Abstract
Ewing sarcoma is a small round blue cell malignancy arising from bone or soft tissue and most commonly affects adolescents and young adults. Metastatic and relapsed Ewing sarcoma have poor outcomes and recurrences remain common. Owing to the poor outcomes associated with advanced disease and the need for a clear research strategy, the Children’s Oncology Group Bone Tumor Committee formed the New Agents for Ewing Sarcoma Task Force to bring together experts in the field to evaluate and prioritize new agents for incorporation into clinical trials. This group’s mission was to evaluate scientific and clinical challenges in moving new agents forward and to recommend agents and trial designs to the Bone Tumor Committee. The task force generated a framework for vetting prospective agents that included critical evaluation of each drug by using both clinical and non-clinical parameters. Representative appraisal of agents of highest priority, including eribulin, dinutuximab, cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitors, anti-angiogenic tyrosine kinase inhibitors, and poly-ADP-ribose polymerase (PARP) inhibitors, is described. The task force continues to analyze new compounds by using the paradigm established.
Keywords: Ewing sarcoma, metastasis, relapse, clinical trials, therapy
Introduction
Ewing sarcoma (ES) is a small round blue cell bone tumor most commonly occurring in adolescent and young adult (AYA) patients. ES is a classic fusion oncoprotein-driven tumor typically associated with a reciprocal translocation involving EWSR1 (chromosome 22) and the ETS family transcription factor FLI1 (chromosome 11) 1. These fusions may arise from chromoplexy events 2. Whereas classically no known genetic predisposition syndromes were linked to ES tumor development, recent evidence suggests that germline mutations in genes regulating DNA damage pathways are associated with an increased risk of developing ES 3. The field continues to learn more about the origins, nature, and behavior of the EWS-FLI1 oncoprotein in the context of individual tumor cells and the micro-environment 4, 5. This knowledge could assist in the future discovery of new therapeutic targets and in developing guidelines for risk stratification and treatment.
Currently, the strongest prognostic factor is stage at initial diagnosis. In North America, survival in localized ES improved to more than 70% 5-year event-free survival (EFS) by treating with alternating cycles of interval-compressed vincristine, doxorubicin, cyclophosphamide and ifosfamide and etoposide (VDC/IE) 6. The Euro-Ewing 99 clinical trial improved outcomes in localized ES with high-risk features by intensification of therapy with high-dose chemotherapy with busulfan and melphalan 7. The addition of IE cycles in earlier studies or intensification of therapy with high-dose chemotherapy has not improved outcomes for patients with metastatic disease 8, 9. As patients with metastatic ES have not benefited from treatment intensification, it suggests that targeted agents will be needed for this population. As an example, an ongoing Children’s Oncology Group (COG) trial is evaluating the insulin-like growth factor 1 receptor (IGF-1R) antibody ganitumab with cytotoxic chemotherapy (ClinicalTrials.gov Identifier: NCT02306161) 10. Patients with relapsed ES also have a dismal prognosis, and survival estimates are about 10% 1. Current efforts in the relapsed setting have focused on targeting EWS-FLI1 itself, targeting DNA damage vulnerabilities, or exploring immunotherapy strategies 11– 20. For the purpose of this report, we consider patients with either newly diagnosed metastatic or relapsed ES to have advanced disease. There is a dire need for new therapies to improve outcomes for patients with advanced ES.
It is in this clinical climate that the COG Bone Tumor Committee established the New Agents for Ewing Sarcoma Task Force. The COG Bone Tumor Committee previously established a successful working group for drug development in osteosarcoma 21. The purpose of this ongoing effort in ES is to bring together experts (basic scientists, experts in preclinical testing, pediatric sarcoma clinicians, and clinical investigators) with the primary goal of identifying potential agents of high priority for clinical evaluation and expeditiously incorporating these agents into clinical trials. Many new agents are being investigated in relapsed ES and many of these are listed in Table 1. In this report, we summarize our work to date, including establishing a framework to prioritize potential agents in this rare disease, characterizing challenges in understanding the disease biology, determining the bar for preclinical data, recommending an appropriate cytotoxic chemotherapy backbone on which to layer novel agents, and highlighting practical considerations for clinical trial development in the advanced ES patient population. We aim to provide clinicians and basic scientist’s insight into our approach in order to broadly facilitate discussions on moving new agents forward in this rare tumor. For additional background on ES more generally, we refer the reader to a recent review article 22.
Table 1. Agents discussed and evaluated by New Agents for Ewing Sarcoma Task Force in 2018.
The agents appearing in a bold typeface are expanded upon in Table 2.
| Drug/Target Class | Example drugs |
|---|---|
| EWSR1-FLI1 Target agents:
Splicing inhibitors Minor groove-binding agents |
TK-216
Mithramycin, Trabectedin and Lurbinectidin |
| Epigenetic therapies | Lysine-specific demethylase 1A (LSD1) inhibitors (seclidemstat and IMG-7289)
Histone deacetylase inhibitors (vorinostat, entinostat, and panobinostat) Bromodomain inhibitors |
| CD99 targeting agents | Clofarabine/Cladribine and CD99 antibody |
| Novel cytotoxic agents | Eribulin, aldoxorubicin and palifosfamide |
| Multi-targeted tyrosine Kinase Inhibitors | Pazopanib, regorafenib and cabozantinib |
| Mammalian target of rapamycin (mTOR) inhibitors | Nab-Rapamycin, temsirolimus |
| DNA damage/Repair | Poly-ADP-ribose polymerase (PARP) inhibitors
(niraparib, olaparib, talazoparib)
Wee1 inhibitors (AZD1775) CHK1 inhibitors (prexasertib) |
| Cell cycle cyclin-dependent kinase (CDK) inhibitors | CDK4/6 Inhibitors (palbociclib, ribociclib, abemaciclib) |
| Transcriptional CDK Inhibitors | CDK7 inhibitor (SY-1365)
CDK12 inhibitor |
| MDM2 Inhibitor | AMG-232, DS-3032b, ALRN-6924 and idasanutlin |
| Insulin-like growth factor 1 receptor (IGF-1R)
inhibitors |
Ganitumab |
| Platelet-derived growth factor receptor (PDGFR)
antibodies |
Olaratumab |
| Other monoclonal antibodies | MORab-004 |
| Metabolic modulators | Nicotinamide phosphoribosyltransferase (NAMPT) inhibitors and Metformin |
| Immunotherapy | GD2 antibody
(dinutuximab)
VIGIL/FANG Chimeric antigen receptor (CAR) T cells |
Conceptual framework for assessing novel agents in advanced Ewing sarcoma
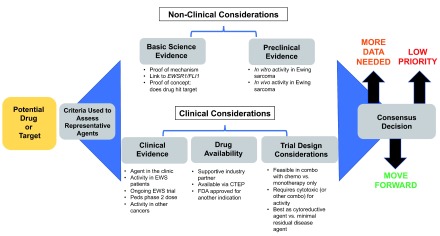
The task force defined key criteria against which to gauge potential new agents of relevance to ES ( Figure 1). These criteria were broadly divided into non-clinical and clinical criteria.
Figure 1. Paradigm for evaluation new agents for Ewing sarcoma.
Task force members proposed agents or targets (detailed in Table 1). These proposals were then each individually discussed using the step-wise approach outlined. Agents deemed worthy to move forward were then re-examined and re-vetted through this work flow as new preclinical or trial data updates became available. CTEP, Cancer Therapy Evaluation Program; EWS, Ewing sarcoma; FDA, US Food and Drug Administration.
Non-clinical criteria
The fundamental non-clinical criterion is the strength of the basic science evidence demonstrating that the target either is critical to ES pathogenesis or has an expression pattern that is relatively specific to ES. Mechanistic dependency may be linked directly to targeting the oncoprotein EWS-FLI or effectors downstream of EWS-FLI1 or micro-environmental factors critical to the tumor. Once a target is identified, it is crucial to have proof-of-concept data that a putative drug is active against the intended target.
In addition to proof-of-concept data, the remaining requirements for preclinical data will likely vary greatly depending on the agent and the model systems available. For example, although ideally animal model data would be obtained in the preclinical setting, acquiring animal data for immunotherapy agents is currently not optimal given the lack of transgenic and well-developed humanized mouse models of ES 23. Although a range of in vitro and in vivo supportive preclinical testing can be considered to help prioritize agents for further development, for a rare tumor such as ES for which relapse is nearly uniformly fatal, extensive preclinical testing need not be required if the rationale for the agent is otherwise strong. Preclinical data in ES have not always predicted clinical response; furthermore, the degree of testing is not standardized and particular attention to pharmacokinetics, pharmacodynamics, and the use of a clinically relevant dose and schedule is recommended to provide translational relevance 24– 28.
Clinical criteria
From a clinical perspective, the paramount criterion is a signal of activity in early-phase testing with agents meeting this criterion prioritized for rapid translation into a trial for relapsed or newly diagnosed metastatic populations. Drug availability, through US Food and Drug Administration (FDA) approval for other indications, inclusion in the Cancer Therapy Evaluation Program (CTEP) portfolio, or collaboration with an engaged industry partner, offers another factor in prioritization. Availability of pediatric dosing schedules is an advantage but not a prerequisite in this AYA cancer 29– 31. Given that single-agent therapy is unlikely to be curative in most circumstances, it is important to consider the feasibility of combination therapy. Other clinical parameters discussed are outlined in Figure 1.
With these non-clinical and clinical criteria in place, we formulated a list of potential agents for advanced ES. Table 1 is an inclusive list of agents considered in 2018. We next methodically considered each agent in a step-wise fashion, applying the aforementioned criteria. We applied these criteria to selected potential agents of greatest interest ( Table 2). Table 2 also provides a benchmark for ganitumab, an agent currently in phase 3 testing in newly diagnosed metastatic ES, and for mammalian target of rapamycin (mTOR) inhibitors currently proposed for the next COG trial for first recurrence.
Table 2. Work-flow summary of the top five promising agents in 2018.
Work-flow summary of eribulin, dinutuximab, tyrosine kinase inhibitors, cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitors, and poly-ADP-ribase polymerase (PARP) inhibitors. Ganitumab (currently in a metastatic Ewing sarcoma clinical trial) and mammalian target of rapamycin (mTOR) inhibitors (currently in a trial proposal) are also included using the task force’s work flow for comparison.
| Drug | Basic
Science Evidence |
Preclinical
Evidence |
Clinical
Evidence |
Drug
Availability |
Trial Design
Consideration |
Consensus
Decision |
|---|---|---|---|---|---|---|
|
Ganitumab
(in trial) |
++ | + | ++ | + | ++ | In trial
(AEWS1221) |
|
mTOR inhibitor
(proposed trial) |
++ | + | + | ++ | ++ | Move forward |
|
Anti-GD2
monoclonal |
++ | + | ++ | ++ | Move forward | |
| Eribulin | + | + | + | ++ | ++ | In early trials |
|
Multi-targeted
tyrosine kinase inhibitor |
+ | + | + | ++ | + | In early trials |
| CDK4/6 inhibitor | ++ | ++ | ++ | + | In early trials | |
| PARP inhibitor | ++ | ++ | - | ++ | - | In early trials |
Table 2 - = negative data, + =some evidence, ++ =significant evidence, blank=no data
Eribulin
Eribulin is a microtubule inhibitor that inhibits polymerization of tubulin subunits and differs from other microtubule inhibitors by preventing lengthening and shortening of microtubules during division. Aggregation of unstable tubulin polymers ultimately results in cellular apoptosis 32– 35. EWS-FLI1 is known to drive the expression of proteins that regulate microtubule stability 15. Preclinical testing of ES cell lines demonstrated apoptosis, induced through the Bcl-2 pathway 36. Eribulin was also evaluated by the Pediatric Preclinical Testing Program (PPTP); four out of five ES xenografts demonstrated a complete response to treatment 37. In sarcoma treatment, eribulin is FDA-approved for adult patients with liposarcoma who previously received an anthracycline 38. The COG performed a phase 1 trial of eribulin in children with advanced solid tumors; one of the evaluable patients with ES experienced a partial response for four cycles 39. An ongoing phase 2 trial (ClinicalTrials.gov Identifier: NCT03441360) is assessing eribulin in patients with relapsed ES 40. An ongoing phase 1/2 trial (ClinicalTrials.gov Identifier: NCT03245450) is evaluating eribulin in combination with irinotecan, although these agents are not expected to have significant overlapping toxicities 41. Furthermore, vincristine and topoisomerase 1 inhibitors act synergistically in xenograft models and in pediatric clinical trials; therefore, eribulin with irinotecan may also have clinical synergy 42, 43.
Eribulin shows strong preclinical data in ES, is an FDA-approved agent for another sarcoma indication, and has led to at least one monotherapy response in relapsed ES. We will recommend further trial development with eribulin as a component of combination therapy pending the results of current trials.
Dinutuximab
GD2 is a disialoganglioside that is expressed on the surface of tumors of neural crest origin, such as neuroblastoma 44. The precise cell of origin for ES is unknown; however, it is thought to be a neural crest or mesenchymal-derived tumor 45– 47. ES tumors have been evaluated for GD2 expression, and results range from no detectable surface expression to diffuse/intense staining in some tumors 48– 51. It is not known whether GD2 expression changes upon relapse or whether levels differ in patients with newly diagnosed metastatic ES. Several available antibodies in the clinic are known to bind to this target, including dinutuximab, which is approved by the FDA for use in neuroblastoma. There is currently no preclinical evidence for utility of dinutuximab in ES.
The clinical evidence supporting dinutuximab borrows heavily from another GD2-positive pediatric cancer, neuroblastoma. The combination of irinotecan, temozolomide, and dinutuximab has been shown to demonstrate significant clinical benefit in children with advanced neuroblastoma 52. Pediatric dosing, schedules, and toxicities are well documented with this therapy combination. Patients with relapsed neuroblastoma are generally younger than patients with relapsed ES, and there is the potential for different toxicity in older patients. As some ES tumors express GD2, determining whether the addition of dinutuximab to chemotherapy extends to ES is a logical clinical trial question. Dinutuximab is FDA-approved and also available in the CTEP portfolio. As demonstrated in neuroblastoma trials, dinutuximab is both feasible and more effective in combination with irinotecan and temozolomide (IT).
The task force determined that the available clinical data in another GD2-positive tumor treated with this combination are sufficiently strong to nominate this approach for clinical evaluation in ES. Irinotecan and temozolomide are agents routinely used in relapsed ES therapy, and a next-step trial could determine the efficacy of dinutuximab added to IT. The combination would be strategically combining immune-based treatment with traditional cytotoxic therapy. Given the current inconsistency in GD2 immunohistochemistry staining, it would not be possible to select patients upfront on the basis of tumor GD2 expression.
Anti-angiogenic tyrosine kinase inhibitors
A large body of work highlights the role of angiogenesis in ES 53– 56. Several tyrosine kinase inhibitors (TKIs) targeting angiogenesis have been developed, although most are multi-targeted TKIs that target other receptor tyrosine kinases (RTKs). RTKs are important regulators of cell growth, proliferation, and survival. Aberrant RTK signaling resulting from amplification, mutation, or overexpression has been implicated in many cancers, including ES 57.
Pazopanib primarily targets VEGFR-1 and -2, PDGFR-α and -β, and c-Kit 58. Pazopanib has gained FDA approval for treatment of refractory soft tissue sarcoma (STS) in adult patients. A phase 3 clinical trial for patients with advanced STS demonstrated improved progression-free survival (PFS) (4.6 months) in the pazopanib arm compared with placebo (1.6 months) 59. The PPTP evaluation of pazopanib revealed a statistically prolonged EFS in ES xenografts but no objective responses 60. Several case reports have demonstrated partial responses in patients with ES; however, resistance seems to develop with prolonged use 61– 63. Cabozantinib targets VEGFR2, c-MET, and AXL and is FDA-approved for the treatment of medullary thyroid cancer and renal cell carcinoma in adults. A phase 2 study of cabozantinib in patients with recurrent ES showed tumor control with 9 (27.7%) partial responses and 10 (30.3%) with stable disease 64. Pediatric phase 2 dosing is established for both pazopanib and cabozantinib 65, 66. Regorafenib is an oral multikinase inhibitor that targets VEGFR-1-3, FGFR1, PDGFR-α and -β, CSFR-1, and c-Kit. It has FDA approval for use in metastatic colorectal carcinoma, gastrointestinal stromal tumor, and hepatocellular carcinoma. SARC024 evaluated regorafenib in 30 patients with ES. The median PFS was 3.6 months, and the median duration of response was 5.5 months 67.
At least three anti-angiogenic TKIs have shown clinical monotherapy activity in relapsed ES. This clinical activity together with preclinical rationale and drug availability should motivate evaluation of this class of agents in combination with cytotoxic chemotherapy or other targeted agents or as a potential maintenance therapy in future trials.
CDK4/6 inhibitors
CDKN2A is a gene that codes for two proteins: p16 and p14arf. Both proteins are tumor suppressors; p16 inhibits cyclin-dependent kinases 4 and 6 (CDK4 and CDK6) by phosphorylating the RB protein, thus preventing cell cycle progression 68. About 13 to 30% of ES tumors have deletions in CDKN2A 69– 71. CDKN2A deletion does not appear to be associated with clinical outcome 72. Owing to the alteration of p16 in a subset of ES, there is clinical interest in CDK4/6 inhibitors that target and inhibit this pathway. The cyclin D1 gene has been shown to be associated with a superenhancer in ES 73. ES cells consequently have an activated cyclin D1/CDK4 pathway and require CDK4 and cyclin D1 for growth. In vivo data demonstrated decreased tumor growth and prolonged survival with CDK4/6 inhibition 73. These data provide a rationale for use even in the absence of CDKN2A deletions 74.
Currently, three CDK4/6 inhibitors have been FDA-approved for advanced breast cancer: abemaciclib, palbociclib, and ribociclib. These agents each have ongoing or completed single-agent pediatric phase 1 trials (ClinicalTrials.gov Identifiers: NCT02644460, NCT01747876, and NCT02255461) 75– 77. As in adults, the predominant toxicity seen in children is hematologic 78. Since single-agent therapy is rarely curative in sarcomas, there is little interest in evaluating single-agent CDK4/6 inhibitors in patients with ES. Combination with conventional cytotoxic chemotherapy agents, many of which are dependent on S-phase cycling, may be challenging because of antagonistic mechanisms 79. Furthermore, the significant myelosuppression seen with CDK4/6 inhibitors complicates combinatorial therapy. Without significant adult data evaluating CDK4/6 inhibitors with chemotherapy, we await the results of a planned trial evaluating palbociclib with IT before recommending a chemotherapy combination approach in advanced ES (ClinicalTrials.gov Identifier: NCT03709680) 80. There are other trials in the US combining CDK4/6 inhibitors with agents, including MEK and mTOR inhibitors (ClinicalTrials.gov Identifiers: NCT03387020, NCT03114527, and NCT02703571) 81– 83. Other preclinical data suggest that dual inhibition of CDK4/6 and IGF-1R may be a combination to consider in ES, as CDK4/6 drug resistance is mediated by activation of IGF-1R signaling 84. In sum, CDK4/6 inhibitors have potential efficacy in ES but identifying the appropriate combination therapy has been challenging. We recommend additional preclinical and clinical data before moving this class of drugs forward in first-relapse or metastatic ES.
PARP inhibitors
Much enthusiasm has surrounded poly-ADP-ribose polymerase 1 (PARP1) inhibitors in ES. PARP plays a significant role in DNA repair, particularly with single-strand DNA damage. Inhibition of PARP proteins can cause persistent single-strand breaks, ultimately resulting in cellular apoptosis. EWS-FLI1 interacts with PARP1, influencing its transcriptional activity, and ES tumors have high levels of PARP mRNA and protein activity 85. Several PARP inhibitors, including olaparib, rucaparib, talazoparib, and niraparib, have received FDA approval for the treatment of ovarian or breast cancer in adult patients. Interest in PARP inhibitors in ES resulted from preclinical data demonstrating sensitivity in ES cell lines 86. A phase 2 trial with olaparib monotherapy quickly opened for adults with relapsed ES. Of the 12 patients enrolled, none had objective responses and four patients had stable disease. The median time to progression was 5.7 weeks 25. The PPTP and others evaluated PARP inhibitors in combination with DNA-damaging chemotherapy and demonstrated activity in ES xenografts 87– 89.
Owing to these promising preclinical data, a series of successor trials have evaluated PARP inhibitors in combination with irinotecan, temozolomide, or IT (ClinicalTrials.gov Identifiers: NCT02116777, NCT01858168, NCT02044120, and NCT02392793) 90– 93. While there have been hints of clinical activity, preliminary presentations of the toxicity data have shown that the myelosuppression of PARP inhibitors with cytotoxic chemotherapy is limiting dose intensity in these combination trials 94. This drug class demonstrates that strong preclinical activity and rationale may not always predict clinical feasibility, particularly in a heavily pretreated, relapsed patient population. At this time, we recommend awaiting results from ongoing clinical trials prior to moving this class of drugs further in advanced ES clinical trials.
The cytotoxic backbone: trial recommendations
We also discussed the chemotherapy backbone onto which selected agents could be added for patients with first recurrent ES. Currently, there is no established standard backbone for patients with recurrent or refractory ES. Topotecan with cyclophosphamide has shown activity in patients with relapsed ES with response rates between 23 and 41% 95– 100. Owing to this promising activity, the COG conducted a pilot trial adding vincristine, topotecan, and cyclophosphamide (VTc) to interval-compressed ES therapy. This combination was tolerable, but hematologic toxicity was between 44 and 63% 101. The Euro Ewing Consortium is evaluating topotecan and cyclophosphamide in the rEECur trial (EudraCT number: 2014-000259-99) for recurrent ES. A randomized COG phase 3 trial, AEWS1031, evaluated the efficacy of adding VTc to the interval-compressed five-drug backbone, and results are pending. Owing to the significant hematologic toxicity of this combination, incorporating additional agents to this backbone may prove challenging but could be considered for the appropriate novel agents.
The combination of vincristine and IT (VIT) has also shown activity in patients with relapsed ES. VIT has demonstrated response rates between 29 and 63% in relapsed or refractory patients, although the actual VIT response rate has not yet been investigated in a prospective randomized trial 102– 105. This chemotherapy has schedule-dependent synergy 106, 107. The combination of drugs also has limited overlapping toxicity. Diarrhea and abdominal pain are the most common dose-limiting toxicities of irinotecan 108, 109. The major dose-limiting toxicity of temozolomide is myelosuppression 110– 112. Currently, the Euro Ewing Consortium is evaluating IT in relapsed ES. IT has a strong history of efficacious combination with monoclonal antibodies or targeted therapies in other diseases 52, 113, 114. Currently, there are ongoing trials for patients with ES combining IT with PARP inhibitors or Vigil autologous vaccine (ClinicalTrials.gov Identifiers: NCT02044120, NCT01858168, and NCT03495921) 90, 92, 93, 115. Owing to the reported efficacy of IT in ES and successful combination with other novel therapeutics, we believe IT (with or without vincristine) may be a preferred and feasible cytotoxic backbone for future combination clinical trials in first recurrent ES.
Although the original addition of IE to VDC did show improved outcomes for localized disease, patients with upfront metastatic ES saw no clinical benefit from this addition 8, 116, 117. Given these data, the group also discussed eliminating or reducing IE cycles for metastatic patients to decrease toxicity, allowing the addition of other targeted agents to VDC in future trials. Furthermore, early data from a single-institution trial including patients with upfront metastatic ES (ClinicalTrials.gov Identifier: NCT01864109) demonstrate the feasibility of incorporating IT into standard upfront therapy for metastatic ES 118. Altering the number of IE cycles and adding IT to the upfront chemotherapy backbone are both considerations for further trial design considering drug synergism and overlapping toxicities of the targeted agents being studied.
Additional key clinical trial design considerations
In addition to establishing recommendations for advanced ES cytotoxic backbones, we examined other important aspects of trial design including patient randomization, maintenance therapy, and specific differences in trial design approach for patients with upfront metastatic versus relapsed ES. Given the rarity of this tumor, we continue to support collaborative, multi-national trials in order to study this cancer in a timely and inclusive manner.
With a goal of testing novel agents in patients with advanced ES, we propose several potential trial designs depending upon the agent and specific population of interest. The newly diagnosed metastatic population presents two potential opportunities for evaluating new agents. For agents expected to combine well with conventional cytotoxic chemotherapy, we recommend a randomized design comparing chemotherapy alone versus chemotherapy plus a novel agent. Depending upon the available evidence supporting the agent, the statistical parameters for such a trial may incorporate a phase 2 design with early stopping rules or a phase 3 trial to show definitive efficacy. For agents difficult to combine with chemotherapy, those that may be administered on a chronic schedule, or those with expected efficacy in minimal residual disease settings, a maintenance design could be considered. In this design, patients in a radiographic complete remission would continue treatment with the novel agent upon completion of frontline therapy. Both approaches are used in the ongoing COG trial of ganitumab. Given the paucity of clinical data for maintenance strategies in ES, this approach is best studied in the context of a randomized study, comparing maintenance with no maintenance or comparing two different promising maintenance strategies. Likewise, the type of maintenance regimen should be carefully considered in light of concerns about toxicity and adherence in an AYA population 119.
For the relapse population, there are two main design considerations. Given that ES often remains chemotherapy-responsive at first relapse, trial designs should incorporate cytotoxic chemotherapy. Therefore, testing of new agents can be performed as a single-arm trial combining a new agent with a standard backbone, and inference about activity of the combination is based on strong historical data for the backbone regimen. Alternatively, new agents may be tested in a randomized manner, either as a definitive comparison against the backbone alone or in a selection design comparing two novel agents added to the same backbone. Promising agents that are unlikely to pair well with chemotherapy (for example, antagonism; overlapping toxicity) may be best evaluated as monotherapy in a second relapse population.
Conclusions and future directions
We have generated a robust group and infrastructure for vetting novel agents for evaluation in patients with ES. Future efforts by our group and other groups may use this paradigm as new agents or concepts become available. We discussed several strategies that were determined to be too early in development for immediate translation into a clinical trial for the newly diagnosed metastatic or first recurrent populations. Examples of these strategies include lysine-specific demethylase 1A (LSD1) inhibition, inhibition of interaction between EWSR1-FLI1 protein with RNA helicase, epigenetic modification of chromatin modeling resulting in EWS-FLI1 suppression, inhibition of transcriptionally active CDKs, and immunotherapy approaches in ES 17, 120– 122. The first two of these approaches are particularly noteworthy as they target fundamental functions of the fusion oncoprotein that drives this disease. The task force will continue to discuss the progress of these and other emerging strategies.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
David J Gordon, Department of Pediatrics, Division of Pediatric Hematology/Oncology, University of Iowa, Iowa City, Iowa, 52242, USA
Antonio Llombart-Bosch, Pathology Department, University of Valencia, Valencia, Spain
Alan W Craft, Northern Institute for Cancer Research, Newcastle University, Newcastle upon Tyne, UK
Funding Statement
This work is supported by the Children’s Oncology Group and the National Cancer Institute of the National Institutes of Health under NCTN Operations Center Grant U10CA180886.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; peer review: 3 approved]
References
- 1. Lawlor ER, Sorensen PH: Twenty Years on: What Do We Really Know about Ewing Sarcoma and What Is the Path Forward? Crit Rev Oncog. 2015;20(3–4):155–71. 10.1615/CritRevOncog.2015013553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Anderson ND, de Borja R, Young MD, et al. : Rearrangement bursts generate canonical gene fusions in bone and soft tissue tumors. Science. 2018;361(6405): pii: eaam8419. 10.1126/science.aam8419 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 3. Brohl AS, Patidar R, Turner CE, et al. : Frequent inactivating germline mutations in DNA repair genes in patients with Ewing sarcoma. Genet Med. 2017;19(8):955–8. 10.1038/gim.2016.206 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 4. Franzetti GA, Laud-Duval K, van der Ent W, et al. : Cell-to-cell heterogeneity of EWSR1-FLI1 activity determines proliferation/migration choices in Ewing sarcoma cells. Oncogene. 2017;36(25):3505–14. 10.1038/onc.2016.498 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 5. Pedersen EA, Menon R, Bailey KM, et al. : Activation of Wnt/β-Catenin in Ewing Sarcoma Cells Antagonizes EWS/ETS Function and Promotes Phenotypic Transition to More Metastatic Cell States. Cancer Res. 2016;76(17):5040–53. 10.1158/0008-5472.CAN-15-3422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Womer RB, West DC, Krailo MD, et al. : Randomized controlled trial of interval-compressed chemotherapy for the treatment of localized Ewing sarcoma: a report from the Children's Oncology Group. J Clin Oncol. 2012;30(33):4148–54. 10.1200/JCO.2011.41.5703 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 7. Whelan J, Le Deley MC, Dirksen U, et al. : High-Dose Chemotherapy and Blood Autologous Stem-Cell Rescue Compared With Standard Chemotherapy in Localized High-Risk Ewing Sarcoma: Results of Euro-E.W.I.N.G.99 and Ewing-2008. J Clin Oncol. 2018;36(31):3110–9. 10.1200/JCO.2018.78.2516 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 8. Grier HE, Krailo MD, Tarbell NJ, et al. : Addition of ifosfamide and etoposide to standard chemotherapy for Ewing's sarcoma and primitive neuroectodermal tumor of bone. N Engl J Med. 2003;348(8):694–701. 10.1056/NEJMoa020890 [DOI] [PubMed] [Google Scholar]
- 9. Ladenstein R, Pötschger U, Le Deley MC, et al. : Primary disseminated multifocal Ewing sarcoma: results of the Euro-EWING 99 trial. J Clin Oncol. 2010;28(20):3284–91. 10.1200/JCO.2009.22.9864 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 10. NCT02306161: National Cancer Institute, Randomized Phase 3 Trial Evaluating the Addition of the IGF-1R Monoclonal Antibody Ganitumab (AMG 479, NSC# 750008) to Multiagent Chemotherapy for Patients With Newly Diagnosed Metastatic Ewing Sarcoma.In: ClinicalTrials.gov (cited 2019, Jan 29). Reference Source [Google Scholar]
- 11. Theisen ER, Pishas KI, Saund RS, et al. : Therapeutic opportunities in Ewing sarcoma: EWS-FLI inhibition via LSD1 targeting. Oncotarget. 2016;7(14):17616–30. 10.18632/oncotarget.7124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gorthi A, Romero JC, Loranc E, et al. : EWS-FLI1 increases transcription to cause R-loops and block BRCA1 repair in Ewing sarcoma. Nature. 2018;555(7696):387–91. 10.1038/nature25748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gorthi A, Bishop AJR: Ewing sarcoma fusion oncogene: At the crossroads of transcription and DNA damage response. Mol Cell Oncol. 2018;5(4):e1465014. 10.1080/23723556.2018.1465014 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 14. Tawbi HA, Burgess M, Bolejack V, et al. : Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): a multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol. 2017;18(11):1493–1501. 10.1016/S1470-2045(17)30624-1 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 15. Zöllner SK, Selvanathan SP, Graham GT, et al. : Inhibition of the oncogenic fusion protein EWS-FLI1 causes G 2-M cell cycle arrest and enhanced vincristine sensitivity in Ewing's sarcoma. Sci Signal. 2017;10(499): pii: eaam8429. 10.1126/scisignal.aam8429 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 16. Stegmaier K, Wong JS, Ross KN, et al. : Signature-based small molecule screening identifies cytosine arabinoside as an EWS/FLI modulator in Ewing sarcoma. PLoS Med. 2007;4(4):e122. 10.1371/journal.pmed.0040122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Erkizan HV, Kong Y, Merchant M, et al. : A small molecule blocking oncogenic protein EWS-FLI1 interaction with RNA helicase A inhibits growth of Ewing's sarcoma. Nat Med. 2009;15(7):750–6. 10.1038/nm.1983 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 18. Grohar PJ, Woldemichael GM, Griffin LB, et al. : Identification of an inhibitor of the EWS-FLI1 oncogenic transcription factor by high-throughput screening. J Natl Cancer Inst. 2011;103(12):962–78. 10.1093/jnci/djr156 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 19. Grohar PJ, Griffin LB, Yeung C, et al. : Ecteinascidin 743 interferes with the activity of EWS-FLI1 in Ewing sarcoma cells. Neoplasia. 2011;13(2):145–53. 10.1593/neo.101202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Boro A, Prêtre K, Rechfeld F, et al. : Small-molecule screen identifies modulators of EWS/FLI1 target gene expression and cell survival in Ewing's sarcoma. Int J Cancer. 2012;131(9):2153–64. 10.1002/ijc.27472 [DOI] [PubMed] [Google Scholar]
- 21. Khanna C, Fan TM, Gorlick R, et al. : Toward a drug development path that targets metastatic progression in osteosarcoma. Clin Cancer Res. 2014;20(16):4200–9. 10.1158/1078-0432.CCR-13-2574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Grünewald TGP, Cidre-Aranaz F, Surdez D, et al. : Ewing sarcoma. Nat Rev Dis Primers. 2018;4(1): 5. 10.1038/s41572-018-0003-x [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 23. Minas TZ, Surdez D, Javaheri T, et al. : Combined experience of six independent laboratories attempting to create an Ewing sarcoma mouse model. Oncotarget. 2017;8(21):34141–63. 10.18632/oncotarget.9388 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 24. DuBois SG, Krailo MD, Lessnick SL, et al. : Phase II study of intermediate-dose cytarabine in patients with relapsed or refractory Ewing sarcoma: a report from the Children's Oncology Group. Pediatr Blood Cancer. 2009;52(3):324–7. 10.1002/pbc.21822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Choy E, Butrynski JE, Harmon DC, et al. : Phase II study of olaparib in patients with refractory Ewing sarcoma following failure of standard chemotherapy. BMC Cancer. 2014;14:813. 10.1186/1471-2407-14-813 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 26. Houghton PJ, Morton CL, Kang M, et al. : Evaluation of cytarabine against Ewing sarcoma xenografts by the pediatric preclinical testing program. Pediatr Blood Cancer. 2010;55(6):1224–6. 10.1002/pbc.22355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Scheinberg T, Lomax A, Martin HN, et al. : PD-1 blockage using pembrolizumab in adolescent and young adult patients with advanced bone and soft tissue sarcoma. J Clin Oncol. 2017;35(15_suppl):3060 10.1200/JCO.2017.35.15_suppl.3060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Langenau DM, Sweet-Cordero A, Wechsler-Reya RJ, et al. : Preclinical Models Provide Scientific Justification and Translational Relevance for Moving Novel Therapeutics into Clinical Trials for Pediatric Cancer. Cancer Res. 2015;75(24):5176–86. 10.1158/0008-5472.CAN-15-1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chuk MK, Mulugeta Y, Roth-Cline M, et al. : Enrolling Adolescents in Disease/Target-Appropriate Adult Oncology Clinical Trials of Investigational Agents. Clin Cancer Res. 2017;23(1):9–12. 10.1158/1078-0432.CCR-16-1367 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 30. Gaspar N, Marshall LV, Binner D, et al. : Joint adolescent-adult early phase clinical trials to improve access to new drugs for adolescents with cancer: Proposals from the multi-stakeholder platform—ACCELERATE. Ann Oncol. 2018;29(3):766–71. 10.1093/annonc/mdy002 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 31. Gore L, Ivy SP, Balis FM, et al. : Modernizing Clinical Trial Eligibility: Recommendations of the American Society of Clinical Oncology-Friends of Cancer Research Minimum Age Working Group. J Clin Oncol. 2017;35(33):3781–7. 10.1200/JCO.2017.74.4144 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 32. Okouneva T, Azarenko O, Wilson L, et al. : Inhibition of centromere dynamics by eribulin (E7389) during mitotic metaphase. Mol Cancer Ther. 2008;7(7):2003–11. 10.1158/1535-7163.MCT-08-0095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jordan MA, Kamath K, Manna T, et al. : The primary antimitotic mechanism of action of the synthetic halichondrin E7389 is suppression of microtubule growth. Mol Cancer Ther. 2005;4(7):1086–95. 10.1158/1535-7163.MCT-04-0345 [DOI] [PubMed] [Google Scholar]
- 34. Towle MJ, Salvato KA, Budrow J, et al. : In vitro and in vivo anticancer activities of synthetic macrocyclic ketone analogues of halichondrin B. Cancer Res. 2001;61(3):1013–21. [PubMed] [Google Scholar]
- 35. Kuznetsov G, Towle MJ, Cheng H, et al. : Induction of morphological and biochemical apoptosis following prolonged mitotic blockage by halichondrin B macrocyclic ketone analog E7389. Cancer Res. 2004;64(16):5760–6. 10.1158/0008-5472.CAN-04-1169 [DOI] [PubMed] [Google Scholar]
- 36. Weiß LM, Hugle M, Fulda S: Eribulin alone or in combination with the PLK1 inhibitor BI 6727 triggers intrinsic apoptosis in Ewing sarcoma cell lines. Oncotarget. 2017;8(32):52445–52456. 10.18632/oncotarget.17190 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 37. Kolb EA, Gorlick R, Reynolds CP, et al. : Initial testing (stage 1) of eribulin, a novel tubulin binding agent, by the pediatric preclinical testing program. Pediatr Blood Cancer. 2013;60(8):1325–32. 10.1002/pbc.24517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Koliou P, Karavasilis V, Theochari M, et al. : Advances in the treatment of soft tissue sarcoma: focus on eribulin. Cancer Manag Res. 2018;10:207–16. 10.2147/CMAR.S143019 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 39. Schafer ES, Rau RE, Berg S, et al. : A phase 1 study of eribulin mesylate (E7389), a novel microtubule-targeting chemotherapeutic agent, in children with refractory or recurrent solid tumors: A Children's Oncology Group Phase 1 Consortium study (ADVL1314). Pediatr Blood Cancer. 2018;65(8):e27066. 10.1002/pbc.27066 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 40. NCT03441360 Eisai Inc.: A Phase 2, Multicenter, Open-label Study to Assess Safety and Preliminary Activity of Eribulin Mesylate in Pediatric Subjects With Relapsed/Refractory Rhabdomyosarcoma (RMS), Non-rhabdomyosarcoma Soft Tissue Sarcoma (NRSTS) and Ewing Sarcoma (EWS).In: ClinicalTrials.gov. (cited 2019, Jan 29). Reference Source [Google Scholar]
- 41. NCT03245450 Eisai Inc.: A Phase 1/2 Single-arm Study Evaluating the Safety and Efficacy of Eribulin Mesilate in Combination With Irinotecan in Children With Refractory or Recurrent Solid Tumors.In: ClinicalTrials.gov. (cited 2019, Jan 29). Reference Source [Google Scholar]
- 42. Pappo AS, Lyden E, Breitfeld P, et al. : Two consecutive phase II window trials of irinotecan alone or in combination with vincristine for the treatment of metastatic rhabdomyosarcoma: the Children's Oncology Group. J Clin Oncol. 2007;25(4):362–9. 10.1200/JCO.2006.07.1720 [DOI] [PubMed] [Google Scholar]
- 43. Thompson J, George EO, Poquette CA, et al. : Synergy of topotecan in combination with vincristine for treatment of pediatric solid tumor xenografts. Clin Cancer Res. 1999;5(11):3617–31. [PubMed] [Google Scholar]
- 44. Martinez C, Hofmann TJ, Marino R, et al. : Human bone marrow mesenchymal stromal cells express the neural ganglioside GD2: a novel surface marker for the identification of MSCs. Blood. 2007;109(10):4245–8. 10.1182/blood-2006-08-039347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hu-Lieskovan S, Zhang J, Wu L, et al. : EWS-FLI1 fusion protein up-regulates critical genes in neural crest development and is responsible for the observed phenotype of Ewing's family of tumors. Cancer Res. 2005;65(11):4633–44. 10.1158/0008-5472.CAN-04-2857 [DOI] [PubMed] [Google Scholar]
- 46. von Levetzow C, Jiang X, Gwye Y, et al. : Modeling initiation of Ewing sarcoma in human neural crest cells. PLoS One. 2011;6(4):e19305. 10.1371/journal.pone.0019305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tirode F, Laud-Duval K, Prieur A, et al. : Mesenchymal stem cell features of Ewing tumors. Cancer Cell. 2007;11(5):421–9. 10.1016/j.ccr.2007.02.027 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 48. Dobrenkov K, Ostrovnaya I, Gu J, et al. : Oncotargets GD2 and GD3 are highly expressed in sarcomas of children, adolescents, and young adults. Pediatr Blood Cancer. 2016;63(10):1780–5. 10.1002/pbc.26097 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 49. Kailayangiri S, Altvater B, Meltzer J, et al. : The ganglioside antigen G D2 is surface-expressed in Ewing sarcoma and allows for MHC-independent immune targeting. Br J Cancer. 2012;106(6):1123–33. 10.1038/bjc.2012.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fisher JP, Flutter B, Wesemann F, et al. : Effective combination treatment of GD2-expressing neuroblastoma and Ewing's sarcoma using anti-GD2 ch14.18/CHO antibody with Vγ9Vδ2+ γδT cells. Oncoimmunology. 2016;5(1): e1025194. 10.1080/2162402X.2015.1025194 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 51. Lipinski M, Hirsch MR, Deagostini-Bazin H, et al. : Characterization of neural cell adhesion molecules (NCAM) expressed by Ewing and neuroblastoma cell lines. Int J Cancer. 1987;40(1):81–6. 10.1002/ijc.2910400115 [DOI] [PubMed] [Google Scholar]
- 52. Mody R, Naranjo A, Van Ryn C, et al. : Irinotecan-temozolomide with temsirolimus or dinutuximab in children with refractory or relapsed neuroblastoma (COG ANBL1221): an open-label, randomised, phase 2 trial. Lancet Oncol. 2017;18(7):946–57. 10.1016/S1470-2045(17)30355-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. DuBois SG, Marina N, Glade-Bender J: Angiogenesis and vascular targeting in Ewing sarcoma: a review of preclinical and clinical data. Cancer. 2010;116(3):749–57. 10.1002/cncr.24844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zhou Z, Bolontrade MF, Reddy K, et al. : Suppression of Ewing's sarcoma tumor growth, tumor vessel formation, and vasculogenesis following anti vascular endothelial growth factor receptor-2 therapy. Clin Cancer Res. 2007;13(16):4867–73. 10.1158/1078-0432.CCR-07-0133 [DOI] [PubMed] [Google Scholar]
- 55. Reddy K, Zhou Z, Schadler K, et al. : Bone marrow subsets differentiate into endothelial cells and pericytes contributing to Ewing's tumor vessels. Mol Cancer Res. 2008;6(6):929–36. 10.1158/1541-7786.MCR-07-2189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Potikyan G, Savene RO, Gaulden JM, et al. : EWS/FLI1 regulates tumor angiogenesis in Ewing's sarcoma via suppression of thrombospondins. Cancer Res. 2007;67(14):6675–84. 10.1158/0008-5472.CAN-06-4140 [DOI] [PubMed] [Google Scholar]
- 57. Fleuren ED, Versleijen-Jonkers YM, Boerman OC, et al. : Targeting receptor tyrosine kinases in osteosarcoma and Ewing sarcoma: current hurdles and future perspectives. Biochim Biophys Acta. 2014;1845(2):266–76. 10.1016/j.bbcan.2014.02.005 [DOI] [PubMed] [Google Scholar]
- 58. Bukowski RM, Yasothan U, Kirkpatrick P: Pazopanib. Nat Rev Drug Discov. 2010;9(1):17–8. 10.1038/nrd3073 [DOI] [PubMed] [Google Scholar]
- 59. van der Graaf WT, Blay JY, Chawla SP, et al. : Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2012;379(9829):1879–86. 10.1016/S0140-6736(12)60651-5 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 60. Keir ST, Morton CL, Wu J, et al. : Initial testing of the multitargeted kinase inhibitor pazopanib by the Pediatric Preclinical Testing Program. Pediatr Blood Cancer. 2012;59(3):586–8. 10.1002/pbc.24016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Alcindor T: Response of refractory Ewing sarcoma to pazopanib. Acta Oncol. 2015;54(7):1063–4. 10.3109/0284186X.2014.971938 [DOI] [PubMed] [Google Scholar]
- 62. Attia S, Okuno SH, Robinson SI, et al. : Clinical Activity of Pazopanib in Metastatic Extraosseous Ewing Sarcoma. Rare Tumors. 2017;7(2):86–8. 10.4081/rt.2015.5992 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 63. Yamamoto Y, Nozawa M, Shimizu N, et al. : Pazopanib for recurrent extraosseous Ewing's sarcoma of the retroperitoneum. Int J Urol. 2014;21(11):1183–4. 10.1111/iju.12546 [DOI] [PubMed] [Google Scholar]
- 64. Italiano A, Penel N, Toulmonde M, et al. : Cabozantinib in patients with advanced osteosarcomas and Ewing sarcomas: a French Sarcoma Group (FSG)/US National Cancer Institute phase II collaborative study. Connective Tissue Oncology Society Annual Meeting Rome, Italy2018. Reference Source [Google Scholar]
- 65. Chuk MK, Widemann BC, Minard CG, et al. : A phase 1 study of cabozantinib in children and adolescents with recurrent or refractory solid tumors, including CNS tumors: Trial ADVL1211, a report from the Children's Oncology Group. Pediatr Blood Cancer. 2018;65(8):e27077. 10.1002/pbc.27077 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 66. Glade Bender JL, Lee A, Reid JM, et al. : Phase I pharmacokinetic and pharmacodynamic study of pazopanib in children with soft tissue sarcoma and other refractory solid tumors: a children's oncology group phase I consortium report. J Clin Oncol. 2013;31(24):3034–43. 10.1200/JCO.2012.47.0914 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 67. Attia S, Bolejack V, Ganjoo KN, et al. : A phase 2 trial of regorafenib (REGO) in patients with advanced Ewing sarcoma and related tumors of soft tissue and bone: SARC0024 trial results. J Clin Oncol. 2017;35(15 suppl):abstr 11005 10.1200/JCO.2017.35.15_suppl.11005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Serrano M, Hannon GJ, Beach D: A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature. 1993;366(6456):704–7. 10.1038/366704a0 [DOI] [PubMed] [Google Scholar]
- 69. Huang HY, Illei PB, Zhao Z, et al. : Ewing sarcomas with p53 mutation or p16/p14ARF homozygous deletion: a highly lethal subset associated with poor chemoresponse. J Clin Oncol. 2005;23(3):548–58. 10.1200/JCO.2005.02.081 [DOI] [PubMed] [Google Scholar]
- 70. Brohl AS, Solomon DA, Chang W, et al. : The genomic landscape of the Ewing Sarcoma family of tumors reveals recurrent STAG2 mutation. PLoS Genet. 2014;10(7):e1004475. 10.1371/journal.pgen.1004475 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 71. Crompton BD, Stewart C, Taylor-Weiner A, et al. : The genomic landscape of pediatric Ewing sarcoma. Cancer Discov. 2014;4(11):1326–41. 10.1158/2159-8290.CD-13-1037 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 72. Lerman DM, Monument MJ, McIlvaine E, et al. : Tumoral TP53 and/or CDKN2A alterations are not reliable prognostic biomarkers in patients with localized Ewing sarcoma: a report from the Children's Oncology Group. Pediatr Blood Cancer. 2015;62(5):759–65. 10.1002/pbc.25340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kennedy AL, Vallurupalli M, Chen L, et al. : Functional, chemical genomic, and super-enhancer screening identify sensitivity to cyclin D1/CDK4 pathway inhibition in Ewing sarcoma. Oncotarget. 2015;6(30):30178–93. 10.18632/oncotarget.4903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Dowless M, Lowery CD, Shackleford T, et al. : Abemaciclib Is Active in Preclinical Models of Ewing Sarcoma via Multipronged Regulation of Cell Cycle, DNA Methylation, and Interferon Pathway Signaling. Clin Cancer Res. 2018;24(23):6028–39. 10.1158/1078-0432.CCR-18-1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. NCT02644460: Cynthia Wetmore, Emory University, Abemaciclib in Children With Newly Diagnosed Diffuse Intrinsic Pontine Glioma, and in Children With Recurrent and Refractory Solid Tumors Including Malignant Brain Tumors. In: ClinicalTrials.gov (cited 2019, Jan 29). Reference Source [Google Scholar]
- 76. NCT01747876: Novartis Pharmaceuticals, A Phase I, Multi-center, Open-label Study of LEE011 in Patients With Malignant Rhabdoid Tumors and Neuroblastoma. In: ClinicalTrials.gov (cited 2019, Jan 29). Reference Source [Google Scholar]
- 77. NCT02255461: Pediatric Brain Tumor Consortium, Phase I Study of CDK 4-6 Inhibitor PD-0332991 (Palbociclib; IBRANCE) in Children With Recurrent, Progressive or Refractory Central NervousSystem Tumors. In: ClinicalTrials.gov (cited 2019, Jan 29). Reference Source [Google Scholar]
- 78. Geoerger B, Bourdeaut F, DuBois SG, et al. : A Phase I Study of the CDK4/6 Inhibitor Ribociclib (LEE011) in Pediatric Patients with Malignant Rhabdoid Tumors, Neuroblastoma, and Other Solid Tumors. Clin Cancer Res. 2017;23(10):2433–41. 10.1158/1078-0432.CCR-16-2898 [DOI] [PubMed] [Google Scholar]
- 79. McClendon AK, Dean JL, Rivadeneira DB, et al. : CDK4/6 inhibition antagonizes the cytotoxic response to anthracycline therapy. Cell Cycle. 2012;11(14):2747–55. 10.4161/cc.21127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. NCT03709680: Pfizer, Phase 1 study to evaluate the safety and pharmacokinetics of palbociclib in combination with irinotecan and temozolomide in pediatric patients with recurrent or refractory solid tumors. In: ClinicalTrials.gov (cited 2019, Jan 29). Reference Source [Google Scholar]
- 81. NCT03387020: Pediatric Brain Tumor Consortium, A Phase I and Surgical Study of Ribociclib and Everolimus (RAD001) in Children With Recurrent or Refractory Malignant Brain Tumors. In: ClinicalTrials.gov (cited 2019, Jan 29), Reference Source [Google Scholar]
- 82. NCT03114527: Fox Chase Cancer Center, Phase II Trial of Ribociclib in Combination With Everolimus in Advanced Dedifferentiated Liposarcoma (DDL) and Leiomyosarcoma (LMS). In: ClinicalTrials.gov (cited 2019, Jan 29). Reference Source [DOI] [PubMed] [Google Scholar]
- 83. NCT02703571: Novartis Pharmaceuticals, A Phase I/II Study of Safety and Efficacy of Ribociclib (LEE011) in Combination With Trametinib (TMT212) in Patients With Metastatic or Advanced Solid Tumors. In: ClinicalTrials.gov (cited 2019,Jan 29). Reference Source [Google Scholar]
- 84. Guenther LM, Dharia NV, Ross L, et al. : A Combination CDK4/6 and IGF1R Inhibitor Strategy for Ewing Sarcoma. Clin Cancer Res. 2019;25(4):1343–57. 10.1158/1078-0432.CCR-18-0372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Brenner JC, Feng FY, Han S, et al. : PARP-1 inhibition as a targeted strategy to treat Ewing's sarcoma. Cancer Res. 2012;72(7):1608–13. 10.1158/0008-5472.CAN-11-3648 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 86. Garnett MJ, Edelman EJ, Heidorn SJ, et al. : Systematic identification of genomic markers of drug sensitivity in cancer cells. Nature. 2012;483(7391):570–5. 10.1038/nature11005 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 87. Vormoor B, Schlosser YT, Blair H, et al. : Sensitizing Ewing sarcoma to chemo- and radiotherapy by inhibition of the DNA-repair enzymes DNA protein kinase (DNA-PK) and poly-ADP-ribose polymerase (PARP) 1/2. Oncotarget. 2017;8(69):113418–30. 10.18632/oncotarget.21300 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 88. Stewart E, Goshorn R, Bradley C, et al. : Targeting the DNA repair pathway in Ewing sarcoma. Cell Rep. 2014;9(3):829–41. 10.1016/j.celrep.2014.09.028 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 89. Smith MA, Reynolds CP, Kang MH, et al. : Synergistic activity of PARP inhibition by talazoparib (BMN 673) with temozolomide in pediatric cancer models in the pediatric preclinical testing program. Clin Cancer Res. 2015;21(4):819–32. 10.1158/1078-0432.CCR-14-2572 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 90. NCT02392793. St. Jude Children's Research Hospital: A Phase I Study of Talazoparib (BMN 673) Plus Irinotecan With or Without Temozolomide in Children With Refractory or Recurrent Solid Malignancies. In: ClinicalTrials.gov (cited 2019, Jan 29). Reference Source [Google Scholar]
- 91. NCT02116777. National Cancer Institute: A Phase 1/2 Study of BMN 673, an Oral Poly(ADP-Ribose) Polymerase Inhibitor, Plus Temozolomide in Children With Refractory or Recurrent Malignancies.In: ClinicalTrials.gov (cited 2019, Jan 29). Reference Source [Google Scholar]
- 92. NCT01858168. Edwin Choy, MD Massachusetts General Hospital: Phase I Study of Olaparib and Temozolomide in Adult Patients With Recurrent/Metastatic Ewing's Sarcoma Following Failure of Prior Chemotherapy.In: ClinicalTrials.gov (cited 2019, Jan 29). Reference Source [Google Scholar]
- 93. NCT02044120. Sarcoma Alliance for Research through Collaboration: ESP1/SARC025 Global Collaboration: A Phase I Study of a Combination of the PARP Inhibitor, Niraparib and Temozolomide and/or Irinotecan in Patients With Previously Treated,Incurable Ewing Sarcoma.In: ClinicalTrials.gov (cited 2019, Jan 29). Reference Source [Google Scholar]
- 94. Federico S, Stewart E, Coleman J, et al. : Phase I study of talazoparib and irinotecan in children and young adults with recurrent/refractory solid tumors. J Clin Oncol. 2017;35(15_suppl):10542 10.1200/JCO.2017.35.15_suppl.10542 [DOI] [Google Scholar]
- 95. Saylors RL, 3rd, Stine KC, Sullivan J, et al. : Cyclophosphamide plus topotecan in children with recurrent or refractory solid tumors: a Pediatric Oncology Group phase II study. J Clin Oncol. 2001;19(15):3463–9. 10.1200/JCO.2001.19.15.3463 [DOI] [PubMed] [Google Scholar]
- 96. Hunold A, Weddeling N, Paulussen M, et al. : Topotecan and cyclophosphamide in patients with refractory or relapsed Ewing tumors. Pediatr Blood Cancer. 2006;47(6):795–800. 10.1002/pbc.20719 [DOI] [PubMed] [Google Scholar]
- 97. Bernstein ML, Devidas M, Lafreniere D, et al. : Intensive therapy with growth factor support for patients with Ewing tumor metastatic at diagnosis: Pediatric Oncology Group/Children's Cancer Group Phase II Study 9457--a report from the Children's Oncology Group. J Clin Oncol. 2006;24(1):152–9. 10.1200/JCO.2005.02.1717 [DOI] [PubMed] [Google Scholar]
- 98. Farhat R, Raad R, Khoury NJ, et al. : Cyclophosphamide and topotecan as first-line salvage therapy in patients with relapsed ewing sarcoma at a single institution. J Pediatr Hematol Oncol. 2013;35(5):356–60. 10.1097/MPH.0b013e318270a343 [DOI] [PubMed] [Google Scholar]
- 99. Kebudi R, Cakir FB, Gorgun O, et al. : A modified protocol with vincristine, topotecan, and cyclophosphamide for recurrent/progressive ewing sarcoma family tumors. Pediatr Hematol Oncol. 2013;30(3):170–7. 10.3109/08880018.2013.767868 [DOI] [PubMed] [Google Scholar]
- 100. Wagner L: Camptothecin-based regimens for treatment of ewing sarcoma: past studies and future directions. Sarcoma. 2011;2011: 957957. 10.1155/2011/957957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Mascarenhas L, Felgenhauer JL, Bond MC, et al. : Pilot Study of Adding Vincristine, Topotecan, and Cyclophosphamide to Interval-Compressed Chemotherapy in Newly Diagnosed Patients With Localized Ewing Sarcoma: A Report From the Children's Oncology Group. Pediatr Blood Cancer. 2016;63(3):493–8. 10.1002/pbc.25837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Casey DA, Wexler LH, Merchant MS, et al. : Irinotecan and temozolomide for Ewing sarcoma: the Memorial Sloan-Kettering experience. Pediatr Blood Cancer. 2009;53(6):1029–34. 10.1002/pbc.22206 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 103. Wagner LM, McAllister N, Goldsby RE, et al. : Temozolomide and intravenous irinotecan for treatment of advanced Ewing sarcoma. Pediatr Blood Cancer. 2007;48(2):132–9. 10.1002/pbc.20697 [DOI] [PubMed] [Google Scholar]
- 104. Wagner LM: Fifteen years of irinotecan therapy for pediatric sarcoma: where to next? Clin Sarcoma Res. 2015;5:20. 10.1186/s13569-015-0035-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Palmerini E, Jones RL, Setola E, et al. : Irinotecan and temozolomide in recurrent Ewing sarcoma: an analysis in 51 adult and pediatric patients. Acta Oncol. 2018;57(7):958–64. 10.1080/0284186X.2018.1449250 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 106. Houghton PJ, Stewart CF, Cheshire PJ, et al. : Antitumor activity of temozolomide combined with irinotecan is partly independent of O6-methylguanine-DNA methyltransferase and mismatch repair phenotypes in xenograft models. Clin Cancer Res. 2000;6(10):4110–8. [PubMed] [Google Scholar]
- 107. Patel VJ, Elion GB, Houghton PJ, et al. : Schedule-dependent activity of temozolomide plus CPT-11 against a human central nervous system tumor-derived xenograft. Clin Cancer Res. 2000;6(10):4154–7. [PubMed] [Google Scholar]
- 108. Furman WL, Crews KR, Billups C, et al. : Cefixime allows greater dose escalation of oral irinotecan: a phase I study in pediatric patients with refractory solid tumors. J Clin Oncol. 2006;24(4):563–70. 10.1200/JCO.2005.03.2847 [DOI] [PubMed] [Google Scholar]
- 109. McGregor LM, Stewart CF, Crews KR, et al. : Dose escalation of intravenous irinotecan using oral cefpodoxime: a phase I study in pediatric patients with refractory solid tumors. Pediatr Blood Cancer. 2012;58(3):372–9. 10.1002/pbc.23075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Newlands ES, Blackledge GR, Slack JA, et al. : Phase I trial of temozolomide (CCRG 81045: M&B 39831: NSC 362856). Br J Cancer. 1992;65(2):287–91. 10.1038/bjc.1992.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Nicholson HS, Krailo M, Ames MM, et al. : Phase I study of temozolomide in children and adolescents with recurrent solid tumors: a report from the Children's Cancer Group. J Clin Oncol. 1998;16(9):3037–43. 10.1200/JCO.1998.16.9.3037 [DOI] [PubMed] [Google Scholar]
- 112. Estlin EJ, Lashford L, Ablett S, et al. : Phase I study of temozolomide in paediatric patients with advanced cancer. United Kingdom Children's Cancer Study Group. Br J Cancer. 1998;78(5):652–61. 10.1038/bjc.1998.555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. DuBois SG, Mosse YP, Fox E, et al. : Phase II Trial of Alisertib in Combination with Irinotecan and Temozolomide for Patients with Relapsed or Refractory Neuroblastoma. Clin Cancer Res. 2018;24(24):6142–9. 10.1158/1078-0432.CCR-18-1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Bagatell R, Norris R, Ingle AM, et al. : Phase 1 trial of temsirolimus in combination with irinotecan and temozolomide in children, adolescents and young adults with relapsed or refractory solid tumors: a Children's Oncology Group Study. Pediatr Blood Cancer. 2014;61(5):833–9. 10.1002/pbc.24874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. NCT03495921. Gradalis, Inc.: A Multi-Center Phase III, Randomized, Open-Label Trial of Vigil (Bi-shRNAfurin and GMCSF Augmented Autologous Tumor Cell Immunotherapy) in Combination With Irinotecan and Temozolomide as a Second-Line Regimen for Ewing's Sarcoma.In: ClinicalTrials.gov (cited 2019, Jan 29). Reference Source [Google Scholar]
- 116. Magnan H, Goodbody CM, Riedel E, et al. : Ifosfamide dose-intensification for patients with metastatic Ewing sarcoma. Pediatr Blood Cancer. 2015;62(4):594–7. 10.1002/pbc.25373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Miser JS, Krailo MD, Tarbell NJ, et al. : Treatment of metastatic Ewing's sarcoma or primitive neuroectodermal tumor of bone: evaluation of combination ifosfamide and etoposide--a Children's Cancer Group and Pediatric Oncology Group study. J Clin Oncol. 2004;22(14):2873–6. 10.1200/JCO.2004.01.041 [DOI] [PubMed] [Google Scholar]
- 118. NCT01864109. Memorial Sloan Kettering Cancer Center: A Phase II Trial of Irinotecan and Temozolomide in Combination With Existing High Dose Alkylator Based Chemotherapy for Treatment of Patients With Newly Diagnosed Ewing Sarcoma.In: ClinicalTrials.gov (cited 2019, Jan 29). Reference Source [Google Scholar]
- 119. Bielack SS, Smeland S, Whelan JS, et al. : Methotrexate, Doxorubicin, and Cisplatin (MAP) Plus Maintenance Pegylated Interferon Alfa-2b Versus MAP Alone in Patients With Resectable High-Grade Osteosarcoma and Good Histologic Response to Preoperative MAP: First Results of the EURAMOS-1 Good Response Randomized Controlled Trial. J Clin Oncol. 2015;33(20):2279–87. 10.1200/JCO.2014.60.0734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Sankar S, Bell R, Stephens B, et al. : Mechanism and relevance of EWS/FLI-mediated transcriptional repression in Ewing sarcoma. Oncogene. 2013;32(47):5089–100. 10.1038/onc.2012.525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Iniguez AB, Stolte B, Wang EJ, et al. : EWS/FLI Confers Tumor Cell Synthetic Lethality to CDK12 Inhibition in Ewing Sarcoma. Cancer Cell. 2018;33(2):202–216.e6. 10.1016/j.ccell.2017.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 122. Harlow ML, Chasse MH, Boguslawski EA, et al. : Trabectedin Inhibits EWS-FLI1 and Evicts SWI/SNF from Chromatin in a Schedule-dependent Manner. Clin Cancer Res. 2019; clincanres.3511.2018. 10.1158/1078-0432.CCR-18-3511 [DOI] [PMC free article] [PubMed] [Google Scholar]