Abstract
Neuropsychological studies in electrical injury patients have reported deficits in attention, learning, and working memory, but the neural substrates of these deficits remain poorly characterized. In this study we sought to examine whether electrical injury subjects demonstrate abnormal patterns of brain activation during working memory and procedural learning tasks. Fourteen electrical injury subjects and fifteen demographically matched healthy control subjects performed a spatial working memory paradigm and a procedural learning paradigm during functional MRI studies. For the spatial working memory task, electrical injury patients exhibited significantly greater activation in the middle frontal gyrus and motor and posterior cingulate cortices. Increased activation in EI subjects also was observed on a visually-guided saccade task in several sensorimotor regions, including the frontal and parietal eye fields and striatum. On the procedural learning task, electrical injury patients exhibited significantly less activation in the middle frontal gyrus, anterior cingulate cortex, and frontal eye fields than controls. This is the first study to document task-dependent, system-level cortical and subcortical dysfunction in individuals who had experienced an electrical shock trauma.
Key words: cognitive functioning, electrical injury, functional magnetic resonance imaging
Introduction
Electrical injuries (EI) can result from contact with an electrical power source. As the point of electrical contact is most often an extremity, damage to the peripheral nervous system commonly occurs through breakdown of cellular membranes via electroporation and electroconformational changes in membrane proteins (Lee, 1997). Neuropsychological studies have shown that cognitive changes occur in EI survivors, even in cases in whom the head was not in direct contact with the electrical power source (Pliskin et al., 1999). Pliskin and colleagues (2006) found that EI patients demonstrated deficits on measures of attention, mental speed, and motor skills that were independent of secondary medical or psychiatric complications. The presence of neuropsychological deficits following peripheral EI suggests an effect of electrical exposure on central nervous system function, although direct evidence of deficits in the neural substrates of higher cognitive function is lacking.
There have been few neuroimaging studies and no functional imaging studies examining brain changes following EI. Neuroimaging studies have yielded inconsistent findings of structural and perfusion abnormalities, including basal ganglia lesions (Sahiner et al., 2002), and hypoperfusion in mesial temporal cortex and the caudate nucleus (Deveci et al., 2002). The use of functional magnetic resonance imaging (fMRI) provides an approach for probing the functional capacity of the neural substrates involved in cognitive functions, and how such systems might be affected following EI. Functional MRI investigations using oculomotor tasks can be informative about regional neurological dysfunction, and potentially provide a translational platform for research in this area. Sensorimotor control of eye movements is provided by frontal, supplementary, and parietal eye fields. The dorsolateral prefrontal cortex, anterior cingulate, and dorsal striatum have also been shown to play a large role in the cognitive control of eye movement activity such as that required for working memory and procedural learning (Luna et al., 2002; Simo et al., 2005). Examination of the functional integrity of neural substrates of oculomotor control therefore provides a potentially useful tool to probe the neuronal circuitry of widely distributed neural systems supporting cognitive processes after trauma to the brain such as that resulting from EI.
The present study is the first to examine differences in patterns of neural activation between EI patients and matched healthy individuals. The oculomotor tasks used in the current study were chosen to evaluate cognitive abilities previously found to be impaired in neuropsychological studies of EI patients, specifically working memory and learning (Pliskin et al., 2006; Pliskin et al., 1999).
Methods
Participants
The participants included groups of 14 adult individuals who sustained an EI from a domestic or commercial power source that required medical intervention, and 15 medically healthy adult individuals that were matched for age and premorbid IQ (Table 1). Exclusion criteria for all subjects included a history of psychiatric treatment; neurological disorder unrelated to EI; a history of learning disability, traumatic head injury, or direct contact of the head with the electricity source; and conventional contraindications to MRI studies.
Table 1.
Demographic and Cognitive Characteristics of the Study Participants
|
Electrical injury mean (SD) |
Controls mean (SD) |
|
|---|---|---|
| Sex | 13 males, 1 female | 10 males, 5 females |
| Age | 38.00 (8.38) | 32.86 (10.52) |
| Years of education | 12.71 (1.68)a | 13.93 (1.27) |
| Estimated IQ | 96.07 (10.48) | 101.46 (10.01) |
| Beck Depression Inventory-II | 21.35 (10.62)a | 5.57 (5.17) |
| Impact of Events Scale | 36.21 (17.22)a | 18.14 (12.35) |
Significant between-group differences (p < 0.05).
All participants underwent a semi-structured clinical interview (Structured Clinical Interview for DSM-IV Axis I Disorders) administered by a psychiatrist or psychologist to assess current and lifetime psychiatric status. In the EI group, post-EI psychiatric disorders were comorbid major depression and posttraumatic stress disorder (PTSD) in six of the subjects. One EI subject reported a history of depressive symptoms preceding the EI. Ten EI subjects reported taking prescription psychiatric medications: seven were taking antidepressants, five of whom also reported taking antianxiety medications, all benzodiazepines; two subjects reported taking antidepressant and antiseizure medication for mood regulation rather than seizure activity; and one subject reported taking antipsychotic and stimulant medication. None of the healthy control subjects met DSM-IV criteria for current or lifetime psychiatric disorder or reported taking psychiatric medications. Following the EI, nine subjects reported chronic peripheral pain. Seven of the subjects reported taking prescription pain medication, 80% of which were opioids. Nine of the EI subjects had unremarkable structural brain MRI examinations, and five were noted to have small unspecified T2 hyperintensities of unknown significance. All healthy control subjects denied any current significant medical condition and all had unremarkable structural MRIs.
At study enrollment, nine of the EI subjects were on disability or unemployed, while the rest were full-time employees. Estimated exposure to voltage during EI ranged from 220 V to 480 V in six of the subjects. Three subjects were presumably exposed to 7200 V. Four subjects reported transient heart irregularities following the EI. Four subjects reported brief loss of consciousness during EI. Ten subjects were admitted to an emergency room immediately following the EI. Average time of the brain scans following the EI was 1408 days (range 268 to 3379). All of the subjects passed effort testing as measured by the Test of Memory Malingering (score on trial 2 > 45). Written informed consent was obtained from all participants. The study was approved by the Institutional Review Boards of the University of Illinois at Chicago.
Neuropsychological assessment
All participants underwent a neuropsychological evaluation to assess premorbid intellectual functioning, effort, attention/working memory, and episodic memory. The neuropsychological battery included: the National Adult Reading Test (NART; Nelson, 1982), Test of Memory Malingering (TOMM; Tombaugh, 1996), Trail Making Test (Reitan, 1958), Stroop Color and Word Test (Golden and Freshwater, 2002), Paced Auditory Serial Addition Test (PASAT; Gronwall and Sampson, 1974), Conner's Continuous Performance Test-II (CPT; Conner, 2002), and the California Verbal Learning Test–2nd Edition (CVLT-II; Delis et al., 2000). Self-report questionnaires included: Beck Depression Inventory-II (BDI-II; Beck et al., 1987) and the Impact of Events Scale (IES; Horowitz et al., 1979) (Tables 1 and 2).
Table 2.
Neuropsychological Test Scores (Raw Values)
| Test |
Electrical injury mean (SD) |
Controls mean (SD) |
|---|---|---|
| Trails Aa | 26.07 (10.5) | 26.43 (9.87) |
| Trails Ba | 71.28 (22.14) | 73.07 (41.7) |
| Stroop Worda | 86.85 (26.7) | 93.15 (20.71) |
| Stroop Colora | 63.92 (11.26) | 72.84 (16.62) |
| PASAT T1 (3 second rate)b | 34.27 (11.2) | 38.64 (12.66) |
| PASAT T4 (1.6-second rate)b | 17.36 (7.89) | 18.5 (10.38) |
| Continuous Performance Test: Omissionsc | 2.88 (3.55) | 4.28 (8.43) |
| Continuous Performance Test: Commissionsc | 10.30 (4.21) | 7.71 (5.44) |
| California Verbal Learning Test-2: Immediate Recall Trial 1d | 5.42e (1.6) | 7.66 (2.38) |
| California Verbal Learning Test-2: Immediate Recall Totald | 46.71e (12.2) | 57.33 (9.35) |
| California Verbal Learning Test-2: Long Delay Free Recalld | 9.92 (3.62) | 12.06 (2.37) |
Time to task completion in seconds.
Number of correct responses.
Number of errors.
Number of words correctly recalled.
Significant between-group difference p < 0.05.
Working memory and procedural learning fMRI paradigms
Two separate paradigms were administered in fMRI studies: an oculomotor delayed response task (ODR) to assess working memory, and a predictive saccade task to assess procedural learning (PRED). Performance on each task was contrasted with a visually guided saccade task (VGS) matched in demands for number and amplitude of required saccade responses. During functional imaging studies, the stimuli were either white or red circles presented against a dark gray background on a rear projection screen. When necessary, visual acuity was brought to within 20/40 by corrective MR-compatible lenses. Consistent compliance with task instructions was established prior to scanning to ensure that all participants understood the tasks and could perform them reliably. Each paradigm was presented in a block-design format.
Spatial working memory paradigm
For each ODR trial, following 1 sec of center fixation, a peripheral cue was presented 4 or 8 degrees to the left or right of center for 50 msec. The subjects were instructed to covertly attend to (not to directly look at) and learn the location of the peripheral target stimulus, while holding central fixation. After 4.5 sec of continued central fixation, the center light was extinguished cueing subjects to direct their gaze to the remembered peripheral target location. After 1.5 sec from disappearance of the central cue, a light at the correct “to be remembered” location appeared for 1 sec to provide feedback about task performance. The central fixation target then reappeared, marking the beginning of the next trial. In the VGS trials, subjects looked to unpredictable targets as they appeared. Subjects performed eight 32-sec blocks of ODR trials alternating with nine 32-sec blocks of VGS trials, as in previous studies from this laboratory (Luna et al., 2002), with the type of trial determined by the color of the central fixation cue. Each block had four 8-sec trials. Thus, while the blocks were similar in motor demands, the ODR block had the crucial requirement to maintain spatial location information in visual working memory during delay periods.
Procedural learning paradigm
This task included three different block types: visually guided saccades (VGS), predictive saccades (PRED), and visual fixation, as in previous studies in our laboratory (Simo et al., 2005). In both the VGS and PRED blocks, targets consisted of a small white dot that stepped between locations in the horizontal plane. For all tasks, subjects were instructed to direct their gaze onto the target on the screen. In the VGS and PRED blocks, the targets were presented at 1 of 7 possible locations (±9, 6, and 3 degrees, and 0 degrees). Three-degree target steps occurred every 1000 msec. In the VGS task, target movement to the left or right was unpredictable, except when at the 9-degree location the target always moved toward center. In the PRED blocks, target position alternated in 3-degree steps between two specific locations. The transition between tasks was not cued, and was made from the last target position of the prior condition. Thus it was left up to subjects to learn to utilize target predictability for initiating responses. When subjects learn target location will be predictable, they quickly learn to initiate saccades with much briefer latency, often before target appearance, and to rely more on prefrontal circuitry and less on sensorimotor systems to initiate responses (Simo et al., 2005). VGS and PRED blocks were balanced in terms of the number of saccades required to perform each task (1 per sec), their amplitude (3 degrees), and their direction.
The task also included five fixation blocks that lasted for 30 sec and were presented at the beginning and after every four saccade blocks to provide rest periods and a baseline condition. In fixation blocks, subjects were instructed to fixate on a crosshair in the middle of the screen.
A commercial MR-compatible limbus tracking system (Cambridge Research Systems, Rochester, Kent, UK) that samples eye position at 1000 Hz was used to record eye movements during the MR studies.
MRI scanning
Brain imaging was performed on a clinical 3.0 Tesla scanner (Signa; General Electric Medical Systems, Waukesha, WI). Single-shot gradient-echo echo-planar imaging (EPI) was performed with a standard quadrature head coil for fMRI studies. Twenty-five axial slices were acquired with the following parameters: TE (echo time) = 25 msec; TR = 2000 msec; 90-degree flip angle; slice thickness = 5 mm; gap = 1 mm; 64 × 64 acquisition matrix; voxel size: 3 × 3 × 5 mm; FOV = 20 × 20.
T1-weighted images were obtained in the axial plane and used to register the functional images in standardized space. These 3-D spoiled gradient recalled images were collected with the following acquisition parameters: TR = 25 msec; TE = 3 msec; 40-degree flip angle; slice thickness = 1.5 mm; gap = 0 mm; 256 × 256 × 124 acquisition matrix; FOV = 24 × 18.
Functional data analysis
Functional data from each subject were preprocessed using FIASCO 5.2 (Functional Imaging Software-Computational Olio; Eddy et al., 1996) software to correct for movement and scanner artifacts, shift the time series 6 sec to compensate for delayed blood oxygen level–dependent response to blocks of stimuli, and generate functional activation maps based on voxel-wise t-tests between conditions in each task for individual subjects. For the ODR paradigm, ODR and VGS blocks were contrasted, and for PRED, two contrasts were examined: VGS blocks with fixation to assess basic sensorimotor processes involved in supporting saccades, and PRED blocks were contrasted with VGS blocks to assess activation associated with predicting target timing and location. AFNI (Analysis of Functional NeuroImages; Cox, 1996) software was used to overlay functional maps of individual subjects onto their anatomical images, warp each subject's data into Talairach space, and conduct between-group statistical analyses.
Prior to group comparison, individual subjects' t-maps were resampled to 3 × 3 × 3-mm voxels (in-plane voxel dimension at acquisition), and transformed to effect size maps expressed as Fisher z′ statistics. Significant group differences in task-related activation were identified with a contiguity threshold that defined volumes of contiguous activated voxels with a volume of at least 270 mm3 in which voxels had individual z-values of 2.58 or greater, and a connection radius of no more than 3.1 mm. These parameters were determined with AFNI AlphaSim Monte Carlo simulations to preserve an experiment-wise type I error rate of p < 0.025.
Regions of interest (ROIs) were used to characterize between-group differences in anatomically defined regions known a priori to support task performance. These were developed in Talairach space in an independent group of 15 healthy individuals. The ROIs, as well as the rationale for ROI definitions, are available at http://ccm.psych.uic.edu/Research/NormalBrain/ROI_rules.htm.
Results
Demographic and cognitive characteristics
There were no significant between-group differences in age and IQ. EI patients performed more poorly on a measure of immediate recall for learned auditory-verbal information presented in a list format. Notably, there was no significant between-group difference on the delayed recall trial of the measure (Table 2).
Eye movement measurements
There were no significant main effects or interaction effects for any saccade measure in relation to saccade direction or target step amplitude. Therefore, data were collapsed across these dimensions for statistical analyses. On the ODR task, there were no significant group differences in latency or accuracy of primary saccades to remembered target locations. For the PRED task, in order to examine the change in saccade latency over the course of the task, primary saccades in the 20 trials in each block were collapsed into three sub-blocks for statistical analysis (block 1 = trials 1–6; block 2 = trials 7–13; and block 3 = trials 14–20). While both groups showed speeding of response latencies over trials (Table 3), there were no significant group differences in saccade latency reduction over trials. In order to examine qualitative aspects of task performance (internally generated predictive saccades versus sensory-driven visually elicited responses), each primary saccade was classified as a predictive saccade when response latency was less than 90 msec. The two groups did not differ in the proportion of predictive saccades (Table 3).
Table 3.
Saccade Measurements Obtained During the fMRI Study
| Oculomotor delayed response paradigm |
Electrical injury mean (SD) |
Controls mean (SD) |
|---|---|---|
| Visually-guided saccades | ||
| Latency (msec) | 209 (48) | 210 (30) |
| Gain | .90 (.13) | .80 (.09) |
| Memory-guided saccades | ||
| Latency (msec) | 345 (88) | 345 (64) |
| Gain | .83 (.13) | .84 (.21) |
| Predictive saccades paradigm | ||
| Visually-guided saccades | ||
| Latency (msec) | 162 (26) | 152 (25) |
| Gain | .87 (.10) | .84 (.15) |
| Predictive saccades | ||
| Latency (msec) Block 1 | 151 (43) | 134 (48) |
| Block 2 | 84 (36) | 52 (92) |
| Block 3 | 69 (37) | 29 (96) |
| Gain of regular saccades | .99 (.12) | .89 (.11) |
| Fast saccades | .78 (.28) | .84 (.14) |
| Predictive saccades | .64 (.15) | .69 (.13) |
| Proportion of predictive saccades | 27.50 (9.2) | 34.00 (17.90) |
Functional imaging
Predictive saccade task
On the VGS versus fixation contrast in the predictive saccade paradigm, EI subjects exhibited greater activation than controls in the frontal eye fields, anterior cingulate cortex, striatum and insula bilaterally, and in left intraparietal sulcus (Table 4).
Table 4.
Brain Regions Showing Statistically Greater Task-Related Activation in EI Subjects or Matched Healthy Control Subjects during the Visually-Guided Saccade Task
| Left hemisphere | Right hemisphere | |||||
|---|---|---|---|---|---|---|
| Volume (mm3) of increased activation | Peak t value | X, Y, Z coordinates | Volume (mm3) of increased activation | Peak t value | X, Y, Z coordinates | |
| EI > healthy | ||||||
| Frontal eye fields | 297 | 3.25 | −46, 4, 23 | 270 | 3.86 | 31, −13, 47 |
| Intraparietal sulcus | 270 | 3.98 | −37, −52, 32 | |||
| Anterior cingulate cortex | 270 | 4.46 | −1, 49, 8 | 189 | 3.94 | 1, 46, 8 |
| Striatum | 783 | 4.02 | −19, 4, 11 | 541 | 4.34 | 28, 4, −3 |
| Insula | 1431 | 4.58 | −37, −52, 32 | 1836 | 4.71 | 34, 25, 8 |
The table shows the t value for the peak activation in each region, its corresponding Talairach coordinates, and volume of voxels showing statistically greater activation in one group relative to the other.
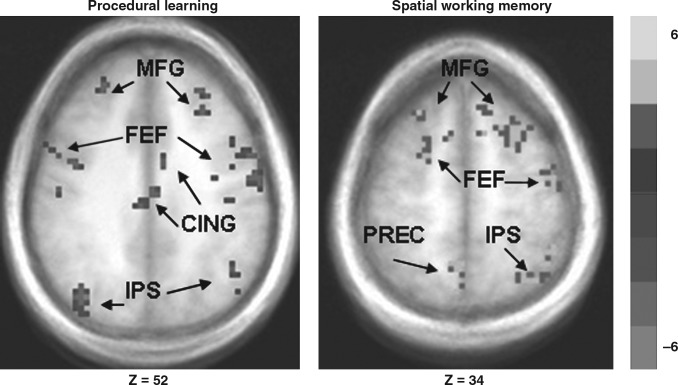
In the comparison of the PRED versus VGS tasks, EI subjects exhibited significantly less activation in the middle frontal gyrus, frontal eye fields, supplementary eye fields, posterior intraparietal sulcus, precuneus, the anterior, motor, and posterior cingulate cortices bilaterally, and in the striatum and thalamus (Fig. 1 and Table 5).
FIG. 1.
Group comparison (t test) maps, thresholded as described in the methods section, during the procedural learning (left, Z = 52) and spatial working memory (right, Z = 34) tasks, overlaid on the overall group average anatomical image. On the procedural learning task, healthy controls displayed greater activation in the frontal eye fields (FEF), middle frontal gyrus (MFG), motor and posterior cingulate cortices (CING), and in the intraparietal sulcal (IPS) region. On the spatial working memory task, greater activation among the EI subjects was seen in the FEF, MFG, IPS, and precuneus (PREC) region.
Table 5.
Brain Regions Showing Statistically Reduced Task-Related Activation in EI Patients Compared to Matched Healthy Control Subjects When Performing Saccades to Predictable Versus Unpredictable Saccade Targets
| Left hemisphere | Right hemisphere | |||||
|---|---|---|---|---|---|---|
| Volume (mm3) of increased activation | Peak t value | X, Y, Z coordinates | Volume (mm3) of increased activation | Peak t value | X, Y, Z coordinates | |
| EI < healthy | ||||||
| Frontal eye fields | 2403 | 4.55 | −46, −4, 47 | 486 | 3.44 | 31, −4, 62 |
| Supplementary eye fields | 162 | 3.96 | −7, 1, 68 | 351 | 4.20 | 1, −4, 65 |
| Intraparietal sulcus | 2079 | 4.54 | −46, −46, 60 | |||
| Middle frontal gyrus | 3321 | 4.65 | −46, 34, 26 | 2295 | 4.55 | 25, −28, 41 |
| Precuneus | 54 | 2.61 | −13, −82, 38 | 1647 | 4.57 | 4, −55, 50 |
| Anterior cingulate cortex | 1404 | 4.10 | −4, 28, 14 | 1323 | 4.51 | 1, 19, 32 |
| Motor cingulate cortex | 243 | 4.21 | −10, 10, 32 | 27 | 1, 13, 29 | |
| Posterior cingulate cortex | 27 | 2.81 | −1, −46, 8 | 81 | 3.14 | 1, −46, 8 |
| Striatum | 189 | 3.29 | −28, −10, −1 | 27 | 2.61 | 28, −10, −3 |
| Insula | 2592 | 5.52 | −49, 1, −9 | 1296 | 4.13 | 31, 19, −1 |
| Thalamus | 27 | 2.85 | −10, −28, 2 | |||
The table shows the t value for the peak activation in each region, its corresponding Talairach coordinates, and volume of voxels showing statistically reduced activation in EI patients.
Peak activation values within each ROI were correlated between the PRED and the VGS tasks. There were no significant correlations in most ROIs, expect for within the frontal eye fields (r = .83, p < 0.05) and supplementary eye fields (r = .68, p < 0.05) for controls only.
Oculomotor delayed response task
Compared with the control group, EI patients demonstrated significantly greater activation during the ODR than the VGS task in the bilateral middle frontal gyrus, the frontal and supplementary eye fields, the intraparietal sulcus and the precuneus. Increased activation relative to controls was also noted in the motor and posterior cingulate cortices and insula (Table 6).
Table 6.
Brain Regions Showing Statistically Greater Task-Related Activation in EI Subjects Compared to Matched Healthy Control Subjects during the Memory-Guided Saccade Task
| Left hemisphere | Right hemisphere | |||||
|---|---|---|---|---|---|---|
| Volume (mm3) of increased activation | Peak t value | X, Y, Z coordinates | Volume (mm3) of increased activation | Peak t value | X, Y, Z coordinates | |
| EI > healthy | ||||||
| Frontal eye fields | 4,644 | 4.15 | −37, −13, 59 | 1,431 | 3.88 | 52, 4, 44 |
| Supplementary eye fields | 783 | 3.60 | −7, 4, 62 | 2,025 | 4.66 | 1, −4, 62 |
| Intraparietal sulcus | 1269 | 4.25 | −46, −67, 38 | 945 | 4.51 | 34, −70, 35 |
| Middle frontal gyrus | 2052 | 5.22 | −16, 40, 53 | 1539 | 4.02 | 25, 43, 35 |
| Precuneus | 216 | 3.31 | −7, − 49, 44 | 405 | 3.53 | 1, −58, 47 |
| Motor cingulate cortex | 270 | 3.84 | −7, 1, 35 | 135 | 3.41 | 10, −19, 41 |
| Posterior cingulate cortex | 540 | 3.81 | −4, −16, 35 | 918 | 4.33 | 7, −7, 41 |
| Insula | 216 | 3.67 | −43, 7, 17 | 513 | 4.55 | 40, −13, 14 |
The table shows the t value for the peak difference between groups in each region, its corresponding Talairach coordinates, and the volume of voxels showing statistically greater activation in EI patients.
Discussion
The results of this first fMRI investigation of functional brain systems in EI indicate the presence of significant functional alterations in sensorimotor and cognitive brain systems. Specifically, the EI group displayed increased activation in neocortical sensorimotor systems during a simple visual sensorimotor task compared to healthy controls, and a greater increase in activation in prefrontal systems during a spatial working memory task. By contrast, EI subjects exhibited less activation in frontostriatal systems compared to controls during a procedural learning task. These patterns of abnormal activation reflect a general disturbance throughout the cortical and subcortical regions involved in cognitive and sensorimotor processes in EI subjects, and could represent the neurologic basis for working memory and learning deficiencies detected by neuropsychological testing (Pliskin et al., 2006; Pliskin et al., 1999).
In the analysis of activation associated with making visually guided saccades versus central fixation, EI subjects exhibited a pattern of increased activation in brain regions supporting sensorimotor and attentional control of eye movement activity. The generation of visually-guided saccades and related visual attention are mediated at the neocortical level by the frontal and parietal eye fields (Merriam et al., 2001; Corbetta et al., 1998), where EI subjects demonstrated significantly greater activation in these regions during saccade generation than healthy controls. The pattern of increased activation in these brain regions during the visually-guided saccades task in EI subjects suggests inefficiency and subsequent compensation in regions supporting sensorimotor and attentional control of saccadic eye movements.
Against this level of increased activation during the sensorimotor control task, EI subjects exhibited a further increase in brain activation during a more cognitively demanding task requiring spatial working memory. These effects were seen in prefrontal and sensorimotor systems. While previous studies have documented increases in activation of working memory in healthy individuals (e.g., Sweeney, 1996), the increases in regional brain function observed in EI subjects were enhanced. Thus, even in the context of increased activation on the simpler sensorimotor saccade task (VGS), EI subjects exhibited a further increase in brain activation during a more cognitively demanding task requiring maintenance of spatial information over time to plan and guide saccades after a delay period. Similarly to observations in the visually-guided saccade control task, this increase in neurophysiological activity may reflect compensatory increases to meet task demands during working memory processes, and thus suggest reduced integrity of brain systems supporting working memory processes after electrical trauma.
The pattern of increased activation during working memory tasks has been described in functional imaging studies of patients with traumatic brain injury (TBI; Scheibel et al., 2003; McAllister et al., 2001). In TBI, this pattern has been postulated to result from diffuse axonal injury that necessitates a more extensive recruitment of neocortical systems to maintain information and behavioral plans over time in working memory, or from deficits in the ability to appropriately match processing resources to processing demands (McAllister et al., 2001). The working memory task in our study imposed only modest working memory demands, requiring subjects to remember a spatial location over a delay period without distraction, competing cognitive processes, or demand for internal manipulation of information to select and plan a response. Thus, EI subjects could show increased activation because increased neuronal activity is required to support these relatively simpler processing demands, or because they have difficulty scaling processing resources to processing demands. Future studies using cognitive tasks that can be varied parametrically are needed to address this issue. Both possibilities are consistent with our observations in EI, and both also indicate the presence of a dysfunction in prefrontal and frontostriatal systems similar to the consequences of TBI.
In contrast to the pattern of increased activation observed on the visually-guided saccade and working memory tasks, EI subjects exhibited decreased activation in the prefrontal and sensorimotor cortex and striatum during the procedural learning task relative to the sensorimotor control task. In addition to findings in the sensorimotor cortex, this decreased activation in EI subjects relative to controls was also observed in frontal systems known to be involved in cognitive aspects of oculomotor control during the visual procedural learning task (e.g., the middle frontal gyrus and supplementary eye fields). Learning to perform a predictive saccade task requires the maintenance and retrieval of spatial-temporal information to guide behavior as response sequences are learned. Our findings with an implicit motor learning task indicate a deficiency at the level of the neural system subserving the ability to learn simple motor routines. Of note, this pattern of reduced activation was observed relative to the visually-guided saccade task, which when analyzed separately in relation to fixation, increased activation was observed for EI patients relative to healthy controls. However, analyses did not reveal significant correlations between effects on the sensorimotor and the procedural learning tasks for the EI group. This suggests that activation patterns in each task are largely independent of each other. The controls' correlations were significant for frontal eye fields and supplementary eye fields between the tasks, showing that increased activation in these regions across the tasks is normally correlated, but the lack of a similar relationship within ROIs for EI patients further supports the idea of disrupted neural systems in EI.
The presence of increased activation in prefrontal and other systems during the working memory task, but decreased activation during the procedural learning task in the same regions, suggests an important differentiation of EI effects on brain systems. One possibility is that these task-dependent differences in brain activation may be due to differences in access to compensatory capacities as related to each task. For example, the widely distributed pattern of enhanced activation observed in the EI group during the working memory task may reflect voluntary compensatory attentional or executive processes that are elicited during the working memory task. In contrast, because learning simple motor sequences involves procedural learning that is more implicit and automatic, enhanced utilization of voluntary executive processes as was seen with the working memory task may be less useful and therefore less used. Therefore, the finding of reduced activation during the procedural learning task in the EI group may reflect inefficiencies in the more automatic processes associated with procedural learning. Importantly, the findings of task-dependent differences in activation also argue against a generalized failure of neuronal activation or in neurovascular coupling in EI as an explanation for the current findings.
As of yet, there is no established neuropathological model to explain how peripheral nervous system electrical exposure could cause neuropsychological and neurophysiological alterations in central nervous system function. The current findings suggest that more translational work in this area is needed to address this issue. Even in the absence of evidence for direct passage of electricity through the brain during the EI, or notable structural abnormalities on MRI, the present findings document alterations in brain functioning after electrical trauma. Considering that demyelinization has been described in peripheral nervous system axons following EI (Lee, 1997), one possible explanation for these findings could be that transmission of electricity to the brain occurs via the spinal cord. Indeed, electric current has been shown to preferentially travel along myelinated axons (Sances et al., 1981), and it is possible that electric current that reaches the spinal cord can directly affect brain systems. Studies that examined the neurobiological mechanisms underlying the analgesic effect of spinal cord stimulation in chronic pain patients have demonstrated the presence of supraspinal processes, including downregulation of glutamatergically-mediated intracortical excitability (Schlaier et al., 2007). Therefore, electric current may produce a complex set of interactions between spinal and supraspinal systems that may contribute to enduring cognitive dysfunction following EI. Future imaging studies that specifically examine the integrity of white matter tracts could be informative in addressing the question of possible involvement of myelinated axons in EI.
There are some potential limitations to the present study that merit comment. First, in a clinical study of this nature with no pre-EI assessment, it is impossible to fully rule out the possibility that some pre-existing factors might be the cause of the observed dysfunctions in the EI patients. However, because our study groups were matched on key demographic variables including age and IQ, reasons to suspect such confounding factors are not strong. Perhaps more importantly, the clinical consequences of EI in terms of psychiatric adjustment and pain were significant, and required treatment with CNS-active medications, including primarily antidepressants and pain medications. These medications can impact brain functioning, and as such may contribute to our findings. However, the nature of our findings, including the simple cognitive tasks and especially the dissociation between findings with working memory and procedural learning tasks (i.e., increased activation on the working memory task versus decreased activation on the procedural learning task), argue against some general artifact along the lines of a sedating or stimulating effect of these medications on brain activity or blood flow as an explanation for our findings. Moreover, we observed no differences in task performance between the subject groups that suggests differences in sedation or engagement with the tasks. The high rate of psychiatric morbidity in our EI sample represents another potential confounding factor. While this alternative explanation cannot be ruled out with complete certainty, the neuropsychological effects of mild to moderate depression in young adults are generally modest (Grant et al, 2001), and studies examining brain activation during working memory tasks in depressed patients suggest task-specific functional abnormalities that are mostly confined to the prefrontal cortex (Harvey et al., 2005) rather than the more widespread abnormalities noted here. Similarly, a review of the few published studies examining brain functioning during working memory tasks in PTSD patients reveals a pattern of reduced activity in the frontal and parietal cortices in PTSD subjects (Weber et al., 2005), whereas our findings indicated increased activation in brain regions involved in working memory. Finally, our findings may not generalize to all EI patients, given that we examined a convenient sample of treatment-seeking EI patients, potentially skewing our results toward the clinical range. Additionally, our conservative inclusion and exclusion criteria could limit the generalizability of the current findings.
Notably, while we did not find significant between-group differences on standard neuropsychological measures of working memory, our fMRI data indicate functional abnormalities in the neural substrates of spatial working memory following EI. One explanation of the observed discrepancy could be that our sample was not large enough to power a robust statistical analysis of the neuropsychological data. Additionally, fMRI may be more sensitive to the alterations in brain function that can result from EI than standard neuropsychological testing alone. Such a differential ability to detect neurobehavioral deficits would suggest that fMRI and neuropsychological approaches may have different clinical utilities. Given the literature showing increased rates of cognitive complaints among EI patients (Pliskin et al., 1998), our findings emphasize the need for further exploration of different assessment approaches for evaluating cognitive functioning following EI and perhaps other forms of brain trauma.
In summary, this is the first functional imaging study to document altered CNS function in individuals who have experienced electrical trauma. Our findings provide the first demonstration that EI is associated with changes in brain function at a network level in neural substrates involved in cognition. These findings come from a sample of EI subjects that sustained peripheral injuries, and thus who have no known direct passage of electricity through the brain. The significant between-group effects in patterns of activation during the working memory and procedural learning tasks suggest a neurologic basis for the previously reported neuropsychological deficits in EI, and are consistent with patient reports of post-injury cognitive difficulties (Pliskin et al., 1998). Future studies are needed to learn about the neural mechanisms of this effect, and indicate a need to evaluate neurocognitive and brain function in patients who have experienced electrical trauma.
Author Disclosure Statement
No conflicting financial interests exist.
References
- Beck A. Steer R. Brown G. Beck Depression Inventory–II. The Psychological Corporation; San Antonio, TX: 1987. [Google Scholar]
- Conner C.K. Manual for the Conner's Continuous Performance Test–II. Multi health Systems; Tanawanda, NY: 2002. [Google Scholar]
- Corbetta M. Frontoparietal cortical networks for directing attention and the eye to visual locations: identical, independent, or overlapping neural systems? Proc. Natl. Acad. Sci. U.S.A. 1998;95:831–838. doi: 10.1073/pnas.95.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R.W. AFNI: Software for analysis and visualization of functional magnetic neuroimages. Comput. Biomed. Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Delis D.C. Kramer J.H. Kaplan E. Ober B.A. California Verbal Learning Test, Second Adult Version Manual. The Psychological Corporation; San Antonio, TX: 2000. [Google Scholar]
- Deveci M. Buzkut M. Arslan N. Sengezer M. Nuclear imaging of the brain in electrical burn patients. Burns. 2002;28:591–594. doi: 10.1016/s0305-4179(02)00070-0. [DOI] [PubMed] [Google Scholar]
- Eddy W.F. Fitzgerald M. Noll D.C. Improved image registration by using Fourier interpolation. Magn. Reson. Med. 1996;36:923–931. doi: 10.1002/mrm.1910360615. [DOI] [PubMed] [Google Scholar]
- Golden C.J. Freshwater S.M. The Stroop Color and Word Test: A Manual for Clinical and Experimental Uses. Stoelting Co.; Chicago, IL: 2002. [Google Scholar]
- Grant M.M. Thase M.E. Sweeney J.A. Cognitive disturbance in outpatient depressed younger adults: evidence of modest impairment. Biol. Psychiatry. 2001;50:35–43. doi: 10.1016/s0006-3223(00)01072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronwall D. Sampson H. The Psychological Effects of Concussion. Auckland University Press; Auckland, New Zealand: 1974. [Google Scholar]
- Harvey P.O. Fossati P. Pochon J.B. Levy R. Lebastard G. Lehericy S. Allilaire J.F. Dubois B. Cognitive control and brain resources in major depression: an fMRI study using the n-back task. NeuroImage. 2005;26:860–869. doi: 10.1016/j.neuroimage.2005.02.048. [DOI] [PubMed] [Google Scholar]
- Horowitz M. Wilner B.A. Alvarez M.A. The Impact of Events Scale: Measure of subjective stress. Psychosom. Med. 1979;41:209–218. doi: 10.1097/00006842-197905000-00004. [DOI] [PubMed] [Google Scholar]
- Lee R.C. Injury by electrical forces: pathophysiology, manifestations, and therapy. Curr. Probl. Surg. 1997:684–764. doi: 10.1016/s0011-3840(97)80007-x. [DOI] [PubMed] [Google Scholar]
- Luna B. Minshew N.J. Garver K.E. Lazar N.A. Thulborn K.R. Eddy W.F. Sweeney J.A. Neocortical system abnormalities in autism: an fMRI study of spatial working memory. Neurology. 2002;59:834–840. doi: 10.1212/wnl.59.6.834. [DOI] [PubMed] [Google Scholar]
- McAllister T.W. Sparling M.B. Flashman L.A. Guerin S.J. Manourian A.C. Saykin A.J. Differential working memory load effects after MTBI. NeuroImage. 2001;14:1004–1012. doi: 10.1006/nimg.2001.0899. [DOI] [PubMed] [Google Scholar]
- Merriam E.P. Colby C.L. Thulborn K.R. Luna B. Olson C.R. Sweeney J.A. Stimulus-response incompatibility activates cortex proximate to three eye fields. NeuroImage. 2001;13:794–800. doi: 10.1006/nimg.2000.0742. [DOI] [PubMed] [Google Scholar]
- Nelson H.E. National Adult Reading Test (NART): Test Manual. NFER-Nelson; Windsor: 1982. [Google Scholar]
- Pliskin N.H. Ammar A.N. Fink J.W. Hill S.K. Malina A.C. Ramati A. Kelley K.M. Lee R.C. Neuropsychological changes following electrical injury. J. Int. Neuropsychol. Soc. 2006;12:17–23. doi: 10.1017/S1355617706060061. [DOI] [PubMed] [Google Scholar]
- Pliskin N.H. Capelli-Schellpfeffer M. Law R.T. Malina A.C. Kelley K.M. Lee R.C. Neuropsychological symptom presentation after electrical injury. J. Trauma. 1998;44:709–715. doi: 10.1097/00005373-199804000-00027. [DOI] [PubMed] [Google Scholar]
- Pliskin N.H. Fink J.W. Malina A. Moran S. Kelley K.M. Capelli-Schellpfeffer M. Lee R. The neuropsychological effects of electrical injury: new insights. Ann. N.Y. Acad. Sci. 1999;888:140–149. doi: 10.1111/j.1749-6632.1999.tb07952.x. [DOI] [PubMed] [Google Scholar]
- Reitan R. The validity of the Trail Making Test as an indicator of organic brain damage. Percept. Mot. Skills. 1958;8:271–271. [Google Scholar]
- Sahiner T. Kurt T. Sinan Bir L. Guzhanoglu O. Akalin O. Celiker A. Ozdemir F. Reversible hyperintense Ts MRI lesions of basal ganglia after an electrical injury. Burns. 2002;28:591–594. doi: 10.1016/s0305-4179(02)00054-2. [DOI] [PubMed] [Google Scholar]
- Sances A. Myklebust J.B. Larson S.J. Darin J.C. Swiontek T. Prieto T. Chilbert M. Cusick J.F. Experimental electrical injury studies. J. Trauma. 1981;21:589–597. doi: 10.1097/00005373-198108000-00001. [DOI] [PubMed] [Google Scholar]
- Scheibel R.S. Pearson D.A. Faria L.P. Kotrla K.J. Aylward E. Bechevalier J. Levin H.S. An fMRI study of executive functioning after diffuse TBI. Brain Inj. 2003;17:919–930. doi: 10.1080/0269905031000110472. [DOI] [PubMed] [Google Scholar]
- Schlaier J.R. Eichhammer P. Langguth B. Doenitz C. Binder H. Hajak G. Brawanski A. Effects of spinal cord stimulation on cortical excitability in patients with chronic neuropathic pain: a pilot study. Eur. J. Pain. 2007;11:863–868. doi: 10.1016/j.ejpain.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Simo L.S. Krisky C.M. Sweeney J.A. Functional neuroanatomy of anticipatory behavior: dissociation between sensory-driven and memory-drive systems. Cereb. Cortex. 2005;15:1982–1991. doi: 10.1093/cercor/bhi073. [DOI] [PubMed] [Google Scholar]
- Sweeney J.A. Mintun M.A. Kwee S. Wiseman M.B. Brown D.L. Rosenberg D.R. Carl J.R. Positron emission tomography study of saccadic eye movements and spatial working memory. J. Neurophysiol. 1996;75:454–468. doi: 10.1152/jn.1996.75.1.454. [DOI] [PubMed] [Google Scholar]
- Tombaugh T.N. Test of Memory Malingering, TOMM. MHS; New York/Toronto: 1996. [Google Scholar]
- Weber D.L. Clark R.C. McFarlane A.C. Moores K.A. Morris P. Egan G.F. Abnormal frontal and parietal activity during working memory updating in post-traumatic stress disorder. Psychiatry Res. 2005;140:27–44. doi: 10.1016/j.pscychresns.2005.07.003. [DOI] [PubMed] [Google Scholar]