Abstract
This study was conducted to determine the effect of Bacillus subtilis ANSB060 biodegradation product (BDP) in reducing the milk aflatoxin M1 (AFM1) content of dairy cows fed a diet contaminated with aflatoxin B1 (AFB1). Twenty-four Chinese Holstein cows (254 ± 19 d in milk; milk production 19.0 ± 1.2 kg d−1) were assigned to three dietary treatments, as follows: (1) control diet (CON), consisting of a basal total mixed ration (TMR); (2) aflatoxin diet (AF), containing CON plus 63 μg of AFB1 kg−1 of diet dry matter; and (3) aflatoxin diet plus BDP (AF + BDP), containing AF plus BDP at 0.2% of diet dry matter. The experiment lasted 12 days, including an AFB1-dosing period from days one to eight, followed by a clearance period from days nine to twelve. Milk samples were collected on days 2, 4, 6, and 8–12, and the plasma was sampled on day 9, before morning feeding. Short-term AFB1 exposure did not affect the milk production and composition. The plasma biochemical indices, except for lactic dehydrogenase (LDH), were also not changed by the AFB1 intake. The plasma LDH level was significantly elevated (p < 0.05) following dietary treatment with AFB1, while no significant difference was observed between the AF + BDP and CON treatments. Adding BDP to the AFB1-contaminaed diet resulted in a significant reduction in AFM1 concentration (483 vs. 665 ng L−1) in the milk, AFM1 excretion (9.14 vs. 12.71 μg d−1), and transfer rate of dietary AFB1 to milk AFM1 (0.76 vs. 1.06%). In conclusion, the addition of BDP could be an alternative method for reducing the dietary AFB1 bioavailability in dairy cows.
Keywords: Bacillus subtilis ANSB060, aflatoxin B1, aflatoxin M1, milk, dairy cows
1. Introduction
Aflatoxins (AF) are harmful secondary metabolites mainly produced by Aspergillus flavus and Aspergillus parasiticus fungi. Among the eighteen different types of aflatoxins, the major naturally-occurring members are aflatoxin B1, B2, G1, and G2. AFB1 is the most prevalent and toxic, and has been classified as a Group I human carcinogen by the International Agency for Research on Cancer (IARC). After ingestion by livestock animals, AFB1 is partly bio-transformed into aflatoxin M1 (AFM1) in the liver by the mitochondrial cytochrome P450 oxidative system, which is then secreted into the milk of lactating animals, including dairy cattle. The carcinogenicity of AFM1 is about 10 times lower than that of AFB1; however, unlike AFB1, AFM1 exerts a direct cytotoxicity on human cells in the absence of metabolic activation. The transfer rate of dietary AFB1 to milk AFM1 mainly depends on the milk yield, and is usually 1%–2% for low-yielding cows (30 kg milk yield per day) and up to ~6% for high-yielding cows (>30 kg milk yield per day) [1]. Milk contamination with AFM1 has attracted worldwide attention because of the high consumption of milk and dairy products by humans, especially children. Considering the health risks associated with the human dietary exposure to AFM1, more than 60 countries have set strict guidelines for maximum residue level (MRL) of AFM1 in milk [2]. While in the United States and China, the maximum allowable concentration of AFM1 in fluid milk is 0.5 μg L–1, the legal limit is much more stringent in the European Union, where the level is set at 0.05 μg L−1. To avoid carry-over, the maximum permissible amount of AFB1 in dairy feed has also been established, ranging from 20 μg kg−1 in the United States, to 10 μg kg−1 in China, and 5 μg kg−1 in the European Union.
The pre-harvest prevention of aflatoxins occurrence and the post-harvest elimination of contamination are the main strategies to reduce aflatoxicosis in human and animals [3]. The application of good agricultural practices (GAPs), such as crop rotation, harvesting at the right time, control of insect damage, and choice of fungal resistant varieties, is helpful for inhibiting fungal growth and aflatoxins production. Meanwhile, strategies for post-harvest decontamination include physical, chemical, or biological methods. Physical treatments like thermal inactivation, irradiation, and extrusion generally do not comply with the cost and productivity requirements for commercialization [4]. The addition of mycotoxin binders to contaminated diets is also a physical method, which has been widely applied to reduce AFB1 absorption in dairy cows. Common types of mycotoxin binders include calcium montmorillonite clay [5], aluminosilicate clay [6], and yeast cell culture [7]. However, some of these adsorbents may also bind minerals, vitamins, and amino acids in feeds [8], as well as reducing the efficiency of the pharmacokinetics of antibiotics [9]. The use of chemical methods comprising ammoniation, ozonation, and peroxidation in food and feeds is limited as a result of the potential toxicity of chemical residues [10]. The biological degradation of aflatoxins, using microorganisms and enzymes, has been considered to be a promising strategy for lessening the negative effects of dietary AFB1 in animals. The major advantage of biological detoxification methods is that the enzymes that come from microbes can transform aflatoxins into non-toxic or less toxic metabolites under mild conditions, with a minor impact on the palatability and nutritive quality of food and feeds [11].
Some species of microbes, including fungal and bacterial strains, such as Armillariella tabescens [12], Pleurotus pulmonarius [13], Rhodococcus erythropolis [14], Bacillus subtilis [15], and Bacillus licheniformis [16], have been reported to biodegrade AFB1 in vitro. However, few studies have evaluated their detoxification efficiency in vivo. B. subtilis ANSB060 was isolated from fish gut, which can degrade AFB1, AFM1, and AFG1 by 81.5%, 60%, and 80.7%, respectively, in liquid culture [15]. In addition, B. subtilis ANSB060 has been shown to effectively alleviate aflatoxicosis in layers [17], broilers [18], ducks [19], and carps [20]. The dietary supplementation of a B. subtilis ANSB060 fermentation product can also reduce the accumulation of AFB1 and AFM1 in the liver of birds, suggesting that B. subtilis ANSB060 can degrade aflatoxins in animal gastrointestinal tracts. B. subtilis is a transient microorganism of the digestive tract, and the bacteria is capable of forming spores resistant to heat and a low gastric pH. Furthermore, B. subtilis is generally recognized as safe (GRAS) status for nutritional and pharmaceutical use. Spores of the Bacillus species have been used as direct-fed microbial feed additives for ruminants [21,22]. Thus, the aim of this study was to investigate the effectiveness of the B. subtilis ANSB060 biodegradation product (BDP) in reducing the AFM1 content in the milk of dairy cows exposed to AFB1.
2. Results and Discussion
2.1. Production Performances
Over the 12-day feeding trial, all of the cows in the three treatments behaved normally, without observed clinical signs of aflatoxicosis. As shown in Table 1, there were no treatment differences on the milk production and components (milk protein, fat, lactose, milk urea nitrogen (MUN), and somatic cell count (SCC)) of the dairy cows. In accordance with the results of our study, the report of Kutz et al. [23] showed that the incorporation of 112 µg kg−1 of AFB1 to the diet did not affect the feed intake, milk production, and component concentrations of dairy cows. Consistently, Maki et al. [5] and Sulzberger et al. [24] also found no changes in the milk yield and composition of dairy cows in early to mid-lactation when administrated with AFB1 at doses of 79 and 100 µg kg−1 in the feed. However, Queiroz et al. [25] reported that dosing the cows with AFB1 at 75 µg kg−1 of diet resulted in a significant reduction of milk protein concentration and milk fat yield. It should be noted that the cows were exposed to naturally contaminated diets, which might have contained multiple mycotoxins in the study of Queiroz et al. [25]. The co-exposure to the mycotoxin combinations may have led to more adverse effects on the cows than purified AFB1, due to the possible additive or synergic effect.
Table 1.
Effect of the dietary addition of aflatoxin B1 (AFB1) with or without Bacillus subtilis ANSB060 biodegradation product (BDP) on the milk production and composition of dairy cows (n = 8).
| Item | Dietary Treatment 1 | SEM | p-Value | ||
|---|---|---|---|---|---|
| CON | AF | AF + BDP | |||
| Milk yield (kg d−1) | 20.18 | 19.33 | 19.32 | 0.83 | 0.71 |
| Fat (%) | 3.43 | 3.34 | 3.37 | 0.18 | 0.94 |
| Protein (%) | 3.34 | 3.48 | 3.31 | 0.09 | 0.40 |
| Lactose (%) | 4.86 | 4.75 | 4.89 | 0.12 | 0.69 |
| MUN 2 (mg dL−1) | 11.38 | 12.07 | 11.88 | 0.53 | 0.65 |
| SCC 3 (log cells μL−1) | 5.37 | 5.70 | 5.50 | 0.26 | 0.67 |
1 CON: control diet, consisting of a basal total mixed ration (TMR); AF: aflatoxin diet, containing CON plus 63 μg of AFB1 kg−1 of diet dry matter; AF + BDP: aflatoxin diet plus BDP, containing AF plus BDP at 0.2% of diet dry matter. 2 Milk urea nitrogen (MUN). 3 Natural logarithmic value of somatic cell count (SCC) μL−1 of milk. SEM: standard error of the mean.
2.2. Plasma Biochemical Indices
The effects of dosing AFB1 with or without BDP on the plasma parameters of dairy cows are presented in Table 2. The short-term addition of AFB1 to the diet did not cause statistically significant changes in the plasma biochemical indices tested in the current study, except for lactic dehydrogenase (LDH), an indicator widely used to evaluate the presence of cells and tissues damage. The plasma LDH level of the dairy cows was remarkably increased in the AF treatment when compared with that of the CON treatment. In contrast, there was no significant difference in the plasma LDH level between the CON and AF + BDP treatments. To our knowledge, few studies have investigated the impact of AFB1 exposure on the plasma LDH content of dairy cows. A contradictory result was present in the literature regarding the response of ewes to AFB1 challenge, in which the serum LDH level was not affected by the incorporation of AFB1 in the diet [26]. The liver is the main site of AFB1 metabolism, and a major target organ for aflatoxicosis. In agreement with the current study, the main indices of liver injury, like serum alkaline phosphatase (ALP), aspartate amino transferase (AST), and γ-glutamyl transferase (GGT), were not changed in the dairy cows following dietary exposure to 100 µg of AFB1 kg−1 of diet [27]. In addition, Xiong et al. [7,27] also found no effects of dietary AFB1 on the liver function parameters of dairy cows. Taken together, the elevated plasma LDH level in AF treatment might not be due to liver damage in this experiment, and further study is needed in order to understand the increased plasma LDH level in the dairy cows exposed to AFB1.
Table 2.
Effect of dietary addition of aflatoxin B1 (AFB1) with or without Bacillus subtilis ANSB060 biodegradation product (BDP) on plasma metabolite parameters of dairy cows (n = 8).
| Item | Dietary Treatment 1 | SEM | p-Value | ||
|---|---|---|---|---|---|
| CON | AF | AF + BDP | |||
| TP (g dL−1) | 7.10 | 7.41 | 7.23 | 0.21 | 0.57 |
| ALB (g dL−1) | 3.19 | 3.19 | 3.05 | 0.07 | 0.34 |
| AST (U L−1) | 89.63 | 92.13 | 83.63 | 3.09 | 0.16 |
| ALP (U L−1) | 33.13 | 34.13 | 32.38 | 1.88 | 0.81 |
| ALT (U L−1) | 42.50 | 43.88 | 39.75 | 1.84 | 0.29 |
| GGT (U L−1) | 39.38 | 39.88 | 37.13 | 2.89 | 0.78 |
| LDH (U L−1) | 945.88 b | 1106.75 a | 1032.88 ab | 36.52 | 0.02 |
a,b Values in the same row with no common superscript differ significantly(p < 0.05). 1 CON: control diet, consisting of a basal total mixed ration (TMR); AF: aflatoxin diet, containing CON plus 63 μg of AFB1 kg−1 of diet dry matter; AF + BDP: aflatoxin diet plus BDP, containing AF plus BDP at 0.2% of diet dry matter. ALP: alkaline phosphatase; AST: aspartate amino transferase; GGT: γ-glutamyl transferase; TP: total protein; ALB: albumin; LDH: lactate dehydrogenase.
2.3. AFM1 Content in Milk
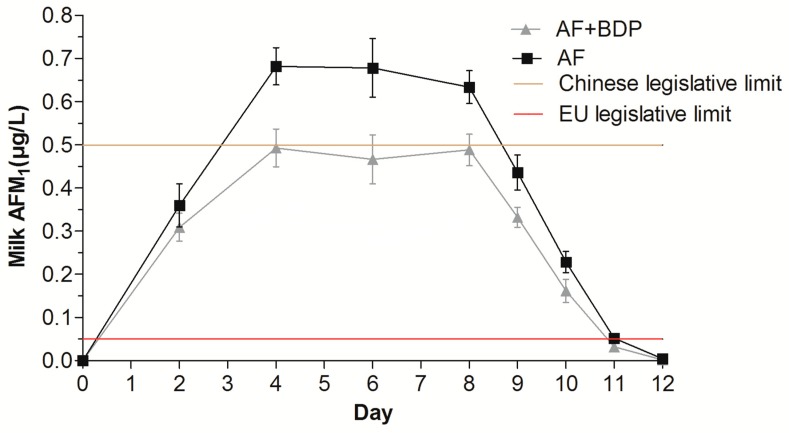
The aflatoxins (AFB1, AFB2, AFG1, and AFG2) contents in the TMR were below the detection limits. Prior to dietary treatment with AFB1, AFM1 was not detected in the milk samples of all of the cows. Also, no AFM1 was found in the milk from the CON treatment during the experimental period. The patterns of milk AFM1 concentration in the AF and AF + BDP treatments are shown in Figure 1. Previous studies have confirmed that AFM1 can be found in the milk of dairy cows [1,28], ewes [29], and goats [30] within 24 h of the first AFB1 administration. In the present study, milk AFM1 reached a mean of 360 and 309 ng L−1 in the AF and AF + BDP treatments, respectively, on the second day of the AFB1-dosing period. The study of Moschini et al. [31] showed that AFB1 could be rapidly absorbed through the rumen wall before reaching the intestine, and could be transformed into AFM1 in the liver of dairy cows as soon as 15 minutes after a single oral intake of 4.9 mg AFB1. A plateau of AFM1 concentration in the milk was observed from day 4 of administration, and the steady state condition was maintained up to the last day of the AFB1-dosing period (day 8). When the AFB1 administration was withdrawn, the milk AFM1 concentration dropped rapidly and was below the Chinese legislative limit (0.5 μg L–1) within 24 h (day 9). The milk AFM1 was not detected by day 12 in both AF and AF + BDP treatments. The time of disappearance of milk AFM1 after the last dietary administration of AFB1 was in agreement with published data. Xiong et al. [7] reported that no AFM1 was detected in the milk of dairy cows after day 3 of the AFB1 withdrawal period, regardless of whether the level of dietary AFB1 was 20 or 40 μg kg−1. Moreover, Queiroz et al. [25] and Ogunade et al. [28] demonstrated that AFM1 cleared from the milk of the dairy cows fed a diet contaminated with AFB1 (75 μg kg−1) 72 h after withdrawing the toxin. These findings suggested that the clearance of AFM1 from milk after the ending of AFB1 administration is not related to the dietary AFB1 content.
Figure 1.
Effect of dietary addition of aflatoxin B1 (AFB1) with or without Bacillus subtilis ANSB060 biodegradation product (BDP) on the concentration of aflatoxin M1 (AFM1) in the milk of dairy cows. AFB1-dosing period = days 1 to 8; clearance period = days 9 to 12. AF: aflatoxin diet, containing control diet (CON), consisting of a basal total mixed ration (TMR) plus 63 μg of AFB1 kg−1 of diet dry matter; AF + BDP: aflatoxin diet plus BDP, containing AF plus BDP at 0.2% of diet dry matter.
The effects of dosing AFB1 with or without BDP on the AFM1 content in the milk of dairy cows are summarized in Table 3. The average AFM1 concentration in milk at steady state was 665 ng L−1 in the AF treatment, 1.33 and 133 times higher than the maximum allowable level set by China (0.5 μg L−1) and the European Union (0.05 μg L−1), respectively, while the mean AFM1 concentration (483 ng L−1) in the AF + BDP treatment was below the Chinese legislative limit. The carryover rate of AFB1 from feed into AFM1 in milk was 1.06% in the AF treatment, and the value was in agreement with the results reported by Maki et al. [5] and Ogunade et al. [28], in which the observed transfer rate was 1.07% and 1.13%, in dairy cows challenged with 100 μg kg−1 and 75 μg kg−1 of AFB1 in their diet, respectively. However, Britzi et al. [1] showed that the carryover rate of AFB1 into AFM1 from high producing cows (milk yield of >35 kg per day) was on average 5.4%. Accumulating evidence has suggested that the milk yield is the main factor contributing to the variability of the transfer rate of feed AFB1 to milk AFM1 in dairy cows [32,33,34]. Britzi et al. [1] described the relationship between the carryover of AFB1 to AFM1 in milk and the milk yield as follows: carry-over% = 0.5154 e0. 0521 × milk yield (r2 = 0. 6224). A linear regression equation was described was proposed by Masoero et al., as follows: carry-over% = −0.326 + 0.077 × milk yield; r2 = 0. 58) [32]. It is noteworthy that the model developed by Masoero et al. [32] fitted the data of the present study well, with the actual transfer rate and estimated transfer rate being 1.06% and 1.16%, respectively.
Table 3.
Effect of the dietary addition of aflatoxin B1 (AFB1) with or without Bacillus subtilis ANSB060 biodegradation product (BDP) on the concentration, excretion, and transfer rate of aflatoxin M1 (AFM1) in the milk of dairy cows at steady-state (days 4 to 8) (n = 8).
| Item | Dietary Treatment 1 | SEM | p-Value | |
|---|---|---|---|---|
| AF | AF + BDP | |||
| AFM1 concentration (ng L−1) | 665 a | 483 b | 9 | <0.01 |
| AFM1 excretion 2 (μg d−1) | 12.71 a | 9.14 b | 1.58 | <0.01 |
| Transfer rate 3 (%) | 1.06 a | 0.76 b | 0.01 | <0.01 |
a,b Values in the same row with no common superscript differ significantly(p < 0.05). 1 CON = basal TMR; AF = basal TMR + 63µg kg−1 AFB1, AF + BDP= basal TMR + 63µg kg−1 AFB1 + 2g kg−1 BDP. 2 AFM1 excretion (μg d−1) = concentration of AFM1 in milk (ng L−1) × milk yield (kg d−1). 3 Transfer rate (%) = excretion of AFM1 (ng d−1)/AFB1 consumption (ng d−1) × 100.
The addition of BDP to the AFB1-contaminated diet resulted in a 27.4% reduction in milk AFM1 concentration, a 28.1% reduction in AFM1 excretion, and a 28.3% reduction in AFB1 transfer from feed to milk. Previous studies have reported that BDP reduced the bioavailability and toxicity of AFB1 in livestock animals. The addition of BPD (0.2%) to an aflatoxins-contaminated diet resulted in a 62.5% and 40.0% reduction of AFB1 and AFM1 residues in the liver of broilers, as well as a 76.7% and 79.9% reduction of AFB1 and AFB2 recovered from duodenal content [18,35]. More recently, Fan et al. [20] also found that incorporating BDP at the level of 0.1% in the diet significantly lowered the AFB1 residues by 94.4% and 92.2% in the hepatopancreas and gonad of Yellow River carps, respectively, during the period of exposure to AFB1. Previous studies have shown that B. subtilis ANSB060 degraded AFB1 by the extracellular constitutively produced enzyme, as the culture supernatant of ANSB060 was able to degrade 78.7% of AFB1 after incubation with the toxin for 72 h [15]. Although it was not clear whether the spores of ANSB060 could germinate in the gastrointestinal tract of dairy cows and whether they displayed an AFB1 degradation activity, the constitutive aflatoxins degrading enzymes in the ANSB060 fermentation product should be responsible for the AFB1 reduction. More research is required to explore this question.
3. Conclusions
Feed contamination with AFB1 is of great concern in the dairy industry, because of the inherent risk of AFM1 residues in milk and milk products intended for human consumption. The inclusion of AFB1 (63 µg kg−1) in the diet increased the milk AFM1 concentration beyond the Chinese action level of 0.5 µg L−1 in lactating dairy cows, under the current study conditions. The addition of Bacillus subtilis ANSB060 biodegradation product to the diet of the cows exposed to AFB1 resulted in a significant reduction in the milk AFM1 concentration (483 vs. 665 ng L−1), AFM1 excretion (9.14 vs.12.71 μg d−1), and AFB1 transfer rate (0.76 vs. 1.06%) from feed to milk. Additional studies are needed in order to investigate the underlying mechanisms involved in the AFB1 detoxification process of B. subtilis ANSB060, and the influences of AFB1 sequestration by BDP in vivo, aiming the commercial application of this product as a biological agent for AFM1 reduction.
4. Materials and Methods
4.1. Animals, Experimental Design and Diets
The cows in this study were cared for according to the protocols approved by the Animal Welfare Committee of the China Agricultural University (ethical approval code: CAU20180630-2; Date: 30 June 2018). Twenty-four Holstein cows, averaging 254 ± 19 day in milk (DIM), were assigned to three groups balanced for milk production, body weight, and parity. Dietary treatments included the following: (1) control diet (CON), consisting of a basal total mixed ration (TMR); (2) aflatoxin diet (AF), containing CON plus 63 μg kg−1 of AFB1 of diet dry matter; (3) aflatoxin diet plus BDP (AF + BDP), containing AF plus BDP at 0.2% of diet dry matter. The BDP consisted of 80% spray-dried fermentation product of B. subtilis ANSB060 and 20% rice husk meal as the carrier. The viable spores of B. subtilis ANSB060 in the BDP were 2 × 109 CFU g−1 by the plate count. The diets were formulated according to the nutrient requirements of the Holstein cows in late lactation producing 20 kg of milk d−1 [36]. The ingredient and chemical compositions of the basal TMR are shown in Table 4. The cows were fed AFB1 and BDP based on an estimated average of 19 kg of dry matter intake (DMI) each day, resulting in a daily intake of 1197 µg of AFB1 (19 kg of DMI × 63 µg of AFB1 kg−1) and 38 g (19 kg of DMI × 0.2%) of BDP. The 12-day trial was divided into an AFB1-dosing phase (days 1 to 8) and a clearing phase (days 9 to 12). The cows were fed the TMR in two equal aliquots daily (at 09:30 and 16:30). During the AFB1-dosing phase, the daily dose of AFB1 and BDP for each animal was divided into two aliquots. Each aliquot of AFB1 was mixed with ground corn, placed into a digestible gelatin capsule, and orally administered with the help of a balling gun. Each aliquot of BDP was mixed with 150 g of ground corn so as to encourage consumption. All of the cows in the three treatments were only fed the basal TMR during the following four-day clearance period. The contents of the mycotoxins (AFB1, AFB2, AFG1, AFG2, deoxynivalenol, T-2 toxin, zearalenone, and ochratoxin A) in TMR were determined as previously described by Li et al. [37]. The health condition of the cows was monitored continuously before and during the entire experiment period.
Table 4.
Ingredients and chemical composition of the basal diet.
| Item | Amount |
|---|---|
| Ingredient composition (% of DM) | |
| Corn silage | 41.5 |
| Alfalfa silage | 14.9 |
| Flaked corn | 12.1 |
| Cottonseed meal | 4.9 |
| Distillers dried grains with soluble (DDGS) | 9.7 |
| Soybean meal (47% CP) | 2.5 |
| Corn gluten feed | 12.2 |
| Mineral and vitamin mix 1 | 2.2 |
| NEL (Mcal kg−1 of DM) | 1.51 |
| Chemical composition (% of DM) | |
| Crude protein (CP) | 15.5 |
| Fat | 3.5 |
| Neutral detergent fiber(NDF) | 42.6 |
| Acid detergent fiber(ADF) | 25.5 |
| Starch | 17.2 |
| Aflatoxins B1, B2, G1, and G2 | ND 2 |
| Deoxynivalenol | ND 2 |
| T-2 toxin | ND 2 |
| Zearalenone | ND 2 |
| Ochratoxin A | ND 2 |
1 The mineral and vitamin mix was formulated with 20% salt, 18% Ca, 10% P, 18 mg kg−1 of Co, 600 mg kg−1 of Cu, 700 mg kg−1 of Mn, 800 mg kg−1 of Zn, 16 mg kg−1 of I, 16 mg kg−1 of Se, 30 mg kg−1 of Fe, 810 mg kg−1 of niacin, 40 mg kg−1 of biotin, 300,000 IU kg−1 of vitamin A, 7600 IU kg−1 of vitamin D3, and 2500 IU kg−1 of vitamin E. 2 ND: not detected. DM: dry matter. CP: crude protein.
4.2. Sample Collection and Analysis
The cows were milked three times (at 08:30, 15:30, and 21:30) daily, and the individual milk yield was recorded at each milking. Milk samples were collected at days 0, 2, 4, 6, 8, 9, 10, 11, and 12, were proportionally mixed according to the milk yield from each milking, and were stored at −20 °C for the analysis of the milk AFM1. Furthermore, one aliquot of the milk samples from day 8 was immediately sent to the Xinjiang Dairy Herd Improvement testing center for an analysis of the milk protein, fat, lactose, milk urea nitrogen (MUN), and somatic cell count (SCC), using a near-infrared reflectance spectroscopy analyzer (Seris300 CombiFOSS; Foss Electric, Hillerød, Denmark). The blood samples were collected from the coccygeal vessel into heparinized evacuated tubes before the morning feeding on day 9, were centrifuged at 2000 rpm for 20 min at 4 °C to prepare the plasma, and were stored at −20 °C for the subsequent analysis. The plasma levels of the total protein (TP); albumin (ALB); and the activities of the enzymes, including aspartate amino transferase (AST), alanine amino transferase (ALT), alkaline phosphatase (ALP), γ-glutamyl transferase (GGT), and lactate dehydrogenase (LDH), were analyzed using an automatic biochemistry autoanalyzer (7160, Hitachi) by the Beijing Sino–UK Institute of Biological Technology (Beijing, China).
4.3. Quantification of Aflatoxin M1 in Milk
The fluid milk samples were incubated at 37 °C for 10 min, and then centrifuged at 5000 rpm for 15 min to separate the fat. Fifty milliliters of supernatant were collected and applied to an immune-affinity column (Clover Technology Group, Beijing, China) at a steady flow rate of 2 mL min−1 using the vacuum system. The column was washed twice with distilled water (10 mL), then eluted with 2 mL of methanol. The eluent was evaporated to dryness at 40 °C under a stream of nitrogen, and reconstituted in 200 μL of distilled water. Chromatographic analyses were performed with an HPLC system (Shimadzu LC-10 AT, Shimadzu, Tokyo, Japan) consisting of a post-column photochemical derivation and a RF-20A fluorescence monitor. The separation of AFM1 was achieved by a reverse phase column (DIKMA, C18, 5μm, 15 × 4.6 mm ID). The mobile phase was isocratic acetonitrile:methanol:water (24:8:68, v/v/v), wherein the flow rate was 1 mL min−1. The wavelengths for excitation and emission were 365 nm and 435 nm, respectively. The injection volume was 20 μL.
4.4. Calculations
AFM1 excretion (μg d−1) = concentration of AFM1 in milk (ng L−1) × milk yield (kg d−1)
Transfer rate (%) = excretion of AFM1 (ng d−1)/AFB1 consumption (ng d−1) × 100%
The SCC values were divided by 1000 and were natural logarithm transformed before analysis.
4.5. Statistical Analysis
The milk yield, milk components, and plasma biochemical parameters were analyzed via one-way ANOVA using SAS (version 9.2; SAS Institute, Cary, NC, USA). Duncan’s multiple range tests were conducted when a significant difference was detected among the treatments. Milk AFM1 content (concentration, excretion, and transfer rate) at steady-state (days 4 to 8) were analyzed by one-way ANOVA with day of collection as repeated measures. The significance level was set at 0.05.
Author Contributions
Formal analysis, Y.Z. and J.Z.; methodology, Y.G., C.J., and L.Z.; project administration, Y.G. and C.W.; supervision, C.J. and L.Z.; writing (original draft), Y.G. and Q.M.; writing (review and editing), L.Z.
Funding
This research was supported by the National Key Research and Development Program of China (No. 2018YFD0500600), the National Natural Science Foundation of China (No. 31772637), and Henan Natural Science Foundation (No. 162300410048).
Conflicts of Interest
All of the authors declare that they have no conflict of interest.
Key Contribution
The study provides an alternative to reducing the milk aflatoxin M1 contamination.
References
- 1.Britzi M., Friedman S., Miron J., Solomon R., Cuneah O., Shimshoni J.A., Soback S., Ashkenazi R., Armer S., Shlosberg A. Carry-over of aflatoxin B1 to aflatoxin M1 in high yielding Israeli cows in mid- and late-lactation. Toxins. 2013;5:173–183. doi: 10.3390/toxins5010173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sadia A., Jabbar M.A., Deng Y., Hussain E.A., Riffat S., Naveed S., Arif M. A survey of aflatoxin M1 in milk and sweets of Punjab, Pakistan. Food Control. 2012;26:235–240. doi: 10.1016/j.foodcont.2012.01.055. [DOI] [Google Scholar]
- 3.Accinelli C., Mencarelli M., Saccà M.L., Vicari A., Abbas H.K. Managing and monitoring of Aspergillus flavus in corn using bioplastic-based formulations. Crop Prot. 2012;32:30–35. doi: 10.1016/j.cropro.2011.10.006. [DOI] [Google Scholar]
- 4.Verheecke C., Liboz T., Mathieu F. Microbial degradation of aflatoxin B1: Current status and future advances. Int. J. Food Microbiol. 2016;237:1–9. doi: 10.1016/j.ijfoodmicro.2016.07.028. [DOI] [PubMed] [Google Scholar]
- 5.Maki C.R., Thomas A.D., Elmore S.E., Romoser A.A., Harvey R.B., Ramirez-Ramirez H.A., Phillips T.D. Effects of calcium montmorillonite clay and aflatoxin exposure on dry matter intake, milk production, and milk composition. J. Dairy Sci. 2016;99:1039–1046. doi: 10.3168/jds.2015-10242. [DOI] [PubMed] [Google Scholar]
- 6.Pate R.T., Paulus Compart D.M., Cardoso F.C. Aluminosilicate clay improves production responses and reduces inflammation during an aflatoxin challenge in lactating Holstein cows. J. Dairy Sci. 2018;101:11421–11434. doi: 10.3168/jds.2018-15024. [DOI] [PubMed] [Google Scholar]
- 7.Xiong J.L., Wang Y.M., Nennich T.D., Li Y., Liu J.X. Transfer of dietary aflatoxin B1 to milk aflatoxin M1 and effect of inclusion of adsorbent in the diet of dairy cows. J. Dairy Sci. 2015;98:2545–2554. doi: 10.3168/jds.2013-7842. [DOI] [PubMed] [Google Scholar]
- 8.Zhu Y., Hassan Y.I., Watts C., Zhou T. Innovative technologies for the mitigation of mycotoxins in animal feed and ingredients—A review of recent patents. Anim. Feed Sci. Tech. 2016;216:19–29. doi: 10.1016/j.anifeedsci.2016.03.030. [DOI] [Google Scholar]
- 9.De Mil T., Devreese M., Broekaert N., Fraeyman S., De Backer P., Croubels S. In vitro adsorption and in vivo pharmacokinetic interaction between doxycycline and frequently used mycotoxin binders in broiler chickens. J. Agric. Food Chem. 2015;63:4370–4375. doi: 10.1021/acs.jafc.5b00832. [DOI] [PubMed] [Google Scholar]
- 10.Loi M., Fanelli F., Liuzzi V.C., Logrieco A.F., Mule G. Mycotoxin biotransformation by native and commercial enzymes: Present and future perspectives. Toxins. 2017;9:111. doi: 10.3390/toxins9040111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vanhoutte I., Audenaert K., De Gelder L. Biodegradation of mycotoxins: Tales from known and unexplored worlds. Front. Microbiol. 2016;7:561. doi: 10.3389/fmicb.2016.00561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cao H., Liu D., Mo X., Xie C., Yao D. A fungal enzyme with the ability of aflatoxin B1 conversion: Purification and ESI-MS/MS identification. Microbiol. Res. 2011;166:475–483. doi: 10.1016/j.micres.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Loi M., Fanelli F., Zucca P., Liuzzi V.C., Quintieri L., Cimmarusti M.T., Monaci L., Haidukowski M., Logrieco A.F., Sanjust E., et al. Aflatoxin B1 and M1 degradation by Lac2 from Pleurotus pulmonarius and redox mediators. Toxins. 2016;8:245. doi: 10.3390/toxins8090245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eshelli M., Harvey L., Edrada-Ebel R., McNeil B. Metabolomics of the bio-degradation process of aflatoxin B1 by actinomycetes at an initial pH of 6.0. Toxins. 2015;7:439–456. doi: 10.3390/toxins7020439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao X., Ma Q., Zhao L., Lei Y., Shan Y., Ji C. Isolation of Bacillus subtilis: Screening for aflatoxins B1, M1, and G1 detoxification. Eur. Food Res. Technol. 2011;232:957–962. doi: 10.1007/s00217-011-1463-3. [DOI] [Google Scholar]
- 16.Rao K.R., Vipin A.V., Hariprasad P., Anu Appaiah K.A., Venkateswaran G. Biological detoxification of aflatoxin B1 by Bacillus licheniformis CFR1. Food Control. 2017;71:234–241. [Google Scholar]
- 17.Ma Q.G., Gao X., Zhou T., Zhao L.H., Fan Y., Li X.Y., Lei Y.P., Ji C., Zhang J.Y. Protective effect of Bacillus subtilis ANSB060 on egg quality, biochemical and histopathological changes in layers exposed to aflatoxin B1. Poult. Sci. 2012;91:2852–2857. doi: 10.3382/ps.2012-02474. [DOI] [PubMed] [Google Scholar]
- 18.Fan Y., Zhao L., Ma Q., Li X., Shi H., Zhou T., Zhang J., Ji C. Effects of Bacillus subtilis ANSB060 on growth performance, meat quality and aflatoxin residues in broilers fed moldy peanut meal naturally contaminated with aflatoxins. Food Chem. Toxicol. 2013;59:748–753. doi: 10.1016/j.fct.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 19.Zhang L., Ma Q., Ma S., Zhang J., Jia R., Ji C., Zhao L. Ameliorating effects of Bacillus subtilis ANSB060 on growth performance, antioxidant functions, and aflatoxin residues in ducks fed diets contaminated with aflatoxins. Toxins. 2016;9:1. doi: 10.3390/toxins9010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fan Y., Liu L., Zhao L., Wang X., Wang D., Huang C., Zhang J., Ji C., Ma Q. Influence of Bacillus subtilis ANSB060 on growth, digestive enzyme and aflatoxin residue in Yellow River carp fed diets contaminated with aflatoxin B1. Food Chem. Toxicol. 2018;113:108–114. doi: 10.1016/j.fct.2018.01.033. [DOI] [PubMed] [Google Scholar]
- 21.Deng K.D., Xiao Y., Ma T., Tu Y., Diao Q.Y., Chen Y.H., Jiang J.J. Ruminal fermentation, nutrient metabolism, and methane emissions of sheep in response to dietary supplementation with Bacillus licheniformis. Anim. Feed Sci. Tech. 2018;241:38–44. doi: 10.1016/j.anifeedsci.2018.04.014. [DOI] [Google Scholar]
- 22.Souza V.L., Lopes N.M., Zacaroni O.F., Silveira V.A., Pereira R.A.N., Freitas J.A., Almeida R., Salvati G.G.S., Pereira M.N. Lactation performance and diet digestibility of dairy cows in response to the supplementation of Bacillus subtilis spores. Livest. Sci. 2017;200:35–39. doi: 10.1016/j.livsci.2017.03.023. [DOI] [Google Scholar]
- 23.Kutz R.E., Sampson J.D., Pompeu L.B., Ledoux D.R., Spain J.N., Vázquez-Añón M., Rottinghaus G.E. Efficacy of Solis, NovasilPlus, and MTB-100 to reduce aflatoxin M1 levels in milk of early to mid lactation dairy cows fed aflatoxin B1. J. Dairy Sci. 2009;92:3959–3963. doi: 10.3168/jds.2009-2031. [DOI] [PubMed] [Google Scholar]
- 24.Sulzberger S.A., Melnichenko S., Cardoso F.C. Effects of clay after an aflatoxin challenge on aflatoxin clearance, milk production, and metabolism of Holstein cows. J. Dairy Sci. 2017;100:1856–1869. doi: 10.3168/jds.2016-11612. [DOI] [PubMed] [Google Scholar]
- 25.Queiroz O.C.M., Han J.H., Staples C.R., Adesogan A.T. Effect of adding a mycotoxin-sequestering agent on milk aflatoxin M1 concentration and the performance and immune response of dairy cattle fed an aflatoxin B1-contaminated diet. J. Dairy Sci. 2012;95:5901–5908. doi: 10.3168/jds.2011-5287. [DOI] [PubMed] [Google Scholar]
- 26.Battacone G., Nudda A., Palomba M., Mazzette A., Pulina G. The transfer of aflatoxin M1 in milk of ewes fed diet naturally contaminated by aflatoxins and effect of inclusion of dried yeast culture in the diet. J. Dairy Sci. 2009;92:4997–5004. doi: 10.3168/jds.2008-1684. [DOI] [PubMed] [Google Scholar]
- 27.Xiong J.L., Wang Y.M., Zhou H.L., Liu J.X. Effects of dietary adsorbent on milk aflatoxin M1 content and the health of lactating dairy cows exposed to long-term aflatoxin B1 challenge. J. Dairy Sci. 2018;101:8944–8953. doi: 10.3168/jds.2018-14645. [DOI] [PubMed] [Google Scholar]
- 28.Ogunade I.M., Arriola K.G., Jiang Y., Driver J.P., Staples C.R., Adesogan A.T. Effects of 3 sequestering agents on milk aflatoxin M1 concentration and the performance and immune status of dairy cows fed diets artificially contaminated with aflatoxin B1. J. Dairy Sci. 2016;99:6263–6273. doi: 10.3168/jds.2016-10905. [DOI] [PubMed] [Google Scholar]
- 29.Battacone G., Nudda A., Palomba M., Pascale M., Nicolussi P., Pulina G. Transfer of aflatoxin B1 from feed to milk and from milk to curd and whey in dairy sheep fed artificially contaminated concentrates. J. Dairy Sci. 2005;88:3063–3069. doi: 10.3168/jds.S0022-0302(05)72987-8. [DOI] [PubMed] [Google Scholar]
- 30.Battacone G., Nudda A., Rassu S.P.G., Decandia M., Pulina G. Excretion pattern of aflatoxin M1 in milk of goats fed a single dose of aflatoxin B1. J. Dairy Sci. 2012;95:2656–2661. doi: 10.3168/jds.2011-5003. [DOI] [PubMed] [Google Scholar]
- 31.Moschini M., Masoero F., Gallo A., Diaz D. Mucosal absorption of aflatoxin B1 in lactating dairy cows. Ital. J. Anim. Sci. 2007;6:324–326. doi: 10.4081/ijas.2007.1s.324. [DOI] [Google Scholar]
- 32.Masoero F., Gallo A., Moschini M., Piva G., Diaz D. Carryover of aflatoxin from feed to milk in dairy cows with low or high somatic cell counts. Animal. 2007;1:1344–1350. doi: 10.1017/S1751731107000663. [DOI] [PubMed] [Google Scholar]
- 33.Van der Fels-Klerx H.J., Camenzuli L. Effects of milk yield, feed composition, and feed contamination with aflatoxin B1 on the aflatoxin M1 concentration in dairy cows’ milk investigated using Monte Carlo Simulation Modelling. Toxins. 2016;8:290. doi: 10.3390/toxins8100290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Eijkeren J.C.H., Bakker M.I., Zeilmaker M.J. A simple steady-state model for carry-over of aflatoxins from feed to cow’s milk. Food Addit. Contam. 2006;23:833–838. doi: 10.1080/02652030600779890. [DOI] [PubMed] [Google Scholar]
- 35.Fan Y., Zhao L., Ji C., Li X., Jia R., Xi L., Zhang J., Ma Q. Protective effects of Bacillus subtilis ANSB060 on serum biochemistry, histopathological changes and antioxidant enzyme activities of broilers fed moldy peanut meal naturally contaminated with aflatoxins. Toxins. 2015;7:3330–3343. doi: 10.3390/toxins7083330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.National Research Council . Nutrient Requirements of Dairy Cattle. 7th revised ed. National Academy Press; Washington, DC, USA: 2001. [Google Scholar]
- 37.Li X., Zhao L., Fan Y., Jia Y., Sun L., Ma S., Ji C., Ma Q., Zhang J. Occurrence of mycotoxins in feed ingredients and complete feeds obtained from the Beijing region of China. J. Anim. Sci. Biotechnol. 2014;5:37. doi: 10.1186/2049-1891-5-37. [DOI] [PMC free article] [PubMed] [Google Scholar]