cell. 2006;22:657–668. doi: 10.1016/j.molcel.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 82.Cooper C., Guo J., Yan Y., Chooniedass-Kothari S., Hube F., Hamedani M.K., Murphy L.C., Myal Y., Leygue E. Increasing the relative expression of endogenous non-coding Steroid Receptor RNA Activator (SRA) in human breast cancer cells using modified oligonucleotides. Nucleic Acids Res. 2009;37:4518–4531. doi: 10.1093/nar/gkp441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mayama T., Marr A.K., Kino T. Differential Expression of Glucocorticoid Receptor Noncoding RNA Repressor Gas5 in Autoimmune and Inflammatory Diseases. Horm. Metab. Res. 2016;48:550–557. doi: 10.1055/s-0042-106898. [DOI] [PubMed] [Google Scholar]
- 84.Mourtada-Maarabouni M., Pickard M.R., Hedge V.L., Farzaneh F., Williams G.T. GAS5, a non-protein-coding RNA, controls apoptosis and is downregulated in breast cancer. Oncogene. 2009;28:195–208. doi: 10.1038/onc.2008.373. [DOI] [PubMed] [Google Scholar]
- 85.Sousa D., Lima R.T., Vasconcelos M.H. Intercellular Transfer of Cancer Drug Resistance Traits by Extracellular Vesicles. Trends Mol. Med. 2015;21:595–608. doi: 10.1016/j.molmed.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 86.Chen M., Xu R., Ji H., Greening D.W., Rai A., Izumikawa K., Ishikawa H., Takahashi N., Simpson R.J. Transcriptome and long noncoding RNA sequencing of three extracellular vesicle subtypes released from the human colon cancer LIM1863 cell line. Sci. Rep. 2016;6:38397. doi: 10.1038/srep38397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Koldemir O., Ozgur E., Gezer U. Accumulation of GAS5 in exosomes is a marker of apoptosis induction. Biomed. Rep. 2017;6:358–362. doi: 10.3892/br.2017.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tripathi V., Ellis J.D., Shen Z., Song D.Y., Pan Q., Watt A.T., Freier S.M., Bennett C.F., Sharma A., Bubulya P.A., et al. The nuclear-retained noncoding RNA MALAT1 regulates alternative splicing by modulating SR splicing factor phosphorylation. Mol. Cell. 2010;39:925–938. doi: 10.1016/j.molcel.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yang L., Lin C., Liu W., Zhang J., Ohgi K.A., Grinstein J.D., Dorrestein P.C., Rosenfeld M.G. ncRNA- and Pc2 methylation-dependent gene relocation between nuclear structures mediates gene activation programs. Cell. 2011;147:773–788. doi: 10.1016/j.cell.2011.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schmidt L.H., Spieker T., Koschmieder S., Schaffers S., Humberg J., Jungen D., Bulk E., Hascher A., Wittmer D., Marra A., et al. The long noncoding MALAT-1 RNA indicates a poor prognosis in non-small cell lung cancer and induces migration and tumor growth. J. Thorac. Oncol. 2011;6:1984–1992. doi: 10.1097/JTO.0b013e3182307eac. [DOI] [PubMed] [Google Scholar]
- 91.Zhang R., Xia Y., Wang Z., Zheng J., Chen Y., Li X., Wang Y., Ming H. Serum long non coding RNA MALAT-1 protected by exosomes is up-regulated and promotes cell proliferation and migration in non-small cell lung cancer. Biochem. Biophys. Res. Commun. 2017;490:406–414. doi: 10.1016/j.bbrc.2017.06.055. [DOI] [PubMed] [Google Scholar]
- 92.Lang H.L., Hu G.W., Zhang B., Kuang W., Chen Y., Wu L., Xu G.H. Glioma cells enhance angiogenesis and inhibit endothelial cell apoptosis through the release of exosomes that contain long non-coding RNA CCAT2. Oncol. Rep. 2017;38:785–798. doi: 10.3892/or.2017.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Redis R.S., Sieuwerts A.M., Look M.P., Tudoran O., Ivan C., Spizzo R., Zhang X., de Weerd V., Shimizu M., Ling H., et al. CCAT2, a novel long non-coding RNA in breast cancer: Expression study and clinical correlations. Oncotarget. 2013;4:1748–1762. doi: 10.18632/oncotarget.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ozawa T., Matsuyama T., Toiyama Y., Takahashi N., Ishikawa T., Uetake H., Yamada Y., Kusunoki M., Calin G., Goel A. CCAT1 and CCAT2 long noncoding RNAs, located within the 8q.24.21 ‘gene desert’, serve as important prognostic biomarkers in colorectal cancer. Ann. Oncol. 2017;28:1882–1888. doi: 10.1093/annonc/mdx248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fosselteder J., Calin G.A., Pichler M. Long non-coding RNA CCAT2 as a therapeutic target in colorectal cancer. Expert Opin. Ther. Targets. 2018;22:973–976. doi: 10.1080/14728222.2018.1541453. [DOI] [PubMed] [Google Scholar]
- 96.Ling H., Spizzo R., Atlasi Y., Nicoloso M., Shimizu M., Redis R.S., Nishida N., Gafa R., Song J., Guo Z., et al. CCAT2, a novel noncoding RNA mapping to 8q24, underlies metastatic progression and chromosomal instability in colon cancer. Genome Res. 2013;23:1446–1461. doi: 10.1101/gr.152942.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Conigliaro A., Costa V., Lo Dico A., Saieva L., Buccheri S., Dieli F., Manno M., Raccosta S., Mancone C., Tripodi M., et al. CD90+ liver cancer cells modulate endothelial cell phenotype through the release of exosomes containing H19 lncRNA. Mol. Cancer. 2015;14:155. doi: 10.1186/s12943-015-0426-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bullock M.D., Silva A.M., Kanlikilicer-Unaldi P., Filant J., Rashed M.H., Sood A.K., Lopez-Berestein G., Calin G.A. Exosomal Non-Coding RNAs: Diagnostic, Prognostic and Therapeutic Applications in Cancer. Noncoding RNA. 2015;1:53–68. doi: 10.3390/ncrna1010053. [DOI] [PMC free article] [PubMed] [Google Scholar]