Abstract
Aflatoxin B1 (AFB1) is a serious threat to the poultry industry. Proanthocyanidins (PCs) demonstrates a broad range of biological, pharmacological, therapeutic, and chemoprotective properties. The aim of this study was to investigate the ameliorative effects of PCs against AFB1-induced histopathology, oxidative stress, and apoptosis via the mitochondrial pathway in the bursa of Fabricius (BF) of broilers. One hundred forty-four one-day old Cobb chicks were randomly assigned into four treatment groups of six replicates (6 birds each replicate) for 28 days. Groups were fed on the following four diets; (1) Basal diet without addition of PCs or AFB1 (Control); (2) basal diet supplemented with 1 mg/kg AFB1 from contaminated corn (AFB1); (3) basal diet supplemented with 250 mg/kg PCs (PCs); and (4) basal diet supplemented with 1 mg/kg AFB1 + 250 mg/kg PCs (AFB1+ PCs). The present study results showed that antioxidant enzymes activities of total superoxide dismutase (T-SOD), catalase (CAT), glutathione peroxidase (GSH-Px), and glutathione S-transferase (GST) in AFB1 treated group were (p < 0.05) decreased, whereas malondialdehyde (MDA) contents were significantly increased in comparison with the control group. Furthermore, we found that dietary PCs treatment ameliorated AFB1-induced oxidative stress in the BF through inhibiting the accumulation of MDA content and enhancing the antioxidant enzymes activities (T-SOD, CAT, GSH-Px, and GST). Similarly, PCs markedly enhanced messenger RNA (mRNA) expression of antioxidant genes (SOD, CAT, GPx1, and GST) in comparison with AFB1 group. Moreover, histological results showed that PCs alleviated AFB1-induced apoptotic cells in the BF of broilers. In addition, both mRNA and protein expression results manifested that mitochondrial-apoptosis-associated genes (Bax, caspase-9, caspase-3, and p53 and cytochrome c) showed up-regulation, while (Bcl-2) showed down-regulation in AFB1 fed group. The supplementation of PCs to AFB1 diet significantly reversed the mRNA and protein expression of these apoptosis-associated genes, as compared to the AFB1 group. Our results demonstrated that PCs ameliorated AFB1-induced oxidative stress by modulating the antioxidant defense system and apoptosis in the BF through mitochondrial pathway in broilers.
Keywords: aflatoxin B1, proanthocyanidins, oxidative stress, apoptosis, bursa of Fabricius, broiler
1. Introduction
Aflatoxins are secondary fungal metabolites, mainly produced by Aspergillus flavus and Aspergillus parasiticus, regarded as detrimental effects to the health of animals and humans [1]. Aflatoxin B1 (AFB1) is well-known among the different aflatoxin types because of its widespread occurrence, high toxicity, and economic implications across the world [2]. According to the directions of the International Agency for Research on Cancer (IARC), AFB1 is a group 1 carcinogen [3]. AFB1 is well known for its hepatotoxic, carcinogenic, teratogenic, immunosuppressive, and other devastating effects in mammals and poultry [4,5,6,7]. Furthermore, oxidative stress has been stated to have an essential role in the AFB1 toxicity mechanism [8]. AFB1 induces the formation of free radicals, hence raising the oxidative damage and lipid peroxidation which, in turn, leads to massive cellular damage which ultimately causes death to animals and humans [9,10].
Apoptosis is the phenomenon of programmed cell death [11]. It happened in multicellular organisms. Apoptosis is an essential process for normal homeostasis of tissue; also it is associated with causing pathogenesis of various diseases [12]. Apoptosis mechanism has been experimentally tested in multiple models that aflatoxins induce apoptosis via cellular toxicity, inhibition of carbohydrate, lipid metabolism, and protein synthesis [13]. In poultry, AFB1 can severely affect the immune system, caused oxidative stress, apoptosis, and histopathological lesions in lymphoid tissues [14,15,16]. At the same time, AFB1 can change the size of the immune organs, hence severely altering the immunological functions in chickens [14,17].
Recently, chemopreventive agents derived from natural plants are getting more attention for their low toxicity and efficient therapeutic uses. One among such phytochemical agent used in toxicity experiments is proanthocyanidins, mainly derived from the grape seed extract. Proanthocyanidins (PCs) demonstrates a broad range of biological effects, including anti-carcinogenic, anti-inflammatory, anti-arthritic, anti-apoptotic, anti-mutagenic, neuroprotective, and anti-allergic properties [18,19,20,21,22,23].
The bursa of Fabricius (BF) is a central lymphoid organ in birds, having the main role in the establishment and maintenance of humoral immunity and B cells compartment [24]. Although, a previous study showed that AFB1 could inhibit the development of BF [14]. However, the therapeutic effects of PCs against AFB1-induced toxic effects on BF of broilers have been rarely studied, especially oxidative stress and apoptosis. Therefore, this prompted us to investigate the protective effects of PCs toward AFB1-induced toxic effects on the BF of broilers. The objective of the current study was to evaluate the ameliorative effects of PCs against AFB1-induced histopathology, oxidative stress, and apoptosis via mitochondrial-mediated apoptosis pathway in the BF of broilers. These findings will be helpful for the dietary use of PCs as a therapeutic agent against AFB1-induced toxicity in both human and animals.
2. Results
2.1. The Relative Weight of Bursa of Fabricius
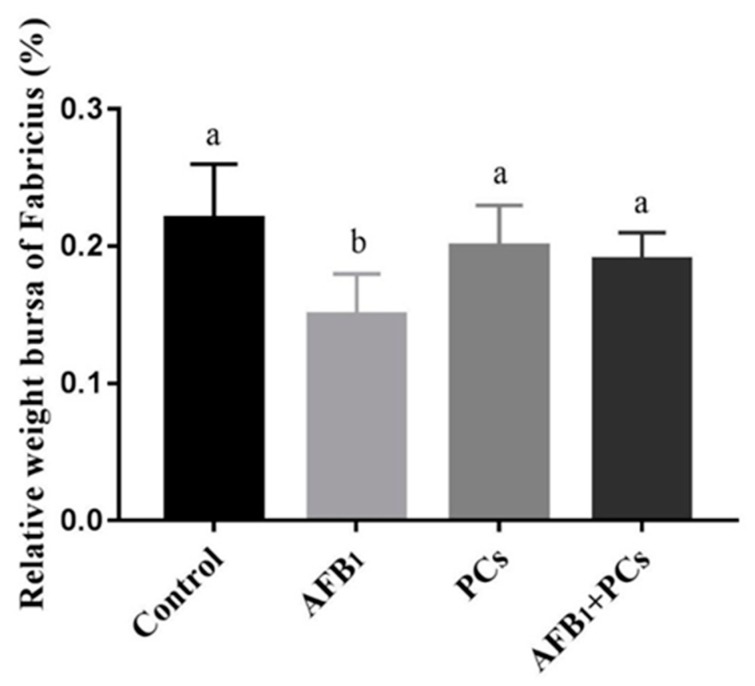
As shown in Figure 1, the relative weight of bursa of Fabricius (BF) in the AFB1 treated group was significantly lowered in contrast with the control group. Compared to the AFB1 group the relative weight of BF in the AFB1+ PCs group was (p < 0.05) increased.
Figure 1.
Effect of proanthocyanidins (PCs) on the relative weight of bursa of Fabricius (BF) in control and experimental broilers exposed to Aflatoxin B1 (AFB1). All data were expressed as mean ± SD (n = 6). Columns with different letters (a,b) indicate a significant difference at (p < 0.05).
2.2. Pathological Observation
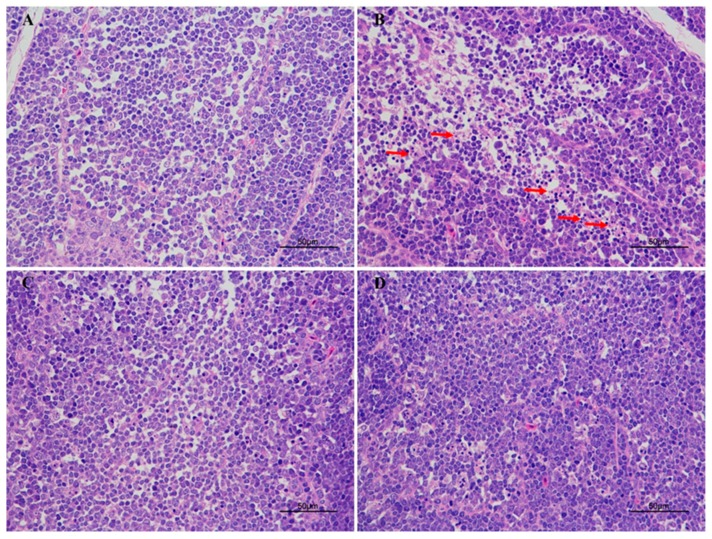
Histological changes in broiler BF tissues were shown in Figure 2. There were no pathological alterations in control and PCs alone fed groups (Figure 2A,C). The BF from AFB1 fed group birds manifested apoptotic cells, as compared to control and experimental groups (Figure 2B). Contrastingly, the supplementation of PCs to AFB1 contaminated diet significantly ameliorated and restored AFB1-induced apoptotic cells in the BF as compared to the AFB1-challenged group (Figure 2D).
Figure 2.
Effect of PCs on AFB1-induced histopathology in the control and experimental broilers. (A) The BF tissue from the control group; (B) the BF tissue, challenged with AFB1, induced apoptotic cells (arrows); (C) BF tissue from group of broilers treated with PCs; and (D) BF tissue from the group of broilers challenged with AFB1 and treated with PCs showing amelioration. The BF sections were stained with hematoxylin and eosin.
2.3. Determination of Oxidative Stress Markers
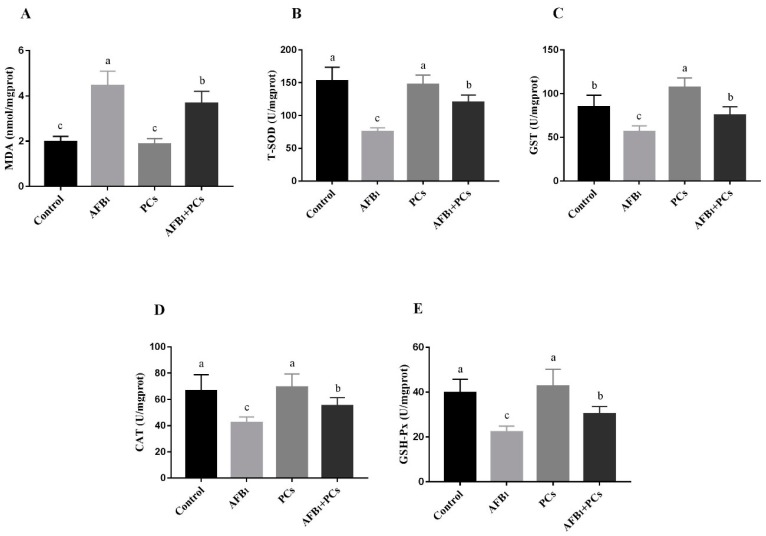
To examine the lipid peroxidation and antioxidant capacity in the BF of experimental broilers, the activities of total superoxide dismutase (T-SOD), glutathione S-transferase (GST), catalase (CAT), and glutathione peroxidase (GSH-Px) and contents of malondialdehyde (MDA) were measured (Figure 3). AFB1 group showed a significant increased MDA content in BF, as compared with the control group. In contrast, compared to the AFB1 group the MDA content was (p < 0.05) decreased in the group treated with AFB1 and PCs. Furthermore, the activities of T-SOD, GST, CAT, and GSH-Px were significantly decreased in AFB1 fed group when compared to the control group. However, the supplementation of PCs to AFB1 diet resulted in a significant improvement in the activity of T-SOD, GST, CAT, and GSH-Px when compared to the AFB1 fed group. In addition, PCs alone treated group exhibited a (p < 0.05) increase in the activity of GST in comparison with the control group (Figure 3C).
Figure 3.
Effect of PCs on AFB1-induced oxidative stress markers in the control and experimental broilers. All data were expressed as mean ± SD (n = 6). Columns with different letters (a–c) indicate a significant difference at p < 0.05. (A) Malondialdehyde (MDA); (B) Total superoxide dismutase (T-SOD); (C) Glutathione S-transferase (GST). (D) Catalase (CAT); (E) Glutathione peroxidase (GSH-Px).
2.4. Relative mRNA Expression of Antioxidant-Related Genes
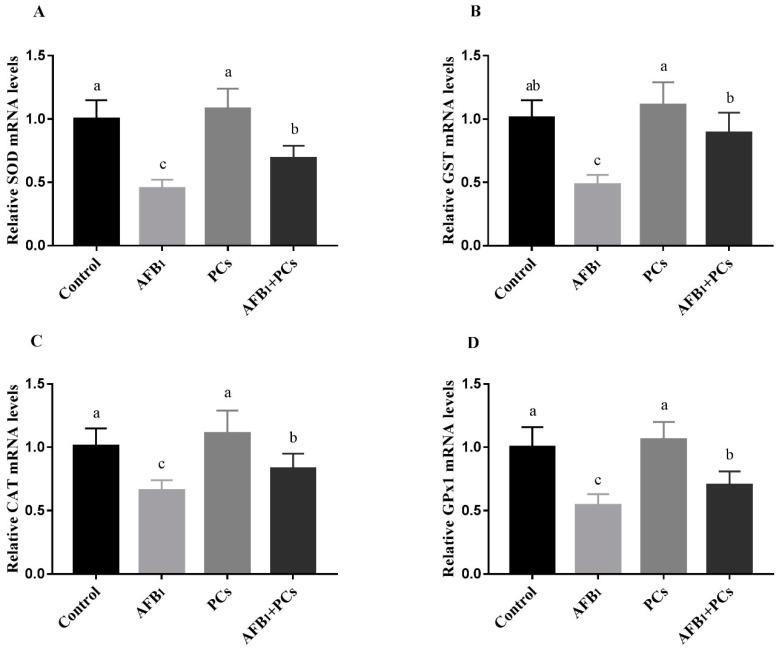
The messenger RNA (mRNA) expression of antioxidant genes SOD, GST, CAT, and GPx1 in BF of broilers are shown in Figure 4. The results of quantitative real-time PCR showed that the mRNA expression levels of SOD, GST, CAT, and GPx1 were (p < 0.05) down-regulated in the AFB1 group, compared to the control group. However, the addition of PCs into the AFB1 contaminated diet resulted in a significant up-regulation on the relative mRNA levels of SOD, GST, CAT, and GPx1, when compared to the AFB1 group.
Figure 4.
Effect of PCs on AFB1-induced oxidative stress-related genes in the control and experimental broilers. All data were expressed as mean ± SD (n = 6). Columns with different letters (a–c) indicate a significant difference at p < 0.05. (A) Superoxide dismutase (SOD); (B) Glutathione S-transferase (GST); (C) Catalase (CAT); (D) Glutathione peroxidase 1 (GPx1).
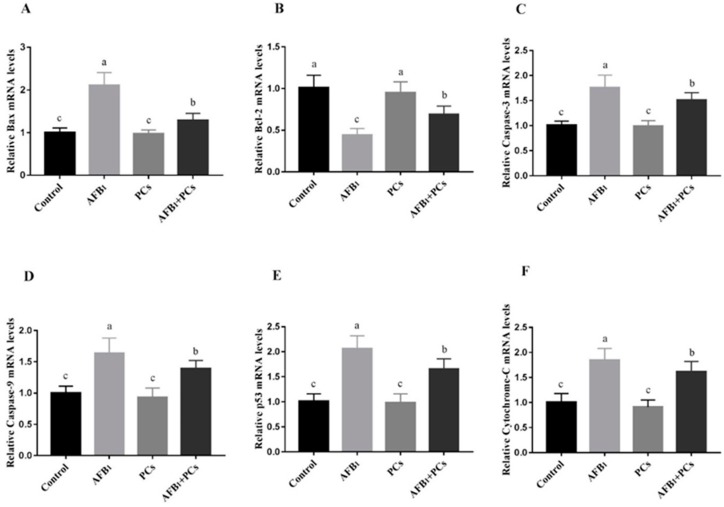
2.5. Relative mRNA Expression Levels of Apoptosis-Associated Genes
The effects of AFB1 and/or PCs on the mRNA expression levels of Bax, Bcl-2, caspase-3, caspase-9, p53, and cytochrome-C in control and experimental groups are depicted in Figure 5. Compared to the control group, the mRNA expression of Bax, caspase-3, caspase-9, p53, and cytochrome-C was significantly up-regulated in the AFB1 treated group. However, the supplementation of PCs to AFB1 diet (p < 0.05) reversed AFB1-induced up-regulation in the mRNA expression of Bax, caspase-3, caspase-9, p53, and cytochrome-C, as compared with the AFB1 group. The Bcl-2 mRNA expression level markedly down-regulated in the AFB1 fed group, when compared to the control group (Figure 5B). In contrast, compared to the AFB1 treated group, there was significant up-regulation in the Bcl-2 mRNA expression in the group co-treated with AFB1 + PCs.
Figure 5.
Effect of PCs on mRNA levels of mitochondrial apoptosis-associated genes in the control and experimental broilers exposed to AFB1. All data were expressed as mean ± SD (n = 6). Columns with different letters (a–c) indicate a significant difference at (p < 0.05). (A) Bcl-2-associated X protein (Bax); (B) B-cell lymphoma 2 (Bcl-2); (C) caspase-3; (D) caspase-9; (E) tumor protein p53 (p53); and (F) cytochrome c.
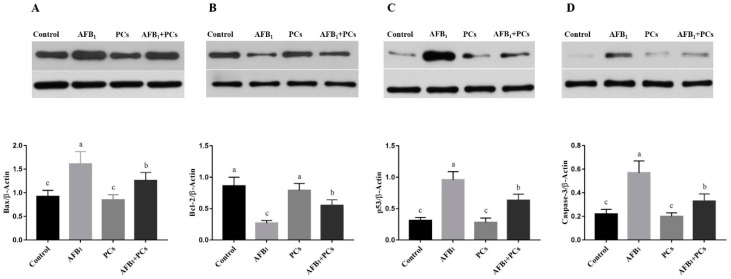
2.6. Protein Expression Levels of Apoptosis-Associated Genes
To explore the protective effects of PCs on AFB1-induced apoptosis, we further determined the protein expression level of Bax, Bcl-2, p53, and caspase-3 in broiler BF by Western blotting (Figure 6). Interestingly, the same trends were noted in Bax, Bcl-2, p53, and caspase-3 protein expressions as indicated in the mRNA expression levels. AFB1 treated group showed significant up-regulation in the protein expression of Bax, p53, and caspase-3 (Figure 6A,C,D). In contrast (p < 0.05) down-regulation was observed in the protein expression of Bcl-2 as compared to the control group (Figure 6B). However, compared to the AFB1 group, the significant down-regulation of Bax, p53, and caspase-3, and (p < 0.05) up-regulation in Bcl-2 were noticed in the group treated with AFB1 and PCs.
Figure 6.
Effect of PCs on protein expression levels of mitochondrial apoptosis-associated genes in the control and experimental broilers. All data were expressed as mean ± SD. Columns with different letters (a–c) indicate a significant difference at (p < 0.05). The lower bands are representing β-Actin for all above validated proteins. (A) Bcl-2-associated X protein (Bax); (B) B-cell lymphoma 2 (Bcl-2); (C) tumor protein p53 (p53); and (D) caspase-3.
3. Discussion
Proanthocyanidins are natural antioxidants with a wide range of pharmacological and medicinal properties. The absorption of PCs initially takes place in the small intestine and, subsequently, liver, thus resulting in a wide range of metabolites that reach to other organs through bloodstream [25]. In poultry the BF is a primary lymphoid organ which is responsible for the proliferation and modification of B cells [26]. Its relative weight usually evaluates the developmental status of BF. In our current study, AFB1 treated group reduced the relative weight of the BF, as compared with the control group. Moreover, our results showed that broilers challenged with AFB1 resulted in apoptotic cells in the BF, these findings were consistent with previous reports [14,27]. Strikingly, the supplementation of PCs to AFB1 contaminated diet ameliorated and restored AFB1-induced apoptotic cells in the BF as compared to the AFB1 challenged group and significantly improved the relative weight of BF altered by AFB1.
Aflatoxins have been reported to trigger the production of reactive oxygen species (ROS) and weaken antioxidant defense system, thus resulting in deoxyribonucleic acid (DNA) and mitochondrial damages, and the induction of apoptosis [28,29]. The cellular damage and lipid peroxidation status can be recognized by measuring the MDA content, as MDA is the main product of polyunsaturated lipid peroxidation [30]. The GST, SOD, GSH-Px, and CAT which are the most important elements of the endogenous antioxidant defense system, play a key role in relieving oxidative damage and free radicals scavenging and maintain the intracellular redox balance [31]. These antioxidant enzymes have been validated to have a critical role in the antioxidant mechanism of the body that can eliminate ROS from the cell, such as, for the first line of defense SOD catalyzes the dismutation of superoxide anion (O2−) to hydrogen peroxide (H2O2) [32]. CAT, a primary antioxidant defense component, catalyzes H2O2 directly to H2O and O2 with the presence of GSH. GSH-Px helps to metabolize H2O2 to non-toxic products and halts lipid peroxidation [33,34]. In the present study, the T-SOD, GST, CAT, and GSH-Px, activities as well as the MDA contents were also measured to assess the oxidative status of BF. Our results indicated that T-SOD, GST, CAT, and GSH-Px, activities were significantly decreased, while MDA content markedly increased in the AFB1 fed group, as compared to the control group. The current study findings are in accordance with the previous studies which revealed that AFB1 could induce oxidative stress in broilers [5,15,35,36]. Furthermore, PCs reduced the MDA contents and markedly increased the antioxidant enzyme activities (T-SOD, GST, CAT, and GSH-Px,), when compared to the AFB1 group. The above results were further confirmed by the mRNA expression analysis of antioxidant genes. Notably, in our study PCs enhanced GST enzyme activity and gene expression which is crucial for the detoxification of AFB1 and its toxic metabolites [37]. The present study findings confirmed that PCs counteracted oxidative stress and enhanced the antioxidant defense system induced by AFB1 in the BF of broilers.
Apoptosis is the phenomenon of programmed cell death that may occur in multicellular organisms [38]. Mitochondrial-dependent apoptotic signaling pathway plays a crucial role in the process of apoptosis. It is well documented that members of the Bcl-2 proteins regulate the mitochondrial-dependent apoptotic pathway including both anti-apoptotic (Bcl-2), and pro-apoptotic (Bax) [39]. Bcl-2 is one of anti-apoptotic protein which protects cells from apoptosis and pro-apoptotic proteins such as Bax that stimulate cell death [40]. Moreover, p53 protein can trigger the transcriptional activation of apoptotic factors such as Bax. Additionally, it can be transferred to the mitochondria, then binds to the Bcl-2 protein, thereby counteracting the anti-apoptotic role of Bcl-2 [41]. The caspase family has an essential function in the apoptosis pathway, and caspase-3 plays a key role in mediating in the mitochondrial pathway [42]. Throughout the transduction of an apoptotic (death) signal into the cell, an alteration occurs in the permeability of the membranes of the mitochondrial cells. As a consequence translocation of the apoptogenic proteins such as cytochrome complex (Cyt c) into the cytoplasm, that activates the initiation of caspase-9 and leads to the caspase-3 activation, thus induction of apoptosis [43,44]. Furthermore, previous studies reported that aflatoxins react antagonistically with different cell proteins, leading to inhibition of carbohydrate and lipid metabolism and protein synthesis, which induces apoptosis [13]. In this study, we determined the expression patterns of apoptosis genes involved in the mitochondrial pathway and therapeutic effects of PCs in preventing AFB1-induced apoptosis in BF of broilers. Our results showed that the mRNA expression levels of Bax, caspase-3, caspase-9, p53, and cytochrome-C were markedly increased while the mRNA expression levels of Bcl-2 were significantly decreased in the AFB1-fed group. Additionally, the same trends were observed in the protein expression profile of Bax, Bcl-2 caspase-3, and p53. The Western blot results showed that exposure to AFB1 significant up-regulation was noted in the protein expression of Bax, caspase-3, and p53, whereas down-regulation in the Bcl-2, when compared to the control and PCs alone treated groups. Similar results were noted in previous studies. Researchers reported that AFB1 exposure could result in apoptosis in the liver [45], BF [14], spleen [15], and thymus [46] of broilers, the mechanism associated with the mitochondrial pathway. However, the inclusion of PCs into AFB1 contaminated diet prevented AFB1-induced up-regulation in Bax, caspase-3, caspase-9, p53, and cytochrome-C and down-regulation in Bcl-2 mRNA expression levels as compared to the AFB1 treated group. Moreover, the supplementation of PCs along with an AFB1-contaminated diet reversed AFB1-induced protein expressions of Bax, Bcl-2, caspase-3, and p53 when compared with the AFB1 group. From these results, it has been suggested that PCs prevented AFB1-induced apoptosis in the BF of broilers by modulating both mRNA and protein expression levels of apoptosis-associated genes through the mitochondrial pathway.
4. Conclusions
In conclusion, our results suggested that AFB1 exerted toxic effects in the BF of broilers, as it induced oxidative stress and apoptosis. Furthermore, histology results showed that PCs ameliorated AFB1-induced apoptotic cells. In addition, PCs inhibited AFB1-induced oxidative stress by reducing the LPO accumulation and enhancing the antioxidant enzymes capacity. Notably, PCs attenuate AFB1-induced excessive apoptosis through the mitochondrial-mediated apoptosis pathway in the BF of broilers. The current study findings will provide helpful insight into the dietary use of PCs as a therapeutic agent against AFB1-induced toxicity in both humans and animals.
5. Material and Methods
5.1. Animal Ethics
All the experimental design and protocols were approved by the Institutional Animal Care and Ethics Committee of Huazhong Agricultural University, Wuhan, China on 7 August 2017 (approval no. HZAUCH-2017-007).
5.2. Aflatoxin B1 Production and Analysis
The Aspergillus flavus strain (NRRL-3357) was obtained from Sun Yat-sun University China. The strain was sub-cultured on potato dextrose agar (E. Merck, Darmstadt, Germany) medium at 30 °C for seven days. AFB1 was produced on corn following the method according to our previous study [47]. After inoculation of corn it was incubated for 15 days to get the approximate AFB1 content of 64 mg/kg. The concentration of AFB1 was determined by high-performance liquid chromatography (HPLC) (Waldbronn, Germany) equipped with fluorescent detector. Chromatographic separation was done using a C18 column (250 × 4.6 mm, 5 µm, Agilent Technologies, Santa Clara, CA, USA) as previously described [47].
5.3. Bird, Diets, and Management
Proanthocyanidins, extracted from grape seed were obtained from Zelang Medical Technology Company (Nanjing, China; purity ≥ 98%). One hundred forty-four one-day old Cobb broilers were purchased from a commercial hatchery (Jingzhou Kang Poultry Co., Ltd., Jingzhou, China). To ensure the proper distribution of artificially AFB1 contaminated feed and PCs in the basal diet. The basal diet was mixed with AFB1 contaminated feed and PCs in Rotex Master Mixer model YSHJ-100 (Shandong Rotex Machinery Co; Ltd, Shandong, China) for 20 min. After acclimatization for three days all the birds were randomly allocated into four groups with six replicates; six birds each replicate (n = 36 per group). Groups were distributed based on the following four dietary treatments: (1) basal diet without addition of PCs or AFB1 (Control); (2) basal diet supplemented with 1 mg/kg AFB1 from contaminated corn (AFB1); (3) basal diet supplemented with 250 mg/kg PCs (PCs); and (4) basal diet supplemented with 1 mg/kg AFB1 + 250 mg/kg PCs (AFB1 + PCs). Birds were reared in cages with electrically-heated units and feed and water were provided ad libitum throughout the experimental period (28 days). The experiment was conducted under controlled environmental conditions. The composition of the basal diet has been presented in Table 1.
Table 1.
Basal diet formulation and nutritional value.
| Ingredient | (%) |
|---|---|
| Corn | 58.3 |
| Soybean meal | 30.2 |
| Fish meal | 5.6 |
| Soybean oil | 2.3 |
| Dicalcium phosphate | 1.2 |
| Lime stone | 1.00 |
| Salt | 0.2 |
| Methionine | 0.2 |
| Premix 1 | 1.00 |
| Total | 100.00 |
| Calculated chemical composition | |
| Crude protein | 21.87 |
| Metabolisable energy (MJ/kg) | 13.45 |
| Lysine | 1.14 |
| Methionine | 0.40 |
| Methionine + Cystine | 0.94 |
| Calcium | 0.95 |
| Available phosphorus | 0.49 |
1 The premix contained (per kg of diet): Fe, 60 mg; Cu, 7.5 mg; Zn, 65 mg; Mn, 110 mg; I, 1.1 mg; Se, 0.4 mg; biotin, 0.04 mg; choline chloride, 400 mg; vitamin A (from retinyl acetate), 4500 IU; vitamin D3 (from cholecalciferol), 1000 IU; vitamin K (menadione sodium bisulphate), 1.3 mg; vitamin B1, 2.2 mg; vitamin B2, 10 mg; vitamin B3, 10 mg; vitamin B5, 50 mg; vitamin B6, 4 mg; vitamin B11, 1 mg; vitamin B12, 0.013 mg.
5.4. Collection of Samples
At the 28 days of age, one bird randomly selected from each cage (six birds from each group). Birds were individually weighed, and euthanized by cervical dislocation, and then bursa of Fabricius (BF) was collected and weighed immediately. The BF was immersed in liquid nitrogen and preserved at −80 °C for further analysis. The relative weight of each collected BF was calculated by the following formula:
| Relative weight = organ weight (g)/body weight (kg) |
5.5. Pathological Observation
For histological examination, the BF tissues were fixed in 10% neutral formalin. The hematoxylin and eosin (H and E) staining were performed according to previously described [35]. The BF sections of all birds were microscopically examined.
5.6. Determination of Oxidative Stress
The BF samples for determination of oxidative stress were prepared as described in our previous study [35]. Briefly, BF tissue samples (0.5 g) were cut into small pieces and homogenized in 4.5 mL ice cold physiological saline. The homogenate was centrifuged at 1000× g for 15 minutes at 4 °C. The supernatant was collected and stored at −80 °C for the following analysis. The contents of malondialdehyde (MDA) and activities of catalase (CAT), glutathione peroxide (GSH-Px), glutathione-S transferase (GST) and total superoxide dismutase (T-SOD), in the BF supernatants were measured spectrophotometrically (Hengping, Shanghai, China) using assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The details of all the determination procedures were performed according to the manufacturer’s protocols.
5.7. Total RNA Extraction and Quantitative Real-Time PCR
Total mRNA from the tissues of BF was extracted with Trizol® (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s instructions. The quality and concentration of RNA samples were measured by nucleic acid concentration analyzer NanoDrop 2000 (Thermo Fisher, Waltham, MA, USA). The complementary DNA (cDNA) was obtained from 1 µg of total ribonucleic acid (RNA) through reverse transcription in a 20 µL mixture reaction using a PrimeScriptTM RT reagent Kit (Takara DRR037A, Dalian, China) following the manufacturer’s protocol. The expression levels of pertaining genes (β-actin, SOD, CAT, GPx1, GST, Bax, Bcl-2, caspase-9, caspase-3, p53, and cytochrome-c) were quantified by quantitative real-time PCR (CFX384, Bio-Rad, Hercules, CA, USA) using the SYBER® Green PCR Master Mix (Applied Biosynthesis, Waltham, UK), following the method according to our previous study [48], and the manufacturer’s instructions. The primer sequences of each gene used in this study are listed in Table 2. The 2−△△Ct method was used for quantification with the β-actin as a reference gene, and the relative abundance was normalized to the control (as 1) [49,50]. The findings were expressed as relative mRNA levels.
Table 2.
Primers used for quantitative real-time PCR.
| Target Gene | Primer | Primer Sequence (5′→3′) | Accession No. |
|---|---|---|---|
| β-Actin | Forward Reverse |
CCCGCAAATGCTCTAAACC CCAATCCTGTCTTGTTTTATGC |
L08165 |
| Bax | Forward Reverse |
TCCTCATCGCCATGCTCAT CCTTGGTCTGGAAGCAGAAGA |
XM_422067 |
| Bcl-2 | Forward Reverse |
CGCCGCTACCAGAGGGACTT CCGGACCCAGTTGACCCCAT |
Z_11961.1 |
| Caspase-9 | Forward Reverse |
CCAACCTGAGAGTGAGCGATT GTACACCAGTCTGTGGGTCGG |
AY057940 |
| Caspase-3 | Forward Reverse |
GGCTCCTGGTTTATTCAGTCTC ATTCTGCCACTCTGCGATTT |
NM_204725.1 |
| p53 | Forward Reverse |
GCCGTGGCCGTCTATAAGAA GGTCTCGTCGTCGTGGTAAC |
NM_205264.1 |
| SOD | Forward Reverse |
CGTCATTCACTTCGAGCAGAAGG GTCTGAGACTCAGACCACATA |
NM_205064 |
| GPx1 | Forward Reverse |
GACCAACCCGCAGTACATCA GAGGTGCGGGCTTTCCTTTA |
NM_001277853.1 |
| CAT | Forward Reverse |
CCACGTGGACCTCTTCTTGT AAACACTTTCGCCTTGCAGT | NM_001031215.1 |
| GST | Forward Reverse |
AGTCGAAGCCTGATGCACTT TCTAGGCGTGGTTTCCTTTG |
L15386.1 |
| Cytochrome-C | Forward Reverse |
CGCAGGCTCCATACTACTCG TTAGGGCACCTCATAGGGCT |
NC_001323.1 |
5.8. Western Blot Analysis
Protein expressions of Bax, Bcl-2, caspase-3, and p53 in the BF of broilers were estimated by western blot according to our previous study [51]. The following antibodies used for the present study were purchased from indicated sources: Anti-Bax, Bcl-2, caspase-3, and p53 (Abclonal Technology, Wuhan, China), and Anti-β-Actin, (Cell Signaling Technology, Boston, MA, USA). The HRP-labeled goat anti-rabbit IgG (Servicebio Technology, Wuhan, China) was used as the secondary antibody. Samples were analyzed in triplicate; a representative blot is shown in respective figures. Proteins bands were detected through chemiluminescence WesternBrightTM ECL substrate kit (Advansta, Menlo Park, CA, USA), then visualized and quantified by a FluroChem FC2 Imaging System (ProteinSimple, California, USA).
5.9. Statistical Analysis
The experimental data were analyzed by one-way ANOVA using IBM SPSS Statistic 22 (IBM Corporation, Armonk, NY, USA). Differences were considered to be significant at p < 0.05, the Duncan’s test was used to separate the significant differences between means. Results were presented as mean ± SD.
Author Contributions
The authors’ responsibilities were as follows: S.A.R. and D.Q.: designed the research; S.A.R., C.Z., Y.F., S.T.W., M.M.K., A.S., and H.Q.: conducted the research; I.R.R., D.M.B., M.H., and N.R.: guided and performed the statistical analysis; S.A.R.: wrote the manuscript and hold primary responsibility for the final content; and all authors read and approved the final manuscript.
Funding
This research was funded by National Key Research and Development Program of China, grant number (2016YFD0501207) and National Natural Science Foundation of China (NSFC), grant number (31772635).
Conflicts of Interest
The authors declare no conflict of interest.
Key Contribution
PCs ameliorated AFB1-induced oxidative stress by modulating the antioxidant defense system and apoptosis in the BF through mitochondrial-mediated apoptosis pathway in broilers.
References
- 1.Marroquin-Cardona A.G., Johnson N.M., Phillips T.D., Hayes A.W. Mycotoxins in a changing global environment--a review. Food Chem. Toxicol. 2014;69:220–230. doi: 10.1016/j.fct.2014.04.025. [DOI] [PubMed] [Google Scholar]
- 2.Mary V.S., Valdehita A., Navas J.M., Rubinstein H.R., Fernandezcruz M.L. Effects of aflatoxin B1, fumonisin B1 and their mixture on the aryl hydrocarbon receptor and cytochrome P450 1A induction. Food Chem. Toxicol. 2015;75:104–111. doi: 10.1016/j.fct.2014.10.030. [DOI] [PubMed] [Google Scholar]
- 3.Craig A.W. IARC-Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. International Agency for Research on Cancer; Lyon, France: 1986. [Google Scholar]
- 4.Trebak F., Alaoui A., Alexandre D., El Ouezzani S., Anouar Y., Chartrel N., Magoul R. Impact of aflatoxin B1 on hypothalamic neuropeptides regulating feeding behavior. Neurotoxicology. 2015;49:165–173. doi: 10.1016/j.neuro.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 5.Zhang N.Y., Qi M., Zhao L., Zhu M.K., Guo J., Liu J., Gu C.Q., Rajput S.A., Krumm C.S., Qi D.S. Curcumin prevents aflatoxin B₁ hepatoxicity by inhibition of cytochrome p450 isozymes in chick liver. Toxins (Basel) 2016;8:327. doi: 10.3390/toxins8110327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stettler P.M., Sengstag C. Liver carcinogen aflatoxin B1 as an inducer of mitotic recombination in a human cell line. Mol. Carcinog. 2001;31:125–138. doi: 10.1002/mc.1047. [DOI] [PubMed] [Google Scholar]
- 7.Meissonnier G.M., Pinton P., Laffitte J., Cossalter A.M., Gong Y.Y., Wild C.P., Bertin G., Galtier P., Oswald I.P. Immunotoxicity of aflatoxin B1: Impairment of the cell-mediated response to vaccine antigen and modulation of cytokine expression. Toxicol. Appl. Pharmacol. 2008;231:142–149. doi: 10.1016/j.taap.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Abdel-Wahhab A.W., Aly S.E. Antioxidants and radical scavenging properties of vegetable extracts in rats fed aflatoxin-contaminated diet. J. Agric. Food Chem. 2003;51:2409–2414. doi: 10.1021/jf0209185. [DOI] [PubMed] [Google Scholar]
- 9.Surai P.F. Natural Antioxidants in Avian Nutrition and Reproduction. 1st ed. Nottingham University Press; Nottingham, UK: 2002. [Google Scholar]
- 10.Shen H.M., Shi C.Y., Lee H.P., Ong C.N. Aflatoxin B1-induced lipid peroxidation in rat liver. Toxicol. Appl. Pharmacol. 1994;127:145–150. doi: 10.1006/taap.1994.1148. [DOI] [PubMed] [Google Scholar]
- 11.Samali A., Fulda S., Gorman A.M., Hori O., Srinivasula S.M. Cellular stress responses: Cell survival and cell death. Int. J. Cell Biol. 2010;2010:245803. doi: 10.1155/2010/245803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brahmi D., Bouaziz C., Ayed Y., Mansour H.B., Zourgui L., Bacha H. Chemopreventive effect of cactus Opuntia ficus indicaon oxidative stress and genotoxicity of aflatoxin B1. Nutr. Metab. 2011;8:73. doi: 10.1186/1743-7075-8-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bbosa G.S., Kitya D., Odda J., Ogwal-Okeng J. Aflatoxins metabolism, effects on epigenetic mechanisms and their role in carcinogenesis. Health (N. Y.) 2013;5:14–34. doi: 10.4236/health.2013.510A1003. [DOI] [Google Scholar]
- 14.Chen K., Fang J., Peng X., Cui H., Chen J., Wang F., Chen Z., Zuo Z., Deng J., Lai W., Zhou Y. Effect of selenium supplementation on aflatoxin B1-induced histopathological lesions and apoptosis in bursa of Fabricius in broilers. Food Chem. Toxicol. 2014;74:91–97. doi: 10.1016/j.fct.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 15.Wang F., Shu G., Peng X., Fang J., Chen K., Cui H., Chen Z., Zuo Z., Deng J., Geng Y., Lai W. Protective effects of sodium selenite against aflatoxin B1-induced oxidative stress and apoptosis in broiler spleen. Int. J. Environ. Res. Public Health. 2013;10:2834–2844. doi: 10.3390/ijerph10072834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu T., Ma Q., Zhao L., Jia R., Zhang J., Ji C., Wang X. Protective effects of sporoderm-broken spores of ganderma lucidum on growth performance, antioxidant capacity and immune function of broiler chickens exposed to low level of aflatoxin B1. Toxins (Basel) 2016;8:278. doi: 10.3390/toxins8100278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peng X., Zhang K., Bai S., Ding X., Zeng Q., Yang J., Fang J., Chen K. Histological lesions, cell cycle arrest, apoptosis and T cell subsets changes of spleen in chicken fed aflatoxin-contaminated corn. Int. J. Environ. Res. Public Health. 2014;11:8567–8580. doi: 10.3390/ijerph110808567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ariga T. The antioxidative function, preventive action on disease and utilization of proanthocyanidins. Biofactors. 2004;21:197–201. doi: 10.1002/biof.552210140. [DOI] [PubMed] [Google Scholar]
- 19.Long M., Chen X., Wang N., Wang M., Pan J., Tong J., Li P., Yang S., He J. Proanthocyanidins protect epithelial cells from zearalenone-induced apoptosis via inhibition of endoplasmic reticulum stress-induced apoptosis pathways in mouse small intestines. Molecules. 2018;23:1508. doi: 10.3390/molecules23071508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uchino R., Madhyastha H., Dhungana S., Nakajima Y., Omura S., Maruyama M. NFkappaB-dependent regulation of urokinase plasminogen activator by proanthocyanidin-rich grape seed extract: Effect on invasion by prostate cancer cells. Blood Coagul. Fibrinolysis. 2010;21:528–533. doi: 10.1097/MBC.0b013e32833a9b61. [DOI] [PubMed] [Google Scholar]
- 21.Attia S.M., Bakheet S.A., Al-Easheed N.M. Proanthocyanidins produce significant attenuation of doxorubicin-induced mutagenicity via suppression of oxidative stress. Oxid. Med. Cell. Longev. 2010;3:404–413. doi: 10.4161/oxim.3.6.14418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahn S.H., Kim H.J., Jeong I., Hong Y.J., Kim M.J., Rhie D.J., Jo Y.H., Hahn S.J., Yoon S.H. Grape seed proanthocyanidin extract inhibits glutamate-induced cell death through inhibition of calcium signals and nitric oxide formation in cultured rat hippocampal neurons. BMC Neurosci. 2011;12:78. doi: 10.1186/1471-2202-12-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bagchi D., Sen C.K., Ray S.D., Das D.K., Bagchi M., Preuss H.G., Vinson J.A. Molecular mechanisms of cardioprotection by a novel grape seed proanthocyanidin extract. Mutat. Res. 2003;523–524:87–97. doi: 10.1016/S0027-5107(02)00324-X. [DOI] [PubMed] [Google Scholar]
- 24.Potworowski E.F. T and B lymphocytes. Organ and age distribution in the chicken. Immunology. 1972;23:199–204. [PMC free article] [PubMed] [Google Scholar]
- 25.Serra A., Macia A., Romero M., Angles N., Morello J.R., Motilva M.J. Distribution of procyanidins and their metabolites in rat plasma and tissues after an acute intake of hazelnut extract. Food Funct. 2011;2:562–568. doi: 10.1039/c1fo10083a. [DOI] [PubMed] [Google Scholar]
- 26.Masteller E.L., Lee K.P., Carlson L.M., Thompson C.B. Expression of sialyl Lewis (x) and Lewis (x) defines distinct stages of chicken B cell maturation. J. Immunol. 1995;155:5550–5556. [PubMed] [Google Scholar]
- 27.Santin E., Paulillo A.C., Maiorka A., Nakaghi L., Macari M., Fisher da Silva A.V., Alessi A.C. Evaluation of the efficacy of saccharomyces cerevisiae cell wall to ameliorate the toxic effects of aflatoxin in broilers. Int. J. Poult. Sci. 2003;2:341–344. [Google Scholar]
- 28.Kujoth G.C., Hiona A., Pugh T.D., Someya S., Panzer K., Wohlgemuth S.E., Hofer T., Seo A.Y., Sullivan R., Jobling W.A. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging et al. Science. 2005;309:481–484. doi: 10.1126/science.1112125. [DOI] [PubMed] [Google Scholar]
- 29.Yang C.F., Liu J., Wasser S., Shen H., Tan C.E.L., Ong C.N. Inhibition of ebselen on aflatoxin B1-induced hepatocarcinogenesis in Fischer 344 rats. Carcinogenesis. 2000;21:2237–2243. doi: 10.1093/carcin/21.12.2237. [DOI] [PubMed] [Google Scholar]
- 30.Del Rio D., Stewart A.J., Pellegrini N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nut. Metab. Cardiovasc. Dis. 2005;15:316–328. doi: 10.1016/j.numecd.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 31.Yener Z., Celik I., Ilhan F., Bal R. Effects of Urtica dioica L. Seed on lipid peroxidation, antioxidants and liver pathology in aflatoxin-induced tissue injury in rats. Food Chem. Toxicol. 2009;47:418–424. doi: 10.1016/j.fct.2008.11.031. [DOI] [PubMed] [Google Scholar]
- 32.Ighodaro O.M., Akinloye O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. AJME. 2018;54:287–293. doi: 10.1016/j.ajme.2017.09.001. [DOI] [Google Scholar]
- 33.Liu X.F., Zhang L.M., Guan H.N., Zhang Z.W., Xu S.W. Effects of oxidative stress on apoptosis in manganese-induced testicular toxicity in cocks. Food Chem. Toxicol. 2013;60:168–176. doi: 10.1016/j.fct.2013.07.058. [DOI] [PubMed] [Google Scholar]
- 34.Liu J., Li N., Ma L., Duan Y., Wang J., Zhao X., Wang S., Wang H., Hong F. Oxidative injury in the mouse spleen caused by lanthanides. J. Alloy. Compd. 2010;489:708–713. doi: 10.1016/j.jallcom.2009.09.158. [DOI] [Google Scholar]
- 35.Rajput S.A., Sun L., Zhang N., Khalil M.M., Gao X., Ling Z., Zhu L., Khan F.A., Zhang J., Qi D. Ameliorative effects of grape seed proanthocyanidin extract on growth performance, immune function, antioxidant capacity, biochemical constituents, liver histopathology and aflatoxin residues in broilers exposed to aflatoxin B1. Toxins (Basel) 2017;9:371. doi: 10.3390/toxins9110371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fan Y., Zhao L., Ji C., Li X., Jia R., Xi L., Zhang J., Ma Q. Protective effects of Bacillus subtilis ANSB060 on serum biochemistry, histopathological changes and antioxidant enzyme activities of broilers fed moldy peanut meal naturally contaminated with aflatoxins. Toxins (Basel) 2015;7:3330–3343. doi: 10.3390/toxins7083330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deng J., Ling Z., Ni-Ya Z., Karrow N.A., Christopher S.K., De-Sheng Q., Lv-Hui S. Aflatoxin B1 metabolism: Regulation by phase I and II metabolizing enzymes and chemoprotective agents. Mutat. Res.-/Rev. Mutat. 2018;778:778–779. doi: 10.1016/j.mrrev.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 38.Carson D.A., Ribeiro J.M. Apoptosis and disease. The Lancet. 1993;341:1251–1254. doi: 10.1016/0140-6736(93)91154-E. [DOI] [PubMed] [Google Scholar]
- 39.Antonsson B. Mitochondria and the Bcl-2 family proteins in apoptosis signaling pathways. Mol. Cell. Biochem. 2004;256–257:141–155. doi: 10.1023/B:MCBI.0000009865.70898.36. [DOI] [PubMed] [Google Scholar]
- 40.Siddiqui W.A., Ahad A., Ahsan H. The mystery of Bcl2 family: Bcl-2 proteins and apoptosis: An update. Arch. Toxicol. 2015;89:289–317. doi: 10.1007/s00204-014-1448-7. [DOI] [PubMed] [Google Scholar]
- 41.Miyashita T., Reed J.C. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell. 1995;80:293–300. doi: 10.1016/0092-8674(95)90412-3. [DOI] [PubMed] [Google Scholar]
- 42.Jin Y., Zhang S., Tao R., Huang J., He X., Qu L., Fu Z. Oral exposure of mice to cadmium (II), chromium (VI) and their mixture induce oxidative-and endoplasmic reticulum-stress mediated apoptosis in the livers. Environ. Toxicol. 2016;31:693–705. doi: 10.1002/tox.22082. [DOI] [PubMed] [Google Scholar]
- 43.Ly J.D., Grubb D.R., Lawen A. The mitochondrial membrane potential (ΔΨm) in apoptosis; an update. Apoptosis. 2003;8:115–128. doi: 10.1023/A:1022945107762. [DOI] [PubMed] [Google Scholar]
- 44.Green D.R., Reed J.C. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 45.Wang X., Muhammad I., Sun X., Han M., Hamid S., Zhang X. Protective role of curcumin in ameliorating AFB1-induced apoptosis via mitochondrial pathway in liver cells. Mol. Biol. Rep. 2018;45:881–891. doi: 10.1007/s11033-018-4234-4. [DOI] [PubMed] [Google Scholar]
- 46.Peng X., Chen K., Chen J., Fang J., Cui H., Zuo Z., Deng J., Chen Z., Geng Y., Lai W. Aflatoxin B1 affects apoptosis and expression of Bax, Bcl-2, and Caspase-3 in thymus and bursa of Fabricius in broiler chickens. Environ. Toxicol. 2016;31:1113–1120. doi: 10.1002/tox.22120. [DOI] [PubMed] [Google Scholar]
- 47.Liu J., Sun L., Zhang N., Zhang J., Guo J., Li C., Rajput S.A., Qi D. Effects of nutrients in substrates of different grains on aflatoxin B1 production by Aspergillus flavus. Biomed. Res. Int. 2016;2016 doi: 10.1155/2016/7232858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rajput S.A., Sun L., Zhang N.-Y., Khalil M.M., Ling Z., Chong L., Wang S., Rajput I.R., Bloch D.M., Khan F.A. Grape seed proanthocyanidin extract alleviates aflatoxin B1-induced immunotoxicity and oxidative stress via modulation of NF-κBand Nrf2 signaling pathways in broilers. Toxins (Basel) 2019;11:23. doi: 10.3390/toxins11010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang S., Yang J., Zhang B., Wu K., Yang A., Li C., Zhang J., Zhang C., Rajput S.A., Zhang N. Deoxynivalenol impairs porcine intestinal host defense peptide expression in weaned piglets and IPEC-J2 cells. Toxins (Basel) 2018;10:541. doi: 10.3390/toxins10120541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sun L.H., Lei M.Y., Zhang N.Y., Gao X., Li C., Krumm C.S., Qi D.S. Individual and combined cytotoxic effects of aflatoxin B1, zearalenone, deoxynivalenol and fumonisin B1 on BRL 3A rat liver cells. Toxicon. 2015;95:6–12. doi: 10.1016/j.toxicon.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 51.Gao X., Xiao Z., Li C., Zhang J., Zhu L., Sun L., Zhang N., Khalil M.M., Rajput S.A., Qi D. Prenatal exposure to zearalenone disrupts reproductive potential and development via hormone-related genes in male rats. Food Chem. Toxicol. 2018;116:11–19. doi: 10.1016/j.fct.2018.04.011. [DOI] [PubMed] [Google Scholar]