Abstract
The reorganization of epithelial sheets into tubes is a fundamental process in the formation of many organs, such as the lungs, kidneys, gut, and neural tube. This process involves the patterning of distinct cell types and the coordination of those cells during the shape changes and rearrangements that produce the tube. A better understanding of the cellular and genetic mechanisms that regulate tube formation is necessary for tissue engineers to develop functional organs in vitro. The Drosophila egg chamber has emerged as an outstanding model for studying tubulogenesis. Synthesis of the dorsal respiratory appendages by the follicular epithelium resembles primary neurulation in vertebrates. This review summarizes work on the patterning and morphogenesis of the dorsal-appendage tubes and highlights key areas where mathematical modeling could contribute to our understanding of these processes.
Introduction
Tissue engineers have used two general approaches to reconstitute tubular organs.1,2 The first method creates a scaffold of artificial or biological material that provides a foundation for directed growth of differentiated cells seeded into the matrix.3 This approach has produced kidney-like filter apparati that show tremendous promise for use in individuals with renal failure.4–6 Similarly, recent efforts have generated primitive beating hearts that allow drug studies, electrophysiological investigations, and other analyses.7–10 These results are exciting and bode well for the development of practical applications. Nevertheless, our ability to create matrices that match the functional requirements of individual patients limit these methods; unfortunately, one size does not fit all.
The second approach involves inducing the differentiation of pluripotent cells with the goal of creating an organ, or parts of an organ, de novo.11 Many studies have focused on the signaling molecules that regulate tissue growth and morphogenesis; others have emphasized the mechanical stimuli that influence the response of cells to growth conditions.12 Recent interest in stem-cell biology has expanded efforts to develop methods for controlling the differentiation of tissues.13 Although the popular press has extolled the virtues of this line of approach, these studies reveal our limited understanding of the mechanisms that define distinct cells types within a tissue. We know even less about the cell biological processes that coordinate cells as they construct an organ. To realize the potential of pluripotent cells, we need basic research on normal developmental processes.
Tube formation occurs through five distinct mechanisms: wrapping, budding, cavitation, cord hollowing, and cell hollowing.14 Polarized epithelial sheets (in which cells tightly adhere to each other and have distinct apical and basal surfaces) use wrapping and budding to produce tubes. During wrapping, cells in a linear patch constrict their apical surfaces and expand their basal surfaces, thereby curving out of the epithelium. Cells on two edges of the patch come together to seal off the tube, producing a pipe-like structure that runs parallel to the epithelial sheet. This process is responsible for creating the primary neural tube in vertebrates, the ventral furrow in insects, and intermediates in other organ structures.15–17 In contrast, budding involves the extension of cells perpendicular to the sheet and produces a tube that resembles a finger poking out of a cloth. This mechanism creates the branched structures of vertebrate lungs, insect trachea, and many other organs.18–20
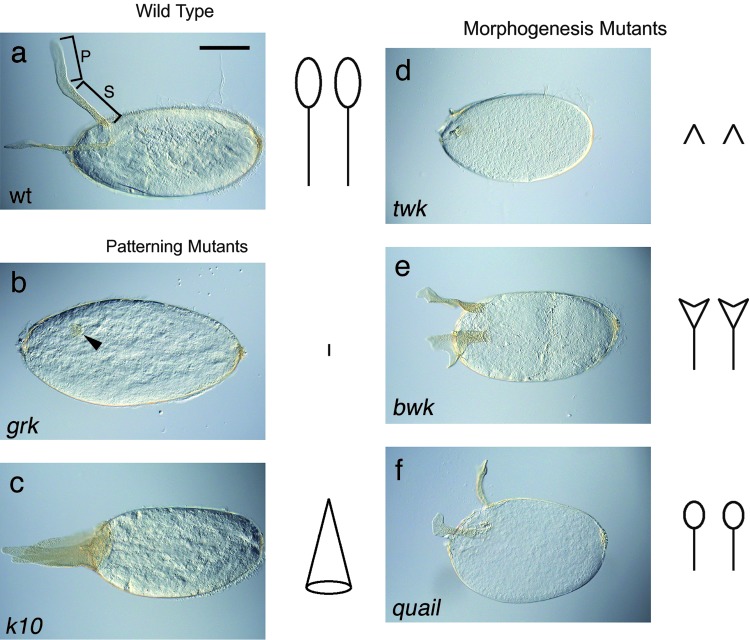
To study tube formation, we are analyzing the signaling pathways that define and control sub-populations within the Drosophila follicular epithelium. Epidermal growth factor (EGF) and bone morphogenetic protein (BMP) signals specify two patches of dorsal–anterior cells that then use a wrapping mechanism to form two closed tubes; no cell division or cell death occurs, simplifying the analysis. Tube formation is rapid (20 min) and is followed by secretion of chorion proteins into the tube lumens to create two dorsolateral eggshell appendages (DAs), which facilitate respiration in the developing embryo.21 Although the follicle cells slough off when the egg is laid, the DAs provide a record of the tube-forming process, much like JELL-O after removal of the mold. Analyses of wild-type and mutant eggs have facilitated identification of dozens of factors that regulate DA formation, giving insight into general features of tubulogenesis (Fig. 1).22
FIG. 1.
Patterning and morphogenesis mutants. In all panels, anterior is to the left, and dorsal is up. Stick figures to the right of each panel represent the dorsolateral eggshell appendages (DAs) for each strain. (a) Wild type. Brackets indicate the stalk (S) and paddle (P). Scale bar = 0.1 mm. (b, c) Patterning mutants alter the number or position of the DAs. (b) Strong ventralizing mutants such as gurken lack DA material; a nub of appendage (arrowhead) is barely visible on the dorsal midline. (c) Dorsalizing mutant fs(1)K10 produces a cone of appendage material. (d, e, f) Morphogenesis mutants produce two correctly positioned DAs with defective shapes. (d) An unusual allele of tramtrack69 creates the two nubs of the twin peaks mutant. Tubes form but fail to elongate. (e) Moose antler appendages give bullwinkle its name. (f) The short appendages of this quail (Villin) mutant are typical phenotypes caused by partial loss of actin regulatory components.
Scope of Review
The purpose of this review is to summarize our understanding of the patterning events and morphogenetic processes that create the DA tubes and to highlight current challenges that could benefit from mathematical modeling.
Patterning the Epithelium
Current knowledge
One of the mysteries of developmental biology is how a small number of signaling pathways can define disparate tissues, control their growth, and induce differentiation yet produce distinct morphologies and functions. In the fly ovary, BMP and EGF signaling regulate germline stem cell behavior, the survival and division of somatic cells, the patterning of anterior, posterior, and dorsal follicle cells, the migration of subsets of follicle cells, and events required for maturation of the egg.23–28 Although the same signaling pathways also regulate embryonic dorsal–ventral patterning, nervous system development, and eye, leg, and wing morphogenesis,29,30 the aforementioned processes evolved to optimize production of oocytes.
Oocyte development takes place in the context of egg chambers, which develop in assembly-line fashion along strings called ovarioles.31 The egg chamber consists of 16 interconnected germline cells—15 nurse cells and a single oocyte—surrounded by a layer of approximately 650 somatically derived follicle cells. The highly polyploid nurse cells synthesize components required by the developing oocyte and future embryo and transport these molecules and organelles into the oocyte through cytoplasmic bridges called ring canals.32 The follicle cells secrete ligands or activators that establish polarity within the oocyte and embryo;33–35 the follicle cells also synthesize the layers and specializations of the eggshell (Fig. 1a).36
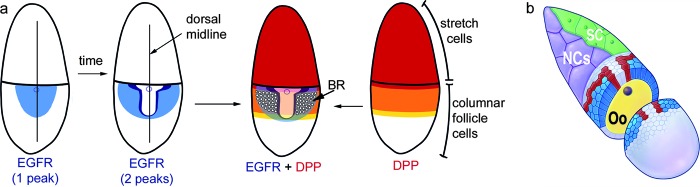
Midway through oogenesis, at stage 10, the oocyte occupies the posterior half of the egg chamber, and the nurse cells occupy the anterior half (Fig. 2a). The follicle cells over the nurse cells have flattened into a thin layer (the stretch cells), whereas the follicle cells over the oocyte are columnar (Fig. 2b). Although no obvious physical differences distinguish groups of cells within the columnar layer, patterning is occurring to designate distinct domains of columnar cells.22,37 Gurken (GRK = EGF) signaling from the underlying oocyte and Decapentaplegic (DPP = BMP) signaling from the anterior stretch cells (Fig. 2a) induce further signaling that refines the EGF and BMP gradients.22,37 The net effect is to produce groups of cells that express specific markers (Fig. 2b, 3A) and synthesize specialized anterior eggshell structures. In particular, two DA primordia lie lateral to the midline. Within each primordium, a hinge-shaped row of approximately 15 “floor” cells borders a patch of approximately 55 “roof” cells. As described below, these cells will reorganize into tubes that secrete the dorsal appendages.38
FIG. 2.
Patterning of the dorsolateral eggshell appendage (DA)-forming cells. (a) Signaling through Epidermal growth factor receptor (blue) and Decapentaplegic (red) pathways combine to specify dorsal-anterior cell fates. Cells expressing Rhomboid (purple), a protease that activates epidermal growth factor ligands, will form the floor of the DA tubes, whereas cells that express high levels of the transcription factor Broad (white dots) will form the roof and sides of the DA tubes. (b) Crosssection of a stage 10B shows the outcome of patterning the epithelium: stretch cells (cut away, in green), nurse cells (purple), centripetal and dorsal midline cells (light gray), floor cells (red), roof cells (blue), oocyte (yellow). Modified from James and Berg70 and Dorman et al.38
FIG. 3.
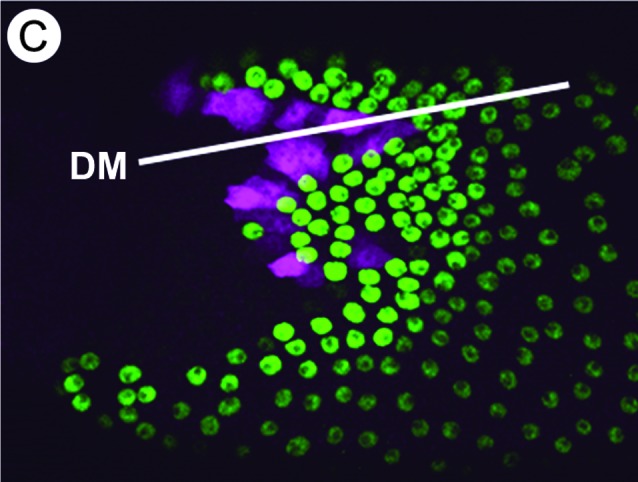
Patterning mutations alter the dorsolateral eggshell appendage (DA) primordia. Stage 10 egg chambers express Broad (green) and rhomboid-lacZ (magenta) to mark the roof and floor cells, respectively. Anterior is to the left. A white line labels the dorsal midline. (A) Wild type. The complete left primordium and part of the right primordium are visible. (B) A partial loss-of-function mutation in Ras85D disrupts epidermal growth factor signaling and depletes dorsal cell fates. The floor cells form one continuous row anterior to the roof cells. Such egg chambers produce eggshells with a single DA. (C) Over-expression of Decapentaplegic pushes the DA primordia toward the posterior.
Future challenges
How do cells combine information from two gradients to specify two DA primordia and, within each primordium, define exactly one row of floor cells bordering a population of roof cells? Studies thus far demonstrate that at least three mechanisms contribute to this process.
First, the exact quantity of both signals determines the position and number of cells fated to make DAs (Fig. 3). For example, partial loss-of-function mutations affecting GRK or its receptor reduces the total number of cells contributing to DA formation, moving each population toward the dorsal midline, whereas over-expressing GRK moves the DAs more laterally on the egg.23,39 Similarly, over-expressing DPP pushes the primordia toward the posterior while loss of function depletes the population and moves the appendages more anteriorly.24,40,41 Both signals are needed to specify the DAs.42,43 Modeling these combined gradients would give insight into the patterning process and provide an interesting puzzle for mathematical biologists. Unlike other situations in which a point source creates a one-dimensional (linear) gradient, the effect of these molecules changes from dorsal to ventral and from anterior to posterior.
A second process that affects the patterning is feedback information induced by the initial signaling pathways. The follicle cells express additional ligands, activators, and inhibitors in response to GRK signaling, and these molecules influence the position and size of the DA primordia.44–47 Similarly, DPP modulates its own activity by inducing an inhibitor that limits responsiveness to this signal.43 Crosstalk between the EGF and BMP pathways provides additional complexity to the system.43,48,49 Our understanding of these molecular interactions would benefit from efforts to describe these processes quantitatively, as a function of space and time.
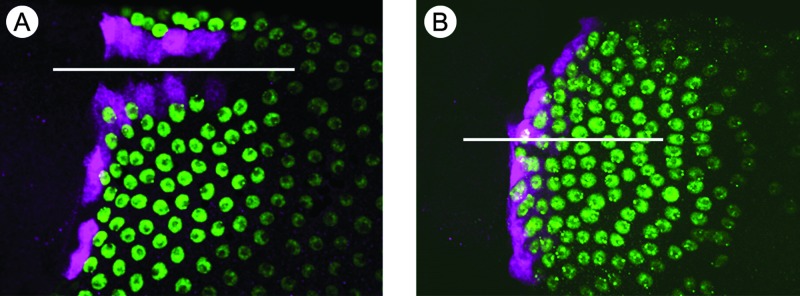
Regardless of how one manipulates the EGF or BMP pathways, the follicle cells always create one row of floor cells adjacent to a group of roof cells50 (Fig. 3). This result suggests that EGF and BMP induce additional signaling pathway(s) that modify the patterning of the epithelium. Indeed, Notch protein is up-regulated in a dorsal–anterior “T” (Fig. 4), and its function there is necessary to establish correct roof- and floor-cell fates.51 It is likely that expression of the Notch gene results from integration of GRK and DPP signaling, and the Notch pattern could serve as a useful assay for testing hypotheses regarding the mechanism of this combinatorial signaling.
FIG. 4.
Notch expression in the “T” prefigures primordium fates. Stage 10 egg chambers express Notch (A, C, in green), Broad (B, D, in green), or rhomboid-lacZ (red). Anterior is to the left. A yellow arrow labels the dorsal midline. (A) At mid stage 10, Notch expression is high in a dorsal anterior “T,” including the centripetal cells, dorsal midline cells, and future floor cells. The floor cells, however, do not yet express the rhomboid-lacZ reporter. (B) In cells expressing high Notch, Broad is degraded. (C) At late stage 10, Notch expression diminishes as the floor-cell marker appears. (D) Broad is expressed at high levels in roof cells, moderate levels in main-body follicle cells, and not at all in cells of the “T.” Modified from Ward et al.51
Shvartsman and colleagues have begun to model these patterning processes using an elegant set of partial differential equations. They predict the effect of GRK signaling on the epithelium and show how DA number and position change by altering this pathway.52 They have also developed a powerful method to describe the GRK gradient using a single dimensionless parameter.53 The challenge now is to develop models that incorporate information from all three signaling pathways.
Morphogenesis of the Tubes
Current knowledge
When epithelial sheets form a tube, participating cells must reorganize their cytoskeleton, contract apical surfaces while expanding basal surfaces, adapt vesicle transport to remodel the membrane, and strengthen adhesion between tube-forming cells while diminishing attachments to neighbors. Analyses of cultured cells, especially canine kidney cells, and studies in several model organisms have revealed essential aspects of these processes.54,55 Nevertheless, many questions remain about the mechanisms that govern tubulogenesis. For example, what are the developmental and environmental cues that regulate temporal aspects of tube formation?56 What factors control the size and shape of the tube?57 How do cells within a tube coordinate their activities?58 DA formation is a powerful system for addressing these questions; the small number of cells that form each tube, the ability to culture egg chambers and image tubulogenesis in real time, the ease of generating mutants, and the unparalleled ability to manipulate gene function provide significant advantages for analyzing morphogenesis. Here I describe wild-type DA tube formation and then focus on two mutants that affect DA size and shape.
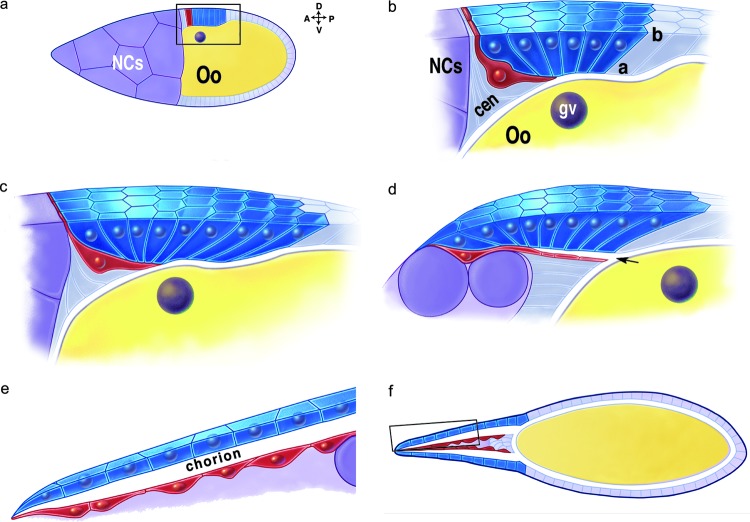
DA tubulogenesis occurs in the context of other egg-maturation events. Shortly after the appearance of markers that identify roof and floor cells (stage 10B), the follicle cells closest to the nurse cell–oocyte boundary migrate centripetally, between the nurse cells and oocyte; these cells eventually secrete the operculum, the anterior face of the eggshell.59 Next, the nurse cells rapidly transfer their contents into the oocyte and initiate a process of programmed cell death.32,60 During this short stage 11 (20hmin), the DA-forming cells begin their morphogenesis (Fig. 5, 6). The roof cells constrict their apices, curving out of the epithelium. Lateral roof cells converge toward the midline, extending the tube toward the anterior of the egg chamber. At the same time, the floor cells elongate and dive beneath the roof cells, zippering their apices to seal off the tube.38,50 The resulting tube is closed off at the anterior–dorsal corner and has a flat floor with horseshoe-shaped roof and sides.
FIG. 5.
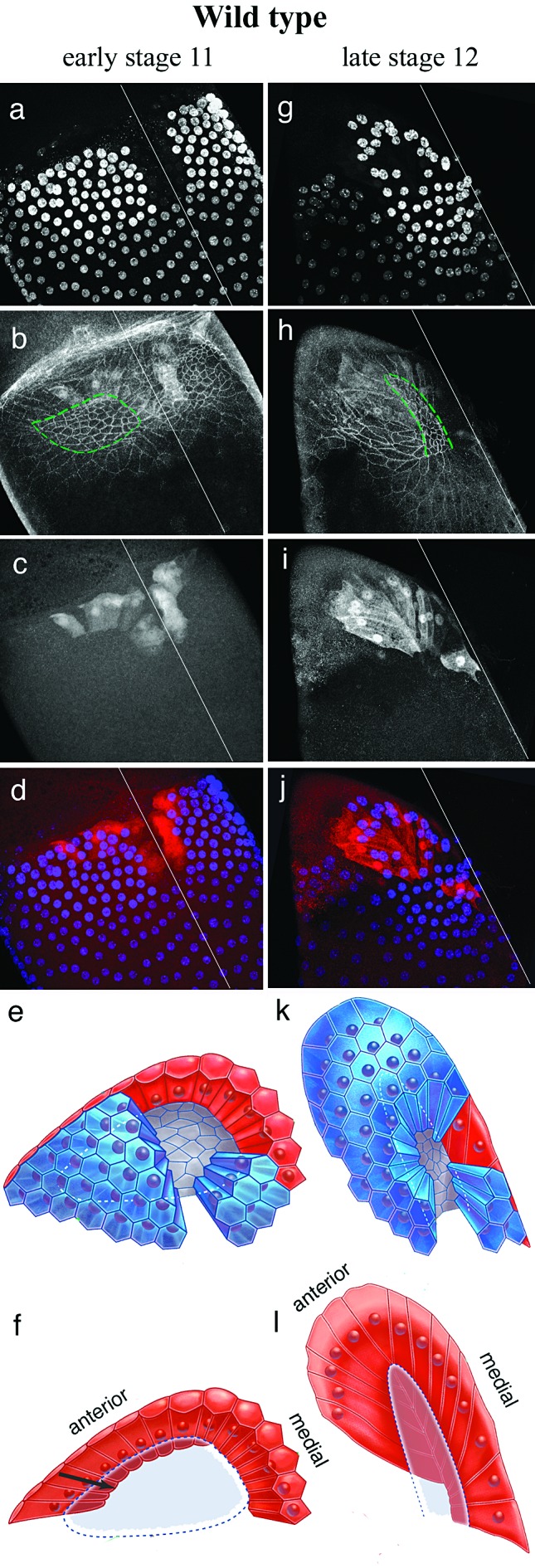
Dorsolateral eggshell appendage (DA) tube formation. In all panels, anterior is toward the top. In a–d and g–j, a white line marks the dorsal midline; the schematic drawings at the bottom are shown in the same orientation as the confocal images above. Roof-forming cells, represented in blue in the schematic drawings, express high levels of Broad (a, g). They constrict their apices (b, h, outlined in green), as visualized using E-cadherin staining. Convergent extension brings lateral roof cells toward the midline (a –> g, e –> k). Floor cells, represented in red in the schematics, express the reporter rhomboid-lacZ (c, i) and extend underneath the high-Broad-expressing roof cells (d, j, f, l). During anterior extension, the roof-cell population narrows and lengthens into a skinny triangle (g, green outline in h); the floor-cell population forms a candy cane (i, j). Roof-cell apical area overlying floor cells is shown in (f, l) as light shading bounded by dotted lines. Modified from Dorman et al.38
FIG. 6.
Lateral views of dorsolateral eggshell appendage (DA) morphogenesis. Schematic drawings of stage 10 to stage 14 egg chambers. (a) Stage 10; Nurse cells (NCs), Oocyte (Oo). Box is enlarged in (b). Floor cell (red) elongates beneath roof cells (blue). Apical (a) is down. (c) Stage 11. Nurse cells begin transferring their contents into the oocyte. Roof-cell apices are tightly constricted. (d) Stage 12. The DA tube elongates over the nurse cells by crawling on the stretch cells (not shown). (e) Stage 13. Enlarged view of (f). Roof cells expand their apices and secrete chorion into the tube lumen. Modified from Dorman et al.38
During stage 12, intercalation of roof cells continues to elongate and narrow the tube. In addition, anterior floor cells extend filopodia as they crawl over the stretch cells (Fig. 6d, 7C). At stage 13, the roof and floor cells stop moving, shorten their lateral surfaces, and expand their apices (Fig. 6e). The roof cells secrete chorion proteins into the tube lumen. By this time, the nurse cells are almost gone. The stretch cells and nurse-cell remnants lie between the two DA tubes (Fig. 6f). At stage 14, 6 h after morphogenesis began, all follicle cells begin to degenerate. They slough off during passage of the mature egg through the oviduct.61
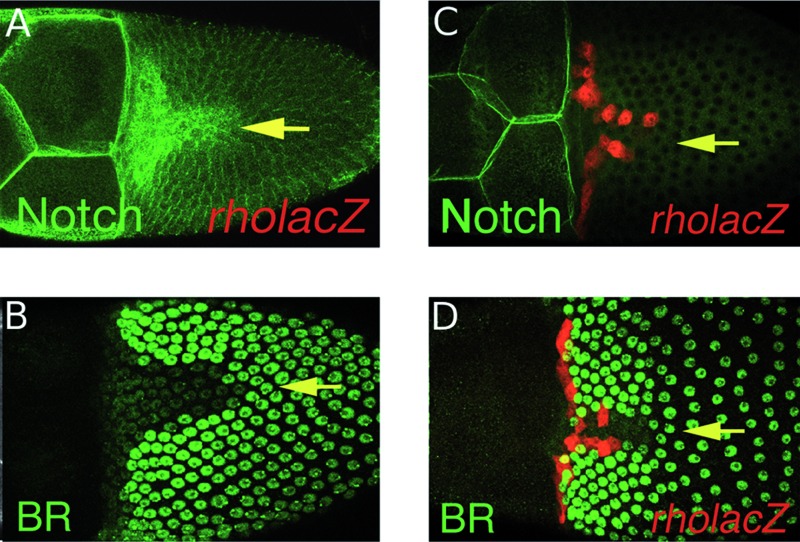
FIG. 7.
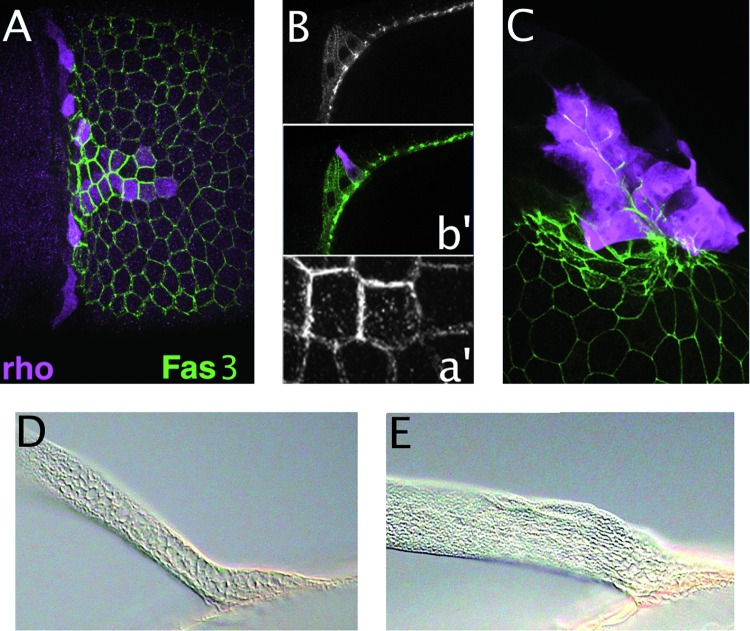
Role of Fasciclin 3 (FAS3) in dorsolateral eggshell appendage (DA) morphogenesis. Top panels: Stage 10-12 egg chambers stained for FAS3 (green) or rhomboid-lacZ (magenta). Anterior is to the left. Lower panels: Magnified views of DA stalks on laid eggs. (A) Stage 10 dorsal view showing high FAS3 levels in the “T,” including the floor cells, and lower levels in main body follicle cells. Inset (a’) shows differential staining on floor-cell surfaces adjacent to roof cells. (B, b’) Lateral view of stage-10 egg chamber highlights differential FAS3 levels. (C) Dorsolateral view of a stage-12 egg chamber showing high FAS3 levels in floor cells and operculum cells. Note filopodia extending from basal surfaces of floor cells. (D) Wild-type DAs have a rounded stalk. (E) Fas3 mutant DAs are broad and flat along their entire length, not just at the paddle. The top panels are taken from Ward and Berg.50
Future challenges
Early during tubulogenesis, the roof-cell apices are tightly constricted and the tube lumen is narrow. By the end of oogenesis, the roof cells have expanded their apices, greatly increasing the volume of the lumen. How do roof cells determine the optimal apical surface area? It is likely that many factors contribute to this regulation, including the position and strength of the adherens junctions, the extent of actin filament formation and cross-linking, the activity of myosin motors, and the rates of vesicle endo- and exocytosis.62,63 Two mutants exhibit striking DA defects and suggest that cell–cell adhesion between floor cells affects roof-cell behavior.
Fasciclin 3 (FAS3) is a homophilic cell adhesion molecule that is expressed at high levels on lateral and apical surfaces of floor cells and at much lower levels in adjoining roof cells50,64 (Fig. 7A–C). When follicle cells lack FAS3,50 the DAs are long and flat, resembling a cricket bat, rather than assuming the stalk and paddle shape of a rowboat oar (Fig. 7D, E). Other cell adhesion molecules, such as E-cadherin, must provide redundant function to hold the cells together, but FAS3 ensures the creation of a round rather than flat tube. How does differential adhesion in floor cells regulate tube shape? Analysis of a second mutant gives clues to this process.
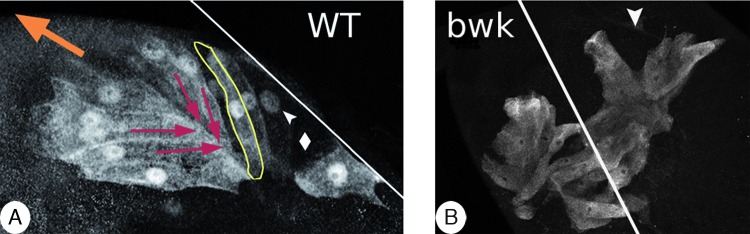
Loss-of-function mutations in bullwinkle cause branched appendages that resemble moose antlers (Fig. 1e). The underlying defect is more complicated than that for Fas3 mutants. bullwinkle encodes a SOX transcription factor that acts in the germline to promote the maturation of the stretch cells, which then express guidance signals that affect the anterior movement of the floor cells.65,66 In the absence of Bullwinkle, floor cells migrate laterally rather than anteriorly38 (Fig. 8). This aberrant movement disrupts adhesion between floor cells and partially tears the floor cells apart from each other. Because roof cells are attached to floor cells, defects in floor-cell conformation affect roof-cell behavior, altering the overall shape of the tube.
FIG. 8.
Adhesion and shapes of wild-type (WT) and bullwinkle (bwk) floor cells. Stage 12 egg chambers expressing the rhomboid-lacZ reporter. Anterior is to the upper left. A white line marks the dorsal midline. (A) Wild type. The orange arrow indicates the direction of tube elongation. A yellow line highlights the shape of a single floor cell. Red arrows indicate the direction in which cell shortening will occur at stage 13. (B) The floor cells in bwk mutant egg chambers migrate laterally rather than anteriorly. An arrowhead marks the site at which the basolateral region of two floor cells have separated from each other. This behavior puts tension on the roof cells, which expand their apices and widen the lumen. Modified from Dorman et al.38
How much adhesion between floor cells is needed to ensure correct tube size and shape? How do adherens junctions between roof and floor cells change as basolateral contacts between floor cells falter? To model these behaviors, we must estimate adhesive properties of cells as well as stresses resulting from cell migration. Kerszberg and Changeux67 have developed a model involving differential adhesion between cells to describe the folding of the vertebrate neural tube. Using cellular automata, a two-dimensional array of cells that follow defined rules of behavior, these authors show that local concentrations of signaling molecules affect expression of cell adhesion molecules, thereby altering the shape and movement of cells. Depending on the choice of parameters specifying the activities of the signaling molecules, the model predicts deformation of the epithelium in a global manner, creating a tube. Zajac et al.68,69 also use differential adhesion to explain cell behaviors, but these authors place more emphasis on the shapes of the cells and require their system to achieve an energy minimum. We now need to use the information available from our morphogenesis mutants to assess these differential adhesion models.
In conclusion, DA formation is an excellent system for studying tube formation. Combinatorial signaling, feedback loops, and crosstalk between pathways create a two-dimensional pattern that changes with time, providing a challenging yet tractable system for modeling the mechanisms that determine cell fate. Tube morphogenesis involves apical constriction and rearrangement of roof cells, coupled with elongation and sealing of floor cells. Adhesion among the floor cells, and between floor cells, roof cells, and stretch cells, affects the size and shape of the tube. Developing models that consider these interactions will improve our understanding of the forces that shape biological tubes.
Acknowledgments
Drs. Jennie B. Dorman and Ellen J. Ward contributed significantly to the work reviewed here; I especially appreciate their insights into these processes. I am grateful to Melissa Knoth Tate and Stanislas Shvartsman for organizing a highly informative meeting on tissue engineering and developmental biology. I am also grateful to the outstanding participants at that meeting whose questions and feedback improved the analysis presented here.
References
- 1.Langer R. Vacanti J.P. Tissue engineering. Science. 1993;260:920. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 2.Hwang N.S. Varghese S. Elisseeff J. Controlled differentiation of stem cells. Adv Drug Deliv Rev. 2008;60:199. doi: 10.1016/j.addr.2007.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park H. Cannizzaro C. Vunjak-Novakovic G. Langer R. Vacanti C.A. Farokhzad O.C. Nanofabrication and microfabrication of functional materials for tissue engineering. Tissue Eng. 2007;13:1867. doi: 10.1089/ten.2006.0198. [DOI] [PubMed] [Google Scholar]
- 4.Humes H.D. Buffington D.A. MacKay S.M. Funke A.J. Weitzel W.F. Replacement of renal function in uremic animals with a tissue-engineered kidney. Nat Biotechnol. 1999;17:451. doi: 10.1038/8626. [DOI] [PubMed] [Google Scholar]
- 5.Minuth W.W. Sorokin L. Schumacher K. Generation of renal tubules at the interface of an artificial interstitium. Cell Physiol Biochem. 2004;14:387. doi: 10.1159/000080348. [DOI] [PubMed] [Google Scholar]
- 6.Heber S. Denk L. Hu K. Minuth W.W. Modulating the development of renal tubules growing in serum-free culture medium at an artificial interstitium. Tissue Eng. 2007;13:281. doi: 10.1089/ten.2006.0199. [DOI] [PubMed] [Google Scholar]
- 7.Eschenhagen T. Didié M. Heubach J. Ravens U. Zimmermann W. Cardiac tissue engineering. Transpl Immunol. 2002;9:315. doi: 10.1016/s0966-3274(02)00011-4. [DOI] [PubMed] [Google Scholar]
- 8.Kubo H. Shimizu T. Yamato M. Fujimoto T. Okano T. Creation of myocardial tubes using cardiomyocyte sheets and an in vitro cell sheet-wrapping device. Biomaterials. 2007;28:3508. doi: 10.1016/j.biomaterials.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 9.Franchini J.L. Propst J.T. Comer G.R. Yost M.J. Novel tissue engineered tubular heart tissue for in vitro pharmaceutical toxicity testing. Microsc Microanal. 2007;13:267. doi: 10.1017/S1431927607070663. [DOI] [PubMed] [Google Scholar]
- 10.Ott H.C. Matthiesen T.S. Goh S. Black L.D. Kren S.M. Netoff T.I. Taylor D.A. Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart. Nat Med. 2008;14:213. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 11.Parenteau N.L. Rosenberg L. Hardin-Young J. The engineering of tissues using progenitor cells. Curr Top Dev Biol. 2004;64:101. doi: 10.1016/S0070-2153(04)64006-3. [DOI] [PubMed] [Google Scholar]
- 12.Becker C. Jakse G. Stem cells for regeneration of urological structures. Eur Urol. 2007;51:1217. doi: 10.1016/j.eururo.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 13.Kurosawa H. Methods for inducing embryoid body formation: in vitro differentiation system of embryonic stem cells. J Biosci Bioeng. 2007;103:389. doi: 10.1263/jbb.103.389. [DOI] [PubMed] [Google Scholar]
- 14.Lubarsky B. Krasnow M.A. Tube morphogenesis: making and shaping biological tubes. Cell. 2003;112:19. doi: 10.1016/s0092-8674(02)01283-7. [DOI] [PubMed] [Google Scholar]
- 15.Lowery L. Sive H. Strategies of vertebrate neurulation and a re-evaluation of teleost neural tube formation. Mech Dev. 2004;121:1189. doi: 10.1016/j.mod.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 16.Sweeton D. Parks S. Costa M. Wieschaus U. Gastrulation in Drosophila: the formation of the ventral furrow and posterior midgut invaginations. Development. 1991;112:775. doi: 10.1242/dev.112.3.775. [DOI] [PubMed] [Google Scholar]
- 17.Gilbert S.F. Developmental Biology. Sunderland, MA: Sinauer Associates, Inc.; 2006. [Google Scholar]
- 18.Warburton D. Schwarz M. Tefft D. Flores-Delgado G. Anderson K.D. Cardoso W.V. The molecular basis of lung morphogenesis. Mech Dev. 2000;92:55. doi: 10.1016/s0925-4773(99)00325-1. [DOI] [PubMed] [Google Scholar]
- 19.Ghabrial A. Luschnig S. Metzstein M.M. Krasnow M.A. Branching morphogenesis of the Drosophila tracheal system. Annu Rev Cell Dev Biol. 2003;19:623. doi: 10.1146/annurev.cellbio.19.031403.160043. [DOI] [PubMed] [Google Scholar]
- 20.Hogan B.L.M. Building organs from buds, branches and tubes. Differentiation. 2006;74:323. doi: 10.1111/j.1432-0436.2006.00107.x. [DOI] [PubMed] [Google Scholar]
- 21.Hinton H.E. Respiratory systems of insect egg shells. Annu Rev Entomol. 1969;14:343. doi: 10.1146/annurev.en.14.010169.002015. [DOI] [PubMed] [Google Scholar]
- 22.Berg C.A. The Drosophila shell game: patterning genes and morphological change. Trends Genet. 2005;21:346. doi: 10.1016/j.tig.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 23.Schüpbach T. Germ line and soma cooperate during oogenesis to establish the dorsoventral pattern of egg shell and embryo in Drosophila melanogaster. Cell. 1987;49:699. doi: 10.1016/0092-8674(87)90546-0. [DOI] [PubMed] [Google Scholar]
- 24.Twombly V. Blackman B.K. Jin H. Graff J.M. Padgett R.W. Gelbart W.M. The TGF-beta signaling pathway is essential for Drosophila oogenesis. Development. 1996;122:1555. doi: 10.1242/dev.122.5.1555. [DOI] [PubMed] [Google Scholar]
- 25.Xie T. Spradling A.C. Decapentaplegic is essential for the maintenance and division of germline stem cells in the Drosophila ovary. Cell. 1998;94:251. doi: 10.1016/s0092-8674(00)81424-5. [DOI] [PubMed] [Google Scholar]
- 26.Duchek P. RØrth P. Guidance of cell migration by EGF receptor signaling during Drosophila oogenesis. Science. 2001;291:131. doi: 10.1126/science.291.5501.131. [DOI] [PubMed] [Google Scholar]
- 27.McDonald J.A. Pinheiro E.M. Kadlec L. Schüpbach T. Montell D.J. Multiple EGFR ligands participate in guiding migrating border cells. Dev Biol. 2006;296:94. doi: 10.1016/j.ydbio.2006.04.438. [DOI] [PubMed] [Google Scholar]
- 28.Gilboa L. Lehmann R. Soma-germline interactions coordinate homeostasis and growth in the Drosophila gonad. Nature. 2006;443:97. doi: 10.1038/nature05068. [DOI] [PubMed] [Google Scholar]
- 29.Shilo B.Z. Signaling by the Drosophila epidermal growth factor receptor pathway during development. Exp Cell Res. 2003;284:140. doi: 10.1016/s0014-4827(02)00094-0. [DOI] [PubMed] [Google Scholar]
- 30.Affolter M. Basler K. The Decapentaplegic morphogen gradient: from pattern formation to growth regulation. Nat Rev Genet. 2007;8:663. doi: 10.1038/nrg2166. [DOI] [PubMed] [Google Scholar]
- 31.Spradling A.C. Developmental genetics of oogenesis. In: Bate M., editor; Martinez-Arias A., editor. The Development of Drosophila melanogaster. Vol. 1. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1993. pp. 1–70. [Google Scholar]
- 32.Mahajan-Miklos S. Cooley L. Intercellular cytoplasm transport during Drosophila oogenesis. Dev Biol. 1994;165:336. doi: 10.1006/dbio.1994.1257. [DOI] [PubMed] [Google Scholar]
- 33.Ray R.P. Schüpbach T. Intercellular signaling and the polarization of body axes during Drosophila oogenesis. Genes Dev. 1996;10:1711. doi: 10.1101/gad.10.14.1711. [DOI] [PubMed] [Google Scholar]
- 34.Roth S. The origin of dorsoventral polarity in Drosophila. Philos Trans R Soc Lond B Biol Sci. 2003;358:1317. doi: 10.1098/rstb.2003.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.LeMosy E.K. Pattern formation: the eggshell holds the cue. Curr Biol. 2003;13:R508. doi: 10.1016/s0960-9822(03)00441-x. [DOI] [PubMed] [Google Scholar]
- 36.Waring G.L. Morphogenesis of the eggshell in Drosophila. Int Rev Cytol. 2000;198:67. doi: 10.1016/s0074-7696(00)98003-3. [DOI] [PubMed] [Google Scholar]
- 37.Dobens L.L. Raftery L.A. Integration of epithelial patterning and morphogenesis in Drosophila ovarian follicle cells. Dev Dyn. 2000;218:80. doi: 10.1002/(SICI)1097-0177(200005)218:1<80::AID-DVDY7>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 38.Dorman J.B. James K.E. Fraser S.E. Kiehart D.P. Berg C.A. bullwinkle is required for epithelial morphogenesis during Drosophila oogenesis. Dev Biol. 2004;267:320. doi: 10.1016/j.ydbio.2003.10.020. [DOI] [PubMed] [Google Scholar]
- 39.Neuman-Silberberg F.S. Schüpbach T. Dorsoventral axis formation in Drosophila depends on the correct dosage of the gene gurken. Development. 1994;120:2457. doi: 10.1242/dev.120.9.2457. [DOI] [PubMed] [Google Scholar]
- 40.Deng W.M. Bownes M. Two signalling pathways specify localized expression of the Broad-Complex in Drosophila eggshell patterning and morphogenesis. Development. 1997;124:4639. doi: 10.1242/dev.124.22.4639. [DOI] [PubMed] [Google Scholar]
- 41.Dobens L.L. Peterson J.S. Treisman J. Raftery L.A. Drosophila bunched integrates opposing DPP and EGF signals to set the operculum boundary. Development. 2000;127:745. doi: 10.1242/dev.127.4.745. [DOI] [PubMed] [Google Scholar]
- 42.Peri F. Roth S. Combined activities of Gurken and Decapentaplegic specify dorsal chorion structures of the Drosophila egg. Development. 2000;127:841. doi: 10.1242/dev.127.4.841. [DOI] [PubMed] [Google Scholar]
- 43.Shravage B.V. Altmann G. Technau M. Roth S. The role of DPP and its inhibitors during eggshell patterning in Drosophila. Development. 2007;134:2261. doi: 10.1242/dev.02856. [DOI] [PubMed] [Google Scholar]
- 44.Morimoto A.M. Jordan K.C. Tietze K. Britton J.S. O′Neill E.M. Ruohola-Baker H. Pointed, an ETS domain transcription factor, negatively regulates the EGF receptor pathway in Drosophila oogenesis. Development. 1996;122:3745. doi: 10.1242/dev.122.12.3745. [DOI] [PubMed] [Google Scholar]
- 45.Wasserman J.D. Freeman M. An autoregulatory cascade of EGF receptor signaling patterns the Drosophila egg. Cell. 1998;95:355. doi: 10.1016/s0092-8674(00)81767-5. [DOI] [PubMed] [Google Scholar]
- 46.Sapir A. Schweitzer R. Shilo B.Z. Sequential activation of the EGF receptor pathway during Drosophila oogenesis establishes the dorsoventral axis. Development. 1998;125:191. doi: 10.1242/dev.125.2.191. [DOI] [PubMed] [Google Scholar]
- 47.Peri F. Bökel C. Roth S. Local Gurken signaling and dynamic MAPK activation during Drosophila oogenesis. Mech Dev. 1999;81:75. doi: 10.1016/s0925-4773(98)00228-7. [DOI] [PubMed] [Google Scholar]
- 48.Chen Y. and Schüpbach, T. The role of brinker in eggshell patterning. Mech Dev. 2006;123:395. doi: 10.1016/j.mod.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 49.Yakoby N. Lembong J. Schüpbach T. Shvartsman S.Y. Drosophila eggshell is patterned by sequential action of feedforward and feedback loops. Development. 2008;135:343. doi: 10.1242/dev.008920. [DOI] [PubMed] [Google Scholar]
- 50.Ward E.J. Berg C.A. Juxtaposition between two cell types is necessary for dorsal appendage tube formation. Mech Dev. 2005;122:241. doi: 10.1016/j.mod.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 51.Ward E.J. Zhou X. Riddiford L.M. Berg C.A. Ruohola-Baker H. Border of Notch activity establishes a boundary between the two dorsal appendage tube cell types. Dev Biol. 2006;297:461. doi: 10.1016/j.ydbio.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 52.Shvartsman S.Y. Muratov C.B. Lauffenburger D.A. Modeling and computational analysis of EGF receptor-mediated cell communication in Drosophila oogenesis. Development. 2002;129:2577. doi: 10.1242/dev.129.11.2577. [DOI] [PubMed] [Google Scholar]
- 53.Goentoro L.A. Reeves G.T. Kowal C.P. Martinelli L. Schüpbach T. Shvartsman S.Y. Quantifying the Gurken morphogen gradient in Drosophila oogenesis. Dev Cell. 2006;11:263. doi: 10.1016/j.devcel.2006.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zegers M. O′Brien L. Yu W. Datta A. Mostov K. Epithelial polarity and tubulogenesis in vitro. Trends Cell Biol. 2003;13:169. doi: 10.1016/s0962-8924(03)00036-9. [DOI] [PubMed] [Google Scholar]
- 55.Nelson W. Tube morphogenesis: closure, but many openings remain. Trends Cell Biol. 2003;13:615. doi: 10.1016/j.tcb.2003.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Affolter M. Bellusci S. Itoh N. Shilo B. Thiery J.P. Werb Z. Tube or not tube: remodeling epithelial tissues by branching morphogenesis. Dev Cell. 2003;4:11. doi: 10.1016/s1534-5807(02)00410-0. [DOI] [PubMed] [Google Scholar]
- 57.Beitel G.J. Krasnow M.A. Genetic control of epithelial tube size in the Drosophila tracheal system. Development. 2000;127:3271. doi: 10.1242/dev.127.15.3271. [DOI] [PubMed] [Google Scholar]
- 58.Ghabrial A.S. Krasnow M.A. Social interactions among epithelial cells during tracheal branching morphogenesis. Nature. 2006;441:746. doi: 10.1038/nature04829. [DOI] [PubMed] [Google Scholar]
- 59.Edwards K.A. Kiehart D.P. Drosophila nonmuscle myosin II has multiple essential roles in imaginal disc and egg chamber morphogenesis. Development. 1996;122:1499. doi: 10.1242/dev.122.5.1499. [DOI] [PubMed] [Google Scholar]
- 60.Nezis I.P. Stravopodis D.J. Papassideri I. Robert-Nicoud M. Margaritis L.H. Stage-specific apoptotic patterns during Drosophila oogenesis. Eur J Cell Biol. 2000;79:610. doi: 10.1078/0171-9335-00088. [DOI] [PubMed] [Google Scholar]
- 61.Nezis I.P. Stravopodis D.J. Papassideri I. Robert-Nicoud M. Margaritis L.H. Dynamics of apoptosis in the ovarian follicle cells during the late stages of Drosophila oogenesis. Cell Tissue Res. 2002;307:401. doi: 10.1007/s00441-001-0498-3. [DOI] [PubMed] [Google Scholar]
- 62.Odell G.M. Oster G. Alberch P. Burnside B. The mechanical basis of morphogenesis. I. Epithelial folding and invagination. Dev Biol. 1981;85:446. doi: 10.1016/0012-1606(81)90276-1. [DOI] [PubMed] [Google Scholar]
- 63.Pilot F. Lecuit T. Compartmentalized morphogenesis in epithelia: from cell to tissue shape. Dev Dyn. 2005;232:685. doi: 10.1002/dvdy.20334. [DOI] [PubMed] [Google Scholar]
- 64.Snow P.M. Bieber A.J. Goodman C.S. Fasciclin III: a novel homophilic adhesion molecule in Drosophila. Cell. 1989;59:313. doi: 10.1016/0092-8674(89)90293-6. [DOI] [PubMed] [Google Scholar]
- 65.Rittenhouse K.R. Berg C.A. Mutations in the Drosophila gene bullwinkle cause the formation of abnormal eggshell structures and bicaudal embryos. Development. 1995;121:3023. doi: 10.1242/dev.121.9.3023. [DOI] [PubMed] [Google Scholar]
- 66.Tran D.H. Berg C.A. bullwinkle and shark regulate dorsal-appendage morphogenesis in Drosophila oogenesis. Development. 2003;130:6273. doi: 10.1242/dev.00854. [DOI] [PubMed] [Google Scholar]
- 67.Kerszberg M. Changeux J. A simple molecular model of neurulation. BioEssays. 1998;20:758. doi: 10.1002/(SICI)1521-1878(199809)20:9<758::AID-BIES9>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 68.Zajac M. Jones G.L. Glazier J.A. Model of convergent extension in animal morphogenesis. Phys Rev Lett. 2000;85:2022. doi: 10.1103/PhysRevLett.85.2022. [DOI] [PubMed] [Google Scholar]
- 69.Zajac M. Jones G.L. Glazier J.A. Simulating convergent extension by way of anisotropic differential adhesion. J Theor Biol. 2003;222:247. doi: 10.1016/s0022-5193(03)00033-x. [DOI] [PubMed] [Google Scholar]
- 70.James K.E. Berg C.A. Temporal comparison of Broad-Complex expression during eggshell-appendage patterning and morphogenesis in two Drosophila species with different eggshell-appendage numbers. Gene Expr Patterns. 2003;3:629. doi: 10.1016/s1567-133x(03)00136-4. [DOI] [PubMed] [Google Scholar]