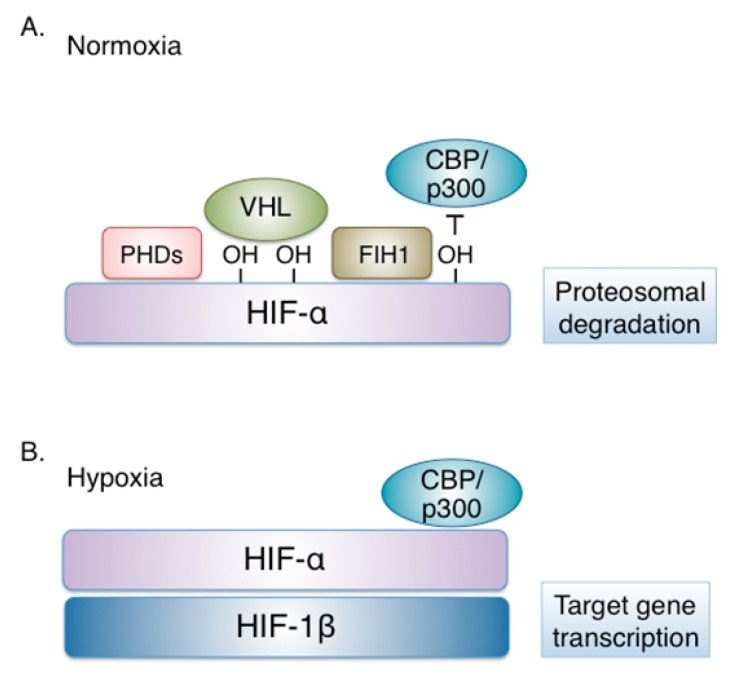
Figure 1.
Regulation of hypoxia-inducible factor (HIF) by oxygen. (A) Under physiological oxygen concentration (Normoxia), HIF-α isoforms are modified by oxygen-dependent prolyl-hydroxylases (PHDs), recognized by the von Hippel–Lindau (VHL) tumor suppressor protein, ubiquitinated and targeted to the proteasome for degradation. In addition, HIF-α modification by FIH (factor-inhibiting HIF), an oxygen-sensitive asparaginyl hydroxylase, disrupts interaction with the transcriptional co-activators p300/CBP and impairs residual HIF transcriptional activity. (B) When oxygen becomes limited (Hypoxia), PHDs and FIH are inactive. The non-hydroxylated HIF-α is stable and dimerizes with HIF-1β. The HIF heterodimer interacts with p300/CBP and activates the transcription of HIF target genes.