Abstract
Cardiovascular diseases are the most prominent cause of death in Western society, especially in the elderly. With the increasing life expectancy, the number of patients with cardiovascular diseases will rise in the near future, leading to an increased healthcare burden. There is a need for new therapies to treat this growing number of patients. The discovery of long non-coding RNAs has led to a novel group of molecules that could be considered for their potential as therapeutic targets. This review presents an overview of long non-coding RNAs that are regulated in vascular disease and aging and which might therefore give insight into new pathways that could be targeted to diagnose, prevent, and/or treat vascular diseases.
Keywords: aging, vascular disease, lncRNA
1. Introduction
The sequencing of the human genome revealed that the majority of the human genome does not code for proteins [1,2]. The large number of non-protein coding transcripts were assumed to be junk RNA, but nowadays it is recognized that these non-coding RNAs (ncRNAs) have important cellular functions [3]. Non-coding RNAs can be divided into small non-coding RNAs, like microRNAs (miRNA), small nucleolar RNAs, piwi-interacting RNAs, and larger long non-coding RNAs (lncRNAs), which are transcripts larger than 200 nt [3,4]. These lncRNAs are a heterogeneous group of transcripts that can exert different functions in cells dependent on their cytoplasmic or nuclear localization in the cell. In the nucleus, they can act as transcription factors, chromatin modifiers, ribonucleoprotein (RNP) complexes or they can influence splicing. In the cytoplasm, they can influence RNA stability or bind to miRNAs, thereby regulating the mRNA targets of the miRNA [3].
1.1. Age and Cardiovascular Disease
Many lncRNAs are found to be regulated during aging and disease. Discovering the role of age-regulated lncRNAs will become increasingly important as life expectancy is increasing [5]. People aged >85 years have been the most rapidly expanding segment of the population over the past decades [5], and since this group is the most susceptible to disease, this will lead to increasing health costs. In the elderly, cardiovascular diseases are the most prominent cause of death [6]. As most cardiovascular diseases are age-dependent, cellular changes that appear with aging are likely to influence the susceptibility to developing cardiovascular diseases. With aging, the vessel walls stiffen, leading to a reduction of dampening of the pulsatile flow and influencing blood pressure and endothelial activation [7,8]. Development of atherosclerosis is already seen at a young age, but as the body ages, the plaques become bigger and less stable, increasing the risk of thrombus formation and infarction [9]. Although aging normally reduces the amount of angiogenesis [10,11], in diabetic patients there is an increase of angiogenesis in the course of the disease in tissues like the retina due to hyperglycemia-induced macro- and microvascular damage [12]. This increased angiogenesis is, however, unwanted since the capillarization occurs in a disordered fashion by highly-permeable vessels, leading to retinal edema, apoptosis of the surrounding cells, and subsequent hypoperfusion and tissue hypoxia.
1.2. LncRNAs in Vascular Disease
Long non-coding RNAs are able to influence different processes in the vascular system. They can influence vessel development, outgrowth, remodeling, tube formation, and angiogenesis by regulation of cellular processes like proliferation, apoptosis, adhesion, migration, and differentiation of endothelial cells (ECs) and vascular smooth muscle cells (SMCs) [13]. This will affect vascular diseases like atherosclerosis, aneurysms, hyper- or hypotension, retinopathies or diseases with vascular components like diabetes. Inflammation also plays an important role in atherosclerosis, so lncRNAs influencing the inflammatory system may also be important for vascular pathologies [14]. The role of lncRNAs in the different vascular cell types has been reviewed before by Simion et al. [13,15] and other reviews have summarized the role of lncRNAs in atherosclerosis [14,16], angiogenesis [15], aneurysms [17], and diabetes [18,19].
2. LncRNA Function in Vascular Diseases
In this review, we will discuss the role of ten lncRNAs which are implicated in vascular diseases and changed with aging and we arranged them based on their function. These lncRNAs also have functions in other cell types, but for now we will only consider the functions studied in the vasculature and in vitro studies in vascular ECs, perivascular/mural cells, such as fibroblasts, SMCs and pericytes, and cells of the immune system that can affect endothelial activation. Long non-coding RNAs can be divided into different groups based on their cellular location (e.g., cytoplasmic or nuclear), genomic location (e.g., sense, antisense, intronic, inter-genetic), mechanism of functioning (e.g., transcriptional regulation, post-transcriptional regulation or other functions) or mechanism of action. Here, we will divide the lncRNAs into four archetypes based on their mechanism of action [20,21]. One lncRNA can be part of more than one of these groups.
Decoy lncRNA: These are RNP-binding lncRNAs, which inhibit the biological function of the RNP by preventing them from binding to their targets. This group also includes competing endogenous RNAs (ceRNAs) which bind to miRNAs and are often claimed to “sponge” off the miRNA. However, because lncRNAs are expressed at low levels and miRNAs are expressed at high levels, it is not completely clear how a lncRNA sponge would have such a big effect on miRNA levels [3].
Guide lncRNA: These lncRNAs bind to a transcription factor or a chromatin modifier and guide it to the target promoter thereby altering the transcription of the target gene.
Scaffold lncRNA: These lncRNAs bind to several RNPs, bringing them together to form ribonucleoprotein complexes, which in some cases can lead to transcriptional activation or repression.
Enhancer lncRNAs: these lncRNAs are transcribed from enhancer regions and bring together enhancers and promoters in the genome through chromosomal looping to activate expression. We did not find published evidence for enhancer lncRNAs that play a role in vascular aging.
A fifth archetype has also been described, called signal lncRNAs. This group of lncRNAs are transcribed in response to a certain stimulus or stimuli in a cell-type-specific manner by RNA polymerase II, but since all lncRNAs are in the end stimulated in one way or the other and subsequently exert their action via one of the mechanism described beneath, we will not discuss these as a separate group.
2.1. Decoy lncRNAs
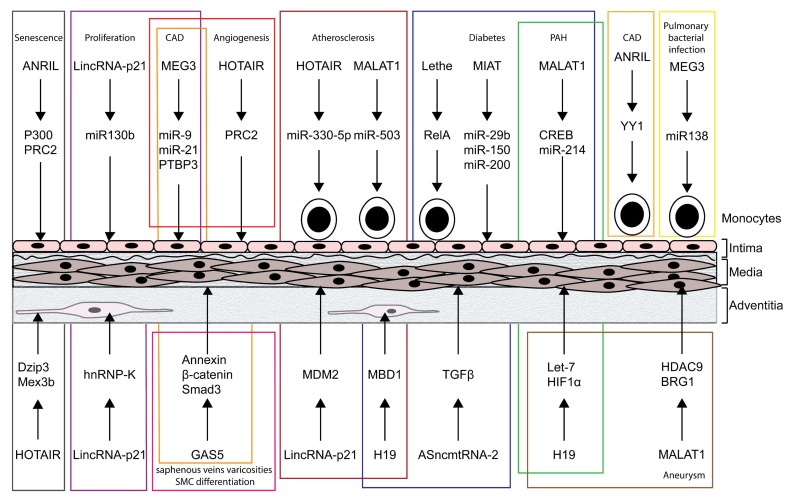
Maternally expressed gene 3 (Meg3) is a nuclear lncRNA implicated in different cardiovascular diseases and found to be upregulated in old human cardiac atria, mouse livers, and senescent human umbilical vein endothelial cells (HUVECs) compared to their young controls [22,23]. Lower Meg3 expression is found in patients with coronary artery disease (CAD) [24], pulmonary arterial hypertension (PAH) [25], and metabolic syndrome [26], but increased expression was found in diabetes type 2 [27] and heart failure patients [28]. One of the molecular mechanisms by which Meg3 can alter cellular functions is by its decoy function. Maternally expressed gene 3 is thought to suppress miR-21 expression in ECs, which is found to be upregulated in CAD patients, and thereby influence RhoB, PTEN, and collagen expression and EC proliferation [24] (Figure 1). The effect of Meg3 on EC proliferation and capillary formation has, however, also been partially attributed to the binding to miR-9 [29]. Hypoxia, an important trigger for angiogenesis, increases the expression of Meg3 [30]. In cardiomyocytes, it has been found that this leads its binding to the protein FUS and increases apoptosis [28], but it has not yet been published if the same mechanism applies to ECs or SMCs. Inflammation has also been found to be influenced by Meg3. The splice variant Meg3–4 is found to bind to the microRNA miR-138, thereby increasing the miR-138 target pro-inflammatory cytokine interleukin 1 beta in macrophages [31].
Figure 1.
Long non-coding RNAs (LncRNAs) associated with vascular disease and age, and exerting a decoy, guide or scaffold function in endothelial cells, vascular smooth muscle cells, fibroblasts and/or immune cells. CAD: coronary artery disease; PAH: pulmonary arterial hypertension.
Another nuclear lncRNA induced by hypoxia is metastasis associated lung adenocarcinoma transcript 1 (MALAT1), also known as NEAT2 [30,32]. The expression of MALAT1 is reduced in replicative senescence [33] and in atherosclerotic plaques [34], while its increased expression is found in diabetes type 2 patients [27]. In addition, genetic variations in the MALAT1 gene are associated with decreased risk for coronary atherosclerotic disease [35] and PAH [36]. Not only in humans, but also in diabetic mice and rats and stressed retinal cells, MALAT1 expression is increased [37,38]. The lncRNA MALAT1 was found to bind to CREB, thereby inhibiting its dephosphorylation by PP2A and the resulting continuous CREB signaling leads to abnormal cell viability and hyperproliferation. In atherosclerotic mice, MALAT1 seems to target miR-503, leading to an increase in FGF2 in monocytes [34]. In HUVECs, the MALAT1 variant that showed a reduced PAH risk was found to interact with miR-214, thereby increasing the expression of X box-binding protein 1, and consequently MALAT1 could inhibit EC proliferation and migration [36].
The lncRNA myocardial infarction associated transcript (MIAT), also called Gomafu, is downregulated in senescent fibroblasts compared to early passage proliferation fibroblasts [39]. Like MALAT1, MIAT is also upregulated in the retinas and peripheral blood mononuclear cells of diabetes patients [27,40], and is furthermore upregulated in peripheral blood leukocytes of ischemic stroke patients [41]. In addition, MIAT is found to be increased in a mouse diabetes model and in vitro after high glucose treatment [40,42], which is at least partially the effect of increased binding of the protein complex NF-κB to the MIAT promotor. Overexpression of MIAT suppresses miR-29b which leads to decreased cell viability and increased apoptosis. Knockdown of MIAT reverses the high glucose-induced apoptosis and decreased cell activity [42]. Furthermore, MIAT is also a decoy for miR-150 and miR-200a, thereby reducing the repression of VEGF by these miRNAs [40,43]. The increased MIAT expression in diabetic patients could therefore be (partially) responsible for the increase in VEGF and pathological angiogenesis.
The lncRNA H19 is a cytoplasmic lncRNA [44,45] which is highly expressed in the embryonic stage, but its expression decreases shortly after birth [46,47,48]. However, in some vascular diseases, the expression of H19 increases again. Increased expression has been shown in aortic aneurysms [49], atherosclerotic lesions [48], in calcific aortic valves [50], and in plasma levels of CAD and ischemic stroke patients [51,52]. On the contrary, decreased levels in ECs of carotid atherosclerotic plaques have been found by Hofmann et al. [45]. Genetic variation in H19 has also been described to lead to an increased risk for CAD [53] and ischemic stroke [54]. In the cardiovascular system, the binding of H19 to the let-7 family seems to be important [55,56]. In PAH, the H19/let-7 interaction was found to lead to an increase in angiotensin receptor AT1R levels and increase in proliferation. A knockdown of H19 in mice could reduce the pulmonary artery remodeling in the PAH model [56]. Moreover, H19 was also suggested to bind to HIF1α, thereby retaining HIF1α in the cytoplasm, and in addition, H19 facilitated the binding of transcription factor Sp1 to the HIF1α promotor, increasing HIF1α expression [49]. Subsequently, HIF1α binds to mouse double minute 2 homolog (MDM2), thereby reducing MDM2 binding to tumor protein p53which would normally lead to p53 degradation, thereby leading to a decrease in SMC proliferation and an increase in apoptosis and aneurysm progression.
Another lncRNA that is inhibited after transition from embryonic to adult stage is lincRNA-p21 [57]. Also genetic variation in lincRNA-p21 has shown to influence CAD and myocardial infarction [58], and levels are decreased in coronary tissue from CAD patients [59] and in atherosclerotic plaques [60], but increased in thoracic aorta aneurysm [61] and diabetes type 2 patients [27]. The nuclear lncRNA lincRNA-p21 is regulated by p53 [62]. In SMCs and macrophages lincRNA-p21 interacts with MDM2, which would otherwise bind to p53 and facilitate p53 degradation [59]. Silencing of lincRNA-p21 in SMCs or mouse carotid increases the binding of MDM2 to p53, but decreases the binding between p300 and p53, leading to reduced transcription of p53 target genes, such as PUMA and Bax, and thereby reducing apoptosis and increasing intima-media thickness. In mouse ECs, lincRNA-p21 is thought to act as a ceRNA for miR-130b, thereby inhibiting proliferation [63].
The lncRNA HOX Antisense Intergenic RNA (HOTAIR) is an antisense lncRNA flanked by HOXC11 and HOXC12. It is expressed both in the cytoplasm and the nucleus and found to be upregulated in senescent fibroblasts [64,65]. Silencing of HOTAIR reduced the levels of senescent markers and lowered β-galactosidase activity. The expression of HOTAIR is decreased in sporadic thoracic aortic aneurysm tissue [66], ECs for atherosclerotic plaques [67], and in the peripheral blood lymphocytes of atherosclerosis patients [68], but increased in patients with diabetes [27] and congenital heart disease [69]. In atheromatous plaques hypermethylation of CpG sites in the HOTAIR gene was found [70]. As inflammation is an important aspect of atherosclerosis, the function of HOTAIR has been studied in monocyte/macrophage cell lines. In a human monocytic cell line HOTAIR expression was found to be increased upon ox-LDL treatment and silencing of HOTAIR reduced cholesterol levels, reactive oxygen species (ROS) production and pro-inflammatory cytokines through the interaction with miR-330-5p [71]. However, ox-LDL has shown to decrease HOTAIR expression in a mouse macrophage cell line and overexpression of HOTAIR led to increases in anti-inflammatory and adipose gene expression and a decrease in pro-inflammatory and lipogenesis gene expression [68]. In this cell line HOTAIR increased the expression of the RNA-binding protein FXR1 and thereby reduced inflammatory response by inhibition of the NF-κB pathway. It is not investigated if this process involved a decoy function of HOTAIR. However, since both the human and mouse cell line are immortalized, they might have an altered cellular phenotype that could influence the acquired results and might not resemble the in vivo situation.
Compared to most lncRNAs mentioned above, there is relatively little known about the lncRNA Lethe. Long non-coding RNA Lethe is a pseudogene of Rps15a and is found to be downregulated with age in mice [72]. It is found to be decreased in the wounds of diabetic mice [73] and is regulated by glucose pro-inflammatory cytokines via NF-κB. Lethe binds to the NF-κB subunit RelA and thereby represses NF-κB binding to chromatin, creating a negative feedback loop and reducing ROS production [72,73].
Growth arrest specific 5 (GAS5) was found to be upregulated with age in the mouse hippocampus [74], but downregulated with age in the rest of the brain and in mouse ovaries [75]. It is unknown what the effect of aging is on GAS5 levels in the cardiovascular system. Polymorphisms of GAS5 have been associated with atherosclerosis risk [76] and the expression is increased in rat and patient atherosclerotic plaques [77]. In patients with hypertension, type 2 diabetes, CAD, and primary varicose great saphenous veins, the expression was, however, decreased [27,78,79,80]. Knockdown of GAS5 in hypertensive rats aggravates the hypertension phenotype, including further increases in blood pressure, vascular remodeling, formation of retinal angiogenesis, and vascular leakage [78]. These effects are thought to be the result of an interaction between GAS5 and β-catenin, which decreases β-catenin nuclear translocation, and thereby the expression of its target genes. Correspondingly, in rats with CAD an abnormally activated Wnt/β-catenin signaling pathway was found and could be decreased by GAS5 overexpression [80]. In vein SMCs, GAS5 was found to bind to Annexin A2, influencing proliferation and thereby possibly the pathogenesis of great saphenous veins varicosities [79]. In addition, GAS5 has also been shown to bind to Smad3 in SMCs, which reduces the binding of Smad3 to gene promoters normally activated by TGF-beta signaling, which results in the inhibition of TGF-beta/Smad3-mediated differentiation of SMCs [81].
2.2. Guide lncRNAs
Besides acting as a decoy lncRNA, Meg3 can also function as a guide lncRNA. The lncRNA Meg3 binds to both PTBP1 and PTBP3 [82,83]. The RNA-binding protein PTBP3 has been shown to bind to the promoters of p53 targets in HUVECs and knockdown influences the transcription of these targets. The EC function, like proliferation and apoptosis, is compromised by knockdown of either Meg3 or PTBP3, an effect which is not seen after PTBP1 knockdown, suggesting an important role of the Meg3–PTBP3 binding in ECs. An effect of Meg3 on p53 target signaling has also been shown in pulmonary SMCs [25] and hepatoma cells [84]. Besides Meg3, lincRNA-p21 also affects p53 target expression. The binding of lincRNA-p21 to hnRNP-K in MEFs leads to promotor binding to and repression of cell death-repressing genes which are part of the p53 transcriptional response [62].
The lncRNA MALAT1 also has a guide function in addition to its decoy function. It forms a complex with HDAC9 and BRG1, which binds to PRC2 and thereby increases H3K27me3 marks on contractile protein gene promotors and represses their expression [85]. In SMCs of aortas from an aneurysm mouse model, increased expression and colocalization of HDAC9, BRG1, and MALAT1 was found and deletion of MALAT1 or HDAC9 restored the contractile protein expression and inhibited aneurysm growth. In addition, HOTAIR also interacts with PRC2, thereby inducing H3K27-methylation which results in suppression in gene expression, for example of HOXD [86,87,88]. Since HOXD genes are found to affect angiogenesis [89,90], this transcriptional regulation might be important for the effect of HOTAIR on vascular diseases. On the other hand, H19 induces H3K9me3 modifications by interacting with MBD1 [91]. This leads to transcriptional control over several genes, including IGF2 which is implicated in diabetes and atherosclerosis [92,93].
A lncRNA that has no decoy function, but functions as a guide lncRNA is antisense non-coding RNA in the INK4 locus (ANRIL). The expression of ANRIL increases with age in rodents [87], but decreases in expression in later passages of some cell lines [94,95]. Genetic susceptibility of the ANRIL locus has been associated with CAD [96,97], but also with atherosclerosis [98], intracranial aneurysms [99], and type 2 diabetes [100]. In fibroblasts, ANRIL has shown to bind to the PRC2 complex, thereby influencing binding of PRC2 to the p15INK4B locus [101]. Loss of ANRIL reduces PRC2 binding to the p15INK4B locus and increases its expression, thereby inducing cellular senescence. The binding to PRC2 and in addition p300 is also thought to increase VEGF expression, which subsequently influences tube formation of retinal ECs in vitro and retinal microvascular permeability in vivo [102]. Furthermore, ANRIL seems to bind to the transcription factor YY1 after TNFα induction, thereby inducing IL6 and IL8 transcription [103]. Since a correlation between ANRIL levels and IL6 and IL8 levels was found in CAD patients and elevated IL8 levels are associated with CAD risk [104], ANRIL may be a potential therapeutic target for CAD.
2.3. Scaffold lncRNAs
Scaffold lncRNAs bind to several RNPs, bringing them together to form ribonucleoprotein complexes. The lncRNA HOTAIR brings together PRC2 with the LSD1/CoREST/REST complex increasing H3K27me3 and decreasing H3K4me2 [87]. Two of the target genes shown to be affected by HOTAIR knockdown, SIRT2 and LRP1B, have been implicated in atherosclerosis [105,106]. The scaffolding of LSD1 might also affect aneurysm formation, as LSD1 has also been reported to affect collagen I expression in osteoblasts [107] and silencing of HOTAIR in HASMCs has been shown to suppress collagen I expression and leads to weakening off the aortic wall [66]. In addition, HOTAIR also acts as a scaffold between E3 ubiquitin ligases Dzip3 and Mex3b and their corresponding substrates Ataxin-1 and Snurportin which might affect senescence [64].
The binding of ANRIL to PRC1 and PRC2 has not only been described as two separate guiding mechanisms, but scaffold action binding of both complexes has also been proposed [20,95,101].
2.4. Other lncRNAs Regulated in Vascular Diseases and Aging
A lncRNA which is induced by aging in mouse aorta and old passage HUVECs [108], but with an unknown functional mechanism is the antisense noncoding mitochondrial RNA ASncmtRNA-2. This lncRNA is transcribed from mitochondrial DNA [109] and is upregulated in diabetic mouse kidneys and in vitro after high-glucose-induced ROS production [110]. Knockdown of ASncmtRNA-2 decreases the expression of the pro-fibrotic factors TGFβ1 and fibronectin, concluding that ASncmtRNA-2 might be important in diabetic glomerular fibrosis. Since TGFβ1 and fibronectin are known to be regulated in aneurysms and ASncmtRNA-2 is expressed in aortas [111], it might be interesting to test if ASncmtRNA-2 plays a role in aneurysm formation.
3. LncRNAs as Biomarkers
Although differential plasma levels of several lncRNAs have been found in patients with vascular diseases, it is still unclear if these lncRNAs are suitable as specific biomarkers, since they are often also implicated in other diseases. For example, increased H19 levels in plasma are found in patients with CAD and it was an independent predictor even after adjustment for known cardiovascular risk factors [51]. However, H19 was also suggested as a biomarker for breast cancer [112] and gastric cancer [113] after discovering increased H19 levels in plasma in these patients. Likewise, GAS5 levels in plasma are found to be decreased in diabetes type 2 patients and are even further decreased in CAD patients [114], but GAS5 levels in plasma are also decreased in non-small cell lung cancer patients [115].
Upregulated levels of lincRNA-p21 in serum could be used to distinguish thoracic aortic aneurysm patients from healthy people [61]. Reduced levels are on the other hand found in CAD patients [54], so lincRNA-p21 has been suggested as a potential biomarker atherosclerosis. However, decreased levels are also found in hepatitis B positive liver fibrosis patients [116] and in colorectal cancer patients [117].
The lncRNA ANRIL could act as a prognostic factor for a more specific group of patients, since higher levels in plasma are associated with in-stent restenosis compared to patients with stent without stenosis [113]. Care should, however, be taken in patients with a higher risk for breast cancer, since increased levels of ANRIL in plasma are also found in patients with breast cancer [118]. Single-nucleotide polymorphisms in ANRIL can also be used as a biomarker, since rs10757274 and rs2383206 are associated with an increased risk of CAD [119,120,121].
4. Conclusions
In summary, we have discussed several lncRNAs which are differentially regulated with aging and are implicated in vascular disease. As aging is an important risk factor for cardiovascular diseases, the lncRNAs implicated in both might be the most promising therapeutic targets. The mechanisms by which these lncRNA influence vascular pathologies are starting to be unraveled, but further research still needs to be done. With a deeper understanding of the mechanisms by which lncRNAs act, novel and effective targets might be identified for the development of therapeutic strategies for vascular diseases. This process may be hampered by the lack of sequence conservation in other species and be delayed because lncRNAs often have multiple transcript variants, which do not always have the same function. An additional limitation might be that some annotated lncRNAs are later on found to encode a short peptide which was overlooked in the past.
Abbreviations
| ANRIL | antisense non-coding RNA in the INK4 locus |
| CAD | coronary artery disease |
| ceRNAs | competing endogenous RNAs |
| ECs | endothelial cells |
| GAS5 | Growth Arrest Specific 5 |
| HOTAIR | HOX Antisense Intergenic RNA |
| HUVECs | human umbilical vein endothelial cells |
| lncRNA | long non-coding RNA |
| MALAT1 | Metastasis Associated Lung Adenocarcinoma Transcript 1 |
| MDM2 | Mouse double minute 2 homolog |
| Meg3 | Maternally expressed gene 3 |
| MIAT | Myocardial infarction associated transcript |
| miRNA | microRNA |
| ncRNA | non-coding RNA |
| PAH | pulmonary arterial hypertension |
| RNP | ribonucleoprotein |
| ROS | reactive oxygen species |
| SMCs | smooth muscle cells. |
Author Contributions
D.I.B., N.L.-V., R.A.B. designed and wrote the review.
Funding
This research was funded by the Deutsche Forschungsgemeinschaft (SFB834), the European Research Council (“NOVA”), the DZHK (German Centre for Cardiovascular Research), and The Netherlands Organisation for Scientific Research (NWO Vidi).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Venter J.C., Adams M.D., Myers E.W., Li P.W., Mural R.J., Sutton G.G., Smith H.O., Yandell M., Evans C.A., Holt R.A., et al. The sequence of the human genome. Science. 2001;291:1304–1351. doi: 10.1126/science.1058040. [DOI] [PubMed] [Google Scholar]
- 2.Okazaki Y., Furuno M., Kasukawa T., Adachi J., Bono H., Kondo S., Nikaido I., Osato N., Saito R., Suzuki H., et al. Analysis of the mouse transcriptome based on functional annotation of 60,770 full-length cdnas. Nature. 2002;420:563–573. doi: 10.1038/nature01266. [DOI] [PubMed] [Google Scholar]
- 3.Boon R.A., Jae N., Holdt L., Dimmeler S. Long noncoding rnas: From clinical genetics to therapeutic targets? J. Am. Coll. Cardiol. 2016;67:1214–1226. doi: 10.1016/j.jacc.2015.12.051. [DOI] [PubMed] [Google Scholar]
- 4.Kapranov P., Cheng J., Dike S., Nix D.A., Duttagupta R., Willingham A.T., Stadler P.F., Hertel J., Hackermuller J., Hofacker I.L., et al. Rna maps reveal new rna classes and a possible function for pervasive transcription. Science. 2007;316:1484–1488. doi: 10.1126/science.1138341. [DOI] [PubMed] [Google Scholar]
- 5.Christensen K., Doblhammer G., Rau R., Vaupel J.W. Ageing populations: The challenges ahead. Lancet. 2009;374:1196–1208. doi: 10.1016/S0140-6736(09)61460-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lozano R., Naghavi M., Foreman K., Lim S., Shibuya K., Aboyans V., Abraham J., Adair T., Aggarwal R., Ahn S.Y., et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the global burden of disease study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zarrinkoob L., Ambarki K., Wahlin A., Birgander R., Carlberg B., Eklund A., Malm J. Aging alters the dampening of pulsatile blood flow in cerebral arteries. J. Cereb. Blood Flow Metab. 2016;36:1519–1527. doi: 10.1177/0271678X16629486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steppan J., Barodka V., Berkowitz D.E., Nyhan D. Vascular stiffness and increased pulse pressure in the aging cardiovascular system. Cardiol. Res. Pract. 2011;2011:263585. doi: 10.4061/2011/263585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hong Y.M. Atherosclerotic cardiovascular disease beginning in childhood. Korean Circ. J. 2010;40:1–9. doi: 10.4070/kcj.2010.40.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakae I., Fujita M., Miwa K., Hasegawa K., Kihara Y., Nohara R., Miyamoto S., Ueda K., Tamaki S., Sasayama S. Age-dependent impairment of coronary collateral development in humans. Heart Vessels. 2000;15:176–180. doi: 10.1007/PL00007269. [DOI] [PubMed] [Google Scholar]
- 11.Rivard A., Fabre J.E., Silver M., Chen D., Murohara T., Kearney M., Magner M., Asahara T., Isner J.M. Age-dependent impairment of angiogenesis. Circulation. 1999;99:111–120. doi: 10.1161/01.CIR.99.1.111. [DOI] [PubMed] [Google Scholar]
- 12.Madonna R., Balistreri C.R., Geng Y.J., De Caterina R. Diabetic microangiopathy: Pathogenetic insights and novel therapeutic approaches. Vasc. Pharmacol. 2017;90:1–7. doi: 10.1016/j.vph.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 13.Simion V., Haemmig S., Feinberg M.W. Lncrnas in vascular biology and disease. Vasc. Pharmacol. 2018 doi: 10.1016/j.vph.2018.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li H., Zhu H., Ge J. Long noncoding rna: Recent updates in atherosclerosis. Int. J. Biol. Sci. 2016;12:898–910. doi: 10.7150/ijbs.14430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu B., Wang S. Angio-lncrs: Lncrnas that regulate angiogenesis and vascular disease. Theranostics. 2018;8:3654–3675. doi: 10.7150/thno.26024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou T., Ding J.W., Wang X.A., Zheng X.X. Long noncoding rnas and atherosclerosis. Atherosclerosis. 2016;248:51–61. doi: 10.1016/j.atherosclerosis.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 17.Duggirala A., Delogu F., Angelini T.G., Smith T., Caputo M., Rajakaruna C., Emanueli C. Non coding rnas in aortic aneurysmal disease. Front. Genet. 2015;6:125. doi: 10.3389/fgene.2015.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leung A., Amaram V., Natarajan R. Linking diabetic vascular complications with lncrnas. Vasc. Pharmacol. 2018 doi: 10.1016/j.vph.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He X., Ou C., Xiao Y., Han Q., Li H., Zhou S. Lncrnas: Key players and novel insights into diabetes mellitus. Oncotarget. 2017;8:71325–71341. doi: 10.18632/oncotarget.19921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang K.C., Chang H.Y. Molecular mechanisms of long noncoding rnas. Mol. Cell. 2011;43:904–914. doi: 10.1016/j.molcel.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Devaux Y., Zangrando J., Schroen B., Creemers E.E., Pedrazzini T., Chang C.P., Dorn G.W., 2nd, Thum T., Heymans S., Cardiolinc n. Long noncoding rnas in cardiac development and ageing. Nat. Rev. Cardiol. 2015;12:415–425. doi: 10.1038/nrcardio.2015.55. [DOI] [PubMed] [Google Scholar]
- 22.Boon R.A., Hofmann P., Michalik K.M., Lozano-Vidal N., Berghauser D., Fischer A., Knau A., Jae N., Schurmann C., Dimmeler S. Long noncoding rna meg3 controls endothelial cell aging and function: Implications for regenerative angiogenesis. J. Am. Coll. Cardiol. 2016;68:2589–2591. doi: 10.1016/j.jacc.2016.09.949. [DOI] [PubMed] [Google Scholar]
- 23.White R.R., Milholland B., MacRae S.L., Lin M., Zheng D., Vijg J. Comprehensive transcriptional landscape of aging mouse liver. BMC Genom. 2015;16:899. doi: 10.1186/s12864-015-2061-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu Z., He Y., Li D., Fang X., Shang T., Zhang H., Zheng X. Long noncoding rna meg3 suppressed endothelial cell proliferation and migration through regulating mir-21. Am. J. Transl. Res. 2017;9:3326–3335. [PMC free article] [PubMed] [Google Scholar]
- 25.Sun Z., Nie X., Sun S., Dong S., Yuan C., Li Y., Xiao B., Jie D., Liu Y. Long non-coding rna meg3 downregulation triggers human pulmonary artery smooth muscle cell proliferation and migration via the p53 signaling pathway. Cell. Physiol. Biochem. 2017;42:2569–2581. doi: 10.1159/000480218. [DOI] [PubMed] [Google Scholar]
- 26.Liu H.Z., Wang Q.Y., Zhang Y., Qi D.T., Li M.W., Guo W.Q., Ma Y.H., Wang L.Y., Chen Y., Gao C.Y. Pioglitazone up-regulates long non-coding rna meg3 to protect endothelial progenitor cells via increasing hdac7 expression in metabolic syndrome. Biomed. Pharmacother. 2016;78:101–109. doi: 10.1016/j.biopha.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 27.Sathishkumar C., Prabu P., Mohan V., Balasubramanyam M. Linking a role of lncrnas (long non-coding rnas) with insulin resistance, accelerated senescence, and inflammation in patients with type 2 diabetes. Hum. Genom. 2018;12:41. doi: 10.1186/s40246-018-0173-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu H., Zhao Z.A., Liu J., Hao K., Yu Y., Han X., Li J., Wang Y., Lei W., Dong N., et al. Long noncoding rna meg3 regulates cardiomyocyte apoptosis in myocardial infarction. Gene Ther. 2018;25:511–523. doi: 10.1038/s41434-018-0045-4. [DOI] [PubMed] [Google Scholar]
- 29.He C., Yang W., Yang J., Ding J., Li S., Wu H., Zhou F., Jiang Y., Teng L., Yang J. Long noncoding rna meg3 negatively regulates proliferation and angiogenesis in vascular endothelial cells. DNA Cell Biol. 2017;36:475–481. doi: 10.1089/dna.2017.3682. [DOI] [PubMed] [Google Scholar]
- 30.Michalik K.M., You X., Manavski Y., Doddaballapur A., Zornig M., Braun T., John D., Ponomareva Y., Chen W., Uchida S., et al. Long noncoding rna malat1 regulates endothelial cell function and vessel growth. Circ. Res. 2014;114:1389–1397. doi: 10.1161/CIRCRESAHA.114.303265. [DOI] [PubMed] [Google Scholar]
- 31.Li R., Fang L., Pu Q., Bu H., Zhu P., Chen Z., Yu M., Li X., Weiland T., Bansal A., et al. Meg3-4 is a mirna decoy that regulates il-1beta abundance to initiate and then limit inflammation to prevent sepsis during lung infection. Sci. Signal. 2018;11 doi: 10.1126/scisignal.aao2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Voellenkle C., Garcia-Manteiga J.M., Pedrotti S., Perfetti A., De Toma I., Da Silva D., Maimone B., Greco S., Fasanaro P., Creo P., et al. Implication of long noncoding rnas in the endothelial cell response to hypoxia revealed by rna-sequencing. Sci. Rep. 2016;6:24141. doi: 10.1038/srep24141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tripathi V., Shen Z., Chakraborty A., Giri S., Freier S.M., Wu X., Zhang Y., Gorospe M., Prasanth S.G., Lal A., et al. Long noncoding rna malat1 controls cell cycle progression by regulating the expression of oncogenic transcription factor b-myb. PLoS Genet. 2013;9:e1003368. doi: 10.1371/journal.pgen.1003368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cremer S., Michalik K.M., Fischer A., Pfisterer L., Jae N., Winter C., Boon R.A., Muhly-Reinholz M., John D., Uchida S., et al. Hematopoietic deficiency of the long non-coding rna malat1 promotes atherosclerosis and plaque inflammation. Circulation. 2018;139:1320–1334. doi: 10.1161/CIRCULATIONAHA.117.029015. [DOI] [PubMed] [Google Scholar]
- 35.Wang G., Li Y., Peng Y., Tang J., Li H. Association of polymorphisms in malat1 with risk of coronary atherosclerotic heart disease in a Chinese population. Lipids Health Dis. 2018;17:75. doi: 10.1186/s12944-018-0728-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhuo Y., Zeng Q., Zhang P., Li G., Xie Q., Cheng Y. Functional polymorphism of lncrna malat1 contributes to pulmonary arterial hypertension susceptibility in Chinese people. Clin. Chem. Lab. Med. 2017;55:38–46. doi: 10.1515/cclm-2016-0056. [DOI] [PubMed] [Google Scholar]
- 37.Yao J., Wang X.Q., Li Y.J., Shan K., Yang H., Wang Y.N., Yao M.D., Liu C., Li X.M., Shen Y., et al. Long non-coding rna malat1 regulates retinal neurodegeneration through creb signaling. EMBO Mol. Med. 2016;8:346–362. doi: 10.15252/emmm.201505725. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38.Liu J.Y., Yao J., Li X.M., Song Y.C., Wang X.Q., Li Y.J., Yan B., Jiang Q. Pathogenic role of lncrna-malat1 in endothelial cell dysfunction in diabetes mellitus. Cell Death Dis. 2014;5:e1506. doi: 10.1038/cddis.2014.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abdelmohsen K., Panda A., Kang M.J., Xu J., Selimyan R., Yoon J.H., Martindale J.L., De S., Wood W.H., 3rd, Becker K.G., et al. Senescence-associated lncrnas: Senescence-associated long noncoding rnas. Aging Cell. 2013;12:890–900. doi: 10.1111/acel.12115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yan B., Yao J., Liu J.Y., Li X.M., Wang X.Q., Li Y.J., Tao Z.F., Song Y.C., Chen Q., Jiang Q. Lncrna-miat regulates microvascular dysfunction by functioning as a competing endogenous rna. Circ. Res. 2015;116:1143–1156. doi: 10.1161/CIRCRESAHA.116.305510. [DOI] [PubMed] [Google Scholar]
- 41.Zhu M., Li N., Luo P., Jing W., Wen X., Liang C., Tu J. Peripheral blood leukocyte expression of lncrna miat and its diagnostic and prognostic value in ischemic stroke. J. Stroke Cerebrovasc. Dis. 2018;27:326–337. doi: 10.1016/j.jstrokecerebrovasdis.2017.09.009. [DOI] [PubMed] [Google Scholar]
- 42.Zhang J., Chen M., Chen J., Lin S., Cai D., Chen C., Chen Z. Long non-coding rna miat acts as a biomarker in diabetic retinopathy by absorbing mir-29b and regulating cell apoptosis. Biosci. Rep. 2017;37 doi: 10.1042/BSR20170036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang H., Ding X.G., Yang J.J., Li S.W., Zheng H., Gu C.H., Jia Z.K., Li L. Lncrna miat facilitated bm-mscs differentiation into endothelial cells and restored erectile dysfunction via targeting mir-200a in a rat model of erectile dysfunction. Eur. J. Cell Biol. 2018;97:180–189. doi: 10.1016/j.ejcb.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 44.Brannan C.I., Dees E.C., Ingram R.S., Tilghman S.M. The product of the h19 gene may function as an rna. Mol. Cell. Biol. 1990;10:28–36. doi: 10.1128/MCB.10.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hofmann P., Sommer J., Theodorou K., Kirchhof L., Fischer A., Li Y., Perisic L., Hedin U., Maegdefessel L., Dimmeler S., et al. Long non-coding rna h19 regulates endothelial cell aging via inhibition of stat3 signaling. Cardiovasc. Res. 2018;115:230–242. doi: 10.1093/cvr/cvy206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Poirier F., Chan C.T., Timmons P.M., Robertson E.J., Evans M.J., Rigby P.W. The murine h19 gene is activated during embryonic stem cell differentiation in vitro and at the time of implantation in the developing embryo. Development. 1991;113:1105–1114. doi: 10.1242/dev.113.4.1105. [DOI] [PubMed] [Google Scholar]
- 47.Kim D.K., Zhang L., Dzau V.J., Pratt R.E. H19, a developmentally regulated gene, is reexpressed in rat vascular smooth muscle cells after injury. J. Clin. Investig. 1994;93:355–360. doi: 10.1172/JCI116967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Han D.K., Khaing Z.Z., Pollock R.A., Haudenschild C.C., Liau G. H19, a marker of developmental transition, is reexpressed in human atherosclerotic plaques and is regulated by the insulin family of growth factors in cultured rabbit smooth muscle cells. J. Clin. Investig. 1996;97:1276–1285. doi: 10.1172/JCI118543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li D.Y., Busch A., Jin H., Chernogubova E., Pelisek J., Karlsson J., Sennblad B., Liu S., Lao S., Hofmann P., et al. H19 induces abdominal aortic aneurysm development and progression. Circulation. 2018;138:1551–1568. doi: 10.1161/CIRCULATIONAHA.117.032184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hadji F., Boulanger M.C., Guay S.P., Gaudreault N., Amellah S., Mkannez G., Bouchareb R., Marchand J.T., Nsaibia M.J., Guauque-Olarte S., et al. Altered DNA methylation of long noncoding rna h19 in calcific aortic valve disease promotes mineralization by silencing notch1. Circulation. 2016;134:1848–1862. doi: 10.1161/CIRCULATIONAHA.116.023116. [DOI] [PubMed] [Google Scholar]
- 51.Zhang Z., Gao W., Long Q.Q., Zhang J., Li Y.F., Liu D.C., Yan J.J., Yang Z.J., Wang L.S. Increased plasma levels of lncrna h19 and lipcar are associated with increased risk of coronary artery disease in a chinese population. Sci. Rep. 2017;7:7491. doi: 10.1038/s41598-017-07611-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang J., Zhao H., Fan Z., Li G., Ma Q., Tao Z., Wang R., Feng J., Luo Y. Long noncoding rna h19 promotes neuroinflammation in ischemic stroke by driving histone deacetylase 1-dependent m1 microglial polarization. Stroke. 2017;48:2211–2221. doi: 10.1161/STROKEAHA.117.017387. [DOI] [PubMed] [Google Scholar]
- 53.Gao W., Zhu M., Wang H., Zhao S., Zhao D., Yang Y., Wang Z.M., Wang F., Yang Z.J., Lu X., et al. Association of polymorphisms in long non-coding rna h19 with coronary artery disease risk in a Chinese population. Mutat. Res. 2015;772:15–22. doi: 10.1016/j.mrfmmm.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 54.Wang J., Cao B., Han D., Sun M., Feng J. Long non-coding rna h19 induces cerebral ischemia reperfusion injury via activation of autophagy. Aging Dis. 2017;8:71–84. doi: 10.14336/AD.2016.0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kallen A.N., Zhou X.B., Xu J., Qiao C., Ma J., Yan L., Lu L., Liu C., Yi J.S., Zhang H., et al. The imprinted h19 lncrna antagonizes let-7 micrornas. Mol. Cell. 2013;52:101–112. doi: 10.1016/j.molcel.2013.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Su H., Xu X., Yan C., Shi Y., Hu Y., Dong L., Ying S., Ying K., Zhang R. Lncrna h19 promotes the proliferation of pulmonary artery smooth muscle cells through at1r via sponging let-7b in monocrotaline-induced pulmonary arterial hypertension. Respir. Res. 2018;19:254. doi: 10.1186/s12931-018-0956-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Groff A.F., Sanchez-Gomez D.B., Soruco M.M.L., Gerhardinger C., Barutcu A.R., Li E., Elcavage L., Plana O., Sanchez L.V., Lee J.C., et al. In vivo characterization of linc-p21 reveals functional cis-regulatory DNA elements. Cell Rep. 2016;16:2178–2186. doi: 10.1016/j.celrep.2016.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tang S.S., Cheng J., Cai M.Y., Yang X.L., Liu X.G., Zheng B.Y., Xiong X.D. Association of lincrna-p21 haplotype with coronary artery disease in a chinese han population. Dis. Mark. 2016;2016:9109743. doi: 10.1155/2016/9109743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu G., Cai J., Han Y., Chen J., Huang Z.P., Chen C., Cai Y., Huang H., Yang Y., Liu Y., et al. Lincrna-p21 regulates neointima formation, vascular smooth muscle cell proliferation, apoptosis, and atherosclerosis by enhancing p53 activity. Circulation. 2014;130:1452–1465. doi: 10.1161/CIRCULATIONAHA.114.011675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cekin N., Ozcan A., Goksel S., Arslan S., Pinarbasi E., Berkan O. Decreased fendrr and lincrna-p21 expression in atherosclerotic plaque. Anatol. J. Cardiol. 2018;19:131–136. doi: 10.14744/AnatolJCardiol.2017.8081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hu W., Wang Z., Li Q., Wang J., Li L., Jiang G. Upregulation of lincrna-p21 in thoracic aortic aneurysms is involved in the regulation of proliferation and apoptosis of vascular smooth muscle cells by activating tgf-beta1 signaling pathway. J. Cell. Biochem. 2018;120:4113–4120. doi: 10.1002/jcb.27696. [DOI] [PubMed] [Google Scholar]
- 62.Huarte M., Guttman M., Feldser D., Garber M., Koziol M.J., Kenzelmann-Broz D., Khalil A.M., Zuk O., Amit I., Rabani M., et al. A large intergenic noncoding rna induced by p53 mediates global gene repression in the p53 response. Cell. 2010;142:409–419. doi: 10.1016/j.cell.2010.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.He C., Ding J.W., Li S., Wu H., Jiang Y.R., Yang W., Teng L., Yang J., Yang J. The role of long intergenic noncoding rna p21 in vascular endothelial cells. DNA Cell Biol. 2015;34:677–683. doi: 10.1089/dna.2015.2966. [DOI] [PubMed] [Google Scholar]
- 64.Yoon J.H., Abdelmohsen K., Kim J., Yang X., Martindale J.L., Tominaga-Yamanaka K., White E.J., Orjalo A.V., Rinn J.L., Kreft S.G., et al. Scaffold function of long non-coding rna hotair in protein ubiquitination. Nat. Commun. 2013;4:2939. doi: 10.1038/ncomms3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li L., Zhang M., Chen W., Wang R., Ye Z., Wang Y., Li X., Cai C. Lncrna-hotair inhibition aggravates oxidative stress-induced h9c2 cells injury through suppression of mmp2 by mir-125. Acta Biochim. Biophys. Sin. (Shanghai) 2018;50:996–1006. doi: 10.1093/abbs/gmy102. [DOI] [PubMed] [Google Scholar]
- 66.Guo X., Chang Q., Pei H., Sun X., Qian X., Tian C., Lin H. Long non-coding rna-mrna correlation analysis reveals the potential role of hotair in pathogenesis of sporadic thoracic aortic aneurysm. Eur. J. Vasc. Endovasc. Surg. 2017;54:303–314. doi: 10.1016/j.ejvs.2017.06.010. [DOI] [PubMed] [Google Scholar]
- 67.Peng Y., Meng K., Jiang L., Zhong Y., Yang Y., Lan Y., Zeng Q., Cheng L. Thymic stromal lymphopoietin-induced hotair activation promotes endothelial cell proliferation and migration in atherosclerosis. Biosci. Rep. 2017;37 doi: 10.1042/BSR20170351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pang J.L., Wang J.W., Hu P.Y., Jiang J.S., Yu C. Hotair alleviates ox-ldl-induced inflammatory response in raw264.7 cells via inhibiting nf-kappab pathway. Eur. Rev. Med. Pharmacol. Sci. 2018;22:6991–6998. doi: 10.26355/eurrev_201810_16170. [DOI] [PubMed] [Google Scholar]
- 69.Jiang Y., Mo H., Luo J., Zhao S., Liang S., Zhang M., Yuan J. Hotair is a potential novel biomarker in patients with congenital heart diseases. Biomed. Res. Int. 2018;2018:2850657. doi: 10.1155/2018/2850657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yamada Y., Horibe H., Oguri M., Sakuma J., Takeuchi I., Yasukochi Y., Kato K., Sawabe M. Identification of novel hyper- or hypomethylated cpg sites and genes associated with atherosclerotic plaque using an epigenome-wide association study. Int. J. Mol. Med. 2018;41:2724–2732. doi: 10.3892/ijmm.2018.3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu J., Huang G.Q., Ke Z.P. Silence of long intergenic noncoding rna hotair ameliorates oxidative stress and inflammation response in ox-ldl-treated human macrophages by upregulating mir-330-5p. J. Cell. Physiol. 2019;234:5134–5142. doi: 10.1002/jcp.27317. [DOI] [PubMed] [Google Scholar]
- 72.Rapicavoli N.A., Qu K., Zhang J., Mikhail M., Laberge R.M., Chang H.Y. A mammalian pseudogene lncrna at the interface of inflammation and anti-inflammatory therapeutics. Elife. 2013;2:e00762. doi: 10.7554/eLife.00762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zgheib C., Hodges M.M., Hu J., Liechty K.W., Xu J. Long non-coding rna lethe regulates hyperglycemia-induced reactive oxygen species production in macrophages. PLoS ONE. 2017;12:e0177453. doi: 10.1371/journal.pone.0177453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Meier I., Fellini L., Jakovcevski M., Schachner M., Morellini F. Expression of the snorna host gene gas5 in the hippocampus is upregulated by age and psychogenic stress and correlates with reduced novelty-induced behavior in c57bl/6 mice. Hippocampus. 2010;20:1027–1036. doi: 10.1002/hipo.20701. [DOI] [PubMed] [Google Scholar]
- 75.Cuomo D., Porreca I., Ceccarelli M., Threadgill D.W., Barrington W.T., Petriella A., D’Angelo F., Cobellis G., De Stefano F., D’Agostino M.N., et al. Transcriptional landscape of mouse-aged ovaries reveals a unique set of non-coding rnas associated with physiological and environmental ovarian dysfunctions. Cell Death Discov. 2018;4:112. doi: 10.1038/s41420-018-0121-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shen Z., She Q. Association between the deletion allele of ins/del polymorphism (rs145204276) in the promoter region of gas5 with the risk of atherosclerosis. Cell. Physiol. Biochem. 2018;49:1431–1443. doi: 10.1159/000493447. [DOI] [PubMed] [Google Scholar]
- 77.Chen L., Yao H., Hui J.Y., Ding S.H., Fan Y.L., Pan Y.H., Chen K.H., Wan J.Q., Jiang J.Y. Global transcriptomic study of atherosclerosis development in rats. Gene. 2016;592:43–48. doi: 10.1016/j.gene.2016.07.023. [DOI] [PubMed] [Google Scholar]
- 78.Wang Y.N., Shan K., Yao M.D., Yao J., Wang J.J., Li X., Liu B., Zhang Y.Y., Ji Y., Jiang Q., et al. Long noncoding rna-gas5: A novel regulator of hypertension-induced vascular remodeling. Hypertension. 2016;68:736–748. doi: 10.1161/HYPERTENSIONAHA.116.07259. [DOI] [PubMed] [Google Scholar]
- 79.Li L., Li X., The E., Wang L.J., Yuan T.Y., Wang S.Y., Feng J., Wang J., Liu Y., Wu Y.H., et al. Low expression of lncrna-gas5 is implicated in human primary varicose great saphenous veins. PLoS ONE. 2015;10:e0120550. doi: 10.1371/journal.pone.0120550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li X., Hou L., Cheng Z., Zhou S., Qi J., Cheng J. Overexpression of gas5 inhibits abnormal activation of wnt/beta-catenin signaling pathway in myocardial tissues of rats with coronary artery disease. J. Cell. Physiol. 2018 doi: 10.1002/jcp.27792. [DOI] [PubMed] [Google Scholar]
- 81.Tang R., Zhang G., Wang Y.C., Mei X., Chen S.Y. The long non-coding rna gas5 regulates transforming growth factor beta (tgf-beta)-induced smooth muscle cell differentiation via rna smad-binding elements. J. Biol. Chem. 2017;292:14270–14278. doi: 10.1074/jbc.M117.790030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shihabudeen Haider Ali M.S., Cheng X., Moran M., Haemmig S., Naldrett M.J., Alvarez S., Feinberg M.W., Sun X. Lncrna meg3 protects endothelial function by regulating the DNA damage response. Nucleic Acids Res. 2018;47:1505–1522. doi: 10.1093/nar/gky1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang L., Yang Z., Trottier J., Barbier O., Wang L. Long noncoding rna meg3 induces cholestatic liver injury by interaction with ptbp1 to facilitate shp mrna decay. Hepatology. 2017;65:604–615. doi: 10.1002/hep.28882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhu J., Liu S., Ye F., Shen Y., Tie Y., Zhu J., Wei L., Jin Y., Fu H., Wu Y., et al. Long noncoding rna meg3 interacts with p53 protein and regulates partial p53 target genes in hepatoma cells. PLoS ONE. 2015;10:e0139790. doi: 10.1371/journal.pone.0139790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lino Cardenas C.L., Kessinger C.W., Cheng Y., MacDonald C., MacGillivray T., Ghoshhajra B., Huleihel L., Nuri S., Yeri A.S., Jaffer F.A., et al. An hdac9-malat1-brg1 complex mediates smooth muscle dysfunction in thoracic aortic aneurysm. Nat. Commun. 2018;9:1009. doi: 10.1038/s41467-018-03394-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Betancur J.G., Tomari Y. Cryptic rna-binding by prc2 components ezh2 and suz12. RNA Biol. 2015;12:959–965. doi: 10.1080/15476286.2015.1069463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tsai M.C., Manor O., Wan Y., Mosammaparast N., Wang J.K., Lan F., Shi Y., Segal E., Chang H.Y. Long noncoding rna as modular scaffold of histone modification complexes. Science. 2010;329:689–693. doi: 10.1126/science.1192002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rinn J.L., Kertesz M., Wang J.K., Squazzo S.L., Xu X., Brugmann S.A., Goodnough L.H., Helms J.A., Farnham P.J., Segal E., et al. Functional demarcation of active and silent chromatin domains in human hox loci by noncoding rnas. Cell. 2007;129:1311–1323. doi: 10.1016/j.cell.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Boudreau N., Andrews C., Srebrow A., Ravanpay A., Cheresh D.A. Induction of the angiogenic phenotype by hox d3. J. Cell Biol. 1997;139:257–264. doi: 10.1083/jcb.139.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Myers C., Charboneau A., Cheung I., Hanks D., Boudreau N. Sustained expression of homeobox d10 inhibits angiogenesis. Am. J. Pathol. 2002;161:2099–2109. doi: 10.1016/S0002-9440(10)64488-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Monnier P., Martinet C., Pontis J., Stancheva I., Ait-Si-Ali S., Dandolo L. H19 lncrna controls gene expression of the imprinted gene network by recruiting mbd1. Proc. Natl. Acad. Sci. USA. 2013;110:20693–20698. doi: 10.1073/pnas.1310201110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zaina S., Pettersson L., Ahren B., Branen L., Hassan A.B., Lindholm M., Mattsson R., Thyberg J., Nilsson J. Insulin-like growth factor ii plays a central role in atherosclerosis in a mouse model. J. Biol. Chem. 2002;277:4505–4511. doi: 10.1074/jbc.M108061200. [DOI] [PubMed] [Google Scholar]
- 93.Casellas A., Mallol C., Salavert A., Jimenez V., Garcia M., Agudo J., Obach M., Haurigot V., Vila L., Molas M., et al. Insulin-like growth factor 2 overexpression induces beta-cell dysfunction and increases beta-cell susceptibility to damage. J. Biol. Chem. 2015;290:16772–16785. doi: 10.1074/jbc.M115.642041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kalwa M., Hanzelmann S., Otto S., Kuo C.C., Franzen J., Joussen S., Fernandez-Rebollo E., Rath B., Koch C., Hofmann A., et al. The lncrna hotair impacts on mesenchymal stem cells via triple helix formation. Nucleic Acids Res. 2016;44:10631–10643. doi: 10.1093/nar/gkw802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yap K.L., Li S., Munoz-Cabello A.M., Raguz S., Zeng L., Mujtaba S., Gil J., Walsh M.J., Zhou M.M. Molecular interplay of the noncoding rna anril and methylated histone h3 lysine 27 by polycomb cbx7 in transcriptional silencing of ink4a. Mol. Cell. 2010;38:662–674. doi: 10.1016/j.molcel.2010.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Broadbent H.M., Peden J.F., Lorkowski S., Goel A., Ongen H., Green F., Clarke R., Collins R., Franzosi M.G., Tognoni G., et al. Susceptibility to coronary artery disease and diabetes is encoded by distinct, tightly linked snps in the anril locus on chromosome 9p. Hum. Mol. Genet. 2008;17:806–814. doi: 10.1093/hmg/ddm352. [DOI] [PubMed] [Google Scholar]
- 97.Yari M., Bitarafan S., Broumand M.A., Fazeli Z., Rahimi M., Ghaderian S.M.H., Mirfakhraie R., Omrani M.D. Association between long noncoding rna anril expression variants and susceptibility to coronary artery disease. Int. J. Mol. Cell. Med. 2018;7:1–7. doi: 10.22088/IJMCM.BUMS.7.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Holdt L.M., Beutner F., Scholz M., Gielen S., Gabel G., Bergert H., Schuler G., Thiery J., Teupser D. Anril expression is associated with atherosclerosis risk at chromosome 9p21. Arterioscler. Thromb. Vasc. Biol. 2010;30:620–627. doi: 10.1161/ATVBAHA.109.196832. [DOI] [PubMed] [Google Scholar]
- 99.Yasuno K., Bilguvar K., Bijlenga P., Low S.K., Krischek B., Auburger G., Simon M., Krex D., Arlier Z., Nayak N., et al. Genome-wide association study of intracranial aneurysm identifies three new risk loci. Nat. Genet. 2010;42:420–425. doi: 10.1038/ng.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Scott L.J., Mohlke K.L., Bonnycastle L.L., Willer C.J., Li Y., Duren W.L., Erdos M.R., Stringham H.M., Chines P.S., Jackson A.U., et al. A genome-wide association study of type 2 diabetes in finns detects multiple susceptibility variants. Science. 2007;316:1341–1345. doi: 10.1126/science.1142382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kotake Y., Nakagawa T., Kitagawa K., Suzuki S., Liu N., Kitagawa M., Xiong Y. Long non-coding rna anril is required for the prc2 recruitment to and silencing of p15(ink4b) tumor suppressor gene. Oncogene. 2011;30:1956–1962. doi: 10.1038/onc.2010.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Thomas A.A., Feng B., Chakrabarti S. Anril: A regulator of vegf in diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2017;58:470–480. doi: 10.1167/iovs.16-20569. [DOI] [PubMed] [Google Scholar]
- 103.Zhou X., Han X., Wittfeldt A., Sun J., Liu C., Wang X., Gan L.M., Cao H., Liang Z. Long non-coding rna anril regulates inflammatory responses as a novel component of nf-kappab pathway. RNA Biol. 2016;13:98–108. doi: 10.1080/15476286.2015.1122164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Boekholdt S.M., Peters R.J., Hack C.E., Day N.E., Luben R., Bingham S.A., Wareham N.J., Reitsma P.H., Khaw K.T. Il-8 plasma concentrations and the risk of future coronary artery disease in apparently healthy men and women: The epic-norfolk prospective population study. Arterioscler. Thromb. Vasc. Biol. 2004;24:1503–1508. doi: 10.1161/01.ATV.0000134294.54422.2e. [DOI] [PubMed] [Google Scholar]
- 105.Zhang B., Ma Y., Xiang C. Sirt2 decreases atherosclerotic plaque formation in low-density lipoprotein receptor-deficient mice by modulating macrophage polarization. Biomed. Pharmacother. 2018;97:1238–1242. doi: 10.1016/j.biopha.2017.11.061. [DOI] [PubMed] [Google Scholar]
- 106.Seki N., Bujo H., Jiang M., Tanaga K., Takahashi K., Yagui K., Hashimoto N., Schneider W.J., Saito Y. Lrp1b is a negative modulator of increased migration activity of intimal smooth muscle cells from rabbit aortic plaques. Biochem. Biophys. Res. Commun. 2005;331:964–970. doi: 10.1016/j.bbrc.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 107.Sun J., Ermann J., Niu N., Yan G., Yang Y., Shi Y., Zou W. Histone demethylase lsd1 regulates bone mass by controlling wnt7b and bmp2 signaling in osteoblasts. Bone Res. 2018;6:14. doi: 10.1038/s41413-018-0015-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bianchessi V., Badi I., Bertolotti M., Nigro P., D’Alessandra Y., Capogrossi M.C., Zanobini M., Pompilio G., Raucci A., Lauri A. The mitochondrial lncrna asncmtrna-2 is induced in aging and replicative senescence in endothelial cells. J. Mol. Cell. Cardiol. 2015;81:62–70. doi: 10.1016/j.yjmcc.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 109.Burzio V.A., Villota C., Villegas J., Landerer E., Boccardo E., Villa L.L., Martinez R., Lopez C., Gaete F., Toro V., et al. Expression of a family of noncoding mitochondrial rnas distinguishes normal from cancer cells. Proc. Natl. Acad. Sci. USA. 2009;106:9430–9434. doi: 10.1073/pnas.0903086106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gao Y., Chen Z.Y., Wang Y., Liu Y., Ma J.X., Li Y.K. Long non-coding rna asncmtrna-2 is upregulated in diabetic kidneys and high glucose-treated mesangial cells. Exp. Ther. Med. 2017;13:581–587. doi: 10.3892/etm.2017.4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Paloschi V., Kurtovic S., Folkersen L., Gomez D., Wagsater D., Roy J., Petrini J., Eriksson M.J., Caidahl K., Hamsten A., et al. Impaired splicing of fibronectin is associated with thoracic aortic aneurysm formation in patients with bicuspid aortic valve. Arterioscler. Thromb. Vasc. Biol. 2011;31:691–697. doi: 10.1161/ATVBAHA.110.218461. [DOI] [PubMed] [Google Scholar]
- 112.Zhang K., Luo Z., Zhang Y., Zhang L., Wu L., Liu L., Yang J., Song X., Liu J. Circulating lncrna h19 in plasma as a novel biomarker for breast cancer. Cancer Biomark. 2016;17:187–194. doi: 10.3233/CBM-160630. [DOI] [PubMed] [Google Scholar]
- 113.Zhou X., Yin C., Dang Y., Ye F., Zhang G. Identification of the long non-coding rna h19 in plasma as a novel biomarker for diagnosis of gastric cancer. Sci. Rep. 2015;5:11516. doi: 10.1038/srep11516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yin Q., Wu A., Liu M. Plasma long non-coding rna (lncrna) gas5 is a new biomarker for coronary artery disease. Med. Sci. Monit. 2017;23:6042–6048. doi: 10.12659/MSM.907118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Liang W., Lv T., Shi X., Liu H., Zhu Q., Zeng J., Yang W., Yin J., Song Y. Circulating long noncoding rna gas5 is a novel biomarker for the diagnosis of nonsmall cell lung cancer. Medicine (Baltimore) 2016;95:e4608. doi: 10.1097/MD.0000000000004608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yu F., Zhou G., Huang K., Fan X., Li G., Chen B., Dong P., Zheng J. Serum lincrna-p21 as a potential biomarker of liver fibrosis in chronic hepatitis b patients. J. Viral Hepat. 2017;24:580–588. doi: 10.1111/jvh.12680. [DOI] [PubMed] [Google Scholar]
- 117.Zhao W., Song M., Zhang J., Kuerban M., Wang H. Combined identification of long non-coding rna ccat1 and hotair in serum as an effective screening for colorectal carcinoma. Int. J. Clin. Exp. Pathol. 2015;8:14131–14140. [PMC free article] [PubMed] [Google Scholar]
- 118.Liu M., Xing L.Q., Liu Y.J. A three-long noncoding rna signature as a diagnostic biomarker for differentiating between triple-negative and non-triple-negative breast cancers. Medicine (Baltimore) 2017;96:e6222. doi: 10.1097/MD.0000000000006222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.McPherson R., Pertsemlidis A., Kavaslar N., Stewart A., Roberts R., Cox D.R., Hinds D.A., Pennacchio L.A., Tybjaerg-Hansen A., Folsom A.R., et al. A common allele on chromosome 9 associated with coronary heart disease. Science. 2007;316:1488–1491. doi: 10.1126/science.1142447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Aleyasin S.A., Navidi T., Davoudi S. Association between rs10757274 and rs2383206 snps as genetic risk factors in iranian patients with coronary artery disease. J. Tehran Heart Cent. 2017;12:114–118. [PMC free article] [PubMed] [Google Scholar]
- 121.Nawaz S.K., Noreen A., Rani A., Yousaf M., Arshad M. Association of the rs10757274 snp with coronary artery disease in a small group of a pakistani population. Anatol. J. Cardiol. 2015;15:709–715. doi: 10.5152/akd.2014.5470. [DOI] [PMC free article] [PubMed] [Google Scholar]