Abstract
Hyperthermia is common following traumatic brain injury (TBI) and has been associated with poor neurologic outcome, and hypothermia has emerged as a potentially effective therapy for TBI, although its mechanism is still unclear. In this study we investigated the effects of temperature modulations on astrocyte survival following traumatic injury and the involved MAPK pathways. Trauma was produced by scratch injury of a monolayer of confluent astrocytes in culture, followed by incubation at hypothermia (30°C), normothermia (37°C), or hyperthermia (39°C). The activation of MAPK pathways including extracellular signal-regulated protein kinase (ERK), c-Jun NH(2)-terminal kinase (JNK), and p38 MAPK were measured at 0, 15, 30, 60, and 120 min after traumatic injury followed by temperature modulation. Apoptosis of astrocytes was assessed by quantitation of cleaved caspase-3 expression 24 h after injury. Our findings showed that only JNK activation at 15 min after trauma was reduced by hypothermia, and this was associated with a marked reduction in apoptosis. Hyperthermia activated both ERK and JNK and increased apoptosis. The specific JNK inhibitor, SP60025, markedly reduced JNK-induced apoptosis at normothermia and hyperthermia, and showed a dose-dependent effect. In conclusion, the JNK pathway appears to mediate traumatic injury–induced apoptosis in astrocytes. Prolonged hyperthermia as a secondary insult worsens apoptosis by increasing JNK activation. Hypothermia protects against traumatic injury via early suppression on JNK activation and subsequent prevention of apoptosis. Manipulation of the JNK pathway in astrocytes may represent a therapeutic target for ameliorating the devastating progression of tissue injury and cell death after TBI.
Key words: apoptosis, astrocyte, hyperthermia, hypothermia, MAPKs, SP60025, traumatic brain injury
Introduction
Traumatic brain injury (TBI) is a leading cause of death and disability in the United States. Current medical therapies exhibit limited efficacy in reducing neurological injury, and the prognosis for patients with severe TBI remains poor. One promising therapy that targets multiple pathological mechanisms caused by TBI is hypothermia. Hypothermia decreases inflammation, excitotoxicity, free radical production, and intracranial pressure, and improves cerebral metabolism after TBI and cerebral ischemia (Dietrich et al., 1994; Fritz and Bauer, 2004; Liu and Yenari, 2007). In contrast, hyperthermia after TBI appears to worsen secondary brain injury and neurologic outcome (Dietrich, 1992; Dietrich et al., 1996; Chatzipanteli et al., 2000; Yu et al., 2001). While most research is focused on the direct protection of neurons, non-neuronal cells such as astrocytes play an important role in the pathogenesis of TBI. As the most abundant glial cell in the brain, astrocytes have been implicated in the maintenance and support of neurons to regulate neuronal communication and survival. Astrocytes also help maintain the integrity of the blood–brain barrier (Abbott, 2002; Haseloff et al., 2005; Siddharthan et al., 2007). Recent work implicates a role for astrocytes in the reduction of neuronal cell death following a variety of cellular insults (Aschner and Kimelberg, 1991; Tanaka et al., 1999; Wilson, 1997; Dringen et al., 2000). However, astrocytes also have potential harmful effects by development of astrogliosis after TBI. The balance between these harmful and protective factors plays an important role in neuron survival. In-vivo studies showed that astrogliosis as a chronic reaction usually occurs 10–30 days after trauma. Astrocytes decrease both in numbers and immunoreactivity acutely at 1 day after TBI. However, 3 days later, they start to reactively respond to TBI by proliferation and hypertrophy, and finally develop astrogliosis (Dihné et al., 2001). Apoptosis in astrocytes can be detected as early as 6 h after trauma in a controlled cortical impact animal model (Beer et al., 2001). In-vitro cultured glial cells also show that immediate early genes are upregulated at 30 min after mechanical injury, and then subside within 3 h (Katano et al., 2001). Understanding the molecular mechanisms that regulate the early survival and apoptosis of astrocytes after trauma may offer an opportunity to improve outcome by preserving the neuroprotective features of these cells.
Protein kinase cascades are key components of signaling pathways and networks that regulate cell survival. Recent studies in both in-vivo and in-vitro models of CNS trauma demonstrate that mitogen-activated protein kinases (MAPKs) are activated in response to mechanical forces and play central roles in injury and repair after trauma by affecting responses to extracellular stress (Raghupathi et al., 2003; Otani et al., 2003; Dash et al., 2002; Neary, 2005). There are three major subtypes of MAPKs: extracelluar signal-regulated protein kinase (ERK), c-Jun NH(2)-terminal kinase (JNK, also known as stress-activated protein kinase, or SAPK), and p38. In this study we investigated the effect of temperature modulations on ERK, JNK, and p38 activation, and survival of astrocytes after traumatic injury in vitro. We hypothesized that hypothermia reduces and hyperthermia increases trauma-induced changes in MAPK activation patterns, and that these changes are associated with astrocyte survival.
Methods
Preparation of astrocyte cultures
Primary astrocyte cultures were obtained from 1-day old newborn Sprague-Dawley rat pup cerebral cortices as previously described by Yu and associates (Yu et al., 1989). Briefly, meninges-free cortices were cut into small cubes (<1 mm3) in a modified Dulbecco's modified Eagle medium (DMEM). After being mechanically dissociated and vortex mixed for 40 sec, the cell suspension was sieved through sterile nylon Nitex filters (pore sizes 75 μm and 10 μm). The filtered cell suspension was seeded (0.25 × 106 cells/dish) in 35-mm Falcon tissue culture dishes. Fresh DMEM supplemented with 15% fetal bovine serum (FBS) was added to yield a final volume of 2 mL/dish. All cultures were incubated at 37°C in a 95%:5% (volume:volume) mixture of atmospheric air and CO2 with 95% humidity. The culture medium was changed every 3 days with DMEM containing 15% FBS for 1 week, and then containing 10% FBS in the remaining time (Yu et al., 1993). At least 95% of the cell populations were astrocytes, as determined by staining astrocyte-specific marker-GFAP. Experiments were conducted on 4- to 6-week-old cultures.
Traumatic injury model of astrocytes
Injury to astrocytes was produced by manually scratching the confluent astrocyte cell cultures as described by Yu and colleagues (Yu et al., 1993; Lau and Yu, 2001). Briefly, each 35-mm dish confluent culture was manually scratched with a sterile plastic pipette tip following a 9 × 9-square grid (with 4-mm spacing between the lines). Following scratch injury, the cultures were washed with PBS to remove cellular debris. The scratching resulted in the removal of approximately 37% of the total cells in the culture dish as determined by counting of calcein-stained live cells (Molecular Probes, Sunnyvale, CA), and by comparison of the protein content in injured to uninjured cultures (Lau and Yu, 2001). Uninjured cells served as controls. Because scratch injury activates astrocytes first at the wound edge and later expands to the entire astrocyte monolayer (Mandell et al., 2001), the entire culture on each dish was used for all experiments.
Temperature modulation
Temperature was changed immediately after trauma by placing the culture dishes in temperature-controlled incubators that were maintained at the desired temperature: 30°C for hypothermia, 37°C for normothermia, and 39°C for hyperthermia. For MAPK activation measurements by Western blot, cells were harvested at 15, 30, 60, and 120 min post-injury. Control dishes were set by placing uninjured culture dishes in each incubator for the same time periods as the injured cells. For assessment of the effect of temperature on cell survival, the temperature was maintained for 24 h, after which both Western blotting for total caspase-3 and cleaved caspase-3, and fluorescein staining with the Caspa Tag™ caspase-3/7 in-situ assay kit (Chemicon, Temecula, CA) were performed on the cultures.
Western immunoblot analysis
After removal of medium, the cells were washed three times with cold PBS. The cells were then lysed by lysis buffer consisting of Tris (pH 7.6), 2.5 mM EDTA, 1 mM DTT, 1.25 μg/mL pepstatin A, 10 μg/mL leupeptin, 25 μg/mL aprotinin, 0.5 mM PMSF, and 0.1 mM Na3VO4. Protein concentration was determined by the Bradford method. Cell lysates of 25 μg of protein were subjected to SDS-PAGE (10% acrylamide gel for MAPKs and 12% acrylamide gel for caspase-3) and Western blot analysis was performed. Primary antibodies including phospho-ERK1/2 (1:2000), total ERK1/2 (1:1000), phospho-JNK (c-JunSer-63; 1:750), total JNK (1:1000), phopho-p38 (1:500), and total p38 (1:1000) were all obtained from Cell Signaling Technology (Beverly, MA).
Cleaved-caspase-3 expression and MAPK inhibition
Apoptosis was determined by cleaved caspase-3 (Asp175) expression in astrocytes 24 h after scratch by Western blot, and fluorescein staining with the Caspa Tag caspase-3/7 in-situ assay kit. Antibodies against cleaved caspase-3 (1:500) and caspase-3 (1:1000; Cell Signaling Technology) were used for Western blot. The green fluorescent signal is a direct measurement of the amount of active caspase-3 or caspase-7 present in the cell at the time the reagent was added. After comparing the difference between hypothermia and hyperthermia in activation of MAPKs and apoptosis, specific inhibitors were used to determine which MAPK pathways were taking part in mediating apoptosis after trauma. The inhibitor was added to the cell culture dishes immediately after injury. Dose-response was determined for the effect of this inhibitor on cleaved caspase-3 expression 24 h after trauma at different temperatures.
Statistical analysis
Each experiment was performed three times with cultures from different seedings. Data were analyzed by Student's t-test for comparisons between two groups, and by ANOVA for repeated measures followed by Dunnett's post-hoc comparisons using SPSS 11.0 statistical software (SPSS Inc., Chicago IL). Data are expressed as mean ± SD.
Results
Effects of temperature on MAPK activation
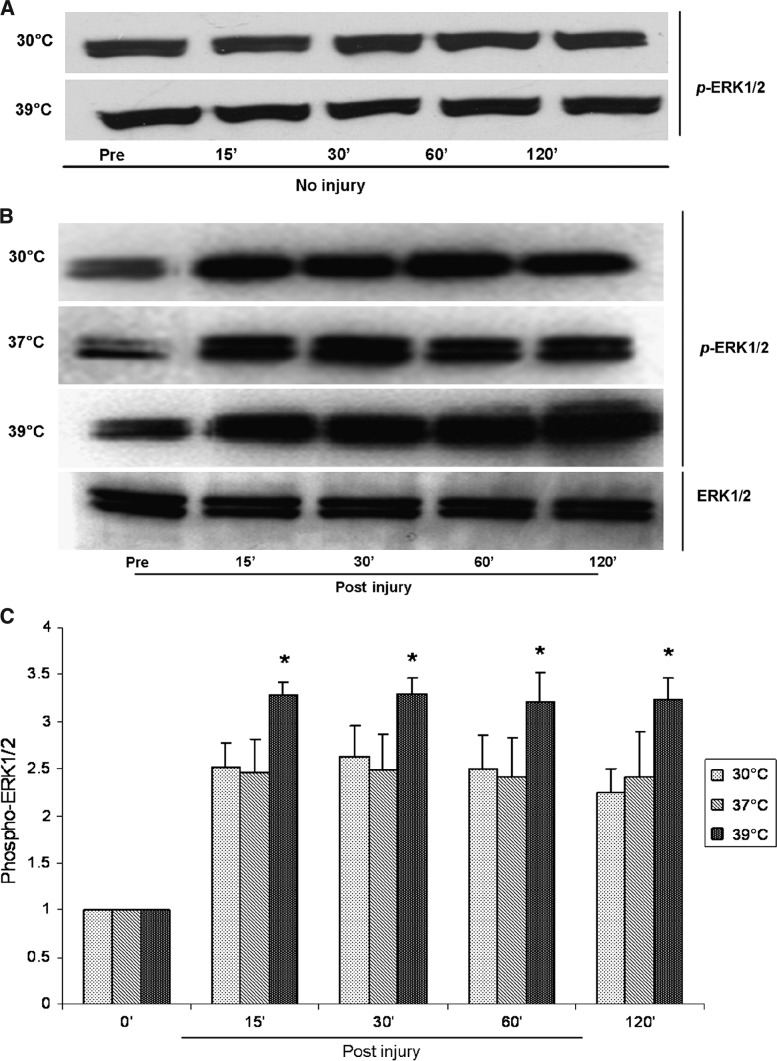
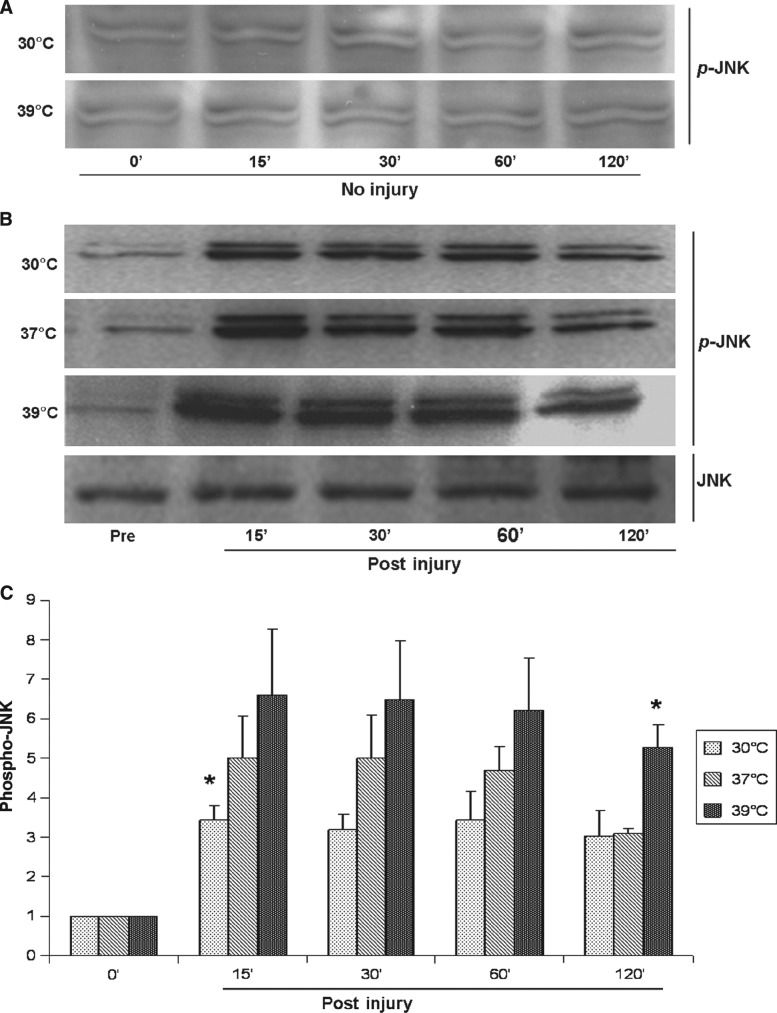
After trauma under normothermic conditions (37°C), both ERK1/2 and JNK (c-JunSer-63) phosphorylation increased at 15 min and remained higher than baseline for 2 h post-injury (Figs. 1 and 2). Trauma induced a 2.4 ± 0.5-fold increase (p = 0.001) in ERK1/2 phosphorylation post-trauma, and a 5.0 ± 1.5-fold increase (p = 0.009) in JNK phosphorylation at 15 min post-trauma. There were no significant changes in p38 phosphorylation at any time point after injury (p > 0.05) (Fig. 3).
FIG. 1.
(A) Representative Western blots show that neither hypothermia nor hyperthermia changed the phosphorylation level of ERK1/2 in uninjured astrocytes. (B) ERK1/2 is rapidly phosphorylated after traumatic injury in astrocytes, while total levels of ERK1/2 remain constant. (C) ERK1/2 phosphorylation was significantly higher post-injury than pre-injury at all temperatures from 15 min to 2 h post-injury. Hyperthermia increased ERK1/2 activation (*p < 0.05 versus normothermia), while hypothermia did not change the phosphorylation level of ERK1/2.
FIG. 2.
(A) Representative Western blots show that neither hypothermia nor hyperthermia changed the phosphorylation level of JNK in uninjured astrocytes. (B) JNK is rapidly phosphorylated after traumatic injury, while total levels of JNK remain constant. (C) JNK phosphorylation was significantly higher post-injury than pre-injury at all temperatures from 15 min to 2 h post-injury. Hypothermia decreased and hyperthermia increased JNK activation (*p < 0.05 versus normothermia).
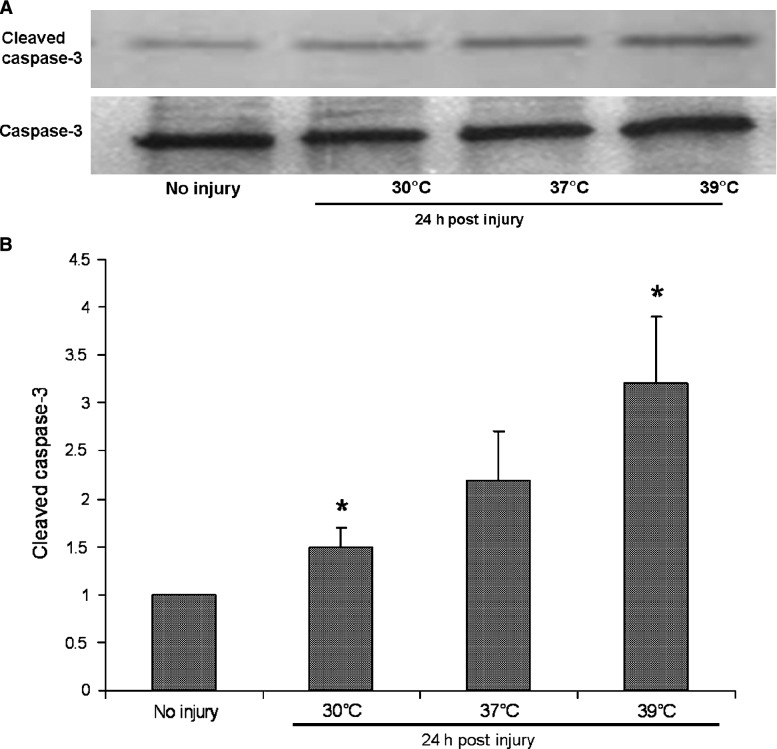
FIG. 3.
(A) Representative Western blots show that cleaved caspase-3 was activated 24 h after traumatic injury under normothermia, and was increased by hyperthermia. No significant activation of cleaved caspase-3 after trauma was observed under hypothermia. The total levels of caspase-3 remained constant. (B) Western blots of cleaved caspase-3 were quantified from three independent culture experiments using standard densitometry technique (*p < 0.05 versus normothermia).
Under hypothermic conditions (30°C), phosphorylated ERK1/2 levels were no different from normothermia at any time point from 15 min to 120 min post-trauma (p > 0.05) (Fig. 1). However, hypothermia significantly reduced the phosphorylation of JNK at 15 min post-trauma compared to normothermia, and there was a trend for this effect to continue until 60 min post-trauma (Fig. 2). The maximal phosphorylation of JNK at 15 min post-trauma was reduced by 42% by hypothermia (3.2 ± 0.5 times uninjured control at 30°C versus 5.0 ± 1.5 times uninjured control at 37°C; p < 0.05). Hypothermia had no effect on the phosphorylation of p38 after trauma (data not shown). Hypothermia had no significant effects on ERK1/2 and JNK activation in uninjured astrocytes (Figs. 1 and 2).
Hyperthermia (39°C) increased the phosphorylation of both ERK1/2 and JNK post-trauma, but had no effect on p38 phosphorylation (p > 0.05). Hyperthermia significantly increased the phosphorylation of ERK1/2 from 15 min to 120 min after trauma (3.2 ± 0.3 to 3.3 ± 0.1 times uninjured control at 39°C versus 2.4 ± 0.5 to 2.5 ± 0.4 times uninjured control at 37°C; p < 0.05) (Fig. 1). Phosphorylated JNK levels with hyperthermia for 15 min to 60 min post-trauma tended to be higher than those seen with normothermia, but they were not statistically significantly different (6.6 ± 2.3 to 6.2 ± 1.9 times uninjured control at 39°C versus 4.8 ± 1.5 to 4.7 ± 0.8 times uninjured control at 37°C; p > 0.05). However, at 120 min post-injury, hyperthermia significantly increased JNK phosphorylation (5.3 ± 0.8 times uninjured control at 39°C versus 3.1 ± 0.2 times uninjured control at 37°C; p = 0.002) (Fig. 2). Hyperthermia has no significant effects on ERK1/2 and JNK activation in uninjured astrocytes (Figs. 1 and 2).
Comparison of the individual components of the MAPK family revealed that traumatic injury in astrocytes induced more pronounced changes in the JNK signaling pathway than in the ERK1/2 and p38 pathways (twice as much as ERK1/2 and five times more than p38). In addition, temperature changes post-trauma had greater effects on JNK compared to either ERK1/2 or p38. The only change in the activity of these kinases seen with hypothermia occurred in JNK, whereas hyperthermia led to a greater increase in JNK than in ERK1/2 (increases of approximately 70% versus 35%), and no change in p38.
The effects of temperature and JNK inhibition on apoptosis after trauma
Temperature had a marked effect on cleaved caspase-3 expression at 24 h post-trauma. Hypothermia reduced and hyperthermia increased cleaved caspase-3 expression compared to normothermia (1.5 ± 0.2 times uninjured control at 30°C versus 2.2 ± 0.5 times uninjured control at 37°C and 3.2 ± 0.7 times uninjured control at 39°C; p = 0.018) (Fig. 3).
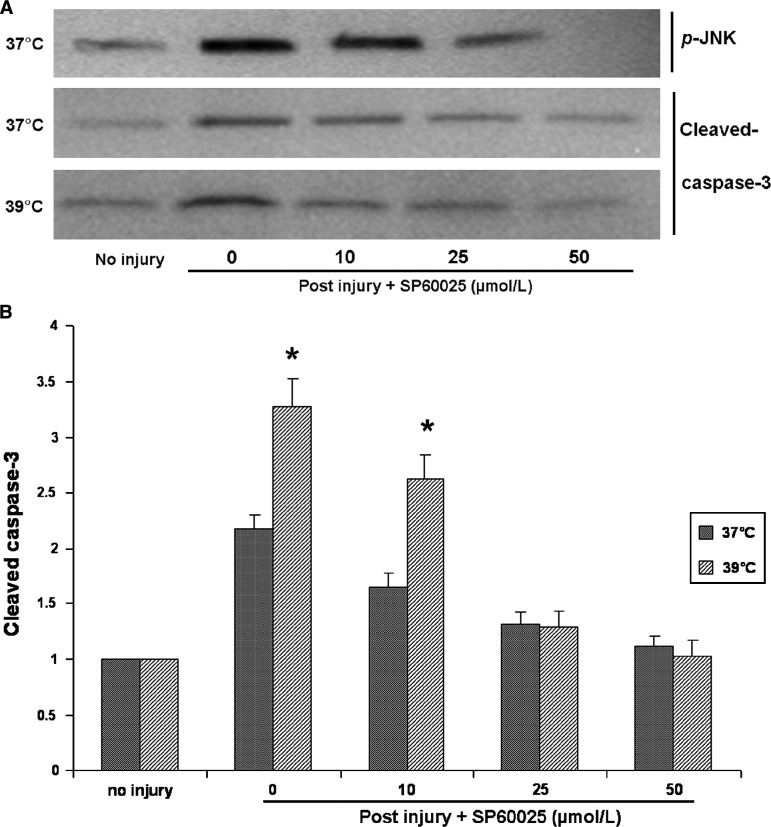
In order to determine the role of JNK in inducing apoptosis after trauma, astrocytes were exposed to the specific JNK inhibitor SP60025 immediately after injury, and cleaved caspase-3 expression was measured 24 h later. SP60025 significantly reduced trauma-induced apoptosis at both 37°C and 39°C, and showed temperature- and dose-dependent effects (Fig. 4). At a low dose of 10 μM, SP60025 reduced expression of cleaved caspase-3 by 46% at 37°C and by 28% at 39°C. Maximal inhibition of JNK was achieved by 50 μM of SP60025 at both temperatures. Similar results were also obtained using a different method of identifying apoptotic cells (by use of the Caspa Tag caspase-3/7 in-situ assay kit), which labels caspase-3-positive cells with fluorescein (Fig. 5).
FIG. 4.
The effect of SP60025 on cleaved caspase-3 activation after trauma. (A) Representative Western blots show that phosphorylation levels of JNK after trauma decreased with increasing doses of SP60025, and that maximal inhibition of JNK activation was achieved by 50 μmol/L SP60025. Cleaved caspase-3 activation 24 h after traumatic injury under either normothermia or hyperthermia was also inhibited with increasing doses of SP60025, and they returned to baseline level at the dose of 50 μmol/L SP60025. (B) Western blots of cleaved caspase-3 were quantified from three independent culture experiments using standard densitometry technique (*p < 0.05 versus normothermia).
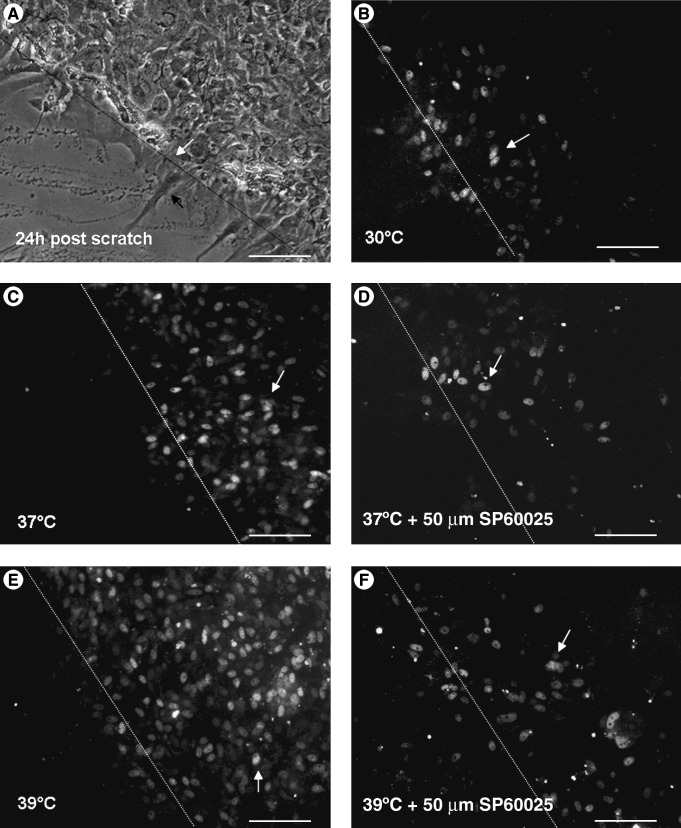
FIG. 5.
Micrographs of caspase-3/7 fluorescein stained cells taken 24 h after scratch injury in astrocytes. (A) At 24 h after scratch injury (the dashed line indicates the edge of the scratch injury, marked by the white arrow), the astrocytes began to extend their processes (indicated by the black arrow). (B) The grey arrows indicate the positively-stained cells. Caspase-3/7 expression under hypothermia (30°C) was significantly lower than that seen at 37°C (C) and 39°C (E). Also, 50 μmol/L of JNK inhibitor (SP60025) dramatically reduced caspase-3/7 expression at 37°C (D) and 39°C (F) after trauma (magnification × 200; bar = 50 μm).
Discussion
Temperature changes after TBI may affect both acute and long-term outcomes. Hyperthermia is common following brain injury and has been associated with poor neurological outcomes. Hypothermia has emerged as a potentially effective therapy for TBI, although its exact mechanisms of action have yet to be elucidated. Intracellular signaling cascades are of particular interest after TBI because they are involved in the regulation of cellular repair, plasticity, and homeostatic functions, which are markedly altered in response to TBI. It is now well documented that MAPK pathways represent highly conserved upstream mechanisms for transducing stress signals in eukaryotic cells (Garrington and Johnson, 1999; Guyton et al., 1996; Mielke and Herdegen, 2000). This study shows that the MAPK pathways for ERK and JNK, but not p38, are activated soon after traumatic injury in astrocytes in vitro, and that temperature markedly affected these responses. Only JNK activation was reduced by hypothermia, and this was associated with a marked reduction in expression of cleaved caspase-3. In contrast, hyperthermia activated both ERK and JNK and increased expression of cleaved caspase-3. These results indicate that JNK activation increases apoptosis after trauma. This hypothesis was further corroborated by the inhibition of apoptosis seen after trauma in astrocytes treated with the JNK inhibitor SP60025.
Most studies show that ERK1/2 pathway activation leads to protective effects after brain injury. ERK has been shown to be involved in preconditioning responses that protect against secondary insults in cultured neurons (Gonzalez-Zulueta et al., 2000), and has been associated with neuroprotection in global cerebral ischemia in-vivo studies (Hu et al., 2000; Sugino et al., 2000). Dash and colleagues (Dash et al., 2002) showed that in rats with cortical impact injury, ERK1/2 inhibition prior to trauma reduced recovery of memory and motor skills. Atkins and colleagues found that hypothermia increased ERK1/2 activation in the ipsilateral hippocampus after TBI in rats (Atkins et al., 2007). While most of these studies were done using in-vivo TBI models or in-vitro cultured neurons, little is known about the effects of trauma on astrocytes, which have recently been found to have protective effects against brain injury. In our astrocyte scratch injury model, ERK1/2 increased 15 min after injury and remained elevated 2 h after injury. Hypothermia suppressed caspase-3 activation after trauma with no significant difference on ERK1/2 activation compared to normothermia, whereas hyperthermia increased caspase-3 activation with increased ERK1/2 phosphorylation seen post-trauma. These results suggest that the protective effects of hypothermia are not mediated by the ERK1/2 pathway. The elevation of ERK1/2 activation by hyperthermia could be a direct effect of hyperthermia, or it could represent a compensatory protective response to the secondary insult of hyperthermia.
While ERK has been found consistently to be activated by trauma, p38 and JNK activation after TBI have been more variable. Experimental evidence suggests that ERK activation is usually associated with protective effects, whereas activation of p38 and JNK are considered to be deleterious (Xia et al., 1995). Therefore, the dynamic balance between growth factor-activated ERK and stress-activated JNK-p38 pathways may be important in determining whether a cell survives or undergoes apoptosis. In a cortical impact model of TBI in mice, phosphorylated ERK and p38, but not JNK, increased rapidly (Mori et al., 2002). In a lateral fluid-percussion model of TBI in rats, phosphorylated ERK and JNK, but not p38, were increased in the hippocampus and cortex (Otani et al., 2002). The differences in MAPK pathway activation seen after TBI may be related to differences in the methods of inducing TBI. In our in-vitro astrocyte scratch injury model, ERK and JNK, but not P38, were activated. A similar pattern of MAPK activation was seen in astrocytes traumatized by the fluid-percussion method (Jayakumar et al., 2008). JNK, also known as stress-activated protein kinase (SAPK), is activated by a variety of cytoplasmic kinases and stressors such as heat, ultraviolet light, hydrogen peroxidase, and protein synthesis inhibitors. In this study, JNK quickly responded to traumatic injury and its phophorylation reached a peak only 15 min after injury, then gradually decreased, but remained elevated at 2 h post-injury. Hypothermia inhibited JNK activation only at 15 min post-injury. These data suggest that JNK rapidly responds to traumatic injury in astrocytes, and that suppression of JNK activation can be achieved with early hypothermia. This is consistent with in-vivo studies that show that the induction of hypothermia must occur within 30–60 min for protective effects to occur in animal models of fluid-percussion brain injury and controlled cortical impact injury (Lyeth et al., 1993; Markgraf et al., 2001). Hyperthermia was associated with sustained JNK phosphorylation for up to 120 min after trauma, which suggests the importance of the duration as well as the severity of hyperthermic periods after TBI. Prolonged hyperthermia worsened apoptosis by increasing JNK activation.
Our results showing that hypothermia is a protective factor inhibiting JNK activation, and hyperthermia is a secondary insult that aggravates JNK activation, suggest that JNK may play a critical role in inducing apoptosis after trauma. In order to investigate the precise role of JNK in mediating apoptosis following traumatic injury and hyperthermia, a specific JNK inhibitor, SP60025, was added after injury followed by normothermia or hyperthermia. Maximal inhibition of injury-induced activation of JNK was achieved by a high dose (50 μM) of SP60025. Cleaved caspase-3 expression under normothermia and hyperthermia also correspondingly decreased with the inhibition of JNK activation, and showed a dose-dependent response to SP60025. When SP60025 was increased to 50 μM, cleaved caspase-3 expression after trauma reduced to baseline levels no different from those seen in uninjured cells, even under hyperthermic conditions. These results demonstrate that JNK mediates trauma-induced apoptosis in astrocytes. Early inhibition of JNK protects traumatically-injured astrocytes from apoptosis, and attenuates the secondary insult of hyperthermia. This finding is similar to the copper-induced astrocyte apoptosis caused by JNK activation (Chen et al., 2008), but this is the first time that activation of JNK has been reported in trauma-induced astrocyte apoptosis.
Astrocytes play a number of important roles in the response to TBI. Both harmful and beneficial effects have been attributed to these cells. Reactive astrocytes have the potential for neural toxicity (Ikeda and Murase, 2004), increased neural inflammation (Rostworowski et al., 1997), and inhibition of axon regeneration (Bush et al., 1999; Wanner et al., 2008). Recent studies point to beneficial roles for reactive astrocytes, such as demarcating areas of damaged tissue and reductions in inflammation (Sofroniew, 2005; Bush et al., 1999; Faulkner et al., 2004; Cui et al., 2001; Myer et al., 2006), stimulation of blood–brain barrier repair (Abbott, 2002; Haseloff et al., 2005; Siddharthan et al., 2007), reduction of edema (Bush et al., 1999), and protection of neurons, oligodendrocytes, and neural function (Bush et al., 1999).
Using the same injury model as that used in this study, it has been reported that astrocytes respond to traumatic injury by sending out foot processes across the cleft, upregulating endogenous VEGF (Krum et al., 2008), and increasing reactivity and survival genes, including GFAP, GDNF, nestin, and vimentin (Vandevord et al., 2008; Moon et al., 2004; Eliasson et al., 1999). By 7 days after scratch injury, the cleft left by the scratch is fully covered over by astrocytes through the gliotic response to trauma. A better understanding of the intracellular changes seen in astrocytes following TBI may lead to means of pharmacologically targeting astrocytes to limit neurovascular injury and promote functional brain repair. Intracellular signaling cascades involving MAPK are of particular interest after TBI because they are involved in the regulation of cell survival, repair, and plasticity (Chang and Karin, 2001; Neary, 2005). Our study shows that ERK and JNK, but not p38 MAPK pathways, are activated soon after traumatic injury in astrocytes, and that JNK activation leads to apoptosis of astrocytes after trauma, an effect that can be prevented with JNK inhibition.
In summary, this study provides evidence that the JNK pathway mediates traumatic injury–induced apoptosis in astrocytes. Prolonged hyperthermia as a secondary insult worsens apoptosis by increasing JNK activation. Hypothermia protects against traumatic injury via early suppression of JNK activation and subsequent prevention of apoptosis. Manipulation of the JNK pathway in astrocytes may represent a therapeutic target for ameliorating the devastating progression of tissue damage seen after TBI.
Author Disclosure Statement
No conflicting financial interests exist.
References
- Abbott N.J. Astrocyte-endothelial interactions and blood-brain barrier permeability. J. Anat. 2002;200:629–638. doi: 10.1046/j.1469-7580.2002.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschner M. Kimelberg H.K. The use of astrocytes in culture as model systems for evaluating neurotoxic-induced-injury. Neurotoxicology. 1991;12:505–517. [PubMed] [Google Scholar]
- Atkins C.M. Oliva A.A. Alonso O.F. Chen S. Bramlett H.M. Hu B. Dietrich W.D. Hypothermia treatment potentiates ERK1/2 activation after traumatic brain injury. European J. Neurosci. 2007;26:810–819. doi: 10.1111/j.1460-9568.2007.05720.x. [DOI] [PubMed] [Google Scholar]
- Beer R. Franz G. Krajewski S. Pike B.R. Hayes R.L. Reed J.C. Wang K.K. Klimmer C. Schmutzhard E. Poewe W. Kampfl A. Temporal and spatial profile of caspase 8 expression and proteolysis after experimental traumatic brain injury. J. Neurochem. 2001;78:862–873. doi: 10.1046/j.1471-4159.2001.00460.x. [DOI] [PubMed] [Google Scholar]
- Bush T.G. Puvanachandra N. Horner C.H. Polito A. Ostenfeld T. Svendsen C.N. Mucke L. Johnson M.H. Sofroniew M.V. Leukocyte infiltration, neuronal degeneration, and neurite outgrowth after ablation of scarforming, reactive astrocytes in adult transgenic mice. Neuron. 1999;23:297–308. doi: 10.1016/s0896-6273(00)80781-3. [DOI] [PubMed] [Google Scholar]
- Chang L. Karin M. Mammalian MAP kinase signaling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- Chatzipanteli K. Alonso O.F. Kraydieh S. Dietrich W.D. Importance of posttraumatic hypothermia and hyperthermia on the inflammatory response after fluid percussion brain injury: biochemical and immunocytochemical studies. J. Cereb. Blood Flow Metab. 2000;20:531–542. doi: 10.1097/00004647-200003000-00012. [DOI] [PubMed] [Google Scholar]
- Chen S.H. Lin J.K. Liu S.H. Liang Y.C. Lin-Shiau S.Y. Apoptosis of cultured astrocytes induced by the copper and neocuproine complex through oxidative stress and JNK activation. Toxicol. Sci. 2008;102:138–149. doi: 10.1093/toxsci/kfm292. [DOI] [PubMed] [Google Scholar]
- Cui W. Allen N.D. Skynner M. Gusterson B. Clark A.J. Inducible ablation of astrocytes shows that these cells are required for neuronal survival in the adult brain. Glia. 2001;34:272–282. doi: 10.1002/glia.1061. [DOI] [PubMed] [Google Scholar]
- Dash P.K. Mach S.A. Moore A.N. The role of extracellular signal-regulated kinase in cognitive and motor deficits following experimental traumatic brain injury. Neuroscience. 2002;114:755–767. doi: 10.1016/s0306-4522(02)00277-4. [DOI] [PubMed] [Google Scholar]
- Dietrich W.D.Alonso O.Busto R.Globus M.Y.>Ginsberg M.D.1994Post-traumatic brain hypothermia reduces histopathological damage following concussive brain injury in the rat Acta Neuropathol. (Berl.) 87250–258. [DOI] [PubMed] [Google Scholar]
- Dietrich W.D. Alonso O. Halley M. Busto R. Delayed posttraumatic brain hyperthermia worsens outcome after fluid percussion brain injury: a light and electron microscopic study in rats. Neurosurgery. 1996;38:533–541. doi: 10.1097/00006123-199603000-00023. [DOI] [PubMed] [Google Scholar]
- Dietrich W.D. The importance of brain temperature in cerebral injury. J. Neurotrauma. 1992;9(Suppl 2):S475–S485. [PubMed] [Google Scholar]
- Dihné M. Block F. Korr H. Töpper R. Time course of glial proliferation and glial apoptosis following excitotoxic CNS injury. Brain Res. 2001;902:178–189. doi: 10.1016/s0006-8993(01)02378-2. [DOI] [PubMed] [Google Scholar]
- Dringen R. Gutterer J.M. Hirrlinger J. Glutathione metabolism in brain metabolic interaction between astrocytes and neurons in the defense against reactive oxygen species. Eur. J. Biochem. 2000;267:4912–4916. doi: 10.1046/j.1432-1327.2000.01597.x. [DOI] [PubMed] [Google Scholar]
- Eliasson C. Sahlgren C. Berthold C.H. Stakeberg J. Celis J.E. Betsholtz C. Eriksson J.E. Pekny M. Intermediate filament protein partnership in astrocytes. J. Biol. Chem. 1999;274:23996–24006. doi: 10.1074/jbc.274.34.23996. [DOI] [PubMed] [Google Scholar]
- Faulkner J.R. Herrmann J.E. Woo M.J. Tansey K.E. Doan N.B. Sofroniew M.V. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J. Neurosci. 2004;24:2143–2155. doi: 10.1523/JNEUROSCI.3547-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz H.G. Bauer R. Secondary injuries in brain trauma: Effects of hypothermia. J. Neurosurg. Anesthesiol. 2004;16:43–52. doi: 10.1097/00008506-200401000-00009. [DOI] [PubMed] [Google Scholar]
- Garrington T.P. Johnson G.L. Organization and regulation of mitogen-activated protein kinase signaling pathways. Curr. Opin. Cell Biol. 1999;11:211–218. doi: 10.1016/s0955-0674(99)80028-3. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Zulueta M. Feldman A.B. Klesse L.J. Kalb R.G. Dillman J.F. Parada L.F. Dawson T.M. Dawson V.L. Requirement for nitric oxide activation of p21(ras)/extracellular regulated kinase in neuronal ischemic preconditioning. Proc. Natl. Acad. Sci. U.S.A. 2000;97:436–441. doi: 10.1073/pnas.97.1.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyton K.Z. Liu Y. Gorospe M. Xu Q. Holbrook N.J. Activation of mitogen-activated protein kinase by H2O2: role in cell survival following oxidant injury. J. Biol. Chem. 1996;271:4138–4142. doi: 10.1074/jbc.271.8.4138. [DOI] [PubMed] [Google Scholar]
- Haseloff R.F. Blasig I.E. Bauer H.C. Bauer H. In search of the astrocytic factor(s) modulating blood-brain barrier functions in brain capillary endothelial cells in vitro. Cell Mol. Neurobiol. 2005;25:25–39. doi: 10.1007/s10571-004-1375-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B.R. Liu C.L. Park D.L. Alteration of MAP kinase pathways after transient forebrain ischemia. J. Cereb. Blood Flow Metab. 2000;20:1089–1095. doi: 10.1097/00004647-200007000-00008. [DOI] [PubMed] [Google Scholar]
- Ikeda H. Murase K. Glial nitric oxide-mediated long-term presynaptic facilitation revealed by optical imaging in rat spinal dorsal horn. J. Neurosci. 2004;24:9888–9896. doi: 10.1523/JNEUROSCI.2608-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayakumar A.R. Rao K.V.R. Kiran S. Panickar K.S. Moriyama M. Reddy P.V.B. Norenberg M.D. Trauma-induced cell swelling in cultured astrocytes. J. Neuropathol. Exp. Neurol. 2008;67:417–427. doi: 10.1097/NEN.0b013e31816fc9d4. [DOI] [PubMed] [Google Scholar]
- Katano H. Fujita K. Kato T. Asai K. Kawamura Y. Masago A. Yamada K. A metabotropic glutamate receptor antagonist, alpha-methyl-4-carboxyphenylglycine, attenuates immediate early gene mRNA expression following traumatic injury in cultured rat cortical glial cells. Neurosci. Lett. 2001;306:101–105. doi: 10.1016/s0304-3940(01)01832-8. [DOI] [PubMed] [Google Scholar]
- Krum J.M. Mani N. Rosenstein J.M. Roles of the endogenous VEGF receptors flt-1 and flk-1 in astroglial and vascular remodeling after brain injury. Exp. Neurol. 2008;212:108–117. doi: 10.1016/j.expneurol.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau L.T. Yu A.C. Astrocytes produce and release interleukin-1, interleukin-6, tumor necrosis factor alpha and interferon-gamma following traumatic and metabolic injury. J. Neurotrauma. 2001;18:351–359. doi: 10.1089/08977150151071035. [DOI] [PubMed] [Google Scholar]
- Liu L. Yenari M.A. Therapeutic hypothermia: Neuroprotective mechanisms. Front. Biosci. 2007;12:816–825. doi: 10.2741/2104. [DOI] [PubMed] [Google Scholar]
- Lyeth B.G. Jiang J.Y. Liu S. Behavioral protection by moderate hypothermia initiated after experimental traumatic brain injury. J. Neurotrauma. 1993;10:57–64. doi: 10.1089/neu.1993.10.57. [DOI] [PubMed] [Google Scholar]
- Mandell J.W. Gocan N.C. Vandenberg S.R. Mechanical trauma induces rapid astroglial activation of ERK/MAP kinase: Evidence for a paracrine signal. Glia. 2001;34:283–295. doi: 10.1002/glia.1062. [DOI] [PubMed] [Google Scholar]
- Markgraf C.G. Clifton G.L. Moody M.R. Treatment window for hypothermia in brain injury. J. Neurosurg. 2001;95:979–983. doi: 10.3171/jns.2001.95.6.0979. [DOI] [PubMed] [Google Scholar]
- Mielke K. Herdegen T. JNK and p38 stress kinases: degenerative effectors of signal transduction cascades in the nervous system. Prog. Neurobiol. 2000;61:45–60. doi: 10.1016/s0301-0082(99)00042-8. [DOI] [PubMed] [Google Scholar]
- Moon C. Ahn M. Kim S. Jin J.K. Sim K.B. Kim H.M. Lee M.Y. Shin T. Temporal patterns of the embryonic intermediate filaments nestin and vimentin expression in the cerebral cortex of adult rats after cryoinjury. Brain Res. 2004;1028:238–242. doi: 10.1016/j.brainres.2004.09.022. [DOI] [PubMed] [Google Scholar]
- Mori T. Wang X. Jung J.C. Sumii T. Singhal A.B. Fini M.E. Dixon C.E. Alessandri A. Lo E.H. Mitogen-activated protein kinase inhibition in traumatic brain injury: in vitro and in vivo effects. J. Cereb. Blood Flow Metab. 2002;22:444–452. doi: 10.1097/00004647-200204000-00008. [DOI] [PubMed] [Google Scholar]
- Myer D.J. Gurkoff G.G. Lee S.M. Hovda D.A. Sofroniew M.V. Essential protective roles of reactive astrocytes in traumatic brain injury. Brain. 2006;129:2761–2772. doi: 10.1093/brain/awl165. [DOI] [PubMed] [Google Scholar]
- Neary J. Protein kinase signaling cascades in CNS trauma. Life. 2005;57:711–718. doi: 10.1080/15216540500319143. [DOI] [PubMed] [Google Scholar]
- Otani N. Nawashiro H. Fukui S. Nomura N. Yano A. Miyazawa T. Shima K. Differential activation of mitogen-activated protein kinase pathways after traumatic brain injury in the rat hippocampus. J. Cereb. Blood Flow Metab. 2002;22:327–334. doi: 10.1097/00004647-200203000-00010. [DOI] [PubMed] [Google Scholar]
- Otani N. Nawashiro H. Tsuzuki N. Katoh H. Miyazawa T. Shima K. Mitogen-activated protein kinases phosphorylation in posttraumatic selective vulnerability in rats. Acta Neurochir. Suppl. 2003;86:287–289. doi: 10.1007/978-3-7091-0651-8_62. [DOI] [PubMed] [Google Scholar]
- Raghupathi R. Muir J.K. Fulp C.T. Pittman R.N. McIntosh T.K. Acute activation of mitogen-activated protein kinases following traumatic brain injury in the rat: implications for posttraumatic cell death. Exp. Neurol. 2003;183:438–448. doi: 10.1016/s0014-4886(03)00166-3. [DOI] [PubMed] [Google Scholar]
- Rostworowski M. Balasingam V. Chabot S. Owens T. Yong V.W. Astrogliosis in the neonatal and adult murine brain post-trauma: elevation of inflammatory cytokines and the lack of requirement for endogenous interferon-gamma. J. Neurosci. 1997;17:3664–3674. doi: 10.1523/JNEUROSCI.17-10-03664.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddharthan V. Kim Y.V. Liu S. Kim K.S. Human astrocytes/astrocyte-conditioned medium and shear stress enhance the barrier properties of human brain microvascular endothelial cells. Brain Res. 2007;1147:39–50. doi: 10.1016/j.brainres.2007.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew M.V. Reactive astrocytes in neural repair and protection. Neuroscientist. 2005;11:400–407. doi: 10.1177/1073858405278321. [DOI] [PubMed] [Google Scholar]
- Sugino T. Nozaki K. Takagi Y. Hattori I. Hashimoto N. Moriguchi T. Nishida E. Activation of mitogen-activated protein kinases after transient forebrain ischemia in gerbil hippocampus. J. Neurosci. 2000;20:4506–4514. doi: 10.1523/JNEUROSCI.20-12-04506.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka J. Toku K. Zhang B. Ishihara K. Sakanaka M. Maeda N. Astrocytes prevent neuronal death induced by reactive oxygen and nitrogen species. Glia. 1999;28:85–96. doi: 10.1002/(sici)1098-1136(199911)28:2<85::aid-glia1>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Vandevord P.J. Leung L.Y. Hardy W. Mason M. Yang K.H. King A.I. Up-regulation of reactivity and survival genes in astrocytes after exposure to short duration overpressure. Neurosci. Lett. 2008;434:247–252. doi: 10.1016/j.neulet.2008.01.056. [DOI] [PubMed] [Google Scholar]
- Wanner I.B. Deik A. Torres M. Rosendahl A. Neary J.T. Lemmon V.P. Bixby J.L. A new in vitro model of the glial scar inhibits axon growth. Glia. 2008;56:1691–1709. doi: 10.1002/glia.20721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J.X. Antioxidant defense of the brain: a role for astrocytes. Can. J. Physiol. Pharmacol. 1997;75:1149–1163. [PubMed] [Google Scholar]
- Xia Z. Dickens M. Rainegaud J. Davis R.J. Greenberg M.E. Opposing effects of ERK and JNK/P38 MAP kinase on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- Yu A.Ch. Gregory G.A. Chan P.H. Hypoxia induced dysfunction and injury of astrocytes in primary cell cultures. J. Cereb. Blood Flow Metab. 1989;9:20–28. doi: 10.1038/jcbfm.1989.3. [DOI] [PubMed] [Google Scholar]
- Yu A.Ch. Lee L. Eng L.F. Astrogliosis in culture: I. The model and the effect of antisense oligonucleotide on the glial fibrillary acidic protein synthesis. J. Neurosci. Res. 1993;34:295–303. doi: 10.1002/jnr.490340306. [DOI] [PubMed] [Google Scholar]
- Yu C.G. Jagid J. Ruenes G. Dietrich W.D. Marcillo A.E. Yezierski R.P. Detrimental effects of systemic hyperthermia on locomotor function and histopathological outcome after traumatic spinal cord injury in the rat. Neurosurgery. 2001;49:152–158. doi: 10.1097/00006123-200107000-00023. [DOI] [PubMed] [Google Scholar]