Abstract
The SOX2 gene located at 3q26 is frequently amplified and overexpressed in multiple cancers, including head and neck squamous cell carcinomas (HNSCC). The tumor-promoting activity and involvement of SOX2 in tumor progression has been extensively demonstrated, thereby emerging as a promising therapeutic target. However, the role of SOX2 in early stages of tumorigenesis and its possible contribution to malignant transformation remain unexplored. This study investigates for the first time SOX2 protein expression by immunohistochemistry and gene amplification by real-time PCR using a large series of 94 laryngeal precancerous lesions. Correlations with the histopathological classification and the risk of progression to invasive carcinoma were established. Nuclear SOX2 expression was frequently detected in 38 (40%) laryngeal dysplasias, whereas stromal cells and normal adjacent epithelia showed negative expression. SOX2 gene amplification was detected in 18 (33%) of 55 laryngeal dysplasias. Univariate Cox analysis showed that SOX2 gene amplification (p = 0.046) and protein expression (p < 0.001) but not histological grading (p = 0.432) were significantly associated with laryngeal cancer risk. In multivariate stepwise analysis including age, tobacco, histology, SOX2 gene amplification and SOX2 expression, SOX2 expression (HR = 3.531, 95% CI 1.144 to 10.904; p = 0.028) was the only significant independent predictor of laryngeal cancer development. These findings underscore the relevant role of SOX2 in early tumorigenesis and a novel clinical application of SOX2 expression as independent predictor of laryngeal cancer risk in patients with precancerous lesions beyond current WHO histological grading. Therefore, targeting SOX2 could lead to effective strategies for both cancer prevention and treatment.
Keywords: cancer risk assessment, larynx, dysplasia, SOX2, immunohistochemistry, gene amplification
1. Introduction
The sex-determining region Y (SRY)-related high-mobility-group (HMG)-box family of transcription factors member SOX2 (Sex-determining region Y-box 2) plays a critical role during embryonic development and organogenesis, thereby showing a very restricted, and precisely regulated, spatial-temporal expression pattern [1,2]. Similar to other pluripotency-associated transcription factors such as NANOG (Nanog Homeobox) and OCT4 (Octamer-binding transcription factor 4, also known as POU5F1), SOX2 has been implicated in sustaining stemness of embryonic stem cells, reprogramming of adult somatic cells to a pluripotent stem cell state and also in multiple tumorigenic processes [3,4,5,6,7,8,9,10].
Amplification of the chromosomal region 3q26-27 is one of the most recurrent genetic alterations in head and neck squamous cell carcinomas (HNSCC) and other carcinomas, which has been associated with tumor progression and poor patient prognosis [11,12]. SOX2 located at 3q26 has emerged as a candidate tumor driver gene within this locus [7,13,14,15]. SOX2 amplification and overexpression has been implicated in many tumor types, but mostly in squamous carcinomas of various localizations (lung, esophagus, head and neck) [13,14,15,16,17,18,19].
The role of SOX2 in HNSCC progression and its impact on prognosis and disease outcome has been subject of intense investigation [7,11,20,21,22,23]. However, the role of SOX2 in the early stages of HNSCC tumorigenesis and its possible contribution to malignant transformation and acquisition of an invasive phenotype remains unexplored.
This study investigates for the first time SOX2 protein expression and gene amplification in the early stages of HNSCC tumorigenesis using a large series of 94 laryngeal precancerous lesions. Correlations with the risk of progression to invasive carcinoma and with the histopathological classification (current gold standard) were established. Our findings uncover the clinical application of SOX2 expression as an independent predictor of laryngeal cancer risk in patients with laryngeal precancerous lesions, showing superior predictive value to the current World Health Organization (WHO) histological classification.
2. Materials and Method
2.1. Patients and Tissue Specimens
Surgical tissue specimens from patients who were diagnosed of laryngeal dysplasia at the Hospital Universitario Central de Asturias between 1996 and 2010 were retrospectively collected, in accordance with approved institutional review board guidelines. All experimental procedures were conducted in accordance to the Declaration of Helsinki and approved by Institutional Ethics Committee of the Hospital Universitario Central de Asturias, and by the Regional CEIC from Principado de Asturias (date of approval: 18 July 2013; approval number: 81/2013) for the project PI13/00259. Informed consent was obtained from all patients. Patients must meet the following criteria to be included in the study: (i) pathological diagnosis of laryngeal dysplasia; (ii) with lesions of the vocal folds (iii) no previous history of head and neck cancer; (iv) complete excisional biopsy of the lesion; (v) a minimum follow-up of five years (or until progression to malignancy occurred); and (vi) patients with a diagnosis of laryngeal dysplasia who developed cancer within the next six months were excluded from the study. A total of 94 patients who met these criteria were included in this study. All the patients were treated with macroscopically complete excisional biopsy of the lesion, either with CO2 laser or with cold instruments. Microscopically surgical margins were not assessed. No other treatments were administered. Follow up with the patients occurred every two months in the first six months after completing the treatment, every three months until the second year, and every six months thereafter.
Representative tissue sections from the original biopsy material were obtained from archival, paraffin embedded blocks and the histological diagnosis and epithelial dysplasia grade was confirmed in all the cases by an experienced pathologist (MSFG). The sections selected for study also contained normal epithelia as internal controls. The premalignant lesions were classified into the categories of low-grade and high-grade dysplasia following the WHO classification (4th Edition) [24].
2.2. Gene Amplification Analysis
The protocol for DNA extraction from paraffin-embedded tissue sections has been described elsewhere [25]. DNA extracted from normal mucosa obtained from non-oncologic patients was used as calibrator sample. Gene amplification was evaluated by real-time PCR (Q-PCR) in an ABI PRISM 7500 Sequence detector (Applied Biosystems, Foster City, CA, USA) using Power SYBR Green PCR Master Mix and oligonucleotides with the following sequences: for the SOX2 gene, Fw, 5′- CTCCGGGACATGATCAGC-3′ and Rv, 5′- CTGGGACATGTGAAGTCTGC-3′; and for the reference gene COL7A1 (located at 3p21), Fw, 5′- ACCCAGTACCGCATCATTGTG-3′ and Rv, 5′- TCAGGCTGGAACTTCAGTGTGT-3′. Samples were analyzed in triplicates and template-free blanks were also included.
The relative gene copy number for SOX2 was calculated using the 2-ΔΔCT method. Calibration curves for the reference gene (COL7A1) and the target gene (SOX2) showed parallel slopes and comparable amplification efficiency across the linear range (1.5–25 ng) (Supplementary Figure S1). The ΔΔCT represents the difference between ΔCT of dysplasia - ΔCT of normal mucosa, with ΔCT being the average CT for the target gene (SOX2) minus the average CT for the reference gene (COL7A1). The optimal cut-off value for SOX2 amplification was identified via a receiver operating characteristic (ROC) curve analysis, using progression to cancer as an end point, and patients were categorized into positive SOX2 amplification (≥1.75) and negative amplification (<1.75) (Supplementary Figure S2A).
2.3. Immunohistochemistry
The formalin-fixed, paraffin-embedded tissues were cut into 3-µm sections and dried on Flex IHC microscope slides (Dako, Glostrup, Denmark). The sections were deparaffinized with standard xylene and hydrated through graded alcohols into water. Antigen retrieval was performed using Envision Flex Target Retrieval solution, high pH (Dako). Staining was done at room temperature on an automatic staining workstation (Dako Autostainer Plus, Dako, Glostrup, Denmark) with Anti-SOX2 rabbit polyclonal antibody (Merck Millipore # AB5603) at 1:1000 dilution using the Dako EnVision Flex + Visualization System (Dako Autostainer). Counterstaining with hematoxylin was the final step.
SOX2 staining was evaluated as the percentage of cells with nuclear staining in the dysplastic epithelium. The optimal cut-off value for SOX2 staining calculated by ROC analysis using progression to cancer as end-point was 12.5% (Supplementary Figure S2B). Scores were classified as negative or positive staining on the basis of values below or above the cut-off value of 12.5%.
2.4. Statistical Analysis
χ2 and Fisher’s exact tests were used for comparison between categorical variables. For time-to-event analysis, Kaplan-Meier curves were plotted. Differences between survival times were analyzed by the log-rank method. Cox proportional hazards models were utilized for univariate and multivariate analyses. The hazard ratios (HR) with 95% confidence interval (CI) and p values were reported. All tests were two-sided. p values of ≤ 0.05 were considered statistically significant.
3. Results
3.1. Patient Characteristics
All patients were men, with a mean age of 65 years (range 36–86 years). All but two patients were active or old smokers. The mean tobacco consumption was 50 packs-year (range 15–150 packs-year). After the diagnosis, all the patients that were active smokers received smoking cessation advice; however, 14 of them continued smoking. Of the 94 patients included in the study, 14 (15%) lesions were classified as low-grade dysplasia, and 80 (85%) as high-grade dysplasia. During the follow-up period, 29 (31%) of 94 patients developed an invasive carcinoma at the biopsy site.
The mean time to cancer diagnosis in the cases that progressed was 27 months (range 8 to 66 months). No significant differences attributable to age were observed (p = 0.501) between the patients who developed cancer (mean, 64 years) and those who did not (mean, 64 years). The mean tobacco consumption for patients who developed an invasive carcinoma was 58 packs per year, compared to 53 packs per year for those who did not develop cancer (p = 0.819).
3.2. SOX2 Protein Expression in Laryngeal Precancerous Lesions
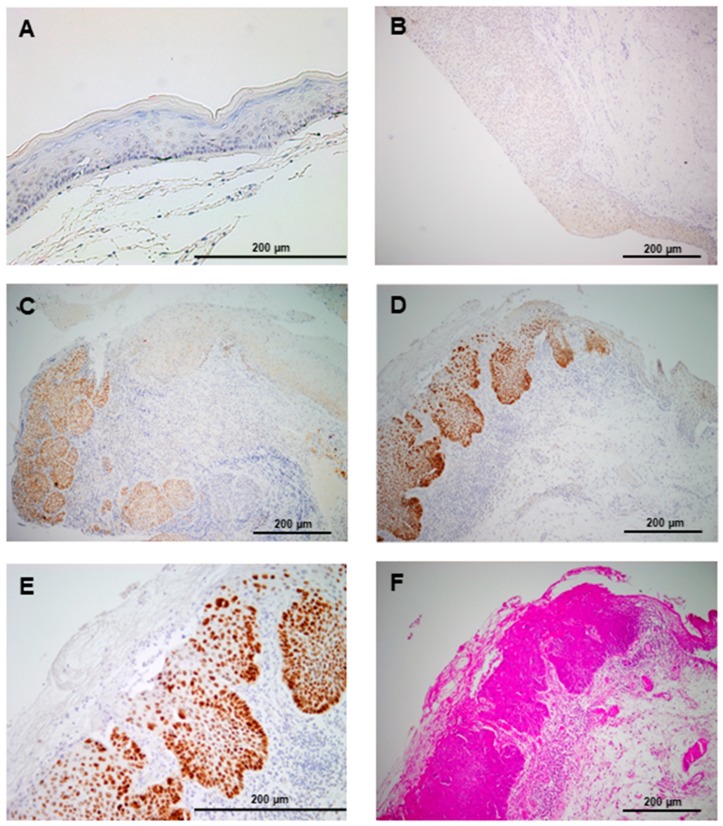
SOX2 protein expression was evaluated by immunohistochemistry in 94 laryngeal dysplasias. Nuclear SOX2 expression was detected in 38 (40%) dysplasias, whereas stromal cells and normal adjacent epithelia showed negative expression (Figure 1).
Figure 1.
Immunohistochemical analysis of SOX2 (Sex-determining region Y-box 2) expression in laryngeal precancerous lesions. Normal adjacent epithelia showed negative staining (A). Representative examples of laryngeal dysplasias showing negative (B), and positive nuclear SOX2 staining (C,D), compared to the negative expression in normal-adjacent epithelia (right side). (E) Higher magnification from D. (F) H&E staining from D.
SOX2 protein expression significantly correlated with the histopathological classification: 2 (14%) of the 14 low-grade dysplasias, and 36 (45%) of the 80 high-grade dysplasias exhibited SOX2-positive expression (Fisher’s exact test p = 0.039).
3.3. SOX2 Gene Amplification during Laryngeal Tumorigenesis
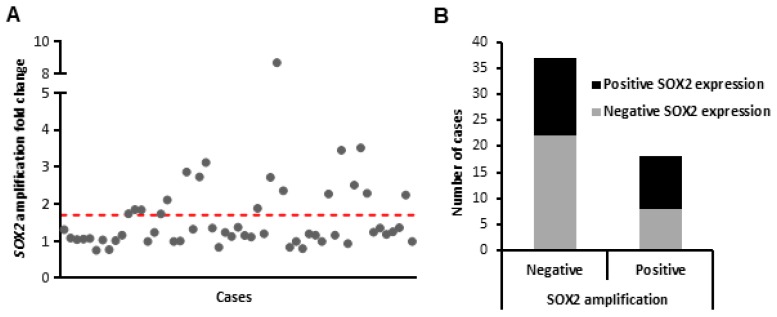
SOX2 gene amplification was assessed by real-time PCR on a set of 55 laryngeal dysplasias, using DNA extracted from the same paraffin tissue blocks. SOX2 gene amplification was detected in 18 (33%) of 55 laryngeal dysplasias, with relative copy numbers ranging from 2-fold to 9-fold (Figure 2A). SOX2 gene amplification increased with the severity of the lesions: 1 (17%) of the 6 low-grade dysplasias and 17 (35%) of the 49 high-grade dysplasias showed SOX2 gene amplification, although the differences did not reach statistical differences (Fisher’s exact test p = 0.651).
Figure 2.
Analysis of SOX2 gene amplification in laryngeal precancerous lesions. SOX2 gene copy number was evaluated by Q-PCR in 55 cases. (A) Data are represented as fold-change in the precancerous lesion relative to the normal mucosa. Red dotted line indicates the threshold established to define positive cases (1.75). (B) Correlations between SOX2 gene amplification and protein expression determined by immunohistochemistry.
When analyzing the correlation between SOX2 gene amplification and protein expression, we found that gene amplification only partially lead to SOX2 protein expression (Figure 2B). Thus, even though 22 out of 55 dysplasias were negative for both SOX2 expression and gene amplification, 15 amplification-negative lesions also showed positive SOX2 expression, indicating that additional mechanisms should contribute to the frequent SOX2 expression detected in laryngeal tumorigenesis.
3.4. Associations with Laryngeal Cancer Risk
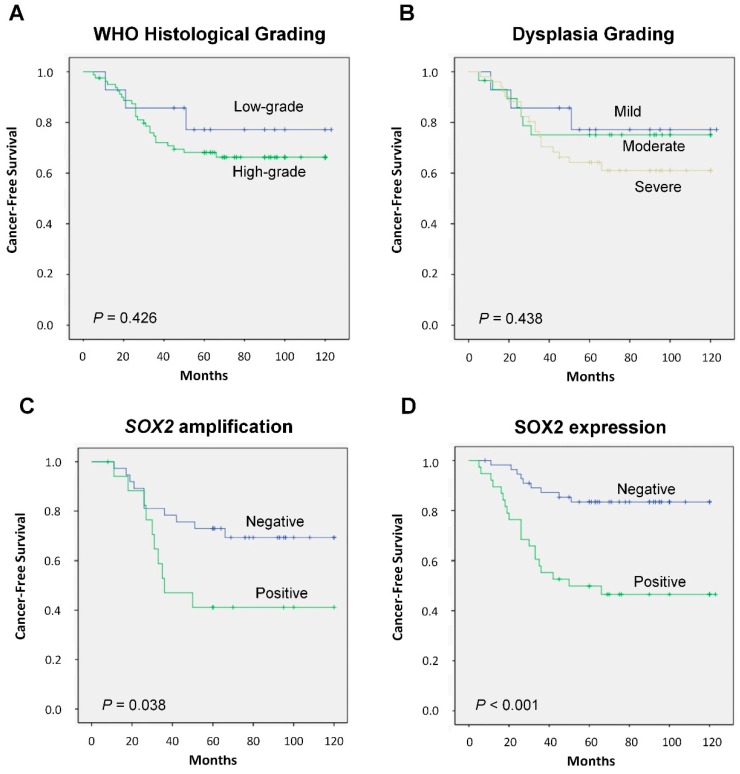
There was no statistically significant correlation in this cohort between the WHO histopathological grade and the risk of progression to laryngeal cancer (p = 0.538; Table 1; log-rank p = 0.426, Figure 3A), although high-grade dysplasias showed a higher cancer risk (HR = 1.615, 95% CI 0.489 to 5.336; p = 0.432; Table 1). Even the previous histological classification based in the dysplasia grading (mild, moderate vs. severe dysplasia) did not predict significantly cancer risk (log-rank p = 0.438, Figure 3B).
Table 1.
Evolution of the premalignant lesions in relation to histopathological diagnosis, SOX2 gene amplification, and protein expression.
| Characteristic | No of Cases (%) | Progression to Carcinoma (%) | p † |
|---|---|---|---|
| Histopathological diagnosis | |||
| Low-grade dysplasia | 14 (15) | 3 (21) | 0.538 |
| High-grade dysplasia | 80 (85) | 26 (32) | |
| SOX2 gene amplification | |||
| Negative | 37 (67) | 11 (30) | 0.081 |
| Positive | 18 (33) | 10 (56) | |
| SOX2 protein expression | |||
| Negative | 56 (60) | 9 (16) | <0.001 |
| Positive (>10% stained nuclei) | 38 (40) | 20 (53) |
† Fisher’s exact test.
Figure 3.
Kaplan-Meier cancer-free survival curves in the cohort of 94 patients with laryngeal dysplasias categorized by WHO (World Health Organization) histological grading (A), dysplasia grading (B) SOX2 gene amplification (C) or SOX2 protein expression (D). p values were estimated using the log-rank test.
In marked contrast, we found that SOX2 gene amplification significantly correlated with an increased laryngeal cancer risk (log-rank p = 0.038; Figure 3C). Furthermore, SOX2 protein expression showed the most robust association with laryngeal cancer risk (log-rank p < 0.001; Figure 3D). Five years after the patients were diagnosed, 20 (53%) of the 38 patients with SOX2-positive expression developed laryngeal cancer, whereas only 9 (16%) of the 56 patients with SOX2-negative expression progressed to invasive carcinoma (p < 0.001; Table 1).
Univariate Cox analysis showed that SOX2 gene amplification and protein expression but not histological grading were significantly associated with laryngeal cancer risk (Table 2). In multivariate stepwise analysis including age, tobacco, histology, SOX2 gene amplification, and SOX2 expression, SOX2 expression (HR = 3.531, 95% CI 1.144 to 10.904; p = 0.028) was the only significant independent predictor of laryngeal cancer development.
Table 2.
Univariate Cox Proportional Hazards Model to Estimate Laryngeal Cancer Risk.
| Characteristic | p | Hazard Ratio | 95% CI |
|---|---|---|---|
| Age (above vs. below the mean) | 0.554 | 1.254 | 0.593–2.653 |
| Smoking (above vs. below the mean) | 0.618 | 1.210 | 0.572–2.558 |
| Histology (high-grade vs. low-grade dysplasia) | 0.432 | 1.615 | 0.489–5.336 |
| SOX2 amplification (positive vs. negative) | 0.046 | 2.410 | 1.017–5.710 |
| SOX2 expression (positive vs. negative) | <0.001 | 4.130 | 1.878–9.086 |
4. Discussion
There is mounting evidence demonstrating the role of SOX2 in tumorigenesis, and its contribution to tumor progression has been extensively documented in multiple cancers [7,8,9,10,19,20,21,22,23]. Accordingly, SOX2 has emerged as a candidate driver gene responsible for the 3q26-associated tumor aggressiveness [11,12,13,14,15,16,17,18]. In HNSCC, SOX2 expression has been found to induce cancer stem cell (CSC)-like properties, including increased self-renewal, tumorigenic potential and chemoresistence [26,27,28,29]. Accordingly, various studies using large cohorts of HNSCC samples have demonstrated that SOX2 expression significantly correlated with tumor recurrence and poor prognosis [20,28,29]. Although it has also been reported that low expression of SOX2 was associated with reduced survival and poor clinical outcome [30,31].
This study further and significantly extends these data investigating for the first time SOX2 protein expression and gene amplification in early stages of laryngeal tumorigenesis to ascertain its role in malignant transformation. Our findings demonstrate that SOX2 protein expression and gene amplification are frequent events in laryngeal tumorigenesis and, more importantly, both emerge as clinically and biologically relevant features in laryngeal cancer development. Even though SOX2 protein expression and SOX2 amplification were found to increase in high-grade dysplasias, SOX2 protein expression and gene amplification but not histological grading were significantly associated with progression to laryngeal cancer. In particular, patients carrying SOX2-expressing dysplastic lesions exhibited a significantly higher cancer incidence than those with negative expression. In other tumor types, it has been reported that SOX2 antagonizes signals promoting differentiation to maintain stemness in CSC subpopulations [32,33]. On this basis, we could speculate that the increased cancer risk in patients harboring SOX2-expressing lesions may reflect the presence of a larger proportion of cells presenting cancer stem-like properties. Supporting this hypothesis, the expression of other CSC markers such as NANOG or Podoplanin has also been found to increase in precancerous lesions and to associate with a higher risk of malignization [34,35,36]. Podoplanin was identified as a marker of tumor-initiating cells in squamous cell carcinomas [37]. Tumorigenicity and capability of recapitulating human squamous cell carcinomas are by definition properties of CSCs. Thus, it has been interpreted that premalignant lesions with podoplanin expression expanding beyond the basal cell layer may represent truly early neoplastic lesions, enriched in CSC and indeed lesions with such clonal expansion carry a higher risk of progression to invasive cancer.
Furthermore, this study uncovers SOX2 expression as a robust independent predictor of laryngeal cancer risk beyond histological evaluation. Histopathological diagnosis of squamous intraepithelial lesions remains the gold standard in clinical practice for cancer risk assessment and decision-making [38]. Quite remarkably, the new and recently established WHO classification as well as the previous dysplasia grading failed to show a significant role in assessing laryngeal cancer risk in this cohort. This emphasizes the still limited value of histological grading to predict outcome, which is certainly affected by inter- and intra-observer variability [39]. Additional objective and reliable markers are therefore needed to improve patient stratification and to more accurately identify those carrying lesions at higher risk of progression who will require the most intense treatment and follow-up [40]. Our results clearly demonstrate that SOX2 protein analysis may provide valuable additional information beyond histological features. Hence, immunohistochemical evaluation of SOX2 is proposed to be incorporated into the clinical practice as a complementary and relatively simple molecular test for cancer risk assessment and decision-making. Nevertheless, routine implementation of SOX2 expression as biomarker will require confirmation in large prospective studies, while also extending analysis to other subsites in the head and neck area.
The present study also revealed important temporal and mechanistic information regarding the early occurrence and frequency of SOX2 expression and gene amplification in laryngeal tumorigenesis. Thus, while both alterations are frequently detected at early stages of tumorigenesis and their frequency increased with the grade of dysplasia, SOX2 gene amplification occurred at a lower frequency and did not perfectly match with protein expression, indicating that additional mechanisms must be contributing to SOX2 expression, such as transcriptional or posttranscriptional regulatory mechanisms. In line with this, it has been demonstrated that various transcription factors frequently altered in HNSCC, such as OCT4, YAP1 (Yes-Associated Protein 1) or the hypoxic factor HIF1α may induce the expression of SOX2 at mRNA level [41,42]. On the other hand, it has also been reported that amplification of PIK3CA and other genes mapping at 3q26 do not necessarily lead to increased expression, indicating that further epigenetic events could be involved in the transcriptional control [43]. Hence, this may explain some cases harboring SOX2-positive amplification that showed negative SOX2 protein expression. These results are in agreement with the TCGA data obtained from 279 HNSCC patients [44] using the platform cBioPortal for Cancer Genomics (http://cbioportal.org/) [45] (Supplementary Figure S3). It has been extensively demonstrated (both experimentally and in silico) that 3q26 amplicon harbors numerous genes found to be frequently and concomitantly co-amplified and overexpressed in multiple cancers [46,47]. Various genes have been highlighted as highly significant oncogenic drivers for HNSCC survival [46], including the four known driver genes PIK3CA, PRKCI, SOX2 and TP63. In silico analysis of TCGA HNSCC data included in Supplementary Figure S4 further illustrates that co-amplification of these four genes occurs frequently in HNSCC, thus showing that 3q26 amplification in HNSCC is not restricted to the SOX2 gene. In addition, cytogenetic analyses demonstrated that 3q26 amplification is an early event in HNSCC tumorigenesis, and PIK3CA amplification and expression has been detected in dysplasias and associated with progression to invasive carcinoma [43].
5. Conclusions
This study provides the first evidence demonstrating the clinical relevance of SOX2 expression and gene amplification in early stages of laryngeal tumorigenesis. Our findings also uncover the clinical application of SOX2 expression as an independent predictor of laryngeal cancer risk in patients with precancerous lesions beyond current WHO histological grading. Hence, targeting SOX2 expression/function may potentially lead to the development of effective molecular-targeted therapies for both cancer prevention and treatment. Further investigation is therefore encouraged.
Acknowledgments
We thank the samples and technical assistance kindly provided by the Principado de Asturias BioBank (PT13/0010/0046), financed jointly by Servicio de Salud del Principado de Asturias, Instituto de Salud Carlos III and Fundación Bancaria Cajastur and integrated in the Spanish National Biobanks Network. We also thank Pablo Martínez-Camblor for his assistance with statistical analyses and Juan Pérez for his excellent administrative support.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6694/11/3/286/s1, Figure S1: Standard curve showing the Log10 DNA amount, in ng per μL, plotted against threshold cycle (Ct) for different dilutions of genomic DNA in PCR-grade water by real-time PCR (A). The table shows the efficiency of amplification for each primer pair, calculated using the slope of the corresponding standard curve (B), Figure S2: Determination of the optimal cut-off values for SOX2 amplification and SOX2 expression by receiver operating characteristic (ROC) curve analysis. ROC curves for SOX2 amplification (A) and SOX2 expression (B) (as percentage of positive nuclei). The optimal cut-off values calculated were 1.75 for SOX2 amplification and 12.5% for SOX2 expression. The area under de curve (AUC), with 95%CI, the p values, and the sensitivity and specificity calculated for each optimal cut-off are shown, Figure S3: Analysis of SOX2 gene amplification and mRNA expression from the TCGA cohort of 279 HNSCC patients [44] using cBioPortal [45]. Schematic representation (A) showing the number of cases with gene amplification and/or mRNA upregulation or no alteration. Correlations between SOX2 expression and gene amplification in this cohort (B), Figure S4: Gene amplification analysis of SOX2 and other driver genes (PIK3CA, PRKCI and TP63) mapping at 3q26 amplicon using the TCGA cohort of 279 HNSCC patients [44] and the platform cBioPortal [45]. Oncoprint illustrates frequent co-amplification of all these genes.
Author Contributions
Conceptualization, J.P.R. and J.M.G.-P.; Data curation, S.T.M. and J.M.G.-P.; Formal analysis, S.T.M., R.R., J.P.R. and J.M.G.-P.; Funding acquisition, J.P.R. and J.M.G.-P.; Investigation, R.G.-D., S.T.M., D.P.M., M.S.-C., A.R. and M.S.F.-G.; Methodology, R.G.-D., S.T.M., L.S.-F. and A.V.; Resources, A.V. and M.S.F.-G.; Supervision, J.M.G.-P.; Validation, F.H.-P.; Visualization, R.G.-D., R.R., J.P.R. and J.M.G.-P.; Writing—original draft, J.M.G.-P.; Writing—review & editing, R.G.-D., S.T.M., R.R. and J.P.R.
Funding
This study was supported by grants from the Plan Nacional de I+D+I 2013-2016 ISCIII PI13/00259, PI16/00280, CIBERONC (CB16/12/00390), and MINECO/FEDER (SAF2016-75286-R), Fundación Merck Salud (17-CC-008), the Instituto de Investigación Sanitaria del Principado de Asturias (ISPA), PCTI-Asturias (GRUPIN14-003), Fundación Bancaria Caja de Ahorros de Asturias-IUOPA and the FEDER Funding Program from the European Union. STM (Sara Borrell Program - CD16/00103) and RR (Miguel Servet II Program - CPII16/00049) were recipients of fellowships from ISCIII.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Graham V., Khudyakov J., Ellis P., Pevny L. SOX2 functions to maintain neural progenitor identity. Neuron. 2003;39:749–765. doi: 10.1016/S0896-6273(03)00497-5. [DOI] [PubMed] [Google Scholar]
- 2.Kiernan A.E., Pelling A.L., Leung K.K., Tang A.S., Bell D.M., Tease C., Lovell-Badge R., Steel K.P., Cheah K.S. Sox2 is required for sensory organ development in the mammalian inner ear. Nature. 2005;434:1031–1035. doi: 10.1038/nature03487. [DOI] [PubMed] [Google Scholar]
- 3.Boyer L.A., Lee T.I., Cole M.F., Johnstone S.E., Levine S.S., Zucker J.P., Guenther M.G., Kumar R.M., Murray H.L., Jenner R.G., et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122:947–956. doi: 10.1016/j.cell.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang J., Rao S., Chu J., Shen X., Levasseur D.N., Theunissen T.W., Orkin S.H. A protein interaction network for pluripotency of embryonic stem cells. Nature. 2006;444:364–368. doi: 10.1038/nature05284. [DOI] [PubMed] [Google Scholar]
- 5.Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K., Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 6.Avilion A.A., Nicolis S.K., Pevny L.H., Perez L., Vivian N., Lovell-Badge R. Multipotent cell lineages in early mouse development depend on SOX2 function. Gene Dev. 2003;17:126–140. doi: 10.1101/gad.224503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weina K., Utikal J. SOX2 and cancer: Current research and its implications in the clinic. Clin. Transl. Med. 2014;3:19. doi: 10.1186/2001-1326-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maurizi G., Verma N., Gadi A., Mansukhani A., Basilico C. Sox2 is required for tumor development and cancer cell proliferation in osteosarcoma. Oncogene. 2018;37:4626–4632. doi: 10.1038/s41388-018-0292-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu F., Qian W., Zhang H., Liang Y., Wu M., Zhang Y., Zhang X., Gao Q., Li Y. SOX2 Is a Marker for Stem-like Tumor Cells in Bladder Cancer. Stem Cell Rep. 2017;9:429–437. doi: 10.1016/j.stemcr.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Santini R., Pietrobono S., Pandolfi S., Montagnani V., D’Amico M., Penachioni J.Y., Vinci M.C., Borgognoni L., Stecca B. SOX2 regulates self-renewal and tumorigenicity of human melanoma-initiating cells. Oncogene. 2014;33:4697–4708. doi: 10.1038/onc.2014.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh B., Stoffel A., Gogineni S., Poluri A., Pfister D.G., Shaha A.R., Pathak A., Bosl G., Cordon-Cardo C., Shah J.P., et al. Amplification of the 3q26.3 locus is associated with progression to invasive cancer and is a negative prognostic factor in head and neck squamous cell carcinomas. Am. J. Pathol. 2002;161:365–371. doi: 10.1016/S0002-9440(10)64191-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heselmeyer K., Macville M., Schröck E., Blegen H., Hellström A.C., Shah K., Ried T. Advanced-stage cervical carcinomas are defined by a recurrent pattern of chromosomal aberrations revealing high genetic instability and a consistent gain of chromosome arm 3q. Genes Chromosome Cancer. 1997;19:233–240. doi: 10.1002/(SICI)1098-2264(199708)19:4<233::AID-GCC5>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 13.Gen Y., Yasui K., Zen Y., Zen K., Dohi O., Endo M., Tsuji K., Wakabayashi N., Itoh Y., Naito Y., et al. SOX2 identified as a target gene for the amplification at 3q26 that is frequently detected in esophageal squamous cell carcinoma. Cancer Genet. Cytogenet. 2010;202:82–93. doi: 10.1016/j.cancergencyto.2010.01.023. [DOI] [PubMed] [Google Scholar]
- 14.Hussenet T., Dali S., Exinger J., Monga B., Jost B., Dembelé D., Martinet N., Thibault C., Huelsken J., Brambilla E., et al. SOX2 is an oncogene activated by recurrent 3q26.3 amplifications in human lung squamous cell carcinomas. PLoS ONE. 2010;5:e8960. doi: 10.1371/journal.pone.0008960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bass A.J., Watanabe H., Mermel C.H., Yu S., Perner S., Verhaak R.G., Kim S.Y., Wardwell L., Tamayo P., Gat-Viks I., et al. SOX2 is an amplified lineage-survival oncogene in lung and esophageal squamous cell carcinomas. Nat. Genet. 2009;41:1238–1242. doi: 10.1038/ng.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hussenet T., du Manoir S. SOX2 in squamous cell carcinoma: Amplifying a pleiotropic oncogene along carcinogenesis. Cell Cycle. 2010;9:1480–1486. doi: 10.4161/cc.9.8.11203. [DOI] [PubMed] [Google Scholar]
- 17.Maier S., Wilbertz T., Braun M., Scheble V., Reischl M., Mikut R., Menon R., Nikolov P., Petersen K., Beschorner C., et al. SOX2 amplification is a common event in squamous cell carcinomas of different organ sites. Hum. Pathol. 2011;42:1078–1088. doi: 10.1016/j.humpath.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 18.Rudin C.M., Durinck S., Stawiski E.W., Poirier J.T., Modrusan Z., Shames D.S., Bergbower E.A., Guan Y., Shin J., Guillory J., et al. Comprehensive genomic analysis identifies SOX2 as a frequently amplified gene in small-cell lung cancer. Nat. Genet. 2012;44:1111–1116. doi: 10.1038/ng.2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodríguez-Pinilla S.M., Sarrio D., Moreno-Bueno G., Rodríguez-Gil Y., Martínez M.A., Hernández L., Hardisson D., Reis-Filho J.S., Palacios J. Sox2: A possible driver of the basal-like phenotype in sporadic breast cancer. Mod. Pathol. 2007;20:474–481. doi: 10.1038/modpathol.3800760. [DOI] [PubMed] [Google Scholar]
- 20.Wang Q., He W., Lu C., Wang Z., Wang J., Giercksky K.E., Nesland J.M., Suo Z. Oct3/4 and Sox2 are significantly associated with an unfavorable clinical outcome in human esophageal squamous cell carcinoma. Anticancer Res. 2009;29:1233–1241. [PubMed] [Google Scholar]
- 21.Neumann J., Bahr F., Horst D., Kriegl L., Engel J., Luque R.M., Gerhard M., Kirchner T., Jung A. SOX2 expression correlates with lymph-node metastases and distant spread in right-sided colon cancer. BMC Cancer. 2011;11:518. doi: 10.1186/1471-2407-11-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang J., Chang D.Y., Mercado-Uribe I., Liu J. Sex-determining region Y-box 2 expression predicts poor prognosis in human ovarian carcinoma. Hum. Pathol. 2012;43:1405–1412. doi: 10.1016/j.humpath.2011.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lundberg I.V., Löfgren Burström A., Edin S., Eklöf V., Öberg Å., Stenling R., Palmqvist R., Wikberg M.L. SOX2 expression is regulated by BRAF and contributes to poor patient prognosis in colorectal cancer. PLoS ONE. 2014;9:e101957. doi: 10.1371/journal.pone.0101957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.WHO Classification of Head and Neck Tumours . In: WHO/IARC Classification of Tumours. 4th ed. El-Naggar A.K., Chan J.K.C., Grandis J.R., Takata T., Slootweg P.J., editors. Volume 9. IARC; Lyon, France: 2017. [Google Scholar]
- 25.Rodrigo J.P., Álvarez-Alija G., Menéndez S.T., Mancebo G., Allonca E., García-Carracedo D., Fresno M.F., Suárez C., García-Pedrero J.M. Cortactin and focal adhesion kinase as predictors of cancer risk in patients with laryngeal premalignancy. Cancer Prev. Res. 2011;4:1333–1341. doi: 10.1158/1940-6207.CAPR-10-0338. [DOI] [PubMed] [Google Scholar]
- 26.Bourguignon L.Y., Wong G., Earle C., Chen L. Hyaluronan-CD44v3 interaction with Oct4-Sox2-Nanog promotes miR-302 expression leading to self-renewal, clonal formation, and cisplatin resistance in cancer stem cells from head and neck squamous cell carcinoma. J. Biol. Chem. 2012;287:32800–32824. doi: 10.1074/jbc.M111.308528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keysar S.B., Le P.N., Miller B., Jackson B.C., Eagles J.R., Nieto C., Kim J., Tang B., Glogowska M.J., Morton J.J., et al. Regulation of head and neck squamous cancer stem cells by PI3K and SOX2. J. Nat. Cancer Inst. 2016;109 doi: 10.1093/jnci/djw189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee S.H., Oh S.Y., Do S.I., Lee H.J., Kang H.J., Rho Y.S., Bae W.J., Lim Y.C., et al. SOX2 regulates self-renewal and tumorigenicity of stem-like cells of head and neck squamous cell carcinoma. Br. J. Cancer. 2014;111:2122–2130. doi: 10.1038/bjc.2014.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schröck A., Bode M., Göke F.J., Bareiss P.M., Schairer R., Wang H., Weichert W., Franzen A., Kirsten R., van Bremen T., et al. Expression and role of the embryonic protein SOX2 in head and neck squamous cell carcinoma. Carcinogenesis. 2014;35:1636–1642. doi: 10.1093/carcin/bgu094. [DOI] [PubMed] [Google Scholar]
- 30.Bochen F., Adisurya H., Wemmert S., Lerner C., Greiner M., Zimmermann R., Hasenfus A., Wagner M., Smola S., Pfuhl T., et al. Effect of 3q oncogenes SEC62 and SOX2 on lymphatic metastasis and clinical outcome of head and neck squamous cell carcinomas. Oncotarget. 2017;8:4922–4934. doi: 10.18632/oncotarget.13986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bayo P., Jou A., Stenzinger A., Shao C., Gross M., Jensen A., Grabe N., Mende C.H., Rados P.V., Debus J., et al. Loss of SOX2 expression induces cell motility via vimentin up-regulation and is an unfavorable risk factor for survival of head and neck squamous cell carcinoma. Mol. Oncol. 2015;9:1704–1719. doi: 10.1016/j.molonc.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Basu-Roy U., Seo E., Ramanathapuram L., Rapp T.B., Perry J.A., Orkin S.H., Mansukhani A., Basilico C. Sox2 maintains self renewal of tumor-initiating cells in osteosarcomas. Oncogene. 2012;31:2270–2282. doi: 10.1038/onc.2011.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Basu-Roy U., Bayin N.S., Rattanakorn K., Han E., Placantonakis D.G., Mansukhani A., Basilico C. Sox2 antagonizes the Hippo pathway to maintain stemness in cancer cells. Nat. Commun. 2015;6:6411. doi: 10.1038/ncomms7411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kawaguchi H., El-Naggar A.K., Papadimitrakopoulou V., Ren H., Fan Y.H., Feng L., Lee J.J., Kim E., Hong W.K., Lippman S.M., et al. Podoplanin: A novel marker for oral cancer risk in patients with oral premalignancy. J. Clin. Oncol. 2008;26:354–360. doi: 10.1200/JCO.2007.13.4072. [DOI] [PubMed] [Google Scholar]
- 35.Rodrigo J.P., García-Carracedo D., González M.V., Mancebo G., Fresno M.F., García-Pedrero J. Podoplanin expression in the development and progression of laryngeal squamous cell carcinomas. Mol. Cancer. 2010;9:48. doi: 10.1186/1476-4598-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodrigo J.P., Villaronga M.Á., Menéndez S.T., Hermida-Prado F., Quer M., Vilaseca I., Allonca E., Pedregal Mallo D., Astudillo A., García-Pedrero J.M. A Novel Role for Nanog As An Early Cancer Risk Marker In Patients With Laryngeal Precancerous Lesions. Sci. Rep. 2017;7:11110. doi: 10.1038/s41598-017-11709-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Atsumi N., Ishii G., Kojima M., Sanada M., Fujii S., Ochiai A. Podoplanin, a novel marker of tumor-initiating cells in human squamous cell carcinoma A431. Biochem. Biophys. Res. Commun. 2008;373:36–41. doi: 10.1016/j.bbrc.2008.05.163. [DOI] [PubMed] [Google Scholar]
- 38.Gale N., Michaels L., Luzar B., Poljak M., Zidar N., Fischinger J., Cardesa A. Current review on squamous intraepithelial lesions of the larynx. Histopathology. 2009;54:639–656. doi: 10.1111/j.1365-2559.2008.03111.x. [DOI] [PubMed] [Google Scholar]
- 39.Nankivell P., Weller M., McConkey C., Paleri V., Mehanna H. Biomarkers in laryngeal dysplasia: A systematic review. Head Neck. 2011;33:1170–1176. doi: 10.1002/hed.21592. [DOI] [PubMed] [Google Scholar]
- 40.Rodrigo J.P., García-Pedrero J.M., Suárez C., Takes R.P., Thompson L.D., Slootweg P.J., Woolgar J.A., Westra W.H., Brakenhoff R.H., Rinaldo A., et al. Biomarkers predicting malignant progression of laryngeal epithelial precursor lesions: A systematic review. Eur. Arch. Otorhinolaryngol. 2012;269:1073–1083. doi: 10.1007/s00405-011-1831-4. [DOI] [PubMed] [Google Scholar]
- 41.Bora-Singhal N., Nguyen J., Schaal C., Perumal D., Singh S., Coppola D., Chellappan S. YAP1 Regulates OCT4 Activity and SOX2 Expression to Facilitate Self-Renewal and Vascular Mimicry of Stem-Like Cells. Stem Cells. 2015;33:1705–1718. doi: 10.1002/stem.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bae K.M., Dai Y., Vieweg J., Siemann D.W. Hypoxia regulates SOX2 expression to promote prostate cancer cell invasion and sphere formation. Am. J. Cancer Res. 2016;6:1078–1088. [PMC free article] [PubMed] [Google Scholar]
- 43.Woenckhaus J., Steger K., Werner E., Fenic I., Gamerdinger U., Dreyer T., Stahl U. Genomic gain of PIK3CA and increased expression of p110alpha are associated with progression of dysplasia into invasive squamous cell carcinoma. J. Pathol. 2002;198:335–342. doi: 10.1002/path.1207. [DOI] [PubMed] [Google Scholar]
- 44.Cancer Genome Atlas Network Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 2015;517:576–582. doi: 10.1038/nature14129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cerami E., Gao J., Dogrusoz U., Gross B.E., Sumer S.O., Aksoy B.A., Jacobsen A., Byrne C.J., Heuer M.L., Larsson E., et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Davidson M.A., Shanks E.J. 3q26-29 Amplification in head and neck squamous cell carcinoma: A review of established and prospective oncogenes. FEBS J. 2017;284:2705–2731. doi: 10.1111/febs.14061. [DOI] [PubMed] [Google Scholar]
- 47.Fields A.P., Justilien V., Murray N.R. The chromosome 3q26 OncCassette: A multigenic driver of human cancer. Adv. Biol. Regul. 2016;60:47–63. doi: 10.1016/j.jbior.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.