Abstract
IN BRIEF Obstacles to realizing the clinical benefits of continuous glucose monitoring (CGM) for daily diabetes management are being overcome with more affordable, user-friendly technologies. This article describes a novel category of CGM known as “flash” that may allow more routine use of continuous data for greater numbers of patients treated in primary care.
Modern diabetes management came of age in the late 20th century, when major trials showed the benefit of close glucose control, A1C became the established standard of care, and smaller and more accurate glucose meters enabled regular self-monitoring of blood glucose (SMBG). Yet, even as A1C and SMBG remain cornerstones of diabetes care, the emergence of metrics that could more predictably guide daily glucose levels, together with recent advances in technology, have fueled demand for a fuller characterization of glycemia, including the duration, direction, magnitude, and frequency of glucose fluctuations (1,2). In this milieu, a small subset of patients, mostly with type 1 diabetes treated in specialty clinics, have relied on continuous glucose monitoring (CGM) to better observe real-time glucose values, trend information, and potentially harmful high and low glucose swings known as glycemic variability.
Studies conducted with newer-generation CGM devices affirm the clinical benefit of accessing continuous data regularly; specifically, subjects experienced improved A1C, decreased time spent in hyper- and hypoglycemia, and a lower incidence of severe hypoglycemia (3–14). Nevertheless, overall adoption of CGM remains at only 8–17%, even among motivated patients using insulin (15–18). Longstanding barriers include cost, concerns about accuracy, alarm fatigue, encumbrances of device wear, lack of standardized data reports, and uncertainty about applying the data to inform treatment decisions (7,19,20). This article revisits these obstacles in the wake of newly introduced flash CGM (FCGM) technology, a novel category of continuous data capture that can be practically implemented in primary care to add context to A1C and provide more actionable information than SMBG alone.
Flash: A New CGM Device Category
Unlike SMBG, which produces a static picture of blood glucose at a single point in time, CGM technology provides near-continuous data by measuring the glucose concentration in the body’s interstitial fluid and extrapolating blood glucose levels for real-time or retrospective analysis. In 2016, the FreeStyle Libre Pro (Abbott Diabetes Care, Alameda, Calif.) was introduced in the United States, representing a new category of CGM called “flash” or “intermittently scanned” CGM (21). Although employing the same chemical glucose oxidase mechanism for glucose measurement as traditional CGM, FCGM uses osmium-based wired enzyme glucose-sensing technology to automatically measure glucose every minute and stores readings at 15-minute intervals. Because this technology does not produce as much drift from blood glucose values as earlier sensors and responds more stably over a longer period, it can be calibrated at the time of manufacturing and does not require recalibration by the patient or clinician. This reduces the burden of calibration for the patient and increases ease of use.
FCGM is now available in two versions. The personal system, FreeStyle Libre, which was approved as a replacement for fingerstick testing by the U.S. Food and Drug Administration (FDA) in September 2017, affords on-demand observation of real-time and trend data, as well as retrospective review of complete profiles by patients at home or providers in their clinics (22). The earlier-approved FreeStyle Libre Pro system is referred to as a professional device because it is owned by the clinic, and patients wear a sensor temporarily without being able to see glucose values until their provider uploads the data for review during an office visit. Both versions include a disposable sensor worn on the back of the arm for up to 14 days (23,24).
As of this writing, these FreeStyle Libre systems are the only FCGM devices approved by the FDA (24,25). The personal system, introduced abroad in 2014 and obtainable without a prescription in some countries, has generated high interest among patients through international forums and has focused attention on the utility of this device category in the United States, where both the personal and professional systems are available for use separately or in tandem (26,27).
Mechanism of Action
Personal and professional FCGM systems provide different routes for making therapeutic or behavioral adjustments that affect glucose control. Personal FCGM enables patients to make “fast” adjustments through on-demand readings obtained by scanning, or “flashing,” the glucose sensor with a handheld reader. Patients can look at the display screen to view last-minute values, trend arrows, and graphs showing data from the previous 8 hours. In the case of people with type 2 diabetes who do not perform SMBG routinely, personal FCGM may afford the first opportunity to visualize glucose trending in response to behavior, such as how glucose rises after eating or falls in response to physical activity (28–30). Additionally, personal FCGM may benefit people with type 1 diabetes who are accustomed to using real-time data for diabetes management but find alarms bothersome (31). It may also benefit those with type 1 diabetes who have not used CGM in the past and are testing their blood glucose infrequently. The immediate availability of real-time data can help this population understand for the first time the granularity of how different foods, exercise, and stress affect their diabetes control. On-demand readings can be used for insulin dosing when glucose is not changing rapidly, physical symptoms match the values on the reader, and there is no “check blood glucose symbol” on the home screen (22,32).
The professional version of FCGM, unlike the personal version, is designed exclusively for retrospective review of continuous data patterns, facilitating “slower,” more deliberative adjustment of therapies and behaviors, such as changing insulin-to-carbohydrate ratios. As previously mentioned, patients have no interaction with the device and cannot see glucose readings during wear (33,34). Data can be retrieved only when the health care professional scans the reader over the sensor and uploads the data to the LibreView desktop software (35). At that point, the provider, usually together with the patient, can view a single-page report, described later in this article, to quickly identify trouble spots such as nocturnal hypoglycemia or decipher A1C values that are higher or lower than would be expected based on SMBG readings alone (36).
Differences Among Currently Available CGM Systems
Addressing known issues affecting CGM uptake—namely, cost, human factors such as wearability and convenience, data display formats, and confidence in using the data for therapy decisions—will be important to encourage the routine use of continuous data (19). Table 1 compares the features of selected CGM systems available in the United States (24,32,37). Although both flash and traditional CGM systems allow users to monitor interstitial glucose levels, there are distinct differences between these technologies that require consideration when evaluating their suitability for individual patients (38).
TABLE 1.
| System Type | Brand Name | Wear Time | Warm-Up Time | Calibration | Frequency of Glucose Readings | Accuracy (MARD) | Software and/or Device Compatibility |
|---|---|---|---|---|---|---|---|
| Professional CGM | Freestyle Libre Pro system | Up to 14 days | 1 hour | None | Every 15 minutes | 12.3% | Data scanned from sensor using Freestyle Libre Pro reader in office |
| Medtronic iPro 2 Enlite CGM sensor and iPro2 digital recorder | Up to 6 days | 1 hour | None, but at least one blood glucose entry every 12 hours is required for system uploads | Every 5 minutes | 13.6% | Data uploaded from sensor recorder using Medtronic CareLink iPro website | |
| Dexcom G4 Platinum Professional Continuous Monitoring System | 7 days | 2 hours | Every 12 hours after the 2-hour start-up calibration | Every 5 minutes | 13.3% | Data uploaded from sensor using Dexcom STUDIO software | |
| Personal CGM | Freestyle Libre system | Up to 14 days | 1 hour | None | Available every minute; automatically records every 15 minutes | 9.4% | Data may be uploaded from reader in health care provider’s office using Freestyle Libre View software |
| Dexcom Platinum G4/G5 sensor with G4 Platinum transmitter | Up to 7 days | 2 hours | Every 12 hours | Every 5 minutes | 9% when used with most current Dexcom software | Stand alone with Dexcom G4 receiver and compatible with Animas Vibe and Tandem t:slim insulin pumps | |
| Dexcom Platinum G4/G5 sensor with G5 Platinum transmitter | Up to 7 days | 2 hours | Every 12 hours | Every 5 minutes | 9% when used with most current Dexcom software | Stand alone with Dexcom G5 receiver, web-based Clarity software (G5 only), and most Apple and Android products and compatible with Tandem t:slim X2 insulin pump | |
| Dexcom G6 sensor and transmitter | Up to 10 days | 2 hours | None | Every 5 minutes | 9.8% overall and 9.6% in children 6–17 years of age | Stand alone with most Dexcom G5 receivers and G6 receiver and most Apple and Android products; both G5 and G6 download to web-based Clarity software | |
| Medtronic Enlite Sensor and MiniLink or Guardian Link transmitter | Up to 6 days | 2 hours | Every 12 hours | Every 5 minutes | 13.6% | Compatible with Medtronic 530G and 630G insulin pumps | |
| Medtronic Guardian Sensor 3 sensor and Guardian Link 3 transmitter | Up to 7 days | 2 hours | Every 12 hours | Every 5 minutes | 10.6% with 2 calibrations/day; 9.6% with 3–4 calibrations/day | Compatible with Medtronic 670G hybrid closed-loop insulin pump system |
At the most fundamental level, FCGM does not passively display data continuously (rather, the user must scan the sensor with the reader to see glucose information displayed), nor does it trigger an alarm to alert users to potential hypo- or hyperglycemia. However, a distinct audible tone is generated if a scan occurs when glucose is <70 or >240 mg/dL, projected to be <70 or >240 mg/dL, the display reads “hi” or “lo” (indicating a reading outside of the measuring range of 40–500 mg/dL) or is projected to read “hi” or “lo,” glucose is rapidly changing, or no trend arrow displays. Traditional CGM devices, by contrast, send data continuously to receivers, phones, and/or insulin pumps and feature programmable alerts and alarms that warn patients of current and impending hyper- or hypoglycemia.
Although fingerstick calibration is unnecessary with FCGM, as with latest-generation traditional CGM systems, blood glucose testing is still mandatory in situations of rapidly changing glucose, when clinical signs are inconsistent with displayed values, for confirmation of sensor-reported hypoglycemia, and during the system warm-up period (39–41).
The retail price of FCGM systems is currently less than traditional CGM systems, addressing the significant barrier of cost (20). As with the Dexcom G5 system (Dexcom, San Diego, Calif.), FCGM is covered under Medicare for beneficiaries with diabetes who use intensive insulin therapy (three or more injections per day), perform fingerstick glucose testing four times per day, and require frequent adjustment of therapy.
Finally, neither FCGM nor the newer Dexcom G6 (Dexcom, San Diego, Calif.) is affected by acetaminophen interference, which has been a historical barrier to CGM use for some patients (42).
FCGM Accuracy
Patients’ continued use of CGM for diabetes management is directly related to their trust in the accuracy and reliability of the data it provides (43,44). The most common numerical metric for assessing CGM accuracy is the aggregate mean absolute relative difference (MARD) between all CGM values and matched reference values. The FDA uses MARD in determining approval of new devices (45). A low MARD percentage indicates that CGM results are closer to the reference readings, whereas a higher MARD percentage indicates larger discrepancies.
A 2015 performance study confirmed the accuracy of FCGM against capillary and venous glucose testing in a wide range of individuals with type 1 or type 2 diabetes (46). Although comparing MARD values among CGM systems is difficult due to a lack of standardization among clinical study methodologies, FDA assessments for product approval indicate comparable accuracy among currently available systems (21,47–49). It is important to confirm sensor readings with blood glucose measurements in situations in which glucose is rapidly changing or is in the hypoglycemic range (<70 mg/dL) or when symptoms do not match sensor glucose values (39).
CGM Comparison Studies
Recent head-to-head accuracy comparisons between FCGM and selected newer-generation traditional systems indicate similar accuracy. A recent study by Aberer et al. (50) comparing the FreeStyle Libre to the Dexcom G4 Platinum (Dexcom, San Diego, Calif.) and Medtronic MiniMed 640G (Medtronic Diabetes, Northridge, Calif.) systems over 12 hours (24 hours after sensor insertion) during mimicked real-life conditions such as meals, exercise, and hypo- and hyperglycemia found that MARDs in the entire glycemic range were 13.2% (± 10.9%), 16.8% (± 12.3%), and 21.4% (± 17.6%) for the systems, respectively. All three sensors performed less accurately during hypoglycemia and best during hyperglycemia, with the FreeStyle Libre exhibiting the lowest MARD across all glycemic ranges. An earlier study by Bonora et al. (51) comparing only the FreeStyle Libre and Dexcom G4 Platinum sensors to SMBG for up to 14 days showed good overall agreement between the two systems, although the comparative performance varied significantly and inexplicably among individual patients, all eight of whom had type 1 diabetes.
To better understand the performance of the FreeStyle Libre and Dexcom G4 systems during glycemic excursions, Boscari et al. (52) collected accuracy data from 22 adults with type 1 diabetes both at home and during a single 6-hour hospital admission to induce glycemic excursions (early post-meal hyperglycemia followed by a quick decrease in blood glucose). Both sensors functioned with similar accuracy during home use, although the accuracy of both significantly worsened during the excursions due to lag time between plasma and interstitial glucose (52,53). A follow-up study with the newer-generation Dexcom G5 Mobile sensor, which, like the FreeStyle Libre, is approved for making diabetes treatment decisions without the need for confirmatory fingerstick testing, found that both systems performed safely and effectively, with an overall at-home MARD of 12.3% (range 5.6–21.4%) for the FreeStyle Libre and 9.8% (range 4.7–18.0%) for the G5 (P <0.001) (54). However, the MARD increased during hypoglycemia and decreased during hyperglycemia with both systems, again pointing to the need for confirming CGM with SMBG when results are in the hypoglycemic range or inconsistent with symptoms.
Effectiveness and Utility
Efficacy studies of FCGM evaluating glucose control, hypoglycemia, and quality of life substantiate its utility. The IMPACT study by Bolinder et al. (55) comparing FCGM to SMBG in European adults with well-controlled type 1 diabetes (n = 239) and awareness of hypoglycemia showed that participants in the FCGM group spent 38% less time in the hypoglycemic range (<70 mg/dL). This reduction was accomplished with no change in total daily insulin dose or deterioration of A1C. Glucose time in range significantly increased in the intervention group; high scores for treatment satisfaction and a scan rate averaging 15 times/day indicated good acceptance of FCGM.
The relationship between glucose control and scanning frequency was explored by Dunn et al. (56), who evaluated de-identified and uploaded data for >50,000 FreeStyle Libre system readers with 279,446 sensors (86.4 million monitoring hours by 63.8 million scans). Users scanned an average of 16.3 times/day. When divided into 20 equal-sized groups by scan rate (n = 2,542 each), estimated A1C levels decreased (P = 0.001) from 8.0% in the group with the lowest scan rate (4.4 times/day) to 6.7% for those with the highest scan rate (48 times/day). Because these were estimated A1C levels from the downloads and measured A1C levels were not reported, there were no pre-FCGM data to report. Scan rates also correlated with hypoglycemia rates; as scan rates increased, hypoglycemia rates below 70, 55, and 45 mg/dL decreased by 15, 40, and 49%, respectively (all P <0.001).
Two recent single-arm studies without control groups demonstrated significant A1C improvement after FCGM initiation. In the first, Dover et al. (57) prospectively evaluated FCGM in 25 participants with type 1 diabetes and reported improved glucose control, fewer episodes of hypoglycemia, and improved quality of life. The mean A1C fell from 8.0 ± 0.14% to 7.5 ± 0.14% (–0.48%, P = 0.001) after 16 weeks of FCGM. The number of people with an A1C of ≤7.5% more than doubled after FCGM use. Those with a baseline >7.5% experienced a greater reduction than participants with A1C <7.5% at baseline (–0.59 ± 0.15% vs. –0.2 ± 0.11%, P = 0.005). FCGM data showed that the number of hypoglycemic episodes (<72 mg/dL) dropped from 17 in the first 2 weeks of use to 12 in the final 2 weeks. Significant improvements were observed in the Diabetes Distress Scale mean score and other quality-of-life indicators. FCGM use was also associated with a significant increase in the administration of prandial insulin in advance of meals according to recommendation versus immediately before or after.
Ish-Shalom et al. (58) reported similar outcomes in the second single-arm study, which enrolled 31 patients with difficult-to-control type 1 (n = 6) or type 2 (n = 25) diabetes. Patients treated with a multiple daily injection regimen whose A1C was ≥7.5% (baseline average 8.9 ± 0.26%) used FCGM to achieve target glucose levels and minimize hypoglycemia. A1C decreased by 1.33 ± 0.29% after 8 weeks, and for those who continued using FCGM after the 12-week study period (n = 27), the change was sustained for 24 weeks (1.21 ± 0.42%, P = 0.009). Questionnaires completed by all 31 participants indicated high satisfaction and desire to continue using the device. This finding is in keeping with a study by Olafsdottir et al. (59), in which 58 adults with type 1 diabetes rated their FCGM experience as positive, with average scores from 8.22 to 9.8 on a scale of 0 to 10.
In a large multicenter study of patients with insulin-requiring type 2 diabetes, Haak et al. (60) compared FCGM to standard fingerstick glucose measurement in 224 participants. Baseline A1C was 8.74% in the FCGM group and 8.8% in the SMBG group. A1C reduction in both groups was comparable overall (–0.29 ± 0.07% vs. –0.31 ± 0.09%, respectively). However, patients <65 years of age in the FCGM group showed significant A1C improvement compared to the control group (–0.53 ± 0.09% vs. –0.20 ± 0.12%, P = 0.0301). Time in hypoglycemia <70 and <55 mg/dL was reduced by 0.47 ± 0.13 hours/day (43%) and 0.22 ± 0.07 hours/day (53%), respectively, for FCGM versus SMBG users. Nocturnal hypoglycemia (<70 mg/dL) declined by 54% in the FCGM group (P = 0.0001), and time in hypoglycemia was decreased by 56% (P = 0.0083) for patients ≥65 years of age. Treatment satisfaction was higher in FCGM users, and no device-related serious adverse events were reported.
Opportunities for CGM in Primary Practices
Despite a decade-long surge in new diabetes medications and technologies, the proportion of people achieving a target A1C <7.0% remains about 50% (61). Examination of two waves of data from a subset of respondents to the National Health and Nutrition Examination Survey (n = 2,677) found a slight downward trend in the achievement of target A1C, from 52.2% during the period of 2007–2010 to 50.9% during the period of 2011–2014 (62). Explanations for this impasse are largely speculative but include increasingly complex therapies, higher out-of-pocket costs, a shift from undiagnosed diabetes to diagnosed diabetes among individuals predisposed to poor glycemic control, and the open question of whether A1C is the best marker of glycemic control for individual patients (62–65).
Limitations of A1C
Although A1C, which measures mean glycemic exposure during the 2–3 months before testing, remains the gold standard for assessing population health and complications risk over time, the assay has limitations that may go unrecognized in clinical practice. Accuracy can be affected by the presence of hemoglobinopathies, inter-individual glycation characteristics, and conditions that affect red blood cell life span (66). Moreover, the range of mean glucose concentrations and glucose profiles correlated with a given A1C level is wider than appreciated. The A1c-Derived Average Glucose study, which assessed the relationship between A1C and glucose levels in ∼500 adults without any known factors affecting A1C, revealed that the 95% predictive interval, or range of corresponding average glucose, increased at each successive A1C level (64,67). Thus, the average glucose of an individual with an A1C of 7% (95% CI 123–185 mg/dL) could in reality be higher than the average glucose of another with an A1C of 8% (95% CI 147–217 mg/dL). Moreover, an average glucose of 154 mg/dL, corresponding to an A1C of 7.0%, can be achieved by blood glucose fluctuating between 120 and 188 mg/dL or between 50 and 258 mg/dL, each requiring markedly different treatment than the other.
A further limitation of A1C is that it does not distinguish people who reach target average glucose levels with frequent glycemic excursions, known as glycemic variability, from those who do so more evenly (68). The role of glycemic variability as an independent risk factor for long-term diabetes complications, including cardiovascular disease, neuropathy, and retinopathy, is the subject of ongoing study (69–77). More immediately, glycemic variability is a strong predictor of hypoglycemia and poor glycemic control regardless of baseline A1C, with associated decrements in cognitive function and quality of life (11,76,78–80).
The Tipping Point?
Readily accessible and affordable CGM has been a promising, although elusive, pathway toward deciphering the meaning of A1C for individual patients. Calls for more personalized diabetes care by professional diabetes organizations, combined with technological innovations such as FCGM, suggest an imminent tipping point toward wider use of continuous data (19,21,64). According to the American Association of Clinical Endocrinologists (AACE) 2010 consensus statement on CGM (80), obvious candidates for CGM are people with type 1 diabetes who have hypoglycemia or hypoglycemia unawareness or who have an A1C above target. In 2016, AACE refined these criteria, specifying patients >65 years of age with type 1 diabetes and comorbidities or at risk for severe hypoglycemia, as well as those with chronic diabetic kidney disease (81). In 2016, AACE also added insulin-treated patients with type 2 diabetes and pregnant women with diabetes as eligible candidates, with the provision that studies will be required to determine cost-effectiveness (2).
In a 2015 white paper (82), the American Association of Diabetes Educators (AADE) advocated CGM for any person with diabetes who is willing to wear a device, regardless of diabetes type, duration, or patient age. Key purposes cited by the AADE expert panel were identifying glycemic excursions, validating therapy adjustments, observing and modulating the effects of physical activity and meals on glucose levels, and using trend information to prevent or mitigate issues associated with glycemic variability. To these ends, the American Diabetes Association stressed the need for education, training, and support when prescribing CGM, particularly with respect to data interpretation (1).
Meeting these evolving standards will depend in part on minimizing the burden of learning different approaches to different CGM systems and re-thinking assumptions that continuous data analysis is appropriate only for patients on intensive insulin therapy. Because FCGM poses comparatively few demands on users, it offers an opportunity to address common impediments, such as cost, frequent alarms, and the complexity of data interpretation, when considering CGM options for patients who have monitored blood glucose erratically, unsuccessfully, or not at all (27,39,57,83).
Using FCGM With Data Management Tools
Many researchers, clinicians, and patients advocate a standardized glucose report, similar to an electrocardiogram, as fundamental to wider acceptance of CGM (21). The ambulatory glucose profile (AGP) report developed by Mazze et al. (84) reflects the ongoing effort to create a universal template for more predictable viewing, easier comprehension, and ready interpretation of glucose data. FCGM personal and professional devices were among the first to feature the AGP, which is gradually being incorporated into other CGM systems (84,85).
AGP-enabled software collapses and plots all collected glucose values as if they occurred in a single 24-hour period. The downloadable report, which can be accessed in modular fashion, begins with a statistical summary showing glucose exposure, glucose variability (coefficient of variation [CV] and SD), the proportion of glucose values in the target range (70–180 mg/dL), the percentage of values above or below target (low, serious low, high, or serious high), and the percentage of time CGM is active. Many clinicians are familiar with SD (square root of the variance) as a metric for glucose variability because this has been provided on glucose downloads for >20 years. The problem with SD is that it is not normalized to the mean. CV, on the other hand, is the SD/mean, meaning it is now possible to compare glucose variability values no matter what the mean is.
Beneath this summary, five distribution curves drawn from the aggregated glucose readings provide an at-a-glance picture of a standard day. In the AGP used with FCGM, a dark blue line represents the median curve and would be mostly flat under optimal conditions. The curves immediately above and below the median curve (25th and 75th percentiles) depict the daily, nightly, and postprandial spans for 50% of the aggregated glucose values; a wider span (indicated by blue shading) indicates high risk for glycemic variability during the associated time period, whereas a narrower span denotes lower risk. Dashed curves represent the 10th and 90th percentiles, showing data above or below 80% of all the data (indicated by gray shading), conveying “occasional excursions.”
AGP With Professional FCGM
Professional FCGM studies can be an effective tool for educating patients about the effects of food choices, exercise, and medications on blood glucose levels and actions that can be taken to improve glycemic control moving forward (86). Because the FCGM sensor can be worn for a full 2-week period without replacement and there is no need for calibration or patient interaction with the device, a professional study affords an uninterrupted and representative view of a patient’s changing glucose levels. For optimum results, patients should be instructed to keep a detailed log of their meals and activities that can be reviewed with the AGP. With minimal training, clinicians can look at the AGP dashboard to visualize and prioritize clinical problems and, through an ongoing process of shared decision-making with patients, introduce interventions to increase glucose time in range without increasing hypoglycemia (33,87). Use of the standardized report also enhances workflow and communication by allowing the entire diabetes care team to work from the same visualization.
A basic review of the AGP report should include time in range, as well as patterns of hypoglycemia, hyperglycemia, and prandial glucose excursions (87). It is ideal to review results and recommendations face-to-face with patients, using the report as a decision aid to illustrate relationships among glucose data, medication, and other therapeutic or behavioral interventions (34,88). After confirming adequate data (at least 10 days of wear) (37), a patient’s daily habits should be reviewed. Asking for 3 days of detailed information before the scheduled appointment and, if possible, a record of unusual days can facilitate this process. Information such as medication regimen, exercise, meals, and/or snacking should be marked directly under the curve on the printed-out AGP sheet. Once the sheet is marked up, asking the patient to briefly describe what he or she sees as possible reasons for glycemic excursions often elicits honest and helpful insights. The daily thumbnail profiles can add further dimension, particularly when regular activities vary from day to day.
Actions should be prioritized according to patterns of hypoglycemia, hyperglycemia, and glucose variability. For example, if the 10% lower line is touching the 70 mg/dL target line—indicating that, at that time of day, 10% of all glucose levels are <70 mg/dL—adjustments should be made to reduce hypoglycemia. Alternatively, if the light blue area is very wide—conveying high glycemic variability—the patient should be asked if he or she can do anything to adjust factors such as the timing or amount of food intake, timing or dosing of medications, or patterns of exercise that may exacerbate glucose fluctuations. Each time period should be examined in turn, keeping in mind the following questions:
Do glucose levels start at target before eating?
After eating, do glucose levels regularly fluctuate upward or downward?
Do upward or downward fluctuations happen overnight?
What normally happens with physical activity?
Are weekend patterns different?
Is there an explanation for glucose variability?
Is there a special situation, such as stress or illness, that requires greater focus?
Does A1C reflect daily glucose control?
At the end of the consultation, the key messages of the AGP analysis should be summarized so that the patient comes away with one or two specific recommendations (87). In all cases, the first priority should be treating hypoglycemia, denoted by the blue curves touching the 70 mg/dL line or lower. A follow-up appointment should be scheduled within 3–6 months (sooner for pharmacologic intervention than for lifestyle change) to assess progress. Insertion and removal of the FCGM sensor, as well as interpretation of the report by a physician, nurse practitioner, or physician’s assistant, are reimbursable by most insurance plans using existing codes. (Readers are referred to AACE’s “New and Updated Codes for Continuous Glucose Monitoring (CGM) in 2018” [89]).
Data Visualization Personal FCGM
Unlike professional FCGM, the personal version provides users with observable feedback after the reader is swiped over the sensor. The sensor, about the size of two stacked U.S. quarters, can be swiped through clothing for displays of real-time glucose values, trend arrows, and graphs showing the past 8 hours of data. The sensor should be scanned at least three times per day, 8 hours apart, for complete data capture, but there is no limit to the number of scans that can be made. From the home screen, users may also add tags to each scan—e.g., carbohydrate intake, insulin, or exercise—or access a glucose history from the past 90 days.
For people accustomed to SMBG, the trend-arrow feature of FCGM is often the most educational, although patients should be advised to use all of the information on the screen when deciding what to do. Arrows for personal FCGM are defined as:
↑rising quickly (>2 mg/dL/minute)
➚rising (1–2 mg/dL/minute)
➙changing slowly (<1 mg/dL/minute)
➘falling (1–2 mg/dL/minute)
↓falling quickly (>2 mg/dL/minute)
Patients who have no experience with continuous data should adopt the use of trend arrows gradually as they gain better understanding of how circumstances such as meals, physical activity, and insulin on board (insulin remaining active from the most recent dose) affect their particular glucose response (90). An example of how trend arrows can help in making treatment decisions is shown in Table 2. Notably, if glucose is rapidly changing, <70 mg/dL, or projected to be <70 mg/dL, or if there is no glucose number or trend arrow, a “check blood glucose” symbol will appear on the home screen, signaling the need to perform SMBG before taking action.
TABLE 2.
Sample of Trend Arrow–Guided Decision-Making (90)
| Patient Profile | Scanning Time | What the Display Shows | What the Patient Does |
|---|---|---|---|
| Jane has a target of 100 mg/dL and a correction factor of 1:50. This means she should take 1 unit of insulin to lower her glucose about 50 mg/dL. | After breakfast |
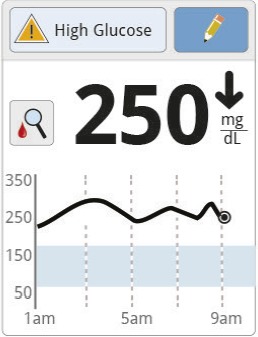 Jane sees a reading of 250 mg/dL trending rapidly downward. There is also a high glucose message and the “check blood glucose” symbol. |
 Seeing the symbol, Jane performs SMBG before deciding what to do. |
| Before lunch |
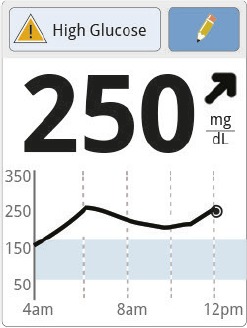 Jane’s glucose is 250 mg/dL and rising. |
Before eating, Jane adds 50 mg/dL to her current reading given the rising trend arrow (250 + 50 = 300 mg/dL). She subtracts her target number (300–100 = 200 mg/dL) and divides by her correction factor (200 ÷ 50 = 4). Jane takes 4 units of insulin. | |
| After lunch |
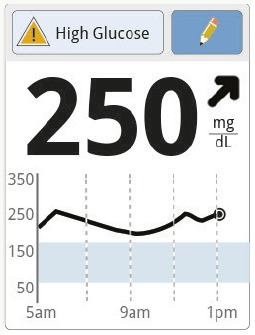 Ninety minutes later, Jane’s glucose is the same. The trend arrow and graph show a continued rise. |
Jane does not take a correction dose because it is within 2 hours of her meal dose. This could lead to “insulin stacking” (adding an insulin dose on top of insulin still active from the previous dose) and low glucose. The insulin she took for her meal may still be active. Instead, Jane decides to wait and scan again later. | |
| Before dinner |
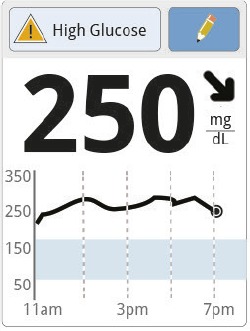 Jane’s current glucose is 250 mg/dL. The trend arrow and graph indicate that her glucose is going down. |
Jane asks herself what might be causing her glucose to go down and what she might do to prevent low glucose, deciding to take less insulin before her meal. She subtracts 50 mg/dL from the current value because of the falling trend arrow (250 – 50 = 200 mg/dL) and then subtracts her target number (200 – 100 = 100 mg/dL). She divides this by her correction factor (100 ÷ 50 = 2). Jane takes 2 units of insulin. |
Users who wish to delve deeper than current glucose values and trends can select the “review history” menu option for 7-, 14-, 30-, and 90-day scroll-through reports showing average glucose, time-in-target trends, daily patterns of hypo- and hyperglycemia, low glucose events, and how often the sensor has been scanned. As with the AGP report, these data can be uploaded from the reader in various formats for analysis by patients at home or with a health care provider during a clinic visit.
Outlook and Case Example
Encouraging clinician and patient acceptance of CGM, used either intermittently or for everyday diabetes management, will depend largely on dispelling historical biases (38). Measures to overcome real or perceived obstacles, particularly in primary care, must address the following questions: How much time and training will be required to teach patients the basics of device operation? What kind of support will be necessary for patients to use real-time data effectively? What resources and workflow adaptations will be needed to facilitate retrospective data analysis (by patients and clinicians together or individually)? How well will continuous data translate into actions and behaviors that realize patient-centered outcomes, including personalized glycemic control, more time in range, greater treatment satisfaction, and better quality of life?
FCGM, which has a track record of successful implementation with minimal training outside the United States, may point the way to feasibility of CGM in primary care (33). Intuitive navigation and data interpretation allow a greater degree of self-management than normally associated with CGM. Figure 1 shows a case example of a patient with type 2 diabetes who reduced A1C and increased time in range based on FCGM guidance alone.
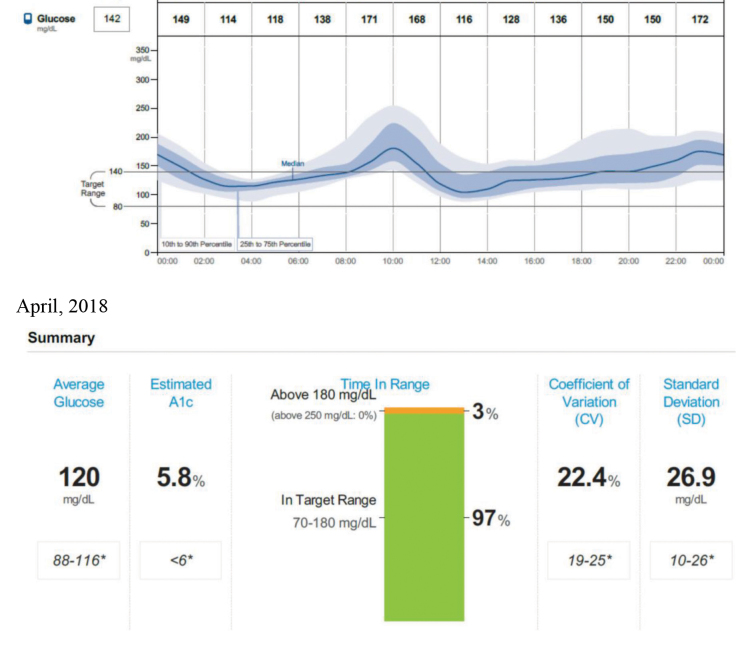
FIGURE 1.
AGP profile and follow-up summary report of a 60-year-old man with type 2 diabetes. He was diagnosed with diabetes 3 years ago. His physician prescribed metformin and canagliflozin. With an A1C usually between 6.5 and 7.5%, and 42% of values within the hyperglycemic range as of August 2017, he began personal FCGM the following December. His medication therapy remained the same. When he returned to the clinic in April 2018, 97% of his glucose values were within target range, and his estimated A1C was 5.8%. He attributed this improvement to controlling his rice intake based on feedback from FCGM.
Summary and Conclusion
Clinical trial and empirical evidence indicate that continuous glucose data analysis, used as an adjunct to A1C, provides more robust and actionable information than SMBG. Although CGM is recognized as a powerful tool for individualizing diabetes care, its optimal utilization has been stymied by cost and reimbursement issues, limited resources to learn or implement new technology, and user factors such as interference with daily life (7,19). FCGM offers a new avenue. Considered an easy, intuitive monitoring system, FCGM is suitable for a variety of patients, ranging from those not on insulin using SMBG with mixed success to people on intensive insulin regimens who find alarms and other features of traditional systems challenging. Professional FCGM offers a low-cost and nearly burden-free opportunity for gaining insight into patterns of high and low blood glucose that can be addressed moving forward. For patients who begin with or transition to personal FCGM, the ability to make in-the-moment adjustments based on real-time glucose readings and trends can be motivating and can lead to more rewarding patient-provider interactions.
Acknowledgments
Acknowledgment
The authors thank Carol A. Verderese for her editorial assistance.
Funding
Abbott Diabetes Care provided funding to support the writing of this article.
Duality of Interest
Both authors have served as consultants to Abbott Diabetes Care.
Author Contributions
I.B.H. and E.E.W. developed the manuscript and reviewed the content. Both authors are guarantors of this work and, as such, take full responsibility for its integrity and accuracy.
References
- 1.American Diabetes Association 6. Glycemic targets: Standards of Medical Care in Diabetes—2018. Diabetes 2018;41(Suppl. 1):S55–S64 [DOI] [PubMed] [Google Scholar]
- 2.Fonseca VA, Grunberger G, Anhalt, et al. Continuous glucose monitoring: a consensus conference of the American Association of Clinical Endocrinologists and American College of Endocrinology. Endocr Pract 2016;22:1008–1021 [DOI] [PubMed] [Google Scholar]
- 3.Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group Effectiveness of continuous glucose monitoring in a clinical care environment: evidence from the Juvenile Diabetes Research Foundation Continuous Glucose Monitoring (JDRF-CGM) trial. Diabetes Care 2010;33:17–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hermanides J, Nørgaard K, Bruttomesso D, et al. Sensor-augmented pump therapy lowers HbA1c in suboptimally controlled type 1 diabetes; a randomized controlled trial. Diabet Med 2011;28:1158–1167 [DOI] [PubMed] [Google Scholar]
- 5.Battelino T, Conget I, Olsen B, et al. ; SWITCH Study Group . The use and efficacy of continuous glucose monitoring in type 1 diabetes treated with insulin pump therapy: a randomised controlled trial. Diabetologia 2012;55:3155–3162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.New JP, Ajjan R, Pfeiffer AF, Freckmann G. Continuous glucose monitoring in people with diabetes: the randomized controlled Glucose Level Awareness in Diabetes Study (GLADIS). Diabet Med 2015;32:609–617 [DOI] [PubMed] [Google Scholar]
- 7.Wong JC, Foster NC, Maahs DM, et al. ; T1D Exchange Clinic Network. Real-time continuous glucose monitoring among participants in the T1D Exchange clinic registry. Diabetes Care 2014;37:2702–2709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riveline JP, Schaepelynck P, Chaillous L, et al. ; EVADIAC Sensor Study Group. Assessment of patient-led or physician-driven continuous glucose monitoring in patients with poorly controlled type 1 diabetes using basal-bolus insulin regimens: a 1-year multicenter study. Diabetes Care 2012;35:965–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rachmiel M, Landau Z, Boaz M, et al. The use of continuous glucose monitoring systems in a pediatric population with type 1 diabetes mellitus in real-life settings: the AWeSoMe Study Group experience. Acta Diabetol 2015;52:323–329 [DOI] [PubMed] [Google Scholar]
- 10.Bergenstal RM, Klonoff DC, Garg SK, et al. ; ASPIRE In-Home Study Group. Threshold-based insulin-pump interruption for reduction of hypoglycemia. N Engl J Med 2013;369:224–232 [DOI] [PubMed] [Google Scholar]
- 11.Weinstock RS, Xing D, Maahs DM, et al. ; T1D Exchange Clinic Network . Severe hypoglycemia and diabetic ketoacidosis in adults with type 1 diabetes: results from the T1D Exchange clinic registry. J Clin Endocrinol Metab 2013;98:3411–3419 [DOI] [PubMed] [Google Scholar]
- 12.Pickup JC, Freeman SC, Sutton AJ. Glycaemic control in type 1 diabetes during real time continuous glucose monitoring compared with self monitoring of blood glucose: meta-analysis of randomised controlled trials using individual patient data. BMJ 2011;343:d3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garg SK, Voelmle MK, Beatson CR, et al. Use of continuous glucose monitoring in subjects with type 1 diabetes on multiple daily injections versus continuous subcutaneous insulin infusion therapy: a prospective 6-month study. Diabetes Care 2011;34:574–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beck RW, Riddlesworth T, Ruedy K, et al. ; DIAMOND Study Group . Effect of continuous glucose monitoring on glycemic control in adults with type 1 diabetes using insulin injections: the DIAMOND randomized clinical trial. JAMA 2017;317:371–378 [DOI] [PubMed] [Google Scholar]
- 15.T1D Exchange Clinic Network Real- time continuous glucose monitoring among participants in the T1D Exchange clinic registry. Diabetes Care 2014;37:2702–2709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller KM, Foster NC, Beck RW, et al. ; T1D Exchange Clinical Network . Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D Exchange clinic registry. Diabetes Care 2015;38:971–978 [DOI] [PubMed] [Google Scholar]
- 17.Anhalt H. Limitations of continuous glucose monitor usage. Diabetes Technol Ther 2016;18:115–117 [DOI] [PubMed] [Google Scholar]
- 18.T1D Exchange Why do some people with T1D stop using a pump and CGM? Available from t1dexchange.org/ pages/why-do-some-people-with-t1d-stop-using-a-pump-and-cgm. Accessed 12 April 2018.
- 19.Petrie JR, Peters AL, Bergenstal RM, Hall RW, Fleming AG, Heinemann L. Improving the clinical value and utility of CGM systems: issues and recommendations: a joint statement of the European Association for the Study of Diabetes and the American Diabetes Association diabetes technology workshop. Diabetes Care 2017;40:1614–1621 [DOI] [PubMed] [Google Scholar]
- 20.Tanenbaum ML, Hanes SJ, Miller KM, Naranjo D, Bensen R, Hood KK. Diabetes device use in adults with type 1 diabetes: barriers to uptake and potential intervention targets. Diabetes Care 2017;40:181–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care 2017;40:1631–1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.U.S. Food and Drug Administration FDA approves first continuous glucose monitoring system for adults not requiring blood sample calibration. September 27, 2017. Available from www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm577890.htm. Accessed 4 April 2018.
- 23.Blum A. Freestyle Libre glucose monitoring system. Clin Diabetes 2018;36:203–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.U.S. Food & Drug Administration Freestyle Libre 14 day flash glucose monitoring system – P160030/S017. Available from www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/Recently-ApprovedDevices/ucm614903.htm Accessed 9 September 2018.
- 25.U.S. Food and Drug Administration Approval order: Freestyle Libre Pro flash glucose monitoring system. PI50021. Silver Spring, Md., Department of Health and Human Services, 2016. Available from www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P150021. Accessed 8 April 2018.
- 26.Leelarathna L, Wilmot EG. Flash forward: a review of flash glucose monitoring. Diabetes Med 2018;35:472–482 [DOI] [PubMed] [Google Scholar]
- 27.Close K, Brown A. Abbott’s FreeStyle Libre transforming glucose monitoring through utter simplicity, fingersticks aside! diaTribe. 9 January 2015. Available from diatribe.org/abbott-freestyle-libre-transforming-glucose-monitoring-through-utter-simplicity-fingersticks. Accessed 2 April 2018.
- 28.Schnell O, Alawi H, Battelino T, et al. Self-monitoring of blood glucose in type 2 diabetes: recent studies. J Diabetes Sci Technol 2013;7:478-488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vigersky RA, Fonda SJ, Chellappa M, Walker S, Ehrhardt NM. Real-time continuous glucose monitoring in patients with type 2 diabetes. Diabetes Care 2012;35:32–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vigersky R, Shrivastav M. Role of continuous glucose monitoring for type 2 in diabetes management and research. J Diabetes Complications 2017;31:280–287 [DOI] [PubMed] [Google Scholar]
- 31.Heinemann L, Freckmann G. CGM versus FGM; or, continuous glucose monitoring is not flash glucose monitoring. J Diabetes Sci Technol 2015;9:947–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Abbott. FreeStyle Libre User’s Manual. Available from www.accessdata.fda.gov/cdrh_docs/pdf16/P160030S017C.pdf. Accessed 9 September 2018.
- 33.Distiller LA, Cranston I, Mazze R. First clinical experience with retrospective flash glucose monitoring (FGM) analysis in South Africa: characterizing glycemic control with ambulatory glucose profile. J Diabetes Sci Technol 2016;10:1294–1302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hirsch IB, Verderese CA. Professional flash continuous glucose monitoring with ambulatory glucose profile reporting to supplement A1C: rationale and practical implementation. Endocr Pract 2017;23:1333–1344 [DOI] [PubMed] [Google Scholar]
- 35. Abbott. FreeStyle Pro Operator’s Manual. Available from https://www.myfreestyle.com/provider/freestyle-libre-pro-resources. Accessed 13 September 2018.
- 36.Rubinow KB, Hirsch IB. Reexamining metrics for glucose control. JAMA 2011;305:1132–1133 [DOI] [PubMed] [Google Scholar]
- 37.Hirsch IB, Battelino T, Peters AL, Chamberlain JJ, Aleppo G, Bergenstal RM. Role of Continuous Glucose Monitoring in Diabetes Treatment. Arlington, Va, American Diabetes Association, 2018 [Google Scholar]
- 38.Rodbard D. Continuous glucose monitoring: a review of successes, challenges, and opportunities. Diabetes Technol Ther 2016;18(Suppl. 2):S3–S13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fokkert MJ, van Dijk PR, Edens MA, et al. Performance of the FreeStyle Libre flash glucose monitoring system in patients with type 1 and type 2 diabetes mellitus. BMJ Open Diabetes Res Care 2017;5:e000320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.U.S. Food and Drug Administration FDA authorizes first fully interoperable continuous glucose monitoring system, streamlines review pathway for similar devices. March 27, 2018. Available from www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm602870.htm. Accessed 5 May 2018.
- 41. Medtronic MiniMed. iPro2 User Guide. Available from www.medtronicdiabetes.com/sites/default/files/library/download-library/user-guides/iPro2-with-Enlite-User-Guide.pdf. Accessed 3 March 2018.
- 42.Maahs DM, DeSalvo D, Pyle L, et al. Effect of acetaminophen on CGM glucose in an outpatient setting. Diabetes Care 2015;38:e158–e159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Polonsky WH, Hessler D. Perceived accuracy in continuous glucose monitoring: understanding the impact on patients. J Diabetes Sci Technol 2015;9:330–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Edelman S, Pettus J, Price D. How current continuous glucose monitoring (CGM) users translate CGM data into diabetes management: results of a survey. Diabetologia 2014;57(Suppl. 1):1020 [Google Scholar]
- 45.Clarke W, Kovatchev B. Statistical tools to analyze continuous glucose monitor data. Diabetes Technol Ther 2009;11(Suppl. 1):S45–S54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bailey T, Bode BW, Christiansen MP, et al. The performance and usability of a factory-calibrated flash glucose monitoring system. Diabetes Technol Ther 2015;17:787–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.U.S. Food and Drug Administration Summary of safety and effectiveness data (SSED). Dexcom G4 PLATINUM continuous glucose monitoring system. Available from www.accessdata.fda.gov/cdrh_docs/pdf12/P120005b.pdf. Accessed 5 May 2018.
- 48.U.S. Food and Drug Administration Summary of safety and effectiveness data (SSED). Medtronic MiniMed 530G (Enlite Sensor). Available from https://www.accessdata.fda.gov/cdrh_docs/pdf15/P150019b.pdf. Accessed 7 May 2018.
- 49. U.S. Food and Drug Administration. Summary of safety and effectiveness data (SSED). FreeStyle Libre flash glucose monitoring system. Available from https://www.accessdata.fda.gov/cdrh_docs/pdf16/P160030B.pdf. Accessed 5 May 2018.
- 50.Aberer F, Hajnsek M, Rumpler M, et al. Evaluation of subcutaneous glucose monitoring systems under routine environmental conditions in patients with type 1 diabetes. Diabetes Obes Metab 2017;19:1051–1055 [DOI] [PubMed] [Google Scholar]
- 51.Bonora B, Maran A, Ciciliot S, Avogaro A, Fadini GP. Head-to-head comparison between flash and continuous glucose monitoring systems in outpatients with type 1 diabetes. J Endocrinol Invest 2016;39:1391–1399 [DOI] [PubMed] [Google Scholar]
- 52.Boscari F, Galasso S, Facchinetti A, et al. FreeStyle Libre and Dexcom G4 Platinum sensors: accuracy comparisons during two weeks of home use and use during experimentally induced glucose excursions. Nutr Metab Cardiovasc Dis 2018;28:180–186 [DOI] [PubMed] [Google Scholar]
- 53.Cobelli C, Schiavon M, Dalla Man C, Basu A, Basu R. Interstitial fluid glucose is not just a shifted-in-time but a distorted mirror of blood glucose: insight from an in silico study. Diabetes Technol Ther 2016;18:505–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boscari F, Galasso S, Acciaroli G, et al. Head-to-head comparison of the accuracy of Abbott FreeStyle Libre and Dexcom G5 mobile [letter]. Nut Metab Dis 2018;28:425–429 [DOI] [PubMed] [Google Scholar]
- 55.Bolinder J, Antuna R, Geelhoed-Duijvestijn P, Kroger J, Weitgasser R. Novel glucose-sensing technology and hypoglycemia in type 1 diabetes: a multicentre, non-masked, randomized controlled trial. Lancet 2016;388:2254–2263 [DOI] [PubMed] [Google Scholar]
- 56.Dunn TC, Xu Y, Hayter G, Ajjan RA. Real-world flash glucose monitoring patterns associated between self-monitoring frequency and glycaemic measures: a European analysis of over 60 million glucose tests. Diabetes Res Clin Pract 2018;137:37–46 [DOI] [PubMed] [Google Scholar]
- 57.Dover AR, Stimson RH, Zammit NN, Gib FW. Flash glucose monitoring improves outcomes in a type 1 diabetes clinic. J Diabetes Sci Technol 2017;11:442–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ish-Shalom M, Wainstein J, Raz I, Mosenzon O. Improvement in glucose control in difficult-to-control patients with diabetes using a novel flash glucose monitoring device. J Diabetes Sci Technol 2016;10:1412–1413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Olafsdottir AF, Attvall S, Sandgren U, et al. A clinical trial of the accuracy and treatment experience of the flash glucose monitor FreeStyle Libre in adults with type 1 diabetes. Diabetes Technol Ther 2017;19:164–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Haak T, Hanaire H, Ajjan R, Hermanns N, Riveline JP, Rayman G. Flash glucose-sensing technology as a replacement for blood glucose monitoring for the management of insulin-treated type 2 diabetes: a multicenter, open-label randomized controlled trial. Diabetes Ther 2017;8:55–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ali MK, Bullard KM, Saaddine JB, Cowie CC, Iperatore G, Gregg EW. Achievement of goals in US diabetes care, 1999–2010. N Engl J Med 2013;368:1613–1624 [DOI] [PubMed] [Google Scholar]
- 62.Carls G, Huynh J, Tuttle E, Yee J, Edelman SV. Achievement of glycated hemoglobin goals in the US remains unchanged through 2014. Diabetes Ther 2017;8:863–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dwyer-Lindgren L, Machenbach JP, van Lenthe FJ, Flaxman AD, Mokdad AH. Diagnosed and undiagnosed diabetes prevalence by country in the US, 1999–2012. Diabetes Care 2016;39:1556–1562 [DOI] [PubMed] [Google Scholar]
- 64.Beck RW, Connor CG, Mullen D, Wesley DM, Bergenstal RM. The fallacy of average: how using A1C alone to assess glycemic control can be misleading. Diabetes Care 2017;40:994–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.U.S. Food and Drug Administration Public workshop: diabetes outcomes measures beyond hemoglobin A1c (HbA1c). Updated August 31, 2016. Available from https://www.fda.gov/Drugs/NewsEvents/ucm499281.htm. Accessed 5 March 2018.
- 66.Wright LA, Hirsch HB. Metrics beyond hemoglobin A1C in diabetes management: time in range, hypoglycemia, and other parameters. Diabetes Technol Ther 2017;19(Suppl. 2):S16–S26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nathan DM, Kuenen J, Borg R, Zhen H, Schoenfeld D, Heine RJ; A1c-Derived Average Glucose Study Group. Translating the A1C assay into estimated average glucose values. Diabetes Care 2008;31:1473–1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Monnier L, Lapinski H, Collette C. Contributions of fasting and postprandial plasma glucose increments to the overall durational hyperglycemia of type 2 diabetic patients: variations with increasing levels of HbA(1c). Diabetes Care 2003;26:881–885 [DOI] [PubMed] [Google Scholar]
- 69.Hirsch IB, Brownlee M. Beyond hemoglobin A1c: need for additional markers of risk for diabetic microvascular complications. JAMA 2010;303:2291–2292 [DOI] [PubMed] [Google Scholar]
- 70.Cryer PE. Severe hypoglycemia predicts mortality in diabetes. Diabetes Care 2012;35:1814–1816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vigersky RA. Escaping the hemoglobin A1c-centric world in evaluating diabetes mellitus interventions. J Diabetes Sci Technol 2015;9:1148–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Monnier L, Mas E, Ginet C, et al. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA 2006;295:1681–1687 [DOI] [PubMed] [Google Scholar]
- 73.Esposito K, Ciotola M, Carleo D, et al. Post-meal glucose peaks at home associate with carotid intima-media thickness in type 2 diabetes. J Clin Endocrinol Metab 2008;93:1345–1350 [DOI] [PubMed] [Google Scholar]
- 74.Monnier L, Colette C, Leiter L, et al. The effect of glucose variability on the risk of microvascular complications in type 1 diabetes. Diabetes Care 2007;30:185–188 [DOI] [PubMed] [Google Scholar]
- 75.Qu Y, Jacober SJ, Zhang Q, et al. Rate of hypoglycemia in insulin-treated patients with type 2 diabetes can be predicted from glycemic variability data. Diabetes Technol Ther 2012;14:1008–1012 [DOI] [PubMed] [Google Scholar]
- 76.Monnier L, Wojtusciszyn A, Colette C, et al. The contribution of glucose variability to asymptomatic hypoglycemia in persons with type 2 diabetes. Diabetes Technol Ther 2011;13:813–818 [DOI] [PubMed] [Google Scholar]
- 77.Ceriello A, Esposito K, Piconi L, et al. Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes 2008;57:1349–1354 [DOI] [PubMed] [Google Scholar]
- 78.Testa MA, Gill J, Su M, et al. Comparative effectiveness of basal-bolus versus premix analog insulin on glycemic variability and patient-centered outcomes during insulin intensification in type 1 and type 2 diabetes: a randomized, controlled, crossover trial. J Clin Endocrinol Metab 2012;97:3504–3514 [DOI] [PubMed] [Google Scholar]
- 79.Cryer PE. Glycemic goals in diabetes: trade-off between glycemic control and iatrogenic hypoglycemia. Diabetes 2014;63:2188–2195 [DOI] [PubMed] [Google Scholar]
- 80.Blevins TC, Bode BW, Garg SK, et al. ; AACE Continuous Glucose Monitoring Task Force; Rothermal C. Statement by the American Association of Clinical Endocrinologists consensus panel on continuous glucose monitoring. Endocr Pract 2010;16:730–745 [DOI] [PubMed] [Google Scholar]
- 81.Fonseca VA, Grunberger G, Anhalt H, et al. ; Consensus Conference Writing Committee. Continuous glucose monitoring: a consensus conference of the American Association of Clinical Endocrinologists and American College of Endocrinology. Endocr Pract 2016;22:1008–1021 [DOI] [PubMed] [Google Scholar]
- 82.American Association of Diabetes Educators White Paper: Continuous Glucose Monitoring Summit. Chicago, Ill, American Association of Diabetes Educators, 2015 [Google Scholar]
- 83.Blumer I. The contemporary role of masked continuous glucose monitoring in a real-time world. J Diabetes Sci Technol 2016;10:790–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mazze RS, Lucido D, Langer O, Hartmann K, Rodbard D. Ambulatory glucose profile: representation of verified self-monitored blood glucose data. Diabetes Care 1987;10:111–117 [DOI] [PubMed] [Google Scholar]
- 85.Bergenstal RM, Ahmann AJ, Bailey T, et al. Recommendations for standardizing glucose reporting and analysis to optimize clinical decision making in diabetes: the ambulatory glucose profile (AGP). Diabetes Technol Ther 2013;15:198–211 [DOI] [PubMed] [Google Scholar]
- 86.Allen NA, Fain JA, Braun B, et al. Continuous glucose monitoring in non-insulin-using individuals with type 2 diabetes: acceptability, feasibility, and teaching opportunities. Diabetes Technol Ther 2009;11:151–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Carlson AL, Mullen DM, Bergenstal RM. Clinical use of continuous monitoring in adults with type 2 diabetes. Diabetes Ther 2017;19(Suppl. 2):S4–S11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rodriguez-Gutierrez R, Gionfriddo MR, Ospina NS, et al. Shared decision making in endocrinology: present and future directions. Lancet Diabetes Endocrinol 2016;4:706–716 [DOI] [PubMed] [Google Scholar]
- 89.American Association of Clinical Endocrinologists New and updated codes for continuous glucose monitoring (CGM) in 2018: revised 7/2/18. Available from www.aace.com/files/socioeconomics/new_revised_codes_2018.pdf. Accessed 5 September 2018.
- 90.Pettus J, Edelman SV. Recommendations for using real-time continuous glucose monitoring (rtCGM) data for insuin adjustments in type 1 diabetes. J Diabetes Sci Technol 2017;11:138–147 [DOI] [PMC free article] [PubMed] [Google Scholar]