Abstract
Mycotoxins are fungal secondary metabolites that pose health risks to exposed individuals, requiring necessary measures to reduce them. Using liquid chromatography-tandem mass spectrometry (LC-MS/MS), mycotoxins were quantified in whole grain sorghum and ting subsequently derived from two sorghum varieties (high and low tannin). The whole grain (WG) ting samples were obtained by fermenting sorghum with Lactobacillus fermentum strains (FUA 3165 and FUA 3321). Naturally (spontaneously) fermented WG-ting under the same conditions were equally analysed. Among the mycotoxins investigated, fumonisin B1 (FB1), B2 (FB2), B3 (FB3), T-2 toxin (T-2), zearalenone (ZEA), alpha-zearalenol (α-ZOL) and beta-zearalenol (β-ZOL) were detected in sorghum. Results obtained showed that mycotoxin concentrations significantly (p ≤ 0.05) reduced after fermentation. In particular, L. fermentum FUA 3321 showed the capability to significantly (p ≤ 0.05) reduce all the mycotoxins by 98% for FB1, 84% for T-2 and up to 82% for α-ZOL, compared to raw low tannin sorghum. Fermenting with the L. fermentum strains showed potential to effectively reduce mycotoxin contamination in whole grain ting. Thus, we recommended L. fermentum FUA 3321 in particular to be used as a potential starter culture in sorghum fermentation.
Keywords: sorghum, ting, fermentation, Lactobacillus fermentum, mycotoxins, food safety
1. Introduction
Mycotoxins are deleterious and of global public health concern, with numerous reported adverse health and economic effects [1,2]. These naturally occurring toxic compounds are frequent contaminants of agricultural commodities, significantly contributing to food losses and exposing the world’s population to a high health risk. In particular, chronic health risks arising from mycotoxin contamination are a main concern for the South African population, since major staple foods are highly contaminated by mycotoxins [1,3,4]. The climatic and environmental conditions in South Africa are characterised by temperate and humid conditions, erratic rainfall and frequent drought episodes, which are ideal conditions for mycotoxigenic fungal growth and proliferation. A detailed review by Misihairabgwi et al. [3] highlighted incidences of these mycotoxins in Southern African foods, as well as the link between mycotoxin contamination and aggravating health-related challenges including infant malnutrition, kwashiorkor and marasmus. Fumonisins (FBs) have been of significant prominence in South Africa since they were first discovered in 1988 [5], and are implicated in outbreaks of equine leukoencephalomalacia, porcine pulmonary edema, neural tube defects and esophageal cancer [5,6,7,8].
Cereals have been identified as a major route of dietary exposure to mycotoxins, which is of global concern, especially when these mycotoxins are carried over to subsequent products derived from these cereals [9,10,11]. Sorghum is an important cereal crop that is prevalent in Africa [12]. However, sorghum, like other cereal crops, is susceptible to fungal proliferation during cultivation, harvest, storage and processing. This colonisation of sorghum by toxigenic fungi could be accompanied by the production of secondary metabolites including mycotoxins [9,10], further aggravated by the favourable tropical climatic conditions that are prevalent in Africa. When ingested, these mycotoxins can cause harmful health effects including cancer and in extreme cases may lead to death [11]. Notable mycotoxins in sorghum include aflatoxins (AFs), fumonisins (FBs), zearalenone (ZEA), deoxynivalenol (DON) and ochratoxin A (OTA) [9,10,11,13,14]. Except for AFs, limited reports exist on mycotoxin occurrence in dietary products in southern African countries [15].
Fermentation is a traditional, age-old technique of transforming sorghum grains, or any other grain, into diverse forms of food that constitute the daily diets of most African populations. This processing technique has been well documented to improve shelf life, nutrient bioavailability and health benefits [16,17]. Porridges are staple foods in Southern African cuisine. An indigenous fermented sorghum-based porridge known as ting is commonly consumed as bogobe or motogo in South Africa, Botswana and other neighbouring Southern African countries [18,19,20]. The product is frequently fed to infants and regularly consumed by adults.
Considering the deleterious effects of mycotoxins, there is a need to explore viable, safe and practicable strategies that can mitigate their presence in foods. Several approaches of pre- and postharvest measures have not necessarily met the desired efficacy, safety levels and nutrient retention. Microbial decontamination provides a viable alternative for the possible removal of these toxic substances in foods under mild conditions, limiting significant losses in the aesthetic quality of food products [21,22,23,24]. Available reports in the literature have suggested fermentation, particularly with lactic acid bacteria (LAB) starter cultures, as an effective and promising technique for reducing the presence of mycotoxins while improving food composition, conferring preservative effects and retaining nutritive value [25,26,27,28,29,30].
With these naturally occurring toxic compounds that frequently contaminate agricultural commodities posing a hazardous risk to humans and animals, this study explored the reduction of these toxins through natural and lactic acid bacteria (LAB) fermentation during ting production from whole grain (WG) sorghum.
2. Results and Discussion
2.1. Presence of Mycotoxins
As observed in Table 1, the method validation results fulfilled the generally acceptable parameters [31]. Of the 14 mycotoxins investigated in this study (Table 1), seven including the fumonisins (FB1, FB2 and FB3), T-2 toxin (T-2), ZEA, alpha-zearalenol (α-ZOL) and beta-zearalenol (β-ZOL) were detected in raw WG-sorghum grain and subsequent WG-ting samples (Table 2). It was observed that the raw low tannin (LT)-sorghum samples generally had higher levels of mycotoxins than the high tannin (HT)-sorghum samples (Table 2). While LT-sorghum samples had FB1, FB2 and FB3 levels of 163, 12 and 400 μg/kg, respectively, the HT-sorghum samples contained neither FB1 nor FB2 though the same sample had FB3 at a level of 148 μg/kg. Higher mycotoxin levels in LT-sorghum samples when compared to their HT counterparts were equally recorded for T-2, α-ZOL and β-ZOL (Table 2). It was established in previous studies that there were higher concentrations of bioactive compounds (such as polyphenols, flavonoids and tannins) in HT-sorghum as compared to LT-sorghum [18,20,30]. Thus, we can reinforce the hypothesis that these compounds may have contributed to the inhibition of mycotoxigenic fungi and microbial action in addition to limiting attendant mycotoxin production, as demonstrated in other studies [32,33].
Table 1.
Identity and characteristics of the mycotoxins investigated by liquid chromatography-tandem mass spectrometry (LC-MS/MS).
| Rt (min) | Mycotoxin Standard | MW | Parent Ion m/z (Precursor) | MS/MS Fragments | CE | R2 (Neat Solvent) | R2 (Sorghum Mix) | ARR (%) | LOD (µg/kg) | LOQ (µg/kg) |
|---|---|---|---|---|---|---|---|---|---|---|
| 2.78 | DON | 296 | 297.10 | 231, 249 | 12 | 0.989 | 0.981 | 89 | 0.11 | 1.74 |
| 6.87 | AFG2 | 330 | 331.00 | 245, 313 | 32 | 0.999 | 0.992 | 82 | 0.01 | 0.04 |
| 7.12 | AFG1 | 328 | 329.00 | 243, 311 | 28 | 0.999 | 0.990 | 85 | 0.01 | 0.04 |
| 7.30 | AFB2 | 314 | 315.00 | 259, 287 | 31 | 0.999 | 0.989 | 91 | 0.59 | 1.98 |
| 7.48 | AFB1 | 312 | 313.00 | 241, 285 | 24 | 0.999 | 0.997 | 90 | 0.01 | 0.04 |
| 7.58 | FB1 | 721 | 722.40 | 352, 334 | 42 | 0.997 | 0.996 | 98 | 0.34 | 0.87 |
| 8.03 | β-ZOL | 322 | 323.00 | 277, 305 | 11 | 0.998 | 0.998 | 90 | 0.21 | 0.71 |
| 8.20 | FB2 | 705 | 706.10 | 336, 318 | 38 | 0.999 | 0.996 | 99 | 0.41 | 0.93 |
| 8.25 | FB3 | 705 | 706.30 | 336, 354 | 35 | 0.999 | 0.997 | 94 | 1.02 | 4.11 |
| 8.28 | OTB | 369 | 370.10 | 205, 324 | 14 | 0.998 | 0.991 | 94 | 0.06 | 0.19 |
| 8.38 | α-ZOL | 322 | 323.10 | 277, 305 | 9 | 0.999 | 0.993 | 87 | 0.65 | 2.17 |
| 8.53 | T-2 | 466 | 467.20 | 245, 305 | 11 | 0.999 | 0.997 | 96 | 0.39 | 0.90 |
| 8.74 | ZEA | 318 | 319.10 | 185, 187 | 21 | 0.999 | 0.991 | 92 | 0.03 | 0.11 |
| 8.78 | OTA | 403 | 404.00 | 239, 221 | 38 | 0.999 | 0.993 | 86 | 0.04 | 0.14 |
ARR—apparent recovery rate; CE—collision energy; Rt—retention time; LOD—limit of detection; LOQ—limit of quantification; MW—molecular weight; m/z—mass-to-charge ratio; MS/MS—tandem mass spectrometry; R2—coefficient of regression; AFB1—aflatoxin B1; AFB2—aflatoxin B2; AFG1—aflatoxin G1; AFG2—aflatoxin G2; DON—deoxynivalenol; FB1—fumonisin B1; FB2—fumonisin B2; FB3—fumonisin B3; OTA—ochratoxin A; OTB—ochratoxin B; T-2—T-2 toxin; ZEA—zearalenone; α-ZOL—alpha-zearalenol; and β-ZOL—beta-zearalenol.
Table 2.
Quantification of mycotoxins (µg/kg) in sorghum and after fermentation to whole grain ting.
| FB1 | FB2 | FB3 | T-2 | ZEA | α-ZOL | β-ZOL | |
|---|---|---|---|---|---|---|---|
| LT-sorghum | |||||||
| Raw LT-sorghum | 162.67 d ± 3.90 | 12.00 b ± 0.99 | 400.00 d ± 2.98 | 7.39 b ± 1.20 | 6.67 c ± 0.50 | 28.00 d ± 0.45 | 37.33 d ± 0.53 |
| 3424 | 34.68 c ± 5.58 | 2.67 a ± 0.45 | 170.67 c ± 2.35 | 2.32 a ± 0.74 | 5.67 b ± 0.28 | 11.00 c ± 0.99 | 24.67 c ± 0.49 |
| 3424 + 3165 | 9.33 b ± 1.40 | 2.31 a ± 0.98 | 155.33 b ± 1.45 | 1.68 a ± 0.78 | 4.00 a ± 0.82 | 7.00 b ± 0.97 | 13.33 b ± 0.75 |
| 3424 + 3321 | 4.00 a ± 2.63 | 1.33 a ± 1.06 | 133.33 a ± 2.19 | 1.17 a ± 0.63 | 4.00 a ± 0.05 | 5.00 a ± 0.92 | 11.83 a ± 0.63 |
| 3424 (3165 + 3321) | 6.67 ab ± 2.10 | 2.00 a ± 0.92 | 156.67 b ± 2.09 | 1.28 a ± 0.75 | 5.00 b ± 0.06 | 9.67 c ± 0.81 | 11.94 a ± 0.07 |
| HT-sorghum | |||||||
| Raw HT-sorghum | – | – | 148.00 d ± 1.93 | 6.67 b ± 1.00 | 6.04 b ± 0.13 | 20.89 c ± 0.82 | 25.33 c ± 0.44 |
| 2872 | – | – | 105.33 c ± 1.80 | 4.06 a ± 0.06 | 5.33 ab ± 0.43 | 10.33 b ± 0.44 | 19.31 b ± 0.44 |
| 2872 + 3165 | – | – | 91.07 b ± 1.74 | 3.94 a ± 0.85 | 4.82 a ± 0.1 | 8.69 a ± 0.21 | 12.00 a ± 0.87 |
| 2872 + 3321 | – | – | 84.06 a ± 2.29 | 3.17 a ± 0.17 | 4.67 a ± 0.3 | 8.67 a ± 0.51 | 10.67 a ± 0.46 |
| 2872 (3165 + 3321) | – | – | 93.38 b ± 1.82 | 3.85 a ± 0.33 | 5.00 a ± 0.71 | 9.00 a ± 0.23 | 18.33 b ± 1.21 |
HT—high tannin; LT—low tannin; 2872—naturally fermented ting from HT-sorghum type; (3165)—fermentation with Lactobacillus fermentum FUA 3165; (3321)—fermentation with L. fermentum FUA 3321; (3165 + 3321)—fermentation with L. fermentum FUA 3165 and L. fermentum FUA 3321; 3424—naturally fermented ting from LT-sorghum type. Each value is a mean ± standard deviation of triplicates. a,b,c,d No common letters within a column under each sample type significantly (p ≤ 0.05) differ. FB1—fumonisin B1, FB2—fumonisin B2; FB3—fumonisin B3, T-2—T-2 toxin; ZEA—zearalenone; α-ZOL—α-zearalenol and β-ZOL—β-zearalenol.
The levels of mycotoxins in the WG-sorghum samples recorded in this study are below the regulatory recommended mycotoxin limits in Southern Africa and the European Union (EU) [31,34,35], indicating that the LT- and HT-sorghum samples are quite “safe” for intended consumers. The relatively low mycotoxin levels observed in this study compared to other available studies in the literature on sorghum [10,11,13,14,27,36] could be the result of effective agricultural practices during its production, which might have limited the initial fungal contamination of the sorghum grains.
2.2. Mycotoxin Reduction
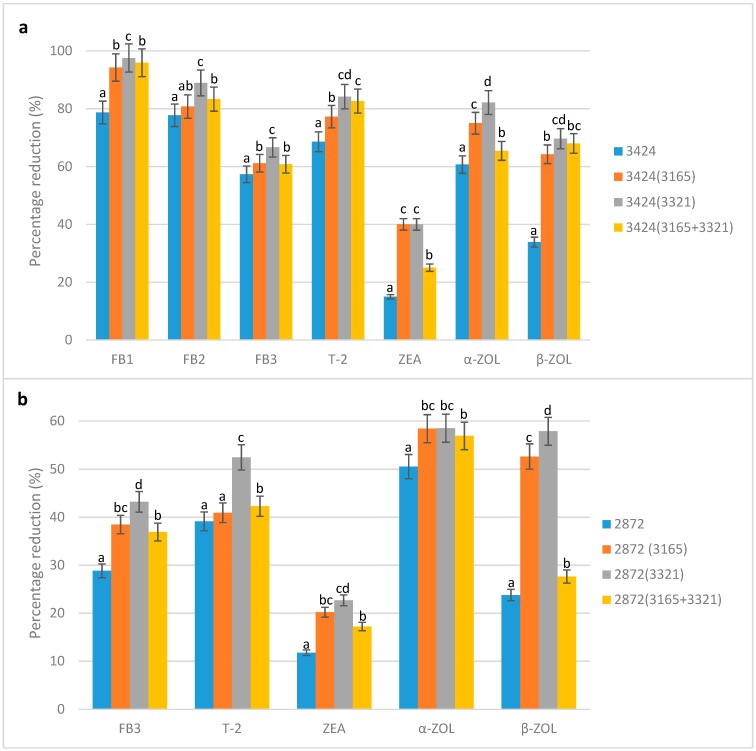
The presence of all the detected mycotoxins was significantly (p ≤ 0.05) reduced after the fermentation of WG-sorghum to WG-ting (Table 2). Residual mycotoxin levels in LT-ting samples were FB1 (4–34.68 μg/kg), FB2 (1.33–2.67 μg/kg), FB3 (133.33–170.67 μg/kg), T-2 (1.17–2.32 μg/kg), ZEA (4.00–5.67 μg/kg), α-ZOL (5–11 μg/kg) and β-ZOL (11.83–24.67 μg/kg). For HT-ting samples, residual mycotoxin levels ranged between 84.06 and 105.33, between 3.17 and 4.06, between 4.67 and 5.33, between 8.67 and 10.33 and between 10.67 and 19.31 μg/kg for FB3, T-2, ZEA, α-ZOL and β-ZOL, respectively (Table 2). Although the sorghum varieties were fermented in different conditions, i.e., 24 h at 34 °C (LT-sorghum type) and 72 h at 28 °C (HT-sorghum type), fermentation with L. fermentum strains both singly and in combination was observed to be more effective at reducing mycotoxins in subsequent WG-ting samples derived from them (Figure 1a,b).
Figure 1.
Reduction of mycotoxin levels in ting from whole grain sorghum. (a) Ting samples obtained from the LT-sorghum type; (b) Ting samples obtained from the HT-sorghum type. 2872—naturally fermented ting from HT-sorghum; 3424—naturally fermented ting from LT-sorghum type; (3165)—fermentation with L. fermentum FUA 3165; (3321)—fermentation with L. fermentum FUA 3321; (3165 + 3321)—fermentation with L. fermentum FUA 3165 and L. fermentum FUA 3321.
Natural fermentation of the LT-ting samples resulted in 79, 78, 57, 69, 15, 61 and 34% reductions in FB1, FB2, FB3, T-2, ZEA, α-ZOL and β-ZOL, respectively (Figure 1a). Lesser reduction levels were, however, observed in the HT-ting samples (Figure 1b). Percentage reduction of the mycotoxins after fermentation with L. fermentum strains was more pronounced and above 60% in the LT-ting samples (Figure 1a) and above 25% in the HT-ting samples. These reductions were relatively low in naturally (spontaneously) fermented WG-ting samples obtained from both LT- and HT-sorghum samples (Figure 1a,b). Although the fermentation time for HT-ting samples was longer, the percentage of mycotoxin reduction in LT-ting samples was higher, implying that initial substrate composition could have influenced the extent of mycotoxin reduction. This is equally reflected in the higher microbial population observed in the LT-ting samples compared to their HT-counterpart in our earlier study [18]. The high percentage FB reduction of over 90% in all of the LAB-fermented LT-ting samples recorded in this study (Figure 1a) is worthy of note. Similar findings have been reported in other African fermented foods such as fermented maize gruel, ogi and mahewu [28,37,38,39]. This is particularly important, considering the deleterious effect of these FBs and their harmful effects to inhabitants of southern Africa and the world at large.
The composition of microbiota largely influences the products of microbial metabolism during fermentation. As observed in our earlier study, relatively low pH values, higher corresponding titratable acidity (TTA) values and significantly (p ≤ 0.05) higher microbial loads were recorded during ting production with the L. fermentum strains [18]. Relatively low pH, high alcohol content, lactic acid and increased production of other relevant metabolites during the fermentation with LABs could have instigated a better mycotoxin reduction during LAB fermentation. Consequent reduction could be attributed to a possible breakdown and/or degradation of mycotoxins to less toxic products by the fermenting microbiota. Fermentation has been identified as an effective process to reduce mycotoxins due to their breakdown by endogenous enzymes and compounds secreted and released into the food matrix by these fermenting organisms [21,29]. The production of bacteriocins as well as antagonistic and proteinaceous compounds might have also contributed to this observation. As observed in our previous study [18], the production of such compounds could have also resulted in the reduction of fungal load with the LAB-fermented ting samples compared to the spontaneously fermented ones.
The significantly (p ≤ 0.05) lower residual mycotoxin levels and corresponding higher percentages of mycotoxin reduction in WG-ting samples obtained using L. fermentum FUA 3321 (singly) demonstrates the highest mycotoxin reduction of all the tested mycotoxins. In a study on FB1 and FB2 reduction by Lactobacillus strains, Zhao et al. [40] attributed their efficacy to an enhanced interaction between mycotoxins and the bacterial cell wall. Likewise, this interaction could be a reason for the effectiveness of L. fermentum FUA 3321 at reducing mycotoxins. Relatively low mycotoxin reduction in HT-ting samples (Figure 1b) compared to LT-ting samples (Figure 1a) and the generally higher reduction with LAB strains (Figure 1a,b) compared to natural fermentation could be attributed to accelerated fermentation and increased microbial action. Not only was L. fermentum FUA 3321 a better fermenting microorganism, as observed in an earlier study [18], a faster decontamination process and rapid metabolism of the strain could have contributed to this observation. Studies have shown that fermentative organisms such as LABs are capable of adsorbing mycotoxins from agricultural products onto their cell wall surface components, thereby effectively decontaminating food [41,42]. As indicated in previous studies [21,28,43], LABs can better detoxify mycotoxins to less toxic forms during cereal fermentation. It could thus be speculated that these toxins have been completely detoxified, hydrolysed and degraded to less toxic forms.
Although effective mycotoxin reduction in this study was highest for L. fermentum FUA 3321 alone, followed by L. fermentum FUA 3165 alone, the combination of L. fermentum FUA 3321 and 3165 and then natural fermentation (Figure 1a,b), this trend was distinctly different for T-2 (Table 2, Figure 1a,b). While L. fermentum FUA 3321 was still the most effective strain, a combination of L. fermentum FUA 3321 and 3165 proved more effective than L. fermentum FUA 3165 when used in isolation. It can thus be suggested that the synergistic effect of the mixed LABs on T-2 reduction was more effective in binary combination (L. fermentum FUA 3165 and L. fermentum FUA 3321) compared to a pure culture of L. fermentum FUA 3165. It could also be speculated that a single strain of L. fermentum FUA 3321 and the binary combination of the strains had relatively higher affinity for the 12,13-epoxy ring responsible for the toxicity of T-2 [44]. On the contrary, the lower percentage reduction of ZEA (Figure 1a,b) could rather be due to the reduced affinity of the strains to the lactone ring of the ZEA molecule.
While these mycotoxins are all known to pose significant threats and illicit toxic effects, their reduction levels vary as recorded in Table 2 and Figure 1. This variation in the extent of mycotoxin reduction can also be attributed to differences in their chemical structure, polarity, dissociation constant and initial mycotoxin levels/concentration, all of which contributed to the reduction levels obtained. Furthermore, several other compounds have the capability of reacting with the cell wall of fermenting strains, making them equal competitors for the mycotoxin detoxification process [45,46]. While all of this might have occurred during both natural and LAB fermentation processes of ting, a better interaction between the cell wall of L. fermentum FUA 3321 and the mycotoxins could have resulted in a significantly (p ≤ 0.05) higher percentage mycotoxin reduction observed. Although not investigated in this study, the size, shape, surface area and the surface charge of the L. fermentum FUA 3321 cells could have also contributed to this observation.
3. Conclusions
Fermentation significantly reduced the levels of mycotoxins in ting samples obtained from whole grain sorghum samples, especially with the use of lactic acid bacteria strains. Although both L. fermentum strains exhibited good mycotoxin reduction ability, the use of L. fermentum FUA 3321 resulted in a better mycotoxin reduction. It can thus be deduced that L. fermentum strains are promising and suitable starter cultures for dietary detoxification of mycotoxins in fermented food commodities. Further research is still needed to investigate the activity of these strains on other fermented foods and to clarify whether the mycotoxins apparently lost during WG-ting preparation are indeed destroyed, hydrolysed or bound to the food matrix to become non-recoverable. Additionally needed and equally important are toxicity studies to ascertain the toxic nature of the decontamination products thereof.
4. Materials and Methods
4.1. Raw Material and Sample Preparation
Sorghum (Sorghum bicolor L.) grain cultivars, i.e., high tannin (HT) and low tannin (LT) obtained and prepared as reported in Adebo et al. [20], were used. The tannin content (TNC) of both raw LT- and HT-sorghum types was investigated, with values recorded as 17.97 and 31.68 mg CE/g. As such, these were subsequently classified as LT- and HT-sorghum types, respectively [18].
4.2. Lactobacillus Strains
Two L. fermentum strains (L. fermentum FUA 3165 and L. fermentum FUA 3321) used were earlier isolated from ting [10] and prepared as described in [18].
4.3. Fermentation of Sorghum into WG-Ting
Ting was processed by mixing 50 g of WG-sorghum flour and sterile distilled water (40 °C) (1:1, w/v) [20]. Fermentation with the LAB strains was conducted as earlier described [18]. Naturally fermented WG-ting samples were also obtained by spontaneously fermenting sorghum under similar conditions (without the strains). All samples were freeze-dried at −55 °C for 24 h prior to analysis.
4.4. Mycotoxin Standards
Mycotoxin standards including AFB1, AFB2, AFG1, AFG2 and DON were purchased from Sigma Aldrich, Germany, while FB1, FB2, FB3, OTA, OTB, T-2, ZEA, α-ZOL and β-ZOL were obtained from the Council for Scientific and Industrial Research (CSIR), South Africa. They were all dissolved in LC-grade methanol (MeOH) and prepared at five different concentration levels ranging between 0.0002 and 0.2 μg/L (OTA, OTB), 0.001 and 1 μg/L (AFB1, AFB2, AFG1, AFG2), 0.0025 and 2.5 μg/L (FB1, FB2, FB3, ZEA, α-ZOL, β-ZOL) and 0.05 and 5 μg/L (DON, T-2). This was subsequently used to obtain a blank (neat solvent) calibration curve for initial mycotoxin quantification.
4.5. Mycotoxin Extraction
A modified Quick Easy Cheap Effective Rugged and Safe (QuEChERS) method earlier adapted by Oueslati et al. [36] and Motloung et al. [47] was followed for mycotoxin extraction. Freeze-dried raw sorghum and ting samples were finely milled and homogenised. Then, 1 g was weighed into an extraction tube containing 5 mL of distilled water, vortexed and left for 30 min. Thereafter, 5 mL (acetonitrile (ACN)/1% formic acid, v/v) extraction solvent was added and sonicated for 20 min. NaCl (0.5 g) and 2 g of MgSO4 anhydrous salt were added; the tube was capped and briefly shaken to avoid agglomeration. The tubes were vortexed, centrifuged for 15 min at 4000 × g and the supernatant layer was filtered (0.22 µm, Millipore, Bedford, MA, USA) into vials for LC-MS/MS analysis.
4.6. Mycotoxin Analysis
For the quantification of the mycotoxins, triplicates of each extract were analysed in multiple reaction monitoring (MRM) mode by injecting 10 µL of each into the LC-MS/MS system that consisted of a UPLC instrument (Shimadzu Kyoto, Japan) equipped with an auto-sampler (SIL-30 AC, Nexera, Kyoto, Japan), communication bus module (CBM-20A), column oven (CTO-30A), degassing unit (DGU-20A5R) and a liquid chromatograph (LC-30AD) interfaced with a triple quadrupole mass spectrometer (LC-MS-8030). A Raptor C18 column (2.7 µm particle size × 100 mm length × 2.1 mm ID, Restek, Bellefonte, PA, USA) was used and the analysis was performed at a constant flow rate of 0.2 mL/min. The mobile phases, solvents A and B, consisted of 0.1% formic acid in water and 0.1% formic acid in ACN:MeOH (50:50, v/v), respectively. The solvent gradients were 10% B for 0.1 min, ramped to 95% B over 8.4 min, held at 95% B for 3 min and then 10% B for 1 min, after which the column was re-equilibrated at this condition for 4.5 min. The column temperature was maintained at 40 °C throughout the chromatographic runs and MS data were acquired in a positive mode. The interface nebulising gas flow rate was 3 L/min, the DL temperature was 250 °C, the heat block temperature was 400 °C and the drying gas flow rate was 15 L/min. Other optimal LC-MS/MS parameters are presented in Table 1.
Apparent recovery rates (ARR) were accessed by spiking 1 g of sorghum “blank samples” with known concentrations of mycotoxins in triplicates and maintaining them at room temperature overnight for solvent evaporation [48]. The mycotoxin was re-extracted and quantified as earlier described in this section. Matrix (sorghum)-matched calibration curves were also prepared from the sorghum matrix and subsequent results were based on the ARR obtained and quantification obtained through the matrix-matched calibration curve. The limits of detection (LOD) and quantification (LOQ) were determined using the method described by Wenzl et al. [49]. Percentage mycotoxin reduction after fermentation was calculated as [(A − B)/A] × 100, where A and B are the initial and final mycotoxin concentrations, respectively [50].
4.7. Statistical Analysis
All analyses were conducted in triplicate and the results presented represent the average of triplicate determinations. An analysis of variance (ANOVA) was performed using SPSS 22 (IBM, New York, NY, USA) [32] and mean values among treatments for each sample type were considered to differ significantly if p ≤ 0.05.
Acknowledgments
Special thanks to Riaan Meyer and Darryl Harris from Shimadzu, South Africa for their technical assistance, Michael Gänzle of the University of Alberta in Canada for donating the L. fermentum strains used in this study as well as Janet Adebiyi and Sefater Gbashi of UJ for analytical assistance.
Author Contributions
Conceptualization, O.A.A, E.K, and P.B.N.; Methodology, O.A.A., E.K., and P.B.N.; Software, O.A.A.; Validation, O.A.A. and P.B.N.; Formal Analysis, O.A.A.; Investigation, O.A.A., E.K., and P.B.N.; Funding and Resources, P.B.N.; Data Curation, O.A.A.; Original Manuscript Draft, O.A.A.; Proofing and Editing, P.B.N. and E.K.
Funding
This research was funded via the National Research Foundation (NRF) Research and Technology Funding and as well as the NRF/LEAP-AGRI funding.
Conflicts of Interest
The authors declare no conflict of interest.
Key Contribution
Fermentation of whole grain sorghum into ting resulted in reduced mycotoxin levels. Better mycotoxin reduction was observed in L. fermentum ting samples, indicating that these strains are suitable starter cultures for dietary detoxification of mycotoxins.
References
- 1.Udomkun P., Wiredu A.N., Nagle M., Bandyopadhyay R., Müller J., Vanlauwe B. Mycotoxins in sub-Saharan Africa: Present situation, socio-economic impact, awareness, and outlook. Food Cont. 2017;72:110–122. doi: 10.1016/j.foodcont.2016.07.039. [DOI] [Google Scholar]
- 2.IARC . Improving Public Health through Mycotoxin Control. In: Pitt J.I., Wild C.P., Baan R.A., Gelderblom W.C.A., Miller J.D., Riley R.T., Wu F., editors. IARC Scientific Publication No. 158. International Agency for Research on Cancer; Lyon, France: 2012. [Google Scholar]
- 3.Misihairabgwi J.M., Ezekiel C.N., Sulyok M., Shephard G.S., Krska R. Mycotoxin contamination of foods in Southern Africa: A 10-year review (2007–2016) Crit. Rev. Food Sci. Nutr. 2019;59:43–58. doi: 10.1080/10408398.2017.1357003. [DOI] [PubMed] [Google Scholar]
- 4.Gbashi S., Madala N.E., De Saeger S., De Boevre M., Adekoya I., Adebo O.A., Njobeh P.B. The socio-economic impact of mycotoxin contamination in Africa. In: Njobeh P.B., Stepman F., editors. Fungi and Mycotoxins—Their Occurrence, Impact on Health and the Economy as well as Pre- and Postharvest Management Strategies. InTech; Rijeka, Croatia: 2019. [Google Scholar]
- 5.Marasas W.F.O. Discovery and occurrence of the fumonisins: A historical perspective. Environ. Health Perspect. 2001;109:239–243. doi: 10.1289/ehp.01109s2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marasas W.F., Riley R.T., Hendricks K.A., Stevens V.L., Sadler T.W., Gelineau-van Waes J. Fumonisins disrupt sphingolipid metabolism, folate transport, and neural tube development in embryo culture and in vivo: A potential risk factor for human neural tube defects among populations consuming fumonisin-contaminated maize. J. Nutr. 2004;134:711–716. doi: 10.1093/jn/134.4.711. [DOI] [PubMed] [Google Scholar]
- 7.Shephard G.S., Burger H.M., Rheeder J.P., Alberts J.F., Gelderblom W.C.A. The effectiveness of regulatory maximum levels for fumonisin mycotoxins in commercial and subsistence maize crops in South Africa. Food Cont. 2019;97:77–80. doi: 10.1016/j.foodcont.2018.10.004. [DOI] [Google Scholar]
- 8.Shephard G.S. Impact of mycotoxins on human health in developing countries. Food Addit. Contam. 2008;25:146–151. doi: 10.1080/02652030701567442. [DOI] [PubMed] [Google Scholar]
- 9.Chala A., Taye W., Ayalew A., Krska R., Sulyok M., Logrieco A. Multimycotoxin analysis of sorghum (Sorghum bicolor L. Moench) and finger millet (Eleusine coracana L. Garten) from Ethiopia. Food Cont. 2014;45:29–35. doi: 10.1016/j.foodcont.2014.04.018. [DOI] [Google Scholar]
- 10.Taye W., Ayalew A., Denjene M., Chala A. Fungal invasion and mycotoxin contamination of stored sorghum grain as influenced by threshing methods. Int. J. Pest Manag. 2018;64:66–76. doi: 10.1080/09670874.2017.1327681. [DOI] [Google Scholar]
- 11.Njobeh P.B., Dutton M.F., Makun H.A. Mycotoxins and human health: Significance, prevention and control. In: Ajay K.M., Ashutosh T., Shivanti B.M., editors. Smart Biomolecules in Medicine. VBRI Press; Allahabad, India: 2010. pp. 132–177. [Google Scholar]
- 12.Odunmbaku L.A., Sobowale S.S., Adenekan M.K., Oloyede T., Adebiyi J.A., Adebo O.A. Influence of steeping duration, drying temperature and duration on the chemical composition of sorghum-starch. Food Sci. Nutr. 2018;6:348–355. doi: 10.1002/fsn3.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Warth B., Parich A., Atehnkeng J., Bandyopadhyay R., Schuhmacher R., Sulyok M., Krska R. Quantitation of mycotoxins in food and feed from Burkina Faso and Mozambique using a modern LC-MS/MS multitoxin method. J. Agric. Food Chem. 2012;60:9352–9936. doi: 10.1021/jf302003n. [DOI] [PubMed] [Google Scholar]
- 14.Makun H.A., Gbodi T.A., Akanya H.O., Salako E.A., Ogbadu G.H. Fungi and some mycotoxins found in mouldy sorghum in Niger State, Nigeria. World J. Agric. Sci. 2009;5:5–17. [Google Scholar]
- 15.Matumba L., Van Poucke C., Ediage E.N., De Saeger S. Keeping mycotoxins away from the food: Does the existence of regulations have any impact in Africa? Crit. Rev. Food Sci. Nutr. 2017;57:1584–1592. doi: 10.1080/10408398.2014.993021. [DOI] [PubMed] [Google Scholar]
- 16.Taylor J.R.N., Schober T.J., Bean S.R. Novel food and non-food uses for sorghum and millets. J. Cereal Sci. 2006;44:252–271. doi: 10.1016/j.jcs.2006.06.009. [DOI] [Google Scholar]
- 17.Adebo O.A., Njobeh P.B., Adebiyi J.A., Gbashi S., Phoku J.Z., Kayitesi E. Fermented pulse-based food products in developing nations as functional foods and ingredients. In: Hueda M.C., editor. Functional Food—Improve Health through Adequate Food. InTech; Rijeka, Croatia: 2017. pp. 77–109. [Google Scholar]
- 18.Adebo O.A., Njobeh P.B., Kayitesi E. Fermentation by Lactobacillus fermentum strains (singly and in combination) enhances the properties of ting from two whole grain sorghum types. J. Cereal Sci. 2018;82:49–56. doi: 10.1016/j.jcs.2018.05.008. [DOI] [Google Scholar]
- 19.Sekwati-Monang B., Gänzle M.G. Microbiological and chemical characterization of ting, a sorghum-based sourdough product from Botswana. Int. J. Food Microbiol. 2011;150:115–121. doi: 10.1016/j.ijfoodmicro.2011.07.021. [DOI] [PubMed] [Google Scholar]
- 20.Adebo O.A., Njobeh P.B., Mulaba-Bafubiandi A.F., Adebiyi J.A., Desobgo Z.S.C., Kayitesi E. Optimization of fermentation conditions for ting production using response surface methodology. J. Food Proc. Preserv. 2018;42:e13381. doi: 10.1111/jfpp.13381. [DOI] [Google Scholar]
- 21.Adebo O.A., Njobeh P.B., Gbashi S., Nwinyi O.C., Mavumengwana V. Review on microbial degradation of aflatoxins. Crit. Rev. Food Sci. Nutr. 2017;57:3208–3217. doi: 10.1080/10408398.2015.1106440. [DOI] [PubMed] [Google Scholar]
- 22.Alberts J.F., Gelderblom W.C.A., Botha A., van Zyl W.H. Degradation of aflatoxin B1 by fungal laccase enzymes. Int. J. Food Microbiol. 2009;135:47–52. doi: 10.1016/j.ijfoodmicro.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 23.Samuel M.S., Sivaramakrishna A., Mehta A. Degradation and detoxification of aflatoxin B1 by Pseudomonas putida. Int. Biodeter. Biodegr. 2014;86:202–209. doi: 10.1016/j.ibiod.2013.08.026. [DOI] [Google Scholar]
- 24.Adebo O.A., Njobeh P.B., Mavumengwana V. Degradation and detoxification of AFB1 by Staphylocococcus warneri, Sporosarcina sp. and Lysinibacillus fusiformis. Food Cont. 2016;68:92–96. doi: 10.1016/j.foodcont.2016.03.021. [DOI] [Google Scholar]
- 25.Karlovsky P., Suman M., Berthiller F., De Meester J., Eisenbrand G., Perrin I., Oswald I.P., Speijers G., Chiodini A., Recker T., et al. Impact of food processing and detoxification treatments on mycotoxin contamination. Mycotoxin Res. 2016;32:179–205. doi: 10.1007/s12550-016-0257-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adebo O.A., Njobeh P.B., Sidu S., Tlou M.G., Mavumengwana V. Aflatoxin B1 degradation by liquid cultures and lysates of three bacterial strains. Int. J. Food Microbiol. 2016;233:11–19. doi: 10.1016/j.ijfoodmicro.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 27.Ezekiel C.N., Abia W.A., Ogara I.M., Sulyok M., Warth B., Krska R. Fate of mycotoxins in two popular traditional cereal-based beverages (kunu-zaki and pito) from rural Nigeria. LWT-Food Sci. Technol. 2015;60:137–141. doi: 10.1016/j.lwt.2014.08.018. [DOI] [Google Scholar]
- 28.Nyamete F.A., Bennink M., Mugula J.K. Potential of lactic acid fermentation in reducing aflatoxin B1 in Tanzania maize-based gruel. Afric. J. Food Agric. Nutr. Dev. 2016;16:11139–11151. doi: 10.18697/ajfand.75.ILRI12. [DOI] [Google Scholar]
- 29.Okeke C.A., Ezekiel C.N., Sulyok M., Ogunremi O.R., Ezeamagu C.O., Sarkanj B., Warth B., Krska R. Traditional processing impacts mycotoxin levels and nutritional value of ogi—A maize-based complementary food. Food Cont. 2018;86:224–233. doi: 10.1016/j.foodcont.2017.11.021. [DOI] [Google Scholar]
- 30.Adebo O.A., Njobeh P.B., Adebiyi J.A., Kayitesi E. Co-influence of fermentation time and temperature on physicochemical properties, bioactive components and microstructure of ting (a Southern African food) from whole grain sorghum. Food Biosci. 2018;25:118–127. doi: 10.1016/j.fbio.2018.08.007. [DOI] [Google Scholar]
- 31.European Commission Regulation No 1881/2006 of 19 December 2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs. [(accessed on 23 December 2017)]; Available online: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CONSLEG:2006R1881:20100701:EN:PDF.
- 32.Atanasova-Penichon V., Barreau C., Richard-Forget F. Antioxidant secondary metabolites in cereals: Potential involvement in resistance to Fusarium and mycotoxin accumulation. Front. Microbiol. 2016;7:1–16. doi: 10.3389/fmicb.2016.00566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Telles A.C., Kupski L., Furlong E.B. Phenolic compound in beans as protection against mycotoxins. Food Chem. 2017;214:293–299. doi: 10.1016/j.foodchem.2016.07.079. [DOI] [PubMed] [Google Scholar]
- 34.South African Government Foodstuffs, Cosmetics and Disinfectants Act (Act 54 of 1972) and Regulations Published under Government Notice No. R. 1145, Dated 8 October 2004. [(accessed on 3 March 2019)]; Available online: http://www.sagl.co.za/Portals/0/Maize%20Crop%202015%202016/Page%2073.pdf.
- 35.European Commission Recommendation of 27 March 2013 on the Presence of T-2 and HT-2 Toxin in Cereals and Cereal Products. [(accessed on 23 December 2017)]; Available online: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2013:091:0012:0015:EN:PDF.
- 36.Oueslati S., Romero-González R., Lasram S., Frenich A.G., Martínez Vidal J.L. Multi-mycotoxin determination in cereals and derived products marketed in Tunisia using ultra-high performance liquid chromatography coupled to triple quadrupole mass spectrometry. Food Chem. Toxicol. 2012;50:2376–2381. doi: 10.1016/j.fct.2012.04.036. [DOI] [PubMed] [Google Scholar]
- 37.Nyamete F.A., Mourice B., Mugula J.K. Fumonisin B1 reduction in lactic acid bacteria fermentation of maize porridges. Tanz. J. Agric. Sci. 2016;15:13–20. [Google Scholar]
- 38.Dawlal P., Brabet C., Thantsha M.S., Buys E.M. Potential of lactic acid bacteria for the reduction of fumonisin exposure in African fermented maize based foods. World Mycotoxin J. 2017;10:309–318. doi: 10.3920/WMJ2017.2184. [DOI] [Google Scholar]
- 39.Dawlal P., Brabet C., Thantsha M.S., Buys E.M. Visualisation and quantification of fumonisins bound by lactic acid bacteria isolates from traditional African maize-based fermented cereals, ogi and mahewu. Food Addit. Contam. Part A. 2019;36:296–307. doi: 10.1080/19440049.2018.1562234. [DOI] [PubMed] [Google Scholar]
- 40.Zhao H., Wang X., Zhang J., Zhang J., Zhang B. The mechanism of Lactobacillus strains for their ability to remove fumonisins B1 and B2. Food Chem. Toxicol. 2016;97:40–46. doi: 10.1016/j.fct.2016.08.028. [DOI] [PubMed] [Google Scholar]
- 41.Garda J., Macedo R.M., Faria R., Bernd L., Dors G.C., Badiale-Furlong E. Alcoholic fermentation effects on malt spiked with trichothecenes. Food Cont. 2005;16:423–428. doi: 10.1016/j.foodcont.2004.05.001. [DOI] [Google Scholar]
- 42.Shetty P.H., Jespersen L. Saccharomyces cerevisiae and lactic acid bacteria as potential mycotoxin decontaminating agents. Trends Food Sci. Technol. 2006;17:48–55. doi: 10.1016/j.tifs.2005.10.004. [DOI] [Google Scholar]
- 43.Mokoena M.P., Chelule P.K., Ggaleni N. Reduction of fumonisin B1 and zearalenone by lactic acid bacteria in fermented maize meal. J. Food Prot. 2005;68:2095–2099. doi: 10.4315/0362-028X-68.10.2095. [DOI] [PubMed] [Google Scholar]
- 44.Adhikari M., Negi B., Kaushik N., Adhikari A., Al-Khedhairy A.A., Kaushik N.K., Choi E.H. T-2 mycotoxin: Toxicological effects and decontamination strategies. Oncotarget. 2017;8:33933–33952. doi: 10.18632/oncotarget.15422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Amezqueta S., Gonzales Peñas E., Murillo-Arbizu M., Lopez de Cerain A. Ochratoxin A decontamination: A review. Food Cont. 2009;20:326–333. doi: 10.1016/j.foodcont.2008.05.017. [DOI] [Google Scholar]
- 46.Meca G., Blaiotta G., Ritieni A. Reduction of ochratoxin A during the fermentation of Italian red wine Moscato. Food Control. 2010;21:579–583. doi: 10.1016/j.foodcont.2009.08.008. [DOI] [Google Scholar]
- 47.Motloung L., De Saeger S., De Boevre M., Detavernier C., Audenaert K., Adebo O.A., Njobeh P.B. Study on mycotoxin contamination in South African food spices. World Mycotoxin J. 2019;11:401–409. doi: 10.3920/WMJ2017.2191. [DOI] [Google Scholar]
- 48.Njobeh P.B., Dutton M.F., Aberg A.T., Haggblom P. Estimation of multi-mycotoxin contamination in South African compound feeds. Toxins. 2012;4:836–848. doi: 10.3390/toxins4100836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wenzl T., Johannes H., Schaechtele A., Robouch P., Storka J. Guidance Document on the Estimation of LOD and LOQ for Measurements in the Field of Contaminants in Feed and Food. [(accessed on 18 May 2018)]; Available online: http://publications.jrc.ec.europa.eu/repository/bitstream/JRC102946/eur%2028099%20en_lod%20loq%20guidance%20document.pdf.
- 50.Adebo O.A., Njobeh P.B., Sidu S., Adebiyi J.A., Mavumengwana V. Aflatoxin B1 degradation by culture and lysate of a Pontibacter specie. Food Cont. 2017;80:99–103. doi: 10.1016/j.foodcont.2017.04.042. [DOI] [Google Scholar]