Abstract
Previous studies by our group have indicated that exercise intervention can ameliorate endothelial dysfunction, which is an early pathophysiological change of prediabetes mellitus. The present study aimed to test the hypothesis that nitric oxide synthases (NOSs), which are expressed in blood vessel endothelium, contribute to the mitigation of vascular endothelium-dependent dysfunction by aerobic exercise in prediabetes mellitus. A prediabetic rat model was established by feeding the rats an additional high-energy diet, and was confirmed by testing blood glucose levels, the area-under-the-curve for the blood glucose tests (P<0.05) and the changes to the histological morphology of the thoracic aorta. Further examination identified that NOS expression changed significantly between the control and prediabetes groups, indicating endothelial dysfunction in the prediabetic rats. Following aerobic exercise, a significant increase in NOS, endothelial (eNOS) mRNA and protein expression (P<0.05), and a significant decrease in NOS, inducible (iNOS) mRNA and protein expression (P<0.05) was identified in the prediabetic rats compared with the control group. No significant change in nitric oxide synthase, brain expression was observed in the prediabetic rat group compared with the control group. Notably, there was also a significant increase and decrease in eNOS and iNOS activity, respectively, in the prediabetes group compared with the control group (P<0.05). Furthermore, nitric oxide (NO) concentration in the vascular endothelium was detected, which revealed a significant increase in NO concentration in the prediabetic rats following aerobic exercise compared with the control (P<0.05). The present study provided results that demonstrated that aerobic exercise ameliorated the vascular endothelium-dependent dysfunction through the NOS/NO signaling pathway, which is primarily regulated by NOS expression and activity, in prediabetes mellitus. The current study provided the theoretical basis for the use of exercise intervention to prevent diabetes mellitus during the early stage.
Keywords: aerobic exercise, nitric oxide synthase, prediabetes mellitus
Introduction
Prediabetes mellitus is a pathological state that represents an elevation of plasma glucose above the normal range but below that of clinical diabetes (1–5). Prediabetes mellitus is characterized by impaired fasting glucose (IFG) and/or impaired glucose tolerance (IGT) (4,5). IFG and IGT are risk factors for type 2 diabetes, and the risk is even greater when IFG and IGT occur together (1,2). The annual risk of prediabetes developing into diabetes is 5–10%, with a similar proportion converting back to normoglycaemia (2,3).
The intact vascular endothelium prevents injury to blood vessels under physiological conditions and in diabetes mellitus hyperglycemia is associated with the development of endothelial dysfunction (4–6). Several studies have suggested that endothelial dysfunction contributes to macro- and micro-vascular complications in diabetes mellitus (7–9). Beneficial effects of exercise training on remedying diabetic endothelial dysfunction by inhibiting oxidative stress and improving nitric oxide (NO) bioavailability in the vascular wall have been reported (10,11). Xie et al (12) also demonstrated that exercise protects endothelium continuity through increasing NO production in collateral-dependent porcine coronary arterioles. Furthermore, NO synthesis depends on physical stimuli that modulate the activity of NO synthase (NOS) (13–15).
At present, three isoforms of NOS are recognized, including NOS, brain (nNOS), macrophage or NOS, inducible (iNOS) and NOS, endothelial (eNOS) (13). Studies have revealed that exercise-induced relaxation of the collateral coronary arteries is associated with the increased expression of eNOS mRNA and protein in healthy dogs and miniature swine (16,17). Miyauchi et al (18) demonstrated that, following exercise, eNOS mRNA expression was remarkably increased and the protein level decreased, while iNOS was not significantly affected in the lungs of animals. Tatchum-Talom et al (19) identified that swimming enhanced hindquarter acetylcholine-induced vasodilatation with an increase in nNOS activation in skeletal muscle, and eNOS in the lung, atria and aorta. Pellegrin et al (20,21) observed that swimming raised eNOS protein expression in mice with hypercholesterolemia and atherosclerosis, and had no effect on eNOS protein levels in normal mice. The results of these studies investigating exercise training suggest that the NOS/NO signaling pathway serves an important role in the regulation of vascular function. However, the contribution of eNOS/NO activity to the mitigation of vascular endothelium-dependent dysfunction by aerobic exercise in prediabetes mellitus requires further investigation.
In the present study, the effects of low-moderate exercise training on vascular pathological changes in prediabetic rats were examined, as well as the potential molecular mechanisms underlying these effects.
Materials and methods
Animals
The present study used 54 2-month-old male Wistar rats (230–235 g) that were obtained from Wushi Experimental Animal Supply Co., Ltd. (Fuzhou, China). All the rats were housed under optimal hygiene conditions at 23–25°C and with 50–60% humidity and a 12 h light/dark cycle. The animals were given a standard rat pellet diet and water ad libitum. The experimental protocol was approved in accordance with the Guide for the Care and Use of Laboratory Animals prepared by the Institutional Animal Care and Use Committee of Fujian Normal University (Fuzhou, China).
Experimental design
Rats were randomly divided into the control group (n=24) and the prediabetes group (n=30). In the control group, animals were fed a standard chow diet, while the prediabetes group received an additional high-energy diet emulsion, as previously described (22). For 1 month, the rats were given the high-energy diet daily. In the first 5 days, animals were intragastrically administered with the emulsion once a day; 1 ml was added to the volume of the emulsion administered each time until day 5. Thereafter, they were administered 5 ml of emulsion. Water was intragastrically administered to the control group at the same volume.
Blood was collected at the end of the tail using a pin-prick technique. Blood glucose levels were assayed using the Accu-Chek test strip (Roche Diabetes Care, Burgess Hill, UK) according to the manufacturer's protocol. The glucose tolerance of the rats was detected at 3 months of age, as described previously (22). The animals underwent fasting for 14–18 h prior to testing. An intraperitoneal (IP) injection with 6 ml 30% glucose (w/v)/kg of body weight was administered to each animal. Glucose levels were measured at 30, 60, 90 and 120 min after glucose loading. The area-under-the-curve (AUC) after 120 min of glucose level testing was calculated using the trapezoid rule.
Following a comprehensive analysis of glucose levels and the AUC of the glucose tolerance test for the prediabetes and control groups, 28 rats comprised the prediabetes group and 24 rats in the control group. Two rats in the prediabetes group succumbed. The animals in each group were randomly divided into two subgroups: The control and exercise intervention groups.
A total of 24 h after the last bout of aerobic exercise training the rats were anesthetized with an IP injection of pentobarbital solution (40 mg/kg; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and sacrificed by cervical dislocation. The thoracic aorta was rapidly removed and loosely adherent tissues were also removed. The aortic tissue was divided into two parts, one part was frozen with liquid nitrogen and stored at −80°C for biochemical analysis, and the other part was immersed in 4% paraformldehyde at room temperature for 24–48 h for histopathological evaluation.
Exercise training
Aerobic exercise training was performed according to the method used by Braga et al (23), but with modifications. All rats were acclimated to treadmill running for 10 min periods for 1 week. On the first day, an electric shock of 1 mA was applied to make the rats start running, following which they would run spontaneously. The maximal aerobic velocity (MAV) was evaluated with an incremental test to exhaustion using a protocol with an initial velocity of 5 m/min being intensified every 5 min with an increase in speed of 5 m/min, until the animal was unable or unwilling to continue. Aerobic exercise training was performed on a treadmill at a low-moderate intensity (50–60% MAV), 1 h/day, 5 days/week for 8 weeks. The experiment was performed in a quiet, well-ventilated room with 30–40% humidity and at ~18±2°C. Sedentary rats in the control group were handled in the same way as the exercise group; however, they did not engage in the regular running.
RNA extraction and reverse transcriptase-polymerase chain reaction (RT-PCR) analysis of NOS mRNA expression
Total RNA was extracted from the thoracic aorta samples using TRIzol solution (Thermo Fisher Scientific, Inc., Waltham, MA, USA), quantified by ultraviolet spectrometric detection using BioPhotometer Plus (Eppendorf, Hamburg, Germany) according to the manufacturer's protocol (24) and reverse transcribed into complementary DNA using a PrimeScript RT Reagent kit (Takara Biotechnology, Co., Ltd., Dalian, China), according to the manufacturer's protocol. qPCR analysis was performed to analyze the mRNA expression of eNOS, iNOS and nNOS using the SYBR Premix Ex Taq II kit (Takara Biotechnology, Co., Ltd.) with a program of 30 sec at 95°C, followed by 40 cycles of 15 sec at 95°C and 1 min at 60°C. The primers were synthesized by Takara Biotechnology, Co., Ltd., including eNOS primers [forward (F): 5′-GGCAGAGGAGTCCAGCGAAC-3′, reverse (R): 5′-TGTGGAACAGACCCCATAGTGC-3′], iNOS primers (F: 5′-GGACCACCTCTATCAGGAA-3′, R: 5′-CCTCATGATAACGTTTCTGGC-3′), nNOS primers (F: 5′-GGCAAACATGACTTCCGAGTGT-3′, R: 5′-CCCCAAGGTAGAGCCATCTG-3′) and GADPH primers (F: 5′-CGACCCCTTCATTGACCTCAAC-3′, R: 5′-AAGACGCCAGTAGACTCCACGAC-3′). The amount of target gene mRNA relative to the internal control gene, GADPH, mRNA was calculated in accordance with the 2−ΔΔCq method (25). Relative mRNA levels are reported as 2−ΔΔCq values. The results of three independent experiments were used for statistical analysis.
Immunohistochemistry for NOS
Following fixation, aortic segments were embedded in paraffin and then sectioned into 5-µm-thick sections. Certain sections were stained using hematoxylin for 3 min and eosin for 30 sec at room temperature for pathological evaluation. Immunohistochemical localization of eNOS, iNOS and nNOS was performed using mouse anti-eNOS (1:1,000; cat. no. ab76198), goat anti-nNOS (1:2,000; cat. no. ab1376) and rabbit anti-iNOS (1:100; cat. no. ab15323) antibodies (Abcam, Cambridge, MA, USA). The sections were incubated at room temperature overnight with the primary antibodies. The immunoreactivity of the specific protein was visualized using the Elite ABC kit (BioGenex Laboratories, San Ramon, CA, USA), according to the manufacturer's protocol. Then, the sections were counter-stained with hematoxylin for 2 min at room temperature, and mounted with coverslips to identify the structure and types of cells in the rat aorta. The negative control was treated with concentrated goat serum (1:10 dilution; Boster Biological Technology, Ltd., Wuhan, China) instead of primary antibodies. The slides were examined under an optical microscope at a magnification of ×100 or ×400.
Western blotting for examining eNOS protein expression
The western blot procedure was performed as previously described (26). Aorta tissue was homogenized and left for 30 min in ice-cold RIPA buffer [50 mM Tris (pH 7.4), 150 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate and 0.1% SDS; all Beyotime Institute of Biotechnology, Jiangsu, China] containing 2 mM phenylmethylsulfonyl fluoride (Beyotime Institute of Biotechnology). Thereafter, protein was extracted by centrifuging the cells at 4°C for 20 min at 13,000 × g, the protein concentration was calibrated using the BCA method and the samples were boiled in SDS-PAGE sample loading buffer (Beyotime Institute of Biotechnology). Sample were resolved by SDS-PAGE (6% acrylamide gel, 35 µg protein per lane) and transferred onto a polyvinylidene difluoride membrane. The transferred blots were blocking with 5% non-fat milk at room temperature for 1 h and then incubated at 4°C overnight with rabbit polyclonal anti-eNOS antibodies (1:500; cat. no. sc-654; Santa Cruz Biotechnology, Inc., Dallas, TX, USA). Mouse monoclonal anti-β-actin antibodies (1:1,000 dilution; cat. no. AF0003; Beyotime Institute of Biotechnology, Jiangsu, China) were used as the internal loading control for overnight at 4°C. Following washing with PSB, the blots were incubated with goat anti-rabbit (cat. no. A0208) and goat anti-rat (cat. no. A0192) horseradish peroxidase-conjugated immunoglobulin G secondary antibodies (1:1,000 dilution; Beyotime Institute of Biotechnology) at room temperature for 2 h. Protein bands were detected with BeyoECL Star Western Blotting Detection reagent (Beyotime Institute of Biotechnology). The relative intensity of eNOS compared with β-actin bands was quantified using the AlphaView® Q software (version 3.0; Proteinsimple; Bio-Techne, Minneapolis, MN, USA).
Determination of NOS activity and NO content
According to the method for NOS activity established by Wu et al (24), the total (t)NOS [constitutive (c)NOS and iNOS] and iNOS activities were assayed in accordance with the NOS typed assay kit (cat. no. A014-1; Nanjing Jiancheng Bioengineering Institute, Nanjing, China). As cNOS is eNOS in the rat aorta, the eNOS activity was calculated as tNOS minus iNOS. The enzyme activities were expressed as units/mg of protein. The results of six independent experiments were used for statistical analysis. NO content was examined with the nitrate reductase method using the NO assay kit (cat. no. A012; Nanjing Jiancheng Bioengineering Institute) according to the manufacturer's protocol.
Statistical analysis
Data are presented as mean ± standard error of the mean. The significant differences in mean values within and between multiple groups were evaluated using one-way analysis of the variance, followed by a Tukey's multiple range test. The tests were performed using SPSS software (version 19.0, IBM Corp., Armonk, NY, USA). P<0.05 indicated that the difference between groups was statistically significant.
Results
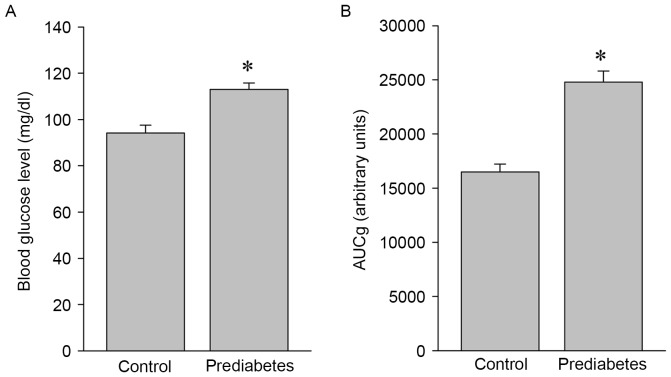
Blood glucose levels and glucose intolerance are increased in prediabetic rats
To investigate the effects of aerobic exercise on the expression and activity of NOS, a prediabetic rat model was developed and used in the present study. Significant increases in blood glucose levels (P<0.05; Fig. 1A) and glucose intolerance (P<0.05; Fig. 1B) were identified in the prediabetes group compared with the control group, indicating that these animals were in a prediabetic state and suitable for the following exercise intervention experiments.
Figure 1.
Blood glucose levels are higher and glucose intolerance is greater in prediabetic rats. (A) Blood glucose levels and (B) the glucose intolerance in the control and prediabetic rats. *P<0.05 vs. the control group. AUCg, area-under-the-curve for glucose tolerance test.
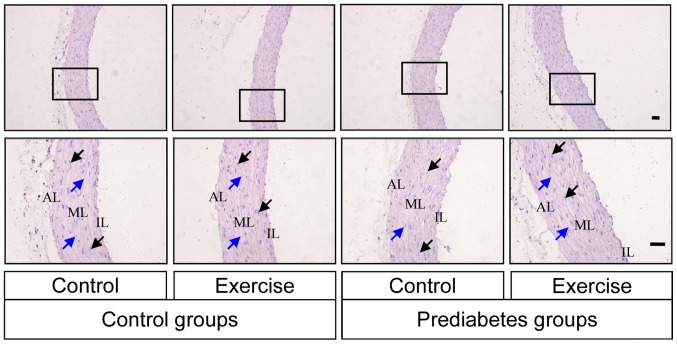
Aerobic exercise improves prediabetes mellitus-induced aortic damage
The histological morphology of the thoracic aorta was examined by HE staining in each group of rats. Intact vascular endothelial layers and smooth muscle layers were identified in the control groups, while, in the prediabetes groups, the vascular tunica intima was damaged (Fig. 2). Vascular endothelium staining was shallow and the internal elastic fibers were partially broken in the prediabetic rats, while the severity of histopathological alterations in the prediabetic rats subjected to aerobic exercise intervention was lower compared with those without exercise intervention. These observations indicating that aerobic exercise improved the damage caused to the thoracic aorta by prediabetes mellitus.
Figure 2.
Aerobic exercise improves prediabetes mellitus-induced aortic damage. The histological morphology of thoracic aorta was examined by hematoxylin and eosin staining in each group of rats. AL, adventitia layer; ML, medial layer; IL, intimal layer. The black and blue arrows indicate the elastic membranes and the smooth muscle nuclei, respectively. Original magnification, ×100 (upper row) or ×400 (lower row).
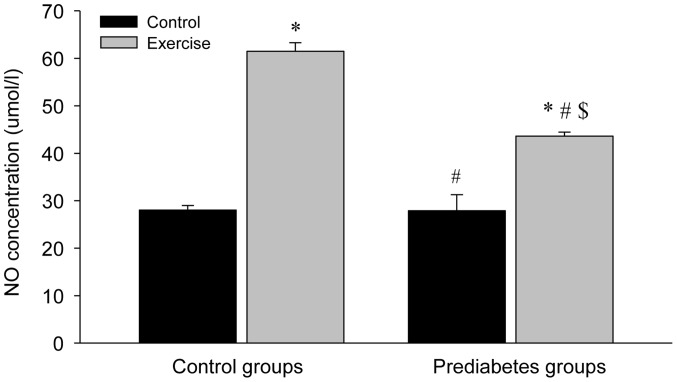
Aerobic exercise increases NO levels in the aorta
The NO levels in the aortas of the different groups were significantly increased following exercise intervention compared with the control groups (P<0.05; Fig. 3). However, the NO concentration in the control group with exercise intervention increased more compared with that in the prediabetes group with exercise intervention (P<0.05). Notably, NO concentrations between the two groups without exercise intervention were similar. These results indicated that increased NO production is associated with the repair of the vascular endothelial injury in prediabetic rats following exercise intervention.
Figure 3.
NO concentrations in aorta increase following aerobic exercise. NO, nitric oxide. *P<0.05 vs. the control/control group, #P<0.05 vs. the control/exercise group, $P<0.05 vs. the prediabetes/control group.
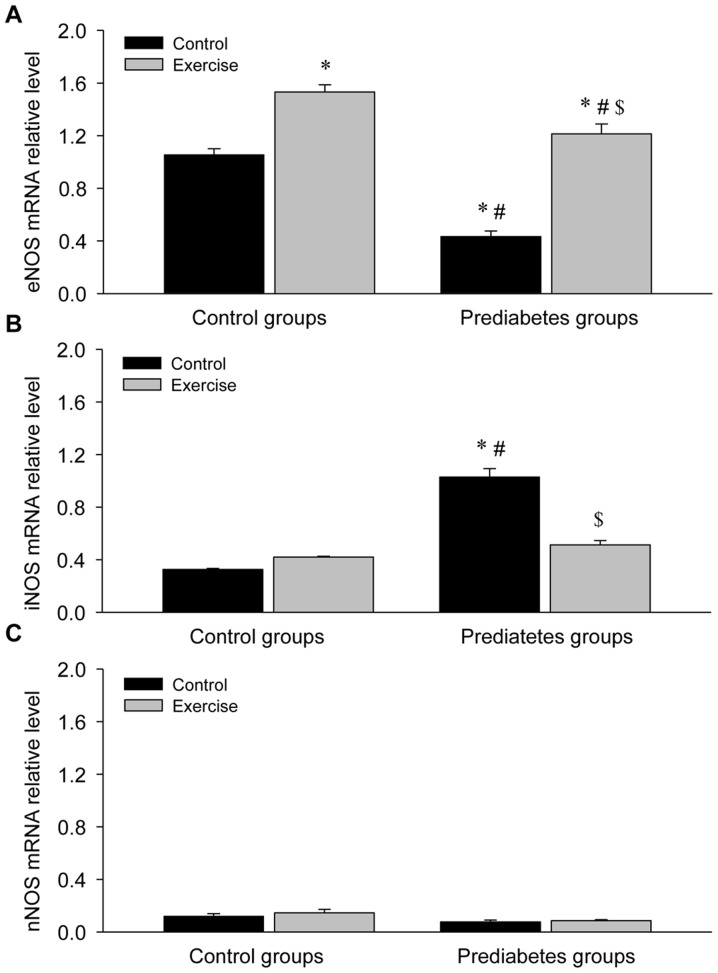
Aerobic exercise increases eNOS and decreases iNOS mRNA expression in the aorta of prediabetic rats
Given the regulatory role of NOS in NO production, the mRNA expression of NOS was detected in the aortas of rats in each group (Fig. 4). This revealed a significant decrease in eNOS mRNA expression (P<0.05; Fig. 4A), a significant increase in iNOS mRNA expression (P<0.05; Fig. 4B) and no obvious change in nNOS mRNA expression (Fig. 4C) in the prediatetes rats compared with the control rats, suggesting a possible mechanism for why there was no significant change in NO levels between these two groups. Following exercise intervention, eNOS mRNA expression increased and iNOS mRNA expression decreased significantly, indicating that eNOS serves an important role in the reversal of endothelial injury following aerobic exercise intervention.
Figure 4.
Aerobic exercise increases eNOS and decreases iNOS mRNA expression in the aortas of prediabetic rats. (A) eNOS, (B) iNOS and (C) nNOS mRNA expression levels. *P<0.05 vs. the control/control group, #P<0.05 vs. the control/exercise group, $P<0.05 vs. the prediabetes/control group. NOS, nitric oxide synthase; eNOS, NOS, endothelial; iNOS, NOS, inducible; nNOS, NOS, brain.
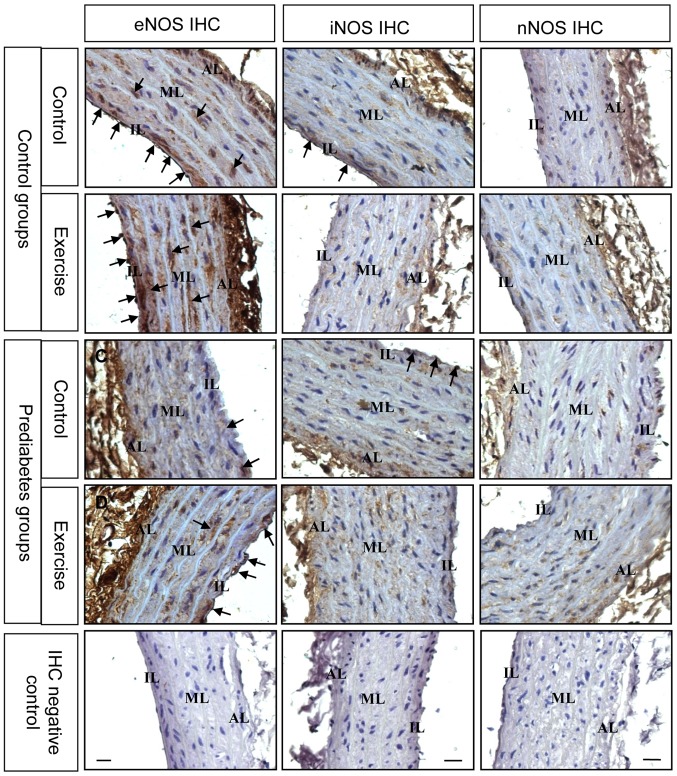
For further determination of the changes in NOS expression, immunostaining was performed to examine NOS expression in the thoracic aorta from each group. The expression and localization of NOS was revealed by arterial cross sections immunolabelled with antibodies directed against different NOS isoforms (Fig. 5). The results demonstrated that prior to exercise intervention, eNOS was decreased in the endothelial and smooth muscle layers of thoracic aorta in the prediabetes group compared with the control group. Following exercise intervention, eNOS immunostaining was stronger in these two groups. Nevertheless, iNOS expression decreased following exercise intervention. Changes in nNOS immunostaining were not evident. These immunostaining results were consistent with the NOS mRNA expression results and further suggest that eNOS serves an important role in the regulation of endothelial dysfunction in prediabetic rats.
Figure 5.
Aerobic exercise increases eNOS and decreases iNOS protein expression in the aortas of prediabetic rats. The black arrows denote the positive signals. Original magnification, ×400. NOS, nitric oxide synthases; eNOS, NOS, endothelial; iNOS, NOS, inducible; nNOS, NOS, brain; AL, adventitia layer; ML, medial layer; IL, intimal layer.
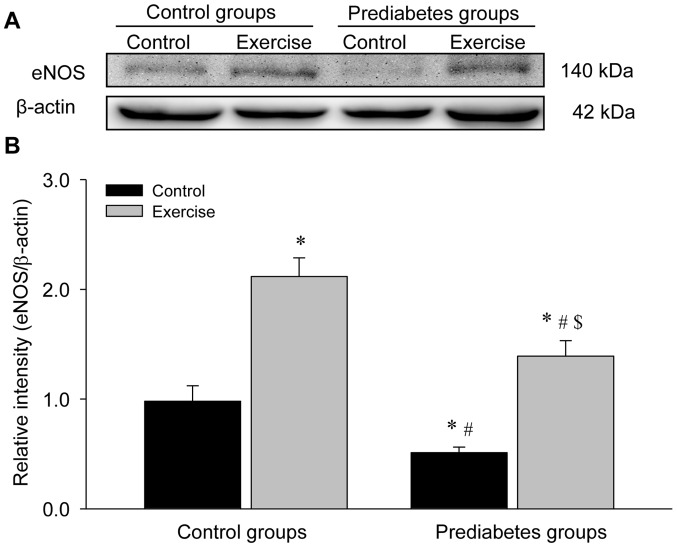
Aerobic exercise increases eNOS protein expression in the aorta of prediabetic rats
The present study detected eNOS protein expression levels through western blot analyses (Fig. 6). The results demonstrated a significant decrease in eNOS protein levels in the sedentary prediabetic rats compared with the sedentary control rats (P<0.05), and exercise intervention significantly increased eNOS protein expression in these two groups (both P<0.05 vs. their respective control groups), which was consist with the identified changes in eNOS mRNA expression.
Figure 6.
Aerobic exercise increases eNOS protein expression in the aorta of prediabetic rats. (A) Representative western blotting images of the protein levels of eNOS. (B) Quantification of the blot intensities of eNOS. *P<0.05 vs. the control/control group, #P<0.05 vs. the control/exercise group, $P<0.05 vs. the prediabetes/control group. eNOS, nitric oxide synthase, endothelial.
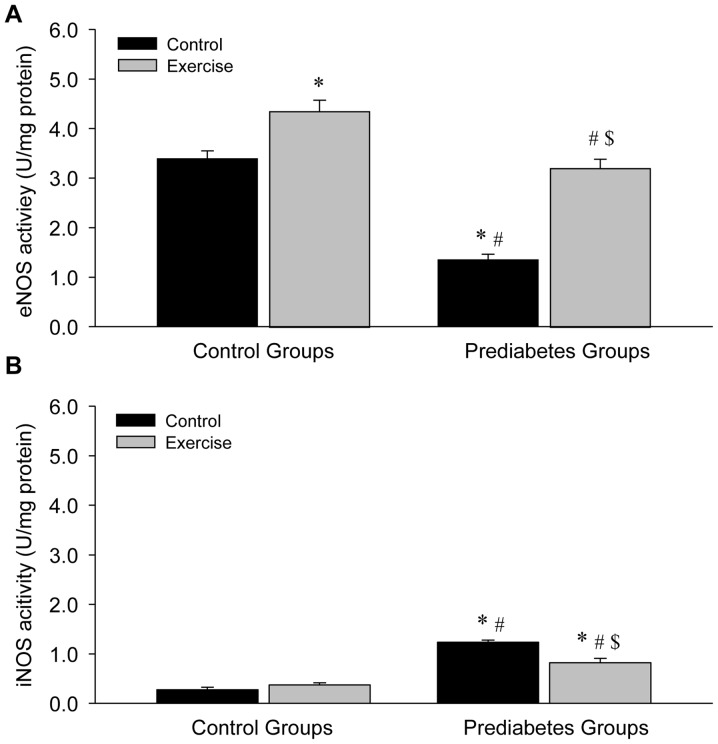
Aerobic exercise increases eNOS and decreases iNOS activity in the aortas of prediabetic rats
NOS activity was examined in the present study in order to further explore the association between exercise intervention and NO production. Compared with the sedentary control group, a significant decrease in eNOS activity (P<0.05; Fig. 7A) and a significant increase in iNOS activity (P<0.05; Fig. 7B) in the vessels were identified in the sedentary prediabetic group. However, following exercise intervention, eNOS activity significantly increased in the exercise and control groups (both P<0.05; Fig. 7A), while iNOS activity significantly decreased in the prediabetes group (P<0.05; Fig. 7B). These changes were similar to the observed changes in NOS expression, indicating that NOS activity depended upon NOS expression.
Figure 7.
Aerobic exercise increases (A) eNOS and decreases (B) iNOS activity in the aortas of prediabetic rats. *P<0.05 vs. the control/control group, #P<0.05 vs. the control/exercise group, $P<0.05 vs. the prediabetes/control group. NOS, nitric oxide synthases; eNOS, NOS, endothelial; iNOS, NOS, inducible.
Discussion
The present study demonstrated that aerobic exercise intervention helped to ameliorate vascular endothelium-dependent dysfunction through the NOS/NO signaling pathway, primarily regulated by NOS expression and activity in prediabetes mellitus. These findings provide important insight that can be used in further investigations of the underlying molecular mechanism of exercise intervention and prevention of diabetes mellitus.
Prediabetes mellitus is a pathological state between normoglycemia and diabetes mellitus, which is characterized by IGT and mild hyperglycemia (1–3). In the present study, a prediabetic rat model was established based on the method of Rato et al (22), and was used to examine blood glucose levels and glucose intolerance. An intact vascular endothelium inhibits atherosclerosis under physiological conditions and vascular compliance is a direct indicator of the functional status of the arteries (27). In the present study, an incomplete vascular endothelium and broken internal elastic fibers were observed in the prediabetic rats, indicating vascular endothelium-dependent dysfunction. These rats were used in the following exercise intervention experiments.
Aerobic exercise intervention is widely considered to be an important non-pharmacological tool for the improvement of vascular endothelial function (10). Previous studies by our group have indicated that regular aerobic exercise intervention promotes the maintenance of vasomotor functions (1–5,9). Xie et al (12) demonstrated that exercise protects the endothelium through increasing NO production, which was consistent with the results of the present study. The present study further demonstrated that aerobic exercise intervention alleviated vascular histopathological alterations through improving the vascular endothelium and elevated NO production through inducing eNOS expression in prediabetic rats.
NO is a key signaling molecule in vascular homeostasis (28), which was originally identified as an endothelium-derived relaxation factor (12). The present study identified that under normal conditions without exercise intervention, marked changes in NO levels were not identified in the prediabetic rats. Notably, the expression of eNOS mRNA and protein decreased significantly following exercise in the prediabetic group. Given the regulatory role of NOS in the process of NO biosynthesis, the expression of different NOS isoforms was detected in order to further understand the molecular mechanism of aerobic exercise intervention in the regulation of vascular endothelium-dependent dysfunction in prediabetic rats. The significant decrease in eNOS mRNA and protein expression identified in prediabetic rats was reversed by exercise intervention in the prediabetic rats, which is consistent with previous reports (16–21). Several studies have indicated that exercise-induced relaxation of the collateral coronary arteries is associated with the increased expression of eNOS mRNA and protein in healthy animals (16,17), and that swimming increases eNOS expression at the protein level in mice prone to hypercholesterolemia and atherosclerosis (20,21). The present study demonstrated that the expression of iNOS mRNA was in contrast to eNOS mRNA expression in the prediabetic group, which led to the homeostasis of NO production. Gielen et al (29) identified that physical exercise decreased the expression of iNOS at the mRNA and protein levels in blood vessels, and Wu et al (24) revealed that high glucose incubation led to a significant decrease in eNOS expression and NO concentration, with increased iNOS mRNA and protein levels in rat thoracic aortic rings. The activity of eNOS and iNOS was also examined in the present study, which revealed increased eNOS activity in prediabetic rats following exercise. These results suggest that the eNOS/NO signaling pathway serves an important role in the regulation of vascular dysfunction in prediabetes mellitus.
In conclusion, the present study demonstrated that aerobic exercise intervention attenuated vascular injury of the thoracic aorta through activating the eNOS/NO signaling pathway in prediabetic rats. However, the specific mechanism of eNOS induction requires further investigation. In addition, the exercise threshold may be important for the regulation of NO production, but the precise identification of this threshold requires further study. The results of the current study demonstrated that aerobic exercise intervention improved endothelial function and reduced aortic histopathological injury, due to activating the eNOS/NO signaling pathway, in prediabetes mellitus, which highlights a mechanism that could be targeted to prevent diabetes mellitus in clinical practice.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science Foundation of China (grant no. 31271255), the Fujian Provincial Natural Science Foundation (grant nos. 2016J01145 and 2016J01150) and the Education Department of Fujian Province Science and Technology Project (grant no. JAT160118).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
SW, YL and ZW designed the study. SW, JL, CZ and GX performed the experiments. SW, ZT, ZZ, YL and ZW analyzed the data. SW, YL and ZW interpreted the data and discussed the results. SW wrote the manuscript and ZW revised it with YL. All authors read and approved the final version of the manuscript for publication.
Ethics approval and consent to participate
The experimental protocol was approved in accordance with the Guide for the Care and Use of Laboratory Animals prepared by the Institutional Animal Care and Use Committee of Fujian Normal University (Fuzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Tabák AG, Herder C, Rathmann W, Brunner EJ, Kivimäki M. Prediabetes: A high-risk state for diabetes development. Lancet. 2012;379:2279–2290. doi: 10.1016/S0140-6736(12)60283-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nathan DM, Davidson MB, DeFronzo RA, Heine RJ, Henry RR, Pratley R, Zinman B, American Diabetes Association. Impaired fasting glucose and impaired glucose tolerance: Implications for care. Diabetes Care. 2007;30:753–759. doi: 10.2337/dc07-9920. [DOI] [PubMed] [Google Scholar]
- 3.Gupta A, Al-Aubaidy HA, Mohammed BI. Glucose dependent insulinotropic polypeptide and dipeptidyl peptidase inhibitors: Their roles in management of type 2 diabetes mellitus. Diabetes Metab Syndr 10 (2 Suppl 1) 2016:S170–S175. doi: 10.1016/j.dsx.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 4.Liu Y, Li J, Zhang Z, Tang Y, Chen Z, Wang Z. Endocrinological analysis of endothelium-dependent vasodilation in middle-aged patients with impaired glucose tolerance during prediabetes mellitus. Exp Ther Med. 2014;7:697–702. doi: 10.3892/etm.2014.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Y, Li J, Zhang Z, Tang Y, Chen Z, Wang Z. Effects of exercise intervention on vascular endothelium functions of patients with impaired glucose tolerance during prediabetes mellitus. Exp Ther Med. 2013;5:1559–1565. doi: 10.3892/etm.2013.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fiorentino TV, Prioletta A, Zuo P, Folli F. Hyperglycemia-induced oxidative stress and its role in diabetes mellitus related cardiovascular diseases. Curr Pharm Des. 2013;19:5695–5703. doi: 10.2174/1381612811319320005. [DOI] [PubMed] [Google Scholar]
- 7.Sena CM, Pereira AM, Seiça R. Endothelial dysfunction-a major mediator of diabetic vascular disease. Biochim Biophys Acta. 2013;1832:2216–2231. doi: 10.1016/j.bbadis.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 8.Fowler MJ, Michael J. Microvascular and macrovascular complications of diabetes. Clin Diabetes. 2008;26:77–82. doi: 10.2337/diaclin.26.2.77. [DOI] [Google Scholar]
- 9.De Vriese AS, Verbeuren TJ, Van de Voorde J, Lameire NH, Vanhoutte PM. Endothelial dysfunction in diabetes. Br J Pharmacol. 2000;130:963–974. doi: 10.1038/sj.bjp.0703393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chakraphan D, Sridulyakul P, Thipakorn B, Bunnag S, Huxley VH, Patumraj S. Attenuation of endothelial dysfunction by exercise training in STZ-induced diabetic rats. Clin Hemorheol Microcirc. 2005;32:217–226. [PubMed] [Google Scholar]
- 11.Chis IC, Coseriu A, Simedrea R, Oros A, Nagy AL, Clichici S. In vivo effects of quercetin in association with moderate exercise training in improving streptozotocin-induced aortic tissue injuries. Molecules. 2015;20:21770–21786. doi: 10.3390/molecules201219802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie W, Parker JL, Heaps CL. Effect of exercise training on nitric oxide and superoxide/H2O2 signaling pathways in collateral-dependent porcine coronary arterioles. J Appl Physiol (1985) 2012;112:1546–1555. doi: 10.1152/japplphysiol.01248.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang W, Wei QW, Wang ZC, Ding W, Wang W, Shi FX. Cell-specific expression and immunolocalization of nitric oxide synthase isoforms and the related nitric oxide/cyclic GMP signaling pathway in the ovaries of neonatal and immature rats. J Zhejiang Univ Sci B. 2011;12:55–64. doi: 10.1631/jzus.B1000174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng K, Sulieman FJ, Li J, Wei Q, Xu M, Shi F. Nitric oxide and thyroid hormone receptor alpha 1 contribute to ovarian follicular development in immature hyper- and hypo-thyroid rats. Reprod Biol. 2015;15:27–33. doi: 10.1016/j.repbio.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 15.Xu M, Wei Q, Zheng K, Mao D, Zheng Y, Li Y, Shi F. Protective effects of Big-leaf mulberry and physiological roles of nitric oxide synthases in the testis of mice following water immersion and restraint stress. Acta Histochem. 2014;116:1323–1330. doi: 10.1016/j.acthis.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 16.Laughlin MH, Pollock JS, Amann JF, Hollis ML, Woodman CR, Price EM. Training induces nonuniform increases in eNOS content along the coronary arterial tree. J Appl Physiol (1985) 2001;90:501–510. doi: 10.1152/jappl.2001.90.2.501. [DOI] [PubMed] [Google Scholar]
- 17.Sessa WC, Pritchard K, Seyedi N, Wang J, Hintze TH. Chronic exercise in dogs increases coronary vascular nitric oxide production and endothelial cell nitric oxide synthase gene expression. Circ Res. 1994;74:349–353. doi: 10.1161/01.RES.74.2.349. [DOI] [PubMed] [Google Scholar]
- 18.Miyauchi T, Maeda S, Iemitsu M, Kobayashi T, Kumagai Y, Yamaguchi I, Matsuda M. Exercise causes a tissue-specific change of NO production in the kidney and lung. J Appl Physiol (1985) 2003;94:60–68. doi: 10.1152/japplphysiol.00269.2002. [DOI] [PubMed] [Google Scholar]
- 19.Tatchum-Talom R, Schulz R, McNeill JR, Khadour FH. Upregulation of neuronal nitric oxide synthase in skeletal muscle by swim training. Am J Physiol Heart Circ Physiol. 2000;279:H1757–H1766. doi: 10.1152/ajpheart.2000.279.4.H1757. [DOI] [PubMed] [Google Scholar]
- 20.Pellegrin M, Berthelot A, Houdayer C, Gaume V, Deckert V, Laurant P. New insights into the vascular mechanisms underlying the beneficial effect of swimming training on the endothelial vasodilator function in apolipoprotein E-deficient mice. Atherosclerosis. 2007;190:35–42. doi: 10.1016/j.atherosclerosis.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 21.Pellegrin M, Miguet-Alfonsi C, Berthelot A, Mazzolai L, Laurant P. Long-term swimming exercise does not modulate the Akt-dependent endothelial nitric oxide synthase phosphorylation in healthy mice. Can J Physiol Pharmacol. 2011;89:72–76. doi: 10.1139/Y10-107. [DOI] [PubMed] [Google Scholar]
- 22.Rato L, Duarte AI, Tomás GD, Santos MS, Moreira PI, Socorro S, Cavaco JE, Alves MG, Oliveira PF. Pre-diabetes alters testicular PGC1-α/SIRT3 axis modulating mitochondrial bioenergetics and oxidative stress. Biochim Biophys Acta. 2014;1837:335–344. doi: 10.1016/j.bbabio.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 23.Braga VA, Couto GK, Lazzarin MC, Rossoni LV, Medeiros A. Aerobic exercise training prevents the onset of endothelial dysfunction via increased nitric oxide bioavailability and reduced reactive oxygen species in an experimental model of menopause. PLoS One. 2015;10:e0125388. doi: 10.1371/journal.pone.0125388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu Y, Xue L, Du W, Huang B, Tang C, Liu C, Qiu H, Jiang Q. Polydatin restores endothelium-dependent relaxation in rat aorta rings impaired by high glucose: A novel insight into the PPARβ-NO signaling pathway. PLoS One. 2015;10:e0126249. doi: 10.1371/journal.pone.0126249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C (T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 26.Wang SB, Xing BS, Yi L, Wang W, Xu YX. Expression of Frizzled 2 in the mouse ovary during oestrous cycle. J Anim Physiol Anim Nutr (Berl) 2010;94:437–445. doi: 10.1111/j.1439-0396.2009.00927.x. [DOI] [PubMed] [Google Scholar]
- 27.Westerterp KR. Perception, passive overfeeding and energy metabolism. Physiol Behav. 2006;89:62–65. doi: 10.1016/j.physbeh.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 28.Ignarro LJ. Nitric oxide as a unique signaling molecule in the vascular system: A historical overview. J Physiol Pharmacol. 2002;53:503–514. [PubMed] [Google Scholar]
- 29.Gielen S, Adams V, Linke A, Erbs S, Möbius-Winkler S, Schubert A, Schuler G, Hambrecht R. Exercise training in chronic heart failure: Correlation between reduced local inflammation and improved oxidative capacity in the skeletal muscle. Eur J Cardiovasc Prev Rehabil. 2005;12:393–400. doi: 10.1097/01.hjr.0000174824.94892.43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.