Abstract
Background
Distance to HIV care may be associated with retention in care (RIC) and viral suppression (VS) in Washington, DC.
Methods
RIC (≥ 2 HIV visits or labs ≥90 days apart), prescribed antiretroviral therapy (ART), VS (<200 copies/mL at last visit) and distance to care were estimated among 3,623 DC Cohort participants receiving HIV care in outpatient clinics in 2015. Logistic regression models and geospatial statistics were computed.
Results
RIC was 73%; 97% were on ART, among whom 77% achieved VS. ZIP code-level clusters of low RIC and high VS were observed in the Northwest; low VS in the Southeast. Those traveling ≥5 miles had 30% lower RIC (aOR=0.71, 95% CI: 0.58, 0.86) and lower VS (aOR=0.70, 95% CI: 0.52, 0.94).
Conclusions
Longer distances were associated with lower RIC and VS. Geospatial clustering of RIC
Keywords: Distance, spatial patterns, retention, viral suppression, care continuum
INTRODUCTION
The ability to achieve optimal HIV outcomes such as retention in care (RIC) and viral suppression (VS) depends on receipt of appropriate HIV medical care and maintenance of healthy behaviors to manage their HIV. Sociodemographic characteristics such as younger age, non-Hispanic black race/ethnicity, poverty, and unstable housing have been well documented as risk factors associated with poor HIV care outcomes in the US.1-8 In addition to individual-level characteristics, area-level characteristics including features of one’s residential neighborhood, such as community socioeconomic status, have been associated with higher rates of newly reported HIV diagnoses and lower RIC and VS.9-11 Other neighborhood features such as access to health care and transportation, food security, housing stability and local policy and programmatic issues related to HIV/AIDS, have been associated with a person’s ability to link to and remain in HIV care.12-18
Despite these findings, few studies have visualized the spatial distribution of HIV and spatial patterns of care and treatment outcomes as a means to reduce gaps in HIV care.19-21 Characterizing where people reside, work and socialize, including physical proximity to one’s HIV care site, may play a role in HIV care.6,22 Decisions about where, with whom, and how to get care may not solely be based on the closest HIV provider. The closest HIV care provider to one’s place of residence, sometimes thought of as the “likely provider” because of their geographic convenience, has not been found to fully explain the variability in distance travelled to HIV care.23 Findings from one study on HIV-infected persons residing in rural and suburban areas of North Carolina showed that newly diagnosed HIV-infected persons who travelled farther from their residence to their HIV diagnosis site, despite physical proximity to a closer testing facility, were diagnosed at a later stage of disease compared with those testing closer to their residence.24 Similarly, selecting an HIV provider may be informed by a variety of factors including perceived community stigma, distrust in the medical system, suggestions by friends and family, prior relationships with provider, provider reputation, provider location relative to where one ‘lives’, and the insurance accepted by provider.25-27
It is not understood whether residential proximity plays a role in HIV care in an urban setting such as Washington, DC – a relatively small geographic area (68 square miles) that has many HIV care providers, generous city-funded benefits subsidizing HIV-related prescription drugs, and transit-rich neighborhoods.27-29 To address this question, we sought to evaluate whether geographic distance to one’s HIV care site is predictive of HIV care patterns. The objectives were twofold. The first objective was to assess whether distance to HIV care was associated with RIC and VS using data from a city-wide cohort of HIV-infected persons in care. The second objective was to assess whether there was clustering of ZIP codes with higher (or lower) RIC and VS. Classifying geographic areas by HIV care outcomes may help identify locally relevant factors that help facilitate or limit HIV care.
METHODS
Study population
The DC Cohort Study, which began enrollment in January 2011, is a longitudinal cohort of HIV-infected persons in care. Details of the DC Cohort study design have been described previously.30 Briefly, participants’ clinical data were abstracted from their electronic medical records (EMR) and entered in a web-based data entry system called Discovere® (Cerner Corporation, Kansas City, MO). DC Cohort data are linked semi-annually to the DC Department of Health (DOH)’s HIV/AIDS, Hepatitis, STD and TB Administration surveillance databases.30 Post-linked data include HIV-related laboratory tests (i.e., CD4 count and viral load (VL) test results) that participants received from both DC Cohort and non-DC Cohort providers, improving our ascertainment of RIC and VS. At the time of this analysis, 13 DC Cohort sites were enrolling participants: eight were hospital-based and five were community-based clinics. Approximately 95% were treatment-experienced. The study protocol was approved by the George Washington University Institutional Review Board (IRB), the DC Department of Health IRB, and the IRBs of the individual study sites.
Eligibility criteria
Participants enrolled between January 1, 2011 and June 15, 2015 were eligible for inclusion in the analysis. Participants were considered LTFU if, after manual review, no lab or EMR data were available for 18 months or longer as of December 15, 2014. Those who withdrew from the study, were LTFU, or resided outside DC were excluded. Three residential ZIP codes with fewer than five participants were also excluded for participant confidentiality.
Outcome variables
RIC and VS were defined using recommendations from the US Department of Health and Human Services.31 RIC was defined as ≥2 clinical encounters and/or HIV-related labs ≥90 days apart in a 12-month period between June 2014 and June 2015. VS was defined as the last VL lab result <200 copies/ml as of June 2015 among participants who were retained. Only participants who met the RIC definition were included in the VS analysis so that we could assess predictors of VS that were independent of RIC. Area-level percent RIC and VS were computed by aggregating person-level outcomes to the ZIP code-level. Depending on the type of analysis, ZIP-code level percent RIC and VS were either treated as a percent or grouped into quartiles where the lowest quartile represented the lowest proportion of participants RIC or VS.
Exposure variables
Distance to care, the primary exposure variable of interest, was computed as the Euclidean distance between the population-weighted ZIP code centroid and provider street address (X,Y coordinate). Residential street addresses for participants were not available in our de-identified dataset, though they were available to the DC DOH. Due to concerns about patient privacy, analyses at geographies of more consistent population size such as census tract or census block were not possible. The DC DOH provided us a limited dataset containing population-weighted centroids. This approach adjusts the location of the geographic centroid of a ZIP code (i.e., the “centermost” location) to the area of a ZIP code where the population resides, thus improving our ascertainment of residence in the absence of full participant street address. For homeless participants whose current residence was a shelter, participant ZIP codes were assigned to the ZIP code of the shelter. Among those enrolled at a DC Cohort site with multiple affiliated clinics located across more than one ZIP code in DC, provider location was assigned to the clinic most frequently reported as the HIV provider on laboratory reports. Among participants whose laboratory results reported a non-DC Cohort provider more frequently than a DC Cohort provider (<1%), provider location was assigned to the non-DC Cohort site. Among participants whose HIV-related laboratory results were split between two non-DC Cohort sites (i.e., ties), provider location was assigned to the site in closest proximity to the participant.
Covariates
Person-level demographics and characteristics such as housing status, mode of HIV transmission, employment status, insurance type, prescription for antiretroviral therapy (ART), history of AIDS, and selected co-morbid conditions based on their higher prevalence in the Cohort (i.e., hypertension, diabetes, asthma, Hepatitis C virus, illicit drug abuse/dependence and depression) were included. A limited number of clinic-level variables such as clinic type (hospital-based versus community-based) and receipt of primary care (yes versus no) was included. Other clinic-level characteristics thought to influence care were not available at the time of this analysis.
Descriptive analyses
Differences in sociodemographic and clinical characteristics and distance to care by RIC and VS status were assessed using χ2 statistics in SAS v9.4 (Cary, NC). Choropleth maps of DC were generated to visualize the proportion of participants, distance to care, RIC and VS by ZIP-code. Provider locations were mapped by clinic type.
Spatial analyses
Maps
The DC Master Address Repository, a comprehensive address database containing key geographies for DC addresses such as X, Y, coordinates, was used to map provider street addresses to geographic coordinates (i.e. geocode) in a Geographic Information System (GIS).32 Geographic coordinates of participant residence were approximated by participant’s ZIP code of residence. US Postal Service ZIP codes were designed to improve mail delivery service. They do not necessarily represent where individuals live as they may include PO Boxes, areas with no residential population and unique areas including university campuses, and other large mail generating organizations such as Walter Reed Medical Center and the Navy Yard.33 In contrast, ZCTAs were designed to represent geographic areas based on underlying Census geography and population, and may better reflect where the population resides.34 To map the DC geography using Tiger shapefiles that are based on ZCTA boundaries as opposed to ZIP code boundaries, we created a crosswalk between residential ZIP codes and ZCTAs using the most frequently occurring ZIP code within a ZCTA. We determined that ZCTA approximated ZIP codes and used this approximation for mapping.35,36
Hot spot analysis
Hot spot analyses (Getis-Ord Gi* statistics) were generated to detect clustering of high or low values using the software Arc GIS v10.3.1 by Environmental Systems Research Institute (Redlands, CA). To identify a statistically significant hot spot or cold spot, Z-scores were calculated for each ZIP code as the difference between the sum of the observed and expected values of RIC or VS with respect to its neighbors (i.e., ZIP codes that shared at least some border), relative to all ZIP codes. The number of neighbors ranged from a minimum of 2 to a maximum of 7. Z-scores were considered statistically significant if these relative differences were too great to be due to chance. A ZIP code assigned a high Z-score surrounded by neighboring ZIP codes with high Z-scores with a p-value <0.05 was defined as a ‘hot spot’ (i.e., clusters of high RIC or high VS). Similarly, a ZIP code assigned a low Z-score surrounded by neighboring ZIP codes with low Z-scores with a p-value <0.05 was defined as a ‘cold spot’ (i.e., clusters of low RIC or low VS). We generated ‘hot spot’ maps based on Z scores to illustrate clusters of RIC and VS (Figure 2). The significance and interpretation of cluster analyses can be influenced by the scale of the analysis, thus were subject to influence from extreme values in RIC or VS in a small number of ZIP codes. We therefore evaluated another indicator of spatial autocorrelation, local Moran’s I, for comparison. Moran’s I is computed similarly to the Gi* in that it compares observed and expected values based on a Z score algorithm, but, unlike Gi*, excludes the value for a given ZIP code when comparing to the average value of its neighbors.
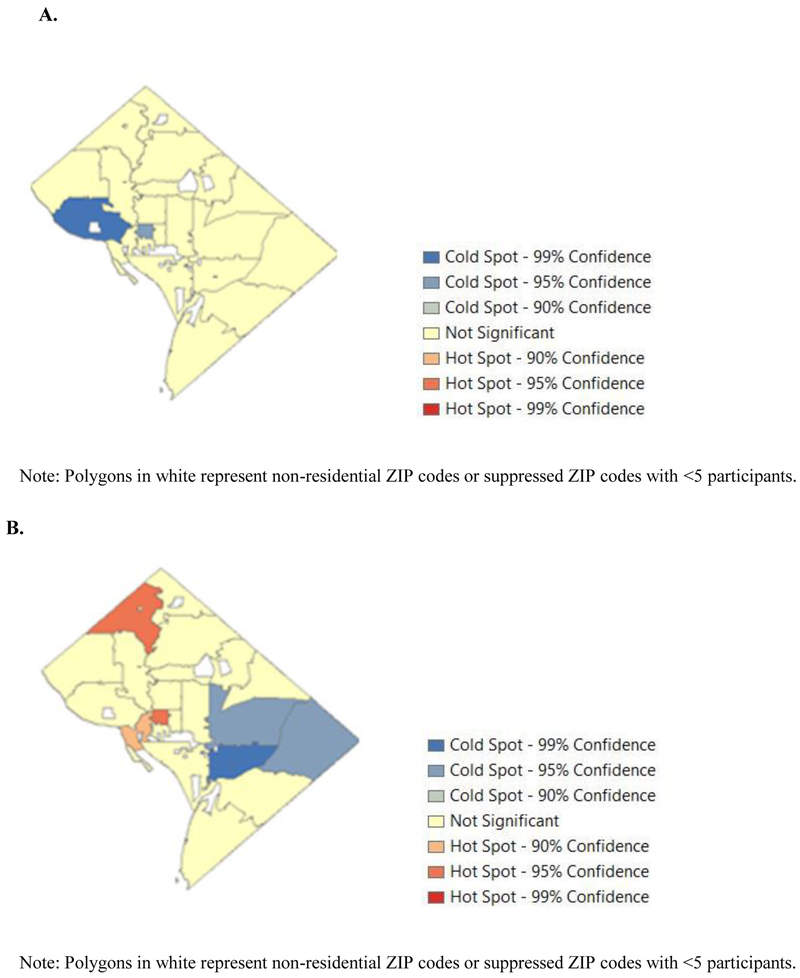
Figure 2. Hot spot analysis of retention-in-care (RIC) and viral suppression (VS) in Washington, DC 2014-2015.
A, RIC. B, VS.
Modeling
Sensitivity analyses were conducted to explore how distance (i.e., the distance from the population-weighted centroid of a participant’s residence to HIV provider) behaved with respect to RIC and VS, using pre-defined categories such as quartiles or specific cutoffs scaled to DC geography. We also treated distance as a continuous measure – including a measure with a quadratic term (distance2) to identify a potential nonlinear relationship. Based on sensitivity analyses, a non-linear relationship was observed in univariable models. Based on this finding, distance was modeled as a non-linear term in multivariable logistic regression models of RIC and VS. Models were adjusted for covariates that were identified as statistically significant at the 0.05 level in descriptive analyses. Hypothesis testing was two-sided and associations were considered significant at the p<0.05 level.
RESULTS
Descriptive analyses
Of the 5,521 participants, 4,476 (81%) were enrolled in the study with at least one year of follow-up by the end of 2014. The proportion of participants who were residents of DC was 91% (n=4,091). Of the 4,091, nearly 90% had ZIP code data corresponding to 23 out of 31 possible ZIP codes. Three of the 23 ZIP codes, comprising less than five participants, were excluded for confidentiality. The remaining 20 ZIP codes were included in subsequent analyses, representing 3,623 participants and reaching all four quadrants of the city.
The proportion of participants who were non-Hispanic (NH) black was 82%; the median age was 50 years (Table 1). Similar to the overall DC Cohort, a majority of participants were publicly insured (74%) and receiving HIV care at community-based clinics (60%) and almost half were men who had sex with men (49%). At enrollment, median duration of HIV diagnosis was 14 years, 97% of participants were treatment experienced, and 62% had a history of AIDS.
Table 1.
Baseline sociodemographic and clinical characteristics of DC Cohort participants by person-level retention-in-care (N=3,623) and viral suppression (N=2,556).
| Overall N (%) |
Retention-in-care1 N (%) |
Viral suppression2 N (%) |
|||||
|---|---|---|---|---|---|---|---|
| No | Yes | P-value | No | Yes | P-value | ||
| 3,623 (100) | 972 (27.0) | 2,651 (73.0) | 580 (23) | 1,976 (77.3) | |||
| Distance ≥ 5 miles | |||||||
| No | 3,173 (87.6) | 774 (79.6) | 2,399 (90.5) | <0.0001 | 503 (86.7) | 1,816 (91.9) | <0.0001 |
| Yes | 450 (12.4) | 198 (20.4) | 252 (9.5) | 77 (13.3) | 160 (8.1) | ||
| Age as of June 2015 | |||||||
| Median (IQR) | 50 (40-57) | 47 (37-54) | 51 (42-58) | <.0001 | 50 (37-55) | 52 (43-58) | <.0001 |
| Sex at birth | |||||||
| Female | 1,114 (30.7) | 324 (29.1) | 790 (70.9) | 0.04 | 221 (38.1) | 534 (27.1) | <.0001 |
| Male | 2,509 (69.3) | 648 (25.8) | 1,861 (74.2) | 359 (61.9) | 1,442 (72.9) | ||
| Race/ethnicity3 | |||||||
| Hispanic | 151 (4.2) | 34 (3.5) | 117 (4.4) | <.0001 | 20 (3.5) | 94 (4.8) | <.0001 |
| NH Black | 2,978 (82.2) | 756 (77.8) | 2,222(83.8) | 529 (91.2) | 1,613 (81.4) | ||
| NH White | 434 (12.0) | 160 (16.5) | 274 (10.3) | 21 (3.6) | 248 (12.5) | ||
| Other/Unknown | 60 (1.7) | 22 (2.3) | 38 (1.4) | 10 (1.3) | 26 (1.3) | ||
| Housing | |||||||
| Permanent | 2,807 (77.5) | 791 (81.4) | 2,016 (76.0) | <0.01 | 455 (78.5) | 1,489 (75.1) | 0.05 |
| Homeless/unstable | 368 (10.2) | 13 (1.3) | 56 (2.1) | 60 (10.3) | 222 (11.2) | ||
| Other/Unknown | 448 (12.4) | 105 (10.8) | 343 (12.9) | 65 (11.2) | 270 (13.7) | ||
| HIV Risk4 | |||||||
| MSM | 1,762 (48.6) | 486 (50.0) | 1,276 (48.1) | <.0001 | 219 (37.8) | 1,009 (51.1) | <.0001 |
| IDU | 603 (16.6) | 111 (11.4) | 492 (18.6) | 115 (19.9) | 361 (18.3) | ||
| Heterosexual | 1,099 (30.3) | 322 (33.1) | 777 (29.3) | 217 (37.5) | 531 (26.9) | ||
| Other/unknown | 159 (4.4) | 53 (5.5) | 106 (4.0) | 28 (4.8) | 73 (3.7) | ||
| Primary insurance5 | |||||||
| Private | 758 (20.9) | 344 (35.4) | 414 (54.6) | <.0001 | 86 (14.8) | 526 (26.6) | <.0001 |
| Public | 2,674 (73.8) | 579 (59.6) | 2,095 (79.0) | 460 (79.3) | 1,351 (68.4) | ||
| Other/ Unknown | 191 (5.3) | 49 (5.0) | 142 (5.4) | 34 (5.9) | 99 (5.0) | ||
| Employment status6 | |||||||
| Employed | 850 (23.5) | 342 (35.2) | 508 (19.2) | <.0001 | 37 (10.4) | 409 (13.1) | <.0001 |
| Unemployed | 1,223 (33.8) | 359 (36.9) | 864 (32.6) | 281 (48.5) | 550 (27.8) | ||
| Other | 1,550 (42.8) | 271 (27.9) | 1,279 (48.2) | 223 (38.4) | 1,017 (51.5) | ||
| Clinic type7 | |||||||
| Hospital | 1,467 (40.5) | 650 (66.9) | 817 (30.8) | <.0001 | 107 (18.4) | 679 (34.3) | <0.001 |
| Community | 2,156 (59.5) | 322 (33.1) | 1,834 (69.2) | 473 (81.6) | 1,297 (65.6) | ||
| Receipt of primary care | |||||||
| Yes | 2,788 (78.0) | 528 (55.5) | 2,260 (86.2) | <.0001 | 535 (93.0) | 1,643 (84.2) | <.0001 |
| No | 786 (22.0) | 424 (44.5) | 362 (13.8) | 40 (7.0) | 309 (15.8) | ||
| History of AIDS | 2,259 (62.4) | 585 (60.2) | 1,674 (63.1) | 0.1 | 378 (65.2) | 1,259 (63.7) | 0.52 |
NOTE: NH=non-Hispanic; MSM = men who have sex with men; IDU = injection drug user; IQR = interquartile range. P-values were computed using Pearson (goodness of fit) χ2statistics for categorical variables and t-tests for continuous variables. P-values ≤ 0.05 alpha level were considered statistically significant.
Retention-in-care was defined as evidence of ≥ 2 HIV-related clinical encounters and/or HIV-related labs ≥90 days apart in 12 months.
Viral suppression was defined as VL <200 copies/mL at last visit among those retained in care and prescribed antiretroviral therapy.
Other race groups include those of multiple race group and unknown.
MSM risk includes persons identified as having both MSM and IDU risk.
Primary insurance type was either public (Medicare, Medicaid, Ryan White/ADAP, or DC Alliance) or private (commercial payer or Tricare).
Other includes retired, student, disabled, termination of student, unknown, and other.
Site type defined as either hospital or community-based.
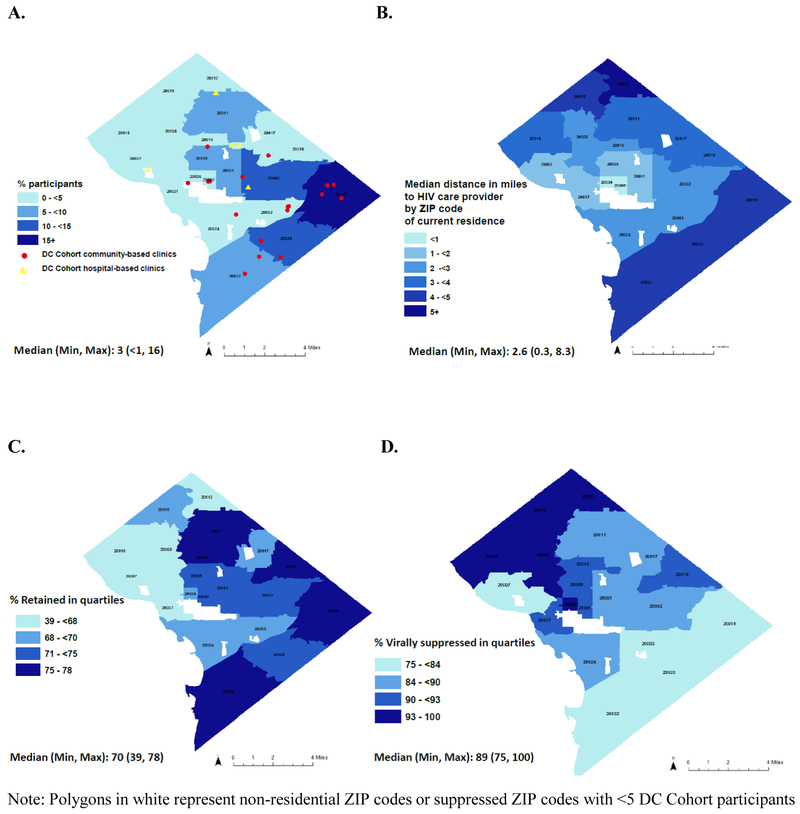
The proportion of participants residing in 20 ZIP codes ranged from <1 to 16% with the highest proportions residing in the Southeast (SE) quadrant (top 3 ZIP codes: 20020, 2019 and 20002) and the lowest proportions residing in the Northwest (NW) quadrant of the city (Figure 1A). DC Cohort sites were in 10 of the 20 ZIP codes with all hospital-based sites located in the NW and over 75% of community-based sites located in the South and Southeast quadrants (Figure 1A). Distance to HIV care ranged from 0.3 to 8.3 miles (median: 2.6), with longer distances, on average, for participants residing in ZIP codes bordering Virginia and Maryland (Figure 1B).
Figure 1. Geography, density, distance to care to HIV care, retention-in-care (RIC) and viral suppression (VS) by ZIP code of residence in Washington, DC 2014-2015.
A, % participants residing in ZIP codes with areas in darker blue representing ZIP codes with higher percentages. Red circles and yellow triangles represent DC Cohort community-based and hospital-based clinics, respectively. B, Median distance to care site with areas in darker blue representing ZIP codes with participants travelling longer distances to care. C, RIC % (Quartiles) with areas in darker blue representing ZIP codes with higher RIC. D, VS% (Quartiles) with areas in darker blue representing ZIP codes with higher VS.
Overall RIC was 73% among 3,623 participants (Table 1). The proportion on ART was nearly 97% among participants RIC (n=2,651). Among those RIC and on ART (n=2,556), 77% achieved VS (n=1,976). ZIP code-level proportion RIC and VS ranged from 39 to 78 (median: 70) and from 75 to 100 (median: 89), respectively (Figures 1C-D).
Differences in age, sex at birth, race/ethnicity, mode of HIV transmission, insurance status, employment status, clinic type and receipt of primary care were observed by overall RIC and VS status (all p<0.05). Participants who were retained were more likely to receive primary care at their HIV clinic and were more likely to receive care at a community-based clinic (p<0.001), while those who were VS were less likely in regard to both (p<0.001). No differences were observed by history of AIDS or duration of infection (data not shown).
Modeling
In sensitivity analyses, a non-linear relationship between distance and outcomes was detected when using the terms, distance (in miles) and distance2. Using these terms for distance, a threshold effect was observed for those travelling 5 or more miles such that those travelling farther had, on average, worse outcomes for RIC and VS. In multivariable models of RIC, participants who travelled ≥5 miles were 30% less likely to be retained (adjusted odds ratio, aOR=0.71, 95% CI: 0.58, 0.86). Correspondingly, the proportion of participants not RIC who travelled farther (≥5 miles) was twice that of those RIC (20% vs 10%; p<0.0001). In multivariable models of VS, participants who travelled ≥5 miles were 30% less likely to achieve VS (aOR=0.70, 95% CI: 0.52, 0.94) (Table 2), with a lower proportion of participants VS travelling farther (≥5 miles) than those without VS (8% vs 13%; p<0.0001). Multivariable regression models, also elucidated some of the patient and community characteristics driving RIC and VS, including sex, race/ethnicity, housing status, and risk factors for HIV transmission (Table 2).
Table 2.
Factors associated with achieving retention-in-care (RIC) and viral suppression (VS) in Washington, DC 2014-2015.
| Retention-in-care N=3,623 |
Viral suppression N=2,658 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Univariable | Multivariable | Univariable | Multivariable | |||||||
| N (%) | OR | 95% CI | aOR | 95% CI | N (%) | OR | 95% CI | aOR | 95% CI | |
| Distance ≥5 miles | ||||||||||
| No | 3,173 (78.5) | Ref | Ref | Ref | Ref | 2,421 (89.7) | Ref | Ref | Ref | Ref |
| Yes | 450 (12.5) | 0.52 | 0.44, 0.62 | 0.71 | 0.58, 0.86 | 237 (9.3) | 0.76 | 0.59, 0.99 | 0.70 | 0.52, 0.94 |
| Age as of June 2015 | ||||||||||
| Med (IQR) | 50 (40-57) | 1.02 | 1.02, 1.03 | 1.02 | 1.01, 1.03 | 49.4 (12.1) | 1.02 | 1.01, 1.03 | 1.02 | 1.01, 1.03 |
| Sex at birth (Female) | 1,114 (30.7) | 0.85 | 0.73, 0.99 | 0.83 | 0.65, 1.06 | 755 (29.5) | 0.60 | 0.49, 0.72 | 0.95 | 0.73, 1.24 |
| Race/ethnicity3 | ||||||||||
| Hispanic | 151 (4.2) | 2.01 | 1.31, 3.09 | 1.65 | 1.02, 2.67 | 114 (4.5) | 0.40 | 0.21, 0.77 | 0.66 | 0.33, 1.31 |
| NH Black | 2,978 (82.2) | 1.72 | 1.39, 2.12 | 1.33 | 1.02, 1.74 | 2,137 (83.6) | 0.26 | 0.16, 0.40 | 0.42 | 0.26, 0.71 |
| NH White | 434 (12.0) | Ref | Ref | Ref | Ref | 269 (10.5) | Ref | Ref | Ref | Ref |
| Other/Unknown | 60 (1.7) | 1.01 | 0.58, 1.77 | 1.18 | 0.62, 2.25 | 36 (1.4) | 0.22 | 0.09, 0.52 | 0.58 | 0.15, 0.96 |
| Housing | ||||||||||
| Permanent | 2,807 (77.5) | Ref | Ref | Ref | Ref | 1,939 (75.9) | Ref | Ref | NA | NA |
| Homeless/Temp | 368 (10.2) | 1.51 | 1.16, 1.97 | 0.82 | 0.60, 1.10 | 282 (11.0) | 1.13 | 0.83, 1.52 | ||
| Other/Unknown | 448 (12.4) | 1.28 | 1.02, 1.62 | 1.02 | 0.79, 1.32 | 335 (13.1) | 1.29 | 0.96, 1.72 | ||
| Employment status4 | ||||||||||
| Employed | 850 (23.5) | Ref | Ref | Ref | Ref | 485 (19.0) | Ref | Ref | Ref | Ref |
| Unemployed | 1,223 (33.8) | 1.62 | 1.35, 1.95 | 0.63 | 0.49, 0.80 | 831 (32.5) | 0.36 | 0.27, 0.47 | 0.58 | 0.42, 0.80 |
| Other | 1,550 (42.8) | 3.18 | 2.63, 3.84 | 1.14 | 0.89, 1.45 | 1,240 (48.5) | 0.85 | 0.64, 1.13 | 1.35 | 0.98, 1.85 |
| HIV Risk5 | ||||||||||
| MSM | 1,762 (48.6) | 1.09 | 0.92, 1.29 | 0.98 | 0.75, 1.28 | 1,228 (48.1) | 1.89 | 1.53, 2.35 | 1.52 | 1.13, 2.04 |
| IDU | 603 (16.6) | 1.84 | 1.44, 2.34 | 1.27 | 0.96, 1.68 | 476 (18.6) | 1.26 | 0.97, 1.64 | 1.23 | 0.93, 1.63 |
| Heterosexual | 1,099 (30.3) | Ref | Ref | Ref | Ref | 748 (29.3) | Ref | Ref | Ref | Ref |
| Other/unknown | 159 (4.4) | 0.85 | 0.59, 1.23 | 1.55 | 1.02, 2.35 | 101 (3.9) | 1.07 | 0.68, 1.71 | 0.72 | 0.43, 1.21 |
| Insurance type6 | ||||||||||
| Private | 758 (20.9) | Ref | Ref | Ref | Ref | 401 (15.7) | Ref | Ref | Ref | Ref |
| Public | 2,674 (73.8) | 3.01 | 2.54, 3.56 | 1.89 | 1.49, 2.38 | 2,022 (79.1) | 0.50 | 0.37, 0.68 | 0.98 | 0.72, 1.33 |
| Other/Unknown | 191 (5.3) | 2.41 | 1.69, 3.43 | 1.15 | 0.76, 1.74 | 133 (5.2) | 0.47 | 0.29, 0.77 | 0.98 | 0.60, 1.62 |
| Clinic type | ||||||||||
| Hospital | 1,467 (40.5) | Ref | Ref | Ref | Ref | 786 (30.8) | Ref | Ref | Ref | Ref |
| Communitv | 2.156 (59.5) | 4.53 | 3.87, 5.30 | 2.72 | 2.16, 3.42 | 1.770 (69.3) | 0.43 | 0.34, 0.54 | 0.44 | 0.32, 0.62 |
| Primary care at site | 2,788 (78.0) | 5.01 | 4.23, 5.94 | 1.98 | 1.58, 2.48 | 2,178 (86.2) | 0.39 | 0.28, 0.56 | 0.68 | 0.44, 1.05 |
| History of AIDS | 2,259 (62.4) | 1.13 | 0.98, 1.32 | NA | NA | 1,637 (64.1) | 0.94 | 0.78, 1.14 | NA | NA |
NOTE: NH=non-Hispanic; MSM = men who have sex with men; IDU = injection drug user; IQR = interquartile range. P-values were considered statistically significant at the 0.05 alpha level.
RIC was defined as evidence of ≥ 2 HIV-related clinical encounters and/or HIV-related labs ≥90 days apart in 12 months.
VS was defined as VL <200 copies/mL at last visit among those retained in care and prescribed antiretroviral therapy.
Other race groups include those of multiple race group and unknown.
Other includes retired, student, disabled, termination of student, unknown, and other.
MSM risk includes persons identified as having both MSM and IDU risk.
Primary insurance type was either public (Medicare, Medicaid, Ryan White/ADAP, or DC Alliance) or private (commercial payer).
Spatial analyses
Geographic clustering of RIC was observed, with a pattern of lower RIC in the NW, and significantly lower RIC (i.e., cold spots) in two ZIP codes (Figure 2C). These cold spots had RICs of 60% (Gi* p<0.01) and 70% (Gi* p<0.05), respectively. In contrast, ZIP codes outside of these areas had an average RIC of 73%. Significant cold spots for VS were similarly detected in the SE quadrant of the city (VS=75-83%, Gi* p<0.05). The mean proportion of VS across ZIP codes was high (90%), with hot spots in the NW (Figure 2B). One of these ZIP codes included 100% VS (Gi* p<0.05) and another 95% (Gi* p<0.10). Only one ZIP code, located in the center of DC, belonged to both a cluster of low RIC and a cluster of high VS. Clustering revealed by Moran’s I was generally consistent with these results, with few exceptions.
Distance traveled differed by cold spot and hot spot status. In terms of distance as a continuous measure, participants in RIC cold spots travelled, on average, 2.5 fewer miles than those residing outside RIC cold spots (0.6 miles versus 3.1 miles; p<0.0001). In terms of distance as a dichotomous variable with a cut off at 5 miles, no participants in RIC cold spots traveled ≥5 miles (0%) compared with 13% outside RIC cold spots (Table 4). In contrast, participants in VS cold spots travelled nearly a mile more, on average, than those outside VS cold spots (3.2 miles versus 2.3 miles; p<0.0001). Less than 10% of those in VS cold spots travelled ≥5 miles (8%) compared with 10% outside VS cold spots (p<0.05). Participants in VS hot spots travelled 0.7 fewer miles than those outside VS hot spots (2.1 miles versus 2.8 miles; p<0.01), with only 3% travelling ≥5 miles compared with 9% outside VS hot spots. This observation was not statistically significant likely due to small cell sizes (p=0.22).
DISCUSSION
This analysis represents one of the first attempts to analyze spatial patterns of HIV care using clinical, laboratory, treatment and surveillance data. Overall, person-level RIC (73%) was slightly higher than national estimates (69%) and similar to estimates from other local and nationally representative studies (54-78%)37-48 Higher estimates of RIC and VS were not surprising as DC Cohort participants represented persons at least minimally engaged in HIV care.49
Factors associated with RIC likely differ from factors associated with VS, even in the setting of high ART coverage. This finding was observed at both the individual-level and the cluster-level in our data. Those RIC were not necessarily less likely to achieve VS; clusters of low RIC did not geographically overlap with clusters of low VS. This paradox may be explained, in part, by a subset of participants who were VS and not indicated to visit an HIV provider as often, thereby appearing to not be retained - an observation noted in other US cohorts.47,48 For those appearing retained, multiple encounters and/or laboratory results may be related to their assessed risk for suboptimal HIV outcomes. Perhaps this group returned to care more often because providers scheduled them at more frequent intervals, based on concerns about patient health or compliance, or about losing contact with the most vulnerable or transient clients. This hypothesis is consistent with our finding that those RIC were more likely to be unemployed, publicly insured, and not receiving primary care at their DC Cohort site compared with those not RIC.
Our near real-time data showed that participants residing in clusters of low VS, areas containing several community-based clinics, had longer distances to care. These results suggest that the ‘closest’ HIV provider may not be the ‘likely’ provider, and indicate that reasons for selecting a particular location(s) for HIV services are complex. One study of HIV-infected persons in DC found that those with poor clinical outcomes also tend to receive care at multiple HIV care providers, known as site migration.50 While approximately 75% of DC Cohort participants received care at only one site, those who do seek care at >1 site may also be traveling farther. In post-hoc sensitivity analyses assessing prevalence of comorbid conditions, we found that participants travelling ≥5 miles were more likely to have Hepatitis C virus and depression, yet no more likely to have hypertension, diabetes, asthma or drug abuse and dependence (data not shown). Thus, it is not clear if the prevalence of comorbid conditions in this population may be related to where people seek care for their HIV.
Seeking care farther away from one’s residence may be due to preference for a particular clinic. Preferences are likely varied and may include factors related to quality of service, accessibility and availability, experience, confidentiality, proximity to pharmacies, co-located primary care services, changes in eligibility for ancillary services, and integrated supportive services such as case management and care navigation.50,51 Seeking care farther away may also be a proxy for other unmeasured factors. Most participants who travelled ≥5 miles resided in the SE quadrant of the city or "East of the river," a reference to the physical and socioeconomic divide created by the Anacostia River. Neighborhoods like Anacostia, Congress Heights and Hillcrest and other neighborhoods in Wards 7 and 8 are marked by higher poverty (i.e., higher percentages of residents living below the federally-defined poverty line), higher housing instability and higher HIV burden than other parts of DC.22 These areas are also part of the SE transit corridor which is hampered by longer commuter times, fewer metro stops, higher bus ridership, higher bus overcrowding and lower bus reliability than other parts of the city.52-54
Limited access to modes of travel and access to destinations could be a potential barrier to care. For example, travel time and convenience for participants are likely to vary for different modes of transportation. In Washington DC, rain transfers are likely required for many participants to reach a DC Cohort site, as several sites including all hospital-based sites are in the NW and many medical specialists are in one area of the city. Moreover, schedules for DC’s metro transit lines vary, contributing to differences in travel time to access a provider for the same distance.54 For instance, a trip to the center of the city could take more than 45 minutes from the SE compared to average travel time of 25-30 minutes from the NW.54-56 Moreover, given that less than 25% of publicly-insured residents from ‘East of the River’ seek primary care in their ZIP code, it is likely this group travels longer distances.57 While it is plausible that these factors may similarly affect HIV care in other urban/metro environments, additional research is needed to better understand how participants travel to their HIV provider and how these factors influence HIV care.
Interpretation of our findings is subject to limitations. First, this analysis focuses on HIV care after testing, diagnosis, and linkage to care. DC Cohort participants represent an in-care population attending outpatient clinics and consenting to participate in an HIV cohort study. This group may not be representative of all HIV-infected DC residents, particularly those who have not been diagnosed or linked to care. Targeting the earlier stages in the care continuum such as HIV testing and linkage to care remains a challenge. Second, we were not able to discriminate whether an HIV-related laboratory result from a given hospital came from the HIV clinic (routine care) or emergency room (acute care). We did not have access to participant street address, potentially introducing measurement error in our computations of distance to care. However, weighting ZIP code centroids to the DC Cohort population mitigated this potential source of misclassification. We note that methods to estimate and operationalize travel patterns are not standardized, and our findings based on Euclidean (i.e., straight line) distance may not be easily interpreted. While such distance is often used as a proxy for access to care, other methods that incorporate travel patterns associated with driving and mass transit lines may improve distance to care estimates, especially in urban areas heavily reliant on public transportation.6,58 Additionally, spatial aggregation of person-level data to the ZIP code-level may have introduced a type of statistical bias known as the modifiable areal unit problem, which makes area-level estimates of RIC and VS dependent on both the shape and size of the ZIP code.59 Lastly, findings on the role of distance and HIV care may not be generalizable to other cities, as DC’s geography, population density and mobility, transportation and HIV/AIDS-related policies and programs may differ from other cities.
The finding that longer distances to care may be a barrier to HIV care provides practical information on a possible barrier to care. Moreover, it underscores the need to increase accessibility, acceptability and uptake of services to improve HIV care. Such interventions should be tailored to specific geographical areas at increased risk for suboptimal RIC and VS.
Table 3.
Distance to care, demographic and clinical characteristics of DC Cohort participants residing in clusters of low retention-in-care (RIC), low viral suppression (VS) and high VS in Washington, DC 2014-2015.
| Participants residing in cluster of neighboring ZIP-codes with low RIC N=3,623 |
Participants residing in cluster of neighboring ZIP-codes with low VS N=2,568 |
Participants residing in cluster of neighboring ZIP-codes with high VS N=2,568 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| In cluster | Not in cluster | p-value | In cluster | Not in cluster | p-value | In cluster | Not in cluster | p-value | |
| 45 (1.2) | 3,578 (98.8) | 811 (31.6) | 1,757 (68.4) | 32 (1.2) | 2,536 (98.8) | ||||
| Distance ≥5 miles | |||||||||
| No | 45 (100) | 3,128 (87.4) | <0.01 | 743 (92.4) | 1,576 (90.0) | <0.05 | 31 (96.9) | 2,288 (90.7) | 0.22 |
| Yes | 0 (0) | 450 (12.6) | 61 (7.6) | 176 (10.0) | 1 (3.1) | 236 (9.3) | |||
| Median (IQR) | 0.6 (0.2, 2.3) | 3.1 (1.6, 4.5) | <.0001 | 3.2 (2.5, 4.5) | 2.3 (1.2, 4.0) | <.0001 | 2.1 (0.6, .0) | 2.8 (1.6, 4.2) | <0.01 |
| Retained | 29 (64.4) | 2,609 (72.4) | 0.18 | 843 (74.1) | 1,808 (72.8) | 0.40 | NA | NA | NA |
| VS | 26 (57.8) | 1,955 (54.6) | 0.67 | 585 (72.1) | 1,396 (79.5) | <0.0001 | 30 (96.8) | 1,951 (84.6) | 0.06 |
| Age as of June 2015 | |||||||||
| Median (IQR) | 52 (47-61) | 50 (40-57) | 0.12 | 51 (43-58) | 51 (42-58) | 0.83 | 51 (42, 58) | 53 (44, 62) | <0.001 |
| Sex at birth | |||||||||
| Male | 42 (93.3) | 2,467 (68.9) | <0.001 | 523 (64.5) | 1,284 (73.1) | <0.001 | 30 (93.7) | 1,777 (70.1) | <0.01 |
| Female | 3 (6.7) | 1.111 (31.1) | 288 (35.5) | 473 (26.9) | 2 (6.3) | 759 (29.9) | |||
| Race/ethnicity3 | |||||||||
| Hispanic | 4 (8.9) | 147 (4.1) | <.0001 | 15 (1.8) | 99 (5.6) | <.0001 | 4 (12.5) | 110 (4.3) | <0.0001 |
| NH Black | 9 (20.0) | 2,969 (83.0) | 752 (92.7) | 1,397 (79.5) | 12 (37.5) | 2,137 (84.3) | |||
| NH White | 28 (62.2) | 406 (11.3) | 37 (4.6) | 232 (13.2) | 15 (46.9) | 254 (10.0) | |||
| Other/Unknown | 4 (8.9) | 56 (1.6) | 7 (0.9) | 29 (1.7) | 1 (3.1) | 35 (1.4) | |||
| Housing | |||||||||
| Permanent | 42 (93.3) | 2,765 (77.3) | 0.04 | 615 (75.8) | 1,335 (76.0) | 0.99 | 26 (81.2) | 1,924 (75.9) | 0.51 |
| Homeless/Unstable | 1 (2.2) | 367 (10.3) | 89 (11.0) | 194 (11.0) | 4 (12.5) | 279 (11.0) | |||
| Other/Unknown | 2 (4.4) | 446 (12.5) | 107 (13.2) | 228 (13.0) | 2 (6.3) | 333 (13.1) | |||
| Employment status4 | |||||||||
| Employed | 21 (46.7) | 829 (23.2) | <.001 | 131 (16.2) | 355 (20.2) | <.0001 | 8 (25.0) | 478 (18.9) | 0.23 |
| Unemployed | 5 (11.1) | 1,218 (34.0) | 317 (39.1) | 523 (29.8) | 6 (18.7) | 834 (32.9) | |||
| Other | 19 (42.2) | 1,531 (42.8) | 363 (44.8) | 878 (50.0) | 18 (56.3) | 1,224 (48.3) | |||
| HIV Risk5 | |||||||||
| MSM | 35 (77.8) | 1,727 (48.3) | <0.101 | 315 (38.8) | 916 (52.1) | <.0001 | 24 (75.0) | 1,207 (47.6) | <0.01 |
| IDU | 3 (6.7) | 600 (16.8) | 185 (22.8) | 185 (22.8) | 2 (6.3) | 752 (29.7) | |||
| Heterosexual | 6 (13.3) | 1,093 (30.5) | 278 (34.3) | 476 (27.1) | 4 (12.5) | 475 (18.8) | |||
| Other/unknown | 1 (2.2) | 158 (4.4) | 33 (4.1) | 71 (4.0) | 2 (6.2) | 99 (3.9) | |||
| Primary insurance type6 | |||||||||
| Private | 27 (55.6) | 733 (20.5) | <.0001 | 99 (12.2) | 302 (17.2) | <0.001 | 13 (40.6) | 388 (15.3) | <0.001 |
| Public | 19 (42.2) | 2,655 (74.2) | 679 (83.7) | 1,355 (77.1) | 19 (59.4) | 2,015 (79.5) | |||
| Other/Unknown | 1 (2.2) | 190 (5.3) | 33 (4.1) | 100 (5.7) | 0 (0) | 133 (5.2) | |||
| Clinic type | |||||||||
| Hospital | 30 (66.7) | 1,437 (40.2) | <0.001 | 237 (29.2) | 549 (31.2) | 0.30 | 16 (50) | 770 (30.4) | <0.05 |
| Community | 14 (33.3) | 2,141 (59.8) | 574 (70.8) | 1,207 (68.8) | 16 (50) | 1,766 (69.6) | |||
| Primary care at site | |||||||||
| Yes | 27 (60.0) | 2,761 (78.2) | <0.01 | 695 (86.9) | 1,495 (86.0) | 0.54 | 25 (78.1) | 2,165 (86.4) | 0.18 |
| No | 18 (40.0) | 768 (21.8) | 105 (13.1) | 244 (14.0) | 7 (21.9) | 342 (13.6) | |||
| History of AIDS | |||||||||
| Yes | 23 (51.1) | 2,236 (62.5) | 0.12 | 520 (64.1) | 1,123 (63.9) | 0.92 | 21 (65.6) | 1,622 (65.6) | 0.85 |
| No | 22 (48.9) | 1,342 (37.5) | 291 (35.9) | 634 (36.1) | 11 (34.4) | 914 (34.4) | |||
NOTE: NH=non-Hispanic; MSM = men who have sex with men; IDU = injection drug user; IQR = interquartile range. P-values were computed using Pearson (goodness of fit) χ2statistics for categorical variables and using t-tests for equal variances for continuous variables. For all tests, p-values were considered statistically significant at the 0.05 alpha level.
RIC was defined as evidence of ≥ 2 HIV-related clinical encounters and/or HIV-related labs ≥90 days apart in 12 months.
Viral suppression (VS) was defined as viral load <200 copies/mL at last visit among those retained in care and prescribed antiretroviral therapy.
Other race groups include those belonging to more than race group and unknown.
Other includes retired, student, disabled, termination of student, unknown, and other.
MSM risk includes persons identified as having both MSM and IDU risk.
Primary insurance type was either public (Medicare, Medicaid, Ryan White/ADAP, or DC Alliance) or private (commercial payer).
Acknowledgements
Data in this manuscript were collected by the DC Cohort Study Group with investigators and research staff located at: Cerner Corporation (Thilakavathy Subramanian, Jeffery Binkley, Rachel Hart, Rob Taylor, Nabil Rayeed, Cheryl Akridge, Stacey Purinton, Kate Shelton, Christy Dueck, Ryan Moog, Jeff Naughton); Children's National Medical Center Adolescent (Lawrence D'Angelo) and Pediatric (Natella Rahkmanina) clinics; The Senior Deputy Director of the DC Department of Health HAHSTA (Michael Kharfen); Family and Medical Counseling Service (Angela Wood); Georgetown University (Princy Kumar); George Washington University Medical Faculty Associates (David Parenti); George Washington University Department of Epidemiology and Biostatistics (Maria Jaurretche, Brittany Lewis, James Peterson, Jeanne Jordan, Kamwing Jair, Keith Crandall, Marcos Perez-Losada, Taylor Maxwell; Howard University Adult Infectious Disease Clinic (Ronald Wilcox), and Pediatric Clinic (Sohail Rana); Kaiser Permanente (Michael Horberg); La Clinica Del Pueblo, (Ricardo Fernandez); MetroHealth (Annick Hebou); National Institutes of Health (Carl Dieffenbach, Henry Masur); Providence Hospital (Jose Bordon); Unity Health Care (Gebeyehu Teferi); Veterans Affairs Medical Center (Debra Benator); Washington Hospital Center (Maria Elena Ruiz); and Whitman-Walker Health (Deborah Goldstein, David Hardy)
Conflicts of Interest and Funding Source: This work was supported by the National Institute of Allergy and Infectious Diseases at the National Institutes of Health under Grant UO1 AI69503-03S2. The authors have no conflicts to declare.
Footnotes
Presented in part at the 2017 Conference on Retroviruses and Opportunistic Infections. Poster presentation #1312. February 14, 2017. Seattle, WA.
REFERENCES
- 1.Shacham E, Estlund AL, Tanner AE, et al. Challenges to HIV management among youth engaged in HIV care. AIDS Care. 2017. February;29(2):189–196. [DOI] [PubMed] [Google Scholar]
- 2.Dailey AF, Johnson AS, Wu B. HIV Care Outcomes Among Blacks with Diagnosed HIV - United States, 2014. MMWR Morb Mortal Wkly Rep. 2017. February 3;66(4):97–103. doi: 10.15585/mmwr.mm6604a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castel AD, Kalmin M, Hart R, et al. Disparities in achieving and sustaining viral suppression among a large cohort of HIV-infected persons in care - Washington, DC. AIDS Care. 2016. DOI: 10.1080/09540121.2016.1189496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolitski RJ, Kidder DP, Pals SL, et al. ; Housing and Health Study Team. Randomized trial of the effects of housing assistance on the health and risk behaviors of homeless and unstably housed people living with HIV. AIDS Behav. 2010;14(3):493–503. doi: 10.1007/s10461-009-9643-x. [DOI] [PubMed] [Google Scholar]
- 5.Ransome Y, Kawachi I, Braunstein S, et al. Structural inequalities drive late HIV diagnosis: The role of black racial concentration, income inequality, socioeconomic deprivation, and HIV testing. Health Place. 2016; 42:148–158. doi: 10.1016/j.healthplace.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eberhart MG, Voytek CD, Hillier A, et al. Travel distance to HIV medical care: a geographic analysis of weighted survey data from the Medical Monitoring Project in Philadelphia, PA. AIDS Behav. 2014. April;18(4):776–82. doi: 10.1007/s10461-013-0597-7. [DOI] [PubMed] [Google Scholar]
- 7.Scribner RA, Simonsen NR, Leonardi C. The Social Determinants of Health Core: Taking a Place-Based Approach. Am J Prev Med. 2017. January;52(1S1): S13–S19. doi: 10.1016/j.amepre.2016.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanna DB Hessol NA, Golub ET, Cocohoba JM, Cohen MH, Levine AM, Wilson TE, Young M, Anastos K, Kaplan RC. Increase in single-tablet regimen use and associated improvements in adherence-related outcomes in HIV-infected women. J Acquir Immune Defic Syndr. 2014. April 15;65(5):587–96. doi: 10.1097/QAI.0000000000000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McFall AM, Dowdy DW, Zelaya CE, et al. ; Women's Interagency HIV Study. Understanding the disparity: predictors of virologic failure in women using highly active antiretroviral therapy vary by race and/or ethnicity. J Acquir Immune Defic Syndr. 2013. November 1;64(3):289–98. doi: 10.1097/QAI.0b013e3182a095e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.An Q, Prejean J, McDavid Harrison K, et al. Association between community socioeconomic position and HIV diagnosis rate among adults and adolescents in the Unites States, 2005 to 2009. Am J Public Health. 2013; 103(1):120–6. DOI: 10.2105/AJPH.2012.300853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eberhart MG, Yehia BR, Hillier A, et al. Individual and community factors associated with geographic clusters of poor HIV care retention and poor viral suppression. J Acquir Immune Defic Syndr. S1 2015; 69 Suppl 1: S37–43. doi: 10.1097/QAI.0000000000000587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eberhart MG, Yehia BR, Hillier A, et al. Behind the cascade: analyzing spatial patterns along the HIV care continuum. J Acquir Immune Defic Syndr. S1 2013;64 Supp 1: S42–S51. doi: 10.1097/QAI.0b013e3182a90112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ransome Y, Kawachi I, Dean LT. Neighborhood Social Capital in Relation to Late HIV Diagnosis, Linkage to HIV Care, and HIV Care Engagement. AIDS Behav. 2017;21(3):891–904. doi: 10.1007/s10461-016-1581-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Terzian AS, Irvine M, McAllister, et al. Effect of HIV housing services on engagement in care and treatment, New York City, 2011. AIDS Behav. 2015. DOI: 10.1007/s10461-015-1003-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castel AD, Befus M, Willis S, et al. Use of the community viral load as a population-based biomarker of HIV burden. AIDS. 2012. January 28;26(3):345–53. doi 10.1097/QAD.0b013e32834de5fe. [DOI] [PubMed] [Google Scholar]
- 16.Goswami ND, Schmitz MM, Sanchez T, et al. Understanding local spatial variation along the care continuum: The potential impact of transportation vulnerability on HIV linkage to care and viral suppression in high-poverty areas, Atlanta, GA. J Acquir Immune Defic Syndr. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sheehan DM, Fennie KP, Mauck DE, et al. Retention in HIV Care and Viral Suppression: Individual- and Neighborhood-Level Predictors of Racial/Ethnic Differences, Florida, 2015. AIDS Patient Care STDS. 2017;31(4):167–175. doi: 10.1089/apc.2016.0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruiz MS, O’Rourke A, Allen ST. Impact Evaluation of a Policy Intervention for HIV Prevention in Washington, DC. AIDS Behav. 2016. 20: 22. doi: 10.1007/s10461-015-1143-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Division of HIV/AIDS Prevention. HIV/AIDS policy and law. Atlanta, Georgia: Centers for Disease Control and Prevention, 2016. Available at https://www.cdc.gov/hiv/policies/index.html. Accessed on May 10, 2017. [Google Scholar]
- 20.Blevins M, Wehbe FH, Rebeiro PF, et al. ; The Caribbean, Central, South America Network for HIV Epidemiology (CCASAnet). Interactive Data Visualization for HIV Cohorts: Leveraging Data Exchange Standards to Share and Reuse Research Tools. PLoS One. 2016; 11(3): e0151201. doi: 10.1371/journal.pone.0151201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scribner RA, Johnson SA, Cohen DA, et al. Geospatial methods for identification of core groups for HIV/AIDS. Sub Use Misuse 2008;43(2):203–21. doi: 10.1080/10826080701690607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.AIDSVu. Emory University, Rollins School of Public Health. Available at http://aidsvu.org/. Accessed on February 24, 2016.
- 23.Cook PA, Downing J, Wheater CP, et al. Influence of socio-demographic factors on distances travelled to access HIV services: enhanced surveillance of HIV patients in north west England. BMC Public Health 2009. June 12; 9:92. doi: 10.1186/1471-2334-9-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cope AB, Powers KA, Serre ML, et al. Distance to testing sites and its association with timing of HIV diagnosis. AIDS Care. 2016. November;28(11):1423–7. doi: 10.1080/09540121.2016.1191599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akullian AN, Mukose A, Levine GA, et al. People living with HIV travel farther to access healthcare: a population-based geographic analysis from rural Uganda. J Int AIDS Soc. 2016; 19(1): 20171. doi: 10.7448/IAS.19.1.20171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Magnus M, Herwehe J, Murtaza-Rossini M, et al. Linking and Retaining HIV Patients in Care: The Importance of Provider Attitudes and Behaviors. AIDS Patient Care STDS. 2013. May; 27(5): 297–303. doi: 10.1089/apc.2012.0423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greenberg AE, Hader SL, Masur H, et al. Fighting HIV/AIDS in Washington, D.C. Health Affairs 28, no.6 (2009):1677–1687. doi: 10.1377/hlthaff.28.6.1677 [DOI] [PubMed] [Google Scholar]
- 28.Brian McKenzie. 2015. “Transit Access and Population Change: The Demographic Profiles of Rail Accessible Neighborhoods in the Washington, DC Area.” SEHSD Working Paper No. 2015-023. U.S. Census Bureau; Washington, DC: Available at https://www.census.gov/content/dam/Census/library/working-papers/2015/demo/SEHSD-WP2015-23.pdf. Accessed May 8, 2017. [Google Scholar]
- 29.Kaiser Family Foundation (KFF). HIV/AIDS Policy Fact Sheet: The HIV/AIDS Epidemic in Washington, D.C. (2012). Available at https://kaiserfamilyfoundation.files.wordpress.com/2013/01/8335.pdf. Accessed May 10, 2017.
- 30.Greenberg AE, Hays H, Castel AD, et al. ; DC Cohort Executive Committee. Development of a large urban longitudinal HIV clinical cohort using a web-based platform to merge electronically and manually abstracted data from disparate medical record systems: technical challenges and innovative solutions. J Am Med Inform Assoc. 2016. May;23(3):635–43. doi: 10.1093/jamia/ocv176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Valdiserri RO, Forsyth AD, Yakovchenko V, et al. Measuring what matters: the development of standard HIV core indicators across the US Department of Health and Human Services. Public Health Rep. 2013; 128: 354–9. PMID: 23997280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DC GIS Program. DC GIS Master Address Repository. Office of Chief Technology Officer (OCTO). Available at http://dcatlas.dcgis.dc.gov/mar/. Accessed April 20, 2017. [Google Scholar]
- 33.ZIP Code FAQs. Available at http://www.zipboundary.com/zipcode_faqs.html. Accessed November 21, 2016.
- 34.ZIP Code Tabulation Areas. Available at https://www.arcgis.com/home/item.html?id=e9fb9ca01f81477ca9644fd598f63c01 Accessed September 28, 2017.
- 35.Erickson SR and Lin YN. Geospatial analysis of statin adherence using pharmacy claims data in the state of Michigan. J Manag Care Spec Pharm. 2014. December;20(12):1208–15. DOI: 10.18553/jmcp.2014.20.12.1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.US Census Bureau. US Census Bureau 2010. ZCTA shapefiles (tl_2010_state_zcta510.shp). Available at https://www.census.gov/geo/maps-data/data/tiger-line.html. Accessed December 1, 2016.
- 37.Cohen SM, Hu X, Sweeney P, et al. HIV viral suppression among persons with varying levels of engagement in HIV medical care, 19 US jurisdictions. J Acquir Immune Defic Syndr. 2014. December 15;67(5):519–27. DOI: 10.1097/QAI.0000000000000349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Althoff KN, Rebeiro P, Brooks JT, et al. Disparities in the quality of HIV care when using US Department of Health and Human Services Indicators. Clin Infect Dis. 2014; 58(8):1185–9. DOI: 10.1093/cid/ciu044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rebeiro PF, Althoff KN, Lau B, et al. North American AIDS Cohort Collaboration on Research and Design. Laboratory Measures as Proxies for Primary Care Encounters: Implications for Quantifying Clinical Retention Among HIV-Infected Adults in North America. Am J Epidemiol. 2015. December 1;182(11):952–60. DOI: 10.1093/aje/kwv181. Epub 2015 Nov 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rebeiro PF, Gange SJ, Horberg MA, et al. ; North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD). Geographic Variations in Retention in Care among HIV-Infected Adults in the United States. PLOS ONE. 2016. January 11;11(1): e0146119. doi: 10.1371/journal.pone.0146119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burchell AN, Gardner S, Ligh L, et al. Implementation and Operational Research: Engagement in HIV care among persons enrolled in a clinical HIV cohort in Ontario, Canada, 2001-2011. J Acquir Immune Defic Syndr. 2015. September 1;70(1):e10–9. DOI: 10.1097/QAI.0000000000000690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yehia BR, Stephens-Shields AJ, Fleishman JA, et al. ; HIV Research Network. The HIV care continuum: changes over time in retention in care and viral suppression. PLOS ONE, 2015; 10(6): e0129376 DOI: 10.1371/journal.one.0129376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hall HI, Frazier EL, Rhodes P, et al. Differences in human immunodeficiency virus care and treatment among subpopulations in the United States. JAMA Intern Med. 2013. July 22;173(14):1337–44. DOI: 10.1001/jamainternmed.2013.6841. [DOI] [PubMed] [Google Scholar]
- 44.Xia Q, Braunstein SL, Wiewel EW, et al. Persons Living with HIV in the United States: Fewer Than We Thought. J Acquir Immune Defic Syndr. 2016. March 19 DOI: 10.1097/QAI.0000000000001008. [DOI] [PubMed] [Google Scholar]
- 45.Greer GA, Tamhane A, Malhotra R, et al. Achieving Core Indicators for HIV Clinical Care Among New Patients at an Urban HIV Clinic. AIDS Patient Care STDS. 2015. September;29(9):474–80. DOI: 10.1089/apc.2015.0028. [DOI] [PubMed] [Google Scholar]
- 46.Korthuis PT, McGinnis KA, Kraemer KL, Gordon AJ, Skanderson M, Justice AC, et al. ; Veterans Aging Cohort Study. Quality of HIV Care and Mortality Rates in HIV-Infected Patients. Clin Infect Dis. 2016. January 15;62(2):233–9. DOI: 10.1093/cid/civ762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Engelhard E, Smit C, Nieuwkerk PT, Reiss P, Kroon FP, Brinkman K and Geerlings SE. Structure and quality of outpatient care for people living with an HIV infection. AIDS Care. 2016. March; 28(8): 1062–1072. doi: 10.1080/09540121.2016.1153590. [DOI] [PubMed] [Google Scholar]
- 48.Horberg MA. HIV Quality Measures and Outcomes: The Next Phase. Clin Infect Dis. 2016;62(2):240–1. [DOI] [PubMed] [Google Scholar]
- 49.Castel AD, Terzian AS, Hart R, Young H, Kalmin MM and Greenberg AE on behalf of the DC Cohort Executive Committee. Use of National Standards to Monitor HIV Care and Treatment in a High Prevalence City - Washington, DC. PLOS ONE. PLoS One. 2017. October 5;12(10):e0186036. doi: 10.1371/journal.pone.0186036. eCollection 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jia Y, Sengupta D, Opoku J, Wu C, Griffin A, West T, et al. Site migration in seeking care services from multiple providers is associated with worse clinical outcomes among HIV-infected individuals in Washington, DC. AIDS Care. 2014;26(11):1346–51. doi: 10.1080/09540121.2014.913762. [DOI] [PubMed] [Google Scholar]
- 51.Amstislavski P, Matthews A, Sheffield S, Maroko AR, Weedon J Medica Yehia BR, Rebeiro P, Althoff KN, Agwu AL, Horberg MA, Samji H, et al. ; North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD). Impact of age on retention in care and viral suppression. J Acquir Immune Defic Syndr. 2015. April 1;68(4):413–9. DOI: 10.1097/QAI.0000000000000489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zippel, Claire. “DC’s Black Residents Increasingly Live East of the Anacostia River.” DC Financial Policy Institute; September 28, 2016, https://www.dcfpi.org/all/dcs-black-residents-increasingly-live-east-of-the-anacostia-river/. Accessed October 17, 2017. [Google Scholar]
- 53.Washington Metropolitan Area Transit Authority (WMATA). Available at http://opendata.dc.gov/. Accessed December 1, 2016.
- 54.The District Department of Transportation, The District Mobility Project; 2017. (Washington, DC: 2017) Available at https://districtmobility.org/documents/district-mobility-project-summary-report.pdf. Accessed October 17, 2017. [Google Scholar]
- 55.Rowlands, Daniel Walter. “See how long it takes to get from each metro station to the downtown core.” Greater Greater Washington, 30 January 2017, https://ggwash.org/view/62151/see-how-long-it-takes-to-get-from-each-metro-station-to-the-downtown-core. Accessed October 17, 2017. [Google Scholar]
- 56.Dunn, Peter. “Time Scale System Map.” Stonebrown Design. http://www.stonebrowndesign.com/time-scale-metro-map.html. Accessed October 17, 2017. [Google Scholar]
- 57.Itkowitz, Colby. "Poor, sick and still traveling long distances for health care in D.C. The Washington Post; 19 September 2017, https://www.washingtonpost.com/local/dc-politics/poor-sick-and-still-traveling-long-distances-for-health-care-in-dc/2017/09/19/e32201c0-9d3e-11e7-9083-fbfddf6804c2_story.html?utm_term=.051a0b53ce83. Accessed October 18, 2017. [Google Scholar]
- 58.Bliss RL, Katz IN, Wright EA et al. Estimating proximity to care: Are straight line and zipcode centroid distances acceptable proxy measures? Medical Care. 2012. January; 50(1):99–106. doi: 10.1097/MLR.0b013e31822944d1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Krieger N, Chen JT, Waterman PD, Soobader MJ, Subramanian SV, and Carson R. Geocoding and monitoring of US socioeconomic inequalities in mortality and cancer incidence: does the choice of area-based measure and geographic level matter?: the Public Health Disparities Geocoding Project. Am J Epidemiol. 2002. September 1;156(5):471–82. [DOI] [PubMed] [Google Scholar]