Abstract
Circulating microRNAs (miRNAs) present in the serum/plasma are characteristically altered in many pathological conditions, and have been employed as diagnostic markers for specific diseases. We examined if plasma miRNA levels are altered in patients with traumatic brain injury (TBI) relative to matched healthy volunteers, and explored their potential for use as diagnostic TBI biomarkers. The plasma miRNA profiles from severe TBI patients (Glasgow Coma Scale [GCS] score ≤8) and age-, gender-, and race-matched healthy volunteers were compared by microarray analysis. Of the 108 miRNAs identified in healthy volunteer plasma, 52 were altered after severe TBI, including 33 with decreased and 19 with increased relative abundance. An additional 8 miRNAs were detected only in the TBI plasma. We used quantitative RT-PCR to determine if plasma miRNAs could identify TBI patients within the first 24 h post-injury. Receiver operating characteristic curve analysis indicated that miR-16, miR-92a, and miR-765 were good markers of severe TBI (0.89, 0.82, and 0.86 AUC values, respectively). Multiple logistic regression analysis revealed that combining these miRNAs markedly increased diagnostic accuracy (100% specificity and 100% sensitivity), compared to either healthy volunteers or orthopedic injury patients. In mild TBI patients (GCS score > 12), miR-765 levels were unchanged, while the plasma levels of miR-92a and miR-16 were significantly increased within the first 24 h of injury compared to healthy volunteers, and had AUC values of 0.78 and 0.82, respectively. Our results demonstrate that circulating miRNA levels are altered after TBI, providing a rich new source of potential molecular biomarkers. Plasma-derived miRNA biomarkers, used in combination with established clinical practices such as imaging, neurocognitive, and motor examinations, have the potential to improve TBI patient classification and possibly management.
Key words: biomarker, microRNA, mild traumatic brain injury, plasma, traumatic brain injury
Introduction
MicroRNAs (miRNAs) are a large class of endogenous, small, non-coding RNAs that regulate gene expression at the post-transcriptional level (Fire et al., 1998). MiRNAs are thought to be involved in regulating expression of more than 30% of messenger RNAs, and some miRNAs have been predicted to regulate up to several hundred target mRNAs (Lewis et al., 2005; Lim et al., 2005; Xie et al., 2005). The most recent Sanger miRBase release (version 15, April 2010), contains 940 described human miRNA sequences (Griffiths-Jones et al., 2006, 2008). However, new miRNA sequences continue to be identified at a rapid rate, suggesting that the final number of miRNAs contained within the human genome remains to be fully determined (Agarwal et al., 2010; Li et al., 2010). In addition to questions surrounding the total number of extant miRNAs, the precise function of the large majority of these miRNAs remains to be fully elucidated. MiRNAs are also garnering increased attention as potential biomarkers for the detection, identification, and classification of cancers and other disease states, at least in part due to the following properties: (1) like many protein coding mRNAs, miRNA expression is enriched in, or specific for, many different cell and tissue-types (Avnit-Sagi et al., 2009; Cordes and Srivastava, 2009; Rogelj and Giese, 2004), (2) miRNA expression levels are altered in disease-specific patterns (Bartels and Tsongalis, 2009; Lu et al., 2005), and (3) miRNAs are stable and detectable in serum, plasma, cerebrospinal fluid, and other bodily fluids (Cogswell et al., 2008; Gilad et al., 2008; Michael et al., 2010; Mitchell et al., 2008).
Recent miRNA profiling studies have detected a relatively large number of miRNAs in circulating plasma or serum, although the number of detected miRNAs can vary widely depending on the detection methodology employed (Chen et al., 2008; Mitchell et al., 2008; Tanaka et al., 2009; Taylor and Gercel-Taylor, 2008). Characteristic changes in the serum or plasma miRNA profiles of several cancers or other conditions have identified unique signatures that could be exploited in a clinical setting (Gilad et al., 2008; Mitchell et al., 2008; Taylor et al., 2008; Wang et al., 2009). For example, the ratio of miR-92a and miR-638 in plasma is a sensitive marker for the detection of acute leukemia (Tanaka et al., 2009). The plasma level of miR-92 is also increased in patients with colorectal cancer, and this increase can be used to detect the disease state with good diagnostic accuracy (area under the curve [AUC] = 0.885; Ng et al., 2009). Tissue-specific miRNAs released into the circulation after damage, such as that which occurs after myocardial infarction, stroke, or drug-induced injury, have also been demonstrated to be potentially useful as diagnostic biomarkers (Ai et al., 2010; Laterza et al., 2009; Wang et al., 2010, 2009).
Traumatic brain injury (TBI) is a major cause of death and disability among young adults in the United States. While altered sensory, motor, and cognitive function, and head computed tomography (CT) scans are routinely used to diagnose TBI and to assess injury severity, the availability of a blood-based diagnostic test would supplement clinical assessment and management of TBI patients. Although in numerous studies researchers have investigated the utility of using changes in serum proteins and the appearance of protein breakdown products as indicators of injury severity (Brophy et al., 2009; Dash et al., 2010a; Hergenroeder et al., 2008a, 2008b; Posmantur et al., 1998; Ringger et al., 2004), circulating miRNAs have not yet been examined to determine if they can be used as biomarkers of TBI. In the present study, we examined if the levels of miRNAs circulating in the plasma are altered following severe TBI, and if these changes could be used to assist in the diagnosis and/or classification of TBI.
Methods
Materials
TaqMan® MicroRNA assays were purchased from Applied Biosystems (Foster City, CA). A Total RNA Isolation kit was purchased from Norgen Biotek (Thorold, Ontario, Canada). Vacutainers for plasma isolation were from BD Biosciences (Franklin Lakes, NJ).
Patient recruitment, management, and sample collection
All protocols regarding the use of human subjects were reviewed and approved by the University of Texas Committee for the Protection of Human Subjects, and were in compliance with the Helsinki Declaration. Written consent was obtained from the patient or patient's next of kin, and the samples were coded to protect confidentiality. All patients presented to the emergency department at Memorial Hermann Hospital, and received standard care with the addition of study blood draws. This study used plasma samples from a total of 47 individuals. Non-trauma healthy volunteers (HV) were consented and enrolled by the University of Texas Clinical Research Unit at Memorial Hermann Hospital (Houston, Texas). Orthopedic injury patients had a radiographically-confirmed fracture, no head trauma, no other known inflammatory process or infection, no history of neurological or psychiatric disorders, and no alcohol or drug dependency. Orthopedic and mild TBI (mTBI) injury subjects were consented, and a one-time blood draw was taken within 10 h of the injury, and prior to any required medical procedures. mTBI subjects over 14 years old included those with a non-penetrating head trauma and Glasgow Coma Scale (GCS) score > 12 (as assessed in the emergency department), manifesting one or more of the following: loss of consciousness, post-traumatic amnesia, altered mental status, focal neurological deficits, or seizure. Manifestations of mTBI were not related to drugs, alcohol, treatments, other injuries, other psychological trauma, or language barrier (Carroll et al., 2004). Severe TBI patients (14–65 years old) admitted to the adult neurotrauma intensive care unit of Memorial Hermann Hospital who had GCS scores ≤8 were recruited, and consented (through their next-of-kin) as previously described (Hergenroeder et al., 2008a). An initial blood draw was obtained at the earliest possible time after admission and patient stabilization, and informed consent was obtained. Patient management was as previously described (Hergenroeder et al., 2010). The blood samples collected were obtained via venipuncture in the mTBI and orthopedic injury patients, and via arterial line in the severe TBI patients. The blood samples were centrifuged for 10 min at 1500g and 4°C, the supernatant was removed to clean tubes, and the serum was further clarified by centrifugation for 10 min at 10,000g and 4°C. The resulting plasma fraction was divided into aliquots and frozen at −80°C until needed.
RNA preparation
For microarray analysis, 20 μL of plasma from each of 10 HV or severe TBI patients (68 ± 8 h post-injury, mean ± SEM) was pooled, and total RNA was isolated using the Total RNA Isolation kit from Norgen Biotek. For qRT-PCR analysis, 100 μL of plasma from individual patients was independently processed for RNA. Time course data (Fig. 1) consisted of 8 HV and 18 severe TBI patients whose samples were collected between 10 and 64 h post-injury. Briefly, 3.5 volumes of lysis solution containing 125 mM β-mercaptoethanol were added to the plasma aliquots and vortexed vigorously. Ethanol was added to 45% (v/v) final, mixed, and the solution was applied to a spin column by centrifugation for 1 min at 14,000g and room temperature. The flow through was discarded, and the columns washed three times with 400 μL of the provided wash solution. The spin columns were centrifuged for 2 min at 14,000g at room temperature to dry, and transferred to clean elution tubes. The purified RNA was eluted with two applications of 50 μL of the provided elution buffer; each round of elution was centrifuged for 2 min at 200g, followed by 1 min at 14,000g at room temperature, and then combined. The pooled samples used for microarray analysis were dried to 30 μL, while the samples for qRT-PCR analysis were kept so that 1 μL of eluted sample was equal to 1 μL of input plasma.
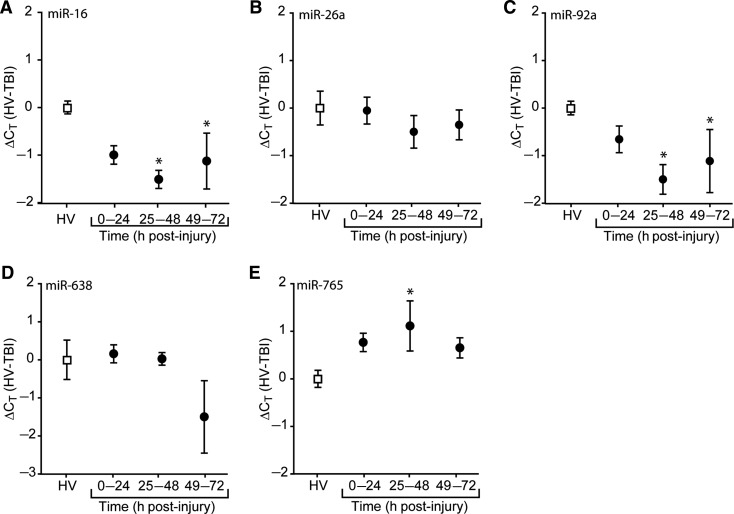
FIG. 1.
Temporal changes in plasma miRNA content after traumatic brain injury. Purified RNA from plasma collected at various time points after injury were assayed using miRNA-specific TaqMan probes directed against (A) miR-16, (B) miR-26a, (C) miR-92a, (D) miR-638, or (E) miR-765. Changes in threshold cycle are relative to the mean of the healthy volunteer (HV) values. Data are presented as the mean ± standard error of the mean of each group (*p < 0.05 by one-way ANOVA, or ANOVA on ranks; ANOVA, analysis of variance; miRNA, microRNA; TBI, traumatic brain injury).
MiRNA microarray
To screen for differences in the plasma abundance of miRNAs between healthy volunteer and severe TBI patients, we utilized a μParaflo™ microarray platform (LC Sciences, Houston, TX), and equal volumes of purified plasma RNAs, essentially as described previously (Redell et al., 2009). The microarrays contained 875 unique mature human miRNA detection probes (repeated four times within each array), complementary to the target miRNAs (Sanger miRBase Release, version 13). The hybridization melting temperatures of the probes were balanced by chemical modification of the detection probes. Hybridization was performed at 34°C in 100 μL 6 × SSPE buffer (900 mM NaCl, 60 mM Na2HPO4, and 6 mM EDTA, pH 6.8) containing 25% formamide. After washing, the hybridized fluorescence signals were detected using a GenePix 4000B scanner (Molecular Devices, Sunnyvale, CA). Data were analyzed by subtracting the background followed by signal normalization using a LOWESS (locally-weighted regression) filter (Bolstad et al., 2003). To be classified as reliably detectable, an miRNA signal had to exhibit an overall signal intensity > 3 × the background standard deviation, a spot coefficient of variation < 0.5, and all of the repeated probe signal intensities were above detection level.
qRT-PCR
The relative plasma abundance of target miRNAs was measured using TaqMan MicroRNA Assays (Applied Biosystems), which employ a looped-primer RT-PCR amplification and fluorescent probe hydrolysis quantification strategy. For each target, the equivalent of 3 μL of plasma RNA for each individual was reverse transcribed in a 10-μL reaction containing: 1 × reverse transcription buffer, 1 × miRNA-specific RT primers, 33.5 units Multiscribe reverse transcriptase, 2.6 U RNase inhibitor, and 1 mM dNTPs. The reactions were carried out for 10 min at 4°C, 30 min at 16°C, 30 min at 42°C, and heat inactivated for 5 min at 85°C. Quantification was performed in a 20-μL reaction containing: 10 μL 2× TaqMan Universal PCR Master Mix, No AmpErase® UNG, 1 μL 20× miRNA-specific primers, 1.33 μL cDNA reaction, and 7.67 μL H2O. Amplification reactions were performed in an iCycler (BioRad, Hercules, CA) programmed for one cycle of 10 min at 95°C, followed by 50 cycles of 15 sec at 95°C and 1 min at 60°C. The resulting data were analyzed using the iCycler iQ optical system software version 3.1 (BioRad). Changes in threshold cycle (CT) were calculated using the formula: ΔCT = mean (HV CT) – (Xn) CT, where HV CT represents the mean CT value calculated using all healthy volunteers, and Xn represents an individual's threshold cycle.
Multiple logistic regression analysis
Changes in threshold cycle (ΔCT) data were used for multiple logistic regression followed by receiver operating characteristic (ROC) curve analysis. ΔCT for control and experimental samples for each miRNA were calculated and combinations were fit to logistic regression curves using SigmaPlot 11.0 (Systat Software, Inc., San Jose, CA). The logit function of the resulting logistic curve for each individual was solved and the values were used to generate an ROC curve to evaluate the diagnostic accuracy of each potential combination.
Statistical analysis
The differences in miRNA abundance between HV and severe TBI samples (background adjusted, log2 transformed, balanced fluorescence values) as detected by microarray analysis were calculated, and changes between the signal intensities were evaluated using Student's t-test. p Values ≤ 0.01 were considered to be significantly different. Data obtained from qRT-PCR experiments were compared using one-way analysis of variance (ANOVA) for time course experiments, or Student's t-test for pair-wise comparisons between HV, severe TBI, mild TBI, or orthopedic injury groups. Results were considered significant at p < 0.05. A Holm-Sidak post-hoc test with a correction for multiple comparisons was used to determine differences between the experimental groups. For data that did not pass the Kolmogorov-Smirnov normality test, a Kruskal-Wallis one-way ANOVA on ranks was used to assess overall significance, and Dunn's method for multiple comparisons was used for post-hoc pair-wise comparisons.
Results
Temporal changes in plasma miRNA levels following severe TBI
In order to identify potential candidate miRNAs whose levels are altered in response to TBI, an miRNA microarray analysis was performed. We screened plasma RNAs from severe TBI patients, as these were expected to give rise to maximal changes in circulating miRNA levels compared to age-, race-, and gender-matched healthy volunteers (HV). Pooled plasma samples were generated from healthy volunteers (n = 10) or severe TBI patients (n = 10), corresponding to 68 ± 8 h post-injury, and total RNA was extracted. This relatively late time point was chosen for the initial screen because previous studies have shown that the levels of several putative biomarkers can be detected in the serum/plasma of TBI patients by 3 days post-injury (Hergenroeder et al., 2008a; Pelinka et al., 2004; Siman et al., 2009). Of the 875 miRNAs represented on the microarray, detectable signals were recorded for 108 miRNAs in HV plasma, with an additional 8 unique miRNAs detected in severe TBI plasma. Of these, 27 were found to have levels that were significantly elevated in the samples from severe TBI patients relative to the levels detected in HV controls (Supplementary Table 1; see online supplementary material at http://www.liebertonline.com). An additional 33 miRNAs were found to be significantly reduced in the injured patient plasma (Supplementary Table 2; see online supplementary material at http://www.liebertonline.com).
Five candidate miRNAs (miR-16, miR-26a, miR-92a, miR-638, and miR-765) were chosen for further analysis based on the change in expression detected by microarray, known expression patterns, and potential roles in regulating biological processes and functions involved in TBI pathophysiology. The relative levels of the candidate mRNAs in individual samples were determined using qRT-PCR. The cycle threshold (CT) for each target was determined as described in the methods section. Data are presented as the change in cycle threshold (ΔCT) for each individual patient subtracted from the mean cycle threshold detected in healthy volunteers. Figure 1 shows the temporal pattern of candidate miRNA abundance following injury compared to the levels detected in HV. The data show that the levels of miR-16 (Fig. 1A, Kruskal-Wallis one-way ANOVA on ranks, H = 11.45, p = 0.010), miR-92a (Fig. 1C, Kruskal-Wallis one-way ANOVA on ranks, H = 11.46, p = 0.010), and miR-765 (Fig. 1E, one-way ANOVA, F(3,22) = 3.49, p = 0.033), were significantly altered in the plasma of persons with severe TBI compared to HV. The levels of miR-26a (Fig. 1B, one-way ANOVA, F(3,22) = 0.46, p = 0.716) and miR-638 (Fig. 1D, Kruskal-Wallis one-way ANOVA on ranks, H = 2.07, p = 0.558) were not significantly altered at the time points examined.
ROC analysis of candidate miRNAs in severe TBI
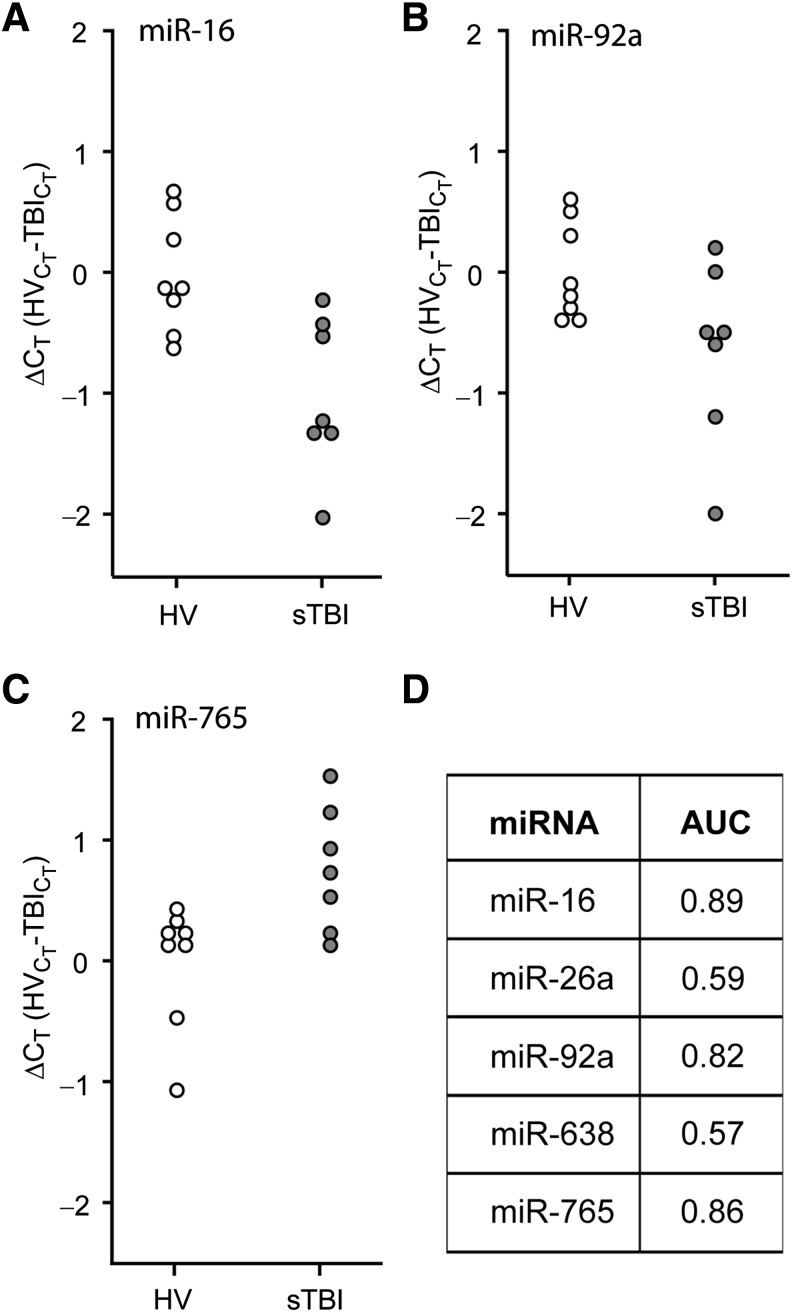
Receiver operating characteristic (ROC) curves were generated using the ΔCT values recorded during the first 24 h of injury (n = 7), and compared to HV (n = 8). This early time point after injury was chosen to determine if the candidate miRNAs could be used to assist in the early diagnosis and stratification of TBI patients. Figure 2A–C are dot histograms for the target miRNAs that showed significantly altered plasma levels in Figure 1, and indicate the distribution of the calculated ΔCT values for each individual relative to the mean CT value of the healthy volunteers. Figure 2D shows that when the ΔCT values were used for ROC analysis, miR-16, miR-92a, and miR-765, were found to be good markers of severe TBI (AUC values of 0.89, 0.82, and 0.86, respectively), while miR-26a and miR-638 were poor markers. To assess if a combination of changes in plasma miRNA levels could be used to increase the diagnostic accuracy for identifying subjects with severe TBI, the ΔCT data were re-analyzed using multiple logistic regression as described in the methods section. While the combinations of miR-765 with either miR-16 or miR-92a were found to have excellent accuracy (Supplemental Table 3; see online supplementary material at http://www.liebertonline.com), 100% sensitivity and 100% specificity (AUC = 1.00) could be achieved in identifying severe TBI patients by combining all three miRNAs.
FIG. 2.
Plasma miRNA levels are altered in severe TBI patients. RNA was purified from plasma samples collected within the first 24 h after injury, and assayed by TaqMan qRT-PCR. Dot histogram plots of the ΔCT values for (A) miR-16, (B) miR-92a, (C) miR-765, and (D) area under the curve (AUC) values obtained from receiver operating characteristic (ROC) analysis for each miRNA are shown (healthy volunteer [HV] n = 8; severe TBI [sTBI] n = 7; miRNA, microRNA; qRT-PCR, quantitative reverse transcriptase polymerase chain reaction; TBI, traumatic brain injury).
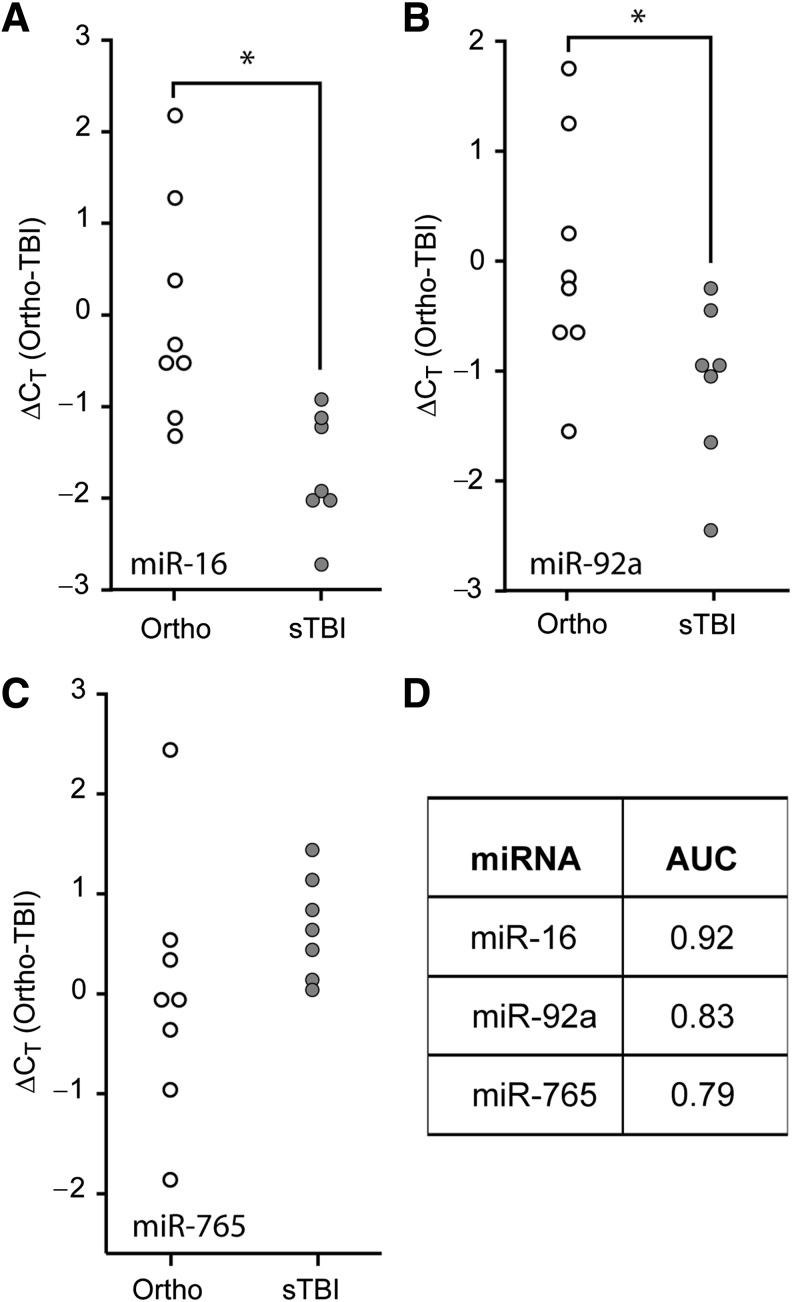
TBIs often present with additional bodily injuries, a condition referred to as polytrauma. As the levels of circulating biomarkers in polytrauma patients may respond to either the central or peripheral injuries, we asked if the diagnostic utility of the candidate miRNAs would be maintained when compared to patients with orthopedic injuries. Similarly to the previous results, miR-16 (Fig. 3A, Student's t-test: p = 0.005) and miR-92a (Fig. 3B, Student's t-test: p = 0.039) were significantly decreased in the first 24 h post-injury in the plasma of severe TBI patients, compared to the levels detected in orthopedic injury patients. However, miR-765 was not significantly altered in the plasma of severe TBI versus orthopedic injury patients (Fig. 3C, Student's t-test: p = 0.211). ROC curve analysis of miR-92a and miR-16 ΔCT data indicated that they remained good indicators of severe TBI when compared to persons having injuries outside the CNS (Fig. 3D). When these data were subjected to multiple logistic regression analysis as described above, the resulting ROC curve had an AUC of 1.00, indicating 100% specificity and 100% sensitivity for the biomarker combination of miR-92a, miR-765, and miR-16, to correctly distinguish severe TBI patients from orthopedic injury patients. No significant differences were observed when the values obtained from the orthopedic injury patients were compared to those detected in healthy volunteers (data not shown).
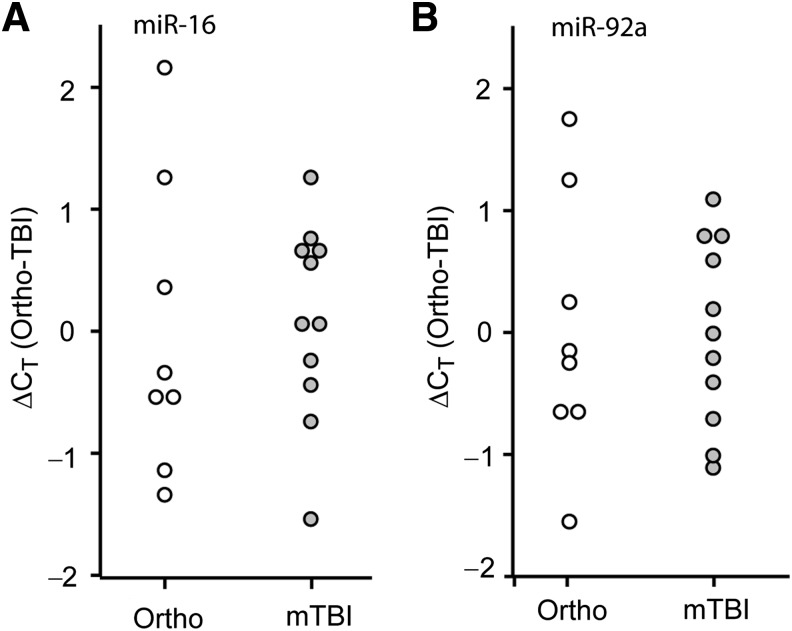
FIG. 3.
Comparison of relative plasma miRNA abundance in severe TBI versus orthopedic injury patients. Purified plasma RNA was assayed for (A) miR-16, (B) miR-92a, or (C) miR-765 relative abundance by TaqMan qRT-PCR assay. Dot histogram plots of the ΔCT values are shown using the mean orthopedic CT as the reference. (D) Area under the curve (AUC) values obtained from ROC analysis for each miRNA, indicating the diagnostic potential of each miRNA target (*p < 0.05 by two-tailed Student's t-test; orthopedic injury [Ortho], n = 8; severe TBI [sTBI], n = 7; miRNA, microRNA; qRT-PCR, quantitative reverse transcriptase polymerase chain reaction; TBI, traumatic brain injury; ROC, receiver operating characteristic).
Determination of diagnostic potential for candidate miRNAs for mild TBI
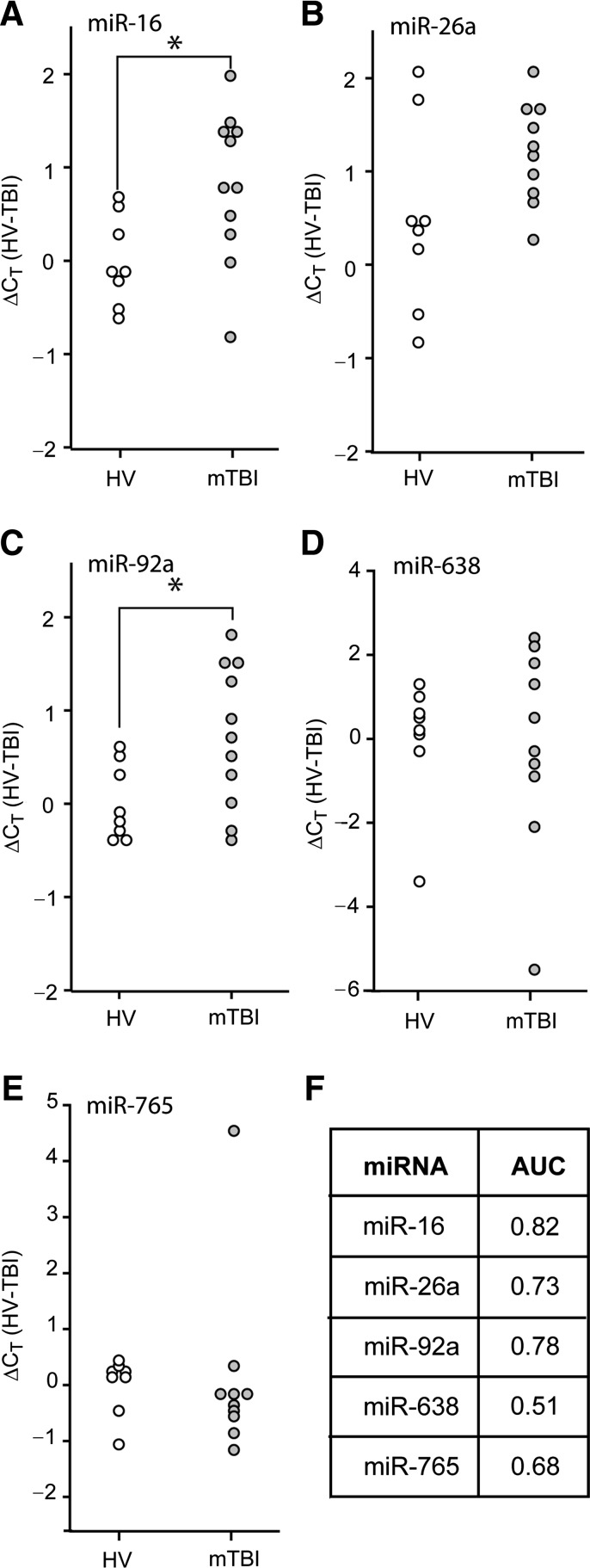
Since the results using RNA purified from plasma collected from severe TBI patients indicated that some miRNAs have good diagnostic potential, we questioned if these candidate miRNAs could be used to diagnose mild TBI (mTBI). Plasma samples from 11 mild TBI patients (GCS score > 12, head CT negative) were collected within 10 h of the reported time of injury, and total RNA was extracted. qRT-PCR was carried out to assess the relative abundance of miR-16, miR-26, miR-92a, miR-638, and miR-765 in these samples relative to those detected in plasma samples from healthy volunteers. Figure 4 shows the calculated ΔCT values (relative to the mean CT value of the healthy volunteers) for each of the mTBI patients and healthy volunteers. Unlike the decreased levels observed for severe TBI patients, the levels of miR-16 and miR-92a were found to be significantly elevated in mTBI patients (Fig. 4A and 4C; Student's t-test: p = 0.020 for miR-16, p = 0.029 for miR-92a). However, these changes only had fair diagnostic accuracy in identifying mTBI patients (AUC values of 0.82 and 0.78, respectively; Fig. 4F). Combining these miRNAs did not offer any additional diagnostic accuracy (AUC = 0.81). There was not a significant difference in ΔCT values between mTBI and orthopedic injury plasma samples (Fig. 5A and 5B). Poor diagnostic accuracy was found for these miRNAs, both individually (AUC for miR-16 = 0.59; AUC for miR-92a = 0.58), and in combination, in differentiating between mTBI and orthopedic injury patients (Supplemental Table 3; see online supplementary material at http://www.liebertonline.com).
FIG. 4.
Diagnostic value of plasma miRNAs for identifying mild TBI patients. RNA was purified from plasma samples collected within the first 10 h after injury, and miRNA relative abundance was determined by qRT-PCR. Dot histogram plots of the ΔCT values for (A) miR-16, (B) miR-26a, (C) miR-92a, (D) miR-638, or (E) miR-765. (F) Area under the curve (AUC) values obtained from ROC analysis for each miRNA (*p < 0.05 by two-tailed Student's t-test; healthy volunteer [HV], n = 8; mild TBI [mTBI], n = 11; miRNA, microRNA; qRT-PCR, quantitative reverse transcriptase polymerase chain reaction; TBI, traumatic brain injury; ROC, receiver operating characteristic).
FIG. 5.
Comparison of relative plasma miRNA levels in mild TBI versus orthopedic injury patients. TaqMan assays for (A) miR-16 or (B) miR-92a were performed using purified RNA from plasma collected within the first 10 h after trauma. Dot histogram plots of the ΔCT values are shown using the mean orthopedic CT as the reference (orthopedic injury [Ortho], n = 8; mild TBI [mTBI], n = 11; TBI, traumatic brain injury; miRNA, microRNA).
Discussion
Recent studies have demonstrated that circulating miRNAs have the potential to be used as diagnostic biomarkers for several different types of cancer, neurodegenerative diseases, and tissue injury (Ai et al., 2010; Cogswell et al., 2008; Cortez and Calin, 2009; De Smaele et al., 2010; Laterza et al., 2009; Wang et al., 2009, 2010). In the present study, we investigated if changes in the levels of plasma miRNAs can be used to assist in determining the severity of traumatic brain injury (TBI). The key findings from this study are: (1) the plasma level of miR-765 is significantly elevated, while miR-16 and miR-92a levels are significantly decreased, as determined by qRT-PCR analysis of plasma of patients with severe TBI compared to healthy volunteers; (2) altered miR-16, miR-92a, and miR-765 plasma levels were also observed when severe TBI patients were compared to orthopedic injury patients; (3) a biomarker signature, consisting of miR-16, miR-92a, and miR-765, had 100% sensitivity and specificity for discriminating severe TBI from HV; (4) in contrast to that seen in severe TBI, the plasma levels of miR-16 and miR-92a were significantly elevated in mild TBI patients; and (5) the plasma levels of miR-16 and miR-92a can be used to discriminate mild TBI patients from HV with a diagnostic accuracy of 0.81.
Previous studies using a variety of detection methods have indicated that from tens to hundreds of distinct, mature miRNA species can be detected in cell-free preparations of circulating fluids, where they are remarkably stable (Chen et al., 2008; Gilad et al., 2008; Lodes et al., 2009; Mitchell et al., 2008; Tanaka et al., 2009; Taylor and Gercel-Taylor, 2008). Consistent with these reports, we detected 108 miRNAs in our healthy volunteer sample, out of the 875 unique mature miRNA target sequences represented on the microarray. For our screening approach, plasma samples from 10 severe TBI patients were pooled in order to account for potential patient-to-patient variability. From this initial screen, five candidate miRNAs (miR-16, miR-26a, miR-92a, miR-638, and miR-765) were chosen for further analysis based on parameters such as microarray fold change, signal intensity in healthy volunteer samples, and potential involvement in TBI pathophysiological processes. For example, transcripts regulated by miR-16 are involved in regulating several biological processes activated after TBI, including cell proliferation, cell cycle progression, and apoptosis (Cimmino et al., 2005; Kaddar et al., 2009; Linsley et al., 2007). Likewise, miR-26a was recently found to be a robust direct regulator of phosphatase and tensin homolog (PTEN) expression (Huse et al., 2009), which is intimately involved in cell survival after injury (Chang et al., 2007; Zhang et al., 2007). Angiogenic processes are also impacted by TBI, and miR-92a has been identified as a negative regulator of angiogenesis (Bonauer et al., 2009; Doebele et al., 2010). Quantification of the candidate miRNAs in RNA purified from individual patient plasma samples revealed wide variations in their plasma levels in both healthy volunteers and TBI patient groups (Fig. 2). Similar patient-to-patient variations have been previously observed in other studies evaluating circulating miRNA levels (Tanaka et al., 2009). Although the sources of the variability observed among healthy volunteers is not presently known, possible contributing factors include the methods employed for initial sample preparation and storage, RNA extraction, and detection/quantification. Since comprehensive analysis of circulating mRNA/miRNA content is still relatively new, some technical aspects regarding sample stability and experimental reliability remain to be adequately resolved. In addition, future large-scale studies will be required to establish normal parameters for the number, identity, and abundance of circulating miRNAs in healthy versus diseased individuals, and to identify an appropriate slate of stably expressed internal references. Standardization of sample processing and the establishment of appropriate internal references will enable more accurate sample-to-sample normalization, and will help minimize intragroup variations. Some investigators have normalized their data using miRNAs or snoRNAs found to have minimal variability across their experimental samples or groups, or have used non-parametric statistical analysis methods to compare highly variable data sets. For example, Tanaka and colleagues rank ordered the miRNAs detected in plasma, and also used the expression of miR-638 as an internal reference, to compare patients with acute leukemia to healthy volunteers (Tanaka et al., 2009). Although our qRT-PCR analysis revealed that the levels of miR-638 (and miR-26a) were not significantly altered after TBI, it still exhibited a high degree of variability that made it unsuitable as a normalization control for our studies. Evaluation of the levels of additional circulating RNA species will be required to identify appropriate internal control candidates that can be utilized for patient-to-patient normalization in a clinical setting.
Using ΔCT as a measure of relative change, we found that miR-16 and miR-92a were significantly decreased, while miR-765 was significantly increased, in the plasma of severe TBI patients compared to healthy volunteers. These markers were found to have good diagnostic value when used individually, and excellent value when used in combination. Our analysis also revealed that both miR-16 and miR-92a have fair diagnostic value in identifying mild TBI. Interestingly, the plasma levels of these miRNAs were found to be elevated in the blood of persons with mild TBI, a finding in contrast to that observed in severe TBI patients, in whom plasma miR-16 and miR-92a levels were found to be decreased. While the reason for this differential response is not yet clear, it illustrates the limitations of utilizing plasma miRNA biomarkers identified in severe TBI patients to diagnose mild TBI. This is likely due to the involvement of multiple pathological processes in severe TBI that may not be present in mild TBI (Saatman et al., 2008). However, we cannot rule out the possibility that changes in plasma miRNAs after mild TBI may develop at later time points to more closely mimic those observed after severe TBI.
The precise mechanisms governing the observed alterations in plasma miRNA levels seen after TBI are not currently known. The increase in miR-765 plasma abundance could be the result of direct release from injured tissues/cells, as has been previously demonstrated (Laterza et al., 2009), although the tissue-specific expression pattern for miR-765 needs to be confirmed. Alternatively, animal studies have shown that an isolated injury to the brain can cause cellular and molecular changes within other organs as a result of sympathetic, hormonal, and/or neuroimmune responses (Kalsotra et al., 2007). In addition to possible peripheral responses to brain injury, severe TBI patients often have trauma to other organs. Although we cannot rule out the contribution of other bodily injuries to the changes we observed, our analysis showed that the candidate miRNAs remained capable of identifying severe TBI patients when patients suffering orthopedic injuries were used as the control group. The identification of brain-specific miRNAs released after injury would further assist in the diagnosis of brain injury in the context of polytrauma.
There is accumulating experimental evidence indicating that circulating miRNAs are contained within lipoprotein microparticles that may help protect them from degradation. These microparticles, including exosomes, microvesicles, and apoptotic bodies, are present in the circulation under both normal and pathological conditions (Simpson et al., 2009). Exosomes are produced by most cell types as well as numerous cancers, and contain a complex mixture of components, including mRNAs, miRNAs, and proteins, but not DNA (Mathivanan and Simpson, 2009). Analysis of exosome contents indicates that their composition is distinct from the generating cell type, suggesting that they may selectively accumulate certain mRNA, miRNA, and protein species (Al-Nedawi et al., 2009; Skog et al., 2008; Valadi et al., 2007). Recent studies have shown that secreted exosomes are important mediators of intercellular communication that function by transferring their contents into recipient cells, where they are biologically active and can modify gene expression, protein translation, and signaling (Al-Nedawi et al., 2008; Kosaka et al., 2010; Skog et al., 2008; Valadi et al., 2007; Yuan et al., 2009; Zernecke et al., 2009). Although the mechanisms of exosome cellular uptake remain poorly defined, Kosaka and colleagues recently showed that exosomes can be secreted using a ceramide-dependent but ESCRT-independent mechanism (Kosaka et al., 2010). It is tempting to speculate that the observed decrease in plasma miR-16 and miR-92a levels after severe TBI may result from increased uptake by recipient cells (Valadi et al., 2007; Zernecke et al., 2009), and that these internalized miRNAs may contribute to or modify the evolution of pathophysiological processes.
The identification of biomarkers of outcome, the occurrence of secondary injury, and injury severity, remains an active and relevant area of research (Dash et al., 2010b). The biomarkers identified herein would complement the current clinical tests used to classify TBI patients, and facilitate hospital resource allocation and patient management. While the use of miRNAs as biomarkers in cancer has been widely examined, this is the first report of alterations in circulating miRNAs after TBI. We detected significant changes in miR-16, miR-92a, and miR-765 plasma levels after TBI, demonstrating that circulating miRNAs are altered after TBI. As the number of patients included this study was small, subsequent studies utilizing larger patient populations are required to substantiate and extend our findings. In addition to the miRNAs validated in this report, the microarray data identified many other miRNA candidates that remain to be evaluated for their diagnostic potential. Further studies examining changes in circulating miRNAs, and their possible connection to TBI pathophysiology, are warranted. The results presented herein indicate that circulating miRNAs hold promise as molecular biomarkers of TBI.
Supplementary Material
Acknowledgments
This research was supported by grants from Mission Connect-Project of the TIRR Foundation (to P.K.D.), the Gillson Longenbaugh Foundation (to P.K.D.), and the American Heart Association (09BGIA2260018 to J.B.R.).
Author Disclosure Statement
No competing financial interests exist.
References
- Agarwal S. Vaz C. Bhattacharya A. Srinivasan A. Prediction of novel precursor miRNAs using a context-sensitive hidden Markov model (CSHMM) BMC Bioinformatics. 2010;11(Suppl. 1):S29. doi: 10.1186/1471-2105-11-S1-S29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ai J. Zhang R. Li Y. Pu J. Lu Y. Jiao J. Li K. Yu B. Li Z. Wang R. Wang L. Li Q. Wang N. Shan H. Li Z. Yang B. Circulating microRNA-1 as a potential novel biomarker for acute myocardial infarction. Biochem. Biophys. Res. Commun. 2010;391:73–77. doi: 10.1016/j.bbrc.2009.11.005. [DOI] [PubMed] [Google Scholar]
- Al-Nedawi K. Meehan B. Rak J. Microvesicles: messengers and mediators of tumor progression. Cell Cycle. 2009;8:2014–2018. doi: 10.4161/cc.8.13.8988. [DOI] [PubMed] [Google Scholar]
- Al-Nedawi K. Meehan B. Micallef J. Lhotak V. May L. Guha A. Rak J. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat. Cell Biol. 2008;10:619–624. doi: 10.1038/ncb1725. [DOI] [PubMed] [Google Scholar]
- Avnit-Sagi T. Kantorovich L. Kredo-Russo S. Hornstein E. Walker M.D. The promoter of the pri-miR-375 gene directs expression selectively to the endocrine pancreas. PLoS ONE. 2009;4:e5033. doi: 10.1371/journal.pone.0005033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels C.L. Tsongalis G.J. MicroRNAs: novel biomarkers for human cancer. Clin. Chem. 2009;55:623–631. doi: 10.1373/clinchem.2008.112805. [DOI] [PubMed] [Google Scholar]
- Bolstad B.M. Irizarry R.A. Astrand M. Speed T.P. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics. 2003;19:185–193. doi: 10.1093/bioinformatics/19.2.185. [DOI] [PubMed] [Google Scholar]
- Bonauer A. Carmona G. Iwasaki M. Mione M. Koyanagi M. Fischer A. Burchfield J. Fox H. Doebele C. Ohtani K. Chavakis E. Potente M. Tjwa M. Urbich C. Zeiher A.M. Dimmeler S. MicroRNA-92a controls angiogenesis and functional recovery of ischemic tissues in mice. Science. 2009;324:1710–1713. doi: 10.1126/science.1174381. [DOI] [PubMed] [Google Scholar]
- Brophy G.M. Pineda J.A. Papa L. Lewis S.B. Valadka A.B. Hannay H.J. Heaton S.C. Demery J.A. Liu M.C. Tepas J.J., III Gabrielli A. Robicsek S. Wang K.K. Robertson C.S. Hayes R.L. alphaII-Spectrin breakdown product cerebrospinal fluid exposure metrics suggest differences in cellular injury mechanisms after severe traumatic brain injury. J. Neurotrauma. 2009;26:471–479. doi: 10.1089/neu.2008.0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll L.J. Cassidy J.D. Holm L. Kraus J. Coronado V.G. Methodological issues and research recommendations for mild traumatic brain injury: the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J. Rehabil. Med. 2004;43(Suppl.):113–125. doi: 10.1080/16501960410023877. [DOI] [PubMed] [Google Scholar]
- Chang N. El-Hayek Y.H. Gomez E. Wan Q. Phosphatase PTEN in neuronal injury and brain disorders. Trends Neurosci. 2007;30:581–586. doi: 10.1016/j.tins.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Chen X. Ba Y. Ma L. Cai X. Yin Y. Wang K. Guo J. Zhang Y. Chen J. Guo X. Li Q. Li X. Wang W. Zhang Y. Wang J. Jiang X. Xiang Y. Xu C. Zheng P. Zhang J. Li R. Zhang H. Shang X. Gong T. Ning G. Wang J. Zen K. Zhang J. Zhang C.Y. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18:997–1006. doi: 10.1038/cr.2008.282. [DOI] [PubMed] [Google Scholar]
- Cimmino A. Calin G.A. Fabbri M. Iorio M.V. Ferracin M. Shimizu M. Wojcik S.E. Aqeilan R.I. Zupo S. Dono M. Rassenti L. Alder H. Volinia S. Liu C.G. Kipps T.J. Negrini M. Croce C.M. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc. Natl. Acad. Sci. USA. 2005;102:13944–13949. doi: 10.1073/pnas.0506654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogswell J.P. Ward J. Taylor I.A. Waters M. Shi Y. Cannon B. Kelnar K. Kemppainen J. Brown D. Chen C. Prinjha R.K. Richardson J.C. Saunders A.M. Roses A.D. Richards C.A. Identification of miRNA changes in Alzheimer's disease brain and CSF yields putative biomarkers and insights into disease pathways. J. Alzheimers Dis. 2008;14:27–41. doi: 10.3233/jad-2008-14103. [DOI] [PubMed] [Google Scholar]
- Cordes K.R. Srivastava D. MicroRNA regulation of cardiovascular development. Circ. Res. 2009;104:724–732. doi: 10.1161/CIRCRESAHA.108.192872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortez M.A. Calin G.A. MicroRNA identification in plasma and serum: a new tool to diagnose and monitor diseases. Expert. Opin. Biol. Ther. 2009;9:703–711. doi: 10.1517/14712590902932889. [DOI] [PubMed] [Google Scholar]
- Dash P.K. Redell J.B. Hergenroeder G. Zhao J. Clifton G.L. Moore A. Serum ceruloplasmin and copper are early biomarkers for traumatic brain injury-associated elevated intracranial pressure. J. Neurosci. Res. 2010a;88:1719–1726. doi: 10.1002/jnr.22336. [DOI] [PubMed] [Google Scholar]
- Dash P.K. Zhao J. Hergenroeder G. Moore A.N. Biomarkers for the diagnosis, prognosis, and evaluation of treatment efficacy for traumatic brain injury. Neurotherapeutics. 2010b;7:100–114. doi: 10.1016/j.nurt.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Smaele E. Ferretti E. Gulino A. MiRNAs as biomarkers for CNS cancer and other disorders. Brain Res. 2010;1338:100–111. doi: 10.1016/j.brainres.2010.03.103. [DOI] [PubMed] [Google Scholar]
- Doebele C. Bonauer A. Fischer A. Scholz A. Reiss Y. Urbich C. Hofmann W.K. Zeiher A.M. Dimmeler S. Members of the microRNA-17-92 cluster exhibit a cell-intrinsic antiangiogenic function in endothelial cells. Blood. 2010;115:4944–4950. doi: 10.1182/blood-2010-01-264812. [DOI] [PubMed] [Google Scholar]
- Fire A. Xu S. Montgomery M.K. Kostas S.A. Driver S.E. Mello C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- Gilad S. Meiri E. Yogev Y. Benjamin S. Lebanony D. Yerushalmi N. Benjamin H. Kushnir M. Cholakh H. Melamed N. Bentwich Z. Hod M. Goren Y. Chajut A. Serum microRNAs are promising novel biomarkers. PLoS ONE. 2008;3:e3148. doi: 10.1371/journal.pone.0003148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths-Jones S. Grocock R.J. Bateman A. Enright A.J. miRBase: microRNA sequences, targets and gene nomenclature. Nucleic Acids Res. 2006;34:D140–D144. doi: 10.1093/nar/gkj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths-Jones S. Saini H.K. Enright A.J. miRBase: tools for microRNA genomics. Nucleic Acids Res. 2008;36:D154–D158. doi: 10.1093/nar/gkm952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hergenroeder G. Redell J.B. Moore A.N. Dubinsky W.P. Funk R.T. Crommett J. Clifton G.L. Levine R. Valadka A. Dash P.K. Identification of serum biomarkers in brain-injured adults: potential for predicting elevated intracranial pressure. J. Neurotrauma. 2008a;25:79–93. doi: 10.1089/neu.2007.0386. [DOI] [PubMed] [Google Scholar]
- Hergenroeder G.W. Moore A.N. McCoy J.P., Jr. Samsel L. Ward N.H., III Clifton G.L. Dash P.K. Serum IL-6: a candidate biomarker for intracranial pressure elevation following isolated traumatic brain injury. J. Neuroinflammation. 2010;7:19. doi: 10.1186/1742-2094-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hergenroeder G.W. Redell J.B. Moore A.N. Dash P.K. Biomarkers in the clinical diagnosis and management of traumatic brain injury. Mol. Diagn. Ther. 2008b;12:345–358. doi: 10.1007/BF03256301. [DOI] [PubMed] [Google Scholar]
- Huse J.T. Brennan C. Hambardzumyan D. Wee B. Pena J. Rouhanifard S.H. Sohn-Lee S.C. Agami R. Tuschl T. Holland E.C. The PTEN-regulating microRNA miR-26a is amplified in high-grade glioma and facilitates gliomagenesis in vivo. Genes Dev. 2009;23:1327–1337. doi: 10.1101/gad.1777409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaddar T. Rouault J.P. Chien W.W. Chebel A. Gadoux M. Salles G. Ffrench M. Magaud J.P. Two new miR-16 targets: caprin-1 and HMGA1, proteins implicated in cell proliferation. Biol. Cell. 2009;101:511–524. doi: 10.1042/BC20080213. [DOI] [PubMed] [Google Scholar]
- Kalsotra A. Zhao J. Anakk S. Dash P.K. Strobel H.W. Brain trauma leads to enhanced lung inflammation and injury: evidence for role of P4504Fs in resolution. J. Cereb. Blood Flow Metab. 2007;27:963–974. doi: 10.1038/sj.jcbfm.9600396. [DOI] [PubMed] [Google Scholar]
- Kosaka N. Iguchi H. Yoshioka Y. Takeshita F. Matsuki Y. Ochiya T. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J. Biol. Chem. 2010;285:17442–17452. doi: 10.1074/jbc.M110.107821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laterza O.F. Lim L. Garrett-Engele P.W. Vlasakova K. Muniappa N. Tanaka W.K. Johnson J.M. Sina J.F. Fare T.L. Sistare F.D. Glaab W.E. Plasma MicroRNAs as sensitive and specific biomarkers of tissue injury. Clin. Chem. 2009;55:1977–1983. doi: 10.1373/clinchem.2009.131797. [DOI] [PubMed] [Google Scholar]
- Lewis B.P. Burge C.B. Bartel D.P. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- Li S.C. Chan W.C. Hu L.Y. Lai C.H. Hsu C.N. Lin W.C. Identification of homologous microRNAs in 56 animal genomes. Genomics. 2010;96:1–9. doi: 10.1016/j.ygeno.2010.03.009. [DOI] [PubMed] [Google Scholar]
- Lim L.P. Lau N.C. Garrett-Engele P. Grimson A. Schelter J.M. Castle J. Bartel D.P. Linsley P.S. Johnson J.M. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature. 2005;433:769–773. doi: 10.1038/nature03315. [DOI] [PubMed] [Google Scholar]
- Linsley P.S. Schelter J. Burchard J. Kibukawa M. Martin M.M. Bartz S.R. Johnson J.M. Cummins J.M. Raymond C.K. Dai H. Chau N. Cleary M. Jackson A.L. Carleton M. Lim L. Transcripts targeted by the microRNA-16 family cooperatively regulate cell cycle progression. Mol. Cell Biol. 2007;27:2240–2252. doi: 10.1128/MCB.02005-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodes M.J. Caraballo M. Suciu D. Munro S. Kumar A. Anderson B. Detection of cancer with serum miRNAs on an oligonucleotide microarray. PLoS ONE. 2009;4:e6229. doi: 10.1371/journal.pone.0006229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J. Getz G. Miska E.A. Varez-Saavedra E. Lamb J. Peck D. Sweet-Cordero A. Ebert B.L. Mak R.H. Ferrando A.A. Downing J.R. Jacks T. Horvitz H.R. Golub T.R. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- Mathivanan S. Simpson R.J. ExoCarta: A compendium of exosomal proteins and RNA. Proteomics. 2009;9:4997–5000. doi: 10.1002/pmic.200900351. [DOI] [PubMed] [Google Scholar]
- Michael A. Bajracharya S.D. Yuen P.S. Zhou H. Star R.A. Illei G.G. Alevizos I. Exosomes from human saliva as a source of microRNA biomarkers. Oral Dis. 2010;16:34–38. doi: 10.1111/j.1601-0825.2009.01604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P.S. Parkin R.K. Kroh E.M. Fritz B.R. Wyman S.K. Pogosova-Agadjanyan E.L. Peterson A. Noteboom J. O'Briant K.C. Allen A. Lin D.W. Urban N. Drescher C.W. Knudsen B.S. Stirewalt D.L. Gentleman R. Vessella R.L. Nelson P.S. Martin D.B. Tewari M. Circulating microRNAs as stable blood-based markers for cancer detection. Proc. Natl. Acad. Sci. USA. 2008;105:10513–10518. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng E.K. Chong W.W. Jin H. Lam E.K. Shin V.Y. Yu J. Poon T.C. Ng S.S. Sung J.J. Differential expression of microRNAs in plasma of patients with colorectal cancer: a potential marker for colorectal cancer screening. Gut. 2009;58:1375–1381. doi: 10.1136/gut.2008.167817. [DOI] [PubMed] [Google Scholar]
- Pelinka L.E. Kroepfl A. Leixnering M. Buchinger W. Raabe A. Redl H. GFAP versus S100B in serum after traumatic brain injury: relationship to brain damage and outcome. J. Neurotrauma. 2004;21:1553–1561. doi: 10.1089/neu.2004.21.1553. [DOI] [PubMed] [Google Scholar]
- Posmantur R.M. Zhao X. Kampfl A. Clifton G.L. Hayes R.L. Immunoblot analyses of the relative contributions of cysteine and aspartic proteases to neurofilament breakdown products following experimental brain injury in rats. Neurochem. Res. 1998;23:1265–1276. doi: 10.1023/a:1020792132629. [DOI] [PubMed] [Google Scholar]
- Redell J.B. Liu Y. Dash P.K. Traumatic brain injury alters expression of hippocampal microRNAs: Potential regulators of multiple pathophysiological processes. J. Neurosci. Res. 2009;87:1435–1448. doi: 10.1002/jnr.21945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringger N.C. O'Steen B.E. Brabham J.G. Silver X. Pineda J. Wang K.K. Hayes R.L. Papa L. A novel marker for traumatic brain injury: CSF alphaII-spectrin breakdown product levels. J. Neurotrauma. 2004;21:1443–1456. doi: 10.1089/neu.2004.21.1443. [DOI] [PubMed] [Google Scholar]
- Rogelj B. Giese K.P. Expression and function of brain specific small RNAs. Rev. Neurosci. 2004;15:185–198. doi: 10.1515/revneuro.2004.15.3.185. [DOI] [PubMed] [Google Scholar]
- Saatman K.E. Duhaime A.C. Bullock R. Maas A.I. Valadka A. Manley G.T. Classification of traumatic brain injury for targeted therapies. J. Neurotrauma. 2008;25:719–738. doi: 10.1089/neu.2008.0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siman R. Toraskar N. Dang A. McNeil E. McGarvey M. Plaum J. Maloney E. Grady M.S. A panel of neuron-enriched proteins as markers for traumatic brain injury in humans. J. Neurotrauma. 2009;26:1867–1877. doi: 10.1089/neu.2009.0882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson R.J. Lim J.W. Moritz R.L. Mathivanan S. Exosomes: proteomic insights and diagnostic potential. Expert. Rev. Proteomics. 2009;6:267–283. doi: 10.1586/epr.09.17. [DOI] [PubMed] [Google Scholar]
- Skog J. Wurdinger T. Meijer D.H. Gainche L. Sena-Esteves M. Curry W.T., Jr. Carter B.S. Krichevsky A.M. Breakefield X.O. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 2008;10:1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M. Oikawa K. Takanashi M. Kudo M. Ohyashiki J. Ohyashiki K. Kuroda M. Down-regulation of miR-92 in human plasma is a novel marker for acute leukemia patients. PLoS ONE. 2009;4:e5532. doi: 10.1371/journal.pone.0005532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D.D. Gercel-Taylor C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol. Oncol. 2008;110:13–21. doi: 10.1016/j.ygyno.2008.04.033. [DOI] [PubMed] [Google Scholar]
- Valadi H. Ekstrom K. Bossios A. Sjostrand M. Lee J.J. Lotvall J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- Wang G.K. Zhu J.Q. Zhang J.T. Li Q. Li Y. He J. Qin Y.W. Jing Q. Circulating microRNA: a novel potential biomarker for early diagnosis of acute myocardial infarction in humans. Eur. Heart J. 2010;31:659–666. doi: 10.1093/eurheartj/ehq013. [DOI] [PubMed] [Google Scholar]
- Wang K. Zhang S. Marzolf B. Troisch P. Brightman A. Hu Z. Hood L.E. Galas D.J. Circulating microRNAs, potential biomarkers for drug-induced liver injury. Proc. Natl. Acad. Sci. USA. 2009;106:4402–4407. doi: 10.1073/pnas.0813371106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X. Lu J. Kulbokas E.J. Golub T.R. Mootha V. Lindblad-Toh K. Lander E.S. Kellis M. Systematic discovery of regulatory motifs in human promoters and 3′ UTRs by comparison of several mammals. Nature. 2005;434:338–345. doi: 10.1038/nature03441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan A. Farber E.L. Rapoport A.L. Tejada D. Deniskin R. Akhmedov N.B. Farber D.B. Transfer of microRNAs by embryonic stem cell microvesicles. PLoS ONE. 2009;4:e4722. doi: 10.1371/journal.pone.0004722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zernecke A. Bidzhekov K. Noels H. Shagdarsuren E. Gan L. Denecke B. Hristov M. Koppel T. Jahantigh M.N. Lutgens E. Wang S. Olson E.N. Schober A. Weber C. Delivery of microRNA-126 by apoptotic bodies induces CXCL12-dependent vascular protection. Sci. Signal. 2009;2:ra81. doi: 10.1126/scisignal.2000610. [DOI] [PubMed] [Google Scholar]
- Zhang Q.G. Wu D.N. Han D. Zhang G.Y. Critical role of PTEN in the coupling between PI3K/Akt and JNK1/2 signaling in ischemic brain injury. FEBS Lett. 2007;581:495–505. doi: 10.1016/j.febslet.2006.12.055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.