Abstract
Aims and Hypothesis
The optimal duration and frequency of short-term continuous glucose monitoring (CGM) to reflect long-term glycemia have not been determined. The Juvenile Diabetes Research Foundation CGM randomized trials provided a large dataset of longitudinal CGM data for this type of analysis.
Methods
The analysis included 185 subjects who had 334 3-month intervals of CGM data meeting specific criteria. For various glucose indices, correlations (r2) were computed for the entire 3-month interval versus selected sampling periods ranging from 3 to 15 days. Other computed agreement measures included median relative absolute difference, values within ± 10% and ± 20% of full value, and median absolute difference.
Results
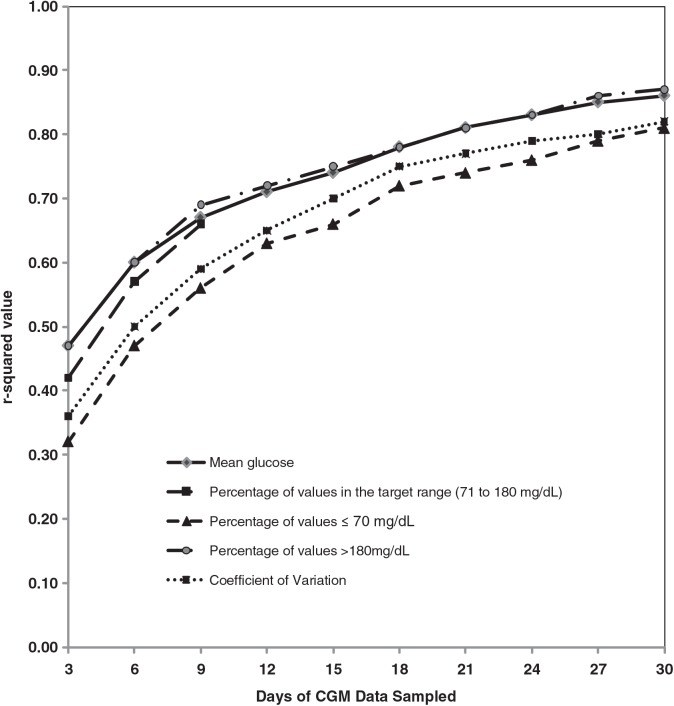
As would be expected, the more days of glucose data that were sampled, the higher the correlation with the full 3 months of data. For 3 days of sampling, the r2 value ranged from 0.32 to 0.47, evaluating mean glucose, percentage of values 71–180 mg/dL, percentage of values >180 mg/dL, percentage of values ≤70 mg/dL, and coefficient of variation; in contrast, for 15 days of sampling, the r2 values ranged from 0.66 to 0.75. The results were similar when the analysis intervals were stratified by age group (8–14, 15–24, and ≥25 years), by baseline hemoglobin A1c level (<7.0% and ≥7.0%), and by CGM device type.
Conclusions and Interpretation
Our data suggest that a 12–15-day period of monitoring every 3 months may be needed to optimally assess overall glucose control. Shorter periods of sampling can be useful, but the correlation with 3-month measures of glycemic control is lower.
Introduction
Episodic use of continuous glucose monitoring (CGM) technology provides clinicians and clinical investigators with a powerful tool to assess heretofore inaccessible outcomes, such as the effects of new treatments on glucose variability, the proportion of glucose values within target ranges, and exposure to hyper- and hypoglycemia.1–4 However, the optimal duration and frequency of such monitoring to accurately reflect long-term glycemia have not been evaluated.
In two 6-month randomized clinical trials and 6-month extension studies, the Juvenile Diabetes Research Foundation CGM Study Group evaluated the effectiveness of real-time CGM in 451 intensively treated patients with type 1 diabetes mellitus.4–8 In these studies, subjects randomized to the CGM group used CGM for 12 months, whereas subjects randomized to the control group used CGM for 6 months after the completion of the randomized trial. These studies provided a large dataset of longitudinal CGM data that were used to estimate how well short-term episodic CGM monitoring correlates with long-term glycemic control.
Research Design and Methods
The study protocol and clinical characteristics of enrolled subjects have been described in detail elsewhere.4,6,9 Major eligibility criteria included age ≥8 years, type 1 diabetes for ≥1 year, use of either an insulin pump or at least three daily insulin injections, and a hemoglobin A1c (HbA1c) level of <10.0%. Subjects were randomly assigned to either a CGM group or a control group for the first 6 months, after which both groups used CGM for an additional 6 months. Three CGM devices were used in these studies: the FreeStyle Navigator™ (Abbott Diabetes Care, Inc., Alameda, CA), the MiniMed Paradigm® REAL-Time insulin pump and the Continuous Glucose Monitoring System (Medtronic MiniMed, Inc., Northridge, CA), and the DexCom™ SEVEN® (DexCom, Inc., San Diego, CA). The CGM devices were calibrated according to the manufacturers' recommendations. Previous studies showed that the median relative absolute difference (RAD) of the three devices ranged from 14% to 20%.10–13 Subjects were encouraged to use the CGM device daily and to make adjustment to their diabetes management based on the sensor glucose readings using algorithms developed by the study group. Follow-up visits occurred at 1, 4, 8, 13, 19, 26, 39, and 52 weeks, with one phone contact between each visit in the first 26 weeks for both randomization groups. In addition, control subjects had follow-up visits at 1 and 4 weeks after the initiation of CGM use and phone contacts 3 days after CGM initiation and 7 days after the 1-week visit.
The dataset utilized for the current analyses included up to 12 months of CGM data for subjects assigned to the CGM group and up to 6 months for subjects assigned to the control group across all baseline HbA1c levels. All CGM data used in this analysis were unblinded where the subjects could see the glucose data and manage their diabetes accordingly. Data from calendar days with at least 12 h of CGM glucose data were included in the analysis. Data in the 3-month interval prior to the 13-, 26-, 39-, and 52-week visits for the CGM group and in the 3-month interval prior to the 39- and 52-week visits for the control group were evaluated. The 3-month interval was chosen to mimic the visit schedule often followed in clinical practice. The analyses were restricted to 3-month intervals where at least 12 h of CGM data per day were available for ≥70% of days. As a result, the analysis dataset included 334 such 3-month intervals from 185 subjects (253 intervals from 115 subjects in the CGM group and 81 intervals from 70 subjects in the control group).
For each 3-month interval, a sampling period was chosen ranging from the 3 days prior to the visit to the 15 days prior to the visit in increments of 3 days (i.e., 3, 6, 9, 12, and 15 days). To have the requisite number of days, each sampling period could be extended by 25% when there were not consecutive analyzable days (i.e., 4 days for the 3-day sampling period, 8 days for the 6-day sampling period, etc.). If any of the extended periods still did not contain a sufficient number of analyzable days, the 3-month interval was excluded from the analysis. For each 3-month interval and sampling period, the following glucose indices were calculated: (1) mean sensor glucose, (2) percentage of values in the target range of 71–180 mg/dL, (3) percentage of values in the hypoglycemic range (≤70 mg/dL), (4) percentage of values in the hyperglycemic range (>180 mg/dL), and (5) coefficient of variation (CV) (defined as the SD divided by mean glucose, a measure of glucose variability). For each glycemic measure, the correlation between the sampling period and the 3-month interval was computed using the squared value of the correlation coefficient based on ranks using the method of Magee14 to account for repeated measures. The r2 values represent how much of the total information the sampled data can provide, with a value of 1.0 denoting 100% information and a value of 0 denoting zero information. The median RAD and the percentage of sampled values within ± 10% and ± 20% of the full values (i.e., values over the 3-month interval) were computed for mean glucose, percentage of values in the target range (71–180 mg/dL), percentage of values >180 mg/dL, and CV. Because of the small percentage of values ≤70 mg/dL, the relative error is artificially large for this measure (e.g., 2% of values below 70 mg/dL vs. 4% is a 100% relative error). Therefore, the absolute difference was evaluated for this measure instead. Similar analyses were performed using 1-month and 2-month analysis intervals. In addition to CV, other measures of glucose variability such as mean amplitude of glycemic excursion and SD were analyzed, and the results were similar (data not shown).
In addition to sampling at the end of the 3-month interval described above (sampling scheme A), two other sampling schemes were also evaluated: scheme B, sampling in the middle of the 3 months; and scheme C, sampling once per month for 3 months in the middle of each month. Using similar methods, the results of sampling in each of two consecutive 3-month intervals were correlated with the full 6 months of data. Analyses were conducted using SAS version 9.1 (SAS Institute, Cary, NC).
Results
The 185 subjects in the analysis ranged in age from 8 to 73 years old. One hundred seven (58%) were female. One hundred seventy-eight (96%) were non-Hispanic white. One hundred sixty-two (88%) were using insulin pumps, and 23 (12%) were treated with multiple daily injections of insulin. Mean HbA1c (±SD) was 7.3 ± 0.8%, with a range of 4.7–9.3% at the initiation of CGM. For the 334 3-month analysis intervals, the median of mean glucose levels was 147 mg/dL, with the median percentage of values in the range of 71–180 mg/dL being 70%, median percentage of values >180 mg/dL being 25%, median ≤70 mg/dL being 3.7%, and median CV being 31%.
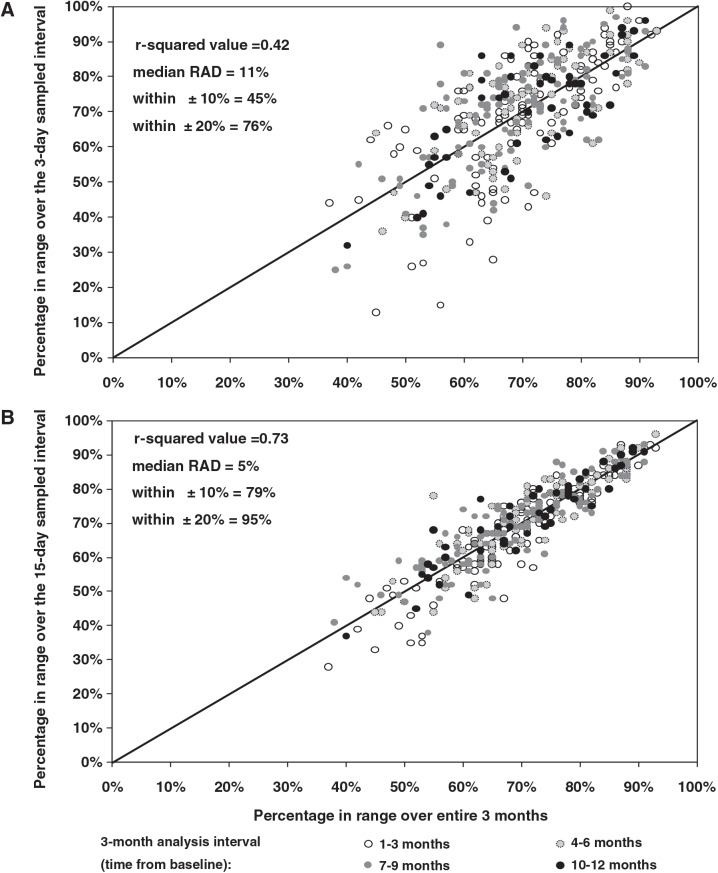
As would be expected, the more days of glucose data that were sampled, the higher the correlation with the full 3 months of data (Table 1 and Figs. 1 and 2). For 3 days of sampling, the r2 value ranged from 0.32 to 0.47, evaluating mean glucose, percentage of values 71–180 md/dL, percentage of values >180 mg/dL, percentage of values ≤70 mg/dL, and CV, whereas for 15 days of sampling, the r2 values ranged from 0.66 to 0.75. Table 1 provides data on the median RAD and the percentage of sampled data within 10% and within 20% of the 3-month value.
Table 1.
Agreement Between Sampled Versus Full Continuous Glucose Monitoring Data (n = 334 Analysis Intervals from 185 Subjects)
| Sampled data versus | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 months of full data | 2 months of full data | 1 month of full data | |||||||||||||
| 3 days | 6 days | 9 days | 12 days | 15 days | 3 days | 6 days | 9 days | 12 days | 15 days | 3 days | 6 days | 9 days | 12 days | 15 days | |
| Mean glucose (mg/dL) | |||||||||||||||
| r2 value | 0.47 | 0.60 | 0.67 | 0.71 | 0.74 | 0.48 | 0.60 | 0.68 | 0.73 | 0.76 | 0.53 | 0.68 | 0.78 | 0.85 | 0.89 |
| Median RADa | 8% | 5% | 5% | 4% | 4% | 7% | 6% | 5% | 4% | 4% | 7% | 5% | 4% | 3% | 3% |
| Within ± 10% | 58% | 79% | 86% | 87% | 89% | 61% | 79% | 87% | 89% | 90% | 64% | 83% | 90% | 96% | 98% |
| Within ± 20% | 92% | 97% | 98% | 99% | 99% | 93% | 97% | 98% | 99% | 99% | 93% | 99% | 100% | 100% | 100% |
| Percentage of values in range (71–180 mg/dL) | |||||||||||||||
| r2 value | 0.42 | 0.57 | 0.66 | 0.71 | 0.73 | 0.42 | 0.57 | 0.69 | 0.74 | 0.76 | 0.46 | 0.62 | 0.75 | 0.83 | 0.87 |
| Median RADa | 11% | 7% | 6% | 5% | 5% | 11% | 7% | 6% | 5% | 4% | 10% | 6% | 5% | 4% | 3% |
| Within ± 10% | 45% | 62% | 71% | 75% | 79% | 44% | 63% | 72% | 77% | 81% | 49% | 65% | 79% | 87% | 89% |
| Within ± 20% | 76% | 90% | 93% | 95% | 95% | 77% | 87% | 93% | 95% | 96% | 80% | 89% | 94% | 98% | 99% |
| Percentage of values >180 mg/dL | |||||||||||||||
| r2 value | 0.47 | 0.60 | 0.69 | 0.72 | 0.75 | 0.47 | 0.60 | 0.70 | 0.74 | 0.77 | 0.50 | 0.66 | 0.77 | 0.84 | 0.88 |
| Median RADa | 34% | 25% | 19% | 17% | 17% | 33% | 25% | 19% | 16% | 15% | 32% | 22% | 17% | 13% | 11% |
| Within ± 10% | 14% | 19% | 23% | 31% | 34% | 15% | 21% | 26% | 34% | 36% | 14% | 22% | 30% | 37% | 47% |
| Within ± 20% | 28% | 41% | 51% | 58% | 60% | 30% | 40% | 52% | 62% | 65% | 29% | 46% | 58% | 73% | 75% |
| Coefficient of variationb | |||||||||||||||
| r2 value | 0.36 | 0.50 | 0.59 | 0.65 | 0.70 | 0.37 | 0.53 | 0.63 | 0.69 | 0.74 | 0.40 | 0.58 | 0.70 | 0.79 | 0.84 |
| Median RADa | 10% | 8% | 7% | 6% | 5% | 11% | 7% | 6% | 5% | 4% | 11% | 7% | 5% | 5% | 4% |
| Within ± 10% | 48% | 62% | 71% | 78% | 84% | 48% | 63% | 75% | 80% | 86% | 47% | 66% | 79% | 89% | 93% |
| Within ± 20% | 81% | 90% | 96% | 99% | 99% | 80% | 91% | 97% | 99% | 99% | 83% | 92% | 99% | 100% | 100% |
| Percentage of values ≤70 mg/dL | |||||||||||||||
| r2 value | 0.32 | 0.47 | 0.56 | 0.63 | 0.66 | 0.34 | 0.51 | 0.59 | 0.66 | 0.70 | 0.38 | 0.58 | 0.69 | 0.78 | 0.85 |
| Median ADc | 2% | 2% | 1% | 1% | 1% | 2% | 1% | 1% | 1% | 1% | 2% | 1% | 1% | 1% | 1% |
| AD within ± 1%d | 26% | 34% | 42% | 48% | 53% | 26% | 36% | 42% | 53% | 53% | 30% | 36% | 47% | 58% | 68% |
| AD within ± 2%d | 49% | 60% | 69% | 75% | 75% | 46% | 61% | 70% | 74% | 79% | 54% | 63% | 77% | 84% | 89% |
Relative absolute difference (RAD) is defined as the absolute difference divided by the full value (expressed as a percentage).
SD divided by mean glucose. It is a measure of glucose variability.
Absolute difference (AD) is the absolute value of the difference between the sampled value and the full value (e.g., 5% for sampled value vs. 4% for full value results in AD = 1%). Because of the small percentage of values ≤70 mg/dL the relative error is artificially large for this measure. AD was given instead of RAD.
Based on AD, not the relative difference (e.g., 5% for sampled value vs. 4% for full value is within ± 1%).
FIG. 1.
Examples of sampled versus full continuous glucose monitoring data over a 3-month interval (n = 334 from 185 subjects). The scatterplots represent data in the (A) 3-day and (B) 15-day samplings of percentage in target range (71–180 mg/dL) compared with the value over a 3-month interval. Each data point denotes one 3-month analysis interval (up to four per subject). RAD, relative absolute difference.
FIG. 2.
The r2 value for the sampled period compared with the 3-month interval. The r2 values between continuous glucose monitoring (CGM) data over the sampled period and the average over the entire 3 months were plotted. Each r2 value was calculated from 334 analysis intervals from 185 subjects.
The results were similar when the analysis intervals were stratified by age group (8–14, 15–24, and ≥25 years), by baseline HbA1c level (<7.0% and ≥7.0%), and by CGM device type. The correlations were consistent over the course of the study (i.e., 1–3, 4–6, 7–9, and 10–12 months).
When the sampled data were compared with only 1 month of full data, the r2 values generally increased by approximately 0.05–0.15 (Table 1). Likewise, when sampling over two consecutive 3-month periods and comparing with 6 months of data, correlations were slightly higher.
Compared with sampling at the end of the 3-month interval, the r2 values slightly increased when sampling in the middle of 3 months or when sampling once per month for 3 months (Table 2).
Table 2.
r2 Values by Different Sampling Schemes (n = 334 Analysis Intervals from 185 Subjects)
| Sampled data vs. 3 months of full data | |||||
|---|---|---|---|---|---|
| 3 daysa | 6 daysa | 9 daysa | 12 daysa | 15 daysa | |
| Mean glucose (mg/dL) | |||||
| Sampling at the end of 3 months | 0.47 | 0.60 | 0.67 | 0.71 | 0.74 |
| Sampling in the middle of 3 monthsb | 0.52 | 0.63 | 0.70 | 0.74 | 0.80 |
| Sampling once per monthc | 0.58 | 0.66 | 0.70 | 0.76 | 0.81 |
| Percentage of values in range (71–180 mg/dL) | |||||
| Sampling at the end of 3 months | 0.42 | 0.57 | 0.66 | 0.71 | 0.73 |
| Sampling in the middle of 3 monthsb | 0.47 | 0.60 | 0.68 | 0.71 | 0.77 |
| Sampling once per monthc | 0.51 | 0.61 | 0.68 | 0.74 | 0.79 |
| Percentage of values >180 mg/dL | |||||
| Sampling at the end of 3 months | 0.47 | 0.60 | 0.69 | 0.72 | 0.75 |
| Sampling in the middle of 3 monthsb | 0.50 | 0.62 | 0.69 | 0.73 | 0.78 |
| Sampling once per monthc | 0.54 | 0.64 | 0.69 | 0.74 | 0.79 |
| Coefficient of variationd | |||||
| Sampling at the end of 3 months | 0.36 | 0.50 | 0.59 | 0.65 | 0.70 |
| Sampling in the middle of 3 monthsb | 0.43 | 0.54 | 0.62 | 0.70 | 0.73 |
| Sampling once per monthc | 0.40 | 0.57 | 0.63 | 0.71 | 0.74 |
| Percentage of values ≤70 mg/dL | |||||
| Sampling at the end of 3 months | 0.32 | 0.47 | 0.56 | 0.63 | 0.66 |
| Sampling in the middle of 3 monthsb | 0.37 | 0.52 | 0.60 | 0.65 | 0.70 |
| Sampling once per monthc | 0.38 | 0.50 | 0.59 | 0.65 | 0.70 |
Total days over the 3-month interval.
n = 333 (excludes one interval that was not eligible for the analysis of sampling in the middle of 3 months).
Sampling once per month (in the middle of month) for 3 months. Divide the number in the cell by 3 to get the number of days that were sampled per month.
Defined as SD divided by mean glucose.
Discussion
In the Juvenile Diabetes Research Foundation CGM randomized clinical trials, CGM systems were used both as an intervention and as an outcome measure. All subjects in those studies wore blinded sensors for 6–7 days at baseline before randomization, and standard blood glucose monitoring control subjects repeated this use at 13 and 26 weeks of the randomized trial. These data provided the means to compare the effect of unblinded CGM use in the experimental group on glucose variability and hypo- and hyperglycemic exposure with corresponding values in the control group. Indeed, in the cohort of patients in the Juvenile Diabetes Research Foundation trials with baseline HbA1c levels <7.0%, the difference between the two treatment groups in the change in the frequency sensor values ≤70 mg/dL from baseline to 6 months was designated as the primary outcome of the study.
We recognized that the choice of a 6-day sampling interval every 3 months was driven primarily by practical considerations related to recruitment, convenience, compliance, and retention of subjects in the study rather than by solid scientific evidence of the reliability of such a sampling schedule. To fill this gap in knowledge, the large CGM dataset that was accumulated during the Juvenile Diabetes Research Foundation CGM clinical trials was used to estimate how well different short-term episodic CGM schedules correlated with a variety of metrics of long-term glycemic control in patients with type 1 diabetes. One of the strengths of this study is that it includes a large number of very well-controlled subjects, as well as subjects with elevated HbA1c levels.
It is not surprising that we found that more frequent and prolonged episodic monitoring correlated better with actual mean 3-month glucose values than shorter and less frequent monitoring. How can these data be used in designing future studies that use CGM as an outcome measure or for clinical care? For both, the data suggest that 2 weeks of CGM data are superior to 1 week in estimating glycemic control over the prior 3-month period and should be the goal if possible. Sampling more frequently will increase the correlation but may lack feasibility. Even sampling for 2 weeks at a time may be impractical, and in some circumstances inserting a sensor in the office and obtaining a single sensor's glucose data may be the best that can be achieved. This is not as good as sampling for 2 weeks, but nevertheless will still give a reasonable estimation of longer-term glycemic control. An important limitation in applying the results of this study is that only subjects with type 1 diabetes were included. Because glycemic excursions tend to be much less labile and more predictable in patients with type 2 diabetes, shorter and less frequent periods of monitoring may be sufficient in this patient group.
Intermittent short-term use for 3–5 days of blinded CGM devices continue to be used in clinical practice for retrospective adjustments in treatment regimens in type 1 diabetes mellitus. Our data suggest, however, that a longer, 12–15-day period of monitoring every 3 months is needed to obtain an accurate assessment of overall glucose control.
Appendix (The Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group)
Clinical Centers
Listed in order of number of patients enrolled with clinical center name, city, and state. Personnel are listed as (PI) for Principal Investigator, (I) for co-Investigator, and (C) for Coordinators:
Diabetes Care Center, University of Washington, Seattle, WA: Irl B. Hirsch, M.D. (PI), Lisa K. Gilliam, M.D., Ph.D. (I), Kathy Fitzpatrick, R.N., M.N., C.D.E. (C), Dori Khakpour, R.D., C.D., C.D.E. (C); Department of Pediatrics, Yale University School of Medicine, New Haven, CT: Stuart A. Weinzimer, M.D. (PI), William V. Tamborlane, M.D. (I), Brett Ives, M.S.N., APRN (C), Joan Bosson-Heenan (C); Adult Section, Joslin Diabetes Center, Boston, MA: Howard Wolpert, M.D. (PI), Greeshma Shetty, M.D. (I), Astrid Atakov-Castillo (C), Judith Giusti, M.S., R.D., L.D.N., C.D.E. (C), Stacey O'Donnell, R.N., C.D.E. (C), Suzanne Ghiloni, R.N., C.D.E. (C); Atlanta Diabetes Associates, Atlanta, GA: Bruce W. Bode, M.D. (PI), Kelli O'Neil, C.D.E. (C), Lisa Tolbert, R.N., M.N., C.D.E. (C); Nemours Children's Clinic, Jacksonville, FL: Tim Wysocki, Ph.D. (co-PI), Larry A. Fox, M.D. (co-PI), Nelly Mauras, M.D. (I), Kimberly Englert, R.N. (C), Joe Permuy, M.S.N., ARNP (C); Division of Pediatric Endocrinology and Diabetes, Stanford University, Stanford, CA: Bruce A. Buckingham, M.D. (PI), Darrell M. Wilson, M.D. (I), Jennifer Block, R.N., C.D.E. (C), Kari Benassi, R.N., N.P. (C); Department of Pediatrics, University of Iowa Carver College of Medicine, Iowa City, IA: Eva Tsalikian, M.D. (PI), Michael Tansey, M.D. (I), Debra Kucera, ARNP, CPNP (C), Julie Coffey, ARNP, CPNP (C), Joanne Cabbage (C); Pediatric Adolescent, and Young Adult Section, Joslin Diabetes Center, Boston, MA: Lori Laffel, M.D., M.P.H. (PI), Kerry Milaszewski, R.N., C.D.E. (C), Katherine Pratt (C), Elise Bismuth, M.D., M.S. (C), Joyce Keady, M.S.N., CPNP (C), Margie Lawlor, M.S., C.D.E. (C); Barbara Davis Center for Childhood Diabetes, University of Colorado, Denver, CO: H. Peter Chase, M.D. (PI), Rosanna Fiallo-Scharer, M.D. (I), Paul Wadwa, M.D. (I), Laurel Messer, R.N., C.D.E. (C), Victoria Gage, R.N. (C), Patricia Burdick (C); Departments of Pediatric Endocrinology and Research and Evaluation, Kaiser Permanente, San Diego and Pasadena, CA: Jean M. Lawrence, Sc.D., M.P.H., MSSA (co-PI), Robert Clemons, M.D. (co-PI), Michelle Maeva, R.N., C.D.E. (C), Bonnie Sattler, M.S., R.D. (C).
Coordinating Center
Jaeb Center for Health Research, Tampa, FL: Roy W. Beck, M.D., Ph.D., Katrina J. Ruedy, MSPH, Craig Kollman, Ph.D., Dongyuan Xing, M.P.H., Judy Sibayan, M.P.H.
University of Minnesota Central Laboratory
Michael Steffes, M.D., Ph.D., Jean M. Bucksa, C.L.S., Maren L. Nowicki, C.L.S., Carol Van Hale, C.L.S., Vicky Makky, C.L.S.
Cost-effectiveness investigators
National Opinion Research Center, University of Chicago, Chicago, IL: Michael O'Grady, Ph.D., Elbert Huang, M.D., M.P.H., Anirban Basu, Ph.D., David O. Meltzer, M.D., Ph.D., Lan Zhao, Ph.D.; University of Michigan, Ann Arbor, MI: Joyce Lee, M.D., M.P.H.
Juvenile Diabetes Research Foundation, Inc.
Aaron J. Kowalski, Ph.D.
Operations Committee
Lori Laffel, M.D., M.P.H. (co-chair), William V. Tamborlane, M.D. (co-chair), Roy W. Beck, M.D., Ph.D., Aaron J. Kowalski, Ph.D., Katrina J. Ruedy, MSPH.
Data and Safety Monitoring Board
Ruth S. Weinstock, M.D., Ph.D. (chair), Barbara J. Anderson, Ph.D., Davida Kruger, M.S.N., APRN, Lisa LaVange, Ph.D., Henry Rodriguez, M.D.
Contributor Information
Collaborators: the Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group
Acknowledgments
The Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group would like to recognize the efforts of the subjects and their families and thank them for their participation. Study funding was provided by the Juvenile Diabetes Research Foundation, Inc. with grant numbers 22-2006-1107, 22-2006-1117, 22-2006-1112, 22-2006-1123, and 01-2006-8031.
Author Disclosure Statement
Continuous glucose monitors and sensors were purchased at a bulk discount price from DexCom, Inc. (San Diego, CA), Medtronic MiniMed, Inc. (Northridge, CA), and Abbott Diabetes Care, Inc. (Alameda, CA). Home glucose meters and test strips were provided to the study by LifeScan, Inc. and Abbott Diabetes Care, Inc. The companies had no involvement in the design, conduct, or analysis of the trial or the manuscript preparation. Below is a listing of relationships of the investigators with companies that make products relevant to the article between July 1, 2006 and present; research funds where listed below were provided to the legal entity that employs the individual and not directly to the individual: B.A.B. reports having received grant support and serving on the Medical Advisory Board for Medtronic MiniMed, Inc., grant support and a speaker honorarium from Abbott Diabetes Care, Inc., and grant support from DexCom, Inc.; C.K. reports having received consulting fees from Medtronic MiniMed, Inc.; L.L. reports having received consulting fees and a speaker honorarium from Abbott Diabetes Care, Inc. and consulting fees and research funding from Medtronic MiniMed, Inc.; W.V.T. reports having received consulting fees from Medtronic MiniMed, Inc.; and S.W. reports having received research support, a speaker honorarium, and travel reimbursement from Medtronic MiniMed, Inc. and a speaker honorarium from Animas Corp./Lifescan, Inc.
References
- 1.Bugler J. An estimation of the amount of data required to measure glycemic variability. Presented at the Advanced Technologies and Treatment for Diabetes Conference; Prague, Czech Republic. 2008. [Google Scholar]
- 2.Fiallo-Scharer R. Diabetes Research in Children Network Study Group: Eight-point glucose testing versus the continuous glucose monitoring system in evaluation of glycemic control in type 1 diabetes. J Clin Endocrinol Metab. 2005;90:3387–3391. doi: 10.1210/jc.2004-2510. [DOI] [PubMed] [Google Scholar]
- 3.Nathan DM. Kuenen J. Borg R. Zheng H. Schoenfeld D. Heine RJ. Translating the A1C assay into estimated average glucose values. Diabetes Care. 2008;31:1473–1478. doi: 10.2337/dc08-0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Tamborlane WV. Beck RW. Bode BW. Buckingham B. Chase HP. Clemons R. Fiallo-Scharer R. Fox LA. Gilliam LK. Hirsch IB. Huang ES. Kollman C. Kowalski AJ. Laffel L. Lawrence JM. Lee J. Mauras N. O'Grady M. Ruedy KJ. Tansey M. Tsalikian E. Weinzimer S. Wilson DM. Wolpert H. Wysocki T. Xing D. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med. 2008;359:1464–1476. doi: 10.1056/NEJMoa0805017. [DOI] [PubMed] [Google Scholar]
- 5.Chase HP. Beck RW. Xing D. Tamborlane WV. Coffey J. Fox LA. Ives B. Keady J. Kollman C. Laffel L. Ruedy KJ. Continuous glucose monitoring in youth with type 1 diabetes: 12-month follow-up of the Juvenile Diabetes Research Foundation continuous glucose monitoring randomized trial. Diabetes Technol Ther. 2010;12:507–515. doi: 10.1089/dia.2010.0021. [DOI] [PubMed] [Google Scholar]
- 6.Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. The effect of continuous glucose monitoring in well-controlled type 1 diabetes. Diabetes Care. 2009;32:1378–1383. doi: 10.2337/dc09-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Bode B. Beck RW. Xing D. Gilliam L. Hirsch I. Kollman C. Laffel L. Ruedy KJ. Tamborlane WV. Weinzimer S. Wolpert H. Sustained benefit of continuous glucose monitoring on HbA1c, glucose profiles, and hypoglycemia in adults with type 1 diabetes. Diabetes Care. 2009;32:2047–2049. doi: 10.2337/dc09-0846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Effectiveness of continuous glucose monitoring in a clinical care environment: evidence from the Juvenile Diabetes Research Foundation continuous glucose monitoring (JDRF-CGM) trial. Diabetes Care. 2010;33:17–22. doi: 10.2337/dc09-1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.JDRF CGM Study Group. JDRF randomized clinical trial to assess the efficacy of real-time continuous glucose monitoring in the management of type 1 diabetes: research design and methods. Diabetes Technol Ther. 2008;10:310–321. doi: 10.1089/dia.2007.0302. [DOI] [PubMed] [Google Scholar]
- 10.Bode B. Gross K. Rikalo N. Schwartz S. Wahl T. Page C. Gross T. Mastrototaro J. Alarms based on real-time sensor glucose values alert patients to hypo- and hyperglycemia: the Guardian Continuous Monitoring System. Diabetes Technol Ther. 2004;6:105–113. doi: 10.1089/152091504773731285. [DOI] [PubMed] [Google Scholar]
- 11.DexCom Inc. DexCom™ STS™ Continuous Glucose Monitoring System User's Guide. San Diego, CA: DexCom Inc.; 2006. pp. 86–102. [Google Scholar]
- 12.Diabetes Research in Children Network (DirecNet) Study Group. The accuracy of the Guardian RT continuous glucose monitor in children with type 1 diabetes. Diabetes Technol Ther. 2008;10:266–272. doi: 10.1089/dia.2007.0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson DM. Beck RW. Tamborlane WV. Dontchev MJ. Kollman C. Chase P. Fox LA. Ruedy KJ. Tsalikian E. Weinzimer SA DirecNet Study Group. The accuracy of the FreeStyle Navigator continuous glucose monitoring system in children with type 1 diabetes. Diabetes Care. 2007;30:59–64. doi: 10.2337/dc06-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Magee L. R2 measures based on Wald and Likelihood Ratio Joint Significance tests. Am Stat. 1990;44:250–253. [Google Scholar]