Abstract
The neural crest is a transient embryonic tissue that initially generates neural crest stem cells, which then migrate throughout the body to give rise to a variety of mature tissues. It was proposed that the fate of neural crest cells is gradually determined via environmental cues from the surrounding tissues. In the present study, neural crest cells were isolated and identified from mouse embryos. Bone morphogenetic protein 4 (BMP4) and Neuregulin (NRG) were employed to induce the differentiation of neural crest cells. Treatment with BMP4 revealed neuron-associated differentiation; cells treated with NRG exhibited differentiation into the Schwann cell lineage, a type of glia. Soft agar clonogenic and neurosphere formation assays were conducted to investigate the effects of N-Myc (MYCN) overexpression in neural crest cells; the number of colonies and neurospheres notably increased after 14 days. These findings demonstrated that the direction of cell differentiation may be affected by altering the factors present in the surrounding environment. In addition, MYCN may serve a key role in regulating neural crest cell differentiation.
Keywords: neural crest cells, bone morphogenetic protein 4, Neuregulin, N-Myc, differentiation
Introduction
Neural crest cells originate from the dorsal margin of the neural plate, and can differentiate into various types of cells and tissues (1,2). Numerous neural crest cells have been isolated and characterized from different organs and tissues (3–7). During embryonic development, neural crest cells arise from the trunk region of the neural crest, migrate ventrally and aggregate adjacently to the dorsal aorta to form the primary sympathetic chain (8). The determination of neural crest cell fate is regulated by environmental factors from the extracellular surroundings (9). In addition, neural crest cells differentiate into various cell lineages according to their position in the embryo, inducing the formation of different cell types, including neurons, melanocytes, glial cells of the peripheral nervous system, endoneurial fibroblasts and endocrine cells (10–12)
Environmental factors may determine the differentiation fate of neural crest cells in vitro; neural crest cells were reported to be induced by a combination of secreted signals (11,12). Bone morphogenetic proteins (BMPs) are a unique group of proteins encoded by the transforming growth factor-β superfamily of genes, and have been reported as key regulators of embryogenesis (13). In addition, BMPs were observed to regulate the establishment of the embryonic body plan, dorsal-ventral patterning and the differentiation of neural cells (14–17). Additionally, BMP signaling has been demonstrated to affect the development of dorsal neural tube cells and formation of neural crest cells during a critical period prior to neural tube closure (18). Neuregulins (NRGs) are members of the epidermal growth factor protein family; it has been reported that NRGs are primarily expressed and secreted by neurons, and act on the surrounding glial cells (19). NRGs were demonstrated to induce the growth and differentiation of glial, epithelial and muscle cells in vitro (20–22). It has been reported that NRG−/− embryos died during embryogenesis and displayed heart malformations (23). NRGs may affect the survival, proliferation, migration, differentiation and myelination potential of Schwann cells (24–29); developing Schwann cells originate from neural crest cells that migrated along developing nerve fibers (10,30–32). Collectively, these findings suggest that environmental factors serve a critical role in neural crest cell differentiation. The present study aimed to determine the mechanism underlying neural crest cell differentiation in response to treatment with BMP4 and NRGs.
Myc activity has been reported to be a critical factor for the development and maintenance of stem cell properties; Myc has been demonstrated to control stem cell functions, including proliferation, differentiation and survival (33). Neural crest cells are generated from neural crest stem cells; as a migratory and multipotent cell population, neural crest cells can give rise to a variety of cell lineages during vertebrate development (34). N-Myc (MYCN) expression was observed in ~25% of neuroblastoma cases (35). A neuroblastoma is a tumor of the peripheral sympathetic nervous system and MYCN overexpression has been proposed as a tumorigenic event in the development of this disease (36,37). Furthermore, MYCN expression may be associated with the self-renewal ability and tumorigenic potential of neuroblastoma cells (36,38). Therefore, another aim of the present study was to determine whether MYCN could regulate the self-renewal ability of neural crest cells, and how the interaction between BMP4 or NGR and MYCN affects the fate of neural crest differentiation.
Materials and methods
Experimental animals
In the present study, 3 male and 9 female C57BL/6J mice (weight, ~22 g; age, ~9 weeks) were employed. All mice were housed under specific pathogen-free conditions as previous described (39). The animal experiments were approved by the Institutional Animal Care and Use Committee of Southwest University.
Cell culture and in vitro differentiation assays
Pregnant female mice (8.5–9 days gestation) were sacrificed via exposure to CO2. The embryos were removed and washed in PBS. A total of 10–12 neural tube sections were excised with a scalpel and planted in 6-well cell culture plates containing Dulbecco's Modified Eagle Medium/Nutrient Mixture F-12 medium (DMEM/F12; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) medium as previously described (32), and photographed at 2, 24 and 48 h with a Nikon TS100 inverted microscope (Nikon Corporation, Tokyo, Japan) at a magnification of ×40 or ×100. Image-Pro Plus 6.0 software (Media Cybernetics, Inc., Rockville, MD, USA) was used for analysis. All experiments were conducted using neural crest cells and their descendants that had not been cultured for >12 passages. For agent-induced differentiation assays, neural crest cells were cultured with 50 ng/ml BMP4 or 130 ng/ml NRG (both R&D Systems, Inc., Minneapolis, MN, USA) for 10 days in 37°C. Neural crest cells treated with 1 µl/ml DMSO (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) served as the negative control.
Immunofluorescence
The tenth passage neural crest cells treated with BMP4, NRG or DMSO were fixed in 4% paraformaldehyde at room temperature for 15 min, permeated with PBS with Tween-20 (0.3% Triton X-100) at room temperature for 5 min and blocked with 10% goat serum (Beyotime Institute of Biotechnology, Haimen, China) at room temperature for 1 h. The cells were then incubated with primary antibodies at 4°C overnight. The primary antibodies were as follows: Rabbit anti-glial fibrillary acidic protein (GFAP; cat. no. ab7260; 1:200; Sigma-Aldrich; Merck KGaA), chicken anti-Nestin (1:1,000; cat. no. NB100-1604; Novus Biologicals, LLC, Littleton, CO, USA), rabbit anti-SRY-related HMG-box 10 (Sox10; 1:300; cat. no. ab155279; Abcam, Cambridge UK) and mouse anti-neuronal-specific class III β-tubulin (TuJ1; 1:300; cat. no. ab78078; Abcam). Following washing with PBS, cells were incubated with secondary antibodies at room temperature for 2 h. All secondary antibodies were purchased from Invitrogen (Thermo Fisher Scientific, Inc.) and used at 1:1,000 dilution. The secondary antibodies were as follows: Alexa Fluor® 488-conjugated goat anti-mouse (cat. no. A-11001), anti-rabbit (cat. no. A-11008) and anti-chicken (cat. no. A-11039), and Alexa Fluor 594-conjugated goat anti-rabbit (cat. no. A-11012) immunoglobulin G. Then, all cells were washed with PBS and counterstained with DAPI (Beyotime Institute of Biotechnology) at room temperature for 20 min to detect nuclei, and images were captured with a Nikon Eclipse TE2000-E fluorescence microscope (Nikon Corporation) at a magnification of ×100 or ×200. Image-Pro Plus 6.0 software was used for analysis.
Retroviral production and transfection
The pBabe-puro/MYCN plasmid (Youbio, Hunan, China) was used to overexpress mouse MYCN in neural crest cells (MYCN-overexpressing neural crest cells), as previously reported (40), and the empty pBabe-puro plasmid as the control. Retroviral production and transfection were conducted as described previously (41). One day after retroviral transfection, the cells were cultured at 37°C in the presence of 2 µg/ml puromycin for 3 days for resistance-based selection.
Soft agar clonogenic and sphere formation assays
For soft agar colony assay, a total of 1,500 pBabe-puro/MYCN or empty pBabe-puro neural crest cells in suspension were mixed with 0.3% low melting point agar containing DMEM supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.). Cells were planted onto 6-well plates (1,500 cells/well) with a solidified bottom layer (0.6% low melting point agar in the same growth medium) for 14 days at 37°C. For sphere formation assay, the medium in the aforementioned wells was replaced with DMEM supplemented with 20 ng/ml epidermal growth factor and 10 ng/ml basic fibroblast growth factor (both Gibco; Thermo Fisher Scientific, Inc.). After the 14-day culture, the colonies or the spheres were examined and photographed using a Nikon TS100 inverted microscope at a magnification of ×100.
Western blot analysis
MYCN-overexpressing neural crest cells treated with BMP4, NRG or DMSO were suspended in RIPA lysis buffer and the total protein concentration determined using the Enhanced BCA protein assay kit (both Beyotime Institute of Biotechnology). Following this, 50 µg/lane of protein were separated by SDS-PAGE on 10% gel, transferred to polyvinylidene difluoride membranes. Membranes were then blocked with 5% no fat milk at room temperature for 1 h and then incubated with anti-MYCN (cat. no. ab24193; 1:1,000; Abcam) or anti-α-tubulin (cat. no. SAB4500087; 1:1,000; Sigma-Aldrich; Merck KGaA). Horseradish peroxidase-conjugated goat anti-mouse (cat. no. 5220–0341) or anti-rabbit (cat. no. 5220-0336) secondary antibodies (both 1:20,000; Kirkegaard & Perry Laboratories; SeraCare Life Sciences, Inc., Milford, MA, USA) were used as secondary antibodies. Proteins were visualized with BeyoECL Plus (Beyotime Institute of Biotechnology).
Results
Cells migrating from the neural tube are neural crest cells, which are characterized by Sox10 and Nestin expression
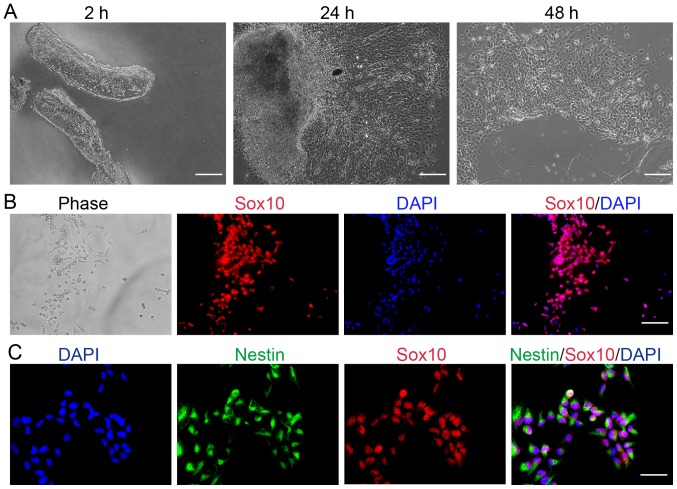
Explant culture has been successfully used to identify the properties of neural crest cells originating from the stem cells of developing rodents (42,43). Therefore, explant culture was selected in the present study to determine whether any cells migrated from the neural tube of mice embryos. After a 2-h explant culture, the neural tube was clearly observed; after 24 h, neural crest cells were detected at the edges of the tissue blocks and some cells had migrated from the neural tube explants into the culture medium (Fig. 1A). The number of cells in the culture increased in what appeared to be a time-dependent manner, suggesting that the cells continued to migrate from the neural tube; after 2 days, more cells floated freely in the medium.
Figure 1.
Cells migrating from the neural tube are neural crest cells, which are characterized by Sox10 and Nestin expression. (A) Cells migrated from the mouse embryo neural tube following culture for 2, 24 and 48 h. 2 h scale bar=250 µm; 24 and 48 h, scale bar=100 µm. (B) Phase images were taken and immunofluorescence analysis were conducted on cells that migrated from the trunk region of the neural tube. Scale bar=100 µm. (C) Immunofluorescence analysis of neural crest cells. Scale bar=50 µm. Sox10, SRY-related HMG-box 10.
Sox10 is a unique HMG-box transcription factor expressed throughout the neural crest and in oligodendrocyte progenitor cells of the central nervous system (9,44,45). In the present study, cells were characterized via immunofluorescence to determine whether cells migrating from the neural tube expressed neural stem cell-associated markers. Compared with the phase image, immunofluorescent analysis revealed that all cells expressed Sox10 (Fig. 1B). Therefore, suggesting that cells migrating from the mouse embryo neural tube, which express Sox10, may be characterized as neural crest cells.
Nestin has been reported as a marker of neural stem or progenitor cells (46,47). Embryonic stem cell-derived neural precursor cells that had been further induced to differentiate into neurons may be selected based on the aforementioned strategy (48). The results of the present study demonstrated that neural crest cells were positive for Nestin (Fig. 1C), suggesting that neural crest cells may possess neural stem cell characteristics.
Neural crest cells maintain the potential of multilineage differentiation
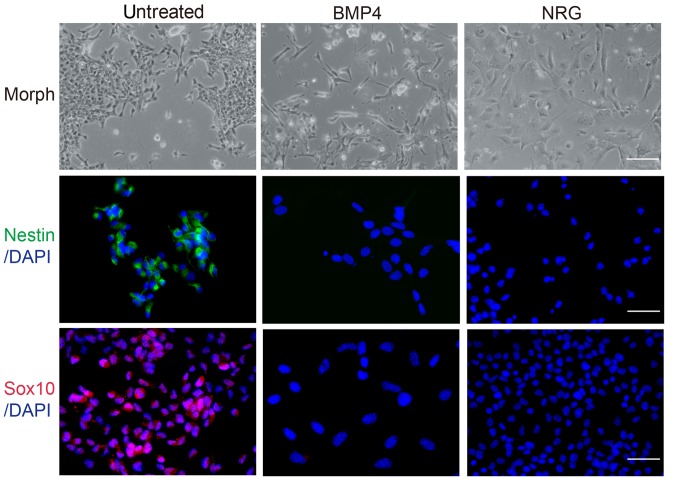
The present study investigated the differentiation potential of neural crest cells in response to a variety of agents. A cell suspension was prepared from individual secondary colonies and plated onto glass coverslips. The results of the immunofluorescence analysis demonstrated that cells expressed the stem cell markers, Nestin and Sox10. In addition, neural crest cells were treated with BMP4 or NRG for 10 days. The majority of DMSO-treated cells exhibited a round and prominent nucleus, and abundant cytoplasm (Fig. 2). Cells treated with BMP4 exhibited neuronal cell morphology (49), with numerous long neuritic processes and small cell bodies that frequently formed aggregates (Fig. 2); however, cells treated with NRG exhibited Schwann-like cell morphology (50,51), with ovoid cell bodies, a prominent nucleus and natural bipolar extensions (Fig. 2). Therefore, these data suggested that BMP4 and NRG treatment may induce neural crest cell differentiation into neurons and Schwann cells.
Figure 2.
Morphology alters and stem cell marker expresion reduces in in neural crest cells following treatment with BMP4 and NRG. Morph, scale bar=100 µm; Nestin and Sox10 immunoflourescence, scale bar=50 µm. Sox10, SRY-related HMG-box 10; BMP4, bone morphogenetic protein 4; NRG, Neuregulin.
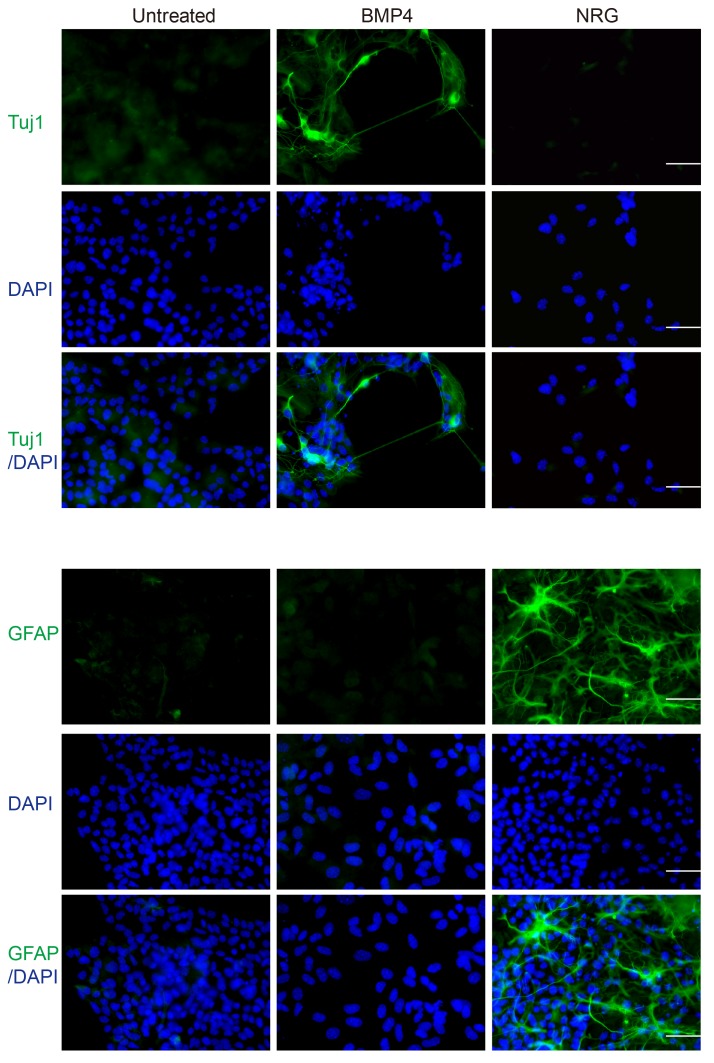
To confirm the cell phenotype following treatment with BMP4 or NRG, immunofluorescence was conducted using anti-Nestin and anti-Sox10 antibodies. Compared with the untreated cell group, treatment with BMP4 or NRG resulted in a marked reduction in the number of cells expressing stem cell markers (Fig. 2), accompanied by a marked increase in the number of cells expressing differentiation markers (Fig. 3). BMP4-treated cells expressed the neuronal marker, Tuj1 (52), whereas NRG-treated cells expressed GFAP, which is a common marker of Schwann and glial cells (53), upon induction of differentiation. On the contrary, untreated cells expressed differentiation markers at markedly lower levels (Fig. 3). These differentiation analyses suggested that neural crest cells may possess the potential for multilineage differentiation and that environmental factors may control their fate.
Figure 3.
BMP4-treated and NRG-treated neural crest cells express differentiation markers. Immunofluorescence analysis of neural crest cells treated with BMP4 or NRG. The nuclei were visualized with DAPI staining. Scale bar=50 µm. BMP4, bone morphogenetic protein 4; NRG, Neuregulin; Tuj1, neuronal-specific class III β-tubulin; GFAP, glial fibrillary acidic protein.
Clonal sphere formation assay of MYCN-overexpressing neural crest cells suggests clonogenic self-renewal potential in vitro
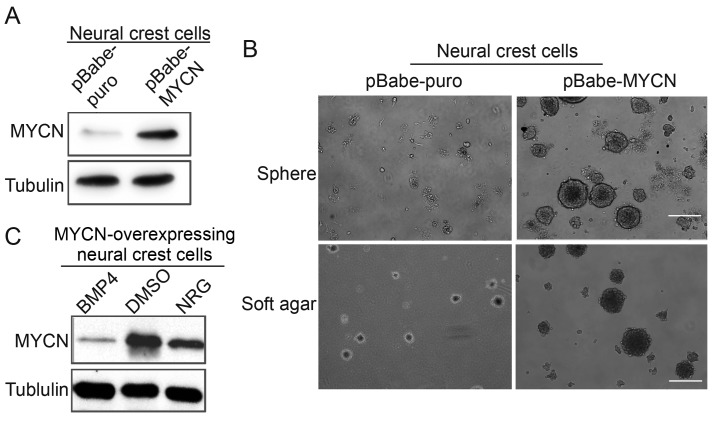
MYCN upregulation was validated by western blotting (Fig. 4A). To investigate the function of MYCN in neural crest cells, the self-renewal and clonogenic abilities of MYCN-overexpressing neural crest cells were analyzed via soft agar and sphere formation assays. The result demonstrated that MYCN-overexpressing neural crest cells exhibited a higher number of colonies and larger colony sizes compared with the control group of neural crest cells (Fig. 4B). Similarly, the neurosphere formation ability of MYCN-overexpressing neural crest cells appeared to be enhanced compared with the neural crest cell control group under the same culture conditions. These results demonstrated that MYCN may promote the self-renewal ability of neural crest cells. The authors further investigated the effects of BMP4 and NRG on MYCN expression in MYCN-overexpressing neural crest cells; the protein expression levels of MYCN were markedly decreased in cells treated with BMP4 or NRG compared with the control cells (Fig. 4C).
Figure 4.
MYCN promotes neurosphere and soft agar colony formation, and BMP4 and NRG reduces MYCN expression. (A) Western blot MYCN expression in transfected neural crest cells. (B) Neurosphere formation and soft agar colonies in transfected neural crest cells after 14 days of culture. Scale bar=100 µm (C) MYCN protein expression following treament with BMP4 or NRG. MYCN, N-Myc; BMP4, bone morphogenetic protein 4; NRG, Neuregulin.
Discussion
Neural crest cells have stem cell characteristics and have the ability to generate various types of cells and tissues during vertebrate development (54). In the present study, neural crest cells were isolated from mouse embryos and characterized by specific stem cell markers, including Sox10 and Nestin. Factors of the extracellular environment may affect the direction of neural crest cell differentiation. It has been reported that partitioning defective 3 homolog regulates the contact between neural crest cells and the timing of Schwann cell differentiation (55). In human neural crest stem cells, aligned electrospun fibers were revealed to promote differentiation towards the Schwann cell lineage (56). A recent study suggested that, during the neural differentiation of embryonic stem cells, miR-29b promoted the differentiation of embryonic stem cells into neural tube epithelial cells and inhibited their differentiation into neural crest cells (57).
It was previously reported that embryonic stem cells cultured with BMP4 differentiated into germ cells (58,59). BMP4 also regulated the proliferation and differentiation in epithelial and mesenchymal tissue compartments of the developing mouse ureter (60). In addition, BMP4 was reported to serve a key role in the differentiation of auditory neuron-like cells from bone-derived mesenchymal stromal cells (61). NRG is a type of polypeptide growth factor that serves a key role in the development and differentiation of the heart and nervous system (23,62). Generally, neural crest cells differentiate into glia, neurons and melanocytes in the mouse embryo (9,63). In the present study, the induction of differentiation via specific agents, BMP4 or NRG, revealed that neural crest cells may differentiate into neurons or Schwann cells, respectively, indicating that neural crest cells may alter their direction of differentiation according to their environments. Therefore, BMP4 and NRG could be considered as key factors in neural crest cell differentiation.
Self-renewal ability is an essential characteristic of stem cells, and enables the generation of daughter cells with the same developmental potential as their parental cells (64,65). Colony and sphere formation assays have been widely used to evaluate the self-renewal ability of individual stem cells (66–68). MYCN was reported as a key factor in the maintenance of embryonic stem cell-derived neural crest stem cells (69). The soft agar clonogenic and sphere formation assays revealed that MYCN-overexpressing neural crest cells were able to self-renew and generate progeny cells with the same self-renewal ability, MYCN-overexpressing neural crest cells developed more colonies compared with the neural crest cells transfected with empty vectors. Neuroblastoma is a cancer of neural crest stem cell lineage, many reports demonstrated that MYCN acted as an oncogene in neuroblastoma (35,36,70). MYCN served important roles in balancing proliferation, differentiation and cell death in neuroblastoma and normal neural crest cells (71,72). Therefore, it was hypothesized that MYCN-overexpressing neural crest cells acquire tumorigenic potential and that MYCN may regulate the development of neuroblastoma that originate from neural crest cells.
Of note, BMP4 could reduce MYCN expression and promote differentiation in neuroblastoma cells (73), and NRG was involved in neuroblastoma cell differentiation (74). These reports suggested BMP4 and NRG regulated neuroblastoma development. In the present study, BMP4 and NRG were shown to suppress MYCN expression in MYCN-overexpressing neural crest cells, which indicated that BMP4 and NRG may inhibit the maintenance of MYCN-induced stemness, suggesting there was cross-talk between MYCN and the BMP4 or NRG signaling pathway. Collectively, the findings of the current study indicated a molecular mechanism through which MYCN may promote the stemness of neural crest cells. Specific agents, including BMP4 and NRG, may decrease MYCN expression and induce neural crest cell differentiation. Therefore, the present study revealed that the direction of cell differentiation would be altered through modifying environmental factors. MYCN could serve a key role in regulating neural crest cell differentiation, and BMP4 and NRG may be regarded as novel inhibitors of MYCN amplification in neuroblastoma.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science Foundation of China (grant no. 31501100), the Chongqing Science and Technology Commission (grant no. cstc2016shmszx80101), the Ph.D. Start-up Foundation of Southwest University (grant no. SWU 2015021) and the Chongqing Research Program of Basic Research and Frontier Technology (grant no. cstc2015jcyjA10120), the China Postdoctoral Science Foundation (grant no. 2016M592624) and the Chongqing Postdoctoral Science Special Foundation (grant no. Xm2016087). The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
SZ performed the all the experiment with the exception of retroviral production and transfection, and wrote the manuscript. WL performed the statistical analyses. HFD and HC conducted the experiments. LY designed the current study and revised this manuscript. All the authors have read and approved the final version of the manuscript.
Ethics approval and consent to participate
The present study was approved by the Institutional Animal Care and Use Committee of Southwest University (Chongqing, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Gonzalez Malagon SG, Lopez Munoz AM, Doro D, Bolger TG, Poon E, Tucker ER, Adel Al-Lami H, Krause M, Phiel CJ, Chesler L, Liu KJ. Glycogen synthase kinase 3 controls migration of the neural crest lineage in mouse and Xenopus. Nat Commun. 2018;9:1126. doi: 10.1038/s41467-018-03512-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang T, Moore M, He F. Pten regulates neural crest proliferation and differentiation during mouse craniofacial development. Dev Dyn. 2018;247:304–314. doi: 10.1002/dvdy.24605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang J, Duan X, Zhang H, Deng Z, Zhou Z, Wen N, Smith AJ, Zhao W, Jin Y. Isolation of neural crest-derived stem cells from rat embryonic mandibular processes. Biol Cell. 2006;98:567–575. doi: 10.1042/BC20060012. [DOI] [PubMed] [Google Scholar]
- 4.Yoshida S, Shimmura S, Nagoshi N, Fukuda K, Matsuzaki Y, Okano H, Tsubota K. Isolation of multipotent neural crest-derived stem cells from the adult mouse cornea. Stem Cells. 2006;24:2714–2722. doi: 10.1634/stemcells.2006-0156. [DOI] [PubMed] [Google Scholar]
- 5.Morrison SJ, White PM, Zock C, Anderson DJ. Prospective identification, isolation by flow cytometry, and in vivo self-renewal of multipotent mammalian neural crest stem cells. Cell. 1999;96:737–749. doi: 10.1016/S0092-8674(00)80583-8. [DOI] [PubMed] [Google Scholar]
- 6.Jiang X, Gwye Y, McKeown SJ, Bronner-Fraser M, Lutzko C, Lawlor ER. Isolation and characterization of neural crest stem cells derived from in vitro-differentiated human embryonic stem cells. Stem Cells Dev. 2009;18:1059–1070. doi: 10.1089/scd.2008.0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chung KF, Sicard F, Vukicevic V, Hermann A, Storch A, Huttner WB, Bornstein SR, Ehrhart-Bornstein M. Isolation of neural crest derived chromaffin progenitors from adult adrenal medulla. Stem Cells. 2009;27:2602–2613. doi: 10.1002/stem.180. [DOI] [PubMed] [Google Scholar]
- 8.Lumb R, Schwarz Q. Sympathoadrenal neural crest cells: The known, unknown and forgotten? Dev Growth Differ. 2015;57:146–157. doi: 10.1111/dgd.12189. [DOI] [PubMed] [Google Scholar]
- 9.Sauka-Spengler T, Bronner-Fraser M. A gene regulatory network orchestrates neural crest formation. Nat Rev Mol Cell Biol. 2008;9:557–568. doi: 10.1038/nrm2428. [DOI] [PubMed] [Google Scholar]
- 10.Joseph NM, Mukouyama YS, Mosher JT, Jaegle M, Crone SA, Dormand EL, Lee KF, Meijer D, Anderson DJ, Morrison SJ. Neural crest stem cells undergo multilineage differentiation in developing peripheral nerves to generate endoneurial fibroblasts in addition to Schwann cells. Development. 2004;131:5599–5612. doi: 10.1242/dev.01429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wakamatsu Y, Maynard TM, Weston JA. Fate determination of neural crest cells by NOTCH-mediated lateral inhibition and asymmetrical cell division during gangliogenesis. Development. 2000;127:2811–2821. doi: 10.1242/dev.127.13.2811. [DOI] [PubMed] [Google Scholar]
- 12.Steventon B, Araya C, Linker C, Kuriyama S, Mayor R. Differential requirements of BMP and Wnt signalling during gastrulation and neurulation define two steps in neural crest induction. Development. 2009;136:771–779. doi: 10.1242/dev.029017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sykaras N, Opperman LA. Bone morphogenetic proteins (BMPs): How do they function and what can they offer the clinician. J Oral Sci. 2003;45:57–73. doi: 10.2334/josnusd.45.57. [DOI] [PubMed] [Google Scholar]
- 14.Sykaras N, Opperman LA. Bone morphogenetic proteins (BMPs): How do they function and what can they offer the clinician? J Oral Sci. 2003;45:57–73. doi: 10.2334/josnusd.45.57. [DOI] [PubMed] [Google Scholar]
- 15.Ripamonti U, Reddi AH. Tissue engineering, morphogenesis, and regeneration of the periodontal tissues by bone morphogenetic proteins. Crit Rev Oral Biol Med. 1997;8:154–163. doi: 10.1177/10454411970080020401. [DOI] [PubMed] [Google Scholar]
- 16.Paralkar VM, Weeks BS, Yu YM, Kleinman HK, Reddi AH. Recombinant human bone morphogenetic protein 2B stimulates PC12 cell differentiation: Potentiation and binding to type IV collagen. J Cell Biol. 1992;119:1721–1728. doi: 10.1083/jcb.119.6.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shah NM, Groves AK, Anderson DJ. Alternative neural crest cell fates are instructively promoted by TGFbeta superfamily members. Cell. 1996;85:331–343. doi: 10.1016/S0092-8674(00)81112-5. [DOI] [PubMed] [Google Scholar]
- 18.Stottmann RW, Klingensmith J. Bone morphogenetic protein signaling is required in the dorsal neural folds before neurulation for the induction of spinal neural crest cells and dorsal neurons. Dev Dyn. 2011;240:755–765. doi: 10.1002/dvdy.22579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raabe TD, Francis A, DeVries GH. Neuregulins in glial cells. Neurochem Res. 1998;23:311–318. doi: 10.1023/A:1022449231651. [DOI] [PubMed] [Google Scholar]
- 20.Wen D, Peles E, Cupples R, Suggs SV, Bacus SS, Luo Y, Trail G, Hu S, Silbiger SM, Levy RB, et al. Neu differentiation factor: A transmembrane glycoprotein containing an EGF domain and an immunoglobulin homology unit. Cell. 1992;69:559–572. doi: 10.1016/0092-8674(92)90456-M. [DOI] [PubMed] [Google Scholar]
- 21.Marchionni MA, Goodearl AD, Chen MS, Bermingham-McDonogh O, Kirk C, Hendricks M, Danehy F, Misumi D, Sudhalter J, Kobayashi K, et al. Glial growth factors are alternatively spliced erbB2 ligands expressed in the nervous system. Nature. 1993;362:312–318. doi: 10.1038/362312a0. [DOI] [PubMed] [Google Scholar]
- 22.Carraway KL, III, Cantley LC. A neu acquaintance for erbB3 and erbB4: A role for receptor heterodimerization in growth signaling. Cell. 1994;78:5–8. doi: 10.1016/0092-8674(94)90564-9. [DOI] [PubMed] [Google Scholar]
- 23.Meyer D, Birchmeier C. Multiple essential functions of neuregulin in development. Nature. 1995;378:386–390. doi: 10.1038/378386a0. [DOI] [PubMed] [Google Scholar]
- 24.Garratt AN, Britsch S, Birchmeier C. Neuregulin, a factor with many functions in the life of a schwann cell. Bioessays. 2000;22:987–996. doi: 10.1002/1521-1878(200011)22:11<987::AID-BIES5>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 25.Jessen KR, Mirsky R. Origin and early development of Schwann cells. Microsc Res Tech. 1998;41:393–402. doi: 10.1002/(SICI)1097-0029(19980601)41:5<393::AID-JEMT6>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 26.Jessen KR, Mirsky R. Schwann cells and their precursors emerge as major regulators of nerve development. Trends Neurosci. 1999;22:402–410. doi: 10.1016/S0166-2236(98)01391-5. [DOI] [PubMed] [Google Scholar]
- 27.Freeman MR. Sculpting the nervous system: Glial control of neuronal development. Curr Opin Neurobiol. 2006;16:119–125. doi: 10.1016/j.conb.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 28.Mirsky R, Jessen KR, Brennan A, Parkinson D, Dong Z, Meier C, Parmantier E, Lawson D. Schwann cells as regulators of nerve development. J Physiol Paris. 2002;96:17–24. doi: 10.1016/S0928-4257(01)00076-6. [DOI] [PubMed] [Google Scholar]
- 29.Falls DL. Neuregulins: Functions, forms, and signaling strategies. Exp Cell Res. 2003;284:14–30. doi: 10.1016/S0014-4827(02)00102-7. [DOI] [PubMed] [Google Scholar]
- 30.Li L, Habbes HW, Eiberger J, Willecke K, Dermietzel R, Meier C. Analysis of connexin expression during mouse Schwann cell development: Connexin29 as a novel marker for the transition of neural crest to precursor cells. Glia. 2007;55:93–103. doi: 10.1002/glia.20427. [DOI] [PubMed] [Google Scholar]
- 31.Aquino JB, Hjerling-Leffler J, Koltzenburg M, Edlund T, Villar MJ, Ernfors P. In vitro and in vivo differentiation of boundary cap neural crest stem cells into mature Schwann cells. Exp Neurol. 2006;198:438–449. doi: 10.1016/j.expneurol.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 32.Buchstaller J, Sommer L, Bodmer M, Hoffmann R, Suter U, Mantei N. Efficient isolation and gene expression profiling of small numbers of neural crest stem cells and developing Schwann cells. J Neurosci. 2004;24:2357–2365. doi: 10.1523/JNEUROSCI.4083-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laurenti E, Varnum-Finney B, Wilson A, Ferrero I, Blanco-Bose WE, Ehninger A, Knoepfler PS, Cheng PF, MacDonald HR, Eisenman RN, et al. Hematopoietic stem cell function and survival depend on c-Myc and N-Myc activity. Cell Stem Cell. 2008;3:611–624. doi: 10.1016/j.stem.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crane JF, Trainor PA. Neural crest stem and progenitor cells. Annu Rev Cell Dev Biol. 2006;22:267–286. doi: 10.1146/annurev.cellbio.22.010305.103814. [DOI] [PubMed] [Google Scholar]
- 35.Huang M, Weiss WA. Neuroblastoma and MYCN. Cold Spring Harb Perspect Med. 2013;3:a014415. doi: 10.1101/cshperspect.a014415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alam G, Cui H, Shi H, Yang L, Ding J, Mao L, Maltese WA, Ding HF. MYCN promotes the expansion of Phox2B-positive neuronal progenitors to drive neuroblastoma development. Am J Pathol. 2009;175:856–866. doi: 10.2353/ajpath.2009.090019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mao L, Ding J, Perdue A, Yang L, Zha Y, Ren M, Huang S, Cui H, Ding HF. Cyclin E1 is a common target of BMI1 and MYCN and a prognostic marker for neuroblastoma progression. Oncogene. 2012;31:3785–3795. doi: 10.1038/onc.2011.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brodeur GM. Neuroblastoma: Biological insights into a clinical enigma. Nat Rev Cancer. 2003;3:203–216. doi: 10.1038/nrc1014. [DOI] [PubMed] [Google Scholar]
- 39.Martinewski A, Correia CS, de Souza NL, Merusse JL. Mouse housing system using pressurized cages intraventilated by direct-current microfans. J Am Assoc Lab Anim Sci. 2012;51:177–180. [PMC free article] [PubMed] [Google Scholar]
- 40.Itahana K, Zou Y, Itahana Y, Martinez JL, Beausejour C, Jacobs JJ, Van Lohuizen M, Band V, Campisi J, Dimri GP. Control of the replicative life span of human fibroblasts by p16 and the polycomb protein Bmi-1. Mol Cell Biol. 2003;23:389–401. doi: 10.1128/MCB.23.1.389-401.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cui H, Schroering A, Ding HF. p53 mediates DNA damaging drug-induced apoptosis through a caspase-9-dependent pathway in SH-SY5Y neuroblastoma cells. Mol Cancer Ther. 2002;1:679–686. [PubMed] [Google Scholar]
- 42.Lo L, Dormand E, Greenwood A, Anderson DJ. Comparison of the generic neuronal differentiation and neuron subtype specification functions of mammalian achaete-scute and atonal homologs in cultured neural progenitor cells. Development. 2002;129:1553–1567. doi: 10.1242/dev.129.7.1553. [DOI] [PubMed] [Google Scholar]
- 43.Greenwood AL, Turner EE, Anderson DJ. Identification of dividing, determined sensory neuron precursors in the mammalian neural crest. Development. 1999;126:3545–3559. doi: 10.1242/dev.126.16.3545. [DOI] [PubMed] [Google Scholar]
- 44.Kelsh RN. Sorting out Sox10 functions in neural crest development. Bioessays. 2006;28:788–798. doi: 10.1002/bies.20445. [DOI] [PubMed] [Google Scholar]
- 45.Kawaguchi J, Nichols J, Gierl MS, Faial T, Smith A. Isolation and propagation of enteric neural crest progenitor cells from mouse embryonic stem cells and embryos. Development. 2010;137:693–704. doi: 10.1242/dev.046896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Selander L, Edlund H. Nestin is expressed in mesenchymal and not epithelial cells of the developing mouse pancreas. Mech Dev. 2002;113:189–212. doi: 10.1016/S0925-4773(02)00023-0. [DOI] [PubMed] [Google Scholar]
- 47.Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60:585–595. doi: 10.1016/0092-8674(90)90662-X. [DOI] [PubMed] [Google Scholar]
- 48.Lee H, Mok KH, Muhandiram R, Park KH, Suk JE, Kim DH, Chang J, Sung YC, Choi KY, Han KH. Local structural elements in the mostly unstructured transcriptional activation domain of human p53. J Biol Chem. 2000;275:29426–29432. doi: 10.1074/jbc.M003107200. [DOI] [PubMed] [Google Scholar]
- 49.Trainor PA. Specification of neural crest cell formation and migration in mouse embryos. Semin Cell Dev Biol. 2005;16:683–693. doi: 10.1016/j.semcdb.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 50.Sugimoto T, Kato T, Sawada T, Horii Y, Kemshead JT, Hino T, Morioka H, Hosoi H. Schwannian cell differentiation of human neuroblastoma cell lines in vitro induced by bromodeoxyuridine. Cancer Res. 1988;48:2531–2537. [PubMed] [Google Scholar]
- 51.Ross RA, Spengler BA, Domenech C, Porubcin M, Rettig WJ, Biedler JL. Human neuroblastoma I-type cells are malignant neural crest stem cells. Cell Growth Differ. 1995;6:449–456. [PubMed] [Google Scholar]
- 52.Lee S, Lee B, Lee JW, Lee SK. Retinoid signaling and neurogenin2 function are coupled for the specification of spinal motor neurons through a chromatin modifier CBP. Neuron. 2009;62:641–654. doi: 10.1016/j.neuron.2009.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jessen KR, Mirsky R. The origin and development of glial cells in peripheral nerves. Nat Rev Neurosci. 2005;6:671–682. doi: 10.1038/nrn1746. [DOI] [PubMed] [Google Scholar]
- 54.Bhatt S, Diaz R, Trainor PA. Signals and switches in Mammalian neural crest cell differentiation. Cold Spring Harb Perspect Biol. 2013;5(pii):a008326. doi: 10.1101/cshperspect.a008326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Blasky AJ, Pan L, Moens CB, Appel B. Pard3 regulates contact between neural crest cells and the timing of Schwann cell differentiation but is not essential for neural crest migration or myelination. Dev Dyn. 2014;243:1511–1523. doi: 10.1002/dvdy.24172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ren YJ, Zhang S, Mi R, Liu Q, Zeng X, Rao M, Hoke A, Mao HQ. Enhanced differentiation of human neural crest stem cells towards the Schwann cell lineage by aligned electrospun fiber matrix. Acta Biomater. 2013;9:7727–7736. doi: 10.1016/j.actbio.2013.04.034. [DOI] [PubMed] [Google Scholar]
- 57.Xi J, Wu Y, Li G, Ma L, Feng K, Guo X, Jia W, Wang G, Yang G, Li P, Kang J. Mir-29b mediates the neural tube versus neural crest fate decision during embryonic stem cell neural differentiation. Stem Cell Reports. 2017;9:571–586. doi: 10.1016/j.stemcr.2017.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Makoolati Z, Movahedin M, Forouzandeh-Moghadam M. In vitro germ cell differentiation from embryonic stem cells of mice: Induction control by BMP4 signaling. Biosci Rep. 2016;36:e00407. doi: 10.1042/BSR20160441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang S, Yuan Q, Niu M, Hou J, Zhu Z, Sun M, Li Z, He Z. BMP4 promotes mouse iPS cell differentiation to male germ cells via Smad1/5, Gata4, Id1 and Id2. Reproduction. 2017;153:211–220. doi: 10.1530/REP-16-0292. [DOI] [PubMed] [Google Scholar]
- 60.Mamo TM, Wittern AB, Kleppa MJ, Bohnenpoll T, Weiss AC, Kispert A. BMP4 uses several different effector pathways to regulate proliferation and differentiation in the epithelial and mesenchymal tissue compartments of the developing mouse ureter. Hum Mol Genet. 2017;26:3553–3563. doi: 10.1093/hmg/ddx242. [DOI] [PubMed] [Google Scholar]
- 61.Peng T, Zhu G, Dong Y, Zeng J, Li W, Guo W, Chen Y, Duan M, Hocher B, Xie D. BMP4: A possible key factor in differentiation of auditory neuron-like cells from bone-derived mesenchymal stromal cells. Clin Lab. 2015;61:1171–1178. doi: 10.7754/Clin.Lab.2015.150217. [DOI] [PubMed] [Google Scholar]
- 62.Britsch S, Li L, Kirchhoff S, Theuring F, Brinkmann V, Birchmeier C, Riethmacher D. The ErbB2 and ErbB3 receptors and their ligand, neuregulin-1, are essential for development of the sympathetic nervous system. Genes Dev. 1998;12:1825–1836. doi: 10.1101/gad.12.12.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Milet C, Monsoro-Burq AH. Neural crest induction at the neural plate border in vertebrates. Dev Biol. 2012;366:22–33. doi: 10.1016/j.ydbio.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 64.Borowicz S, Van Scoyk M, Avasarala S, Karuppusamy Rathinam MK, Tauler J, Bikkavilli RK, Winn RA. The soft agar colony formation assay. J Vis Exp. 2014;92:e51998. doi: 10.3791/51998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Franken NA, Rodermond HM, Stap J, Haveman J, van Bree C. Clonogenic assay of cells in vitro. Nat Protoc. 2006;1:2315–2319. doi: 10.1038/nprot.2006.339. [DOI] [PubMed] [Google Scholar]
- 66.Hamburger AW, Salmon SE. Primary bioassay of human tumor stem cells. Science. 1977;197:461–463. doi: 10.1126/science.560061. [DOI] [PubMed] [Google Scholar]
- 67.Cui H, Ma J, Ding J, Li T, Alam G, Ding HF. Bmi-1 regulates the differentiation and clonogenic self-renewal of I-type neuroblastoma cells in a concentration-dependent manner. J Biol Chem. 2006;281:34696–34704. doi: 10.1074/jbc.M604009200. [DOI] [PubMed] [Google Scholar]
- 68.Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- 69.Zhang JT, Weng ZH, Tsang KS, Tsang LL, Chan HC, Jiang XH. MycN is critical for the maintenance of human embryonic stem cell-derived neural crest stem cells. PLoS One. 2016;11:e0148062. doi: 10.1371/journal.pone.0148062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ke XX, Zhang D, Zhao H, Hu R, Dong Z, Yang R, Zhu S, Xia Q, Ding HF, Cui H. Phox2B correlates with MYCN and is a prognostic marker for neuroblastoma development. Oncol Lett. 2015;9:2507–2514. doi: 10.3892/ol.2015.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Newman EA, Chukkapalli S, Bashllari D, Thomas TT, Van Noord RA, Lawlor ER, Hoenerhoff MJ, Opipari AW, Opipari VP. Alternative NHEJ pathway proteins as components of MYCN oncogenic activity in human neural crest stem cell differentiation: Implications for neuroblastoma initiation. Cell Death Dis. 2017;8:3208. doi: 10.1038/s41419-017-0004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Althoff K, Beckers A, Bell E, Nortmeyer M, Thor T, Sprüssel A, Lindner S, De Preter K, Florin A, Heukamp LC, et al. A Cre-conditional MYCN-driven neuroblastoma mouse model as an improved tool for preclinical studies. Oncogene. 2015;34:3357–3368. doi: 10.1038/onc.2014.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ferlemann FC, Menon V, Condurat AL, Rößler J, Pruszak J. Surface marker profiling of SH-SY5Y cells enables small molecule screens identifying BMP4 as a modulator of neuroblastoma differentiation. Sci Rep. 2017;7:13612. doi: 10.1038/s41598-017-13497-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schlitter AM, Dorneburg C, Barth TF, Wahl J, Schulte JH, Brüderlein S, Debatin KM, Beltinger C. CD57(high) neuroblastoma cells have aggressive attributes ex situ and an undifferentiated phenotype in Patients. PLoS One. 2012;7:e42025. doi: 10.1371/journal.pone.0042025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.