Abstract
Background: Saliva has been recently proposed as an alternative to classic biofluid analyses due to both availability and reliability regarding the evaluation of various biomarkers. Biosensors have been designed for the assessment of a wide spectrum of compounds, aiding in the screening, diagnosis, and monitoring of pathologies and treatment efficiency. This literature review aims to present the development in the biosensors research and their utility using salivary assessment. Methods: a comprehensive literature search has been conducted in the PubMed database, using the keywords “saliva” and “sensor”. A two-step paper selection algorithm was devised and applied. Results: The 49 papers selected for the present review focused on assessing the salivary biomarkers used in general diseases, oral pathologies, and pharmacology. The biosensors proved to be reliable tools for measuring the salivary levels of biochemical metabolic compounds such as glucose, proteinases and proteins, heavy metals and various chemical compounds, microorganisms, oncology markers, drugs, and neurotransmitters. Conclusions: Saliva is a biofluid with a significant clinical applicability for the evaluation and monitoring of a patient’s general health. Biosensors designed for assessing a wide range of salivary biomarkers are emerging as promising diagnostic or screening tools for improving the patients’ quality of life.
Keywords: saliva, biofluid, biosensor, analytes, systematic review
1. Introduction
Nowadays, human biological samples are used not only for the screening and diagnosis of various pathologies but also for assessing the compliance to treatment and the therapeutic efficacy. Depending on the types of investigations required, several options are available, varying from usual specimens (blood, plasma, saliva, sputum, urine, and feces) to more specific ones (cerebrospinal fluid, bone marrow, etc.). Blood collection is typically invasive and uncomfortable for the patients, being associated with high levels of anxiety, whereas urine or feces testing is considered to be privacy-invading [1,2,3].
In recent years, saliva has been explored as an alternative for the evaluation of homeostasis and the detection of pathologic conditions [4]. Most of the saliva (90%) is produced by the three major salivary glands (parotid, submandibular, and sublingual glands), whereas a small amount (10%) is produced by the minor salivary glands, distributed in the labial, buccal, lingual, and palatal areas of the oral mucosa [3,5]. Even though saliva is composed of 99% water, it also contains numerous constituents diffused from blood through para-cellular or trans-cellular pathways [5,6]. Saliva performs multiple functions, including digestion (the lubrication and binding of the alimentary bolus and the initiation of starch digestion), gustatory sensation (by solubilisation of dry food), protection (mechanical mobilization of alimentary debris), and antibacterial activity (lysis of the bacterial cell wall due to lysozyme) [4].
The oral cavity is host to a large number of commensal bacteria, known as the oral microbiome. Bacteria that can be found on the different surfaces in the oral cavity are organised in large communities that thrive and support one another, known as the biofilm. Inside the biofilm, the different species of bacteria communicate with one another, thus enabling the host colonisation, offering defence against competing bacteria, or adapting when changes are made in the environment around them. The pathologic effect of the oral microbiome occurs when the defensive mechanisms of the host are overtaken or impaired due to general pathologies, such as autoimmune diseases or diabetes [7,8]. In a healthy individual, a number of over 700 different bacterial species were identified, of which more than half have never been cultivated. The number of bacterial species and the composition of the microbiome may vary not only from one person to another but also in different areas of the oral cavity [9]. Moreover, the oral microbiome can undergo changes under certain conditions, such as during radiation therapy [10], or in the presence of dental prosthetic rehabilitations [11,12]. On the poorly maintained or unadapted removable prosthesis, an increased accumulation of bacteria has been identified [12]. In the case of fixed rehabilitations, the high accumulation of bacteria can be associated with the surface roughness or the material chosen for the prosthetic piece. The corrosion effect of saliva on the metallic crowns could increase the bacterial accumulation. The corrosive proccess was investigated in vitro by Uriciuc et al. using CoCrMo and CoCrW alloys immersed into artificial saliva. CoCr-based alloys with tungsten (W) content presented a linear and stable anticorrosion tendency and are more suitable for using as a single-casted alloy in prosthetics dental structures [11,13].
Given the modifications that may occur in certain pathologies, practitioners should consider setting a baseline of the salivary values of these patients. In this regard, saliva should be collected after a thorough professional cleaning of the oral cavity, carried out by a dental practitioner. The longitudinal study conducted by Esin et al. revealed a reduction of the microbial load (Streptococcus mutans and Lactobacillus spp.) three months after professional cleaning; this data sustains our suggestion regarding the salivary profiling of certain patients [14]. Furthermore, in order to reduce the alimentary influence, the samples should be collected à jeun before any food intake.
The use of human saliva for the diagnosis of different pathologies and for the monitoring of treatment outcomes is enabled by several advantages. Saliva collection is easy and does not require medical training; thus, it is feasible to both patients and practitioners. The sampling is fast and cost efficient, and saliva storage and shipping are easier than that of most biological samples. Moreover, unlike blood samples that may need additional media to prevent clotting, saliva is not susceptible to transformations. Finally, the contamination risk for medical personnel is lower, since no needles are involved [3,4].
Biosensors are defined as medical devices capable of detecting or measuring chemical or biological reactions by generating signals when in contact with an analyte [15]. Since their proof of concept in 1906, biosensors have become attractive to researchers and medical practitioners as alternatives to regular, expensive, and time-consuming investigations [6,15].
The aim of the present paper was to perform a comprehensive analysis of the existing literature on the ability of biosensors to detect various compounds in human saliva and on their reliability as an alternative to traditional laboratory investigations.
2. Materials and Method
A systematic review based on the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) checklist was carried out. A search in the electronic data base PubMed was conducted using the association of the keywords “saliva” and “sensor”.
In the first step of the review, the titles and abstracts of the returned articles were analysed. The potentially eligible articles had to be published in the last 10 years, between the 1st of January 2009 and the 31st of December 2018. Furthermore, the papers had to focus on the detection of parameters in saliva using biosensors. Only human studies were included. Lastly, the articles had to be written in English.
In the second step of the review, the full text of the potentially eligible articles was analysed. The full text of the final papers had to be available for reading or purchasing. Detailed information about the sensors, their characteristics, and applicability to human saliva had to be reported. The accepted forms of papers were basic research, cross-sectional studies, or cohort studies. A basic research study is defined as a research conducted in the laboratory for the characterization and evaluation of a medical device. A cross-sectional study is an observational study conducted on a set number of subjects, evaluating the medical device at a specific point in time. All the final articles had to have the references listed and must have been cited at least once. The number of citations was determined using the Web of Science Core Collection search tool and, where the articles were not available, the Google Scholar search engine was employed.
3. Results
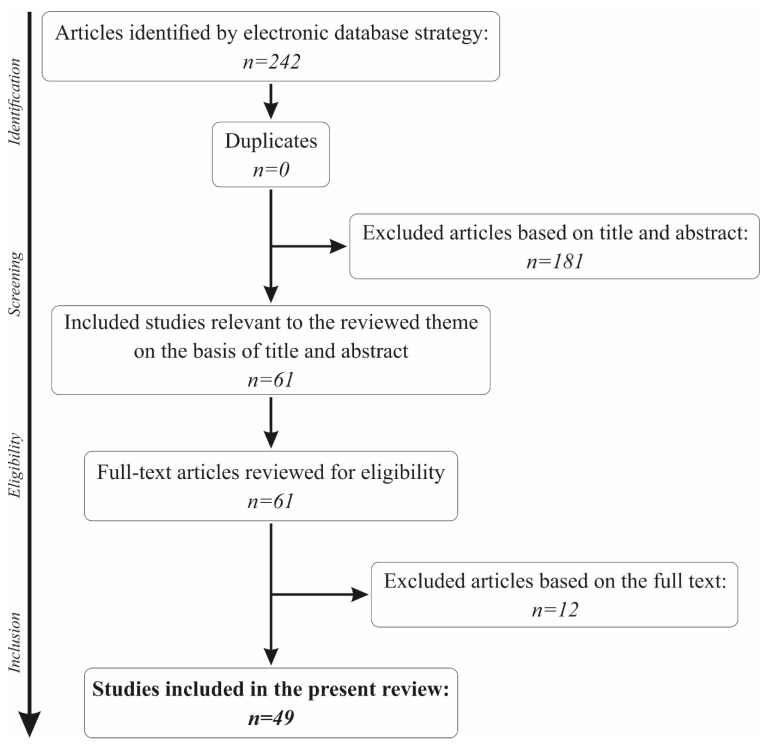
The initial search in the PubMed database using the keywords “saliva” and “sensors” returned a total of 242 results. Out of the total number, 46 had to be excluded for being published before the 1st of January 2009, bringing the number to 196 results. Another 85 papers were eliminated for not being human studies, and 2 other papers were not written in English. The titles and abstracts of the remaining 109 articles were further analysed, and 48 were excluded for not making a reference to the use of biosensors in saliva.
A total of 61 articles remained for further analysis of the full text. Another 12 articles were removed from the present review for not making a reference to the main topic. Thus, a total of 49 papers were included in the present review. The full selection algorithm is visually represented in Figure 1.
Figure 1.
The paper selection algorithm for the present review.
Out of the total number of final articles, 4 were cross-sectional studies, whereas 45 were basic studies. Furthermore, 5 described the use of biosensors for the diagnosis of oral pathologies, 2 described their use in pharmacological monitoring, and 42 described their applicability in general pathologies screening, diagnosis, or follow-up.
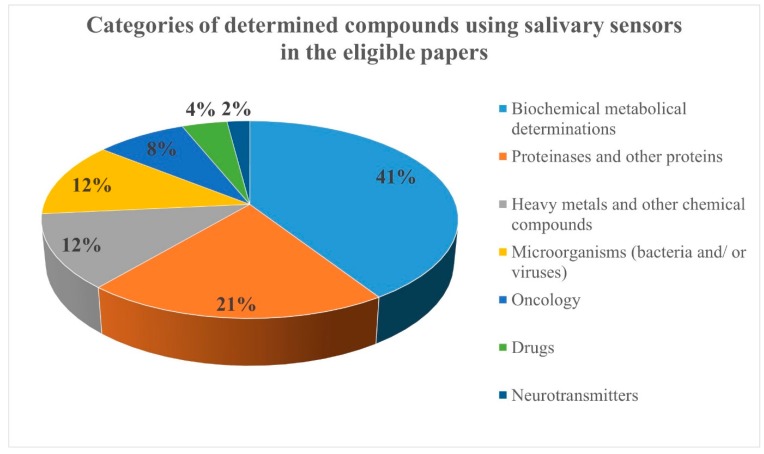
Due to the large number of eligible articles, the compounds determined using salivary sensors were organized in seven categories: biochemical metabolic compounds (20 papers), proteinases and other proteins (10 papers), heavy metals and other chemical compounds (6 papers), microorganisms (bacteria and/or viruses; 6 papers), oncology markers (4 papers), drugs (2 papers), and neurotransmitters (1 paper) (Figure 2).
Figure 2.
The categories of determined compounds using salivary sensors in the eligible papers.
The final selected articles are listed in Table 1, with information on their authors, the original journal and year of publication, the analysed sensors and the assessed compounds, the purpose of determination, their indication, and the number of citations. These papers were sorted by the seven proposed categories and the year of publication.
Table 1.
The final eligible papers for the present review.
| No | Authors | Publication | Year | Type of Paper | Sensor | Determined Compounds | Purpose of Determination | Indication | Number of Times Cited |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Ye et al. [16] | Talanta | 2013 | Basic research | CuO nanoneedle/graphene/carbon nanofiber modified glassy carbon electrode | Glucose | Diagnosis/Monitoring of diabetes mellitus | General pathologies | 59 |
| 2 | Li et al. [17] | Sci Rep | 2015 | Basic research | Electrochemical sensor using anodized cupric oxide nanowires | Glucose | Diagnosis/Monitoring of diabetes mellitus | General pathologies | 36 |
| 3 | Wang et al. [18] | Anal Chem | 2016 | Basic research | Core-shell IrO2@NiO nanowires | Glucose | Diagnosis/Monitoring of diabetes mellitus | General pathologies | 16 |
| 4 | Du et al. [2] | J Diabetes Sci Technol | 2016 | Basic research | Screen-printed sensor chip | Glucose | Diagnosis/Monitoring of diabetes mellitus | General pathologies | 10 * |
| 5 | Arakawa et al. [19] | Biosens Bioelectron | 2016 | Basic research | Mouthguard glucose sensor | Glucose | Diagnosis/Monitoring of diabetes mellitus | General pathologies | 31 |
| 6 | Soni et al. [20] | Anal Chim Acta | 2017 | Cross-sectional study | Paper based sensor and smartphone RGB analysis | Glucose | Diagnosis/Monitoring of diabetes mellitus | General pathologies | 2 |
| 7 | Dominguez et al. [21] | Sensors (Basel) | 2017 | Cross-sectional study | Spectrophotometric detection | Glucose | Diagnosis/Monitoring of diabetes mellitus | General pathologies | 1 |
| 8 | Anderson et al. [22] | Sensors (Basel) | 2017 | Basic research | Colloidal AgNPs/MoS2-based nonenzymatic glucose biosensor | Glucose | Diagnosis/Monitoring of diabetes mellitus | General pathologies | 4 |
| 9 | Bell et al. [23] | Nanotechnology | 2017 | Basic research | Randomly oriented CuO nanowire networks | Glucose | Diagnosis/Monitoring of diabetes mellitus | General pathologies | 4 |
| 10 | Velmurugan et al. [24] | J Colloid Interface Sci | 2017 | Basic research | CuO modified screen printed carbon electrode (SPCE) | Glucose | Diagnosis/Monitoring of diabetes mellitus | General pathologies | 10 |
| 11 | Kim et al. [25] | Biosens Bioelectron | 2017 | Basic research | Molecularly imprinted polymer binding on a conducting polymer layer | Glucose | Diagnosis/Monitoring of diabetes mellitus | General pathologies | 22 |
| 12 | Dutta et al. [26] | Biosens Bioelectron | 2017 | Basic research | Methylene blue, hydrazine and platinum nanoparticles | Glucose | Diagnosis/Monitoring of diabetes mellitus | General pathologies | 20 |
| 13 | Santana-Jiménez et al. [27] | Sensors (Basel) | 2018 | Basic research | Paper-based sensors | Glucose | Diagnosis/Monitoring of diabetes mellitus | General pathologies | 2 |
| 14 | Mitchell et al. [28] | Analyst | 2009 | Basic research | Surface plasmon resonance (SPR) immunosensor | Cortisol | Detection and quantification of cortisol | General pathologies | 35 |
| 15 | Pires et al. [29] | Biomed Mater Eng | 2014 | Basic research | Chemiluminescent organic-based immunosensor | Cortisol | Detection and quantification of cortisol | General pathologies | 6 |
| 16 | Usha et al. [30] | Biosens Bioelectron | 2017 | Basic research | Lossy mode resonance-based fiber optic | Cortisol | Detection and quantification of cortisol | General pathologies | 11 |
| 17 | Frasconi et al. [31] | Anal Bioanal Chem | 2009 | Basic research | Surface plasmon resonance (SPR) immunosensor | Cortisol and cortisone | Detection and quantification of cortisol and cortisone | General pathologies | 27 |
| 18 | Ballesta Claver et al. [32] | Analyst | 2009 | Basic research | Electrochemiluminescent biosensor | Blood lactate | Detection and quantification of blood lactate | General pathologies | 41 |
| 19 | Kim et al. [33] | Biosens Bioelectron | 2015 | Basic research | Screen-printing technology on a flexible polyethylene terephthalate substrate | Uric acid | Detection and quantification of uric acid | General pathologies | 70 |
| 20 | Ciui et al. [34] | Sens Actuators B Chem (in press) | 2019 | Basic research | Cavitas electrochemical sensor in mouthguard | N-epsilon (carboxymethyl)lysine (CML) | Monitoring of CML | General pathologies | 1 * |
| 21 | Lee et al. [35] | ACS Appl Mater Interfaces | 2011 | Basic research | Molecularly imprinted thin films | Salivary proteins | Detection and quantification of salivary proteins (a-amylase) | General pathologies | 29 |
| 22 | Attia et al. [36] | Analyst | 2014 | Basic research | Nano-optical sensor | Salivary proteins | Detection and quantification of salivary proteins (a-amylase) | General pathologies | 22 |
| 23 | Mohseni et al. [37] | Biosens Bioelectron | 2016 | Basic research | Carboxymethyldextran hydrogel sensor chip with immobilized monoclonal MMP-9 antibodies | Matrix metalloproteinases (MMP-9) | Diagnosis of chronic periodontal disease | Oral Pathologies | 13 |
| 24 | Ritzer et al. [38] | Nat Commun | 2017 | Basic research | Diagnostic chewing gum | Matrix metalloproteinases (MMP-1, MMP-8, MMP-9) | Diagnosis of inflammatory implant diseases | Oral Pathologies | 8 |
| 25 | Wang et al. [39] | J Pharm Biomed Anal | 2012 | Basic research | Homogeneous fluorescent sensor | Human serum albumin | Detection and quantification of human serum albumin | General pathologies | 19 |
| 26 | Gorodkiewicz et al. [40] | Folia Histochem Cytobiol | 2012 | Basic research | Surface Plasmon Resonance Imaging (SPRI) biosensor | Cystatin | Detection and quantification of cystatin | Oral pathologies | 2 |
| 27 | Gorodkiewicz et al. [41] | Protein Pept Lett | 2010 | Basic research | Surface plasmon resonance imaging (SPRI) biosensor | Cathepsin D (CatD) and cathepsin E (CatE) | Monitoring of cathepsin D and cathepsin E activity | General pathologies | 16 |
| 28 | Gorodkiewicz et al. [42] | Anal Biochem | 2012 | Basic research | Surface plasmon resonance imaging (SPRI) biosensor | Cathepsin G | Monitoring of Cathepsin G activity | General pathologies | 21 |
| 29 | Wei et al. [43] | Clin Cancer Res | 2009 | Basic research | Electrochemical (EC) sensor | IL-8 mRNA and IL-8 protein | Oncology (early cancer diagnostic) | General pathologies | 111 |
| 30 | Majidi et al. [44] | Talanta | 2016 | Basic research | Two ultrasensitive electrochemical sensor and aptasensor | Tryptophan | Selective analysis of tryptophan in biological samples | General pathologies | 15 |
| 31 | Puchnin et al. [45] | Biosens Bioelectron | 2017 | Basic research | Calixarene tubes | Potassium iodine (KI) | Detection and Monitoring of KI | General pathologies | 3 |
| 32 | Minami et al. [46] | Biosens Bioelectron | 2016 | Basic research | Organic field-effect transistors | Nitrate | Detection and quantification of nitrate ions | General pathologies | 20 |
| 33 | Hassan et al. [47] | Anal Sci | 2009 | Basic research | Potentiometric membrane sensor | Thiocyanate | Detection and quantification of thiocyanate | General pathologies | 10 |
| 34 | Zheng et al. [1] | Nanoscale | 2015 | Basic research | Sandwich-structured SERS probe with a gold nanohole array pattern | Silver and mercury | Detection of heavy metals intoxication | General pathologies | 36 |
| 35 | Timofeeva et al. [48] | Talanta | 2016 | Basic research | PVC membrane electrode | Caffeine | Monitoring of drug metabolizing system activity in hepatocytes | General pathologies | 15 |
| 36 | Zilberman et al. [49] | Biosens Bioelectron | 2015 | Basic research | Portable optoelectronic microfluidic sensor | Ammonia and carbon dioxide | Oncology (screening of stomach cancer) | General pathologies | 18 |
| 37 | Ahmed et al. [50] | Anal Chem | 2013 | Basic research | Impedimetric sensors | Pathogenic microorganisms (Streptococcus pyogenes) | Diagnosis of Streptococcus pyogenes infections | General pathologies | 46 * |
| 38 | Wignarajah et al. [51] | Anal Chem | 2015 | Basic research | Multiplex colorimetric biosensor | Pathogenic microorganisms (Porphyromonas gingivalis proteases) | Diagnosis of chronic periodontal disease | Oral Pathologies | 18 |
| 39 | Hoyos-Nogués et al. [52] | Biosens Bioelectron | 2016 | Basic research | Peptide-based biosensor (hLf1-11) | Pathogenic microorganisms (Streptococcus sanguinis) | Inflammatory implant diseases | Oral Pathologies | 16 |
| 40 | Xue et al. [53] | Sensors (Basel) | 2014 | Basic research | Immunoassay utilizing microchannels within a multicapillary glass plate | Pathogenic microorganisms (detection of viral antibodies) | Diagnosis of viral infections | General pathologies | 8 |
| 41 | Jin et al. [54] | Biosens Bioelectron | 2018 | Cross-sectional study | Microfluidic system (SLIM) | Pathogenic microorganisms (bacteria and viruses) | Ultrasensitive pathogen detection | General pathologies | 1 |
| 42 | Zaitouna et al. [55] | Anal Chim Acta | 2015 | Basic research | Electrochemical peptide based sensor enhanced with extra amino acids | Anti-HIV antibodies | Human Immunodeficiency Virus (HIV) | General pathologies | 7 |
| 43 | Song et al. [56] | Anal Chim Acta | 2018 | Cross-sectional study | 3DN-CNTs sensor | Cyfra 21-1 | Oncology (diagnosis of oral squamous cell carcinoma) | General pathologies | 1 |
| 44 | Chen et al. [57] | Anal Chim Acta | 2014 | Basic research | Fluorescent biosensor | c-erbB-2 oncogene tumor marker | Oncology (early breast cancer diagnostic) | General pathologies | 18 * |
| 45 | Cho et al. [58] | ACS Nano | 2012 | Basic research | Surface-enhanced fluorescent optical sensor | Vascular endothelial growth factor-165 (VEGF165) | Oncology (early cancer diagnostic) | General pathologies | 87 * |
| 46 | Yu et al. [59] | Anal Chem | 2014 | Basic research | Capillary-based 3D fluoroimmunosensor | Carcinoembryonic antigen | Oncology (early cancer diagnostic) | General pathologies | 30 |
| 47 | Machini et al. [60] | Biosens Bioelectron | 2016 | Basic research | Electrochemical sensor using binuclear oxo-manganese complex | Acetazolamide | Detection of doping-associated substances | Pharmacology | 5 |
| 48 | Yu et al. [61] | Talanta | 2018 | Basic research | Electrochemical aptamer-based sensor (E-AB) | Ampicillin | Determination of optimal therapeutic concentration and the most effective method of drug administration | Pharmacology | 12 |
| 49 | Hagen et al. [62] | ACS Chem Neurosci | 2013 | Basic research | Electronic based (FET) biosensor | Orexin A | Detection and quantification of orexin A | General pathologies | 7 |
* Articles available only in the Google Scholar database.
A comprehensive list containing the characterization information about each of the sensors from the final eligible papers, such as the limit of detection, selectivity, and sensitivity, alongside the author’s name, determined compounds, sensor information, and method of detection, can be found in Table 2.
Table 2.
The characterization information on the sensors from the final eligible papers.
| No | Authors | Determined Compounds | Sensor | Method of Detection | Limit of Detection | Selectivity | Sensitivity |
|---|---|---|---|---|---|---|---|
| 1 | Ye et al. [16] | Glucose | CuO nanoneedle/graphene/carbon nanofiber modified glassy carbon electrode | Amperometric detection | 912.7 A·mM−1·cm−2 | N/A † | N/A |
| 2 | Li et al. [17] | Glucose | Electrochemical sensor using anodized cupric oxide nanowires | Electrochemical detection | 0.3/μM | N/A | 2217.4/μA·cm−2 mM−1 |
| 3 | Wang et al. [18] | Glucose | Core-shell IrO2@NiO nanowires | Electrochemical detection | 0.31 μM | N/A | 1539.0 μA·mM−1·cm−2 |
| 4 | Du et al. [2] | Glucose | Screen-printed sensor chip | Amperometric detection | 1.1–45 mg/dL | N/A | N/A |
| 5 | Arakawa et al. [19] | Glucose | Mouthguard glucose sensor | Electrochemical detection | 5 mmol/L | N/A | N/A |
| 6 | Soni et al. [20] | Glucose | Paper based sensor and smartphone RGB analysis | Colorimetric evaluation using an RGB sensor | 24.6 mg/dL | N/A | 0.0012 pixels s−1/mg·dL−1 |
| 7 | Dominguez et al. [21] | Glucose | Spectrophotometric detection | Colorimetric evaluation using an RGB sensor | 0.17 mg/dL | N/A | N/A |
| 8 | Anderson et al. [22] | Glucose | Colloidal AgNPs/MoS2-based nonenzymatic glucose biosensor | Electrochemical detection | 0.03 μM | N/A | 9044.6 μA·mM−1·cm−2 |
| 9 | Bell et al. [23] | Glucose | Randomly oriented CuO nanowire networks | Amperometric detection | 0.05 mM Glucose (Gl) at +0.6 V | N/A | 0.1 nA/mM Gl in the 0–7 mM Gl range and 2.1 nA/mM Gl above 7 mM Gl |
| 10 | Velmurugan et al. [24] | Glucose | CuO modified screen printed carbon electrode (SPCE) | Electrochemical detection | 0.1 μM | N/A | 308.71 μA·mM−1 cm−2 |
| 11 | Kim et al. [25] | Glucose | Molecularly imprinted polymer binding on a conducting polymer layer | Potentiometric measurements | 1.9 (±0.15) × 10−7 M | N/A | N/A |
| 12 | Dutta et al. [26] | Glucose | Methylene blue, hydrazine and platinum nanoparticles | Oxidation current measurements | 2.2 pg/mL | N/A | N/A |
| 13 | Santana-Jiménez et al. [27] | Glucose | Paper-based sensors | Naked-eye detection | 47 μM | N/A | 1.81 A.U./mg |
| 14 | Mitchell et al. [28] | Cortisol | Surface plasmon resonance (SPR) immunosensor | Surface plasmon resonance | 49 pg/mL | N/A | 162 RU.mL/ng |
| 15 | Pires et al. [29] | Cortisol | Chemiluminescent organic-based immunosensor | Organic photodetection | 80 pg/mL | N/A | 685 pg/mL |
| 16 | Usha et al. [30] | Cortisol | Lossy mode resonance-based fiber optic | Fiber optic real-time detection | 25.9 fg/ml | N/A | N/A |
| 17 | Frasconi et al. [31] | Cortisol and cortisone | Surface plasmon resonance (SPR) immunosensor | Surface plasmon resonance | Cortisol: 4 μg·L−1 Cortisone: 10 μg·L−1 |
N/A | N/A |
| 18 | Ballesta Claver et al. [32] | Blood lactate | Electrochemiluminescent biosensor | Electrochemiluminescence detection | N/A | N/A | N/A |
| 19 | Kim et al. [33] | Uric acid | Screen-printing technology on a flexible polyethylene terephthalate substrate | Potentiometric measurements | N/A | 350 μM | 1.08 μA/mM |
| 20 | Ciui et al. [34] | N-epsilon (carboxymethyl)lysine (CML) | Cavitas electrochemical sensor in mouthguard | Electrochemical detection | 0.81 μM | N/A | N/A |
| 21 | Lee et al. [35] | Salivary proteins | Molecularly imprinted thin films | Quartz crystal microbalance detection | 0.1 mg/mL | N/A | N/A |
| 22 | Attia et al. [36] | Salivary proteins | Nano-optical sensor | Spectrofluorimetric detection | 5.7 × 10−1 mol/L−1 | N/A | N/A |
| 23 | Mohseni et al. [37] | Matrix metalloproteinases (MMP-9) | Carboxymethyldextran hydrogel sensor chip with immobilized monoclonal MMP-9 antibodies | Surface plasmon resonance | 8 pg/mL | N/A | High (recovery rate of ~94%) |
| 24 | Ritzer et al. [38] | Matrix metalloproteinases (MMP-1, MMP-8, MMP-9) | Diagnostic chewing gum | Peptide sensors | N/A | N/A | N/A |
| 25 | Wang et al. [39] | Human serum albumin | Homogeneous fluorescent sensor | Fluorescence resonance energy transfer | 3.9 ng/mL | N/A | N/A |
| 26 | Gorodkiewicz et al. [40] | Cystatin | Surface Plasmon Resonance Imaging (SPRI) biosensor | Surface Plasmon Resonance Imaging | 0.1 μg/mL | N/A | N/A |
| 27 | Gorodkiewicz et al. [41] | Cathepsin D (CatD) and cathepsin E (CatE) | Surface plasmon resonance imaging (SPRI) biosensor | Surface Plasmon Resonance Imaging | 0.12 ng mL−1 | N/A | N/A |
| 28 | Gorodkiewicz et al. [42] | Cathepsin G | Surface plasmon resonance imaging (SPRI) biosensor | Surface Plasmon Resonance Imaging | 0.23 ng/mL | N/A | High (recovery rate of 100%) |
| 29 | Wei et al. [43] | IL-8 mRNA and IL-8 protein | Electrochemical (EC) sensor | Electrochemical detection | IL-8 mRNA −3.9 fM and IL-8 protein: 7.4 pg/mL | ~90% | ~90% |
| 30 | Majidi et al. [44] | Tryptophan | Two ultrasensitive electrochemical sensor and aptasensor | Electrochemical detection and Electrochemical impedance spectroscopy | MWCNT-AuSPE: 3.6 × 10−10 mol L−1 and Apt-MWCNT-AuSPE: 4.9 × 10−12 mol L−1 | N/A | N/A |
| 31 | Puchnin et al. [45] | Potassium iodine (KI) | Calixarene tubes | Ion-selective field effect detection | ~3×10−8 M | N/A | N/A |
| 32 | Minami et al. [46] | Nitrate | Organic field-effect transistors | Organic field-effect detection | 45 ppb | N/A | High (recovery rate of 97.4 ± 1.8%) |
| 33 | Hassan et al. [47] | Thiocyanate | Potentiometric membrane sensor | Potentiometric measurements | 5.6 × 10−6 mol/L | N/A | −57.5 ± 0.5 mV decade−1 |
| 34 | Zheng et al. [1] | Silver and mercury | Sandwich-structured SERS probe with a gold nanohole array pattern | Surface-enhanced Raman scattering detection | 0.17 nM of Silver 2.3 pM of Mercury |
N/A | N/A |
| 35 | Timofeeva et al. [48] | Caffeine | PVC membrane electrode | Flow potentiometric measurements | 1.2 mg−1L | N/A | 52 ± 1 mV dec−1 |
| 36 | Zilberman et al. [49] | Ammonia and carbon dioxide | Portable optoelectronic microfluidic sensor | Optoelectronic detection | N/A | N/A | |
| 37 | Ahmed et al. [50] | Pathogenic microorganisms (Streptococcus pyogenes) | Impedimetric sensors | Impedance-based electrochemical measurements | N/A | High (4% charge transfer resistance) |
N/A |
| 38 | Wignarajah et al. [51] | Pathogenic microorganisms (Porphyromonas gingivalis proteases) | Multiplex colorimetric biosensor | Colorimetric detection | HNE: 1 pg/mL Cathepsin G: 100 fg/mL |
N/A | N/A |
| 39 | Hoyos-Nogués et al. [52] | Pathogenic microorganisms (Streptococcus sanguinis) | Peptide-based biosensor (hLf1-11) | Electrochemical impedance spectroscopy | 8.6 × 102 CFU·mL−1 | N/A | 3.85 ± 1.3 kΩ per bacteria concentration decade |
| 40 | Xue et al. [53] | Pathogenic microorganisms (detection of viral antibodies) | Immunoassay utilizing microchannels within a multicapillary glass plate | Fluorescence detection | 0.05 ng/mL | N/A | High (recovery ratio between 93.7%–112.2%) |
| 41 | Jin et al. [54] | Pathogenic microorganisms (bacteria and viruses) | Microfluidic system (SLIM) | Isothermal optical detection | N/A | N/A | 78.6% |
| 42 | Zaitouna et al. [55] | Anti-HIV antibodies | Electrochemical peptide-based sensor enhanced with extra amino acids | Electrochemical detection | 1 nM | N/A | Selectivity factor: 7.8 |
| 43 | Song et al. [56] | Cyfra 21-1 | 3DN-CNTs sensor | Fluorescence detection | 0.5 ng/mL | N/A | N/A |
| 44 | Chen et al. [57] | c-erbB-2 oncogene tumor marker | Fluorescent biosensor | Fluorescence detection | 20 fM | High (discrimination factor ~ 1) | RSD = 1.46% (n = 8) |
| 45 | Cho et al. [58] | Vascular endothelial growth factor-165 (VEGF165) | Surface-enhanced fluorescent optical sensor | Fluorescence detection | 25 pg/mL | N/A | N/A |
| 46 | Yu et al. [59] | Carcinoembryonic antigen | Capillary-based 3D fluoroimmunosensor | Fluorescence detection | 0.2 ng/mL | N/A | High (recovery ratio between 92.82%–118.81) |
| 47 | Machini et al. [60] | Acetazolamide | Electrochemical sensor using binuclear oxo-manganese complex | Electrochemical detection | 4.76 × 10−9 mol L−1 | N/A | N/A |
| 48 | Yu et al. [61] | Ampicillin | Electrochemical aptamer-based sensor (E-AB) | Electrochemical aptamer detection | ACV: 1 μ MSWV: 30 μM |
N/A | N/A |
| 49 | Hagen et al. [62] | Orexin A | Electronic based (FET) biosensor | Field-effect detection | sub-picomolar levels | N/A | N/A |
† N/A = non-applicable/not available information.
4. Discussion
As revealed by the present literature review, the attention given to the applicability of biosensors for salivary determinations has increased in the recent years. The applications of these diagnostic tools have a wide spectrum, from general pathologies to dentistry and pharmacology.
4.1. Biochemical Metabolic Compounds
4.1.1. Glucose
In the recent medical literature, there is a growing interest in metabolic products in saliva for monitoring the treatment effectiveness in various pathologies. The main focus was on the identification and quantification of glucose for monitoring diabetes. At the moment, the regular monitoring of blood glucose in diabetic patients involves the finger prick test, which is not only painful but also has been linked to major scarring in the fingers. Saliva, on the other hand, is easily collected without any of the aforementioned disadvantages. Furthermore, a strong correlation has been established between blood and the salivary levels of glucose in healthy patients, as well as in patients suffering from diabetes mellitus type 1 and 2. Thus, saliva could be an alternative biological fluid suitable for the monitoring of diabetes [2,63,64].
The use of sensors for detecting salivary glucose was reported in 2013 by Ye et al. who developed a CuO nanoneedle/graphene/carbon nanofiber modified glassy carbon electrode biosensor. The sensor was tested on saliva collected from healthy volunteers, and the findings indicated a rapid response as well as a high sensibility (minimum detection limit of 912.7 AmM−1 cm−2) and repeatability [16].
Other glucose-detecting sensors were developed by Li et al. in 2015 (electrochemical sensor using anodized cupric oxide nanowires, which was tested for calibration against serum glucose concentration), by Wang et al. in 2016 (core-shell IrO2@NiO nanowire), and by Du et al. in 2016 (a screen-printed sensor chip) [2,17,18].
A novel idea was the development of a constant-monitoring sensor designed for detecting surges of glucose intake in a patient over a set period of time. In that regard, Arakawa et al. proposed a sensor encased in a mouthguard wearable over a longer period of time. The mouthguard included a platinum and silver/silver chloride electrode, with glucose oxidase (GOD) immobilised by entrapment with Poly (MPC-co-EHMA) glucose sensor and a wireless transmitter. The mouthpiece was tested on a phantom jaw, using artificial saliva, a proved high sensitivity, and the ability to detect glucose in concentrations ranging from 5 to 1000 mmol/L [19].
Another personal-use device was developed by Soni et al. as a paper-based enzymatic sensor and a smartphone auxiliary device in order to reduce the dependability of expensive auxiliary devices for glucose determination in saliva. The paper-based sensor was designed to change colour in contact with glucose, the saturation being directly proportional to the concentration of glucose in the sample. Afterwards, the sensor was scanned with a special RGB-analysing software through a smartphone camera. The system was tested on both healthy and diabetic subjects, and a strong correlation between the salivary and blood glucose was reported (0.44 in healthy subjects, 0.64 in prediabetic patients, and 0.94 in diabetic patients) [20].
Other biochemical sensors for glucose included spectrophotometric detection using a low-cost colorimeter (Dominguez et al. 2017) [21]; using a colloidal AgNPs/MoS2-based nonenzymatic glucose biosensor (Anderson et al. 2017) [22]; tested on both saliva and sweat with similar performances; using randomly oriented CuO nanowire networks (Bell et al. 2017) [23]; using CuO-modified screen-printed carbon electrodes (SPCE; Velmurugan et al. 2017) [24]; using molecularly imprinted polymer binding on a conducting polymer layer (Kim et al. 2017) [25]; tested on both saliva and blood, methylene blue, hydrazine and platinum nanoparticles (Dutta et al. 2017); and using paper-based sensors (Santana-Jiménez et al. 2018) [26,27], standing to prove a high interest in the development of these medical devices.
4.1.2. Cortisol and Cortisone
Cortisol is known to be a steroid hormone correlated with the stress levels, as well as other pathologies, such as Cushing’s syndrome and Addison’s disease. A directly proportional correlation was established between the circadian variations of cortisol concentration in blood and saliva, which led to the development of sensors able to detect and quantify cortisol salivary levels [28,65,66].
The first biosensor able to detect cortisol was developed by Mitchell et al., as a surface plasmon resonance (SPR) immunosensor. After being tested on both a buffer solution and on human saliva from healthy volunteers the sensor was proved to be highly sensitive (lower detection limit of 162 RU.mL/ng) [28].
Another SPR immunosensor was used by Frasconi et al. for the detection of both cortisol and cortisone. The sensors had a low response time (15−20 min), a high reusability (up to 100 times), and a low detection limit (3 μg L−1). The sensor was tested on both saliva and urine, using the proposed method and a reference liquid chromatography/tandem mass spectrometry method. [31].
Two other biosensors were used for the detection of cortisone by Pires et al. in 2014 (a chemiluminescent organic-based immunosensor) and by Usha et al. in 2017 (a lossy mode resonance-based fiber optic) [29,30].
4.1.3. Other Biochemical Metabolic Compounds
Ballesta Claver et al. used an electrochemiluminescent biosensor for the detection of blood lactate in critical-state patients for preventing heart attacks in patients with diabetes mellitus in sports medicine as well as for food analysis [32].
A screen-printing technology on a flexible polyethylene terephthalate substrate was reported by Kim et al. for the detection and quantification of uric acid. The sensor was included in a wearable mouthguard connected to a wireless device through Bluetooth for data collection. The sensor was reported to have high selectivity (detection of 350 μM of uric acid in a solution with relevant physiological interferents), sensitivity (1.08 μA/mM), and stability; moreover, the idea of extending this continuous monitoring to other metabolites or substances was iterated [33].
Lastly, an electrochemical biosensor mounted on a mouthguard used for the monitoring of an advanced glycation end product, N-Carboxymethyl-lysine, was proposed by Ciui et al. The sensor proved high selectivity and sensitivity in a phosphate buffer with a limit of detection of 166 ng/mL (equivalent to 0.81 μM) over a range of 0.5–2500 μg/mL (equivalent to 2.45 μM–12.24 mM) of N-Carboxymethyl-lysine. Thus, the sensor proved a good reproducibility and a good selectivity against interferences from normal salivary constituents within physiological values. The short timescale required for the measurements, a long storage stability, and the ease of use are important advantages of the new mouthguard sensor [34].
4.2. Proteinases And Other Proteins
Salivary proteins have a major role in the digestive function of saliva. The concentration and activity of salivary amylase, one of the main components involved in oral digestion, has been correlated with the oral cancer, tobacco use, and cardiovascular disease. Hence, over the last years there was an increasing interest in developing sensors for salivary amylase detection and quantification. Lee et al. developed a biosensor consisting of molecularly imprinted thin films that, after being tested on saliva from five healthy subjects, proved an accuracy between 93.89% and 95.52% [35]. Another nano-sensor used for salivary amylase was developed by Attia et al. The sensors were applied on different starch-containing foods and showed high sensitivity (detection limit of 5.7 × 10−11 mol/L−1) [36].
Matrix metalloproteinases (MMP) are endopeptidases known for their ability to physiologically or pathologically cleave the components of the connective tissues. These enzymes, when activated by bacterial pathogens, have been linked to the destruction of periodontal soft tissues in periodontitis; the increase in collagenases salivary levels has been demonstrated to occur before the structural damage, allowing for an early diagnosis [37,38]. In this regard, a surface plasmon resonance immunosensor has been developed by Mohseni et al. in 2016 based on a carboxymethyldextran hydrogel sensor chip with immobilized monoclonal MMP-9 antibodies for the detection of MMP-9. The sensor was tested on healthy saliva with different protein concentrations (10−200 ng/mL), revealing that the final device had a detection limit of 8 pg/mL [36].
Another sensor for detecting MMP-1, MMP-8, and MMP-9 was proposed by Ritzer et al. as particle-bound protease cleavable linkers, delivered as a diagnostic chewing gum, used for the early detection of peri-implantitis. The sensor was designed to cleave in the presence of MMPs, releasing a strongly bitter taste. The device was tested on 33 patients (14 healthy and 19 patients with signs of mucositis/peri-implantitis), and the reaction was significantly different in the two groups after both a 5−10 min. incubation (1.5 ± 0.8% versus 4.2 ± 3.34%) and a 60 min. incubation (7.6 ± 4.4% versus 17.1 ± 11.1%) [38].
Human serum albumin (HSA) is the most abundant protein in the human body, accounting for approximately 60% of the total plasma proteins. Under pathological conditions, such as stomatitis associated with chemotherapy or type 2 diabetes, the salivary concentrations of HSA rise above the normal 0.5 g/L [39,67]. Hence, the direct detection of HSA concentration in saliva using a homogeneous fluorescent sensor has been proposed by Rongsheng et al. This device was tested on saliva samples from healthy volunteers, aged between 18 and 65 years, and a detection time between 40 and 50 min. was reported. Furthermore, no cross-reactivity was observed against other plasma proteins, such as human insulin, human C-reactive protein (CRP), or human IgG [31].
Cystatins are a family of proteins implied in regulatory and protective processes in the body. Their quantification in human blood and urine was used for the diagnosis of several diseases (cancer, kidney failure), whereas a low concentration in human saliva (normal values between 0.36 and 4.8 μg/mL) may indicate a predisposition to caries or the presence of active carious processes [40,68]. Gorodkiewicz et al. proposed a surface plasmon resonance imaging (SPRI) biosensor for the detection of cystatin in blood, saliva, and urine. The sensor was tested on six saliva samples, alongside blood plasma and urine, and detected the protein in all samples with concentrations within the normal physiological limits [32].
Gorodkiewicz et al. also proposed a second SPRI immunosensor for the detection of cathepsin D (CatD) and cathepsin E (CatE), as well as a third sensor for the detection of cathepsin G (CatG) [41,42]. CatD and CatE are aspartic peptidases, and their increased concentrations are a prognostic marker of cancer. The selectivity of the SPRI immunosensor was tested against cathepsin B (CatB); the sensor was not influenced by the presence of CatB, even if the concentration was increased 1000-fold [33].
CatG plays a role in the early immune response, as well as in coagulation and normal tissue degradation. An increased activity of CatG was associated with obstructive pulmonary disease, cancer, or emphysema [42,69]. The developed SPRI immunosensor used a specifically synthesised CatG inhibitor, MARS-115, which showed no response to other cathepsins. The sensor was tested for six saliva samples and accurately identified and quantified the peptidase [34].
An electrochemical sensor for the early detection of oral cancer was proposed by Wei et al., focusing on the salivary biomarkers interleukin (IL)-8 mRNA and IL-8 protein. After being tested on both oral cancer patients and healthy subjects, good specificity was proven through amperometric detection (IL-8 mRNA: −904 nA versus S100A8: −103 nA current; IL-8 protein: −298 nA versus IL-1 h protein: −50 nA) [43].
Lastly, the detection of L-tryptophan, a standard amino acid, was reported by Majidi et al., using two screen printed electrodes modified with a multiwall carbon nanotube (MWCNT-AuSPE) and then armed with Trp aptamer molecules (Apt-MWCNT-AuSPE). The sensors were tested against interferants (amino acids, glucose, and heavy metal ions), but the sensors produced no overlapping signals, even if the physiological concentrations exceeded by twenty-five fold [44].
4.3. Miscellaneous Chemical Compounds
The detection and monitoring of potassium iodine (KI) in saliva using a calixarene-based tubes ISFET (ion-selective field effect transistor) system was proposed by Puchnin et al., considering the high concentration of the drug in the salivary glands. The ISFET sensor modified with self-assembly Calixtube Monolayers allows the formation of inclusion complexes with KI, proving the specific discrimination between KI and other iodides due to the specific dimension of the molecule. The sensor detects the KI not the cation or the anion. KI is used for the treatment of dermatological inflammatory diseases. The sensor was tested for selectivity in artificial saliva, both spiked with KI and KI-free, proving distinctive responses in both situations. The detection limit was approximately 3 × 10−8 M, showing promises for clinical applications [45].
Minami et al. developed a nitrate biosensor based on the extended-gate type organic field-effect transistor. Nitrate can be found as an additive in processed food; it is also used for the prevention of cardiovascular disease. A high intake of this substance can cause different forms of cancer [46,70]. The sensor was applied on diluted human saliva, obtained from a healthy volunteer, in order to test its specificity. The recovery for the added nitrate solution was estimated at 97.4 ± 1.8%, comparable to other commercially available colorimetric-based devices [38].
A potentiometric membrane sensor was designed by Hassan et al. for the detection of thiocyanate, a compound excreted in urine and saliva and considered a biomarker for smokers and non-smokers. The sensor was tested on saliva and urine samples from both smokers and non-smokers and proved low detection limits (5.6 × 10−6 mol/L) and a rapid response time (10 s) [47].
Another sensor was developed for the detection of silver and mercury by Zheng et al. The sandwich-structured SERS probe with a gold nanohole array pattern proved a limit of detection for silver of 0.17 nM and a limit of detection for mercury of 2.3 pM [1].
Caffeine was identified in human saliva for the purpose of monitoring the drug metabolizing system activity in hepatocytes by Timofeeva et al. using a PVC membrane electrode biosensor. The device was tested on saliva provided by volunteers before and four hours after the ingestion of a caffeine pill, and it was proved that the normal metabolites in saliva did not interfere with the detection process, even in excess of 100- to 200-fold. Moreover, the sensor was tested using the already established HPLC (high performance liquid chromatography) method, showing insignificant differences between the two methods at a 95% confidence level [48].
Lastly, Zilberman et al. reported the development of a portable optoelectronic microfluidic sensor used for the detection of ammonia and carbon dioxide in saliva, secreted by Helicobacter pylori, used in the screening of stomach cancer. The sensor was tested on both healthy, unaltered saliva, as well as on saliva spiked with carbon dioxide and ammonia, showing good sensibility and selectivity [49].
4.4. Bacteria and Viruses
The direct determination of pathogens, such as bacteria and viruses, is of great importance not only in the early diagnosis of diseases but also in the monitoring the treatment efficiency.
Ahmed et al. proposed an impedimetric biosensor for the detection of a group A Streptococcus, S. pyogenes, incriminated for causing suppurative infections and possibly leading to streptococcal shock-like syndrome. The sensor’s properties were tested on human saliva, spiked with S. pyogenes (107 cells/mL), and the bacteria showed a 4% charge transfer resistance, proving a high selectivity [50].
A microfluidic system (SLIM) for the detection of both bacteria and viruses was developed by Jin et al. The system was tested on 10 saliva samples that were previously positively diagnosed with a herpes zoster infection by real-time PCR. All of the samples were confirmed using the SLIM system and showed a sensitivity of 78.6% [54].
Xue et al. used an immunoassay based on microchannels within the multicapillary glass plate for the detection of viral antibodies and the diagnosis of viral infections. Recovery trials were conducted by adding standard h-IgA to six saliva samples collected from healthy volunteers and revealed that the system had a recovery ratio between 93.7%–112.2%, proving its high sensibility. Similar results were obtained by applying the system to environmental swabs and blood plasma [53].
Zaitouna et al. used an electrochemical peptide-based sensor enhanced with extra amino acids for the detection of anti-HIV antibodies. The peptide sensor spiked with amino acids had a selectivity factor of 7.8 and a limit of detection of 1 nM [55].
The detection of pathogens is also relevant in the early diagnosis of inflammatory periodontal diseases in both natural teeth and implants. Porphyromonas gingivalis proteases were determined by Wignarajah et al. using a multiplex colorimetric biosensor for detecting the chronic periodontal disease. The lower detection limit was reported at 1 pg/mL for HNE (Human Neutrophil Elastase) and 100 fg/mL for Cathepsin G, and the detection speed ranged between 20 and 30 s. [51].
Lastly, Hoyos-Nogués et al. used a peptide-based biosensor for the detection of Streptococcus sanguinis, a pathogen linked to inflammatory diseases involving dental implants. The sensor was tested in artificial saliva with varying concentrations of S. sanguinis, (10 to 105 CFU·mL−1); the limit of detection was 8.6·102 CFU·mL−1, with a sensitivity of 3.85 ± 1.3 kΩ per bacteria concentration decade [52].
4.5. Oncology Markers
The use of salivary biosensors also has applications in oncology, helping for the screening and early diagnosis of different cancers, by the detection of biomarkers. In this regard, Song et al. reported the development of a fluorescence-based immunosensor comprised of a hierarchical three-dimensional network of carbon nanotubes on a Si pillar substrate (3DN-CNTs). The sensor was used for the detection of a Cytokeratin-19 antigen (Cyfra 21-1) for the accurate diagnosis of oral squamous cell carcinoma (OSCC). The testing was carried out on 11 saliva samples (4 healthy and 7 from patients with OSCC), resulting in a limit of detection of 0.5 ng/mL and proving to be valid for clinical samples between 1 and 62.5 ng/mL [56].
Another fluorescent biosensor was designed by Chen et al., for the detection of the c-erbB-2 oncogene that could be useful in the early diagnosis of breast cancer. The device was tested for unstimulated saliva samples, with a detection limit of the oncogene of 20 fM and a small discrimination factor (approximately 1), proving its high specificity [57].
Cho et al. developed a surface-enhanced fluorescent optical sensor for the detection of the vascular endothelial growth factor-165 (VEGF165), a marker of cancer angiogenesis. The properties of the biosensor were tested on eight samples of stimulated saliva and blood plasma (four healthy and four with various forms of cancer), showing proportionate responses at VEGF165 concentrations from 25 pg/mL to 25 μg/mL [58].
Lastly, Yu et al. developed a capillary-based 3-D fluoro-immunosensor capable of detecting and quantifying the salivary levels of carcinoembryonic antigen which were involved in a wide array of cancers, with a limit of detection of 0.2 ng/mL and a relative mean recovery rate between 92.82% and 118.81% [59].
4.6. Drugs
The determination of drug intake using salivary biosensors could be useful for detecting illicit drugs intake. Machini et al. developed an electrochemical sensor based on a binuclear oxo-manganese complex for the detection of acetazolamide, a drug associated with doping in sports. The sensor testing in saliva samples revealed a detection limit of 4.76 × 10−9 mol·L−1 and maximum recovery errors of +2%, similar to the ones obtained in blood plasma and urine [60].
Biosensors and the determination of different compounds in saliva can also be applied in pharmacology for the evaluation of the efficiency of a treatment or the need for dosage adjustments. Yu et al. developed an electrochemical aptamer-based sensor for the detection of ampicillin in different biological fluids, including saliva. Ampicillin determination could be useful for determining the correct therapeutic concentration and the best way of administration. The sensor’s response was evaluated using two techniques: alternating current voltammetry (ACV) and square wave voltammetry (SWV), and the limit of detection varied from 1 μM (ACV) to 30 μM (SWV) [61].
4.7. Neurotransmitters
The detection of orexin A, an indicator used in the assessment of cognitive performance and fatigue, was accomplished by Hagen et al. using an electronic based (FET) biosensor. The compound was detected at concentrations of 10 fM in human saliva specimens and serum with the lowest detection limit established at sub-picomolar levels [62].
4.8. Future Perspectives
Considering the substantial data obtained through fundamental research on existing biosensors, several future perspectives can be drawn on these medical devices. Sensors need to be easily manufactured, at a low price, in order to be available to the wide population. Moreover, salivary biosensors need to be miniaturised and integrated in medical devices, such as mouthguards, or even included in dental prosthesis, dental restorations, or tooth surface retentions. However, this idea raises a few questions. Firstly, the mechanical retention of the device needs to be investigated and a correct long-term protocol for the adhesion of the sensor must be elaborated. Several steps may be necessary for the preparation of the teeth or prosthodontics pieces, such as the etching of the desired surfaces or the creation of micro-retentions using a sandblaster or burs. Secondly, the interference of food intake and microbiome with the readings must be controlled, as it may influence the data interpretation. Furthermore, the analysis of results has to exclude or take into account the oral pathology which can influence these dates recorded by salivary biosensor. Lastly, the durability and lifespan of the devices must be established in order for the medical personnel to plan the replacement of the sensor.
Wireless transmission of the data has already been achieved using Bluetooth connections. In theory, this could enable a continuous flow of the recorded information to already widespread and available mobile devices, allowing for a continuous monitoring by the patient or medical personnel. Such a large amount of data could be easily interpreted by doctors and alerts could be transmitted in real time as spikes in the normal readings. This would allow for the evaluation of drugs administration plans, the assessment of treatment efficiency, and the analysis of lifestyle.
Lastly, given the complex composition of saliva and its importance to the homeostasy of the oral cavity, further research might be needed in order to develop biosensors for all its components (Table 3) [71,72].
Table 3.
The main salivary constituents and the biosensors developed for their determination.
| Category | Compounds | Yes | No |
|---|---|---|---|
| Electrolytes | Sodium | x | |
| Potassium | x | ||
| Calcium | x | ||
| Magnesium | x | ||
| Phosphate | x | ||
| Iodine | x | ||
| Chloride | x | ||
| Bicarbonate | x | ||
| Mucus | Mucoplysaccharides | x | |
| Glycoproteins | x | ||
| Antibacterial compounds | Thiocyanate | x | |
| Hydrogen peroxide | x | ||
| Immunoglobulin A | x | ||
| Immunoglobulin G | x | ||
| Immunoglobulin M | x | ||
| Limphocytes | x | ||
| Monocites | x | ||
| Enzymes | α-amylase | x | |
| Lipase | x | ||
| Kallikrein | x | ||
| Lysozyme | x | ||
| Lactoperoxidase | x | ||
| Lactoferrin | x | ||
| Cells | Desquamated epithelial cells | x | |
| Bacteria | x | ||
| Nitrogenous products | Urea | x | |
| Uric acid | x | ||
| Ammonia | x | ||
| Amino acids | |||
| Glucides | Glucose | x | |
| Epidermal growth factors | x | ||
| Proteins | x |
5. Conclusions
As shown in the present literature review, a great number of studies focused on the development of easy-to-use sensors with applicability on saliva, useful for general medicine, dental care, and pharmacology. As underlined by the authors, further clinical trials are required prior to the applicability of biosensors to the wide population. Furthermore, given the individual variations in salivary compounds, the need of setting a baseline for each patient should be analyzed.
Regarding the devices developed for long-term patient surveillance, such as mouthguards, clinical studies should investigate the potential toxic effects of the materials or of the sensors themselves. Even though biocompatible materials are being used by developers and researchers, further investigations should be conducted to prove that the device is suitable to be worn in the oral cavity.
As technology progresses, the high number of compounds that can be reliably detected in saliva is expected to increase. This could enhance the clinical applicability, provide reliable diagnostic or screening tools for the doctors, and improve the patients’ quality of life.
Acknowledgments
This study was financed by the COFUND-ERA-HDHL ERANET Project, European and International Cooperation Subprogram 3.2 Horizon 2020, PNCDI III Program Biomarkers for Nutrition and Health “Innovative technological approaches for validation of salivary AGEs as novel biomarkers in evaluation of risk factors in diet-related diseases”, grant no 25/1.09.2017.
Author Contributions
Conceptualization, A.I., R.S.C., R.S. and C.C.; methodology, A.I., V.A., C.N.F., M.T. and C.C.; software, V.A., C.N.F., and A.-M.B.; validation, B.C., M.T., R.S. and C.C.; formal analysis, A.B.B., A.-M.B. and N.B.P.; investigation, V.A., C.N.F., A.B.B. and N.B.P.; resources, V.A., C.N.F., A.B.B. and N.B.P.; data curation, B.C. and M.T.; writing—original draft preparation, V.A., C.N.F. and A.B.B.; writing—review and editing, A.I., V.A., C.N.F. and A.B.B.; visualization, B.C. and M.T.; supervision, A.I., R.S., R.S.C. and C.C.; project administration, A.I.; funding acquisition, A.I.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Zheng P., Li M., Jurevic R., Cushing S.K., Liu Y., Wu N. A gold nanohole array based surface-enhanced Raman scattering biosensor for detection of silver(I) and mercury(II) in human saliva. Nanoscale. 2015;7:11005–11012. doi: 10.1039/C5NR02142A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Du Y., Zhang W., Wang M.L. An On-Chip Disposable Salivary Glucose Sensor for Diabetes Control. J. Diabetes Sci. Technol. 2016;10:1344–1352. doi: 10.1177/1932296816642251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaczor-Urbanowicz K.E., Martin Carreras-Presas C., Aro K., Tu M., Garcia-Godoy F., Wong D.T. Saliva diagnostics—Current views and directions. Exp. Biol. Med. 2017;242:459–472. doi: 10.1177/1535370216681550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tiwari M. Science behind human saliva. J. Nat. Sci. Biol. Med. 2011;2:53–58. doi: 10.4103/0976-9668.82322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iorgulescu G. Saliva between normal and pathological. Important factors in determining systemic and oral health. J. Med. Life. 2009;2:303–307. [PMC free article] [PubMed] [Google Scholar]
- 6.Bandodkar A.J., Wang J. Non-invasive wearable electrochemical sensors: A review. Trends Biotechnol. 2014;32:363–371. doi: 10.1016/j.tibtech.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 7.Kilian M., Chapple I.L.C., Hannig M., Marsh P.D., Meuric V., Pedersen A.M.L., Tonetti M.S., Wade W.G., Zaura E. The oral microbiome—An update for oral healthcare professionals. Br. Dent. J. 2016;221:657–666. doi: 10.1038/sj.bdj.2016.865. [DOI] [PubMed] [Google Scholar]
- 8.Marcotte H., Lavoie M.C. Oral Microbial Ecology and the Role of Salivary Immunoglobulin A. Microbiol. Mol. Biol. Rev. 1998;62:71–109. doi: 10.1128/mmbr.62.1.71-109.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berezow A.B., Darveau R.P. Microbial Shift and Periodontitis. Periodontology 2000. 2011;55:36–47. doi: 10.1111/j.1600-0757.2010.00350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sonis S.T. The Chicken or the Egg? Changes in Oral Microbiota as Cause or Consequence of Mucositis During Radiation Therapy. EBioMedicine. 2017;18:7–8. doi: 10.1016/j.ebiom.2017.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hao Y., Huang X., Zhou X., Li M., Ren B., Peng X., Cheng L. Influence of Dental Prosthesis and Restorative Materials Interface on Oral Biofilms. Int. J. Mol. Sci. 2018;19:3157. doi: 10.3390/ijms19103157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marchi-Alves L.M., Freitas D., de Andrade D., de Godoy S., Toneti A.N., Mendes I.A.C. Characterization of Oral Microbiota in Removable Dental Prosthesis Users: Influence of Arterial Hypertension. BioMed Res. Int. 2017;2017 doi: 10.1155/2017/3838640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uriciuc W.A., Vermeșan H., Boșca A.B., Ilea A. Interaction of Saliva with Cobalt-Chromium-Based Dental Alloys in Casted Prosthetic Pieces. Curr. Trends Biomed. Eng. Biosci. 2018;14:555882. [Google Scholar]
- 14.Esin S., Pasini M., Miceli M., Cosseddu G., Giuca M.R., Batoni G. Longitudinal study on the effect of oral hygiene measures on the salivary count of microbial species with cariogenic potential. J. Biol. Regul. Homeost. Agents. 2018;32:1407–1420. [PubMed] [Google Scholar]
- 15.Bhalla N., Jolly P., Formisano N., Estrela P. Introduction to biosensors. Essays Biochem. 2016;60:1–8. doi: 10.1042/EBC20150001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ye D., Liang G., Li H., Luo J., Zhang S., Chen H., Kong J. A novel nonenzymatic sensor based on CuO nanoneedle/graphene/carbon nanofiber modified electrode for probing glucose in saliva. Talanta. 2013;116:223–230. doi: 10.1016/j.talanta.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 17.Li Z., Chen Y., Xin Y., Zhang Z. Sensitive electrochemical nonenzymatic glucose sensing based on anodized CuO nanowires on three-dimensional porous copper foam. Sci. Rep. 2015;5:16115. doi: 10.1038/srep16115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang J., Xu L., Lu Y., Sheng K., Liu W., Chen C., Li Y., Dong B., Song H. Engineered IrO2@NiO Core-Shell Nanowires for Sensitive Non-enzymatic Detection of Trace Glucose in Saliva. Anal. Chem. 2016;88:12346–12353. doi: 10.1021/acs.analchem.6b03558. [DOI] [PubMed] [Google Scholar]
- 19.Arakawa T., Kuroki Y., Nitta H., Chouhan P., Toma K., Sawada S.-I., Takeuchi S., Sekita T., Akiyoshi K., Minakuchi S., et al. Mouthguard biosensor with telemetry system for monitoring of saliva glucose: A novel cavitas sensor. Biosens. Bioelectron. 2016;84:106–111. doi: 10.1016/j.bios.2015.12.014. [DOI] [PubMed] [Google Scholar]
- 20.Soni A., Jha S.K. Smartphone based non-invasive salivary glucose biosensor. Anal. Chim. Acta. 2017;996:54–63. doi: 10.1016/j.aca.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 21.Dominguez R.B., Orozco M.A., Chávez G., Márquez-Lucero A. The Evaluation of a Low-Cost Colorimeter for Glucose Detection in Salivary Samples. Sensors. 2017;17:2495. doi: 10.3390/s17112495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anderson K., Poulter B., Dudgeon J., Li S.-E., Ma X. A Highly Sensitive Nonenzymatic Glucose Biosensor Based on the Regulatory Effect of Glucose on Electrochemical Behaviors of Colloidal Silver Nanoparticles on MoS2. Sensors. 2017;17:1807. doi: 10.3390/s17081807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bell C., Nammari A., Uttamchandani P., Rai A., Shah P., Moore A.L. Flexible electronics-compatible non-enzymatic glucose sensing via transparent CuO nanowire networks on PET films. Nanotechnology. 2017;28:245502. doi: 10.1088/1361-6528/aa7164. [DOI] [PubMed] [Google Scholar]
- 24.Velmurugan M., Karikalan N., Chen S.-M. Synthesis and characterizations of biscuit-like copper oxide for the non-enzymatic glucose sensor applications. J. Colloid Interface Sci. 2017;493:349–355. doi: 10.1016/j.jcis.2017.01.044. [DOI] [PubMed] [Google Scholar]
- 25.Kim D.-M., Moon J.-M., Lee W.-C., Yoon J.-H., Choi C.S., Shim Y.-B. A potentiometric non-enzymatic glucose sensor using a molecularly imprinted layer bonded on a conducting polymer. Biosens. Bioelectron. 2017;91:276–283. doi: 10.1016/j.bios.2016.12.046. [DOI] [PubMed] [Google Scholar]
- 26.Dutta G., Nagarajan S., Lapidus L.J., Lillehoj P.B. Enzyme-free electrochemical immunosensor based on methylene blue and the electro-oxidation of hydrazine on Pt nanoparticles. Biosens. Bioelectron. 2017;92:372–377. doi: 10.1016/j.bios.2016.10.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santana-Jiménez L.A., Márquez-Lucero A., Osuna V., Estrada-Moreno I., Dominguez R.B. Naked-Eye Detection of Glucose in Saliva with Bienzymatic Paper-Based Sensor. Sensors. 2018;18:1071. doi: 10.3390/s18041071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mitchell J.S., Lowe T.E., Ingram J.R. Rapid ultrasensitive measurement of salivary cortisol using nano-linker chemistry coupled with surface plasmon resonance detection. Analyst. 2009;134:380–386. doi: 10.1039/B817083P. [DOI] [PubMed] [Google Scholar]
- 29.Pires N.M.M., Dong T. Measurement of salivary cortisol by a chemiluminescent organic-based immunosensor. Biomed. Mater. Eng. 2014;24:15–20. doi: 10.3233/BME-130778. [DOI] [PubMed] [Google Scholar]
- 30.Usha S.P., Shrivastav A.M., Gupta B.D. A contemporary approach for design and characterization of fiber-optic-cortisol sensor tailoring LMR and ZnO/PPY molecularly imprinted film. Biosens. Bioelectron. 2017;87:178–186. doi: 10.1016/j.bios.2016.08.040. [DOI] [PubMed] [Google Scholar]
- 31.Frasconi M., Mazzarino M., Botrè F., Mazzei F. Surface plasmon resonance immunosensor for cortisol and cortisone determination. Anal. Bioanal. Chem. 2009;394:2151–2159. doi: 10.1007/s00216-009-2914-6. [DOI] [PubMed] [Google Scholar]
- 32.Ballesta Claver J., Valencia Mirón M.C., Capitán-Vallvey L.F. Disposable electrochemiluminescent biosensor for lactate determination in saliva. Analyst. 2009;134:1423–1432. doi: 10.1039/b821922b. [DOI] [PubMed] [Google Scholar]
- 33.Kim J., Imani S., de Araujo W.R., Warchall J., Valdés-Ramírez G., Paixão T.R.L.C., Mercier P.P., Wang J. Wearable salivary uric acid mouthguard biosensor with integrated wireless electronics. Biosens. Bioelectron. 2015;74:1061–1068. doi: 10.1016/j.bios.2015.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ciui B., Tertis M., Feurdean C.N., Ilea A., Sandulescu R., Wang J., Cristea C. Cavitas electrochemical sensor toward detection of N-epsilon (carboxymethyl)lysine in oral cavity. Sens. Actuators B Chem. 2019;281:399–407. doi: 10.1016/j.snb.2018.10.096. [DOI] [Google Scholar]
- 35.Lee M.-H., Thomas J.L., Tseng H.-Y., Lin W.-C., Liu B.-D., Lin H.-Y. Sensing of digestive proteins in saliva with a molecularly imprinted poly(ethylene-co-vinyl alcohol) thin film coated quartz crystal microbalance sensor. ACS Appl. Mater. Interfaces. 2011;3:3064–3071. doi: 10.1021/am2005724. [DOI] [PubMed] [Google Scholar]
- 36.Attia M.S., Zoulghena H., Abdel-Mottaleb M.S.A. A new nano-optical sensor thin film cadmium sulfide doped in sol-gel matrix for assessment of α-amylase activity in human saliva. Analyst. 2014;139:793–800. doi: 10.1039/C3AN01645E. [DOI] [PubMed] [Google Scholar]
- 37.Mohseni S., Moghadam T.T., Dabirmanesh B., Jabbari S., Khajeh K. Development of a label-free SPR sensor for detection of matrixmetalloproteinase-9 by antibody immobilization on carboxymethyldextran chip. Biosens. Bioelectron. 2016;81:510–516. doi: 10.1016/j.bios.2016.03.038. [DOI] [PubMed] [Google Scholar]
- 38.Ritzer J., Lühmann T., Rode C., Pein-Hackelbusch M., Immohr I., Schedler U., Thiele T., Stübinger S., Rechenberg B.V., Waser-Althaus J., et al. Diagnosing peri-implant disease using the tongue as a 24/7 detector. Nat. Commun. 2017;8:264. doi: 10.1038/s41467-017-00340-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang R.E., Tian L., Chang Y.-H. A homogeneous fluorescent sensor for human serum albumin. J. Pharm. Biomed. Anal. 2012;63:165–169. doi: 10.1016/j.jpba.2011.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gorodkiewicz E., Breczko J., Sankiewicz A. Surface Plasmon Resonance Imaging biosensor for cystatin determination based on the application of bromelain, ficin and chymopapain. Folia Histochem. Cytobiol. 2012;50:130–136. doi: 10.5603/FHC.2012.0019. [DOI] [PubMed] [Google Scholar]
- 41.Gorodkiewicz E., Regulska E. SPR imaging biosensor for aspartyl cathepsins: Sensor development and application for biological material. Protein Pept. Lett. 2010;17:1148–1154. doi: 10.2174/092986610791760450. [DOI] [PubMed] [Google Scholar]
- 42.Gorodkiewicz E., Sieńczyk M., Regulska E., Grzywa R., Pietrusewicz E., Lesner A., Lukaszewski Z. Surface plasmon resonance imaging biosensor for cathepsin G based on a potent inhibitor: Development and applications. Anal. Biochem. 2012;423:218–223. doi: 10.1016/j.ab.2012.01.033. [DOI] [PubMed] [Google Scholar]
- 43.Wei F., Patel P., Liao W., Chaudhry K., Zhang L., Arellano-Garcia M., Hu S., Elashoff D., Zhou H., Shukla S., et al. Electrochemical sensor for multiplex biomarkers detection. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2009;15:4446–4452. doi: 10.1158/1078-0432.CCR-09-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Majidi M.R., Omidi Y., Karami P., Johari-Ahar M. Reusable potentiometric screen-printed sensor and label-free aptasensor with pseudo-reference electrode for determination of tryptophan in the presence of tyrosine. Talanta. 2016;150:425–433. doi: 10.1016/j.talanta.2015.12.064. [DOI] [PubMed] [Google Scholar]
- 45.Puchnin K., Andrianova M., Kuznetsov A., Kovalev V. Field-effect transition sensor for KI detection based on self-assembled calixtube monolayers. Biosens. Bioelectron. 2017;98:140–146. doi: 10.1016/j.bios.2017.06.050. [DOI] [PubMed] [Google Scholar]
- 46.Minami T., Sasaki Y., Minamiki T., Wakida S.-I., Kurita R., Niwa O., Tokito S. Selective nitrate detection by an enzymatic sensor based on an extended-gate type organic field-effect transistor. Biosens. Bioelectron. 2016;81:87–91. doi: 10.1016/j.bios.2016.02.036. [DOI] [PubMed] [Google Scholar]
- 47.Hassan S.S.M., Badr I.H.A., Kamel A.H., Mohamed M.S. A novel poly(vinyl chloride) matrix membrane sensor for batch and flow-injection determinations of thiocyanate, cyanide and some metal ions. Anal. Sci. Int. J. Jpn. Soc. Anal. Chem. 2009;25:911–917. doi: 10.2116/analsci.25.911. [DOI] [PubMed] [Google Scholar]
- 48.Timofeeva I., Medinskaia K., Nikolaeva L., Kirsanov D., Bulatov A. Stepwise injection potentiometric determination of caffeine in saliva using single-drop microextraction combined with solvent exchange. Talanta. 2016;150:655–660. doi: 10.1016/j.talanta.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 49.Zilberman Y., Sonkusale S.R. Microfluidic optoelectronic sensor for salivary diagnostics of stomach cancer. Biosens. Bioelectron. 2015;67:465–471. doi: 10.1016/j.bios.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 50.Ahmed A., Rushworth J.V., Wright J.D., Millner P.A. Novel impedimetric immunosensor for detection of pathogenic bacteria Streptococcus pyogenes in human saliva. Anal. Chem. 2013;85:12118–12125. doi: 10.1021/ac403253j. [DOI] [PubMed] [Google Scholar]
- 51.Wignarajah S., Suaifan G.A.R.Y., Bizzarro S., Bikker F.J., Kaman W.E., Zourob M. Colorimetric Assay for the Detection of Typical Biomarkers for Periodontitis Using a Magnetic Nanoparticle Biosensor. Anal. Chem. 2015;87:12161–12168. doi: 10.1021/acs.analchem.5b03018. [DOI] [PubMed] [Google Scholar]
- 52.Hoyos-Nogués M., Brosel-Oliu S., Abramova N., Muñoz F.-X., Bratov A., Mas-Moruno C., Gil F.-J. Impedimetric antimicrobial peptide-based sensor for the early detection of periodontopathogenic bacteria. Biosens. Bioelectron. 2016;86:377–385. doi: 10.1016/j.bios.2016.06.066. [DOI] [PubMed] [Google Scholar]
- 53.Xue S., Zeng H., Yang J., Nakajima H., Uchiyama K. A compact immunoassay platform based on a multicapillary glass plate. Sensors. 2014;14:9132–9144. doi: 10.3390/s140509132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jin C.E., Koo B., Lee E.Y., Kim J.Y., Kim S.-H., Shin Y. Simple and label-free pathogen enrichment via homobifunctional imidoesters using a microfluidic (SLIM) system for ultrasensitive pathogen detection in various clinical specimens. Biosens. Bioelectron. 2018;111:66–73. doi: 10.1016/j.bios.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zaitouna A.J., Maben A.J., Lai R.Y. Incorporation of extra amino acids in peptide recognition probe to improve specificity and selectivity of an electrochemical peptide-based sensor. Anal. Chim. Acta. 2015;886:157–164. doi: 10.1016/j.aca.2015.05.037. [DOI] [PubMed] [Google Scholar]
- 56.Song C.K., Oh E., Kang M.S., Shin B.S., Han S.Y., Jung M., Lee E.S., Yoon S.-Y., Sung M.M., Ng W.B., et al. Fluorescence-based immunosensor using three-dimensional CNT network structure for sensitive and reproducible detection of oral squamous cell carcinoma biomarker. Anal. Chim. Acta. 2018;1027:101–108. doi: 10.1016/j.aca.2018.04.025. [DOI] [PubMed] [Google Scholar]
- 57.Chen J., Lin J., Zhang X., Cai S., Wu D., Li C., Yang S., Zhang J. Label-free fluorescent biosensor based on the target recycling and Thioflavin T-induced quadruplex formation for short DNA species of c-erbB-2 detection. Anal. Chim. Acta. 2014;817:42–47. doi: 10.1016/j.aca.2014.01.053. [DOI] [PubMed] [Google Scholar]
- 58.Cho H., Yeh E.-C., Sinha R., Laurence T.A., Bearinger J.P., Lee L.P. Single-step nanoplasmonic VEGF165 aptasensor for early cancer diagnosis. ACS Nano. 2012;6:7607–7614. doi: 10.1021/nn203833d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yu Q., Wang X., Duan Y. Capillary-based three-dimensional immunosensor assembly for high-performance detection of carcinoembryonic antigen using laser-induced fluorescence spectrometry. Anal. Chem. 2014;86:1518–1524. doi: 10.1021/ac402973n. [DOI] [PubMed] [Google Scholar]
- 60.Machini W.B.S., Teixeira M.F.S. Analytical development of a binuclear oxo-manganese complex bio-inspired on oxidase enzyme for doping control analysis of acetazolamide. Biosens. Bioelectron. 2016;79:442–448. doi: 10.1016/j.bios.2015.12.026. [DOI] [PubMed] [Google Scholar]
- 61.Yu Z.-G., Lai R.Y. A reagentless and reusable electrochemical aptamer-based sensor for rapid detection of ampicillin in complex samples. Talanta. 2018;176:619–624. doi: 10.1016/j.talanta.2017.08.057. [DOI] [PubMed] [Google Scholar]
- 62.Hagen J., Lyon W., Chushak Y., Tomczak M., Naik R., Stone M., Kelley-Loughnane N. Detection of orexin A neuropeptide in biological fluids using a zinc oxide field effect transistor. ACS Chem. Neurosci. 2013;4:444–453. doi: 10.1021/cn300159e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gupta S., Nayak M.T., Sunitha J.D., Dawar G., Sinha N., Rallan N.S. Correlation of salivary glucose level with blood glucose level in diabetes mellitus. J. Oral Maxillofac. Pathol. JOMFP. 2017;21:334–339. doi: 10.4103/jomfp.JOMFP_222_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kumar S., Padmashree S., Jayalekshmi R. Correlation of salivary glucose, blood glucose and oral candidal carriage in the saliva of type 2 diabetics: A case-control study. Contemp. Clin. Dent. 2014;5:312–317. doi: 10.4103/0976-237X.137925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dorn L.D., Lucke J.F., Loucks T.L., Berga S.L. Salivary cortisol reflects serum cortisol: Analysis of circadian profiles. Ann. Clin. Biochem. 2007;44:281–284. doi: 10.1258/000456307780480954. [DOI] [PubMed] [Google Scholar]
- 66.Gozansky W.S., Lynn J.S., Laudenslager M.L., Kohrt W.M. Salivary cortisol determined by enzyme immunoassay is preferable to serum total cortisol for assessment of dynamic hypothalamic-pituitary-adrenal axis activity. Clin. Endocrinol. 2005;63:336–341. doi: 10.1111/j.1365-2265.2005.02349.x. [DOI] [PubMed] [Google Scholar]
- 67.Leboffe L., di Masi A., Trezza V., Polticelli F., Ascenzi P. Human serum albumin: A modulator of cannabinoid drugs. IUBMB Life. 2017;69:834–840. doi: 10.1002/iub.1682. [DOI] [PubMed] [Google Scholar]
- 68.Dowd F.J. Saliva and dental caries. Dent. Clin. N. Am. 1999;43:579–597. [PubMed] [Google Scholar]
- 69.Burster T., Macmillan H., Hou T., Boehm B.O., Mellins E.D. Cathepsin G: Roles in antigen presentation and beyond. Mol. Immunol. 2010;47:658–665. doi: 10.1016/j.molimm.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jackson J., Patterson A.J., MacDonald-Wicks L., McEvoy M. The role of inorganic nitrate and nitrite in CVD. Nutr. Res. Rev. 2017;30:247–264. doi: 10.1017/S0954422417000105. [DOI] [PubMed] [Google Scholar]
- 71.Humphrey S.P., Williamson R.T. A review of saliva: Normal composition, flow, and function. J. Prosthet. Dent. 2001;85:162–169. doi: 10.1067/mpr.2001.113778. [DOI] [PubMed] [Google Scholar]
- 72.Nosek T.M. Essentials of Human Physiology. Gold Standard Multimedia Incorporated; Tampa, FL, USA: 1998. [Google Scholar]